Abstract
SARS-CoV-2 uses membrane bound Angiotensin-Converting Enzyme 2 (ACE2) as a key host receptor for its entry. However, inconsistent results are available in terms of shedding of membrane ACE2 and circulating levels of soluble ACE2 during SARS-CoV-2. To ascertain soluble ACE2 as an effective biomarker for the prediction of COVID-19 outcome, in the present study, we investigated the levels of plasma ACE2 during the early phase of infection in COVID-19 patients. The study involved a total of 42 COVID-19 patients along with 10 healthy controls. Plasma levels of ACE2 was determined using ELISA at the time of admission and on day 7 post admission. The association of sACE2 with D-dimer a marker for hyper-coagulation was performed using a dependence test. Compared to healthy controls, SARS-CoV-2 cases has shown a huge increase in the sACE2 at the time of admission. During the course of infection, we found a significant increase (P ≤ 0.001) in sACE2 in severe cases compared to moderate. There was a strong increase in sACE2 in cases with hypertension and diabetes mellitus. Interestingly, a strong positive correlation (P ≤ 0.001) was obtained between sACE2 and D-dimer. Thus, an excessive shedding of ACE2 during the early phase is a common phenomenon in severe form of the SARS-CoV-2. Along with D-dimer, the sACE2 levels could serve as a clinical biomarker for the prediction of disease outcome. However further studies are needed to ascertain its role in host-virus interplay.
Keywords: sACE2, COVID-19, SARS-CoV-2, D-Dimer, Comorbidities, Biomarker
Abbreviations: ACE2, Angiotensin-Converting Enzyme 2; sACE2, soluble Angiotensin-Converting Enzyme 2; cACE2, cellular/membrane bounded ACE2; SARS-CoV, Severe Acute Respiratory Syndrome Coronavirus; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus-2; COVID-19, Coronavirus 2019; RAAS, Renin Angiotensin Aldosterone System; Ang I, Angiotensin I; CVD, Cardiovascular Disease; ALI, Acute Lung Injury; ARDS, Acute Respiratory Distress Syndrome; RT-PCR, Reverse Transciption-Polymerase Chain Reaction; EDTA, Ethylene Diamine Tetra Acetic; ELISA, Enzyme-Linked ImmunoSorbent Assay; IQR, Inter-Quartile Range; HT, Hypertension; DM, Diabetes Mellitus; ADAM17, A Disintegrin and Metalloproteinases 17; ECs, Endothelial Cells
1. Introduction
Angiotensin-Converting Enzyme 2 (ACE2) is an important host receptor for the severe acute respiratory syndrome coronavirus (SARS-CoV), HCoV-NL63, and novel coronavirus SARS-CoV-2 that cause coronavirus disease 19 (COVID-19) [1]. SARS-CoV-2 utilizes the catalytic site of ACE2 receptor for viral entry via an endocytosis process, thereby downregulation of membrane-bound ACE2 [2]. ACE2 is a glycoprotein and ubiquitously expressed in various tissues in humans, specifically in the lung epithelial, oral, and nasal mucosa, indicating a possible viral entry route for SARS-CoVs [3]. This may be the reason for the high incidence of pneumonia and bronchitis in severe COVID-19 infections. Indeed, the expression of ACE2 was also reported in the heart, brain, blood vessel, adrenal gland, testicle liver as well as kidney and gastrointestinal tract which opens a possibility of fecal-oral transmission of the virus and gastrointestinal complications in pediatrics cases [4]. Recently, a study revealed the cryo-EM structure of SARS-CoV-2 spike protein and documented that the affinity for SARS-CoV-2 for ACE2 is 10-20-fold higher than SARS-CoV [5], a conceivable explanation for the higher infectivity rate of SARS-CoV-2.
ACE2 is part of the renin-angiotensin-aldosterone system (RAAS) which has multiple physiological processes. The major function of ACE2 is to oppose the effect of RAAS by conversion of angiotensin I (Ang I) to produce Ang 1–9 and Ang II into Ang 1–7, peptides which trigger vasodilation and have anti-fibrotic, anti-inflammatory, and anti-proliferative properties [6]. In healthy controls, ACE2 exists in its membrane-bounded form with a very low level of ACE2 in circulation [7]. However, in cardiovascular (CVD) patients, increased shedding of ACE2 was observed and higher circulating levels were associated with suppression of membrane-bounded ACE2 [8]. Besides, elevated levels of plasma ACE2 have been found in various inflammatory diseases, including renal failure, CVDs, diabetes as well as pathological conditions like acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) [9,10]. In this perspective, few studies have reported an elevated levels of plasma ACE2 in COVID-19 cases and its significance in predicting disease outcome [11,12]. In this line, in the present study we report a significant increase in the plasma ACE2 in severe form of COVID-19 with a strong positive association with hyper coagulation compared to moderate form.
2. Method
2.1. Study participant & plasma collection
Forty-two patients with a positive reverse transcription-polymerase chain reaction (RT-PCR) test for SARS-CoV-2 on nasopharyngeal swabs were included. All the cases were hospitalized 7–9 days after the occurrence of symptoms. Blood samples were collected in ethylenediaminetetraacetic acid (EDTA) tubes at Mahatma Gandhi Medical College and Research Institute (MGMCRI), a tertiary care hospital in South-Eastern India. Additional 10 healthy controls (HC) were enrolled in the study. After getting informed consent, 2 ml of blood samples were collected from the study participants. The sampling was done at two-time points for COVID-19 cases with the first sampling done on the day of admission (DOA) within 24 h followed by second sampling on day 7 post admission. In the case of healthy volunteers, 2 mL of blood was drawn at a one-time point. Plasma was separated from whole blood by centrifugation at 3000g for 15 min at 4 °C, then aliquoted and frozen at −80 °C until use. Demographic profiles, clinical and laboratory data were collected from the study participants. The study was approved by the Institutional Human Ethical Review Committee (ECR/451/Inst/PO/2013/RR-16). COVID-19 patients were classified based clinical severity as per the clinical management protocol of COVID-19, Ministry of Health and Family Welfare. Moderate cases were defined by respiratory rate of ≥24/mins, complaints of breathlessness and SpO2 of ≤93% in room air. Similarly, severe cases were defined by respiratory rate of ≥30/mins, complaints of breathlessness and SpO2 of ≤90% in room air [13,14].
