Abstract
Introduction
The impact of COVID-19 infection on surgical patients is largely described by small-cohort studies. This study characterized the risk factors for postoperative mortality among patients with preoperative COVID-19 infection.
Methods
Data were abstracted from the electronic medical record for patients who tested positive for COVID-19 before surgery, excluding procedures related to extracorporeal membrane oxygenation (case, March 2020–April 2021). Mortality was compared with that for patients from the American College of Surgeons National Surgical Quality Improvement Program database (control, January 2018‒February 2020) with chi-square, t test, and multivariable regression.
Results
There were 5,209 patients in the control cohort. Among 1,072 patients with positive COVID-19 testing before surgery, 589 had surgeries with specialties tracked by the American College of Surgeons National Surgical Quality Improvement Program (General Surgery, Gynecology, Neurosurgery, Orthopedics, Thoracic, Vascular). Patients with previous COVID-19 infection were younger (age 48 vs 59 years, p<0.001), were more likely to be Black (42% vs 28%, p<0.001), and underwent fewer elective surgeries (55% vs 83%, p<0.001). Postoperative mortality was greater among the case cohort (4.4% vs 1%, p<0.001). On multivariable logistic regression, postoperative mortality increased with age (OR=1.02), emergent surgeries (OR=2.6), and previous COVID-19 infection (OR=3.8). Among patients with previous COVID-19 infection, postoperative mortality was associated with male sex (OR=2.7), higher American Society of Anesthesiologists Physical Status Classification Score (OR=4.8), and smoking history (OR=3.7).
Conclusions
Although data abstraction was limited by the electronic medical record, postoperative mortality is nearly 6 times higher for patients infected with COVID-19 within 2 weeks before surgery when adjusting for patient- and procedure-level factors. Among those with previous COVID-19 infection, postoperative mortality is associated with male sex, American Society of Anesthesiologists Physical Status Classification Score, and smoking history.
INTRODUCTION
Obesity, increasing age, and male sex have been identified as independent risk factors for susceptibility to and severity of coronavirus disease 2019 (COVID-19) infection. In a study of over 2,000 patients from over 150 hospitals in 13 states with confirmed COVID-19, multivariable analysis found that intensive care unit (ICU) admission was associated with increased age, male sex, obesity, immunosuppression, and diabetes. In-hospital mortality was associated with increased age, male sex, immunosuppression, and several other comorbidities.1 Other studies have similarly found increased age, obesity, diabetes, and hypertension to put patients with COVID-19 at increased risk for mortality.2, 3, 4, 5
However, the implications of patients’ clinical course after a previous infection with COVID-19 are unknown. As patients recover from viral infections, the expected course of community-acquired pneumonia, heart failure exacerbation, or any other nonviral disease process for an organ system previously affected by the COVID-19 virus is unknown. For surgical patients, this presents a difficult challenge of timing for elective procedures. Elective surgeries were delayed significantly during the beginning of the pandemic because hospital systems attempted to reduce patient exposure to healthcare settings.6 As operative volume resumes, it is difficult to appropriately inform patients about their risk of postoperative complications for those with previous COVID-19 infection.7 The question of whether patients with a history of COVID-19 infection should have their surgeries delayed and, if so, for how long remains largely unanswered.
Small-cohort studies have studied the risk of mortality for patients with a perioperative COVID-19 infection undergoing surgery. Early in the pandemic, a study by Lei et al.8 found that among 34 asymptomatic patients with COVID-19 who underwent elective surgery, there was a 20% postoperative death rate, 44% ICU admission rate, and 100% postoperative pneumonia rate. Another study around the same time by Nahshon and colleagues9 found that among 64 patients unexpectedly diagnosed with COVID-19, 51 of whom were diagnosed postoperatively, there was a 27.5% postoperative mortality rate. Several months later, Carrier et al.10 found that among 44 patients who had recovered from COVID-19 at the time of surgery, the mortality rate was 23% among the symptomatic and 5.6% among the asymptomatic. One quarter of patients had pulmonary complications, and altogether, there was a 16% 30-day mortality.10 Knisely and colleagues11 found that perioperative mortality rate was 17% in those who had a perioperative diagnosis of COVID-19, compared with 1.4% in those without COVID-19. Similarly, ICU admission rate was 36% among those with COVID-19 compared with 16.4% among the controls.
