Abstract
Introduction
Due to Corona Virus disease -19, India saw a surge of mucormycosis cases, associated with high death rate. India, during the month of May to July 2021 saw a surge of mucormycosis from all states, with close to 50,000 cases just in a span of 3 months.
Objective
To examine the histopathological appearances of rhino-orbital/rhino-maxillary/sino-nasal mucormycosis in the backdrop of the ongoing COVID 19 pandemic.
Material and methods
The study involved analysis of 60 biopsy samples of suspected rhino-maxillary /rhino-orbital mucormycosis received from post-COVID-19 patients. A preliminary review of the slides showing hyphal forms of fungal organisms with un-doubtful tissue / mucosal invasion was included. All samples were examined under Hematoxylin and Eosin stains along with special fungal stains. Data thus obtained were analyzed statistically. Special stains for fungus namely Periodic Acidic Schiff (PAS) and Gomori Methenamine silver (GMS) were utilized to confirm and/or to differentiate the fungal organisms and to highlight the cell wall of the fungus.
Results
The mean age of the patients with mucormycosis was 51.68 years and 72 (83.33%) of them were males. Acute type of inflammation was noted in 44 (73.33%), granulomatous inflammation in 14 (23.33%) of cases. Bony invasion and perineural invasion was observed in 5 (8.33%) and 55 (91.67%) cases, respectively. The dominant fungus were mucorales in 58 (96.67%), aspergillous, along with mucorales in 12 (20%) and combination of mucorales and candida identified in 8 (13.33%) cases.
Conclusion
Besides all the histological appearance of angioinvasion, bone, and soft tissue invasion, a notable aspect was the shift in inflammatory pattern, which was more granulomatous in nature, with a decrease in fungal load correlating with the drop of COVID second wave. This proves that as immunity develops, the host's response to secondary opportunistic infections changes.
Keywords: Mucorales, Mucormycosis / microbiology, COVID-19, SARS-CoV-2, Mycoses / complications
1. Introduction
India is affected with resurging waves of Corona Virus disease -19 (COVID 19), ever since the World Health Organization (WHO) declared it as a pandemic in March 2020 [1], [2]. The infection continues relentlessly to become an ongoing public health problem affecting millions of people [1].
The COVID 19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), chiefly infects but not limited to the lungs, causing pneumonia, cardiovascular and even neurological disorders. About 7% of the individuals may develop co-infection with other microorganisms such as fungi or bacteria, further complicating the disease [3]. Clinical spectrum of COVID infection may range from the patient being totally symptom free to severe lung disease, occasionally requiring hospital admission or monitoring in the Intensive care units (ICU) in roughly about 5% of patients [4]. Severe lung infection may sometimes mandate the use of immunomodulatory drugs to manage immune dysregulation as well as cytokine storm [4]. The above conditions, like ICU admission, dependent ventilation, administration of corticosteroids along with other causes of immunosuppression may render an individual vulnerable for the development of fungal opportunistic infections [5], [6].
Fungi of the order mucorales belonging to the phyomycetes class are implicated in a potentially invasive and often fatal opportunistic infection called the Mucormycosis [7]. Due to COVID-19, India saw a surge of mucormycosis, which was associated with high death rate. It was further complicated with reduced availability of antifungal medications. India, during the month of May to July 2021 saw unexpectedly high reports of mucormycosis from all states, with a close to 50,000 cases just in a span of 3 months [8], [9]. The highest incidence of mucormycosis came from West of India followed by South India [9].
According to a report, mucormycosis may develop in 0.25% of COVID-19 patients, anywhere between 15 and 30 days, post infection [6]. This particular disease also received a lot of media attention as “Black fungus” owing to the color of the necrotic tissue [6], [10].
The Department of surgical Pathology involved in this research, during the second wave of the pandemic, started to receive several emergency biopsies of mucormycosis. Varied histopathological observations led to introspect and correlate the spectrum of these appearances with the pandemic, which was coursing through. The question that needed to be answered was, if the immunity developing in the community played a role in the varied histological picture that was observed?
Untreated rhinosinus mucormycosis can develop to cavernous sinus thrombosis and cerebral invasion and the primary aspects of effective care of this fatal infection include early identification, surgical debridement, appropriate antifungal medication, and control of risk factors such as diabetes mellitus. Histoptahological examination plays a key role in diagnosing the mucormycosis [11]. Therefore, the current research was initiated with the objective to examine the histopathological images of rhino-orbital / rhino-maxillary/sino-nasal mucormycosis in the backdrop of ongoing COVID 19 pandemic.