2.2. Estimation of ACE2 by ELISA
A quantitative Human ACE2 sandwich ELISA kit (Fine Biotech, China) was used to determine the plasma concentration of ACE2 following the manufacturers instructions. A 10 μL plasma sample was used in this experiment. Briefly, the samples were added to a pre-coated antibody (capture) and incubated for 90 min at 37 °C. Subsequently, biotin-conjugated antibody (detection) was added and incubated at 37 °C for 60 min. Further incubation was done with HRP-streptavidin conjugate at 37 °C for 30 min, followed by the addition of TMB subrate which reacted with HRP and produced a blue coloured end product. The reaction was finally stopped by the addition of acidic stop solution, which developed a yellow colour, which was read at 450 nm (SpectraMax M5, Molecular Devices, California, USA). The density of yellow is directly proportional to the amount of ACE2 in the sample. All analyses and calibration were carried out in duplicate. The concentration of ACE2 in the tested samples was estimated against the standard curve using the serially diluted standards (7.815–500 ng/ml) provided in the kit. Concentrations are reported as ng/mL.
2.3. Estimation of D-dimer
In vitro quantification of fibrin degradation product D-dimer in human plasma was determined by nephelometric immunoassay that utilizes antibody coated latex particles [15]. D-dimer particles aggregates with the latex coated antibodies, which increases light scattering. The increase of scattering light is proportional to the amount of D-dimer in the sample. In the present study, 80 μL plasma samples were used to measure the concentration of D-dimer by automated cartridge based specific protein analyzer Mispa i3 (Mispa i3, Agappe Diagnostics, Switzerland).
2.4. Statistical analysis
The normality test (Shapiro-Wilk Test) was performed to analyze the data and the results were expressed as median (IQR) or n (%). A comparison within the groups was performed using a two-tailed related sample t-test (Wilcoxon Signed Rank Test). Similarly, a comparison between the groups was performed using a two-tailed Independent Sample t-test (Mann-Whitney U test). The correlation was evaluated using Spearman's Rho Correlation. All the statistical analyses were carried out at a 5% level of significance and results with the P ≤ 0.05 were considered statistically significant. The data were analyzed using SPSS 21.0.
3. Results
3.1. Demographic profile clinical characteristics
Clinical characteristics of the 42 COVID-19 patients sampled at recruitment are shown in Table 1 .The most common symptoms during the time of admission were cough, cold, fever, breathlessness, loss of taste and smell. Similarly, the most common pre-existing comorbidities were hypertension (HT) and diabetes mellitus (DM).
Table 1.
Clinical & demographic profile of study participants.
| Group |
Moderate |
Severe |
All COVID-19 |
Healthy Control |
|
|---|---|---|---|---|---|
| Study Participants | 20 | 22 | 42 | 10 | |
| Gender | Male | 15 (75) | 14 (63.6) | 29 (69) | 5 (50) |
| Female | 5 (25) | 8 (36.4) | 13 (30.9) | 5 (50) | |
| CORAD Score | CORADS 4 | 6 (30) | 3 (13.6) | 9 (21.4) | 0 (0) |
| CORADS 5 | 14 (70) | 19 (86.4) | 33 (78.6) | ||
| CTSS | ≤10 | 12 (60) | 0 (0) | 12 (28.6) | 0 (0) |
| >10 - ≤ 20 | 8 (40) | 16 (72.7) | 24 (57.1) | ||
| >20 - ≤ 25 | 0 (0) | 6 (27.3) | 6 (14.3) | ||
| Symptoms | Fever | 15 (75) | 15 (68.2) | 30 (71.4) | 0 (0) |
| Cold | 2 (10) | 1 (4.5) | 3 (7.1) | ||
| Cough | 7 (35) | 9 (40.9) | 16 (38.1) | ||
| Breathlessness | 9 (45) | 12 (54.5) | 21 (50) | ||
| Loss of taste & Smell | 2 (10) | 8 (36.4) | 10 (23.8) | ||
| Common Comorbidities | Hypertension | 5 (25) | 9 (40.9) | 14 (33.3) | 0 (0) |
| Diabetes Mellitus |
5 (25) |
9 (40.9) |
14 (33.3) |
||
| Deceased | 0 (0) | 3 (13.6) | 3 (7.1) | 0 (0) | |
| Platelet Count | 191500 (150750–252750) | 223000 (183000–275000) | 199000 (155500–265000) | 227189 (189500–275000) | |
| Ferritin | 243.5 (47–445.8) | 303 (151.5–713) | 287 (118–546.5) | 256 (204.4–296) | |
| D-Dimer (DOA) μg/mL | 0.561 (0.361–0.729) | 0.878 (0.682–1.891) | 0.732 (0.467–1.157) | 0.312 (0.289–0.355) | |
| D-Dimer (Day 7) μg/mL | 0.66 (0.395–1.768) | 1.085 (0.889–1.523) | 0.975 (0.51–1.523) | – | |
Data are represented in n (%) and median (IQR).
3.2. Elevated level of sACE2 at the time of admission
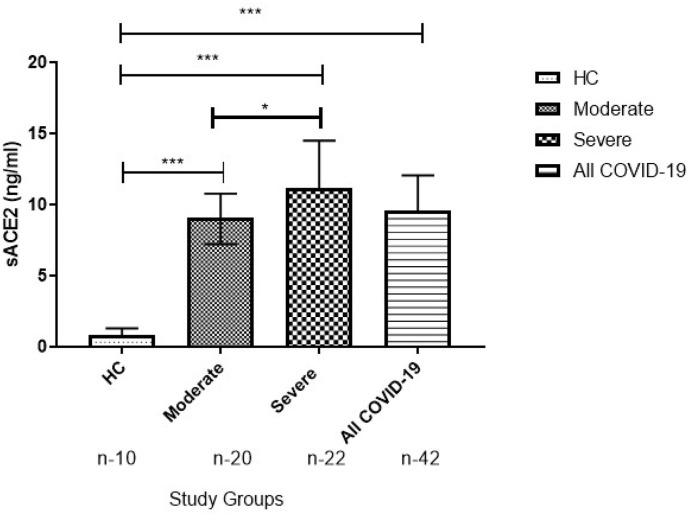
Compared to HC, there was a significant increase in the levels of ACE2 in the moderate, severe, and all COVID-19 groups at the time of admission. Parallelly, the ACE2 levels were higher in the severe group compared to the moderate group, suggesting early multisystem effect in patients with severe SARS-CoV-2 infection (Fig. 1 ).
