Abstract
Introduction
The safety of BCG revaccination is uncertain and there is no data on its use in patients with COVID-19.
Methods
COVID-19 convalescent adults confirmed by SARS-CoV-2 RT-PCR in South-America were 1:1 randomized in the first 14 days of symptoms to BCG intradermal vaccine or placebo and evaluated for adverse events on days 7, 14, 21, and beyond 40 days. Clinical Trial Registration: NCT04369794.
Results
151 placebo and 148 BCG patients were included in the final analysis, with an average age of 40.7 years. No severe adverse event to BCG was reported. On day 7, 130 (87.8%) of the BCG recipients had local reaction, average size of 10.6 ± 6.4 mm, compared to only 2 (1.3%) placebos. Lesions gradually shrunk in size (mean 10.5 mm, 9.7 mm, and 6.8 mm at 14, 21, and beyond 40 days, respectively. The number of symptoms in any of the visits was not different between groups, and anosmia resolved earlier (25.7% vs. 37.1% at 7 days, OR = 1.70, 1.01–2.89, p = 0.035) in the BCG recipients.
Conclusion
The BCG revaccination is safe in convalescent COVID-19 adults of a tuberculosis endemic region, regardless of tuberculin or IGRA test results. Local adverse events were similar though occurred earlier to that previously reported in children.
Keywords: BCG vaccine, COVID-19, SARS-CoV-2, BCG Lesion, Outcomes, Randomized controlled trial, Safety
1. Introduction
The bacillus Calmette-Guérin (BCG) vaccine was developed over 100 years ago and its main target is the prevention of severe forms of tuberculosis in children [1]. In the Brazilian vaccination calendar, it is offered at birth [2]. After the vaccine intradermal application, a local lesion evolves into a vaccine scar, and other local and systemic adverse events may occur [3]. Despite previously confirmed off-target effects and cross-protection of BCG revaccination [4], it is not yet recommended due to the lack of enough studies proving its safety, mainly in adults’ active inflammatory conditions [5].
Due to its non-specific immunomodulatory and anti-inflammatory effects, the BCG vaccine has the potential to increment the results of specific vaccines and treatments against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [6].
Our research group performed a randomized controlled trial to evaluate the efficacy and safety of BCG revaccination in subjects with coronavirus disease 2019 (COVID-19). We will also follow-up our patients to evaluate the effect of BCG injection during acute infection on post-COVID conditions (long COVID) and on future re-infection with SARS-CoV-2 (undergoing study) as the virus becomes endemic.
Since the safety of BCG revaccination is uncertain and there is no data on whether its use is safe in patients with COVID-19, the aim of this article is to present findings on adverse events in adults who underwent BCG revaccination during the period of COVID-19 convalescence (BATTLE trial).
2. Methods
2.1. Study design
This is a multicenter, prospective, randomized 1:1 (https://www.randomization.com), double-blind, placebo-controlled study approved by the National Research Ethics Commission (CONEP) under number 31049320.7.1001.5404 and registered on ClinicalTrials.gov, number NCT04369794.
2.2. Participants and settings
Individuals older than 18 years, with a positive real-time reverse transcriptase-polymerase chain reaction (RT-PCR) result for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), were recruited from 2 outpatient health systems, the University of Campinas Community Health Center (CECOM-UNICAMP), Campinas, and the Paulínia Municipal Primary Health Care units (Campinas metropolitan area), Sao Paulo state, Brazil.
2.3. Vaccination
The intervention group received BCG 10 international unit (IU, 0.1 mL), and the placebo group received 0.1 mL of saline 0.9% solution, both given intradermally to the left deltoid to facilitate monitoring of the vaccine lesion. The syringe was the same for placebo and BCG groups, filled with the same volume, and both vaccine and placebo had the same color (transparent).
2.4. Assessments
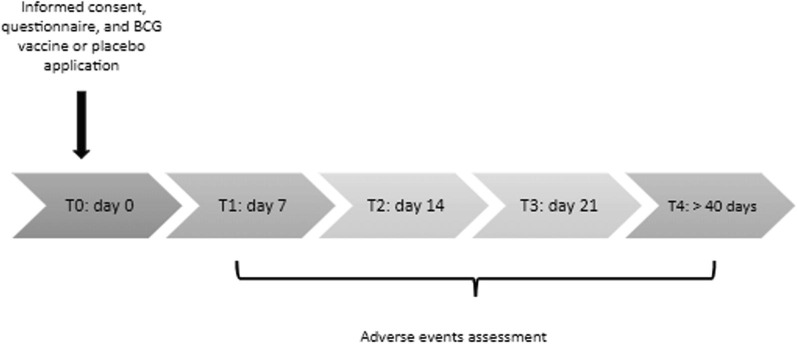
The Supplemental Fig. 1 shows the study timeline. The established COVID-19 BATTLE trial protocol determined T0 as the day of BCG or placebo vaccine, informed consent, and patient demographics collection. T1, T2, T3, and T4 corresponded to 7, 14, 21, and over 40 days after the vaccine, respectively, and subjects underwent questionnaire on adverse events (collected by the RedCap® app), vaccine lesion monitoring, characterizing, measuring, and photographing (Supplemental Fig. 2).
