Abstract
Background and hypothesis
Following the first episode of psychosis, some patients develop poor social and occupational outcomes, while others display a pattern of preserved functioning. Evidence from preclinical, genetic, and biochemical studies suggest a role for high oxidative stress in poor functional outcomes among patients. The measurement of intracortical glutathione (GSH) using magnetic resonance spectroscopy (MRS) enables investigating the relationship between central antioxidant tone and functional outcomes at the time of first-episode psychosis (FEP). We hypothesized that patients with higher central antioxidant tone at first presentation will have better functional outcomes in early stages of illness.
Study design
We scanned 57 patients with FEP and 30 matched healthy controls and estimated GSH resonance using 7-Tesla MRS. We minimized the confounding effects of illness chronicity, long-term treatment exposure, and metabolic complications by recruiting patients with <2 weeks of lifetime antipsychotic exposure on average and followed up this cohort for the next 1 year to determine functional outcomes.
Study results
Patients who achieved employment/education or training status (EET) in the first year, had higher GSH at the baseline than healthy controls. Social and occupational functioning assessment scale (SOFAS) scores were also significantly higher in patients with higher GSH levels at the outset, after adjusting for various confounds including baseline SOFAS. Patients who were not in EET did not differ from healthy subjects in their GSH levels.
Conclusion
Our observations support a key role for the central antioxidant tone in the functional outcomes of early psychosis.
Keywords: antioxidant, employment, schizophrenia, functioning, anterior cingulate
Introduction
For patients with schizophrenia, the probability of functional recovery is highest at the early stages of the illness, around the time of the first psychotic episode; 1–3 when a chronic pattern of the illness gets established, the chances of functional recovery greatly diminish, with only a small subgroup (~13%) recovering at this stage.4,5 Currently, we do not know what mechanistic processes underlie these diminishing returns in recovery rates over time. Several clinical characteristics (e.g., the presence of negative, disorganized symptoms, cognitive deficits6) have been observed in association with poor functional outcomes; in particular, the degree of functioning at first presentation (baseline or premorbid) explains a significant amount of variance in long-term functional outcomes.7–9 Despite their explanatory power, these clinical associations do not offer an actionable mechanistic marker that can be harnessed for therapeutic purposes. There is an urgent need to understand the neurobiological factors that contribute to differences in functional outcomes in early stages of illness.
One promising approach to study variable outcomes in psychosis is quantifying the relative burden of oxidative stress experienced by patients during the first psychotic episode.10 Fournier and colleagues utilized a data-driven stratification procedure on a cohort of patients with early psychosis and identified a subgroup with superior functional outcomes; this subgroup was characterized by lower levels of oxidative stress markers (especially glutathione peroxidase) in the blood.11 Lower baseline blood levels of glutathione (GSH), a major antioxidant, predict later cognitive deficits12 as well as brain volume loss in early psychosis.13 While peripheral markers of oxidative stress correlate with concentration of the primary intracortical antioxidant glutathione,14 a direct link between central glutathione levels and functional outcome in first-episode psychosis (FEP) is yet to be demonstrated. Wood et al.15 reported a 22% increase in medial temporal GSH levels in first-episode psychosis; in a sub-sample from this study, treatment-related increase in GSH was associated with a gain in global functioning scores.16 In a small group of individuals with various mental health difficulties indicating a high-risk state for psychosis, we recently demonstrated higher GSH levels measured using magnetic resonance spectroscopy (MRS) from the dorsal anterior cingulate cortex (ACC) in those with better social and occupational functioning.17 Given the prior reports relating higher ACC GSH levels in high-risk state with baseline functioning,17 and in early stages of psychosis with later treatment response18 and resistance,19 we hypothesized that ACC GSH levels measured at the onset of first-episode psychosis, before treatment initiation, predicts later functional outcomes in the first year of early intervention.
Early functional outcome status is a well-established indicator of long-term course of schizophrenia.20,21 An exciting translational possibility of linking GSH levels with functional outcomes in psychosis, is the availability of targeted treatments that can improve intracortical GSH in patients. Several clinical trials have reported on the safety and efficacy of the glutathione precursor N-acetylcysteine (NAC) in patients with psychosis.22 These trials (6 RCTs)22 indicate that NAC produces a modest, but significant improvement in cognitive deficits and negative symptoms (critical determinants of poor functional outcomes), when used as an adjunct to antipsychotics. Thus, in patients with psychosis, a deficit in intracortical GSH is likely to be a potentially modifiable pathway of poor outcomes.
Methods
Participants
The sample for the present analysis consisted of 72 new referrals to the PEPP (Prevention and Early Intervention for Psychosis Program) at London Health Sciences Center. After exclusions were made due to missing/poor quality scan data (n = 15; n = 11 withdrew from scan, n = 4 incorrect voxel placement due to movement in scanner), our final sample consisted of 57 patients (48 males/9 females) (table 1). All participants provided written, informed consent prior to participation as per approval provided by the Western University Health Sciences Research Ethics Board, London, Ontario. Inclusion criteria for study participation were as follows: individuals experiencing first-episode psychosis, with not more than 14 days of cumulative lifetime antipsychotic exposure, no major head injuries (leading to a significant period of unconsciousness or seizures), or known neurological disorders, and no concurrent substance use disorder. There were no explicit instructions to abstain from substances to participate in the study. Patients on non-antipsychotic prescription medication were not excluded (see supplementary table 1 for medication class by group).
Table 1.
