Abstract
Objective
Describe a case series of vaccine-induced immune thrombotic thrombocytopenia (VITT) after COVID-19 vaccination in Brazil that included ChAdOx1 nCoV-19, Ad26.COV2.S and BNT162b2 vaccines, describing their clinical and laboratory characteristics.
Methodology
Descriptive case series study using Bio-Manguinhos/Fiocruz/AstraZeneca Brazil and National Immunization Program/Ministry of Health (NIP/MoH) data on COVID-19 AEFI surveillance.
We obtained patient-level data from pharmacovigilance for AEFI surveillance and used both the NIP/MoH and Bio-Manguinhos/Fiocruz pharmacovigilance databases to create the study database.
Thirty-nine cases of suspect VITT were included, 36 after ChAdOx1 nCoV-19, one after BNT162b2 and two after Ad26.COV2.S vaccine. All cases were based on meeting the Brighton Collaboration criteria for VITT. The primary outcomes were clinical and laboratory features, site of thrombosis, and anti-PF4 ELISA, when available.
Results
Thirty-nine cases met the criteria, 38 of which were classified as level 1 and one as level 3 according to Brighton Collaboration. Most cases had the central nervous system (CNS) as the main site of thrombosis (21/39) and happened after the vaccine first dose (34/39). The median age of the cases was 41 years old (23 to 86 yo). Most of the cases (61.5%) occurred in women. The median interval between vaccination and onset of symptoms was 8 days (0–37 days). The platelet count and D-dimer count had median values of 34,000/µL and 19,235 µg FEU/L, respectively. The ELISA anti-PF4 antibody was positive in 18 samples. The overall mortality rate was 51% and was higher in cases of CNS thrombosis with intracerebral bleeding.
Conclusion
Our case series shows that Brazilian VITT cases have similar clinical and laboratory profiles as demonstrated in the literature. Brazil has administered more than 300 million doses of COVID-19 vaccines (more than 110 million from ChAdOx1 nCoV-19). VITT seems to be a very rare but serious adverse event following COVID-19 immunization, especially adenoviral vector immunization.
Keywords: Thrombosis, Thrombocytopenia, COVID-19, Vaccine-induced immune thrombotic thrombocytopenia
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has overwhelmed health systems globally in the past two years [1]. By the end of November 2021, more than 250 million cases of COVID-19 were reported worldwide, with approximately 5.1 million deaths [2]. Brazil was the third most affected country in terms of mortality rate in countries with more than 10 million inhabitants and reported more than 22 million cases and about 610,000 deaths [3], [4]. In parallel highly effective vaccines were developed at unprecedented speed using different and innovative technologies, playing an essential role in increasing population immunity and preventing severe disease.
Vaccination against COVID-19 started in Brazil, in January 2021, initially with CoronaVac (Sinovac) and ChAdOx1 nCoV-19 (Oxford/AstraZeneca) vaccines, and then with the inclusion of BNT162b2 (Pfizer/BioNTech) and Ad26.COV2.S (Janssen) vaccines. Although all vaccines undergo studies assessing safety profiles in the preregistration clinical trial phases, rare and very rare adverse events may not be detected. Therefore, the postmarketing surveillance of adverse events is an important activity to ensure the safety and assessment of the benefit-risk profile of these products. Pharmacovigilance activities were planned to address this scenario and quickly identify adverse events following immunization (AEFI), especially those classified as severe and of special interest. After the launch of the vaccination, there was a rapid emergence of new safety issues. It was up to pharmacovigilance teams to detect these reports and investigate causality.
Bio-Manguinhos/Fiocruz, a public pharmaceutical industry related to the Ministry of Health [5] is responsible for the production and distribution of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) in Brazil. Its Pharmacovigilance Unit/ /Clinical Advisory Unit established a multidisciplinary Pharmacovigilance Committee for ChadOx1 nCov-19to monitor and discuss rare and serious AEFI reports contributing to the surveillance. This committee brought together experts from Bio-Manguinhos, Fiocruz and AstraZeneca Brazil and ad hoc specialists, as well as NIP/MoH, National Regulatory Agency (Anvisa), and local surveillance teams. Virtual weekly meetings were held to review and assess causality of AEFI.
First described in March 2021, in Europe [5], vaccine-induced immune thrombotic thrombocytopenia (VITT) affects approximately one in 50,000–100,000 people vaccinated with ChAdOx1 nCoV-19 (Oxford/AstraZeneca). The European Medicines Agency (EMA) assigned a possible causal association between these cases and COVID-19 vaccines. Subsequently, reports of VITT after Ad26.COV2. S (Janssen) vaccine were described, with an incidence of 0.3 in 100,000 doses [6]. Recently, few VITT cases have been reported after mRNA vaccines, but no causal relationship has been postulated thus far [7], [8].
VITT pathophysiology resembles autoimmune heparin-induced thrombocytopenia, characterized by the presence of high titers of anti-platelet factor 4 (PF4)/polyanion IgG autoantibodies and platelet activation [9]. These antibodies activate monocytes and neutrophils and induce neutrophil extracellular trap formation, endothelial damage, and platelet aggregation, favouring thromboembolic phenomena [10]. Clinically, VITT is defined by the onset of symptoms on average 5 to 30 days (or ≤ 42 days in patients with isolated deep vein thrombosis or pulmonary embolism) after vaccination against SARS-CoV-2, the presence of thrombosis, thrombocytopenia (platelet count below 150,000/µL), strikingly high D-dimer, and antibodies to PF4 detected by enzyme-linked immunosorbent assay (ELISA) [11]. Thrombosis mainly affects atypical sites, such as the cerebral venous sinus (CVS) or splanchnic circulation [10]. Physicians should have a low threshold for recognizing VITT signs, and treatment should be promptly instituted while waiting for serological confirmation. Identifying anti-PF4 antibodies in plasma and performing positive functional tests to assess platelet activation are crucial to confirm VITT diagnosis.
