Abstract
Assessment of right ventricular (RV) function is crucial for the evaluation of the dyspnoeic patient and/or with systemic venous congestion and provides powerful prognostic insights. It can be performed using different imaging modalities including standard and advanced echocardiographic techniques, cardiac magnetic resonance imaging, computed tomography, and radionuclide techniques, which should be used in a complementary fashion. Each modality has strengths and weaknesses based on which the choice of their use and in which combination may vary according to the different clinical scenarios as will be detailed in this review. The conclusions from multiple studies using different imaging techniques are concordant: RV function can be reliably assessed and is a critical predictor of clinical outcomes.
Keywords: multi-modality imaging, right ventricle, myocardial function, arterial-ventricular coupling
Graphical Abstract
Graphical Abstract.
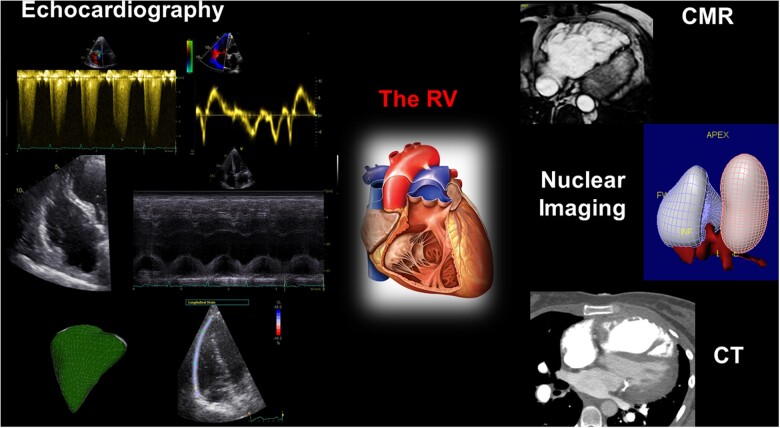
Multi-modality imaging plays a key role in the assessment of right ventricular (RV) morphology, size, and function.
Introduction
The role of the heart is to provide sufficient cardiac output to meet the metabolic needs of the body at rest and during exercise. To achieve this, both the left ventricle (LV) and right ventricle (RV) must augment their function to meet demand. It may be said that ‘you are only as good as your worst ventricle’ because the ventricles act in series as such that the output of one ventricle cannot exceed the other. Heart failure (HF) syndromes have been historically based on LV dysfunction as the source of limitation, and most therapeutic approaches aimed at improving LV remodelling and contractility. However, there are many settings in which an increased load of the RV and/or a decrease in RV contractility can result in a failure to appropriately augment cardiac output.
Excess loading and limitations in contractile force are therefore the key factors that may combine to limit RV output. Regarding RV load, it is critical to note that the RV sits ‘upstream’ of the systemic circulation, the LV, the left atrium, and pulmonary circulation. Dysfunction at any of these levels can result in increased pulmonary artery pressures and RV after-load, such as in HF with preserved or reduced ejection fraction (EF), valvular heart disease, and pulmonary vascular disease. With an RV mass and a contractile reserve of approximately one-third of the LV, RV output is highly sensitive to increases in after-load,1 and can decrease dramatically particularly during physical activity when increases in RV after-load, work and wall stress are far greater than for the LV.2 Thus, the concept of ventricular–arterial interaction is particularly important for the RV. Increase in RV after-load can be initially accommodated by an increase in RV contractility such that output is maintained. However, when sustained increases in loading cannot be matched by RV contractile reserve, ‘uncoupling’ will occur as the RV is incapable of meeting the demands imposed on it. At first, the uncoupling presents during exercise or acute illness, but is then detectable at rest in more advanced disease stages.
These important physiological principles explain why assessment of the RV function is crucial for the evaluation of the dyspnoeic patient and/or with systemic venous congestion, and can provide powerful prognostic insights. RV function has been shown to be a critical determinant of survival in pulmonary vasculature diseases, such as pulmonary arterial hypertension,3,4 and also conditions that have traditionally been regarded as primarily LV pathologies such as congestive HF5–7 and acute myocardial infarction.8–10 The RV can be assessed using a range of imaging modalities including Doppler, 2D and 3D and strain echocardiographic techniques, magnetic resonance imaging (CMR), computed tomography (CT), and radionuclide techniques. Recent technological advances have improved the feasibility and accuracy of each imaging modality for the assessment of RV function, but still there is no single approach able to provide all the necessary information, and their use in a complementary fashion is therefore advised (Graphical Abstract). As summarized in Table 1 and will be detailed in this review, each modality has strengths and weaknesses, based on which the choice of their use and in which combination may vary according to the different clinical scenarios. The conclusions from multiple studies using different imaging techniques are concordant: RV function can be reliably assessed and is a critical predictor of clinical outcomes.
Table 1.
Strengths, limitations, and value in the clinical setting of each imaging modality for the assessment of RV function
| Parameter | Strengths | Limitations | Use/value in the clinical setting |
|---|---|---|---|
| Echocardiography | |||
| TAPSE |
|
|
|
| S′ (TDI) |
|
|
|
| FAC |
|
|
|
| RIMP (TDI) |
|
|
|
| dp/dt |
|
|
|
| 2DE longitudinal strain |
|
|
|
| 3DE EF |
|
|
|
| 3D shape |
|
|
|
| RV–PA coupling |
|
|
|
| Myocardial work |
|
|
|
| CMR | |||
| EF |
|
|
|
| Nuclear imaging | |||
| EF |
|
|
|
| CT | |||
| EF |
|
|
|
CMR, cardiac magnetic resonance imaging; CT, computed tomography; EF, ejection fraction; FAC, fractional area change; IVS, interventricular septum; PA, pulmonary artery; RA, right atrium; RVOT, right ventricular outflow tract; TAPSE, tricuspid annular plane systolic excursion; TDI, Tissue Doppler imaging; RIMP, RV index of myocardial performance.
RV assessment by echocardiography
2D echocardiography
In daily clinical practice, RV evaluation is mainly performed by 2D echocardiography. However, there is currently little uniformity in echocardiographic approach to the RV, owing to potential lack of awareness and/or conformity with recommendations.11
Qualitative evaluation of the RV anatomy should be performed from multiple acoustic windows and is particularly important in the context of ischaemic heart disease or arrhythmogenic right ventricular cardiomyopathy (ARVC, for detection of wall motion abnormalities), and suspicion of constriction or pressure/volume overload (assessment of septal motion patterns).
