Abstract
Rationale
The coronavirus disease 2019 (COVID-19) pandemic and consequent lockdown measures have had a large impact on people's lives. Recent evidence suggests that self-rated health (SRH) scores remained relatively stable or increased during the pandemic.
Objective
For the current project, we examine potential changes in the variance decomposition of SRH before and during the COVID-19 pandemic in the Netherlands.
Methods
We analyse data from the Netherlands Twin Register to examine pre-pandemic SRH scores (N = 16,127), pandemic SRH scores (N = 17,451), and SRH difference scores (N = 7464). Additionally, we perform bivariate genetic analyses to estimate genetic and environmental variance components in pre-pandemic and pandemic SRH, and estimate the genetic correlation to assess potential gene-environment interaction.
Results
The majority of the sample (66.7%) reported the same SRH before and during the pandemic, while 10.8% reported a decrease, and 22.5% an increase. Individuals who reported good/excellent SRH before the pandemic were most likely to report unchanged SRH during the pandemic, and individuals with bad/mediocre/reasonable SRH more often reported increased SRH. The bivariate longitudinal genetic model reveals no significant change in variance decomposition of SRH from before to during the pandemic, with a heritability estimate of 45% (CI 36%–52%). We found that the genetic correlation could be constrained to 1, and a moderate unique environmental correlation (rE = 0.49, CI = 0.37 to 0.60).
Conclusions
We theorize that the increases in SRH are explained by uninfected individuals evaluating their health more positively than under normal circumstances (partly through social comparison with infected individuals), rather than actual improvements. As the same genes are expressed under different environmental exposures, these results imply no evidence for gene-environment interaction. While different environmental factors might influence SRH at the two time-points, the influence of environmental factors does not become relatively more important during the pandemic.
Keywords: Self-rated health, Covid-19, Lockdown, Twin study, Pandemic
Abbreviations: SRH, Self-Rated Health
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has had, and continues to have, an enormous impact worldwide. Even when not infected, people suffer from the consequences the preventive measures have on their daily life. Many efforts have been taken to slow down infection rates, such as social distancing policies, and the shutting down of schools, restaurants, and other public facilities. While these regulations are necessary to ensure sufficient capacity in intensive care (IC) units, they also have large economic and public health consequences. An important question, in this regard, is what the effect of the COVID-19 pandemic is on population health and well-being (Holmes et al., 2020). Answering this question requires world-wide research in various populations and settings due to differences in policies and (lockdown) measures for different populations. The consequences for the populations’ health and well-being are expected to vary from country to country not only due to differences in COVID-19 prevalence and regulations, but also because differences in well-being and health care already existed before the commencement of the pandemic (Helliwell et al., 2020).
In the Netherlands, COVID-19 spread rapidly in March 2020, leading to a so-called intelligent lockdown with stay-at-home and social distancing measures starting on March 12, 2020. During this intelligent lockdown, people were allowed to leave their homes and go outside for walks or work-outs, but public spaces such as shops, schools, bars, and restaurants were closed and people were asked to work from home. In this way, people were much more restricted than in their usual pre-pandemic lives, but the government also appealed to a self-discipline principle that allowed them to retain some authority over their lives. Studies that investigated the health consequences of the COVID-19 pandemic in the Netherlands have mainly focussed on the consequences for mental health. For example, in a study in Dutch older adults, it was found that measures for social distancing led to higher levels of loneliness, but that mental health remained relatively stable (van Tilburg et al., 2020). In another study, mental health status during the pandemic was compared to retrospective reports of pre-pandemic mental health in a population-representative sample, with 80% of the participants reporting no change in mental health since the beginning of the pandemic. Moreover, being male and having high pre-pandemic levels of positive well-being seemed to act as protective factors for well-being (Gijzen et al., 2020).
While mental health is a very important aspect of people's health, it does not cover the concept of health in its entirety. While it is obvious that many people with a current or past COVID-19 infection suffer from health consequences, people who have not been infected might also suffer from indirect health consequences, for example due to changes in diet, exercise, and sleeping patterns, stress and loneliness (Bu et al., 2020; Cancello et al., 2020; Kowal et al., 2020; Martínez-de-Quel et al., 2021; Poelman et al., 2021). An interesting question in the context of the COVID-19 pandemic and consequent lockdown measures is to what extent it has affected people's Self-Rated Health (SRH). Typically measured using a single Likert scale question, SRH is a reliable and valid measure of subjective health as measured by other indicators in many population groups (Ahmad et al., 2014): it is a good predictor of mortality and chronic or severe diseases (Idler and Benyamini, 1997; Jylhä, 2009), and higher SRH is associated with better mental health and well-being (Baselmans et al., 2019; Van Lente et al., 2012). Thus, SRH predominantly measures people's subjective perceptions of health, but is also associated with objective health status. In addition, SRH is widely used to study trends and socio-economic inequalities in population health (Assari and Lankarani, 2015). Existing literature suggests that the concept of SRH is useful both as a spontaneous assessment of health and for more enduring evaluations of one's health. Measures of SRH are responsive to changes in health status such as changes in mental well-being, but it also seems to be a relatively stable measure over time, supporting the role for an enduring self-concept of health (Perruccio et al., 2010). Changes in SRH have been linked to several factors, such as changes in income (Gunasekara et al., 2011), different physical and psychosocial work factors (Dieker et al., 2019), and lifestyle characteristics such as changed physical activity or dietary habits (Pisinger et al., 2009).
With respect to the COVID-19 pandemic, a study in large German sample examined changes in SRH from before to during the pandemic (Peters et al., 2020). More than half of the participants (56%) reported no changes in their SRH, while 32% reported improved SRH, and 12% reported a decrease. Most participants who reported worsened SRH had been tested for COVID-19. Similarly, in a study with French respondents, more people reported to be in very good health during lockdown compared to between 2017 and 2019 (Recchi et al., 2020). The authors refer to this finding as an “eye of the hurricane” paradox, where individuals who are not infected by the COVID-19 virus might evaluate their health more positively than they normally would. A number of studies have assessed factors that potentially predict changes in SRH in (the beginning of) the pandemic. Bierman and colleagues find that baseline SRH and baseline psychological distress are associated with SRH during the pandemic, with individuals reporting greater distress and lower SRH before the pandemic also reporting lower SRH during the pandemic (Bierman et al., 2021a). A similar result was found in a study by Szwarcwald and colleagues, where the proportion of individuals reporting decreased SRH during pandemic was larger for individuals reporting bad baseline SRH compared to those reporting good baseline SRH (Szwarcwald et al., 2021).
