Abstract
The development of DNA-sensing platforms based on new synthetized Methylene Blue functionalized carbon nanodots combined with different shape gold nanostructures (AuNs), as a new pathway to develop a selective and sensitive methodology for SARS-CoV-2 detection is presented. A mixture of gold nanoparticles and gold nanotriangles have been synthetized to modify disposable electrodes that act as an enhanced nanostructured electrochemical surface for DNA probe immobilization. On the other hand, modified carbon nanodots prepared a la carte to contain Methylene Blue (MB-CDs) are used as electrochemical indicators of the hybridization event. These MB-CDs, due to their structure, are able to interact differently with double and single-stranded DNA molecules. Based on this strategy, target sequences of the SARS-CoV-2 virus have been detected in a straightforward way and rapidly with a detection limit of 2.00 aM. Moreover, this platform allows the detection of the SARS-CoV-2 sequence in the presence of other viruses, and also a single nucleotide polymorphism (SNPs). The developed approach has been tested directly on RNA obtained from nasopharyngeal samples from COVID-19 patients, avoiding any amplification process. The results agree well with those obtained by RT-qPCR or reverse transcription quantitative polymerase chain reaction technique.
Keywords: SARS-CoV-2, AuNs, MB-CDs, Carbon nanodots, DNA biosensor
Graphical Abstract
1. Introduction
The rapid spread of COVID-19 or coronavirus disease 2019, caused by the virus SARS-CoV-2, has produced a revolution in the development of new strategies for its detection. The transmission of the virus includes airborne, fomite, contact, droplet, bloodborne and fecal-oral transmissions, which produces the quick spread of the virus [1]. An infected person could be highly contagious even during the incubation period [2]. For this reason, rapid diagnostic testing is crucial for virus detection and prevent isolation. Therefore, to avoid the spread of the virus, it is important to accurately and quickly diagnose COVID-19. Nowadays, there are different strategies available for SARS-CoV-2 detection based on anti-SARS-CoV-2 antibodies (serological tests), SARS-CoV-2 antigens (antigen tests), or on the amplification of different regions of the virus genome by using RT-qPCR or quantitative reverse transcription polymerase chain reaction. This last approach, is the “gold standard” technique for SARS-CoV-2 detection [3], [4]; however, it is not easy to apply RT-qPCR as a mass screening technique due to its high cost, laboriousness and the need for highly qualified personnel and expensive equipment [4]. On the contrary, antigen tests can be used for mass screening due to high specificity, their portability and low cost, but they are usually less sensitive than PCR methods [5]. Lastly, despite the sensitivity of serological they are also not suitable for mass testing since, in general, they require long periods and trained personnel, long time for data reporting and their cost [6]. So, a simple, accurate, fast, and reliable SARS-CoV-2 detection method is fundamental to manage this pandemic situation. Hence, many investigations are aimed to develop new approaches that combine RT-qPCR sensitivity and simplicity of antigen tests. In this regard, DNA biosensors can be presented as an alternative since virus detection is based on a specific region of its genetic code, as in RT-qPCR, but with the advantage of being simple, quick and also qualified personnel is not required. In particular, electrochemical biosensors have become an ideal technique to detect SARS-CoV-2 because it presents advantages such as portability, low cost, and miniaturization, which are positive to their quick deployment in healthcare centers or outbreak places where it is difficult to access professional testing laboratories. These DNA sensors detect DNA or RNA sequences using probe strands, immobilized on the electrode surface, which are complementary to the target DNA. One of the most common strategies to detect the hybridization of probe and analyte, due to its simplicity, is the use of electrochemical indicators. These compounds are redox molecules that interact strongly with DNA and present different affinity binding to double-stranded (dsDNA) than single-stranded (ssDNA) DNA. Despite the use of different redox indicators, as phenothiazines (methylene blue [7], [8], [9], [10], [11], Azure A [12]) or ruthenium complex [13], [14], has been reported for DNA biosensor development, there is still a great interest in the design of new electrochemical indicators highly selective to develop improved and more sensitive DNA biosensors.
In this regard, the use of nanomaterials or hybrid systems opens new possibilities for DNA hybridization detection [15]. Among nanomaterials, carbon nanomaterials and, especially, carbon nanodots (CDs) are interesting regarding their easy synthesis with non-pollutant and nature abundant precursors besides their possibility to interact with DNA [16]. CDs are the allotropic species of carbon last discovered [17]. They can be synthesized using simple hydrothermal methods, and the functional groups surrounding the carbon graphitic core can be chosen using the proper precursors. Currently, a new synthetic process is being developed, enhancing the possibility of introducing specific moieties in the nanostructure carbon dimensional network, endowing it with specific functionalities. For instance, M. Prato et al.[18] have recently demonstrated, how choosing the proper precursors for CDs synthesis, specific electroactive moieties can be inserted in the carbon dots nanostructure, modifying their electronic and electrochemical properties. The same concept has been used in this work using phenothiazine molecules. In particular, we present the synthesis of CDs functionalized with Methylene Blue (a redox indicator) inserted in their structure since both of them, CDs and MB, can interact with DNA[19], [20], [21], [22]. Despite there are some controversy in the type of interaction mode of CDs with DNA, all authors point out to a stronger interaction of CDs with double-stranded DNA than with single-stranded DNA. In this sense, the objective of the chemically modification of CDs with MB is to prepare a new and improved redox indicator (MB-CDs) to selectively detect the hybridization process in electrochemical DNA biosensors, by combining the ability of both molecules to interact strongly and preferentially with double-stranded DNA. Compared to MB alone, MB-CDs has the advantage that a higher number of methylene blue molecules (inserted in the carbon nanodots structure) will be retained in the double-stranded DNA. This fact would lead to a higher electrochemical signal and therefore to a higher selectivity.
Nanomaterials have also been used as electrode modifiers to improve sensitivity and selectivity in electrochemical DNA biosensors development [2], [6], [23], [24], [25], [26], [27], [28]. Despite there are many strategies centered on the use of nanomaterials in biosensor´s design, most of them include many steps and complicated procedures, being still an area of great interest. Among nanomaterials, gold nanomaterials are considered as perfect materials for electrochemical biosensors consequence of their properties such as high conductivity, high surface area, catalytic activity and biocompatibility [29], [30]. Despite the considerable interest demonstrated for gold nanostructures of different shapes in electrochemical DNA biosensor development, particularly, spherical gold nanoparticles [31], [32], and the differences that the shape causes in the behaviour, the combination of gold nanomaterials of different shapes has not been widely exploited yet. This combination of gold nanomaterials of different shapes can provide many advantages. For instance, the edges and vertices of non-spherical particles (gold nanotriangles, nanocubes) can provide higher surface energy, so there are more reaction sites available, resulting in more reactive facets [33], [34]. On the other hand, spherical nanoparticles (AuNPs) can be used to improve stability and generate homogenous recovery on the surface of the electrode [35].
Taking into account the above described, in this work, we present a new selective and sensitive DNA electrochemical biosensor for SARS-CoV-2 detection, based on the combination of two different nanomaterials, Methylene Blue functionalized carbon nanodots (MB-CDs) and a mixture of different shape gold nanomaterials (gold nanoparticles (AuNPs) and gold nanotriangles (AuNTs)). MB-CDs are used as selective redox indicators and gold nanostructures (AuNs) as electrode modifiers to improve sensitivity.
2. Experimental section
2.1. Chemicals
Sodium phosphate dibasic dihydrate (Na2HPO4·2H2O), sodium phosphate monobasic monohydrate (NaH2PO4·H2O), sodium chloride (NaCl), sulfuric acid (H2SO4), sodium thiosulphate (Na2S2O3), tetrachloroauric acid (HAuCl4), L-arginine, [7-(dimethylamino)phenothiazin-3-ylidene]-dimethylazanium chloride (methylene blue chloride), DNA oligonucleotides (listed in Table 1), 3,3′-diamino-n-methyldipropylamine, double-stranded calf thymus DNA (dsDNA), and 1,4-Dithiothreitol (DTT) were purchased from Merck (www.merckgroup.com/es-es). Water is purified with a Millipore Milli-Q-System (18.2 MΩ cm) and was used for the preparation of all solutions.
Table 1.
Oligonucleotide sequences used in this work.
| SARS-CoV-2 ORF1ab sequences | Named | |
|---|---|---|
| Thiol- probe | 5′- SH-C6H10-CCATAACCTTTCCACATACCGCAGACGG −3′ | Probe-SH |
| Thiol- probe TAMRA | 5′- SH-C6H10-CCATAACCTTTCCACATACCGCAGACGG -TAMRA-3′ | ProbeTAMRA-SH |
| Complementary | 5′- CCGTCTGCGGTATGTGGAAAGGTTATGG −3′ | SARS-CoV-2 |
| Interferent 1 | 5′-C CAGGT GGAAC ATCAT CCGGT GATGC-3′ | SARS-CoV |
| Interferent 2 | 5′-TTAGTCATCTGCGGGAATGCAGCATTATCT-3′ | Influenza A |
| Non-Complementary | 5′- GACCGTCGAAGTAAAGGGTTCCATA −3′ | SARS-CoV-2N |
| Mutated | 5′- CCGTCTGCGGTATCTGGAAAGGTTATGG −3′ | SARS-CoV-2SP |
In this work, SARS-CoV-2 has been detected by targeting a specific viral gene region coding for the open reading frame 1 ab (ORF1ab) gene. Table 1 shows all the oligonucleotide sequences used in this work. The DNA sequences comprises the probe sequence (a single stranded sequence, thiolated or modified with an hexalquilthiol at 5′-end, complementary to target analyte; named as Probe-SH), the target sequence (specific sequence from ORF1ab of SARS-CoV-2, denoted as SARS-CoV-2), a mutated sequence (particularly a single nucleotide polymorphism sequence named as SARS-CoV-2SP), a non-complementary sequence (SARS-CoV-2N) and potential interferent sequences from other viruses such as SARS-CoV and Influenza A (SARS-CoV and Influenza A).
