Abstract
Introduction
Symptoms referable to central and peripheral nervous system involvement are often evident both during the acute phase of COVID-19 infection and during long-COVID. In this study, we evaluated a population of patients with prior COVID-19 infection who showed signs and symptoms consistent with neurological long-COVID.
Methods
We prospectively collected demographic and acute phase course data from patients with prior COVID-19 infection who showed symptoms related to neurological involvement in the long-COVID phase. Firstly, we performed a multivariate logistic linear regression analysis to investigate the impact of demographic and clinical data, the severity of the acute COVID-19 infection and hospitalization course, on the post-COVID neurological symptoms at three months follow-up. Secondly, we performed an unsupervised clustering analysis to investigate whether there was evidence of different subtypes of neurological long COVID-19.
Results
One hundred and nine patients referred to the neurological post-COVID outpatient clinic. Clustering analysis on the most common neurological symptoms returned two well-separated and well-balanced clusters: long-COVID type 1 contains the subjects with memory disturbances, psychological impairment, headache, anosmia and ageusia, while long-COVID type 2 contains all the subjects with reported symptoms related to PNS involvement. The analysis of potential risk-factors among the demographic, clinical presentation, COVID 19 severity and hospitalization course variables showed that the number of comorbidities at onset, the BMI, the number of COVID-19 symptoms, the number of non-neurological complications and a more severe course of the acute infection were all, on average, higher for the cluster of subjects with reported symptoms related to PNS involvement.
Conclusion
We analyzed the characteristics of neurological long-COVID and presented a method to identify well-defined patient groups with distinct symptoms and risk factors. The proposed method could potentially enable treatment deployment by identifying the optimal interventions and services for well-defined patient groups, so alleviating long-COVID and easing recovery.
Keywords: Long-COVID, Post-COVID neurological symptoms
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of Coronavirus disease 2019 (COVID-19), was isolated in December 2019 in Wuhan (China) and then caused a significant global medical issue, with a growing number of cumulative confirmed cases [1,2]. The clinical spectrum of the SARS-CoV-2 infection ranges from mild respiratory symptoms to severe multiorgan disease [3]. In this scenario, a neurological involvement has been reported from the first cases in observational studies; specifically, a study of more than 200 patients hospitalized in 3 COVID-19–focused hospitals in Wuhan demonstrated that more than one-third of patients experienced a variety of neurologic manifestations, including altered mental status and acute cerebrovascular diseases, most commonly in those with severe respiratory illness [4]. Reports have also implicated isolated, sudden onset of anosmia and ageusia as early indicators of SARS-CoV-2 infection, suggesting that early neurological involvement may be relevant [5]. Neurological disorders related to SARS-CoV-2, which can involve both the central nervous system (CNS) and the peripheral nervous system (PNS), can manifest themselves both as neurological sequelae (post-infectious complications) and as symptoms in the course of infection (para-infectious complications). According to recent studies, more than a third of patients present neurological symptoms during infection [6].
With the progression of the pandemic, there is evidence to suggest the emergence of an associated secondary syndrome, labelled as either post-COVID or long-COVID syndrome, in which recovering SARS-CoV-2 patients suffer from persistent and, often, debilitating symptoms extending several months past their initial diagnosis [7]. Recent data suggested that upwards of 20% of SARS-CoV-2 positive individuals go on to develop long-COVID syndrome [7]. The Guideline published by the National Institute for Health and Care Excellence (NICE), the Scottish Intercollegiate Guidelines Network, and the Royal College of General Practitioners has defined long-COVID as “signs and symptoms developed during or following a disease consistent with COVID-19 and which continue for more than four weeks but they are not explained by alternative diagnoses” [8]. According to the NICE guidelines on long-COVID-19 the definitions of post-acute COVID-19 include i) acute COVID (symptoms for up to 4 weeks); ii) ongoing symptomatic COVID (symptoms between 4 and 12 weeks after the start of acute symptoms) and iii) post-COVID syndrome (symptoms developed during or after an infection and continuing for more than 12 weeks) [9]. Subsequent evidence has shown that neurological involvement is well established in long-COVID. COVID-19-associated neurological disorder has been categorized into five types which include encephalopathies, inflammatory syndromes, stroke, peripheral neuropathies, and various other CNS disorders [10].
