Abstract
The persistence of COVID-19 symptoms weeks or months after an initial SARS-CoV-2 infection has become one of the most burdensome legacies of the pandemic. This condition, known as long COVID syndrome, affects many persons of all age groups and is associated with substantial reductions of quality of life. Several mechanisms may be involved in long COVID syndrome, including chronic inflammation, metabolic perturbations, endothelial dysfunction, and gut dysbiosis. These pathogenic mechanisms overlap with those of the aging process and may aggravate pre-existing degenerative conditions. This review discusses bioactive foods, supplements, and nutraceuticals as possible interventions against long COVID syndrome.
Keywords: Geriatrics, Gerontology, Frailty, Sarcopenia, Fatigue, Nutrition, Bioactive foods, Therapy
Key points
-
•
Long-term persistence of COVID-19–related symptoms after SARS-CoV-2 infection impacts quality of life.
-
•
Several mechanisms may be involved in the persistence of COVID-19 symptoms, including chronic inflammation, immunometabolic processes, endothelial dysfunction, and gut dysbiosis.
-
•
Bioactive foods, supplements, and nutraceuticals may be used for the management of long-term COVID-19 clinical sequelae.
Introduction
SARS-CoV-2 infection affects multiple organs owing to the ubiquitous distribution of its target receptor ACE-2.1 Increasing interest has been paid to the postacute phase of COVID-19. The time to recover from an acute COVID-19 episode ranges from 2 to 6 weeks depending on disease severity.2 However, a substantial share of people reports clinical sequelae weeks or months after symptom onset. This condition, known as postacute COVID-19 syndrome, postacute sequelae of COVID-19, or long COVID syndrome, encompasses long-lasting signs and symptoms, such as cough, dyspnea, fatigue, difficulties with memory and concentration ("brain fog"), sleep disorders, gastrointestinal complaints, and musculoskeletal problems.3 , 4
A recent systematic review showed that more than half of COVID-19 survivors (mostly middle-aged men hospitalized during the acute phase) had at least one long-lasting symptom at 6 months.5 Results from a digital questionnaire in a cohort of middle-aged nonhospitalized patients with COVID-19 from Denmark showed that persistent symptoms lasting for more than 4 weeks were experienced by 36% of participants who had been symptomatic during the acute phase.6 Recently, our group showed that persisting symptoms were common in older adults who had been hospitalized for COVID-19, with approximately 50% of the sample presenting with 3 or more symptoms after 3 months of hospital discharge.7 Symptom persistence was more frequent in those who experienced fatigue during acute COVID-19.
The mechanisms of chronic COVID-19 manifestations have not been yet unveiled. Several hypotheses are currently being tested.4 , 8 These include viral persistence associated with chronic inflammation, autoimmune processes, alterations in immunometabolic pathways, dysbiosis, endothelial damage, and unresolved organ damage.3 , 4 , 8 It is noteworthy that some of the immunologic and systemic features of long COVID resemble signs of accelerated or premature aging, and may aggravate pre-existing age-associated degenerative conditions, such as sarcopenia and cognitive decline.9, 10, 11
Specific treatments for long COVID are currently lacking. Patient management mostly relies on symptomatic treatments and recommendations to conduct an active and healthy lifestyle. A plethora of dietary supplements and natural bioactive substances have been tested for their potential to contrast long COVID.12 , 13 In this review, the possible actions of nutraceuticals and dietary supplements on mechanisms associated with long COVID are described. A brief overview of ongoing studies investigating the effects of specific nutraceuticals in patients with long COVID attending the outpatient service at the Fondazione Policlinico A. Gemelli IRCCS (Rome, Italy)14 is presented.
Discussion
Amino Acids
Amino acids play a crucial role in cell metabolism and modulate several biological processes (eg, inflammation, glucose homeostasis, redox balance) that may be involved in post-COVID clinical sequelae.4 , 8 , 15 Amino acids support the increased metabolic demands of activated immune cells by contributing critical intermediates to biosynthetic and energy-producing pathways, such as glycolysis, tricarboxylic acid (TCA) cycle, and oxidative phosphorylation pathways.15
Glutamine replenishes TCA cycle intermediates that have been consumed in biosynthetic processes in T cells, macrophages, and plasma cells through anaplerosis.16, 17, 18 In immune cells, glutamine is also used for glutathione and in hexosamine biosynthetic pathways and supplies amino groups for other amino acids via transamination.19
The branched-chain amino acids (BCAAs) leucine, isoleucine, and valine are essential amino acids with well-established effects on protein synthesis and glucose homeostasis. BCAAs mainly act through the stimulation of cellular anabolic signaling pathways (eg, phosphoinositide 3-kinase-protein kinase B, mammalian target of rapamycin [mTOR]).20 BCAAs may exert direct and indirect effects on immune function.15 In vitro models have shown that BCAAs are essential for proliferating lymphocytes to synthesize proteins and nucleotides in response to stimulation.21 BCAAs can be incorporated into and oxidized by immune cells. The amount of BCAAs incorporated into proteins is higher in lymphocytes, followed by eosinophils and neutrophils.21 In immune cells, BCAAs are also sources of the coenzyme A (CoA) derivatives acetyl-CoA and succinyl-CoA, which enter the TCA cycle and support mitochondrial bioenergetics.15 BCAAs may also improve gut mucosal surface defense by stimulating soluble immunoglobulin A secretion.22
Arginine is a semiessential amino acid involved in multiple biological processes. Its main activities on the immune system derive from its conversion to nitric oxide (NO) by NO synthase (NOS) and its metabolism through arginase, that competes with NOS for arginine availability in most immune cells.23 NO is known to have direct and indirect antiviral activity through the generation of immunomodulatory oxidized phospholipids.24, 25, 26 NO also exerts potent anti-inflammatory effects via inhibition of leukocyte recruitment.27 , 28 Several studies in animal models have shown that the mechanisms of NO action on leukocyte function involve the activation of guanylyl cyclase, followed by the generation of cyclic GMP that suppresses the expression of P-selectin, a crucial mediator in leukocyte adhesion.29 Reduced arginine bioavailability may impair T-cell response and function.30 In patients with COVID-19, a decrease in plasma arginine levels with concomitant increase in arginase activity was associated with impaired T-cell proliferative capacity, which can be restored in vitro by arginine supplementation.31 Arginine, through its action on NO synthesis, may also modulate endothelial and respiratory functions and exert antithrombotic and cytoprotective activities.32 Preliminary data suggest that oral arginine supplementation added to standard therapy reduced the need of respiratory support and the duration of in-hospital stay in patients with severe COVID-19.33 Because of its modulating activities on inflammation and endothelial function, arginine has also been indicated as a plausible agent to contrast COVID-19 sequelae.
Collectively, available evidence suggests that amino acids are crucial to modulate immune response and several other mechanisms involved in both acute and chronic COVID-19. In older adults, the physiologic changes associated with the aging process (eg, increased splanchnic extraction, anabolic resistance, chronic inflammation) may further increase the need of adequate amino acid supplies.34 Protein/amino acid malnutrition in older adults is associated with immune dysfunction and sarcopenia, the latter being aggravated by reduced mobility and inflammatory processes induced by COVID-19.35
Given these premises, it is reasonable to focus on the achievement of an adequate amino acid intake in COVID-19 survivors, also through oral supplementation. In particular, amino acid intake should be monitored in older people who are at greater risk of malnutrition as a specific consequence of SARS-CoV-2 infection.
Hydroxy-Beta-Methylbutyrate
Beta-hydroxy-beta-methyl butyrate (HMB) is the active metabolite of leucine.36 HMB, through the stimulation of mTOR-dependent pathways, modulates several physiologic processes, including protein metabolism, insulin activity, skeletal muscle hypertrophy, cell apoptosis, and muscle stem cell proliferation and differentiation.37
Oral supplementation of HMB (3 g/d) mitigates the decline in muscle mass and preserves muscle function in older adults and frail people, especially during hospital stay and recovery.38 Interestingly, HMB may modulate the immune and inflammatory response, especially under stressful conditions.39 HMB supplementation was found to have short-term anti-inflammatory and anticatabolic effects and to improve pulmonary function in patients with chronic obstructive pulmonary disease in an intensive care unit setting.40
Possible effects of HMB on brain have also been explored, starting from the evidence that HMB crosses the blood-brain barrier in rats.41 HMB was found to promote neurite outgrowth in vitro,42 and long-term HMB supplementation in rats attenuated age-related dendritic shrinkage of pyramidal neurons in the medial prefrontal cortex,43 thus suggesting possible beneficial effects on cognition.44 , 45
Based on its well-established effects on muscle mass preservation and its potential biological activities on physiologic systems perturbed by COVID-19, HMB may represent a useful support for older patients suffering from long-term COVID-19 sequelae.
Tricarboxylic Acid Cycle Intermediates
Malic acid, citric acid, and succinic acid are TCA cycle intermediates with a well-established role in mitochondrial energy metabolism. Once considered byproducts of cellular metabolism to be used for the biosynthesis of macromolecules (eg, lipids, nucleotides, proteins), TCA cycle intermediates are now recognized as important mitochondrial signaling molecules regulating multiple cellular functions.46 Indeed, TCA cycle metabolites may also modulate chromatin remodeling, DNA methylation, posttranslational protein modifications, platelet activity, and immunity.15 , 47 , 48
Supplementation with TCA cycle intermediates may help preserve mitochondrial biogenesis and function and fulfill the increased metabolic demands of older people recovering from COVID-19. In this context, TCA cycle metabolites may support the beneficial effects of amino acid supplementation in the prevention of mitochondrial dysfunction, oxidative damage, and muscle loss.49 Novel formulas combining mixtures of amino acids plus TCA intermediates, such as citric, succinic, and malic acid, and B group vitamins, were tested for their effects on mitochondrial bioenergetics and antioxidant response both in vitro and in animal models of aging.50 , 51 In murine neural stem cells and human-induced pluripotent stem cells, mitochondrial function, oxidant scavenging mechanisms, and neuronal stem cells differentiation were boosted via activation of the mechanistic target of rapamycin complex 1 and nuclear factor erythroid 2 like 2 (Nrf2)-mediated gene expression.50 In a mouse model of accelerated muscular and cognitive aging, the combination of amino acids plus TCA cycle metabolites and cofactors preserved mitochondrial efficiency, muscle mass, and physical and cognitive abilities by acting on proliferator-activated receptor γ coactivator 1α and NRF2 in skeletal muscle and hippocampus.51
Further investigation is warranted to evaluate the potential effects of TCA cycle metabolites (alone or combined with other nutrients) to contrast long-term consequences of COVID-19.
