Abstract
Background
Thrombosis in COVID-19 worsens mortality. In our study, we sought to investigate how the dose and type of anticoagulation (AC) can influence patient outcomes.
Methods
This is a single-center retrospective analysis of critically ill intubated patients with COVID-19, comparing low-molecular-weight heparin (LMWH) and unfractionated heparin (UFH) at therapeutic and prophylactic doses. Of 218 patients, 135 received LMWH (70 prophylactic, 65 therapeutic) and 83 UFH (11 prophylactic, 72 therapeutic). The primary outcome was mortality. Secondary outcomes were thromboembolic complications confirmed on imaging and major bleeding complications. Cox proportional-hazards regression models were used to determine whether the type and dose of AC were independent predictors of survival. We performed Kaplan-Meier survival analysis to compare the cumulative survivals.
Results
Overall, therapeutic AC, with either LMWH (65% vs 79%, P = .09) or UFH (32% vs 46%, P = .73), conveyed no survival benefit over prophylactic AC. UFH was associated with a higher mortality rate than LMWH (66% vs 28%, P = .001), which was also evident in the multivariable analysis (LMWH vs UFH mortality, hazard ratio: 0.47, P = .001) and in the Kaplan-Meier survival analysis. Thrombotic and bleeding complications did not depend on the AC type (prophylactic LMWH vs UFH: thrombosis P = .49, bleeding P = .075; therapeutic LMWH vs UFH: thrombosis P = .5, bleeding P = .17). When comparing prophylactic with therapeutic AC, the rate of both thrombotic and bleeding complications was higher with the use of LMWH compared with UFH. In addition, transfusion requirements were significantly higher with both therapeutic LMWH and UFH.
Conclusions
Among intubated critically ill COVID-19 intensive care unit patients, therapeutic AC, with either LMWH or UFH, conveyed no survival benefit over prophylactic AC. AC with LMWH was associated with higher cumulative survival compared with AC with UFH.
Keywords: COVID-19, SARS-CoV-2, Low-molecular-weight heparin (LMWH), Unfractionated heparin (UFH), Thromboprophylaxis, Anticoagulation (AC)
ARTICLE HIGHLIGHTS.
-
•
Type of Research: Single-center retrospective cohort study.
-
•
Key Findings: Of 218 patients, 135 received low-molecular-weight heparin (LMWH) and 83 received unfractionated heparin. Among intubated critically ill COVID-19 intensive care unit patients, therapeutic anticoagulation (AC), with either LMWH or unfractionated heparin, conveyed no survival benefit over prophylactic AC. AC with LMWH was associated with higher cumulative survival compared with AC with unfractionated heparin.
-
•
Take Home Message: In intubated, critically ill, COVID-19 adult ICU patients, therapeutic AC, with either LMWH or UFH, had no survival benefit or greater organ-support-free days over prophylactic AC. This finding is in concordance with the NIH (National Institutes of Health) guidelines supporting the use of prophylactic AC over therapeutic AC in those critically ill unless AC is contraindicated or there is a documented VTE. It seems that the initiation of therapeutic AC after severe COVID-19 has developed may be too late to alter the consequences of established disease processes. Furthermore, AC with LMWH is preferable as it was shown to be associated with higher cumulative survival than AC with UFH.
The COVID-19 pandemic has led to a global health crisis with more than 471 million cases and 6 million deaths.1 The high mortality associated with COVID-19 is partially related to microvascular2 and macrovascular thromboembolic complications,3, 4, 5 attributed to SARS-CoV-2-induced thromboinflammation6 and hypercoagulability.7 The International Society on Thrombosis and Hemostasis recommended using prophylactic doses of low-molecular-weight heparin (LMWH) for all hospitalized patients with COVID19 unless they have active bleeding or low platelet count (<25 × 109/L),8 whereas further guidelines also stated to consider a 50% increase in the dose of thromboprophylaxis in obese patients.9 Recent randomized controlled clinical trials have demonstrated increased survival to discharge in noncritically ill patients with COVID-19, whereas no benefit was seen in critically ill patients.10 , 11 In this study, we sought to identify whether the different types of anticoagulation (AC), LMWH vs unfractionated heparin (UFH), and AC level, prophylactic vs therapeutic, can have an impact on patient mortality and the development of thrombotic and bleeding complications. We also evaluated the correlation between the type of AC and COVID-19 inflammatory markers such as C-reactive protein (CRP) and IL-6 to demonstrate any potential anti-inflammatory properties of LMWH and UFH in critically ill patients affected by SARS-CoV-2.12
Methods
Ethics statement
This study was a retrospective chart review of a COVID-19 patient database. Stony Brook University Committee on Research in Human Subjects approved the study protocol and supervised all study procedures according to state and federal regulations, with a waiver of informed consent.