Exclusion criteria were BCG contraindication (pregnant, immunosuppressed, transplanted, cancer, immunosuppressants use, HIV positive), beyond 14 days of COVID-19 symptoms or follow-up beyond 2 days sooner or later the planned.
The systemic adverse events evaluated were the musculoskeletal (myalgia and arthralgia), neurological (headache, drowsiness, and mental confusion), gastrointestinal (ageusia, nausea, vomiting, and diarrhea), respiratory (cough, runny nose, sore throat, nasal congestion, and anosmia), and general events (fever, chills, fatigue/ tiredness, chest pain, cyanosis, and paleness).
The local adverse events evaluated were erythema, papule, pustule, pain, itching, crust, flaking, small ulcer, and scar.
2.5. Statistical analyses
All statistical analyses were performed using R version 4.1.2 on RStudio platform 2021.09.2 + 382 “Ghost Orchid” release and using the packages tidyverse and ggpubr. Wilcoxon rank sum test (unpaired) was used for continuous variables. Fisher exact test was used for categorical analysis. P-value of<0.05 was considered significant. Error bars throughout all figures represent one standard deviation unless otherwise specified.
Sensitivity analysis: since the two groups had baseline imbalance after randomization (higher frequency of chronic pulmonary disease in the BCG group), we also calculated odds ratios after excluding all patients with chronic pulmonary disease and compared the new result to the crude analysis in a graph.
3. Results
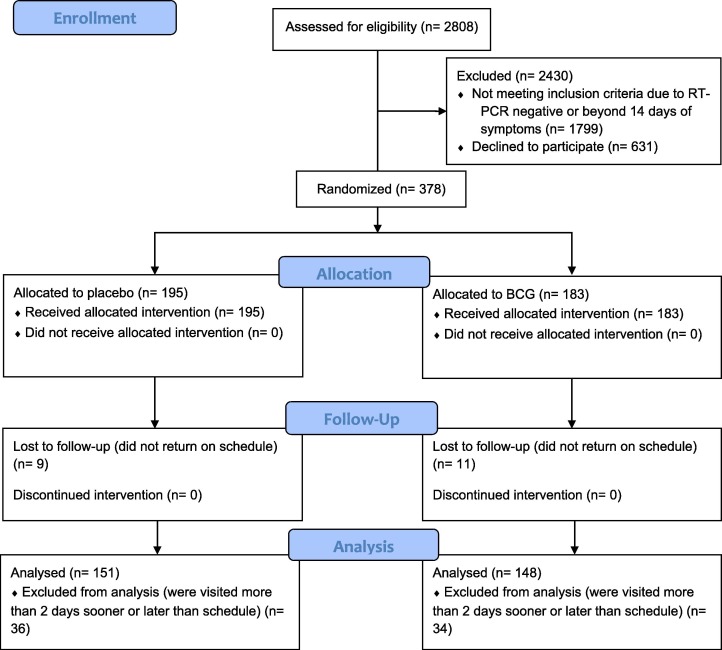
A total of 2808 patients were approached, 378 were randomized and after 20.9% were excluded due to follow-up protocol breach, 299 were included in the final analysis, 151 underwent placebo and 148 BCG vaccine, (Fig. 1 ). The average age of studied patients was 40.7 years; 19.8% of them were asymptomatic at the time of admission and the rest had mild symptoms.
Fig. 1.
Study flowchart (BCG = Bacillus Calmette-Guérin; n = number of participants).
There were no significant differences between the two groups regarding demographics or baseline symptoms, nonetheless the BCG recipients had a higher frequency of chronic pulmonary disease (6.8% vs. 1.3%, p = 0.039) (Table 1 ).
Table 1.