The quantitative assessment of the RV size should include several measurements. RV-free wall thickness can be measured at end-diastole in the subcostal four-chamber view or parasternal view: >5 mm is considered abnormal after exclusion of the trabeculae. RV basal, mid-cavitary diameters, and base-to-apex length are also recommended, together with the RV outflow tract (RVOT) diameter (measured above aortic valve) in the parasternal long-axis view, as well as RVOT proximal and distal diameters in the parasternal short-axis view.12 These measurements are particularly important as part of the criteria for the diagnosis of ARVC. However, the operator should ensure a correct alignment of the views to provide accurate and reproducible values. RV end-systolic area (ESA) and end-diastolic area (EDA) are obtained by manually tracing the ventricular endocardium in the RV-focused apical four-chamber view, excluding the trabeculae: normal values can be found in the ASE/EACVI recommendation paper.12
The quantitative evaluation of RV performance is quite challenging and can be estimated with different parameters. Fractional area change (FAC) is calculated by the formula [(EDA − ESA)/EDA] × 100, and showed fair correlation with RVEF assessed by CMR: a value <35% is considered abnormal.13 The ability of FAC to predict major adverse cardiac events has been demonstrated in various cardiovascular conditions including myocardial infarction and pulmonary hypertension.9,14 2D RVEF is not recommended for clinical use due to the inaccuracy of the method.12 The tricuspid annular plane systolic excursion (TAPSE) quantifies the excursion of the lateral tricuspid annulus from end-diastole to end-systole and reflects only the longitudinal contraction of the RV-free wall. This simple and reproducible measure is considered abnormal when <17 mm. Many assumptions and drawbacks should be considered while using this parameter (angle- and load-dependent, inaccurate in patients post-cardiac surgery or in case of RV pacing/RV apex rotation, etc.). Nevertheless, its prognostic value has been demonstrated in several conditions such as HF, coronary artery disease, pulmonary embolism, pulmonary hypertension, and in critically ill patients.15,16 Tissue Doppler imaging (TDI)-derived tricuspid lateral annular systolic velocity (S′) is another parameter of RV longitudinal function, which correlates with CMR-derived RVEF13: a value <10 cm/s is considered abnormal but may decrease with age. The limitations are similar to those for TAPSE and should be taken into account. RV index of myocardial performance (RIMP) is a ratio between isovolumic contraction and relaxation times and the ejection time. This parameter reflects global RV systolic function and was shown to correlate well with CMR-derived RVEF. RIMP <0.55 is considered abnormal. Another measure of RV function is the RV dp/dt, which represents the rate of change of pressure and is calculated as the slope of the spectrum of tricuspid regurgitation between 1 and 2 m/s: a value <400 is considered abnormal. This parameter is less used due to its high load dependency. Finally, a less load dependent parameter of RV performance is the right isovolumic myocardial acceleration (IVA), which is the peak isovolumic myocardial velocity divided by the time to reach this peak velocity: it is considered abnormal when <2.2 m/s.
Assessment of RV systolic function during exercise stress echocardiography is a promising diagnostic and prognostic tool. Exercise-induced reduction in TAPSE demonstrated important incremental prognostic value in asymptomatic patients with primary mitral regurgitation.17 Attenuated increase in FAC and S′ with exercise indicated subclinical RV dysfunction in athletes with RV arrhythmias.18
2D speckle tracking echocardiography (STE) has recently emerged, enabling a real-time tracking of the frame-to-frame myocardial motion, and overcoming most of the limitations inherent in conventional echocardiography. In particular, STE is less angle- and load-dependent, and less influenced by passive tethering, thus allowing accurate quantification of regional and global myocardial function, reflecting more closely myocardial contractility.19 RV peak systolic longitudinal strain and strain rate are assessed in the (RV-focused) apical four-chamber view, where RV-free wall and septum are each divided into three segments (basal, middle, and apical). Regional strain values are generated, and the RV four-chamber longitudinal strain (average of the six segments) and RV-free wall strain (average of the three segments) are derived.19 Current recommendations propose a cut-off value of −20% for RV four-chamber longitudinal strain and of −23% for RV-free wall strain as abnormal. However, some studies showed that a lower cut-off for RV four-chamber strain of −19% was associated with poor prognosis. Overall, RV longitudinal strain has shown important prognostic value in various conditions, including pulmonary hypertension, valvular heart disease, LV dysfunction, and HF.20 Recent studies suggest that the mechanical dispersion of the strain curves could also play a role in the risk stratification of patients with ARVC.21
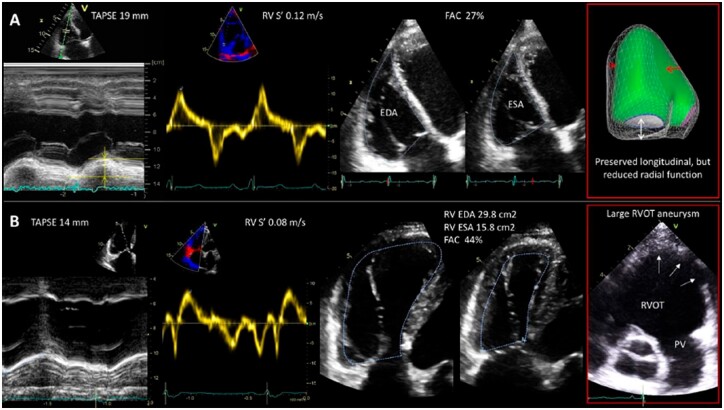
Importantly, assessment of the RV size and systolic function by conventional echocardiography should always be performed in a clinical context, including RV loading conditions. Accurate evaluation requires multi-parametric approach and use of different echocardiographic views to ensure correct interpretation of the findings, especially when there is discrepancy between echocardiographic parameters, as illustrated in Figure 1.
Figure 1.
Importance of multi-parametric approach in accurate interpretation of RV systolic function by conventional echocardiography. (A) In a patient with pulmonary hypertension, TAPSE, and S′ values are normal, however FAC is reduced. 3D surface-rendered model of the RV demonstrates preserved longitudinal contraction (white arrow), but impaired radial contraction (red arrows) due to reduced RV-free wall motion and flattening of the interventricular septum. FAC considers not only longitudinal but also radial RV contraction and in this case is able to reflect the reduced RV systolic function. (B) In a patient with repaired Tetralogy of Fallot, TAPSE, and S′ are reduced (common post-cardiac surgery), but FAC is normal. 3D-derived RV EF is reduced (40%). This is explained by large dyskinetic area in the RVOT (last panel, arrows) which was not included neither in TAPSE nor in S′ or FAC measurements.
3D echocardiography
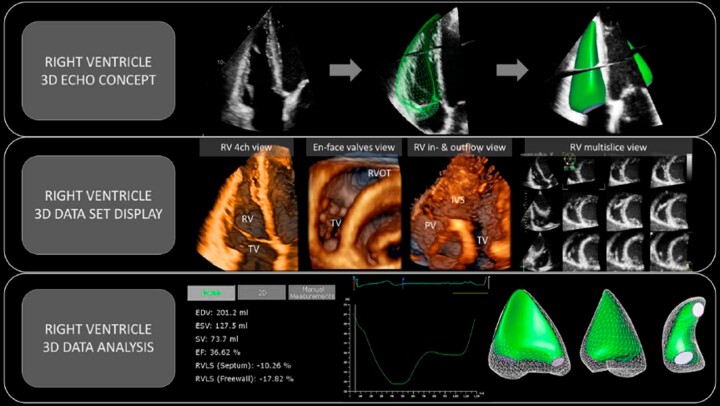
Unlike 2D echocardiography, 3D echocardiography enables acquisition of full-volume datasets from either a single beat capture or few consecutive beats with sub-volumes stitched together, allowing for higher temporal and spatial resolution. This full-volume dataset incorporates all three components of the RV (inflow, apical portion, and outflow) and allows for a detailed analysis of RV size, shape, function, and contraction patterns (Figure 2). In particular, RV full-volume dataset can be post-processed using dedicated software packages to obtain tracing of the RV endocardial surface at end-diastole and end-systole and measurement of RV volumes and EF, without using any geometrical assumptions or formulas.
