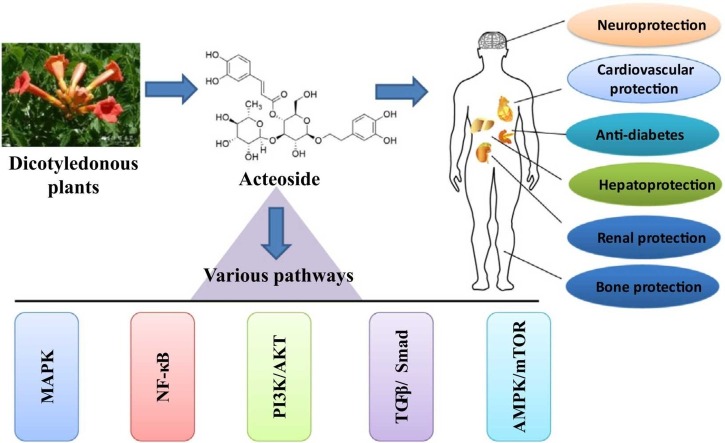
Abstract
Acteoside (AC), a phenylpropanoid glycoside isolated from many dicotyledonous plants, has been demonstrated various pharmacological activities, including anti-oxidation, anti-inflammation, anti-cancer, neuroprotection, cardiovascular protection, anti-diabetes, bone and cartilage protection, hepatoprotection, and anti-microorganism. However, AC has a poor bioavailability, which can be potentially improved by different strategies. The health-promoting characteristics of AC can be attributed to its mediation in many signaling pathways, such as MAPK, NF-κB, PI3K/AKT, TGFβ/Smad, and AMPK/mTOR. Interestingly, docking simulation study indicates that AC can be an effective candidate to inhibit the activity of SARS-CoV2 main protease and protect against COVID-19. Many clinical trials for AC have been investigated, and it shows great potentials in drug development.
Keywords: Acteoside, Anti-oxidation, Anti-inflammation, Neuroprotection
Graphical Abstract
1. Introduction
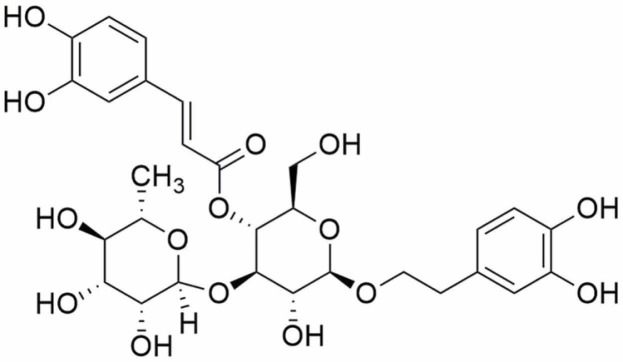
Acteoside (AC, also named as verbascoside or kusagin), [β-(3,4-dihydroxyphenylethyl)-O-α-L-rhamnopyr-anosyl-(1→3)-β-D-(4-O-caffeoyl)-glucopyranoside], is abundant in many dicotyledonous plants, such as Oleaceae, Bignoniaceae, Verbenaceae, and Labiatae [1]. Chemically, AC ( Fig. 1), a formula of C29H36O15 and a molecular weight of 624 daltons, is a phenylpropanoid glycoside featured as a binding of caffeic acid and hydroxytyrosol to a glucose moiety by forming ester and glycosidic bonds, respectively [2]. As a bioactive natural ingredient, AC is an important secondary metabolite in medicinal plants [3]. The biosynthesis, including natural and transgenic technology-modified, of AC has been reviewed [4]. AC is susceptible to be hydrolyzed in gastrointestinal ducts and degraded into several products before being absorbed into the blood [5]. AC is chemically stable at pH3 after 24 h in two human intestinal cell lines (HT-29 and Caco-2). However, it may be transformed (up to 62.4 %) into isoverbascoside or other oxidative products at pH7. This chemical transformation can significantly decrease the activity in the anti-oxidant assays in HT-29 and Caco-2 cells [6].
Fig. 1.
The chemical structure of AC.
It has been demonstrated the association between the biological effects of phenolic compounds and their affinity and distribution in the lipid membranes. AC has a great affinity for phospholipid membranes, particularly to those formed by phospholipids with negative charge. The caffeoyl moiety of AC locates deep inside the phospholipid palisade in phosphatidylcholine (PC) but not phosphatidylglycerol (PG) membranes. AC forms a stabilized phospholipid complex at a high concentration and perturbs PC membranes resulting in formation of different membrane domains. Interestingly, AC increases vesicle curvature and decreases the particle size of PG unilamellar vesicles through increasing the phospholipid head group area [7]. AC exhibits protective activity against free radical-induced oxidation of low-density lipoprotein (LDL), blocking the development of atherosclerosis [8]. The anti-oxidative activity against DPPH radicals and the inhibitory activity against Cu2+-induced LDL lipid peroxidation by AC have been reported with IC50 values of 19.89 μg/mL and 63.31 μg/mL, respectively [9]. The neuroprotective activity of AC is also reported by inhibiting the activity of NF-κB signaling in Aβ-treated N2a cells [10]. Recently, the reviews on AC have been studied [1], [2], [11], [12], [13]. For example, Khan [11] focuses on the anticancer activity of AC. Srivastava [12] reports the comprehensive review of phytochemistry and pharmacology of Duranta erecta Linn, which can be a significant source of AC. In addition, Srivastava also discusses some pharmacological activities of AC [12]. From the sophisticate literature research, we find that AC has been reported various pharmacological activities, including anti-oxidation [14], anti-inflammation [15], anti-cancer [11], neuroprotection [16], cardiovascular protection [17], anti-diabetes [18], bone and cartilage protection [19], hepatoprotection [20], and anti-microorganism [21]. In this review, we will mainly focus on these fields and summarize the research progress of AC, including the pharmacokinetic profiles. This article will fully elucidate the comprehensive pharmaceutics and pharmacology of AC, providing a novel platform for discussing and developing AC as a potential candidate in the therapeutic management of diseases.
2. Literature search strategy
The search terms “acteoside”, “verbascoside”, and “kusagin” were employed to obtain the necessary information from electronic database, including Pubmed, Web of Science, Sciencedirect, Wiley, Springerlink, Taylor & Francis, and Oxford Journals. Traditional uses, biological activities, pharmacokinetic characteristics, and clinical trials were summarized.
3. Pharmacokinetic profiles
The pharmacokinetic properties of AC after single intravenous administration can be suitably interpreted by a two-compartmental pharmacokinetic model. The distribution and elimination of half-lives of unbound AC are 5 min and 28 min, respectively, in the blood [22]. After intravenous administration of AC for 15 min in rats, the binding of AC to the protein in the rat plasma is 75.5 % and the bioavailability is only 0.12 %. Furthermore, the C max values by oral (100 mg/kg) and intravenous (3 mg/kg) administration are 0.13 μg/mL and 48.6 μg/mL, respectively. The T 1/2 values are 92.1 min and 10.7 min, respectively [5] ( Table 1). Another study reports that the plasma level of AC is 135.3 ng/mL in rats after oral administration of 10 g/kg Plantago asiatica decoction. The C max value of AC is 135.4 ng/mL, the T max value is 13.3 min, and the AUC is 187.1 μg h/L [23]. In beagle dogs, the absolute bioavailability of AC is about 4 %. After single oral administration of 10 mg/kg, 20 mg/kg, and 40 mg/kg AC, the values of AUC are 47.28 mg min/L, 87.86 mg min/L, and 183.14 mg min/L, respectively. The C max values are 0.42 μg/mL, 0.72 μg/mL, and 1.44 μg/mL, respectively [24].
Table 1.
The metabolism and biological effects of AC in different models.
| Category | Models | Biological effects | Ref. |
|---|---|---|---|
| Metabolism | Rats | The bioavailability is 0.12 %. Cmax can be 0.13 μg/mL (oral, 100 mg/kg) or 48.6 μg/mL (intravenous, 3 mg/kg). | [5] |
| Beagle dogs | The absolute bioavailability is about 4 %. Cmax is 0.42 μg/mL (oral, 10 mg/kg). | [24] | |
| CKD rats | Cmax is 0.31 μg/mL. | [25] | |
| DN rats | Cmax can be 69.08 μg/L or 70.53 μg/L. | [26] | |
| Caco-2, HT-29 | The total accumulation efficiency is about 0.10 %. | [27], [28] | |
| Anti- inflammation | RBL-2H3 | Inhibits the activity of cPLA2 with a Ki value of 5.9 μM. | [15] |
| Raw264.7 | Inhibits iNOS/NO expression by attenuating AP-1activation. | [39] | |
| RAW 264.7, THP-1 | Inhibits free fatty acid-induced COX-2/PGE2 expression. | [40] | |
| A549 | Decreases NF-κB, IL-1β, IL-6, IL-8, and caspase-3/-8/-9, increases NRF2, HO-1, NQO-1, and GCLC. | [42] | |
| U937 | Induces SHP-1 phosphorylation, attenuates the activation of TAK1/JNK/AP-1, and decreases COX and NOS expression. | [44] | |
| Dendritic cells | Increases IL-10 expression by activating AhR expression. | [46] | |
| KU812 | Suppresses MAPK/JNK signaling and inhibits CCL1–4, FCER1A, and NFATC1 expression. | [48] | |
| HMC-1 | Decreases STAT5/6, IL-6, IL-13, TNFα, and IL-1β expression, down regulates MDM2, and up regulates p53. | [50] | |
| TRPV3- HEK293 |
Selectively inhibits 2-APB-activated human TRPV3 channel with an IC50 value of 14.1 μM. | [53] | |
| Anti-oxidative stress | SY5Y | Protects against Aβ-induced ROS generation and mitochondrial dysfunctions and apoptosis. | [57] |
| Rats | Alleviates I/R-induced increased production of SOD, GSH-Px, TAS, and TT and decreased levels of TOS, OSI, and MDA. | [59] | |
| Sheep oocytes | Reduces ROS production and lipid peroxidation and protects mitochondrial functions. | [64] | |
| Anti-cancers | B16F10 | Attenuates tyrosinase activity and inhibit melanin biosynthesis by activating ERK signaling and down regulating the expression of MITF, tyrosinase, and TRP-1. | [69] |
| 4T1 | Inhibits the proliferative activity (IC50 = 117 μM). | [71] | |
| GBM cells | Inhibits metastasis and promotes cell apoptosis and autophagy by let-7 g-5p/HMGA2/Wnt/β-catenin signaling. | [73] | |
| Caco-2, HCT-116 | Induces G1 cell cycle arrest and increases cell apoptosis by PI3K/AKT signaling. | [78] | |
| Du-145, PC-3 | Decreases HMGB1/RAGE/TGFβI/II/Smad2/3, inhibits EMT. | [79] | |
| Mouse | Inhibits metastasis by decreasing NF-κB/MMP-2 signaling and promotes cell apoptosis by Bcl-2/Bcl-XL. | [82] | |
| JHH-7 | Increases p53 expression and decreases KLK-1, -2, -4, -9, and -10 expression. | [84] | |
| Neuro-protection | Mice | Protects against I/R-induced expression of HIF-1α, NF-κB, and VEGF. | [94] |
| Rats | Decreases Aβ 1–40 production, inhibits Aβ 1–42 oligomerization | [96] | |
| PC12 | Activates NRF2/HO-1 signaling, protects neuron against Aβ-induced injury. | [98] | |
| BV-2 | Acts as an inhibitor of NF-κB and an agonist of AMPK. | [99] | |
| Rats | Increases Glut1, Glut3, and Glut4 expression, reduces ROS production, and protects against ICV-STZ-induced learning and cognitive impairment. | [102] | |
| Zebrafish | Protects against 6-OHDA-induced movement disorders and dopaminergic neuron death. | [105] | |
| RGC-5 | Protects against H2O2-induced injury by mediating CASC2/miR-155/mTOR signaling. | [110] | |
| Cardio-vascular protection | Rat serum | Inhibits ACE activity with an IC50 value of 365 μM. | [113] |
| H9c2 | Increases mitochondrial biogenesis, inhibits apoptosis. | [115] | |
| Rats | Improves the lipid profiles and the organ coefficients by AMPK/mTOR signaling. | [118] | |
| Anti-diabetes | Caco-2 | Inhibits SGLT1-mediated glucose absorption. | [120] |
| βtc3 cells | Increases insulin biosynthesis and secretion by inhibiting oxidative stress and ERS. | [121] | |
| Bone and cartilage protection | Rats | Inhibits MMP-13, MMP-3, and MMP-1 expression by decreasing MAPK/NF-κB signaling. | [19] |
| Mice | Reduces osteoclastogenesis by attenuation of NF-κB pathway and stimulation of PI3K/AKT pathway. | [125] | |
| MC3T3-E1 | Enhances proliferation and differentiation by increasing IGF-1/BMP/PI3K/mTOR signaling | [127] |
Elucidation of the pharmacokinetic properties is important for the prediction of drug disposition in vivo, and it is useful for drug therapeutic effects evaluation, dose adjustment, and drug’s rational use. In chronic kidney disease (CKD) and normal rats, the AUC0-t values of AC after oral administration of Rehmannia glutinosa extract are 61.15 ± 10.56 μg min/mL and 32.69 ± 7.63 μg min/mL, respectively. The values of C max are 0.31 ± 0.05 μg/mL and 0.19 ± 0.05 μg/mL, respectively. The T max values are 24.00 ± 13.42 min and 15.00 ± 7.45 min, respectively [25] (Table 1). AC is the main effective compound from TLR (leaves of Rehmannia) and DTG (Dihuangye total glycoside capsule) extracts. After oral administration of TLR and DTG, the AUC0-t values of AC in diabetic nephropathy (DN) rats are 324.59 μg/L and 396.81 μg/L, respectively, lower than those in the control rats (1161.45 μg/L and 1465.13 μg/L, respectively). The C max values in DN rats are 69.08 μg/L and 70.53 μg/L, respectively, lower than those in the control rats (109.77 μg/L and 443.03 μg/L, respectively) [26].
In Caco-2 cell models, the rapid absorbance of AC with peak accumulation occurs within 30 min, and the total accumulation efficiency is about 0.10 %. The digestive recovery (bioaccessibility) of AC is 35.5 ± 0.55 % in vitro, indicating potential sensitivity to the gastrointestinal conditions [27]. Consistently, the total accumulation efficiency of AC reaches about 0.12 % in HT-29 cell models [28]. A study reports that AC can be absorbed rapidly in the normal healthy colonic mucosal sections from different individual patients, reaching the highest tissue concentration within 15–30 min. The total accumulation efficiency is also about 0.12 %, which is equal to the mean intracellular level of 0.29 μg/cm2 tissue. Furthermore, the absorption is time-dependently associated with the different colonic segments. Specifically, AC is absorbed in the proximal tract within 5–15 min (0.50 μg/cm2) mainly, in the descending tract within 30 min (0.38 μg/cm2), and in the sigmoid colon within 60 min (0.34 μg/cm2). This discrepancy can be attributed to the specific functional difference the colon segments [29].
