SUMMARY
SETTING :
All 11 tuberculosis (TB) diagnostic and treatment units in Kyankwanzi and Kiboga Districts in Uganda.
OBJECTIVES:
To determine the frequency of, factors associated with and barriers related to incomplete anti-tuberculosis treatment sputum monitoring.
DESIGN:
Data were abstracted from anti-tuberculosis treatment and laboratory registers of sputum smear-positive patients who started treatment between January 2009 and December 2011 in the study districts. Patients missing documentation for any smear results at 2 or 3, 5, and 6 or 8 months were classified as having incomplete monitoring. Health providers and patients were interviewed about barriers to sputum monitoring.
RESULTS:
Overall, 272 (55%) of 492 patients had incomplete monitoring: 16% (78/492) at 2 or 3 months, 39% (181/465) at 5 months and 28% (119/428) at 6 or 8 months of treatment. More sputum results were recorded in laboratory than in TB treatment registers. Incomplete monitoring was significantly associated with being male, living in Kyankwanzi District and not receiving directly observed treatment. Patients’ inability to produce sputum, long laboratory waiting times, and insufficient patient and provider education were primary reasons for incomplete monitoring.
CONCLUSION:
Over half of patients missed at least one smear result during treatment, which has implications for treatment monitoring and treatment outcomes in Uganda.
Keywords: tuberculosis, monitoring, sputum smear, Uganda
RESUME
CONTEXTE :
Toutes les 11 unités de diagnostic et de traitement de la tuberculose (TB) des districts de Kyankwanzi et Kiboga en Ouganda.
OBJECTIFS :
Déterminer la fréquence de suivi incomplet des frottis de crachats au cours du traitement de la TB ainsi que les facteurs et les obstacles qui y sont associés.
SCHEMA :
Les données ont été extraites des registres de traitement de la TB et de laboratoire pour les patients à frottis de crachats positifs qui ont débuté leur traitement entre janvier 2009 et décembre 2011 dans les districts de l’ étude. Les patients n’ayant pas de documents de résultats de frottis de crachats aux 2ème ou 3ème, 5ème, et 6ème ou 8ème mois ont été considérés comme ayant un suivi incomplet. Les prestataires de soins et les patients ont été interviewés à propos des obstacles au suivi des frottis de crachats.
RÉSULTATS :
Dans l’ensemble, 272 (55%) des 492 patients ont eu un suivi incomplet : 16% (78/492) à 2 ou 3 mois, 39% (181/465) à 5 mois et 28% (119/428) à 6 ou 8 mois de traitement. Il y a eu davantage de résultats de frottis de crachats enregistrés au laboratoire que dans les registres de traitement de TB. Un suivi incomplet a été significativement associé avec le sexe masculin, le district de Kyankwanzi et le fait de ne pas recevoir de traitement directement observé. L’incapacité des patients à produire des crachats, un long temps d’attente au laboratoire et une éducation insuffisante du patient et du prestataire de soins ont été les premières raisons d’un suivi incomplet.
CONCLUSION :
Il manquait au moins un résultat de frottis en cours de traitement chez plus de la moitié des patients, ce qui a des implications en termes de suivi du traitement et de résultat final du traitement en Ouganda.
RESUMEN
MARCO DE REFERENCIA:
Las 11 unidades que ofrecen servicios de diagnόstico y tratamiento de la tuberculosis (TB) en los distritos de Kyankwanzi y Kiboga de Uganda.
OBJETIVOS:
Determinar la frecuencia de una supervisiόn incompleta del esputo durante el tratamiento antituberculoso, las características que se asocian con esta deficiencia y los obstáculos que la ocasionan.
MÉTODOS:
Se obtuvieron los datos de los registros de tratamiento y los registros de laboratorio de los pacientes con TB y baciloscopia positiva que comenzaron tratamiento de enero del 2009 a diciembre del 2011 en los distritos estudiados. La supervisiόn se clasificό como incompleta en los pacientes que carecían de algún resultado de baciloscopia del segundo y el tercer, el quinto, y el sexto u octavo mes. Se entrevistaron los profesionales de salud y los pacientes sobre los obstáculos a la supervisiόn de la baciloscopia del esputo.