Fig. 1.
Elevated level of sACE2 at the time of admission in COVID-19. Mann-Whitney U test was used to compare between groups and data are represented in Median (IQR). P-value ≤ 0.05 is considered significant. “n” represents the number of samples in each panel. ∗∗∗ Indicates the P ≤ 0.001 when compared to HC at the time of Admission.
3.3. Increased shedding of sACE2 during the early course of COVID-19
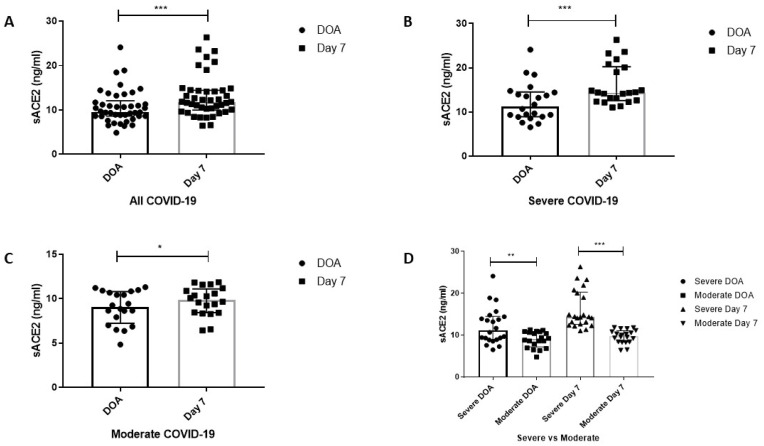
The plasma level of ACE2 in the study participant is represented in Table 2 . The plasma level of sACE2 was significantly elevated in all the COVID-19 groups (all COVID-19, severe and moderate) on day 7 post admission (Fig. 2 A–C). Consistently, the sACE2 levels were significantly higher in the severe group compared to the moderate group (Fig. 2D), indicating persistent shedding of membrane-bounded ACE2 from the early course of infection.
Table 2.
Plasma Level of sACE2 (ng/mL).
| Study Group | DOA | Day 7 |
|---|---|---|
| Healthy Control (n-4) | 0.828 (0.394–1.328) | |
| Moderate (n-20) | 9.072 (7.245–10.8) | 9.873 (8.44–11.1)∗ |
| Severe (n-22) | 11.21 (8.955–14.53) | 14.38 (12.6–20.27)∗∗∗ |
| All COVID-19 (n-42) | 9.597(8.63–12.08) | 11.85(9.976–14.43)∗∗∗ |
Since the data was found to be non-parametric, the Related Sample t-Test (Wilcoxon Signed Rank Test) was used to compare the data within the groups. The results were expressed as median (IQR).
∗Indicates the P ≤ 0.05 when compared to DOA within the groups.
∗∗∗ Indicates the P ≤ 0.001 when compared to DOA within the groups.
Fig. 2.
Circulating Plasma levels of sACE2 in COVID-19 groups. (A) Plasma levels of sACE2 in all COVID-19 cases. (B) Plasma levels of sACE2 in severe COVID-19 cases. (C) Plasma levels of sACE2 in moderate COVID-19 cases. (D) Plasma levels of sACE2 between severe and moderate COVID-19 group. The results are expressed in median (IQR). Wilcoxon Signed Rank Test and Mann-Whitney U Test were used to compare data within the groups and between groups, respectively. P-value ≤ 0.05 is considered significant.
∗Indicates the P ≤ 0.05; ∗∗ Indicates the P ≤ 0.01; ∗∗∗ Indicates the P ≤ 0.001.
3.4. Plasma levels of sACE2 based on gender
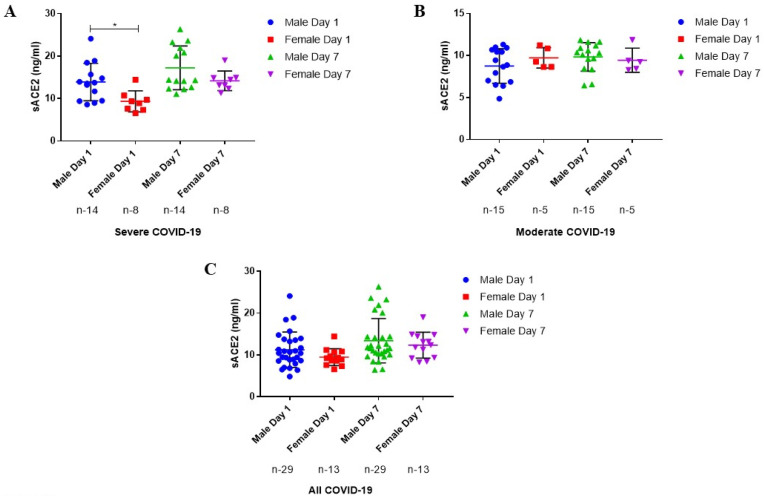
We further assessed the gender based plasma levels of sACE2. The sACE2 levels were found to be significantly elevated in male patients with severe COVID-19 than female at the time of admission (Fig. 3 A). However, no significant changes in the plasma levels of sACE2 was recorded in moderate and all COVID-19 groups (Fig. 3 B&C).
Fig. 3.
Plasma Levels of sACE2 based on Gender. (A) Plasma levels of sACE2 in severe COVID-19 cases. (B) Plasma levels of sACE2 in Moderate COVID-19 cases. (C) Plasma levels of sACE2 in all COVID-19 cases. The results are expressed in median (IQR). Mann-Whitney U Test were used to compare data between groups, respectively. P-value ≤ 0.05 is considered significant.