The low bioavailability of AC might be related to its low absorption, poor bioaccessibility, and efflux transport. A study shows that the bioaccessibility of AC is 50.1 ± 3.04 % in the digestion model in vitro. The absorption of AC is 0.461–0.698 % in Caco-2 cell models. The value of P app is 4.75 × 10-7 cm/s, indicating low permeability. Moreover, the active efflux by P-glycoprotein (P-gp) is also involved in the permeation mechanism [30]. Consistently, the low permeability of AC in Caco-2 cell models with the P app value of 1.15 × 10-7 cm/s is reported. However, the efflux and active transport are not observed [31]. Promisingly, the bioavailability of AC can be improved by encapsulation with liposomes and chitosan, as shown by the improved pharmacokinetic parameters of chitosan-coated AC liposome (CS-AC-Lip). The C max value for CS-AC-Lip is 0.76 μg/mL at 1.83 h (T max). In contrast, the value for AC is 0.44 μg/mL at 0.54 h (T max) [32]. Another study shows that the oral bioavailability of AC can be enhanced to 1.43-fold by the P-gp inhibitor EGCG. However, EGCG has no effects on the storage and digestion stability of AC [33].
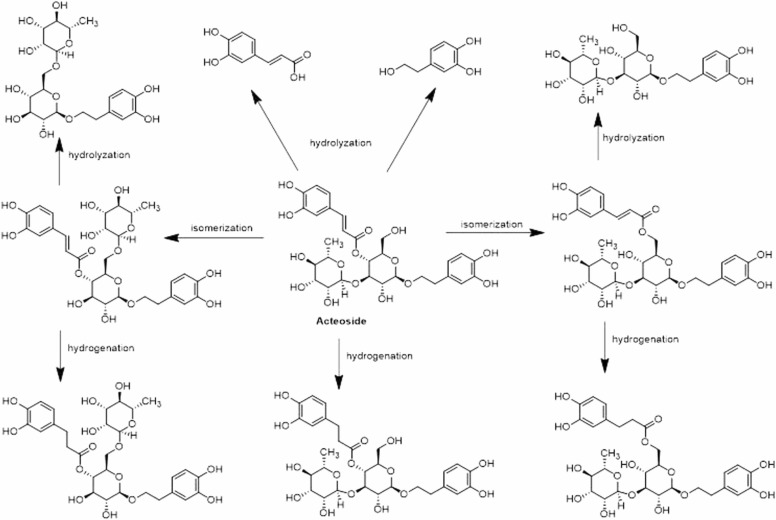
The oxidization, glucuronidation, sulfation, and methyl conjugation are involved in the metabolic processes of AC ( Fig. 2). 19 metabolites of the parent compound and 16 metabolites of the degradation products are verified. AC acts as a prodrug and is hydrolyzed into degradation products, particularly caffeic acid and hydroxytyrosol [34]. Another study shows that 44 metabolites are detected and identified. Among them, 35 compounds are the parent drug metabolites [35]. When incubated with human or rat intestinal bacteria, AC produces 14 metabolites by hydrolyzation, which is the main metabolic pathway. Of these metabolites, they include 8 degradation metabolites, 2 isomers in intestinal bacteria and intestinal enzyme samples, and 4 parent metabolites only found in intestinal enzyme samples [36].
Fig. 2.
The metabolic pathways of AC. AC can be transformed by the reactions of isomerization, hydrolyzation, and hydrogenation.
The toxicity prediction has been explored. The maximum tolerance dose of AC is 0.443 mg/kg/day in human, and the oral acute toxicity and oral chronic toxicity are 2.527 mol/kg and 3.783 mg/kg, respectively, in rats [37]. In V79 cells, AC at the dose of more than 50 μg/mL exhibits cytotoxic effects with IC50 values of 73.80 μg/mL for MTT assays and of 41.42 μg/mL for NRU assays. However, AC does not show any genotoxic activity and phototoxicity [38].
4. Anti-inflammation and immune-mediation
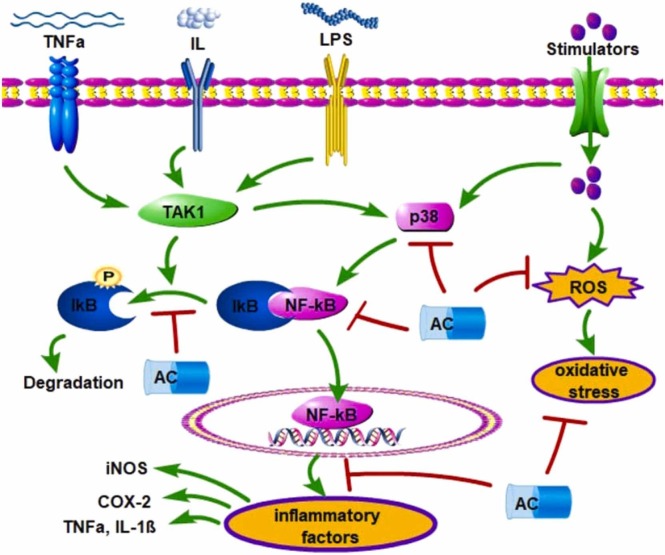
Inflammatory responses include activation of phospholipase A2 (PLA2). Cytosolic Ca2+-dependent PLA2 (cPLA2) and secretory PLA2 (sPLA2) are the members of the PLA2 superfamily. It has been demonstrated that AC can dose-dependently block melittin-induced release of [3H]arachidonic acid by inhibiting the activity of cPLA2, rather than sPLA2, with a K i value of 5.9 μM in RBL-2H3 cells [15]. Nitric oxide (NO) produced by NO synthase (NOS) exhibits double effects. Constitutive NOS (cNOS)-produced NO is essential for the maintenance of cellular functions, and inducible NOS (iNOS)-produced NO is a mediator of acute or chronic inflammation. AC has been demonstrated to inhibit LPS-induced NO release and suppress iNOS mRNA and protein expression by selectively attenuating the activation of AP-1 in Raw264.7 macrophage cells [39]. It is interesting to find that AC can selectively interact with the catalytic cavity of COX-2 and inhibit the production of PGE2, without affecting COX-1 activity in free fatty acid-treated RAW 264.7 and THP-1 macrophage cells [40] ( Fig. 3, Table 1).
Fig. 3.
AC exhibits the inhibitory activity against inflammatory responses and oxidative stress. NF-κB signaling can be activated by the upstream factors, such as ILs, TNFα, LPS, and p38 MAPK. Active NF-κB enters the nucleus to promote the transcriptional expression of inflammatory factors. Extracellular stimulators can also induce ROS production, leading to oxidative stress. These can be effectively abolished by AC treatment.
Anti-inflammation therapy has been considered as an effective strategy for clinical management of mucositis. In methotrexate (MTX)-induced mice mucositis, AC may ameliorate the histological severity scores by 75 %, 78 %, and 88 % in the duodenum, jejunum, and ileum, respectively on day 5, compared with those in the MTX group. In addition, AC decreases the crypt depth by 49 %, 51 %, and 33 %, respectively, and increase the villus height by 19 %, 38 %, and 10 %, respectively. The possible mechanism might be associated with the inhibitory activity of AC against metallothionein by 50 % and against myeloperoxidase by 60 % and 30 % in the duodenum and jejunum, respectively [41]. AC has been shown to dose-dependently ameliorate TNFα-induced IL-1β, IL-6, IL-8, and caspase-3/-8/-9 expression, ROS generation, and cell apoptosis. In addition, AC increases the expression of NRF2, HO-1, NADPH quinone oxidoreductase (NQO-1), and glutamate cysteine ligase catalytic subunit (GCLC) and decreases the activity of NF-κB signaling pathway in TNFα-treated A549 cells [42]. In lipopolysaccharide (LPS)-induced acute lung injury, AC exhibits protective activity against the histopathological changes, the increase of proinflammatory cytokines, and the enhancement of NF-κB signaling pathway [43]. LPS can dephosphorylate and activate Src homology region 2 domain-containing phosphatase-1 (SHP-1), which is a negative regulator of many signaling pathways, such as MAPK and NF-κB/AP-1. AC may induce phosphorylation of SHP-1, attenuate the activation of TGFβ-activated kinase-1 (TAK1)/JNK/AP-1, and decrease the production of COX and NOS in LPS-treated U937 cells [44] (Fig. 3, Table 1).
Regulatory B cells, a source of IL-10 production, has been well known for maintaining immune tolerance. AC has been shown to ameliorate experimental Sjogren’s syndrome by increasing Breg cells biological functions and decreasing the activity of T effector cells [45]r. In LPS-treated dendritic cells, AC significantly decreases the production of IL-12 and TNFα and increases the levels of IL-10 by activating the expression of AhR, which promotes the generation of Foxp3+ regulatory T cells, leading to protection against pulmonary inflammation in asthmatic mice [46]. Type I allergy can be induced by antigens, which stimulate the generation of antibodies that bind to mast cells or basophils. In immunoglobulin E (IgE)-regulated RBL-2H3 and KU812 cells, act as type I allergy cell models, the release of β-hexosaminidase and histamine is induced, the level of intracellular calcium is increased, and the production of TNFα and IL-4 is enhanced [47]. These can be significantly attenuated by AC treatment. The microarray analysis reveals that AC-mediated allergy inhibition might be related to suppression of MAPK/JNK signaling and down regulation of CCL1–4, FCER1A, and NFATC1 expression in KU812 cells [48]. IL-32 is a cytokine that promotes the productions of IL-1β, IL-6, IL-8, and TNFα and plays a critical role in the development of allergic rhinitis. In IL-32-treated THP-1 cells, AC attenuates inflammatory cytokines expression and alleviates NF-κB signaling and caspase-1 expression. Additionally, AC also suppresses IL-32-activated macrophage-like cells differentiation [49] (Fig. 3).
AC shows significant anti-inflammation and anti-proliferation in thymic stromal lymphopoietin (TSLP)-induced human mast cells, as indicated by decreased expression of STAT5/6, IL-6, IL-13, TNFα, and IL-1β. The possible mechanism might be associated with down regulation of murine double minute 2 (MDM2) and up regulation of p53 by AC in TSLP-induced HMC-1 cells [50] (Table 1). Neutrophils is a factor in the host defense system against infection. Inhibition of neutrophil recruitment and functions becomes a useful therapeutic strategy. In LPS-stimulated neutrophils, AC has been shown to selectively inhibit the expression of CD11b and CXCR2 and attenuate p38 MAPK phosphorylation, decreasing TLR2- and TLR4-mediated apoptosis [51]. Atopic dermatitis is a skin disease characterized by chronic inflammation. AC may be effective against atopic dermatitis, as shown that AC can relieve the scratching behaviors and skin lesion in 2,4-dinitrochlorobenzene (DNCB)-treated mice. The possible mechanism might be associated with the inhibitory activity of AC against DNCB-induced inflammatory cytokines production and NF-κB signaling. In human monocyte THP-1 models, AC suppresses the expression of CD54 and CD86 induced by DNCB at the cell surface [52]. Another study shows that AC can selectively inhibits 2-APB-activated human TRPV3 channel with an IC50 value of 14.1 ± 3.3 μM and mouse TRPV3 channel, which stimulates thymic stromal lymphopoietin (TSLP) and pro-inflammatory cytokines [53].
5. Anti-oxidative stress
AC has been identified as one of the main constituents responsible for the anti-oxidative activity of Abeliophyllum distichum. At the dose of 25 μg/mL, AC has been shown the scavenging activity against DPPH, ∙OH, and O2 - radicals by 82.84 %, 89.46 %, and 30.31 %, respectively [14]. Consistently, AC is also demonstrated the anti-oxidant activity in ABTS radical cation decoloration assay, DPPH radical scavenging assay, and ferric reducing anti-oxidant power assay [54]. Another study shows that AC exhibits a high activity in DPPH radical scavenging with an IC50 value of 0.09 μg/mL. In addition, AC shows more potent in scavenging H2O2 with an IC50 value of 2.6 μg/mL than ascorbic acid (IC50 = 4.0 μg/mL) [55]. The anti-oxidative activity can be divided two groups, including the direct and the indirect anti-oxidative activity. The former can be evaluated by the free radical scavenging assays, and the later can be determined by the expression of anti-oxidant enzymes. Interestingly, one study reports that AC shows no effects on the expression of anti-oxidant enzymes but the direct scavenging activity [56].
Oxidative damage has become one of the main pathological mechanisms of amyloid β-peptide (Aβ)-induced AD. AC exhibits anti-oxidative stress and protects against Aβ-induced ROS generation and mitochondrial dysfunctions, inhibiting mitochondrial apoptosis in SY5Y cells [57] (Table 1). Cerebral ischemia-reperfusion (I/R) injury often induces oxidative stress. It has been shown that AC can protect against I/R-induced oxidative stress, as indicated by decreased levels of reactive oxygen species (ROS) and malondialdehyde (MDA) and increased productions of superoxide dismutase (SOD) and catalase (CAT). AC significantly ameliorates I/R-induced pathological changes by regulating PKR/eIF2α signaling [58]. Similarly, AC also alleviates I/R-induced pathological damages and exhibits anti-oxidative activity by increasing the production of SOD, GSH-Px, TAS, and TT and decreasing the levels of TOS, OSI, and MDA in colon mucosa of rats [59]. Exposure to glutamate can induce oxidative stress and stimulate apoptosis in PC12 cells. Treatment with AC may alleviate glutamate-induced the loss of cell viability, the apoptosis rate, the decreased productions of SOD and GSH-Px, and the increased levels of lipid peroxide formation, ROS generation, and intracellular calcium influx, resulting in neuroprotection against excitotoxicity [60].
Oocytes and embryos are vulnerable to oxidative stress in the culture environment in vitro. Supplementation with AC has been shown beneficial effects on the development of matured equine oocytes by intracytoplasmic sperm injection and embryos in vitro [61]. AC also protects oocytes during in vitro maturation of embryo development and quality against di-(2-ethylhexy) phthalate (DEHP)-induced oxidative stress [62]. Consistently, AC acts as an anti-oxidant to improve the quality of porcine oocytes and the subsequent mature of pre-implantation embryos in vitro [63]. In sheep oocytes, AC at the nanomolar concentration may increase the formation and quality of blastocyst and promote the development of embryo in vitro by reducing ROS production and lipid peroxidation and protecting mitochondrial functions and gene expression from oxidative stress [64] (Table 1).
Protein glycation is non-enzymatic modification and can be one of the important sources of ROS production. Glycation may produce intermediates, such as glyoxal or methylglyoxal (MGO), to induce the formation of advanced glycation end products (AGE) irreversibly. AC has been shown to exhibit anti-glycation activity in high glucose-treated models [65]. Aging is characterized by a decline in physiological functions, and oxidative stress contributes to aging. Natural compounds have screened for developing potential candidates against aging [66]. AC has been exhibited the protective activity against D-galactose-induced mice aging, as shown by improved spatial learning and memory impairment, decreased escape latency, increased zone time, and improved platform crossing times. The possible mechanisms might be associated with the anti-oxidative activity of AC in alleviating the production of AGE and 8-hydroxy-2’-deoxyguanosine [67].