In this study, we characterize a population of patients with prior COVID-19 infection who showed signs and symptoms consistent with neurological long-COVID. Further, we analyze the patterns of symptoms persisting three months after the end of the acute phase of COVID-19 infection and discuss the presence of subtypes of neurological long-COVID, and their potential risk factors.
2. Methods
2.1. Population overview
In November 2020 we established the neurological long-COVID outpatient clinic at the Neurological Clinic of San Martino Hospital in Genoa, to assess patients with post-COVID neurological symptoms. Patients were referred from the long-COVID outpatient clinic of the Pneumology Department [11] and included patients with a previous admission to the Pneumology Department, intensive care unit (ICU) or Infectious Department, and patients who had not required admission during the acute phase of the infection.
2.2. Data collection
Patient data were collected prospectively from 1st November 2020 to 31st December 2021 focusing on both demography and characteristics of the course of acute infection. The diagnosis of COVID-19 was confirmed in all patients with PCR on naso-pharyngeal swab, even in asymptomatic patients at onset. Specifically, we collected data about: i) pre-infection comorbidities, smoking habits, BMI; ii) presentation of the acute COVID-19 infection (i.e. upper respiratory tract symptoms, gastroenteritis, pneumonia), number of symptoms at the onset of the acute infection (i.e. anosmia, ageusia, cough, rhinitis, fever, myalgia, gastrointestinal symptoms), therapy used to treat the acute infection (e.g. corticosteroid, heparin, antibiotics, remdesivir, tocilizumab), type of administration device for oxygen therapy (i.e. nasal cannula, face mask, non-invasive ventilation - NIV, transtracheal catheter, tracheostomy), duration of ventilation (i.e. less than 5 days, between 5 and 10 days, between 11 and 15 days, between 16 and 20 days, more than 20 days), use of prone ventilation, number of extra-neurological complications (i.e. cardiovascular, pulmonary thromboembolism, renal failure, sepsis), length of hospital stay (i.e. shorter or longer than 30 days); iii) severity of acute phase during hospitalization (defined by the type of oxygen therapy used, length of hospital stay, need for home care or long term care after discharge). Regarding the neurological aspects of long-COVID, data were collected on the main symptoms reported by patients and on any secondary complaints. The symptoms were divided as follows: anosmia and ageusia, headache, dizziness, memory disorder, psychological disorder, fatigue, PNS involvement disorder, sleep disorder, other symptoms not classifiable in the above groups.
2.3. Statistical analysis
Firstly, we performed a multivariate logistic linear regression analysis to investigate the impact of demographic and clinical data, the severity of the acute COVID-19 infection and hospitalization course, on the long-COVID neurological symptoms at three months follow-up. Specifically, we built a linear regression model to predict the five most common symptoms (symptoms of PNS involvement, memory disturbances, psychological impairment, headache, anosmia and/or ageusia) from a set of variables including both demographic and clinical (sex, BMI, age, smoking habit, number of comorbidities at onset), the severity of the acute COVID-19 infection and hospitalization course (number of COVID-19 symptoms at onset, number of non-neurological complications during COVID-19 acute phase, severity of COVID-19 at onset, dyspnea at onset, smell loss at onset, time of onset of the neurological complications).
Secondly, we performed a k-means clustering analysis to investigate whether there was evidence of different subtypes of neurological long-COVID [12]. As a first step, we divided the dataset in a training set of 76 subjects (70%) and a validation set of 33 subjects (30%). The number of ideal clusters was obtained via a silhouette analysis with Dice distance. The clustering analysis was performed on the training set, on the five most common symptoms reported as the main complaint by patients (symptoms of PNS involvement, memory disturbances, psychological impairment, headache, anosmia and/or ageusia). The variables tested as potential risk-factors to assess the separability of such clusters were: sex, BMI, age, smoking habit, number of comorbidities at onset, number of COVID-19 symptoms at onset, number of non-neurological complications during the acute phase of the infection, severity of COVID-19 at onset, dyspnea at onset, smell loss at onset, time of onset of the neurological complications. Finally, the validation set was used to test the method on unseen subjects.
