Abstract
Atrazine, a herbicide widely used in corn production, is a frequently detected groundwater contaminant. Nine gram-positive bacterial strains able to use this herbicide as a sole source of nitrogen were isolated from four farms in central Canada. The strains were divided into two groups based on repetitive extragenic palindromic (rep)-PCR genomic fingerprinting with ERIC and BOXA1R primers. Based on 16S ribosomal DNA sequence analysis, both groups were identified as Nocardioides sp. strains. None of the isolates mineralized [ring-U-14C]atrazine. There was no hybridization to genomic DNA from these strains using atzABC cloned from Pseudomonas sp. strain ADP or trzA cloned from Rhodococcus corallinus. S-Triazine degradation was studied in detail in Nocardioides sp. strain C190. Oxygen was not required for atrazine degradation by whole cells or cell extracts. Based on high-pressure liquid chromatography and mass spectrometric analyses of products formed from atrazine in incubations of whole cells with H218O, sequential hydrolytic reactions converted atrazine to hydroxyatrazine and then to the end product N-ethylammelide. Isopropylamine, the putative product of the second hydrolytic reaction, supported growth as the sole carbon and nitrogen source. The triazine hydrolase from strain C190 was isolated and purified and found to have a Km for atrazine of 25 μM and a Vmax of 31 μmol/min/mg of protein. The subunit molecular mass of the protein was 52 kDa. Atrazine hydrolysis was not inhibited by 500 μM EDTA but was inhibited by 100 μM Mg, Cu, Co, or Zn. Whole cells and purified triazine hydrolase converted a range of chlorine or methylthio-substituted herbicides to the corresponding hydroxy derivatives. In summary, an atrazine-metabolizing Nocardioides sp. widely distributed in agricultural soils degrades a range of s-triazine herbicides by means of a novel s-triazine hydrolase.
The agricultural herbicide atrazine (2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine; see Fig. 1 for structure) is used extensively in many parts of the world to control a variety of weeds, primarily in the production of corn. There is some evidence to suggest that atrazine may be an endocrine-disrupting chemical (10, 28). Trace levels of atrazine residues are frequently detected in surface and well water samples (19, 32, 41). Once in aquifers, it is persistent (1, 48), and thus there is considerable interest in developing agricultural management practices that minimize the potential for atrazine pollution of surface water and groundwater resources.
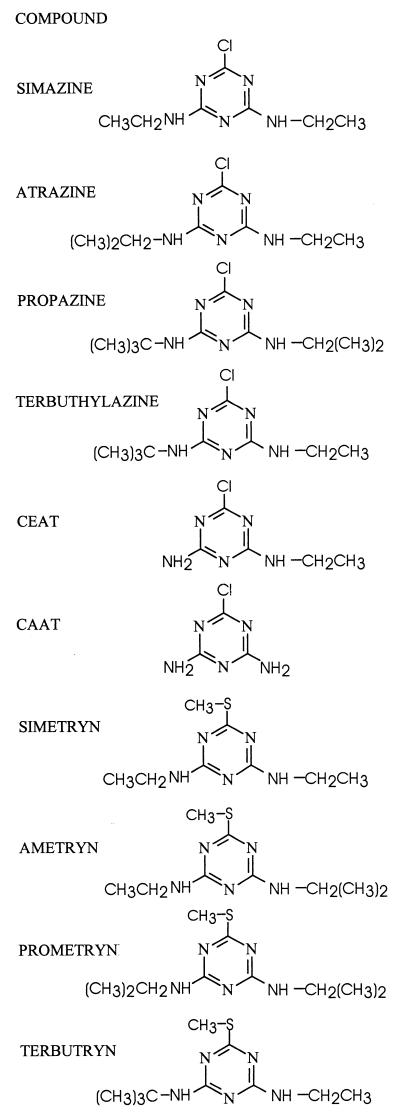
FIG. 1.
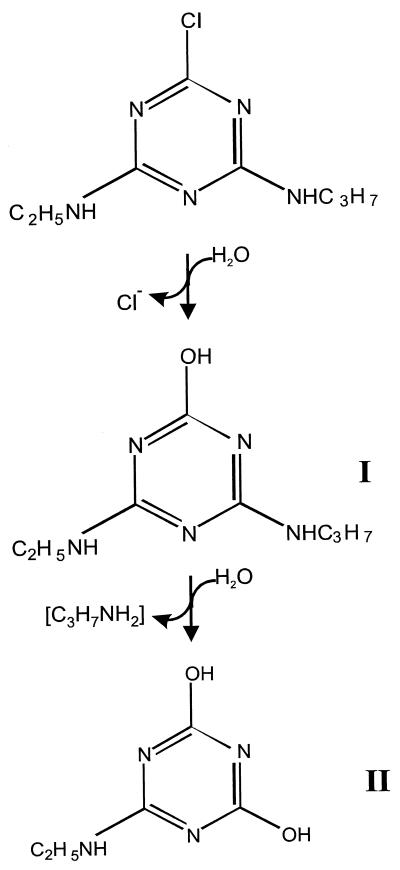
Structures of s-triazine compounds used in this study.
A variety of fungi (15, 24, 29) and bacteria (5, 7, 27, 33) which dealkylate or dechlorinate atrazine but do not mineralize the s-triazine ring have been isolated. Recently, there have been several reports of rapid atrazine mineralization in agricultural soils (4, 17, 44, 46), and a variety of atrazine-mineralizing bacteria, including members of the genera Pseudomonas, Acinetobacter, and Agrobacterium, have been isolated from soils that have come in contact with this chemical (3, 26, 27, 36, 42, 49). These bacteria commonly initiate atrazine degradation by a hydrolytic dechlorination reaction. The genes encoding an atrazine chlorohydrolase (atzA) and two amidohydrolytic reactions (atzB and atzC), which together convert atrazine to the ring cleavage substrate cyanuric acid, have been cloned from the Pseudomonas sp. strain ADP (6, 11, 38). Cyanuric acid is converted by another set of amidohydrolase enzymes to biuret and urea, which are then mineralized (9). The genes encoding these enzymes are widespread, highly conserved, and plasmid borne in isolates that have been examined (13, 14, 23).