Patients’ demographics.
| Demographics | BCG (n = 148) | Placebo (n = 151) | p-value |
|---|---|---|---|
| Age (mean ± SD) | 40.4 ± 13.6 | 42.8 ± 11.8 | 0.14 |
| BMI | 29.3 ± 18.5 | 26.7 ± 4.2 | 1 |
| Female gender N (%) | 86 (58.1) | 96 (63.6) | 0.29 |
| Symptomatic on admission N (%) | 118 (79.7) | 122 (80.8) | 0.88 |
| Childhood BCG Scar N (%) | 139 (93.9) | 140 (92.7) | 0.83 |
| Diabetes N (%) | 10 (6.8) | 8 (5.3) | 1 |
| Hypertension N (%) | 30 (20.3) | 27 (17.9) | 1 |
| Cardiopathy N (%) | 4 (2.7) | 1 (0.7) | 0.20 |
| Obesity N (%) | 15 (10.1) | 10 (6.6) | 1 |
| Chronic kidney disease N (%) | 0 (0) | 0 (0) | – |
| Chronic pulmonary disease N (%) | 10 (6.8) | 2 (1.3) | 0.039 |
| Sinusitis N (%) | 25 (16.9) | 23 (15.2) | 0.77 |
| Respiratory allergies N (%) | 20 (13.5) | 17 (11.3) | 0.76 |
| Hemoglobinopathies N (%) | 1 (0.7) | 0 (0) | 0.48 |
| Autoimmune disorder N (%) | 0 (0%) | 2 (1.3) | 0.50 |
| Others N (%) | 47 (31.8) | 51 (33.8) | 0.58 |
BCG = Bacillus Calmette-Guérin; n = number of participants; Others = rinitis, hypothyroidism, gastritis, depression, arthrosis, dyslipidemia, glaucoma, among others; SD = standard deviation.
Most common symptoms on admission were anosmia (44.5%), ageusia (36.1%), fatigue (33.1%), cough (32.1%), headache (20.7%), and nasal congestion (15.1%).
3.1. Symptom progression
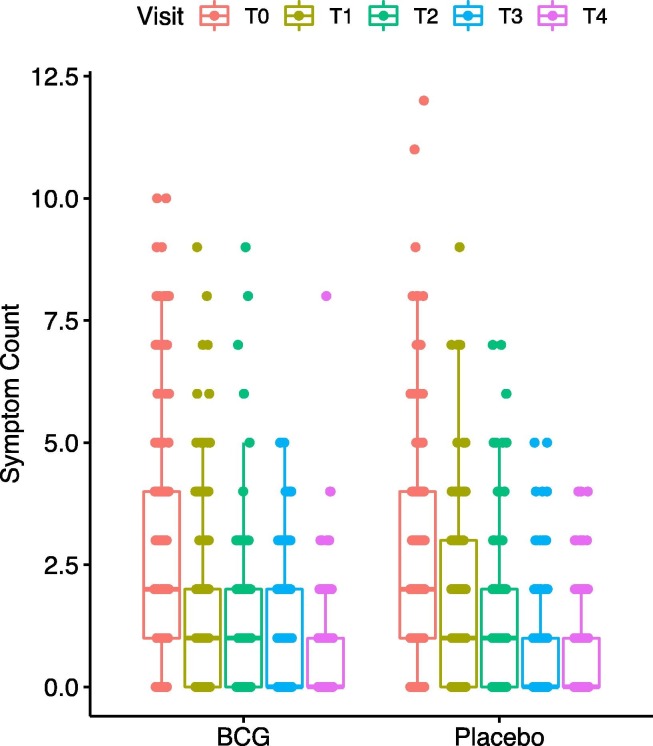
No severe adverse event was reported. Although there was no significant difference between the two groups on the number of symptoms in any of the visits (Fig. 2 ), dyspnea was more common (8.1% vs. 2.6%, at 7 days, OR = 0.31, 0.07–1.05, p = 0.042), and fatigue lasted longer (13.5% vs 5.3% at 21 days, OR = 0.36, 0.13–0.89, p = 0.017), while anosmia resolved earlier (25.7% vs. 37.1% at 7 days, OR = 1.70, 1.01–2.89, p = 0.035) in the BCG recipients compared to placebo, Table 2 .
Fig. 2.
Number of symptoms on each visit.
Table 2.