2.2. COVID-19 patient samples
RNA from inactivated swab nasopharyngeal samples obtained from COVID-19 patients’ and donated by Hospital Ramón y Cajal (Madrid) was extracted with the QIAamp Viral RNA Qiagen kit. The total RNA obtained was eluted in water free of RNase, and its concentration was measured using a Nanodrop prior to its storage at – 80 °C. To avoid any cross-contamination between samples and/or during their manipulation by the operator, all the procedure were performed in P2-biosecurity cabinets with spatial and temporal separation between COVID-19 positive and negative samples. Specifically, the positive sample was analysed by RT-qPCR with a Ct value of 13. Negative sample has a Ct value above the threshold (~35).
2.3. Instrumentation
A Nüve OT012 autoclave was used to sterilize all material and solutions used in this work.
MB-CDs were synthetised using a CEM Discover LabMate™ microwave synthesis reactor. Afterwerds, they were purify by using a 0.10 µm nylon syringe filters and Spectra/Por® 6, MWCO, 0.1–0.5 kDa dialysis membranes, (General Laboratory Supplies (SGL)).
Fluorometric and spectrophotometric measurements for MB-CDs characterization and DNA interaction studies were carried out using a Cary Eclipse Varian spectrofluorometer and a Thermo Scientific™ Multiskan™ GO spectrophotometer. 96-well microplates, supplied by JET-BIOFIL, were used for absorbance titrations.
Characterization of the synthetized MB-CDs and biosensing platform were carried out by different techniques such as Fourier transform infrared spectroscopy (FTIR), Atomic Force Microscopy (AFM), fluorescence microscopy and Transmission electron microscopy (TEM) among others.The equipment required for these experiments are a Brucker IFS60v spectrometer for Fourier transform infrared (FTIR) spectra (wavelength range from 5,000 to 500 cm−1), a FESEM Auriga, Carl Zeiss equipped with an energy-dispersive X-ray spectrometer was used for SEM electrode characterization. In this case experiments were carried out working on a 2.5 kV voltage (low voltage) and 10 pA current mode to avoid any damage of the sample.
A Nanotec Electrónica AFM system, using cantilevers of silicon (PPP-FM Nanosensors, 75 kHz resonant frequency and 2.8 N/m nominal spring constant), in non-contact mode, was used to acquire AFM images. In this case Highly Oriented Pyrolytic Graphite (HOPG) substrates were used to carried out AFM experiments and WSxM software was used for data acquisition and image processing. A microscope Axioskop 2 MAT (ZEISS), implemented with a short mercury arc lamp HBO 50 W/AC L1 (OSRAM), was used for Fluorescence microscopy meassurements. TEM images were acquired by usning A JEOL JEM 2100 electron microscope and Lacey carbon support film copper grids (400 mesh).
Electrochemical studies were carried out using a potentiostat PGSTAT 30 and screen-printed electrode connector (Metrohm). Gold screen-printed electrodes (AuSPE), which integrates a silver pseudoreference electrode, a gold auxiliary electrode and a gold working electrode were used for electrochemical studies. Software package GPES 4.9 was used for data acquisition.
2.4. Procedures
2.4.1. Synthesis of phenothiazine modified carbon nanodots (MB-CDs)
The reaction mixture containing 0.5 mmol (87 mg) of L-arginine, 0.3 mmol Methylene Blue chloride (96 mg), 0.5 mmol 3,3′-diamino-N-methyldipropylamine(80.6 µL) and 5.5 mol of water (100 µL) Milli-Q were irradiated in a microwave system keeping a maximum pressure of 20 bar for 180 s and constant temperature of 235 ºC. The obtained product was dissolved in 10 mL of water and the solution was filtered with a filter of 0.1 µm porous. After that, the obtained solution was dialyzed for 7 days, using a membrane (0.1–0.5 kDa). The obtained solution was stored at 4 ºC. A solution of MB-CDs containing 2.80 mg mL−1 was obtained.
2.4.2. Synthesis of gold nanostructures (AuNs)
Gold nanostructures (AuNs), a mixture of gold nanoparticles (AuNPs) and gold nanotriangles (AuNTs), were prepared by a seed-mediated growth procedure [36] based on the reduction of HAuCl4 by Na2S2O3 in an aqueous medium. Briefly, these gold nanostructures (AuNs) were prepared as follows: 30 mL of 0.5 mM Na2S2O3 was added to 25 mL of 2 mM HAuCl4, with vigorous stirring. 9 min later, 12.5 mL of Na2S2O3 (0.5 mM) was added with vigorous stirring of the solution. The solution(yellow) turned clear brown and in a few minutes deep red. The seeds are formed during these previous 9 min. The growth of seeds and nanotriangles formation occurs after the second addition of thiosulfate. Finally, 45 min of stirring is maintainied to obtain the mixture of triangular and spherical gold nanoparticles.
2.4.3. Preparation of DNA and SARS-CoV-2 DNA oligonucleotides stock solutions
1.0 mg mL−1 stock solutions of double-stranded (dsDNA) were prepared in water. Using the molar absorptivity of DNA at 260 nm (6,600 L mol−1 cm−1) its concentration was determined. Single-stranded DNA (ssDNA) or denatured dsDNA was prepared boiling for 30 min eppendorfs containing dsDNA and using an ice-bath to cooling them. The prepared solutions were stored frozen at −20 ºC.
Thiol-modified probe stock solution was prepared using a NAP-10 column of Sephadex G-25 and DTT for thiol-modified oligonucleotide reduction. A 10.0 µM stock solution of the thiol-modified probe (Probe-SH) was obtained in pH 7.0 phosphate buffer (PB) 10 mM.
100 µM stock solutions of the analytes: complementary sequence (SARS-CoV-2) and non-complementary sequence (SARS-CoV-2N) were prepared in pH 7.0 10 mM PB with 0.4 M NaCl. Stock solutions of 10 µL were stored at −20 ºC.
2.4.4. Interaction between DNA and MB-CDs
Absorbance and fluorescence titrations of MB-CDs and DNA were carried out fixing MB-CDs concentration (1.40 mg mL−1) and changing the concentration of ssDNA and dsDNA from 0 µM to 230 µM. Milli-Q- (18.2 MΩ cm) purified water was employed as solvent. All solutions were left to incubate for about 30 min before measuring.
The binding constants, Kb, were calculated following the equation 1 described by Becker y Meehan[37],
[ADN] / (εa- εf) = [ADN] / (εb- εf) + 1 / Kb (εa- εf) Eq. 1.
where εf and εb are the molar absorptivity of free and bound forms of MB-CDs, respectively and εa is the molar absorptivity of MB-CDs in presence of the different concentrations of DNA.
In the case of cyclic voltametry studies, AuSPE were activated in 0.1 M sulfuric acid. Afterwards, AuSPE surfaces were rinsed with water and modified with 10.0 µL of 1.0 mg mL−1 solution of ds or ssDNA DNA by drop casting. Then the solution was evaporated for 24 h at room temperature and the DNA modified electrode (ds-ssDNA/AuSPE) was rinsed with purified Milli-Q water. Ultimately, the electrodes were placed in a 0.280 mg mL−1 MB-CDs solution and cyclic voltammograms (CV) were carried out.
2.4.5. SARS-CoV-2 biosensor development
Activated AuSPEs were modified with the synthetized gold nanostructures (AuNs), a mixture of AuNPs and AuNTs, by spraying them onto the working electrode surface for 60 s with an airbrush. For allowing fast evaporation of the solvent, the electrodes were heated at 50 ºC on a hot plate during the process. Finally, the nanostructured electrodes (AuSPE/AuNs) were rinsed with plenty of Milli-Q water.
Nanostructured DNA biosensing platforms were prepared by adding 10.0 µL of 10.0 µM thiol-modified probe (Probe-SH) by drop-casting on the gold nanostructures (Probe-SH/AuNs/AuSPE). Then, platforms were kept at room temperature for 24 h and rinsed with water (Milli-Q) to remove non absorbed materials.
Probe-SH/AuNs/AuSPE was subsequently hybridized (in humid chamber at 40 ºC for 1 h) with 10.0 µL of the analyte sequence: a complementary sequence (SARS-CoV-2), a non-complementary sequence (SARS-CoV-2N) or a mutated sequence (SARS-CoV-2SP). Finally, the platforms were rinsed with Milli-Q water to remove unabsorbed materials.
Electrochemical detection was followed by using MB-CDs as an electrochemical indicator. 10.0 µL of MB-CDs (1.40 mg mL−1) were dropped on to the surface of the biosensing platform before and after hybridization with the target during 30 min. Then, the platforms were rinsed with Mili-Q water and differential pulse voltammograms (DPVs) with a scan rate of 10 mVs−1 were immediately recorded using pH 7.0 0.1 M PB as electrolyte.
2.4.6. Detection of SARS-CoV-2 sequences in human serum samples
A DNA molecule containing the sequence of the SARS-CoV-2 was detected in human serum to study the matrix effect in the biosensor response. Firstly, spiked serum samples with SARS-CoV-2 at a final concentration of 1.00 pM, using human serum as solvent, were prepared. Then, 10.0 µL of this solution were incubated with the probe immobilized on the biosensing platform to carry out the hybridization process. Next, the platforms were rinsed with water to remove the material unadsorbed, and electrochemical detection was carried out using MB-CDs as the electrochemical indicator as we described above. Finally, SARS-CoV-2 concentration was determined by interpolating the electrochemical signal of the spiked serum (subtracting the electrochemical signal of the human serum non-spiked) in the calibration plot (I = 0.2205 log [SARS-CoV-2] + 1.762).