Demographic and Clinical Characteristics of Healthy Controls Versus Total Patient Population, and NEET Patients Versus EET Patients
| Variable | Healthy Control n = 30 | All Patients n = 57 |
HC vs Patient | EET Patient n = 31 | NEET Patient n = 26 | EET vs NEET |
|---|---|---|---|---|---|---|
| Demographic Data | ||||||
| Sex(Male/Female) | 19/11 | 48/9 | x2 = 4.83, P = .028 | 25/6 | 23/3 | x2 = 0.65, P = .42 |
| Age [M (SD)} | 21.57 (3.45) | 22.75 (4.28) | t(85) = 1.5, P = .14 | 22.16 (3.92) | 23.46 (5.29) | t(55) = –1.10, P = .274 |
| ParentalNS-SEC[M (SD)} | 3.20(1.42) | 3.34 (1.27) | t(80) = 0.47, P = .64 | 3.31 (1.11) | 3.42 (1.38) | t(51) = 0.47, P = .64 |
| Avg Months to NEET assessment | N/A | 9.00 (4.38) | N/A | 8.90 (3.96) | 10.04 (4.79) | t(55) = 1.79, P = .08 |
| CAST Score | 6.13 (0.73) | 12.38 (5.94) | t(75) = –5.72, P < .01 | 12.00 (5.88) | 12.76(6.09) | t(45) = 0.49, P = .625 |
| AUDIT-C | 2.77 (2.10) |
2.25 (2.63) |
t(79) = 0.91 P = .37 |
2.79 (2.69) | 1.61 (2.46) | t(49) = 1.61, p=0.12 |
| Smoker (Yes/ No) | 0/30 | 11/46 | x2 = 6.62, P = .01 | 5/26 | 6/20 | x2 = 0.44, P = .51 |
| Clinical Data | ||||||
| DUP [M (SD)] | N/A | 5.63 (5.74) | N/A | 5.60 (5.80) | 5.67 (5.82) | t(53) = –0.04, P = .97 |
| Antipsychotic Defined Daily Dose Equivalents | N/A | 1.80 (2.81) | N/A | 1.62 (3.03) | 1.79 (2.09) | t(55) = –0.06, P = .96 |
| Other Psychotropic Medication (yes/ no) | N/A | 13/57 | N/A | 9/31 | 4/26 | x2 = 1.42, P = .22 |
| Baseline SOFAS [M (SD)] | 82.92(4.20) | 41.04 (12.48) | t(77) = 16.30, P < .001 | 44.38 (14.31) | 37.00 (8.45) | t(52) = 2.22, P = .03 |
| Follow-up SOFAS [M (SD)] | N/A | 59.34 (13.70) | N/A | 67.68 (10.08) | 48.37 (9.44) | t(42) = 6.47, P < .001 |
| PANSS-8 Total [M (SD)] | 8.0 (0.00) | 24.98 (5.76) | t(82) = 13.8, P < .01 | 24.07(4.95) | 26.13 (6.57) | t(52) = –0.83, P = .41 |
| PANSS-8 Positive [M (SD)} | 3.0 (0.00) | 12.35 (2.32) | t(82) = 18.6, P < .01 | 12.03 (2.44) | 12.75 (2.15) | t(52) = –0.94, P = .35 |
| PANSS-8 Negative [M (SD)} | 3.0 (0.00) | 7.22 (4.17) | t(82) = 5.67, P < .01 | 6.63 (3.76) | 7.96 (4.61) | t(52) = –0.86, P = .39 |
| CGI- Severity | 1.00 | 5.19 (0.97) | t(81) = 23.15, P < .01 | 5.10 (1.13) | 5.29 (0.75) | t(52) = –0.85, P = .40 |
M, Mean; SD, standard deviation; NS-SEC, national statistics socio-economic classification; CAST, Cannabis Abuse Screening Test; DUP, Duration of untreated Psychosis in Months; NEET, Not engaged in employment education or training; EET, Engaged in employment education or training; SOFAS, Social and Occupational Functioning Assessment Score; PANSS-8, Positive and Negative Syndrome Scale—8 Item Scale; PANSS-8 Positive, Positive and Negative Syndrome Scale total score for positive symptom items; PANSS-8 Negative, Positive and Negative Syndrome Scale total score for negative symptom items; CGI-Severity, Clinical Global Impression—Severity.
The mean lifetime total defined daily dose days (DDD × days on medication) for antipsychotic use was 1.80 days with 27 patients (47.4%) being completely antipsychotic naive at the time of scanning. Of those who had started antipsychotic treatment, (N = 30; 52.6%), the median total defined daily dose days was 2.81 days (range of 0.4–14 DDD days). Patient consensus diagnosis was established using the best estimate procedure described by Leckman et al.23 following 6 months of treatment. Diagnoses were made based on the Structured Clinical Interview for DSM-5.
Healthy control subjects (n = 30) were recruited through posters and word-of-mouth advertising. Healthy control subjects had no personal history of mental illness, no current use of medications, and no first-degree relatives with a history of psychotic disorders. Healthy controls were group matched to the FEP cohort for age and parental socio-economic status (the National Statistics Socioeconomic Classification: five-class version).24 Similar to their FEP counterparts, those with a history of substance use disorders in the past 12 months, significant head injury, or neurological disorders were excluded.
Clinical Measures
A clinical examination was conducted during the baseline assessment (the same day imaging was performed) by a research psychiatrist for patients or a trained rater for healthy controls. The clinical battery was used to assess patient symptom severity, substance use and to ensure that control subjects were free from current psychiatric disorders and history of either psychotic illness or neurologic disorder. To address substance use in the 6 months prior to our initial scan, the 6-item Likert-type Cannabis Abuse Screening Test (CAST)25 was used, in addition to self-reports of alcohol consumption using the Alcohol Use Disorders Identification Test—3 Item version (AUDIT)26 and regular nicotine use (yes or no).
To assess symptoms of psychosis the Positive and Negative Syndrome Scale—8 Item (PANSS-8) was used.27 The PANSS-8 is an abbreviated version of the 30 Item PANSS clinical assessment of symptomology in schizophrenia and psychosis with acceptable internal consistency and highly correlated with the full PANSS.27 Items are scored on a 1 (absent) to 7 (extreme) Likert-type scale, assessing both positive (P1–delusions, P2–Conceptual disorganization, & P3–hallucinations), and negative domains (N1–Blunted or flat affect, N4–passive social withdrawal, and N6–impoverishment of speech).
Additionally, the Social and Occupational Functioning Assessment Scale (SOFAS) was administered at baseline and follow-up. The SOFAS is a single-item measure of functioning scored between 1 (persistent inability to maintain minimum functioning without external support) and 100 (superior functioning in a wide range of activities). The SOFAS ratings of social and occupational functioning were made independent of symptom severity. In our study, SOFAS was taken with consideration for current functioning (rather than highest level of functioning over the past year).