RESULTADOS:
En general, 272 de los 492 pacientes presentaron una supervisiόn incompleta (55%), en el 16% faltaron baciloscopias del esputo al segundo o tercer mes (78/492), en el 39% al quinto mes (181/465) y en el 28% al sexto u octavo mes de tratamiento (119/428). Se observaron más informes de resultados de la baciloscopia en los registros de laboratorio que en los registros de tratamiento antituberculoso. La supervisión incompleta se asociό de manera significativa con el sexo masculino, el distrito de Kyankwanzi y la falta de observaciόn directa. Entre las principales razones referidas de la deficiencia se encontraron la incapacidad de producir muestras de esputo, la espera prolongada en el laboratorio y la educaciόn insuficiente de los pacientes y los trabajadores de salud.
CONCLUSIÓN:
En más de la mitad de los pacientes faltό como mínimo un resultado de baciloscopia durante el tratamiento, lo cual tiene consecuencias en la supervisiόn del tratamiento y los desenlaces terapéuticos de Uganda.
GLOBALLY, THERE WERE AN ESTIMATED 8.6 million incident tuberculosis (TB) cases and 1.3 million deaths due to TB annually in 2012;1 26% of these were in sub-Saharan Africa.1 Appropriate treatment of TB is challenging as it requires multiple drugs administered for at least 6–8 months.2
The World Health Organization (WHO) recommends the use of sputum smear microscopy to evaluate the response of TB disease to treatment and to monitor treatment outcomes.3 In smear-positive pulmonary TB (PTB) patients, it is recommended that sputum be monitored at months 2 or 3 at the completion of the intensive phase, at month 5, and at treatment completion (months 6 or 8),4 to inform treatment decisions.3,4 TB patients who remain sputum smear-positive at the end of the intensive phase should submit another specimen for culture and drug susceptibility testing (DST).3,4 A positive sputum result at any stage during treatment may indicate drug-resistant Mycobacterium tuberculosis that is not responding to first-line treatment and would require a change in treatment regimen.4
Uganda ranks among the world’s 22 high TB burden countries.1 Despite the high TB rates, the Uganda National TB and Leprosy Programme (NTLP) reported a low cure rate (34%) among newly registered smear-positive PTB patients in 2011.5 This low rate may be related to poor sputum smear monitoring, as a final sputum result is needed to designate a patient as ‘cured’; previous studies from India, Malawi and Rwanda have found that 62–78% of TB patients had incomplete smear monitoring, which impacted the cure rate.6–8 The extent of and factors that influence sputum smear monitoring during anti-tuberculosis treatment are unknown in Uganda.
To help address this gap, the present study was conducted to assess monitoring of TB patients by smear microscopy in two districts in Uganda. This study aimed to determine the frequency of, factors associated with and barriers related to incomplete sputum monitoring among smear-positive PTB patients. Specifically, the study sought to 1) document the proportion of registered smear-positive PTB patients who did not have the recommended number of smear microscopy examinations at recommended intervals during treatment, 2) compare the documentation of smear results recorded in TB treatment and laboratory registers, 3) describe the characteristics associated with incomplete smear microscopy monitoring during treatment, and 4) explore barriers to complete smear microscopy monitoring among smear-positive PTB patients.
METHODS
Study setting
The study was conducted in two rural districts of Kiboga and Kyankwanzi in Central Uganda. All 11 TB diagnostic and treatment units (DTUs) in the two districts (eight subcounty-level health centres [HC], two county-level HCs and one district hospital) were included. The two districts have relatively low cure rates—63% in Kiboga and 52% in Kyankwanzi—compared to the national target of 85%.4 A brief review of programmatic data in these two districts showed that a substantial proportion of smears were not performed at the end of the intensive phase of treatment (35% in 2009 and 42% in 2010), similar to other districts in Uganda.
Directly observed treatment (DOT) was provided at the facility by health workers or at home by volunteer treatment supporters (TSs) who are volunteers selected for the patient by the community. The TSs observe the patient take the medication daily and record this in the TB card, which is kept by the patient. Sub-County Health Workers (SCHWs) are responsible for fortnightly supervision visits and for supplying the TB drugs to the TSs. To ensure sputum monitoring is completed at 2 or 3, 5 and 6 or 8 months of anti-tuberculosis treatment, SCHWs must collect and transport sputum specimens to the DTUs, update the results in the SCHW register and patient TB cards and inform the patient of the results and actions to take.
