To the Editor:
The first report of a rise in blood pressure (BP) associated with vaccination for coronavirus disease 2019 (COVID-19) was provided by Meylan et al. [1]. These authors published a series of subjects vaccinated with ‘Comirnaty’ (by Pfizer-BioNtech) or ‘Spikevax’ (by Moderna) [1]. Unexpectedly, hours or days after vaccination, BP in these subjects rose considerably up to levels of 220/115 mmHg [1]. Although subsequent studies substantially confirmed this finding, evidence remains limited [2,3]. The potential basic mechanisms of the BP rise associated with COVID-19 vaccination are elusive, although some possibilities look reasonable. For example, the down-regulation of ACE2 receptors due to their internalization into the cells after the contact with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein, either alone (i.e., produced by vaccines) [4] or combined with the entire virus (i.e., during SARS-CoV-2 infection) [5], [6], [7], would result in a loss of ACE2 enzymatic activity at the outer cell surface. Consequently, angiotensin II would be transformed into angiotensin1–7 to a much lesser extent, with enhancement of the typical unwanted effects of angiotensin II (vasoconstriction, inflammation, thrombosis) [5], [6], [7].
An interesting approach to investigate the potential effects of SARS-CoV-2 infection on BP could be the observation of patients hospitalized for severe COVID-19 [8]. Thus, we conducted a prospective case-control study in hospitalized patients with confirmed diagnosis of SARS-CoV-2 infection (by RNA reverse-transcriptase-polimerase-chain-reaction assays from nasopharyngeal swab specimens) and imaging features for COVID-19 pneumonia [9]. It was not a retrospective enrollment of patients, but a prospective study according to a prespecified protocol. The protocol was approved by the Ethic Committee of our Institution and patients provided their informed consent to participate. We also predefined a control group of patients who had been hospitalized for bacterial pneumonia and whose diagnostic tests for COVID-19 were negative along the entire hospitalization period.
Participants were consecutively recruited in a 2:1 allocation ratio. The primary outcome was the rate of persistent raise in BP requiring a new or intensified anti-hypertensive treatment during hospitalization. BP values ≥ 140 mmHg systolic or 90 mmHg diastolic for at least two consecutive days defined the persistent rise in BP. The secondary outcome was the differences between the two groups in the average BP during hospitalization. We estimated that a total of 58 cases and 29 controls would provide an 85% power to detect a clinically relevant 30% increase in the proportion of uncontrolled hypertension between patients with COVID-19 pneumonia and patients with bacterial pneumonia.
We collected demographic, laboratory, and clinical management data at admission and throughout the entire in-hospital stay. Laboratory parameters were assessed using standard techniques. We used the PaO2/FIO2 ratio to estimate the severity of respiratory dysfunction. We defined comorbidities according to documented medical history, as collected by investigators at study site-level, including interrogation of electronic health record data. All clinical evaluations were performed by the attending physician during the clinical interview and through interrogation of medical records. BP was measured in the morning according to current Guidelines [10]. Previous cardiac events included history of heart failure (defined by at least one prior hospitalization for acute heart failure requiring intravenous therapy) and coronary artery disease (as defined by at least one of the following criteria: (1) presence of any epicardial coronary vessels with >75% stenosis tested on coronary angiography; (2) history of acute coronary syndrome; (3) coronary revascularization, either percutaneous transluminal coronary angioplasty or coronary artery by-pass grafting). Cerebrovascular disease included previous history of stroke or transient ischemic attack.
Table 1 shows the main characteristics of patients. Mean age was 64 and 66 years for COVID-19 and bacterial pneumonia cases, respectively. Clinical features and prevalence of comorbidities were well-balanced between cases and controls. Of note, patients with COVID-19 and bacterial pneumonia had similar BP at admission (systolic: 121 vs 118 mmHg, p = 0.426; diastolic: 76 vs 74 mmHg, p = 0.401).
Table 1.
Main characteristics of patients according to different types of pneumonia (mean ± standard error or percentages, when appropriate).