6. Anti-cancers
The anti-cancer effects of AC have been reviewed that it exhibits anti-angiogenesis, anti-proliferation, anti-invasion, anti-metastasis, and apoptosis-promotion in various cancers [11]. AC has been shown cellular toxicity to cancer cells independently of the status of p53, Rb1, and H-Ras by mediating many signaling pathways, including anti-oxidant, immune, and proteostatic system. The anti-cancer effects of AC are associated with the achieved concentration in the tumor [68]. Abnormal stimulation of melanin synthesis may lead to esthetic issues and development of malignant melanoma. The melanin biosynthesis is under the control of tyrosinase, tyrosinase-related protein-1 (TRP-1), and TRP-2, which are mediated by microphthalmia-associated transcription factor (MITF) and ERK signaling. AC can attenuate the activity of tyrosinase and inhibit the biosynthesis of melanin in B16F10 cells by activating ERK signaling and down regulating the expression of MITF, tyrosinase, and TRP-1 [69] (Table 1). Estrogen and its receptor ER are involved in the suppression of many tumors, including melanoma. AC has been reported to exhibit estrogenic effects, and it inhibits melanoma growth and inflammation by mediating ERβ/Ras/Raf-1/STAT3 signaling [70].
AC inhibits the proliferative activity of breast cancer 4T1 cells with an IC50 value of 117 μM. Additionally, AC up regulates the expression of Bax and caspase-3 and down regulates the expression of Bcl-2, leading to cell apoptosis. Furthermore, at the dose of 130 μM, AC increases TLR4 expression [71]. RNA metabolism regulation by microRNA or long noncoding RNA has been implicated in regulation of gene expression in cancer development [72]. In glioblastoma, AC inhibits cell viability, invasion, migration, and tumor growth and promotes cell apoptosis and autophagy by mediating the expression let-7 g-5p/HMGA2/Wnt/β-catenin signaling pathway [73] ( Fig. 4). Exosomes are the small vesicles characterized by single membrane with 30–200 nm in diameter. Tumor-derived exosomes play an important role in carcinogenesis, proliferation, and metastasis. Many studies reports that PI3K/AKT signaling pathway has been involved in the regulation of cancer development, and it has become the potential target for screening natural candidates [74], [75], [76]. In GBM cells AC has been reported to increase the expression of miR-7–5p, which then inhibits the expression of epidermal growth factor receptor (EGFR) and subsequently inactivates PI3K/AKT signaling pathway, resulting in inhibition of cell proliferation, migration, invasion, and microtubule formation [77]. Treatment failure of colorectal cancer (CRC) with 5-Fluorouracil (5-FU) can be attributed to the intrinsic and acquired resistance. AC may re-sensitize CRC cells to 5-FU and inhibit cellular proliferation in vitro. Specifically, 5-FU, by cooperating with AC, induces G1 cell cycle arrest and increases cell apoptosis by targeting the activity of PI3K/AKT signaling pathway in Caco-2 and HCT-116 cells [78] (Table 1).
Fig. 4.
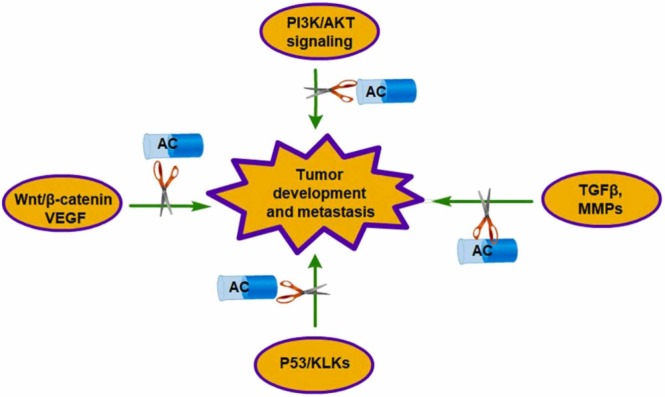
The inhibitory effects of AC against tumor development and metastasis. The activation of PI3K/AKT, Wnt/β-catenin, VEGF, and p53/KLKs signaling might promote the development of tumor development. Up regulation of TGFβ/SMAD and MMPs expression induces tumor metastasis.
In prostate cancer Du-145 and PC-3 cells, AC inhibits cell proliferation, migration, and invasion by down regulating the expression of HMGB1/RAGE axis and their downstream TGFβI/II/Smad2/3 signaling pathway. Epithelial-mesenchymal transition (EMT) is involved in the carcinogenesis and metastasis of various cancers. AC inhibits the expression of EMT transcription factors, such as Slug and Snail, and enhances the expression of E-cadherin [79]. In GBM cells, AC significantly suppresses c-Met-induced EMT in vivo and in vitro. Mechanistically, AC may interact with c-Met protein directly and then promote its degradation by the ubiquitination-proteasome pathway [80]. In human fibrosarcoma HT-1080 cells, AC has been reported to inhibit PMA-induced MMP-9 expression, invasion, and migration by decreasing the Ca2+-dependent CaMK/ERK and JNK/NF-κB signaling pathways [81]. In a xenograft human oral squamous cell carcinoma (OSCC) mouse model, AC significantly inhibits the metastasis by down regulation of NF-κB/MMP-2 signaling and promotes cell apoptosis by mediation of Bcl-2/Bcl-XL expression in vivo [82]. HIPK2 expression is negatively correlated with CRC invasion and Dukes stage. AC dose-dependently enhances the protein expression of pro-apoptosis factors p-p53 and Bax and decreases the expression of Bcl-2 [83]. AC can inhibit the tube formation and cell migration of HUVECs and produce stronger inhibition by combination with sorafenib in HCC cell lines. In JHH-7 cells, AC increases the expression of p53 and decreases the expression of kallikrein-related peptides (KLK1, 2, 4, 9, and 10) [84] (Fig. 4, Table 1).
Two mutagenic tests (chromosome aberrations test and sister chromatid exchanges test) have been used to identified that AC shows no mutagenic activity in rabbit lymphocytes [85]. Oxidative DNA damage has been involved in the pathogenesis of many diseases, including cancers. γ-H2AX and p53 are the two important factors that play distinct roles in DNA damage responses. It has been demonstrated that AC reduces the phosphorylation of γ-H2AX and p53 in 150 μM FeSO4- and 600 μM H2O2-treated NIH 3T3 cells [86]. AC is considered as an anti-genotoxic compound against H2O2-induced damage and shows no genotoxic activity in the somatic mutation and recombination test of Drosophila melanogaster [87]. Consistently, in the Drosophila wing spot test, AC exhibits no genotoxic activity at any tested concentrations [88]. Although one study reports an increase in CAs and SCEs and a decrease in the mitotic index in AC-treated in human normal lymphocytes in vitro, accompanied by up regulation of PARP-1 and p53 expression [89].
7. Neuroprotective activity
The neuroprotective activity of AC has been demonstrated that AC may significantly reduce MPP+-induced collapse of mitochondrial membrane potential, activation of caspase-3, and cell apoptosis in PC12 cells [16]. AC can be found as one of the main ingredients from Achyranthes aspera Linn., which has been shown anti-oxidative and anti-inflammatory activities to alleviate I/R-induced cerebral pathological changes [90]. The neuroprotective activity of AC is consistently associated with its anti-oxidative activity, which are demonstrated by DPPH scavenging assay (IC50 = 58.1 μM), xanthine oxidase assay (IC50 = 24.4 μM), and hydroxyl radical scavenging assay (IC50 = 357 μM) in HepG2 and SH-SY5Y cells. Furthermore, AC significantly inhibits the activity of tyrosinase, MAO-A (IC50 = 3.44 μM), and acetylcholinesterase [91]. It has been demonstrated that AC, derived from Chinese traditional medicine Radix Rehmanniae, can reduce neurological deficit score and delay the onset of multiple sclerosis. The possible mechanisms might be associated with the inhibitory effects of AC on ONOO--induced excessive mitophagy, which contributes to inflammation, peripheral activation, and central nervous system (CNS) infiltration of encephalitogenic CD4+ T cells and CD11b+ activated microglia/macrophages [92].
Hypoxic-ischemic brain damage often results in neurological dysfunction and acute death in neonates. It has been demonstrated that AC may exhibit neuroprotective activity, as shown by decreased neurofunctional latency, reduced brain infarct volume, and ameliorated neuronal degeneration. The possible underlying mechanism might be associated with the inhibitory activity of AC against the formation of autophagosome [93]. Cerebral I/R injury often causes neurological, motor, and cognitive deteriorating dysfunctions, along with alteration of brain morphology and biochemical factors expression. AC can effectively protect against I/R-induced pathological changes by regulating the expression of HIF-1α, NF-κB, and VEGF [94] ( Fig. 5) (Table 1). The differentially expressed genes (DEGs) were analyzed to predict the possible targets of AC in rats with middle cerebral artery occlusion. CCL2, CXCL10, and ICAM1 have been identified as the potential targets, and their expression are increased in MACO rats and OGD/R-induced cells. AC exhibits protective activity in cell proliferation promotion and apoptosis inhibition by mediating IL-10/STAT3 signaling [95].
Fig. 5.
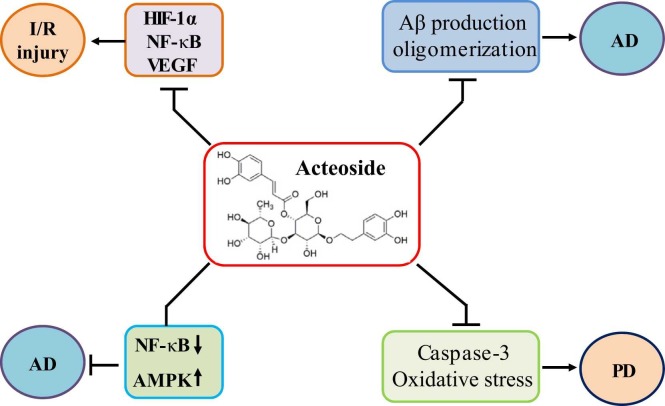
AC shows neuroprotective activity. AC ameliorates I/R injury by attenuating the expression of HIF-1α, NF-κB, and VEGF. AC protects against AD development by activating AMPK, attenuating NF-κB, and decreasing Aβ production and oligomerization. In addition, AC inhibits caspase-3 expression and oxidative stress, leading to amelioration of PD development.
Amyloid β peptide (Aβ) plays a critical role in Alzheimer’s disease (AD). Aβ 1-42 production is associated with memory impairment and neuronal degeneration in AD patients. AC has been shown to increase Aβ 1-40 degradation, decrease Aβ 1–40 production, and inhibit Aβ 1-42 oligomerization, leading to amelioration of Aβ 1-42-induced cognitive dysfunction in rats [96] (Fig. 5) (Table 1). The inhibitory effects of AC on Aβ42 aggregation have been investigated with an IC50 value of 8.9 μM, and the two catechol moieties of AC might be responsible for such inhibitory activity [97]. Aβ-induced neuronal injury is associated with oxidative stress, which has become a potential therapeutic strategy for AD management. Recently, AC has been shown to activate NRF2/HO-1 signaling pathway and protect neuron against Aβ-induced injury in PC12 cells. NRF2 siRNA or HO-1 inhibitors may significantly abolish the protective activity of AC. Furthermore, ERK inhibitor PD98059 and PI3K inhibitor LY294002 also block the neuroprotection of AC [98] (Table 1).
Microglia-driven inflammation contributes to neuron damage and neurodegeneration, promoting the progression of AD. A computational network model has been analyzed and verified that AC acts as an inhibitor of NF-κB and an agonist of AMPK in AlCl3-induced AD and LPS-treated BV-2 cells. AC inhibits M1 polarization, stimulates M2 polarization, and ameliorates mitochondrial dysfunction by mediating NF-κB and AMPK/PGC-1α/UCP2 signaling pathways [99] (Fig. 5). In PC12 cells, melittin can enhance the intracellular Ca2+ mobilization but not cause the intracellular Ca2+ release. In addition, melittin also induces arachidonic acid (AA) activation by stimulating the secretion of Ca2+-dependent phospholipase A2 (PLA2) and catecholamine. However, these melittin-induced pathological changes can be significantly ameliorated by AC [100]. In mice treated with a combination of D-galactose and AlCl3, AC may effectively improve learning and memory impairment. This might be associated with the inhibitory activity of AC against the expression of nitric oxide, nitric oxide synthase, and caspase-3 in the hippocampus [101]. Intracerebroventricular administration of streptozotocin (ICV-STZ) may induce cognitive impairment, glucose metabolism dysregulation, and oxidative stress. AC up regulates the protein expression of Glut1, Glut3, and Glut4 and reduces the production of ROS, protecting against ICV-STZ-induced learning and cognitive impairment [102].
The progressive degeneration of the dopaminergic neurons is the main pathological mechanism of Parkinson’s disease (PD). Caspase-3 inhibition has been demonstrated to protect against dopaminergic neurons degeneration (Fig. 5). AC is reported to interact with and inhibit caspase-3 activity by forming hydrogen bonds with Thr177, Ser178, Gly238, Ser339, Arg341, and Trp348 in the active site [103]. In the network pharmacology and experimental study of PD, the protective activity of AC against PD is associated with the induction of mitophagy and inhibition of neuronal apoptosis [104]. In 6-hydroxydopamine (6-OHDA)-induced PD zebrafish models, AC has been demonstrated to penetrate the blood-brain-barrier (BBB). AC can protect against 6-OHDA-induced movement disorders and dopaminergic neuron death by up regulating the activity of NRF2/ARE signaling pathway in zebrafish [105]. Due to the short half-life and low bioavailability, AC can be constructed into a nanomicelle composite AC-PLA-mPEG-CTA-pDNA-NGF (APPDN) with a size of about 160 nm and being capable of penetrating the BBB easily. It has been shown that APPDN may effectively inhibit Lewy body formation in MPTP-induced PD mice and block α-syn aggregation in MPP+-lesioned PD cell models [106].
L-Glutamate is one of the main excitatory neurotransmitters. AC, isolated from the leaves of Callicarpa dichotoma, exhibits neuroprotective effects against glutamate-induced intracellular Ca2+ influx, oxidative stress, and mitochondrial dysfunction [107]. γ-aminobutyric acid (GABA) dysregulation has been involved in the pathogenesis of epilepsy. AC is reported to exhibit anti-convulsant activity in pentylenetetrazole (PTZ)-induced mice by up regulating GABA and GABAA receptor expression. In addition, AC does not produce central side effects, such as motor incoordination and locomotor deficits [108]. Chronic spinal cord injury often induces skeletal muscle atrophy, accompanied by decreased secretion of axonal growth factors. AC can stimulate the secretion of pyruvate kinase isoform M2 (PKM2) and the proliferation of skeletal muscle cells, resulting in recovery of skeletal muscle weight reduction and motor function impairment [109]. Loss of retinal ganglion cells (RGCs) may lead to optic nerve atrophy and vision loss. AC has been demonstrated to protect RGC-5 cells from H2O2-induced injury by mediating CASC2/miR-155/mTOR signaling [110] (Table 1). Furthermore, AC can inhibit autophagy-mediated apoptosis of RGCs through regulating the activity of PI3K/AKT pathway and the expression of caveolin 1 [111]. Glaucoma progressively impairs the eyesight and causes blindness, and protection of RGCs from apoptosis can be a useful strategy. AC has been demonstrated to inhibit autophagy-induced RGCs apoptosis by regulating the expression of optineurin and PI3K/AKT/mTOR signaling pathway [112].