However, when adjusting for other surgical factors, the specific impact of preoperative COVID-19 infection on postoperative outcomes is not yet known. Therefore, this study aimed to characterize the risk factors for postoperative mortality among patients who had a COVID-19 infection before surgery. It was hypothesized that factors contributing to complications for medical patients with COVID-19, namely obesity, male sex, and increased age, would contribute to postoperative mortality for surgical patients with COVID-19 as well. In addition, it was hypothesized that having a history of a COVID-19 infection would independently increase the risk of postoperative mortality.
METHODS
Study Sample
The control group data were identified from the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database. ACS-NSQIP is a nationally validated, risk-adjusted, outcomes-based program to measure and improve the quality of surgical care. The case cohort consisted of patients with a COVID-19‒positive test before surgery (March 2020–April 2021). For patients with multiple surgeries, the first surgery during that cohort was used for analysis. The control cohort was comprised patients who had surgery any time during January 2018‒February 2020. The case group data were abstracted from the electronic medical record for patients who had a positive COVID-19 diagnosis at any time before surgery. This was determined by ICD-10 codes. Only surgeries with specialties tracked by the ACS-NSQIP were included. Patients whose primary procedure was extracorporeal membranous oxygenation were excluded.
Measures
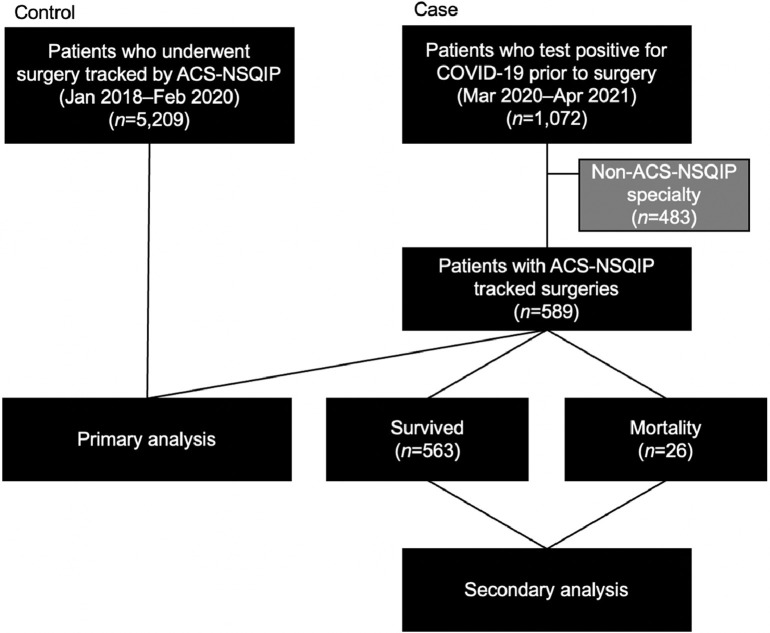
A primary analysis focused on determining the independent association of a previous COVID-19 infection with postoperative mortality, and a secondary analysis focused on determining the factors associated with postoperative mortality among patients previously infected with COVID-19. The case cohort is shown in Figure 1 . The primary analysis compared the factors associated with mortality between the case and control cohorts. The primary outcome of mortality was compared by patient-level (history of positive COVID-19 test, demographics, BMI, history of smoking within the last year) and procedure-level (surgical service, emergent versus elective, American Society of Anesthesiologists [ASA] Physical Status Classification Score, case duration, wound classification) characteristics using chi-square and 2-sample t tests. Multivariable logistic regression was performed to evaluate adjusted associations with mortality. Within the case cohort, a secondary analysis was performed to determine the factors associated with postoperative mortality among patients previously infected with COVID-19.