2.4.7. Detection of SARS-CoV-2 in COVID-19 patients
For biosensor measurements, 5 µL of the obtained RNA sample (see section of COVID-19 samples) were deposited on the electrode modified with the capture probe (Probe-SH/AuNs/AuSPE) and were hybridized at 40 ºC for 1 h. Finally, MB-CDs were accumulated on the hybridized DNA layer by direct adsorption and DPVs were recorded.
Results are presented as the mean of the peak current measured at −0.400 V ± standard deviation (SD) (n = 3 electrodes). The statistical analysis was obtained using R software [38]. A Student T-test for independent samples, with a confidence interval of 95 % has been used.
3. Results and discussion
In a DNA electrochemical biosensor, the immobilization of the probe and the election of the redox indicator to use are two crucial steps for the sensitive and selective subsequent hybridization event detection. In this work for this purpose, we combine Methylene Blue modified Carbon nanodots (MB-CDs) with a mixture of spherical and triangular gold nanoparticles. The mixture of different shape gold nanostructures (AuNs) increases not only the electrode surface area by nanostructuration but also the number of active sites and consequently the amount of immobilized thiolated DNA probe, improving the biosensing layer development. The MB functionalized carbon nanodots are electrochemically active, due to the presence of the dye. In addition, they can also interact efficiently with DNA, being good candidates to act as an efficient electrochemical indicator of the hybridization event for the development of a nanostructured electrochemical DNA biosensor for selective and sensitive detection of SARS-CoV-2 virus.
3.1. Synthesis and characterization of nanomaterials
3.1.1. Synthesis and characterization of Methylene Blue Carbon Nanodots (MB-CDs)
MB-CDs were synthetized in a one-step procedure, using all precursors together, by microwave irradiation using L-arginine, 3,3′-diamino-N-methyldipropylamine and Methylene Blue chloride as precursors, as we describe in detail in experimental section. It is worth to note that they have not being prepared by just mixing pre-synthesized carbon nanodots and methylene blue. Fig. 1a shows the TEM micrograph of the synthesized MB-CDs. They present rounded shape CDs with a medium size of 8 nm. In order to probe the different composition of MB-CDs compared with similar CDs synthesized previously in absence of Methylene Blue during the synthesis, FT-IR experiments of unmodified CDs, MB-CDs and Methylene Blue were carried out (Fig. 1b). Methylene Blue spectrum showed a band at 3,402 cm−1, which is associated to ν(O-H) due to adsorbed water. Bands around 2,917 and 1,603 cm−1 correspond to the stretching vibration of C-H and to the stretching skeleton vibration of the benzene ring and the N–H bonding, respectively. The bands at 1,490 cm−1 and 1,394 cm−1 are associated to the C–N stretching vibration of aromatic amines; the absorption peaks near 1,141 cm−1 are ascribed to the C–N stretching vibration in the aliphatic chain; the split peak close to 1,338 cm−1 is ascribed to the –CH3 symmetric and asymmetric stretching vibration. CDs show the typical stretching band of NH2 and OH as a broad band at 3,418 cm−1. The bands near to 2,929 cm−1 come from the C–H bond stretching vibration. C C stretching vibrations are observed at 1,640 cm−1.
Fig. 1.
(a) TEM micrograph of MB-CDs. (b) FT-IR spectra of Methylene Blue (blue curve), unmodified CDs (black curve) and MB-CDs (red curve). (c) UV-Visible spectra of 12.5 µM Methylene Blue (blue curve), 140 ng mL−1 of unmodified CDs (black curve) and 140 ng mL−1 MB-CDs (red curve) in MilliQ-water. (d) Cyclic voltammograms of: 50 µM Methylene Blue (blue curve) and 600 ng mL−1 of MB-CDs (red curve), in pH 7 0.1 M PB at 100 mV s−1. Ref Ag.
The tiny band at 1,555 cm−1 corresponds to the stretching vibration of C– N, while the bands at 1,431 cm−1 and 1,381 cm−1 are due to the C–N bonds. In the case of MB-CDs, the absorption increase of bands at 3,418, 1,431 and 1,381 cm−1 is consistent with the introduction of new amine groups present in the Methylene Blue structure. Furthermore, the observed increase of the band at 2,929 cm−1 is ascribed to the increase of C-H bonds, probably caused by the insertion of the Methylene blue aromatic ring in the nanostructure. The same effect is observed in the band at 1,640 cm−1 as a consequence of the increase of aromatic structure related to the insertion of Methylene Blue phenothiazine ring. The result obtained by FT-IR suggests that Methylene Blue is now covalently inserted into the CDs nanostructure.
Elemental analysis of MB-CDs and CDs were also carried out. The experimental data obtained show differences between them as a consequence of the insertion of Methylene Blue molecules in CDs structure. MB-CDs composition was: %C: 50.93, %H: 8.23, %N: 20,36, %S: 0,92, while CDs composition was: %C: 50.14, %H: 9.01, %N: 21.68, S: 0,00. As can be observed, the presence of near 1 % of sulphur suggests that methylene blue phenothiazine ring is inserted in the nanomaterial structure.
In order to further prove the insertion of Methylene Blue molecules in the CDs structures UV-Visible spectroscopy and cyclic voltammetry experiments were carried out. As can be observed at spectrum in Fig. 1c, MB-CDs (red spectrum) show a big absorption band at 300 nm, which could be due to various contribution as n–π * transition of a phenothiazine ring (blue spectrum) or the π-π * transition of the C C conjugated units associated to the CDs (black spectrum). The broad band assigned to the Methylene Blue covalently attached to the CDs is centred at 625 nm (red spectrum), showing two peaks at 590 and 640 nm. This band can be correlated with the maximum absorption peak (664 nm) and the shoulder (610 nm) shown by the Methylene Blue solution (blue spectrum). The shift observed is probably due to the phenothiazine ring modification that takes place during the MB-CDs synthesis process, suggesting the ring insertion in the covalent net of the CDs. Regarding the cyclic voltammograms (CVs) shown at Fig. 1d, one can observe that in the case of Methylene Blue one redox pair is observed at formal potential of −0.390 V (vs. Ag) with two electrons involved in the process. However, in the case of MB-CDs, two redox pairs at formal potential of −0.310 and −0.490 V (vs. Ag), each of them exchanging one electron, are observed. This electrochemical behaviour is consistent with the methylene blue insertion in the MB-CDs structure, and is mainly caused by modifications of the electronic structure of phenothiazine ring moiety after its insertion into the CDs nanostructure.
X-ray diffraction analysis of MB-CDs was carried out, showing a clear amorphous material as it is deduced from diffractogram (see Fig. 1 of Supporting Information,).
3.1.2. DNA and MB-CDs Interaction
Since phenothiazines have been successfully employed as redox indicators of the hybridization event[39], and to check if MB-CDs improve that property, we have studied the MB-CDs interaction with double and single-stranded DNA by spectroscopic and electrochemical techniques.
It is well known that the interaction of DNA with small molecules can be studied by changes caused in their electronic spectrum [40]. Hence, we recorded the UV–visible spectra of MB-CDs in the absence and in the presence of increasing concentrations (from 0 µM to 230 µM) of dsDNA or ssDNA ( Fig. 2). The absorption spectra of MB-CDs in pure water upon addition of increasing concentrations of dsDNA or ssDNA show a progressive absorbance decrease or hypochromic effect in the absorption maximum of MB-CDs (600 nm), as well as a slight shift towards higher wavelengths (bathochromic effect). These effects are more evident in the case of dsDNA. The observed DNA-induced spectral variations are reported to be caused by the strong interaction with purine and pyrimidine bases [37]. They suggest that DNA interacts with MB-CDs and this interaction is stronger in the case of dsDNA. From the changes in the absorption spectra, we also estimated the respective binding constants. These constants are used to quantify the strength of interaction. Following Equation 1 of experimental section, the binding constants (Kb) were found to be 6.45 10 4 M−1 and 1.96 10 4 M−1 for ds and ssDNA, respectively. These values are similar to those reported in the literature for compounds that interact with DNA strongly [42]. Hence, they confirm that there is a strong interaction between MB-CDs and DNA, being stronger with dsDNA than with ssDNA. From the tritation data, plotting the absorbance vs [MB-CDs] / [DNA] the stoichiometry between MB-CDs and ds-DNA was also calculated (β. 2 SI). The ratio [MB-CDs] / [DNA] was found to be 20. This value is similar to those reported in the literature by compounds that interact strongly with DNA [43].
Fig. 2.
Absorption spectra of MB-CDs in the absence (black line) and in the presence of increasing amounts of dsDNA (a) or ssDNA (b) in water solution. c) Cyclic voltammograms of MB-CDs in pH 7.0 0.1 M PB solution at a nacked AuSPE (black curve), a dsDNA/AuSPE (blue curve) and a ssDNA/AuSPE (red curve). Scan rate: 100 mV s−1. d) DPVs of MB-CDs in pH 7.0 0.1 M PB after accumulation in AuSPE (black curve), ssDNA/AuSPE (red curve) and dsDNA/AuSPE (blue curve).