3. Results
During the first and second waves of the pandemic, 3818 patients referred to the Pneumology Department, ICU or the Infectious Department with an acute SARS-CoV-2 infection. Of the 508 patients evaluated at the long-COVID outpatient clinic of the Pneumology Department, 109 patients referred to the neurological post-COVID outpatient clinic from 1st November 2020 to 31st December 2021.
3.1. Demographic characteristics and clinical data
Table 1 summarizes the demographic characteristics and aspects of the course of acute infection of the total amount of 109 patients.
Table 1.
Demography and clinical data.
| Sex (male:female) | 1:1 |
|---|---|
| Age: mean (range) | 57.17 years (18–80) |
| Smoking habit | |
| non-smoker | 55% |
| smoker | 10% |
| ex-smoker | 35% |
| BMI | |
| underweight mean ± SD |
3% 16.6 ± 0.57 |
| healthy weight mean ± SD |
51% 22.75 ± 1.5 |
| overweight mean ± SD |
46% 29.6 ± 4.1 |
| Manifestation of acute COVID | |
| pneumonia | 81% |
| upper respiratory tract symptoms | 16% |
| gastrointestinal symptoms | 1% |
| Number of symptoms of acute infection† | |
| no symptoms | 2.8% |
| 1 symptom | 5.5% |
| 2 symptoms | 36.7% |
| 3 symptoms | 27.5% |
| 4 symptoms | 14.7% |
| 5 symptoms | 9.1% |
| 6 symptoms | 2.7% |
| 7 symptoms | 1% |
| Oxygen therapy | |
| no oxygen therapy | 31.2% |
| nasal cannula/mask | 20.2% |
| NIV | 18.3% |
| intubation/tracheostomy | 30.3% |
| Prone ventilation | 0,23 |
| Days of admission stay | |
| no admission | 18.3% |
| < 30 days | 36.7% |
| > 30 days | 45% |
| Severity of acute phase | |
| low | 50.5% |
| moderate | 18.9% |
| severe | 30.6% |
BMI: body max index, NIV: non invasive ventilation.
anosmia, ageusia, cough, rhinitis, fever, myalgia, gastrointestinal symptoms.
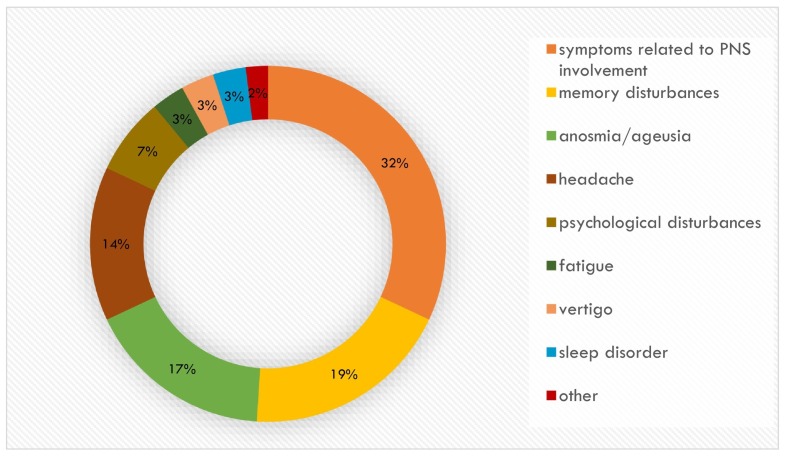
Fig. 1 shows the percentage distribution of neurological symptoms complained of by patients, reported as both the principal symptoms and secondary symptoms.
Fig. 1.
Percentage of neurological symptoms.