We obtained baseline SOFAS on the same day MRI data were acquired. The patients enrolled in the PEPP clinic were followed up over the next 12 months, and we ascertained their NEET status and follow-up SOFAS scores between 6 to 12 months. The functional assessment was based on multiple sources of information: patient interviews, information from the psychiatrist providing the clinical care, PEPP case managers, and where required, information from family members documented in clinical charts. Due to the need for multiple information sources, not all patients were assessed at the same time point after their illness onset, but the vocational (NEET) status of the cohort between the window of 6 to 12 months was captured, in addition to baseline and follow-up assessment of cross-sectional functioning using SOFAS. Patients were classified as NEET (vocationally inactive) if they were unemployed and not in any form of schooling/education for more than half of the time since the onset of treatment for psychosis. Individuals who were engaged in work or school for more than half of the duration of treatment were classified as EET (vocationally active). This definition considers a longer time frame (up to 6 months) than the 1-week period used by the Organisation for Economic Co-Operation and Development (OECD),28 in line with its use in early intervention services for psychosis.29,30 A consensus was reached within the research study team when discrepancies noted in reported functioning between patient’s accounts and those of care providers.
MRS Assessment
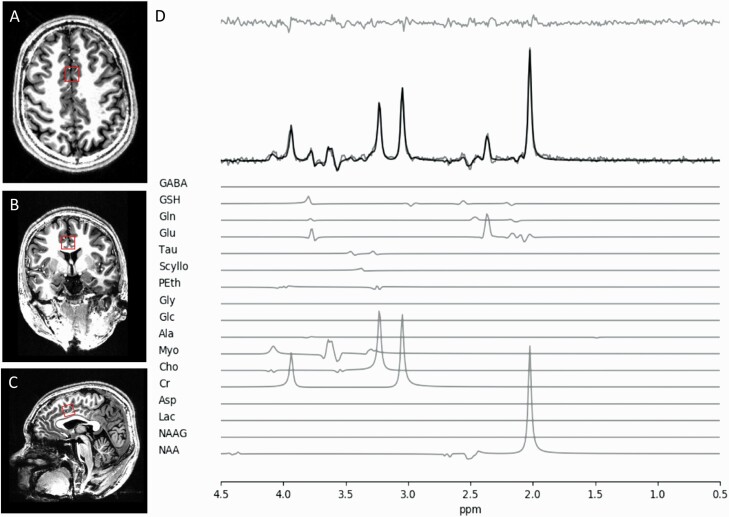
The complete MRS protocol for this study has been described in overlapping sample (37/72 patients overlap) previously published from this research project by Dempster and colleagues.18 Metabolite concentrations were estimated using single-voxel 1H-MRS data acquired with a Siemens MAGNETOM 7.0T head-only MRI scanner (Siemens, Erlangen, Germany) using an 8-channel transmit/32-channel receive head coil at the Center for Functional and Metabolic Mapping of Western University in London, Ontario. A 2.0 × 2.0 × 2.0 cm (8 cm3) voxel was placed on the bilateral dorsal ACC using a two-dimensional anatomical images acquired in the sagittal direction (37 slices, TR = 8000 ms, TE = 70 ms, flip-angle (α) = 120°, thickness = 3.5 mm, field of view = 240 × 191 mm). The posterior end of the voxel was set to coincide with the precentral gyrus and the caudal face of the voxel coincided with the most caudal location not part of the corpus callosum. The angulation of the voxel was determined to be tangential to the corpus callosum (figure 1). A total of 32 water-suppressed spectra were acquired using a semi-LASER 1H-MRS pulse sequence (TR = 7500 ms, TE = 100 ms) during each scan session, while participants were at rest. A long echo time was used during this study as it was demonstrated by Wong and colleagues that optimal GSH quantification alongside a higher spectral quality of glutamate signal is obtained when using long echo time for the semi-LASER sequence at 7-Tesla field31 (also see32). An additional benefit of a long echo time is the removal of any macromolecular contribution, increasing the precision of our spectral fitting and quantification procedures.
Fig. 1.
Illustrative example of the voxel placement on the dorsal ACC. A) Dorsal Brain View, B) Posterior view, C) Sagittal view, and D) A representative spectrum.
Phase and frequency corrections were applied to our MRS data using MATLAB tools developed by Near et al.33 after which they were averaged into a single representative spectrum. The spectrum then underwent QUALITY Deconvolution and Eddy Current Correction (QUECC)34 and Hankel Singular Value Decomposition (HSVD)35 post-processing for line shape deconvolution and residual water signal removal. The software fitMAN36 was used for spectral fitting, which included 17 brain metabolites in its echo time-specific prior knowledge fitting template. Barstool37 was used for quantification and included corrections for T1 and T2 relaxations of gray matter, white matter, and CSF via partial volume segmentation calculations. MRS quality was assess using Cramer-Rao lower bounds (CRLB) for each metabolite fit. No significant differences were observed in spectral linewidth or SNRNAA was seen among the three groups of interest (healthy controls, FEP-NEET, and FEP-EET) (see supplementary table 2). Cramer-Rao lower bound (CRLB) thresholds for study inclusion was <10% for glutamate and <25% for glutathione, though the majority of CRLB values were much closer to half of that threshold (supplementary table 3).
Statistical Analyses
We used IBM SPSS Statistics version 24 for all analyses. First, the primary hypothesis of a relationship between NEET status in the first year and glutathione was tested using a one-way ANOVA, with healthy controls, NEET and EET patients as 3 groups of interest. Second, within the patient group, a bivariate correlation between follow-up SOFAS scores and GSH measurement was conducted, with bootstrapping for generating p-values and confidence intervals. We also tested if GSH levels retain their ability to predict follow-up SOFAS, after adjusting for the variance explained by baseline functioning (SOFAS at the time of MRS scanning), age, and sex as covariates. Finally, we excluded patients without a clear diagnosis of schizophrenia by 6-12 months assessment and tested the relationship between GSH and SOFAS and NEET.
