Abstract
Emerging infectious diseases are the infections that could be newly appeared or have existed demographic area with rapidly increasing in some geographic range. Among various types of emerging infectious diseases like Ebola, chikungunya, tuberculosis, SARS, MERS, avian flu, swine flu, Zika, and so on, very recently we have witnessed the emergence of recently recognized coronavirus infection as Covid-19 pandemic caused by SARS-CoV-2, which rapidly spread around the world. Various emerging factors precipitating disease emergence include environmental, demographic, or ecological that increase the contact of people with unfamiliar microbial agents or their host or promote dissemination. Here in this chapter, we reviewed the various emerging considerations of infectious diseases including factors responsible for emerging and re-emerging infectious diseases as well as drug delivery challenges to treat infectious diseases and various strategies to deal with these challenges including nanotheranostics. Nanotheranostics are showing potential toward real-time understanding, diagnosis, and monitoring the response of the chemotherapy during treatment with reduced nontarget toxicity and enhanced safety level in the recent research studies.
Keywords: Infectious diseases, Diagnosis, Theranostics, Antimicrobial agents, Drug delivery
1. Introduction
Infectious diseases are one of the leading causes of mortality and morbidity with a significant impact on health and economy of all the countries throughout the world. They are existing for centuries and continuously challenging the development and health of all living beings including humans. Further, the situation is worsened by the emergence of some new or unrecognized or even sometimes old infectious parasites causing epidemic, which can affect the population globally. In last three decades, about 30 new infectious agents have emerged which affected the human population all over the world. Emerging infectious diseases are the newly recognized diseases like COVID-19 or already existing diseases like influenza, but their occurrences are rapidly increasing in some geographical range [1], [2], [3].
Emerging infectious diseases have gained the attention of medical communities and pharmaceutical research scientists since the influenza pandemic occurred in 1919. However, the recently occurred pandemic condition COVID-19 due to the newly discovered airborne virus, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), had taught the severity of infectious diseases to the research community. Emerging infectious diseases are mostly spread to the human by zoonotic hosts due to the change in behaviors like increased traveling, change in food habits, and/or modification of the physical environment. These diseases have characteristics of emergence and re-emergence after specific time intervals due to the development of antimicrobial resistance, scarcity of target, termination of dormancy period, etc. To fight against these diseases modern scientific tools like theranostic delivery systems provide the good alternative to current treatment options which can deliver the antimicrobial agent as well as diagnostic agents with ability to target infectious microorganisms [3], [4], [5].
In last few decades nanomaterials are emerging as a promising tool for theranostic applications attributed to their multifunctionality and unique nanometric architecture and are being explored in, simultaneous, diagnosis and treatment of cancer, neurodegenerative disorders, cardiovascular diseases and also in infectious diseases. Although, affordability, efficacy, reproducibility, and safety are the major concern associated with nanotechnology-based medicines, i.e., nanomedicines, yet some bioinspired smartly engineered nanomaterials including functionalized dendrimers, carbon nanotubes, nanoparticles (NPs), nanocrystals, quantum dots, liposomes, etc. are emerging as promising nanometric platform to be engineered smartly for various theranostic applications which could be extended to infectious diseases [6], [7], [8], [9], [10]. Selection of an appropriate therapeutic agent with significant efficacy, nanocarrier(s) with sufficient stability, suitable modification of nanomaterials to get sustained and targeted drug release with a promising imaging agent is essential for design of an effective nanotheranostics [9], [11], [12], [13].
Nanotheranostics or theranostic medicines are based on the use of nanomaterials and nanotechnology, strategically to combine diagnostic and therapeutic modalities on single platform. They can offer advantages of patient-specific personalized medicines, improved therapeutic index, reduction in side effects and hence can overcome the challenges or limitations associated with conventional diagnostic and therapeutic methods. Theranostics has particularly been explored by scientists in cancer and neurodegenerative disorders. Attributed to prior research on application of theranostics in brain disorders, scientists are trying to further investigate this knowledge in infectious brain diseases also. The possible application of theranostics has been reviewed by scientists to simultaneously diagnose and treat infections caused in central nervous system by pathogenic amoebae with possibility of reduction in disease burden by sensitive and well-timed diagnosis with augmentation of effective therapeutic strategy (Fig. 1 ). Even scientists are also advocating exploration of nanomedicines and nanotheranostics in treatment of COVID-19, for which currently no specific treatment option is available, attributed to ability of nanomedicines and nanotheranostics to emerge as a promising tool for targeted delivery of therapeutic moieties like drug molecules, immunomodulators, vaccines, siRNA or other genetic materials, peptides, proteins etc. along with diagnostic/imaging agent. The scientists have further supported the exploration and optimization of intranasal nanotheranostics to treat COVID-19 attributed to the fact that intranasal administration has given promising results in other infectious respiratory infections (Fig. 2, Fig. 3 ) [13], [14].
Fig. 1.

Schematic depiction of proposed theranostic approaches against infections caused by brain-eating amoebae with some nanomaterials which could be explored as suitable nanotheranostics due to their small size, unique physicochemical properties, and attributes for drug delivery applications.
(Reproduced with permission from Anwar A, Siddiqui R, Khan NA. Importance of theranostics in rare brain-eating amoebae infections. ACS Chem Nerosci 2019;10(1):6–12.)
Fig. 2.
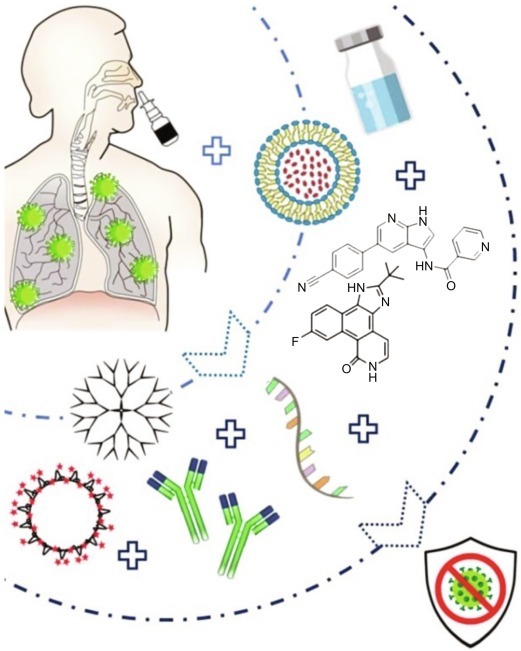
Diagrammatic presentation of nanotechnology-based treatment approaches for intranasal delivery where nanocarrier may be conjugated to therapeutic agents like siRNA, peptides, peptide inhibitors, or antibodies, or may be administered as virus-like NPs formulated as emulsion or solution and maybe easily administered to the patients via a nasal spray to treat SARS-CoV-2 infection efficiently.
(Reproduced with permission from Itani R, Tobaiqy M, Al Faraj A. Optimizing use of theranostic nanoparticles as a life-saving strategy for treating COVID-19 patients. Theranostics 2020;10(13):5932–42.)
Fig. 3.

Schematic presentation of various types of nanomaterials that could be explored and optimized for intranasal pulmonary administration of therapeutic agents.
(Reproduced with permission from Itani R, Tobaiqy M, Al Faraj A. Optimizing use of theranostic nanoparticles as a life-saving strategy for treating COVID-19 patients. Theranostics 2020;10(13):5932–42.)