Individual differences in SRH are accounted for by both genetic and environmental factors unique to the individual, with heritability estimates ranging from 25% to 64% (Romeis et al., 2000; Silventoinen et al., 2007). The variation in heritability estimates may reflect population differences or changes in the relative role of genetic and environmental factors across the lifespan. In a large longitudinal study of Finnish twins, the heritability of SRH peaked at 63% at age 16, but declined to 33% at age 25 (Silventoinen et al., 2007). The study also found that genetic factors were primarily responsible for moderate correlations between health ratings at different life stages. In contrast, Mosing et al. (2009) observed a heritability of 46% in a sample of elderly Australian twins, and observed increasing heritability and genetic variance of SRH in older age groups among Swedish twins. It is important to keep in mind that heritability reflects the relative influence of genetic factors. This means that if environmental variance increases, the relative influence of genetic factors will decrease. In case of a large environmental change, such as a pandemic, heritability estimates may thus change. Additionally, new genetic variation might emerge in different environmental situations, e.g. the presence of stressors in an environment might lead to stress-specific genetic variation (De Geus et al., 2007), a phenomenon that is known as gene by environment (GxE) interaction.
In this paper, we examine the effect of the COVID-19 pandemic on SRH during the first intelligent lockdown in the Netherlands in persons that did not have noticeable COVID-19 symptoms. SRH scores from before the pandemic are compared to scores during the first months of the intelligent lockdown. Moreover, a genetically informative design is applied that allows us to decompose variance in, and covariance between, SRH at the two time-points. More specifically, we assess whether the total genetic and environmental variance changes (quantitative gene-environment interaction). If the pandemic leads to an increase in unique environmental variance, and genetic effects remain stable, then the relative influence of environmental variance will increase while the relative influence of genetic factors on individual differences (the heritability) will decrease. An increase in environmental variance is expected if the environmental changes brought by the pandemic do not impact everybody in the same way (e.g. people with different professions and different household compositions may be differently impacted by work-from-home policies, effectively amplifying existing differences between individuals). Additionally, the genes and environmental factors that influence SRH under “normal conditions” may be at least partially different from those influencing SRH under a different, perhaps more stressful, environment during the pandemic. We therefore also examine whether different genes influence SRH during the pandemic by assessing the genetic correlation (qualitative gene-environment interaction).
2. Materials and methods
2.1. Sample
All study participants were registered with the Netherlands Twin Register (NTR) (Ligthart et al., 2019). Every couple of years, NTR participants are asked to fill out a survey including questions about their health, lifestyle, personality, well-being, and other life domains. For the present study, we compared pre-pandemic SRH data collected between 2014 and 2020 to pandemic SRH data collected in April and May 2020 (the first lockdown in the Netherlands). Data were collected in twins and multiples and family members who were 16 years or older. For the pre-pandemic sample, we used data collected in two questionnaires: one collected in 2014–2015, and one collected in 2019–2020. The means and variances of SRH were very similar for the observations from 2014 to 2015 (M = 3.98, SD = 0.72, variance = 0.52) and the observations from 2019 to 2020 (M = 3.95, SD = 0.72, variance = 0.52). In predicting pandemic SRH from pre-pandemic SRH, adding the number of years between the pre-pandemic and pandemic data-points as a predictor significantly improved the prediction model (p = .005), but the change in R2 (0.001) was negligible. When participants filled out both pre-pandemic questionnaires, we used data from the last questionnaire. For twin analyses (see below), we only included twin pairs where both twins had data available from the same survey.
Sample characteristics can be found in Table 1 . Since the focus of our study was to examine the effect of the pandemic in general, and not the disease itself, we excluded individuals who tested positive for COVID-19 or had an expected COVID-19 diagnosis based on the Menni model (Menni et al., 2020) (more information in measures section). In total, 517 participants were excluded due to (expected) COVID-19 infection, of whom 217 had data on both time-points. As seen in Table 1, these individuals were on average younger than the general sample and scored lower on SRH. In total, pre-pandemic SRH data were available for 16,127 participants (5602 males and 10,525 females). After excluding cases, pandemic SRH data were available for 17,451 participants (5065 males and 12,386 females). Of these people, 7464 had data available at both time-points (2214 males and 5250 females).
Table 1.
Descriptive statistics for SRH.
| N (males/females) | M(SD) age | M(SD) SRH | Var (Range) SRH | ||
|---|---|---|---|---|---|
| Excluded COVID cases | 517 (241/330) | 36.02 (12.90) | 4.01 (.81) | .66 (1–5) | |
| Pre-pandemic questionnaire | 16127 (5602/10525) | 41.47 (16.37) | 3.97 (.72) | .52 (1–5) | |
| Pandemic questionnaire (excl. cases) | 17451 (5064/12387) | 44.63 (14.80) | 4.12 (.68) | .46 (1–5) | |
| Overlap (excl. cases)* | 7464 (2214/5250) | 44.63 (16.44) | 3.99 (.71) | .51 (1–5) | |
| Overlap (excl. cases)** | 7464 (2214/5250) | 47.46 (15.13) | 4.11 (.70) | .49 (1–5) | |
| Non-overlap (only pre-pandemic) | 8623 (3353/5270) | 38.91 (15.85) | 3.96 (.72) | .52 (1–5) | |
| Non-overlap (only pandemic) | 8798 (2433/6365) | 40.62 (13.16) | 4.14 (.66) | .43 (1–5) | |
| Difference scores | .12 (.61) | .38 (−3 to 3) | |||
Note. N= Sample Size, M = Mean, SD=Standard Deviation, Var = Variance, SRH= Self-Rated Health.
* pre-pandemic descriptives.
** pandemic descriptives.
With respect to missingness, 8623 individuals responded to a pre-pandemic survey but did not respond to the pandemic survey, and 8798 individuals failed to respond to the pre-pandemic survey but did respond to the pandemic survey (see Table 1). Logistic regression indicates that missingness for the pandemic survey was not completely at random, with pre-pandemic SRH (β = −0.16, SE = 0.02, p < .001), age (β = −0.02, SD = 0.001, p < .001), and gender (β = −0.51, SE = 0.04, p < .001) predicting missingness for the pandemic survey. However, individuals who had pre-pandemic data available but did not respond to the pandemic questionnaire scored very similar on SRH (M = 3.96, SD = 0.72) as individuals who filled out both questionnaires (M = 3.99, SD = 0.71). The group that filled out both questionnaires had a lower percentages of males (29.7%) and were slightly older (M = 44.63, SD = 16.44) compared to the group that only responded to the pre-pandemic surveys (38.9% males, M age = 38.91, SD age = 15.85). With respect to missingness for the pre-pandemic survey, we could predict this missingness with age (β = −0.04, SD = 0.001, p <.001), but not with pandemic SRH (β = −0.03, SD = 0.02, p = .22) or sex (β = −0.06, SD = 0.04, p = .08). Compared to respondents with both pandemic and pre-pandemic data, individuals that did not have pre-pandemic data were younger (M = 40.62, SD = 13.16) than individuals who had data at both time-points (M = 47.46, SD = 15.13).