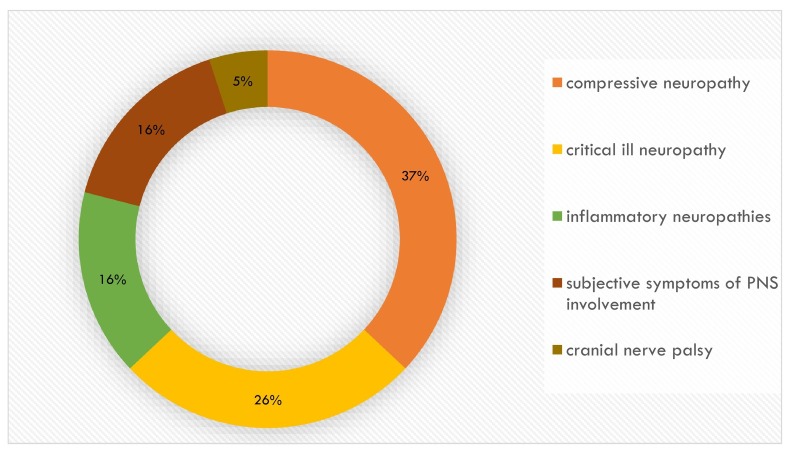
Fig. 2 shows the distribution of the subtypes of disorders related to PNS involvement. We observed i) acute and chronic neuropathies with likely inflammatory genesis, ii) cranial mononeuropathies, iii) patients with subjective symptoms of PNS involvement (e.g. acral paresthesia) in the absence of detectable electroneurographic damage), iv) neuropathies and plexopathies with compressive genesis (i.e. involvement of ulnar, radial, peroneal nerves, and brachial plexopathies).
Fig. 2.
Subtypes of disorders related to PNS involvement.
3.2. Statistical results
We performed a multivariate linear logistic regression analysis to investigate the impact of both demographic and clinical data, the severity of the acute COVID-19 infection and hospitalization course, on the long-COVID neurological symptoms at three months follow-up. We did not find any significant (Bonferroni-corrected for multiple comparison) correlations between any of these variables.
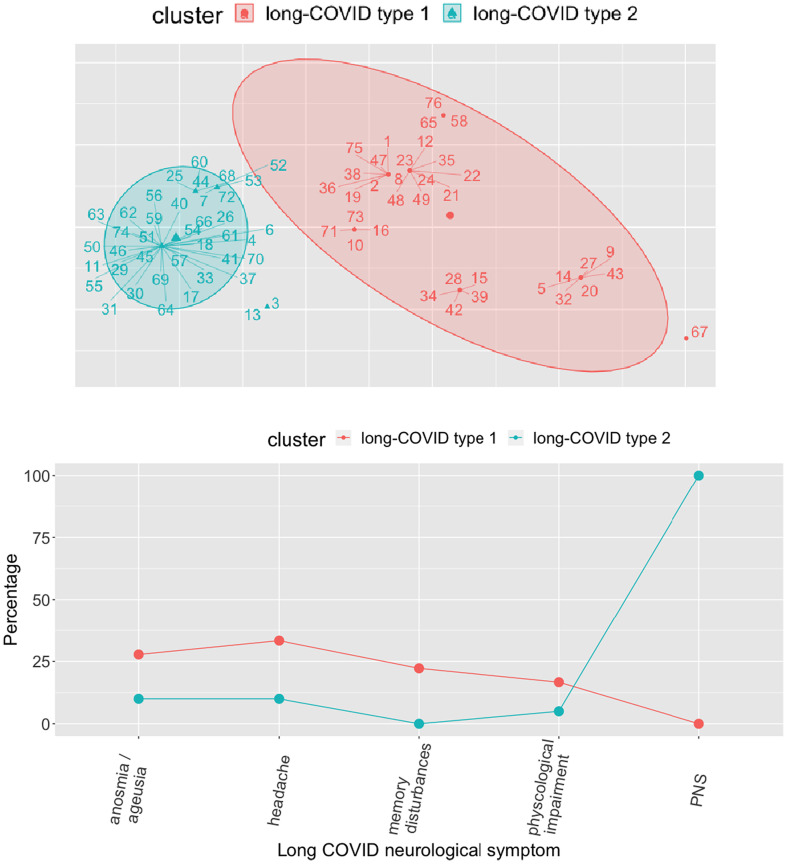
Clustering analysis on the five most common neurological symptoms returned two well-separated and well-balanced (40 vs 36 patients) clusters (see Fig. 3 , top panel). By analyzing the centroid profiles of the two clusters, we observed that the largest cluster (the blue one in Fig. 3, comprised of 40 patients) contains all the subjects with reported symptoms related to PNS involvement. This cluster of patients is hereafter defined as the long-COVID type 2 subset. On the other hand, the second cluster (the red one in Fig. 3, comprised of 36 patients) contains mostly subjects with reported symptoms of memory disturbances, psychological impairment, headache, anosmia and ageusia, which we will define as the long-COVID type 1 subset.
