Abstract
Background
The antibody titer is known to wane within months after receiving two doses of the Pfizer-BioNTech BNT162b2 mRNA SARS-CoV-2 vaccine. However, knowledge of the cellular immune response dynamics following vaccination is limited. This study to aimed to determine antibody and cellular immune responses following vaccination, and the incidence and determinants of breakthrough infection.
Methods
This prospective cohort study a 6-month follow-up period was conducted among Japanese healthcare workers. All participants received two doses of BNT162b2 vaccine. Anti-SARS-CoV-2 antibody titers and T-cell immune responses were measured in serum samples collected at several timepoints before and after vaccination.
Results
A total of 608 participants were included in the analysis. Antibody titers were elevated 3 weeks after vaccination and waned over the remainder of the study period. T-cell immune responses showed similar dynamics. Six participants without predisposing medical conditions seroconverted from negative to positive on the IgG assay for nucleocapsid proteins, indicating breakthrough SARS-CoV-2 infection. Five of the six breakthrough infections were asymptomatic.
Conclusions
Both humoral and cellular immunity waned within 6 months after BNT162b2 vaccination. The incidence of asymptomatic breakthrough infection within 6 months after vaccination was approximately one percent.
UMIN Clinical Trials Registry ID
UMIN000043340.
Keywords: COVID-19, SARS-CoV-2, BNT162b2 vaccine, mRNA vaccines, Immune response, Breakthrough infections, Healthcare workers
1. Introduction
Although 2 years have passed since the first cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection were reported, the pandemic is ongoing. It was hoped that vaccination would end the pandemic; however, the antibody titer wanes within months after the first two doses of vaccine [1] and cases of breakthrough infection have occurred [2]. The Pfizer-BioNTech BNT162b2 mRNA SARS-CoV-2 vaccine is a messenger RNA vaccine that is widely used in Japan and worldwide. Most large cohort studies describing antibody dynamics after two doses of BNT162b2 vaccine were conducted in Western countries [3]. However, the immune response may be affected by racial and demographic factors. Although some reports about declining immune response or cellular immunity during the time period among Japanese vaccinees are available, the sample size was limited [4], [5], [6]. We conducted a prospective cohort study in more than 600 Japanese healthcare workers to determine humoral and cellular immune responses to the vaccine in a Japanese population. We have previously described the humoral and cellular responses immediately after two doses of BNT162b2 vaccine and their relationship to adverse reactions [7], [8]. Here, we describe the dynamics of the antibody titer and the cellular immune response during the 6 months after the initial two doses of BNT162b2 vaccine. Additionally, we describe cases of suspected breakthrough infection based on the development of antibodies to SARS-CoV-2 nucleocapsid proteins.
2. Methods
2.1. Participants
Healthcare workers working on the Keio University Shinanomachi Campus (Tokyo, Japan) who were vaccinated against SARS-CoV-2 between February 16 and March 9, 2021 were recruited for the study. The campus has a university hospital with 960 beds and a medical school. Written informed consent was obtained from all participants before mass vaccination. Mass vaccination was carried out using the BNT162b2 vaccine (Comirnaty intramuscular injection, Pfizer, New York, USA), which was stored and prepared according to the instructions given in the package insert. Each participant received two doses of vaccine, administered 3 weeks apart.
2.2. Study registration and ethics approval
The study protocol was registered with UMIN Clinical Trials Registry on February 16, 2021 (UMIN ID: UMIN000043340). The study was approved by the ethics committee of the Keio University School of Medicine (approval no. 20200330).