2.2. Measures
SRH was measured with the single item ‘In general, how would you rate your health?‘. In both questionnaires, there were five answer options which are scored on a five point scale with 1 = “bad”, 2 = “mediocre”, 3 = “reasonable”, 4 = “good”, and 5 = “excellent”. This single item assessment of SRH is recommended by the World Health Organization (WHO) and validated across many studies and contexts (Ahmad et al., 2014; Bardage et al., 2005; Chandola and Jenkinson, 2000).
COVID-19 infection status was assessed by two methods: First, by asking participants if they had been tested for COVID-19, and if so, whether an infection was confirmed (0 = No, 1 = Yes). Since there was limited testing in the Netherlands at the time of data collection, it is likely that many people remained undiagnosed at that time. Therefore, we also enquired the extent to which participants experienced a range of symptoms since February 20 (on a 5-point scale) and used the Menni self-reported symptom-based prediction model (Menni et al., 2020) to predict whether a person likely had COVID-19 (see original paper for more details). We excluded individuals if they reported having been tested positive, or were predicted to have been infected based on the Menni model.
2.3. Statistical analyses
2.3.1. Pre-pandemic to pandemic comparison
For the pre-pandemic and pandemic SRH, we computed the means, variances, and min-max range. For the subset of participants with data available for both surveys, we calculated within-person difference scores by subtracting the pre-pandemic questionnaire scores from the pandemic questionnaire scores for SRH. Means were compared in a genetically unrelated subsample using a paired-samples t-test. Statistical tests were performed in R (R Core Team, 2013).
2.3.2. Bivariate genetic models
In a bivariate genetic model for twin data, we quantified the contribution of genetic and environmental factors to pre-pandemic and pandemic SRH (excluding COVID-19 cases) and the stability of SRH over time. These models rely on the fact that monozygotic (MZ) twins share (nearly) 100% of their genes, while dizygotic (DZ) twins share on average 50% of their segregating genes. This makes it possible to decompose (co)variation in a set of traits into four potential sources: additive genetic (A) factors (shared 100% by MZ twins and 50% by DZ) twins, dominant genetic (D) factors (shared 100% by MZ twins and 25% by DZ twins), common environmental factors (C) (shared completely by both types of twins) and unique environmental (E) factors (unshared environmental factors and measurement error). When the MZ correlation is less than twice the DZ correlation, an ACE model is used. When the MZ correlation is twice or more the DZ correlation, and ADE model is used. Based on earlier research on SRH in the Netherlands, we expect the twin correlations to reflect an ADE model (De Moor et al., 2007). Twin correlations and cross-twin cross-trait correlations were estimated in saturated models in which all parameters (means, covariates, variances, and covariances) were freely estimated.
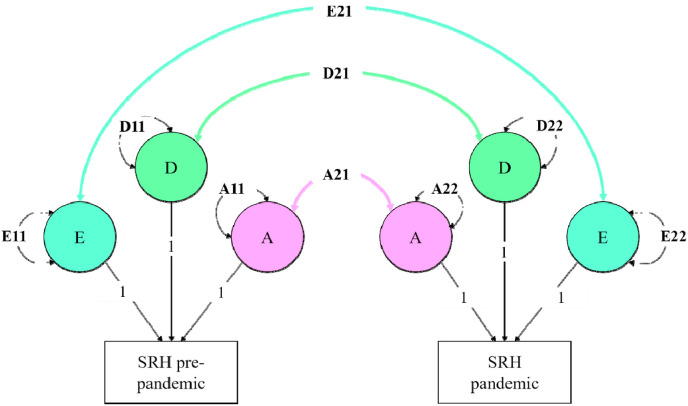
We performed the bivariate genetic analyses using the variance component approach (Verhulst et al., 2019) in OpenMx (Boker et al., 2011) (see Fig. 1 ). Since SRH was measured on an ordinal scale, we fitted threshold models to the data, with gender and age as covariates. These models assume that categorical variables have an underlying liability with a continuous and standard normal distribution. We used 1 threshold to divide the liability distribution into two discrete categories, one representing less than good health and one representing good/excellent health. The contribution of the A and D variance components was estimated using full-information maximum-likelihood estimation and tested for significance by dropping these components one by one. By fitting the model with and without the constraints of interest, a log-likelihood ratio test (LRT) can be used to compare the nested sub-models models. The more parsimonious model is rejected if the log-likelihood statistic exceeds the chosen p-value threshold. We chose a p-value threshold of p = .005, in line with the reasoning described in Benjamin et al. (2018).
Fig. 1.
Variance decomposition of SRH into additive genetic (A), dominant genetic (D), and unique environmental (E) variance components.
We tested for potential gene-environment interaction in two steps. First, we constrained the genetic correlation to 1 and compared the fit of the model where the genetic correlation could be freely estimated to the fit of the model where the genetic correlation was constrained to 1 with a log-likelihood ratio test. If the fit of the constrained model is significantly worse than the fit of the unconstrained model, it indicates the genetic correlation cannot be constrained to 1 and thus that different genes influence SRH at the two time-points, pointing at qualitative gene-environment interaction (Falconer, 1952). That is, given a change in environmental conditions, we can test in the longitudinal data if the environmental change triggers a change in the genes that are expressed. In the same model, we also tested for quantitative gene-environment interaction by comparing the contribution of the variance components during the pandemic compared to pre-pandemic. If the amount of genetic/environmental variance changes significantly from pre-pandemic to pandemic, it indicates that genes interact with environmental change in the form of quantitative gene-environment interaction. We tested this using a log-likelihood ratio test where a model where the genetic variance components were constrained to be equal were compared to the unconstrained model. This constraint was applied by setting the variance explained by genetic factors pre-pandemic (A11) equal to the variance explained by genetic factors during the pandemic (A22).