Target population and data sources
We identified all critically ill intubated patients with COVID-19 admitted to Stony Brook University Hospital between February 7, 2020, and May 17, 2020. The diagnosis of COVID-19 was based on the positive reverse transcription-polymerase chain reaction test for SARS-CoV-2. Aside from the difference in the AC type and dose, the patients were treated in the same manner for all aspects of COVID-19 disease. We selected the study population based on the following criteria: age ≥18 years, reverse transcription-polymerase chain reaction proven COVID-19, initiation, and administration of a chemical AC regimen for at least 24 hours, and respiratory failure requiring endotracheal intubation. Patients were excluded from the study if AC was never started, or the administered AC was other than LMWH or UFH. As many people were intubated soon after their presentation to the emergency department, we elected to exclude those who were receiving oral AC before hospital admission to avoid any bias in our analysis that targeted to compare the effectiveness of LMWH vs UFH.
Electronic medical record review
We reviewed each electronic medical record and collected the following data: demographics (age, sex, body mass index [BMI]), dates of admission, intubation, comorbidities (hypertension, chronic obstructive pulmonary disease, congestive heart failure, diabetes mellitus, chronic kidney disease [CKD]), laboratory data (D-dimer, CRP, creatinine, IL-6), Sequential Organ Failure Assessment (SOFA) score that was calculated based on lab values obtained at the time of intubation and for 24 hours subsequently, thromboembolic complications, both venous (deep vein thrombosis [DVT], pulmonary embolism [PE]) and arterial (myocardial infarction [MI], stroke, peripheral thrombosis), clinically significant bleeding defined as upper or lower gastrointestinal bleeding requiring transfusion of at least two units of red blood cells, hemoglobin <7 mg/dL, intracranial bleeding, other major bleeding requiring transfusion, including massive hemoptysis, hematuria, retroperitoneal hematoma, intraperitoneal or intrathoracic bleeding, heparin-induced thrombocytopenia, and mortality. A total of 30 computed tomography angiography scans and 20 venous duplex ultrasound scans were performed in the whole cohort after clinical suspicion for venous thromboembolism. For all patients, 5-month follow-up data were available. All patients were included in the Kaplan-Meier analysis.
AC protocol
All patients admitted to Stony Brook University Hospital were placed at least on a thromboprophylaxis regimen on admission, unless medically contraindicated. Our institution implemented an aggressive anticoagulation protocol, which included dose escalation based on daily measured D-dimer levels. Patients with D-dimer <1000 ng/mL received enoxaparin 40 mg daily, and those with D-dimer ≥1000 ng/mL but <3000 ng/mL received enoxaparin 40 mg twice a day. Finally, those with D-dimer ≥3000 ng/mL received therapeutic anticoagulation with enoxaparin 1 mg/kg twice a day or intravenous heparin drip at a starting rate of 18 units/kg/h to achieve a goal partial thromboplastin time of 60-90. Therapeutic AC was also initiated whenever it was medically warranted, such as atrial fibrillation or suspected and confirmed venous thromboembolic disease (DVT, PE). Because of the absence of patient randomization, the type of AC was based on physicians’ preference and was characterized by wide heterogeneity as the patients were admitted in five different intensive care units that were managed by both medicine and surgery intensivists. When these patients were becoming critically ill, our institution was in the rapidly escalating pandemic curve. Although UFH was used more commonly in patients with known CKD or new acute kidney injury, LMWH was also used in patients despite creatinine elevation.
Data analysis
Statistics
Statistical analyses were performed using SPSS 21.0 software (SPSS Inc) and in-house developed coding in MATLAB. The significance level for all tests was .05. All reported P values were calculated two-sided. The primary end point was mortality. Secondary end points were the development of thromboembolic and bleeding complications. Data were reported as group means and the two-tailed Student’s t-statistic for several labs (D-dimer, CRP, creatinine, IL-6). We used the χ2 test to compare categorical variables. The two-sample t-test or the Mann-Whitney U test was used for continuous variables as indicated based on normal distribution vs skewness of factors. Nonparametric Mann-Whitney U-test analysis was performed to compare the means of maximum D-dimer, CRP, creatinine, and IL-6. Survival and its association with measured factors were evaluated using Kaplan-Meier models. The log-rank test was used to compare survival between groups. To determine whether the type and the level of AC were independent predictors of survival, we used Cox proportional-hazards regression models. On the basis of the univariable analysis, we determined significant factors to be involved in the multivariable Cox regression model. These factors included age, sex, type of AC (UFH vs LMWH), level of AC (prophylactic vs therapeutic), SOFA score, and steroid use. The entry level for multivariable analysis was P < .1. This model provided hazard ratios to estimate which parameters are independent predictors of survival. There were no missing data regarding survival measures.