Figure 2.
3D echocardiographic assessment of the RV. (Upper) Standard 2D echocardiography cannot consider contribution of the RV parts not captured in the scanning plane. 3D echocardiography allows to incorporate all RV components in a single dataset (green surface-rendered model) and enables accurate assessment. (Middle) The 3D full-volume dataset of the RV can be cropped, rotated, and displayed in 3D volume rendering mode or multi-slice display, and provide information targeted to exam question (e.g. assessment of RV morphology, valves, RVOT, or interventricular septum). The multi-slice view is useful during data acquisition to ensure that the whole RV is encompassed in the dataset and can also be used for assessment of RV wall motion abnormalities. (Lower) Post-processing using dedicated software packages provides RV volumes and EF. It also generates the volume curve which details emptying and filling of the RV during cardiac cycle. Different views of surface-rendered 3D model of the RV enable visual assessment of the RV dynamics and contribution of different components of the RV pump function.
RV volumes and EF derived from 3D echocardiography are known to correlate well with CMR data both in children and adults.22,23 High accuracy and reliability of 3D echocardiography has also been confirmed in a meta-analysis comparing the performance of different imaging modalities using CMR as reference method: 3D echocardiography was observed to slightly overestimate RVEF24 and underestimate RV volumes.23 Thus, CMR and 3D echo values and reference ranges cannot be used interchangeably. Large 3D echocardiography studies conducted in healthy subjects have provided sex-, age-, and body size-specific reference values for RV volumes and EF.25 Importantly, 3D echocardiographic assessment of RV volumes and EF is recommended by current guidelines as a method of choice and a RVEF >45% is considered as the lower limit of normal.12 It is important to note that accurate 3D echocardiographic quantification of RV volumes and EF requires experienced personnel and the fact that only 12% of European centres use 3D assessment of RV systolic function on a regular basis highlights the need for further training.26 Principal technical limitations of 3D echocardiography include dependency on image quality and potential incomplete visualization of the whole cardiac chamber in case of severe RV dilation. Multi-beat acquisition also relies on regular heart rate and patients’ co-operation with breath holding. However, 3D echocardiography offers multiple operational and safety benefits including its wide availability, portability, absence of ionizing radiation, and safe examination of patients with intracardiac devices. Recent improvements in echo techniques and data analysis, such as single-beat 3D data acquisition with high volume rate, advanced software solutions for the volumetric analysis of the RV on board of the echocardiographic scanners, semiautomated algorithms allowing for fast, and reproducible volumetric analysis, have defined the 3D echocardiography as an established and versatile technique for the assessment of the RV performance.
Regarding its prognostic value, 3D echocardiography-derived RVEF has been shown to be independently associated with cardiac and all-cause mortality and major adverse cardiac events (MACE) in patients with various cardiovascular conditions.27,28 RVEF offers improvements in prediction of adverse clinical outcomes compared with other parameters including LV systolic and diastolic function or clinical risk factors. Partition values of the RVEF for mild (EF = 40–45%), moderate (30–40%), or severe (<30%) RV dysfunction have been also prognostically validated to stratify the risk of cardiac death and MACE.27 Prognostic value of RVEF was shown to be superior to conventional echocardiographic parameters of RV systolic function for predicting mortality.28
Quantification of the individual motion components of the RV using 3D echocardiography enables a comprehensive characterization of RV contraction pattern, which could be abnormal even in the context of normal RVEF.28,29 Recently developed software package enables to decompose the wall motion of the 3DE-derived RV model along three anatomical axes: longitudinal (from tricuspid annulus to the apex), radial (perpendicular to the interventricular septum), and anteroposterior (parallel to the interventricular septum) axes and quantify contribution of each motion component separately.30 The inward motion of the free wall (radial shortening) and interestingly, the anteroposterior shortening (mainly caused by the stretching of the RV-free wall insertion points during the LV contraction) was shown to be as important as longitudinal shortening in healthy volunteers.31 Also, RV pressure overload could be detected early if radial motion component was lost, while RV volume overload showed more prominent impact on the longitudinal RV component.32 However, data on the prognostic role of different components of RV systolic function remain scarce. A recent study on a large cohort of patients with left-sided heart disease demonstrated that in a subgroup of patients with preserved RVEF, reduced relative contribution of the anteroposterior component was significantly and independently associated with adverse outcomes, while RVEF or LVEF was not.29
Finally, the role of RV remodelling and RV shape alterations are widely recognized in RV pressure and volume overload conditions. However, till recently there was no evidence supporting association of poor outcomes in patients with adverse RV shape changes independent of those predicted by size and functional parameters. A unique approach by 3D echocardiography to provide RV shape indices based on analysis of the RV curvature was recently developed and evaluated in normal subjects and in patients with pulmonary arterial hypertension.33 In patients with pressure overload, the curvature of the RV inflow tract was a more robust predictor of death than RVEF, RV volumes, or other regional curvature indices. Recently the reference values of 3D echocardiographic values of regional curvature indices were reported in a large group of healthy subjects to facilitate future studies on adverse RV remodelling.33,34
Right ventricular arterial coupling and myocardial work
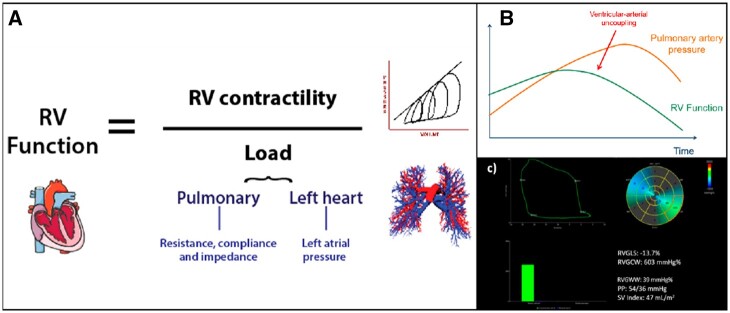
Many conventional measures of RV function, such as FAC, EF, and TAPSE, reflect the interaction between loading conditions and contractility. However, an accurate measure of (load-independent) contractility represents the ideal means of determining the patient’s prognosis and possibly the need for intervention. A load-independent RV function assessment can theoretically be achieved by two means—either a direct load-independent surrogate of contractility or by incorporating load into the measurement of RV function (Figure 3).
Figure 3.