3. Results
3.1. Pre-COVID to COVID-19 comparison
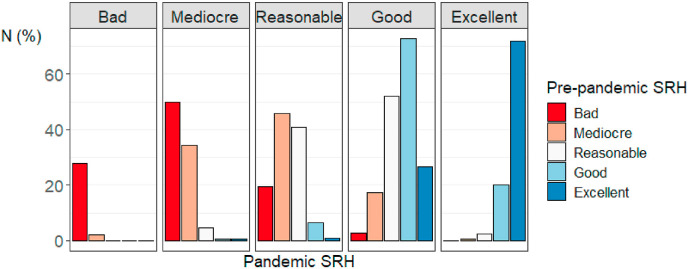
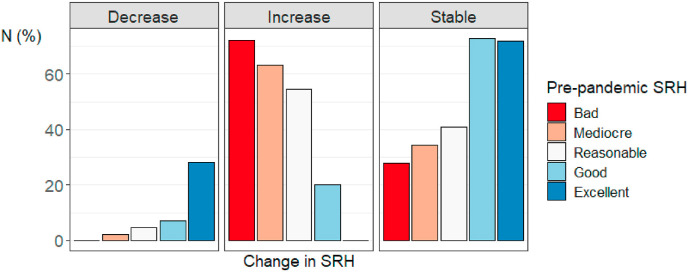
Descriptive statistics for the full sample for SRH at both time-points and the SRH difference scores can be found in Table 1. Excluding (suspected) COVID cases, 807 participants (10.8%) scored lower, 4975 participants (66.7%) scored the same, and 1682 participants (22.5%) scored higher on the pandemic SRH measure vs. the pre-pandemic SRH measure. Fig. 2 and Table 2 depict percentages of respondents per pre-pandemic SRH category (the different colours) categorized by their pandemic SRH score (the different columns). To illustrate, the red bar in the column “mediocre” visualizes the percentage of individuals that indicated feeling bad before the pandemic, but indicated feeling mediocre during the pandemic (50%). Fig. 3 shows the percentage of individuals from each pre-pandemic SRH category with decreased, increased, and stable SRH during the pandemic. Respondents who indicated having good or excellent SRH before the pandemic were relatively most stable, with 72.8% (N = 3353) and 72% (N = 1098) of participants scoring in these respective categories scoring in the same category during the pandemic. About half of the respondents indicating bad (50%, N = 18), mediocre (45.7%, N = 101) or reasonable SRH (52.1%, N = 559) before the pandemic scored one category higher on SRH during the pandemic. For those with decreased SRH levels during the pandemic, the most common decrease was from excellent to good (N = 406, 26.6% of individuals with excellent pre-pandemic SRH). In a genetically unrelated sample of participants who provided data at both time-points, mean SRH scores were significantly lower in the pre-pandemic questionnaire (M = 3.96, SD = 0.72) compared to the pandemic questionnaire (M = 4.09, SD = 0.68) (M diff = -0.12, t(4025) = −12.67, p < 2.2 × 10−16). Supplementary Figure 1 provides histograms of the distribution of pre-pandemic SRH, pandemic SRH, and SRH difference scores. These figures reveal that SRH is not normally distributed at both time-points, but that the difference scores are approximately normally distributed (as assumed by a paired-samples t-test).
Fig. 2.
Percentage of individuals per pre-pandemic self-rated health (SRH) category categorized by pandemic SRH score.
Table 2.
Cross table of pre-pandemic and pandemic self-rated health (SRH) scores.
| Pandemic SRH | Bad | Mediocre | Reasonable | Good | Excellent | Total | |
|---|---|---|---|---|---|---|---|
| Pre-pandemic SRH | Bad | 10 (27.8%) | 18 (50%) | 7 (19.4%) | 1 (2.8%) | 0 (0%) | 36 |
| Mediocre | 5 (2.3%) | 76 (34.4%) | 101 (45.7%) | 38 (17.2%) | 1 (0.5%) | 221 | |
| Reasonable | 0 (0%) | 49 (4.6%) | 438 (40.8%) | 559 (52.1%) | 27 (2.5%) | 1073 | |
| Good | 2 (0%) | 21 (0.5%) | 302 (6.6%) | 3353 (72.8%) | 930 (20.2%) | 4608 | |
| Excellent | 0 (0%) | 8 (0.5%) | 14 (0.9%) | 406 (26.6%) | 1098 (72%) | 1526 | |
| Total | 17 | 172 | 862 | 4357 | 2056 | 7464 |
Fig. 3.
The percentage of individuals from each pre-pandemic self-rated health (SRH) category with decreased, increased, and stable SRH during the pandemic.
3.2. Bivariate genetic models
The overall phenotypic correlation between SRH at the two time-points in our sample is .72 (CI 0.66 to 0.77). The twin correlations and cross-twin cross-trait correlations from the saturated model are displayed in Table 3 . The pre-pandemic MZ correlation (rMZ = 0.54, CI = 0.42 to 0.64) and pandemic MZ correlation (rMZ = 0.44, CI = 0.31 to.56) were larger than twice the pre-pandemic DZ correlation (rDZ = 0.12, CI = −0.11 to 0.33) and pandemic DZ correlation (rDZ = 0.15, CI = −0.09 to 0.38), indicating both the presence of additive (A) and dominant genetic influences (D).
Table 3.
Twin correlations.
| MZ | ||
|---|---|---|
| SRH pre-pan | SRH pan | |
| SRH pre-pan | .54 (.42–.64) | |
|
SRH pan |
.37 (.23–.50) |
.44 (.31–.56) |
|
DZ | ||
|
SRH pre-pan |
SRH pan |
|
| SRH pre-pan | .12 (−.11 to .33) | |
| SRH pan | .22 (−.06 to .47) | .15 (−.09 to .38) |
SRH pre-pan = Self-rated health pre-pandemic, SRH pan =Self-rated during the pandemic, MZ = monozygotic, DZ = dizygotic.
The full model fitting results can be found in Table 4 . Dropping the D component, resulting in an AE model, did not lead to significantly worse model fit compared to the full ADE model (Δ-2LL(Δdf) = 2.47(3), p = .48). Additionally, constraining the genetic correlation to 1 also did not result in a significantly worse model fit (Δ-2LL(Δdf) = 1.27(1), p = .26), indicating an absence of qualitative gene-environment interaction. Lastly, constraining the variance components to be equal also did not result in a worse model fit (Δ-2LL(Δdf) = 2.05(1), p = .15), indicating the absence of quantitative gene-environment interaction. In this final model, the heritability for both traits was A = 0.45 (CI 0.36 to 0.52), indicating that 45% of individual differences in SRH could be explained by genetic factors, both before and during the pandemic. The other 55% could be explained by unique environmental differences (E = 0.55, CI = 0.48 to 0.64).We found a moderate unique environmental correlation (rE = 0.49, CI = 0.37 to 0.60) indicating that partly different environmental factors influence SRH at the two time-points.
Table 4.
Bivariate model fitting results and parameter estimates.
| Model fitting results |
Standardized parameter estimates |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SRH pre-pandemic |
SRH pandemic |
|||||||||||
| Model | vs. | -2LL | df | χ2 | Δ df | p | A | D | E | A | D | E |
| 1. Saturated | – | 4057.41 | 5617 | – | – | – | – | – | – | – | – | – |
| 2. ADE | 1 | 4069.94 | 5628 | 12.53 | 11 | 0.33 | .01 | .53 | .46 | .16 | .27 | .57 |
| 3. AE | 2 | 4072.41 | 5631 | 2.47 | 3 | 0.48 | .52 | – | .48 | .42 | – | .58 |
| 4. AE, rA = 1 | 3 | 4073.68 | 5632 | 1.27 | 1 | 0.26 | .51 | – | .49 | .38 | – | .62 |
| 5. AE, rA = 1, A11 = A21 | 4 | 4075.73 | 5633 | 2.05 | 1 | 0.15 | .45 | - | .55 | .45 | - | .55 |
Note. Best fitting model is presented in bold. Vs. = versus, -2LL = −2 Log Likelihood, df = degrees of freedom, χ2 = chi-square statistic, Δ df = difference in degrees of freedom, p = p-value, SRH = self-rated health, A = proportion of variance due to additive genetic factors, D = proportion of variance due to dominant genetic effects E = proportion of variance due to unique environmental effects.