We are interested in agricultural management practices that influence the persistence of pesticides, and we have recently initiated a study examining the relationship of herbicide treatment history with atrazine persistence and biodegradation pathways in agricultural soils and watersheds (43). Persistence generally declines in response to herbicide use, suggesting that exposure of soil to the herbicide enhances the abundance and activity of atrazine-degrading bacteria (4, 34, 35). The objective of the work reported here was to gain a better understanding of atrazine-degrading microorganisms by characterizing the diversity, identity, and atrazine degradation mechanism of bacteria isolated from agricultural soils that have a history of exposure to atrazine.
MATERIALS AND METHODS
Sampling sites and enrichment, isolation, characterization, and maintenance of atrazine-degrading bacteria.
The bacteria described in this paper were isolated from four farms located in central Canada. These consisted of a loam (site 1 at 45°22′N, 75°43′W, described in reference 44; pH 5.9; 3.0% organic matter) located near the city of Ottawa, Ontario; a sandy loam located near Winchester, Ontario (site 2 at 45°05′N, 75°40′W, described in reference 20; pH 5.7; 2.6% organic matter); a clay located near Harrow, Ontario (site 3 at 42°05′N, 82°50′W, described in reference 16; pH 5.6; 2.0% organic matter); and a loam (site 4 at 45°35′N, 73°10′W; pH 6.0; 1.4% organic matter) located near Saint Hyacinthe, Quebec. All four soils had been cropped to corn and had been treated with atrazine for weed control according to normal farming practice. Five replicate soil cores were obtained from each sample site, pooled, homogenized, and stored without drying at 4°C prior to being used for enrichment and isolation of atrazine-degrading bacteria.
Enrichment preparations consisting of a mineral salts medium containing 25 mg of atrazine/liter as the sole nitrogen and carbon source were inoculated with soil (25% wt/vol) and incubated aerobically with shaking at 30°C (45). Bacteria which formed clear zones on solidified media containing atrazine as the sole nitrogen source were purified and characterized as previously described (45).
Chemicals and analytical methods.
The structures of s-triazine compounds used in this study are presented in Fig. 1. We have adopted the nomenclature of Cook et al. (9) for the amino-substituted s-triazines. Analytical-grade triazine herbicides and metabolites were gifts from Novartis Crop Protection Canada Inc. (Guelph, Ontario, Canada) or were purchased from ChemService Inc. (West Chester, Pa.). Stock solutions were prepared in methanol. When added to cultures or extracts, the solvent was allowed to evaporate before the aqueous solutions were added to containers. [ring-U-14C]atrazine (specific activity, 4.5 mCi/mmol; radioactive purity, 95%) was purchased from Sigma Chemical Co. (St. Louis, Mo.). Water containing 18O (enriched 95 to 98%) was purchased from Cambridge Isotope Laboratories, Inc. (Andover, Mass.). Parent compounds and transformation products were analyzed by reverse-phase high-pressure liquid chromatography (HPLC) on a C18 column using an instrument equipped with a UV detector (set at 220 nm) coupled in series with a radioactivity detector (43). The size of radioactive peaks is expressed as the integrated peak area. The solvent consisted of 70% methanol–30% 5 mM Na2HPO4 (pH 9.0) (solvent system 1) or 50% methanol–50% 10 mM ammonium acetate (solvent system 2). Radioactivity of samples was measured in Universol scintillation cocktail (ICN, Costa Mesa, Calif.) with a Beckman model LS5801 (Beckman, Irvine, Calif.) liquid scintillation counter using an external standard for quench correction. Mass spectra were determined by electron impact on a Finnigan-MAT 8230 mass spectrometer at an ionizing voltage of 70 eV. Metabolites were isolated and purified in preparation for mass spectral analysis by fractionating culture filtrates by HPLC, evaporating under a stream of nitrogen, and taking up the final sample in methanol. Protein was quantified by the Bradford assay (8).
Characterization of triazine herbicide degradation.
Cell extracts were prepared by sonicating (four times, 2.5 min each, with 30-s rest intervals) cells resuspended in 10 mM sodium phosphate buffer (pH 7.2) and removing the undisrupted cells by centrifugation (12,000 × g, 12 min). In some cases, cell extracts were incubated anaerobically in serum vials sealed with grey butyl rubber stoppers by repeatedly evacuating the headspace under vacuum and backfilling to atmospheric pressure with nitrogen gas. The possible incorporation of H218O into atrazine was determined with whole cells resuspended in 100 μl of H216O or H218O and incubated aerobically overnight at 30°C with 100 μg of atrazine. Aqueous samples for chemical analysis of the parent compound and metabolites were prepared by adding methanol (50% final concentration) to cell suspensions or cell extracts and removing precipitated debris by centrifugation (14,000 × g, 4 min). Metabolites to be analyzed by mass spectroscopy were first isolated by HPLC fractionation using the method described below. In some cases, dechlorination of atrazine, CEAT, ametryn, and prometryn were measured spectrophotometrically at 240, 260, 250, and 252 nm, respectively. The experimentally determined values for extinction coefficients for these substrates and their dechlorinated products were as follows: atrazine, ɛ240 = 11.2 mM−1 cm−1; hydroxyatrazine, ɛ240 = 7.2 mM−1 cm−1; CEAT, ɛ260 = 5.2 mM−1 cm−1; OEAT, ɛ260 = <0.1 mM−1 cm−1; ametryn, ɛ250 = 12 mM−1 cm−1; hydroxyametryn, ɛ250 = 1.5 mM−1 cm−1; prometryn, ɛ252 = 8.9 mM−1 cm−1; and hydroxyprometryn, ɛ252 = 1.7 mM−1 cm−1. Deamination of AAAT was measured spectrophotometrically at 235 nm as described previously (31). Typical assays contained 0.5 ml of 10 mM potassium phosphate (pH 7.0) with 0.2 mM atrazine and 2 to 50 μl of cell extract and were incubated at 25°C.
DNA manipulations.