During the period of observation, the 8-month regimens of first-line treatment for new and retreatment TB consisted of respectively 2HRZE/6HE and 2HRZES/1HRZE/5HRE.*4
Data collection
A retrospective review of programmatic TB records was conducted during August–October 2013 using a pre-tested standardised data abstraction tool to capture demographic, clinical and laboratory information (including completeness of smear testing) from treatment and laboratory TB registers for all registered smear-positive PTB patients who started treatment between 1 January 2009 and 31 December 2011. We also compared the number of sputum smears documented in the TB treatment and laboratory registers. A sputum smear was defined as not having been performed if the results were not documented in the TB treatment register.
To explore barriers to complete smear microscopy monitoring, interviews were conducted with TB patients and service providers using pre-tested semi-structured questionnaires. A convenience sample of TB patients (approximately 10% of all TB patients) who were smear-positive at diagnosis and had been on anti-tuberculosis treatment for ≥2 months were invited to participate in exit interviews. Health care providers, such as medical and clinical officers, nurses and nursing assistants, SCHWs and laboratory staff providing TB services at the study facilities, were purposively recruited for interviews. All departments offering TB services were considered. Three providers from each of the eight sub-county-level HCs, five from county-level HCs and 10 from the district hospital were recruited. Interviews were conducted to collect information from patients and providers on their knowledge about sputum monitoring, availability of laboratory equipment and reagents, and patient access to the facility. Providers were also interviewed about their availability, attitudes towards sputum collection, and experience with recording and reporting. Information from interviews was intended to provide a context for the quantitative data.
Definitions
Patients who were smear-positive at the start of anti-tuberculosis treatment were classified as having incomplete sputum smear microscopy monitoring if they did not complete any one of the recommended sputum microscopy examinations during treatment at months 2 or 3, 5, and 6 or 8, as evidenced by lack of documentation of smear results in the TB treatment register during those months. Other variables collected from registers included age, sex, facility type, district, type of patient (new and retreatment), human immunodeficiency virus (HIV) status, antiretroviral treatment (ART), DOTstatus, smear result at follow-up and treatment outcome. DOT status at initiation of treatment was based on documentation in the TB treatment registers.4 Patients’ outcomes were classified according to WHO definitions.5 Successful treatment outcomes included cured and treatment completed, while unsuccessful outcomes included failure, death and default. Patients who transferred out of the facility were excluded.
Data analysis
Data were managed with EpiData version 3.1 (EpiData Association, Odense, Denmark) and Epi Info version 6 (Centers for Disease Control and Prevention [CDC], Atlanta, GA, USA), and analysed using Epi Info and SAS version 9.3 (Statistical Analysis System, Cary, NC, USA) software. The proportion of registered smear-positive PTB patients who did not undergo the recommended number of smear microscopy examinations during treatment was calculated at months 2 or 3, 5, and 6 or 8. The percentage difference in documented smears for patients in the TB treatment and laboratory registers was calculated by subtracting the totals in the TB treatment and laboratory registers and dividing by the total number in the TB treatment registers at months 2 or 3, 5, and 6 or 8. The associations between patient characteristics and incomplete sputum smear microscopy monitoring were analysed using odds ratios (ORs) and 95% confidence intervals (CIs). Fisher’s exact test was used for comparisons when cell counts were fewer than five cases. Statistical significance was defined as P ˂ 0.05. Factors were entered into logistic regression models based on biological plausibility, previous literature and statistical significance in bivariate analysis (P ˂ 0.10) to determine variables associated with incomplete smear microscopy monitoring. The Hosmer-Lemeshow test was used to evaluate goodness-of-fit, and the likelihood ratio test was used to select the best fitting model. Data from interviews were tabulated and descriptive statistics were used to calculate frequencies and proportions. Qualitative data were recorded, sorted and coded into themes. The content was analysed to supplement quantitative data.
Ethics issues
Ethical approval was obtained from the Uganda Virus Research Institute, Entebbe, and the Ethics Committee of the Uganda National Council for Science and Technology, Kampala, Uganda. Additional review by the CDC institutional review board was not required, as CDC investigators were determined not to be engaged in human subjects research. Informed consent was provided by each respondent prior to interview.
