Abstract
Background
Motor abnormalities are strong transdiagnostic indicators of psychopathology risk that reflect emerging neural network abnormalities. Indeed, motor signs, such as motor slowing and agitation, are widely recognized as core features of both psychosis and depression. However, it is unclear whether these reflect shared or distinct etiology.
Methods
A sample of 11 878 adolescents completed self-reported clinical measures of rated psychotic-like experiences (PLEs) and depression. Familial risk for psychopathology and the presence of motor signs were drawn from parental reports, including developmental motor delays (eg, sitting, walking), and adolescent motor signs (eg, dyscoordination, psychomotor retardation, and psychomotor agitation). Finally, motor network connectivity in theoretically relevant networks (cortico-striatal, cortico-thalamic, and cortico-cerebellar) were related to symptoms and familial risk for psychopathology.
Results
Developmental motor delays related to increased PLEs, increased depression symptoms, and greater familial risk. Familial risk for both PLEs and depression showed higher rates of developmental motor delays than all other groups. Adolescent motor signs, however, showed unique patterns of relationships to symptoms and familial risk such that dyscoordination reflected risk for PLEs, both psychomotor agitation and retardation reflected depression risk, and psychomotor agitation reflected transdiagnostic risk. Cortico-striatal connectivity was related to depression and PLEs, but cortico-cerebellar connectivity was linked to PLEs only.
Conclusions
Motor signs may be a transdiagnostic marker of vulnerability for psychopathology. Early developmental motor delays could belie pluripotent, familial risk features. Unique items, eg, dyscoordination specifically related to PLEs, possibly reflecting processes inherent in distinct emerging forms of psychopathology.
Keywords: motor development, psychomotor agitation, psychomotor retardation, coordination, psychotic-like experience, depression
Introduction
Motor signs appear in the original descriptions of both psychosis1 and depression,2 and may provide insight into shared and distinct etiological mechanisms of these disorders.3 Conceptual work in risk for psychopathology,4 suggests that a pluripotent period of transdiagnostic symptoms precedes the emergence of specific disorders.5 Over development, these pluripotent symptoms that mark general risk for psychopathology undergo heterotypic trajectories (ie, trajectories from an early sign or prodrome that “branch” into different disorders5,6) resulting in diagnostic-specific symptoms.5 For motor signs, early developmental motor signs (eg, delays in walking) may reflect transdiagnostic risk for psychosis and depression,7–11 then later, adolescent motor signs may reflect the emergence of pathophysiologies that are specific to particular disorders.3 As a result, examining motor signs transdiagnostically (in both depression and psychosis) and over development may provide new insight into the transdiagnostic and unique features of emerging psychopathology.
Motor signs have traditionally been examined separately in psychosis10,12–14 and depression.15–18 Despite these separate literatures, conceptual models of psychosis and depression19–21 indicate that these features may reflect both shared and distinct pathophysiologies. Psychosis and depression may both be related to long-term dysregulation of neurotransmitter systems that impact connectivity in inter-related but parallel circuits, eg, affective, associative, and motor.18–20,22 This neurotransmitter dysregulation is thought to be driven by signaling from cortical structures to the nucleus accumbens which initiates cascading events that modify neurotransmitter availability, sensitivity and connectivity among these parallel functional networks.18–21 This dysregulation has been associated with features of neurotransmitters (eg, availability, metabolism, production), structural features of the circuit (eg, interneuron), and the interplay between these features in the tuning and regulation of neurotransmitters in feedback loops.18–21 In contrast, some propose that early aberrant development of these circuits will persist and later give rise to motor, cognitive, and affective symptoms.19,21 This developmental tuning hypothesis suggests that early developmental motor signs may be useful markers of disturbances in circuits that are centrally involved in the shared pathophysiology of these disorders as an early pluripotent risk factor.
Indeed there is growing evidence that developmental motor signs reflect genetic, early neurological development and familial environment, and/or environmental risk factors.23 Depression studies suggest that specific motor signs are at least partially heritable17 and more prevalent among individuals with first-degree relatives with depression.23 In addition, early developmental motor signs predict later psychopathology7–11 and relate to the distress surrounding psychotic-like experiences (PLEs) in early adolescence.24 As a result, developmental motor signs may signal transdiagnostic, pluripotent risk for depression and psychosis.
In addition to this pluripotent impact of early developmental motor signs, specific adolescent motor signs may provide unique insight into the nature of circuit disruption as heterotypic trajectories emerge and distinct disorders begin to present. Indeed, research suggests that psychomotor agitation, ie, hyperkinetic movements,18 and psychomotor retardation, ie, hypokinetic movements,18,20 reflect distinct underlying pathophysiology. Therefore, particular types of motor signs may provide unique insight into specific pathophysiology. It is also possible that adolescent motor signs track shared vulnerability to psychosis and depression. Motor signs predict onset, severity, and course for both psychosis10,25,26 and depression diagnoses.15,16 Yet transdiagnostic work in this area is lacking.
In functional connectivity literature, particular motor deficits have been linked to specific motor circuits, including cortico-striatal, cortico-cerebellar, and cortico-cortical networks in psychosis risk and psychosis.25,27–32 Depression, in contrast, has been associated only with alterations in striatal function and cortico-cortical circuitry alterations, which have translated into treatment targets for neuromodulation interventions.19,21 However, individuals diagnosed with depression with psychotic features show motor dyscoordination that may indicate some overlap with the cortico-cerebellar network.33 Specific motor signs and connectivity within these networks may indicate particular heterotypic trajectories related to both psychosis and depression in adolescence.
Despite the potential for motor signs to reflect transdiagnostic risk for psychopathology, examining psychosis and depression separately has limited our insight into early pluripotent risk and the emergence of diagnostic specificity. Although a recent study by Damme et al. (2021) examined the relevance of motor signs to depression in Adolescent Brain Cognitive Development (ABCD) study, a publicly available developmental dataset, no study has examined the relevance of motor signs to PLEs or explored the possibility of shared vulnerability between PLEs and depression. The current study examines the relationship between developmental and adolescent motor signs and motor system connectivity to symptoms and shared familial risk for both depression and PLEs. This approach will allow us to compare the relevance of motor signs to symptoms and familial vulnerability for both PLEs and depression.
First, we tested whether the severity of PLEs and depression symptoms was linked to both developmental and adolescent motor signs. We hypothesized that the presence of motor signs would be related to greater PLEs and depression symptoms for developmental motor delays but that the adolescent motor signs may show specificity to PLEs or depression symptoms.