4. Discussion
4.1. Self-rated health during the COVID-19 pandemic in the Netherlands
In this study, we examined changes in SRH from before the COVID-19 pandemic to during the beginning of the pandemic in a Dutch sample. When we compared the average SRH before the pandemic with the average SRH during the pandemic, we find that (on average) SRH has increased. We observed individual differences in how people's reports of SRH changed. While the majority of the sample (66.7%) did not report a change in their SRH, about one in ten (10.8%) reported a decrease, and about two in ten (22.5%) reported an increase.
The finding that most people's SRH did not change suggests that individuals were quite resilient during the beginning of the COVID-19 pandemic. Importantly, these results only pertain to the beginning of the pandemic, and it is possible that changes in SRH might only reveal themselves over a longer period of time. Having said this, we did find that more participants report an increase rather than a decrease in SRH, which is consistent with previous studies on SRH in the beginning of the pandemic (Peters et al., 2020; Recchi et al., 2020). More specifically, we found that individuals who already reported good or excellent SRH before the pandemic were most likely to report unchanged SRH during the pandemic, and that individuals with bad/mediocre/reasonable SRH were most likely to report increases in SRH. These effects might partially reflect floor and ceiling effects, where we would not be able to detect increases in health for individuals indicating excellent pre-pandemic health, and where we would not be able to detect decreases in health for individuals indicating bad pre-pandemic health. However, since we found a similar result for those with bad pre-pandemic scores as those with mediocre and reasonable scores, and a similar result for those with excellent and good scores, it is unlikely that floor or ceiling effects are the primary explanation for these results. When comparing pre-pandemic SRH in the Netherlands to SRH of other European countries based on Eurostat data, the Netherlands scores higher than most other European Union countries with 77.2% of males and 72.6% of females indicating good or very good self-perceived health in 2019 (Eurostat, 2020). Similarly, the 2019 OECD report indicates the Netherlands (together with Japan, Spain, and Switzerland) to have the best overall health outcome globally based on life expectancy, avoidable mortality, chronic disease morbidity, and SRH (OECD Indicators, 2019). In the context of earlier research identifying a positive association between baseline SRH and pandemic SRH (Bierman et al., 2021a), the relatively high baseline SRH in the Netherlands might have served as a protective mechanism for maintaining good health during the pandemic. However, since our current dataset does not allow for such cross-country evaluations, we can only speculate on this point.
There are different explanations for why individuals might evaluate their health more positively during the pandemic. First, it is possible that people adapted different health habits (e.g. an altered diet, changed physical activity patterns) that improved their health, thus leading to an increase in SRH. The current literature on this topic is mixed. For example, while there are studies reporting increases in physical activity during the lockdown (Ding et al., 2020, Romero-Blanco et al., 2020), the majority of studies report decreased physical activity and increased sedentary behaviour during the COVID-19 lockdowns (Trott et al., 2021). Additionally, it is possible that health conditions that were present in the pre-pandemic measure (i.e., disease or illness), were no longer present or improved at the time of the pandemic measure. Since we did not include objective disease indicators, we cannot rule out the possibility that the observed average increase in SRH reflects objective health increases. However, given that many diseases or illnesses have longer lasting effect, it is more to be expected that health deteriorates over time than that is improves. If, for example, we compare the number of individuals with one or more chronic illnesses (associated with COVD-19 related death) before the pandemic to during the pandemic, this number increases from 1103 to 1247 during the pandemic (see supplementary analyses). While this does not tell us anything about symptom severity, it is at least an indication that the number of individuals with a chronic condition did not decrease.
Another explanation is that people's perception of their health might have changed, even if their objective health did not change. The item we used to measure health was designed to measure subjective, rather than objective, health. While this is an approximation of one's objective health, there are also other factors that contribute to subjective health, such as the context in which one finds themselves. As mentioned in the introduction, a previous study explained the apparent increase in SRH during the pandemic as an “eye of the hurricane” paradox, where individuals who are not infected by the virus evaluate themselves more positively than under normal circumstances (Recchi et al., 2020). A mechanism that might contribute to this paradox is social comparison: people partly rate their health based on how healthy they perceive their peers (Cheng et al., 2007). With respect to the pandemic, individuals who remain uninfected by the virus might rate their health more positively than before, as they can now compare themselves to those who have been infected. This is in line with our finding that it was especially those with bad/mediocre/reasonable pre-pandemic SRH that indicated higher pandemic SRH, while respondents indicating good/excellent pre-pandemic SRH more often remained stable. While we did not collect data on changes in health patterns or comparative SRH ratings (i.e. where people explicitly rate themselves as compared to those around them), it would be an interesting direction for future research to elucidate which mechanisms might be at play.
Second, we examined the genetic and environmental sources of individual differences in SRH across the two time-points. Our results indicate that the genetic architecture of SRH does not change from before to during the first lockdown. We report heritability estimates of 45% (CI 35–52%), which is well within the range of findings from previous research (Mosing et al., 2009; Romeis et al., 2000; Silventoinen et al., 2007). It seems that the early stages of the pandemic did not moderate the strength of the relative influence of genes and the environment on SRH. As mentioned in our introduction, a change in variance decomposition was to be expected if people were impacted dissimilarly by the pandemic, leading to an increase of environmental variance. However, the fact that we did not find an increase in total environmental variance does not necessarily mean that the pandemic impacted all respondents in the same way. The unchanged variance may be explained by the high baseline levels of SRH, which potentially served as a protective mechanism for environmental change, even if environmental circumstances did not change similarly for different respondents. Additionally, the genetic correlation indicates that it were still the same genes that influenced differences in SRH at the two time-points. Lastly, environmental correlations indicate that it is (partly) different environmental factors that influence differences in SRH during the pandemic compared to before the pandemic.
While our bivariate genetic model indicates that partially different environmental factors influence SRH during the pandemic compared to before the pandemic, it does not provide information about which particular factors might be different. Previous research suggests that older cohorts are more likely to report changes in behaviour during the COVID-19 pandemic in terms of stress, sleep, physical activity, diet and alcohol intake compared to younger cohorts (Bann et al., 2020; Nwachukwu et al., 2020). Moreover, differences between males and females have been found to be larger during the lockdown compared to before lockdown, with females reporting more atypical sleep levels and higher stress levels (Bann et al., 2020; Cellini et al., 2021). In this way, the pandemic might have caused existing differences between age and gender groups to become enlarged. With respect to potential environmental factors uniquely influencing SRH during the pandemic, existing research has pointed out several COVID-related stressors that might impact people's health. Examples include worry and psychological distress about risk for COVID-19 and the consequences of the pandemic (Blix et al., 2021; Cunningham et al., 2021), working in a high-risk profession, e.g. healthcare (De Kock et al., 2021), and social distancing with consequent impaired social connectedness (Bierman et al., 2021b). While population-level environmental variance did not change significantly during the pandemic compared to before the pandemic, different environmental factors became important in explaining individual differences in SRH during the pandemic. Research into identifying these specific environmental factors is important since it can be used to inform policy makers on SRH variation during crisis-situations like the COVID-19 pandemic.