Fig. 3.
Results of cluster analysis.
Top panel, clusters on the set of five neurological symptoms. Blue cluster (long-COVID type 2) = symptoms of PNS involvement; red cluster (long-COVID type 1) = memory disturbances, psychological impairment, headache, anosmia and ageusia. Bottom panel, centroid profiles of the two clusters. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
We then investigated whether there were any potential risk-factors among the demographic, clinical presentation, COVID 19 severity and hospitalization course variables. We found that the number of comorbidities at onset (p = 1.12 × 10−5, Bonferroni-corrected), BMI (p = 0.007, Bonferroni-corrected), the number of COVID-19 symptoms (p = 2.81 × 10−5, Bonferroni-corrected), the number of non-neurological complications (p = 1.86 × 10−5, Bonferroni-corrected) and a more severe course of the acute infection (p = 0.0001, Bonferroni-corrected) were all, on average, higher for the long-COVID type 2 cluster.
Further, we tested these results on the validation set: i) we assessed, for each validation subject, whether the values of the five “risk-factors” variables (number of comorbidities at onset, BMI, number of COVID-19 symptoms, number of non-neurological complications and course of the acute infection) were smaller or greater than the average values as computed on the training set; ii) if at least 3 over 5 of such values were greater than the average of the initial dataset, such subjects were classified as “severe”; otherwise they were classified as “mild”; iii) we evaluated the percentage of severe subjects that are predicted in the long-COVID type 2 cluster and the percentage of mild subjects predicted in the long-COVID type 1 cluster. We found that a proportion of 92% of mild subjects are predicted in the long-COVID type 1 cluster, and 62% of severe subjects are predicted in the long-COVID type 2 cluster. These results indicate an overall accuracy of 0.85 (with a sensitivity of 0.88 and specificity of 0.71).
As a final step, we performed the whole analysis removing the 13 subjects (8 in the training set; 5 in the validation set) with critical ill neuropathy (CIN). Results remained similar. Specifically, we obtained two cluster with 36 vs 32 subjects with the same set of risk-factors, more severe subjects in the long-COVID type 2 cluster, and similar centroid profiles. In this analysis, a proportion of 92% of mild subjects are predicted in the long-COVID type 1 cluster, while 70% of severe subjects are predicted in long-COVID type 2 cluster (overall accuracy 0.89).
4. Discussion
In this work, we analyzed a population of patients with previous COVID-19 infection complaining of neurological symptoms who were referred to our neurological outpatient clinic. These patients had symptoms consistent with either CNS or PNS involvement. From a general viewpoint, we observed an average age of onset of symptoms of about 57 years, as already reported in the literature [13], and an equal distribution of neurological disorders in the two sexes; in this regard, some works report a greater, albeit slight, predominance of the female sex [14]. As for the neurological symptoms reported by patients, our case series is wide and includes patients complaining of anosmia and ageusia, headache, vertigo, memory disturbances, psychological symptoms, sleep disorders and symptoms related to peripheral nervous system involvement. Among these symptoms, memory disorders, symptoms related to PNS involvement, psychological symptoms and headaches are the most reported by our patients, in line with what is reported in the literature [13,14]. It is worth noting the lower representation in our case series of patients complaining of fatigue, a disorder reported in a high percentage of cases in the literature [13,14]. Furthermore, fatigue, as well as sleep disturbance, was always reported by our patients as a secondary symptom and was never observed as the main reason for accessing our outpatient clinic [13,14].