Micronutrients
Nutritional surveys indicate that vitamin and mineral deficiencies are highly prevalent in older adults. Micronutrient deficiencies are associated with increased risk of noncommunicable diseases, such as fatigue, cardiometabolic disease, musculoskeletal disorders, and cognitive impairment.52 Micronutrients, including selenium, iron, zinc, and magnesium, are also critical for the proper functioning of the immune system.53
Selenium
Selenium is an essential trace element and is mainly present in the form of selenoproteins. Selenium is involved in several physiologic processes, including neurologic, endocrinologic, cardiovascular, and immune functions.54
Several studies have investigated the role of selenium in modulating the immune response, and for this reason, a putative role for selenium has been evoked in the context of COVID-19.55 Evidence in human, animal, and cellular models suggests that selenium plays an important role in response to viral infections, including respiratory viruses,56 while its deficiency seems to increase host susceptibility to infections.55 , 57 In viral infections, selenoproteins inhibit type I interferon responses and viral transcriptional activators.54 , 55 Selenium supplementation stimulates innate immune system and increases CD4+ T cells and natural killer cell response.58 Selenium also modulates the secretion of proinflammatory interleukins by inhibiting nuclear factor κB (NF-κB).55 Selenium deficiency has been correlated to greater secretion of interleukin-6 (IL-6) in older adults,59 whereas higher levels of selenium are associated with reduced C-reactive protein (CRP) circulating levels.60 Low plasma concentration of selenium was also correlated with increased tissue damage and organ failure, prothrombotic activity, and overall mortality in patients in intensive care, which could explain a correlation with severity of symptoms in patients with COVID-19 and possible COVID-19 sequelae in long haulers.3 , 61, 62, 63 Several studies have reported that circulating selenium concentrations were lower in patients with COVID-19 as compared with healthy controls and associated with COVID-19 severity and mortality.64 , 65
Low selenium levels may increase individual susceptibility to SARS-CoV-2 infection, influence the severity of the disease,66 , 67 and be related to postacute sequelae and long-lasting symptoms.68
Iron
Iron is essential for all living organisms, being a crucial component of hundreds of proteins involved in fundamental biological processes, including oxygen transport, energy production, and nucleic acid synthesis.69 During viral infections, the rapid viral proliferation determines a competition for iron between the invading pathogen and the host.70 Moreover, iron homeostasis influences both the innate and the adaptive host immune response. In fact, hypoferremia is linked to altered B- and T-lymphocyte proliferation and function71 and is associated with higher levels of proinflammatory cytokines and reactive oxygen species.72 Low-iron levels have been associated with the severity of respiratory insufficiency, acute multiorgan failure, and mortality in patients with COVID-19.73, 74, 75 Iron status parameters, such as ferritin, transferrin, and hepcidin, which respectively serve to store, transport, and absorb iron in the human body, may be considered risk factors and clinical biomarkers for COVID-19 prognosis.76 In particular, meta-analyses have correlated high-ferritin levels with COVID-19 severity and mortality.77, 78, 79 Ferritin stimulates the expression of proinflammatory cytokines, such as IL-1β, IL-6, IL-12, and tumor necrosis factor-α (TNF-α),71 and a gradual reduction of its circulating levels has been demonstrated in patients with COVID-19 after infusion of monoclonal antibodies targeting IL-6 receptor.200 Preliminary data from a prospective observational cohort study in patients with mild to critical COVID-19 show that alterations in iron homeostasis may persist for at least 2 months after acute infection and are associated with nonresolving lung disorders and impaired physical performance.80 Iron deficiency was associated with elevated levels of inflammation markers, such as IL-6 and CRP.80 Finally, low-iron levels could theoretically impair the efficacy of COVID-19 vaccination,71 underlining the importance of monitoring iron levels, especially in older adults with long COVID.
Based on the available evidence, iron supplementation, especially in older adults with low iron and hemoglobin levels, could be particularly useful for reducing the level of inflammation, mitigating persistent symptoms (such as fatigue and dyspnea), and improving immune response to vaccination.
Zinc
Zinc is the second most abundant trace metal in the human body after iron and is essential for the maintenance of immune health, cell homeostasis, and reproduction.81 , 82 Zinc deficiency is quite common in the general population and is also associated with immune dysfunction, affecting both the innate and the adaptive immune systems.83 , 84 Inadequate zinc intake has been associated with increased risk of upper- and lower-respiratory tract infections, especially in older adults,85 , 86 whereas supplementation with zinc gluconate seems to reduce duration and severity of common cold symptoms.87
Zinc may inhibit coronavirus RNA polymerase activity in vitro and viral replication in cultured cells.88 Zinc has also an immunomodulatory, antioxidant, and anti-inflammatory role that could influence the trajectory of SARS-CoV-2 infection, and its supplementation could ameliorate lung function, enhance mucociliary clearance, and reduce ventilator-induced lung injury in critical patients with COVID-19.85 Lower serum zinc levels were found in patients with severe COVID-19 with acute respiratory distress syndrome and associated with a higher rate of complications and in-hospital length of stay.64 , 85 Zinc deficiency could also be connected to the onset and persistence of symptoms such as anosmia and dysgeusia, which can last for a variable amount of time after resolution of acute infection.89
Zinc supplementation was tested for its potential role in contrasting the consequences of SARS-CoV-2 infection. However, available data on the efficacy of zinc supplementation for the management of patients with COVID-19 are scarce and contradictory.64 , 90 , 91 A prospective randomized clinical trial, COVID A to Z study, evaluated the efficacy of a treatment with zinc gluconate, ascorbic acid, or their combination in shortening the duration of COVID-19 symptoms.92 The study failed to demonstrate a significant improvement in symptoms, and the study was stopped for futility.
Further studies are needed to evaluate the effects of zinc or mixtures of micronutrients, including zinc in both acute COVID-19 and in long COVID-19 syndrome.
Magnesium
Magnesium is essential for several physiologic functions and biochemical reactions and may exert important anti-inflammatory and antioxidant functions.93 , 94
Although severe magnesium deficiency with clinical symptoms is quite rare, hypomagnesemia is frequent in patients in the intensive care unit, regardless of the cause of admission, and is associated with increased mortality, higher need for ventilator support and incidence of sepsis, and longer hospital stays.95 Although there are no significant data on magnesium homeostasis in patients with COVID-19, it has been suggested that magnesium deficiency could modulate the progress and severity of SARS-CoV-2 infection.96 Magnesium supplementation protects organs and tissues from damage through multiple mechanisms and could potentially influence the natural history of COVID-19.96
It is well known that magnesium exerts anticholinergic, antihistaminic, and anti-inflammatory activities, reduces the risk of airway hyperreactivity and wheezing, and promotes bronchodilation by inhibiting bronchial smooth muscle contraction via blockage of calcium channels.94 , 97 A systematic review demonstrated that a single infusion of 1.2 or 2 g of magnesium sulfate in adults with exacerbations of asthma was associated with a reduction in hospitalization rate and improved lung function in patients who did not respond to standard treatment (ie, oxygen, nebulized short-acting β2-agonist, and intravenous corticosteroids).98 In addition, magnesium reduces the secretion of transforming growth factor β1, thereby preventing lung fibrosis,99 a rare but fearsome consequence of COVID-19.
Magnesium plays a role in modulating the innate and adaptive immune system.93 , 94 Subclinical magnesium deficiency is correlated with low-grade chronic inflammation, which can be crucial for the prognosis of COVID-19 and the persistence of COVID-19 symptoms.93 It has been demonstrated that magnesium sulfate supplementation downregulates inflammatory response and oxidative stress by inhibiting chemokine and cytokines, such as IL-1, IL-6, IL-8, TNF-α, and CRP, which are often significantly elevated in severe COVID-19 cases.93 , 94 Magnesium may also modulate the adaptive immune system, influencing the proliferation and the activation of CD4+ and CD8+ T lymphocytes.96 Furthermore, magnesium exerts an important role in maintaining endothelial function and vascular integrity.100 In response to an acute inflammation stimulus, such as SARS-CoV-2 infection, the endothelium increases the secretion of prothrombotic factors. Accumulating evidence suggests that the endothelium might be directly affected in patients with COVID-19.101 Magnesium supplementation, especially in older adults, could help prevent the risk of thromboembolism in COVID-19 and long-term consequences in COVID-19 survivors.102 In critically ill patients with COVID-19, magnesium sulfate supplementation can represent a supportive treatment with promising beneficial effects.94 A retrospective observational study demonstrated that in hospitalized patients with COVID-19 aged 50 years and older, a combination of oral vitamin D3, magnesium, and vitamin B12 supplementation for up to 14 days significantly reduced the need of oxygen therapy compared with controls.103
Despite the lack of clinical trials and well-powered studies in patients with COVID-19, magnesium supplementation seems to find a potential application in controlling respiratory symptoms, but also in modulating inflammation, cardiovascular and neurologic disorders, and electrolyte abnormalities. The monitoring of magnesium status should be encouraged, as it may influence immune homeostasis and help decrease morbidity and mortality in patients with COVID-19.93
Bromelain
Bromelain is a proteolytic enzyme derived from the fruit and stem of pineapple. Bromelain is commonly used as an anti-inflammatory agent, although its potential biological activity spans from respiratory, digestive, immune, and circulatory systems to anticancer and antimicrobial therapy.104
Bromelain regulates inflammatory processes through the modulation of NF-κB and cyclooxygenase 2 pathways that regulate the synthesis of inflammatory cytokines and prostaglandin E2 and thromboxane A2, respectively.105 Moreover, bromelain has fibrinolytic and antithrombotic properties106 , 107 and may also exert analgesic effects by regulating the synthesis of pain mediators, such as bradykinin.108 The decrease in bradykinin levels induced by bromelain may also indirectly act on inflammatory response through the regulation of vascular permeability and reduction in edema. Bromelain showed immunomodulatory effects both in vitro and in vivo.109 Bromelain may simultaneously enhance and inhibit T-cell responses by acting on accessory cells and directly on T cells. Moreover, bromelain enhanced T-cell–dependent, antigen-specific, B-cell antibody responses in vivo.109
In vitro studies suggest that bromelain may also inhibit SARS-CoV-2 infection by targeting ACE-2, transmembrane serine protease 2, and SARS-CoV-2 S-protein.110
Considering its multiple potential activities on mechanisms involved in acute and chronic COVID-19 phases, bromelain could represent a candidate therapeutic agent for the management of COVID-19. In this context, bromelain may also increase the absorption of curcumin and other bioactive compounds with possible anti–COVID-19 activities after oral administration.111 Currently, data about the properties of bromelain on persistent symptoms related to long COVID-19 are lacking, but it can be hypothesized that its biological activities might help in the process of recovery from chronic fatigue, joint pain, and myalgia.104
Troxerutin
Troxerutin is a flavonoid derived from rutin that can be found in tea, coffee, cereals, fruits, and vegetables. Troxerutin exerts multiple biological functions, including antioxidant, anti-inflammatory, nephroprotective, antithrombotic, antidiabetic, and neuroprotective effects.112 Following ingestion, flavonoids are partially digested by microbiome, which facilitates the absorption by small or large bowel.
Troxerutin exerts its antioxidant functions through direct free radical scavenging activity113 and by enhancing the activity of glutathione and antioxidant enzymes, such as superoxide dismutase, catalase, and glutathione peroxidase.114 Through its antioxidant activities, troxerutin might preserve epithelial cells, fibroblasts, and lymphocytes from oxidative stress-induced apoptosis.113
Troxerutin was found to protect against gentamycin-induced acute kidney injury in rats through the downregulation of inflammatory cytokines (IL-10, TNF-α, and IL-6), the reduction of apoptotic cell death, and the induction of renal tissue regenerative capacity, by acting on p38 mitogen-activated protein kinase signaling.115
Troxerutin protects the hippocampus against amyloid-beta induced oxidative stress and apoptosis by reducing the acetylcholinesterase activity and oxidative stress.114 In a rat model of traumatic brain injury, troxerutin exerted multiple protective effects through the regulation of endothelial NOS activity.116 Moreover, troxerutin demonstrated anxiolytic- and antidepressant-like activities in rodents.117
In rat models of type 1 and type 2 diabetes, troxerutin preserved male fertility and protected against diabetic cardiomyopathy through the reduction of reactive oxygen species and its activity of NF-κB, serine/threonine kinase 1, and insulin receptor substrate 1.118 , 119
Data on the activity of troxerutin in COVID-19 are currently lacking. However, its antioxidant and anti-inflammatory properties might be helpful for both the acute and the chronic consequences of SARS-CoV-2 infection.