Results
Study population
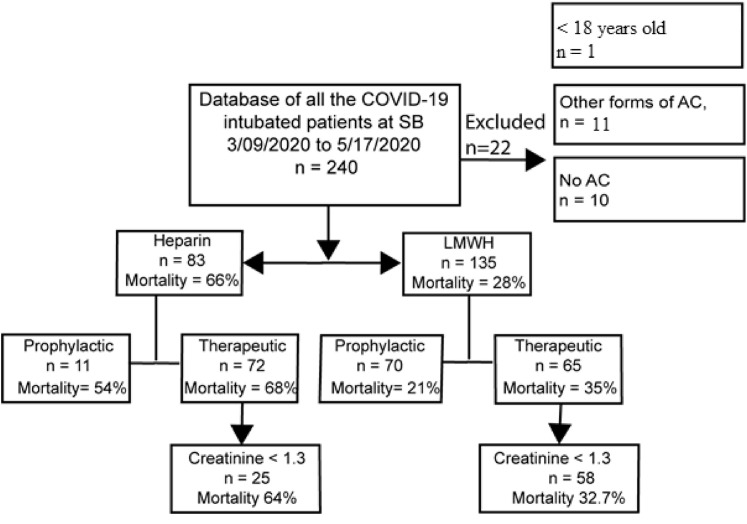
Our study included 240 intubated patients from Stony Brook University Hospital intensive care units (ICUs) between February 7, 2020, and May 17, 2020. Twenty-two patients were excluded after implementation of the exclusion criteria, leaving 218 patients for analysis. We found 135 patients who received LMWH and 83 UFH. There was no significant difference in mean ages (P = .7), BMI (P = .7), and sex (P = .062) between the LMWH and UFH groups. This cohort was divided based on therapeutic AC (65 on LMWH and 72 on UFH) and prophylactic AC dosing (once or twice daily thromboprophylaxis with 70 on LMWH and 11 on UFH) (Fig 1 ). There was no significant difference in the SOFA scores, calculated on the day of intubation, between LWMH and UFH (P = .5) in those who received therapeutic AC. However, there was a statistically significant difference in the prophylactic dose groups, with the SOFA score being slightly higher in those who received LMWH (P = .04) (Table I ).
Fig 1.
Patient selection algorithm. AC, Anticoagulation; LMWH, low-molecular-weight heparin; SB, stony brook.
Table I.
Characteristics of patients who were on low-molecular-weight heparin (LMWH) vs unfractionated heparin (UFH) prophylactic vs therapeutic level of AC
| LMWH | UFH | P value | |
|---|---|---|---|
| SOFA prophylactic (mean ± SD) | 5.8 ± 0.23 | 4.09 ± 0.95 | .04 |
| SOFA therapeutic (mean ± SD) | 5.8 ± 0.24 | 7.6 ± 0.25 | .5 |
| Max D-dimer for prophylactic (mean ± SE) | 3472 ± 458 | 3223 ± 989 | .64 |
| Max D-dimer for therapeutic (mean ± SE) | 12,672 ± 1618 | 11,743 ± 1470 | .67 |
| Admit creatinine for prophylactic (mean ± SE) | 0.99 ± 0.11 | 1.34 ± 0.25 | .14 |
| Admit creatinine for therapeutic (mean ± SE) | 0.98 ± 0.06 | 1.9 ± 0.25 | .001 |
| Max creatinine for prophylactic (mean ± SE) | 1.46 ± 0.18 | 3.18 ± 0.7 | .017 |
| Max creatinine for therapeutic (mean ± SE) | 1.37 ± 0.1 | 4.63 ± 0.34 | .001 |
| Max CRP, prophylactic (mean ± SE) | 36.18 ± 4.9 | 39.49 ± 13.7 | .97 |
| Max CRP, therapeutic (mean ± SE) | 40.4 ± 4.3 | 39.7 ± 3.8 | .88 |
| Max interleukin 6 (Vivacor), prophylactic (mean ± SE) | 218 ± 70 | 1949 ± 1134 | .003 |
| Max interleukin 6 (Vivacor), therapeutic (mean ± SE) | 428 ± 120 | 284 ± 73 | .6 |
AC, Anticoagulation; CRP, C-reactive protein; SD, standard deviation; SE, standard error; SOFA, Sequential Organ Failure Assessment.