8. Cardiovascular protection
NO may induce vasodilation by stimulating the expression of cGMP. NO pathway inhibitors have been shown to abolish the induction of AC (at the dose of 30 μM) in enhancing phenylephrine-induced contractions in endothelium-intact rat arteries. The underlying mechanism of AC on attenuation of endothelial NO-regulated aortic relaxation might be associated with the inhibition of Ca2+-dependent NO generation in the endothelia [17]. Angiotensin (Ang)-converting enzyme (ACE) has been considered as the critical protease to synthesize Ang II in the renin-angiotensin-aldosterone system. AC has been demonstrated to inhibit the activity of ACE with an IC50 value of 365 μM dose-dependently [113]. Consistently, AC is one of the major bioactive constituents from the extract of Plantago asiatica L. seeds and alleviates hypertension in spontaneously hypertensive rats by inhibiting the expression of ACE [114]. Septic cardiomyopathy is a common dysfunction in the sepsis of organ with a ventricular dilation and decreased contractility. In LPS-induced sepsis, AC greatly enhances cardiac functions by amelioration of inflammation and oxidative stress. Specifically, AC increases mitochondrial biogenesis and restores sepsis-induced mitochondrial changes, inhibiting apoptosis in cardiomyocytes [115] ( Fig. 6, Table 1).
Fig. 6.
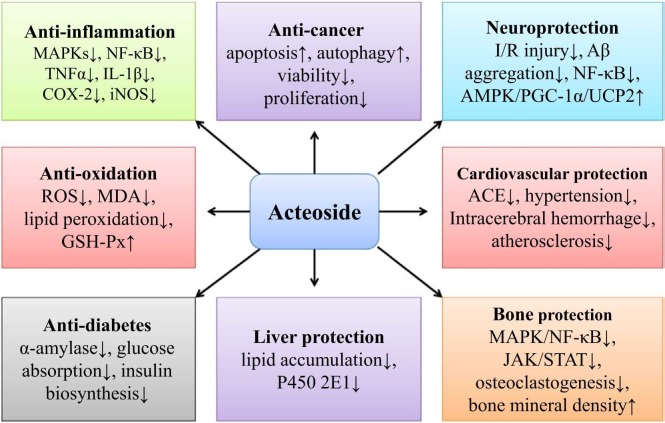
The various biological activities of AC are discussed. These activities involve anti-inflammation, anti-oxidation, anti-cancer, neuroprotection, cardiovascular protection, anti-diabetes, liver protection, and bone protection.
Intracerebral hemorrhage (ICH), a devastating cerebrovascular disease, is associated with high morbidity and mortality. A secondary neuronal injury can be stimulated by inflammation, hematoma toxicity, and microglial activation. Treatment with AC shows protective effects against ICH-induced pathological changes by inhibiting NLRP3 expression and increasing neuronal viability [116]. Another study reports that AC may improve the behavioral score, decrease the hematoma volume, alleviate the brain edema, and inhibit neuronal apoptosis in mice ICH models. Consistently, the possible mechanism might be associated with the inhibition of TLR-4-regulated inflammation [117]. High fat diets (HFD)-induced dyslipidemia contributes to atherosclerosis development. AC has been shown the protective activity against HFD-induced atherosclerosis, as indicated by amelioration of the serum productions of inflammatory cytokines and improvement of the lipid profiles and the organ coefficients in rats. The molecular mechanisms might include the attenuation of AMPK/mTOR signaling pathway by AC [118] (Table 1).
9. Anti-diabetes
Reduction of glucose absorption rate can be an effective strategy to control blood glucose concentrations in diabetes. α-Amylase acts as an important factor to regulate the digestion of carbohydrates and glucose absorption. AC has been found to interact with α-amylase and inhibit its activity with an IC50 value of 125.21 ± 7.87 mg/mL [18]. Hyperglycemia might be associated with chronic hyperalgesia and allodynia. AC has been found as one of the active compounds in the extract from the leaves of Ligustrum vulgare to treat diabetic neuropathy, decreasing hyperalgesia and allodynia. Controversially, the extract shows no effects on the blood glucose concentrations [119]. Sodium-dependent glucose cotransporter 1 (SGLT1), mainly expressed in the brush border membrane of the small intestine, can transport both glucose and galactose into enterocytes. SGLT1/GLUT2 coupling becomes one of the critical mechanisms in maintaining the blood glucose concentration. AC has been exhibited the inhibitory activity in SGLT1-mediated glucose absorption in Caco-2 cells [120]. Endoplasmic reticulum stress (ERS) caused by metabolic insults often results in a decrease of insulin biosynthesis and secretion. AC has been demonstrated to protect β cells against oxidative stress and ERS in clonal and human β cells, as shown by attenuated expression of PERK/eIF2α signaling [121] (Fig. 6, Table 1).
10. Bone and cartilage protection
Osteoarthritis is characterized by low chronic inflammatory joint diseases. AC has been shown to ameliorate IL-1β-induced MMP-13, MMP-3, and MMP-1 expression by decreasing the activity of MAPK/NF-κB signaling pathway in the primary rat chondrocytes [19] (Fig. 6, Table 1). Consistently, AC inhibits IL-1β-induced IL-6, IL-12, TNFα, and IFNγ expression, enhances chondrocyte proliferation, and attenuates cell apoptosis by down regulating the activity of JAK/STAT pathway. In addition, AC also ameliorates synovial inflammation in osteoarthritic rats [122]. The expression of P2X7R, MMP-13, substance P, and PGE2 are higher in rat OA chondrocytes, and these alterations can be reversed by treatment with AC through inactivation of NF-κB signaling pathway [123]. In collagen-induced arthritis mice, the cartilage destruction, synovial hyperplasia, and the expression of MMP-9, MMP-13, and ADAMTS-4/-5 can be significantly alleviated by Fufang Shatai Heji, which includes AC as one of the major active compounds. Molecular docking study indicates that AC can interact with Glu111 and Phe110 in the active pocket of MMP-9 [124].
In an ovariectomized (OVX) mice model, AC significantly enhances bone mineral density and bone biomechanical properties, improving the bone microarchitecture. AC effectively reduces osteoclastogenesis, and the possible mechanisms might be associated with attenuation of NF-κB pathway and stimulation of PI3K/AKT pathway [125]. Inflammatory cytokines are involved in the mediation of osteoclastogenesis, inducing the development of bone resorption and osteoporosis. AC may function as an anti-resorptive agent and inhibit osteoclast differentiation by inactivating the expression of RANKL, p38 MAPK, NF-κB, c-Fos, and NFATc1, resulting in reduction of bone loss [126]. Diabetes mellitus (DM) may cause the dysregulation of bone metabolism, resulting in bone loss and osteoporosis (OP). AC can enhance the proliferative activity and differentiation of high glucose-treated MC3T3-E1 cells. The underlying mechanism might be associated with the promotion of IGF-1/BMP/PI3K/mTOR signaling pathway by AC. Furthermore, the molecular docking study shows that AC can fit well with the active site of IGF-1R by forming 3 hydrogen bonds with Thr1080, Phe1044, and Lys1030 [127] (Table 1). In a rat model of OP combined with AD, AC can increase the mean bone mineral density, improve the trabecular bone mass and microarchitecture, and increase the ability of learning and memory by inhibition of oxidative stress, mediation of acetylcholine metabolism, and protection of neurons in hippocampus [128].
11. Anti-microorganism
The anti-bacterial activity of AC against Staphylococcus aureus has been investigated, and AC can inhibit leucine incorporation and affect protein synthesis [21] (Fig. 6). The eukaryotic-like serine/threonine phosphatase (Stp1), a protein phosphatase, has been associated with the mediation of various virulence factors of Staphylococcus aureus. Deficiency of Stp1 gene exhibits reduction of pathogenicity of S. aureus, indicating its potential roles in anti-virulence drug development. AC has been screened and identified as an effective inhibitor of Stp1 by competitive and allosteric mechanisms. M39, G41, H42, R161, and N162 have been associated with the affinity of AC for competitive binding, while R122, S136, D137, N142, and V145 have been involved in the allosteric binding [129]. Sortase A (SrtA) in Gram-positive pathogenic bacteria is responsible for anchoring to the cell wall and considered as the potential drug target. The bioactivity of SrtA from S. aureus is Ca2+-dependent due to the interaction between Ca2+ and residues E105, E108, D112, and E171. In contrast, SrtA from Streptococcus suis is Ca2+-independent, and Ca2+ position is replaced by a lysine residue. Specifically, K111 forms a salt bridge with D180. AC has been identified as a novel SrtA inhibitor with an IC50 value of 36.3 μM, and the endogenous SrtA activity indicates 86 % inhibition against S. aureus and 98 % against Streptococcus suis [130]. However, whether the discrepancy is associated with Ca2+ or the salt bridge is still needed to be further elucidated.
Respiratory syncytial virus (RSV) has become one of the main causes of lower respiratory tract infections. AC is the potential effective component of the herbs Plantago asiatica and Clerodendrum trichotomum. Both AC and the two herbs extract are active against RSV infection in vivo and in vitro [131]. When fighting against viral infections, T lymphocytes are essential for acquired immunity in the human body. AC exhibits inhibitory activity against vesicular stomatitis virus by increasing ERK activity and up regulating the expression of T-bet/IFN-γ in the CD4+ and CD8+ subsets of T cells particularly [132]. Pneumolysin is an important virulence factor for Streptococcus pneumoniae to infect the host and produce inflammatory responses. AC has been known not to exhibit bacteriostatic activity but to ameliorate pneumolysin-induced cytotoxicity. Molecular dynamic simulation and mutational analysis suggests that AC can interact with the cleft between domain 3 and domain 4 in pneumolysin, leading to inhibition of pneumolysin oligomerization and hemolytic activity [133].
Docking simulation shows that AC exhibits the best binding affinity among the tested compounds by forming the hydrogen bonds with Gly496 of the SARS-CoV2-CTD. However, AC does not inhibit ACE2, which plays a protective activity against acute respiratory distress syndrome and acute lung injury in vivo and is a value for the promising anti-SARS-CoV2 potentials [134]. A study reports that AC mainly binds to the residues F140, G138, Q189, and G170 by forming hydrogen bonds in the highly conserved and catalytic region of SARS-CoV2 main protease (3CLpro/Mpro) with a significant binding energy of − 42.686 kcal/mol [37]. Another docking study indicates that AC exhibits the highest activity of the tested compounds against SARS-CoV2 main protease by forming hydrogen bonds with Cys44, Met49, Asn142, Hie164, Glu166, and Thr190. In vitro on SARS-CoV2 Egyptian strain, AC shows similar biological activity to the positive drug GC376 and has an IC50 value of 43 nM [135].
AC, isolated from Stachytarpheta cayennensis, has been demonstrated to be effective against Leishmania infection (an EC50 value of 19 μM) by inhibiting the activity of arginase (K i = 0.7 μM), which acts as an enzyme in the biosynthesis pathway of polyamines that contribute to the synthesis of the anti-oxidant compound trypanothione and the infectivity of parasite [136]. Deficiency of arginase substrate, L-arginine, may lead to oxidative stress, which can induce apoptosis and kill Leishmania. AC has been shown the inhibitory activity against extracellular promastigotes of Leishmania amazonensis [136]. In addition, AC selectively inhibits the parasite arginase and acts on the intracellular amastigotes of L. amazonensis (an EC50 value of 32 μM), which resists to the NO production induced by LPS and IFN-γ. However, AC does not affect the expression of enzyme and cytokines by murine macrophages [137].
12. Hepatoprotection activity
The pathological development of alcoholic hepatitis may involve oxidative stress, inflammatory responses, metabolic dysregulation, and apoptosis. These can be effectively inhibited by AC treatment, which significantly attenuates the activation of NF-κB signaling in HepG2 cells [20]. The effects of AC on lipid accumulation in HepG2 cells have been investigated, and the results from RNA-seq assays indicate that AC ameliorates the lipid accumulation by mediating the glycolysis, AMPK signaling, and fatty acid degradation [138]. These might be associated with the anti-oxidative activity of AC. In carbon tetrachloride (CCl4)-induced mice, AC consistently decreases MDA production and increases GSH generation. AC also protects against FeCl2-ascorbate-induced lipid peroxidation. Specifically, AC down regulates the bioactivity of cytochrome P450 2E1, including p-nitrophenol and aniline hydroxylation [139] (Fig. 6).
13. Renal protection
Leukocyte accumulation in the glomeruli is associated with the progression of crescentic anti-GBM nephritis. AC has been reported to suppress the accumulation of the total leukocytes, ED-1-positive cells, CD4-positive cells, CD8-positive cells, IL-2 receptor-positive cells, and Ia-positive cells in the rat glomeruli [140]. In addition, AC decreases the expression of ICAM-1, which can be activated by inflammatory cytokines in HUVECs and rat mesangial cells [141]. T helper type 22 (Th22) lymphocyte is involved in the progression of IgA nephropathy (IgAN) by activating the expression of CCL20, CCL22, and CCL27. It has been demonstrated that AC inhibits the proliferation of Th22 cells, suppresses the expression of CCL20, CCL22, and CCL27, and relieves the productions of inflammatory cytokines, such as IL-1, IL-6, and TNFα in mesangial cells. The expression of TGF-β1 is associated with mesangial cell fibrosis. AC can also significantly decrease the expression of TGF-β1 in mesangial cells, ameliorating the progression of IgAN [142].
14. Skin protection
Ultraviolet (UV) radiation-stimulated ROS is involved in photo-damage, and long-term oxidative stress can induce skin photo-aging by increasing MMPs synthesis and collagen degradation. AC has been shown the protective activity against UV-stimulated ROS and thymus and activation-regulated chemokine (TARC) synthesis by up regulation of NRF2 signaling and down regulating NF-κB pathway. In addition, AC may effectively decrease the expression of MMP-1 by activating TGFβ/Smad signaling and inhibiting MAPK/AP-1 pathway [143]. In X-ray radiation-treated human skin fibroblasts, AC significantly inhibits the production of ROS, the up regulation of pro-caspase-3 expression, and the down regulation of Bcl-2 expression by ameliorating the activity of ERK and JNK pathways [144].
ECM remodeling plays a critical role in wound healing, MMP-2 is an ECM degrading enzyme involved in cell migration and proliferation during wound healing. AC has been shown to activate the expression of the precursor of MMP-2 (proMMP-2) but not MMP-2 in normal human dermal fibroblasts. The possible mechanism might be associated with activation of membrane-type 1 MMP expression and PI3K signaling pathway by AC [145]. Treatment with AC can increase the migration of keratinocytes and decrease the expression of TNFα, IL-6, IL-12p70, MCP-1 and IFNγ in LPS-treated N9 cells [146].