With the aim of corroborating the results obtained above and based on MB-CDs electroactivity, we carried out electrochemical studies to analyze their interaction with DNA and the possibility of using them as an electrochemical indicator of the hybridization event in the development of DNA electrochemical biosensors. Fig. 2c shows the CV response of MB-CDs at a bare AuSPE (black line), or DNA modified gold electrode: dsDNA/AuSPE (blue line) or ssDNA/AuSPE (red line), using pH 7.0 0.1 M PB solution as electrolyte. MB-CDs show the above-mentioned peaks at −0.310 and −0.490 V vs. Ag. As can be observed, after electrode modification with DNA a decrease in the peak current is observed, being more evident in the case of single-stranded DNA. Furthermore, it is also observed a formal potential (E⁰´) shift (around 10 mV) of the peak centered at −0.310 V to a more negative value for dsDNA. These results point out to a strong interaction between MB-CDs and the DNA immobilized on the electrode surface[44], [45] and agree with those obtained by spectroscopic studies, confirming again that MB-CDs interact with DNA and the interaction strength is different with ssDNA and dsDNA. In addition, if after accumulation of MB-CDs by absorption either on a bare or DNA modified electrode (see Experimental section), the electrode is rinsed with purified water and immersed in a solution containing only the electrolyte solution in the electrochemical cell, a voltammetric response characteristic of redox couples confined on the surface of the electrode is observed for the dsDNA/AuSPE, ssDNA/AuSPE and AuSPE (data not shown). This response is ascribed to the oxidation of the MB-CDs adsorbed on the electrode surface or accumulated in the DNA layer, being higher in the last case, as is evident in Fig. 2d where the Differential Pulse Voltammogram (DPV) of MB-CDs confined on the electrode surface is depicted. It confirms that the incorporated MB-CDs remains tightly bound to the DNA layer, suggesting again a stronger interaction with the dsDNA.
The results presented above point to the utility of MB-CDs as new redox indicator of the hybridization event in the development of a DNA biosensor.
3.1.3. Synthesis and characterization of gold nanostructures
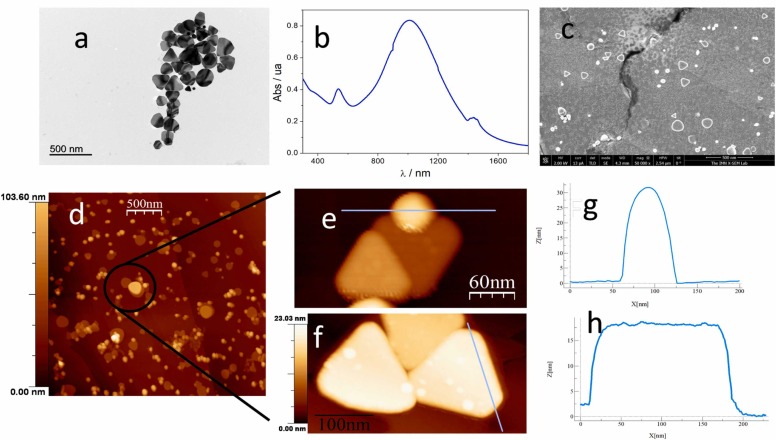
A mixture of spherical (AuNPs) and triangular (AuNTs) gold nanoparticles were prepared following the seed-mediated growth procedure and using as precursors HAuCl4 and Na2S2O3 (see Experimental section). The as prepared gold nanostructures (AuNs) were characterized by UV–visible, Atomic Force Microscopy (AFM), Scanning Electron Microscopy (SEM) and Transmission electron microscopy (TEM) to determine their morphology, size and properties. Morphology and dimensions were estimated by TEM. As shown in Fig. 3a, spherical and triangular gold nanoparticles are observed, the triangular nanoparticles have diameters in a range from 50 to 300 nm and present an average size of lateral length of 150.0 nm ± 0.5 nm, whereas spherical nanoparticles have an average size of 15 nm. Fig. 3b shows the UV–visible-NIR (near infrared) spectrum of gold nanostructures. Two bands at 539 and 1015 nm ascribed to the formation of spherical (539 nm) and triangular nanoparticles (1015 nm) are observed and confirm their synthesis. The AFM study of both nanoparticles on HOPG substrates provides additionally information to confirm their spherical and triangular morphology (Fig. 3e and f, which are a magnification of Fig. 3d). The profile of Figs. 3e and 3f (blue line, Figs. 3g and 3h), shows a gold nanotriangles average length and height of 149 nm and 17.6 nm, respectively. An average diameter of 12.0 nm for spherical gold nanoparticles are also observed. The morphologic characteristics observed by SEM (see Fig. 3c) are in good agreement with those observed by AFM and TEM. Therefore, based on the results obtained, we can confirm the successful synthesis of a mixture of triangular and spherical gold nanoparticles.
Fig. 3.
a) TEM image, b) UV–visible-NIR spectra, c) SEM image and d) AFM image of gold nanostructures (AuNs). e) Magnification of AuNPs and f) AuNTs and their respective profiles g) and h).
3.2. SARS-CoV-2 biosensor development
In this work we present a simple and efficient electrochemical biosensing platform based on the nanostructuration of screen-printed electrodes (AuSPEs) with gold nanostructures of different shapes (AuNs) and their further modification with the corresponding thiolated capture probe, as a base to develop a sensitive and selective methodology to detect SARS-CoV-2 sequences (see Scheme 1).
Scheme 1.
Design of the biosensor.
Hybridization between the target sequence and the probe is conducted to detect a particular sequence of SARS-CoV-2 by using the prepared MB-CDs as electrochemical indicator, allowing also the quantification of DNA molecule containing a region of the SARS-CoV-2 sequence.
The biosensing platform development was characterized by cyclic voltammetry (CV), SEM and AFM. The Cyclic voltammogram (CVs) of an AuSPE after modification with gold nanostructures (blue line of Fig. 4a) shows an increase in the peak current of the characteristic peaks of the gold confirming the modification. From the reduction peak of gold oxide, the electroactive surface area was estimated to be 0.0169 cm2. This value, as expected, is higher than 0.0084 cm2 obtained for the bare electrode. As can be observed in Fig. 4b, the SEM image of the naked electrode shows a rough surface principally composed of big gold particles. After the modification of the electrode with the gold nanostructures, its surface appears covered with a homogeneous distribution of nanostructures (Fig. 4c). AFM image of the AuSPE after modification with the nanostructures (Fig. 4d) shows triangular and spherical particles, corroborating SEM results and the electrode modification.
Fig. 4.
a) Cyclic voltammograms from −0.5 to 1.5 V (vs Ag) of a AuSPE (black line) and an AuNs/AuSPE (blue line) in 0.1 M H2SO4. SEM image of a AuSPE (b) and AuNs/AuSPE (c). d) Topography AFM image (derivative) of the prepared AuNs/AuSPE. e) Cyclic voltammograms (CVs) of MB-CDs in 0.1 M PB pH 7.0 at AuNs/AuSPE (black curve) and Probe-SH/AuNs/AuSPE (blue line). Scan rate: 100 mV s−1. f) Fluorescence image of ProbeTAMRA-SH/AuNs/AuSPE.
The immobilization of the DNA thiolated probe on electrodes previously modified with the gold nanostructures was assessed by CV and fluorescence microscopy. CVs of MB-CDs at a Probe-SH/AuNs/AuSPE, (see blue line of Fig. 4e) shows that after electrode modification with the probe, as we also observed for ds and ssDNA modified electrodes, only the peak of MB-CDs at around −0.490 V appears well defined, probably due to some limitations in the charge transfer caused by the DNA immobilized on the electrode surface and compatible with the immobilization of the capture probe on the AuNs/AuSPE. Probe immobilization was also confirmed by fluorescence microscopy (see Fig. 4f). In this case, a probe labelled with the fluorophore tetramethylrhodamine (TAMRA) was used. It was named as ProbeTAMRA-SH. Optical microscope images of AuNs/AuSPE before and after probe immobilization are almost identical as can be observed in Fig. 3a, b of SI. However, fluorescence images are only observed for electrodes modified with the probe labelled with TAMRA (Fig. 4f). As one would expect, the AuSPE modified only with the gold nanostructures (AuNs/AuSPE) does not show any fluorescence contrast (Fig. 3, Appendix A).
3.3. SARS-CoV-2 DNA sequences detection
The developed biosensor was applied to the detection of an specific sequence from the open reading frame (ORF1ab) of SARS-CoV-2, used as a model. As a capture probe, we use a complementary sequence to a characteristic sequence of the ORF1ab gen of the virus modified with a thiol on 5′ end (Probe-SH, see Table 1) to achieve the probe immobilization on the gold nanostructures. Different strategies have been studied to obtain standed–up and compact probe monolayer. In particular, we have carried out probe immobilization either by employing a mixture of mercaptohexanol (MCH) and the thiolated probe or using adsorption of the thiolated probe, without MCH, but employing a long time (24 h). The hybridization effectiveness was higher when a long time is employed. In this case, we observe a higher difference between the biosensor signal before and after hybridization of the target with the probe (see). These results confirm the importance of immobilization time in the self-assembly of the capture probe monolayer and are in agreement with those previously reported by us [46].
After immobilization of the DNA probe, the hybridization with the target analyte was carried out on the biosensor surface and detected via the increase of the electrochemical signal from the MB-CDs accumulated on the double DNA layer formed after hybridization on the electrode surface. Differential Pulse Voltammetry was employed as electrochemical signal in these studies to reach a higher sensitivity and discriminate better between signal and current background. Therefore, analyte detection was carried out through the change in the oxidation peak current of the MB-CDs accumulated at DNA probe modified electrode (Probe-SH/AuNs/AuSPE) before and after hybridization with the analyte. In the first hybridization test, a complementary (SARS-CoV-2) and, as control, a non-complementary sequence (SARS-CoV-2N) of the DNA probe were employed as the target DNA. As a control, the peak current of the MB-CDs accumulated at an electrode modified only with the gold nanostructures (AuNs/AuSPE) was also checked. As can be observed in Fig. 5, Appendix A, MB-CDS hardly adsorbs on the electrode surface. The detailed procedures for hybridization and redox indicator accumulation steps are described in the Experimental Section (see SARS-CoV-2 biosensor development section). Fig. 5 shows the DPVs (a) and bar diagrams (c) obtained before and after hybridization.