Lactoferrin
Lactoferrin is an iron-binding glycoprotein, which acts as a host defense molecule against microbes. Lactoferrin is mainly secreted from exocrine glands and is present in granules in neutrophils following infections.120 Lactoferrin prevents microbial and viral adhesion to human cells through its iron-chelating ability, which reduces availability of iron used for microbial growth, aggregation, and biofilm formation.121 The activity of lactoferrin is mostly bacteriostatic, but a lethal effect has been demonstrated. One of its derivates, lactoferricin, tends to inactivate the lipid A from lipopolysaccharide of gram-negative bacteria. Lactoferrin may also have a bactericidal effect on gram-positive bacteria. Lactoferrin exerts its antiviral activity through different mechanisms at the mucosal level, including binding to heparan sulfate glycosaminoglycans, viral particles, and viral receptors, thus limiting viral entry.122
In vitro, bovine lactoferrin blocks human cell apoptosis induced by influenza A virus.123 In vitro and in vivo studies showed that lactoferrin may also inhibit cytomegalovirus124 and herpes simplex virus infection.125 The antiviral activity of lactoferrin has been demonstrated against a multitude of viruses, including respiratory syncytial virus, poliovirus, hepatitis B virus, hepatitis C virus, alphaviruses, hantavirus, human papillomavirus, adenovirus, and enterovirus.126 Given its immunomodulatory and antiviral properties, the use of lactoferrin was also proposed to contrast SARS-CoV-2 infection.127 In vitro and in silico assays showed that lactoferrin may have an antiviral activity against SARS-CoV-2 through direct attachment to both SARS-CoV-2 spike S glycoprotein and cell surface components.128 Lactoferrin may also modulate the inflammatory cascade through the reduction of IL-6, TNF-α, and ferritin levels.129 A preliminary study testing the antiviral effect of oral and intranasal liposomal bovine lactoferrin in asymptomatic and patients with mild to moderate COVID-19 showed that lactoferrin treatment induced fast symptom resolution compared with standard care.130 Treatment with lactoferrin also elicited a significant decrease in serum ferritin, IL-6, and d-dimer.
Based on available evidence, lactoferrin may represent a promising molecule to be used in both patients with acute COVID-19 or patients with postacute COVID-19 syndrome. Further investigations are needed to corroborate the positive preliminary findings obtained in patients with COVID-19, and to determine whether lactoferrin supplementation alone or with other bioactive compounds may result in additive beneficial effects against acute and postacute COVID-19.
Probiotics
The gastrointestinal system may be affected during acute and postacute COVID-19.131 Gastrointestinal symptoms, such as diarrhea, nausea, vomiting, and loss of appetite, are commonly experienced by people with COVID-19. SARS-CoV-2 RNA may be detected in stools even after viral clearance in the respiratory tract.132 Moreover, alterations in gut microbiota were associated with COVID-19 severity, possibly as a consequence of a "leaky gut" phenomenon and the release of microbial products and toxins into systemic circulation.133 , 134 Long-term antibiotic therapies, hospitalization, stress, comorbidities, and the direct action of SARS-CoV-2 on ACE2 receptor at the gastrointestinal level are associated with gut dysbiosis. Perturbations in gut microbiota may persist months after acute COVID-19 and may be associated with long-term complications.135
Probiotics are “live microbes that, when administered in adequate amounts, confer health benefits on their hosts.”136 Probiotics (mainly Bifidobacteria and Lactobacilli) may exert relevant immunomodulatory functions on the gut-lung axis.137 Probiotics increase host mucosal defense through the stimulation of immunoglobulin A secretion, induce polarization of the immune response toward Th1, modulate cytokine production, and produce metabolites, such as short chain fatty acids, that contribute to set the tone of the immune system.138 Probiotics may have also anti-inflammatory,139 antioxidant,140 and antiviral properties.141 Interestingly, an in silico molecular docking approach suggested that metabolites from Lactobacillus plantarum may have potential antiviral activity against SARS-CoV-2 by targeting helicase nsp13.142 Probiotics may also have synergistic antiviral activities when combined with compounds with antimicrobial properties. For instance, Lacticaseibacillus paracasei DG strain enhances the lactoferrin anti–SARS-CoV-2 response in Caco-2 cells.143
Numerous studies are currently testing the use of different mixtures of probiotics as adjunctive treatment for the management of both acute and postacute COVID-19.141 , 144 , 145 Preliminary evidence suggests that probiotics administration may reduce secondary infections in people with severe COVID-19.146 The consumption of a specific bacterial formulation containing strains of Streptococcus thermophilus, Lactobacillus acidophilus, Lactobacillus helveticus, Lactobacillus paracasei, L plantarum, Lactobacillus brevis, and Bifidobacterium lactis was associated with remission of gastrointestinal symptoms and reduced risk of respiratory failure in hospitalized patients with COVID-19.145 In COVID-19 outpatients, the administration of a mixture of Lactiplantibacillus plantarum and Pediococcus acidilactici strains improved viral clearance and reduced both respiratory and gastrointestinal symptoms compared with placebo.147
Further studies are needed to assess the effects of specific probiotic formulations and/or the combination of probiotics with other bioactive compounds on long-term COVID-19 sequelae.
The use of probiotics may be particularly recommended in older patients with COVID-19, in whom age-related factors, including frailty, multimorbidity, and malnutrition, may aggravate gut dysbiosis.
Vitamin D
Vitamin D plays a crucial role in the regulation of calcium and bone metabolism.148 Accumulating evidence from preclinical and observational studies suggests that vitamin D could be involved in many extraskeletal pathways, including immune and muscle function, reproduction, and energy metabolism.148 Vitamin D may modulate different aspects of the innate and adaptive immune response.149 Both vitamin D receptor and enzymes involved in vitamin D metabolism are expressed by several immune cell types, including lymphocytes, monocytes, macrophages, and dendritic cells.149
Vitamin D may support innate immunity by stimulating the synthesis of several antimicrobial peptides, including cathelicidins and defensivins.150 Vitamin D may also regulate the activity of antigen-presenting cells, T- and B-cell differentiation, and the balance between proinflammatory and anti-inflammatory cytokines.151 Low vitamin D levels were associated with increased risk of numerous immune-related conditions, including type 1 diabetes,152 multiple sclerosis,153 and rheumatoid arthritis.154 Furthermore, people with vitamin D deficiency tend to be more prone to develop acute respiratory infections.155
Vitamin D deficiency has been associated with increased risk of SARS-CoV-2 infection and worse clinical outcomes, including more severe lung impairment, need for respiratory support and intensive care, and mortality.156 , 157 Hence, vitamin D supplementation has been tested as a candidate adjunctive treatment for COVID-19. In asymptomatic or mildly symptomatic individuals with SARS-CoV-2 and vitamin D deficiency, a 7-day administration of high-dose vitamin D (60,000 IU of cholecalciferol per day) accelerated viral clearance compared with placebo.158 Vitamin D supplementation taken before or during COVID-19 was associated with better 3-month survival in older adults hospitalized for COVID-19 compared with nonsupplemented peers.159 However, a recent living systematic review found insufficient evidence to determine the benefits of vitamin D supplementation as a treatment of COVID-19, owing to the heterogeneity of studies, including different supplementation strategies, formulations, vitamin D status of participants, and reported outcomes.160
As for postacute COVID-19, data on the association between vitamin D levels and the persistence of symptoms are scarce. In a small group of COVID-19 survivors from Dublin, persistent fatigue and reduced exercise tolerance were not associated with vitamin D levels.161
Because of the multiple effects of vitamin D on biological pathways associated with long COVID and its relevance for the overall health status, vitamin D supplementation should be considered to avoid the detrimental consequences of vitamin deficiency. This holds true in particular for vulnerable people, such as older COVID-19 survivors, in whom vitamin D may also contrast geriatric conditions, such as frailty and sarcopenia, that are associated with negative health outcomes and may further reduce the quality of life.162
Pollen-Based Herbal Extracts
Pollen, the male gametophyte of the flower, has long been used for therapeutic purposes by various ancient civilizations.163 Pollen contains a mixture of amino acids, carbohydrates, minerals, and bioactive substances that vary depending on the plant source and geographic origin, climatic conditions, soil type, and the activity of bees.164
Purified cytoplasm of pollen (PCP) is a nonhormonal herbal remedy used to manage vasomotor symptoms and sleep and mood disorders in menopausal women.165 Even if the mechanisms of action of PCP are not fully understood, this compound seems to act as a selective serotonin reuptake inhibitor (SSRI)-like agent to increase serotonin availability in hypothalamic serotonergic neurons. This explains, at least partly, its efficacy in controlling thermoregulation, and sleep and mood disorders in menopausal woman, without the adverse effects of currently prescribed SSRIs.166 Another putative mechanism action of PCP could be based on the precursors of serotonin. Indeed, tryptophan and methyl serotonins have been identified in low concentrations in PCP extracts, even if their contribution to intraneuronal serotonin synthesis could be negligible.166 Studies conducted in women with symptomatic menopause demonstrated that PCP supplementation improved sleep quality and quality of life and reduced hot flushes, fatigue, and muscle and joint pain.165 , 167 The most common symptoms complained of by COVID-19 long haulers are fatigue, muscle weakness, and sleep difficulties, with a higher risk of developing anxiety or depression. Given the accumulating evidence on the effects of PCP in contrasting these symptoms in menopausal women, it could be speculated that PCP may be used as a remedy to mitigate persisting symptoms in long COVID patients.