2. Material and methods
This was an observational study conducted in the Department of General Pathology of a tertiary care teaching hospital from April – June 2021. Institutional Ethics committee approval was obtained, prior to the start of the research. (PSG/IHEC/2021/App/Exp/160).
The study involved analysis of all biopsy samples of suspected rhino-maxillary /rhino-orbital mucormycosis received in post COVID -19 patients. Clinical details were retrieved from hospital information system. The demographic details like, patients age, sex, diabetic status, glycemic control and COVID 19 status were obtained. A preliminary review of the slides showing hyphal forms of fungal organisms with un-doubtful tissue / mucosal invasion were included. Samples received from non-COVID patients were excluded from the study.
Tissues samples were examined macroscopically, processed as per the standard protocols and routine Hematoxylin & Eosin (H&E) staining was done to inspect the samples. Special stains for fungus namely Periodic Acidic Schiff (PAS) and Gomori Methenamine silver (GMS) were utilized to confirm and/or to differentiate the fungal organisms and to highlight the cell wall of the fungus.
Mucorales genera were identified based on the characteristic histological findings like non-pigmented, wide (5–20 µm), thin walled, ribbon-like hyphae with pauci septations or aseptate, and right angle branching. On H&E staining, under 40 x magnification,hyphae will be empty looking as has been described in the literature [12], [13]. Aspergillus species was demonstrated by nonpigmented (hyaline), narrow, septated hyphae with acute-angle branching. Candida species were diagnosed based on the morphological findings such as yeasts (3–5 µm) forms of smaller size admixed with pseudohyphae and/or hyphae forms [14].
Two pathologists independently analyzed the histopathological slides and reached consensus. Parameters noted were site of biopsy, type of inflammation as acute, chronic, mixed suppurative and granulomatous. Inflammation was graded as 1- mild, 2-moderate and 3 - dense. Further, the presence of necrosis, fibrino purulent exudates, angio, soft tissue, neural and bony invasion were studied.
The data thus obtained was analyzed using R software version 4.1.2 and Microsoft Excel 2016 for Windows. Categorical variables were represented in the form of frequency table and Continuous variables as Mean ± SD/ Median (Min, Max) form.
3. Results
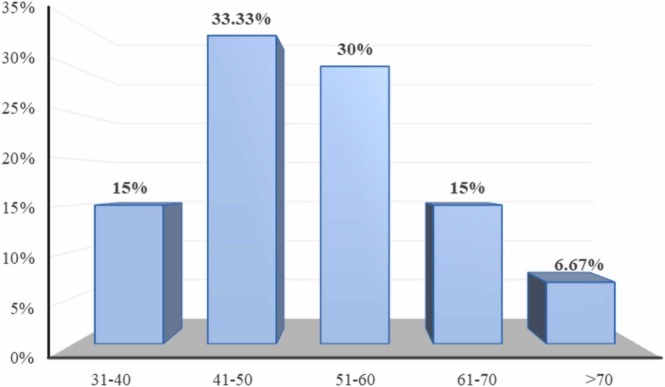
Between the study periods of 3 months (April - June 2021) biopsy samples from sixty patients with post COVID infections were studied. The samples received were from patients with a wide age range, ranging from 30 years to more than 70 years and the mean age was 51.68 ± 10.7 years. There was a predominance of males (83.33%) affected with mucormycosis. The age and sex distribution is represented on Figs. 1 & 2.
Fig. 1.
Distribution of subjects according to age.
Fig. 2.
Distribution of subjects according to gender.
All patients were confirmed COVID 19 positive and were diabetics. with an elevated glycosylated hemoglobin (HbA1C) in 88% of the individuals. Macroscopic examination revealed the tissue samples to be predominantly gray-white to black in color. Tissue necrosis was observed in 71.67% of cases.
The predominant type of inflammatory response observed was mixed suppurative (73.33%), followed by acute (21.7%) and the least was chronic type (5%). Inflammatory infiltrate was mainly comprised of neutrophils, eosinophils, lymphocytes, macrophages and plasma cells. Neutrophilic infiltration was severe in 50 cases, moderate in 8 cases and mild in 2 cases. Patchy reactive lymphoid aggregates and multinucleated giant cell response were noted in a few cases.
Inflammatory exudates in the samples were noted in 56.67% of cases while abscess formation was noted only in 3.33% of cases (n = 2).