2.3. Sample collection
Serial serum samples were collected from each participant at five timepoints. The first sample collection timepoint was before or on the day of the first vaccination. The second was between April 15 and 28, 2021, approximately 3 weeks after the second dose. The third was between May 20 and June 2, 2021, approximately 8 weeks after the second dose. The fourth was between June 28 and July 9, 2021, approximately 3 months after the second dose. The final sample collection timepoint was between September 28 and October 8, 2021, approximately 6 months after the second dose. Each participant completed a structured questionnaire at the time of each sample collection. At the time of the first sample collection, participants provided information on their age, sex, height, body weight, use of systemic steroids or other immunosuppressants, ongoing cancer chemotherapy, and history of immunodeficiency, cancers, autoimmune diseases, diabetes, and COVID-19. At the time of subsequent sample collections participants provided information on history of a COVID-19 episode, COVID-19-like illness, SARS-CoV-2 polymerase chain reaction (PCR) testing, and close contact with COVID-19 patients since the preceding sample collection. Participants who did not receive two doses of vaccine and those who did not who did not provide samples at all five timepoints were excluded from the analysis.
2.4. Measurement of antibody titers
Antibody titers to SARS-CoV-2 spike protein (S-IgG) and anti-SARS-CoV-2 neutralizing antibody titers were measured in all samples using three commercially available chemiluminescence enzyme immunoassays (CLEIA). First, IgG antibody titers against the SARS-CoV-2 spike protein S1 subunit receptor-binding domain (RBD) were measured using SARS-CoV-2 IgG II Quant reagents (Abbott Laboratories, Abbott Park, IL, USA) and an Alinity Analyzer (Abbott Laboratories, Abbott Park, IL, USA) (Alinity RBD-IgG) according to the manufacturer’s instructions. Second, the IgG antibody titers against the anti-SARS-CoV-2 spike protein were measured using HISCL SARS-CoV-2 S-IgG reagents (Sysmex Corporation, Kobe, Japan) and HISCL Analyzer (Sysmex Corporation, Kobe, Japan) and (HISCL S-IgG) according to the manufacturer’s instructions. Third, SARS-CoV-2 neutralizing antibodies were measured using the STACIA SARS-CoV-2 Neutralization Antibody Test (MBL Corporation, Nagoya, Japan) and STACIA Analyzer (LSI Medience Corporation, Tokyo, Japan) (STACIA Neut-Ab), which assess neutralizing ability by measuring the inhibitory activity of ACE 2 enzyme and RBD coated beads. Titers below the limit of detection (0.1 U/mL) were treated as 0.1 U/mL.
2.5. Measurement of cellular immunity
T-cell immunity against SARS-CoV-2 was assessed using an interferon gamma release assay (IGRA). Based on a limited reagent supply, samples were only collected from the first 600 participants to enroll in the study, and whole blood samples were collected in lithium heparin tubes at the first, third, and final sample collection timepoints. Samples were transferred to four QuantiFERON SARS-CoV-2 tubes (Qiagen, Hilden, Germany): coated with antigen 1, antigen 2, phytohemagglutinin (positive control), and no peptide (negative control). Antigen 1 is an epitope of CD4+ T cells derived from the S1 subunit, and antigen 2 is an epitope of CD4+ and CD8+ T cells derived from the S1 and S2 subunits. The tubes were incubated at 37 °C for 16–24 h and enzyme-linked immunosorbent assays were performed using the QuantiFERON SARS-CoV-2 ELISA kit (Qiagen, Hilden, Germany) according to the package insert using an AP-96 auto microplate enzyme immunoassay reader (Kyowa Medex, Tokyo, Japan). Quality control was conducted daily. The dynamics of the interferon gamma levels for antigen 1 (IFN for Ag1) and antigen 2 (IFN for Ag2) were investigated after correction for the negative control.
2.6. Detection of suspected breakthrough infections among participants
In order to detect all the breakthrough infections including asymptomatic or undiagnosed cases during the study period, we measured the IgG antibody titer against the nucleocapsid protein of SARS-CoV-2, which is not affected by the vaccine which codes for the spike protein regions of SARS-CoV-2. In order to measure the immunoglobulin G (IgG) antibody titer against the nucleocapsid protein (N-IgG), we used HISCL SARS-CoV-2 N-IgG reagents (Sysmex Corporation, Kobe, Japan) and the HISCL Analyzer (Sysmex Corporation, Kobe, Japan) according to the manufacturer’s instructions. We defined suspected breakthrough infections as follows: A negative N-IgG test result before and after vaccination, followed by a positive N-IgG test result during the 6 months after vaccination. The N-IgG test positivity was determined according to the manufacturer’s cut-off (10 SU/mL). Among participants with suspected breakthrough infection, their clinical characteristics, and S-IgG, neutralizing antibody titers, and IFN for Ag1 and Ag2 before the suspected breakthrough infection were compared with those of participants without suspected breakthrough infection.