Figure 1.
CONSORT diagram.
ACS-NSQIP, American College of Surgeons National Surgical Quality Improvement Program; Apr, April; Feb, February; Jan, January; Mar, March.
Statistical Analysis
The primary outcome of mortality was compared by patient-level (days from positive COVID-19 test to surgery, demographics, BMI, history of smoking within the last year) and procedure-level (surgical service, emergent versus elective, ASA, case duration, wound classification) covariates using chi-square and 2-sample t tests. A priori power calculations were performed to ensure a sufficient sample size to detect significance. As with the case‒control cohort, multivariable logistic regression was performed to identify adjusted associations with mortality. All analysis was done in R Studio,12 with p<0.05 considered significant. This study was approved by the University of Alabama at Birmingham IRB.
RESULTS
There were 5,209 patients in the control cohort and 1,072 patients who had a positive COVID-19 test before surgery. A total of 483 patients who underwent non‒ACS-NSQIP surgeries were excluded, resulting in 589 patients in the case cohort. A total of 14 patients were included who had surgery during both the control period and the case period. Estimating a small effect size at 0.2,13 the sample size the case population required to reach 80% power is 204, which this study exceeds.
Primary Analysis
Overall, the case and control cohorts in aggregate were 39% male and 30% Black, with a mean age of 58 years (SD=15 years). Most patients had no history of smoking (78%). The mean BMI was 30 (SD=7.9). Most surgeries were with General Surgery (44%) and ASA 3 (73%). Most surgeries were elective (80%) and either clean (44%) or clean/contaminated (46%). On average, cases were 2.5 hours long. Overall postoperative mortality was 1.4%.
Patients with a previous COVID-19 infection were younger (aged 48 vs 59 years, p<0.001), were more likely to be Black (42% vs 28%, p<0.001), had higher BMI (31 vs 30, p=0.02), and were more likely to have a history of smoking (46% vs 19%, p<0.001) than the control cohort (Table 1 ). Although there were differences in ASA, there were no statistically significant differences in mean ASA between the case and control groups (2.86 vs 2.87, p=0.84). Patients undergoing surgery with a previous COVID-19 infection were less likely to be undergoing elective surgery (55% vs 83%, p<0.001). Surgeries were also more likely to be contaminated (8% vs 4%) or dirty/infected (11% vs 5%, p<0.001) in the case cohort than in the control group. Surgeries in the case cohort were shorter, lasting on average an hour shorter than surgeries in the control cohort (1.6 vs 2.6 hours, p<0.001). Surgeries were more often with Orthopedics (30% vs 18%), Gynecology (20% vs 13%), and Vascular (10% vs 7%, p<0.001) and less often with General Surgery (23% vs 47%). Postoperative mortality was greater among the case cohort (4.4% vs 1%, p<0.001).
Table 1.