Right ventricular—arterial interaction. RV function, when measured by tools such as ejection fraction, area change, and strain, is the result of the interaction between the intrinsic muscular force of the RV (contractility) and after-load (often simplified as pulmonary artery pressure)—see panel A. These measures therefore do not enable us to determine whether the RV is matching its work requirements relative to load or whether it is approaching the point of uncoupling (B) at which cardiac output starts to fail relative to demand. RV myocardial work (MW) can be calculated based on non-invasively derived pressure–strain loops for the RV, in this case in a patient with raised pulmonary arterial pressures (C). RVGCW, right ventricular global constructive work; RVGWW, right ventricular global wasted work.
Load-independent RV function measures
Only few measures have been proposed as surrogate approximates of RV contractility. Two decades ago, Vogel et al.35 demonstrated a close relationship between IVA and invasively measures of contractility (preload recruitable stroke work and dP/dTmax) under a range of varied loading conditions. The theory was intriguing given that IVA is a measure of myocardial motion during the isovolumic contraction period, prior to the ventricle being exposed to its after-load. Whilst the concept is appealing, the measure has not been well validated or widely accepted, perhaps due to difficulties with reproducibility. As mentioned above, STE is another approach promoted as being an approximate of contractility: longitudinal strain is a measure of deformation and, both in theory and in practice, should be interpreted relative to load. However, based on animal and human studies,36 the change in strain relative to time (strain rate) might be a closer approximate of contractility. There is some support for this premise, but strain rate has also failed to make its way into clinical routine due to issues with reproducibility and its impact on a measure with small dynamic range.
Load-adjusted RV function measures
An alternate means of dissecting the underlying RV contractility is to express it as the relationship between RV function and load. This is akin to the approach in which contractility can be determined invasively from pressure volume–volume relationships. In a series of elegant studies, Guazzi et al.37 have demonstrated the prognostic utility of expressing TAPSE relative to pulmonary artery systolic pressure (PASP) estimates as a surrogate of contractility. A variant on this concept incorporates the ratio of RV area to PASP and has been validated against a gold-standard hybrid using CMR volumes and invasive pressure measures, both at rest and throughout exercise.38 The utility of these measures during exercise provides an additional strength in that the additional load and work of exercise challenges the contractile reserve of the ventricle. In essence, this provides an insight into whether the compensated RV is starting to fail.
Right ventricular myocardial work
Recently, a non-invasive method to assess LV myocardial work (MW has been proposed incorporating STE-derived LV global longitudinal strain and non-invasive brachial cuff blood pressure measurements. This approach has been validated against pressure–volume loops derived invasively and showed its value in different patient populations as a comprehensive measure of LV function, which considers both LV after-load and the possible presence of post-systolic shortening/dyssynchrony.39 The same method can be applied for the RV, using the pulmonary pressures as an estimate of myocardial force, and STE-derived RV longitudinal strain to estimate changes in segment length (Figure 3). RV MW therefore provides an integrative analysis of RV function incorporating RV longitudinal strain with pulmonary pressures, as measure of RV after-load, although an accurate estimation of the last one can be challenging in some cases. Cardiac cycle timings are determined by pulmonic and tricuspid valve opening and closure events, identified through either direct visualization of 2D images or by pulsed-wave Doppler interrogation. Any myocardial lengthening occurring during systole and any shortening during isovolumic relaxation is recorded as RV wasted work (RVGWW, mmHg%); therefore, any inefficient post-systolic shortening will not contribute to estimate the RV constructive work (RVGCW, mmHg%), defined as the work contributing to the shortening of the cardiac myocytes during systole and the lengthening during isovolumic relaxation. A recent study showed a significant correlation of these novel indices derived non-invasively by pressure–strain loops, and in particular RVGCW, with invasively measured stroke volume index.40 The importance of considering after-load in the assessment of RV function has been demonstrated by studies on RV–pulmonary arterial coupling.37 This is particularly relevant even when using RV longitudinal strain, which remains after-load-dependent even more than LV longitudinal strain due to the thinner walls and lower ventricular elastance of the RV. Furthermore, RVMW synchronizes pulmonic and tricuspid valvular events with RV longitudinal strain, accounting also for possible post-systolic shortening (often seen in the setting of pulmonary hypertension) and septal dyssynchrony due to ventricular interdependence.41 RVMW indices have the potential of delivering a more precise estimate of RV systolic function and may enhance the non-invasive understanding of the pathophysiology of patients with HF, pulmonary hypertension, and any other form of RV involvement, also providing a new tool to non-invasively characterize their response to therapies. Further validation of this approach (currently also based on a single-vendor software) is necessary to support its application in the clinical practice and studies on its potential prognostic value are warranted.
RV assessment by CMR
CMR is currently considered the gold standard for assessment of RV function and structure. It allows indeed high-resolution time-resolved 3D visualization of the complex anatomical shape of the RV without most of the limitations that hinder other imaging modalities such as limited acoustic windows, body size or deformation, and anatomical changes of the RV due to pathology or surgery. The standard technique for imaging volumetric and functional data of the RV is currently 2D steady-state-free precession (SSFP) cine imaging with retrospective ECG gating allowing to fully cover the RV with 10–12 slices acquired in 5–12 short breath-holds. Alternatively, in patients unable to breath-hold or in patients with multiple arrhythmias, images can also be acquired in free-breathing real-time images, but with less spatial and temporal resolution. Recently, using parallel imaging techniques or under-sampling with iterative reconstruction it is also possible to acquire true 3D cine breath-hold imaging.
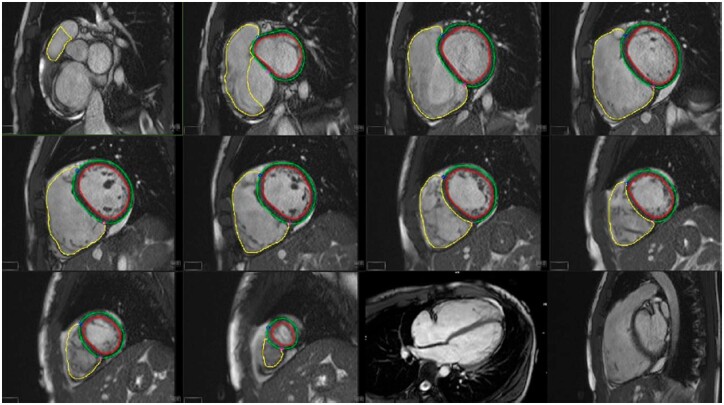
The most commonly used approach for measurement of both RV and LV volumes is to acquire serial slices in short-axis view, covering both ventricles from apex to base (Figure 4). Imaging in axial plane of multiple axially rotated long-axis slices may allow better identification of the tricuspid valve plane and more precise evaluation of RV volumes and EF than imaging in short-axis plane especially in congenital heart diseases. However, this requires separate acquisitions for the RV and lengthens the exam duration.
Figure 4.