4.2. Limitations
Our findings should be interpreted in light of some limitations. First, as we mentioned earlier in the discussion, SRH ratings in general are partly due to comparison to other people. During the pandemic part of the ‘other people’ suddenly became ill of COVID-19. This resulted in an overall increase in SRH for those not affected at the moment of measurement. Of course, this does not have to reflect an absolute increase in health but probably reflects the relative change of self-rated health in comparison to others. Following this logic, the issue is not that we are measuring something different at both time-points, but that different mechanisms influence the construct at the two time-points (a reasoning consistent with our finding that different environmental factors influence SRH at the two time-points). In addition, these findings may be somewhat limited by the representativeness of our sample. The sample used for this study was a subset of NTR participants that had both pre-pandemic and pandemic SRH data available. This particular subset unfortunately included more women (±70%) than men (±30%). Moreover, almost 60% of the sample indicated they attended higher vocational school or university, while in the average Dutch population, only about 30% of the population attends higher vocational school/university (Maslowski, 2018). Since both education attainment and gender are associated with SRH, caution must be applied in interpreting our findings. Additionally, pre-pandemic SRH, gender, and age were associated with missing SRH pandemic data, and age was also associated with missing pre-pandemic data. However, since the differences between the overlapping and non-overlapping sample on these variables were very minor, we do not expect this had a large influence on our results. With respect to the potential influence of these confounders on our results, we ran supplementary analyses where we regressed gender, age, the presence of chronic illnesses, and educational attainment on pre-pandemic SRH, pandemic SRH, and SRH difference scores (see Supplementary Analyses). While all these factors were significant predictors of SRH at both time-points, none of the variables predicted SRH difference scores. Furthermore, although it has been observed that people from disadvantaged sociodemographic groups are more likely to change their SRH score over time (Zajacova and Dowd, 2011), we do expect less of an effect of such inequalities in our analyses in a Dutch population based sample, because of the health care system in the Netherlands which provides basic health insurance to all citizens at affordable costs. Lastly, it is well possible that the influence of the pandemic and accompanying lockdowns on SRH changes over time. The results of this study pertain to the first lockdown in the Netherlands and thus reflect the immediate impact of environmental change in the form of a lockdown. Both the immediate impact and the longer term impact are interesting topics for the study of SRH, and we encourage researchers with multiple time point data during the pandemic to further explore individual differences in SRH during the pandemic.
5. Conclusions
These findings re-confirm that in the study of complex human traits, such as SRH, it is important to not only examine mean changes, but also examine individual differences. The finding that many people's SRH remained unchanged shows that there was quite a resilient response to the first stages of the COVID-19 pandemic in the Netherlands, likely driven by more positive perceptions of health during the pandemic, instead of actual health improvements. Moreover, the finding that the variance decomposition in terms of the relative influence of genetic and environmental factors does not change significantly between these two time-points indicates that, at least during the first lockdown, environmental influences did not become relatively more important. It would be interesting to see if this remains stable during longer time-frames, or whether as more time passes, the pandemic does start to moderate the strength of the relative influence of genes and the environment. Either way, our results indicate that while some people may be affected by the challenges posed by COVID-19 to the perception of their health, others are not.
Credit author contributions
Margot van de Weijer: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Visualization. Lianne de Vries: Conceptualization, Methodology, Writing – review & editing. Dirk Pelt: Conceptualization, Methodology, Writing – review & editing. Lannie Ligthart: Investigation, Data curation, Writing – review & editing. Gonneke Willemsen: Writing – review & editing, Funding acquisition. Dorret Boomsma: Writing – review & editing, Funding acquisition. Eco de Geus: writing – data curation, review and editing, Funding acquisition. Meike Bartels: Conceptualization, Writing – review & editing, Project administration, Supervision, Funding acquisition.
Declaration of competing interest
None.
Acknowledgements
This work is supported by the NWO Corona Fast-Track grant (440.20.022), and the European Research Council Consolidator Grant (ERC-2017-COG 771057 WELL-BEING PI Bartels).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.socscimed.2022.115156.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ahmad F., Jhajj A.K., Stewart D.E., Burghardt M., Bierman A.S. Single item measures of self-rated mental health: a scoping review. BMC Health Serv. Res. 2014 doi: 10.1186/1472-6963-14-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assari S., Lankarani M.M. Does multi-morbidity mediate the effect of socioeconomics on self-rated health? Cross-country differences. Int. J. Prev. Med. 2015 doi: 10.4103/2008-7802.164413. 2015-September. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bann D., Villadsen A., Maddock J., Hughes A., Ploubidis G.B., Silverwood R.J., Patalay P. medRxiv; 2020. Changes in the Behavioural Determinants of Health during the Coronavirus (COVID-19) Pandemic: Gender, Socioeconomic and Ethnic Inequalities in 5 British Cohort Studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardage C., Pluijm S.M.F., Pedersen N.L., Deeg D.J.H., Jylhä M., Noale M., Blumstein T., Otero Á. Self-rated health among older adults: a cross-national comparison. Eur. J. Ageing. 2005;2:149–158. doi: 10.1007/s10433-005-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselmans B.M.L., van de Weijer M.P., Abdellaoui A., Vink J.M., Hottenga J.-J., Willemsen G., Nivard M.G., de Geus E.J.C., Boomsma D.I., Bartels M. A genetic investigation of the well-being spectrum. Behav. Genet. 2019;49:286–297. doi: 10.1007/s10519-019-09951-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin D.J., Berger J.O., Johannesson M., Nosek B.A., Wagenmakers E.J., Berk R., Bollen K.A., Brembs B., Brown L., Camerer C., Cesarini D., Chambers C.D., Clyde M., Cook T.D., De Boeck P., Dienes Z., Dreber A., Easwaran K., Efferson C., Fehr E., Fidler F., Field A.P., Forster M., George E.I., Gonzalez R., Goodman S., Green E., Green D.P., Greenwald A.G., Hadfield J.D., Hedges L.V., Held L., Hua Ho T., Hoijtink H., Hruschka D.J., Imai K., Imbens G., Ioannidis J.P.A., Jeon M., Jones J.H., Kirchler M., Laibson D., List J., Little R., Lupia A., Machery E., Maxwell S.E., McCarthy M., Moore D.A., Morgan S.L., Munafó M., Nakagawa S., Nyhan B., Parker T.H., Pericchi L., Perugini M., Rouder J., Rousseau J., Savalei V., Schönbrodt F.D., Sellke T., Sinclair B., Tingley D., Van Zandt T., Vazire S., Watts D.J., Winship C., Wolpert R.L., Xie Y., Young C., Zinman J., Johnson V.E. Redefine statistical significance. Nat. Human Behav. 2018 doi: 10.1038/s41562-017-0189-z. [DOI] [PubMed] [Google Scholar]
- Bierman A., Upenieks L., Glavin P., Schieman S. Accumulation of economic hardship and health during the COVID-19 pandemic: social causation or selection? Soc. Sci. Med. 2021;275 doi: 10.1016/J.SOCSCIMED.2021.113774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierman A., Upenieks L., Schieman S. 2021. Socially Distant? Social Network Confidants, Loneliness, and Health during the COVID-19 Pandemic; pp. 299–313. 10.1177/23294965211011591. [Google Scholar]
- Blix I., Birkeland M.S., Thoresen S. Worry and mental health in the Covid-19 pandemic: vulnerability factors in the general Norwegian population. BMC Publ. Health. 2021;211 21:1–10. doi: 10.1186/S12889-021-10927-1. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boker S., Neale M., Maes H., Wilde M., Spiegel M., Brick T., Spies J., Estabrook R., Kenny S., Bates T., Mehta P., Fox J. OpenMx: an open source extended structural equation modeling framework. Psychometrika. 2011;76:306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu F., Steptoe A., Fancourt D. Who is lonely in lockdown? Cross-cohort analyses of predictors of loneliness before and during the COVID-19 pandemic. Publ. Health. 2020;186:31–34. doi: 10.1016/j.puhe.2020.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancello R., Soranna D., Zambra G., Zambon A., Invitti C. Determinants of the lifestyle changes during COVID-19 pandemic in the residents of Northern Italy. Int. J. Environ. Res. Publ. Health. 2020;17:6287. doi: 10.3390/ijerph17176287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellini N., Conte F., De Rosa O., Giganti F., Malloggi S., Reyt M., Guillemin C., Schmidt C., Muto V., Ficca G. Changes in sleep timing and subjective sleep quality during the COVID-19 lockdown in Italy and Belgium: age, gender and working status as modulating factors. Sleep Med. 2021;77:112–119. doi: 10.1016/j.sleep.2020.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandola T., Jenkinson C. Validating self-rated health in different ethnic groups. Ethn. Health. 2000;5:151–159. doi: 10.1080/713667451. [DOI] [PubMed] [Google Scholar]
- Cheng S.-T., Fung H., Chan A. Maintaining self-rated health through social comparison in old age. J. Gerontol. B Psychol. Sci. Soc. Sci. 2007;62:P277–P285. doi: 10.1093/geronb/62.5.P277. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2013. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Cunningham T.J., Fields E.C., Garcia S.M., Kensinger E.A. The relation between age and experienced stress, worry, affect, and depression during the spring 2020 phase of the COVID-19 pandemic in the United States. Emotion. 2021. [DOI] [PubMed]
- De Geus E.J.C., Kupper N., Boomsma D.I., Snieder H. Bivariate genetic modeling of cardiovascular stress reactivity: does stress uncover genetic variance? Psychosom. Med. 2007;69:356–364. doi: 10.1097/PSY.0b013e318049cc2d. [DOI] [PubMed] [Google Scholar]
- De Kock J.H., Latham H.A., Leslie S.J., Grindle M., Munoz S.-A., Ellis L., Polson R., O'Malley C.M. A rapid review of the impact of COVID-19 on the mental health of healthcare workers: implications for supporting psychological well-being. BMC Publ. Health. 2021;211 21:1–18. doi: 10.1186/S12889-020-10070-3. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moor M.H.M., Stubbe J.H., Boomsma D.I., De Geus E.J.C. Exercise participation and self-rated health: do common genes explain the association? Eur. J. Epidemiol. 2007;22:27–32. doi: 10.1007/s10654-006-9088-8. [DOI] [PubMed] [Google Scholar]
- Dieker A.C.M., IJzelenberg W., Proper K.I., Burdorf A., Ket J.L., van der Beek A., Hulsegge G. The contribution of work and lifestyle factors to socioeconomic inequalities in self-rated health - a systematic review. Scand. J. Work. Environ. Health. 2019;45:114–125. doi: 10.5271/SJWEH.3772. [DOI] [PubMed] [Google Scholar]
- Ding D., del Pozo Cruz B., Green M.A., Bauman A.E. Is the COVID-19 lockdown nudging people to be more active: a big data analysis. Br. J. Sports Med. 2020;54(20):1183–1184. doi: 10.1136/bjsports-2020-102575. [DOI] [PubMed] [Google Scholar]
- Eurostat Self-perceived health statistics - statistics explained. 2020. https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Self-perceived_health_statistics#Self-perceived_health [WWW Document]. URL. accessed 8.24.21.