Beetroot Juice
Beetroot juice, which is obtained from the red beetroot (Beta vulgaris), has spurred much attention owing to its potential beneficial effects on cardiovascular health, metabolic homeostasis, inflammation, and pulmonary function.168, 169, 170 The multiple health benefits of beetroot juice have traditionally been attributed to its high nitrate (NO3 −) content. Following ingestion, beetroot juice (as well as other nitrate-rich foods, such as leafy green vegetables) increases NO availability through the nitrate-nitrite-NO pathway.171 Dietary nitrate administration in the form of beetroot juice has been extensively studied in sport nutrition, owing to its purported beneficial effects on physical performance.172 In this context, beetroot juice may improve both endurance and power exercise performance through increasing NO bioavailability, which in turn modulates mitochondrial respiration, reduces oxygen cost of exercise, and improves muscular and cerebral perfusion.173 , 174 Recent evidence has shown that the stimulation of the nitrate-nitrite-NO pathway by beetroot juice consumption may improve endothelial function, as measured by flow-mediated dilation, in persons with or at risk of endothelial dysfunction, including older adults,175 people with peripheral artery disease,176 pregnant women,177 and persons with hypercholesterolemia178 and hypertension.179 Moreover, beetroot juice ingestion was associated with a reduction in blood pressure in normotensive180 and hypertensive people,181 and in older adults.182 Interestingly, higher baseline blood pressure, being overweight or obese, and male sex were associated with better response to beetroot juice supplementation.183 Beetroot juice consumption may also increase NO bioavailability in upper and lower airways, which boosts innate immune defense and protects against respiratory tract infections.184 In people with chronic obstructive pulmonary disease, nitrate-rich beetroot juice improves exercise tolerance as measured by the Borg Rating of Perceived Exertion scale.185
Accumulating evidence in preclinical models of inflammatory diseases suggests that inorganic nitrate and nitrite may modulate the inflammatory response by acting on neutrophil and monocyte/macrophage recruitment and activation.171 Only a few studies have investigated the effects of beetroot juice ingestion on the inflammatory response in humans. In healthy older volunteers and individuals with hypercholesterolemia, both acute and medium-term supplementation with beetroot juice was associated with a reduction in proinflammatory monocyte-platelets aggregates.178 , 186 Moreover, a decrease in neutrophil activation was observed within 3 hours of beetroot juice supplementation.186
Besides nitrate, beetroot juice also contains a plethora of bioactive ingredients with antioxidant and anti-inflammatory properties, including polyphenols, carotenoids, betalains, and organic acids.169 , 187 In particular, betalains, a class of natural pigments that includes the red-violet betacyanins and the yellow-orange betaxanthins,188 have gained much attention owing to their ability of stimulating the activity of antioxidant enzymes and inhibiting the inflammatory cascade.169 , 187 Betanin, the most abundant betalain found in beetroots, may directly scavenge reactive oxygen species189 and increase the expression of catalase, superoxide dismutase, and glutathione peroxidase through the activation of Nrf2/antioxidant response element pathway.190 Moreover, betanin and its derivatives may exert anti-inflammatory effects through the inhibition of COX2191 and lipoxygenase 1.192 Betanin may also inhibit NF-κB, a master regulator of the inflammatory response.193 Very few studies have investigated the anti-inflammatory effect of betanins in humans.194 Ten-day supplementation with betalain-rich beetroot extracts reduced the concentration of TNF-α and IL-6 in people with osteoarthritis.195 Consumption of both raw beetroot juice and cooked beets reduced intracellular adhesion molecule-1, vascular endothelial adhesion molecule-1, CRP, IL-6, E-selectin, and TNF-α in adults with hypertension.181 Although both supplementation forms were effective, people consuming beetroot juice showed greater improvement in systemic inflammatory parameters, as well as in blood pressure and endothelial function.181 Collectively, these findings suggest that beetroot juice may have multiple beneficial effects on crucial physiologic functions that could be exploited against COVID-19 and its systemic consequences.196
Further studies are needed to investigate the effects of supplementation with beetroot juice and/or its derivatives in patients with COVID-19 across disease stages and during recovery.
Ongoing Studies
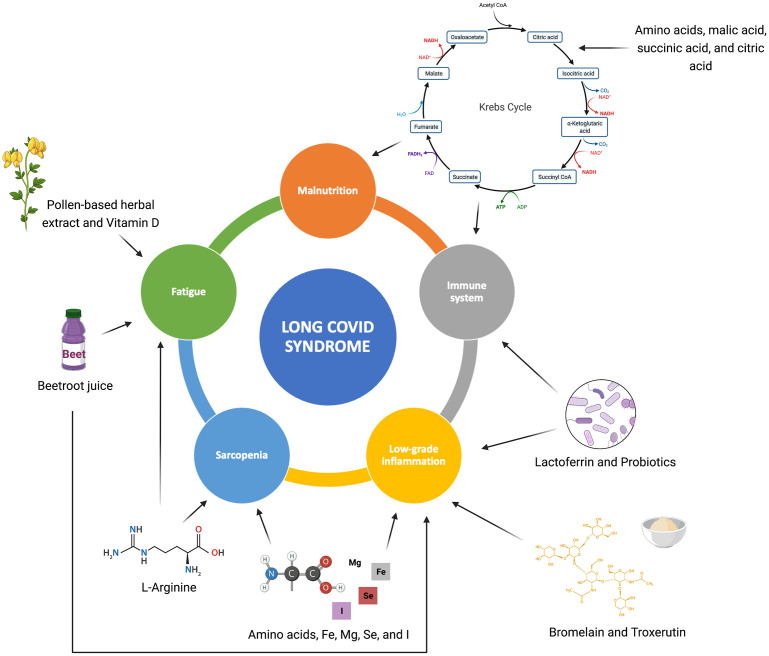
Based on the putative beneficial effects against several processes involved in long COVID, a panel of bioactive foods, nutraceuticals, and supplements is being tested in the outpatient service at the Fondazione Policlinico A. Gemelli IRCSS (Rome, Italy) (Fig. 1 )
Fig. 1.
Long COVID pathophysiological processes potentially targeted by the nutraceuticals and dietary supplements described in the study.
A combination of amino acids and malic, succinic, and citric acid (Amino-Ther Pro; Professional Dietetics, Milan, Italy) has been tested in patients with long COVID with malnutrition and fatigue. Preliminary data on the first 20 patients show improvement in nutritional status and better performance on the handgrip strength and five-repetition chair-stand test after 2 months of treatment (The Gemelli Against COVID-19 Post-Acute Care Study Group, 2022).
In a second pilot study, patients with persistent fatigue received a combination of amino acids and micronutrients (including magnesium, selenium, iron) (ApportAl; PharmaNutra, Pisa, Italy). After 6 weeks of treatment, muscle mass and strength improved with a significant reduction of inflammatory markers (CRP and IL-6) (The Gemelli Against COVID-19 Post-Acute Care Study Group, 2022).
l-Arginine plus vitamin C (Bioarginina C; Farmaceutici Damor, Naples, Italy) has been tested in COVID-19 survivors with persistent fatigue. Preliminary results on the first 46 patients treated with Bioarginina C indicate reduction in fatigue, better physical performance, and better quality of life (The Gemelli Against COVID-19 Post-Acute Care Study Group, 2022).
A combination of bromelain and troxerutin (BromeREX; Pharma G, Anzio, Italy) has been tested in patients with long COVID with increased inflammation. In the first 20 participants treated with bromelain and troxerutin, attenuation of post–COVID-19 symptoms and significant reduction of serum CRP levels were observed (The Gemelli Against COVID-19 Post-Acute Care Study Group, 2022).
A combination of lactoferrin and L paracasei (Pirv-F20; Farmagens Health Care, Rome, Italy) has been administered to persons with long COVID. Preliminary data suggest a significant reduction in inflammatory status and improvement in fatigue, dyspnea, and joint and muscle pain (The Gemelli Against COVID-19 Post-Acute Care Study Group, 2022).
A pollen-based herbal extract (Femal; Shionogi, Milan, Italy) has been prescribed to women with persistent fatigue, sleep disorders, and cognitive distress (brain fog) following an acute COVID-19 episode. A substantial improvement in persistent symptoms and quality has been observed (The Gemelli Against COVID-19 Post-Acute Care Study Group, 2022).
A beetroot juice (Aureli Mario S.S. Agricola, Ortucchio, Italy) with a well-characterized phytochemical profile197 has been tested in COVID-19 survivors with fatigue. Changes in physical performance (6-minute walking test, handgrip strength), endothelial function, inflammatory status, urinary and fecal metabolomics, and metagenomics are being evaluated. Preliminary data suggest that beetroot juice supplementation induces favorable immune and metabolic changes (The Gemelli Against COVID-19 Post-Acute Care Study Group, 2022).
Results from the authors’ ongoing studies may indicate novel strategies for the management of long COVID as part of multimodal interventions. An example of such interventions combining personalized nutrition counseling and physical activity was successfully in the Sarcopenia and Physical fRailty IN older people: multi-componenT Treatment strategies" (SPRINTT) project.198 A multimodel intervention inspired by SPRINTT is currently being implemented at the authors’ outpatient clinic.199
Summary
Long COVID affects a large share of persons of all age groups but may be especially burdensome for vulnerable older adults. Long COVID has a complex pathophysiology encompassing inflammatory and autoimmune processes, perturbations in metabolic pathways, and alterations in endothelial function and redox homeostasis.
The management of long COVID requires a multidimensional approach that should include a comprehensive nutritional assessment. Several food bioactive compounds, nutraceuticals, and supplements may target specific pathways involved in long COVID and may, therefore, be used as an adjunctive therapy to manage the condition.
Clinics care points
-
•
Long COVID affects a large share of persons of all age groups but may be especially burdensome for vulnerable older adults.
-
•
Long COVID has a complex pathophysiology, and its management requires a multidimensional approach that should include a nutritional assessment.
-
•
Food bioactive compounds and nutraceuticals may target specific pathways involved in long COVID.
Disclosure
Unconditional support to research activities at the postacute outpatient service, Fondazione Policlinico Universitario Agostino Gemelli IRCCS (Rome, Italy), was provided by Professional Dietetics, PharmaNutra, Farmaceutici Damor, Pharma G, Farmagens Health Care, Shionogi, Aureli Mario S.S. Agricola. The study was also supported by Ministero della Salute - Ricerca Corrente 2022. The funders had no role in the writing of the article nor decision to submit the article for publication.
Acknowledgments
The author is grateful to thank "The Gemelli Against COVID-19 Post-Acute Care Study Group".The gemelli against COVID-19 postacute care study group is composed as follows: Steering committee: Landi Francesco, Gremese Elisa. Coordination: Bernabei Roberto, Fantoni Massimo, Gasbarrini Antonio. Field Investigators: Gastroenterology team: Settanni Carlo Romano. Geriatric team: Benvenuto Francesca, Bramato Giulia, Brandi Vincenzo, Carfì Angelo, Ciciarello Francesca, Lo Monaco Maria Rita, Martone Anna Maria, Marzetti Emanuele, Napolitano Carmen, Pagano Francesco, Rocchi Sara, Rota Elisabetta, Salerno Andrea, Tosato Matteo, Tritto Marcello, Calvani Riccardo, Catalano Lucio, Picca Anna, Savera Giulia. Infectious disease team: Cauda Roberto, Tamburrini Enrica, Borghetti A, Di Gianbenedetto Simona, Murri Rita, Cingolani Antonella, Ventura Giulio, Taddei E, Moschese D, Ciccullo A. Internal Medicine team: Stella Leonardo, Addolorato Giovanni, Franceschi Francesco, Mingrone Gertrude, Zocco MA. Microbiology team: Sanguinetti Maurizio, Cattani Paola, Marchetti Simona, Posteraro Brunella, Sali M. Neurology team: Bizzarro Alessandra, Lauria Alessandra. Ophthalmology team: Rizzo Stanislao, Savastano Maria Cristina, Gambini G, Cozzupoli GM, Culiersi C. Otolaryngology team: Passali Giulio Cesare, Paludetti Gaetano, Galli Jacopo, Crudo F, Di Cintio G, Longobardi Y, Tricarico L, Santantonio M. Pediatric team: Buonsenso Danilo, Valentini P, Pata D, Sinatti D, De Rose C. Pneumology team: Richeldi Luca, Lombardi Francesco, Calabrese A. Psychiatric team: Sani Gabriele, Janiri Delfina, Giuseppin G, Molinaro M, Modica M. Radiology team: Natale Luigi, Larici Anna Rita, Marano Riccardo. Rheumatology team: Paglionico Annamaria, Petricca Luca, Gigante Luca, Natalello G, Fedele AL, Lizzio MM, Tolusso B, Alivernini S. Vascular team: Santoliquido Angelo, Santoro Luca, Nesci A, Popolla V.
References
- 1.Gupta A., Madhavan M.V., Sehgal K., et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19) https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report Available at: Accessed April 13, 2022.