Symptoms and vaccine lesion progression.
| BCG |
Placebo |
p |
BCG |
Placebo |
p |
BCG |
Placebo |
p |
BCG |
Placebo |
p |
BCG |
Placebo |
p |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N(%) | T0 | T1 | T2 | T3 | T4 | ||||||||||
| Cough | 54(36.5) | 42(27.8) | 0.141 | 33(22.3) | 30(19.9) | 0.671 | 24(16.2) | 17(11.3) | 0.241 | 18(12.2) | 10(6.6) | 0.111 | 14(9.5) | 9(6) | 0.281 |
| Fever | 2(1.4) | 1(0.7) | 0.621 | 0(0) | 1(0.7) | 11 | 0(0) | 0(0) | – | 0(0) | 0(0) | – | 0(0) | 0(0) | – |
| Chills | 6(4.1) | 4(2.6) | 0.531 | 1(0.7) | 2(1.3) | 11 | 0(0) | 1(0.7) | 11 | 0(0) | 1(0.7) | 11 | 0(0) | 0(0) | – |
| Fatigue | 46(31.1) | 53(35.1) | 0.461 | 24(16.2) | 22(14.6) | 0.751 | 24(16.2) | 13(8.6) | 0.0541 | 20(13.5) | 8(5.3) | 0.0171 | 16(10.8) | 15(9.9) | 0.851 |
| OR = 0.49 (0.22 – 1.05) | OR = 0.36 (0.13–0.89) | ||||||||||||||
| Coryza | 12(8.1) | 9(6.0) | 0.501 | 5(3.4) | 7(4.6) | 0.771 | 8(5.4) | 7(4.6) | 0.801 | 6(4.1) | 2(1.3) | 0.171 | 4(2.7) | 3(2.0) | 0.721 |
| Nasal Congestion | 22 (14.9) | 23 (15.2) | 11 | 8(5.4) | 11(7.3) | 0.641 | 6(4.1) | 7(4.6) | 11 | 3(2.0) | 4(2.6) | 11 | 1(0.7) | 3(2.0) | 0.621 |
| Myalgia | 23(15.5) | 28(18.5) | 0.541 | 13(8.8) | 21(13.9) | 0.201 | 4(2.7) | 12(7.9) | 0.0691 | 10(6.8) | 7(4.6) | 0.461 | 5(3.4) | 3(2.0) | 0.501 |
| Arthralgia | 14(9.5) | 15(9.9) | 11 | 3(2.0) | 11(7.3) | 0.0521 | 1(0.7) | 5(3.3) | 0.211 | 3(2.0) | 3(2.0) | 11 | 1(0.7) | 4(2.6) | 0.371 |
| Headache | 31(20.9) | 31(20.5) | 11 | 24(16.2) | 28(18.5) | 0.651 | 16(10.8) | 16(10.6) | 11 | 14(9.5) | 11(7.3) | 0.531 | 18(12.2) | 9(6.0) | 0.071 |
| Sore throat | 16(10.8) | 23(15.2) | 0.301 | 9(6.1) | 7(4.6) | 0.621 | 5(3.4) | 4(2.6) | 0.741 | 4(2.7) | 6(4.0) | 0.751 | 0(0) | 3(2.0) | 0.241 |
| Anosmia | 70(47.3) | 63(41.7) | 0.351 | 38(25.7) | 56(37.1) | 0.0351 | 32(21.6) | 37(24.5) | 0.591 | 23(15.5) | 30(19.9) | 0.371 | 16(10.8) | 26(17.2) | 0.141 |
| OR = 1.70 (1.01–2.89) | |||||||||||||||
| Ageusia | 59 (39.9) | 49 (32.5) | 0.191 | 35(23.6) | 43(28.5) | 0.361 | 25(16.9) | 31(20.5) | 0.461 | 24(16.2) | 24(15.9) | 11 | 12(8.1) | 18(11.9) | 0.341 |
| Nausea | 9(6.1) | 10(6.6) | 11 | 2(1.4) | 4(2.6) | 0.681 | 1(0.7) | 1(0.7) | 11 | 1(0.7) | 1(0.7) | 11 | 1(0.7) | 0(0) | 0.501 |
| Vomiting | 0(0) | 0(0) | – | 0(0) | 0(0) | – | 0(0) | 0(0) | – | 1(0.7) | 0(0) | 0.501 | 0(0) | 0(0) | – |
| Diarrhea | 13(8.8) | 5(3.3) | 0.0541 | 3(2.0) | 4(2.6) | 11 | 4(2.7) | 1(0.7) | 0.211 | 2(1.4) | 1(0.7) | 0.621 | 2(1.4) | 0(0) | 0.241 |
| Chest pain | 11(7.4) | 7 (4.6) | 0.341 | 4(2.7) | 2(1.3) | 0.441 | 1(0.7) | 3(2.0) | 0.621 | 1(0.7) | 1(0.7) | 11 | 2(1.4) | 1(0.7) | 0.621 |
| Dyspnea | 20(13.5) | 14(9.3) | 0.281 | 12(8.1) | 4(2.6) | 0.0421 | 6(4.1) | 5(3.3) | 0.771 | 3(2.0) | 4(2.6) | 11 | 4(2.7) | 2(1.3) | 0.441 |
| OR = 0.31 (0.07 – 1.05) | |||||||||||||||