1.1. Infectious diseases
Infectious diseases can transmit from animal to human or one person to another person by means of inoculation, airborne or waterborne transmission. Most infectious diseases are caused by live microorganisms like viruses, fungi, bacteria, etc. Pathogenicity of these microorganisms depends on their type and extent of damage caused by them on entering their human host. These agents are mostly entered into host body through nose, eyes, skin, mouth, and genital openings. The infection caused by the microorganisms is described by different mechanisms like metabolic product of infectious agents, intracellular or other body fluids extracted by causative agents, release of toxic materials, or presence of enzymes that interfere with the normal body function of humans [2]. These products of infectious agents are responsible for producing the pathogenicity to the human host by invading different human systems or organs and causes a tissue damage.
Currently around 40 infectious diseases were identified which include recently identified virus called SARS-CoV-2, which is responsible for the COVID-19 pandemic. Other major infectious diseases include Ebola, chikungunya, avian flu, swine flu, Zika virus, etc. which have emerged in last few decades. Main reasons behind the multiple incidents of emergence and re-emergence of infectious diseases in last two decades could be increase in the population, increased traveling in distal areas of world, close contact with different wild animals, etc. This was resulted in rapid spread of infectious agents and caused global pandemic or epidemic. However, a different angle to describe the increased incidences of infectious agents is “bioterrorism.” This has ability to emerge the infectious disease by deliberate introduction of infectious agents into the human, animal, or plant species like anthrax, smallpox, tularemia, etc.
1.2. Tropical infectious diseases
The infectious diseases which flourish in the hot and humid environment of the tropical regions are named tropical infectious diseases. As infectious agents thrive more in a tropical environment and hence tropical regions of the world have been severely affected compared to the temperate zone of the world. The primary reason behind the high incidences of infectious diseases in tropical countries lies in their environmental factors like hot and humid conditions, biological factors like good biodiversity of pathogens and social factors like impoverished community, lack of sanitation, and awareness regarding disease. These diseases are caused by the infections of arthropods and parasitic microorganisms like viruses, bacteria, or fungi and are mostly transmitted by air, sexual contact, drinking of contaminated water or food [15]. Sometimes insects like mosquitoes serve as carrier for transmission of infectious agents and inject microorganisms into human body through their bite [16].
The infectious disease that is occurred specifically in some countries of Africa, Asia, and Latin America where people do not have access to clean water and lack proper sanitation are classified as neglected tropical diseases (NTD). However, some diseases like malaria, HIV/AIDS, diarrhea, tuberculosis, hepatitis, etc. occurred all over the world. Here is the list of commonly occurred infectious diseases which are grouped according to their causative agents.
-
(i)
Bacterial diseases: Tuberculosis, typhus, disease caused by drug-resistant gram-negative bacteria, typhoid fever, bubonic plague, shigellosis, and the NTDs mycetoma, buruli ulcer, trachoma, leprosy, and yaws.
-
(ii)
Fungal diseases: the NTD mycetoma and cryptococcosis.
-
(iii)
Protozoan diseases: Leishmaniases (visceral, cutaneous, post kala-azar dermal, and mucocutaneous); cryptosporidiosis, malaria, and the NTDs Chagas disease, human African trypanosomiasis (HAT).
-
(iv)
Viral diseases: Zika disease, Lassa fever, Marburg virus disease, Ebola, HIV/AIDS, yellow fever, Rift Valley fever, COVID-19 (SARS-CoV-2), and the neglected dengue fever, chikungunya, and rabies.
-
(v)
Helminths or metazoan worms: NTDs including cysticercosis, echinococcosis, food-borne trematodiases (clonorchiasis, opisthorchiasis, fasciolosis), dracunculiasis (Guinea worm), schistosomiasis, soil-transmitted helminthiases (ascariasis, hookworm, trichuriasis, strongyloidiasis), lymphatic filariasis, and onchocerciasis [16], [17].
2. Emerging concerns of infectious diseases
Right through the beginning of human history, mankind has continuously faced emergence and re-emergence of various infectious diseases. Most infectious diseases are caused by virus, bacteria, fungus, and protozoa. Factors that are responsible for the emergence and re-emergence of infectious diseases include resistance to antimicrobial agents, scarcity of effective antimicrobial drugs, lack of targeted drug delivery systems, physicochemical properties of antimicrobial agents, and dormancy of infection.
2.1. Emerging and re-emerging infectious diseases
Emerging infectious diseases are diseases that are occurring first time and are responsible for the significant illness in one or more regions of the world. Infectious diseases those seen in developing or poor countries (tropical region) with specific virulence factors and those seen in developed countries (temperate region), are indiscriminate infection. Emerging infectious diseases include those infections which are recently shown in a particular region or within a specific population and its incident rate and geographical range are rapidly increasing soon [18]. Emerging diseases are supposed to be caused by:
-
a.
Previously unknown or not detected infectious agent.
-
b.
Previously known agent which is spread to the new geographical location and infect new population.
-
c.
Known microbial agents whose role in development of disease is not recognized.
-
d.
Re-emergence of the disease with increased incident rate, which was significantly decreased in past, but disease is reappeared, and its incidences are increasing [19].
Main reasons behind the appearance of emergence and re-emergence include infection by unknown agent, evolution of new agent, mutation of microorganism, and acquisition of resistance [20]. Emerging and re-emerging infectious diseases could be contributed by plenty of determinants which could be classified into these factors into three groups, i.e., agent factors, host factors, and environmental factors.
-
•
Agent factors: Microbial adaptation and change, development of resistance against antimicrobial agents
-
•
Host factors: Human demographic change, human behavior, poverty
-
•
Environmental factors: Deforestation, global warming, economic development
Emerging respiratory infections have captured the attention of scientific/medical community including public's fascination and concern for many years. The emergence of multiresistant bacterial strains belonging to the ESKAPE pathogen group (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter species) and high rates of viral spread, associated mostly with tropical and subtropical regions have massively contributed to this public health problem [21]. Various types of emerging infectious diseases are found all over the world, which have been classified below based on their mode of transmission.
-
•
Zoonotic diseases spread from animals to human involves anthrax, rabies, salmonellosis, trichinosis brucellosis, listeriosis, psittacosis, hantavirus pulmonary syndrome, and Q-Fever. Vaccine-preventable diseases, e.g., measles, mumps, and pertussis are also examples of zoonotic diseases.
-
•
Patient care system-associated infections include central-line-associated bloodstream infections, catheter-associated urinary tract infections, and surgical-site infections like Clostridium difficile infection.
-
•
Vector-borne infectious disease means an infection transmitted by blood-feeding anthropods, such as mosquitoes, ticks, and fleas to humans and other animals, which include Lyme disease, malaria, chikungunya, dengue fever, relapsing fever, plague, tularemia (rabbit fever), typhus (flea-borne, endemic), West Nile virus, Zika virus disease, Rocky Mountain spotted fever.
-
•
Food-borne diseases included cryptosporidiosis (Cryptosporidium), cyclosporiasis (Cyclospora spp.), Escherichia coli infection, Giardiasis (Giardia), Listeriosis (Listeria monocytogenes), Campylobacteriosis (Campylobacter), norovirus infection (aka Norwalk virus, calicivirus, viral gastroenteritis), Shigellosis (Shigella), salmonellosis (Salmonella), toxoplasmosis (Toxoplasma gondii), Vibrio infection (Vibrio parahaemolyticus), scombroid fish poisoning, yersiniosis (Yersinia species).