RESULTS
Sputum smear monitoring during treatment
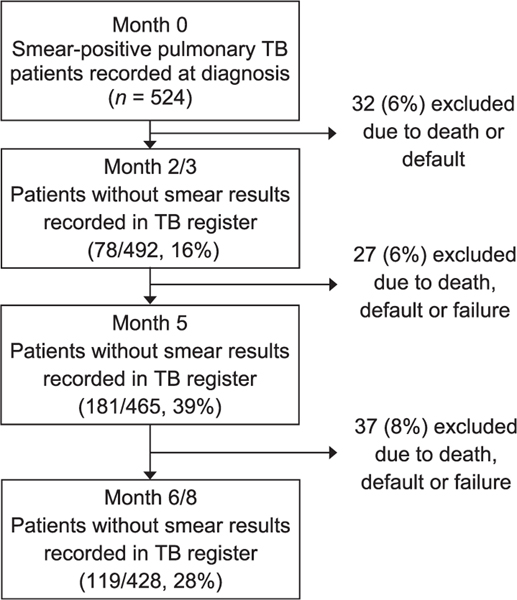
Of the 524 smear-positive TB patients registered for anti-tuberculosis treatment in the 11 study sites during 2009–2011, 32 died or defaulted before the end of the intensive phase, leaving 492 still on treatment at the end of 2 or 3 months of therapy (Figure). After excluding those patients who had died, defaulted from or failed treatment, there were 465 patients still on treatment at 5 months and 428 still on treatment at 6 or 8 months. Overall, 55% (272/492) of registered patients with at least 2 or 3 months of treatment had incomplete sputum smear microscopy during treatment. Of these, 16% (78/492) were at month 2 or 3, 39% (181/465) at month 5 and 28% (119/428) at months 6 or 8.
Figure.
Number and proportion of smear-positive TB patients with incomplete sputum smear microscopy monitoring during anti-tuberculosis treatment, Kiboga and Kyankwanzi Districts, Uganda, 2009–2011. TB = tuberculosis.
Sputum results were more often recorded in laboratory registers than in TB treatment registers, with increasing discrepancy during months 5 (293 vs. 284) and 6 or 8 (339 vs. 309) of treatment, with differences of respectively 3.2% and 9.7% (Table 1). Seventeen smears were performed during months outside the recommended follow-up schedule for smear monitoring.
Table 1.
Comparison of smear microscopy results in laboratory and TB treatment registers among smear-positive pulmonary TB patients during follow-up*
| Smear results documented in registers during treatment, n | ||||
|---|---|---|---|---|
|
| ||||
| Diagnosis | Month 2 or 3 | Month 5 | Month 6 or 8 | |
| Smears recorded in laboratory register, n | — | 413 | 293 | 339 |
| Smears recorded in TB unit register, n | 524 | 414 | 284 | 309 |
| Difference, %† | — | 0.2 | 3.2 | 9.7 |
Three sputum follow-up smears performed at month 4 of treatment, 4 follow-up sputum smears performed at month 6 of treatment and 10 follow-up sputum smears performed at month 7 of treatment were excluded from the analysis.
([Number in laboratory register−number in treatment register]/number in treatment register).
TB = tuberculosis.
Factors associated with incomplete sputum smear microscopy monitoring
Patient characteristics are shown in Table 2. At the bivariate level, incomplete sputum smear microscopy monitoring was significantly associated with sex (P = 0.03), district of residence (P ˂ 0.001), DOT (P = 0.03) and treatment outcomes (P ˂ 0.01).
Table 2.