Characteristics of the Case and Control Cohorts
| Factors | Control (January 2018‒February 2020) (n=5,209) | Case (March 2020‒April 2021) (n=589) | Overall (N=5,798) | p-value |
|---|---|---|---|---|
| Age, years | ||||
| Mean (SD) | 58.6 (14.6) | 48.2 (17.8) | 57.5 (15.3) | <0.001 |
| Median (min, max) | 60.4 (18.0, 101) | 48.3 (14.1, 89.2) | 59.6 (14.1, 101) | |
| Race, n (%) | ||||
| White | 3,627 (69.6) | 292 (49.6) | 3,919 (67.6) | <0.001 |
| Black | 1,478 (28.4) | 247 (41.9) | 1,725 (29.8) | |
| Asian | 52 (1.0) | 12 (2.0) | 64 (1.1) | |
| Other | 52 (1.0) | 38 (6.5) | 90 (1.6) | |
| Sex, n (%) | ||||
| Female | 3,165 (60.8) | 361 (61.3) | 3,526 (60.8) | 0.837 |
| Male | 2,044 (39.2) | 228 (38.7) | 2,272 (39.2) | |
| BMI | ||||
| Mean (SD) | 30.3 (7.74) | 31.2 (8.89) | 30.4 (7.87) | 0.0184 |
| Median (min, max) | 29.2 (13.7, 90.9) | 29.4 (14.7, 69.8) | 29.2 (13.7, 90.9) | |
| Missing, n (%) | 19 (0.4) | 0 (0) | 19 (0.3) | |
| History of smoking, n (%) | ||||
| None | 4,215 (80.9) | 317 (53.8) | 4,532 (78.2) | <0.001 |
| Within the past year | 994 (19.1) | 272 (46.2) | 1,266 (21.8) | |
| ASA, n (%) | ||||
| 1 | 52 (1.0) | 16 (2.7) | 68 (1.2) | <0.001 |
| 2 | 925 (17.8) | 127 (21.6) | 1,052 (18.1) | |
| 3 | 3,883 (74.5) | 368 (62.5) | 4,251 (73.3) | |
| 4 | 343 (6.6) | 74 (12.6) | 417 (7.2) | |
| 5 | 6 (0.1) | 2 (0.3) | 8 (0.1) | |
| Missing | 0 (0) | 2 (0.3) | 2 (0.0) | |
| Case type, n (%) | ||||
| Emergent | 4,296 (82.5) | 328 (55.7) | 4,624 (79.8) | <0.001 |
| Elective | 913 (17.5) | 261 (44.3) | 1,174 (20.2) | |
| Wound classification, n (%) | ||||
| Clean | 2,290 (44.0) | 286 (48.6) | 2,576 (44.4) | <0.001 |
| Clean/contaminated | 2,450 (47.0) | 197 (33.4) | 2,647 (45.7) | |
| Contaminated | 191 (3.7) | 45 (7.6) | 236 (4.1) | |
| Dirty/infected | 278 (5.3) | 61 (10.4) | 339 (5.8) | |
| Case duration, hours | ||||
| Mean (SD) | 2.61 (1.65) | 1.62 (1.32) | 2.51 (1.65) | <0.001 |
| Median (min, max) | 2.18 (0.200, 13.8) | 1.30 (0.0333, 8.90) | 2.08 (0.0333, 13.8) | |
| Specialty, n (%) | ||||
| General surgery | 2,429 (46.6) | 136 (23.1) | 2,565 (44.2) | <0.001 |
| Orthopedics | 910 (17.5) | 179 (30.4) | 1,089 (18.8) | |
| Gynecology | 657 (12.6) | 120 (20.4) | 777 (13.4) | |
| Neurosurgery | 447 (8.6) | 42 (7.1) | 489 (8.4) | |
| Vascular | 363 (7.0) | 61 (10.4) | 424 (7.3) | |
| Thoracic | 386 (7.4) | 11 (1.9) | 397 (6.8) | |
| Plastics | 17 (0.3) | 40 (6.8) | 57 (1.0) | |
| Mortality, n (%) | ||||
| Survived | 5,155 (99.0) | 563 (95.6) | 5,718 (98.6) | <0.001 |
| Mortality | 54 (1.0) | 26 (4.4) | 80 (1.4) |
Note: Boldface indicates statistical significance (p<0.05).
ASA, American Society of Anesthesiologists Physical Status Classification Score; max, maximum; min, minimum.