∗Indicates the P ≤ 0.05.
3.5. Levels of sACE2 in COVID-19 patients with & without comorbidities
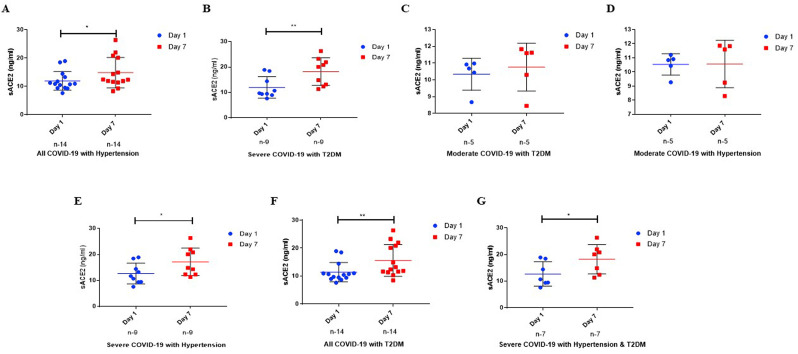
Interestingly, a substantial increase in sACE2 levels was noted in severe and all COVID-19 group patients with hypertension (HT) (P ≤ 0.05 & Fig. 4 A&C) or diabetes milletus (DM) (P ≤ 0.01 & Fig. 4D&F) or both HTand DM (P ≤ 0.05 & Fig. 4G) on Day 7 compared to day 1. However, no such difference in the plasma levels of sACE2 was recorded in all the study groups when other co-morobidites (coronery heart disease, Parkinson disease, breast cancer and hypothyroidism) were included.
Fig. 4.
Plasma Levels of sACE2 based on Comorbidities. (A) Plasma levels of sACE2 in severe COVID-19 cases with Hypertension. (B) Plasma levels of sACE2 in Moderate COVID-19 cases with Hypertension. (C) Plasma levels of sACE2 in all COVID-19 cases with hypertension. (D) Plasma levels of sACE2 in severe COVID-19 cases with diabetes. (E) Plasma levels of sACE2 in Moderate COVID-19 cases with diabetes. (F) Plasma levels of sACE2 in all COVID-19 cases with diabetes. (G) Plasma levels of sACE2 in all COVID-19 cases with both hypertension & diabetes. The results are expressed in median (IQR). Mann-Whitney U Tests were used to compare data between groups, respectively. P-value ≤ 0.05 is considered significant.
∗Indicates the P ≤ 0.05; ∗∗Indicates the P ≤ 0.01.
3.6. Correlation between sACE2 and D-dimer
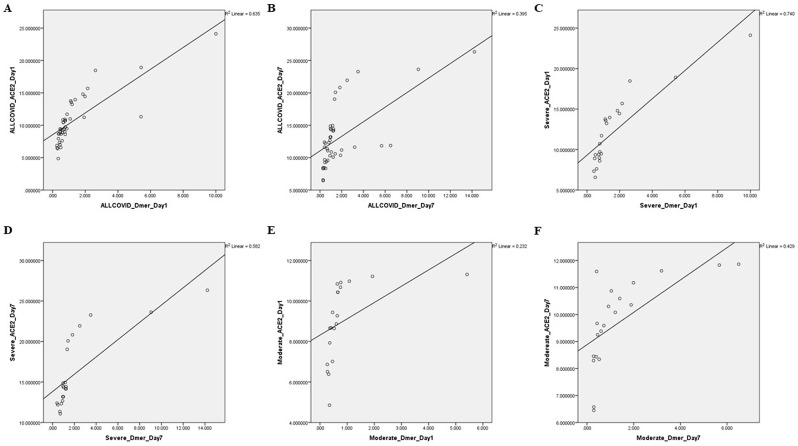
D-Dimer is a marker of hyper-coagulation and intravascular thrombosis formation. Several reports have shown the elevated level of D-Dimer in COVID-19 patients, especially a very high in non-survivor/severe cases compared to survivor/moderate/mild cases [[16], [17], [18]]. To check whether sACE2 has any implication on D-Dimer, we performed a dependence test between sACE2 and D-Dimer. To note, sACE2 showed a strong and significant positive correlation with D-Dimer at Day 1 as well as Day 7 in all COVID-19, severe and non-severe COVID-19 cases as represented in Table 3 & Fig. 5 , suggesting sACE2 could be implicated in hyper-coagulation, a critical factor observed in SARS-CoV-2 infected patients.
Table 3.
Correlation between sACE2 & D-Dimer.
| sACE2 vs D-Dimer | ||||
|---|---|---|---|---|
| Groups | Day 1 | Fig No. | Day 7 | Fig No. |
| All COVID-19 | R = 0.917 & P ≤ 0.001 | A | R = 0.670 & P ≤ 0.001 | B |
| Severe COVID-19 | R = 0.964 & P ≤ 0.001 | C | R = 0.924 & P ≤ 0.001 | D |
| Moderate COVID-19 | R = 0.947 & P ≤ 0.001 | E | R = 0.835 & P ≤ 0.001 | F |
Fig. 5.
Association between ACE2 and D-Dimer in COVID-19 Groups. Correlation between ACE2 and D-Dimer in all COVID-19 group during the (A) Day 1 (R = 0.917 & P ≤ 0.001) and (B) Day 7 (R = 0.670 & P ≤ 0.001); Correlation between ACE2 and D-Dimer in Severe COVID-19 group during the (C) Day 1 (R = 0.964 & P ≤ 0.001) and (D) Day 7 (R = 0.924 & P ≤ 0.001); Correlation between ACE2 and D-Dimer in Moderate COVID-19 group at the (E) Day 1 (R = 0.947 & P ≤ 0.001) and (F) Day 7 (R = 0.835 & P ≤ 0.001). Since the data was non-parametric, Spearman's Rho Correlation was used to compare the data. P-value ≤ 0.05 is considered significant.