The first VITT report in Brazil was in May 2021 in a pregnant woman [12]. The situation caused social commotion, and the Brazilian regulatory agency (Anvisa) decided to preventively suspend the administration of the COVID-19 viral vector vaccines ChAdOx1 nCov-19 (Oxford/AstraZeneca) and Ad26.COV2.S (Janssen) in pregnant women. This recommendation was adopted by NIP/MoH and remains in force, despite the lack of other evidence of an association between VITT and pregnancy [13] and the Category B recommendation for Safety in ChAdOx1 nCov-19 Vaccine label.
The suspect reports of VITT received were discussed in Bio-Manguinhos PV comitee. This work resulted in the publication of a technical document by NIP/MoH containing recommendations and guidelines for the investigation of VITT after COVID-19 vaccination in Brazil [14].
Another difficulty regarding VITT diagnosis was the absence in Brazil of a laboratory network to perform the ELISA anti-PF4 test. All tests had to be sent abroad, thus limiting access to this diagnostic tool. Therefore, to provide broad access to anti-PF4 ELISA and functional platelet tests, NIP/MoH also implemented a nationwide collaborative project (NIP/MoH/Fiocruz/Hemorio) to offer the test in Brazil, free of charge [14]. The tests are being performed at Hemorio, a reference blood centre in Rio de Janeiro.
This study describes a case series of thrombosis with thrombocytopenia syndrome reported to the NIP/MoH and/or to the Pharmacovigilance of Bio-Manguinhos/Fiocruz or AstraZeneca concerning clinical, laboratory, and sociodemographic characteristics. The study also describes possible factors associated with the occurrence of cases and the differences between the groups with and without fatal outcomes. In addition, it discusses the VITT management process in Brazil, identifies weaknesses in the identification, diagnosis, and management of these cases, which may be related to unfavourable outcomes, and helps in the development of future guidelines and actions to improve work processes.
2. Methods
This study reports a case series of suspected vaccine-induced thrombosis with thrombocytopenia syndrome (VITT) following COVID-19 vaccination during the mass vaccination campaign of NIP/MoH between March and November 2021.
Individual case safety reports (ICSR) of ChAdOx1 nCoV-19 (Oxford/AstraZeneca) from the pharmacovigilance database of Bio-Manguinhos/Fiocruz and/or AstraZeneca Brazil and ICSR of BNT162b2 (Pfizer/BioNTech) and Ad26.COV2.S (Janssen) vaccines from the NIP/MoH database were analysed. Health professionals reported spontaneous ICSRs through the NIP/MoH system (e-SUS Notifica), and users and/or health professionals also reported incidents to Customer Service at Bio-Manguinhos/Fiocruz and AstraZeneca. Sociodemographic, clinical and vaccine data were collected from these databases. Laboratory and imaging tests for ICSR were performed during routine care. All collected data were registered in electronic spreadsheets.
2.1. VITT case definition
Cases of VITT were defined according to the VITT Case Definition Level of Certainty Determination of Brighton Collaboration. The purpose of the Brighton Collaboration criteria is to standardize the case information and allow evaluation between different case series. Initially, the case criteria did not include temporal association with the vaccination, but updates were made, and the last published paper included new elements, including the anti-PF4 test [15].
Thereafter, cases under investigation were categorized based on the British Expert Panel classification. This group uses 5 clinical and laboratory features to categorize the cases into four types (definite, probable, possible and unlikely) [11].
2.2. Eligibility criteria
The inclusion criteria were as follows: proven vaccination with one of the COVID-19 vaccines available from the NIP/MoH, information on the application date and the manufacturer of the vaccine received and an assessment that met the minimum criteria of level 3 of VITT by the Brighton Collaboration case definition [15] .
Cases were excluded if it was not possible to retrieve the clinical/laboratory data necessary for evaluation due to inadequate filling of the notification (level 4 Brighton Collaboration) or if they were classified as level 5 according to Brighton Collaboration VITT criteria [15].
2.3. Laboratory tests
Initially, the tests were performed according to the availability of the institution that assisted the patient or in private laboratories. After the establishment of the MoH national flow for the diagnosis of VITT, the samples were sent to Hemorio for dosage of anti-PF4/ELISA and D-dimer. This unit used two solid phase enzyme-linked immunosorbent assay (ELISA) tests to detect anti-PF4. The first one was for IgG anti-PF4 screening, and the second one was for IgG/IgM/IgA anti-PF4 screening, both manufactured by Immucor (PF4 IgG Assay and PF4 Enhanced Assay, Waukesha, WI, USA). When samples were available, a confirmatory functional test, the serotonin-release assay (SRA) with added PF4, was also performed. The test was performed at McMaster Platelet Immunology Laboratory, Canada.
2.4. Statistical analysis
The analysis included sociodemographic variables such as age, sex and information regarding the vaccination process, including vaccine manufacturer, application date, and dose of vaccine (first or second).