Procedures for preparation of genomic DNA, blotting, and hybridizations were exactly as previously described (45). Probes for the genes atzA, atzB, atzC, and trzA were prepared from the plasmids pMD4, pATZ-2, pTD-2, and pSWP1, respectively (6, 11, 38, 40). A 0.9-kb probe for trzA was prepared by cutting plasmid pSWP1 with NcoI and KpnI. Purified plasmids were digested with restriction enzymes (pMD4, ApaI and PstI; pATZ-2, BglII and EcoRI; pTD-2, ClaI and HincII; pSWP1, NcoI and KpnI) by standard procedures (39). After being electrophoretically separated in 1% low-melting-point multipurpose agarose (Roche Molecular Biochemicals, Laval, Quebec, Canada), a 0.6-kb internal fragment of the atzA gene, a 1.2-kb internal fragment of the atzB gene, a 0.75-kb internal fragment of the atzC gene, and a 0.9-kb internal fragment of atzA were extracted using an agarose gel DNA extraction kit (Roche Molecular Biochemicals). The purified fragments were labeled with digoxigenin (DIG) by random priming using the DIG high primer DNA labeling and detection starter kit II (Roche Molecular Biochemicals) as specified by the manufacturer.
PCR genomic fingerprinting was done with ERIC (21) and BOXA1R (47) primers as previously described (45). PCR amplification, cloning, sequencing, and analysis of the entire 16S rRNA gene were done exactly as previously described (45). The following bacteria were included in the phylogenetic analysis: Aeromicrobium erythreum, accession number AF005021; Aeromicrobium fastidiosum, Z78209; Actinoplanes utahensis, X80823; Nocardioides jensenii, AF005006; Nocardioides plantarum, AF005008; Nocardioides simplex, AF005009; Rhodococcus globerulus, X81931; Nocardioides sp. strain C157, AF253509; Nocardioides sp. strain C190, AF253510; and Streptomyces lividans, X86354.
Purification of the triazine hydrolase.
Cells from 4 liters of Nocardioides culture which had been grown to stationary phase were pelleted by centrifugation (4,000 × g, 10 min, 4°C). The 4-g pellet was resuspended with 8 ml of ice-cold 10 mM potassium phosphate (KPi) (pH 7.0) (buffer A). This cell suspension was passed three times through a chilled French pressure cell (15,000 lb/in2), and whole cells and debris were removed by centrifugation (12,000 × g, 10 min, 4°C). The supernatant was subjected to ultracentrifugation (105,000 × g, 1 h, 4°C), and the supernatant from this treatment (crude soluble fraction) was removed and used as a source of triazine hydrolase for further purification. The crude soluble fraction was pumped (3 ml/min) onto a TSK-DEAE anion-exchange column (30.0 by 2.5 cm) (Waters/Millipore, Milford, Mass.) that had been equilibrated with buffer A. After the column was washed with 200 ml of buffer A, a linear gradient of 0 to 1.0 M NaCl (pH 7.0) in buffer A was run (5 ml/min) to elute bound material from the column. Fractions containing triazine hydrolase activity were pooled and used for further purification. The pooled DEAE fractions containing hydrolase activity were brought to 1 M (NH4)2SO4 by the addition of solid (NH4)2SO4 and were pumped at 3 ml/min onto a TSK-phenyl column (2.15 by 15 cm) (HP-Genenchem, South San Francisco, Calif.) that had been equilibrated with buffer A containing 1 M (NH4)2SO4. After the column was washed with 200 ml of equilibration buffer, a linear gradient of 1.0 to 0 M (NH4)2SO4 in buffer A was run at 5 ml/min to elute bound material from the column. Peak active fractions were pooled and diluted 10-fold with buffer A. The pooled diluted phenyl fractions were again subjected to ion-exchange chromatography on a TSK-DEAE column as described above. Peak active fractions were stored at 4°C.
Size exclusion chromatography of crude extracts and purified hydrolase fractions was carried out with a Biosep SEC3000 column (21.5 by 600 mm) (Phenomenex) equilibrated and run with buffer A containing 0.2 M NaCl at 6 ml/min. Pooled DEAE and phenyl fractions were concentrated using Centricon-30 microconcentrators (Amicon, Beverly, Mass.), and 0.1- to 0.2-ml aliquots of this concentrate were injected onto the column. Fractions containing hydrolase activity were pooled. For estimation of the native molecular size of the hydrolase, the column was calibrated with prepared protein standards (Bio-Rad).
Protein determinations and SDS-PAGE.
Protein concentrations in partially purified and purified enzyme preparations were determined by the spectrophotometric method of Kalb and Bernlohr (22). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 4 to 12% acrylamide gradient gel) of proteins was performed by the method of Laemmli (25), and fragments were sized using the Bio-Rad mid-range protein standard kit.
RESULTS
Identity of atrazine-degrading bacteria.
Nine gram-positive atrazine-degrading bacteria which formed clear zones on the atrazine agar medium were isolated and characterized; four isolates were obtained from site 1, three were from site 2, and one each was from sites 3 and 4. Colonies were uniform in appearance, and after 3 weeks of growth on atrazine-mineral salts (AMS) medium, they formed 2-mm-diameter colonies with the following properties: buff, dull, opaque appearance, circular form, convex elevation, entire margin, and viscid consistency without mycelial growth. Cellular morphology was consistent; all isolates were short, nonmotile, nonpleiotrophic gram-positive rods when recovered from AMS agar.
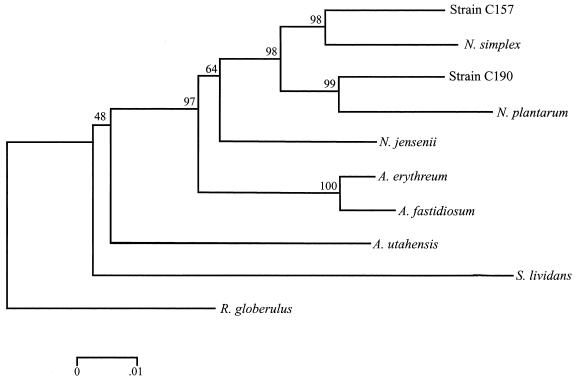
Rep-PCR fingerprinting with the ERIC and the BOXA1R primers revealed one group of two siblings and a second group of seven siblings. Strains C157 and C158, isolated from site 1, had identical ERIC and the BOXA1R fingerprints. The other seven isolates, strains C194 and C196 from site 1, strains C189 and C190 from site 2, strain C183 from site 3, and strain C188 from site 4, likewise had identical ERIC and the BOXA1R fingerprints. The 16S rRNA gene from a representative isolate of each of the two PCR fingerprint types, strains C157 and C190, was sequenced to obtain information on their identity. When aligned with the sequences available in the GenBank database, strains C157 and C190 were identified as Nocardioides sp. (Fig. 2). Strain C190 was most similar to Nocardioides plantarum, and strain C157 most closely related to Nocardioides simplex.