4. Discussion
The role of ACE2 as a key receptor for the fusion and endocytosis of SARS-CoV2 has been well documented [19,20]. Despite being a viral receptor, ACE2 acts as a crucial regulator of RAAS by counterbalancing the harmful effect of the ACE/RAAS signalling pathway by its downstream ACE2/Angiotensin (1–7)/MAS axis [21].
In the normal physiological process, ACE2 exists in cellular/membrane-bounded form (cACE2) with lower levels of ACE2 in circulation (sACE2). However, in a pathological condition, especially in CVD cases, elevated levels of sACE2 were strongly associated with the downregulation of cACE2 [8]. The main finding of our study is that the level of sACE2 was elevated in severe cases compared to moderate cases and healthy controls at the time of admission, which is a mean of day 7 post onset of symptoms. Importantly, we also noted an enormous increase in Day 7 compared to baseline (day of admission) in the patient with severe infection compared to non-severe COVID-19 patients. This shows the persistent shedding of sACE2 from the early course of infection. Our results were consistent with recent studies, investigating the plasma level of ACE2 in SARS-CoV-2 [11,22,23]. Lier et al., found a marked elevation of ACE2 in critically ill COVID-19 patients admitted to the intensive care unit [22]. Similarly, Faygas et al., reported a higher level of ACE2 in critically ill and severe COVID-19 patients when compared to non-COVID-19 sepsis patients regardless of co-morbidities. Besides, the circulating ACE2 levels were further increased during the hospital stay in critically ill COVID-19 patients [11]. Reindl-Schwaighofer et al. also found elevated levels of ACE2 activity in the hospital-treated patient with severe COVID-19 [23]. Unlike other soluble proteins which restores to the baseline levels during the recovery phase of the virus infection, the ACE2 remained higher even after one month of post COVID-19 [24,25]. Moreover, they also noted a strong association between plasma ACE2 level with disease severity and co-morbidities like hypertension, heart disease as well as kidney disease [12]. In reference to this, serum levels of ACE2 activity were also correlated with COVID-19 severity and predicted mortality [11]. These observations with our results indicate that the release of ACE2 into circulation is increased in COVID-19 patients. Despite the above observation, controversial results on ACE2 levels have been reported in COVID-19 patients like unchanged [26,27] or even very low level of sACE2 [28] when compared to control. Since the early prognosis of disease outcome and effective disease management are the only way to prevent severe complications or death, the discovery of novel blood-based biomarkers in COVID-19 is urgently necessary. In this view, we have recently reviewed a set of potential biomarkers for the early prognosis of COVID-19 disease outcomes [29]. Indeed, several studies recommend that sACE2/human recombinant sACE2 could be a promising potential therapy for COVID-19 management [[30], [31], [32], [33]]. But, further clinical studies are required to confirm the clinical value of recombinant ACE2 administrated in COVID-19 patients.
Previous studies showed the virus-induced shedding of ACE2 in SARS-CoV-1 which may be due to infected tissues and/or cells that influence ACE2 shedding into circulation [34,35]. Though the actual role of soluble ACE2 is not known yet, its excessive shedding into circulation may be as part of protective mechanism by the host in order to combat the incoming virus. This property is currently being exploited by developing soluble ACE2 as a therapeutic target to decoy virus. On the contrary, a study demonstrated that the activity of ADAM17 (A Disintegrin and metalloproteinases 17) is enhanced by the internalization of SARS-CoV-1-ACE2 in the ACE2 cytoplasmic dependent manner [36], thereby resulting in proteolytical cleavage of membrane-bounded/cACE2 [37]. This indicates that the internalization of virus mediated-ACE2 prompts positive feedback, which is harmful to tissues. For instance, depletion of ACE2 leads to elevation of Ang II and activation of A1T receptor, which in turn activates ADAM17. Thus, a substantial reduction of ACE2 is indirectly involved in its cleavage by ADAM17 and further depletion of ACE2 in the tissue and release its soluble form (sACE2) into circulation [38,39]. Similarly, a catalytically active form of TMPRSS2 can interact with ACE2 and enzymatically cleave to generate sACE2. Apart from this, TMPRSS11D, HNP/TMPRSS1, and ADAM10 were shown to cleave ACE2 receptors [35,40]. Moreover, other stimuli such as cytokine and elevated levels of Ang II can increase the shedding of ACE2 into circulation [35,41]. Meanwhile, elevated levels of Ang II promote thrombosis through the thrombin-dependent pathway as well as the pro-inflammatory response by AT1R [42,43]. Recently, a review described that ACE2 expressed on the surface of endothelial cells (ECs) is cleaved by ADAM17 [44]. Indeed, the infected ECs and activation of macrophages in the pulmonary alveoli are considered the main source of pro-inflammatory cytokines that lead to cytokine storms [45]. On the other hand, ACE2 mediated SARS-CoV-2 infection of ECs triggers the activation of ECs followed by endothelial damage [46,47]. This could be a possible reason for the release of sACE2 into circulation due to the hyper-permeability of pulmonary vascular ECs. Thus, further studies on serine proteases and matrix metalloproteases involve in the shedding of ACE2 during COVID-19 infection could provide some light endothelial dysfunction and the pathogenesis of COVD-19.
Recent studies have shown that the major risk factor for fatal infection is male gender, increased age, and comorbidities like hypertension, diabetes, and heart disease [48,49]. A recent review by Malik et al. has described the pathophysiology of COVID-19 and its pathophysiology on various pre-existing pathological conditions such as cardiovascular complications (cardiovascular disease and myocarditis, acute myocardial infraction, chronic myocardial infraction, cardiomyopathy), neurological consequences (Parkinson's diseases, Autism spectrum disease, Guillain-Barre Syndorme, acute cerebrovascular disease, encephalitis, encephalopathy), cancer, hemophagocytic lymphohistiocytosis, hypertension, diabetes milletus, and other infections (mucormycosis and ganagrene) [50]. The article provided a link with ACE2 and cardiac injury among COVID-19 cases. Interestingly an in vivo study documented an ACE2 dependent cardiac injury in SARS-CoV2 infected mouse models [50,51]. In the present study, we observed an elevated level of sACE2 in male patients with severe COVID-19 compared to female patients, indicating that male patients are more susceptible to SARS-CoV-2 infection with increasing disease severity. Also, the levels of sACE2 were found to be substantially increased in patients with hypertension, diabetes, and both the comorbidities in severe and all COVID-19 group at day 7 compared to the baseline (time of admission). This shows that plasma levels of sACE2 are significantly increased in patients with pre-existing conditions (hypertension & diabetes), especially in severe COVID-19 cases. This emphasizes the importance of ACE2 in severe COVID-19 disease associated with pre-existing diseases.