Results
When comparing healthy controls to FEPs, no statistically significant differences existed for age, parental socioeconomic status or alcohol use, although males (χ 2 = 4.83, P = .028), and smokers (χ 2 = 6.62, P = .01) were over-represented and self-identified cannabis use frequency (CAST scores: t = 5.72, P < .01) was higher in the patient sample (table 1). No significant differences in baseline demographic, medication, cannabis or alcohol use, or clinical differences were identified between EET and NEET patients, apart from baseline SOFAS scores, which were higher among EET patients (t = 2.23, P = .031). Alcohol, tobacco, and cannabis use were not associated with GSH, baseline SOFAS, or follow-up SOFAS scores (see supplementary table 4). Both EET and NEET groups showed a mean gain in SOFAS scores over the follow-up period, but the gain was higher in the EET group (EET = 21.66 (14.49), and NEET = 12.00 (14.99)) t = 2.07, P = .045). Including demographic data of subjects without useable MRS data (n=15) did not affect this profile (supplementary table 5).
Group Differences in GSH Levels
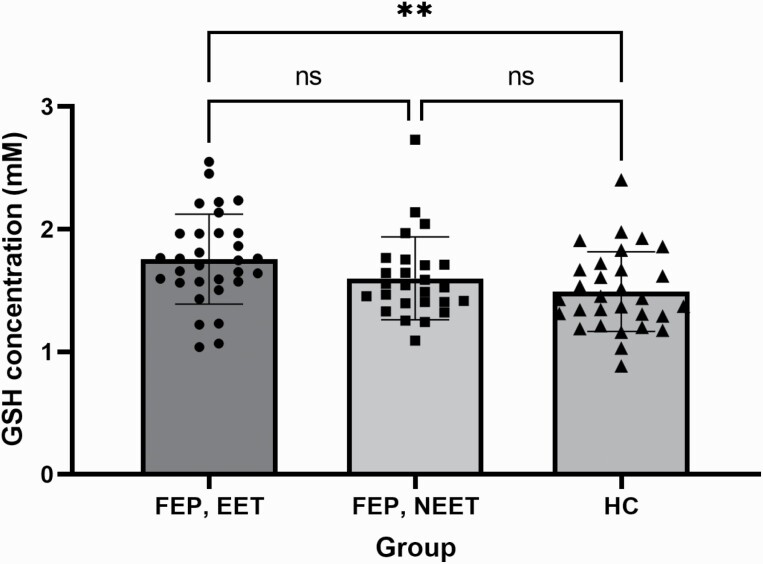
Patients (as a whole group) showed significantly higher glutathione levels versus controls (FEP= 1.68 (0.36), HC = 1.49(0.32); t (85) = 2.46, P = .016). As sex was differently distributed between patients and controls, we included sex as a covariate along with diagnosis but noted no significant effect of sex [F(1,84) = 0.62, P = .43], but patient status continued to predict higher GSH levels [F(1,84) = 6.61, P = .012], after adjusting for sex. When comparing the 2 subgroups of patients (NEET/EET) and controls in a one-way ANOVA, glutathione levels were significantly different among the 3 groups (FEP-NEET, FEP-EET and HC) at the P < .05 level [F(2,84) = 4.55, P = .01]. Post hoc comparisons using the Sidak test indicated that the mean GSH levels for FEP-EET subjects was significantly higher than healthy controls [[Mean(SD) of GSH: FEP-EET = 1.76(0.37), HC = 1.49(0.32); P = .01]. However, FEP-NEET subjects [M (SD) = 1.60 (0.34)] did not significantly differ from the healthy controls (P = .25) or FEP-EET (P = .57) (figure 2).
Fig. 2.
Relationship between Glutathione concentrations (GSH) levels among healthy controls, patients involved in vocational activity (EET) and patients not involved in employment, education, or training (NEET).
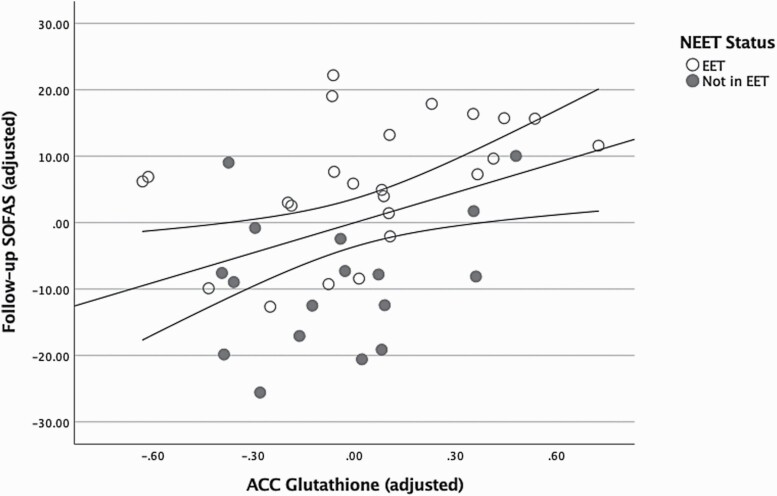
Analyses restricted to the FEP group revealed GSH measured at baseline predicted the follow-up SOFAS scores (R2 = 0.17, F(1,42) = 8.45, P = .006). When covariates age, sex and baseline SOFAS scores were added, the regression model continued to be significant and GSH was the only variable that predicted follow-up SOFAS (R2 = 0.29, F(4,36) = 3.67, P = .013; note n = 41 for this analysis as 3 subjects lacked baseline SOFAS scores). GSH levels (β = 0.36, P = .016) showed the strongest association out of all independent predictors for follow-up SOFAS, after adjusting for the variance explained by baseline functioning (β = 0.19, P = .22), age (β = –0.21, P = .15) and sex (β = 0.17, P = .27). The correlation between adjusted GSH levels and follow-up SOFAS is displayed, stratified by NEET status, in figure 3. (See supplementary table 6 for further regression models demonstrating specificity of GSH - follow-up SOFAS relationship and table 7 for expanded list of available metabolites and associations with SOFAS scores)
Fig. 3.