-
•
Intersecting epidemics of HIV, sexually transmitted infections, chronic viral hepatitis, tuberculosis, and COVID-19.
-
•
Mostly the oral diseases like dental caries, oral candidiasis, periodontitis, and peri-implantitis are caused by the specific type of bacteria named microbial dysbiosis.
2.2. Antimicrobial resistance (AMR)
Antimicrobial resistance (AMR) is a phenomenon where microorganisms initially susceptible to antimicrobials agents, become resistant, and antimicrobials slowly lose their potency toward previously susceptible microorganisms [22]. Microorganisms that develop AMR are sometimes referred to as “superbugs.” AMR threatens the effective prevention and treatment of an ever-increasing range of infections caused by bacteria, parasites, viruses, and fungi. AMR occurs when bacteria, viruses, fungi, and parasites change over time and no longer respond to medicines making infections harder to treat and increasing the risk of disease spread, severe illness, and death. As a result, the medicines become ineffective and infections persist in the body, increasing the risk of spread to others.
In 2015 the World Health Organization (WHO) during its world health assembly adopted the Global Action Plan (GAP) on AMR. The GAP lists “improved awareness and understanding of antimicrobial resistance through effective communication, education, and training” as its first objective. Resistance is mostly developed by the mutation in genetic makeup of microorganisms. Microorganisms have only one feature of survival so that their entire genome was designed in such a way that they continuously create mechanism of survival. Resistance to antimicrobial agents was mostly developed because of administration of antimicrobial agents in a subtherapeutic dose or administration of wrong antibiotics or poor management of antibiotic treatment [23]. Most usual reasons behind the development of resistance include enzymatic inactivation, modification of target site, biofilm formation, alteration in activity of efflux pump, intracellular localization, etc. [24].
Intracellular obstinacy of pathogen is an important concern in development of resistance, because it provides ability to pathogen to get hold of degradative mechanisms of macrophages and other polymorphonuclear cells. Thus, pathogens can easily persisted, replicated, and disseminate in cellular environment as they found suitable conditions for growth [25]. Currently, multiple chemotherapeutic agents are losing their activity against various pathogens in which malaria and tuberculosis are the diseases that showed death due to the antibacterial resistance developed by the pathogens. Basically, this was more commonly seen in some highly virulent pathogens such as K. pneumoniae, P. aeruginosa, S. aureus, A. baumannii, E. faecium, Enterobacter species, etc. [26], [27]. These pathogens are either spread by hospital environment or through community transmission. By seeing its criticality worldwide call has been issued to carefully use the existing antimicrobials and to develop antibiotic adjuvants that hit bacterial nonessential targets.
2.3. Scarcity of molecular targets
Availability of the target molecules plays an important role in development of new antiinfective agents. One of the reasons for emergence of resistance is the number of drugs per target. There is a wide variation in topology of targets; some targets provide wide selectivity for drug binding (glucocorticoid receptors has 61 approved drugs) while some targets provide limited selectivity for drug binding (kinase inhibitors have only few targeting drugs) [16], [28].
Recently, small-molecule chemical probes are being explored as they can be used to interrogate the biological relevance of a target in a disease model [29] and are beneficial as essential tools in the early stages of drug discovery. Chemogenomics libraries, selective small-molecule pharmacological can be used to illuminate new target, as the hit molecules selected from the set of these agents via phenotypic screening gives the idea that the attack on selected target by pharmacological agent will result in phenotypic perturbation [30].
2.4. Carrier and dormancy of infection
All microorganisms, when exposed to periodic stresses that inhibit growth, especially bacteria and fungi, enters into a hardy, nonreplicating state, often termed quiescence, or dormancy. During dormancy period of an infection, slow-growing pathogen can tolerate both immune insults and prolonged antibiotic exposure [31]. In pathophysiology of emerging and re-emerging diseases, dormancy period of pathogen play an important role in deciding treatment regimen. For example, some strains of malaria-like Plasmodium vivax and P. ovale can remain in dormant stage in human hepatocyte cells for weeks to years, which can potentially cause the disease once their dormancy period will complete [32].
Similarly, in case of latent tuberculosis infection (LTBI), dormancy is a potentially reversible state so that the mycobacterial cell remains viable and can restore its ability to divide. In an infected organism, the Mtb recovery from dormancy may result in LTBI reactivation and subsequent development of active TB. The risk of LTBI reactivation is manifold increase in the immunocompromised individuals [33]. Quiescent and dormant pathogens have comparatively very less metabolic activity than the normal pathogens so that they are more likely to remain unaffected by antibiotics. Also, the development of identification tests for dormant microbes is difficult. To tackle this situation new antiinfective agents are required which can identify the dormant and quiescent microbes and eliminate them from human body.
2.5. Physicochemical properties of antimicrobial drugs
To get the maximum benefit of the antimicrobial agents, it is very essential to administer the right amount of dose and ensure that drug reaches the site where pathogens are located. The physicochemical properties of the antimicrobial agents which decide its efficacy include solubility, molecular weight, lipophilicity, number of hydrogen bond donors and acceptor, etc. [34]. To kill the microbes, it is necessary for antibiotic molecules to penetrate inside the microorganism. Normally, antibiotics used porins protein for easy penetration inside the pathogens. This mechanism is commonly seen in the gram-negative bacteria, which are surrounded by the outer membrane. The drug must cross the cell membrane, which is made of lipid bilayer and to cross this barrier, antibiotic molecule requires certain degree of lipophilicity. Therefore, antibiotic molecules should have ability to penetrate inside the pathogen cells to elicit optimum therapeutic effect [35].
Once antibiotic enters in intracellular compartment, there are chances of being expelled out by efflux pump. Some microorganisms such as Chlamydia trachomatis, Mycobacterium tuberculosis, Trypanosoma cruzi and some viruses are situated inside host cell and hinder the penetration of antibiotic drugs. Moreover, some pathogens reside in extreme intracellular conditions like Leishmania spp. localized in acidic organelles and M. tuberculosis found in necrotic granulomas. Thus, to target the pathogen it has been important to prepare a drug which can penetrate inside the pathogen by nonvascularized lipid-rich caseum. Microbes also tend to infect the organs which are protected by tissue or blood barrier for example Taenia solium, Cryptococcus neoformans found in central nervous system while C. trachomatis lies in the eye. To treat these types of infection, drugs need to administer in blood and to reach the target site, drugs should have ability to pass through the endothelial cells and pumped back to the systemic circulation [36].
Treatment cost is also the critical factor which directly affects the financial burden on patients with limited resources. As infectious diseases are more likely to occur in poor-income countries, cost plays an important role in disease control. For example, in case of malaria, it is aimed to reduce the treatment cost up to US$1. To achieve this goal there is a need to develop short and cost-effective synthesis procedure, excluding the complex dosage forms, and advancement in supply chain. Drugs that can easily be transported without need of cold chain and can tolerate temperatures up to 40°C and high humidity are required.
2.6. Poor patient compliance
Emerging diseases are generally tending to infect large population, and hence to reach out to the large population, treatment must be patient compliant, and drug should be administered with minimum medical support. Patient compliance is “patient's behaviors (in terms of taking medication, following diets, or executing lifestyle changes) coincide with physician recommendations for health and medical advice.” Various factors affect patient compliance, i.e., patient-centered factors, therapy-related factors, social and economic factors, healthcare system factors, and disease factors. Patients’ perceptions about infectious diseases and their adherence to treatment protocol and dosage regimen are important in fighting with resistance development, particularly in case of diseases like tuberculosis, and hence efforts should be made to improve the patient compliance by devising new formulation approaches which can improve patient compliance [37], [38].