Next, the rates of developmental and adolescent motor signs were compared by familial risk group (the presence of a first-degree relative PLEs, depression, both, or neither depression nor PLEs) to examine a combination of heritable risk and family environment. We hypothesized that motor signs would be more prevalent among individuals with familial vulnerability for either PLEs or depression reflecting transdiagnostic, shared risk. However, given the novelty of analyses, no specific predictions were made. Finally, three critical motor circuits (cortico-striatal, cortico-cerebellar, cortico-cortical (thalamic)) were related to symptoms and familial risk. We expect that PLEs and familial risk would be related to connectivity in all three circuits but that cortico-striatal connectivity would also be related to risk for depression.
Methods and Materials
Participants
The Adolescent Brain Cognitive Development (ABCD) study included 21 sites across the United States who collected 11 878 participants (aged 9–11 years) with a broad demographic diversity range. The ABCD Study aimed to understand factors that may alter healthy development. In the ABCD study, clinical assessments were comprehensive and included various measures, such as psychopathology symptoms and familial risk for psychopathology (ie, first-degree relatives). The present paper takes advantage of this comprehensive approach by examining how each of these clinical characterizations of PLEs and depression might relate to motor symptoms. More details on the ABCD Study and measures are included in the Supplemental Materials. All research protocols were in line with the ethical guidelines laid out by each respective Institutional Review Board (doi:10.15154/1522838). These guidelines included obtaining both the parents’ informed consent and the children’s assent. The sample for the current study only included one individual by family (randomly selected), resulting in 9856 participants.
Clinical Assessments
The Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present and Lifetime Version (K-SADS-PL) is a semistructured child interview designed to assess present and lifetime psychopathology34 in the ABCD study. This was administered as a partental-report digital measure. K-SADS-PL measures affective and psychotic impairments on both diagnosis-specific and global levels and is highly reliable and well-validated.35 For depression symptoms, the sum of depression symptoms was calculated as a total count of symptoms that were endorsed (current or in the past), resulting in a possible score of 0–35 (M = 1.04; SD = 2.86); see Supplemental Materials. PLEs were assessed using the Prodromal Questionnaire—Brief Child version (PQ-BC), a 21-item self-report questionnaire, which asked children about specific PLEs, which were endorsed with a binary response (ie, yes or no). If endorsed, an intensity rating was also provided (where 0 indicated not bothersome). Total scores were calculated as the total number of endorsed symptom where the intensity was greater than 0. As a result, the score could range from 0 to 21 (M = 3.74; SD = 7.63); higher total scores indicate more PLEs endorsed. Finally, the Family History Assessment Module Screener (FHAM-S),36 a parental-report questionnaire, was used to assess the presence and number of first-degree relatives with depression and/or PLEs. Individuals were categorized into one of the following familial risk categories: presence of depression only (n = 1661), presence of PLE only (n = 43), both depression and PLE (n = 102), or neither depression/PLE reported (n = 4030).
Motor Items
Several items within clinical scales that assess past and current motor abnormalities are based on parent report. These items include (a) early motor developmental delays (ABCD Developmental History Questionnaire), (b) lifetime symptoms coordination (Child Behavioral Checklist; CBCL), (c) psychomotor agitation (K-SADS), and (d) psychomotor retardation (K-SADS). Across all scales, the responses were simplified to a binary response as to whether the motor sign was absent or present for each motor sign. Psychomotor agitation and retardation items were selected from both the depression and bipolar disorder sections. Psychomotor agitation and retardation items were considered present if they were endorsed as occurring in their lifetime (ie, as either current or lifetime symptom).
Motor Networks
Motor networks were defined based on neurobiological models of shared pathophysiology19,21,33 and motor deficits in psychopathology.30 Resting-state data were taken from the curated ABCD fMRI data for Gordon Network.37 Grand averages were created across left and right hemispheres, then across the hand motor network and the mouth motor network to reduce the total number of connectivity networks examined, resulting in cortico-striatal,19,30,38 cortico-cerebellar,27,28,39 and cortico-thalamic (cortico-cortical)25,30 networks. The cortico-striatal network included motor network connectivity to caudate, putamen, and nucleus accumbens. The network first averaged within hemisphere across the hand and the mouth motor networks, which then were averaged across hemispheres and finally were averaged across striatal regions in an additional final step.
For details on the acquisition, see Casey et al.[40] and Hagler et al.[41] for preprocessing pipelines. All analyses excluded individuals collected on the improperly harmonized Phillips scanners and accounted for particular scanner machines as a random effect in analyses.42 In addition, maximum framewise displacement was accounted for in all analyses to account for any remaining artifacts related to motion.
Data Analyses
First, we examined the current symptoms level by calculating the total number of PLEs and depression symptoms endorsed on the K-SADS and the symptom total from the Prodromal Questionnaire—Brief Version (PQ-BC; Karcher and Barch, 2019). Next, the familial risk for PLEs and depression symptoms were qualified by whether a parent/guardian endorsed psychosis and/or depression in a first-degree relative during the Family History Assessment Module Screener (FHAM-S). For the current analyses, these were examined both as a categorical risk factor: (1) first-degree relative with PLEs only, (2) first-degree relative with depression only, (3) first-degree relative with both PLEs and depression, or (4) no family history of either PLEs or depression. Finally, these symptoms and familial risk groups were associated with Gordon resting-state functional motor networks to striatal, thalamic, and cerebellar regions in separate mixed-effects models. For the symptoms analyses, the mixed-effects model nested data by site, and predicted connectivity by depression symptoms, PLE symptoms, age, sex, maximum framewise displacement, and stimulant use status. For the familial risk analyses, the mixed-effects model nested data by site, and predicted connectivity by familial risk group, age, sex, maximum framewise displacement, and stimulant use status.
All analyses account for sex and age due to the developmental nature of the sample. Analyses also accounted for current stimulant medication use because of its known effect on motor behavior. All analyses exclude individuals that were currently on antipsychotics43 because of their impact on both motor and psychiatric symptoms (n = 7). Resting-state analyses also account for maximum framewise displacement and scanner as nuisance covariates. Analyses were conducted in R v.4.1.0 and SPSS v.27; original curated data is available via the National Data Archive; syntax available via GitHub.