Association between anterior cingulate cortex (ACC) glutathione concentrations and 6-12 months follow-up Social and Occupational Functioning Assessment Scale (SOFAS), adjusted for age, sex, and baseline SOFAS. Total sample size = 41 (3 subjects lacked baseline SOFAS scores).
Analysis Restricted to First Episode Schizophrenia Only
Finally, we repeated the above analysis in a subset of patients with a consensus diagnosis of schizophrenia/schizoaffective disorder by 6–12 months, after excluding 5 subjects with major depressive disorder, schizophreniform or bipolar disorder (FES n = 39). Glutathione levels continued to differ significantly among the 3 groups (FES-NEET, FES-EET and HC) at the P < .05 level [F(2,79) = 4.16, P = .02]. Post hoc comparisons using the Sidak test indicated that the mean GSH levels for FES-EET subjects was still significantly higher than healthy controls [[Mean(SD) of GSH in FES-EET = 1.75(0.36), HC = 1.49(0.32); P = .016]. As in the fuller FEP sample, FES-NEET subjects [M(SD) = 1.59(0.35)] did not significantly differ from the healthy controls (P = .26) or FES-EET (P = .67).
Within the FES group, GSH measure at baseline was positively correlated with follow-up SOFAS scores r(39) = 0.38 [bootstrap 95% CI: 0.15–0.61], P = .017. GSH levels, in combination with age, sex and baseline SOFAS scores, continued to predict follow-up SOFAS in the FES group at a trend level, (R2 = 0.24, F(4,32) = 2.47, P = .06; note that the total n = 37 for this analysis as 2 subjects lacked baseline SOFAS). GSH level (β = 0.32, P = .05) continued to be the most prominent independent predictor of the follow-up SOFAS, after adjusting for the variance explained by baseline functioning, age and sex (β = 0.10–0.23, all P > .16).
Prognostic Relevance Based on Binarized GSH Levels
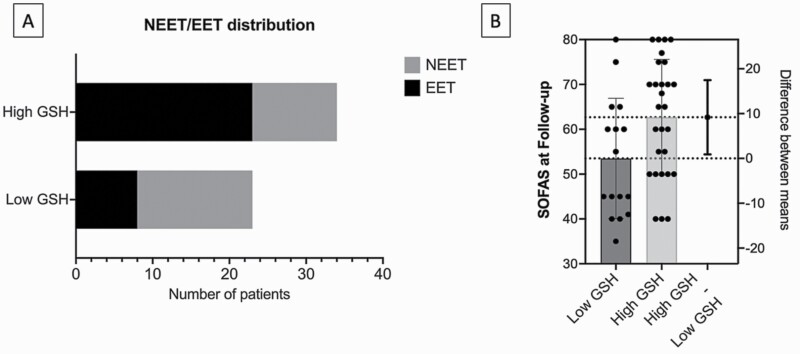
To provide clinically relevant information to a patient with FEP whose GSH values have been quantified as described in this study, we performed a median split analysis of the patient group based on ACC GSH values of the entire sample (median = 1.586 mM). We then compared the low-GSH and high-GSH FEP groups on the clinically relevant variables of NEET status and SOFAS at follow-up. The proportion of patients with FEP who were vocationally active (i.e., EET) significantly differed based on their baseline GSH status, with high-GSH FEP reporting 68% (23 of 34) in EET, while low-GSH FEP reporting 35% (8 of 23) in EET (Fisher’s exact P = .018) (figure 4a). This translated to an odds ratio of 3.92 (95%CI: 1.34–11.2), and a relative risk of 1.95 (95%CI: 1.13–3.7) in favor of being in employment, education, or training if an individual with FEP belonged to the high, instead of low-GSH group at the outset. The mean follow-up SOFAS scores for high-GSH FEP was also significantly higher than low-GSH FEP [Mean(SD) of SOFAS: high-GSH = 62.7(12.9), low-GSH = 53.5 (13.4); t (42) = 2.24; P = .03] (figure 4b).
Fig. 4.
A) Visualization of vocational NEET/EET distribution of First Episode Psychosis patients, based on high versus low GSH grouping B) Comparison of mean SOFAS score among high vs low GSH grouping.
Discussion
Using 7T MRS to measure GSH concentration from ACC in untreated FEP subjects followed up over 1 year period, we report 2 major findings: (1) GSH levels are higher in patients in an education, employment, or training status than healthy controls; and (2) the baseline GSH levels predict later socio-occupational functioning over and above what can be predicted from baseline functioning, indicating a specific mechanistic role for the central antioxidant in the outcome trajectories following first psychotic episode. Patients with GSH levels that were lower than the median values in the sample were at approximately two times higher risk of being vocationally inactive, with approximately 10 points lower scores in SOFAS. This relationship was specific to GSH, as other metabolites did not relate to vocational functioning (supplementary table 3)
Our observed relationship between lower GSH levels and lower SOFAS scores and NEET status is consistent with several prior reports. In an overlapping sample, we previously reported a predictive relationship between low GSH and delayed response to antipsychotics.18 Lack of early response is a critical indicator of long-term poor outcomes in schizophrenia.38–40 In line with studies linking lower GSH to higher residual symptom burden,41 negative symptoms42 and cognitive deficits43 in schizophrenia, our observation highlights a prominent “pathoplastic” role for antioxidant status in shaping the outcomes of this illness. In fact, a recent 7T-MRS study of the ACC reported that patients who fail first-line antipsychotic treatments are more likely to have an intracortical GSH-deficit.44 In our study, the presence of higher ACC GSH in FEP may indicate the overall superior treatment responsiveness in this cohort, given their untreated status. Such higher GSH levels have been previously reported in other brain regions in FEP.15,16 Our observations also establish that the predictive value of GSH relates to “stable” functioning that emerges 6–12 months later; not the SOFAS scores during acute illness which may be determined predominantly by the florid nature of the presenting psychotic symptoms.