15. Miscellaneous section
Many traditional herbs have been used for treating the collapse and exhaustion. AC has been isolated as an active compound from Pedicularis densispica and found that it can prolong the time to exhaustion of rats in a treadmill exercise, attenuate the expression of 5-HT and TPH2, and enhance the expression of 5-HT1B [147]. Pancreatic lipase accounts for 50–70 % hydrolysis of the total dietary fats, controlling the fat absorption or obesity. AC, isolated from the Chinese tea Ligustrum purpurascens kudingcha, can act as a non-competitive lipase inhibitor with a binding constant K a value of 1.88 × 104 M and by forming hydrogen bonds with Lys271, Leu272, and Thr68 residues of lipase [148]. In lacaune ewes, particularly in the first 20 days after parturition, foods supplemented AC can improve the production of milk. Additionally, AC also improve the blood lipid profile and liver functions. This might be associated with anti-oxidative activity of AC [149].
Varicocele within the scrotum is considered as a factor contributing to male-factor infertility. Specifically, varicocele may induce testicular dysfunction, including inflammation, oxidative stress, hormonal imbalance, and apoptosis. AC can significantly enhance sperm viability, increase Johnson’s score, and decrease apoptotic index. These might be associated with up regulation of the hypothalamus-pituitary-gonadal axis by AC [150]. AC exhibits anti-estrogenic effects in ERα- and Erβ-transfected HeLa cells. In addition, AC can reverse the effects of E2 by ERα [151]. H+ K+-ATPase plays a critical role in gastric acid secretion. AC has been identified as the active component of Tectoma grandis for protecting against cold restraint- and pyloric ligation-induced gastric ulcer. AC inhibits H+ K+-ATPase activity with an IC50 value of 60.98 μg/mL [152]. In RBL-2H3 cells, AC inhibits the increase of intracellular Ca2+ mobilization, which is associated not with intracellular Ca2+ release of PLC activity but with the influx of extracellular Ca2+, and inhibit melittin (1 μM)-induced histamine release by blocking the Ca2+-independent PLA2 pathway [153].
Higher level of uric acid in the blood is associated with hyperuricemia and gout. Xanthine oxidase (XOD) is the key enzyme in the purine metabolic pathway. AC at the dose of 54 mg/kg can effectively reduce the serum level of uric acid in the hyperuricemic rats, and the IC50 value of inhibiting XOD activity is 81.15 μg/mL. Mechanically, the phenyl rings of AC can combine to the molybdopterin domain (a hydrophobic pocket) with a low binding energy of − 6.0 kcal/mol by forming hydrogen bonds with several residues, altering the hydrogen-bond network in the active center of XOD and changing its conformation [154]. Carbonic anhydrase (CA) is associated with carbon dioxide and ion transport, respiration, fluid balance, and acid-base balance and has been involved in many diseases, such as glaucoma, diabetes, and cancers. Targeting CA can be the potential therapeutic strategy. AC has been shown the inhibitory activity against human CA I and II isoenzymes with IC50 values of 1.73 μM and 1.90 μM, respectively. The docking study indicates the binding of AC to CA I and II with the K i values of 2.00 μM and 1.49 μM, respectively [155].
16. Future perspective
Herbal medicines are good for the clinical management of chronic diseases, such as anxiety or depression. AC is one the main effective compound of the powdered leaves of Aloysia polystachya, which was involved in a phase-2 clinical trial for treating anxiety symptoms [156]. A single-center, double-blind, and randomized phase II study of AC on the efficacy and tolerability for modulating the platelet aggregation (PA) has been investigated. 100 subjects are included and 50 mg or 100 mg AC is administered. Two weeks of treatment with 50 mg AC does not modify PA values. However, two-week treatment with 100 mg AC significantly decreases the values of PA. No serious adverse effects are reported in this study. These indicates that AC at the dose of 100 mg might reduce PA values in patients with cardiovascular risk factors [157]. AC ameliorates PA values not by arachidonic acid but by ADP stimulation in the blood from aspirin-treated patients. In vitro study, AC may mildly attenuate PA triggered by arachidonic acid and ADP [158]. The anti-oxidative activity of polyphenols may be altered at different temperature and pH conditions due to alterations in chemical structure. Alternatively, mixture of parent form and its structure transformation make them to interact with each other in the biological activity for synergy or antagonism or additive effects, and these may be dependent on the assays used and/or the mixture composition.
Funding Statement
This study was financially supported by National Natural Science Foundation of China (82060407) and Jiangxi Provincial Natural Science Foundation (20212ACB206002).
CRediT authorship contribution statement
Longhuo Wu provided the idea of this paper. Yaosheng Xiao and Qun Ren conducted the experiments and revised and finalized the paper. All authors approved the final paper.
Conflict of Interests
The authors declare no conflict of interests.
Data Availability
The data used to support the findings of this study are included within the article.
References
- 1.Alipieva K., Korkina L., Orhan I.E., Georgiev M.I. Verbascoside – a review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014;32(6):1065–1076. doi: 10.1016/j.biotechadv.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 2.He J., Hu X.P., Zeng Y., Li Y., Wu H.Q., Qiu R.Z., Ma W.J., Li T., Li C.Y., He Z.D. Advanced research on acteoside for chemistry and bioactivities. J. Asian Nat. Prod. Res. 2011;13(5):449–464. doi: 10.1080/10286020.2011.568940. [DOI] [PubMed] [Google Scholar]
- 3.Huang J., Zhao D., Cui C., Hao J., Zhang Z., Guo L. Research progress and trends of phenylethanoid glycoside delivery systems. Foods. 2022;11:769. doi: 10.3390/foods11050769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y.Q., Zhu J.L., Shao L.Y., Guo M.M. Current advances in acteoside biosynthesis pathway elucidation and biosynthesis. Fitoterapia. 2020;142 doi: 10.1016/j.fitote.2020.104495. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y.T., Lin L.C., Sung J.S., Tsai T.H. Determination of acteoside in Cistanche deserticola and Boschniakia rossica and its pharmacokinetics in freely-moving rats using LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006;844(1):89–95. doi: 10.1016/j.jchromb.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 6.D'Imperio M., Cardinali A., D'Antuono I., Linsalata V., Minervini F., Redan B.W., Ferruzzi M.G. Stability–activity of verbascoside, a known antioxidant compound, at different pH conditions. Food Res. Int. 2014;66:373–378. [Google Scholar]
- 7.Funes L., Laporta O., Cerdán-Calero M., Micol V. Effects of verbascoside, a phenylpropanoid glycoside from lemon verbena, on phospholipid model membranes. Chem. Phys. Lipids. 2010;163(2):190–199. doi: 10.1016/j.chemphyslip.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Visioli F., Bellomo G., Montedoro G., Galli C. Low density lipoprotein oxidation is inhibited in vitro by olive oil constituents. Atherosclerosis. 1995;117(1):25–32. doi: 10.1016/0021-9150(95)05546-9. [DOI] [PubMed] [Google Scholar]
- 9.Kim K.H., Kim S., Jung M.Y., Ham I.H., Whang W.K. Anti-inflammatory phenylpropanoid glycosides from Clerodendron trichotomum leaves. Arch. Pharm. Res. 2009;32(1):7–13. doi: 10.1007/s12272-009-1112-6. [DOI] [PubMed] [Google Scholar]
- 10.Chen S., Liu H., Wang S., Jiang H., Gao L., Wang L., Teng L., Wang C., Wang D. The neuroprotection of verbascoside in Alzheimer’s disease mediated through mitigation of neuroinflammation via blocking NF-κB-p65 signaling. Nutrients. 2022;14(7):1417. doi: 10.3390/nu14071417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan R.A., Hossain R., Roy P., Jain D., Mohammad Saikat A.S., Roy Shuvo A.P., Akram M., Elbossaty W.F., Khan I.N., Painuli S., Semwal P., Rauf A., Islam M.T., Khan H. Anticancer effects of acteoside: mechanistic insights and therapeutic status. Eur. J. Pharm. 2022;916 doi: 10.1016/j.ejphar.2021.174699. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava M., Shanker K. Duranta erecta Linn: a critical review on phytochemistry, traditional uses, pharmacology, and toxicity from phytopharmaceutical perspective. J. Ethnopharmacol. 2022;293 doi: 10.1016/j.jep.2022.115274. [DOI] [PubMed] [Google Scholar]
- 13.Brimson J.M., Onlamoon N., Tencomnao T., Thitilertdecha P. Clerodendrum petasites S. Moore: The therapeutic potential of phytochemicals, hispidulin, vanillic acid, verbascoside, and apigenin. Biomed. Pharmacother. = Biomed. Pharmacother. 2019;118 doi: 10.1016/j.biopha.2019.109319. [DOI] [PubMed] [Google Scholar]
- 14.Lee H.D., Kim J.H., Pang Q.Q., Jung P.M., Cho E.J., Lee S. Antioxidant activity and acteoside analysis of Abeliophyllum distichum. Antioxidants. 2020;9(11):1148. doi: 10.3390/antiox9111148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song H.S., Choi M.Y., Ko M.S., Jeong J.M., Kim Y.H., Jang B.H., Sung J.H., Kim M.G., Whang W.K., Sim S.S. Competitive inhibition of cytosolic Ca2+-dependent phospholipase A2 by acteoside in RBL-2H3 cells. Arch. Pharm. Res. 2012;35(5):905–910. doi: 10.1007/s12272-012-0516-x. [DOI] [PubMed] [Google Scholar]
- 16.Sheng G.Q., Zhang J.R., Pu X.P., Ma J., Li C.L. Protective effect of verbascoside on 1-methyl-4-phenylpyridinium ion-induced neurotoxicity in PC12 cells. Eur. J. Pharm. 2002;451(2):119–124. doi: 10.1016/s0014-2999(02)02240-9. [DOI] [PubMed] [Google Scholar]
- 17.Lau C.W., Chen Z.Y., Wong C.M., Yao X., He Z., Xu H., Huang Y. Attenuated endothelium-mediated relaxation by acteoside in rat aorta: role of endothelial [Ca2+]i and nitric oxide/cyclic GMP pathway. Life Sci. 2004;75(10):1149–1157. doi: 10.1016/j.lfs.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Lu Y.Q., Zhou W.Y., Feng Y., Li Y., Liu K., Liu L.Z., Lin D.X., He Z.D., Wu X.L. Acteoside and acyl-migrated acteoside, compounds in Chinese Kudingcha tea, inhibit alpha-amylase in vitro. J. Med. Food. 2017;20(6):577–585. doi: 10.1089/jmf.2016.3910. [DOI] [PubMed] [Google Scholar]
- 19.Lim H., Kim D.K., Kim T.H., Kang K.R., Seo J.Y., Cho S.S., Yun Y., Choi Y.Y., Leem J., Kim H.W., Jo G.U., Oh C.J., Oh D.S., Chun H.S., Kim J.S. Acteoside counteracts interleukin-1 beta-induced catabolic processes through the modulation of mitogen-activated protein kinases and the NF kappa B cellular signaling pathway. Oxid. Med. Cell. Longev. 2021;2021:8684725. doi: 10.1155/2021/8684725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khullar M., Sharma A., Wani A., Sharma N., Sharma N., Chandan B.K., Kumar A., Ahmed Z. Acteoside ameliorates inflammatory responses through NFkB pathway in alcohol induced hepatic damage. Int. Immunopharmacol. 2019;69:109–117. doi: 10.1016/j.intimp.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 21.Avila J.G., de Liverant J.G., Martínez A., Martínez G., Muñoz J.L., Arciniegas A., de Vivar A. Romo. Mode of action of Buddleja cordata verbascoside against Staphylococcus aureus. J. Ethnopharmacol. 1999;66(1):75–78. doi: 10.1016/s0378-8741(98)00203-7. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y.T., Tsai T.R., Lin L.C., Tsai T.H. Liquid chromatographic method with amperometric detection to determine acteoside in rat blood and brain microdialysates and its application to pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007;853(1–2):281–286. doi: 10.1016/j.jchromb.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 23.Li Y.J., Gan L., Li G.Q., Deng L., Zhang X.S., Deng Y.L. Pharmacokinetics of plantamajoside and acteoside from Plantago asiatica in rats by liquid chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2014;89:251–256. doi: 10.1016/j.jpba.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W., Huo S.X., Wen Y.L., Xing H., Chen X.J. Pharmacokinetics of acteoside following single dose intragastric and intravenous administrations in dogs. Chin. J. Nat. Med. 2015;13(8):634–640. doi: 10.1016/S1875-5364(15)30060-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhao M., Qian D., Liu P., Shang E.X., Jiang S., Guo J., Su S.L., Duan J.A., Du L., Tao J. Comparative pharmacokinetics of catalpol and acteoside in normal and chronic kidney disease rats after oral administration of Rehmannia glutinosa extract. Biomed. Chromatogr.: BMC. 2015;29(12):1842–1848. doi: 10.1002/bmc.3505. [DOI] [PubMed] [Google Scholar]
- 26.Dai X.X., Su S.L., Cai H.D., Wei D.D., Zheng T.Y., Zhu Z.H., Yan H., Shang E.X., Guo S., Qian D.W., Duan J.A. Comparative pharmacokinetics of acteoside from total glycoside extracted from leaves of Rehmannia and Dihuangye total glycoside capsule in normal and diabetic nephropathy rats. Biomed. Chromatogr. 2017;31(12) doi: 10.1002/bmc.4013. [DOI] [PubMed] [Google Scholar]
- 27.Cardinali A., Linsalata V., Lattanzio V., Ferruzzi M.G. Verbascosides from olive mill waste water: assessment of their bioaccessibility and intestinal uptake using an in vitro digestion/Caco-2 model system. J. Food Sci. 2011;76(2):H48–H54. doi: 10.1111/j.1750-3841.2010.01996.x. [DOI] [PubMed] [Google Scholar]
- 28.Cardinali A., Pati S., Minervini F., D’Antuono I., Linsalata V., Lattanzio V. Verbascoside, isoverbascoside, and their derivatives recovered from olive mill wastewater as possible food antioxidants. J. Agric. Food Chem. 2012;60(7):1822–1829. doi: 10.1021/jf204001p. [DOI] [PubMed] [Google Scholar]