| Somnolence | 13 (8.8) | 17(11.3) | 0.571 | 8(5.4) | 5(3.3) | 0.411 | 5(3.4) | 5(3.3) | 11 | 3(2.0) | 2(1.3) | 0.681 | 3(2.0) | 3(2.0) | 11 |
| Mental confusion | 4(2.7) | 2(1.3) | 0.441 | 2(1.4) | 1(0.7) | 0.621 | 1(0.7) | 1(0.7) | 11 | 1(0.7) | 1(0.7) | 11 | 1(0.7) | 3(2.0) | 0.621 |
| Median number of symptoms | 2 | 2 | 0.451 | 1 | 1 | 0.101 | 1 | 1 | 0.981 | 0 | 0 | 0.361 | 0 | 0 | 0.881 |
| Vaccine lesion | |||||||||||||||
| Erythema | – | 122(82.4) | 1(0.7) | <0.00011 | 117(79.1) | 2(1.3) | <0.00011 | 106(71.6) | 3(2.0) | <0.00011 | 90(60.8) | 0(0) | <0.00011 | ||
| Erythema size | – | 11.4 ± 5.9 | 5.0 | 10.6 ± 4.2 | 1.0 ± 1.4 | 10.0 ± 5.1 | 3.0 ± 1.0 | 7.2 ± 3.0 | |||||||
| Papule | – | 93(62.8) | 1(0.7) | <0.00011 | 60(40.5) | 3(2.0) | <0.00011 | 55(37.2) | 1(0.7) | <0.00011 | 23(15.5) | 0(0) | <0.00011 | ||
| Papule size | – | 10.4 ± 5.4 | 5.0 | 9.2 ± 2.8 | 1.5 ± 2.1 | 9.2 ± 5.4 | 4.0 | 6.9 ± 3.9 | – | ||||||
| Pustule | – | 12(8.1) | 1(0.7) | 0.00141 | 28(18.9) | 0(0) | <0.00011 | 10(6.8) | 0(0) | 0.00081 | 6(4.1) | 0(0) | 0.0141 | ||
| Pustule size | – | 13.4 ± 6.9 | 2.5 | 11.9 ± 5.2 | – | 9.6 ± 3.3 | – | 7.0 ± 9.9 | – | ||||||
| Local Pain | – | 9(6.1) | 0(0) | 0.00161 | 6(4.1) | 0(0) | 0.0141 | 7(4.7) | 0(0) | 0.00681 | 0(0) | 0(0) | – | ||
| Pain size | – | 13.2 ± 6.3 | – | 11.0 ± 1.4 | – | 9.6 ± 4.0 | – | 8.3 ± 3.6 | 5.0 | ||||||
| Itching | – | 19(12.8) | 0(0) | <0.00011 | 11(7.4) | 0(0) | 0.00031 | 12(8.1) | 0(0) | 0.00021 | 7(4.7) | 0(0) | 0.0071 | ||
| Itching size | – | 10.1 ± 5 0.5 |
– | 10.3 ± 0.8 | – | 11.4 ± 5.7 | – | 7.0 ± 1.2 | – | ||||||
| Ulcer | – | 0(0) | 0(0) | – | 2(1.4) | 0(0) | 0.241 | 6(4.1) | 0(0) | 0.0141 | 7(4.7) | 0(0) | 0.0071 | ||
| Ulcer size | – | 15 ± 0 | – | 8.7 ± 2.5 | – | 7.4 ± 1.4 | – | ||||||||
| Crust | – | 3(2.0) | 0(0) | 0.121 | 13(8.8) | 0(0) | <0.00011 | 19(12.8) | 0(0) | <0.00011 | 33(22.3) | 0(0) | <0.00011 | ||
| Crust size | – | 25 ± 0 | – | 8.9 ± 2.4 | – | 9.6 ± 1.6 | – | 7.0 ± 2.0 | – | ||||||
| Scar | – | 0(0) | 0(0) | – | 1(0.7) | 0(0) | 0.501 | 4(2.7) | 2(1.3) | 0.441 | 33(22.3) | 0(0) | <0.00011 | ||
| Scar size | – | 10 | – | 11.7 ± 11.7 | 3.0 ± 0 | 6.7 ± 2.0 | – | ||||||||
| Peeling | – | 2(1.4) | 0(0) | 0.241 | 12(8.1) | 0(0) | 0.00021 | 16(10.8) | 0(0) | <0.00011 | 32(21.6) | 0(0) | <0.00011 | ||
| Peeling size | – | 12 | – | 11.0 ± 2.5 | – | 10.1 ± 5.5 | – | 7.6 ± 3.0 | – | ||||||
| Vesicle | – | 0(0) | 0(0) | – | 0(0) | 0(0) | – | 0(0) | 0(0) | – | 0(0) | 0(0) | – | ||
| Lymphadenopathy | – | 0(0) | 0(0) | – | 0(0) | 0(0) | – | 0(0) | 0(0) | – | 0(0) | 0(0) | – | ||
| Lesion size overall | – | 10.6 ± 6.4 | 3.3 ± 1.5 | 10.5 ± 4.2 | 3.8 ± 1.5 | 9.7 ± 4.9 | 3.3 ± 1.0 | 6.8 ± 2.9 | 2.0 | ||||||
Legend: 1Wilcoxon rank sum test; p = p-value; BCG = Bacillus Calmette-Guérin; N = number of participants.