2.7. Statistical analysis
Summary statistics of the participants were constructed using frequencies and proportions for categorical variables and means and standard deviations (SD) for continuous variables. We compared antibody titers and interferon gamma levels at each sample collection timepoint using paired t-tests. The characteristics of participants with and without suspected breakthrough infections were compared using Fisher exact tests for categorical variables, and Mann-Whitney U tests for continuous variables because of the small number of participants with suspected breakthrough infections. All statistical analyses were performed using JMP, version 15 and SAS software, version 9.4 (SAS Institute, Cary, NC, USA). Statistical significance was set at p < 0.05.
3. Results
Of the 673 healthcare workers and university staff members who enrolled in the study, three who did not receive two doses of vaccination and 62 who did not provide samples at all five timepoints were excluded from the analysis, leaving a total of 608 participants in the analysis (Table 1 ).
Table 1.
Baseline participant characteristics.
| Variable |
Value (N = 608) |
|---|---|
| Age, years, mean ± SD | 44.4 ± 10.7 |
| Sex, n (%) | |
| Male | 170 (28.0) |
| Female | 438 (72.0) |
| Body mass index (kg/m2), mean ± SD | 21.9 ± 3.2 |
| History, n (%) | |
| COVID-19 | 7 (1.1) |
| Diabetes | 3 (0.5) |
| Malignancy | 10 (1.6) |
| Autoimmune disease | 17 (2.8) |
| Immunosuppressant use, n (%) | |
| Systemic steroids | 7 (1.2) |
| Other | 10 (1.6) |
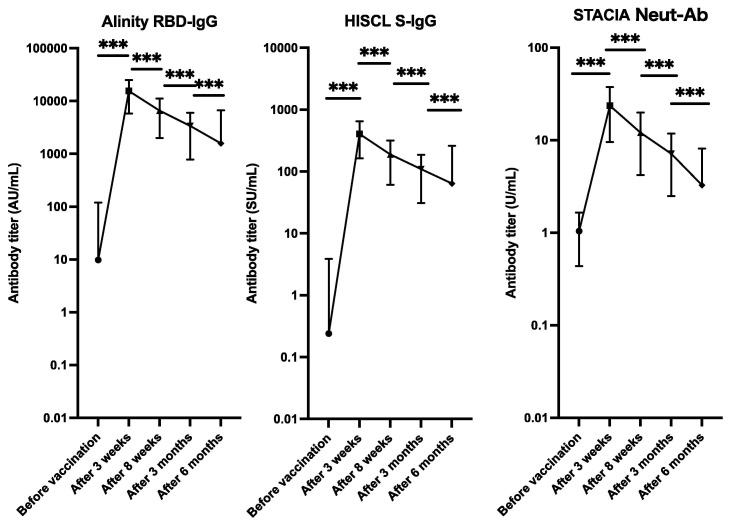
Before vaccination, the mean Alinity RBD-IgG, HISCL S-IgG, and STACIA Neut-Ab titers were 9.8 ± 109.1 AU/mL, 0.2 ± 3.6 SU/mL, and 1.0 ± 0.6 U/mL, respectively. Three weeks after vaccination the mean Alinity RBD-IgG, HISCL S-IgG, and STACIA Neut-Ab titers increased to 15,443.5 ± 9,655.2 AU/mL, 406.0 ± 242.7 SU/mL, and 23.6 ± 14.1 U/mL, respectively. The antibody titers waned and 6 months after vaccination the mean Alinity RBD-IgG, HISCL S-IgG, and STACIA Neut-Ab titers had decreased to 1,576.8 ± 5080.2 AU/mL, 63.9 ± 195.9 SU/mL, and 3.3 ± 4.9 U/mL, respectively (Fig. 1 ). Correlation analysis between the Alinity RBD-IgG, HISCL S-IgG, and STACIA Neut-Ab test results of all samples collected in the study demonstrated high correlations (Supplement Fig. S1).