- 29.Cardinali A., Fiorenza M., Floriana R., Isabella D.A., Lucantonio D., Mario G.F., Vito L. Assessment of verbascoside absorption in human colonic tissues using the using chamber model. Food Res. Int. 2013;54(1):132–138. [Google Scholar]
- 30.Zhou F., Huang W.S., Li M.Q., Zhong Y.H., Wang M.M., Lu B.Y. Bioaccessibility and absorption mechanism of phenylethanoid glycosides using simulated digestion/Caco-2 intestinal cell models. J. Agric. Food Chem. 2018;66(18):4630–4637. doi: 10.1021/acs.jafc.8b01307. [DOI] [PubMed] [Google Scholar]
- 31.Gao Y., Zong C.J., Liu F., Fang L., Cai R.L., Shi Y., Chen X., Qi Y. Evaluation of the intestinal transport of a phenylethanoid glycoside-rich extract from Cistanche deserticola across the Caco-2 cell monolayer model. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0116490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou F., Xu T., Zhao Y.J., Song H.X., Zhang L.Q., Wu X.D., Lu B.Y. Chitosan-coated liposomes as delivery systems for improving the stability and oral bioavailability of acteoside. Food Hydrocoll. 2018;83:17–24. [Google Scholar]
- 33.Zhou F., Huang W.S., Xu T., Wu L.P., Chen Q., Peng J.Y., Liu X., Lu B.Y. Natural P-gp inhibitor EGCG improves the acteoside absorption in Caco-2 cell monolayers and increases the oral bioavailability of acteoside in rats. Food Chem. Toxicol. 2020;146 doi: 10.1016/j.fct.2020.111827. [DOI] [PubMed] [Google Scholar]
- 34.Qi M., Xiong A.Z., Li P.F., Yang Q.M., Yang L., Wang Z.T. Identification of acteoside and its major metabolites in rat urine by ultra-performance liquid chromatography combined with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2013;940:77–85. doi: 10.1016/j.jchromb.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 35.Su D., Li W., Xu Q.M., Liu Y.L., Song Y.G., Feng Y.L. New metabolites of acteoside identified by ultra-performance liquid chromatography/quadrupole-time-of-flight MSE in rat plasma, urine, and feces. Fitoterapia. 2016;112:45–55. doi: 10.1016/j.fitote.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Cui Q., Pan Y., Xu X., Zhang W., Wu X., Qu S., Liu X. The metabolic profile of acteoside produced by human or rat intestinal bacteria or intestinal enzyme in vitro employed UPLC-Q-TOF-MS. Fitoterapia. 2016;109:67–74. doi: 10.1016/j.fitote.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Kallingal A., Kundil V.T., Ayyolath A., Karlapudi A.P., Joseph T.M., Variyar E.J. Molecular modeling study of tectoquinone and acteoside from Tectona grandislinn: a new SARS-CoV-2 main protease inhibitor against COVID-19. J. Biomol. Struct. Dyn. 2022;40(4):1764–1775. doi: 10.1080/07391102.2020.1832580. [DOI] [PubMed] [Google Scholar]
- 38.Henn J.G., Steffens L., Sperotto N.D.D., Ponce B.D., Verissimo R.M., Boaretto F.B.M., Hassemer G., Peres V.F., Schirmer H., Picada J.N., Saffi J., Moura D.J. Toxicological evaluation of a standardized hydroethanolic extract from leaves of Plantago australis and its major compound, verbascoside. J. Ethnopharmacol. 2019;229:145–156. doi: 10.1016/j.jep.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Lee J.Y., Woo E.R., Kang K.W. Inhibition of lipopolysaccharide-inducible nitric oxide synthase expression by acteoside through blocking of AP-1 activation. J. Ethnopharmacol. 2005;97(3):561–566. doi: 10.1016/j.jep.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Ma S.H., Yada K., Lee H., Fukuda Y., Iida A., Suzuki K. Taheebo polyphenols attenuate free fatty acid-induced inflammation in murine and human macrophage cell lines as inhibitor of cyclooxygenase-2. Front. Nutr. 2017;4:63. doi: 10.3389/fnut.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reinke D., Kritas S., Polychronopoulos P., Skaltsounis A.L., Aligiannis N., Tran C.D. Herbal substance, acteoside, alleviates intestinal mucositis in mice. Gastroenterol. Res. Pract. 2015;2015 doi: 10.1155/2015/327872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng J.N., Zhuo J.Y., Nie J., Liu Y.L., Chen B.Y., Wu A.Z., Li Y.C. Phenylethanoid glycosides from callicarpa Kwangtungensis Chun attenuate TNF-alpha-induced cell damage by inhibiting NF-kappa B pathway and enhancing Nrf2 pathway in A549 cells. Front. Pharm. 2021;12 doi: 10.3389/fphar.2021.693983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jing W., Chunhua M., Shumin W. Effects of acteoside on lipopolysaccharide-induced inflammation in acute lung injury via regulation of NF-κB pathway in vivo and in vitro. Toxicol. Appl. Pharm. 2015;285(2):128–135. doi: 10.1016/j.taap.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Pesce M., Franceschelli S., Ferrone A., De Lutiis M.A., Patruno A., Grilli A., Felaco M., Speranza L. Verbascoside down-regulates some pro-inflammatory signal transduction pathways by increasing the activity of tyrosine phosphatase SHP-1 in the U937 cell line. J. Cell. Mol. Med. 2015;19(7):1548–1556. doi: 10.1111/jcmm.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu M., Yu S., Chen Y., Meng W., Chen H., He J., Shen J., Lin X. Acteoside promotes B cell-derived IL-10 production and ameliorates autoimmunity. J. Leukoc. Biol. 2022 doi: 10.1002/JLB.3MA0422-510R. [DOI] [PubMed] [Google Scholar]
- 46.Chang J.H., Chuang H.C., Hsiao G., Hou T.Y., Wang C.C., Huang S.C., Li B.Y., Lee Y.L. Acteoside exerts immunomodulatory effects on dendritic cells via aryl hydrocarbon receptor activation and ameliorates Th2-mediated allergic asthma by inducing Foxp3(+) regulatory T cells. Int. Immunopharmacol. 2022;106 doi: 10.1016/j.intimp.2022.108603. [DOI] [PubMed] [Google Scholar]
- 47.Yamada P., Iijima R., Han J., Shigemori H., Yokota S., Isoda H. Inhibitory effect of acteoside isolated from Cistanche tubulosa on chemical mediator release and inflammatory cytokine production by RBL-2H3 and KU812 cells. Planta Med. 2010;76(14):1512–1518. doi: 10.1055/s-0030-1249775. [DOI] [PubMed] [Google Scholar]
- 48.Motojima H., Villareal M.O., Iijima R., Han J., Isoda H. Acteoside inhibits type I (TM) allergy through the down-regulation of Ca/NFAT and JNK MAPK signaling pathways in basophilic cells. J. Nat. Med. 2013;67(4):790–798. doi: 10.1007/s11418-013-0753-4. [DOI] [PubMed] [Google Scholar]
- 49.Nam S.Y., Kim H.M., Jeong H.J. Attenuation of IL-32-induced caspase-1 and nuclear factor-kappa B activations by acteoside. Int. Immunopharmacol. 2015;29(2):574–582. doi: 10.1016/j.intimp.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 50.Yoou M.S., Kim H.M., Jeong H.J. Acteoside attenuates TSLP-induced mast cell proliferation via down-regulating MDM2. Int. Immunopharmacol. 2015;26(1):23–29. doi: 10.1016/j.intimp.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Dimitrova P., Alipieva K., Stojanov K., Milanova V., Georgiev M.I. Plant-derived verbascoside and isoverbascoside regulate Toll-like receptor 2 and 4-driven neutrophils priming and activation. Phytomed.: Int. J. Phytother. Phytopharm. 2019;55:105–118. doi: 10.1016/j.phymed.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 52.Li Y., Yu H., Jin Y., Li M., Qu C. Verbascoside alleviates atopic dermatitis-like symptoms in mice via its potent anti-inflammatory effect. Int. Arch. Allergy Immunol. 2018;175(4):220–230. doi: 10.1159/000486958. [DOI] [PubMed] [Google Scholar]
- 53.Sun X., Qi H., Wu H., Qu Y., Wang K. Anti-pruritic and anti-inflammatory effects of natural verbascoside through selective inhibition of temperature-sensitive Ca(2+)-permeable TRPV3 channel. J. Dermatol. Sci. 2020;97(3):229–231. doi: 10.1016/j.jdermsci.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 54.Gonçalves S., Anabela R., Neusa M., Tomás G. Antioxidant activity and verbascoside content in extracts from two uninvestigated endemic Plantago spp. Ind. Crops Prod. 2015;65:198–202. [Google Scholar]
- 55.Pierre Luhata L., Usuki T. Free radical scavenging activities of verbascoside and isoverbascoside from the leaves of Odontonema strictum (Acanthaceae) Bioorg. Med. Chem. Lett. 2022;59 doi: 10.1016/j.bmcl.2022.128528. [DOI] [PubMed] [Google Scholar]
- 56.Treml J., Vecerova P., Herczogova P., Smejkal K. Direct and indirect antioxidant effects of selected plant phenolics in cell-based assays. Molecules. 2021;26:2534. doi: 10.3390/molecules26092534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang H., Xu Y., Yan J., Zhao X., Sun X., Zhang Y., Guo J., Zhu C. Acteoside protects human neuroblastoma SH-SY5Y cells against beta-amyloid-induced cell injury. Brain Res. 2009;1283:139–147. doi: 10.1016/j.brainres.2009.05.101. [DOI] [PubMed] [Google Scholar]
- 58.Xia D.J., Zhang Z., Zhao Y.L. Acteoside attenuates oxidative stress and neuronal apoptosis in rats with focal cerebral ischemia reperfusion injury. Biol. Pharmaceut. Bull. 2018;41(11):1645–1651. doi: 10.1248/bpb.b18-00210. [DOI] [PubMed] [Google Scholar]
- 59.Değer A.N., Özyiğit F., Koçak F.E., Bayhan Z., Zeren S., Arık Ö., Değer H. Corrective effect of verbascoside on histomorphological differences and oxidative stress in colon mucosa of rats in which colon ischemia-reperfusion injury was induced. Turk. J. Gastroenterol.: Off. J. Turk. Soc. Gastroenterol. 2021;32(7):548–549. doi: 10.5152/tjg.2021.19747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ji S.L., Cao K.K., Zhao X.X., Kang N.X., Zhang Y., Xu Q.M., Yang S.L., Liu Y.L., Wang C. Antioxidant activity of phenylethanoid glycosides on glutamate-induced neurotoxicity. Biosci. Biotechnol. Biochem. 2019;83(11):2016–2026. doi: 10.1080/09168451.2019.1637243. [DOI] [PubMed] [Google Scholar]
- 61.Martino N.A., Marzano G., Nicassio M., Minervini F., Cardinali A., Lacalandra G.M., Hinrichs K., Dell'aquila M.E. Effects of verbascoside treatment during oocyte in vitro maturation on blastocyst development and bioenergetic/oxidative status after ICSI in the horse. J. Equine Vet. Sci. 2016;41:66. [Google Scholar]
- 62.Marzano G., Mastrorocco A., Zianni R., Mangiacotti M., Chiaravalle A.E., Lacalandra G.M., Minervini F., Cardinali A., Macciocca M., Vicenti R., Fabbri R., Hinrichs K., Dell’Aquila M.E., Martino N.A. Altered morphokinetics in equine embryos from oocytes exposed to DEHP during IVM. Mol. Reprod. Dev. 2019;86(10):1388–1404. doi: 10.1002/mrd.23156. [DOI] [PubMed] [Google Scholar]
- 63.Kim K.J., Chun J.L., Lee K.B., Lee J.H., Park K.S., Han K.W., Lee B.M., Kim E.Y., Kim J.M., Kim M.K. Effect of acteoside on the re-localization and abnormal morphology of mitochondria in porcine oocytes during in vitro maturation. J. Assist. Reprod. Genet. 2016;33(7):939–948. doi: 10.1007/s10815-016-0729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martino N.A., Ariu F., Bebbere D., Uranio M.F., Chirico A., Marzano G., Sardanelli A.M., Cardinali A., Minervini F., Bogliolo L., Dell’Aquila M.E. Supplementation with nanomolar concentrations of verbascoside during in vitro maturation improves embryo development by protecting the oocyte against oxidative stress: a large animal model study. Reprod. Toxicol. 2016;65:204–211. doi: 10.1016/j.reprotox.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 65.Liu Y.H., Lu Y.L., Han C.H., Hou W.C. Inhibitory activities of acteoside, isoacteoside, and its structural constituents against protein glycation in vitro. Bot. Stud. 2013;54:6. doi: 10.1186/1999-3110-54-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen C.Y., Jiang J.G., Yang L., Wang D.W., Zhu W. Anti-ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: pharmacological mechanisms and implications for drug discovery. Br. J. Pharm. 2017;174(11):1395–1425. doi: 10.1111/bph.13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiong L.N., Mao S.Q., Lu B.Y., Yang J.J., Zhou F., Hu Y.Z., Jiang Y.R., Shen C.X., Zhao Y.J. Osmanthus fragrans flower extract and acteoside protect against d-galactose-induced aging in an ICR mouse model. J. Med. Food. 2016;19(1):54–61. doi: 10.1089/jmf.2015.3462. [DOI] [PubMed] [Google Scholar]
- 68.Cheimonidi C., Samara P., Polychronopoulos P., Tsakiri E.N., Nikou T., Myrianthopoulos V., Sakellaropoulos T., Zoumpourlis V., Mikros E., Papassideri I., Argyropoulou A., Halabalaki M., Alexopoulos L.G., Skaltsounis A.L., Tsitsilonis O.E., Aligiannis N.N., Trougakos I.P. Selective cytotoxicity of the herbal substance acteoside against tumor cells and its mechanistic insights. Redox Biol. 2018;16:169–178. doi: 10.1016/j.redox.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Son Y.O., Lee S.A., Kim S.S., Jang Y.S., Chun J.C., Lee J.C. Acteoside inhibits melanogenesis in B16F10 cells through ERK activation and tyrosinase down-regulation. J. Pharm. Pharm. 2011;63(10):1309–1319. doi: 10.1111/j.2042-7158.2011.01335.x. [DOI] [PubMed] [Google Scholar]
- 70.Wu Y.Y., Zeng M.N., Xu R.Q., Zhang B.B., Wang S.C., Li B.K., Kan Y.X., Cao B., Zheng X.K., Feng W.S. Inhibitory activity of acteoside in melanoma via regulation of the ERA-Ras/Raf1-STAT3 pathway. Arch. Biochem. Biophys. 2021;710 doi: 10.1016/j.abb.2021.108978. [DOI] [PubMed] [Google Scholar]
- 71.Daneshforouz A., Nazemi S., Gholami O., Kafami M., Amin B. The cytotoxicity and apoptotic effects of verbascoside on breast cancer 4T1 cell line. BMC Pharm. Toxicol. 2021;22(1):72. doi: 10.1186/s40360-021-00540-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cai W., Xiong Chen Z., Rane G., Satendra Singh S., Choo Z., Wang C., Yuan Y., Zea Tan T., Arfuso F., Yap C.T., Pongor L.S., Yang H., Lee M.B., Cher Goh B., Sethi G., Benoukraf T., Tergaonkar V., Prem Kumar A. Wanted DEAD/H or alive: helicases winding up in cancers. J. Natl. Cancer Inst. 2017;109(6):djw278. doi: 10.1093/jnci/djw278. [DOI] [PubMed] [Google Scholar]