The dominant fungus identified in the specimens were mucorales in 58 samples (96.67%), aspergillous, along with mucorales was identified in 12 samples (20%) where as a combination of mucorales and candida was noted in 8 cases.(13.33%).
Microscopic sections revealed wide (5–20 µm), thin walled, ribbon – like hyphae with pauci septations and right angle branching morphologically consistent with mucorales on H&E staining. Sections with fragmented, folded and thick-walled spherical structures were also observed. Special stains were done (PAS &GMS) in some of the cases with sparse fungal load to demonstrate the fragmented/ sparse fungal wall and also the vessel wall invasion. by the fungus.
Amount of fungal hyphal elements was identified more in the necrotic tissues. Inflammatory exudates predominantly of neutrophils, areas of haemorrhage and thrombosis were exhibited in the surrounding tissue structures.
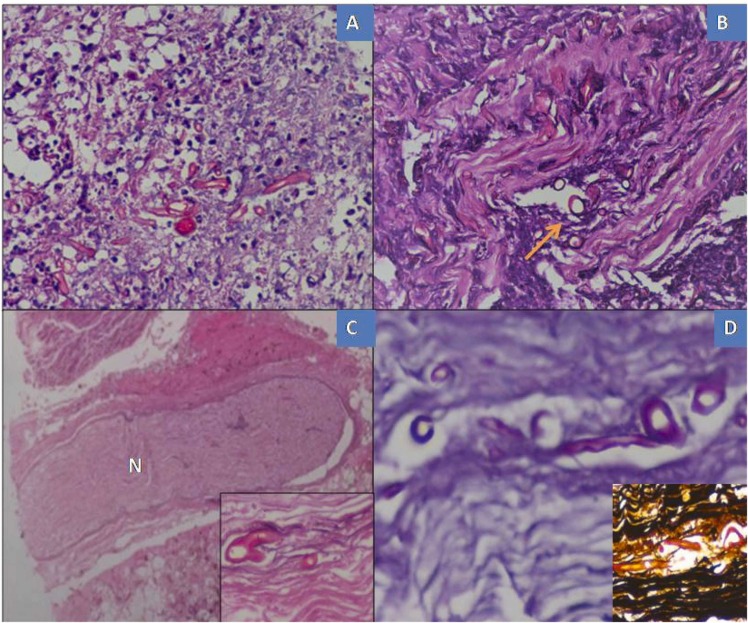
The histological findings of mucorales genera and special stains (PAS &GMS) are represented on Fig. 3A–D.
Fig.: 3.
Histological findings of mucorales genera. (A)Microscopy sections of nasal mucosa with dense fungal growth by broad aseptate hyphae of mucor and areas of necrosis(B) Angioinvasion by the fungal organisms (indicated by yellow arrow), (haematoxylin-eosin stain, X40). (C) Neural bundle (N) invasion (inset view, x40)& (D) PAS stain & Gomori methenamine silver stain (inset) highlights the fungal organisms, X40. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Sections with granulomatous inflammation consisting chiefly of multinucleate giant cells and surrounded by cuff of neutrophils, eosinophils, lymphocytes and plasma cells. These were noted in 23.33% of cases and were predominantly of rhino- maxillary specimens. Cytoplasm of giant cells containing fragmented fungal organism and refractile crystalline bodies was observed in a few cases, represented on Fig. 4A & B. No central necrosis was observed in the granulomas. The number of fungal organisms noted in these cases was less when compared to non-granulomatous inflammatory response type cases.
Fig. 4.
Cytoplasm of giant cells containing fragmented fungal organism and refractile crystalline bodies (A)Section shows granulomas formed by multinucleate giant cells and a few fragmented fungal organisms (indicated by red arrow). (B) Refractile crystalline bodies (indicated by yellow arrow). (C) Combined infection by mucor & aspergillus. (D)Fruiting bodies with black color pigmentation (haematoxylin-eosin stain, X40). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Angio invasion was identified in 71.67% of samples. It was evident by the presence of fragmented fungal elements invading the blood vessel wall or inside their lumen with thrombi formation. Adjacent tissue shows areas of ischemic necrosis. Special stains were done in difficult cases to highlight the elastic lamina of the vessel wall and also to identify the fragmented fungal structures.
Tissue necrosis was identified by granular basophilic material with karyorrhectic debris. Tissue invasion was noted in 85% of the samples with extensive areas of mucosal tissue necrosis. Bony invasion seen in 10% of the samples and were evident by the presence of hyphal structures in the trabecular marrow spaces.