In addition to this, it has also been important to reduce the complexity in dosing and dosing frequency. To improve the patient compliance recently single-dose treatment for malaria has been identified.
3. Drug delivery in infectious diseases
Treatment of infectious diseases mostly depends on antimicrobial drugs of natural, semisynthetic, or synthetic origin. Mechanism of action of some antimicrobial agents along with their limitations in treatment of infectious diseases is summarized in Table 1 . Structure of some frequently used antimicrobial drugs in treatment of various infectious diseases is given in Fig. 4 . Normally, it has been noted that most of the microorganisms affect the specific organ(s) like lower respiratory tract, upper respiratory tract, urinary tract, liver, etc. for instance, SARS-CoV-2 virus, which is responsible for COVID-19 disease, causes infection in the upper or lower part of the respiratory tract; and such behavior of microorganisms is named as Tropism. Most effective approach to overcome the infection is to develop a drug delivery system, which can target the specific organ where parasite resides or produces infection related symptoms, for maximum therapeutic effect with reduced side effects caused by the antimicrobial agents. However, vaccines provide highest level of protection because it helps to eliminate the risk of infection in future [39], [40]. In past, vaccination has been used as effective tool to decrease or sometimes to eliminate the prevalence of infectious diseases, for example, widespread polio vaccine programs were helpful in elimination of endemic conditions caused by polio virus [1], [41]. Various drug delivery and treatment strategy used by scientists to control infectious diseases are shown diagrammatically in Fig. 5 .
Table 1.
Mechanisms of action and limitations of antimicrobial drugs currently used in treatment of various infectious diseases.
| S. No. | Drug | Category | Mechanism of action | Limitation |
|---|---|---|---|---|
| 1 | Rifampicin | Antitubercular Drug | Inhibit bacterial DNA-dependent RNA polymerase | Development of resistance against various antitubercular drugs |
| 2 | Enfuvirtide | Antiretroviral drugs | To prevent the fusion of HIV to the CD4 or another target cell | Resistance and viral evolution |
| 3 | Zidovudine | Antiviral drug | After phosphorylation it competes with endogenous nucleotides for incorporation into the viral DNA | Emergence of resistant mutants, megaloblastic anemia, and leukopenia |
| 4 | Remdesivir | Antiviral drugs | A nucleoside analog and inhibits the RNA-dependent RNA polymerase (RdRp) | Increased liver enzyme levels that may indicate possible liver damage |
| 5 | Metronidazole | Antiprotozoal drugs | Inhibits protein synthesis by interacting with DNA and causing a loss of helical DNA structure and strand breakage | Use is limited to only certain bacterial and parasitic infections, also causes GIT disturbance |
| 6 | Sulfamethoxazole | Sulfonamides | Inhibit with intermediary metabolism of microorganism | Allergic reaction, GIT disturbance |
| 7 | Azithromycin | Macrolide antibiotics | Inhibits bacterial protein synthesis | Allergic reaction |
| 8 | Cephalosporins | Beta-lactamase inhibitors | It inhibits synthesis of the bacterial cell wall | GIT disturbance and superinfection |
| 9 | Diloxanide furoate | Antiamoebic drugs | Uncertain, may inhibit protein synthesis or destroys the trophozoites of E. histolytica that eventually form into cysts | Abdominal cramps and skin rashes |
| 10 | Amoxicillin. | Beta-lactam antimicrobials | Inhibit bacterial cell wall synthesis | Cause superinfections and crystalluria |
| 11 | Cephalexin | Cephalosporins | Inhibit bacterial cell wall synthesis | Tendinopathy and drug–drug interactions |
| 12 | Ciprofloxacin | Fluoroquinolone | Inhibits DNA replication by inhibiting bacterial DNA topoisomerase and DNA-gyrase | Lower serum aminotransferase's level |
| 13 | Clindamycin | Lincomycin antibiotics | Inhibit bacterial protein synthesis by binding to the 50s ribosomal subunit of bacteria | Causes Clostridium difficile-associated diarrhea |
Fig. 4.
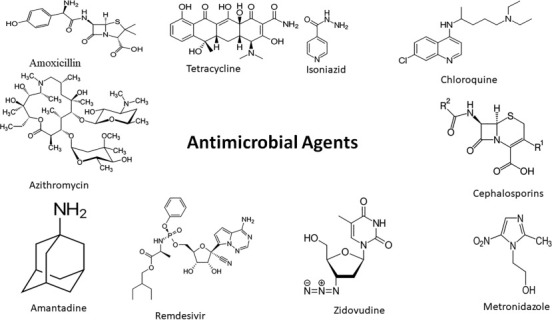
Structure of some frequently used antimicrobial drugs in treatment of various infectious diseases.
Fig. 5.

Diagrammatic representation of various drug delivery and treatment strategy used by scientists to control infectious diseases.
4. Challenges associated with treatment of infectious diseases
In treatment of infectious diseases, vectors play an important role by transferring the pathogen among human population, and hence relationship among human, vector and pathogen should be studied thoroughly. Mostly used therapeutic agents for treatment of infectious diseases are antimicrobials, but there are some challenges in delivery of these agents like low intracellular drug concentration, drug efflux by efflux pump, enzymatic degradation, and most severe is development of antimicrobial resistance. Depending on the nature of microorganism there are some parasites, which reside in extracellular part such as epithelial surface and some of them reside in intracellular part such as reticuloendothelial system. Extracellular microorganisms release the toxins or proteins which leads to the production of antibodies and are easily killed by opsonization followed by phagocytosis. However, intracellular infections are difficult to treat. One of the characteristics of intracellular infection is formation of microbial biofilm, which is responsible for the virulence factor and further development in chronic infections. These microbial characteristics are responsible for the development of drug resistance, and thus conventional antimicrobial therapy did not work. This condition requires combinatorial therapies (combination of two different therapies like combination of chemotherapy and radiotherapy) or multitargeted drug delivery systems [42], [43], [44]. Other challenges associated with treatment of infectious diseases are lack of economic prospects for investment in this field, and hence approaches aiming at the rational design of compounds, such as structure-based drug discovery (SBDD), fragment screening, target-based drug discovery, and drug repurposing should be given special interest [45].
5. Drug delivery challenges in infectious diseases
Current treatment of infectious diseases contains administration of the single or combination of bactericidal and/or bacteriostatic drugs to achieve the complete eradication of infective agent with least possible chance to develop an antibiotic resistance. There are some limitations in the traditional treatment of antimicrobial drugs such as high toxicity, low efficacy, long duration of treatment, emergence of multidrug resistance and extensive drug resistance along with less patient compliance. To solve these challenges associated with conventional use of antimicrobial agents, scientists are exploring different strategies, one of them is targeted delivery of antimicrobial agents [39], [40], [46], [47].
The targeted delivery of antibiotics or antimicrobial agents has shown significant reduction in treatment time and increase in the effectiveness of drug molecules in many preclinical and clinical studies. Moreover, targeted drug delivery systems can limit the emergence of infectious diseases caused by antibiotic-resistant pathogens. Additionally, shorter duration of treatment also improves the patient compliance. Development of new drug delivery systems along with development of the new antiinfective molecules are the top strategies in potentially preventing infectious diseases [5], [47], [48], [49], [50].