On logistic regression (Figure 2 ), Asian patients were grouped into Other owing to low COVID-19 positivity. Postoperative mortality increased with age (OR=1.02, 95% CI=1.0, 1.03), greater ASA (OR=3.88, 95% CI=2.47, 6.09), dirty/contaminated surgeries (OR=4.08, 95% CI=1.93, 8.61), emergent surgeries (OR=2.62, 95% CI=1.49, 4.59), and previous COVID-19 infection (OR=3.83, 95% CI=2.07, 7.11). When isolating elective surgeries only, age (OR=1.03, 95% CI=1.0, 1.07), greater ASA (OR=2.79, 95% CI=1.19, 6.56), dirty/contaminated surgeries (OR=7.29, 95% CI=1.8, 29.52), increased case duration (OR=1.22, 95% CI=1.02, 1.46), and previous COVID-19 infection (OR=3.25, 95% CI=1.03, 10.3) were associated with mortality. Among emergent surgeries, greater ASA (OR=4.2, 95% CI=2.43, 7.26) and history of previous COVID-19 infection (OR=3.93, 95% CI=1.75, 8.84) were associated with mortality, similar to the findings among elective surgeries. When including only patients who underwent surgery <14 days from the day of COVID-19 diagnosis, the OR of mortality associated with a previous infection increased to 5.7 (95% CI=2.59, 12.58).
Figure 2.
Factors associated with postoperative mortality.
Note: *p<0.05, **p<0.01, and ***p<0.001.
Secondary Analysis
Overall, there were 587 patients who were diagnosed with COVID-19 before undergoing surgery whose surgeries were done by specialties tracked by ACS-NSQIP. The population was 40% male and 42% Black, with a mean age of 48 years (SD=18 years). The mean BMI was 31 (SD=8.9). Patients tested positive 0‒298 days before surgery (median=20 days, mean=48.5 days) (Table 2 ). Most procedures were with orthopedics (30%) and general surgery (23.5%). Most surgeries were elective (54%). Overall postoperative mortality was 4.5%.
Table 2.
Characteristics of Patients Undergoing Surgery After a COVID-19 Infection
| Factors | Survived (n=563) | Mortality (n=26) | Overall (N=589) | p-value |
|---|---|---|---|---|
| Age, years | ||||
| Mean (SD) | 48.0 (17.8) | 52.3 (18.8) | 48.2 (17.8) | 0.264 |
| Median (min, max) | 47.8 (14.1, 89.2) | 50.0 (20.0, 81.9) | 48.3 (14.1, 89.2) | |
| Race, n (%) | ||||
| White | 274 (48.7) | 18 (69.2) | 292 (49.6) | 0.155 |
| Black | 239 (42.5) | 8 (30.8) | 247 (41.9) | |
| Asian | 12 (2.1) | 0 (0) | 12 (2.0) | |
| Other | 38 (6.7) | 0 (0) | 38 (6.5) | |
| Sex, n (%) | ||||
| Female | 351 (62.3) | 10 (38.5) | 361 (61.3) | 0.0252 |
| Male | 212 (37.7) | 16 (61.5) | 228 (38.7) | |
| BMI | ||||
| Mean (SD) | 31.1 (8.80) | 33.4 (10.5) | 31.2 (8.89) | 0.29 |
| Median (min, max) | 29.3 (14.7, 69.8) | 30.4 (20.2, 62.1) | 29.4 (14.7, 69.8) | |
| History of smoking, n (%) | ||||
| None | 308 (54.7) | 9 (34.6) | 317 (53.8) | 0.0706 |
| Within the past year | 255 (45.3) | 17 (65.4) | 272 (46.2) | |
| ASA, n (%) | ||||
| 1 | 16 (2.8) | 0 (0) | 16 (2.7) | <0.001 |
| 2 | 127 (22.6) | 0 (0) | 127 (21.6) | |
| 3 | 355 (63.1) | 13 (50.0) | 368 (62.5) | |
| 4 | 62 (11.0) | 12 (46.2) | 74 (12.6) | |
| 5 | 1 (0.2) | 1 (3.8) | 2 (0.3) | |
| Missing | 2 (0.4) | 0 (0) | 2 (0.3) | |
| Case type, n (%) | ||||
| Elective | 323 (57.4) | 5 (19.2) | 328 (55.7) | <0.001 |
| Emergent | 240 (42.6) | 21 (80.8) | 261 (44.3) | |
| Wound classification, n (%) | ||||
| 1 | 279 (49.6) | 7 (26.9) | 286 (48.6) | 0.00843 |