.The cluster analysis that we performed on symptoms persisting three months after the end of the acute phase of COVID-19 infection was aimed at finding patterns of symptoms that characterize possible subtypes of neurological long-COVID. Then, we performed a correlation analysis on some characteristics of patients in order to identify possible risk factors for the development of a particular subtype of neurological long-COVID.
The analysis showed the presence of two subtypes of neurological long-COVID in our population: i) a type 1 long-COVID consisting mostly of patients with memory impairment, psychological disorders, headache, anosmia and ageusia; ii) a type 2 long-COVID consisting mostly of patients with symptoms attributable to PNS involvement. An analysis of demographic and clinical risk factors showed a correlation between the severity of acute infection and the risk of developing a type 2 long-COVID (i.e., PNS involvement).
Several studies evaluated the presence of neurological symptoms that have begun during either the acute phase or long-COVID, but to our knowledge this is the first study that has investigated the presence of neurological long-COVID subtypes and analyzed their possible risk factors. What is currently known is that SARS-CoV-2 can involve the nervous system through: i) a direct effect of the virus entry into the CNS; (ii) para-infectious or post-infectious immune-mediated disease; (iii) a secondary involvement of the CNS following the systemic effects of COVID-19 [17].
Type-1 long-COVID is characterized by memory impairment, psychological disorders, headache, anosmia and ageusia. The effect of COVID-19 on cognitive functions, even in patients with mild symptoms, plays a multisystemic role: the effect of pulmonary disfunction and subsequent hypoxemia, coagulopathy and thrombosis, the blood–brain barrier breakdown, microglial activation, and direct neuronal injury are the mechanisms that are hypothesized to be at the origin of cognitive decline [18]. Recent findings suggested a significant longitudinal changes in the brain structure and larger cognitive decline due to SARS-CoV-2 infection, in particular a greater reduction in grey matter thickness and tissue-contrast in the orbitofrontal cortex and parahippocampal gyrus, greater changes in markers of tissue damage in regions functionally-connected to the primary olfactory cortex, and a greater reduction in global brain size [19].
Several studies evaluated the psychiatric and neuropsychiatric repercussions associated with infections of COVID-19 such as depression, anxiety, and post-traumatic stress disorder [ 20]. In our cohort of patients, 7% had a neuropsychiatric disorder as their primary symptom. Specifically, six patients presented with anxiety associated with depression and two patients manifested post-traumatic stress disorder. These symptoms are mainly due to the broad social impact of the pandemic situation and the government responses to social distancing and quarantines, or to experiences that are specific to individuals infected, such as the concern with the prognosis of their illness, stigma or traumatic memories of serious illnesses [21]. In addition to these social factors, the development of stress due to difficulty in breathing due to infection can trigger the release of corticotropic hormones, hypothalamic stimulation and the production of glucocorticoids with interference on brain metabolism [22].
Headache occurs as one of the initial symptoms of infection in COVID-19, and it may persist for several months after the end of the acute phase of the infection [23]. [24] The possible pathophysiological mechanisms of headache include activation of peripheral trigeminal nerve endings by the SARS-CoV-2 directly or through the vasculopathy and/or increased circulating pro-inflammatory cytokines and hypoxia [25].
Anosmia has already been reported during SARS and other coronavirus infections; however, it represents a rare occurrence. Interestingly, in COVID-19 patients ageusia and anosmia are not accompanied by nasal obstruction or other rhinitis symptoms. Therefore, this is probably due to the direct damage of the virus on the olfactory and gustatory receptors [26]. Previous studies have observed olfactory cleft and olfactory bulb abnormalities, associated with a high percentage of olfactory bulb degeneration [27]. These findings account for the persistence of anosmia and ageusia in patients with long-COVID.
From the analysis of the mechanisms underlying the genesis of most symptoms characterizing the long-COVID type 1, it seems plausible that they are the result of direct invasion of the virus within the nervous system. This explains why these symptoms can occur even in patients with a mild course during the acute phase of infection.