Perineural / neural invasion was identified in 8.33% of samples with the invasion of the nerve bundles by fragmented fungal hyphae observed in the periorbital soft tissues.
Cases with combined mucor, candida and or aspergillus show similar tissue reactions with the predominance of neutrophilic response. One case of mucor with aspergillus niger showed aspergilloma. It is composed of an extracellular fungal ball surrounded by granulation tissue and an outer layer of fibrosis. Many black pigmented fruiting bodies and oxalate crystalloids were noticed in combined mucor and aspergillus infection. We did not encounter Splendore- Hoeppli phenomenon, bony/vascular invasion by Aspergillus/ Candida species.
Table 1 summarizes the invasion pattern of the infection and Table 2 summarizes the type and grading of inflammation. The distribution of cases exhibiting inflammatory exudate and abscess is summarized in Table 3. Black pigmented fruiting bodies noticed in combined mucor and aspergillus infection is represented on Fig. 4C and D.
Table 1.
Distribution of invasion pattern of the infection.
| Variables | Sub category | Number of subjects (%) |
|---|---|---|
| Tissue invasion | Scanty tissue | 4 (6.67%) |
| Absent | 5 (8.33%) | |
| Present | 51 (85%) | |
| Angio Invasion | Scanty tissue | 4 (6.67%) |
| Absent | 13 (21.67%) | |
| Present | 43 (71.67%) | |
| Bony invasion | Absent | 54 (90%) |
| Present | 6 (10%) | |
| Perineural invasion | Absent | 55 (91.67%) |
| Present | 5 (8.33%) |
Table 2.
Distribution of type and grading of inflammation along with type of organism.
| Variables | Sub category | Number of subjects (%) |
|---|---|---|
| Granuloma | Absent | 46 (76.67%) |
| Present | 14 (23.33%) | |
| Type of Inflammation | Acute | 13 (21.67%) |
| Chronic | 3 (5%) | |
| Mixed | 44 (73.33%) | |
| Necrosis | Scanty tissue | 1 (1.67%) |
| Absent | 16 (26.67%) | |
| Present | 43 (71.67%) | |
| Grading of inflammation | Mild | 2 (3.33%) |
| Moderate | 8 (13.33%) | |
| Severe | 50 (83.33%) | |
| Mixed/Only mucormycosis | Aspergillus | 12 (20%) |
| Mucormycosis | 58 (96.67%) | |
| Candida | 8 (13.33%) |
Table 3.
Distribution of cases exhibiting inflammatory exudate and abscess.
| Variables | Sub category | Number of subjects (%) |
|---|---|---|
| Inflammatory exudate | Absent | 26 (43.33%) |
| Present | 34 (56.67%) | |
| Abscess | Absent | 58 (96.67%) |
| Present | 2 (3.33%) |
Our study group showed pathognomonic fungal morphology with the evidence of tissue necrosis, angio invasion and perineural invasion. Tissue inflammatory responses encountered were predominantly suppurative inflammation, mainly of neutrophils and granulomas formation. Fungal ball formation and sporule formation were less noticed. No Splendore-Hoeppli phenomenon was observed. All these case findings were described as histopathological characteristics to diagnose mucormycosis and were similar to non- COVID mucormycosis. Except the formation of granulomas were observed in immunocompetent individuals of non-COVID mucormycosis.
4. Discussion
India was one of the worst hit countries with COVID and by the end of July 2021 had reported about 31 million cases [10]. It is now clear that COVID 19 weakens the host’s immune system, making way for opportunistic secondary infections. Patients having recovered from COVID may develop fungal infections after a few weeks or months. COVID associated mucormycosis (CAM) may form about 0.3% of all co-infections [10].
Many states in India saw a sudden up surge of mucormycosis cases during the COVID second wave and it was declared an epidemic in the midst of an ongoing pandemic. The most common type noted clinically was that of Rhino-orbital-cerebral mucormycosis (CAROCM) [10], [15].
Mucormycosis comprises a group of infections caused by the fungi belonging to the order Mucorales and family Mucoraceae. Rhizopus oryzae is the most common cause of infection. They are seen in soil and decaying matter and are transmitted by airborne asexual spores [16].
Mucormycosis infiltrates blood vessels and bone trabeculae as wide, aseptate or slightly septate ribbon-like hyphae ranging from 5 µ to 20 µm. Septate hyphae with acute-angle branching ranging from 3 to 5 µm appear as Aspergillus sp. or other hyaline moulds. These organisms can be spotted on microscopy in sites of suppurative tissue necrosis [17].