Results
Participants
Motor signs were reported in Table 1. In terms of familial vulnerability, 0.4% of the sample had a relative with PLEs only, 16.9% had a relative with depression only, and 1.0% had relative(s) with both PLEs and depression. See Supplemental Materials for more information.
Table 1.
Motor Signs Endorsed in Sample
| Motor Symptom | % Endorsed |
|---|---|
| Any Motor Signs | 31.5% |
| 2+ Motor Signs | 9.7 |
| 2 Motor Signs | 7.1 |
| 3 Motor Signs | 2.2 |
| All Motor Signs | 0.4 |
| Developmental Motor Delay | 4.8 |
| Dyscoordination | 12.1 |
| Psychomotor Agitation | 15.3 |
| Psychomotor Retardation | 3.0 |
PLEs and Depression Symptoms
Developmental Motor Milestones Delays
Total reported PLEs were higher in the group with developmental motor delays, t(5777) = 2.30, P = .02, M = 5.04, SEM = 0.38, compared to individuals who did not experience motor delays, M = 4.13, SEM = 0.19. Total depression symptoms were significantly higher in the delayed group t(5771) = 4.32, P < .0001, M = 2.30, SEM = 0.15, compared to individuals with no motor delays, M = 1.62, SEM = 2.39. See table 2 for all symptom means by motor signs.
Table 2.
Psychotic-like Experiences and Depression Symptoms by Motor Signs Endorsed
| Motor Symptom | PLEs Symptoms | MDD Symptoms | ||
|---|---|---|---|---|
| Mean (SEM) | Endorsed | Not Endorsed | Endorsed | Not Endorsed |
| Developmental Motor Delay | 5.04 (0.38) | 4.13 (0.19) | 2.30 (0.15) | 1.62 (2.39) |
| Dyscoordination | 5.58 (0.31) | 4.08 (0.20) | 3.16 (0.12) | 1.36 (0.08) |
| Psychomotor Agitation | 5.80 (0.48) | 4.14 (0.40) | 7.79 (0.20) | 1.74 (0.17) |
| Psychomotor Retardation | 5.70 (0.94) | 4.47 (0.21) | 11.59 (0.38) | 3.70 (0.15) |
SEM – Standard Error of the Mean; PLEs – Psychotic-Like Experiences (total from the Prodromal Questionnaire—Brief Version); MDD – Total Current and Lifetime Depression symptoms endorsed on the Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS-PL).
Dyscoordination
Total reported PLEs were higher in individuals who were categorized as dyscoordinated, t(5854) = 5.25, P < .001, M = 5.58, SEM = 0.31, compared to individuals who were not, M = 4.08, SEM = 0.20. Depression symptoms were higher in individuals who were categorized as dyscoordinated, t(5848) = 15.76, P < .001, M = 3.16, SEM = 0.12, compared to individuals who were not, M = 1.36, SEM = 0.08.
Psychomotor Agitation
Total reported PLEs were elevated in individuals who were categorized as having lifetime psychomotor agitation, t(1565) = 3.63, P = .0003, M = 5.80, SEM = 0.48, compared to individuals who did not report psychomotor agitation, M = 4.14, SEM = 0.40. Total depression symptoms were elevated in individuals who were categorized as having current or lifetime psychomotor agitation, t(1565) = 4.69, P < .001, M = 7.79, SEM = 0.20, compared to those who were not, M = 1.74, SEM = 0.17.
Psychomotor Retardation
Total PLEs did not relate to increased reporting of psychomotor retardation, P = .16. Total depression symptoms were higher in individuals who were categorized as having lifetime psychomotor retardation, t(1564) = 28.94, P < .001, M = 11.59, SEM = 0.38, compared to those without psychomotor retardation, M = 3.70, SEM = 0.15.
Motor Abnormalities and Symptoms
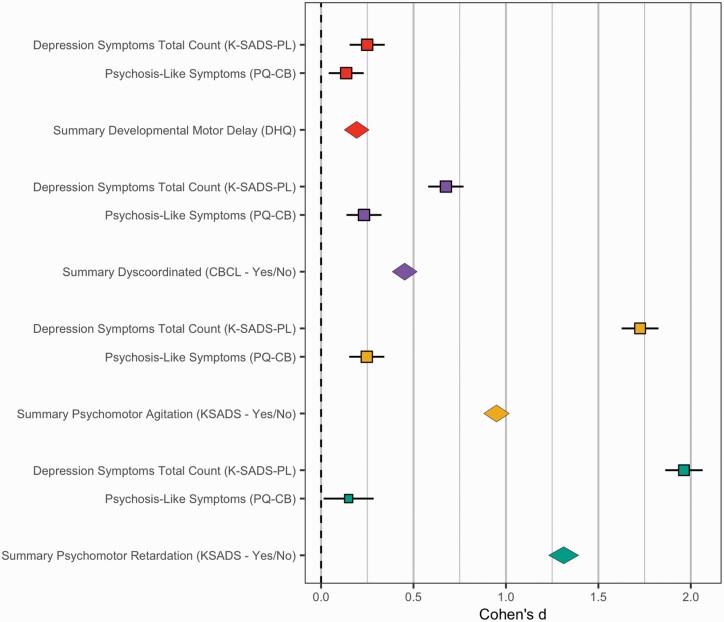
In a general linear model with all motor signs predicting total PLEs and depression symptoms in separate models, accounting for sex, age, and stimulant use, total depression symptoms related to developmental motor delay, t(1536) = 2.53, P = .012, dyscoordination, t(1536) = 6.14, P < .00001, psychomotor retardation, t(1536) = 14.93, P < .00001, and psychomotor agitation, t(1536) = 24.16, P < .00001. Total PLEs related only to psychomotor agitation, t(1537) = 2.76, P = .006. See figure 1 for comparisons of effect sizes that are not corrected for covariance or model covariance.
Fig. 1.