Clinical characteristics were also evaluated, including site of thrombosis, time between vaccination and AEFI, the time between AEFI and hospital admission, presence of comorbidities, previous use of heparin and risk factors for thrombosis. Information regarding the presence of symptoms suggestive of thrombosis, such as headache, blurred vision, seizure, altered level of consciousness, focal neurological sign, abdominal pain, chest pain, dyspnoea, and pain/edema/pallor of lower or upper limbs. The type of treatment implemented was also analysed, as well as the length of hospital stay and outcome (death/recovery).
Laboratory test results and imaging exams were evaluated according to their availability: platelet count, D-dimer, image of thrombosis and its topography, anti-PF4 antibodies and functional tests of platelet aggregation.
Descriptive frequencies, percentages, medians, and standard deviations were used to characterize cases of VITT according to age, sex, manufacturer, time elapsed between vaccination and symptoms, anti-PF4 positivity and clinical outcome. The clinical characteristics of suspected VITT cases and information about vaccination were summarized in frequency and contingency tables, containing absolute frequencies and percentages. Once the previous use of heparin can affect some assays used for VITT diagnosis, this information was also collected if available.
Laboratory results are presented using descriptive statistics such as median, minimum, maximum, mean and standard deviation values.
To characterize the study sample, the sociodemographic variables of the participants were presented. Qualitative variables such as sex are summarized in frequency distribution tables. For quantitative variables, such as age, measures of central tendency and dispersion were calculated.
The study protocol was prepared in accordance with Brazilian clinical research standards, according to the 466 Resolution (December 12, 2012) of the National Health Council, which regulates research involving human beings, and other related national regulations; and it complied with the Good Clinical Practice (GCP) guidelines.
Statistical analysis was performed with IBM SPSS Statistics [16] where the analysis of the study was carried out.
3. Results
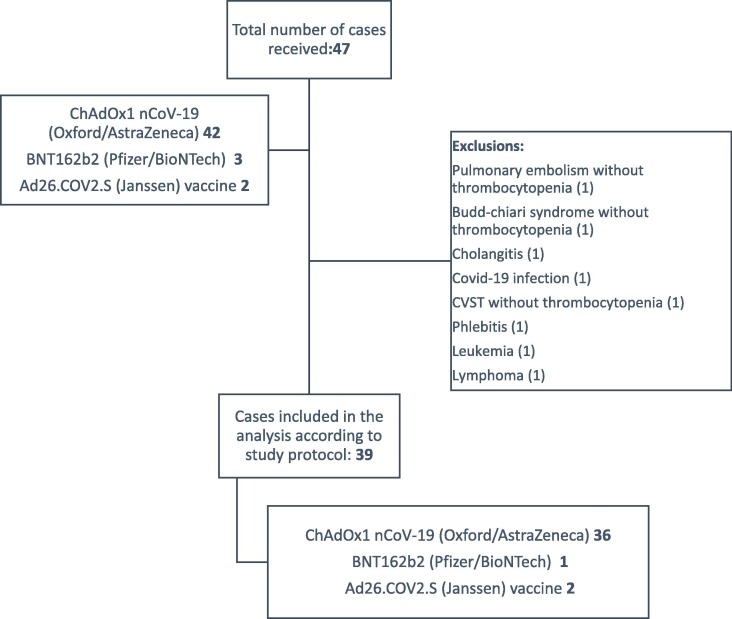
In the study recruitment period, from March to November 2021, 47 reports of suspected VITT cases were received by the researchers. Of these, 42 were attributed to ChAdOx1 nCoV-19 (Oxford/AstraZeneca), 2 to Ad26.COV2. S (Janssen) and 3 to BNT162b2 (Pfizer/BioNTech) vaccines. A total of 8 cases were excluded because they were classified as level 5 according to Brighton Collaboration criteria (not a VITT case); two of these cases occurred after BNT162b2 (Pfizer/BioNTech) vaccination, and six cases after ChAdOx1 nCoV-19 (Oxford/AstraZeneca) vaccine. Fig. 1 shows the flowchart of suspected VITT cases.
Fig. 1.
Flowchart of suspected VITT cases.
Among the 39 cases of suspected VITT included, most of them occurred after the first dose of the vaccine (n = 34; 87.2%): 31 after the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) vaccine, two after the Ad26.COV2. S (Janssen), and one after the BNT162b2 (Pfizer/BioNTech) vaccine. The remaining cases occurred after the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) vaccine, four after the second dose and one with an unknown dose. There were no cases of suspected VITT after the booster dose. Regarding case classification, 38 (97.4%) were classified as level 1 of VITT diagnostic score according to the updated Brighton Collaboration [15], and one case (2.6%) was classified as level 3. According to criteria proposed by the British Society of Haematology expert panel [11], [12] cases (30.8%) were classified as definite cases, 13 (33.3%) as probable, 13 (33.3%) as possible cases and one (2.6%) as unlikely.
Table 1 shows the sociodemographic and main clinical and laboratory characteristics of this case series. In summary, among the 39 cases, 24 were women (61.5%). The median age of the group was 41 years (23–86). The interval between vaccination and onset of symptoms ranged from 0 to 37 days, with a median of 8 days.
Table 1.