Fig. 5.
DPVs (a) and bar diagrams (b) of the biosensor response (Probe-SH/AuNs/AuSPE) in pH 7.0 0.1 M PB solution, before (black curve) and after hybridization with a complementary sequence, SARS-CoV-2 (blue curve), and a non-complementary sequence, SARS-CoV2N (red curve), after accumulation of MB-CDs. c) DPVs response of Probe-SH/AuNs/AuSPE in pH 7.0 0.1 M PB solution after hybridization with different concentrations (from 10.0 aM to 10.0 nM) of the complementary sequence, SARS-CoV-2, after MB-CDs accumulation. d) Calibration curve obtained. Error bars correspond to the standard deviation of three different biosensors (n = 3).
It can be observed that the biosensor response (measured at peak 1, around - 0.400 V) to the totally complementary sequence is around 5,5 times higher than that obtained for the blank. However, near no response to a noncomplementary sequence is observed. These results confirm that the proposed biosensor works well on recognizing specific SARS-CoV-2 sequences complementaries to the probe used. Moreover, as can be observed in Fig. 5b, the biosensor response increases as the concentration of SARS-CoV-2 sequence increases. This increase is linear to the logarithm of SARS-CoV-2 concentration (Fig. 5d) and fits the linear equation I = 0.2205 log [SARS-CoV-2] + 1.762 (R = 0.994). The limit of detection (LOD) and quantification (LOQ) were estimated using the 3 Sb.m−1 and 10 Sb.m−1 criteria, respectively, where Sb is the standard deviation of the background signal (Probe-SH/AuNs/AuSPE) and m is the slope of the calibration plot. Values of 2.00 and 6.60 aM were calculated for the limit of detection (LOD) and quantification (LOQ), respectively. The relative standard deviation (RSD) was estimated to be 1 %. We also evaluate the repeatability of the biosensor by measuring its response 5 times and it was found to be 0.30 %. Moreover, the stability of the biosensor was evaluated and we observed that the biosensor can detect the analyte (SARS-CoV-2sequnce) over a period of sixty days.
To prove the gold nanomaterial effect on the biosensor performance, we also developed the biosensor using the same methodology but avoiding gold nanostructures (AuNs). In this case, the response also increases linearly (y = 0.0772 [SARS-CoV-2] + 1.188 (R = 0.971)) with SARS-CoV-2 concentration (see), but the detection and quantification limits were much higher (LOD and LOQ of 0.650 and 2.18 pM, respectively). These results confirm that the use of gold nanomaterials plays a crucial role on the proposed methodology, probably not only improving the probe immobilization but also the electrochemical signal transduction. Furthermore, to confirm also the synergistic effect of MB-CDs, a control experiment using MB as electrochemical indicator was carried out. As expected, the obtained LOD and LOQ were higher (1.22 and 3.91 pM, respectively) and the sensitivity lower (0.0477µApM−1), confirming the efficiency of using MB-CDs (see).
The detection limit obtained with the developed biosensor is lower than the reported in the literature for other electrochemical biosensors previously reported to detect SARS-CoV-2 (see Table 2). Moreover, our biosensor is easier and simple to develop than most of those previously described with high potential in practical applications for SARS-CoV-2 detection. Furthermore, as can be observed in Table 2 the analysis time in the present work is 90 min, lower than the reported for others authors.
Table 2.
Electrochemical biosensors previously reported to detect SARS-CoV-2.
| Target analyte | Fundament | Method | L.O.D. | Analysis Time | Reference |
|---|---|---|---|---|---|
| N-gene | Electropolymerized polyaniline (PANI) nanowires | DPV | 3.5 fM | 1.5 h | [39] |
| N-gene | Gold nanoparticles capped with antisense oligonucleotides | Signal conditioning circuit | 6.9 copies/µL | 5 min | [40] |
| S and Orf1ab genes | Four-way junction (4-WJ) hybridization | SWV | 5.0 ag/µL | 1 h | [41] |
| ORF1ab | MoS2 | DPV | 1.01 pM | 2 h | [22] |
| ORF1ab | Catalytic hairpin assembly | DPV | 26 fM | 3 h | [23] |
| ORF1ab | Gold nanomaterial and MB-CDs | DPV | 2.2 aM | 1.5 h | Present work |
To evaluate the selectivity of the biosensor we obtained its response to the SARS-CoV-2 sequence (50.0 pM) in the presence of other sequences of different virus such as SARS-CoV-1 and Influenza A (H7N9), at the same concentration (50.0 pM). As can be observed in Fig. 6, the biosensor response, in presence of both virus, is practically the same than that obtained in the absence of potential interferent sequences. These results, prove that the proposed biosensor can detect a SARS-CoV-2 sequence even in the presence of other virus sequences.
Fig. 6.
Bar diagrams of the biosensor response (Probe-SH/AuNs/AuSPE) in pH 7.0 0.1 M PB solution, after hybridization with: a 50.0 pM complementary sequence (SARS-CoV-2) (third bar), a mixture of 50.0 pM of SARS-CoV-2 and 50.0 pM of Influenza A sequences (first bar), a mixture of 50.0 pM of SARS-CoV-2 and 50.0 pM of SARS-CoV sequences (second bar) and a 50.0 pM mutated sequence (SARS-CoV-2SP) (last bar).
3.4. Single Nucleotide Polymorphism (SNP) detection
Currently, the detection of SARS-CoV-2 variants has an enormous interest. These variants are associated with mutations in its sequence. Therefore, the development of new sensing platforms able to detect point mutations such as Single Nucleotide Polymorphisms (SNPs) is a great deal of interest. Hence, we studied the ability of the developed device to detect SNPs in SARS-CoV-2 sequence (see Table 1).
Fig. 6 shows the biosensor response to a 50.0 pM SARS-CoV-2 sequence containing a Single Nucleotide Polymorphism (SARS-CoV-2SP). As we expected, the response to the mutated sequence gives a smaller signal (1.15 ± 0.2 µA), than that observed for the complementary sequence (2.187 µA). The presence of the mutation in the target sequence give a distorted double-helix after hybridation that interacts with the redox indicator in a less extention [47]. As consequence a smaller electrochemical signal is obtained. Based on the signal obtained for both sequences and tacking into account the error associated to each signal, one could confirm that the difference in signals obtained verify the selectivity of the developed biosensor.
3.5. SARS-CoV-2 detection in human serum samples
The biosensor response in a media more complex than a simple buffer was evaluated. For this purpose, we choose human serum. The response of the biosensor to a sample of human serum spiked with SARS-CoV-2 sequence (50.0 pM of SARS-CoV-2) gave an average signal of 2.14 ± 0.05 µA. Using the calibration curve, the SARS-CoV-2 concentration in the spiked serum sample was calculated to be 51.8 pM with a RSD of 8 % and a recovery of 103.6 %. This result concludes that the proposed methodology works well even in a matrix different and complex than a simple phosphate buffer.
3.6. Detection in COVID-19 patient samples
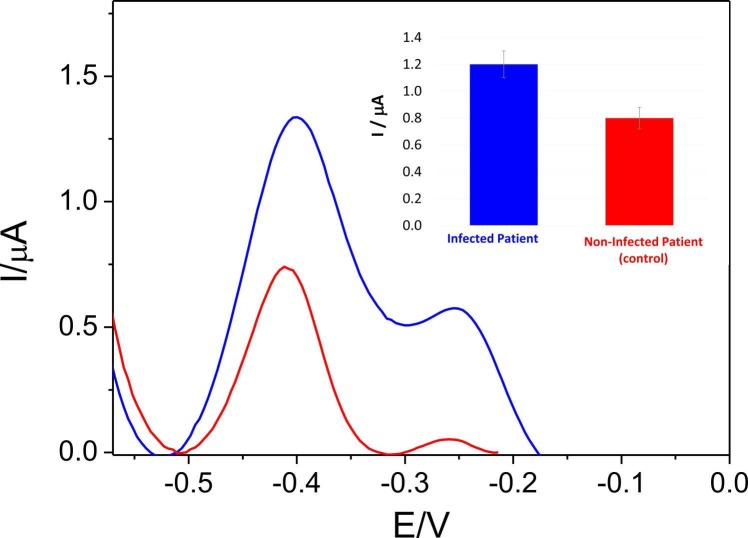
Based on the good results presented above, we took a step forward and applied our biosensor to detect the RNA of SARS-CoV-2 directly in nasopharyngeal samples of COVID-19 patients avoiding any amplification process. The samples were provided and previously analysed at Hospital Ramon y Cajal by RT-qPCR to validate the biosensor. Particularly, a nasopharyngeal sample of an infected patient (viral load of 13 Cts, measured by RT-qPCR) and a sample from a non-infected patient, used as control, were analysed. Fig. 7 shows the signal obtained for both samples. As can be observed, a higher biosensor response (1.2 ± 0.2 µA) was obtained in the case of the infected patient sample compared to the non-infected patient sample (0.8 ± 0.08 µA). Moreover, statistical analysis shows that samples from the infected patient are significantly different than the control (T-test p-values of 0.022 or confidence interval of 97.8 %). Hence, we believe that the proposed biosensor has the adequate sensitivity to be employed as a screening method to rapidly discriminate between infected (viral load at least 13Cts) and non-infected patient samples, without the need of any amplification process.