- Falconer D.S. The problem of environment and selection. Am. Nat. 1952 doi: 10.1086/281736. [DOI] [Google Scholar]
- Gijzen M., Shields-Zeeman L., Kleinjan M., Kroon H., van der Roest H., Bolier L., Smit F., de Beurs D. OSF Prepr; 2020. The Bittersweet Effects of COVID-19 on Mental Health: Results of an Online Survey Among a Sample of the Dutch Population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekara F.I., Carter K., Blakely T. Change in income and change in self-rated health: systematic review of studies using repeated measures to control for confounding bias. Soc. Sci. Med. 2011;72:193–201. doi: 10.1016/J.SOCSCIMED.2010.10.029. [DOI] [PubMed] [Google Scholar]
- Helliwell J.F., Huang H., Wang S., Norton M. World Happiness Report 2020. 2020. Social environments for world happiness. [Google Scholar]
- Holmes E.A., O'Connor R.C., Perry V.H., Tracey I., Wessely S., Arseneault L., Ballard C., Christensen H., Cohen Silver R., Everall I., Ford T., John A., Kabir T., King K., Madan I., Michie S., Przybylski A.K., Shafran R., Sweeney A., Worthman C.M., Yardley L., Cowan K., Cope C., Hotopf M., Bullmore E. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatr. 2020;7:547–560. doi: 10.1016/S2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idler E.L., Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J. Health Soc. Behav. 1997 doi: 10.2307/2955359. [DOI] [PubMed] [Google Scholar]
- Jylhä M. What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Soc. Sci. Med. 2009;69:307–316. doi: 10.1016/j.socscimed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Kowal M., Coll‐Martín T., Ikizer G., Rasmussen J., Eichel K., Studzińska A., Koszałkowska K., Karwowski M., Najmussaqib A., Pankowski D., Lieberoth A., Ahmed O. Who is the most stressed during the COVID‐19 pandemic? Data from 26 countries and areas. Appl. Psychol. Health Well-Being. 2020;12:946–966. doi: 10.1111/aphw.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligthart L., van Beijsterveldt C.E.M., Kevenaar S.T., de Zeeuw E., van Bergen E., Bruins S., Pool R., Helmer Q., van Dongen J., Hottenga J.-J., van’t Ent D., Dolan C.V., Davies G.E., Ehli E.A., Bartels M., Willemsen G., de Geus E.J.C., Boomsma D.I. The Netherlands twin register: longitudinal research based on twin and twin-family designs. Twin Res. Hum. Genet. 2019:1–14. doi: 10.1017/thg.2019.93. [DOI] [PubMed] [Google Scholar]
- Martínez-de-Quel Ó., Suárez-Iglesias D., López-Flores M., Pérez C.A. Physical activity, dietary habits and sleep quality before and during COVID-19 lockdown: a longitudinal study. Appetite. 2021;158 doi: 10.1016/j.appet.2020.105019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowski R. De Sociale Staat Van Nederland 2018. 2018. Onderwijs. [Google Scholar]
- Menni C., Valdes A.M., Freidin M.B., Sudre C.H., Nguyen L.H., Drew D.A., Ganesh S., Varsavsky T., Cardoso M.J., El-Sayed Moustafa J.S., Visconti A., Hysi P., Bowyer R.C.E., Mangino M., Falchi M., Wolf J., Ourselin S., Chan A.T., Steves C.J., Spector T.D. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat. Med. 2020;26:1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosing M.A., Zietsch B.P., Shekar S.N., Wright M.J., Martin N.G. Genetic and environmental influences on optimism and its relationship to mental and self-rated health: a study of aging twins. Behav. Genet. 2009;39:597–604. doi: 10.1007/s10519-009-9287-7. [DOI] [PubMed] [Google Scholar]
- Nwachukwu I., Nkire N., Shalaby R., Hrabok M., Vuong W., Gusnowski A., Surood S., Urichuk L., Greenshaw A.J., Agyapong V.I.O. COVID-19 pandemic: age-related differences in measures of stress, anxiety and depression in Canada. Int. J. Environ. Res. Publ. Health. 2020;17:6366. doi: 10.3390/ijerph17176366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD Indicators . 2019. Health at a Glance , Health at a Glance. [DOI] [Google Scholar]
- Perruccio A.V., Badley E.M., Hogg-Johnson S., Davis A.M. Characterizing self-rated health during a period of changing health status. Soc. Sci. Med. 2010;71:1636–1643. doi: 10.1016/J.SOCSCIMED.2010.07.042. [DOI] [PubMed] [Google Scholar]
- Peters A., Rospleszcz S., Greiser K.H., Dallavalle M., Berger K. The impact of the COVID-19 pandemic on self-reported health. Dtsch. Arztebl. Int. 2020;117:861–867. doi: 10.3238/arztebl.2020.0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisinger C., Toft U., Aadahl M., Glümer C., Jørgensen T. The relationship between lifestyle and self-reported health in a general population: the Inter99 study. Prev. Med. 2009;49:418–423. doi: 10.1016/J.YPMED.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Poelman M.P., Gillebaart M., Schlinkert C., Dijkstra S.C., Derksen E., Mensink F., Hermans R.C.J., Aardening P., de Ridder D., de Vet E. Eating behavior and food purchases during the COVID-19 lockdown: a cross-sectional study among adults in The Netherlands. Appetite. 2021;157 doi: 10.1016/j.appet.2020.105002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recchi E., Ferragina E., Helmeid E., Pauly S., Safi M., Sauger N., Schradie J. The “eye of the hurricane” paradox: an unexpected and unequal rise of well-being during the covid-19 lockdown in France. Res. Soc. Stratif. Mobil. 2020;68 doi: 10.1016/j.rssm.2020.100508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis J.C., Scherrer J.F., Xian H., Eisen S.A., Bucholz K., Heath A.C., Goldberg J., Lyons M.J., Henderson W.G., True W.R. Heritability of self-reported health. Health Serv. Res. 2000;5:995–1010. [PMC free article] [PubMed] [Google Scholar]
- Romero-Blanco C., Rodríguez-Almagro J., Onieva-Zafra M.D., Parra-Fernández M.L., Prado-Laguna M.D.C., Hernández-Martínez A. Physical activity and sedentary lifestyle in university students: changes during confinement due to the COVID-19 pandemic. Int. J. Environ. Res. Public Health. 2020;17(18):6567. doi: 10.3390/ijerph17186567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silventoinen K., Posthuma D., Lahelma E., Rose R.J., Kaprio J. Genetic and environmental factors affecting self-rated health from age 16-25: a longitudinal study of Finnish twins. Behav. Genet. 2007;37:326–333. doi: 10.1007/s10519-006-9096-1. [DOI] [PubMed] [Google Scholar]
- Szwarcwald C.L., Damacena G.N., de Azevedo Barros M.B., Malta D.C., de Souza Júnior P.R.B., Azevedo L.O., Machado Í.E., Lima M.G., Romero D., Gomes C.S., Werneck A.O., da Silva D.R.P., Gracie R., de Fátima de Pina M. Factors affecting Brazilians’ self-rated health during the COVID-19 pandemic. Cad. Saúde Pública. 2021;37 doi: 10.1590/0102-311X00182720. [DOI] [PubMed] [Google Scholar]
- Trott M., Tully M., Shin J., Barnett Y., Butler L., McDermott D., Schuch F., Smith L. Changes in physical activity and sedentary behaviours from before to during the COVID-19 pandemic lockdown: a systematic review. BMJ Open Sport Exerc. Med. 2021;7(1):e000960. doi: 10.1136/bmjsem-2020-000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lente E., Barry M.M., Molcho M., Morgan K., Watson D., Harrington J., McGee H. Measuring population mental health and social well-being. Int. J. Publ. Health. 2012;57:421–430. doi: 10.1007/s00038-011-0317-x. [DOI] [PubMed] [Google Scholar]
- van Tilburg T.G., Steinmetz S., Stolte E., van der Roest H., de Vries D.H. Loneliness and mental health during the COVID-19 pandemic: a study among Dutch older adults. J. Gerontol. Ser. B. 2020;XX:1–7. doi: 10.1093/geronb/gbaa111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst B., Prom-Wormley E., Keller M., Medland S., Neale M.C. Type I error rates and parameter bias in multivariate behavioral genetic models. Behav. Genet. 2019;49:99–111. doi: 10.1007/s10519-018-9942-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajacova A., Dowd J.B. Reliability of self-rated health in US adults. Am. J. Epidemiol. 2011;174:977–983. doi: 10.1093/AJE/KWR204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.