Fig. 1.
Dynamics of the anti-SARS-CoV-2 antibody response after vaccination with two doses of the BNT162b2 vaccine. The plot shows the dynamics of the mean antibody titer at the five sample-collection time-points. The error bars indicate the standard deviation. Alinity RBD-IgG; antibody titer for the receptor-binding domain of SARS-CoV-2 was measured using SARS-CoV-2 IgG II Quant reagents and Alinity System (Abbott Laboratories, Illinois, USA), HISCL S-IgG: antibody titer for anti-SARS-CoV-2 spike protein measured using HISCL SARS-CoV-2 S-IgG reagents and an HISCL Analyzer (Sysmex Corporation, Kobe, Japan); STACIA Neut-Ab: neutralizing ability for SARS-CoV-2 using STACIA SARS-CoV-2 Neutralization Antibody Test reagents (MBL Corporation, Nagoya, Japan), and a STACIA Analyzer (LSI Medience Corporation, Tokyo, Japan). Paired t-tests were used to calculate the p values (*** p < 0.001). The Y-axes are on a logarithmic scale.
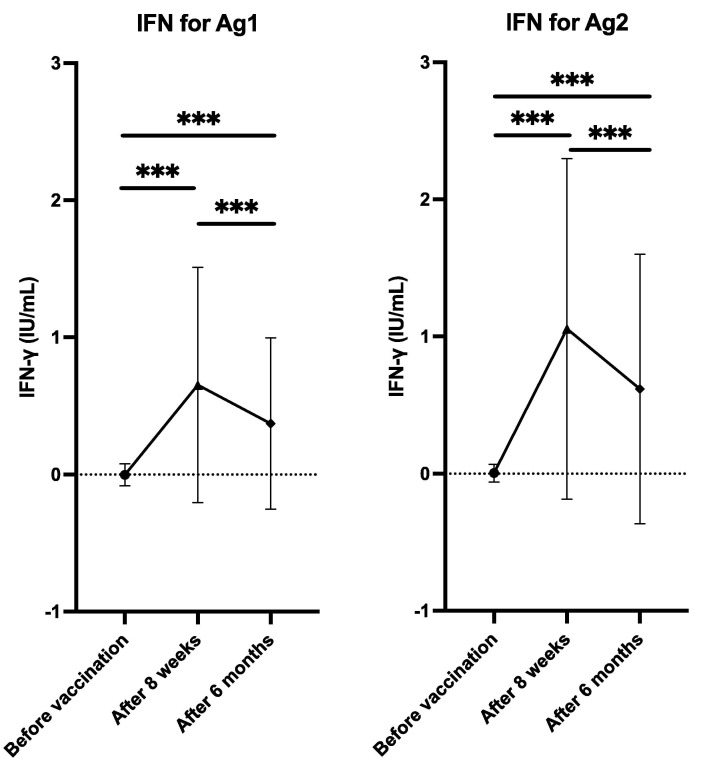
Samples from 536 of the 608 participants were tested using the QuantiFERON SARS-CoV-2 assay. The QuantiFERON SARS-CoV-2 assay results showed similar time dynamics to the SARS-CoV-2 antibody assays. Before vaccination, the mean IFN for Ag1 and IFN for Ag2 was 0.00 ± 0.08 IU/mL 0.01 ± 0.07 IU/mL, respectively. Eight weeks after vaccination, IFN for Ag1 and IFN for Ag2 increased to 0.66 ± 0.87 IU/mL and 1.06 ± 1.25 IU/mL, respectively. However, by 6 months after vaccination, IFN for Ag1 and IFN for Ag2 had decreased to 0.37 ± 0.63 IU/mL and 0.62 ± 0.99 IU/mL, respectively, (Fig. 2 ).
Fig. 2.