Clinical and laboratory features of case series.
| Variable |
Alive |
Deceased |
All cases |
|---|---|---|---|
| (*3 cases without outcome information) | |||
| Age-yr | |||
| Median | 45.5 (IQR 19.3) | 37 (IQR 18) | 41 (IQR 21.3) |
| Range | 23–75 | 28–86 | 23–86 |
| Total | 16 | 19 | 38*3 |
| Sex n/total (%) | |||
| Female | 12/16 (75%) | 10/20 (50%) | 24/39 (61.5%) |
| Male | 4/16 (25%) | 10/20 (50%) | 15/39 (38.5%) |
| Total | 16 | 20 | 39 |
|
Days from vaccination to symptoms*1 | |||
| Median | 9 (IQR 8) | 8 (IQR 3) | 8 (IQR 6) |
| Range | 0–37 | 0–11 | 0–37 |
| Total | 13 | 20 | 36*4 |
|
Days since onset of VITT symptoms and hospital admission | |||
| Median | 0 (IQR 5) | 4 (IQR 6) | 3 (IQR 7) |
| Range | 0–8 | 0–12 | 0–12 |
| Total | 8 | 19 | 29*5 |
|
Platelet count – per µL | |||
| Median | 46,500 (IQR 49,500) | 23,000 (IQR 35,500) | 34,000 (IQR 45,000) |
| Range | 2,000–147,000 | 8,000–113,000 | 2,000–147,000 |
| Total | 16 | 20 | 39 |
| D-dimer (FEU) | |||
| Median | 10,700 (IQR 18,560) | 41,000 (IQR 87,845) | 19,235 (IQR 33,356) |
| Range | 1,119–40,800 | 4,000–128,000 | 1,119–128,000 |
| Total | 12 | 13 | 28*6 |
| Anti-PF4 Elisa*2 | |||
| Positive | 6 | 12 | 18 |
| Negative | 8 | 1 | 12 |
| Total | 14 | 13 | 30*7 |
*1 interval between vaccine and symptoms (assuming the date of the dose applied closely to event onset).
*2 nine results were not available.
*3 one without age information.
*4 this information was not available in 3 cases.
*5 this information was not available in 10 cases.
*6 this information was not available in 11 cases.
*7 this information was not available in 09 cases.
Another piece of information that was evaluated was the interval between the onset of suspected VITT symptoms and the date of hospital admission. This interval ranged from 0 to 12 days, with a median of 3 days.
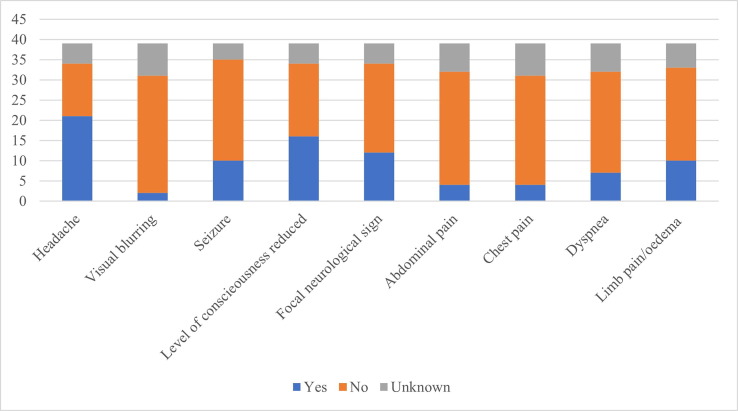
In Fig. 2 , symptoms and signs suggestive of thrombosis are presented. The most common symptoms were headache, blurred vision, seizure, change in the level of consciousness, focal neurological sign, abdominal pain, chest pain, dyspnoea pain, edema and pallor of limbs.
Fig. 2.
Warning signs and symptoms of suspected VITT (n = 39).
Considering the lowest platelet count available for each patient, the minimum value was 2,000/µL, and the maximum value was 147,000/µL, with a median of 34,000/µL platelets.
The D-dimer values were available in 28 cases. The minimum value observed was 1,119/FEU (reference ≤ 500 FEU), and the maximum value was 128,000/FEU, with a median of 19,235/FEU. Table 2 compares the platelet count and D-dimer level with the patient’s outcome.
Table 2.
Platelet count and D-dimer per outcome.
| N | Deceased (n) | Alive (n) | |
|---|---|---|---|
| Platelet count | |||
| Until 9,999/µL | 3 | 2 | 1 |
| 10,000 to 29,999/µL | 14 | 11 | 3 |
| 30,000 to 49,999/µL | 10* | 2 | 5 |
| 50,000 to 149,999/µL | 12 | 5 | 7 |
| D-Dimer | |||
| Until 1,999 FEU | 2* | 0 | 1 |
| 2,000 to 3,999 FEU | 2 | 0 | 2 |
| 4,000 to 9,999 FEU | 5* | 2 | 2 |
| 10,000 to 100,000 FEU | 16* | 8 | 7 |
| Higher than 100,000 FEU | 3 | 3 | 0 |
| Not available | 11 | 7 | 4 |
3 outcomes missing.
The anti-PF4 test was performed in 30 cases, of which 18 were positive (60%). In 10 out of those 18 cases, the functional platelet activating assay (with added PF4) was performed; all of them were positive. For the 12 participants who tested negative for anti-PF4, the functional platelet activating assay (with added PF4) was performed in nine cases, all of which tested negative.
Of the 18 VITT cases with positive anti-PF4, 16 received the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) vaccine (88,9%), and two received Ad26.COV2.S (Janssen) vaccine (11.1%). All these cases occurred after the first dose of the vaccine. The median interval between vaccination and symptoms in this group was 8 days (0–11), the median platelet count was 30,000/µL, and the median D-dimer was 20,000 FEU. Thrombosis was described in all cases (18), and the sites of thrombosis included cerebral thrombosis (15 cases), limb thrombosis (5 cases), pulmonary embolism (1 cases), one case of splanchnic vein thrombosis, and one case of placental thrombosis. Some of these patients had multiple sites of thrombosis.