Fig. 7.
DPVs and bar diagrams (inset) of the biosensor response (Probe-SH/AuNs/AuSPE) in pH 7.0 0.1 M PB solution, after hybridization with a nasopharinge sample of: an infected patient (blue curve) and non infected patient of COVID-19 (red curve).
4. Conclusions
The combination of DNA-sensing architectures based on gold nanostructures of different shapes (a mixture of gold nanoparticles (AuNPs) and gold nanotriangles (AuNTs)) and new prepared MB-CDs has been proved as a successful platform to develop a new electrochemical biosensor for the sensitive and selective detection of DNA sequences related to SARS-CoV-2. Both nanostructures have been synthetized and exhaustively characterized by many techniques before being employed to modify electrodes and prepare an improved nanostructured electrochemical platform for DNA detection. On the other hand, MB-CDs have been synthetized and employed as a new redox indicator. Hybridization event is detected by measuring changes in the electroactivity of the MB-CDs accumulated into dsDNA by means of Differential Pulse Voltammetry. The developed DNA sensor is able to detect a sequence of SARS-CoV-2 with a detection limit of 2.0 aM and in the presence of other potential interferent sequences corresponding to other viruses. Moreover, it can detect a single nucleotide polymorphism. The robustness of the method has been evaluated by detecting SARS-CoV-2 in nasopharyngeal samples from COVID-19 patients avoiding any amplification process.
CRediT authorship contribution statement
Rafael del Caño and Teresa Pineda have synthetized and characterized gold nanomaterials, Emiliano Martinez-Periñan and Alvaro Martínez-Sobrino have synthetized and characterized Carbon nanodots, Clara Pina-Coronado and Laura Gutiérrez Galvez have carried out the experiments regarding the biosensor development, Daniel García-Nieto and Mónica Luna: have performed the AFM and Fluorescence Microscopy characterization, Micaela Rodríguez-Peña and Mónica Luna have perforemd the SEM characterization and analysis, Tania García-Mendiola, Felix Pariente, and Encarnación Lorenzo have supervised the experimental results regarding the biosensor, Tania García-Mendiola and Encarnación Lorenzo have elaborated the manuscript, Melanie Abreu, Rafael Cantón and Juan Carlos Galán have obtained the samples from patients and performed the qPCR, Paula Milán-Rois and Milagros Castellanos have extracted and quantified the viral RNA from patient’s samples, Álvaro Somoza has supervised experimental results regarding RNA samples and review & editing the manuscript, Rodolfo Miranda coordinated the groups and revised the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge the support from the Comunidad de Madrid (TRANSNANOAVANSENS-CM, S2018/NMT-4349, NANOCOV-CM, SI3/PJI/2021–00341) and Ministerio de economia y competitividad de España (PID2020–116728RB-100, CTQ2015–71955-REDT (ELECTROBIONET)). IMDEA Nanociencia acknowledges support from the Programme for Centres of Excellence in R&D ‘Severo Ochoa’ (CEX2020–001039-S, MINECO). Authors also acknowledge REACT EU NANOCOV-CM project. RdC acknowledges support from Fundación IMDEA Nanociencia, Banco Santander, UAM (convocatoria CRUE- SANTANDER-CSIC, reference 10.01.03.02.41).
Biographies
Clara Pina-Coronado obtained the Degree in Chemistry in 2021 from the Universidad Autónoma de Madrid. Currently she is student of the Master degree in Applied Chemistry at the Universidad Autónoma de Madrid. Her current research focuses on the development of electrochemical (bio)sensors for detection of virus.
Álvaro Martínez-Sobrino received his bachelor’s degree and Master degree in chemistry from Universidad Autónoma de Madrid in 2020 and 2021 respectively. He worked in the Sensor and Biosensor group of Universidad Autónoma de Madrid during his final degree and Master final research projects
Laura Gutiérrez Gálvez obtained the Degree in Chemistry in 2019 from the Universidad Autónoma de Madrid and the Master degree in Applied Chemistry in 2020 from the Universidad Autónoma de Madrid. Since 2020 she is a PhD student at the Applied Chemistry Programme of the Universidad Autónoma de Madrid in the Sensor and Biosensor group lead by Professor Encarnación Lorenzo. Her current research focuses on the development of electrochemiluminescent (bio)sensors for detection of virus and cancer biomarkers.
Rafael Del Caño received his Ph.D. from the University of Córdoba (UCO), in 2019. He is currently postdoctoral researcher at Autonomous University of Madrid (UAM). His current research direction is the synthesis of different nanomaterials, their characterization and electrochemical detection applications.
Emiliano Martínez-Periñán received his bachelor’s degree and master’s degree in chemistry from Universidad de Cádiz. He obtained his PhD degree from Universidad Autónoma de Madrid in 2016. After that, he had been working as a visiting postdoc researcher at Manchester Metropolitan University under the direction of Professor Craig E. Banks. Then, he obtained a Juan de la Cierva-Formación fellowship from the Spanish Ministry of economy and innovation at the Electroanalysis and Electrochemical Biosensors group of Universidad Complutense de Madrid lead by Professor José Manuel Pingarrón. Nowadays he is working as associated professor in the department of Analytical Chemistry and Instrumental analysis of Universidad Autónoma de Madrid. He is part of the Sensor and Biosensor group lead by Professor Encarnación Lorenzo. His research interests include electrosynthesis, nanotechnology, the use of different analysis techniques coupled with electrochemistry and electrochemical sensors.
Daniel García-Nieto received his bachelor's degree in Chemistry and master´s degree in Biotechnology from Universidad de Granada in 2017 and 2018, respectively. He was a research assistant from 2019 to 2021 at the Instituto de Micro y Nanotecnología (IMN-CSIC). His research interests comprise the study and synthesis of different nanomaterials (such as nanoparticles and biomaterials) for use in biomedicine (diagnosis and treatment of different diseases) and nanotechnology (advanced biosensors).
Micaela Rodríguez-Peña received her degree in Physics and master´s degree “Nanophysics and advanced materials” from Universidad Complutense de Madrid in 2017 and 2018, respectively. From then on she enjoys a position as research assistant at the Instituto de Micro y Nanotecnología (IMN-CSIC) in Madrid. Concurrently she continues her formation with PhD studies at UCM focused on photocatalytic materials.
Monica Luna earned her bachelor's (Physics) and PhD degrees from Universidad Autónoma de Madrid in 1993 and 1999, respectively. Afterwards, she was awarded a Fulbright Postdoctoral Fellowship to perform research at Lawrence Berkeley National Laboratory (LBNL, California) and subsequently, a Ramón y Cajal Research Contract at the Instituto de Micro y Nanotecnology (IMN-CSIC, Madrid), where she currently enjoys a permanent position as research scientist since 2010. Her research interests are focused on advanced Scanning Probe Microscopy Instrumentation for non-invasive nanomaterials characterization in the fields of Nanomedicine, Photocatalysis and 2D solar devices.
Paula Milán Rois obtained her biology degree at the Universidad Autónoma de Madrid. After that she obtained her master’s degree “Biomolecules and Cell Dynamics” also at Universidad Autónoma de Madrid. Nowadays she is conducting her predoctoral studies at IMDEA Nanoscience. Her thesis deals with the ncRNAs treatment and detection of cancer with gold or magnetic nanoparticles. Moreover, she takes part in other projects such as Duchenne Muscular Dystrophy treatment, COVID diagnosis or lncRNA cancer’s therapy.
Milagros Castellanos Molina received her bachelor's degree in Biology (UCM) and her PhD degree (UAM) in 2005 and 2011, respectively. At present, she is associate scientist at IMDEA Nanoscience (Madrid, Spain). Her research interests include the successful application of cutting-edge nanobiotools for the detection and treatment of several diseases such as COVID-19 or cancer.
Melanie Abreu obtained her bachelor's degree in Pharmaceutical at the Complutense University of Madrid (2008–2014). She is specialist in clinical microbiology (2015–2019). Currently she is a faculty member in the Microbiology Department of the Hospital Ramón y Cajal.
Rafael Cantón obtained his PhD degree in 1994. He was trained as Clinical Microbiology Specialist at the Microbiology Department at the Ramón y Cajal University Hospital (Madrid, Spain), in which he is the Head of the Department. He is also Associated Professor at Complutense University (Madrid). His research activity on antimicrobial resistance, novel techniques on antimicrobial susceptibility testing and chronic respiratory infections is developed at the Institute Ramón y Cajal for Health Research (IRYCIS), in which he coordinates the Microbiology, Immunology and Infection Area. He has been Chairman of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and President of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC).
Juan Carlos Galán. He is specialist in Microbiology (1997). He received his PhD degree in Pharmacy (2002). Head of virology area in Microbiology Department in Ramon y Cajal hospital (2011). Head of a group in National Biomedical Network in Public Health (2018). Coordinator of reference laboratory for flu and SARS-CoV-2 detection in Madrid (2009 and 2020). Coordinator and participant in the protocols for SARS-CoV-2 detection for Spanish Society of Microbiology and Infectious Disease. His interests are implementation of new diagnosis strategies for detection of emerging infectious diseases and to know it spreading among the population
Teresa Pineda is currently Full Professor in the Department of Physical chemistry and Applied Thermodynamics at the University of Cordoba. She received her degree in Chemistry in 1987 and her PhD degree in 1991 from the University of Cordoba. From 1991–1993, she made a post-doctoral stage at the Department of Biochemistry in University of Tennessee-Knoxville. Her research interest is centred in the field of surface chemistry trough the modification of gold and carbon nanoparticles and macroscopic 2D substrates with the object to bring together the basic science concepts with the problems encountered when using these nanomaterials in different fields as health and medicine.