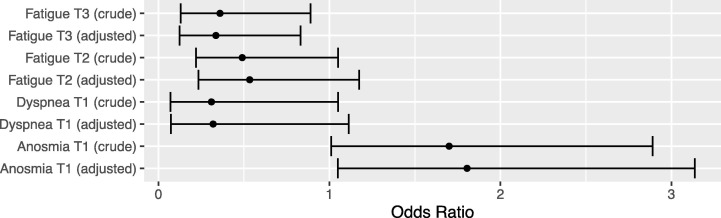
In the sensitivity analysis we excluded all patients with chronic pulmonary disease and compared the new odds ratio to the crude analysis (Fig. 3 ). The odds ratios slightly changed for dyspnea on T1 and the p-value was no longer significant (OR = 0.31, 95% CI = 0.07 – 1.11, p = 0.061). In the T2 visit, fatigue was not significantly different between groups (OR = 0.53, 95% CI = 0.23 – 1.17, p = 0.10). The rest of the analysis did not change significantly.
Fig. 3.
Sensitivity analysis odds ratio adjusted for chronic pulmonary disease.
3.2. Local BCG lesion
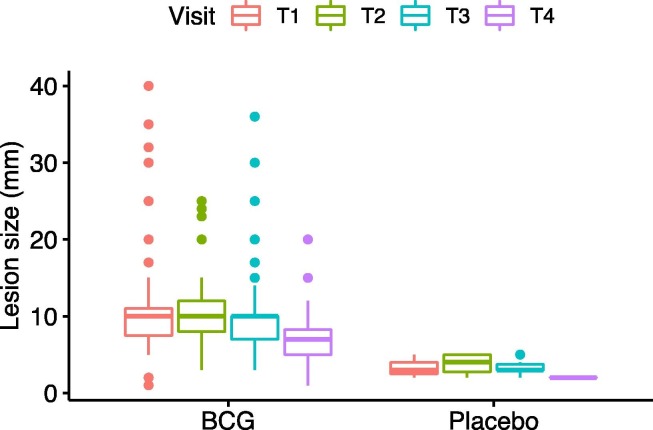
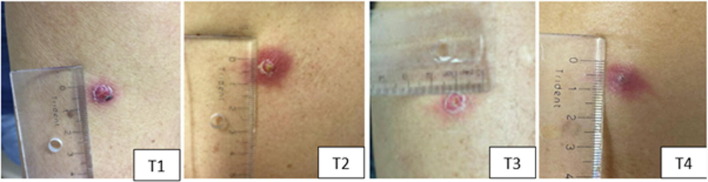
On the first visit following injection, 130 (87.8%) of the BCG recipients had local reaction at the site of injection with average size of 10.6 ± 6.4 mm, whereas only 2 (1.3%) placebo recipients had reaction. Most skin lesions were local erythema (82.4%), papule (62.8%), and pustule (8.1%). Mild itching (12.8%) and mild local pain (6.1%) were also reported. In the following weeks, the lesion gradually shrunk in size (10.5 mm, 9.7 mm, and 6.8 mm on T2, T3, and T4, respectively, Fig. 4 ). Some lesions crusted over and peeling was observed, but in a few BCG recipients (4.7%), the lesion turned into small ulcer reaching maximum average size of 8.7 ± 2.5 mm on third week following injection. Details of lesion types and sizes are available in Table 2.