The small to medium effect size difference in patients vs. healthy controls comparison underscores the considerable heterogeneity in intracortical GSH levels in psychosis, in line with several 3T MRS studies that did not report a notable group difference in the ACC45 as well as other regions.46,47 This indicates that a more nuanced interpretation is required with respect to GSH levels in this illness.48 A meta-analysis of variance that includes the current sample, indicates that inter-individual variability in ACC GSH is increased in patients with psychosis.49 The observed heterogeneity may relate to individual differences (i.e., subgroups of patients with high or low GSH levels) or stage-specific differences (acute excess, followed by later deficit) .48 While several exogenous factors may also affect antioxidant levels, there is no evidence for a major role for these confounders in explaining aberrations in glutathione pathway in psychosis.50
An important strength of our study is its longitudinal nature; with the temporal information we can establish that higher GSH levels at the outset predict superior socio-occupational functioning seen over the next 1 year among patients. But a major limitation is the availability of a single time point of GSH levels and lack of data on adherence to treatment throughout the observation period. To establish a more conclusive causal inference, we need longitudinal follow-up studies that capture multiple time points from untreated early stages of psychosis to a stable phase when functional outcome trajectories become more established. Long-term follow-up of experimental studies with patients in early stages of psychosis that receive adjunctive NAC will also be illuminating in this regard. We also lacked peripheral and genotyping measures of antioxidant capacity in this cohort; while these measures are more accessible than 7T-MRS, they do not consistently reflect intracortical GSH.19,51,52 Finally, our inferences are limited to illness-related vocational functioning and not generalizable to vocational functioning in healthy subjects, which may not depend on one’s GSH levels.
It is important to note that both schizophrenia and antipsychotics can affect T2 of neurometabolite signal amplitudes,53 but this effect may not affect all metabolites54 and may be age, region, and tissue-dependent.55 In our study, we estimated metabolite concentrations based on the ratio of metabolite signal amplitude and water amplitude for all reported metabolites. For our reported GSH specific results to be ascribed to a T2-related effect at long-TE, a specific T2 related GSH-water differential must have occurred. Nevertheless, we recommend study-specific measurement of metabolite T2 relaxation times in future studies.
We conclude that in patients with first-episode psychosis in whom intracortical GSH levels are higher during the acute phase of psychosis, functional outcomes are superior to those with lower levels, over the next 1 year. Thus, ACC GSH measure may be a useful indicator of resilience to oxidative stress and functional recovery after first episode of psychosis. Taken together with prior MRS studies from established cases of schizophrenia, many of which indicate a profile of treatment failures and residual symptoms in patients with intracortical GSH-deficit, our observation makes a compelling case to investigate the role of pre-emptive antioxidant interventions in early stages of psychosis.
Supplementary Material
Acknowledgments
We thank Mr. Trevor Szekeres, Mr. Scott Charlton, Mr. Joseph Gati for their assistance in data acquisition and archiving, Drs. Rob Bartha and Dickson Wong for MRS analysis. We thank all research team members of the NIMI lab and all the staff members of the PEPP London team, particularly Drs. Kara Dempster, Julie Richard, Priya Subramanian and Hooman Ganjavi for their assistance in patient recruitment and supporting clinical care. We gratefully acknowledge the participants and their family members for their contributions. We also thank Dr. Srividya Iyer, the Douglas Research Centre, McGill University for discussions regarding NEET. We dedicate this work to the memory of late Dr. Raj Harricharan of London, Ontario.
Contributor Information
Michael MacKinley, Lawson Health Research Institute, London, ON, Canada; Robarts Research Institute, Western University, London, ON, Canada.
Sabrina D Ford, Robarts Research Institute, Western University, London, ON, Canada; Victoria Hospital, London Health Sciences Centre, London, ON, Canada.
Peter Jeon, Lawson Health Research Institute, London, ON, Canada; Robarts Research Institute, Western University, London, ON, Canada; Department of Medical Biophysics, Western University, London, ON, Canada.
Jean Théberge, Robarts Research Institute, Western University, London, ON, Canada; Department of Medical Biophysics, Western University, London, ON, Canada; Department of Psychiatry, Schulich School of Medicine and Dentistry, Western University, London, ON, Canada.
Lena Palaniyappan, Lawson Health Research Institute, London, ON, Canada; Robarts Research Institute, Western University, London, ON, Canada; Victoria Hospital, London Health Sciences Centre, London, ON, Canada; Department of Medical Biophysics, Western University, London, ON, Canada; Department of Psychiatry, Schulich School of Medicine and Dentistry, Western University, London, ON, Canada.
Conflict of Interest
LP reports personal fees from Otsuka Canada, SPMM Course Limited, UK, Canadian Psychiatric Association; book royalties from Oxford University Press; investigator-initiated educational grants from Janssen Canada, Sunovion and Otsuka Canada outside the submitted work. All other authors report no relevant conflicts.
Funding
This study was funded by Canadian Institutes of Health Research Foundation Grant (375104/2017) to LP; Academic Medical Organization of Southwestern Ontario (AMOSO) Opportunities fund to LP; partial salary support of PJ by Natural Science and Engineering Research Council of Canada Discovery Grant (RGPIN-2016-05055) to JT, Schulich School of Medicine and Dentistry Dean’s Scholarship to PJ; Parkwood Institute Studentship and the Jonathan and Joshua Memorial Scholarship to MM. Data acquisition was supported by the Canada First Excellence Research Fund to BrainSCAN, Western University (Imaging Core); Innovation fund for Academic Medical Organization of Southwest Ontario; Bucke Family Fund, The Chrysalis Foundation and The Arcangelo Rea Family Foundation (London, Ontario). LP acknowledges salary support from the Tanna Schulich Chair of Neuroscience and Mental Health. This research was enabled in part by support provided by Compute Canada Resources for Research Groups Allocation (www.computecanada.ca).
Author Contributions
MM recruited patients, collected, and compiled demographic, functional outcome and treatment data and assisted in acquiring the scans. SF recruited patients, compiled demographic data and assisted in acquiring the scans. PJ acquired the MRS scans, performed the MRS quantification and analysis, supervised by JT. LP obtained the research funds, designed the project with JT and supervised MM, SF and PJ. LP conceived the analysis and wrote the first draft with MM and SF. All authors contributed to the writing and have approved the final version of the manuscript.
Institutional Review Board Statement
This study was conducted according to the guidelines of the declaration of Helsinki and approved by the Research Ethics Board of the University of Western Ontario (Project #108268; October 19, 2020).