The spores of mucormycosis may gain entry into the human body via inhalation, ingestion or inoculation into an open wound, subsequently germinating inside the host into angioinvasive hyphae [2], [10].
The symptoms that most patients with CAROCM experience include facial pain and swelling, loss of vision, periorbital edema, etc. When the spores gain entry via inhalation, they enter the paranasal sinuses which later spread into the orbit and cerebrum. The fungi cause vascular invasion, thrombosis and necrosis [2], [10], [18], [19].
Various hypothesis proposed for the pathogenesis of CAROCM include the COVID associated lymphopenia, increase in pro-inflammatory markers, pulmonary damage, and hyperferretinemia, all of which favor fungal growth [2], [10], [20] Additionally, diabetic ketoacidosis, free radical induced endothelial inflammation and hepcidin activation are all suggested in the pathogenesis of COVID mucormycosis [21].
Certain predisposing factors identified for CAROCM include uncontrolled diabetes mellitus, use of systemic corticosteroids, prophylactic broad-spectrum antibiotics that disrupt the nasal commensals enabling invasion of pathogenic fungi, immune-suppressive medications and diseases, organ transplant and hematological malignancies [2], [9], [10], [18]. Interestingly, inhalation of steam, which was widely advised my many practitioners in India to relieve nasal congestion in COVID, was found to damage the delicate nasal mucosa facilitating mucorale invasion [22].
Chouhan et al. in their research found 41 patients who satisfied the diagnostic criteria of CAROCM and accordingly, had a male preponderance (n = 28), 36 patients had a history of steroid use, 40 patients were diabetics and majority of them had received oxygen while in the hospital [18]. The current study had a similar male preponderance. (88.33%) Two systematic reviews conducted in the past on CAROCM have identified diabetes mellitus as the most common comorbidity followed by hypertension. Corticosteroid usage was identified in majority of the cases [23], [24]. Similar results were obtained by Sharma et al. in 23 patients with mucormycosis affecting the paranasal sinuses [25]. The biopsy samples obtained from the patients in the present research were also diabetics.
Frater et al. have also described histopathological findings in mucormycosis, where entirely neutrophilic response was seen in 50% of the cases, 25% had pyogranulomatous response, 5% showed only granulomatous response and 20% did not exhibit any inflammation. They further observed angioinvasion in 100% of cases and perinural inflammation in 90% of cases. They opined that since loose facial sheath surrounds the nerve, perineural invasion of the fungi could take place with ease [26].
Arora et al. studied the histopathological features of COVID associated rhino cerebral mucormycosis and the median age of the subjects was 57 years. Majority of the patients were males, steroids had been used in 45% of the cases and diabetes mellitus was the predisposing factor in 98% of the cases. Out of the 37-biopsy samples, soft tissue invasion was noted in 59%, necrosis with no cellular response was noted in 43%, acute suppuration in 5% of cases (n = 2), granulomatous inflammation in 11% of the cases [27]. The results of the present study closely match the above study with age, sex and predisposing factor being similar. Soft tissue invasion was seen in a higher percentage with 85% of the specimens, abscess formation in the present study was also noted in only two cases (3.33%).
In a research by Jain et al. of COVID associated rhino cerebral mucormycosis, angioinvasion was noted in 100% of the cases (n = 95), soft tissue invasion in 58% of cases, bone invasion in 6.3% of case and nine cases showed presence of peripheral nerves and 3 out of those 9 cases showed perineural invasion. They also observed giant cell reaction in 33.3% of cases while necrosis was evident in more than 50% of the cases [15]. The results of the present study is similar to the above study with respect to invasions, except that the percentage of involvement was much higher in the present study. This could be due to a higher percentage of the patients with immunocompromised status.
Jain et al. also observed that out of 90 cases of COVID associated rhino cerebral mucormycosis, mixed infection with aspergillus was noted in one patient and with candida in two cases [15]. Pal et al. also found four cases of mucormycosis along with aspergillosis, in 99 patients of COVID associated mucormycosis. [23] In the present study, mixed infection with aspergillus was noted in 20% of cases and with candida in 13.33% of cases. Present research identified higher percentage of candida and aspergillus mixed infection, which could be due to the environmental and geographical difference in the distribution of the fungi. One case of mucormycosis with aspergillus niger, in the present research showed aspergilloma comprising of extracellular fungal ball surrounded by granulation tissue and outer layer of fibrosis.