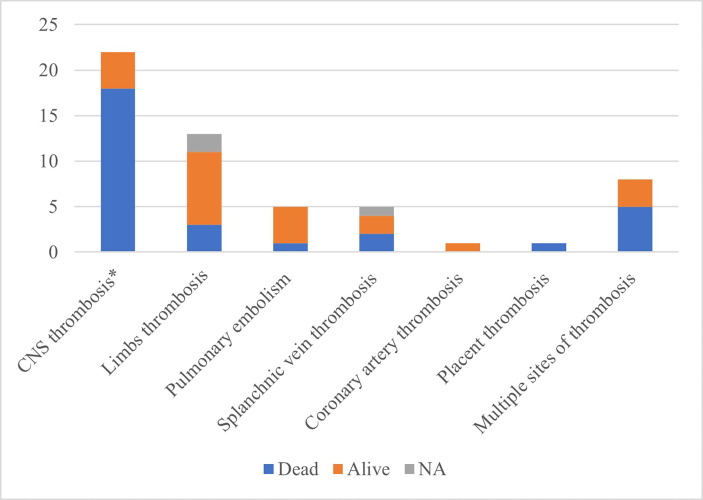
Regarding confirmed thrombosis imaging, it was present in 35 cases, 18 in the central nervous system (CNS) (2 arterial and 16 venous thromboses), 13 limb thromboses, 5 pulmonary embolisms, 5 splanchnic vein thromboses, 1 coronary artery and 1 case of placental thrombosis. There was a description of multiple site thrombosis in 8 cases. In 4 cases, there was no confirmatory image of thrombosis, but the other radiology exam was highly suggestive of central vein sinus thrombosis (CVST), with the presence of intracerebral bleeding.
The outcomes of the 39 patients were as follows: 20 progressed to death for an overall mortality of 51%. The thrombotic site with the highest number of deaths was CNS thrombosis, with 18 deaths in 21 patients (85% lethality rate). Two deaths occurred after splanchnic vein thrombosis. CNS thrombosis was complicated by secondary intracranial haemorrhage in 16 patients, with 15 deaths (94%). Fig. 3 compares the site of thrombosis with patient outcome.
Fig. 3.
Comparison between the site of thrombosis and outcome. *4 cases intracerebral bleeding highly suggestive of CVST. NA – not available
The treatment for this group of patients varied widely, including intravenous immunoglobulin, plasma exchange therapy, systemic glucocorticoids, anticoagulation with non-heparin-based anticoagulant and rituximab infusion, and sometimes more than one treatment was used for the same patient. A total of 11 patients used intravenous immunoglobulin, and eight of these patients recovered. Plasma exchange was used in two patients; however, one patient died. Anticoagulation with non-heparin-based anticoagulant was administered to 12 patients, and 10 of these patients recovered. Systemic glucocorticoids were used in seven patients, and one patient used rituximab. There was no information available about the treatment of nine patients. Nine patients did not receive any kind of specific treatment, and five of these patients died.
Of the 39 patients, 17 (43.6%) had a previous risk factor for thrombosis, 12 (30.8%) had no risk factors, and 10 (25.6%) had unavailable information. The risk factors presented were the use of oral contraceptives (5), smoking (4), obesity (3), previous thrombosis (2), pregnancy (1), family history of thrombophilia (1), family history of thrombosis (1), in treatment for cancer (1) and systemic lupus erythaematousus (1).
Thirteen patients reported no previous use of heparin in the past three months. In the remaining 26 cases there was no previous heparin use registered in clinical records, so this information could not be confirmed.
4. Discussion
Vaccine-induced immune thrombotic thrombocytopenia emerged as a new adverse event after COVID-19 vaccines, especially those of viral vector platforms. Surveillance of this AEFI has been crucial in this scenario, as the world is in the middle of a mass vaccination campaign. VITT surged as a safety signal, defined as information on a new or known adverse event potentially caused by a medicine/vaccine, and that warrants further investigation [17]. Signals are generated from several sources, such as spontaneous reports, clinical studies, and the scientific literature.
Pharmacovigilance actions to investigate this adverse event were crucial to quickly understand more about it, allowing safety actions to be taken, alerting health professionals and users about the issue but also demonstrating that the benefit-risk profile of vaccination remains favourable.
NIP/MoH was created in 1973 and is responsible for the Brazilian national immunization policy. It is one of the largest vaccination programs in the world, recognized nationally and internationally, and it serves the entire Brazilian population, currently estimated at 211.8 million people [18]. The program, which has extensive expertise in mass vaccination, is carrying out vaccinations against COVID-19. As of November 2021, more than 300 million doses of COVID-19 vaccine had been applied in the country [19].
The benefit-risk profile of COVID-19 vaccines remains favourable, even with the rare occurrence of cases of thrombosis with thrombocytopenia syndrome associated with the use of vaccines. Knowing the profile of cases reported to NIP/MoH and to the pharmacovigilance teams of Bio-Manguinhos and AstraZeneca allows dissemination of knowledge about this AEFI, which may lead to an improvement in the diagnosis and treatment of this syndrome.
As with any case series, our study has limitations, as it was not a controlled trial and did not include all the cases that may have occurred in Brazil during the period. Another limitation is regarding selection bias. As the study used Bio-Manguinhos/Fiocruz PV database, most of the cases included were with ChAdOx1 nCoV-19 vaccine (Oxford/AstraZeneca). Some cases with other COVID-19 vaccines in use in Brazil that reached the national Elisa anti-PF4 diagnosis lab was included by NIP/MoH pharmacovigilance team, who was the responsible for this cases information.