Dynamics of changes in cellular immunity after vaccination with two doses of the BNT162b2 vaccine. The plot shows the dynamics of the mean interferon gamma levels in the blood stimulated by antigen 1 and 2 (IFN for Ag1 and IFN for Ag2) of QuantiFERON SARS-CoV-2 tubes (Qiagen, Hilden, Germany). The error bars indicate the standard deviation. Paired t-tests were used to calculate the p values (*** p < 0.001).
The mean N-IgG titer was 0.6 ± 9.5 SU/mL before vaccination. The titer was not affected by the vaccination and was 0.7 ± 8.4 SU/mL, 0.4 ± 4.7 SU/mL, and 0.3 ± 3.5 SU/mL 3 weeks, 8 weeks, and 3 months after vaccination, respectively (Supplement Fig. S2). Six months after vaccination, the mean titer was 0.9 ± 8.9 SU/mL. Six participants turned positive serologically and were thus defined as having suspected breakthrough infection.
All six participants with suspected breakthrough infection were female, without pre-existing illnesses or immunosuppressant use. One participant was diagnosed with COVID-19 according to PCR test results performed 4 months after vaccination. One participant had close contact with COVID-19 patients 5 months after vaccination, but her PCR test result was negative. The other four participants were asymptomatic and had no history of close contact with patients with COVID-19 (Table 2 ). Compared to participants without suspected breakthrough, the antibody titers before the breakthrough infections (measured 3 months after vaccination) were similar, but titers after the breakthrough infections (measured 6 months after vaccination) were significantly higher. However, the IFN for Ag 1 and Ag 2 results were not affected by the breakthrough infections (Table 3 ).
Table 2.
Antibody titers in participants with suspected breakthrough SARS-CoV-2 infection.
| Age (years) | Sex |
Antibody titer 3 months after vaccination |
Antibody titer 6 months after vaccination |
History of possible SARS-CoV-2 infection 3 to 6 months after vaccinationa |
|||||
|---|---|---|---|---|---|---|---|---|---|
|
STACIA Neut-Ab, U/mL |
N-IgG, SU/mL |
STACIA Neut-Ab, U/mL |
N-IgG, SU/mL | COVID-19 | COVID-19-like illness | SARS-CoV-2 PCR testing | Close contact with COVID-19 patients | ||
| 44 | F | 6.45 | 0.5 | 51.38 | 19.8 | − | − | − | − |
| 27 | F | 9.86 | 0.1 | 52.92 | 22.3 | − | − | + | − |
| 47 | F | 10.73 | 0 | 42.21 | 79.9 | − | − | − | − |
| 31 | F | 9.92 | 5.4 | 20.55 | 173.3 | − | − | − | − |
| 50 | F | 7.68 | 0.1 | 53.73 | 55.3 | + | + | + | + |
| 45 | F | 5.17 | 0.1 | 53.24 | 83.0 | − | − | + | + |
COVID-19, coronavirus disease; N-IgG, immunoglobulin G antibody against the SARS-CoV-2 nucleocapsid antigen; Neut-Ab, neutralization antibody test; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Based on answers provided in the questionnaire.
Table 3.
Difference in antibody titers and QuantiFERON results in participants with suspected breakthrough infections compared to the other participants.