A multicentric study by Patel A et al. compared mucormycosis with and without COVID association and identified 287 cases, 65.2% of cases were COVID associated and 62.7% of them were diabetics. They found rhino-orbital type to be the most common type and they did not find any mixed fungi [28]. Similar results are also described by Ramadorai et al. [29]. Pakdel et al. in their study found Rhinomaxillary-orbital type to be most common (47%) followed by sino-orbital type (33%) and sino-nasal type was least common type (7%) [30]. In the present research, there was a higher percentage of patients with elevated glycosylated hemoglobin.(88%) The sino-nasal type was the most common site of involvement (76%) followed by rhino-orbital involvement.
Cornely et al. have described the histopathological picture of mucormycosis and accordingly the acute lesions show hemorrhagic necrosis, angioinvasion, coagulative necrosis, neutrophilic infiltration and perineural invasion. Chronic lesion on the other hand, shows a pyogranulomatous inflammation with giant cells along with a deeply eospinophilic material surrounding the pathogen, the Splendore-Hoeppli phenomenon [12]. The mucormycosis specimens in the current research showed similar classic features such as angioinvasion, granulomatous inflammation along with perineural invasion.
Mucormycosis cases in India during the second wave was estimated to be 70 times higher than the worldwide incidence and there was a sudden increase during the period between April and July 2021 [15], [28]. The same trend was noticed in the present research as well. Interestingly and additionally, the present research identified a change in the histopathological appearance of mucormycosis during the month of May 2021 which was predominantly necrotic tissue with abundant fungal organisms. When the pandemic started to decline, during the month of June 2021, the histopathology showed more of granulomatous type of inflammation with fewer organisms.
Literature does not mention the kind of difference in histopathological appearance of mucormycosis during the rise and fall of the second wave of COVID 19 that was evident in the present research. The varying pattern of inflammation observed in the present study could explain the virulence of the organisms, type of viral strain and the immune status of the patient. The prognosis is generally good for patients with enhanced immune mechanisms, which leads to the formation of granulomatous inflammation with low fungal load. Presentation of mucormycosis infection with granulomatous inflammation is very rare and only a few anecdotes are available [3], [31]. In the current research, 23% of the cases showed granulomatous inflammation with fungi in the cytoplasm. Interestingly majority of these cases were reported in the late month of May and June when the pandemic infection was showing a downtrend.
Certain immune-related factors in COVID-19 patients may allow subsequent opportunistic fungal infections such as mucormycosis to develop. COVID-19-related immunological dysregulation causes a reduction in T lymphocytes, CD4 +T, and CD8 +T cells, potentially altering innate immunity. Interleukin (IL) 4, IL-10, IL-17, and Interferon-gamma (IFN-g) are cytokines produced by CD4 + and CD8 + cells in response to fungal hyphae. The 'cytokine storm' may be exacerbated by the delayed IFN-γ response, extended hyperinflammatory state, and reduced CD4 and CD8 cell counts, which may enhance the severity of COVID-19 infection [14].
5. Limitations
This study was a histopathology based research and hence comparison with culture was not done, although almost all the samples showed positive culture for mucorales. A few limitations that were noted in the present research include the absence of recording and correlating clinical signs and symptoms of mucormycosis, and correlation with radiographic findings. Additionally, hospital or intensive care unit admission, receipt of oxygen, systemic disorders and other co-morbidities that the patients had, additional medications and drug history, vaccination status of the patients, evaluation of management and outcome/death were not included. Further research should be directed towards inclusion of these additional parameters to obtain a corroborative result.
6. Conclusion
The present research was formulated, noticing an abrupt rise of mucormycosis cases during the second wave of COVID-19. This debilitating infection was predominantly associated with diabetes mellitus. The expected histopathological appearance of angioinvasion, bone and soft tissue invasion was very well evident along with a higher percentage of perineural invasion during the rise of the pandemic. The unanticipated feature was the alteration of inflammatory pattern, which was more of granulomatous type with reduction in tissue and vascular invasion coinciding the decline of the COVID second wave. This substantiates that as immunity evolves, the host response varies for secondary opportunistic infections.
Authors contribution
Nidhya Ganesan: Concept, design, data collection & analysis, manuscript writing and final draft approval. Shanthakumari S: Concept, design, manuscript writing and final draft approval.