- 3.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehandru S., Merad M. Pathological sequelae of long-haul COVID. Nat Immunol. 2022;23(2):194–202. doi: 10.1038/s41590-021-01104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groff D., Sun A., Ssentongo A.E., et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4(10) doi: 10.1001/JAMANETWORKOPEN.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bliddal S., Banasik K., Pedersen O.B., et al. Acute and persistent symptoms in non-hospitalized PCR-confirmed COVID-19 patients. Sci Rep. 2021;11(1) doi: 10.1038/S41598-021-92045-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tosato M., Carfì A., Martis I., et al. Prevalence and predictors of persistence of COVID-19 symptoms in older adults: a single-center study. J Am Med Dir Assoc. 2021;22(9):1840–1844. doi: 10.1016/j.jamda.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merad M., Blish C.A., Sallusto F., et al. The immunology and immunopathology of COVID-19. Science. 2022;375(6585):1122–1127. doi: 10.1126/science.abm8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douaud G., Lee S., Alfaro-Almagro F., et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022 doi: 10.1038/S41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mongelli A., Barbi V., Zamperla M.G., et al. Evidence for biological age acceleration and telomere shortening in COVID-19 survivors. Int J Mol Sci. 2021;22(11) doi: 10.3390/IJMS22116151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartleson J.M., Radenkovic D., Covarrubias A.J., et al. SARS-CoV-2, COVID-19 and the ageing immune system. Nat Aging. 2021;1(9):769–782. doi: 10.1038/s43587-021-00114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghidoli M., Colombo F., Sangiorgio S., et al. Food containing bioactive flavonoids and other phenolic or sulfur phytochemicals with antiviral effect: can we design a promising diet against COVID-19? Front Nutr. 2021;8 doi: 10.3389/FNUT.2021.661331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galanakis C.M., Aldawoud T.M.S., Rizou M., et al. Food ingredients and active compounds against the coronavirus disease (COVID-19) pandemic: a comprehensive review. Foods. 2020;9(11) doi: 10.3390/FOODS9111701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landi F., Gremese E., Bernabei R., et al. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613–1620. doi: 10.1007/s40520-020-01616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly B., Pearce E.L. Amino assets: how amino acids support immunity. Cell Metab. 2020;32(2):154–175. doi: 10.1016/j.cmet.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Newsholme P., Curi R., Pithon Curi T.C., et al. Glutamine metabolism by lymphocytes, macrophages, and neutrophils: its importance in health and disease. J Nutr Biochem. 1999;10(6):316–324. doi: 10.1016/s0955-2863(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 17.Palmieri E.M., Gonzalez-Cotto M., Baseler W.A., et al. Nitric oxide orchestrates metabolic rewiring in M1 macrophages by targeting aconitase 2 and pyruvate dehydrogenase. Nat Commun. 2020;11(1) doi: 10.1038/S41467-020-14433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam W.Y., Jash A., Yao C.H., et al. Metabolic and transcriptional modules independently diversify plasma cell lifespan and function. Cell Rep. 2018;24(9):2479–2492. doi: 10.1016/J.CELREP.2018.07.084. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruzat V., Rogero M.M., Keane K.N., et al. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients. 2018;10(11) doi: 10.3390/NU10111564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nie C., He T., Zhang W., et al. Branched chain amino acids: beyond nutrition metabolism. Int J Mol Sci. 2018;19(4) doi: 10.3390/IJMS19040954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calder P.C. Branched-chain amino acids and immunity. J Nutr. 2006;136(1 Suppl) doi: 10.1093/JN/136.1.288S. [DOI] [PubMed] [Google Scholar]
- 22.Ma N., Guo P., Zhang J., et al. Nutrients mediate intestinal bacteria-mucosal immune crosstalk. Front Immunol. 2018;9(JAN) doi: 10.3389/FIMMU.2018.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adebayo A., Varzideh F., Wilson S., et al. l-Arginine and COVID-19: an Update. Nutrients. 2021;13(11) doi: 10.3390/NU13113951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2(10):907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 25.Gharavi N.M., Baker N.A., Mouillesseaux K.P., et al. Role of endothelial nitric oxide synthase in the regulation of SREBP activation by oxidized phospholipids. Circ Res. 2006;98(6):768–776. doi: 10.1161/01.RES.0000215343.89308.93. [DOI] [PubMed] [Google Scholar]
- 26.Zhivaki D., Kagan J.C. Innate immune detection of lipid oxidation as a threat assessment strategy. Nat Rev Immunol. 2021 doi: 10.1038/S41577-021-00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubes P., Suzuki M., Granger D.N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 29.Ahluwalia A., Foster P., Scotland R.S., et al. Antiinflammatory activity of soluble guanylate cyclase: cGMP-dependent down-regulation of P-selectin expression and leukocyte recruitment. Proc Natl Acad Sci U S A. 2004;101(5):1386–1391. doi: 10.1073/pnas.0304264101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geiger R., Rieckmann J.C., Wolf T., et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 2016;167(3):829–842. doi: 10.1016/j.cell.2016.09.031. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reizine F., Lesouhaitier M., Gregoire M., et al. SARS-CoV-2-induced ARDS associates with MDSC expansion, lymphocyte dysfunction, and arginine shortage. J Clin Immunol. 2021;41(3):515–525. doi: 10.1007/s10875-020-00920-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mangoni A.A., Rodionov R.N., Mcevoy M., et al. New horizons in arginine metabolism, ageing and chronic disease states. Age Ageing. 2019;48(6):776–782. doi: 10.1093/ageing/afz083. [DOI] [PubMed] [Google Scholar]
- 33.Fiorentino G., Coppola A., Izzo R., et al. Effects of adding L-arginine orally to standard therapy in patients with COVID-19: a randomized, double-blind, placebo-controlled, parallel-group trial. Results of the first interim analysis. EClinicalMedicine. 2021:40. doi: 10.1016/J.ECLINM.2021.101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calvani R., Miccheli A., Landi F., et al. Current nutritional recommendations and novel dietary strategies to manage sarcopenia. J Frailty Aging. 2013;2(1):38–53. [PMC free article] [PubMed] [Google Scholar]
- 35.Piotrowicz K., Gąsowski J., Michel J.P., et al. Post-COVID-19 acute sarcopenia: physiopathology and management. Aging Clin Exp Res. 2021;33(10):2887–2898. doi: 10.1007/s40520-021-01942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaczka P., Michalczyk M.M., Jastrzab R., et al. Mechanism of action and the effect of beta-hydroxy-beta-methylbutyrate (HMB) supplementation on different types of physical performance - a systematic review. J Hum Kinet. 2019;68(1):211–222. doi: 10.2478/hukin-2019-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landi F., Calvani R., Picca A., et al. Beta-hydroxy-beta-methylbutyrate and sarcopenia: from biological plausibility to clinical evidence. Curr Opin Clin Nutr Metab Care. 2019;22(1):37–43. doi: 10.1097/MCO.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 38.Bear D.E., Langan A., Dimidi E., et al. β-Hydroxy-β-methylbutyrate and its impact on skeletal muscle mass and physical function in clinical practice: a systematic review and meta-analysis. Am J Clin Nutr. 2019;109(4):1119–1132. doi: 10.1093/ajcn/nqy373. [DOI] [PubMed] [Google Scholar]
- 39.Arazi H., Taati B., Suzuki K. A review of the effects of leucine metabolite (β-Hydroxy-β-methylbutyrate) supplementation and resistance training on inflammatory markers: a new approach to oxidative stress and cardiovascular risk factors. Antioxidants (Basel, Switzerland) 2018;7(10) doi: 10.3390/ANTIOX7100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsieh L.C., Chien S.L., Huang M.S., et al. Anti-inflammatory and anticatabolic effects of short-term β-hydroxy-β-methylbutyrate supplementation on chronic obstructive pulmonary disease patients in intensive care unit. Asia Pac J Clin Nutr. 2006;15(4):544–550. https://pubmed.ncbi.nlm.nih.gov/17077073/ Available at. Accessed April 13, 2022. [PubMed] [Google Scholar]
- 41.Santos-Fandila A., Zafra-Gómez A., Barranco A., et al. Quantitative determination of β-hydroxymethylbutyrate and leucine in culture media and microdialysates from rat brain by UHPLC-tandem mass spectrometry. Anal Bioanal Chem. 2014;406(12):2863–2872. doi: 10.1007/s00216-014-7694-y. [DOI] [PubMed] [Google Scholar]
- 42.Salto R., Vílchez J.D., Girón M.D., et al. β-hydroxy-β-methylbutyrate (HMB) promotes neurite outgrowth in neuro2a cells. PLoS One. 2015;10(8) doi: 10.1371/JOURNAL.PONE.0135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kougias D.G., Nolan S.O., Koss W.A., et al. Beta-hydroxy-beta-methylbutyrate ameliorates aging effects in the dendritic tree of pyramidal neurons in the medial prefrontal cortex of both male and female rats. Neurobiol Aging. 2016;40:78–85. doi: 10.1016/j.neurobiolaging.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Hankosky E.R., Sherrill L.K., Ruvola L.A., et al. Effects of β-hydroxy-β-methyl butyrate on working memory and cognitive flexibility in an animal model of aging. Nutr Neurosci. 2017;20(7):379–387. doi: 10.1080/1028415X.2016.1145376. [DOI] [PubMed] [Google Scholar]
- 45.Kougias D.G., Hankosky E.R., Gulley J.M., et al. Beta-hydroxy-beta-methylbutyrate (HMB) ameliorates age-related deficits in water maze performance, especially in male rats. Physiol Behav. 2017;170:93–99. doi: 10.1016/j.physbeh.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 46.Martínez-Reyes I., Chandel N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun. 2020;11(1) doi: 10.1038/S41467-019-13668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wellen K.E., Hatzivassiliou G., Sachdeva U.M., et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324(5930):1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Q.C., Zhao Y., Bian H.M. Anti-thrombotic effect of a novel formula from Corni fructus with malic acid, succinic acid and citric acid. Phytother Res. 2014;28(5):722–727. doi: 10.1002/ptr.5052. [DOI] [PubMed] [Google Scholar]
- 49.Ruocco C., Segala A., Valerio A., et al. Essential amino acid formulations to prevent mitochondrial dysfunction and oxidative stress. Curr Opin Clin Nutr Metab Care. 2021;24(1):88–95. doi: 10.1097/MCO.0000000000000704. [DOI] [PubMed] [Google Scholar]
- 50.Bifari F., Dolci S., Bottani E., et al. Complete neural stem cell (NSC) neuronal differentiation requires a branched chain amino acids-induced persistent metabolic shift towards energy metabolism. Pharmacol Res. 2020;158 doi: 10.1016/J.PHRS.2020.104863. [DOI] [PubMed] [Google Scholar]