For example, Methicillin-resistant S. aureus (MRSA) has ability to cause severe infections. There are multiple antibiotics used to treat MRSA including vancomycin (gold standard) along with linezolid, daptomycin, teicoplanin and recently discovered ceftaroline. Linezolide have shown the ability to isolate MRSA, and hence its use is preferred over vancomycin in nosocomial pneumonia [51], [52].
6. Strategies to deal with challenge
Three basic strategies to combat the infectious diseases include (i) controlling source of infection, (ii) cutting the transmission chain, and (iii) isolating the susceptible patients. Among them, control of infection source requires early treatment and isolation of patient along with fast treatment of patients, which require fast and sensitive testing kit. However, conventional methods for the treatment of infectious disease mainly depend on combination of various antibiotics. Commonly seen side effects of conventional therapy include long-term treatment, poor patient compliance, and various antibiotic-dependent adverse effects. New strategies for the development of antiinfective medicine contain synergistic combination of antibiotics, which can improve drug efficacy, reduced side effects and can decrease time of treatment compared to the conventional antibiotics [25], [49], [53].
The main problem that scientists face with infectious diseases during the development of new drug is, lack of relevant predictive cellular or animal models of human diseases. Along with this, insufficient knowledge regarding microorganisms’ biology and little or no practice of developing small molecules are the main reasons which affect the development of new antiinfective agents. However, latest knowledge regarding the microorganism's characteristics, which are responsible for the resistance development such as biofilm production, molecular interfering with host immune response, efflux pump, etc., have helped in decreasing the resistance development toward the antibiotics [25], [49].
During last four decades, a renaissance of scientific research strategies targeting host factors, rather than pathogen components directly, has opened novel treatment approaches termed host-directed therapies [54]. Host-directed therapies may act as adjunct treatment options for bacterial, viral, and parasitic infectious diseases. It includes commonly used drugs for communicable diseases with good safety profiles, immunomodulatory agents, biologics (e.g., monoclonal antibodies), nutritional products, and cellular therapy using the patient's own immune or bone marrow mesenchymal stromal cells [55].
6.1. Optimization of bioavailability and pharmacokinetic parameters
Optimization of bioavailability and pharmacokinetic parameters, which ensures the right amount of drug is delivered to the right place for right duration is necessary for the stoppage of resistance development toward drug [42]. The newer quorum sensing antibiotics are found to be better pathogen blockers compared to the traditional antibiotic because they are not targeted to kill the pathogens. Even if these antibiotics entered the intracellular space, their availability is limited due to the lack of the sufficient permeability toward the cell envelop and this condition further worsens in case of gram-negative bacteria [49], [56]. Ligand decorated nanoformulations [17], [40] of various antibiotics could be explored to reduce toxicity, increase drug localization index with better macrophage uptake, potential immunomodulatory effect, and antimicrobial activity as alternative to available formulations of antibiotics. Various biopharmaceutical parameters of chemotherapeutics agents should be considered in relation to multidrug resistance microbial agents such as tuberculosis, leishmaniasis, etc. [4].
6.2. Nanotechnology mediated delivery of antimicrobial drugs
Infectious agents those inhabitants to intracellular spaces are more prone to develop the multiple resistance strain. To overcome this problem antibiotics against the pathogens like Salmonella typhimurium, M. tuberculosis, and S. aureus which reside in intracellular compartment were given in a special nano-sized drug carrier system. Different nanocarriers have been explored by scientists for the intracellular targeting of infectious pathogens including metallic NPs, polymeric NPs, lipid-based NPs, dendrimers, QDs, carbon nanotubes, nanocrystals, 3D printed nanomedicine, etc. [57], [58], [59], [60], [61], [62].
Nanocarrier systems have immense potential to provide targeted delivery of antibiotic molecules. Nucleotides attached drug delivery carriers are newer approach in intracellular targeting of antibiotic molecules which has high selectivity and specificity to kill or modify bacteria. Some other approaches used for targeted drug delivery and reducing the occurrences of drug resistance are exploration of cell-specific molecules, antigen-targeted molecules, and attachment of intracellular markers [63], [64], [65]. To eradicate the influenza infection, one nanoparticulate formulation of diphyllin and bafilomycin has emerged as an excellent tool which has ability to inhibit the vacuolar ATPase [66]. Another application of nanotechnological formulation is in vaccine manufacturing, for instance, m-RNA based lipid nanoparticle-based vaccine is currently under clinical trials for the newly emerging disease COVID-19 [41].
To increase intracellular targeting of antiparasitic drugs to macrophages via surface engineered nanocarrier such as dendrimers could be a fruitful strategy for various infectious diseases The grafting or multimeric presentation of immunostimulator and macrophage-activator dipeptide on the surface of nanostructured dendrimers increases the drug tolerance after delivery with carrier system and increased uptake of bioactive by macrophages [17], [40].
6.3. Antimicrobial peptides
One of the newer approaches is antimicrobial peptides (AMPs) which is said to be the future therapeutic alternatives of antibiotics. AMPs have unique features like broad-spectrum antibacterial activity along with antibiofilm and immunomodulatory action. Evolution in the recombinant DNA technology encourages the production and advancement in drug delivery systems like formation of polymer-peptide conjugates, organometallic complexes, etc. encourage the therapeutic use of AMPs molecules and tailoring them for specific applications. In the last decade, United States Food and Drug Administration (USFDA) has successfully approved multiple peptide molecules for antimicrobial activity [67].
AMPs possess many advantages like high specificity, good selectivity, potential antimicrobial activity, less tissue accumulation, and high penetration. Chemically AMPs are the semisynthetic peptide molecules that are derived from the abundantly available natural peptide molecules. Main characteristics of AMPs include ability to bypass the resistance induced by pathogens against antibiotics and broad-spectrum activity against microbes [67], [68].
7. Current scenario and future perspectives
Continuing efforts are needed to develop more effective strategies, particularly for critically ill patients suffering from severe infectious diseases. To curb emerging infectious diseases, it is important to develop effective strategies for prevention and treatment of causing agent persistence in immune-privileged sites, proper optimization of postexposure prophylaxis, and to increase therapeutic breadth by discovering newer antibiotics or using promising strategies like nanotechnology, nanotheranostics, targeted delivery systems for attacking the disease-causing parasites present in cells effectively without producing adverse effects [69], [70].
As antibody-based approaches are identified and advanced, promising small-molecule antivirals currently in clinical-stage development should continue to be evaluated for various infectious diseases, with consideration of their added value in combination approaches with bundled supportive care, their penetration in tissues of interest, the absence of interaction with glycoprotein-based vaccines, and filoviral breadth. The sporadic emergence of new human pathogens, shifts in geographic distributions, and on top of this, the development of antimicrobial (antibiotics, antiviral, antifungal, and antiparasitic actives) resistance emphasizes the urgent need to maintain and intensify our efforts in antiinfective research to ensure as much preparedness as possible. Targeting and releasing antimicrobial drugs into infected reservoirs could be achieved by development of multifunctional nanocargoes encapsulated with drug. It seems to be promising strategy to circumvent the challenge of multidrug resistance [25], [49], [70].