Fig. 4.
Average lesion size on each visit.
4. Discussion
Since the emergence of COVID-19 as a global pandemic, several researchers have been interested in the BCG vaccine as a preventive strategy against COVID-19 [7], [8], [9], [10]; however, we have tested BCG as an adjuvant treatment in patients already infected with SARS-CoV-2, which is the uniqueness of the BATTLE clinical trial. Moreover, our study evaluated the BCG revaccination safety in adult patients of all ages, a theme little explored in the literature [4]. Detailed description of this study is published separately along with results of serology analysis [13]. The aim of current article is to focus on BCG adverse effects including description of the lesion size and progression. Furthermore, the analysis of the data is different in this article: patients were excluded if they were not visited within 2 days of the scheduled follow-up, whereas in the other article patients were analyzed based on the “week” they were visited, not the exact day. The advantage of excluding patients who were not visited within 2 days of follow-up is that the time-analysis is more exact. The disadvantage, however, is the potential for exclusion bias; for example, patients may have missed their scheduled appointment due to the worsening of their symptoms, therefore, excluding these patients might have excluded those who had worse symptoms.
The BCG main local adverse events described in the literature are based on children vaccinated against tuberculosis and include ulcer greater than 1 cm, cold or hot subcutaneous abscess, granuloma, lymphadenopathy > 3 cm suppurated or not, keloid scar, and lupoid reaction. Also, systemic tuberculin lesions can affect the skin, the osteoarticular system, lymph nodes, and organs [3].
Through this study it was observed that revaccination with BCG was safe in adults, no severe adverse event was described and there was no immediate or mediate intervention needed after the vaccine application, with no hospitalizations due to BCG adverse events. Similarly, the phase III ACTIVATE trial showed that BCG revaccination was also safe and effective for elderly patients, though only those with negative skin tuberculin tests were studied [4]. It is noteworthy that participants of the current study underwent BCG revaccination without tuberculin or Interferon Gama Release Assay (IGRA) testing.
Therefore, the current study brings new information concerning the safety of adult BCG revaccination in a tuberculosis endemic country, independent of tuberculin or IGRA test results that were considered exclusion criteria in other recent studies [4], [11]. Another important uniqueness of our study is the fact that patients were COVID-19 convalescent, which might leave them more susceptible to adverse events, beyond potential cross-protection.
While fatigue tended to last longer, anosmia was reduced in BCG recipients. Also, BCG group had a higher frequency of chronic pulmonary disease (6.8% vs 1.3%, p = 0.039), which might explain higher dyspnea frequency in the first follow-up visit (8.1% vs. 2.6%, p = 0.042). We also performed sensitivity analysis to compare crude results to the adjusted results (after excluding patients with chronic pulmonary disease). The sensitivity analysis showed that this difference in dyspnea was likely due to difference in baseline comorbidity of chronic respiratory illness (crude p = 0.042 versus adjusted p = 0.061, Fig. 3). However, it is still wise to be cautious about higher chance of dyspnea during the first week after BCG injection.
The main local events were erythema and papules. Pustules were significant at 14 days along with local itching that was present up to 21 days. After 21 days, the crust and flaking predominate, closing with the significant presence of scar after 40 days in most cases.
In our patients the lesion reached its peak size on first week following injection and slowly shrunk in size after that. Crust and ulcer developed on the second week but were more common on the third week. In comparison, BCG lesion in newborns is expected to form later, with the peak size at 12 weeks reaching average diameter (average horizontal and vertical length) of 4.5 mm. When comparing lesion size, it is important to keep in mind that newborns receive half the amount of adult BCG dose (0.05 mL compared to 0.1 mL in adults) [3], [12]. Such difference in the time of onset and size of the lesion might relate to the adult's immune system maturity added to previous BCG and other Mycobacteria spp. exposure.
A prospective randomized design including temporal analysis of RT-PCR SARS-CoV-2 confirmed patients are important strengths of the study. Regarding limitations, in addition to a relatively small study and exclusion of patients that did not attach to the pre-stipulated follow-up, BCG vaccine is naturally unblinded due to the skin reactions that occur within days at the site of injection. While positive IGRA or tuberculin skin test patients were not excluded, future studies should compare the outcomes according to those tests results. Also, once the BATTLE trial is the first study on the issue, due to safety concerns, severe COVID, hospitalized or immunocompromised patients were excluded and the sample size was relatively limited, in addition, we closely monitored patients on a weekly basis.
5. Conclusion
The BCG revaccination is unlikely to cause serious adverse events in convalescent COVID-19 adults in a tuberculosis endemic region, regardless of tuberculin or IGRA test results. Local adverse events were similar though occurred earlier to that reported in children.