- 51.Brunetti D., Bottani E., Segala A., et al. Targeting multiple mitochondrial processes by a metabolic modulator prevents sarcopenia and cognitive decline in SAMP8 mice. Front Pharmacol. 2020;11 doi: 10.3389/FPHAR.2020.01171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruins M.J., Van Dael P., Eggersdorfer M. The role of nutrients in reducing the risk for noncommunicable diseases during aging. Nutrients. 2019;11(1) doi: 10.3390/NU11010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calder P.C., Carr A.C., Gombart A.F., et al. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12(4) doi: 10.3390/NU12041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Avery J.C., Hoffmann P.R. Selenium, selenoproteins, and immunity. Nutrients. 2018;10(9) doi: 10.3390/NU10091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J., Saad R., Taylor E.W., et al. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020:37. doi: 10.1016/j.redox.2020.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bermano G., Meplan C., Mercer D.K., et al. Selenium and viral infection: are there lessons for COVID-19? Br J Nutr. 2021;125(6):618–627. doi: 10.1017/S0007114520003128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hiffler L., Rakotoambinina B. Selenium and RNA virus interactions: potential implications for SARS-CoV-2 infection (COVID-19) Front Nutr. 2020;7 doi: 10.3389/FNUT.2020.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kiremidjian-Schumacher L., Roy M., Wishe H.I., et al. Supplementation with selenium and human immune cell functions. II. Effect on cytotoxic lymphocytes and natural killer cells. Biol Trace Elem Res. 1994;41(1–2):115–127. doi: 10.1007/BF02917222. [DOI] [PubMed] [Google Scholar]
- 59.Giovannini S., Onder G., Lattanzio F., et al. Selenium concentrations and mortality among community-dwelling older adults: results from IlSIRENTE study. J Nutr Health Aging. 2018;22(5):608–612. doi: 10.1007/s12603-018-1021-9. [DOI] [PubMed] [Google Scholar]
- 60.Razeghi Jahromi S., Moradi Tabriz H., Togha M., et al. The correlation between serum selenium, zinc, and COVID-19 severity: an observational study. BMC Infect Dis. 2021;21(1) doi: 10.1186/S12879-021-06617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dhawan R.T., Gopalan D., Howard L., et al. Beyond the clot: perfusion imaging of the pulmonary vasculature after COVID-19. Lancet Respir Med. 2021;9(1):107–116. doi: 10.1016/S2213-2600(20)30407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet (London, England) 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skalny A.V., Timashev P.S., Aschner M., et al. Serum zinc, copper, and other biometals are associated with COVID-19 severity markers. Metabolites. 2021;11(4) doi: 10.3390/METABO11040244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Majeed M., Nagabhushanam K., Gowda S., et al. An exploratory study of selenium status in healthy individuals and in patients with COVID-19 in a south Indian population: the case for adequate selenium status. Nutrition. 2021:82. doi: 10.1016/J.NUT.2020.111053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bae M., Kim H. Mini-review on the roles of vitamin C, vitamin D, and selenium in the immune system against COVID-19. Molecules. 2020;25(22) doi: 10.3390/MOLECULES25225346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khatiwada S., Subedi A. A mechanistic link between selenium and coronavirus disease 2019 (COVID-19) Curr Nutr Rep. 2021;10(2):125–136. doi: 10.1007/s13668-021-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schomburg L. Selenium deficiency due to diet, pregnancy, severe illness, or covid-19-a preventable trigger for autoimmune disease. Int J Mol Sci. 2021;22(16) doi: 10.3390/IJMS22168532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Drakesmith H., Prentice A. Viral infection and iron metabolism. Nat Rev Microbiol. 2008;6(7):541–552. doi: 10.1038/nrmicro1930. [DOI] [PubMed] [Google Scholar]
- 70.Liu W., Zhang S., Nekhai S., et al. Depriving iron supply to the virus represents a promising adjuvant therapeutic against viral survival. Curr Clin Microbiol Reports. 2020;7(2):13–19. doi: 10.1007/s40588-020-00140-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Girelli D., Marchi G., Busti F., et al. Iron metabolism in infections: focus on COVID-19. Semin Hematol. 2021;58(3):182–187. doi: 10.1053/j.seminhematol.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ganz T., Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol. 2015;15(8):500–510. doi: 10.1038/nri3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shah A., Frost J.N., Aaron L., et al. Systemic hypoferremia and severity of hypoxemic respiratory failure in COVID-19. Crit Care. 2020;24(1) doi: 10.1186/S13054-020-03051-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hippchen T., Altamura S., Muckenthaler M.U., et al. Hypoferremia is associated with increased hospitalization and oxygen demand in COVID-19 patients. Hemasphere. 2020;4(6) doi: 10.1097/HS9.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao K., Huang J., Dai D., et al. Serum iron level as a potential predictor of coronavirus disease 2019 severity and mortality: a retrospective study. Open Forum Infect Dis. 2020;7(7) doi: 10.1093/OFID/OFAA250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lv Y., Chen L., Liang X., et al. Association between iron status and the risk of adverse outcomes in COVID-19. Clin Nutr. 2021;40(5):3462–3469. doi: 10.1016/j.clnu.2020.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Henry B.M., De Oliveira M.H.S., Benoit S., et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 78.Cheng L., Li H., Li L., et al. Ferritin in the coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Lab Anal. 2020;34(10) doi: 10.1002/JCLA.23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang I., Pranata R., Lim M.A., et al. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14 doi: 10.1177/1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sonnweber T., Boehm A., Sahanic S., et al. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients’ performance: a prospective observational cohort study. Respir Res. 2020;21(1) doi: 10.1186/S12931-020-01546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pal A., Squitti R., Picozza M., et al. Zinc and COVID-19: basis of current clinical trials. Biol Trace Elem Res. 2021;199(8):2882–2892. doi: 10.1007/s12011-020-02437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Samad N., Sodunke T.E., Abubakar A.R., et al. The implications of zinc therapy in combating the COVID-19 global pandemic. J Inflamm Res. 2021;14:527–550. doi: 10.2147/JIR.S295377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fukada T., Hojyo S., Hara T., et al. Revisiting the old and learning the new of zinc in immunity. Nat Immunol. 2019;20(3):248–250. doi: 10.1038/s41590-019-0319-z. [DOI] [PubMed] [Google Scholar]
- 84.Read S.A., Obeid S., Ahlenstiel C., et al. The role of zinc in antiviral immunity. Adv Nutr. 2019;10(4):696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Skalny A.V., Rink L., Ajsuvakova O.P., et al. Zinc and respiratory tract infections: perspectives for COVID-19 (review) Int J Mol Med. 2020;46(1):17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barnett J.B., Hamer D.H., Meydani S.N. Low zinc status: a new risk factor for pneumonia in the elderly? Nutr Rev. 2010;68(1):30–37. doi: 10.1111/j.1753-4887.2009.00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Singh M., Das R.R. Zinc for the common cold. Cochrane Database Syst Rev. 2013;2013(6) doi: 10.1002/14651858.CD001364.PUB4. [DOI] [PubMed] [Google Scholar]
- 88.te Velthuis A.J.W., van den Worml S.H.E., Sims A.C., et al. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11) doi: 10.1371/JOURNAL.PPAT.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Propper R.E. Smell/Taste alteration in COVID-19 may reflect zinc deficiency. J Clin Biochem Nutr. 2021;68(1):3. doi: 10.3164/jcbn.20-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carlucci P.M., Ahuja T., Petrilli C., et al. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J Med Microbiol. 2020;69(10):1228–1234. doi: 10.1099/jmm.0.001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yao J.S., Paguio J.A., Dee E.C., et al. The Minimal effect of zinc on the survival of hospitalized patients with COVID-19: an observational study. Chest. 2021;159(1):108–111. doi: 10.1016/j.chest.2020.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thomas S., Patel D., Bittel B., et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw Open. 2021;4(2) doi: 10.1001/JAMANETWORKOPEN.2021.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wallace T.C. Combating COVID-19 and building immune resilience: a potential role for magnesium nutrition? J Am Coll Nutr. 2020;39(8):685–693. doi: 10.1080/07315724.2020.1785971. [DOI] [PubMed] [Google Scholar]
- 94.Tang C.F., Ding H., Jiao R.Q., et al. Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. Eur J Pharmacol. 2020;886 doi: 10.1016/J.EJPHAR.2020.173546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Upala S., Jaruvongvanich V., Wijarnpreecha K., et al. Hypomagnesemia and mortality in patients admitted to intensive care unit: a systematic review and meta-analysis. QJM. 2016;109(7):453–459. doi: 10.1093/qjmed/hcw048. [DOI] [PubMed] [Google Scholar]
- 96.Iotti S., Wolf F., Mazur A., et al. The COVID-19 pandemic: is there a role for magnesium? Hypotheses and perspectives. Magnes Res. 2020;33(2):21–27. doi: 10.1684/mrh.2020.0465. [DOI] [PubMed] [Google Scholar]
- 97.Britton J., Pavord I., Richards K., et al. Dietary magnesium, lung function, wheezing, and airway hyperreactivity in a random adult population sample. Lancet. 1994;344(8919):357–362. doi: 10.1016/s0140-6736(94)91399-4. [DOI] [PubMed] [Google Scholar]
- 98.Kew K.M., Kirtchuk L., Michell C.I. Intravenous magnesium sulfate for treating adults with acute asthma in the emergency department. Cochrane Database Syst Rev. 2014;2014(5) doi: 10.1002/14651858.CD010909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang Q., Zhang P., Liu T., et al. Magnesium isoglycyrrhizinate ameliorates radiation-induced pulmonary fibrosis by inhibiting fibroblast differentiation via the p38MAPK/Akt/Nox4 pathway. Biomed Pharmacother. 2019;115 doi: 10.1016/J.BIOPHA.2019.108955. [DOI] [PubMed] [Google Scholar]
- 100.Chacko S.A., Song Y., Nathan L., et al. Relations of dietary magnesium intake to biomarkers of inflammation and endothelial dysfunction in an ethnically diverse cohort of postmenopausal women. Diabetes Care. 2010;33(2):304–310. doi: 10.2337/dc09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bermejo-Martin J.F., Almansa R., Torres A., et al. COVID-19 as a cardiovascular disease: the potential role of chronic endothelial dysfunction. Cardiovasc Res. 2020;116(10):E132–E133. doi: 10.1093/cvr/cvaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Darooghegi Mofrad M., Djafarian K., Mozaffari H., et al. Effect of magnesium supplementation on endothelial function: a systematic review and meta-analysis of randomized controlled trials. Atherosclerosis. 2018;273:98–105. doi: 10.1016/j.atherosclerosis.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 103.Tan C.W., Ho L.P., Kalimuddin S., et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B 12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19) Nutrition. 2020:79–80. doi: 10.1016/j.nut.2020.111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chakraborty A.J., Mitra S., Tallei T.E., et al. Bromelain a potential bioactive compound: a comprehensive overview from a pharmacological perspective. Life (Basel) 2021;11(4) doi: 10.3390/LIFE11040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bhui K., Prasad S., George J., et al. Bromelain inhibits COX-2 expression by blocking the activation of MAPK regulated NF-kappa B against skin tumor-initiation triggering mitochondrial death pathway. Cancer Lett. 2009;282(2):167–176. doi: 10.1016/j.canlet.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 106.Taussig S.J., Batkin S. Bromelain, the enzyme complex of pineapple (Ananas comosus) and its clinical application. An update. J Ethnopharmacol. 1988;22(2):191–203. doi: 10.1016/0378-8741(88)90127-4. [DOI] [PubMed] [Google Scholar]
- 107.Metzig C., Grabowska E., Eckert K., et al. Bromelain proteases reduce human platelet aggregation in vitro, adhesion to bovine endothelial cells and thrombus formation in rat vessels in Vivo. In Vivo (Brooklyn) 1999;13(1):7–12. [PubMed] [Google Scholar]