“Nanotheranostics” is the use of nanotechnology to develop nanomedicines for combined diagnostics and therapeutics. These are made up of colloidal NPs ranging from 1 to 1000 nm. “Nanotheranostics” is defined as a unified nanotherapeutic platform in which therapeutic and diagnostic functions are combined within a unique system and permits simultaneous detection, spatial targeting as well as tracking the response of therapy [71]. In recent years, various conjugated systems such as bioorganic, bioinorganic, functional inorganic and organic, biological NPs, and so on, have been developed and applied to localized nanomedicine in various types of disease conditions due to their better contrasting and therapeutic ability [72]. Recently nanotheranostics are applicable in various cancerous conditions because these systems have better multimode contrasting ability for deep tissue visualization, a large surface area to load therapeutic molecules, easy surface functionalization, high biocompatibility, smooth and easy circulation, specific biodistribution, high tumor-binding ability [73], [74].
Nanotheranostics is regarded as a promising approach for the management of various infectious diseases. Different classes of receptor molecules are used for targeting bacterial pathogens and the role of various types of NPs serve as efficient carrier agents for these receptors [75]. Besides bacteria, theranostic platforms also exist for parasitic and viral infections [76]. Bioengineered nanomaterials with suitable physicochemical characteristics for site-specific therapeutic delivery, highly sensitive nanobiosensors for detection of very low virus concentration, and real-time protections using the nanorobots can provide roadmaps toward the imminent breakthroughs in theranostics of a variety of diseases including the COVID-19 [77].
8. Conclusions and summary
As described throughout this chapter, various emerging and re-emerging infectious diseases spread globally via several factors. Although a lot of challenges are associated with treatment of these diseases with various antimicrobial agents, yet there is need for novel pharmaceutical formulations and strategies for treatment of infectious diseases. One of such promising strategies seems to be use of nanomedicines and nanotheranostics, which are showing promising potential in treatment of various diseases as being revealed by many in vitro and preclinical investigations as well as in some clinical studies. Various nanoformulation strategies have been explored by scientists, which showed significant improvement in bioavailability, permeability, and toxicity profile of traditional antibiotics leading to increased patient acceptance and more effectiveness. Due to these advantages now, it has been important to continue the nanoparticulate-related investigations for the treatment of infective diseases attributed to its great potential to give good results in difficult-to-treat infections.
Acknowledgment
The authors (K.J., P.R.P., and S.N.) are thankful for support of Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India. NIPER-Raebareli communication number for this publication is NIPER-R/Communication/243.
References
- 1.Jain K., Jain N.K. Vaccines for visceral leishmaniasis: a review. J Immunol Methods. 2015;422:1–12. doi: 10.1016/j.jim.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 2.Nii-Trebi N.I. Emerging and neglected infectious diseases: insights, advances, and challenges. Biomed Res Int. 2017:5245021. doi: 10.1155/2017/5245021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley B.T., Bryan A. Emerging respiratory infections: the infectious disease pathology of SARS, MERS, pandemic influenza, and Legionella. Semin Diagn Pathol. 2019;36(3):152–159. doi: 10.1053/j.semdp.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardhi V.P., Jain K. Impact of binary/ternary solid dispersion utilizing poloxamer 188 and TPGS to improve pharmaceutical attributes of bedaquiline fumarate. J Drug Deliv Sci Technol. 2021;62(102349):1–13. [Google Scholar]
- 5.Jain V., Jain K. Molecular targets, and pathways for the treatment of visceral leishmaniasis. Drug Discov Today. 2018;23(1):161–170. doi: 10.1016/j.drudis.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Jain K., Gupta U., Jain N.K. Dendronized nanoconjugates of lysine and folate for treatment of cancer. Eur J Pharm Biopharm. 2014;87(3):500–509. doi: 10.1016/j.ejpb.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Jain K. Nanohybrids of dendrimers and carbon nanotubes: a benefaction or forfeit in drug delivery? Nanosci Nanotechnol-Asia. 2019;9(1):21–29. [Google Scholar]
- 8.Joshi K., Chandra A., Jain K., Talegaonkar S. Nanocrystalization: an emerging technology to enhance the bioavailability of poorly soluble drugs. Pharm Nanotechnol. 2019;7(4):259–278. doi: 10.2174/2211738507666190405182524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madamsetty V.S., Mukherjee A., Mukherjee S. Recent trends of the bio-inspired nanoparticles in Cancer Theranostics. Front Pharmacol. 2019;10:1264. doi: 10.3389/fphar.2019.01264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauro R., Nandave M., Jain V.K., Jain K. Advances in dendrimer-mediated targeted drug delivery to the brain. J Nanopart Res. 2021;23(3):1–20. [Google Scholar]
- 11.Bagre A.P., Jain K., Jain N.K. Alginate coated chitosan core shell nanoparticles for oral delivery of enoxaparin: in vitro and in vivo assessment. Int J Pharm. 2013;456(1):31–40. doi: 10.1016/j.ijpharm.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 12.Jain K. In: Biopolymer-based composites. Jana S., Maiti S., Jana S., editors. Woodhead Publishing; 2017. Chapter 7: Dendrimers: smart nanoengineered polymers for bioinspired applications in drug delivery; pp. 169–220. [Google Scholar]
- 13.Itani R., Tobaiqy M., Al F.A. Optimizing use of theranostic nanoparticles as a life-saving strategy for treating COVID-19 patients. Theranostics. 2020;10(13):5932–5942. doi: 10.7150/thno.46691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anwar A., Siddiqui R., Khan N.A. Importance of Theranostics in rare brain-eating amoebae infections. ACS Chem Nerosci. 2019;10(1):6–12. doi: 10.1021/acschemneuro.8b00321. [DOI] [PubMed] [Google Scholar]
- 15.De Rycker M., Baragana B., Duce S.L., Gilbert I.H. Challenges and recent progress in tropical disease drug discovery. Nature. 2018;559:498–506. doi: 10.1038/s41586-018-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain K., Jain N.K. Novel therapeutic strategies for treatment of visceral leishmaniasis. Drug Discov Today. 2013;18(23–24):1272–1281. doi: 10.1016/j.drudis.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Jain K., Verma A.K., Mishra P.R., Jain N.K. Characterization and evaluation of amphotericin B loaded MDP conjugated poly(propylene imine) dendrimers. Nanomedicine. 2015;11(3):705–713. doi: 10.1016/j.nano.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 18.McArthur D.B. Emerging infectious diseases. Nurs Clin North Am. 2019;54(2):297–311. doi: 10.1016/j.cnur.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baylor College of Medicine Emerging infectious diseases. 2021. https://www.bcm.edu/departments/molecular-virology-and-microbiology/emerging-infections-and-biodefense/;