Patient characteristics and factors associated with incomplete sputum smear microscopy monitoring (n = 492)
| Incomplete smears (n= 272) n (%) | Complete smears (n = 220) n (%) | Unadjusted χ2 P value* | OR (95%CI) | aOR (95%CI) | |
|---|---|---|---|---|---|
| Sociodemographic factors | |||||
| Age, years | |||||
| 0–24 | 46 (16.9) | 43 (19.6) | — | — | |
| 25–34 | 83 (30.5) | 69 (31.4) | 0.66 | 1.1 (0.7–1.9) | |
| 35–44 | 68 (25.0) | 49 (22.3) | 0.36 | 1.3 (0.8–2.3) | |
| 45–54 | 39 (14.3) | 30 (13.6) | 0.55 | 1.2 (0.7–2.3) | |
| ≥55 | 36 (13.2) | 29 (13.2) | 0.65 | 1.2 (0.6–2.2) | |
| Sex | |||||
| Female | 75 (27.6) | 81 (36.8) | — | — | — |
| Male | 197 (72.4) | 139 (63.2) | 0.03 | 1.5 (1.1–2.2) | 1.5 (1.0–2.2) |
| Facility type | |||||
| Hospital | 90 (33.1) | 70 (31.8) | — | — | |
| Health Centre IV | 65 (23.9) | 51 (23.2) | 0.97 | 1.0 (0.6–1.6) | |
| Health Centre III | 117 (43.0) | 99 (45.0) | 0.69 | 0.9 (0.6–1.4) | |
| District | |||||
| Kiboga | 148 (54.4) | 154 (70.0) | — | — | — |
| Kyankwanzi | 124 (45.6) | 66 (30.0) | ˂0.001 | 2.0 (1.3–2.8) | 2.0 (1.4–2.9) |
| Clinical factors | |||||
| Type of patient | |||||
| New | 258 (94.9) | 201 (91.4) | — | — | |
| Retreatment | 14 (5.1) | 19 (8.6) | 0.13 | 0.6 (0.3–1.2) | |
| DOT | |||||
| On DOT | 188 (69.1) | 172 (78.2) | — | — | — |
| No DOT | 84 (30.9) | 48 (21.8) | 0.03 | 1.6 (1.1–2.4) | 1.7 (1.1–2.7) |
| HIV status | |||||
| Negative | 168 (61.8) | 146 (66.4) | — | — | |
| Positive | 90 (33.1) | 72 (32.7) | 0.67 | 1.1 (0.7–1.6) | |
| Unknown/missing | 14 (5.1) | 2 (0.9) | |||
| Patients on ART* (n = 162) | |||||
| No | 54 (60.1) | 41 (56.9) | — | — | |
| Yes | 33 (36.6) | 30 (41.6) | 0.58 | 0.8 (0.4–1.6) | |
| Unknown/missing | 3 (3.3) | 1 (1.5) | |||
| Treatment outcomes† | |||||
| Successful | 222 (81.6) | 202 (91.8) | — | — | |
| Unsuccessful | 50 (18.4) | 18 (8.2) | ˂0.01 | 2.5 (1.4–4.5) |
ART status based on documentation in the tuberculosis register.
Successful treatment outcome was defined as cured and completed; unsuccessful treatment outcome included died, defaulted and treatment failure. Patients who died or defaulted from treatment during the intensive phase were censored from the analysis; these included only the 492 patients who were on treatment starting at month 2. Treatment outcomes variable was excluded from multivariable analysis due to lack of interpretability when adjusting for outcome as an independent variable.
OR = odds ratio; CI = confidence interval; aOR = adjusted OR; DOT = directly observed treatment; HIV = human immunodeficiency virus; ART = antiretroviral treatment.
In multivariate analysis, patients with incomplete sputum smear microscopy monitoring were more likely to be male (adjusted OR [aOR] 1.5, 95%CI 1.0–2.2) and were twice as likely to be from Kyankwanzi District as Kiboga District (aOR 2.0, 95%CI 1.4–2.9). Not receiving DOT was also significantly associated with incomplete sputum smear microscopy monitoring (aOR 1.7, 95%CI 1.1–2.7). There was no significant association between facility, type of TB patient, HIV status, ART and cotrimoxazole preventive therapy and having incomplete sputum smear microscopy monitoring (Table 2).
Barriers to complete smear microscopy monitoring
A total of 39 health provider and 64 patient interviews were conducted. Patient and provider responses to reasons for incomplete sputum smear microscopy monitoring are shown in Table 3. From both the patients’ and providers’ perspectives, the most common reasons for incomplete sputum monitoring were patients’ lack of transport to return for the appointment and inability to produce sputum (i.e., lack of sputum). Long waiting times and lack of laboratory reagents were among the other factors. In order of frequency, providers’ responses as to why they believed patients missed sputum monitoring included long waiting times to obtain results, inadequate information given by providers to patients about sputum monitoring at the start of anti-tuberculosis treatment and providers’ lack of interest in monitoring TB patients. The most frequent challenges providers faced while transcribing smear results into the TB registers included sputum results from the laboratory not being given out on the day they were requested, forgetting to record results in the registers and not knowing how to record results in the register.