Félix Pariente is currently Full Professor of Analytical Chemistry at the Universidad Autónoma de Madrid (UAM). He was born in Madrid in 1954. He received his B.S. and Ph.D. degrees in Chemistry in 1976 and 1988, respectively. Between 1992 and 1996 he spent several periods as visiting scientist at the University of Cornell in USA. In 1998 he obtains the degree of permanent assistant professor in the UAM. His research interest includes the design and development of enzyme biosensors and genosensors as well as processes involving electrocatalysis with application to the design of fuel cells and new analytical methods.
Alvaro Somoza obtained his Ph.D. at the Universidad Autónoma de Madrid in 2004. Then, he joined the group of Prof. Eric T. Kool at Stanford University, where he worked on the preparation of modified ribonucleosides to study the role of hydrogen bonding interactions between RNA strands in RNA interference. In 2009 he joined IMDEA Nanociencia and, in 2014, was promoted to Research Professor. His research projects are focused on the use of modified oligonucleotides and nanostructures for the detection and treatment of different diseases.
Tania García-Mendiola received her bachelor's degree in Chemistry and her PhD degree from Universidad Autónoma de Madrid in 2003 and 2009, respectively. At present, she is associate professor in the Department of Analytical Chemistry and Instrumental Analysis at the UAM. Her research interests include the design of new electrochemical sensors and DNA biosensors and the use of nanomaterials to improve the analytical properties of the developed devices.
Rodolfo Miranda got his Ph.D in Physics from the Universidad Autónoma de Madrid (UAM) in 1981 for a work on the role of defects on surfaces under the supervision of Prof. J.M. Rojo. He worked in Munich and Berlin with Gerhard Ertl (NL in Chemistry 2007), before being appointed Full Professor of Condensed Matter Physics at the UAM in 1990. Prof. Miranda has been Vice-chancellor of Research and Scientific Policy (1998–2002) of the UAM, Executive Secretary of the R+D Commission of the Conference of Rectors of Spanish Universities (CRUE) (2000–2002) and Director of the Materials Science Institute “Nicolas Cabrera”. Prof. Miranda is Fellow of the American Physical Society since 2007, Head of the Surface Science Lab of the UAM (LASUAM) and Director of the Madrid Institute for Advanced Studies in Nanoscience (IMDEA-Nanociencia) from February 2007. Prof. Miranda’s research interests range from low dimensional magnetism or molecular self-organization on surfaces to the mechanisms of epitaxial growth, the growth and properties of graphene or the use of magnetic nanoparticles in nanomedicine.
María Encarnación Lorenzo is currently Full Professor in the Department of Analytical Chemistry and Instrumental Analysis at the Universidad Autónoma de Madrid. She received her degree in Chemistry in 1978 and her PhD degree in 1985 from the Universidad Autónoma de Madrid. Afterwards, she made a post-doctoral stage at the Department of Chemistry at Dublin City University. In 1990 she was visiting scientist (NATO Program) to the Department of Chemistry in Cornell University. In 1998 the members of the faculty of Tokio University of Agriculture and Technology invited her as visiting professor to the Department of Applied Chemistry. Actually, she is member of management committee of the Spanish Analytical Chemistry Society. Her research interest is the development of sensors and biosensors for the detection of analytes of environmental, clinical and food interest. She is the author/coauthor of more than 100 original research publications and several book chapters in the area of analytical chemistry.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.snb.2022.132217.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020, (2020). 〈https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020〉 (accessed June 11, 2021).
- 2.Chaibun T., Puenpa J., Ngamdee T., Boonapatcharoen N., Athamanolap P., O’Mullane A.P., Vongpunsawad S., Poovorawan Y., Lee S.Y., Lertanantawong B. Rapid electrochemical detection of coronavirus SARS-CoV-2. Nat. Commun. 2021;12:1–10. doi: 10.1038/s41467-021-21121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J., Song J.U., Shim S.R. Comparing the diagnostic accuracy of rapid antigen detection tests to real time polymerase chain reaction in the diagnosis of SARS-CoV-2 infection: a systematic review and meta-analysis. J. Clin. Virol. 2021;144 doi: 10.1016/J.JCV.2021.104985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samacoits A., Nimsamer P., Mayuramart O., Chantaravisoot N., Sitthi-amorn P., Nakhakes C., Luangkamchorn L., Tongcham P., Zahm U., Suphanpayak S., Padungwattanachoke N., Leelarthaphin N., Huayhongthong H., Pisitkun T., Payungporn S., Hannanta-anan P. Machine Learning-Driven and Smartphone-Based Fluorescence Detection for CRISPR Diagnostic of SARS-CoV-2. ACS Omega. 2021;6:2727–2733. doi: 10.1021/ACSOMEGA.0C04929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Márquez-Ipiña A.R., González-González E., Rodríguez-Sánchez I.P., Lara-Mayorga I.M., Mejía-Manzano L.A., Sánchez-Salazar M.G., González-Valdez J.G., Ortiz-López R., Rojas-Martínez A., Santiago G.T., Alvarez M.M. Serological test to determine exposure to SARS-CoV-2: ELISA based on the receptor-binding domain of the spike protein (S-RBDN318-V510) expressed in escherichia coli. Diagnostics. 2021;Vol. 11:271. doi: 10.3390/DIAGNOSTICS11020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suleman S., Shukla S.K., Malhotra N., Bukkitgar S.D., Shetti N.P., Pilloton R., Narang J., Nee Tan Y., Aminabhavi T.M. Point of care detection of COVID-19: advancement in biosensing and diagnostic methods. Chem. Eng. J. 2021;414 doi: 10.1016/J.CEJ.2021.128759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rafiee-Pour H.A., Behpour M., Keshavarz M. A novel label-free electrochemical miRNA biosensor using methylene blue as redox indicator: application to breast cancer biomarker miRNA-21. Biosens. Bioelectron. 2016;77:202–207. doi: 10.1016/J.BIOS.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Lin X., Ni Y., Kokot S. An electrochemical DNA-sensor developed with the use of methylene blue as a redox indicator for the detection of DNA damage induced by endocrine-disrupting compounds. Anal. Chim. Acta. 2015;867:29–37. doi: 10.1016/J.ACA.2015.02.050. [DOI] [PubMed] [Google Scholar]
- 9.Zhang D., Peng Y., Qi H., Gao Q., Zhang C. Label-free electrochemical DNA biosensor array for simultaneous detection of the HIV-1 and HIV-2 oligonucleotides incorporating different hairpin-DNA probes and redox indicator. Biosens. Bioelectron. 2010;25:1088–1094. doi: 10.1016/J.BIOS.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 10.Erdem A., Kerman K., Meric B., Akarca U.S., Ozsoz M. Novel hybridization indicator methylene blue for the electrochemical detection of short DNA sequences related to the hepatitis B virus. Anal. Chim. Acta. 2000;422:139–149. doi: 10.1016/S0003-2670(00)01058-8. [DOI] [Google Scholar]
- 11.Lin C., Wu Y., Luo F., Chen D., Chen X. A label-free electrochemical DNA sensor using methylene blue as redox indicator based on an exonuclease III-aided target recycling strategy. Biosens. Bioelectron. 2014;59:365–369. doi: 10.1016/J.BIOS.2014.03.053. [DOI] [PubMed] [Google Scholar]
- 12.García-Mendiola T., Cerro M.R., López-Moreno J.M., Pariente F., Lorenzo E. Dyes as bifunctional markers of DNA hybridization on surfaces and mutation detection. Bioelectrochemistry. 2016;111:115–122. doi: 10.1016/j.bioelechem.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 13.García T., Revenga-Parra M., Añorga L., arana S., Pariente F., Lorenzo E. Disposable DNA biosensor based on thin-film gold electrodes for selective salmonella detection. Sens. Actuators B: Chem. 2012;161:1030–1037. doi: 10.1016/J.SNB.2011.12.002. [DOI] [Google Scholar]
- 14.García T., Revenga-Parra M., Abruña H.D., Pariente F., Lorenzo E. Single-mismatch position-sensitive detection of DNA based on a bifunctional ruthenium complex. Anal. Chem. 2008;80:77–84. doi: 10.1021/AC071095R. [DOI] [PubMed] [Google Scholar]
- 15.Sun H., Ren J., Qu X. Carbon nanomaterials and DNA: from molecular recognition to applications. Acc. Chem. Res. 2016;49:461–470. doi: 10.1021/ACS.ACCOUNTS.5B00515. [DOI] [PubMed] [Google Scholar]
- 16.García-Mendiola T., Bravo I., López-Moreno J.M., Pariente F., Wannemacher R., Weber K., Popp J., Lorenzo E. Carbon nanodots based biosensors for gene mutation detection. Sens. Actuators B: Chem. 2018;256:226–233. doi: 10.1016/J.SNB.2017.10.105. [DOI] [Google Scholar]