- 20.Menon S., Mathew M.R., Sam S., Keerthi K., Kumar K.G. Recent advances and challenges in electrochemical biosensors for emerging and re-emerging infectious diseases. J Electroanal Chem (Lausanne) 2020;878 doi: 10.1016/j.jelechem.2020.114596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomes B., Augusto M.T., Felício M.R., Hollmann A., Franco O.L., Gonçalves S., et al. Designing improved active peptides for therapeutic approaches against infectious diseases. Biotechnol Adv. 2018;36(2):415–429. doi: 10.1016/j.biotechadv.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Othieno J.O., Obadiah Njagi O., Azegele A. Opportunities and challenges in antimicrobial resistance behavior change communication. One Health. 2020;11 doi: 10.1016/j.onehlt.2020.100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rennie R.P. Current and future challenges in the development of antimicrobial agents. Handb Exp Pharmacol. 2012;211:45–65. doi: 10.1007/978-3-642-28951-4_4. [DOI] [PubMed] [Google Scholar]
- 24.Dominguez D.C., Meza-Rodriguez S.M. In: Pharmaceuticals and personal care products: waste management and treatment technology, emerging contaminants and micro pollutants. MNV P., Vithanage M., Kapley A., editors. 2019. Development of antimicrobial resistance: future challenges; pp. 383–408. [Google Scholar]
- 25.Kiani M.H., Imran M., Raza A., Shahnaz G. Multifunctionalized nanocarriers targeting bacterial reservoirs to overcome challenges of multi drug-resistance. Daru. 2020;28(1):319–332. doi: 10.1007/s40199-020-00337-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerezales M., Ocampo-Sosa A.A., Alvarez Montes L., Diaz Rios C., Bustamante Z., Santos J., et al. High prevalence of extensively drug-resistant Acinetobacter baumannii at a children hospital in Bolivia. Pediatr Infect Dis. 2018;37:1118–1123. doi: 10.1097/INF.0000000000001962. [DOI] [PubMed] [Google Scholar]
- 27.Liu C., Chang Y., Xu Y., Luo Y., Wu L., Mei Z., et al. Distribution of virulence associated genes and antimicrobial susceptibility in clinical Acinetobacter baumannii isolates. Oncotarget. 2018;9:21663–21673. doi: 10.18632/oncotarget.24651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan M.A., Jain V.K., Rizwanullah M., Ahmad J., Jain K. PI3K/AKT/mTOR pathway inhibitors in triple-negative breast cancer: a review on drug discovery and future challenges. Drug Discov Today. 2019;24(11):2181–2191. doi: 10.1016/j.drudis.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Mullard A. A probe for every protein. Nat Rev Drug Discov. 2019;18:733–736. doi: 10.1038/d41573-019-00159-9. [DOI] [PubMed] [Google Scholar]
- 30.Castaldi M.P., Hendricks J.A., Zhang A.X. Design, synthesis and strategic use of small chemical probes toward identification of novel targets for drug development. Curr Opin Chem Biol. 2020;56:91–97. doi: 10.1016/j.cbpa.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Rittershaus E.S., Baek S.H., Sassetti C.M. The normalcy of dormancy: common themes in microbial quiescence. Cell Host Microbe. 2013;13(6):643–651. doi: 10.1016/j.chom.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rycker M.D., Baragaña B., Duce S.L., Gilbert I.H. Challenges and recent progress in drug discovery for tropical diseases. Nature. 2018;559(7715):498–506. doi: 10.1038/s41586-018-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batyrshina Y.R., Schwartz Y.S. Modeling of mycobacterium tuberculosis dormancy in bacterial cultures. Tuberculosis. 2019;117:7–17. doi: 10.1016/j.tube.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Ozumchelouei E.J., Hamidian A.H., Zhang Y., Yang M. Physicochemical properties of antibiotics: a review with an emphasis on detection in the aquatic environment. Water Environ Res. 2020;92(2):177–188. doi: 10.1002/wer.1237. [DOI] [PubMed] [Google Scholar]
- 35.Silver L.L. A gestalt approach to gram-negative entry. Bioorg Med Chem. 2016;24:6379–6389. doi: 10.1016/j.bmc.2016.06.044. [DOI] [PubMed] [Google Scholar]
- 36.Selvi B.A., Hlaing Y.C.S., Infante K., Kaner M., Gualano M., Patel D., et al. Physicochemical characterization, solubilization, and stabilization of a macrolide antibiotic. J Drug Deliv Sci Technol. 2020;57 [Google Scholar]
- 37.Muh Zainal S., Sapar S., Irwandy. The effect of patients’ perception about tuberculosis (TB) against treatment compliance. Enferm Clin. 2020;30(2):416–419. [Google Scholar]
- 38.Jin J., Sklar G.E., Oh V.M., Li S.C. Factors affecting therapeutic compliance: a review from the patient's perspective. Ther Clin Risk Manag. 2008;4(1):269–286. doi: 10.2147/tcrm.s1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shoji Y., Shimada J., Mizushima Y. Drug delivery system to control infectious diseases. Curr Pharm Des. 2002;8:455–465. doi: 10.2174/1381612023395934. [DOI] [PubMed] [Google Scholar]
- 40.Jain K., Verma A.K., Mishra P.R., Jain N.K. Surface-engineered dendrimeric nanoconjugates for macrophage-targeted delivery of amphotericin B: formulation development and in vitro and in vivo evaluation. Antimicrob Agents Chemother. 2015;59(5):2479–2487. doi: 10.1128/AAC.04213-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pilkington E.H., Suys E.J.A., Trevaskis N.L., Wheatley A.K., Zukancic D., Algarni A., et al. From influenza to COVID-19: lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater. 2021;131:16–40. doi: 10.1016/j.actbio.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koo H., Allan R.N., Howlin R.P., Stoodley P., Hall-Stoodley L. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol. 2017;15(12):740–755. doi: 10.1038/nrmicro.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmad J., Gautam A., Komath S., Bano M., Garg A., Jain K. Topical nano-emulgel for skin disorders: formulation approach and characterization. Recent Pat Antiinfect Drug Discov. 2019;14(1):36–48. doi: 10.2174/1574891X14666181129115213. [DOI] [PubMed] [Google Scholar]
- 44.Ojha B., Jain V.K., Gupta S., Talegaonkar S., Jain K. Nanoemulgel: a promising novel formulation for treatment of skin ailments. Polym Bull. 2021 doi: 10.1007/s00289-021-03729-3;1-25. [DOI] [Google Scholar]
- 45.De Godoy A.S., Fernandes R.S., ACC A., Bueno R.V., de Moraes Roso Mesquita N.C., RVC G., Oliva G. Structural and mechanistic insight from antiviral and antiparasitic enzyme drug targets for tropical infectious diseases. Curr Opin Struct Biol. 2019;59:65–72. doi: 10.1016/j.sbi.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 46.Jain K., Kesharwani P., Gupta U., Jain N.K. Dendrimer toxicity: let's meet the challenge. Int J Pharm. 2010;394(1–2):122–142. doi: 10.1016/j.ijpharm.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 47.Dua K., Rapalli V.K., Shukla S.D., Singhvi G., Shastri M.D., Chellappan D.K., et al. Multi-drug resistant mycobacterium tuberculosis & oxidative stress complexity: emerging need for novel drug delivery approaches. Biomed Pharmacother. 2018;107:1218–1229. doi: 10.1016/j.biopha.2018.08.101. [DOI] [PubMed] [Google Scholar]
- 48.Sharma A., Jain K., Flora S.J.S. Vitamins based novel target pathways/molecules as possible emerging drug targets for the Management of Tuberculosis. Med Chem. 2018;14(3):212–224. doi: 10.2174/1573406413666171103093235. [DOI] [PubMed] [Google Scholar]