References
- 1. Henry LP, Amminger GP, Harris MG, et al. The EPPIC follow-up study of first-episode psychosis: longer-term clinical and functional outcome 7 years after index admission. J Clin Psychiatry. 2010;71:716–728. [DOI] [PubMed] [Google Scholar]
- 2. Addington J. The promise of early intervention. Early Interv. Psychiatry 2007;1:294–307. [DOI] [PubMed] [Google Scholar]
- 3. Dama M, Shah J, Norman, R, et al. Short duration of untreated psychosis enhances negative symptom remission in extended early intervention service for psychosis. Acta Psychiatr Scand. 2019;140:65–76. [DOI] [PubMed] [Google Scholar]
- 4. Jääskeläinen E, Juola, P, Hirvonen N, et al. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr Bull. 2013;39:1296–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Norman RMG, MacDougall A, Manchanda R, Harricharan R. An examination of components of recovery after five years of treatment in an early intervention program for psychosis. Schizophr Res. 2018;195:469–474. [DOI] [PubMed] [Google Scholar]
- 6. Santesteban-Echarri O, Paino M, Rice S, et al. Predictors of functional recovery in first-episode psychosis: a systematic review and meta-analysis of longitudinal studies. Clin Psychol Rev. 2017;58:59–75. [DOI] [PubMed] [Google Scholar]
- 7. Díaz-Caneja CM, Pina-Camacho L, Rodríguez-Quiroga A, et al. Predictors of outcome in early-onset psychosis: a systematic review. NPJ Schizophr. 2015;1:14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Keeffe D, Hannigan A, Doyle R, et al. The iHOPE-20 study: relationships between and prospective predictors of remission, clinical recovery, personal recovery and resilience 20 years on from a first episode psychosis. Aust N Z J Psychiatry. 2019;53:1080–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Velthorst E, Fett AKJ, Reichenberg A, et al. The 20-year longitudinal trajectories of social functioning in individuals with psychotic disorders. Am J Psychiatry. 2017;174:1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murray AJ, Rogers JC, Katshu MZUH, Liddle PF, Upthegrove R. Oxidative stress and the pathophysiology and symptom profile of schizophrenia spectrum disorders. Front Psychiatry. 2021;12:1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fournier M, Monin A, Ferrari C, et al. Implication of the glutamate–cystine antiporter xCT in schizophrenia cases linked to impaired GSH synthesis. npj Schizophr. 2017;3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martínez-Cengotitabengoa M, Micó JA, Arango C, et al. Basal low antioxidant capacity correlates with cognitive deficits in early onset psychosis. A 2-year follow-up study. Schizophr Res. 2014;156:23–29. [DOI] [PubMed] [Google Scholar]
- 13. Fraguas D, Gonzalez-Pinto A, Micó JA, et al. Decreased glutathione levels predict loss of brain volume in children and adolescents with first-episode psychosis in a two-year longitudinal study. Schizophr Res. 2012;137:58–65. [DOI] [PubMed] [Google Scholar]
- 14. Lijing X, Mekle R, Fournier M, et al. Genetic polymorphism associated prefrontal glutathione and its coupling with brain glutamate and peripheral redox status in early psychosis. Schizophr Bull. 2016;42:1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wood SJ, Berger GE, Wellard RM, et al. Medial temporal lobe glutathione concentration in first episode psychosis: a 1H-MRS investigation. Neurobiol Dis. 2009;33:354–357. [DOI] [PubMed] [Google Scholar]
- 16. Berger GE, Wood SJ, Wellard RM, et al. Ethyl-eicosapentaenoic acid in first-episode psychosis. A 1H-MRS study. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 2008;33:2467–2473. [DOI] [PubMed] [Google Scholar]
- 17. Jeon P, Limongi R, Ford SD, et al. Glutathione as a molecular marker of functional impairment in patients with at-risk mental state: 7-Tesla 1H-MRS Study. Brain Sci 2021;11:941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dempster K, Jeon P, MacKinley M, et al. Early treatment response in first episode psychosis: a 7-T magnetic resonance spectroscopic study of glutathione and glutamate. Mol Psychiatry. 2020;25:1640–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coughlin JM, Yang K, Marsman A, et al. A multimodal approach to studying the relationship between peripheral glutathione, brain glutamate, and cognition in health and in schizophrenia. Mol Psychiatry. 2021;26:3502–2511. doi: 10.1038/s41380-020-00901-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harrison G, Croudace T, Mason P, Glazebrook C, Medley I. Predicting the long-term outcome of schizophrenia. Psychol Med. 1996;26:697–705. [DOI] [PubMed] [Google Scholar]
- 21. Harrison G, Hopper K, Craig T, et al. Recovery from psychotic illness: a 15- and 25-year international follow-up study. Br. J. Psychiatry J. Ment. Sci. 2001;178:506–517. [DOI] [PubMed] [Google Scholar]
- 22. Yolland COB, Hanratty D, Neill E, et al. Meta-analysis of randomised controlled trials with N -acetylcysteine in the treatment of schizophrenia. Aust N Z J Psychiatry. 2020;54:453–466. [DOI] [PubMed] [Google Scholar]
- 23. Leckman JF, Sholomskas D, Thompson D, Belanger A, Weissman MM. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry. 1982;39:879–883. [DOI] [PubMed] [Google Scholar]
- 24. Rose D, Pevalin DJ.. A Researcher’s Guide to the National Statistics Socio-economic Classification. London, UK: Sage Publications; 2003. [Google Scholar]
- 25. Legleye S. The cannabis abuse screening test and the DSM-5 in the general population: optimal thresholds and underlying common structure using multiple factor analysis. Int J Methods Psychiatr Res. 2018;27:e1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saunders JB, Aasland OG, Babor TF, De La Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 1993;88:791–804. [DOI] [PubMed] [Google Scholar]
- 27. Lin C-H, Lin H-S, Lin S-C, et al. Early improvement in PANSS-30, PANSS-8, and PANSS-6 scores predicts ultimate response and remission during acute treatment of schizophrenia. Acta Psychiatr Scand. 2018;137:98–108. [DOI] [PubMed] [Google Scholar]
- 28. OECD (2021), Youth not in employment, education or training (NEET) (indicator). doi: 10.1787/72d1033a-en. [DOI]
- 29. Iyer S, Mustafa S, Gariépy G, et al. A NEET distinction: youths not in employment, education or training follow different pathways to illness and care in psychosis. Soc Psychiatry Psychiatr Epidemiol. 2018;53:1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maraj A, Mustafa S, Joober R, et al. Caught in the “NEET Trap”: the intersection between vocational inactivity and disengagement from an early intervention service for psychosis. Psychiatr. Serv. Wash. DC 2019;70:302–308. [DOI] [PubMed] [Google Scholar]
- 31. Wong D, Schranz AL, Bartha R. Optimized in vivo brain glutamate measurement using long-echo-time semi-LASER at 7 T. NMR Biomed. 2018;31:e4002. [DOI] [PubMed] [Google Scholar]
- 32. Jeon P. Functional magnetic resonance spectroscopy in first-episode schizophrenia: measuring glutamate and glutathione dynamics at 7-Tesla. Electron Thesis Diss Repos. 2019.