| 2 | 189 (33.6) | 8 (30.8) | 197 (33.4) | |
| 3 | 40 (7.1) | 5 (19.2) | 45 (7.6) | |
| 4 | 55 (9.8) | 6 (23.1) | 61 (10.4) | |
| Case duration | ||||
| Mean (SD) | 1.64 (1.34) | 1.23 (0.792) | 1.62 (1.32) | 0.0187 |
| Median (min, max) | 1.32 (0.0333, 8.90) | 0.992 (0.483, 3.28) | 1.30 (0.0333, 8.90) | |
| Specialty, n (%) | ||||
| General surgery | 126 (22.4) | 10 (38.5) | 136 (23.1) | 0.068 |
| Orthopedics | 175 (31.1) | 4 (15.4) | 179 (30.4) | |
| Gynecology | 117 (20.8) | 3 (11.5) | 120 (20.4) | |
| Vascular | 59 (10.5) | 2 (7.7) | 61 (10.4) | |
| Neurosurgery | 39 (6.9) | 3 (11.5) | 42 (7.1) | |
| Plastics | 38 (6.7) | 2 (7.7) | 40 (6.8) | |
| Thoracic | 9 (1.6) | 2 (7.7) | 11 (1.9) | |
| TTS | ||||
| Mean (SD) | 51.0 (65.6) | 39.5 (62.2) | 50.5 (65.5) | 0.365 |
| Median (min, max) | 22.0 (0, 298) | 10.0 (0, 198) | 21.0 (0, 298) |
Note: Boldface indicates statistical significance (p<0.05).
ASA, American Society of Anesthesiologists Physical Status Classification Score; max, maximum; min, minimum; TTS, time to surgery.
Among patients with a previous COVID-19 infection (Table 2), those who died postoperatively were more male (62% vs 38%, p=0.02), had greater ASA (3.5 vs 2.8, p<0.001), underwent emergent surgery (81% vs 43%, p<0.001), had contaminated (19% vs 7%) or dirty/infected (23% vs 10%, p=0.008) wound classification, and had shorter case duration (1.2 vs 1.6 hours, p=0.02). Mortality varied by specialty (Thoracic 18%, Neurosurgery 7%, General Surgery 7%, Plastics 5%, Vascular 3%, Gynecology 3%, orthopedics 2%, p=0.07). Patients who tested positive for COVID-19 >14 days from surgery had a lower mortality (2.9%) than those who tested positive within the 2 weeks before surgery (6.5%, p=0.05).
On logistic regression (Appendix Figure 1, available online), patients with a race listed as Asian or Other were excluded owing to zero mortality (n=50). Among the resulting 537 cases, postoperative mortality was lower for Black patients (OR=0.33, 95% CI=0.11, 0.98) and among surgeries with longer case duration (OR=0.61, 95% CI=0.38, 1). Postoperative mortality was higher among male patients (OR=2.7, 95% CI=0.94, 7.87), patients with higher ASA (OR=4.81, 95% CI=2.02, 11.48), and patients who had smoked within the past year (OR=3.7, 95% CI=1.27, 10.8).
DISCUSSION
Postoperative mortality is almost 6 times higher for patients infected with COVID-19 within the 2 weeks before surgery when adjusting for patient- and procedure-level factors. Among those with previous COVID-19 infection, the risk of postoperative mortality is associated with male sex, higher ASA, and a history of smoking within the past year.
This is the first, large volume analysis comparing the risk factors associated with postoperative mortality that comprehensively accounts for confounding factors. At the start of the pandemic, surgical volume was restricted to emergent cases only, which included patients who tested positive for COVID-19 at the time of surgery. Small-cohort studies that focused on patients who tested positive for COVID-19 were also likely, including a cohort with worse clinical presentation and burden of comorbidities at the time of surgery owing to the nature of who was receiving surgeries at the time. Therefore, small-cohort studies likely included significant confounding.