- 49.Loretz B., Oh Y.K., Hudson S., Gu Z., Lehr C.M. Drug delivery for fighting infectious diseases: a global perspective. Drug Deliv Transl Res. 2021;11(4):1316–1322. doi: 10.1007/s13346-021-01009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jain K., Shukla R., Yadav A., Ujjwal R.R., Flora S.J.S. 3D printing in development of nanomedicines. Nanomaterials (Basel) 2021;11(2):420. doi: 10.3390/nano11020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guillard P., Blanchardie’re A., Cattoir V., Fischer M., Verdon R., Saint-Lorant G. Antimicrobial stewardship and linezolid. Int J Clin Pharm. 2014;36:1059–1068. doi: 10.1007/s11096-014-9995-9. [DOI] [PubMed] [Google Scholar]
- 52.Schuetz A.N. Laboratory antimicrobial stewardship during an outbreak of linezolid- and vancomycin-resistant enterococcus faecium bacteremia. Clin Infect Dis. 2019;69(2):266–267. doi: 10.1093/cid/ciy1023. [DOI] [PubMed] [Google Scholar]
- 53.Wang C., Liu M., Wang Z., Li S., Deng Y., He N. Point-of-care diagnostics for infectious diseases: from methods to devices. Nano Today. 2021;37 doi: 10.1016/j.nantod.2021.101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zumla A., Rao M., Wallis R.S., Kaufmann S.H.E., Rustomjee R., Mwaba P., et al. Host-directed therapies for infectious diseases: current status, recent progress, and future prospects. Lancet Infect Dis. 2016;16(4):e47–e63. doi: 10.1016/S1473-3099(16)00078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crevel R. Improving host-directed therapy for tuberculous meningitis by linking clinical and multi-omics data. Tuberculosis. 2021;128 doi: 10.1016/j.tube.2021.102085. [DOI] [PubMed] [Google Scholar]
- 56.Ropponen H.K., Richter R., Hirsch A.K., Lehr C.M. Mastering the gram-negative bacterial barrier-chemical approaches to increase bacterial bioavailability of antibiotics. Adv Drug Deliv Rev. 2021;172:339–360. doi: 10.1016/j.addr.2021.02.014. [DOI] [PubMed] [Google Scholar]
- 57.Montanari E., Oates A., Di Meo C., Meade J., Cerrone R., Francioso A., et al. Hyaluronan-based nanohydrogels for targeting intracellular S. aureus in human keratinocytes. Adv Healthc Mater. 2018;7(12) doi: 10.1002/adhm.201701483. [DOI] [PubMed] [Google Scholar]
- 58.Hibbitts A., O'Leary C. Emerging nanomedicine therapies to counter the rise of methicillin-resistant Staphylococcus aureus. Materials (Basel) 2018;11(2):321. doi: 10.3390/ma11020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee B., Lee D.G. Synergistic antibacterial activity of gold nanoparticles caused by apoptosis-like death. J Appl Microbiol. 2019;127(3):701–712. doi: 10.1111/jam.14357. [DOI] [PubMed] [Google Scholar]
- 60.Tenland E., Pochert A., Krishnan N., Umashankar Rao K., Kalsum S., Braun K., et al. Effective delivery of the anti-mycobacterial peptide NZX in mesoporous silica nanoparticles. PLoS One. 2019;14(2) doi: 10.1371/journal.pone.0212858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khademi F., Yousefi-Avarvand A., Derakhshan M., Abbaspour M.R., Sadri K., Tafaghodi M. Formulation and optimization of a new cationic lipid-modified PLGA nanoparticle as delivery system for mycobacterium tuberculosis hspx/esxs fusion protein: an experimental design. Iran J Pharm Res. 2019;18(1):446–458. [PMC free article] [PubMed] [Google Scholar]
- 62.Jain K., Patel A.S., Pardhi V.P., Flora S.J.S. Nanotechnology in wastewater management: a new paradigm towards wastewater treatment. Molecules. 2021;26(6):1797. doi: 10.3390/molecules26061797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jain K., Jain N.K. Surface engineered dendrimers as antiangiogenic agent and carrier for anticancer drug: dual attack on cancer. J Nanosci Nanotechnol. 2014;14(7):5075–5087. doi: 10.1166/jnn.2014.8677. [DOI] [PubMed] [Google Scholar]
- 64.Afsana J.V., Haider N., Jain K. 3D printing in personalized drug delivery. Curr Pharm Des. 2018;24(42):5062–5071. doi: 10.2174/1381612825666190215122208. [DOI] [PubMed] [Google Scholar]
- 65.Tandel H., Bhatt P., Jain K., Shahiwala A., Misra A. In-vitro and in-vivo tools in drug delivery research for optimum clinical outcomes. CRC Press; 2018. In-vitro and in-vivo tools in emerging drug delivery scenario: challenges and updates; pp. 1–24. [Google Scholar]
- 66.Hu C.J., Chen Y.T., Fang Z., Chang W.S., Chen H. Antiviral efficacy of nanoparticulate vacuolar ATPase inhibitors against influenza virus infection. Int J Nanomed. 2018;13:8579–8593. doi: 10.2147/IJN.S185806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Priya A., Swetha T.K., Pandian S.K. In: Microbial and natural macromolecules synthesis and applications. Das S., Dash H.R., editors. Academic Press; 2021. Antimicrobial peptides as a potent therapeutic regimen to quench biofilm-mediated antimicrobial resistance; pp. 531–570. [Google Scholar]
- 68.Nuti R., Goud N.S., Saraswati A.P., Alvala R., Alvala M. Antimicrobial peptides: a promising therapeutic strategy in tackling antimicrobial resistance. Curr Med Chem. 2017;24(38):4303–4314. doi: 10.2174/0929867324666170815102441. [DOI] [PubMed] [Google Scholar]
- 69.de Souza M.E., Verdi C.M., de Andrade E.N.C., Santos R.C.V. In: Applications of targeted nano drugs and delivery systems. Mohapatra S., Ranjan S., Dasgupta N., Mishra R., Thomas S., editors. Elsevier; 2019. Antiviral and antimicrobial (antibacterial) potentiality of nano drugs; pp. 327–342. [Google Scholar]
- 70.Iversen P.L., Kane C.D., Zeng X., Panchal R.G., Warren T.K., Radoshitzky S.R., et al. Recent successes in therapeutics for Ebola virus disease: no time for complacency. Lancet Infect Dis. 2020;20(9):e231–e237. doi: 10.1016/S1473-3099(20)30282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sonali V.M.K., Singh R.P., Agrawal P., Mehata A.K., Pawde D.M., et al. Nanotheranostics: emerging strategies for early diagnosis and therapy of brain cancer. Nanotheranostics. 2018;2(1):70–86. doi: 10.7150/ntno.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prasad R., Jain N.K., Conde J., Srivastava R. Localized nanotheranostics: recent developments in cancer nanomedicine. Mate Today Adv. 2020;8 [Google Scholar]
- 73.Sharma I., Bhattacharjee S. In: Nanobiotechnology microbes and plant assisted synthesis of nanoparticles, mechanisms and applications. Ghosh S., Webster T.J., editors. Elsevier; 2021. Nanotheranostics and biocompatibility; pp. 51–60. [Google Scholar]
- 74.Hossen S., Hossain M.K., Basher M.K., Mia M.N.H., Rahman M.T., Uddin M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: a review. J Adv Res. 2018;15:1–18. doi: 10.1016/j.jare.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jagtap P., Sritharan V., Gupta S. Nanotheranostic approaches for management of bloodstream bacterial infections. Nanomedicine. 2017;13(1):329–341. doi: 10.1016/j.nano.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 76.Coelho E.A.F., Chávez-Fumagalli M.A., Costa L.E., Tavares C.A.P., Soto M., Goulart L.R. Theranostic applications of phage display to control leishmaniasis: selection of biomarkers for serodiagnostics, vaccination, and immunotherapy. Rev Soc Bras Med Trop. 2015;48(4):370–379. doi: 10.1590/0037-8682-0096-2015. [DOI] [PubMed] [Google Scholar]
- 77.Hassanzadeh P. Nanotheranostics against COVID-19: from multivalent to immune-targeted materials. J Control Release. 2020;10(328):112–126. doi: 10.1016/j.jconrel.2020.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]