| Participants with suspected breakthrough infection (N = 6) |
Other participantsa (N = 602) |
P valueb | |
|---|---|---|---|
| Age, years (median, IQR) | 44.5 (30–47.75) | 45 (36–53) | 0.38 |
| Sex | |||
| Male (%) | 0 (0) | 170 (28.2) | 0.19 |
| Female (%) | 6 (1 0 0) | 432 (71.8) | |
| Antibody titer 3 months after vaccination (median, IQR) | |||
| Alinity RBD-IgG, AU/mL | 3758.3 (2396.4–5162.2) | 2740.0 (1780.8–4231.3) | 0.23 |
| HISCL S-IgG, SU/mL | 124.6 (72.3–146.5) | 92.0 (61.8–132.1) | 0.32 |
| STACIA Neut-Ab, U/mL | 8.8 (6.1–10.1) | 6.2 (4.3–8.6) | 0.12 |
| Antibody titer 6 months after vaccination (median, IQR) | |||
| Alinity RBD-IgG, AU/mL | 32407.1 (18973.3–76786.0) | 885.8 (573.1–1466.3) | <0.01 |
| HISCL S-IgG, SU/mL | 1590.6 (860.7–2720.3) | 37.0 (22.5–59.8) | <0.01 |
| STACIA Neut-Ab, U/mL | 51.2 (36.8–53.4) | 2.3 (1.6–3.5) | <0.01 |
| QuantiFERON SARS-CoV-2 results 8 weeks after vaccination (median, IQR) | |||
| IFN for Ag1, IU/mL | 0.16 (0.06–0.80) | 0.35 (0.14–0.80) | 0.35 |
| IFN for Ag2, IU/mL | 0.27 (0.10–1.13) | 0.62 (0.26–1.32) | 0.23 |
| QuantiFERON SARS-CoV-2 results 6 months after vaccination (median, IQR) | |||
| IFN for Ag1, IU/mL | 0.15 (0.07–0.73) | 0.15 (0.06–0.40) | 0.96 |
| IFN for Ag2, IU/mL | 0.18 (0.05–0.83) | 0.26 (0.10–0.69) | 0.48 |
Ag, antigen; IFN, interferon; IQR, interquartile range; Neut-Ab, neutralization antibody test; RBD-IgG, receptor-binding domain immunoglobulin G; S-IgG, anti-spike protein immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus.
Participants who did not provide samples at both timepoints were excluded.
Fisher exact tests were used for categorical values, and Mann-Whitney U tests were used for continuous values.
4. Discussion
Our prospective cohort study of over 600 healthcare workers revealed a decline in humoral and cellular immunity within 6 months after two doses of BNT162b2 vaccine by evaluating antibody titers and antigen-specific IFN-induction. Our finding of waning immunity during the 6 months following vaccination is consistent with the results of a previous study by Coppeta, et al. [9]. Our result also supports reports on waning effectiveness after the first two vaccine doses, highlighting the urgency of administering a booster dose to induce stronger immunity against SARS-CoV-2 [10], [11], [12].
Three commercially available CLEIA-based SARS-CoV-2 antibody measurement kits were used. The IgG antibody titers against RBD or S1 peptides were well correlated with neutralizing antibody levels based on their ability to inhibit the formation of the complex of RBD and angiotensin-converting enzyme 2. Thus, measurement of anti-RBD or anti-S1 IgG titers might be substituted for the measurement of antibody activity to neutralize SARS-CoV-2 infection.
As most breakthrough infections are reported to be mild or asymptomatic, such patients might not suspect SARS-CoV-2 infections and miss diagnostic opportunities [13]. The measurement of N-IgG made it possible to detect five asymptomatic breakthrough infections in the present study. One of the five participants underwent PCR testing 4 months after vaccination due to close contact with COVID-19 patients, but the result was negative. Although the negativity may be attributed to the lower sensitivity of PCR tests compared with antibody tests, as previously reported [14], the negative result might have been attributable to the faster mean rate of viral load decline and viral clearance in vaccinated individuals compared to unvaccinated individuals [15], [16].
Among the participants with suspected breakthrough infection, the median antibody titer 3 months after vaccination was similar to that of those without breakthrough infections. This suggests that breakthrough infections may occur not only in poor responders but also in good responders within 6 months after BNT162b2 vaccination. Among the participants with suspected breakthrough infections, the higher median antibody titer using STACIA Neut-Ab 6 months after vaccination (51.2 U/mL) was higher than that 3 weeks after vaccination (31.9 U/mL). This provides evidence that asymptomatic breakthrough infections exert a sufficient booster effect, consistent with observations previously obtained from breakthrough infections confirmed by PCR tests [17].
Notably, the IGRA results were not affected by breakthrough infections in the present study. Kato et al. [4] have also reported that antibody titers and IGRA had discrepant results after breakthrough infection. As the reason for the discrepancy between the antibody titers and the IGRA results has not been determined, further studies are warranted.