| Patients characteristics | COVID-19 Pneumonia(n = 58) | Bacterial Pneumonia(n = 29) | p |
|---|---|---|---|
| Age (years) | 64±1.9 | 66±2.9 | 0.472 |
| Females, % | 38 | 41 | 0.194 |
| Body Mass Index, Kg/m2 | 27.2±0.63 | 24.8±1.07 | 0.046 |
| Comorbidities | |||
| Hypertension, % | 55 | 48 | 0.544 |
| Diabetes, % | 17 | 21 | 0.153 |
| Current smoker, % | 28 | 31 | 0.112 |
| Cardiac events, % | 16 | 17 | 0.837 |
| Cerebrovascular disease, % | 10 | 7 | 0.600 |
| Chronic obstructive pulmonary disease, % | 5 | 28 | 0.003 |
| Laboratory data at admission | |||
| White blood cell count, x103 | 7.07±0.37 | 10.34±0.97 | <0.001 |
| Serum creatinine, mg/dl | 0.83±0.03 | 0.97±0.10 | 0.078 |
| K+, mEq/l | 4.32±0.06 | 4.37±0.11 | 0.656 |
| Haemoglobin, g/dl | 12.6±0.18 | 12.0±0.28 | 0.062 |
| PaO2/FIO2 ratio, mm | 312±12 | 285±13 | 0.185 |
| Blood pressure and heart rate | |||
| Systolic BP at admission, mmHg | 121±2.3 | 118±3.2 | 0.426 |
| Diastolic BP at admission, mmHg | 76±1.4 | 74±1.6 | 0.401 |
| Heart rate at admission, bpm | 79±2.0 | 81±2.2 | 0.480 |
| Systolic BP during hospitalization, mmHg | 126±1.9 | 118±2.2 | 0.016 |
| Diastolic BP during hospitalization, mmHg | 79±1.1 | 70±0.9 | <0.0001 |
| Short-term systolic BP variability*, mmHg | 13±0.7 | 10±0.9 | 0.043 |
| Short-term diastolic BP variability*, mmHg | 8.1±0.5 | 6.6±0.3 | 0.060 |
| Mean heart rate during hospitalization, bpm | 74±1.0 | 76±1.7 | 0.400 |
| Outcome | |||
| Persistent raise in BP requiring drug therapy, % | 45 | 10 | 0.001 |
Legend: BP = blood pressure; * = estimated by standard deviation of blood pressure during hospitalization.
Conversely, mean systolic/diastolic BP recorded during hospitalization showed a significant difference between patients with COVID-19 pneumonia and patients with bacterial pneumonia (systolic: 126 vs 118 mmHg, p = 0.016; diastolic: 79 vs 70 mmHg, p < 0.0001).
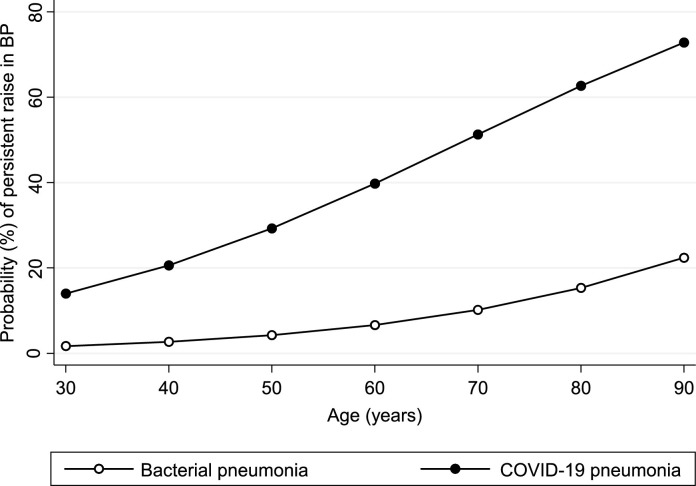
During hospitalization, 28 patients exhibited a persistent raise in BP requiring antihypertensive treatment. Specifically, 25 and 3 patients met the primary endpoint among COVID-19 and bacterial pneumonia, respectively (p = 0.001). Thus, COVID-19 pneumonia was associated with a 7-fold increased risk of uncontrolled hypertension when compared with bacterial pneumonia (odds ratio: 6.99, 95% confidence interval: 1.89 to 25.80, p = 0.004). Similar results were obtained after adjustment for age (Fig. 1 , p = 0.019). Predictors of uncontrolled hypertension (Table 2 ) in the group with COVID-19 were age (p = 0.006), history of hypertension (p = 0.002), diabetes (p = 0.043), and previous cardiac events (p = 0.027). Notably, these features have been associated with ACE2 receptor deficiency, potentially linked to a reduced generation of the potent vasodilator angiotensin1–7, during the active phase of the disease [5,7].
Fig. 1.
Probability of persistent raise in blood pressure according to type of pneumonia and age (see text for details).
Table 2.