- 108.Kumakura S., Yamashita M., Tsurufuji S. Effect of bromelain on kaolin-induced inflammation in rats. Eur J Pharmacol. 1988;150(3):295–301. doi: 10.1016/0014-2999(88)90010-6. [DOI] [PubMed] [Google Scholar]
- 109.Engwerda C.R., Andrew D., Ladhams A., et al. Bromelain modulates T cell and B cell immune responses in vitro and in vivo. Cell Immunol. 2001;210(1):66–75. doi: 10.1006/cimm.2001.1807. [DOI] [PubMed] [Google Scholar]
- 110.Sagar S., Rathinavel A.K., Lutz W.E., et al. Bromelain inhibits SARS-CoV-2 infection via targeting ACE-2, TMPRSS2, and spike protein. Clin Transl Med. 2021;11(2) doi: 10.1002/CTM2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kritis P., Karampela I., Kokoris S., et al. The combination of bromelain and curcumin as an immune-boosting nutraceutical in the prevention of severe COVID-19. Metab Open. 2020;8:100066. doi: 10.1016/j.metop.2020.100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zamanian M., Bazmandegan G., Sureda A., et al. The protective roles and molecular mechanisms of troxerutin (vitamin p4) for the treatment of chronic diseases: a mechanistic review. Curr Neuropharmacol. 2021;19(1):97–110. doi: 10.2174/1570159X18666200510020744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Panat N.A., Maurya D.K., Ghaskadbi S.S., et al. Troxerutin, a plant flavonoid, protects cells against oxidative stress-induced cell death through radical scavenging mechanism. Food Chem. 2016;194:32–45. doi: 10.1016/j.foodchem.2015.07.078. [DOI] [PubMed] [Google Scholar]
- 114.Farajdokht F., Amani M., Bavil F.M., et al. Troxerutin protects hippocampal neurons against amyloid beta-induced oxidative stress and apoptosis. EXCLI J. 2017;16:1081–1089. doi: 10.17179/excli2017-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Salama S.A., Arab H.H., Maghrabi I.A. Troxerutin down-regulates KIM-1, modulates p38 MAPK signaling, and enhances renal regenerative capacity in a rat model of gentamycin-induced acute kidney injury. Food Funct. 2018;9(12):6632–6642. doi: 10.1039/c8fo01086b. [DOI] [PubMed] [Google Scholar]
- 116.Ahmadi Z., Mohammadinejad R., Roomiani S., et al. Biological and therapeutic effects of troxerutin: molecular signaling pathways come into view. J Pharmacopuncture. 2021;24(1):1–13. doi: 10.3831/KPI.2021.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Azarfarin M., Farajdokht F., Babri S., et al. Effects of troxerutin on anxiety- and depressive-like behaviors induced by chronic mild stress in adult male rats. Iran J Basic Med Sci. 2018;21(8):781–786. doi: 10.22038/IJBMS.2018.26915.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zavvari Oskuye Z., Mirzaei Bavil F., Hamidian G.H.R., et al. Troxerutin affects the male fertility in prepubertal type 1 diabetic male rats. Iran J Basic Med Sci. 2019;22(2):197–205. doi: 10.22038/ijbms.2018.32678.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yu Y., Zheng G. Troxerutin protects against diabetic cardiomyopathy through NF-κB/AKT/IRS1 in a rat model of type 2 diabetes. Mol Med Rep. 2017;15(6):3473–3478. doi: 10.3892/mmr.2017.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Siqueiros-Cendón T., Arévalo-Gallegos S., Iglesias-Figueroa B.F., et al. Immunomodulatory effects of lactoferrin. Acta Pharmacol Sin. 2014;35(5):557–566. doi: 10.1038/aps.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bruni N., Capucchio M.T., Biasibetti E., et al. Antimicrobial activity of lactoferrin-related peptides and applications in human and veterinary medicine. Molecules. 2016;21(6) doi: 10.3390/MOLECULES21060752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Valenti P., Antonini G. Lactoferrin: an important host defence against microbial and viral attack. Cell Mol Life Sci. 2005;62(22):2576–2587. doi: 10.1007/s00018-005-5372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pietrantoni A., Dofrelli E., Tinari A., et al. Bovine lactoferrin inhibits influenza A virus induced programmed cell death in vitro. Biometals. 2010;23(3):465–475. doi: 10.1007/s10534-010-9323-3. [DOI] [PubMed] [Google Scholar]
- 124.Beljaars L., Van Der Strate B.W.A., Bakker H.I., et al. Inhibition of cytomegalovirus infection by lactoferrin in vitro and in vivo. Antivir Res. 2004;63(3):197–208. doi: 10.1016/j.antiviral.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 125.Andersen J.H., Jenssen H., Sandvik K., et al. Anti-HSV activity of lactoferrin and lactoferricin is dependent on the presence of heparan sulphate at the cell surface. J Med Virol. 2004;74(2):262–271. doi: 10.1002/jmv.20171. [DOI] [PubMed] [Google Scholar]
- 126.Berlutti F., Pantanella F., Natalizi T., et al. Antiviral properties of lactoferrin--a natural immunity molecule. Molecules. 2011;16(8):6992–7012. doi: 10.3390/molecules16086992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chang R., Ng T.B., Sun W.Z. Lactoferrin as potential preventative and adjunct treatment for COVID-19. Int J Antimicrob Agents. 2020;56(3) doi: 10.1016/J.IJANTIMICAG.2020.106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Campione E., Lanna C., Cosio T., et al. Lactoferrin against SARS-CoV-2: in vitro and in silico evidences. Front Pharmacol. 2021;12 doi: 10.3389/FPHAR.2021.666600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rosa L., Cutone A., Lepanto M.S., et al. Lactoferrin: a natural glycoprotein involved in iron and inflammatory homeostasis. Int J Mol Sci. 2017;18(9) doi: 10.3390/IJMS18091985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Campione E., Lanna C., Cosio T., et al. Lactoferrin as antiviral treatment in COVID-19 management: preliminary evidence. Int J Environ Res Public Health. 2021;18(20) doi: 10.3390/IJERPH182010985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhong P., Xu J., Yang D., et al. COVID-19-associated gastrointestinal and liver injury: clinical features and potential mechanisms. Signal Transduct Target Ther. 2020;5(1) doi: 10.1038/S41392-020-00373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu Y., Guo C., Tang L., et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yeoh Y.K., Zuo T., Lui G.C.Y., et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70(4):698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hussain I., Cher G.L.Y., Abid M.A., et al. Role of gut microbiome in COVID-19: an insight into pathogenesis and therapeutic potential. Front Immunol. 2021;12 doi: 10.3389/FIMMU.2021.765965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu Q., Mak J.W.Y., Su Q., et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut. 2022;71(3):544–552. doi: 10.1136/gutjnl-2021-325989. [DOI] [PubMed] [Google Scholar]
- 136.Hill C., Guarner F., Reid G., et al. Expert consensus document. the international scientific association for probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 137.Dang A.T., Marsland B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019;12(4):843–850. doi: 10.1038/s41385-019-0160-6. [DOI] [PubMed] [Google Scholar]
- 138.Yahfoufi N., Mallet J.F., Graham E., et al. Role of probiotics and prebiotics in immunomodulation. Curr Opin Food Sci. 2018;20:82–91. [Google Scholar]
- 139.Cristofori F., Dargenio V.N., Dargenio C., et al. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: a door to the body. Front Immunol. 2021;12 doi: 10.3389/FIMMU.2021.578386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang Y., Wu Y., Wang Y., et al. Antioxidant properties of probiotic bacteria. Nutrients. 2017;9(5) doi: 10.3390/NU9050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mirashrafi S., Moravejolahkami A.R., Balouch Zehi Z., et al. The efficacy of probiotics on virus titres and antibody production in virus diseases: a systematic review on recent evidence for COVID-19 treatment. Clin Nutr ESPEN. 2021;46:1–8. doi: 10.1016/J.CLNESP.2021.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rather I.A., Choi S.B., Kamli M.R., et al. Potential adjuvant therapeutic effect of lactobacillus plantarum probio-88 postbiotics against SARS-COV-2. Vaccines. 2021;9(10) doi: 10.3390/VACCINES9101067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Salaris C., Scarpa M., Elli M., et al. Lacticaseibacillus paracasei DG enhances the lactoferrin anti-SARS-CoV-2 response in Caco-2 cells. Gut Microbes. 2021;13(1) doi: 10.1080/19490976.2021.1961970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nguyen Q.V., Chong L.C., Hor Y.Y., et al. Role of probiotics in the management of COVID-19: a computational perspective. Nutrients. 2022;14(2) doi: 10.3390/NU14020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.d’Ettorre G., Ceccarelli G., Marazzato M., et al. Challenges in the management of SARS-CoV2 infection: the role of oral bacteriotherapy as complementary therapeutic strategy to avoid the progression of COVID-19. Front Med. 2020;7 doi: 10.3389/FMED.2020.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li Q., Cheng F., Xu Q., et al. The role of probiotics in coronavirus disease-19 infection in Wuhan: a retrospective study of 311 severe patients. Int Immunopharmacol. 2021;95 doi: 10.1016/J.INTIMP.2021.107531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gutiérrez-Castrellón P., Gandara-Martí T., Abreu Y Abreu A.T., et al. Probiotic improves symptomatic and viral clearance in Covid19 outpatients: a randomized, quadruple-blinded, placebo-controlled trial. Gut Microbes. 2022;14(1) doi: 10.1080/19490976.2021.2018899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bouillon R., Marcocci C., Carmeliet G., et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev. 2019;40(4):1109–1151. doi: 10.1210/er.2018-00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Charoenngam N., Holick M.F. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020;12(7):1–28. doi: 10.3390/nu12072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gombart A.F. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4(9):1151–1165. doi: 10.2217/fmb.09.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rak K., Bronkowska M. Immunomodulatory effect of vitamin D and its potential role in the prevention and treatment of type 1 diabetes mellitus-a narrative review. Molecules. 2018;24(1) doi: 10.3390/MOLECULES24010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Littorin B., Blom P., Schölin A., et al. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide diabetes incidence study in Sweden (DISS) Diabetologia. 2006;49(12):2847–2852. doi: 10.1007/s00125-006-0426-x. [DOI] [PubMed] [Google Scholar]
- 153.Mokry L.E., Ross S., Ahmad O.S., et al. Vitamin D and risk of multiple sclerosis: a mendelian randomization study. Plos Med. 2015;12(8) doi: 10.1371/JOURNAL.PMED.1001866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sainaghi P.P., Bellan M., Carda S., et al. Hypovitaminosis D and response to cholecalciferol supplementation in patients with autoimmune and non-autoimmune rheumatic diseases. Rheumatol Int. 2012;32(11):3365–3372. doi: 10.1007/s00296-011-2170-x. [DOI] [PubMed] [Google Scholar]
- 155.Monlezun D.J., Bittner E.A., Christopher K.B., et al. Vitamin D status and acute respiratory infection: cross sectional results from the United States national health and nutrition examination survey, 2001-2006. Nutrients. 2015;7(3):1933–1944. doi: 10.3390/nu7031933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Baktash V., Hosack T., Patel N., et al. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad Med J. 2021;97(1149):442–447. doi: 10.1136/postgradmedj-2020-138712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chiodini I., Gatti D., Soranna D., et al. Vitamin D status and SARS-CoV-2 infection and COVID-19 clinical outcomes. Front Public Heal. 2021;9 doi: 10.3389/FPUBH.2021.736665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rastogi A., Bhansali A., Khare N., et al. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study) Postgrad Med J. 2022;98(1156):87–90. doi: 10.1136/postgradmedj-2020-139065. [DOI] [PubMed] [Google Scholar]