FIG. 2.
Phylogenetic tree based on the 16S ribosomal DNA sequence data showing the relationships of strains C157 and C190 with the most closely related bacteria identified in the GenBank database. Included in the analysis are Actinoplanes utahensis, Aeromicroblum fastidiosum, N. simplex, N. plantarum, Nocardioides albus, N. jensenii, S. lividans, and R. globerulus as an outlier. The bar indicates 0.01 substitutions per nucleotide position.
Pathway and mechanism of atrazine degradation, and s-triazine substrate specificity.
Atrazine was incompletely degraded by these bacteria; none of them converted [ring-U-14C]atrazine to carbon dioxide (data not shown). Strain C190 was chosen for detailed study.
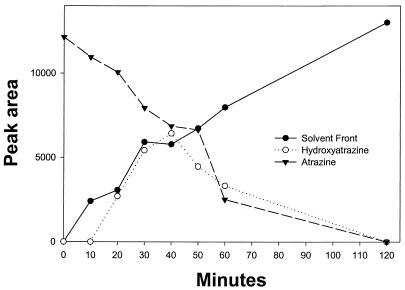
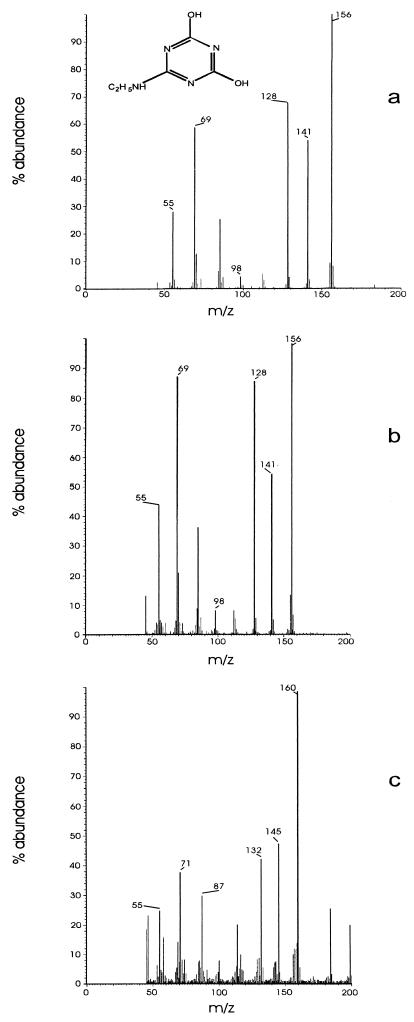
Dense (adjusted to an A600 of 2) cell suspensions degraded 20 mg of atrazine/liter in an overnight incubation (data not shown). Atrazine degradation by cell suspensions or by cell extracts did not require oxygen (data not shown). HPLC analyses of resting cell suspensions of strain C190 incubated with 10 mg of [ring-U-14C]atrazine/liter revealed a metabolite (I) which coeluted with hydroxyatrazine (retention times, 4.7 min [solvent system 1] and 5.9 min [solvent system 2]) and then an end product metabolite (II) which was more hydrophilic and eluted at the solvent front in both solvent systems (Fig. 3). Deethyl- and deisopropylatrazine were not detected in culture filtrates. Metabolites I and II were purified by HPLC fractionation and were subjected to solid-probe mass spectrometry. Metabolite I had a molecular ion (M+, base peak) at m/z 197 and major peaks at m/z 182, 155, 140, 127, 112, 97, 84, 71, and 58 and had a mass spectrum corresponding exactly to that of an analytical-grade hydroxyatrazine standard (data not shown). Metabolite II had a mass spectrum identical to that of an analytical standard of N-ethylammelide (molecular formula, C5H8N4O2), with a molecular ion at m/z 156 (M+, base peak) and major fragments at m/z 141 (loss of CH3), 128 (loss of CH), 112 (loss of O) and 98 (loss of N) (Fig. 4). The source of the hydroxyl groups in the N-ethylammelide was determined by incubating a resting cell suspension of strain C190 for 24 h with atrazine in H218O. The mass spectrum of the recovered metabolite was similar to that of N-ethylammelide but with major peaks larger by 4 or 2 mass units (Fig. 4), consistent with a molecular formula of C5H8N418O2. The molecular ion was at m/z 160 (M+, base peak), with major peaks at m/z 145 (loss of CH3), 132 (loss of CH), 114 (loss of 18O), and 100 (loss of N). The mass spectrum of analytical N-ethylammelide incubated for 24 h at 30°C in H218O was identical to that of the analytical standard, indicating that exchange had not taken place (data not shown). These results indicate that both hydroxyl groups in the N-ethylammelide originate from water, as illustrated in Fig. 5.
FIG. 3.
Disposition of radioactivity during metabolism of [ring-U-14C]atrazine by whole cells of strain C190. Radioactivity is expressed as the area integrated under peaks.
FIG. 4.
Mass spectra of an N-ethylammelide analytical standard (a) and of the end product of atrazine metabolism recovered from culture filtrates of Nocardioides strain C190 incubated in H216O (b) or in H218O (c).
FIG. 5.
Proposed pathway of atrazine metabolism by Nocardioides sp. strain C190. Metabolite I is hydroxyatrazine, and metabolite II is N-ethylammelide.
Strain C190's ability to grow on components of atrazine in solidified mineral salts medium was consistent with the proposed pathway. Two grams of isopropylamine/liter but not 2 g of ethylamine/liter supported growth as the sole carbon and nitrogen source. Two grams of cyanuric acid/liter did not support growth as the sole nitrogen source (data not shown).