- 73.Jia W.Q., Zhu J.W., Yang C.Y., Ma J., Pu T.Y., Han G.Q., Zou M.M., Xu R.X. Verbascoside inhibits progression of glioblastoma cells by promoting Let-7g-5p and down-regulating HMGA2 via Wnt/beta-catenin signalling blockade. J. Cell. Mol. Med. 2020;24(5):2901–2916. doi: 10.1111/jcmm.14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iksen, Pothongsrisit S., Pongrakhananon V. Targeting the PI3K/AKT/mTOR signaling pathway in lung cancer: an update regarding potential drugs and natural products. Molecules. 2021;26(13):4100. doi: 10.3390/molecules26134100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Narayanankutty A. Inhibitory potential of dietary nutraceuticals on cellular PI3K/Akt signaling: implications in cancer prevention and therapy. Curr. Top. Med. Chem. 2021;21(20):1816–1831. doi: 10.2174/1568026621666210716152224. [DOI] [PubMed] [Google Scholar]
- 76.Ong P.S., Wang L.Z., Dai X., Tseng S.H., Loo S.J., Sethi G. Judicious toggling of mTOR activity to combat insulin resistance and cancer: current evidence and perspectives. Front. Pharm. 2016;7:395. doi: 10.3389/fphar.2016.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang H., Feng J., Ao F., Tang Y., Xu P., Wang M., Huang M. Tumor-derived exosomal microRNA-7-5p enhanced by verbascoside inhibits biological behaviors of glioblastoma in vitro and in vivo. Mol. Ther. Oncolytics. 2021;20:569–582. doi: 10.1016/j.omto.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Attia Y.M., El-Kersh D.M., Wagdy H.A., Elmazar M.M. Verbascoside: identification, quantification, and potential sensitization of colorectal cancer cells to 5-FU by targeting PI3K/AKT pathway. Sci. Rep. 2018;8:16939. doi: 10.1038/s41598-018-35083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu C.H., Chen C.H., Hsieh P.F., Lee Y.H., Kuo W.W., Wu R.C., Hung C.H., Yang Y.L., Lin V.C. Verbascoside inhibits the epithelial-mesenchymal transition of prostate cancer cells through high-mobility group box 1/receptor for advanced glycation end-products/TGF-β pathway. Environ. Toxicol. 2021;36(6):1080–1089. doi: 10.1002/tox.23107. [DOI] [PubMed] [Google Scholar]
- 80.Hei B., Wang J., Wu G., Ouyang J., Liu R.E. Verbascoside suppresses the migration and invasion of human glioblastoma cells via targeting c-Met-mediated epithelial-mesenchymal transition. Biochem. Biophys. Res. Commun. 2019;514(4):1270–1277. doi: 10.1016/j.bbrc.2019.05.096. [DOI] [PubMed] [Google Scholar]
- 81.Hwang Y.P., Kim H.G., Choi J.H., Park B.H., Jeong M.H., Jeong T.C., Jeong H.G. Acteoside inhibits PMA-induced matrix metalloproteinase-9 expression via CaMK/ERK- and JNK/NF-κB-dependent signaling. Mol. Nutr. Food Res. 2011;55 Suppl. 1:S103–S116. doi: 10.1002/mnfr.201000336. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Y.Q., Yuan Y., Wu H.M., Xie Z.Y., Wu Y.N., Song X.M., Wang J.J., Shu W., Xu J.Y., Liu B., Wan L.Z., Yan Y.A., Ding X., Shi X.H., Pan Y.C., Li X.K., Yang J.R., Zhao X.H., Wang L. Effect of verbascoside on apoptosis and metastasis in human oral squamous cell carcinoma. Int. J. Cancer. 2018;143(4):980–991. doi: 10.1002/ijc.31378. [DOI] [PubMed] [Google Scholar]
- 83.Zhou L., Feng Y., Jin Y., Liu X., Sui H., Chai N., Chen X., Liu N., Ji Q., Wang Y., Li Q. Verbascoside promotes apoptosis by regulating HIPK2-p53 signaling in human colorectal cancer. BMC Cancer. 2014;14:747. doi: 10.1186/1471-2407-14-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma D., Wang J., Liu L., Chen M.Q., Wang Z.Y. Acteoside as a potential therapeutic option for primary hepatocellular carcinoma: a preclinical study. BMC Cancer. 2020;20(1):936. doi: 10.1186/s12885-020-07447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perucatti A., Genualdo V., Pauciullo A., Iorio C., Incarnato D., Rossetti C., Vizzarri F., Palazzo M., Casamassima D., Iannuzzi L., Iannuzzi A. Cytogenetic tests reveal no toxicity in lymphocytes of rabbit (Oryctolagus cuniculus, 2n = 44) feed in presence of verbascoside and/or lycopene. Food Chem. Toxicol.: Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018;114:311–315. doi: 10.1016/j.fct.2018.02.053. [DOI] [PubMed] [Google Scholar]
- 86.Jang T.W., Choi J.S., Park J.H. Protective and inhibitory effects of acteoside from Abeliophyllum distichum Nakai against oxidative DNA damage. Mol. Med. Rep. 2020;22(3):2076–2084. doi: 10.3892/mmr.2020.11258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anter J., Tasset I., Demyda-Peyrás S., Ranchal I., Moreno-Millán M., Romero-Jimenez M., Muntané J., Luque de Castro M.D., Muñoz-Serrano A., Alonso-Moraga Á. Evaluation of potential antigenotoxic, cytotoxic and proapoptotic effects of the olive oil by-product “alperujo”, hydroxytyrosol, tyrosol and verbascoside. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014;772:25–33. doi: 10.1016/j.mrgentox.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 88.Santos-Cruz L.F., Ávila-Acevedo J.G., Ortega-Capitaine D., Ojeda-Duplancher J.C., Perdigón-Moya J.L., Hernández-Portilla L.B., López-Dionicio H., Durán-Díaz A., Dueñas-García I.E., Castañeda-Partida L., García-Bores A.M., Heres-Pulido M.E. Verbascoside is not genotoxic in the ST and HB crosses of the Drosophila wing spot test, and its constituent, caffeic acid, decreases the spontaneous mutation rate in the ST cross. Food Chem. Toxicol.: Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2012;50(3–4):1082–1090. doi: 10.1016/j.fct.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 89.Santoro A., Bianco G., Picerno P., Aquino R.P., Autore G., Marzocco S., Gazzerro P., Lioi M.B., Bifulco M. Verminoside- and verbascoside-induced genotoxicity on human lymphocytes: involvement of PARP-1 and p53 proteins. Toxicol. Lett. 2008;178(2):71–76. doi: 10.1016/j.toxlet.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 90.Viswanatha G.L., Venkataranganna M.V., Prasad N.B.L., Shylaja H. Achyranthes aspera Linn. alleviates cerebral ischemia-reperfusion-induced neurocognitive, biochemical, morphological and histological alterations in Wistar rats. J. Ethnopharmacol. 2019;228:58–69. doi: 10.1016/j.jep.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 91.Burgos C., Munoz-Mingarro D., Navarro I., Martin-Cordero C., Acero N. Neuroprotective potential of verbascoside isolated from Acanthus mollis L. leaves through its enzymatic inhibition and free radical scavenging ability. Antioxidants. 2020;9(12):1207. doi: 10.3390/antiox9121207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li W.T., Deng R.X., Jing X.S., Chen J.P., Yang D., Shen J.G. Acteoside ameliorates experimental autoimmune encephalomyelitis through inhibiting peroxynitrite-mediated mitophagy activation. Free Radic. Biol. Med. 2020;146:79–91. doi: 10.1016/j.freeradbiomed.2019.10.408. [DOI] [PubMed] [Google Scholar]
- 93.Wei W., Lu M., Lan X., Liu N., Wang H., Du J., Sun T., Li Y., Yu J. Neuroprotective effect of Verbascoside on hypoxic-ischemic brain damage in neonatal rat. Neurosci. Lett. 2019;711 doi: 10.1016/j.neulet.2019.134415. [DOI] [PubMed] [Google Scholar]
- 94.Viswanatha G.L., Shylaja H., Nandakumar K., Rajesh S., Male C. Acteoside isolated from Colebrookea oppositifolia attenuates I/R brain injury in Wistar rats via modulation of HIF-1 alpha, NF-kappa B, and VEGF pathways. Inflammopharmacology. 2021;29(5):1565–1577. doi: 10.1007/s10787-021-00851-6. [DOI] [PubMed] [Google Scholar]
- 95.Wu W.J., Wu G., Cao D.Y. Acteoside presents protective effects on cerebral ischemia/reperfusion injury through targeting CCL2, CXCL10, and ICAM1. Cell Biochem. Biophys. 2021;79(2):301–310. doi: 10.1007/s12013-020-00965-8. [DOI] [PubMed] [Google Scholar]
- 96.Shiao Y.J., Su M.H., Lin H.C., Wu C.R. Acteoside and isoacteoside protect amyloid beta peptide induced cytotoxicity, cognitive deficit and neurochemical disturbances in vitro and in vivo. Int. J. Mol. Sci. 2017;18(4):895. doi: 10.3390/ijms18040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kurisu M., Miyamae Y., Murakami K., Han J., Isoda H., Irie K., Shigemori H. Inhibition of amyloid beta aggregation by acteoside, a phenylethanoid glycoside. Biosci. Biotechnol. Biochem. 2013;77(6):1329–1332. doi: 10.1271/bbb.130101. [DOI] [PubMed] [Google Scholar]
- 98.Wang H.Q., Xu Y.X., Zhu C.Q. Upregulation of heme oxygenase-1 by acteoside through ERK and PI3 K/Akt pathway confer neuroprotection against beta-amyloid-induced neurotoxicity. Neurotox. Res. 2012;21(4):368–378. doi: 10.1007/s12640-011-9292-5. [DOI] [PubMed] [Google Scholar]
- 99.Li Y.Q., Chen Y., Jiang S.Q., Shi Y.Y., Jiang X.L., Wu S.S., Zhou P., Wang H.Y., Li P., Li F. An inhibitor of NF-κB and an agonist of AMPK: network prediction and multi-omics integration to derive signaling pathways for acteoside against Alzheimer’s disease. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.652310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Song H.S., Ko M.S., Jo Y.S., Whang W.K., Sim S.S. Inhibitory effect of acteoside on melittin-induced catecholamine exocytosis through inhibition of Ca2+-dependent phospholipase A(2) and extracellular Ca2+ influx in PC12 cells. Arch. Pharm. Res. 2015;38(10):1913–1920. doi: 10.1007/s12272-015-0601-z. [DOI] [PubMed] [Google Scholar]
- 101.Peng X.M., Gao L., Huo S.X., Liu X.M., Yan M. The mechanism of memory enhancement of acteoside (Verbascoside) in the senescent mouse model induced by a combination of D-gal and AlCl3. Phytother. Res.: PTR. 2015;29(8):1137–1144. doi: 10.1002/ptr.5358. [DOI] [PubMed] [Google Scholar]
- 102.Chen J.Y., Gao L., Zhang Y., Su Y., Kong Z., Wang D.Q., Yan M. Acteoside-improved streptozotocin-induced learning and memory impairment by upregulating hippocampal insulin, glucose transport, and energy metabolism. Phytother. Res. 2021;35(1):392–403. doi: 10.1002/ptr.6811. [DOI] [PubMed] [Google Scholar]
- 103.Yuan J.W., Ren J.P., Wang Y., He X., Zhao Y.W. Acteoside binds to caspase-3 and exerts neuroprotection in the rotenone rat model of Parkinson’s disease. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0162696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aimaiti M., Wumaier A., Aisa Y., Zhang Y., Xirepu X., Aibaidula Y., Lei X.Y., Chen Q., Feng X.Z., Mi N. Acteoside exerts neuroprotection effects in the model of Parkinson’s disease via inducing autophagy: network pharmacology and experimental study. Eur. J. Pharm. 2021;903 doi: 10.1016/j.ejphar.2021.174136. [DOI] [PubMed] [Google Scholar]
- 105.Li M.Q., Zhou F., Xu T., Song H.X., Lu B.Y. Acteoside protects against 6-OHDA-induced dopaminergic neuron damage via Nrf2-ARE signaling pathway. Food Chem. Toxicol. 2018;119:6–13. doi: 10.1016/j.fct.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 106.Xue Y.Y., Wang N., Zeng Z., Huang J.P., Xiang Z.M., Guan Y.Q. Neuroprotective effect of chitosan nanoparticle gene delivery system grafted with acteoside (ACT) in Parkinson’s disease models. J. Mater. Sci. Technol. 2020;43:197–207. [Google Scholar]
- 107.Koo K.A., Kim S.H., Oh T.H., Kim Y.C. Acteoside and its aglycones protect primary cultures of rat cortical cells from glutamate-induced excitotoxicity. Life Sci. 2006;79(7):709–716. doi: 10.1016/j.lfs.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 108.Viswanatha G.L., Shylaja H., Kishore D.V., Venkataranganna M.V., Prasad N.B.L. Acteoside isolated from Colebrookea oppositifolia Smith attenuates epilepsy in mice via modulation of gamma-aminobutyric acid pathways. Neurotox. Res. 2020;38(4):1010–1023. doi: 10.1007/s12640-020-00267-0. [DOI] [PubMed] [Google Scholar]
- 109.Kodani A., Kikuchi T., Tohda C. Acteoside improves muscle atrophy and motor function by inducing new myokine secretion in chronic spinal cord injury. J. Neurotrauma. 2019;36(12):1935–1948. doi: 10.1089/neu.2018.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xi X.T., Ma J., Chen Q.B., Wang X.W., Xia Y., Wen X.W., Yuan J., Li Y. Acteoside attenuates hydrogen peroxide-induced injury of retinal ganglion cells via the CASC2/miR-155/mTOR axis. Ann. Transl. Med. 2022;10(1):5. doi: 10.21037/atm-21-5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xi X., Chen Q., Ma J., Wang X., Xia Y., Wen X., Cai B., Li Y. Acteoside protects retinal ganglion cells from experimental glaucoma by activating the PI3K/AKT signaling pathway via caveolin 1 upregulation. Ann. Transl. Med. 2022;10(6):312. doi: 10.21037/atm-22-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen Q.B., Xi X.T., Zeng Y., He Z.D., Zhao J.F., Li Y. Acteoside inhibits autophagic apoptosis of retinal ganglion cells to rescue glaucoma-induced optic atrophy. J. Cell. Biochem. 2019;120(8):13133–13140. doi: 10.1002/jcb.28586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oh H., Kang D.G., Kwon T.O., Jang K.K., Chai K.Y., Yun Y.G., Chung H.T., Lee H.S. Four glycosides from the leaves of Abeliophyllum distichum with inhibitory effects on angiotensin converting enzyme. Phytother. Res.: PTR. 2003;17(7):811–813. doi: 10.1002/ptr.1199. [DOI] [PubMed] [Google Scholar]
- 114.Tong R.C., Qi M., Yang M., Li P.F., Wang D.D., Lan J.P., Wang Z.T., Yang L. Extract of Plantago asiatica L. seeds ameliorates hypertension in spontaneously hypertensive rats by inhibition of angiotensin converting enzyme. Front. Pharm. 2019;10:403. doi: 10.3389/fphar.2019.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhu X., Sun M., Guo H., Lu G., Gu J., Zhang L., Shi L., Gao J., Zhang D., Wang W., Liu J., Wang X. Verbascoside protects from LPS-induced septic cardiomyopathy via alleviating cardiac inflammation, oxidative stress and regulating mitochondrial dynamics. Ecotoxicol. Environ. Saf. 2022;233 doi: 10.1016/j.ecoenv.2022.113327. [DOI] [PubMed] [Google Scholar]