Volumetric assessment of RV function by CMR. Traces of the LV endocardium (red) epicardium (green) and the RV endocardium (yellow) are contoured in a short-axis stack with reference to the long-axis plane. Volumes are then imputed using a sum-of-disks method. In this case, an RV ejection fraction of 42% was measured in a young athlete presenting with symptomatic ventricular tachycardia, focal regions of RV akinesia, and widespread T-wave inversion meeting task-force criteria for ARVC.
For estimation of RV end-diastolic and end-systolic volumes and RVEF, the endocardial borders of both the LV and RV are traced in end-diastole and end-systole; and end-diastolic and systolic volumes are computed using Simpsons method by summing areas of traced cavity volume times slice thickness. Tracing can be performed manually or automatically. Recently deep-learning methods have been also proposed allowing automatic contour detection of the RV. To measure RV mass, epicardial contouring needs also to be performed. Epicardial RV volume is then subtracted from endocardial contours and multiplied by density of 1.06 to yield mass.
Because of the excellent differentiation of blood from RV tissue, the method is highly accurate. The main source of error arises from differentiating RV from right atrial volume in the most basal slice, which is somewhat more difficult for the RV than for the LV, because of the thinner RV-free wall and the larger excursion of the tricuspid annular plane as compared with the mitral one. The normal values for RV volumes, mass, and EF and their interpretation have been established by a multitude of studies in large populations such as the MESA study, the Framingham population, or the UK Biobank both in adults and children, and for non-Caucasian populations42–44 RV volumes and mass depend on body size and are thus generally indexed to body surface area. Indexed RV volumes and mass are generally greater in men than in women, depend on race and decrease with increasing age. Higher amounts of physical exercise are associated with larger RV mass and volumes, however unchanged RVEF. Obesity by opposition is also associated with an increase in BSA-indexed RV ESV and mass. By contrast to volumes, RVEF is less influenced by age, but is generally greater in women than in men.44,45 In children, an exponential relation between body surface area and RV volumes and mass was found and therefore reported in percentiles and/or z-scores.
Longitudinal RV function can also be evaluated by estimation of TAPSE or by RV feature tracking strain imaging computed on four-chamber cine imaging. Other approaches for computation of RV strains require specific sequences such as DENSE or FastHARP, which have the advantage of higher spatial resolution. However, tagging is less useful for the RV strain computation because of the thin RV wall relative to tag spacing (6–7 mm), allowing only computation of longitudinal but not of circumferential or radial RV strains.
CMR radiomics is also a novel technique for advanced image phenotyping based on the analysis of multiple quantifiers of shape and tissue texture, which has been applied also to the RV.46 Its application in the clinical practice is still limited due to reproducibility issues, for which machine learning models are being currently applied to improve the performance.
Finally, CMR also has the unique advantage of allowing RV myocardial tissue characterization. Fat infiltration in the RV, which is of particular interest in diagnosis or ARVC can be identified by T1 weighted black-blood spin echo imaging and confirmed by fat saturation techniques. RV fibrosis and necrosis may be present in a multitude of RV diseases and can be identified by late-gadolinium imaging (LGE) after injection of contrast. Also, it was also recently shown that after injection of contrast agents CMR also allows for estimation of pulmonary transit time and blood volume,45 allowing for assessment of pulmonary congestion in various situations of RV pressure overload.
Clinical applications
The evaluation of RV function and structure by CMR has shown clinical relevance in multiple diseases, and particularly in congenital heart diseases, in ARVC, in pulmonary hypertension, in ischaemic diseases, and HF.
CMR plays a particular role in determining the aetiology of RV dilatation. An example is the diagnosis and evaluation of severity of atrial septal defects. Also, by assessing global and regional RV dilatation and by revealing structural abnormalities such as fat infiltration and RV fibrosis CMR may confirm diagnosis of ARVC. The current task force criteria rely on CMR volume and EF measurement to define major and minor criterial for ARVC.47 The identification of fat or LGE is not currently part of ARVC diagnosis but may be particularly useful in orientating the diagnosis. Two studies demonstrated that presence of such anomalies had additional prognostic value over ECG findings in ARVC patients or mutation carriers.48
CMR also plays a particular important role in various congenital heart diseases, from patients with either congenitally corrected transposition of RV or which have undergone atrial (Mustard or Senning) switch repair surgery to patients with complex congenital heart disease such as functional univentricular hearts with various palliation surgeries. In this setting the evaluation of RV fibrosis by LGE imaging has also been shown to provide prognostic value. CMR also plays a fundamental role in the follow-up of patients with tetralogy of Fallot (TOF). Indeed, relief of RVOT obstruction in TOF often involves disruption of pulmonary valve integrity, which leads to residual pulmonary regurgitation of various degrees in most patients. While this condition is often well tolerated, when severe pulmonary regurgitation is longstanding, it leads to progressive dilatation of the RV, causing RV failure and arrhythmias. Because of its high precision, and non-invasiveness CMR is ideally suited for longitudinal follow-up in patients with repaired TOF. It was shown that moderate or severe LV or RV systolic dysfunction by CMR, but not pulmonary regurgitation fraction or RV diastolic dimensions, is independently associated with impaired clinical status in long-term survivors of TOF repair.49 Also, the repair of pulmonary regurgitation in tetralogy of Fallot was shown to improve RV dilatation and clinical status. Although patients improve RV volumes after repair of pulmonary regurgitation, normalization was achieved only when dilatation was not excessive, with indexed RV end-diastolic volumes > 150–170 mL/m2 and systolic volumes >80–90 m/m2.49 Therefore, CMR is currently recommended in all patients with TOF to serially follow RV volumes and eventually provide timely indication for surgery.
Moreover, CMR also plays an important role Ebsteins’ anomaly. In this condition, which is characterized by severe tricuspid regurgitation due to anterior displacement of the septal leaflet of the tricuspid valve, it is particularly well suited to precisely distinguish the functional RV from the atrialized part of the RV and to measure their respective sizes.50 Similarly in other settings of tricuspid regurgitation, CMR also allows precise evaluation of RV dilatation and systolic function, which is of fundamental role to give surgery indication.
CMR was recently shown to play an important role in pulmonary hypertension. In this setting several studies have shown that RV function assessment by CMR predicts outcome and allows to evaluate response to therapy.51 Similarly, several recent studies have also highlighted the role of CMR–RV function on outcome of cardiomyopathies and ischaemic heart disease (including RV infarction), as well as in both HF with preserved or reduced EF.6,52
RV assessment by nuclear imaging
Decades ago, radionuclide techniques have been the first imaging modalities used to provide accurate and reproducible measurements of RV structure and function. Nowadays, nuclear imaging still plays a role in selected subgroup of patients, being able to assess RV perfusion and metabolism as well as morphology and EF, and therefore providing new opportunities for comprehensive evaluation of RV from a single study.