Conflict of Interest
Authors have declared that no financial / conflict of interest to disclose.
Acknowledgement
We express our gratitude to departments of Otorhinolaryngology, Medicine and Ophthalmology, PSG Institute of medical Sciences and Research, Coimbatore for their effort during the pandemic and associating us in patient management.
References
- 1.Selarka L., Sharma S., Saini D., Sharma S., Batra A., Waghmare V.T., et al. Mucormycosis and COVID-19: an epidemic within a pandemic in India. Mycoses. 2021;64(10):1253–1260. doi: 10.1111/myc.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt K., Agolli A., Patel M.H., Garimella R., Devi M., Garcia E., et al. High mortality co-infections of COVID-19 patients: mucormycosis and other fungal infections. Discoveries. 2021;9(1) doi: 10.15190/d.2021.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kakamad F.H., Mahmood S.O., Rahim H.M., Abdulla B.A., Abdullah H.O., Othman S., et al. Post covid-19 invasive pulmonary Aspergillosis: a case report. Int J. Surg. Case Rep. 2021;82 doi: 10.1016/j.ijscr.2021.105865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Tawfiq J.A., Alhumaid S., Alshukairi A.N., Temsah M.H., Barry M., Al Mutair A., et al. COVID-19 and mucormycosis superinfection: the perfect storm. Infection. 2021;49(5):833–853. doi: 10.1007/s15010-021-01670-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avatef Fazeli M., Rezaei L., Javadirad E., Iranfar K., Khosravi A., Amini Saman J., et al. Increased incidence of rhino-orbital mucormycosis in an educational therapeutic hospital during the COVID-19 pandemic in western Iran: an observational study. Mycoses. 2021;64(11):1366–1377. doi: 10.1111/myc.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thakar A., Lal D. “Black fungus”: a perspective on the coronavirus disease 2019 (COVID-19)-associated rhino-orbital mucormycosis epidemic in India. Int Forum Allergy Rhinol. 2021;8:1278–1279. doi: 10.1002/alr.22855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meher R., Wadhwa V., Kumar V., Shisha Phanbuh D., Sharma R., et al. COVID associated mucormycosis: A preliminary study from a dedicated COVID Hospital in Delhi. Am. J. Otolaryngol. 2022;43(1) doi: 10.1016/j.amjoto.2021.103220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muthu V., Rudramurthy S.M., Chakrabarti A., Agarwal R. Epidemiology and pathophysiology of COVID-19-associated mucormycosis: india versus the rest of the world. Mycopathologia. 2021;186(6):739–754. doi: 10.1007/s11046-021-00584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biswal M., Gupta P., Kanaujia R., Kaur K., Kaur H., Vyas A., et al. Evaluation of hospital environment for presence of Mucorales during COVID-19 associated mucormycosis outbreak in India–A multi-centre study. J. Hosp. Infect. 2022;S0195–6701(22) doi: 10.1016/j.jhin.2022.01.016. 00031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aranjani J.M., Manuel A., Abdul Razack H.I., Mathew S.T. COVID-19-associated mucormycosis: evidence-based critical review of an emerging infection burden during the pandemic’s second wave in India. PLoS Negl. Trop. Dis. 2021;15(11) doi: 10.1371/journal.pntd.0009921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta D.P., Gupta S., Shah C.K., Sreevidya S.R. Clinical study of surge of mucormycosis in COVID-19 pandemic: a Tertiary Care Center Study [published online ahead of print, 2021 Aug 4] Indian J. Otolaryngol. Head. Neck Surg. 2021:1–8. doi: 10.1007/s12070-021-02784-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornely O.A., Alastruey-Izquierdo A., Arenz D., Chen S.C.A., Dannaoui E., Hochhegger B., et al. Mucormycosis ECMM MSG Global Guideline Writing Group Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019;19(12):e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karanth M., Taniere P., Barraclough J., et al. A rare presentation of zygomycosis (mucormycosis) and review of the literature. J. Clin. Pathol. 2005;58:879–881. doi: 10.1136/jcp.2004.021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guarner J., Brandt M.E. Histopathologic diagnosis of fungal infections in the 21st century. Clin. Microbiol Rev. 2011;24(2):247–280. doi: 10.1128/CMR.00053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain K., Surana A., Choudhary T.S., Vaidya S., Nandedkar S., Purohit M. Clinical and histology features as predictor of severity of mucormycosis in post-COVID-19 patients: an experience from a rural tertiary setting in Central India. SAGE Open Med. 2022;10 doi: 10.1177/20503121221074785. 20503121221074785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anyapu O., Parvatini S., Kalivarapu P., Kuna R., Suvvari J. Surge and scare of mucormycosis in the backdrop of COVID-19 pandemic. J. Clin. Diagn. Res. 2022;16(3):EC15–EC18. [Google Scholar]