Therefore, our objective was to describe the cases and their characteristics without making extrapolations or inferences about the general population.
VITT frequency varies across countries. In the United Kingdom, a nation that has sensitive surveillance of this adverse event, the overall incidence after the first dose or unknown dose was 15 cases per million doses. Data show that there is a higher incidence rate in the young adult groups after the first dose compared to the older groups (20.8 per million doses in people aged 18–49 years compared to 10.9 per million doses in people aged 50 and over). There was no gender predominance in data from the United Kingdom [20]. In our sample, we had a median age of 41 years, and 24 (61%) cases were in women. As it is a case series, it is not possible to calculate rates of the AEFI, but considering the number of cases observed and the high number of doses administered in Brazil during this period (approximately 300 million doses of COVID-19 vaccines, which included more than 110 million doses of the ChAdOx1 nCoV-19 vaccine [Oxford/AstraZeneca]) [19], we can hypothesize that Brazil presented a lower frequency of VITT than observed in European countries.
VITT is a potentially serious adverse event with high mortality, varying according to country from 5% in Australia to 17–55% in the United Kingdom (UK) [20], [21]. In the UK, by the end of September 2021, 419 cases had been reported, of which 89% occurred after the first dose [20]. Our numbers show a global lethality rate of 51.3%, being higher in cases involving the CNS, associated with bleeding and lower platelet count and higher D-dimer values. A recent review showed a VITT overall mortality of 35.9% after ChAdOx1 nCoV-19 (Oxford/AstraZeneca) vaccination. Age ≤ 60 years, platelet count < 25 × 103/µL, fibrinogen < 150 mg/dL, the presence of intracerebral haemorrhage (ICH), and CVST were significantly associated with death and were selected as predictors for mortality [22].
Other groups also proposed diagnostic and causality criteria for VITT. The WHO has published a guideline that proposes a classification based on the Brighton Collaboration criteria but adds elements of temporality with the vaccine and complementary laboratory tests (for example, the anti-PF4 ELISA) to corroborate the causal association with the vaccine [23].
Another group, formed by British researchers who monitored a large number of patients, proposed another definition for VITT cases after vaccination. It takes into account five criteria: temporality with the vaccine (between 5 and 30 days or up to 42 days if isolated deep vein thrombosis and pulmonary embolism), presence of thrombocytopenia, evidence of thrombosis, D-dimer value and dosage of anti-PF4 [11]. When all five criteria are met, the case is a definite VITT. As the number of criteria met decreases, so does diagnostic certainty. In this classification, the numerical value of the D-dimer (greater than4,000 FEU) is important to measure the degree of certainty.
According to current evidence, VITT usually appears 5 to 42 days after the first dose, and some studies have shown a predominance in women under the age of 60 years [11], [24], [25]. Cases occurring after the second dose, or more than 60 days after the first dose, have also been reported, although they are even rarer.
In our series, from the positive anti-PF4 samples, 10 were confirmed by a functional test, which is considered the gold standard for VITT (and for heparin-induced thrombocytopenia/HIT). In the other 8 cases, it was not possible to perform functional tests. On the other hand, we did not find any positive results on functional tests when the anti-PF4 screening was negative. This finding indicates that ELISA can be a reliable way to detect anti-PF4, and this is very important because functional tests are complex and not easy to implement. Furthermore, in two out of the 10 positive anti-PF4 cases (confirmed by functional assay), the antibody was detected only by the enhanced ELISA (IgG/IgM/IgA), which can indicate the need to use this test as a screening for VITT, instead of IgG anti-PF4.
Our data showed a median interval between vaccination and symptoms of 8 days (IQR 6), and it is important to clarify that sometimes the symptoms related to vaccine reactogenicity may overlap with thrombosis symptoms. As this study used secondary databases, in some cases, it was not possible to precisely define the date of onset of VITT symptoms, which may confound this interval in some cases. Therefore, we cannot make any inferences to population level, only describe the data in our sample.
As some researchers have shown, the early diagnosis and treatment of VITT have a high impact on outcome. The UK achieved an important reduction in their mortality rate from approximately 50% to 17% after implementation of guidelines for early recognition and treatment of the syndrome [11]. Another example is Australia, which also reduced its lethality to approximately 5% after guidelines were distributed to healthcare professionals.
The assessment of the efficacy of therapeutic routes was not possible in our series due to the small number of patients and methodological limitations; however, patients who used nonheparin anticoagulants and intravenous immunoglobulin had a favourable evolution.
5. Conclusions
Our case series shows that Brazilian VITT cases have clinical and laboratory features similar to those previously published. Given the dimensions of the country and the number of doses applied to date, VITT seems to be a very rare adverse event following COVID-19 vaccines, especially those of viral vector platforms.
Improving the pharmacovigilance of this adverse event, together with diffusion of technical information on diagnosis and treatment of the syndrome to assistance teams, is the strategy adopted by NIP/MoH, Bio-Manguinhos/Fiocruz, and AstraZeneca to minimize risks and reduce case lethality. This is also important to maintain public confidence in the vaccine, ensuring that security issues are addressed with transparency and speed. It is expected that with this strategy, Brazil would achieve a decrease in lethality rates for this AEFI, as observed in other countries.
This work will contribute to future research on VITT in Brazil and improve COVID-19 immunization actions nationally and internationally.