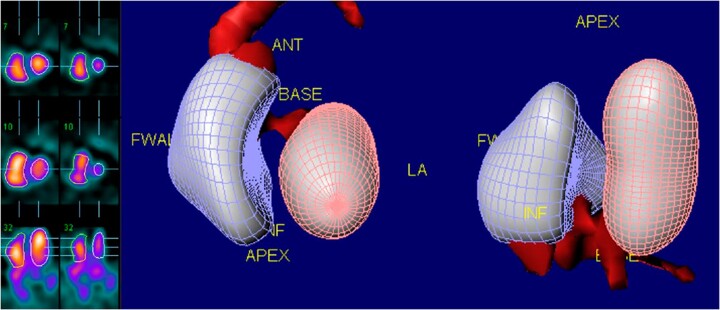
Assessment of RVEF using radionuclide techniques can be performed with either first pass (FPRNA) or equilibrium techniques (planar ERNA, SPECT ERNA), in which the radioisotopes in the blood pool can be imaged. Both these approaches have been extensively validated and, since RVEF is derived from end-systolic and end-diastolic count densities, it is independent of the geometric assumption required for other modalities.53 SPECT equilibrium radionuclide angiography, due to its 3D view, improves spatial separation and resolution of cardiac chambers and has been shown to provide accurate and reproducible assessment of RV volumes and EF (Figure 5).53
Figure 5.
Example of SPECT radionuclide angiography. Regional and global evaluation of kinesis as well as quantitative evaluation of right and left ventricular ejection fraction and volumes are feasible and reproducible.
Nuclear-imaging techniques are emerging as clinically useful tools to assess RV perfusion and metabolism, to detect isolated RV infarction of RV or LV infarction with RV involvement. To improve visualization of RV, it’s possible to use a dedicated computed post-processing technique that can mask activity of LV. Previous studies showed that SPECT provides accurate detection of RV ischaemia in patients with suspected coronary artery disease, identified by reversible defects in the RV and interventricular septum and decreased RVEF during stress. Furthermore, PET allows non-invasive quantification of regional myocardial blood flow and coronary flow reserve in RV mostly using nitrogen-13 ammonia.
Finally, the evaluation of shift in fatty acid metabolism, assessed by SPECT with b-methyl-p-iodine-123 iodophenyl-pentadecanoid acid (BMIPP) and 99Tc sestamibi and by PET with F-18 2-fluoro 2-deoxyglucose (FDG) or carbon-11 labelled palmitate, demonstrated changes in metabolism of RV in response to various stimuli, in particular in patients with RV pressure overload.54
Although nuclear assessment of the RV cannot be considered as the first-choice imaging modality, in selected clinical situations, it can overcome limitations of other techniques (e.g. dependency on geometric assumption for function evaluation of RV as required by other imaging systems) or replace other modalities, when they are not indicated, as in the case of CMR in patients with implantable devices.
RV assessment by cardiac CT
With the recent improvements in the temporal and spatial resolution of the scanners, cardiac CT can be used to assess RV volume and function,55 of particular interest for patients undergoing CT for other clinical indications, such percutaneous intervention of the tricuspid valve or congenital defects or arrhythmias ablation. However, a retrospective ECG-gated acquisition throughout the cardiac cycle is required and ECG-tube current modulation is often implemented to minimize radiation exposure. Previous studies that compared CT measurements of RV volume and function with CMR as the reference standard, showed variable results in terms of agreement,23,24 partially due to the difference in RV segmentation methods.
Conclusions
A systematic and thorough evaluation of RV size, shape, and function provides important diagnostic and prognostic information in most of patients with cardiovascular disease and should be performed by using in a complementary fashion the different imaging modalities available, knowing their strengths and weaknesses. For RV function assessment, echocardiography represents the first line imaging modality in most of patients, and correction of the various measurements for after-load (or the use of less load-dependent approaches) should be attempted, especially when an increase in pulmonary pressures is suspected. CMR, although considered the gold standard for RV dimension and function assessment, is often limited by time, costs, and availability, but should be performed particularly when echocardiography is not conclusive, when detailed anatomical information (such as in congenital heart disease) are necessary and when tissue characterization is required. Cardiac CT is currently used to provide additional RV evaluation in patients undergoing CT for other clinical indications. Finally, nuclear imaging could be performed when information on RV perfusion and metabolism are of importance and the expertise is available.
Conflict of interest: Nina Ajmone Marsan received speaker fees from GE Healthcare and Abbott Vascular and has been in the Medical Advisory Board of Philips Ultrasound. Other authors have no conflict of interest to disclose.
Data availability
No new data were generated or analysed in support of this research.
Contributor Information
Elena Surkova, Cardiac Division, Department of Echocardiography, Royal Brompton Hospital, Part of Guy’s and St Thomas’ NHS Foundation Trust, London, UK.
Bernard Cosyns, Department of Cardiology, Brussels University Hospital, Brussels, Belgium.
Bernhard Gerber, Division of Cardiology, Department of Cardiovascular Diseases, Cliniques Universitaires St. Luc, Pôle de Recherche Cardiovasculaire (CARD), Institut de Recherche Expérimentale et Clinique (IREC), Université Catholique de Louvain, Av Hippocrate, 10/2806 Brussels, Belgium.
Alessia Gimelli, Fondazione Toscana Gabriele Monasterio, Via Moruzzi, 1, Pisa 56124, Italy.
Andre La Gerche, Clinical Research Domain, Baker Heart and Diabetes Institute, Melbourne, Australia.
Nina Ajmone Marsan, Department of Cardiology, Leiden University Medical Centre, Abinusdreef 2, 2300RC Leiden, The Netherlands.