- 33. Near J, Edden R, Evans CJ, et al. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn Reson Med. 2015;73:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bartha R, Drost D, Menon R, Williamson PC. Spectroscopic lineshape correction by QUECC: combined QUALITY deconvolution and eddy current correction. Magn Reson Med. 2000;44:641–645. [DOI] [PubMed] [Google Scholar]
- 35. van den Boogaart A, Ala-Korpela M, Jokisaari J, Griffiths JR. Time and frequency domain analysis of NMR data compared: an application to 1D 1H spectra of lipoproteins. Magn Reson Med. 1994;31:347–358. [DOI] [PubMed] [Google Scholar]
- 36. Bartha R, Drost DJ, Williamson PC. Factors affecting the quantification of short echo in-vivo 1H MR spectra: prior knowledge, peak elimination, and filtering. NMR Biomed. 1999;12:205–216. [DOI] [PubMed] [Google Scholar]
- 37. Wong D. MRI Investigations of Metabolic and Structural Brain Changes in Alzheimer’s Disease and Vitamin D Deprivation. Electron Thesis Diss Repos. 2019.
- 38. Lambert M, Naber D, Schacht A, et al. Rates and predictors of remission and recovery during 3 years in 392 never-treated patients with schizophrenia. Acta Psychiatr Scand. 2008;118:220–229. [DOI] [PubMed] [Google Scholar]
- 39. Derks EM, Fleischhacker WW, Boter H, et al. Antipsychotic drug treatment in firstepisode psychosis: should patients be switched to a different antipsychotic drug after 2, 4, or 6 weeks of nonresponse? J Clin Psychopharmacol. 2010;30:176–180. [DOI] [PubMed] [Google Scholar]
- 40. Carbon M, Correll CU. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci. 2014;16:505–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kumar J, Liddle EB, Fernandes CC, et al. Glutathione and glutamate in schizophrenia: a 7T MRS study. Mol Psychiatry. 2020;25:873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matsuzawa D, Obata T, Shirayama Y, et al. Negative correlation between brain glutathione level and negative symptoms in schizophrenia: a 3T 1H-MRS Study. PLoS One. 2008;3:e1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang AM, Pradhan S, Coughlin JM, et al. Assessing brain metabolism with 7-T proton magnetic resonance spectroscopy in patients with first-episode psychosis. JAMA Psychiatry 2019;76:314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang K, Longo L, Narita Z, et al. A multimodal study of a first episode psychosis cohort: potential markers of antipsychotic treatment resistance. Mol Psychiatry. 2021. doi: 10.1038/s41380-021-01331-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Das TK, Javadzadeh A, Dey A, et al. Antioxidant defense in schizophrenia and bipolar disorder: a meta-analysis of MRS studies of anterior cingulate glutathione. Prog Neuropsychopharmacol Biol Psychiatry. 2019;91:94–102. [DOI] [PubMed] [Google Scholar]
- 46. Lesh TA, Maddock RJ, Howell A, et al. Extracellular free water and glutathione in firstepisode psychosis—a multimodal investigation of an inflammatory model for psychosis. Mol Psychiatry. 2021;26:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Iwata Y, Nakajima S, Plitman E, et al. Glutathione levels and glutathione-glutamate correlation in patients with treatment-resistant schizophrenia. Schizophr. Bull. Open 2021;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Palaniyappan L, Park MTM, Jeon P, et al. Is there a glutathione centered redox dysregulation subtype of schizophrenia? Antioxidants 2021;10:1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Palaniyappan L, Sabesan P, Li X, Luo Q. Schizophrenia increases variability of the central antioxidant system: a meta-analysis of variance from MRS studies of glutathione. Front Psychiatry. 2021;12:2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ballesteros A, Jiang P, Summerfelt A, et al. No evidence of exogenous origin for the abnormal glutathione redox state in schizophrenia. Schizophr Res. 2013;146:184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gawryluk J. W. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123–130. [DOI] [PubMed] [Google Scholar]
- 52. Zhang Y, Catts VS, Weickert CS. Lower antioxidant capacity in the prefrontal cortex of individuals with schizophrenia. Aust N Z J Psychiatry. 2017;52:690–698 doi: 10.1177/0004867417728805. [DOI] [PubMed] [Google Scholar]
- 53. Bracken BK, Rouse ED, Renshaw PF, Olson DP. T2 relaxation effects on apparent N-acetylaspartate concentration in proton magnetic resonance studies of schizophrenia. Psychiatry Res. 2013;213:142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tunc-Skarka N, Weber-Fahr W, Hoerst M, et al. MR spectroscopic evaluation of N-acetylaspartate’s T2 relaxation time and concentration corroborates white matter abnormalities in schizophrenia. NeuroImage 2009;48:525–531. [DOI] [PubMed] [Google Scholar]
- 55. Kirov II, Fleysher L, Fleysher R, et al. Age dependence of regional proton metabolites T2 relaxation times in the human brain at 3 T. Magn Reson Med. 2008;60:790–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.