- 17.Kandasamy S., Muthuraju S., Vasugi A., Chandrasekar M., Murugan R., Inbasekaran P., R P. Clinicopathological study of mucormycosis in COVID-19 patients: experience from a tertiary care center in South India. Cureus. 2022;14(3) doi: 10.7759/cureus.23016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chouhan M., Solanki B., Shakrawal N. Rhino-orbital-cerebral mucormycosis: fungal epidemic in a viral pandemic. J. Laryngol. Otol. 2021;135(11):981–986. doi: 10.1017/S0022215121002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reid G., Lynch J.P., 3rd, Fishbein M.C., Clark N.M. Mucormycosis. Semin Respir. Crit. Care Med. 2020;41(1):99–114. doi: 10.1055/s-0039-3401992. [DOI] [PubMed] [Google Scholar]
- 20.Rocha I.C.N., Hasan M.M., Goyal S., Patel T., Jain S., Ghosh A., et al. COVID-19 and mucormycosis syndemic: double health threat to a collapsing healthcare system in India. Trop. Med. Int. Health. 2021;26(9):1016–1018. doi: 10.1111/tmi.13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jose A., Singh S., Roychoudhury A., Kholakiya Y., Arya S., Roychoudhury S. Current understanding in the pathophysiology of SARS-CoV-2-associated rhino-orbito-cerebral mucormycosis: a comprehensive review. J. Maxillofac. Oral. Surg. 2021;20(3):373–380. doi: 10.1007/s12663-021-01604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar S.K., Chandran S., Mohanty A., Jha M.K. Black fungus: possible causes of surge in cases in the second pandemic in India. J. Fam. Med. Prim. Care. 2021;10(11):4322–4323. doi: 10.4103/jfmpc.jfmpc_1183_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pal R., Singh B., Bhadada S.K., Banerjee M., Bhogal R.S., Hage N., et al. COVID-19-associated mucormycosis: an updated systematic review of literature. Mycoses. 2021;64(12):1452–1459. doi: 10.1111/myc.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh A.K., Singh R., Joshi S.R., Misra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab. Syndr. 2021;15(4) doi: 10.1016/j.dsx.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma S., Grover M., Bhargava S., Samdani S., Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J. Laryngol. Otol. 2021;135(5):442–447. doi: 10.1017/S0022215121000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frater J.L., Hall G.S., Procop G.W. Histologic features of zygomycosis: emphasis on perineural invasion and fungal morphology. Arch. Pathol. Lab. Med. 2001;125(3):375–378. doi: 10.5858/2001-125-0375-HFOZ. [DOI] [PubMed] [Google Scholar]
- 27.Arora R., Goel R., Khanam S., Kumar S., Shah S., Singh S., et al. Rhino-orbito-cerebral-mucormycosis during the COVID-19 second wave in 2021 - a preliminary report from a single hospital. Clin. Ophthalmol. 2021;15:3505–3514. doi: 10.2147/OPTH.S324977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel A., Agarwal R., Rudramurthy S.M., Shevkani M., Xess I., Sharma R., et al. Multicenter epidemiologic study of coronavirus disease–associated mucormycosis, India. Emerg. Infect. Dis. 2021;27(9):2349–2359. doi: 10.3201/eid2709.210934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramadorai A., Ravi P., Narayanan V. Rhinocerebral mucormycosis: a prospective analysis of an effective treatment protocol. Ann. Maxillofac. Surg. 2019;9(1):192–196. doi: 10.4103/ams.ams_231_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pakdel F., Ahmadikia K., Salehi M., Tabari A., Jafari R., Mehrparvar G., et al. Mucormycosis in patients with COVID-19: a cross-sectional descriptive multicentre study from Iran. Mycoses. 2021;64(10):1238–1252. doi: 10.1111/myc.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chi Z., Zhang C., Wang W. Granulomatous mucormycosis of the temporal bone extending into temporomandibular joint and infratemporal fossa: a case report. Ear Nose Throat J. 2021 doi: 10.1177/01455613211022079. 1455613211022079. [DOI] [PubMed] [Google Scholar]