Boldface P values represent statistical significance.
Primary outcomes
Mortality
In the univariable survival analysis, sex (P = .61), BMI (P = .699), hypertension (P = .441), diabetes mellitus (P = .583), CKD stage 3a-5 (P = .153), congestive heart failure (P = .253), chronic obstructive pulmonary disease (P = .284), and steroid use (P = .053) were not predictors of outcome. On the other hand, age above 70 years (P = .001), SOFA score above 7 (P = .002), and use of UFH instead of LMWH AC (P < .0001) proved to be predictors of mortality.
Multivariable analysis showed that patients who received UFH had higher mortality compared with patients who received LMWH, and this finding was independent of age, sex, or SOFA score (mortality LMWH vs UFH hazard ratio [HR], 0.47; 95% confidence interval [CI], 0.30-0.74; P = .001). Furthermore, male sex (HR, 1.68; 95% CI, 1.01-2.78; P = .044) and age over 70 years (HR, 2.15; 95% CI, 1.36-3.39; P < .001) were also predictors of higher mortality. By contrast, SOFA score greater than 7 (HR, 1.33; 95% CI, 0.86-2.06; P = .188) and steroid use (HR, 1.50; 95% CI, 0.69-3.3; P = .303) did not reach statistical significance in the multivariable analysis (Table II ).
Table II.
Multivariable analysis
| Variable | Comparison level | Hazard ratio (95% CI) | P value |
|---|---|---|---|
| Sex | Male vs female | 1.68 (1.01-2.78) | .044 |
| Anticoagulation type | LMWH vs UFH | 0.47 (0.30-0.74) | .001 |
| Age | More than 70 vs less than 70 years old | 2.15 (1.36-3.39) | .001 |
| SOFA | More than 7 vs less than 7 | 1.33 (0.86-2.06) | .188 |
| Steroids | On vs off steroids | 1.50 (0.69-3.30) | .303 |
CI, Confidence interval; LMWH, low-molecular-weight heparin; SOFA, Sequential Organ Failure Assessment; UFH, unfractionated heparin.
Boldface P values represent statistical significance.
We performed a subgroup analysis for creatinine level less than 1.3 measured at the initiation of the therapeutic dose of AC between those who received LMWH and UFH, to account for the potential selection bias, as UFH is more commonly used in patients with decreased renal function compared with using LMWH. Similar to the general cohort, mortality once more was significantly lower in the LMWH group when compared with the UFH group (32.7% vs 64%, P = .002).
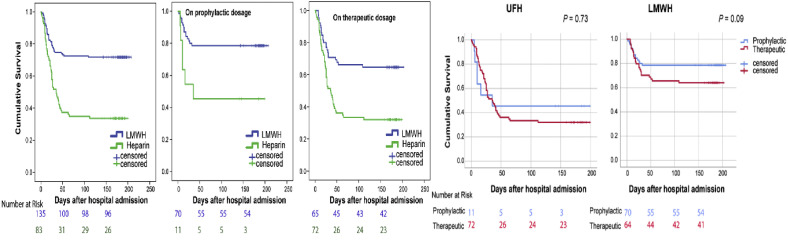
In Kaplan-Meier survival analysis, patients who received LMWH had higher cumulative survival than patients who received UFH in both prophylactic and therapeutic groups (P = .001) (Fig 2 ). The cumulative survival difference between prophylactic LMWH and therapeutic LMWH was not statistically significant (P = .09). Similarly, the cumulative survival difference between prophylactic UFH and therapeutic UFH did not reach statistical significance (P = .73) (Fig 2).
Fig 2.
Anticoagulation (AC) with low-molecular-weight heparin (LMWH) is associated with significantly higher cumulative survival compared with unfractionated heparin (UFH)-based AC, regardless of the AC level, prophylactic or therapeutic. There was no difference in cumulative survival when comparing prophylactic UFH to therapeutic UFH. Similarly, there was no difference in cumulative survival when comparing prophylactic LMWH to therapeutic LMWH.
In our patient population, the most frequent cause of death was multisystem organ failure (75 of 93 patients, 31 of 38 in the LMWH group, and 44 of 55 in the UFH group), primarily driven by hypoxic respiratory failure. Other less common causes of death were MI, lethal arrhythmias, and massive PE.