Genomic DNAs of all strains were tested in a dot blot assay for the ability to hybridize to DIG-labeled sequences from the atzA, atzB, and atzC genes of Pseudomonas strain ADP and trzA from Rhodococcus corallinus. None of the strains hybridized with any of the probes (data not shown). There is a 405-bp region of atzA that is highly conserved in all atrazine-hydrolyzing gram-negative bacteria and in Clavibacter michiganensis ATZ1 and that is detectable by PCR amplification with specific primers (13). These primers failed to amplify genomic DNA of strain C190, whereas they did for Pseudomonas strain ADP (data not shown).
Strain C190 degraded a range of chloro- and methylthio-substituted s-triazines (Fig. 1; Table 1). All substrates were degraded by anaerobic cell suspensions (data not shown). The methylthio-substituted herbicides were degraded by both whole cells and cell extract more rapidly than were the corresponding chlorinated analogs. Metabolites recovered from cultures were more hydrophilic than the parent compounds and had mass spectral characteristics consistent with the loss of the methylthio group (47 mass units) and the acquisition of a hydroxy group (17 mass units; total net loss, 30 mass units) (Table 2). In the case of ametryn and terbutryn, the metabolites had characteristics identical to those metabolites produced from the corresponding chloro-substituted herbicides. Taken together, the results indicate that the methylthio group of these herbicides is removed hydrolytically, yielding the corresponding hydroxytriazine products.
TABLE 1.
Relative rates of triazine hydrolysis by whole cells, cell extracts, and purified triazine hydrolase
| Substrate | Relative rate of triazine hydrolysisa
|
Enzyme value
|
||
|---|---|---|---|---|
| Whole cells | Cell extract | Km (μM) | Vmax (μmol/min/mg of protein) | |
| Atrazine | 100 | 100 | 25 | 31 |
| Simazine | 95 | 119 | NDb | ND |
| Propazine | 17 | 5 | ND | ND |
| Terbuthylazine | 50 | 60 | ND | ND |
| Ametryn | 193 | 194 | 17 | 18 |
| Simetryn | 167 | 198 | ND | ND |
| Prometryn | 212 | 75 | 310 | 98 |
| Terbutryn | 123 | 32 | ND | ND |
| CEAT | ND | ND | 131 | 0.35 |
Given as percentages of the atrazine rate.
ND, not done.
TABLE 2.
Characteristics of methylthio-s-triazine substrates and of metabolites accumulated after overnight incubation of suspensions of Nocardioides sp. strain C190a
| Substrate | Characteristics of substrate
|
Characteristics of product
|
||
|---|---|---|---|---|
| HPLC retention time (min) | m/z (% intensity) | HPLC retention time (min) | m/z (% intensity) | |
| Atrazine | 7.2 | 215 (M+, 75), 200 (100), 173 (20), 132, 18, 71 (24), 58 (58) | 4.6 | 197 (M+, 100), 182 (90), 155 (45), 127 (36), 112 (80), 69 (52), 58 (92) |
| Terbuthylazine | 7.9 | 229 (40), 214 (M+, 100), 173 (50), 132 (30), 71 (42) | 5.4 | 211 (80), 196 (M+, 100), 169 (12), 155 (80), 126 (72), 112 (30), 69 (30), 58 (42) |
| Simetryn | 8.1 | 213 (M+, 100), 198 (24), 170 (50), 155 (42), 96 (35), 71 (53), 68 (84) | 4.0 | 183 (M+, 10), 149 (22), 111 (15), 85 (45), 70 (55), 55 (100) |
| Ametryn | 8.7 | 227 (M+, 100), 212 (64), 185 (35), 170 (40), 99 (25), 68 (60), 58 (64) | 4.6 | 197 (M+, 100), 182 (90), 155 (35), 127 (25), 112 (62), 69 (70), 57 (96) |
| Prometryn | 9.3 | 241 (M+, 100), 226 (60), 199 (30), 184 (72), 106 (25), 58 (64) | 4.8 | 211 (M+, 100), 196 (94), 169 (42), 154 (58), 112 (65), 69 (45), 58 (88) |
| Terbutryn | 15.0 | 241 (M+, 70), 226 (90), 185 (100), 170 (55), 106 (32), 68 (60) | 5.8 | 211 (M+, 62), 196 (100), 155 (72), 126 (64), 69 (28) |
The initial substrate concentration was 10 mg/liter.
Isolation and purification of triazine hydrolase enzyme.
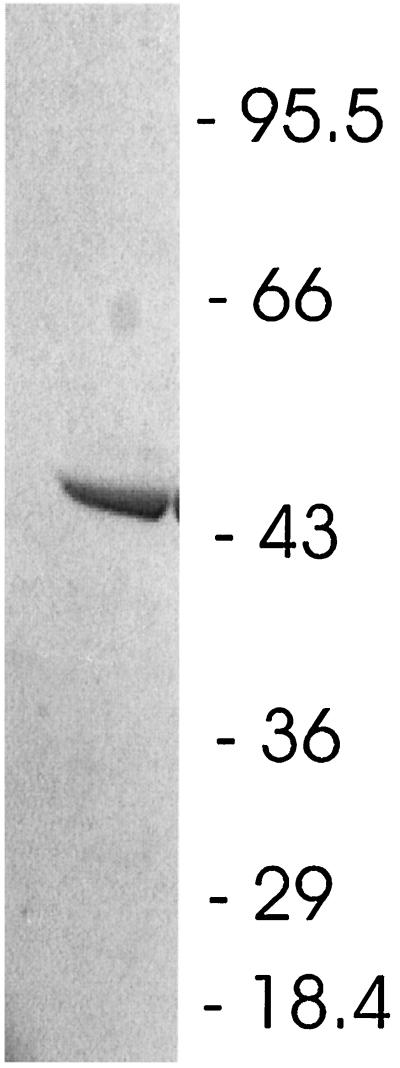
Purification of the triazine hydrolase was accomplished by chromatography of the crude soluble fraction on a DEAE anion-exchange column, followed by chromatography of active fractions on TSK-phenyl and DEAE columns (Table 3). The final preparation had a specific activity approximately 10 times that of the starting material and yielded a single band of approximately 52,000 Da when subjected to SDS-PAGE (Fig. 6).
TABLE 3.