This study had several limitations. First, STACIA Neut-Ab, which was used as the neutralization assay, could only detect the antibody activity to inhibit RBD binding to human ACE 2. Although the STACIA Neut-Ab results for monoclonal antibodies correlated well with the neutralization assay results using in vitro SARS-CoV-2 infection in cells, neutralization is thought to incompletely represent in vivo humoral immunity against SARS-CoV-2 with polyclonality [18]. Therefore, ideally the presence of neutralizing antibody should be verified using SARS-CoV-2 infected cells or animals. Second, cross-reactivity of the N-IgG antibody test has been reported. False-positive results might be obtained due to cross-reactivity to coronavirus NL63 and 229E infections; therefore, N-IgG-positive conversion does not always signify breakthrough SARS-CoV-2 infection. However, it is unlikely that any of the participants in this study had false-positive results because they had elevated HISCL S-IgG titers 6 months after vaccination and this antibody is specific to SARS-CoV-2 infection [19]. Third, the incidence of breakthrough infections could not be explained simply by waning humoral and cellular immunity. Occupational contact with COVID-19 patients, social activity, and prevalence of SARS-CoV-2 infection may have affected the results. Especially, during the period between the fourth and fifth sample collection time-points, there was a high incidence of SARS-CoV-2 recorded in Japan during the Olympic and Paralympic games in Tokyo in July and August 2021. Therefore, further studies with a larger sample size and variety of participants are required to determine the relationship between immunity acquired by vaccination and breakthrough infections. Finally, in Japan, Alpha (B.1.1.7) variant infections were dominant during the third (winter, 2021) and fourth (spring, 2021) waves of the SARS-CoV-2 epidemic, and infections due to the Delta (B.1.617.2) variant were dominant during the fifth (summer, 2021) wave of the SARS-CoV-2 epidemic. Therefore, the rate of suspected breakthrough infections in our study was in the setting of an epidemic of the Delta variant. Currently, the Omicron (B.1.1.529) variant, which can escape the immunity of BNT162b2 vaccination [20], is dominant worldwide, including in Japan. Thus, the incidence of breakthrough infections may have increased.
In conclusion, the study showed waning of both humoral and cellular immunity within 6 months after two doses of BNT162b2 vaccine among Japanese healthcare workers and the occurrence of suspected asymptomatic breakthrough infection in approximately one percent of participants within 6 months after vaccination with two doses of vaccine. These results support the need for booster vaccination to prevent infection.
5. Authors’ contributions
YU conceived and designed the study. YU, AT, TA, AO, AyS, WY, and MW recruited the participants. TK, YT, AkS, MN, YY, TA, AO, YT and AyS collected the data. YU, YS, NH and MW MM analyzed and interpreted the data; YU wrote the manuscript. HY, HN, YS, NH, MW, and MM discussed the data and critically reviewed and revised the manuscript. All authors approved the final version of the manuscript for publication.
Funding
This work was supported by the Japan Agency for Medical Research and Development [grant number: JP21fk0108469]; research funds of the Keio University School of Medicine; and a grant from the Public Foundation of the Vaccination Research Center, Japan.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [Naoki Hasegawa reports financial support was provided by the Japan Agency for Medical Research and Development (AMED). Yoshifumi Uwamino reports financial support was provided by the Public Foundation of the Vaccination Research Center, Japan. Mitsuru Murata reports equipment, drugs, or supplies was provided by MBL Corporation. Mitsuru Murata reports equipment, drugs, or supplies was provided by Sysmex Corp. Mitsuru Murata reports equipment, drugs, or supplies was provided by Qiagen. Yoshifumi Uwamino, Mitsuru Murata, Masatoshi Wakui has patent pending to Keio University].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.06.016.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L., Haas E.J., et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385(24):e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coppeta L., Somma G., Ferrari C., Mazza A., Rizza S., Trabucco Aurilio M., et al. Persistence of anti-S titre among healthcare workers vaccinated with BNT162b2 mRNA COVID-19. Vaccines (Basel) 2021;9(9):947. doi: 10.3390/vaccines9090947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato H, Miyakawa K, Ohtake N, et al. Vaccine-induced humoral and cellular immunity against SARS-CoV-2 at 6 months post BNT162b2 vaccination. medRxiv [Preprint]. Posted. https://doi.org/10.1101/2021.10.30.21265693.