Motor signs by lifetime depression and psychotic-like symptoms. Developmental Motor Delays—Red, Dyscoordination Symptoms—Purple, Psychomotor Retardation—Yellow, Psychomotor Agitation—Green. In this figure, the Cohen’s d reflects the difference between individuals who endorsed a motor sign compared to those who did not. As a result, the 0 point reflects the prevalence of motor signs from individuals that did not endorse a motor sign. If the error bars overlap with the 0 point, this indicates that the group did not differ compared to the no motor sign group. Values to the right of the 0 point indicate elevated symptom levels or rates compared to the normative/control sample. Values to the left indicate reduced symptom levels or rates compared to this normative group. The standard deviation of Cohen’s d was estimated in an open source algorithm, freely available in R Michaela package using validated formulas from meta-analytic literature.44 Data was visualized with the metaviz package.45
Familial Risk Group
Developmental Motor Milestone Delays
There was a significant impact of the family risk group (first-degree relatives) on the endorsement of motor delays, χ2(3) = 13.29, P = .004. Sample distributions, odds ratios, and relative risk are in table 3.
Table 3.
Motor Signs Endorsement Rates by Familial Risk Group
| Familial Risk Group | |||||
|---|---|---|---|---|---|
| Percentage Motor Sign Endorsed | Neither | PLE | MDD | Both | |
| Developmental Motor Delay | 7.30% | 11.60% | 9.68% | 14.85% | |
| Dyscoordination | 9.74% | 25.58% | 1.86% | 25.49% | |
| Psychomotor Agitation | 17.98% | 12.50% | 40.15% | 43.18% | |
| Psychomotor Retardation | 4.03% | 8.98% | 16.67% | 13.79% | |
| Motor Sign Endorsed Risk (OR/RR)* | Neither | PLE | MDD | Both | |
| Developmental Motor Delay | — | 1.66/1.58 | 1.35/1.32 | 2.20/2.02 | |
| Dyscoordination | — | 3.19/2.63 | 0.18/0.19 | 3.17/2.62 | |
| Psychomotor Agitation | — | 0.65/0.75 | 3.06/2.32 | 3.47/2.40 | |
| Psychomotor Retardation | — | 2.35/2.23 | 4.76/4.13 | 3.19/2.63 | |
*Odds Ratio (OR) and Relative Risk (RR) were calculated in reference to the group with Neither familial risk for depression (MDD) nor psychotic-like experiences (PLE); Familial risk groups based in ABCD Family History Assessment: PLE – Endorsed first-degree relative with psychotic-like experiences; MDD – Endorsed first-degree relative with depression; Both – Endorsed first-degree relative with depression and psychotic-like experiences; neither – Neither MDD nor PLE history endorsed.
Dyscoordination
There was a significant impact of the family risk group on dyscoordination, χ2(3) = 83.88, P < .001.
Psychomotor Agitation
There was a significant difference in endorsing lifetime psychomotor agitation based on a familial risk group illness, χ2(3) = 92.50, P < .001.
Psychomotor Retardation
There was a significant difference in endorsing current psychomotor retardation based on family history of psychiatric illness, χ2(3) = 32.07, P < .001.
Motor Abnormalities and Familial Risk Group
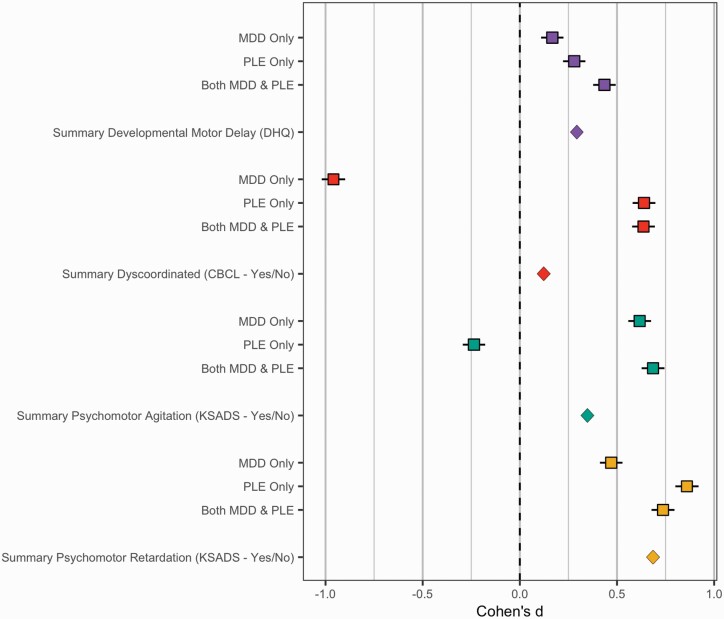
In a logistic regression model with all motor signs predicting familial risk for PLEs and depression, accounting for sex, age, and stimulant use status. Among all motor signs, dyscoordination, χ2(3) = 15.45, P = .001, and psychomotor agitation, χ2(3) = 52.18, P < .001, but no other motor signs were uniquely related to familial risk group when accounting for other motor signs in the same model (Ps > .16). Individuals with familial risk of both PLEs and depression experienced more dyscoordination (B = 0.72, SEM = 0.36, P = .05) and psychomotor agitation (B = 0.84, SEM = 0.35, P = .02) than the group with no reported risk. Familial risk for PLEs only group was associated with more dyscoordination (B = 1.42, SEM = 0.58, P = .014) but did not differ from the group with no reported risk in psychomotor agitation (B = .92, SEM = 0.84, P = .27) than the group with no reported risk. Familial risk for depression only was associated with more dyscoordination (B = 0.049, SEM = 0.15, P = .001) and psychomotor agitation (B = .92, SEM = 0.13, P < .001) than the group with no reported risk. See figure 2 for comparisons of effect sizes that are not corrected for covariance or model covariance.
Fig. 2.
Motor signs by familial risk group. Developmental Motor Delays—Red, Dyscoordination Symptoms—Purple, Psychomotor Retardation—Yellow, Psychomotor Agitation—Green. In this figure, the Cohen’s d reflects the difference between individuals who have psychiatric risk (either MDD only, PLE only, or both MDD & PLE) compared to individuals that had no familial risk. As a result, the 0 point reflects the prevalence of motor signs among individuals that had no familial risk. If the error bars overlap with the 0 point, then this would indicate that the group did not differ compared to this normative group. Values to the right of the 0 point indicate elevated symptom levels or rates compared to the normative/control sample. Values to the left of the zero point indicate reduced symptom levels or rates compared to this normative group. Effect Sizes above were transformed from Odds Ratios into Cohen’s D (see Results section) and standard error using the Michaela package in R. Error bars reflect the standard error using validated formulas from meta-analytic literature,44 and was visualized with the metaviz package.44
Motor Network Connectivity
Depression and Psychosis Symptoms
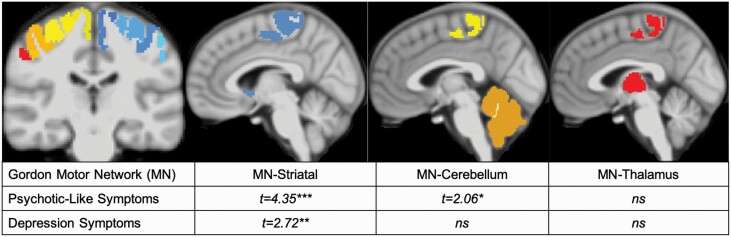
Cortico-striatal connectivity was related to both PLEs, t(3931) = 4.35, P < .001, and depression, t(3931) = 2.72, P = .006. Cortico-cerebellar connectivity did relate to PLEs, t(3932) = 2.064, P = .04, but did not related to depression, t(3932) = 0.45, P = .65. Cortico-thalamic connectivity was not related to PLEs (P = .37) or depression (P = .43), figure 3.