A strong positive association between sACE2 and D-dimer observed in the present study shows that ACE2 may be involved in hyper-coagulation and thrombin formation. Coagulation is a cellular process that involves the interaction of ECs, platelet with coagulation factors. Upon SARS-CoV-2 infection, endothelial dysregulation results from ACE2-mediated binding of SARS-CoV-2, hyper-inflammation, and upregulation of pro-thrombin [52]. In addition, ECs lose their vascular integrity and undergoes apoptosis, which leads to exposure of the thrombogenic basement membrane and activation of various clotting factors [53]. The activated ECs trigger coagulation by expressing vWF, fibrinogen, and P-selectin, through which platelet binds and gets activated [54]. Activated platelets produce VEGF, which induces ECs to express tissue factor (main activator of the coagulation pathway) that stimulates the fibrinolytic system, thereby enhancing the degradation of fibrin via plasmin and releasing D-dimer into circulation [55,56]. In reference to this, a study hypothesized that excess release of D-dimer into circulation during SARS-CoV-2 infection may be due to coagulopathy induced by EC's apoptosis [57]. However, further studies are needed to elucidate the precise role of ACE2 in endovascular thrombotic processes, which could provide some light on COVID-19 disease pathogenesis.
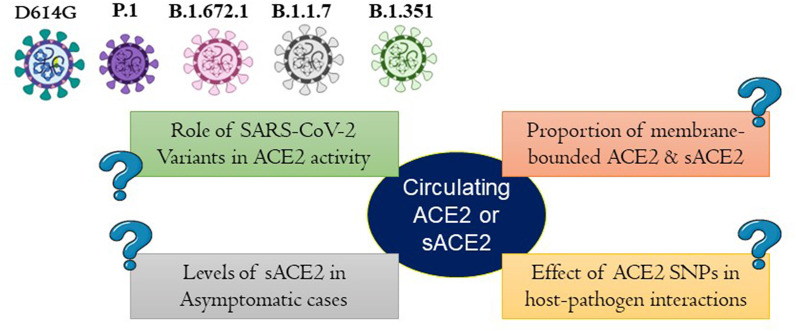
Some of the important questions needs to be addressed (Fig. 6 ) to decipher the precise role of sACE2 in COVID-19 disease virulence (1) Role of SARS-CoV-2 variant in modulating ACE2 activity (2) Proportion of membrane-bounded ACE2 and sACE2 during SARS-CoV-2 infeciton (3) Role of sACE2 in Asymptomatic cases (4) Effect of ACE2 SNPs in host-pathogen interaction.
Fig. 6.
The precise role of sACE2 in COVID-19 disease virulence.
5. Conclusion
To conclude, the plasma level of ACE2 along with D-Dimer could be an indicator for the early prediction of COVID-19 disease severity. However further studies are required to ascertain it. The study results lays a foundation for further investigation of soluble ACE2 functions in endothelial dysfunction and hyper-coagulation during SARS-CoV-2 infection.
Author contributions
Vignesh Mariappan: Formal Analysis, Investigation, Methodology, Writing-Original Draft. Pajanivel Ranganadin: Writing-Review & Editing, Resources, Validation. Lokesh shanmugam: Writing-Review & Editing, Resources, Validation. S.R. Rao: Writing-Review & Editing, Funding acquisition. Agieshkumar Balakrishna Pillai: Conceptualization, Investigation, Methodology, Writing-Review & Editing, Validation, Funding acquisition.
Declaration of competing interest
The author declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknoweldgement
The study was carried out with the funding support from the “Office of the Vice President-Research, Innovation and Development” SBV under “COVID-19 Research grant for R&D proposals in management of COVID-19 pandemic” Sri Balaji Vidyapeeth. Authors greatly acknowledge the funding support and Research infrastructure of SBV. We would like to thank the Central Lab (Department of Microbiology) MGMCRI for D-dimer quantification. The authors also thank the medical staff of the MGMCRI for the collection of blood samples.