Table 3.
Patient and provider opinions on possible reasons for incomplete sputum smear microscopy monitoring
| Items | n |
|---|---|
| Patient perspective: reasons why patient sputum monitoring was not performed (n = 46, multiple responses allowed) | |
| Did not have transport | 15 |
| Not able to produce sputum | 10 |
| Long waiting times for previous results | 8 |
| Time it took to get to the health facility | 5 |
| Power shortages | 3 |
| Did not know | 3 |
| Laboratory staff was away | 2 |
| Provider perspective: reasons why providers did not perform sputum monitoring (n = 36, multiple responses allowed) | |
| Patient failed to produce sputum | 28 |
| Patient did not come back to the clinic | 26 |
| Lack of materials (e.g., reagents, slides, microscope, cups) | 7 |
| Power shortages | 4 |
| Patient came when the laboratory was closed | 4 |
| Laboratory staff was not available | 2 |
| Provider perspective: reasons about why patients missed sputum monitoring (n = 25) | |
| Long waiting times for sputum results | 9 |
| Patients were given insufficient education about the importance of sputum monitoring at start of treatment | 6 |
| Some health workers were not interested in TB patients | 3 |
| Health workers in out-patient department did not submit requests for sputum | 2 |
| Patients did not return and were represented by relatives instead | 2 |
| Laboratory staff did not want to examine sputum | 2 |
| Health workers did not know when sputum follow-up should be performed | 1 |
| Challenges providers faced when recording sputum results in the unit tuberculosis register (n = 36) | |
| Results were not available on the same day. | 20 |
| Provider forgot to record results in the unit register | 10 |
| Provider did not know how to fill in the register | 5 |
| Provider did not know that it was necessary | 1 |
DISCUSSION
This study found that over half (55%) of registered PTB patients had missed at least one smear result by the end of treatment. This finding is slightly higher than those in other studies, including one from India, in which 38% of PTB patients did not undergo all sputum monitoring examinations.6 Poor sputum microscopy monitoring in resource-limited settings is of public health importance because it indicates that response to treatment may not be ascertained and addressed appropriately, and that multidrug-resistant TB cases were being missed, resulting in the transmission of drug-resistant organisms in the community.
The discrepancies between the sputum smear results recorded in the TB treatment register and those in the laboratory register increased throughout treatment. Some sputum examinations were not performed on schedule, further contributing to incomplete monitoring. A study in Malawi found that 71–82% of smears were examined at unscheduled times during treatment,7 which can impact the clinician’s ability to make informed treatment decisions. Results also suggest that there were recording gaps and poor linkages between the laboratory and the TB clinic. The approximately 10% difference between the registers in the number of smears recorded at 6 or 8 months means that cure rates in these districts may be underreported. To improve the transfer of data from the TB laboratory registers to the TB treatment registers, health providers should ensure transcription of smear results from the laboratory request form into the treatment register and escort patients between the TB clinic and laboratory within the same facility, as this has previously been shown to improve recording of such data.9 In addition, the NTLP should introduce electronic registers to improve the quality of records in the TB registers by reducing the missing documentation of sputum results.
As incomplete sputum smear monitoring was more common in male patients, patients from Kyankwanzi District and those not on DOT, these groups should be prioritized for more complete smear monitoring by health care staff. Kyankwanzi was recently established as a new district in 2010, and as a result tended to have health providers with lower qualifications than in Kiboga, which has a more established health system. Health providers in Kyankwanzi may therefore require additional training on sputum monitoring. DOT is the standard of care in Uganda and is the most effective strategy for making sure patients take their TB medicines. However, in this study, more than a quarter of the patients were not initiated on DOT. Regular interaction between patients and providers during DOT allows for more frequent opportunities to ensure laboratory monitoring is done.9 Further investigations are needed to understand why some patients did not receive DOT per national guidelines.