Fig. 3.
Motor network to symptoms. MN – Motor Network; PQ-CB – Prodromal Questionnaire Childhood Brief Version; KSADS – Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present and Lifetime Version; ***P < .001, **P = .006, *P < .05.
Family History of Psychopathology
Familial risk groups did not relate to functional connectivity (Ps > .12).
Discussion
Motor signs may be a transdiagnostic marker of vulnerability for both PLEs and depression. Delays in developmental motor milestones reflected a transdiagnostic, pluripotent marker of vulnerability for both disorders, and familial risk showed elevated likelihood of developmental motor delays over the other risk groups. In adolescent motor signs, heterotypic, specific patterns emerge distinguishing between disorders. Although motor signs were also related to elevated symptoms or familial risk groups, the adolescent motor behaviors showed distinct relationships to psychopathology across symptoms and familial risk. These differences may reflect different contributions to the development of motor signs, distinguishing between emerging specific symptoms and a combination of early genetic, familial environment, and their interactions (familial risk group). In network analyses, cortico-striatal dysconnectivity related to both PLEs and depression symptoms but not familial risk. Cortico-cerebellar connectivity was related to PLEs but not depression symptoms, consistent with the behavioral endorsement of related dyscoordination behaviors being specific to higher PLEs. Across analyses, motor signs build a rich account of shared and distinct vulnerability for psychopathology.
Prior to analyses, we hypothesized that the presence of motor signs would be related to greater symptoms. The findings largely supported this hypothesis with the exception that psychomotor retardation did not relate to PLEs. However, a richer, nuanced pattern of findings emerged that was consistent with the pluripotent model of risk; early motor signs (ie, developmental motor delays) related to elevated depressive and PLE symptoms and familial risk of these disorders. In addition, individuals with familial risk for both depression and PLEs showed an increased rates of developmental motor delays compared to both the healthy controls and the other risk groups. This significant increase in the shared familial risk group may indicate distinct contributions to the pathophysiology of developmental motor delays despite shared vulnerability. This possibility is supported by extant research stating that early developmental motor delays reflect many heterogeneous sources.23 And so, this shared vulnerability may, in fact, be driven by a number of distinct, potential mechanisms.46 This finding provides further evidence of the importance of early development to later risk for psychopathology.
Adolescent motor signs also showed distinct patterns across symptom dimensions. PLEs were only higher among individuals who experienced dyscoordination and psychomotor agitation. In contrast, depression symptoms were elevated among individuals who experienced dyscoordination, psychomotor retardation, and psychomotor agitation. This shared vulnerability for psychomotor agitation may indicate shared pathophysiology in depression and PLEs.19
Although motor signs were expected to be more prevalent in all familial risk groups, different familial risks related to different adolescent motor signs. Familial risk for PLEs were specific to increased dyscoordination and depression familial risk was specific to increased psychomotor agitation. These findings are at odds with a large literature suggesting that motor slowing is observed in psychosis and psychosis risk12,47–50 as well as depression51–53 measured by tasks and instrumentation. These findings, however, are consistent with a growing literature that has described dyscoordination as relating to psychosis symptoms and risk,54–57 but there is also some evidence that dyscoordination is related to depression.15,58,59 It should be noted, however, that the current sample is younger and clinically healthier than the samples of the cited studies and reflects tendencies toward PLEs, which may show unique patterns from psychosis. As a result, these findings may reflect how these patterns are altered at lower risk levels. It is also noteworthy that developmental motor delays showed a lower incidence rate in depression risk than the community sample, and psychomotor agitation showed a lower incidence rate in PLE risk than the community sample. This effect may reflect the heterogeneity in the community sample and the potential for motor signs to be relevant to unmeasured risk features (eg, anxiety, mania).
In motor network analyses, cortico-striatal connectivity showed shared relevance to depression and PLEs, consistent with a model of pluripotent, transdiagnostic symptoms. This cortico-striatal dysconnectivity is consistent with major neurobiological models of both depression and psychosis, which may suggest decreased striatal dopamine productivity,18–21 tuning of excitability to dopamine,19,21 or interneuron dysconnectivity19 within this cortico-striatal network. Cortico-cerebellar connectivity was related to PLEs alone. This finding is consistent with a growing body of work that emphasizes the role of cerebellar connectivity in the development and features of psychosis.27,28,32,39 The familial risk did not relate to altered functional connectivity, which may indicate that connectivity may reflect temporally proximal, tuning processes rather than temporally distal, early contributions of genetic and environmental variability on neurodevelopment.