References
- 1.Liu D.X., Liang J.Q., Fung T.S. Human coronavirus-229E, -OC43, -NL63, and -HKU1 (Coronaviridae) Encyclopedia Virol. 2021:428–440. doi: 10.1016/B978-0-12-809633-8.21501-X. [DOI] [Google Scholar]
- 2.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mariappan V., SR R., Balakrishna Pillai A. Angiotensin-converting enzyme 2: a protective factor in regulating disease virulence of SARS-COV-2. IUBMB Life. 2020;72:2533–2545. doi: 10.1002/iub.2391. [DOI] [PubMed] [Google Scholar]
- 4.Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., Guo Q., Sun X., Zhao D., Shen J., Zhang H., Liu H., Xia H., Tang J., Zhang K., Gong S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amraei R., Rahimi N. COVID-19, renin-angiotensin system and endothelial dysfunction. Cells. 2020;9:E1652. doi: 10.3390/cells9071652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ra L., Fj W., I H., Ma Y., J R., Lm B., Ai S. Angiotensin-converting enzyme 2 catalytic activity in human plasma is masked by an endogenous inhibitor. Exp. Physiol. 2008;93 doi: 10.1113/expphysiol.2007.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramchand J., Patel S.K., Kearney L.G., Matalanis G., Farouque O., Srivastava P.M., Burrell L.M. Plasma ACE2 activity predicts mortality in aortic stenosis and is associated with severe myocardial fibrosis. JACC Cardiovase Image. 2020;13:655–664. doi: 10.1016/j.jcmg.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Narula S., Yusuf S., Chong M., Ramasundarahettige C., Rangarajan S., Bangdiwala S.I., van Eikels M., Leineweber K., Wu A., Pigeyre M., Paré G. Plasma ACE2 and risk of death or cardiometabolic diseases: a case-cohort analysis. Lancet. 2020;396:968–976. doi: 10.1016/S0140-6736(20)31964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia H. Pulmonary angiotensin-converting enzyme 2 (ACE2) and inflammatory lung disease. Shock. 2016;46:239–248. doi: 10.1097/SHK.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 11.Fagyas M., Fejes Z., Sütő R., Nagy Z., Székely B., Pócsi M., Ivády G., Bíró E., Bekő G., Nagy A., Kerekes G., Szentkereszty Z., Papp Z., Tóth A., Kappelmayer J., Nagy B. Circulating ACE2 activity predicts mortality and disease severity in hospitalized COVID-19 patients. Int. J. Infect. Dis. 2021;115:8–16. doi: 10.1016/j.ijid.2021.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kragstrup T.W., Singh H.S., Grundberg I., Nielsen A.L.-L., Rivellese F., Mehta A., Goldberg M.B., Filbin M.R., Qvist P., Bibby B.M. Plasma ACE2 predicts outcome of COVID-19 in hospitalized patients. PLoS One. 2021;16 doi: 10.1371/journal.pone.0252799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.202104231216553337715COVIDManagementAlgorithm_22Apr21.pdf, (n.d.). https://dghs.gov.in/WriteReadData/Orders/202104231216553337715COVIDManagementAlgorithm_22Apr21.pdf (accessed April 6, 2022).
- 14.UpdatedDetailedClinicalManagementProtocolforCOVID19adultsdated24052021.pdf, (n.d.). https://www.mohfw.gov.in/pdf/UpdatedDetailedClinicalManagementProtocolforCOVID19adultsdated24052021.pdf (accessed April 6, 2022).
- 15.Ahmed H.M., Abd El Kareem R.M., Ali F.M., Sayed A.R., Mohamed Y.A. Clinical, laboratory, and radiological characteristics of COVID-19-infected children admitted to pediatric intensive care unit: a single-center experience. Beni-Suef Univ J. Basic Appl. Sci.. 2021;10:79. doi: 10.1186/s43088-021-00168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J.-J., Dong X., Cao Y.-Y., Yuan Y.-D., Yang Y.-B., Yan Y.-Q., Akdis C.A., Gao Y.-D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 17.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemostasis. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson C.B., Farzan M., Chen B., Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022;23:3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samavati L., Uhal B.D. ACE2, much more than just a receptor for SARS-COV-2. Front. Cell. Infect. Microbiol. 2020;10:317. doi: 10.3389/fcimb.2020.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Lier D., Kox M., Santos K., van der Hoeven H., Pillay J., Pickkers P. Increased blood angiotensin converting enzyme 2 activity in critically ill COVID-19 patients. ERJ Open Res. 2021;7:848–2020. doi: 10.1183/23120541.00848-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reindl-Schwaighofer R., Hödlmoser S., Eskandary F., Poglitsch M., Bonderman D., Strassl R., Aberle J.H., Oberbauer R., Zoufaly A., Hecking M. ACE2 elevation in severe COVID-19. Am. J. Respir. Crit. Care Med. 2021;203:1191–1196. doi: 10.1164/rccm.202101-0142LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel S.K., Juno J.A., Lee W.S., Wragg K.M., Hogarth P.M., Kent S.J., Burrell L.M. Plasma ACE2 activity is persistently elevated following SARS-CoV-2 infection: implications for COVID-19 pathogenesis and consequences. Eur. Respir. J. 2021;57 doi: 10.1183/13993003.03730-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundström A., Ziegler L., Havervall S., Rudberg A.-S., von Meijenfeldt F., Lisman T., Mackman N., Sandén P., Thålin C. Soluble angiotensin-converting enzyme 2 is transiently elevated in COVID-19 and correlates with specific inflammatory and endothelial markers. J. Med. Virol. 2021;93:5908–5916. doi: 10.1002/jmv.27144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kintscher U., Slagman A., Domenig O., Röhle R., Konietschke F., Poglitsch M., Möckel M. Plasma angiotensin peptide profiling and ACE (Angiotensin-Converting enzyme)-2 activity in COVID-19 patients treated with pharmacological blockers of the renin-angiotensin system. Hypertension. 2020;76:e34–e36. doi: 10.1161/HYPERTENSIONAHA.120.15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rieder M., Wirth L., Pollmeier L., Jeserich M., Goller I., Baldus N., Schmid B., Busch H.-J., Hofmann M., Kern W., Bode C., Duerschmied D., Lother A. Serum ACE2, angiotensin II, and aldosterone levels are unchanged in patients with COVID-19. Am. J. Hypertens. 2021;34:278–281. doi: 10.1093/ajh/hpaa169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rojas M., Acosta-Ampudia Y., Monsalve D.M., Ramírez-Santana C., Anaya J.-M. How important is the assessment of soluble ACE-2 in COVID-19? Am. J. Hypertens. 2021;34:296–297. doi: 10.1093/ajh/hpaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mariappan V., Manoharan P.S., R P., Shanmugam L., Rao S.R., Pillai A.B. Potential biomarkers for the early prediction of SARS-COV-2 disease outcome. Microb. Pathog. 2021;158 doi: 10.1016/j.micpath.2021.105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassler L., Wysocki J., Gelarden I., Tomatsidou A., Gula H., Nicoleascu V., Randall G., Henkin J., Yeldandi A., Batlle D. A novel soluble ACE2 protein totally protects from lethal disease caused by SARS-CoV-2 infection. bioRxiv. 2021 doi: 10.1101/2021.03.12.435191. 2021.03.12.435191. [DOI] [Google Scholar]