Type 2 long-COVID is characterized by symptoms attributable to PNS involvement. But why should PNS involvement be correlated with a worse clinical course in the acute phase of infection?
In our case series, the involvement of the PNS is variable. Excluding CINs, a condition that correlates with ICU stay for any pathology and in our case series accounts for 26% of diagnoses in the neuropathy cluster, we observed i) acute and chronic neuropathies with likely inflammatory genesis (16%), ii) cranial mononeuropathies (5%), iii) patients with subjective symptoms of PNS involvement (e.g. acral paresthesia (16%), iv) neuropathies and plexopathies with compressive genesis (37%). Acute (5%) and chronic neuropathies (11%) with likely inflammatory genesis seem to be the expression of either direct damage on the nerve by SARS-CoV-2 or the establishment of an autoimmune process against the nerve due to a molecular mimicry mechanism. Indeed SARS-CoV-2 has been found to be responsible for the genesis of Guillain-Barré Syndrome as reported in the literature since the beginning of the pandemic [28]. We also observed cranial mononeuropathies (i.e. VI and VIII cranial nerve) as already reported in the literature during SARS-CoV-2 infection [29]. In this cases it is currently not known whether cranial neuropathies as early neurologic manifestations of COVID-19 infection arise from direct viral infiltration of the nervous system or as an autoimmune response.
Conversely, mononeuropathies and compression plexopathies are probably related to the long hospitalization and the consequent iatrogenic damage resulting from bed rest, from the maintenance of inadequate postures for long periods, from the execution of frequent arterial sampling for blood gas analysis, from the positioning of ventilation support devices, from the use of prone ventilation. Both ischemic and mechanical factors are involved in the development of compression neuropathy. Experimental studies suggest a dose response curve such that the greater the duration and amount of pressure, the more significant is neural dysfunction [30]. Taken together, the results of our observations agree with what has already been reported in the literature: peripheral neuropathy in patients with COVID-19 is frequent and predominantly due to immune mechanisms and to the compression of peripheral nerves resulting from prolonged bedding in ICU [31]. Therefore, unlike what we have observed for the long-COVID type 1 in which even contact with the virus is enough to determine the neurological complication, in most of the disorders of the long-COVID type 2 the damage is caused by prolonged hospitalization or by inflammatory immune- mediated process. These observations could explain why long-COVID type 2 correlates with a worse clinical course in the acute phase of infection.
In conclusion, in this work we analyzed the characteristics of neurological long-COVID and presented a method to identify well-defined patient groups with distinct symptoms and risk factors. The proposed method could potentially enable treatment deployment by identifying the optimal interventions and services for well-defined patient groups, so alleviating long-COVID and easing recovery.
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8) doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223) doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020 doi: 10.1136/bmj.m1091. Published online March 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6) doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lechien J.R., Chiesa-Estomba C.M., de Siati D.R., et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Otorhinolaryngol. 2020;277(8) doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niazkar H.R., Zibaee B., Nasimi A., Bahri N. The neurological manifestations of COVID-19: a review article. Neurol. Sci. 2020;41(7) doi: 10.1007/s10072-020-04486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iqbal F.M., Lam K., Sounderajah V., Clarke J.M., Ashrafian H., Darzi A. Characteristics and predictors of acute and chronic post-COVID syndrome: a systematic review and meta-analysis. EClinicalMedicine. 2021;36 doi: 10.1016/j.eclinm.2021.100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.London: National Institute for Health and Care Excellence (UK) 2020. COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. [PubMed] [Google Scholar]