It is important to note several limitations in this study. First, PLEs assessed by the PQ-BC and in the family history questionnaire may reflect a more common cognitive profile of nonclinical psychosis60 rather than true risk for psychosis.24,61 For this reason, the relevance of motor signs to true psychosis risk may be underestimated, and future research is needed to examine first-degree relatives of individuals with psychosis and individuals at clinical high-risk for psychosis.60,61 Next, these analyses may overestimate the sensitivity of psychomotor agitation and psychomotor retardation as any current psychomotor agitation and psychomotor slowing are included in the sum of depression symptoms. However, the presence of psychomotor motor signs was independent of the familial risk measure, which also indicated elevated rates of motor signs in depression. Additionally, motor signs measures were largely based on parental-report items, which may have limited sensitivity to motor signs relative to controlled laboratory assessments and may reflect only a few motor behaviors. Future research should take advantage of the many motor behavioral measures that are easily accessible and readily available, eg, force variability, velocity scaling,48,62 and examine broader array of motor signs, eg catatonia. Existing literature demonstrates that behavioral measures are more sensitive to motor symptoms than observation or self-report measures alone.63,64 Motor network connectivity analyses may somewhat mitigate this concern as the motor assessment is independent of parental observation. The motor network connectivity metrics were limited to predefined regions and processing pipelines of the curated ABCD dataset. Although this approach increases the transparency and replicability of the findings; the connectivity reflect a broader motor network than the cortico-striatal, cortico-cerebellar, and cortico-cortical, described in the psychosis30,65 and trandiagnostic models of motor signs.66 Future imaging studies should examine a more circumscribed network during both motor tasks and resting state for potential added specificity.67 In Addition, future studies should examine the impact of sequence features and global signal reduction on these circuits.68 In addition, future studies should examine the impact of sequence features and global signal reduction on these circuits.68 Next, it is also notable that the effect sizes are small; however, the current effect sizes (Odds Ratios: 1.35–3.47) are within a similar range to other risk markers of psychopathology (eg, familial depression, depression risk genes; Odds Ratios: 1.15–1.99).15,69–72 Finally, depression and PLEs are heterogeneous and reflect a number of complex profiles. Future studies should consider examining specific symptom clusters rather than aggregating over this heterogeneous group.
In conclusion, motor signs provided transdiagnostic relevance to depression and PLEs. Developmental motor delays provide insights into pluripotent, transdiagnostic risk, and adolescent development signs provided insight into emerging specificity. The relative degree to which particular motor signs related to depression and PLEs provided an informative pattern of findings, suggesting particular relevance of certain items to vulnerability. Motor network differences also related to emerging PLEs and depression symptoms but did not reflect familial vulnerability, suggesting that neural networks may reflect active processes relevant to psychopathology rather than a stable risk state. This study also demonstrated the utility of exploring pluripotent symptom features across development to increase the sensitivity of shared and distinct measures of mechanisms underlying psychopathology.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Mental Health (VAM Grant R01MH094650, R01MH112545–01, R01MH103231, R01MH112545, R01MH094650, R01MH118741, R21/R33MH103231; SW Grant Swiss National Science Foundation grants 182469, 184717; T32MH126368 to KSFD; R01MH119771 to SS). Data used in the preparation of this work were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9–10 and follow them over ten years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, U24DA041147, U01DA041093, and U01DA041025. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/wp-content/uploads/2019/04/Consortium_Members.pdf. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. DOIs can be found at https://nda.nih.gov/generalquery.html?q=query=studies%20%7Eand%7E%20orderBy=id%20%7Eand%7E%20orderDirection=Ascending.
Contributor Information
Katherine S F Damme, Department of Psychology, Northwestern University, Evanston, IL, USA; Institute for Innovations in Developmental Sciences (DevSci), Northwestern University, Evanston/Chicago, IL, USA.
Jadyn S Park, Department of Psychology, Northwestern University, Evanston, IL, USA; Department of Psychiatry, Northwestern University, Chicago, IL, USA.
Sebastian Walther, University Hospital of Psychiatry, Translational Research Center, University of Bern, Bern, Switzerland.
Teresa Vargas, Institute for Innovations in Developmental Sciences (DevSci), Northwestern University, Evanston/Chicago, IL, USA; Department of Psychiatry, Northwestern University, Chicago, IL, USA.
Stewart A Shankman, Department of Psychiatry, Northwestern University, Chicago, IL, USA.
Vijay A Mittal, Department of Psychology, Northwestern University, Evanston, IL, USA; Institute for Innovations in Developmental Sciences (DevSci), Northwestern University, Evanston/Chicago, IL, USA; Department of Psychiatry, Northwestern University, Chicago, IL, USA; Medical Social Sciences, Northwestern University, Chicago, IL, USA; Institute for Policy Research (IPR), Northwestern University, Chicago, IL, USA.
References
- 1. Kraepelin E. Dementia praecox and paraphrenia. Huntington, New York: Robert E. Krieger Publishing Co. Inc.; 1919; 1971. [Google Scholar]
- 2. Hippocrates. Corpus Hippocraticum. 1525 BCE. Venice Italy: Adine Press; 1525. [Google Scholar]
- 3. Damme TV, Simons J, Sabbe B, van West D. Motor abilities of children and adolescents with a psychiatric condition: A systematic literature review. World J Psychiatry. 2015;5(3):315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mittal VA, Wakschlag LS. Research domain criteria (RDoC) grows up: Strengthening neurodevelopment investigation within the RDoC framework. J Affect Disord. 2017;216:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hartmann JA, McGorry PD, Destree L, et al. Pluripotential Risk and clinical staging: theoretical considerations and preliminary data from a transdiagnostic risk identification approach. Front Psychiatry. 2021;11:1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry. 1999;40(1):57–87. [PubMed] [Google Scholar]
- 7. Filatova S, Koivumaa-Honkanen H, Hirvonen N, et al. Early motor developmental milestones and schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2017;188:13–20. [DOI] [PubMed] [Google Scholar]
- 8. Fish B. The detection of schizophrenia in infancy. J Nerv Mental Dis. 1957;125:1–24. [DOI] [PubMed] [Google Scholar]
- 9. Fish B. Neurobiologic antecedents of schizophrenia in children. Evidence for an inherited, congenital neurointegrative defect. Arch Gen Psychiatry. 1977;34(11):1297–1313. [DOI] [PubMed] [Google Scholar]