- 17.Xu X., Ray R., Gu Y., Ploehn H.J., Gearheart L., Raker K., Scrivens W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004;126:12736–12737. doi: 10.1021/JA040082H. [DOI] [PubMed] [Google Scholar]
- 18.Rigodanza F., Đorđević L., Arcudi F., Prato M. Customizing the electrochemical properties of carbon nanodots by using quinones in bottom-up synthesis. Angew. Chem. Int Ed. Engl. 2018;57:5062–5067. doi: 10.1002/ANIE.201801707. [DOI] [PubMed] [Google Scholar]
- 19.Wu H., Pang L.-F., Wei N., Guo X.-F., Wang H. Nucleus-targeted N-doped carbon dots via DNA-binding for imaging of hypochlorous in cells and zebrafish. Sens. Actuators B: Chem. 2021;333 doi: 10.1016/j.snb.2021.129626. [DOI] [Google Scholar]
- 20.Martínez-Periñán E., Martínez-Sobrino Á., Bravo I., García-Mendiola T., Mateo-Martí E., Pariente F., Lorenzo E. Neutral red-carbon nanodots for selective fluorescent DNA sensing. Anal. Bioanal. Chem. 2022 doi: 10.1007/s00216-022-03980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farjami E., Clima L., v Gothelf K., Ferapontova E.E. DNA interactions with a Methylene Blue redox indicator depend on the DNA length and are sequence specific. Analyst. 2010;135:1443–1448. doi: 10.1039/C0AN00049C. [DOI] [PubMed] [Google Scholar]
- 22.García-Mendiola T., Requena-Sanz S., Martínez-Periñán E., Bravo I., Pariente F., Lorenzo E. Influence of carbon nanodots on DNA-Thionine interaction. Application to breast cancer diagnosis. Electrochim. Acta. 2020;353 doi: 10.1016/j.electacta.2020.136522. [DOI] [Google Scholar]
- 23.Kashefi-Kheyrabadi L., Nguyen H.V., Go A., Baek C., Jang N., Lee J.M., Cho N.H., Min J., Lee M.H. Rapid, multiplexed, and nucleic acid amplification-free detection of SARS-CoV-2 RNA using an electrochemical biosensor. Biosens. Bioelectron. 2022;195 doi: 10.1016/J.BIOS.2021.113649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan Z., Yao B., Ding Y., Xu D., Zhao J., Zhang K. Rational engineering the DNA tetrahedrons of dual wavelength ratiometric electrochemiluminescence biosensor for high efficient detection of SARS-CoV-2 RdRp gene by using entropy-driven and bipedal DNA walker amplification strategy. Chem. Eng. J. 2022;427 doi: 10.1016/J.CEJ.2021.131686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan Z., Yao B., Ding Y., Zhao J., Xie M., Zhang K. Entropy-driven amplified electrochemiluminescence biosensor for RdRp gene of SARS-CoV-2 detection with self-assembled DNA tetrahedron scaffolds. Biosens. Bioelectron. 2021;178 doi: 10.1016/J.BIOS.2021.113015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raziq A., Kidakova A., Boroznjak R., Reut J., Öpik A., Syritski V. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021;178 doi: 10.1016/j.bios.2021.113029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martínez-Periñán E., García-Mendiola T., Enebral-Romero E., del Caño R., Vera-Hidalgo M., Vázquez Sulleiro M., Navío C., Pariente F., Pérez E.M., Lorenzo E. A MoS2 platform and thionine-carbon nanodots for sensitive and selective detection of pathogens. Biosens. Bioelectron. 2021;189 doi: 10.1016/j.bios.2021.113375. [DOI] [PubMed] [Google Scholar]
- 28.Peng Y., Pan Y., Sun Z., Li J., Yi Y., Yang J., Li G. An electrochemical biosensor for sensitive analysis of the SARS-CoV-2 RNA. Biosens. Bioelectron. 2021;186 doi: 10.1016/j.bios.2021.113309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagheri Hashkavayi A., Raoof J.B., Azimi R., Ojani R. Label-free and sensitive aptasensor based on dendritic gold nanostructures on functionalized SBA-15 for determination of chloramphenicol. Anal. Bioanal. Chem. 2016;408:2557–2565. doi: 10.1007/S00216-016-9358-6. 2016 408:10. [DOI] [PubMed] [Google Scholar]
- 30.Ferapontova E.E., Shleev S., Ruzgas T., Stoica L., Christenson A., Tkac J., Yaropolov A.I., Gorton L. Direct electrochemistry of proteins and enzymes. Perspect. Bioanal. 2005;1:517–598. doi: 10.1016/S1871-0069(05)01016-5. [DOI] [Google Scholar]
- 31.Aydın E.B., Aydın M., Sezgintürk M.K. Highly selective and sensitive sandwich immunosensor platform modified with MUA-capped GNPs for detection of spike receptor binding domain protein: a precious marker of COVID 19 infection. Sens Actuators B Chem. 2021;345 doi: 10.1016/J.SNB.2021.130355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fressatti M.G. v, Cabral B.P., de Oliveira J.H., Buzzetti P.H.M., Radovanovic E., Monteiro J.P., Girotto E.M. Device for streptavidin detection using LSPR and electrochemical transductions on the same platform. Artic. J. Braz. Chem. Soc. 2021;32:777–785. doi: 10.21577/0103-5053.20200229. [DOI] [Google Scholar]
- 33.Park Sungho, Wasileski Sally A., Weaver* M.J. Electrochemical infrared characterization of carbon-supported platinum nanoparticles: a benchmark structural comparison with single-crystal electrodes and high-nuclearity carbonyl clusters. J. Phys. Chem. B. 2001;105:9719–9725. doi: 10.1021/JP011903X. [DOI] [Google Scholar]
- 34.Abedi R., Bakhsh Raoof J., Bagheri Hashkavayi A., Asghary M. Highly sensitive and label-free electrochemical biosensor based on gold nanostructures for studying the interaction of prostate cancer gene sequence with epirubicin anti-cancer drug. Microchem. J. 2021;170 doi: 10.1016/J.MICROC.2021.106668. [DOI] [Google Scholar]
- 35.Mahato K., Nagpal S., Shah M.A., Srivastava A., Maurya P.K., Roy S., Jaiswal A., Singh R., Chandra P. Gold nanoparticle surface engineering strategies and their applications in biomedicine and diagnostics. 3 Biotech. 2019;9:1–19. doi: 10.1007/S13205-019-1577-Z/FIGURES/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pelaz B., Grazu V., Ibarra A., Magen C., del Pino P., de La Fuente J.M. Tailoring the synthesis and heating ability of gold nanoprisms for bioapplications. Langmuir. 2012;28:8965–8970. doi: 10.1021/la204712u. [DOI] [PubMed] [Google Scholar]
- 37.Meehanss T., Gamperl H., Beckers J.F. Characterization of reversible, physical binding of Benzo[a]pyrene derivatives to DNA. J. Biol. Chem. 1982;257:10479–10485. doi: 10.1016/S0021-9258(18)34043-2. [DOI] [PubMed] [Google Scholar]
- 38.R Core Development Team, R: a language and environment for statistical computing, 3.2.1, Document Freely Available on the Internet at: Http://Www. r-Project. Org. (2015). http://www.r-project.org/ (accessed July 29, 2021).
- 39.Mediavilla M., Martínez-Periñán E., Bravo I., García-Mendiola T., Revenga-Parra M., Pariente F., Lorenzo E. Electrochemically driven phenothiazine modification of carbon nanodots. Nano Res. 2021;11:6405. doi: 10.1007/S12274-018-2165-Y. [DOI] [Google Scholar]
- 40.Breslin D.T., Yu C., Ly D., Schuster G.B. Structural modification changes the DNA binding mode of cation-substituted anthraquinone photonucleases: association by intercalation or minor groove binding determines the DNA cleavage efficiency†. Biochemistry. 1997;36:10463–10473. doi: 10.1021/BI9702750. [DOI] [PubMed] [Google Scholar]
- 41.Pyle A.M., Rehmann J.P., Meshoyrer R., Turro N.J., Barton J.K., Kumar C. v. Mixed-ligand complexes of ruthenium(II): factors governing binding to DNA. J. Am. Chem. Soc. 2002;111:3051–3058. doi: 10.1021/JA00190A046. [DOI] [Google Scholar]
- 42.Bai G.Y., Dong B., Lü Y.Y., Wang K.Z., Jin L.P., Gao L.H. A comparative study of the interaction of two structurally analogue ruthenium(II) complexes with DNA. J. Inorg. Biochem. 2004;98:2011–2015. doi: 10.1016/J.JINORGBIO.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 43.García-Mendiola T., Bayon-Pizarro V., Zaulet A., Fuentes I., Pariente F., Teixidor F., Viñas C., Lorenzo E. Metallacarboranes as tunable redox potential electrochemical indicators for screening of gene mutation. Chem. Sci. 2016;7:5786–5797. doi: 10.1039/C6SC01567K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pang D.W., Abruña H.D. Micromethod for the Investigation of the Interactions between DNA and redox-active molecules. Anal. Chem. 1998;70:3162–3169. doi: 10.1021/ac980211a. [DOI] [PubMed] [Google Scholar]
- 45.Pang D. ‐W., Qi Y. ‐P., Wang Z. ‐L., Cheng J. ‐K., Wang J. ‐W. Electrochemical oxidation of DNA at a gold microelectrode. Electroanalysis. 1995;7:774–777. doi: 10.1002/elan.1140070817. [DOI] [Google Scholar]
- 46.García T., Fernández-Barrena M.G., Revenga-Parra M., Núñez A., Casero E., Pariente F., Prieto J., Lorenzo E. Disposable sensors for rapid screening of mutated genes. Anal. Bioanal. Chem. 2010;398:1385–1393. doi: 10.1007/S00216-010-4029-5/FIGURES/5. [DOI] [PubMed] [Google Scholar]
- 47.García T., Revenga-Parra M., Abruña H.D., Pariente F., Lorenzo E. Single-mismatch position-sensitive detection of DNA based on a bifunctional ruthenium complex. Anal. Chem. 2008;80:77–84. doi: 10.1021/ac071095r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material