Secondary outcomes
Thromboembolic and bleeding events
There was no significant difference in thrombotic complications, both venous (DVT, PE) and arterial (MI, stroke, peripheral thrombosis), and bleeding complications (upper and lower GI bleed, hemothorax, mediastinal, and tracheostomy site bleeding) when comparing LMWH and UFH in the therapeutic groups (LMWH vs UFH: thrombosis P = .5, bleeding P = .17). We observed similar results when we compared LMWH and UFH in the prophylactic groups (LMWH vs UFH: thrombosis P = .49, bleeding P = .075). However, we found that the transfusion requirements were significantly higher in those who received therapeutic LMWH and UFH (P = .001). Notably, there was no difference in the prophylactic LMWH vs prophylactic UFH groups (P = .17) (Table III ). For the VTE diagnosis, a total of 30 computed tomography angiography scans and 20 venous duplex ultrasound scans were performed after clinical suspicion for PE and DVT, respectively.
Table III.
Comparing the complications of low-molecular-weight heparin (LMWH) vs unfractionated heparin (UFH) in prophylactic vs therapeutic AC doses
| Complications | Prophylactic (UFH: 11, LMWH: 70) | Therapeutic (UFH: 72, LMWH: 65) | P value |
|---|---|---|---|
| Thromboembolic | |||
| UFH | 9% | 18.06% | .45 |
| LMWH | 4.2% | 13.8% | .051 |
| Bleeding | |||
| UFH | 18.1% | 31.9% | .35 |
| LMWH | 4.2% | 21.54% | .002 |
| HIT | |||
| UFH | 0 | 4.17% | |
| LMWH | 0 | 1.54% | |
| Received transfusion | |||
| UFH | 36.3% | 72.2% | .0008 |
| LMWH | 18.5% | 38.46% | .002 |
| Therapeutic UFH (n = 72) | Therapeutic LMWH (n = 65) | ||
|---|---|---|---|
| Thromboembolic | 18.06% | 13.8% | .5 |
| PE/DVT | 9.7% | 9.2% | .9 |
| Arterial complications | 9.7% | 9.2% | .9 |
| Bleeding | 31.9% | 21.54% | .17 |
| HIT | 4.17% | 1.54% | .36 |
| Received transfusion | 72.2% | 38.46% | .001 |
| Prophylactic UFH (n = 11) | Prophylactic LMWH (n = 70) | ||
|---|---|---|---|
| Thromboembolic | 9% | 4.2% | .49 |
| Bleeding | 18.1% | 4.2% | .075 |
| HIT | 0% | 0% | |
| Received transfusion | 36.3% | 18.5% | .17 |
AC, Anticoagulation; DVT, deep vein thrombosis; HIT, heparin-induced thrombocytopenia; PE, pulmonary embolism.
Boldface P values represent statistical significance.
To establish the safety profile of the administration of therapeutic AC in the management of severe COVID-19, we further compared prophylactic vs therapeutic AC for the rates of thrombotic and bleeding complications. We found that the rate of thrombotic complications was higher with the use of therapeutic compared with prophylactic LMWH (LMWH P = .051) and the same between prophylactic and therapeutic doses of UFH (UFH P = .45). Bleeding complications were higher in the therapeutic LMWH than prophylactic LMWH (P = .002) but not different between the prophylactic and therapeutic UFH groups (P = .35). The rate of blood transfusions was higher with therapeutic than prophylactic AC for both LMWH and UFH (LMWH P = .002, UFH P = .0008) (Table III).
Laboratory results: CRP and D-dimer levels
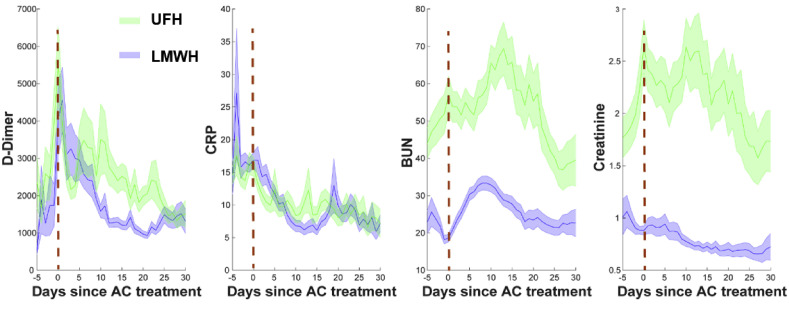
There was no significant difference in the maximum CRP levels between the therapeutic and prophylactic groups among those who received LMWH and UFH (Table I). Notably, the CRP peak in both groups occurred early in the hospital course and reduced after AC treatment, both in those who received LMWH and UFH (Fig 3 ).
Fig 3.