References
- 1. MacNee W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part one. Am J Respir Crit Care Med 1994;150:833–52. [DOI] [PubMed] [Google Scholar]
- 2. La Gerche A, Heidbuchel H, Burns AT, Mooney DJ, Taylor AJ, Pfluger HBet al. Disproportionate exercise load and remodeling of the athlete’s right ventricle. Med Sci Sports Exerc 2011;43:974–81. [DOI] [PubMed] [Google Scholar]
- 3. van de Veerdonk MC, Kind T, Marcus JT, Mauritz GJ, Heymans MW, Bogaard HJet al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 2011;58:2511–9. [DOI] [PubMed] [Google Scholar]
- 4. Fine NM, Chen L, Bastiansen PM, Frantz RP, Pellikka PA, Oh JKet al. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ Cardiovasc Imaging 2013;6:711–21. [DOI] [PubMed] [Google Scholar]
- 5. Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani Ret al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol 2001;37:183–8. [DOI] [PubMed] [Google Scholar]
- 6. Gulati A, Ismail TF, Jabbour A, Alpendurada F, Guha K, Ismail NAet al. The prevalence and prognostic significance of right ventricular systolic dysfunction in nonischemic dilated cardiomyopathy. Circulation 2013;128:1623–33. [DOI] [PubMed] [Google Scholar]
- 7. Motoki H, Borowski AG, Shrestha K, Hu B, Kusunose K, Troughton RWet al. Right ventricular global longitudinal strain provides prognostic value incremental to left ventricular ejection fraction in patients with heart failure. J Am Soc Echocardiogr 2014;27:726–32. [DOI] [PubMed] [Google Scholar]
- 8. Antoni ML, Scherptong RW, Atary JZ, Boersma E, Holman ER, van der Wall EEet al. Prognostic value of right ventricular function in patients after acute myocardial infarction treated with primary percutaneous coronary intervention. Circ Cardiovasc Imaging 2010;3:264–71. [DOI] [PubMed] [Google Scholar]
- 9. Zornoff LA, Skali H, Pfeffer MA, St John Sutton M, Rouleau JL, Lamas GAet al. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol 2002;39:1450–5. [DOI] [PubMed] [Google Scholar]
- 10. Verma A, Pfeffer MA, Skali H, Rouleau J, Maggioni A, McMurray JJet al. Incremental value of echocardiographic assessment beyond clinical evaluation for prediction of death and development of heart failure after high-risk myocardial infarction. Am Heart J 2011;161:1156–62. [DOI] [PubMed] [Google Scholar]
- 11. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran Ket al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713. [DOI] [PubMed] [Google Scholar]
- 12. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande Let al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–70. [DOI] [PubMed] [Google Scholar]
- 13. Focardi M, Cameli M, Carbone SF, Massoni A, De Vito R, Lisi Met al. Traditional and innovative echocardiographic parameters for the analysis of right ventricular performance in comparison with cardiac magnetic resonance. Eur Heart J Cardiovasc Imaging 2015;16:47–52. [DOI] [PubMed] [Google Scholar]
- 14. Anavekar NS, Skali H, Bourgoun M, Ghali JK, Kober L, Maggioni APet al. Usefulness of right ventricular fractional area change to predict death, heart failure, and stroke following myocardial infarction (from the VALIANT ECHO Study). Am J Cardiol 2008;101:607–12. [DOI] [PubMed] [Google Scholar]
- 15. Kjaergaard J, Akkan D, Iversen KK, Kober L, Torp-Pedersen C, Hassager C.. Right ventricular dysfunction as an independent predictor of short- and long-term mortality in patients with heart failure. Eur J Heart Fail 2007;9:610–6. [DOI] [PubMed] [Google Scholar]
- 16. Ghio S, Pica S, Klersy C, Guzzafame E, Scelsi L, Raineri Cet al. Prognostic value of TAPSE after therapy optimisation in patients with pulmonary arterial hypertension is independent of the haemodynamic effects of therapy. Open Heart 2016;3:e000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kusunose K, Popović ZB, Motoki H, Marwick TH.. Prognostic significance of exercise-induced right ventricular dysfunction in asymptomatic degenerative mitral regurgitation. Circ Cardiovasc Imaging 2013;6:167–76. [DOI] [PubMed] [Google Scholar]
- 18. La Gerche A, Claessen G, Dymarkowski S, Voigt JU, De Buck F, Vanhees Let al. Exercise-induced right ventricular dysfunction is associated with ventricular arrhythmias in endurance athletes. Eur Heart J 2015;36:1998–2010. [DOI] [PubMed] [Google Scholar]
- 19. Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen Tet al. ; Reviewers: this document was reviewed by members of the ESDC . Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2018;19:591–600. [DOI] [PubMed] [Google Scholar]
- 20. Longobardo L, Suma V, Jain R, Carerj S, Zito C, Zwicke DLet al. Role of two-dimensional speckle-tracking echocardiography strain in the assessment of right ventricular systolic function and comparison with conventional parameters. J Am Soc Echocardiogr 2017;30:937–46.e6. [DOI] [PubMed] [Google Scholar]
- 21. Kirkels FP, Lie OH, Cramer MJ, Chivulescu M, Rootwelt-Norberg C, Asselbergs FWet al. Right ventricular functional abnormalities in arrhythmogenic cardiomyopathy: association with life-threatening ventricular arrhythmias. JACC Cardiovasc Imaging 2021;14:900–10. [DOI] [PubMed] [Google Scholar]
- 22. Muraru D, Spadotto V, Cecchetto A, Romeo G, Aruta P, Ermacora Det al. New speckle-tracking algorithm for right ventricular volume analysis from three-dimensional echocardiographic data sets: validation with cardiac magnetic resonance and comparison with the previous analysis tool. Eur Heart J Cardiovasc Imaging 2016;17:1279–89. [DOI] [PubMed] [Google Scholar]
- 23. Sugeng L, Mor-Avi V, Weinert L, Niel J, Ebner C, Steringer-Mascherbauer Ret al. Multimodality comparison of quantitative volumetric analysis of the right ventricle. J Am Coll Cardiol Imaging 2010;3:10–8. [DOI] [PubMed] [Google Scholar]
- 24. Pickett CA, Cheezum MK, Kassop D, Villines TC, Hulten EA.. Accuracy of cardiac CT, radionucleotide and invasive ventriculography, two- and three-dimensional echocardiography, and SPECT for left and right ventricular ejection fraction compared with cardiac MRI: a meta-analysis. Eur Heart J Cardiovasc Imaging 2015;16:848–52. [DOI] [PubMed] [Google Scholar]
- 25. Maffessanti F, Muraru D, Esposito R, Gripari P, Ermacora D, Santoro Cet al. Age-, body size-, and sex-specific reference values for right ventricular volumes and ejection fraction by three-dimensional echocardiography: a multicenter echocardiographic study in 507 healthy volunteers. Circ Cardiovasc Imaging 2013;6:700–10. [DOI] [PubMed] [Google Scholar]
- 26. Ajmone Marsan N, Michalski B, Cameli M, Podlesnikar T, Manka R, Sitges Met al. EACVI survey on standardization of cardiac chambers quantification by transthoracic echocardiography. Eur Heart J Cardiovasc Imaging 2020;21:119–23. [DOI] [PubMed] [Google Scholar]
- 27. Muraru D, Badano LP, Nagata Y, Surkova E, Nabeshima Y, Genovese Det al. Development and prognostic validation of partition values to grade right ventricular dysfunction severity using 3D echocardiography. Eur Heart J Cardiovasc Imaging 2020;21:10–21. [DOI] [PubMed] [Google Scholar]
- 28. Surkova E, Muraru D, Genovese D, Aruta P, Palermo C, Badano LP.. Relative prognostic importance of left and right ventricular ejection fraction in patients with cardiac diseases. J Am Soc Echocardiogr 2019;32:1407–15.e3. [DOI] [PubMed] [Google Scholar]