- 159.Annweiler C., Beaudenon M., Simon R., et al. Vitamin D supplementation prior to or during COVID-19 associated with better 3-month survival in geriatric patients: extension phase of the GERIA-COVID study. J Steroid Biochem Mol Biol. 2021;213 doi: 10.1016/J.JSBMB.2021.105958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Stroehlein J.K., Wallqvist J., Iannizzi C., et al. Vitamin D supplementation for the treatment of COVID-19: a living systematic review. Cochrane Database Syst Rev. 2021;5(5) doi: 10.1002/14651858.CD015043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Townsend L., Dyer A.H., McCluskey P., et al. Investigating the relationship between vitamin D and persistent symptoms following SARS-CoV-2 infection. Nutrients. 2021;13(7) doi: 10.3390/NU13072430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Remelli F., Vitali A., Zurlo A., et al. Vitamin D deficiency and sarcopenia in older persons. Nutrients. 2019;11(12) doi: 10.3390/NU11122861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Genazzani A., Panay N., Simoncini T., et al. Purified and specific cytoplasmic pollen extract: a non-hormonal alternative for the treatment of menopausal symptoms. Gynecol Endocrinol. 2020;36(3):190–196. doi: 10.1080/09513590.2020.1722994. [DOI] [PubMed] [Google Scholar]
- 164.Komosinska-Vassev K., Olczyk P., Kaźmierczak J., et al. Bee pollen: chemical composition and therapeutic application. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/297425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.LELLO S., CAPOZZI A., XHOLLI A., et al. The benefits of purified cytoplasm of pollen in reducing menopausal symptoms in peri- and post-menopause: an Italian multicentre prospective observational study. Minerva Obstet Gynecol November. 2021 doi: 10.23736/S2724-606X.21.04964-2. [DOI] [PubMed] [Google Scholar]
- 166.Hellström A.C., Muntzing J. The pollen extract Femal--a nonestrogenic alternative to hormone therapy in women with menopausal symptoms. Menopause. 2012;19(7):825–829. doi: 10.1097/gme.0b013e31824017bc. [DOI] [PubMed] [Google Scholar]
- 167.Fait T., Sailer M., Regidor P.A. Prospective observational study to evaluate the efficacy and safety of the pollen extract Sérélys ® in the management of women with menopausal symptoms. Gynecol Endocrinol. 2019;35(4):360–363. doi: 10.1080/09513590.2018.1538347. [DOI] [PubMed] [Google Scholar]
- 168.Mirmiran P., Houshialsadat Z., Gaeini Z., et al. Functional properties of beetroot (Beta vulgaris) in management of cardio-metabolic diseases. Nutr Metab (Lond) 2020;17(1) doi: 10.1186/S12986-019-0421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Milton-Laskibar I., Alfredo Martínez J., Portillo M.P. Current knowledge on beetroot bioactive compounds: role of nitrate and betalains in health and disease. Foods (Basel, Switzerland) 2021;10(6) doi: 10.3390/FOODS10061314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lundberg J.O., Weitzberg E., Gladwin M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 171.Kapil V., Khambata R.S., Jones D.A., et al. The noncanonical pathway for in vivo nitric oxide generation: the nitrate-nitrite-nitric oxide pathway. Pharmacol Rev. 2020;72(3):692–766. doi: 10.1124/pr.120.019240. [DOI] [PubMed] [Google Scholar]
- 172.Jones A.M., Thompson C., Wylie L.J., et al. Dietary nitrate and physical performance. Annu Rev Nutr. 2018;38:303–328. doi: 10.1146/annurev-nutr-082117-051622. [DOI] [PubMed] [Google Scholar]
- 173.Larsen F.J., Weitzberg E., Lundberg J.O., et al. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 2007;191(1):59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 174.Arazi H., Eghbali E. Possible effects of beetroot supplementation on physical performance through metabolic, neuroendocrine, and antioxidant mechanisms: a narrative review of the literature. Front Nutr. 2021;8 doi: 10.3389/FNUT.2021.660150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Walker M.A., Bailey T.G., McIlvenna L., et al. Acute dietary nitrate supplementation improves flow mediated dilatation of the superficial femoral artery in healthy older males. Nutrients. 2019;11(5) doi: 10.3390/NU11050954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pekas E.J., Wooden T.S.K., Yadav S.K., et al. Body mass-normalized moderate dose of dietary nitrate intake improves endothelial function and walking capacity in patients with peripheral artery disease. Am J Physiol Regul Integr Comp Physiol. 2021;321(2):R162–R173. doi: 10.1152/ajpregu.00121.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Volino-Souza M., De Oliveira G.V., Alvares T.S. A single dose of beetroot juice improves endothelial function but not tissue oxygenation in pregnant women: a randomised clinical trial. Br J Nutr. 2018;120(9):1006–1013. doi: 10.1017/S0007114518002441. [DOI] [PubMed] [Google Scholar]
- 178.Velmurugan S., Gan J.M., Rathod K.S., et al. Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Am J Clin Nutr. 2016;103(1):25–38. doi: 10.3945/ajcn.115.116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kapil V., Khambata R.S., Robertson A., et al. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertens. 2015;65(2):320–327. doi: 10.1161/HYPERTENSIONAHA.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vanhatalo A., Bailey S.J., Blackwell J.R., et al. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol. 2010;299(4) doi: 10.1152/ajpregu.00206.2010. [DOI] [PubMed] [Google Scholar]
- 181.Asgary S., Afshani M.R., Sahebkar A., et al. Improvement of hypertension, endothelial function and systemic inflammation following short-term supplementation with red beet (Beta vulgaris L.) juice: a randomized crossover pilot study. J Hum Hypertens. 2016;30(10):627–632. doi: 10.1038/jhh.2016.34. [DOI] [PubMed] [Google Scholar]
- 182.Kelly J., Fulford J., Vanhatalo A., et al. Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. Am J Physiol Regul Integr Comp Physiol. 2013;304(2) doi: 10.1152/AJPREGU.00406.2012. [DOI] [PubMed] [Google Scholar]
- 183.Ocampo D.A.B., Paipilla A.F., Marín E., et al. Dietary nitrate from beetroot juice for hypertension: a systematic review. Biomolecules. 2018;8(4) doi: 10.3390/BIOM8040134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kroll J.L., Werchan C.A., Rosenfield D., et al. Acute ingestion of beetroot juice increases exhaled nitric oxide in healthy individuals. PLoS One. 2018;13(1) doi: 10.1371/JOURNAL.PONE.0191030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Alshafie S., El-Helw G.O., Fayoud A.M., et al. Efficacy of dietary nitrate-rich beetroot juice supplementation in patients with chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. Clin Nutr ESPEN. 2021;42:32–40. doi: 10.1016/j.clnesp.2021.01.035. [DOI] [PubMed] [Google Scholar]
- 186.Raubenheimer K., Hickey D., Leveritt M., et al. Acute effects of nitrate-rich beetroot juice on blood pressure, hemostasis and vascular inflammation markers in healthy older adults: a randomized, placebo-controlled crossover study. Nutrients. 2017;9(11) doi: 10.3390/nu9111270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Clifford T., Howatson G., West D.J., et al. The potential benefits of red beetroot supplementation in health and disease. Nutrients. 2015;7(4):2801–2822. doi: 10.3390/nu7042801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Polturak G., Aharoni A. La Vie en Rose”: biosynthesis, sources, and applications of betalain pigments. Mol Plant. 2018;11(1):7–22. doi: 10.1016/j.molp.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 189.Kanner J., Harel S., Granit R. Betalains--a new class of dietary cationized antioxidants. J Agric Food Chem. 2001;49(11):5178–5185. doi: 10.1021/jf010456f. [DOI] [PubMed] [Google Scholar]
- 190.Krajka-Kuźniak V., Paluszczak J., Szaefer H., et al. Betanin, a beetroot component, induces nuclear factor erythroid-2-related factor 2-mediated expression of detoxifying/antioxidant enzymes in human liver cell lines. Br J Nutr. 2013;110(12):2138–2149. doi: 10.1017/S0007114513001645. [DOI] [PubMed] [Google Scholar]
- 191.Reddy M.K., Alexander-Lindo R.L., Nair M.G. Relative inhibition of lipid peroxidation, cyclooxygenase enzymes, and human tumor cell proliferation by natural food colors. J Agric Food Chem. 2005;53(23):9268–9273. doi: 10.1021/jf051399j. [DOI] [PubMed] [Google Scholar]
- 192.Vidal P.J., López-Nicolás J.M., Gandía-Herrero F., et al. Inactivation of lipoxygenase and cyclooxygenase by natural betalains and semi-synthetic analogues. Food Chem. 2014;154:246–254. doi: 10.1016/j.foodchem.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 193.Silva DVT da, Baião D. dos S., Ferreira V.F., et al. Betanin as a multipath oxidative stress and inflammation modulator: a beetroot pigment with protective effects on cardiovascular disease pathogenesis. Crit Rev Food Sci Nutr. 2022;62(2):539–554. doi: 10.1080/10408398.2020.1822277. [DOI] [PubMed] [Google Scholar]
- 194.Moreno-Ley C.M., Osorio-Revilla G., Hernández-Martínez D.M., et al. Anti-inflammatory activity of betalains: a comprehensive review. Hum Nutr Metab. 2021;25:200126. [Google Scholar]
- 195.Pietrzkowski Z., Nemzer B., Sporna A., et al. Influence of betalain-rich extract on reduction of discomfort associated with osteoarthritisa) • New Medicine 1/2010. Czytelnia Medyczna Borgis. 2009;(1) http://www.czytelniamedyczna.pl/3333,influence-of-betalainrich-extract-on-reduction-of-discomfort-associated-with-ost.html Available at. Accessed April 13, 2022. [Google Scholar]
- 196.Volino-Souza M., Oliveira GV de, Conte-Junior C.A., et al. Covid-19 quarantine: impact of lifestyle behaviors changes on endothelial function and possible protective effect of beetroot juice. Front Nutr. 2020;7 doi: 10.3389/FNUT.2020.582210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Giampaoli O., Sciubba F., Conta G., et al. Red Beetroot’s NMR-based metabolomics: phytochemical profile related to development time and production year. Foods (Basel, Switzerland) 2021;10(8) doi: 10.3390/FOODS10081887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Landi F., Cesari M., Calvani R., et al. The “Sarcopenia and Physical fRailty IN older people: multi-componenT Treatment strategies” (SPRINTT) randomized controlled trial: design and methods. Aging Clin Exp Res. 2017;29(1):89–100. doi: 10.1007/s40520-016-0715-2. [DOI] [PubMed] [Google Scholar]
- 199.Salini S., Russo A., Biscotti D., et al. Professional sport players recover from coronavirus and return to competition: hopes for resuming a normal life after COVID-19 for older people. J Gerontol Geriatr. 2020;68(Special issue 4):212–215. [Google Scholar]
- 200.Hashimoto S., Yoshizaki K., Uno K., et al. Prompt Reduction in CRP, IL-6, IFN-γ, IP-10, and MCP-1 and a Relatively Low Basal Ratio of Ferritin/CRP Is Possibly Associated With the Efficacy of Tocilizumab Monotherapy in Severely to Critically Ill Patients With COVID-19. Front Med (Lausanne) 2021;8:734838. doi: 10.3389/fmed.2021.734838. Published 2021 Sep 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading
- 1.Hashimoto Shoji, Yoshizaki Kazuyuki, Uno Kazuko, et al. Prompt Reduction in CRP, IL-6, IFN-γ, IP-10, and MCP-1 and a Relatively Low Basal Ratio of Ferritin/CRP Is Possibly Associated With the Efficacy of Tocilizumab Monotherapy in Severely to Critically Ill Patients With COVID-19. Frontiers in medicine. 2021;8 doi: 10.3389/fmed.2021.734838. [DOI] [PMC free article] [PubMed] [Google Scholar]