- 10. Mittal VA, Walker EF. Movement abnormalities predict conversion to Axis I psychosis among prodromal adolescents. J Abnorm Psychol. 2007;116(4):796–803. [DOI] [PubMed] [Google Scholar]
- 11. Vameghi R, Amir Ali Akbari S, Sajjadi H, Sajedi F, Alavimajd H. Correlation between mothers’ depression and developmental delay in infants aged 6–18 months. Glob J Health Sci. 2015;8(5):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bachman P, Niendam TA, Jalbrzikowski M, et al. Processing speed and neurodevelopment in adolescent-onset psychosis: cognitive slowing predicts social function. J Abnorm Child Psychol. 2012;40(4):645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kindler J, Michel C, Schultze-Lutter F, et al. Functional and structural correlates of abnormal involuntary movements in psychosis risk and first episode psychosis. Schizophr Res. 2019;212:196–203. [DOI] [PubMed] [Google Scholar]
- 14. Peralta V, Cuesta MJ. Neuromotor abnormalities in neuroleptic-naive psychotic patients: antecedents, clinical correlates, and prediction of treatment response. Compr Psychiatry. 2011;52(2):139–145. [DOI] [PubMed] [Google Scholar]
- 15. Damme KSF, Park JS, Vargas T, Walther S, Shankman SA, Mittal VA. Motor abnormalities, depression risk, and clinical course in adolescence. Biol Psychiatry: Global Open Sci. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Finazzi ME, Mesquita ME, Lopes JR, Fu IL, Oliveira MG, Del Porto JA. Motor activity and depression severity in Adolescent outpatients. 2010. https://www.karger.com/Article/PDF/262178?casa_token=iUXSBhNbdK8AAAAA:qMRKvBYo1e84mv5v7DHl0nd4qMWxYJyKlIuO5VU9EttCOJ6RWzm3mj_uR8AzelMKZMsOsQRT-Q. Accessed May 6, 2020. [DOI] [PubMed]
- 17. Leventhal AM, Pettit JW, Lewinsohn PM. Characterizing major depression phenotypes by presence and type of psychomotor disturbance in adolescents and young adults. Depress Anxiety. 2008;25(7):575–592. [DOI] [PubMed] [Google Scholar]
- 18. Sobin C, Sackeim HA. Psychomotor symptoms of depression. Am J Psychiatry. 1997;154(1):4–17. [DOI] [PubMed] [Google Scholar]
- 19. Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17(8):524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Obeso JA, Rodriguez-Oroz MC, Stamelou M, Bhatia KP, Burn DJ. The expanding universe of disorders of the basal ganglia. Lancet. 2014;384(9942):523–531. [DOI] [PubMed] [Google Scholar]
- 21. Robison AJ, Thakkar KN, Diwadkar VA. Cognition and reward circuits in Schizophrenia: synergistic, not separate. Biol Psychiatry. 2020;87(3):204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schrijvers D, Hulstijn W, Sabbe BG. Psychomotor symptoms in depression: a diagnostic, pathophysiological and therapeutic tool. J Affect Disord. 2008;109(1-2):1–20. [DOI] [PubMed] [Google Scholar]
- 23. Beauchaine TP, Constantino JN. Redefining the endophenotype concept to accommodate transdiagnostic vulnerabilities and etiological complexity. Biomark Med. 2017;11(9):769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karcher NR, Barch DM, Avenevoli S, et al. Assessment of the prodromal questionnaire-brief child version for measurement of self-reported psychoticlike experiences in Childhood. JAMA Psychiatry. 2018;75(8):853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dean DJ, Walther S, Bernard JA, Mittal VA. Motor clusters reveal differences in risk for psychosis, cognitive functioning, and thalamocortical connectivity: evidence for vulnerability subtypes. Clin Psychol Sci. 2018;6(5):721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferruccio NP, Tosato S, Lappin JM, et al. Neurological Signs at the first psychotic episode as correlates of long-term outcome: results from the AESOP-10 study. Schizophr Bull. 2021;47(1):118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bernard JA, Orr JM, Mittal VA. Cerebello-thalamo-cortical networks predict positive symptom progression in individuals at ultra-high risk for psychosis. Neuroimage Clin. 2017;14:622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bernard JA, Mittal VA. Cerebellar-motor dysfunction in schizophrenia and psychosis-risk: the importance of regional cerebellar analysis approaches. Front Psychiatry. 2014;5:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martinot M, Bragulat V, Artiges E, et al. Decreased presynaptic dopamine function in the left caudate of depressed patients with affective flattening and psychomotor retardation. Am J Psychiatry. 2001;158(2):314–316. [DOI] [PubMed] [Google Scholar]
- 30. Mittal VA, Bernard JA, Northoff G. What can different motor circuits tell us about Psychosis? An RDoC perspective. Schizophr Bull. 2017;43(5):949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Viher PV, Docx L, Van Hecke W, et al. Aberrant fronto-striatal connectivity and fine motor function in schizophrenia. Psychiatry Res Neuroimaging. 2019;288:44–50. [DOI] [PubMed] [Google Scholar]
- 32. Walther S, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Viher PV. Aberrant Hyperconnectivity in the motor system at rest is linked to motor abnormalities in Schizophrenia Spectrum Disorders. Schizophr Bull. 2017;43(5):982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Owoeye O, Kingston T, Scully PJ, et al. Epidemiological and clinical characterization following a first psychotic episode in major depressive disorder: comparisons with schizophrenia and bipolar I disorder in the Cavan-Monaghan First Episode Psychosis Study (CAMFEPS). Schizophr Bull. 2013;39(4):756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-SADS-PL. J Am Acad Child Adolesc Psychiatry. 2000;39(10):1208–1208. [DOI] [PubMed] [Google Scholar]
- 35. Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. [DOI] [PubMed] [Google Scholar]
- 36. Rice JP, Reich T, Bucholz KK, et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19(4):1018–1023. [DOI] [PubMed] [Google Scholar]
- 37. Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb Cortex. 2016;26(1):288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fusar-Poli P, Howes OD, Allen P, et al. Abnormal frontostriatal interactions in people with prodromal signs of psychosis: a multimodal imaging study. Arch Gen Psychiatry. 2010;67(7):683–691. [DOI] [PubMed] [Google Scholar]
- 39. Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24(2):203–218. [DOI] [PubMed] [Google Scholar]
- 40. Casey BJ, Cannonier T, Conley MI, et al. ; ABCD Imaging Acquisition Workgroup . The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018;32:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hagler DJ Jr, Hatton S, Cornejo MD, et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage. 2019;202:116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nielson DM, Pereira F, Zheng CY, et al. Detecting and harmonizing scanner differences in the ABCD study-annual release 1.0. BioRxiv. 2018:309260.
- 43. Peluso MJ, Lewis SW, Barnes TR, Jones PB. Extrapyramidal motor side-effects of first- and second-generation antipsychotic drugs. Br J Psychiatry. 2012;200(5):387–392. [DOI] [PubMed] [Google Scholar]
- 44. Schild AH, Voracek M. Finding your way out of the forest without a trail of bread crumbs: development and evaluation of two novel displays of forest plots. Res Synth Methods. 2015;6(1):74–86. [DOI] [PubMed] [Google Scholar]
- 45. Lam PH, Chiang JJ. Michaela—Open r Package for Converting Effect Sizes. https://github.com/phoebehlam/michaela. Accessed November 12, 2021.