- 29. Surkova E, Kovacs A, Tokodi M, Lakatos BK, Merkely Bet al. Contraction patterns of the right ventricle associated with different degrees of left ventricular systolic dysfunction. Circ Cardiovasc Imaging 2021;14:e012774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lakatos B, Tősér Z, Tokodi M, Doronina A, Kosztin A, Muraru Det al. Quantification of the relative contribution of the different right ventricular wall motion components to right ventricular ejection fraction: the ReVISION method. Cardiovasc Ultrasound 2017;15:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lakatos BK, Nabeshima Y, Tokodi M, Nagata Y, Tősér Z, Otani Ket al. Importance of nonlongitudinal motion components in right ventricular function: three-dimensional echocardiographic study in healthy volunteers. J Am Soc Echocardiogr 2020;33:995–1005.e1. [DOI] [PubMed] [Google Scholar]
- 32. Moceri P, Duchateau N, Baudouy D, Schouver ED, Leroy S, Squara Fet al. Three-dimensional right-ventricular regional deformation and survival in pulmonary hypertension. Eur Heart J Cardiovasc Imaging 2018;19:450–8. [DOI] [PubMed] [Google Scholar]
- 33. Addetia K, Maffessanti F, Yamat M, Weinert L, Narang A, Freed BHet al. Three-dimensional echocardiography-based analysis of right ventricular shape in pulmonary arterial hypertension. Eur Heart J Cardiovasc Imaging 2016;17:564–75. [DOI] [PubMed] [Google Scholar]
- 34. Addetia K, Maffessanti F, Muraru D, Singh A, Surkova E, Mor-Avi Vet al. Morphologic analysis of the normal right ventricle using three-dimensional echocardiography-derived curvature indices. J Am Soc Echocardiogr 2018;31:614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vogel M, Schmidt MR, Kristiansen SB, Cheung M, White PA, Sorensen Ket al. Validation of myocardial acceleration during isovolumic contraction as a novel noninvasive index of right ventricular contractility: comparison with ventricular pressure-volume relations in an animal model. Circulation 2002;105:1693–9. [DOI] [PubMed] [Google Scholar]
- 36. Hodzic A, Bobin P, Mika D, Ly M, Lefebvre F, Lechene Pet al. Standard and strain measurements by echocardiography detect early overloaded right ventricular dysfunction: validation against hemodynamic and myocyte contractility changes in a large animal model. J Am Soc Echocardiogr 2017;30:1138–47.e4. [DOI] [PubMed] [Google Scholar]
- 37. Guazzi M, Dixon D, Labate V, Beussink-Nelson L, Bandera F, Cuttica MJet al. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging 2017;10:1211–21. [DOI] [PubMed] [Google Scholar]
- 38. Claessen G, La Gerche A, Voigt J-U, Dymarkowski S, Schnell F, Petit Tet al. Accuracy of echocardiography to evaluate pulmonary vascular and RV function during exercise. JACC Cardiovasc Imaging 2016;9:532–43. [DOI] [PubMed] [Google Scholar]
- 39. Russell K, Eriksen M, Aaberge L, Wilhelmsen N, Skulstad H, Remme EWet al. A novel clinical method for quantification of regional left ventricular pressure-strain loop area: a non-invasive index of myocardial work. Eur Heart J 2012;33:724–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Butcher SC, Fortuni F, Montero-Cabezas JM, Abou R, El Mahdiui M, van der Bijl Pet al. Right ventricular myocardial work: proof-of-concept for non-invasive assessment of right ventricular function. Eur Heart J Cardiovasc Imaging 2021;22:142–52. [DOI] [PubMed] [Google Scholar]
- 41. Marcus JT, Gan CT, Zwanenburg JJ, Boonstra A, Allaart CP, Gotte MJet al. Interventricular mechanical asynchrony in pulmonary arterial hypertension: left-to-right delay in peak shortening is related to right ventricular overload and left ventricular underfilling. J Am Coll Cardiol 2008;51:750–7. [DOI] [PubMed] [Google Scholar]
- 42. Petersen SE, Aung N, Sanghvi MM, Zemrak F, Fung K, Paiva JMet al. Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. J Cardiovasc Magn Reson 2017;19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tandri H, Daya SK, Nasir K, Bomma C, Lima JA, Calkins Het al. Normal reference values for the adult right ventricle by magnetic resonance imaging. Am J Cardiol 2006;98:1660–4. [DOI] [PubMed] [Google Scholar]
- 44. Foppa M, Arora G, Gona P, Ashrafi A, Salton CJ, Yeon SBet al. Right ventricular volumes and systolic function by cardiac magnetic resonance and the impact of sex, age, and obesity in a longitudinally followed cohort free of pulmonary and cardiovascular disease: the Framingham heart study. Circ Cardiovasc Imaging 2016;9:e003810. [DOI] [PubMed] [Google Scholar]
- 45. Ricci F, Barison A, Todiere G, Mantini C, Cotroneo AR, Emdin Met al. Prognostic value of pulmonary blood volume by first-pass contrast-enhanced CMR in heart failure outpatients: the PROVE-HF study. Eur Heart J Cardiovasc Imaging 2018;19:896–904. [DOI] [PubMed] [Google Scholar]
- 46. Priya S, Aggarwal T, Ward C, Bathla G, Jacob M, Gerke Aet al. Radiomics side experiments and DAFIT approach in identifying pulmonary hypertension using cardiac MRI derived radiomics based machine learning models. Sci Rep 2021;11:12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DAet al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010;121:1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Te Riele AS, Bhonsale A, James CA, Rastegar N, Murray B, Burt JRet al. Incremental value of cardiac magnetic resonance imaging in arrhythmic risk stratification of arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol 2013;62:1761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Geva T, Sandweiss BM, Gauvreau K, Lock JE, Powell AJ.. Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. J Am Coll Cardiol 2004;43:1068–74. [DOI] [PubMed] [Google Scholar]
- 50. Hosch O, Sohns JM, Nguyen TT, Lauerer P, Rosenberg C, Kowallick JTet al. The total right/left-volume index: a new and simplified cardiac magnetic resonance measure to evaluate the severity of Ebstein anomaly of the tricuspid valve: a comparison with heart failure markers from various modalities. Circ Cardiovasc Imaging 2014;7:601–9. [DOI] [PubMed] [Google Scholar]
- 51. van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MDet al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J 2007;28:1250–7. [DOI] [PubMed] [Google Scholar]
- 52. Bourantas CV, Loh HP, Bragadeesh T, Rigby AS, Lukaschuk EI, Garg Set al. Relationship between right ventricular volumes measured by cardiac magnetic resonance imaging and prognosis in patients with chronic heart failure. Eur J Heart Fail 2011;13:52–60. [DOI] [PubMed] [Google Scholar]
- 53. Corbett JR, Akinboboye OO, Bacharach SL, Borer JS, Botvinick EH, DePuey EGet al. ; Quality Assurance Committee of the American Society of Nuclear Cardiology . Quality assurance committee of the American Society of Nuclear C. Equilibrium radionuclide angiocardiography. J Nucl Cardiol 2006;13:e56–e79. [DOI] [PubMed] [Google Scholar]
- 54. Sankaralingam S, Lopaschuk GD.. Cardiac energy metabolic alterations in pressure overload-induced left and right heart failure (2013 Grover Conference Series). Pulm Circ 2015;5:15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dupont MVM, Drăgean CA, Coche EE.. Right ventricle function assessment by MDCT. AJR Am J Roentgenol 2011;196:77–86. [DOI] [PubMed] [Google Scholar]