Predictors of uncontrolled hypertension in the group with COVID-19 pneumonia (see text for details).
| Variable | Comparison | OR (95% CI) | p |
|---|---|---|---|
| Age | 10 years | 1.91 (1.21–3.06) | 0.006 |
| Sex | Male vs Female | 2.89 (0.79–10.57) | 0.109 |
| Body mass index | 10 Kg/m2 | 1.34 (0.43–4.17) | 0.616 |
| Comorbidities | |||
| Hypertension | Yes vs No | 7.27 (2.14–24.77) | 0.002 |
| Diabetes | Yes vs No | 5.64 (1.05–30.19) | 0.043 |
| Cardiac events | Yes vs No | 11.67 (1.33–20.72) | 0.027 |
| Cerebrovascular disease | Yes vs No | 0.81 (0.12–5.28) | 0.827 |
| Chronic obstructive pulmonary disease | Yes vs No | 2.61 (0.23–30.57) | 0.445 |
| Laboratory data at admission | |||
| White blood cell count | 103 | 1.06 (0.88–1.28) | 0.554 |
| Serum creatinine | 1 mg/dl | 2.39 (0.18–31.35) | 0.506 |
| K+ | 1 ng/mL | 1.07 (0.35–3.33) | 0.901 |
| Haemoglobin | 1 g/dl | 0.87 (0.59–1.28) | 0.475 |
| PaO2/FIO2 ratio | 10 mm | 0.98 (0.92–1.04) | 0.467 |
| Blood pressure | |||
| Systolic BP at admission | 10 mmHg | 1.22 (0.91–1.62) | 0.178 |
| Diastolic BP at admission | 10 mmHg | 1.15 (0.74–1.77) | 0.540 |
| Heart rate at admission | 10 bpm | 0.96 (0.67–1.36) | 0.804 |
Legend: BP = blood pressure; OR=odds ratio; CI=confidence interval.
To the best of our knowledge, this case-control study is the first to indicate that COVID-19 pneumonia is associated with a rise in BP in hospitalized patients. These preliminary data should be confirmed in larger case series. The potential basic mechanisms underlying this phenomenon require further research.
References
- 1.Meylan S., Livio F., Foerster M., Genoud P.J., Marguet F., Wuerzner G., Center C.C.V. Stage III hypertension in patients after mRNA-based SARS-CoV-2 vaccination. Hypertension. 2021;77:e56–e57. doi: 10.1161/HYPERTENSIONAHA.121.17316. Dallas, Tex: 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zappa M., Verdecchia P., Spanevello A., Visca D., Angeli F. Blood pressure increase after Pfizer/BioNTech SARS-CoV-2 vaccine. Eur J Intern Med. 2021;90:111–113. doi: 10.1016/j.ejim.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angeli F., Reboldi G., Trapasso M., Santilli G., Zappa M., Verdecchia P. Blood pressure increase following COVID-19 vaccination: a systematic overview and meta-analysis. J Cardiovasc Dev Dis. 2022;9 doi: 10.3390/jcdd9050150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angeli F., Spanevello A., Reboldi G., Visca D., Verdecchia P. SARS-CoV-2 vaccines: lights and shadows. Eur J Intern Med. 2021;88:1–8. doi: 10.1016/j.ejim.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angeli F., Zappa M., Reboldi G., Trapasso M., Cavallini C., Spanevello A., Verdecchia P. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection: one year later. Eur J Intern Med. 2021;93:28–34. doi: 10.1016/j.ejim.2021.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verdecchia P., Cavallini C., Spanevello A., Angeli F. COVID-19: aCE2centric Infective disease? Hypertension. 2020;76:294–299. doi: 10.1161/HYPERTENSIONAHA.120.15353. Dallas, Tex: 1979. [DOI] [PubMed] [Google Scholar]
- 8.Angeli F., Verdecchia P., Reboldi G. Pharmacotherapy for hypertensive urgency and emergency in COVID-19 patients. Expert Opin Pharmacother. 2022;23:235–242. doi: 10.1080/14656566.2021.1990264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson S., Kay F.U., Abbara S., Bhalla S., Chung J.H., Chung M., Henry T.S., Kanne J.P., Kligerman S., Ko J.P., Litt H. Radiological society of north america expert consensus document on reporting chest CT findings related to COVID-19: endorsed by the society of thoracic radiology, the American college of radiology, and RSNA. Radiol Cardiothorac Imaging. 2020;2 doi: 10.1148/ryct.2020200152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stergiou G.S., Palatini P., Parati G., O'Brien E., Januszewicz A., Lurbe E., Persu A., Mancia G., Kreutz R. European society of hypertension C, the European society of hypertension working group on blood pressure m and cardiovascular V. 2021 European society of hypertension practice guidelines for office and out-of-office blood pressure measurement. J Hypertens. 2021;39:1293–1302. doi: 10.1097/HJH.0000000000002843. [DOI] [PubMed] [Google Scholar]