- 116.Zhou H., Zhang C., Huang C. Verbascoside attenuates acute inflammatory injury caused by an intracerebral hemorrhage through the suppression of NLRP3. Neurochem. Res. 2021;46(4):770–777. doi: 10.1007/s11064-020-03206-9. [DOI] [PubMed] [Google Scholar]
- 117.Lai X., Xiong Y., Zhou J., Yang F., Peng J., Chen L., Zhong W. Verbascoside attenuates acute inflammatory injury in experimental cerebral hemorrhage by suppressing TLR4. Biochem. Biophys. Res. Commun. 2019;519(4):721–726. doi: 10.1016/j.bbrc.2019.09.057. [DOI] [PubMed] [Google Scholar]
- 118.Fan Y., Zhang K. Verbascoside inhibits the progression of atherosclerosis in high fat diet induced atherosclerosis rat model. J. Physiol. Pharm.: Off. J. Pol. Physiol. Soc. 2021;72(3):329–337. doi: 10.26402/jpp.2021.3.03. [DOI] [PubMed] [Google Scholar]
- 119.Czerwińska M.E., Gąsińska E., Leśniak A., Krawczyk P., Kiss A.K., Naruszewicz M., Bujalska-Zadrożny M. Inhibitory effect of Ligustrum vulgare leaf extract on the development of neuropathic pain in a streptozotocin-induced rat model of diabetes. Phytomed.: Int. J. Phytother. Phytopharm. 2018;49:75–82. doi: 10.1016/j.phymed.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 120.Shimada H., Urabe Y., Okamoto Y., Li Z., Kawase A., Morikawa T., Tu P., Muraoka O., Iwaki M. Major constituents of Cistanche tubulosa, echinacoside and acteoside, inhibit sodium-dependent glucose cotransporter 1-mediated glucose uptake by intestinal epithelial cells. J. Funct. Foods. 2017;39:91–95. [Google Scholar]
- 121.Galli A., Marciani P., Marku A., Ghislanzoni S., Bertuzzi F., Rossi R., Di Giancamillo A., Castagna M., Perego C. Verbascoside protects pancreatic beta-cells against ER-stress. Biomedicines. 2020;8(12):582. doi: 10.3390/biomedicines8120582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Qiao Z.G., Tang J.X., Wu W., Tang J., Liu M. Acteoside inhibits inflammatory response via JAK/STAT signaling pathway in osteoarthritic rats. BMC Complement. Altern. Med. 2019;19(1):264. doi: 10.1186/s12906-019-2673-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ma H., Qin S., Zhao S. Osteoarthritis is prevented in rats by verbascoside via nuclear factor kappa B (NF-κB) pathway downregulation. Med. Sci. Monit.: Int. Med. J. Exp. Clin. Res. 2020;26 doi: 10.12659/MSM.921276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fan K.J., Li Y.W., Wu J., Wang Q.S., Xu B.X., Teng H., Chen S.J., Wang T.Y. Pharmacology and molecular docking study of cartilage protection of Chinese herbal medicine Fufang Shatai Heji (STHJ) by inhibiting the expression of MMPs in collagen-induced arthritis mice. Ann. Palliat. Med. 2022;2:466–479. doi: 10.21037/apm-21-1765. [DOI] [PubMed] [Google Scholar]
- 125.Yang L.L., Zhang B., Liu J.J., Dong Y.H., Li Y.T., Li N., Zhao X.J., Snooks H., Hu C.L., Ma X.Q. Protective effect of acteoside on ovariectomy-induced bone loss in mice. Int. J. Mol. Sci. 2019;20(12):2794. doi: 10.3390/ijms20122974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee S.Y., Lee K.S., Yi S.H., Kook S.H., Lee J.C. Acteoside suppresses RANKL-mediated osteoclastogenesis by inhibiting c-Fos induction and NF-kappa B pathway and attenuating ROS production. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gong W., Zhang N.D., Cheng G., Zhang Q.L., He Y.Q., Shen Y., Zhang Q., Zhu B., Zhang Q.Y., Qin L.P. Rehmannia glutinosa libosch extracts prevent bone loss and architectural deterioration and enhance osteoblastic bone formation by regulating the IGF-1/PI3K/mTOR pathway in streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2019;20(16):3964. doi: 10.3390/ijms20163964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen Y., Li Y.Q., Fang J.Y., Li P., Li F. Establishment of the concurrent experimental model of osteoporosis combined with Alzheimer's disease in rat and the dual-effects of echinacoside and acteoside from Cistanche tubulosa. J. Ethnopharmacol. 2020;257 doi: 10.1016/j.jep.2020.112834. [DOI] [PubMed] [Google Scholar]
- 129.Yang Y., Wang X., Gao Y., Wang H., Niu X. Insight into the dual inhibitory mechanism of verbascoside targeting serine/threonine phosphatase Stp1 against Staphylococcus aureus. Eur. J. Pharmaceut. Sci.: Off. J. Eur. Fed. Pharmaceut. Sci. 2021;157 doi: 10.1016/j.ejps.2020.105628. [DOI] [PubMed] [Google Scholar]
- 130.Chen F.G., Xie F., Yang B.L., Wang C.C., Liu S.G., Zhang Y.L. Streptococcus suis sortase A is Ca2+ independent and is inhibited by acteoside, isoquercitrin and baicalin. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0173767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chathuranga K., Kim M.S., Lee H.C., Kim T.H., Kim J.H., Gayan Chathuranga W.A., Ekanayaka P., Wijerathne H., Cho W.K., Kim H.I., Ma J.Y., Lee J.S. Anti-respiratory syncytial virus activity of Plantago asiatica and Clerodendrum trichotomum extracts in vitro and in vivo. Viruses. 2019;11(7):604. doi: 10.3390/v11070604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Song X., He J., Xu H., Hu X.P., Wu X.L., Wu H.Q., Liu L.Z., Liao C.H., Zeng Y., Li Y., Hao Y., Xu C.S., Fan L., Zhang J., Zhang H.J., He Z.D. The antiviral effects of acteoside and the underlying IFN-γ-inducing action. Food Funct. 2016;7(7):3017–3030. doi: 10.1039/c6fo00335d. [DOI] [PubMed] [Google Scholar]
- 133.Zhao X.R., Li H.E., Wang J.F., Guo Y., Liu B.W., Deng X.M., Niu X.D. Verbascoside alleviates Pneumococcal Pneumonia by reducing pneumolysin oligomers. Mol. Pharm. 2016;89(3):376–387. doi: 10.1124/mol.115.100610. [DOI] [PubMed] [Google Scholar]
- 134.Khattab A.R., Teleb M., Kamel M.S. In silico study of potential anti-SARS cell entry phytoligands from Phlomis aurea: a promising avenue for prophylaxis. Future Virol. 2021;16(11):761–775. doi: 10.2217/fvl-2021-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Abdallah H.M., El-Halawany A.M., Sirwi A., El-Araby A.M., Mohamed G.A., Ibrahim S.R.M., Koshak A.E., Asfour H.Z., Awan Z.A., Elfaky M.A. Repurposing of some natural product isolates as SARS-COV-2 main protease inhibitors via in vitro cell free and cell-based antiviral assessments and molecular modeling approaches. Pharmaceuticals. 2021;14(3) doi: 10.3390/ph14030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maquiaveli C.C., Lucon-Júnior J.F., Brogi S., Campiani G., Gemma S., Vieira P.C., Silva E.R. Verbascoside inhibits promastigote growth and arginase activity of Leishmania amazonensis. J. Nat. Prod. 2016;79(5):1459–1463. doi: 10.1021/acs.jnatprod.5b00875. [DOI] [PubMed] [Google Scholar]
- 137.Maquiaveli C.D.C., Rochetti A.L., Fukumasu H., Vieira P.C., da Silva E.R. Antileishmanial activity of verbascoside: selective arginase inhibition of intracellular amastigotes of Leishmania (Leishmania) amazonensis with resistance induced by LPS plus IFN-γ. Biochem. Pharm. 2017;127:28–33. doi: 10.1016/j.bcp.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 138.Sun L., Yu F., Yi F., Xu L.J., Jiang B.P., Le L., Xiao P.G. Acteoside from Ligustrum robustum (Roxb.) blume ameliorates lipid metabolism and synthesis in a HepG2 cell model of lipid accumulation. Front. Pharm. 2019;10:602. doi: 10.3389/fphar.2019.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee K.J., Woo E.R., Choi C.Y., Shin D.W., Lee D.G., You H.J., Jeong H.G. Protective effect of acteoside on carbon tetrachloride-induced hepatotoxicity. Life Sci. 2004;74(8):1051–1064. doi: 10.1016/j.lfs.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 140.Hayashi K., Nagamatsu T., Ito M., Hattori T., Suzuki Y. Acteoside, a component of Stachys sieboldii MIQ, may be a promising antinephritic agent (2): effect of acteoside on leukocyte accumulation in the glomeruli of nephritic rats. Jpn. J. Pharm. 1994;66(1):47–52. doi: 10.1254/jjp.66.47. [DOI] [PubMed] [Google Scholar]
- 141.Hayashi K., Nagamatsu T., Ito M., Yagita H., Suzuki Y. Acteoside, a component of Stachys sieboldii MIQ, may be a promising antinephritic agent (3): effect of aceteoside on expression of intercellular adhesion molecule-1 in experimental nephritic glomeruli in rats and cultured endothelial cells. Jpn. J. Pharm. 1996;70(2):157–168. doi: 10.1254/jjp.70.157. [DOI] [PubMed] [Google Scholar]
- 142.Gan L., Li X.Z., Zhu M.Y., Chen C., Luo H.M., Zhou Q.L. Acteoside relieves mesangial cell injury by regulating Th22 cell chemotaxis and proliferation in IgA nephropathy. Ren. Fail. 2018;40(1):364–370. doi: 10.1080/0886022X.2018.1450762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gao W., Zheng S., Hwang E., Yi T.H., Wang Y.S. Effects of phenylethanol glycosides from Orobanche cernua Loefling on UVB-Induced skin photodamage: a comparative study. Photochem. Photobiol. Sci. 2021;20(5):599–614. doi: 10.1007/s43630-021-00038-6. [DOI] [PubMed] [Google Scholar]
- 144.Yang J.H., Yan Y., Liu H.B., Wang J.H., Hu J.P. Protective effects of acteoside against X-ray-induced damage in human skin fibroblasts. Mol. Med. Rep. 2015;12(2):2301–2306. doi: 10.3892/mmr.2015.3630. [DOI] [PubMed] [Google Scholar]
- 145.Si N., Kanazawa H., Okuyama K., Imada K., Wang H.J., Yang J., Zhao H.Y., Bian B.L., Ito A., Sato T. Involvement of catechols in acteoside in the activation of promatrix metalloproteinase-2 and membrane type-1-matrix metalloproteinase expression via a phosphatidylinositol-3-kinase pathway in human dermal fibroblasts. Biol. Pharmaceut. Bull. 2018;41(10):1530–1536. doi: 10.1248/bpb.b18-00107. [DOI] [PubMed] [Google Scholar]
- 146.Sperotto N.D.D., Steffens L., Verissimo R.M., Henn J.G., Peres V.F., Vianna P., Chies J.A.B., Roehe A., Saffi J., Moura D.J. Wound healing and anti-inflammatory activities induced by a Plantago australis hydroethanolic extract standardized in verbascoside. J. Ethnopharmacol. 2018;225:178–188. doi: 10.1016/j.jep.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 147.Zhu M., Zhu H., Tan N., Wang H., Chu H., Zhang C. Central anti-fatigue activity of verbascoside. Neurosci. Lett. 2016;616:75–79. doi: 10.1016/j.neulet.2016.01.042. [DOI] [PubMed] [Google Scholar]
- 148.Wu X.L., He W.Y., Zhang H.P., Li Y., Liu Z.G., He Z.D. Acteoside: a lipase inhibitor from the Chinese tea Ligustrum purpurascens kudingcha. Food Chem. 2014;142:306–310. doi: 10.1016/j.foodchem.2013.07.071. [DOI] [PubMed] [Google Scholar]
- 149.Casamassima D., Palazzo M., Martemucci G., Vizzarri F., Corino C. Effects of verbascoside on plasma oxidative status and blood and milk production parameters during the peripartum period in Lacaune ewes. Small Rumin. Res. 2012;105(1):1–8. [Google Scholar]
- 150.Han L., Xiang S., Rong B., Liang Y., Zhao S. Verbascoside attenuates experimental varicocele-induced damage to testes and sperm levels through up-regulation of the hypothalamus-pituitary-gonadal (HPG) axis. Pharm. Biol. 2021;59(1):715–722. doi: 10.1080/13880209.2021.1933085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Papoutsi Z., Kassi E., Mitakou S., Aligiannis N., Tsiapara A., Chrousos G.P., Moutsatsou P. Acteoside and martynoside exhibit estrogenic/antiestrogenic properties. J. Steroid Biochem. Mol. Biol. 2006;98(1):63–71. doi: 10.1016/j.jsbmb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 152.Singh N., Shukla N., Singh P., Sharma R., Rajendran S.M., Maurya R., Palit G. Verbascoside isolated from Tectona grandis mediates gastric protection in rats via inhibiting proton pump activity. Fitoterapia. 2010;81(7):755–761. doi: 10.1016/j.fitote.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 153.Ryu J.S., Jang B.H., Jo Y.S., Kim S.J., Eom T.I., Kim M.C., Ko H.J., Sim S.S. The effect of acteoside on intracellular Ca(2+) mobilization and phospholipase C activity in RBL-2H3 cells stimulated by melittin. Arch. Pharm. Res. 2014;37(2):239–244. doi: 10.1007/s12272-013-0208-1. [DOI] [PubMed] [Google Scholar]
- 154.Wan Y., Zou B., Zeng H.L., Zhang L.N., Chen M., Fu G.M. Inhibitory effect of verbascoside on xanthine oxidase activity. Int. J. Biol. Macromol. 2016;93:609–614. doi: 10.1016/j.ijbiomac.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 155.Aggul A.G., Taslimi P., Kuzu M., Uzun N., Bilginer S., Gulcin I. Oleuropein and verbascoside – their inhibition effects on carbonic anhydrase and molecular docking studies. J. Oleo Sci. 2021;70(9):1275–1283. doi: 10.5650/jos.ess21106. [DOI] [PubMed] [Google Scholar]
- 156.Carmona F., Coneglian F.S., Batista P.A., Aragon D.C., Angelucci M.A., Martinez E.Z., Pereira A.M.S. Aloysia polystachya (Griseb.) Moldenke (Verbenaceae) powdered leaves are effective in treating anxiety symptoms: a phase-2, randomized, placebo-controlled clinical trial. J. Ethnopharmacol. 2019;242 doi: 10.1016/j.jep.2019.112060. [DOI] [PubMed] [Google Scholar]
- 157.Campo G., Pavasini R., Biscaglia S., Ferri A., Andrenacci E., Tebaldi M., Ferrari R. Platelet aggregation values in patients with cardiovascular risk factors are reduced by verbascoside treatment. A randomized study. Pharm. Res. 2015;97:1–6. doi: 10.1016/j.phrs.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 158.Campo G., Marchesini J., Bristot L., Monti M., Gambetti S., Pavasini R., Pollina A., Ferrari R. The in vitro effects of verbascoside on human platelet aggregation. J. Thromb. Thrombolysis. 2012;34(3):318–325. doi: 10.1007/s11239-012-0757-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.