Funding
Coordination for the Improvement of Higher Education Personnel - CAPES: 88887.506617/2020-00 and National Council for Scientific and Technological Development – CNPq, Research Productivity: 304747/2018-1. The funder had no involvement in study design, data collection, data analysis, manuscript preparation, and/or publication decisions.
Ethical approval
University of Campinas ethics committee approval number: 4.173.069.
Informed consent
Obtained per protocol.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [Leonardo O. Reis reports financial support was provided by Coordination of Higher Education Personnel Improvement.].
Acknowledgment
To the involved institution(s), the patients, and those that provided and cared for study patients.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.06.039.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary Figure 1.
Study timeline.
Supplementary Figure 2.
Vaccine lesion monitoring.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.World Health Organization. Global Tuberculosis Report, https://www.who.int/tb/publications/global_report/en/; 2020 [accessed 10 September 2021].
- 2.Brazil, Ministério da Saúde. National Vaccination Calendar, “https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/c/calendario-de-vacinacao”; 2020 [accessed 10 September 2021].
- 3.Brazil, Technical Note No. 10/2020 of the state of São Paulo. BCG vaccine vaccination: indications, contraindications, adverse events and conducts, https://www.prefeitura.sp.gov.br/cidade/secretarias/upload/saude/nota_tecnica_10_2020_bcg.pdf“; 2020 [Accessed 21 September 2021].
- 4.Giamarellos-Bourboulis E.J., Tsilika M., Moorlag S., Antonakos N., Kotsaki A., Domínguez-Andrés J., et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell. 2020;183(2):315–323.e9. doi: 10.1016/j.cell.2020.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bannister S., Sudbury E., Villanueva P., Perrett K., Curtis N. The safety of BCG revaccination: a systematic review. Vaccine. 2021;39(20):2736–2745. doi: 10.1016/j.vaccine.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Pavan Kumar N., Padmapriyadarsini C., Rajamanickam A., Marinaik S.B., Nancy A., Padmanaban S., et al. Effect of BCG vaccination on proinflammatory responses in elderly individuals. Sci Adv. 2021;7(32) doi: 10.1126/sciadv.abg7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Junqueira-Kipnis A.P., Dos Anjos L.R.B., Barbosa L.C.S., da Costa A.C., Borges K.C.M., Cardoso A.D.R.O., et al. BCG revaccination of health workers in Brazil to improve innate immune responses against COVID-19: a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21(1):881. doi: 10.1186/s13063-020-04822-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madsen A.M.R., Schaltz-Buchholzer F., Benfield T., Bjerregaard-Andersen M., Dalgaard L.S., Dam C., et al. Using BCG vaccine to enhance non-specific protection of health care workers during the COVID-19 pandemic: a structured summary of a study protocol for a randomized controlled trial in Denmark. Trials. 2020;21(1):799. doi: 10.1186/s13063-020-04714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yitbarek K., Abraham G., Girma T., Tilahun T., Woldie M. The effect of Bacillus Calmette-Guérin (BCG) vaccination in preventing severe infectious respiratory diseases other than TB: implications for the COVID-19 pandemic. Vaccine. 2020;38(41):6374–6380. doi: 10.1016/j.vaccine.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsilika M., Taks E., Dolianitis K., Kotsaki A., Leventogiannis K., Damoulari C., et al. ACTIVATE-2: a double-blind randomized trial of BCG vaccination against COVID19 in individuals at risk. medRxiv. 2021 doi: 10.1101/2021.05.20.21257520. [DOI] [Google Scholar]
- 11.Szigeti R., Kellermayer R. Natural unblinding of BCG vaccination trials. Vaccine. 2021;39(15):2017–2019. doi: 10.1016/j.vaccine.2021.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiaffino F., Lee G.O., Paredes-Olortegui M., Cabrera L., Penataro-Yori P., Gilman R.H., et al. Evolution of the Bacillus Calmette-Guerin scar and its association with birth and pregnancy characteristics in a prospective cohort of infants in iquitos. Peru Am J Perinatol. 2019;36(12):1264–1270. doi: 10.1055/s-0038-1676614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jalalizadeh M., Buosi K., Dionato F.A.V., Dal Col L.S.B., Giacomelli C.F., Ferrari K.L., et al. Randomized clinical trial of BCG vaccine in patients with convalescent COVID-19: clinical evolution, adverse events, and humoral immune response. J Intern Med. 2022 doi: 10.1111/joim.13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.