- 31.Zoufaly A., Poglitsch M., Aberle J.H., Hoepler W., Seitz T., Traugott M., Grieb A., Pawelka E., Laferl H., Wenisch C., Neuhold S., Haider D., Stiasny K., Bergthaler A., Puchhammer-Stoeckl E., Mirazimi A., Montserrat N., Zhang H., Slutsky A.S., Penninger J.M. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir. Med. 2020;8:1154–1158. doi: 10.1016/S2213-2600(20)30418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnamurthy S., Lockey R.F., Kolliputi N. Soluble ACE2 as a potential therapy for COVID-19. Am. J. Physiol. Cell Physiol. 2021;320:C279–C281. doi: 10.1152/ajpcell.00478.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monteil V., Dyczynski M., Lauschke V.M., Kwon H., Wirnsberger G., Youhanna S., Zhang H., Slutsky A.S., Hurtado Del Pozo C., Horn M., Montserrat N., Penninger J.M., Mirazimi A. Human soluble ACE2 improves the effect of remdesivir in SARS-CoV-2 infection. EMBO Mol. Med. 2021;13 doi: 10.15252/emmm.202013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glowacka I., Bertram S., Herzog P., Pfefferle S., Steffen I., Muench M.O., Simmons G., Hofmann H., Kuri T., Weber F., Eichler J., Drosten C., Pöhlmann S. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J. Virol. 2010;84:1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jia H.P., Look D.C., Tan P., Shi L., Hickey M., Gakhar L., Chappell M.C., Wohlford-Lenane C., McCray P.B. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;297:L84–L96. doi: 10.1152/ajplung.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haga S., Yamamoto N., Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T., Yamamoto N., Sasazuki T., Ishizaka Y. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc. Natl. Acad. Sci. U. S. A. 2008;105:7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambert D.W., Yarski M., Warner F.J., Thornhill P., Parkin E.T., Smith A.I., Hooper N.M., Turner A.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J. Biol. Chem. 2005;280:30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oudit G.Y., Pfeffer M.A. Plasma angiotensin-converting enzyme 2: novel biomarker in heart failure with implications for COVID-19. Eur. Heart J. 2020;41:1818–1820. doi: 10.1093/eurheartj/ehaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zipeto D., Palmeira J. da F., Argañaraz G.A., Argañaraz E.R. ACE2/ADAM17/TMPRSS2 interplay may Be the main risk factor for COVID-19. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.576745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Senapati S., Banerjee P., Bhagavatula S., Kushwaha P.P., Kumar S. Contributions of human ACE2 and TMPRSS2 in determining host-pathogen interaction of COVID-19. J. Genet. 2021;100:12. doi: 10.1007/s12041-021-01262-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel V.B., Clarke N., Wang Z., Fan D., Parajuli N., Basu R., Putko B., Kassiri Z., Turner A.J., Oudit G.Y. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J. Mol. Cell. Cardiol. 2014;66:167–176. doi: 10.1016/j.yjmcc.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 42.Senchenkova E.Y., Russell J., Esmon C.T., Granger D.N. Roles of Coagulation and fibrinolysis in angiotensin II-enhanced microvascular thrombosis. Microcirculation. 2014;21:401–407. doi: 10.1111/micc.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eguchi S., Kawai T., Scalia R., Rizzo V. Understanding angiotensin II type 1 receptor signaling in vascular pathophysiology. Hypertension. 2018;71:804–810. doi: 10.1161/HYPERTENSIONAHA.118.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.-C., Turner A.J., Raizada M.K., Grant M.B., Oudit G.Y. Angiotensin-Converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Escher R., Breakey N., Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb. Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagy B., Fejes Z., Szentkereszty Z., Sütő R., Várkonyi I., Ajzner É., Kappelmayer J., Papp Z., Tóth A., Fagyas M. A dramatic rise in serum ACE2 activity in a critically ill COVID-19 patient. Int. J. Infect. Dis. 2021;103:412–414. doi: 10.1016/j.ijid.2020.11.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banerjee A., Pasea L., Harris S., Gonzalez-Izquierdo A., Torralbo A., Shallcross L., Noursadeghi M., Pillay D., Sebire N., Holmes C., Pagel C., Wong W.K., Langenberg C., Williams B., Denaxas S., Hemingway H. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Lancet. 2020;395:1715–1725. doi: 10.1016/S0140-6736(20)30854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmad Malik J., Ahmed S., Shinde M., Almermesh M.H.S., Alghamdi S., Hussain A., Anwar S. The impact of COVID-19 on comorbidities: a review of recent updates for combating it. Saudi J. Biol. Sci. 2022;29:3586–3599. doi: 10.1016/j.sjbs.2022.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun S.-H., Chen Q., Gu H.-J., Yang G., Wang Y.-X., Huang X.-Y., Liu S.-S., Zhang N.-N., Li X.-F., Xiong R., Guo Y., Deng Y.-Q., Huang W.-J., Liu Q., Liu Q.-M., Shen Y.-L., Zhou Y., Yang X., Zhao T.-Y., Fan C.-F., Zhou Y.-S., Qin C.-F., Wang Y.-C. A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe. 2020;28:124–133. doi: 10.1016/j.chom.2020.05.020. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin Y., Ji W., Yang H., Chen S., Zhang W., Duan G. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduct. Targeted Ther. 2020;5:293. doi: 10.1038/s41392-020-00454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sturtzel C. Endothelial cells. Adv. Exp. Med. Biol. 2017;1003:71–91. doi: 10.1007/978-3-319-57613-8_4. [DOI] [PubMed] [Google Scholar]
- 54.Pober J.S., Sessa W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 55.Nachman R.L., Rafii S. Platelets, petechiae, and preservation of the vascular wall. N. Engl. J. Med. 2008;359:1261–1270. doi: 10.1056/NEJMra0800887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iba T., Levy J.H., Levi M., Connors J.M., Thachil J. Coagulopathy of coronavirus disease 2019. Crit. Care Med. 2020;48:1358–1364. doi: 10.1097/CCM.0000000000004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guler N., Siddiqui F., Fareed J. Is the reason of increased D-dimer levels in COVID-19 because of ACE-2-induced apoptosis in endothelium? Clin. Appl. Thromb. Hemost. 2020;26 doi: 10.1177/1076029620935526. 1076029620935526. [DOI] [PMC free article] [PubMed] [Google Scholar]