- 5.Nomura Y., Sawahata M., Nakamura Y., Koike R., Katsube O., Hagiwara K., et al. Attenuation of antibody titers from 3 to 6 months after the second dose of the BNT162b2 vaccine depends on sex, with age and smoking risk factors for lower antibody titers at 6 months. Vaccines (Basel) 2021;9(12):1500. doi: 10.3390/vaccines9121500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeuchi JS, Fukunaga A, Yamamoto S, et al. SARS-CoV-2 specific T cell and humoral immune responses upon vaccination with BNT162b2. medRxiv [Preprint]. Posted. https://doi.org/10.1101/2021.11.06.21265632.
- 7.Uwamino Y., Kurafuji T., Sato Y., Tomita Y., Shibata A., Tanabe A., et al. Young age, female sex, and presence of systemic adverse reactions are associated with high post-vaccination antibody titer after two doses of BNT162b2 mRNA SARS-CoV-2 vaccination: an observational study of 646 Japanese healthcare workers and university staff. Vaccine. 2022;40(7):1019–1025. doi: 10.1016/j.vaccine.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uwamino Y, Wakui M, Yatabe Y, Nakagawa T, Sakai A, Kurafuji T, et al. Anti-SARS-CoV-2 cellular immunity in 571 vaccinees assessed using an interferon-γ release assay. medRxiv [Preprint]. https://doi.org/10.1101/2021.12.14.21267039.
- 9.Coppeta L., Ferrari C., Somma G., Mazza A., D’Ancona U., Marcuccilli F., et al. Reduced titers of circulating anti-SARS-CoV-2 antibodies and risk of COVID-19 infection in healthcare workers during the nine months after immunization with the BNT162b2 mRNA vaccine. Vaccines (Basel) 2022;10(2):141. doi: 10.3390/vaccines10020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falsey A.R., Frenck R.W., Walsh E.E., Kitchin N., Absalon J., Gurtman A., et al. SARS-CoV-2 Neutralization with BNT162b2 vaccine dose 3. N Engl J Med. 2021;385(17):1627–1629. doi: 10.1056/NEJMc2113468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., et al. Protection of BNT162b2 Vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., et al. Covid-19 Breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis. 2020;71(16):2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singanayagam A., Hakki S., Dunning J., Madon K.J., Crone M.A., Koycheva A., et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis. 2022;22(2):183–195. doi: 10.1016/S1473-3099(21)00648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coppeta L., Balbi O., Grattagliano Z., Mina G.G., Pietroiusti A., Magrini A., et al. First dose of the BNT162b2 mRNA COVID-19 vaccine reduces symptom duration and viral clearance in healthcare workers. Vaccines (Basel) 2021;9(6):659. doi: 10.3390/vaccines9060659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bates T.A., McBride S.K., Winders B., Schoen D., Trautmann L., Curlin M.E., et al. Antibody response and variant cross-neutralization after SARS-CoV-2 breakthrough infection. JAMA. 2022;327(2):179. doi: 10.1001/jama.2021.22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeshita M., Nishina N., Moriyama S., Takahashi Y., Uwamino Y., Nagata M., et al. Incomplete humoral response including neutralizing antibodies in asymptomatic to mild COVID-19 patients in Japan. Virology. 2021;555:35–43. doi: 10.1016/j.virol.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noda K., Matsuda K., Yagishita S., Maeda K., Akiyama Y., Terada-Hirashima J., et al. A novel highly quantitative and reproducible assay for the detection of anti-SARS-CoV-2 IgG and IgM antibodies. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-84387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cele S., Jackson L., Khoury D.S., Khan K., Moyo-Gwete T., Tegally H., et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602(7898):654–656. doi: 10.1038/s41586-021-04387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.