Evolution of the critical inflammation markers and organ function laboratory values over the intensive care unit period in COVID-19 intubated patients treated by unfractionated heparin (UFH) (green, n = 83) and low-molecular-weight heparin (LMWH) (blue, n = 134), regardless of AC level. AC, Anticoagulation; BUN, blood urea nitrogen; CRP, C-reactive protein.
Prophylactic AC with LMWH was associated with a significantly lower max IL-6 level in COVID-19 intubated patients when compared with the UFH group (P = .003), although our data were limited (n = 6). However, there was no statistically significant difference in the maximum IL-6 level between LMWH and UFH in those who received therapeutic AC (n = 35) (P = .6) (Table I).
Maximum D-dimer levels were not statistically different between the therapeutic and prophylactic groups among patients who received LMWH and UFH (Table I). The D-dimer peak occurred early in the ICU course matching the time of AC initiation, and following the same trend as CRP, gradually down-trended over the hospital course (Fig 3).
Discussion
Our study found that in critically ill intubated patients hospitalized with COVID-19, therapeutic AC did not affect the cumulative survival compared with prophylactic AC, with either LMWH or UFH. Our findings are in line with the most lately published National Institutes of Health (NIH) guidelines, according to which therapeutic doses of heparin have no significant benefit in patients with COVID-19 admitted to the ICU, unless VTE is confirmed.13
We analyzed patients who suffered from COVID-19 during the first wave of the pandemic, when no official guidelines for AC in COVID-19 existed. Our decision to implement an aggressive AC protocol, similar to a protocol proposed by the European Society of Cardiology,14 was based on our observation that severe arterial and venous thromboembolic events emerged despite the use of routine thromboprophylaxis. Although the escalation of AC to high-intensity thromboprophylaxis and furthermore to therapeutic AC in our cohort of patients was associated with significantly improved organ function and overall survival,15 and that this practice might be able to balance the negative effects of obesity on the overall patient mortality,16 the most updated NIH guidelines recommend against this practice and advice over de-escalation of AC once patients get admitted to the ICU.13 The cornerstone of these recommendations is based on two randomized controlled clinical trials by the REMAP-CAP/ACTIV-4a/ATTACC investigators who showed that although the strategy to administer therapeutic AC increased the probability of survival to hospital discharge with reduced use of cardiovascular or respiratory organ support as compared with the administration of prophylactic AC in noncritically ill patients,10 this benefit disappeared in critically ill patients who received ICU level of care.11 We also support the hypothesis that has been made by the clinical trial investigators that the initiation of therapeutic AC after severe COVID-19 has developed may be too late to alter the consequences of established disease processes.11
We also found that in critically ill intubated patients hospitalized with COVID-19, UFH was associated with higher mortality rate compared with those who received LMWH, regardless of the AC level, prophylactic or therapeutic. LMWH has been the mainstay AC regimen in most studies, as it was shown early in the COVID-19 pandemic to be associated with better prognosis, especially in severe COVID-19 patients with sepsis-induced coagulopathy score ≥4 or D-dimer >6-fold of upper limit of normal.17 Our observation regarding the superiority of LMWH agrees with the findings from a large intention to treat trial in which UFH was not associated with significant survival benefit, administered at prophylactic or therapeutic doses while LMWH improved survival when given as prophylaxis.14
The rate of thromboembolic complications was higher with the use of therapeutic LMWH compared with prophylactic LMWH, whereas the rate was similar between therapeutic and prophylactic UFH groups. This can be explained by the fact that in our institution we do not regularly monitor the anticoagulant effect of LMWH with antifactor Xa levels, which could lead to subtherapeutic levels.18 , 19 This is further supported by a recent observation in which patients with COVID-19 who were administered antifactor Xa-guided LMWH were achieving appropriate levels compared with the weight-based approach.20 There was no observed superiority of LMWH vs UFH, when these were compared with prophylactic and therapeutic doses, respectively, in preventing imaging confirmed macrovascular thromboembolic complications. These findings should be interpreted with caution though. Because of the risk of viral contamination and the instability of critically ill patients with COVID-19 who frequently precluded transportation, we did not routinely screen all our patients for the presence of subclinical PE or DVT, and thus we have probably underestimated the true VTE rate. It has been shown in studies that adopted a more systematic screening approach that there was a higher VTE incidence compared with the ones that implemented imaging on clinical suspicion only.21
Previous studies have also shown that elevated D-dimer levels are a marker of COVID-19 hypercoagulability and disease severity, linked with worse mortality.22 , 23 Our analysis found that the peak of the D-dimers matched the time of intubation, and although there was no difference in the maximum D-dimer level between LMWH and UFH, regardless of AC dose, their levels significantly decreased after AC escalation, during the patients’ hospital course. Our findings were supported by another retrospective study in which the early implementation of AC was associated with down trending D-dimer levels and improved 30-day mortality in patients suffering from severe COVID-19.24 Based on the most updated NIH guidelines, therapeutic AC is currently recommended in adults with D-dimer levels above the upper limit, but only for those who require low-flow oxygen and do not require ICU level of care.13