In this study, comparing patients who were previously diagnosed with COVID-19 and underwent surgery with those who underwent surgery before the COVID-19 pandemic and represent a single institution's baseline outcomes helped to quantify the increased risk associated with a preoperative COVID-19 diagnosis. Comorbidities associated with worse medical and postoperative outcomes for those with COVID-19 and for those without are very similar: namely increased age, increased BMI, and medical comorbidities. These factors were included to establish the surgical risks among a demographic that is increasingly prevalent: those previously diagnosed with COVID-19.
Notably, there were more Black patients in the case cohort (42%) than the state demographics (26%) and certainly in studies in established literature (12%–15%),11 many of which do not include race.9 , 14 There are significant disparities between those typically cared for (28.3% Black, control cohort) and those operated on who had been previously infected with COVID-19 (42% Black). This suggests an increased risk of acquiring COVID-19 among Black populations, likely owing to structural socioecologic inequities, with less compelling association with postoperative outcomes. The protective association seen on the multivariable logistic regression is likely because of the high prevalence of COVID-19 among the Black population. Additional research is needed to better understand and eliminate these disparities.
Although time to surgery (TTS) was not a significant contributory factor to postoperative mortality, there was a trend toward improved mortality with increased TTS, as shown in Appendix Figure 2 (available online). Among those undergoing elective surgery, in which the TTS is able to be controlled, increasing the duration from COVID-19 infection to operative intervention may improve postoperative outcomes. Particularly for patients with significant comorbidities or a history of smoking, who are independently at risk of increased postoperative mortality after COVID-19 infection, delaying surgery may provide the patient with survival benefits. Additional analysis will allow for optimized surgical timing to reduce the complications associated with previous COVID-19 infection while balancing the harms of delaying surgical care.
Limitations
There were several limitations to this study. First, this is a retrospective cohort study only capturing perioperative data for surgeries documented with the ACS-NSQIP database as well as in the electronic medical record. Data captured are prone to human error. Whereas the ACS-NSQIP database is nationally validated and collected by trained clinical reviewers, data from the patients’ medical charts are prone to errors in documentation. In addition, testing positive for COVID-19 was determined by ICD-10 coding, which could similarly be prone to error as well as falsely represent a new infection when there may be instances of convalescence or false positives. Third, patients who have previously tested positive at another facility or who had tested positive without requiring hospital admission were not included in the study cohort. In addition, outcomes were based on the patient's first surgical encounter during the cohort period. Patients with multiple surgeries may have had higher acuity or more complex procedures done later in the period that was not included. However, the inclusion of the first surgery is most representative of the relationship between testing positive for COVID-19 and surgical outcomes. Finally, this analysis is a preliminary study of the factors associated with mortality. Future studies will include additional data on comorbidities and other postoperative outcomes such as venous thromboembolism, postoperative length of stay, and 30-day readmissions.
CONCLUSIONS
Previous infection with COVID-19 is independently associated with an almost fourfold increase in postoperative mortality when adjusting for patient- and procedure-level factors. For patients who underwent surgery within 2 weeks of contracting COVID-19, the risk increased almost sixfold. Among those with previous COVID-19 infection, the risk of postoperative mortality is predicted by male sex, higher ASA, and a history of smoking within the past year. There may be utility in increasing the time from COVID-19 diagnosis to surgery to reduce the risk of complications.
CRediT authorship contribution statement
Connie C. Shao: Funding acquisition, Formal analysis, Conceptualization, Writing – original draft. M. Chandler McLeod: Supervision, Conceptualization. Suneetha Thogaripally: Data curation. Michael J. Mugavero: Data curation. Lauren T. Gleason: Writing – review & editing. Isabel C. Dos Santos Marques: Writing – review & editing. Daniel I. Chu: . Drew J. Gunnells: Conceptualization.