Health care providers and patients commonly attributed incomplete sputum monitoring to patients’ lack of transport, patients’ inability to produce sputum, insufficient education of patients about sputum monitoring and long waiting times to receive sputum results. In Uganda, all cadres of staff are supposed to receive training on monitoring anti-tuberculosis treatment using sputum smear microscopy;4 however, the fact that a third of service providers had not received training for over 3 years suggests a knowledge gap among staff in basic monitoring schedules. Similarly, there was limited understanding among staff about how to document sputum smear results. One nursing officer said, ‘I do not know how to fill a TB register’, although recording and reporting of results is supposed to be a key component of the job responsibilities in the TB clinic, and instructions to guide health workers on how to record sputum results are listed in the registers. To improve sputum monitoring and recording and reporting practices, the NTLP should consider strengthening the supervision of health facility staff and SCHWs by the District and Zonal TB managers and using results from this study to inform the development of training materials, which are currently underway. Other strategies include mentoring providers on how to help patients produce sputum and improving patient awareness of the importance of smear monitoring through additional education. Policy makers and district managers should prioritise DOT, ensuring funding to support SCHWs delivering sputum to DTUs, adequate TB laboratory supplies and that laboratory staff are on duty to reduce waiting times.
This study had some limitations. First, it was conducted only in rural settings, thus limiting its generalisability to similar settings in Uganda. Second, based on study definitions, 17 patients who returned for sputum smear monitoring during months outside the recommended intervals were defined as having ‘incomplete monitoring’, although they had been tested. To ensure proper anti-tuberculosis treatment, it is important for all TB patients to be monitored according to national guidelines. Third, data gathered during interviews were based on responses from patients and service providers who were available at the time of the interview, and as a result may not be representative. However, the strength of the study is that it quantifies the extent of incomplete sputum monitoring and provides evidence for local and national TB staff to prioritise and improve sputum monitoring protocols.
In conclusion, incomplete monitoring of sputum smears was common in general, but more common in male patients and in the new district of Kyankwanzi. Male patients should be targeted to increase completeness of monitoring. District TB staff should engage TB providers and patients to improve recording practices, laboratory services, patient health provider education and uptake of facility- or community-based DOT to all TB patients.4
Acknowledgements
This project was supported by the US Centers for Disease Control and Prevention(CDC; Atlanta, GA, USA) through the United States Agency for International Development (USAID; Washington DC, USA). Some authors from this publication are employed by the CDC.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Conflicts of interest: none declared.
H = isoniazid; R = rifampicin; Z = pyrazinamide; E = ethambutol; S = streptomycin. Numbers before the letters indicate the duration in months of the phase of treatment.
References
- 1.World Health Organization. Global tuberculosis report, 2013. WHO/HTM/TB/2013.11. Geneva, Switzerland: WHO, 2013. [Google Scholar]
- 2.Fauci AS, Braunwald E, Kasper D, et al. Harrison’s principles of internal medicine. 17th ed. New York, NY, USA: McGraw-Hill Professional, 2008. [Google Scholar]
- 3.World Health Organization. Treatment of tuberculosis: guidelines. 4th ed. WHO/HTM/TB/2009.420. Geneva, Switzerland: WHO, 2010. [PubMed] [Google Scholar]
- 4.Uganda Ministry of Health. Manual of the National Tuberculosis and Leposy Programme. 2nd ed. Kampala, Uganda: MoH, 2010. www.who.int/hiv/pub/guidelines/uganda_tb.pdf Accessed December 2015. [Google Scholar]
- 5.World Health Organization. Global TB control report, 2012. WHO/HTM/TB/2012.6. Geneva, Switzerland: WHO, 2012. [Google Scholar]
- 6.Satyanarayana S, Nagaraja SB, Kelamane S, et al. Did successfully treated pulmonary tuberculosis patients undergo all follow-up sputum smear examinations? Public Health Action 2011; 1: 27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harries AD, Gausi F, Salaniponi F. When are follow-up sputum smears actually examined in patients treated for new smear-positive pulmonary tuberculosis? Int J Tuberc Lung Dis 2004; 8: 440–444. [PubMed] [Google Scholar]
- 8.Kayigamba FR, Bakker MI, Mugisha V, et al. Adherence to tuberculosis treatment, sputum smear conversion and mortality: a retrospective cohort study in 48 Rwandan clinics. PLOS ONE 2013; 8: e73501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adatu F, Odeke R, Mugenyi M, et al. Implementation of the DOTS strategy for tuberculosis control in rural Kiboga District, Uganda, offering patients the option of treatment supervision in the community, 1998–1999. Int J Tuberc Lung Dis 2003; 7 (Suppl 1): S63–S71. [PubMed] [Google Scholar]