Purification of triazine hydrolase from Nocardioides sp. strain C190
| Purification step | Total protein (mg) | Total activity (IU) | Sp act (IU/mg of protein) | Recovery (%) | Purification (fold) |
|---|---|---|---|---|---|
| Crude soluble | 5.94 | 14.2 | 2.39 | 100 | |
| TSK-DEAE | 5.6 | 8.9 | 1.59 | 66 | |
| TSK-phenyl | 0.95 | 4.4 | 4.60 | 31 | 1.9 |
| TSK-DEAE | 0.08 | 1.8 | 22.5 | 12 | 9.4 |
FIG. 6.
SDS-PAGE gel of purified triazine hydrolase. The numbers correspond to the molecular masses (in kilodaltons) of protein standards.
Enzyme characterization.
The native molecular size of the triazine hydrolase was estimated by size exclusion chromatography to be approximately 75,000 Da. From these results, it is unclear whether the enzyme is composed of a single subunit with the total size of 52,000 Da or is a dimer with a total size of 104,000 Da.
In assays designed to test the effect of different metal salts or divalent metal chelators, MnSO4 and EDTA showed no effect on enzyme activity at 100 μM and 500 μM, respectively. However, assays containing 100 μM MgSO4, CuSO4, CoSO4, or ZnSO4 showed inhibition of hydrolase activity by these metal salts (approximately 20, 50, 70, and 80% inhibition, respectively).
In order to estimate the substrate specificity of the triazine hydrolase, Michaelis-Menten constants were estimated from least-squares regression of Lineweaver-Burk plots using the structurally related s-triazines atrazine, ametryn, prometryn, and CEAT (Table 1). The enzyme also had activity toward the s-triazines terbuthylazine and propazine, but we were unable to determine kinetic constants using these compounds because of their low aqueous solubilities. The enzyme displayed no detectable dechlorination activity in assays containing the triazine CAAT and no detectable deamination activity toward CAAT or AAAT. We did not test whether these compounds were competitive inhibitors of the dechlorination of atrazine.
DISCUSSION
In this study, nine atrazine-degrading gram-positive isolates were obtained from four agricultural soils, clustered into two groups by rep-PCR fingerprinting, and identified as Nocardioides sp. strains on the basis of 16S ribosomal DNA sequencing. The fact that these atrazine-degrading Nocardioides sp. strains were isolated from four independent farms in central Canada suggests that these organisms are geographically widespread. Nocardioides species have previously been shown to degrade a variety of toxic organic pollutants, including 2,4,6-trinitrophenol (picric acid), 2,4,5-trichlorophenoxyacetic acid, and the organophosphorus insecticide coumaphos (18, 30, 37).
The Nocardioides sp. strains converted atrazine through hydroxyatrazine to the end product N-ethylammelide by means of an atrazine chlorohydrolase and a hydroxyatrazine N-isopropylaminehydrolase (Fig. 5). This proposed pathway is supported by several lines of evidence: ring-labeled atrazine was not mineralized, atrazine degradation did not require oxygen, the herbicide was converted to hydroxyatrazine and then to N-ethylammelide by whole cells, the two hydroxyl groups in the N-ethylammelide end product originated from water, and isopropylamine but not ethylamine or cyanuric acid was metabolized. Previously reported atrazine-degrading gram-positive bacteria N-dealkylate atrazine by means of a P450-like monooxygenase (33). The absence of detection of deethyl- or deisopropylatrazine in supernatants of cultures that have degraded atrazine further suggests that Nocardioides does not transform atrazine by this mechanism. R. corallinus NRRLB-15444R produces a triazine chlorohydrolase, but atrazine is not transformed by this enzyme (31). Remarkably, the Nocardioides atrazine-degradation pathway appears to be identical to that recently reported for Clavibacter michiganensis ATZ1, part of an atrazine-mineralizing consortium isolated from an agricultural soil (12). Strain ATZ1 was identified on the basis of fatty acid methyl ester analysis, and it has a similarity index of 0.584 with C. michiganensis when compared with the MIDI database (2). Although the pathways of atrazine degradation are identical, the genes encoding the enzymes are not; atzABC genes were undetectable by hybridization in Nocardioides, whereas C. michiganensis possesses sequences homologous to these genes (13). atzABC genes have been found in all atrazine chlorohydrolase-expressing bacteria examined to date, including Pseudomonas, Alcaligenes, Ralstonia, Agrobacterium, and Pseudaminobacter (12, 13, 42, 45). Although the hydrolytic mechanism of s-triazine transformation is conserved in Nocardioides, our results indicate that there is hitherto-undetected diversity in the genes encoding the enzyme.
In a related study, two of the soils which yielded the Nocardioides sp. (sites 1 and 4) also yielded a Pseudaminobacter sp. which mineralized atrazine, the “upper pathway” consisting of hydrolases encoded by atzABC (45). It was noted in that report that the alkylamine-degrading Pseudaminobacter sp. would have the selective advantage of being able to utilize atrazine as a carbon source upon the acquisition of atzABC. The results reported in this study indicate that these very same soils contain at least two distinct populations which can use atrazine as both a carbon and a nitrogen source. It is noteworthy that although the genes encoding the atrazine-degrading enzymes are not shared by these bacteria, the metabolic strategy of hydrolytically cleaving growth-supporting alkylamine groups from the s-triazine ring is conserved.
The ability of strain C190 to degrade methylthio-substituted s-triazine herbicides makes it distinct from Pseudomonas strain ADP, which cannot (26). Recently, a range of genera including Clavibacter, Alcaligenes, and Agrobacterium expressing homologs of AtzA were found to vary significantly in their s-triazine substrate specificity, in spite of sharing a nearly identical 500-bp conserved region in the chlorohydrolase genes (J. L. Seffernick, M. J. Sadowsky, and L. P. Wackett, Abstr. 99th Gen. Meet. Am. Soc. Microbiol. 1999, abstr. K-62, p. 412, 1999). Analysis of the chlorohydrolase-encoding gene from strain C190 will help elucidate sequences specifying substrate specificity and hydrolytic activity. We are currently attempting to locate and clone this gene.
In summary, our results indicate that a Nocardioides sp. isolated from atrazine-treated agricultural soils degrades a variety of s-triazine herbicides by means of a novel chlorohydrolase and that there is hitherto-undetected diversity in atrazine chlorohydrolase-encoding genes.