Declaration of Competing Interest
Patricia Mouta Nunes de Oliveira ,Catherine Crespo Cordeiro, Debora Lima Abreu, Gabriellen Vitiello Teixeira, Janaina Reis Xavier, Letícia Kegele Lignani, Livia Neves Waite Freitas, Maria de Lourdes de Sousa Maia, Michael Bernardes Ramos, Patricia Mouta Nunes de Oliveira, Paulo Roberto Gomes Takey, Renata Saraiva Pedro, Tainá dos Santos Pereira, and Vitor Cardoso da Gama are employees of the Immunobiological Technology Institute/Oswaldo Cruz Foundation (Bio-Manguinhos/Fiocruz), Marketing Authorization Holder of the covid-19 vaccine (recombinant)* in Brazil. Bárbara Emoingt Furtado and André Santa Maria reports a relationship with AstraZeneca Brasil that includes: employment. employee of AstraZeneca Brasil , Marketing Authorization Holder of the covid-19 vaccine (recombinant)* in Brazil. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors thank the entire Clinical Advisory Unit of Bio-Manguinhos/Fiocruz, Bio-Manguinhos/Fiocruz team, the MoH, local surveillance teams and local immunization teams, the Brazilian Health Regulatory Agency (Anvisa) and healthcare professionals that made the vaccination campaign possible and successful in Brazil.
The Brazilian VITT investigative Collaborative Group includes several professionals who worked at assistance, investigation and epidemiological surveillance of the reported adverse events following immunization here described. The authors are grateful to the members of the collaborative group.
The Brazilian VITT investigative collaborative group :
-
▪
Anna Paula Bise Viegas and Liliam Cristiana Júlio: Reference Center for Special Immunobiologicals of Santa Catarina/Brazil.
-
▪
Ana Paula Pietrowski Bertuol: Health Department of the Federal District/Brasília/Brazil.
-
▪
Vitor Alves Cruz and Adriana de Oliveira Sousa Matos: Municipal Health Department of Goiânia/Goiás/Brazil.
-
▪
Eder Gatti Fernandes:Epidemiological Surveillance Center “Prof. Alexandre Vranjac”, São Paulo/São Paulo/Brazil.
-
▪
Risoleide Marques de Figueiredo and Rosane Ferreira: Rio de Janeiro State Department of Health, Rio de Janeiro/Brazil.
-
▪
Cláudia Weingaertner Palm and Marion Burger: Municipal Health Department of Curitiba/Paraná/Brazil.
-
▪
Diogenes Seraphim Ferreira, Georgia Karina Morgenstern and Tsukiyo Obu Kamoi: Hospital das Clínicas of Paraná Federal University, Curitiba/Paraná/Brazil.
-
▪
Sandra Maria Deotti Carvalho: National Immunization Program/ Brazilian Ministry of Health/ Brazil.
-
▪
Isis Mattos de Carvalho, Juliana Jenifer da Silva Araújo Cunha, Nadja Greffe, Nathalya Macedo Nascimento Costa and Thaina Genuino de Souza: Municipal Health Department of Immunization of Rio de Janeiro/Rio de Janeiro/Brazil.
-
▪
Gabriele Tantos Nunes, Tatiana Garcez: Hemorio/ Rio de Janeiro/ Brazil.
-
▪
Aline Moura Ferraz Pereira, Assistant Hepatologist, Curitiba/Paraná/Brazil.
-
▪
Bruna Souza Sabioni: Assistant Hematologist. Petropólis/Rio de Janeiro/Brazil.
-
▪
Juliana Vassalo Rodrigues: Assistant Hematologist, Niterói/Rio de Janeiro/Brazil.
-
▪
Marielle Fraga Salenave: Assistant Hematologist, Brasília/ Distrito Federal/ Brazil.
-
▪
Fabio Augusto Schutz: Assistant Oncologist, São Paulo / São Paulo / Brazil.
-
▪
Jacques Kaufman: Assistant Hematologist, Niterói/Rio de Janeiro/Brazil
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.06.014.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 20 de fevereiro de 2020;382(8):727–33. [DOI] [PMC free article] [PubMed]
- 2.World Health Organization. WHO Coronavirus (COVID-19) Dashboard [Internet]. 2021 [citado 13 de setembro de 2021]. Disponível em: https://covid19.who.int.
- 3.Ministério da Saúde. Painel Coronavírus [Internet]. 2021 [citado 13 de setembro de 2021]. Disponível em: https://covid.saude.gov.br/.
- 4.Johns Hopkins University & Medicine. COVID-19 Map - Johns Hopkins Coronavirus Resource Center [Internet]. 2021 [citado 13 de setembro de 2021]. Disponível em: https://coronavirus.jhu.edu/map.html.
- 5.Fiocruz. Immunobiological Technology Institute (Biomanguinhos) [Internet]. Fiocruz. [citado 7 de dezembro de 2021]. Disponível em: https://portal.fiocruz.br/en/unidade/immunobiological-technology-institute-biomanguinhos.