ACKNOWLEDGMENTS
This study was approved by the University of Alabama at Birmingham IRB under protocol number IRB-300005755.
This work was supported by a grant from the National Institute on Minority Health and Health Disparities (U54MD000502).
No financial disclosures were reported by the authors of this paper.
Footnotes
This article is part of a supplement entitled Obesity-Related Health Disparities: Addressing the Complex Contributors, sponsored by the National Institute on Minority Health and Health Disparities (NIMHD), part of the National Institutes of Health (NIH), an agency of the U.S. Department of Health and Human Services (HHS).
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2022.01.035.
SUPPLEMENT NOTE
This article is part of a supplement entitled Obesity-Related Health Disparities: Addressing the Complex Contributors, which is sponsored by the National Institute on Minority Health and Health Disparities (NIMHD), National Institutes of Health (NIH), U.S. Department of Health and Human Services (HHS). The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of NIMHD, NIH, or HHS.
Appendix. SUPPLEMENTAL MATERIAL
REFERENCES
- 1.Kim L, Garg S, O'Halloran A, et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the U.S. Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET) Clin Infect Dis. 2021;72(9):e206–e214. doi: 10.1093/cid/ciaa1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolff D, Nee S, Hickey NS, Marschollek M. Risk factors for Covid-19 severity and fatality: a structured literature review. Infection. 2021;49(1):15–28. doi: 10.1007/s15010-020-01509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverio A, Di Maio M, Citro R, et al. Cardiovascular risk factors and mortality in hospitalized patients with COVID-19: systematic review and meta-analysis of 45 studies and 18,300 patients. BMC Cardiovasc Disord. 2021;21(1):23. doi: 10.1186/s12872-020-01816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peña JE, Rascón-Pacheco RA, Ascencio-Montiel IJ, et al. Hypertension, diabetes and obesity, major risk factors for death in patients with COVID-19 in Mexico. Arch Med Res. 2021;52(4):443–449. doi: 10.1016/j.arcmed.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu W, Rohli KE, Yang S, Jia P. Impact of obesity on COVID-19 patients. J Diabetes Complications. 2021;35(3) doi: 10.1016/j.jdiacomp.2020.107817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shao C. The COVID trolley dilemma. Am J Surg. 2020;220(3):545–549. doi: 10.1016/j.amjsurg.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shao C, Broman K. Association for Academic Surgery; Los Angeles, CA: 2021. Informed consent during the COVID-19 pandemic: addressing known unknowns.https://www.aasurg.org/blog/informed-consent-during-the-covid-19-pandemic-addressing-known-unknowns/ Published June 22Accessed March 1, 2022. [Google Scholar]
- 8.Lei S, Jiang F, Su W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;21 doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nahshon C, Bitterman A, Haddad R, Hazzan D, Lavie O. Hazardous postoperative outcomes of unexpected COVID-19 infected patients: a call for global consideration of sampling all asymptomatic patients before surgical treatment. World J Surg. 2020;44(8):2477–2481. doi: 10.1007/s00268-020-05575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrier FM, Amzallag É, Lecluyse V, et al. Postoperative outcomes in surgical COVID-19 patients: a multicenter cohort study. BMC Anesthesiol. 2021;21(1):15. doi: 10.1186/s12871-021-01233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knisely A, Zhou ZN, Wu J, et al. Perioperative morbidity and mortality of patients with COVID-19 who undergo urgent and emergent surgical procedures. Ann Surg. 2021;273(1):34–40. doi: 10.1097/SLA.0000000000004420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.R Core Team; Vienna, Austria: 2020. R: A Language and Environment for Statistical Computing [Computer Program] [Google Scholar]
- 13.Cohen J. 2nd ed. L. Erlbaum Associates; Hillsdale, NJ: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 14.Collaborative COVIDSurg. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study [published correction appears in Lancet. 2020;396(10246):238] Lancet. 2020;396(10243):27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.