ACKNOWLEDGMENTS
This work was partially funded by Novartis Crop Protection.
We thank J. Purdy and H. Buser of Novartis Crop Protection for their excellent collaboration. We are grateful to M. L. de Souza and L. P. Wackett for providing Pseudomonas sp. strain ADP, pMD4, and pATZ-2. We thank H. Bork for excellent technical assistance and C. F. Drury, R. Lalande, and E. G. Gregorich for providing us with soil samples.
REFERENCES
- 1.Agertved J, Rugge K, Barker J F. Transformation of the herbicides MCPP and atrazine under natural aquifer conditions. Ground Water. 1992;30:500–506. [Google Scholar]
- 2.Alvey S, Crowley D E. Survival and activity of an atrazine-mineralizing bacterial consortium in rhizosphere soil. Environ Sci Technol. 1996;30:1596–1603. [Google Scholar]
- 3.Assaf N A, Turco R F. Accelerated biodegradation of atrazine by a microbial consortium is possible in culture and soil. Biodegradation. 1994;5:29–35. doi: 10.1007/BF00695211. [DOI] [PubMed] [Google Scholar]
- 4.Barriuso E, Houot S. Rapid mineralization of the S-triazine ring of atrazine in soils in relation to soil management. Soil Biol Biochem. 1996;28:1341–1348. [Google Scholar]
- 5.Behki R M, Topp E, Dick W, Germon P. Metabolism of the herbicide atrazine by Rhodococcus strains. Appl Environ Microbiol. 1993;59:1955–1959. doi: 10.1128/aem.59.6.1955-1959.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boundy-Mills K L, de Souza M L, Mandelbaum R T, Wackett L P, Sadowsky M J. The atzB gene of Pseudomonas sp. strain ADP encodes the second enzyme of a novel atrazine degradation pathway. Appl Environ Microbiol. 1997;63:916–923. doi: 10.1128/aem.63.3.916-923.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouquard C, Ouazzani J, Promé J-C, Michel-Briand Y, Plésiat P. Dechlorination of atrazine by a Rhizobium sp. isolate. Appl Environ Microbiol. 1997;63:862–866. doi: 10.1128/aem.63.3.862-866.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 9.Cook A M, Beilstein P, Grossenbacker H, Hutter R. Ring cleavage and degradative pathway of cyanuric acid in bacteria. Biochem J. 1985;231:25–30. doi: 10.1042/bj2310025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crain A D, Guillette L J, Rooney A A, Pickford D B. Alterations in steroidogenesis in alligators (Alligator mississippiensis) exposed naturally and experimentally to environmental contaminants. Environ Health Perspect. 1997;105:528–533. doi: 10.1289/ehp.97105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Souza M L, Sadowsky M J, Wackett L P. Atrazine chlorohydrolase from Pseudomonas sp. strain ADP: gene sequence, enzyme purification, and protein characterization. J Bacteriol. 1996;178:4894–4900. doi: 10.1128/jb.178.16.4894-4900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Souza M L, Newcombe D, Alvey S, Crowley D E, Hay A, Sadowsky A M J, Wackett L P. Molecular basis of a bacterial consortium: interspecies catabolism of atrazine. Appl Environ Microbiol. 1998;64:178–184. doi: 10.1128/aem.64.1.178-184.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Souza M L, Seffernick J, Martinez B, Sadowski M, Wackett L P. The atrazine catabolism genes atzABC are widespread and highly conserved. J Bacteriol. 1998;180:1951–1954. doi: 10.1128/jb.180.7.1951-1954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Souza M L, Wackett L P, Sadowsky M J. The atzABC genes encoding atrazine catabolism are located on a self-transmissible plasmid in Pseudomonas sp. strain ADP. Appl Environ Microbiol. 1998;64:2323–2326. doi: 10.1128/aem.64.6.2323-2326.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnelly P K, Entry J A, Crawford D L. Degradation of atrazine and 2,4-dichlorophenoxyacetic acid by mycorrhizal fungi at three nitrogen concentrations in vitro. Appl Environ Microbiol. 1993;59:2642–2647. doi: 10.1128/aem.59.8.2642-2647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drury C F, Tan C S. Long-term (35 years) effects of fertilization, rotation and weather on corn yields. Can J Plant Sci. 1995;75:355–362. [Google Scholar]
- 17.Gan J, Becker R L, Koskinen W C, Buhler D D. Degradation of atrazine in two soils as a function of concentration. J Environ Qual. 1996;25:1064–1072. [Google Scholar]
- 18.Golovleva L A, Pertsova R N, Evtushenko L I, Baskunov B P. Degradation of 2,4,5-trichlorophenoxyacetic acid by a Nocardioides simplex culture. Biodegradation. 1990;1:263–271. doi: 10.1007/BF00119763. [DOI] [PubMed] [Google Scholar]
- 19.Goodrich J A, Lykins B W, Clark R M. Drinking water from agriculturally contaminated ground water. J Environ Qual. 1991;20:707–717. [Google Scholar]
- 20.Gregorich E G, Reynolds W D, Culley J L B, McGovern M A, Curnoe W E. Changes in soil physical properties with depth in a conventionally tilled soil after no-tillage. Soil Tillage Res. 1993;26:289–299. [Google Scholar]
- 21.Hulton C S J, Higgins C F, Sharp P M. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimurium and other enteric bacteria. Mol Microbiol. 1991;5:825–834. doi: 10.1111/j.1365-2958.1991.tb00755.x. [DOI] [PubMed] [Google Scholar]
- 22.Kalb V J, Bernlohr R W. A new spectrophotometric assay for proteins in cell extracts. Anal Biochem. 1977;82:362–371. doi: 10.1016/0003-2697(77)90173-7. [DOI] [PubMed] [Google Scholar]
- 23.Karns J S, Eaton R W. Genes encoding s-triazine degradation are plasmid-borne in Klebsiella pneumoniae strain 99. J Agr Food Chem. 1997;45:1017–1022. [Google Scholar]
- 24.Kaufman D D, Blake J. Degradation of atrazine by soil fungi. Soil Biol Biochem. 1970;2:73–80. [Google Scholar]