- 6.See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, et al. US Case Reports of Cerebral Venous Sinus Thrombosis With Thrombocytopenia After Ad26.COV2.S Vaccination, March 2 to April 21, 2021. JAMA. 30 de abril de 2021;325(24):2448–56. [DOI] [PMC free article] [PubMed]
- 7.Sangli S., Virani A., Cheronis N., Vannatter B., Minich C., Noronha S., et al. Thrombosis With Thrombocytopenia After the Messenger RNA-1273 Vaccine. Ann Intern Med outubro de. 2021;174(10):1480–1482. doi: 10.7326/L21-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holm S, Kared H, Michelsen AE, Kong XY, Dahl TB, Schultz NH, et al. Immune complexes, innate immunity, and NETosis in ChAdOx1 vaccine-induced thrombocytopenia. Eur Heart J. 14 de outubro de 2021;42(39):4064–72. [DOI] [PMC free article] [PubMed]
- 9.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. New England J Med. 3 de junho de 2021;384(22):2092–101. [DOI] [PMC free article] [PubMed]
- 10.Wolf M.E., Luz B., Niehaus L., Bhogal P., Bäzner H., Henkes H. Thrombocytopenia and Intracranial Venous Sinus Thrombosis after “COVID-19 Vaccine AstraZeneca” Exposure. J Clin Med janeiro de. 2021;10(8):1599. doi: 10.3390/jcm10081599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavord S., Scully M., Hunt B.J., Lester W., Bagot C., Craven B., et al. Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. N Engl J Med. 2021;385(18):1680–1689. doi: 10.1056/NEJMoa2109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendes-de-Almeida DP, Martins-Gonçalves R, Morato-Santos R, De Carvalho GAC, Martins SA, Palhinha L, et al. Intracerebral hemorrhage associated with vaccine-induced thrombotic thrombocytopenia following ChAdOx1 nCOVID-19 vaccine in a pregnant woman. Haematologica. 1o de novembro de 2021;106(11):3025–8. [DOI] [PMC free article] [PubMed]
- 13.COVAX. COVAX: Maternal immunization working group webinar expert consultation on post-vaccine thrombosis thrombocytopenia syndrome & impact on maternal immunization - Meeting Report , 9 June 2021 [Internet]. 2021 [citado 14 de dezembro de 2021]. Disponível em: https://media.tghn.org/medialibrary/2021/08/COVAX_MIWG_Webinar_Report_June_2021_23Jul2021.pdf.
- 14.Ministério da Saúde. Nota Técnica - no 933-2021 - CGPNI-DEIDT-SVS-MS - Atualização das orientações para a investigação da Síndrome de Trombose com Trombocitopenia no contexto da vacinação contra a covid-19 no Brasil. [Internet]. 2021 [citado 14 de dezembro de 2021]. Disponível em: https://www.gov.br/saude/pt-br/coronavirus/vacinas/plano-nacional-de-operacionalizacao-da-vacina-contra-a-covid-19/notas-tecnicas/nota-tecnica-no-933-2021-cgpni-deidt-svs-ms.pdf/view.
- 15.Chen RT. Updated Proposed Brighton Collaboration process for developing a standard case definition for study of new clinical syndrome X, as applied to Thrombosis with Thrombocytopenia Syndrome (TTS) [Internet]. 2021 [citado 4 de outubro de 2021]. Disponível em: https://brightoncollaboration.us/wp-content/uploads/2021/05/TTS-Interim-Case-Definition-v10.16.3-May-23-2021.pdf.
- 16.IBM Corp. IBM SPSS Statistics for Windows. Released 2011. Armonk, NY; 2011.
- 17.CZARSKA-THORLEY D. Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 27-30 September 2021 [Internet]. European Medicines Agency. 2021 [citado 7 de dezembro de 2021]. Disponível em: https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-27-30-september-2021.
- 18.Ministério da Saúde. Plano Nacional de Operacionalização da Vacinação contra a Covid-19 [Internet]. 2021 [citado 7 de dezembro de 2021]. Disponível em: https://www.gov.br/saude/pt-br/coronavirus/publicacoes-tecnicas/guias-e-planos/plano-nacional-de-vacinacao-covid-19.
- 19.Ministério da Saúde. COVID-19 Vacinação [Internet]. [citado 7 de dezembro de 2021]. Disponível em: https://infoms.saude.gov.br/extensions/DEMAS_C19_Vacina_v2/DEMAS_C19_Vacina_v2.html.
- 20.Medicines & Healthcare products, Regulatory Agency. Coronavirus vaccine - weekly summary of Yellow Card reporting [Internet]. GOV.UK. 2021 [citado 7 de dezembro de 2021]. Disponível em: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting.
- 21.Australian Government Department of Health Therapeutic GoodsAdministration. COVID-19 vaccine weekly safety report - 14-10-2021 [Internet]. Therapeutic Goods Administration (TGA). Australian Government Department of Health; 2021 [citado 7 de dezembro de 2021]. Disponível em: https://www.tga.gov.au/periodic/covid-19-vaccine-weekly-safety-report-14-10-2021.
- 22.Hwang J, Park SH, Lee SW, Lee SB, Lee MH, Jeong GH, et al. Predictors of mortality in thrombotic thrombocytopenia after adenoviral COVID-19 vaccination: the FAPIC score. Eur Heart J. 21 de setembro de 2021;ehab592. [DOI] [PMC free article] [PubMed]
- 23.World Health Organization. Guidance for clinical case management of thrombosis with thrombocytopenia syndrome (TTS) following vaccination to prevent coronavirus disease (COVID-19) [Internet]. 2021. Disponível em: https://www.who.int/publications/i/item/WHO-2019-nCoV-TTS-2021.1. [PubMed]
- 24.Cines DB, Bussel JB. SARS-CoV-2 Vaccine–Induced Immune Thrombotic Thrombocytopenia. New England Journal of Medicine. 10 de junho de 2021;384(23):2254–6. [DOI] [PMC free article] [PubMed]
- 25.Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, et al. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. New England J Med. 10 de junho de 2021;384(23):2202–11. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.