Despite the survival benefit of AC administration to patients with COVID 19, a major contributor to morbidity and mortality is clinically significant bleeding. In our cohort when comparing LMWH with UFH, there was no difference in bleeding risk. This finding has been supported by studies that showed equivalent bleeding risk among patients receiving either LMWH or UFH,25 although others indicate that LMWH is associated with less risk for major bleeding, mostly attributed to more predictable anticoagulant response.9
On the other hand, when comparing level of AC, we found that those who received therapeutic LMWH had higher bleeding complications compared with those on prophylactic, whereas there was no difference in the UFH group between prophylactic or therapeutic doses. Although the development of clinically significant bleeding with therapeutic AC is intuitive from a physiologic perspective because higher doses can lead to impaired clotting, some studies have shown no statistically significant difference in major bleeding events between prophylactic and therapeutic AC in patients with COVID-19.26 , 27 Nevertheless, our findings seem to be in line with other studies in which therapeutic AC was associated with an increased risk of major bleeding28 although this observation could be due to a relative bias toward administering higher doses of AC to sicker patients with higher D-dimer levels,29 which was also the case in our cohort. In our study population, the transfusion requirements were significantly higher in both LMWH and UF therapeutic dose groups compared with prophylactic groups. Even though previous studies have shown no difference in transfusion requirements with therapeutic AC,26 , 30 anemia, which is common in patients requiring ICU level of care, can be attributed not only to bleeding events but also to decreased erythropoiesis by the cytokine-induced inflammatory status and the frequent venipunctures.4
Limitations
Our study has a retrospective, observational, and opportunistic design based on a single center experience that was feasible in the setting of a new evolving phenomenon during which our understanding of SARS-CoV-2 was expanding. The fact that PE is common in patients with severe COVID-19 infection and imaging was underused might have led to underdiagnosis of thromboembolic complications. Our study did not include patients who did not receive AC, and thus we cannot make safe assumptions as to if some of the mortalities could be attributed clearly to adverse effects of AC. The preferential administration of UFH to patients with elevated creatinine could also introduce a potential selection bias. In our attempt to control this, we evaluated patients with acceptable renal function. Still, the small sample size precluded further conclusions in those who received prophylactic AC dose. Even though we found a statistically significant difference in the survival between LMWH and UFH in patients with creatinine <1.3, this comparison needs to be made with caution due to the possibility for unobserved differences between the groups.
Conclusions
Among intubated critically ill COVID-19 ICU patients, therapeutic AC, with either LMWH or UFH, conveyed no survival benefit or greater organ support-free days over prophylactic AC. AC with LMWH was associated with higher cumulative survival compared with AC with UFH.
Author Contributions
Conception and design: PV, PD, AT, SM, MB
Analysis and interpretation: PV, PD, SG, AT, SM, MB
Data collection: PV, PD, LA, NC, AA, AO, SG, JS, SM, MB
Writing the article: PV, PD, AT, SM, MB
Critical revision of the article: PV, PD, SG, AT, SM, MB
Final approval of the article: PV, PD, LA, NC, AA, AO, SG, JS, CM, JR, NL, AT, SM, MB
Statistical analysis: PV, PD, SG, SM
Obtained funding: This work was supported by the SUNY Seed Grant 1160738–1-87777. No industry or other external funding was used for this research.
Overall responsibility: MB
PV and PD contributed equally to this work as first authors. SM and MB contributed equally to this work as last authors.
From the Eastern Vascular Society
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Worldometer. https://www.worldometers.info/coronavirus/
- 2.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadkarni G.N., Lala A., Bagiella E., Chang H.L., Moreno P.R., Pujadas E., et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connors J.M., Levy J.H. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18:1559–1561. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spyropoulos A.C., Levy J.H., Ageno W., Connors J.M., Hunt B.J., Iba T., et al. Subcommittee on perioperative, critical care thrombosis, haemostasis of the Scientific, Standardization Committee of the International Society on Thrombosis and Haemostasis. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ATTACC Investigators, ACTIV-4a Investigators, REMAP-CAP Investigators. Lawler P.R., Goligher E.C., Berger J.S., et al. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021;26:790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.REMAP-CAP Investigators, ACTIV-4a Investigators, ATTACC Investigators. Goligher E.C., Bradbury C.A., McVerry B.J., et al. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021;26:777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi C., Wang C., Wang H., Yang C., Cai F., Zeng F., et al. The potential of low molecular weight heparin to mitigate cytokine storm in severe COVID-19 patients: a retrospective cohort study. Clin Transl Sci. 2020;13:1087–1095. doi: 10.1111/cts.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NIH. Therapeutic management of hospitalized adults with COVID-19. Available at: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults---therapeutic-management/?utm_source=site&utm_medium=home&utm_campaign=highlights. Accessed April 5, 2021.