- 25.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Mandelbaum R T, Allan D L, Wackett L P. Isolation and characterization of a Pseudomonas sp. that mineralizes the s-triazine herbicide atrazine. Appl Environ Microbiol. 1995;61:1451–1457. doi: 10.1128/aem.61.4.1451-1457.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirgain I, Green G A, Monteil H. Degradation of atrazine in laboratory microcosms—isolation and identification of the biodegrading bacteria. Environ Toxicol Chem. 1993;12:1627–1634. [Google Scholar]
- 28.Moore A, Waring C P. Mechanistic effects of a triazine pesticide on reproductive endocrine function in mature male Atlantic salmon (Salmo salar L.) Parr. Pestic Biochem Physiol. 1998;62:41–50. [Google Scholar]
- 29.Mougin C, Laugero C, Asther M, Chaplain V. Biotransformation of s-triazine herbicides and related degradation products in liquid cultures by the white rot fungus Phanerochaete chrysosporium. Pestic Sci. 1997;49:169–177. [Google Scholar]
- 30.Mulbry W. Characterization of a novel organophosphorus hydrolase from Nocardioides simplex NRRL B-24074. Microbiol Res. 2000;154:285–288. doi: 10.1016/S0944-5013(00)80001-4. [DOI] [PubMed] [Google Scholar]
- 31.Mulbry W W. Purification and characterization of an inducible s-triazine hydrolase from Rhodococcus corallinus NRRL B-15444R. Appl Environ Microbiol. 1994;60:613–618. doi: 10.1128/aem.60.2.613-618.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller S R, Berg M, Ulrich M M, Schwarzenbach R P. Atrazine and its primary metabolites in Swiss lakes: input characteristics and long-term behavior in the water column. Environ Sci Technol. 1997;31:2104–2113. [Google Scholar]
- 33.Nagy I, Compernolle R, Ghys K, Vanderleyden J, Demot R. A single cytochrome P-450 system is involved in degradation of the herbicides EPTC (S-ethyl dipropylthiocarbamate) and atrazine by Rhodococcus sp. strain NI86/21. Appl Environ Microbiol. 1995;61:2056–2060. doi: 10.1128/aem.61.5.2056-2060.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostrofsky E B, Traina S J, Tuovinen O H. Variation in atrazine mineralization rates in relation to agricultural management practice. J Environ Qual. 1997;26:647–657. [Google Scholar]
- 35.Pussemier L, Goux S, Vanderheyden V, Debongnie P, Tresinie I, Foucart G. Rapid dissipation of atrazine in soils taken from various maize fields. Weed Res. 1997;37:171–179. [Google Scholar]
- 36.Radosevich M, Traina S J, Hao Y L, Tuovinen O H. Degradation and mineralization of atrazine by a soil bacterial isolate. Appl Environ Microbiol. 1995;61:297–302. doi: 10.1128/aem.61.1.297-302.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajan J, Valli K, Perkins R E, Sariaslani F S, Barns S M, Reysenbach A L, Rehm S, Ehringer M, Pace N R. Mineralization of 2,4,6-trinitrophenol (picric acid): characterization and phylogenetic identification of microbial strains. J Ind Microbiol. 1996;16:319–324. doi: 10.1007/BF01570041. [DOI] [PubMed] [Google Scholar]
- 38.Sadowsky M J, Tong Z, de Souza M, Wackett L P. AtzC is a new member of the amidohydrolase protein superfamily and is homologous to other atrazine-metabolizing enzymes. J Bacteriol. 1998;180:152–158. doi: 10.1128/jb.180.1.152-158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 40.Shao Z Q, Seffens W, Mulbry W, Behki R M. Cloning and expression of the s-triazine hydrolase gene (trzA) from Rhodococcus corallinus and development of Rhodococcus recombinant strains capable of dealkylating and dechlorinating the herbicide atrazine. J Bacteriol. 1995;177:5748–5755. doi: 10.1128/jb.177.20.5748-5755.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solomon K R, Baker D B, Richards R P, Dixon K R, Klaine S J, La Point T W, Kendall R J, Weisskopf C P, Giddings J M, Giesy J P, et al. Ecological risk assessment of atrazine in North American surface waters. Environ Toxicol Chem. 1996;15:31–76. doi: 10.1002/etc.2050. [DOI] [PubMed] [Google Scholar]
- 42.Struthers J K, Jayachandran K, Moorman T B. Biodegradation of atrazine by Agrobacterium radiobacter J14a and use of this strain in bioremediation of contaminated soil. Appl Environ Microbiol. 1998;64:3368–3375. doi: 10.1128/aem.64.9.3368-3375.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Topp E, Gutzman D W, Bourgoin B, Millette J, Gamble D S. Rapid mineralization of the herbicide atrazine in alluvial sediments and enrichment cultures. Environ Tox Chem. 1995;14:743–747. [Google Scholar]
- 44.Topp E, Tessier L, Gregorich E G. Dairy manure incorporation stimulates rapid atrazine mineralization in an agricultural soil. Can J Soil Sci. 1996;76:403–409. [Google Scholar]
- 45.Topp E, Zhu H, Nour S M, Houot S, Lewis M, Cuppels D. Characterization of an atrazine-degrading Pseudaminobacter sp. isolated from Canadian and French agricultural soils. Appl Environ Microbiol. 2000;66:2773–2782. doi: 10.1128/aem.66.7.2773-2782.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanderheyden V, Debongnie P, Puissemier L. Accelerated degradation and mineralization of atrazine in surface and subsurface soil materials. Pestic Sci. 1997;49:237–242. [Google Scholar]
- 47.Versalovic J, Schneider M, de Bruijn F J, Lupski J R. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol Cell Biol. 1994;5:25–40. [Google Scholar]
- 48.Widmer S K, Spalding R F. A natural gradient transport study of selected herbicides. J Environ Qual. 1995;24:445–453. [Google Scholar]
- 49.Yanze-Kontchou C, Gschwind N. Mineralization of the herbicide atrazine as a carbon source by a Pseudomonas strain. Appl Environ Microbiol. 1994;60:4297–4302. doi: 10.1128/aem.60.12.4297-4302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]