- 9.Venkatesan P. NICE guideline on long COVID. The lancet. Respir. Med. 2021;9(2) doi: 10.1016/S2213-2600(21)00031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paterson R.W., Brown R.L., Benjamin L., et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10) doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aloè T., Benedetti L., Garbarino S., et al. Vol 3. 2021. Three-Month Outpatient Follow-up of Discharged COVID-19 Patients: A Single Center Cross-Sectional Study.www.medicalandresearch.com [Google Scholar]
- 12.Manning C.D. Cambridge University Press; New York: 2008. RPSH. Introduction to Information Retrieval. [Google Scholar]
- 13.Lombardo M.D.M., Foppiani A., Peretti G.M., et al. Long-Term Coronavirus Disease 2019 complications in inpatients and outpatients: a one-year follow-up cohort study. Open Forum Infect. Dis. 2021;8(8) doi: 10.1093/ofid/ofab384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boesl F., Audebert H., Endres M., Prüss H., Franke C. A neurological outpatient clinic for patients with post-COVID-19 syndrome — a report on the clinical presentations of the first 100 patients. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.738405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J., Tan B., Wu S., Gui Y., Suo J., Li Y. Evidence of central nervous system infection and neuroinvasive routes, as well as neurological involvement, in the lethality of SARS-CoV-2 infection. J. Med. Virol. 2021;93(3):1304–1313. doi: 10.1002/jmv.26570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker H.A., Safavynia S.A., Evered L.A. The ‘third wave’: impending cognitive and functional decline in COVID-19 survivors. Br. J. Anaesth. 2021;126(1):44–47. doi: 10.1016/j.bja.2020.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Douaud G., Lee S., Alfaro-Almagro F., et al. SARS-CoV-2 is associated with changes in brain structure in UK biobank. Nature. 2022;7 doi: 10.1038/s41586-022-04569-5. Published online March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Sousa Moreira J.L., Barbosa S.M.B., Vieira J.G., et al. The psychiatric and neuropsychiatric repercussions associated with severe infections of COVID-19 and other coronaviruses. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021;106 doi: 10.1016/j.pnpbp.2020.110159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers J.P., Chesney E., Oliver D., et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H., Xue Q., Xu X. Involvement of the nervous system in SARS-CoV-2 infection. Neurotox. Res. 2020;38(1):1–7. doi: 10.1007/s12640-020-00219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martelletti P., Bentivegna E., Spuntarelli V., Luciani M. Long-COVID headache. SN Comprehens. Clin. Med. 2021;3(8):1704–1706. doi: 10.1007/s42399-021-00964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uygun Ö., Ertaş M., Ekizoğlu E., et al. Headache characteristics in COVID-19 pandemic-a survey study. J. Headache Pain. 2020;21(1):121. doi: 10.1186/s10194-020-01188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolay H., Gül A., Baykan B. COVID-19 is a Real Headache! Headache: J. Head face Pain. 2020;60(7):1415–1421. doi: 10.1111/head.13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaira L.A., Salzano G., Deiana G., de Riu G. Anosmia and Ageusia: common findings in COVID-19 patients. Laryngoscope. 2020;130(7):1787. doi: 10.1002/lary.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kandemirli S.G., Altundag A., Yildirim D., Tekcan Sanli D.E., Saatci O. Olfactory bulb MRI and paranasal sinus CT findings in persistent COVID-19 anosmia. Acad. Radiol. 2021;28(1):28–35. doi: 10.1016/j.acra.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caress J.B., Castoro R.J., Simmons Z., et al. COVID-19–associated Guillain-Barré syndrome: the early pandemic experience. Muscle Nerve. 2020;62(4):485–491. doi: 10.1002/mus.27024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costello F., Dalakas M.C. Cranial neuropathies and COVID-19. Neurology. 2020;95(5):195–196. doi: 10.1212/WNL.0000000000009921. [DOI] [PubMed] [Google Scholar]
- 30.Mackinnon S.E. Pathophysiology of nerve compression. Hand Clin. 2002;18(2):231–241. doi: 10.1016/S0749-0712(01)00012-9. [DOI] [PubMed] [Google Scholar]
- 31.Finsterer J., Scorza F.A., Scorza C.A., Fiorini A.C. Peripheral neuropathy in COVID-19 is due to immune-mechanisms, pre-existing risk factors, anti-viral drugs, or bedding in the intensive care unit. Arq. Neuropsiquiatr. 2021;79(10):924–928. doi: 10.1590/0004-282x-anp-2021-0030. [DOI] [PubMed] [Google Scholar]