- 14.Atallah B., Mallah S.I., AlMahmeed W. Anticoagulation in COVID-19. Eur Heart J Cardiovasc Pharmacother. 2020;6:260–261. doi: 10.1093/ehjcvp/pvaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tassiopoulos A.K., Mofakham S., Rubano J.A., Labropoulos N., Bannazadeh M., Drakos P., et al. D-dimer-driven anticoagulation reduces mortality in intubated COVID-19 patients: a cohort study with a propensity-matched analysis. Front Med (Lausanne) 2021;8:631335. doi: 10.3389/fmed.2021.631335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drakos P., Volteas P., Naeem Z., Asencio A.A., Cleri N.A., Alkadaa L.N., et al. Aggressive anticoagulation may decrease mortality in obese critically ill COVID-19 patients. Obes Surg. 2022;32:391–397. doi: 10.1007/s11695-021-05799-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsh J., Warkentin T.E., Shaughnessy S.G., Anand S.S., Halperin J.L., Raschke R., et al. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(Suppl):64S–94S. doi: 10.1378/chest.119.1_suppl.64s. [DOI] [PubMed] [Google Scholar]
- 19.Bigos R., Solomon E., Dorfman J.D., Ha M. A Weight- and Anti-Xa-Guided Enoxaparin Dosing Protocol for venous thromboembolism prophylaxis in intensive care unit trauma patients. J Surg Res. 2021;265:122–130. doi: 10.1016/j.jss.2021.02.034. [DOI] [PubMed] [Google Scholar]
- 20.Dutt T., Simcox D., Downey C., McLenaghan D., King C., Gautam M., et al. Thromboprophylaxis in COVID-19: anti-FXa-the missing factor? Am J Respir Crit Care Med. 2020;202:455–457. doi: 10.1164/rccm.202005-1654LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenner W.J., Kanji R., Mirsadraee S., Gue Y.X., Price S., Prasad S., et al. Thrombotic complications in 2928 patients with COVID-19 treated in intensive care: a systematic review. J Thromb Thrombolysis. 2021;51:595–607. doi: 10.1007/s11239-021-02394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lippi G., Favaloro E.J. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. 2020;120:876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu A., Liu Y., Zayac A.S., Olszewski A.J., Reagan J.L. Intensity of anticoagulation and survival in patients hospitalized with COVID-19 pneumonia. Thromb Res. 2020;196:375–378. doi: 10.1016/j.thromres.2020.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He P., Liu Y., Wei X., Jiang L., Guo W., Guo Z., et al. Comparison of enoxaparin and unfractionated heparin in patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention: a systematic review and meta-analysis. J Thorac Dis. 2018;10:3308–3318. doi: 10.21037/jtd.2018.05.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paranjpe I., Fuster V., Lala A., Russak A.J., Glicksberg B.S., Levin M.A., et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonmarker S., Hollenberg J., Dahlberg M., Stackelberg O., Litorell J., Everhov Å.H., et al. Dosing of thromboprophylaxis and mortality in critically ill COVID-19 patients. Crit Care. 2020;24:653. doi: 10.1186/s13054-020-03375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fraissé M., Logre E., Pajot O., Mentec H., Plantefève G., Contou D. Thrombotic and hemorrhagic events in critically ill COVID-19 patients: a French monocenter retrospective study. Crit Care. 2020;24:275. doi: 10.1186/s13054-020-03025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musoke N., Lo K.B., Albano J., Peterson E., Bhargav R., Gul F., et al. Anticoagulation and bleeding risk in patients with COVID-19. Thromb Res. 2020;196:227–230. doi: 10.1016/j.thromres.2020.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Billett H.H., Reyes-Gil M., Szymanski J., Ikemura K., Stahl L.R., Lo Y., et al. Anticoagulation in COVID-19: effect of enoxaparin, heparin, and apixaban on mortality. Thromb Haemost. 2020;120:1691–1699. doi: 10.1055/s-0040-1720978. [DOI] [PMC free article] [PubMed] [Google Scholar]