- 46. Hadders-Algra M. The neuronal group selection theory: promising principles for understanding and treating developmental motor disorders. Dev Med Child Neurol. 2000;42(10):707–715. [DOI] [PubMed] [Google Scholar]
- 47. Bervoets C, Docx L, Sabbe B, et al. The nature of the relationship of psychomotor slowing with negative symptomatology in schizophrenia. Cogn Neuropsychiatry. 2014;19(1):36–46. [DOI] [PubMed] [Google Scholar]
- 48. Damme KSF, Osborne KJ, Gold JM, Mittal VA. Detecting motor slowing in clinical high risk for psychosis in a computerized finger tapping model. Eur Arch Psychiatry Clin Neurosci. 2020;270(3):393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Walther S, Federspiel A, Horn H, et al. Alterations of white matter integrity related to motor activity in schizophrenia. Neurobiol Dis. 2011;42(3):276–283. [DOI] [PubMed] [Google Scholar]
- 50. Walther S, Hügli S, Höfle O, et al. Frontal white matter integrity is related to psychomotor retardation in major depression. Neurobiol Dis. 2012;47(1):13–19. [DOI] [PubMed] [Google Scholar]
- 51. Caligiuri MP, Ellwanger J. Motor and cognitive aspects of motor retardation in depression. J Affect Disord. 2000;57(1-3):83–93. [DOI] [PubMed] [Google Scholar]
- 52. Hart RP, Kwentus JA. Psychomotor slowing and subcortical-type dysfunction in depression. J Neurol Neurosurg Psychiatry. 1987;50(10):1263–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sabbe B, Hulstijn W, van Hoof J, Tuynman-Qua HG, Zitman F. Retardation in depression: assessment by means of simple motor tasks. J Affect Disord. 1999;55(1):39–44. [DOI] [PubMed] [Google Scholar]
- 54. Dean DJ, Kent JS, Bernard JA, et al. Increased postural sway predicts negative symptom progression in youth at ultrahigh risk for psychosis. Schizophr Res. 2015;162(1-3):86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dickson H, Roberts RE, To M, Wild K, Loh M, Laurens KR. Adolescent trajectories of fine motor and coordination skills and risk for schizophrenia. Schizophr Res. 2020;215:263–269. [DOI] [PubMed] [Google Scholar]
- 56. Kent JS, Hong SL, Bolbecker AR, et al. Motor deficits in schizophrenia quantified by nonlinear analysis of postural sway. PLoS One. 2012;7(8):e41808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Osborne KJ, Bernard JA, Gupta T, et al. Beat gestures and postural control in youth at ultrahigh risk for psychosis. Schizophr Res. 2017;185:197–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Draghi TTG, Cavalcante Neto JL, Tudella E. Symptoms of anxiety and depression in schoolchildren with and without developmental coordination disorder. J Health Psychol. 2021;26(10):1519–1527. doi: 10.1177/1359105319878253 [DOI] [PubMed] [Google Scholar]
- 59. Hill EL, Brown D. Mood impairments in adults previously diagnosed with developmental coordination disorder. J Ment Health. 2013;22(4):334–340. [DOI] [PubMed] [Google Scholar]
- 60. Kelleher I, Cannon M. Psychotic-like experiences in the general population: characterizing a high-risk group for psychosis. Psychol Med. 2011;41(1):1–6. [DOI] [PubMed] [Google Scholar]
- 61. Verdoux H, van Os J. Psychotic symptoms in non-clinical populations and the continuum of psychosis. Schizophr Res. 2002;54(1-2):59–65. [DOI] [PubMed] [Google Scholar]
- 62. Shankman SA, Mittal VA, Walther S. An Examination of psychomotor disturbance in current and remitted MDD: an RDoC Study. J Psychiatr Brain Sci. 2020;5:e200007. doi: 10.20900/jpbs.20200007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cortese L, Caligiuri MP, Malla AK, Manchanda R, Takhar J, Haricharan R. Relationship of neuromotor disturbances to psychosis symptoms in first-episode neuroleptic-naive schizophrenia patients. Schizophr Res. 2005;75(1):65–75. [DOI] [PubMed] [Google Scholar]
- 64. van Harten PN, Walther S, Kent JS, Sponheim SR, Mittal VA. The clinical and prognostic value of motor abnormalities in psychosis, and the importance of instrumental assessment. Neurosci Biobehav Rev. 2017;80:476–487. [DOI] [PubMed] [Google Scholar]
- 65. Northoff G, Hirjak D, Wolf RC, Magioncalda P, Martino M. All roads lead to the motor cortex: psychomotor mechanisms and their biochemical modulation in psychiatric disorders. Mol Psychiatry. 2021;26(1):92–102. [DOI] [PubMed] [Google Scholar]
- 66. Magioncalda P, Martino M, Conio B, et al. Intrinsic brain activity of subcortical-cortical sensorimotor system and psychomotor alterations in schizophrenia and bipolar disorder: A preliminary study. Schizophr Res. 2020;218:157–165. [DOI] [PubMed] [Google Scholar]
- 67. Damme KSF, Pelletier-Baldelli A, Cowan HR, Orr JM, Mittal VA. Distinct and opposite profiles of connectivity during self-reference task and rest in youth at clinical high risk for psychosis. Hum Brain Mapp. 2019;40(11):3254–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Scalabrini A, Vai B, Poletti S, et al. All roads lead to the default-mode network—global source of DMN abnormalities in major depressive disorder. Neuropsychopharmacology. 2020;45(12):2058–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Funder DC, Ozer DJ. Evaluating effect size in psychological research: sense and nonsense. Adv Methods Pract Psychol Sci. 2019;2(2):156–168. [Google Scholar]
- 70. Flint J, Kendler KS. The genetics of major depression. Neuron. 2014;81(3):484–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stringaris A, Cohen P, Pine DS, Leibenluft E. Adult outcomes of youth irritability: a 20-year prospective community-based study. Am J Psychiatry. 2009;166(9):1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pagliaccio D, Alqueza KL, Marsh R, Auerbach RP. Brain Volume abnormalities in Youth at high risk for depression: adolescent Brain and cognitive development study. J Am Acad Child Adolesc Psychiatry. 2019;59(10):1178–1188. doi: 10.1016/j.jaac.2019.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.