Abstract
Coronavirus disease 2019 (COVID-19) represents a global healthcare crisis that has led to morbidity and mortality on an unprecedented scale. While studies on COVID-19 vaccines are ongoing, the knowledge about the reactogenic symptoms that can occur after vaccination and its generator mechanisms can be critical for healthcare professionals to improve compliance with the future vaccination campaign. Because sleep and immunity are bidirectionally linked, sleepiness or sleep disturbance side effects reported after some of the COVID-19 vaccines advise an academic research line in the context of physiological or pathological neuroimmune interactions. On the recognized basis of inflammatory regulation of hypothalamic neurons in sickness behavior, we hypothesized that IL-1β, INF-γ and TNF-α pro-inflammatory cytokines inhibit orexinergic neurons promoting sleepiness after peripheral activation of the innate immune system induced by the novel COVID-19 vaccines. In addition, based on knowledge of previous vaccines and disease manifestations of SARS-CoV-2 infection, it also suggests that narcolepsy must be included as potential adverse events of particular interest to consider in pharmacovigilance studies.
Keywords: Orexin, COVID-19 vaccines, Reactogenicity, Narcolepsy, Sleep
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has led to more than 434 million confirmed cases and more than 5 million deaths. Afterward, global efforts focus on developing safe and efficacious vaccines for its prevention [1]. Currently, there are a few provisionally licensed vaccines against COVID-19, 146 candidate vaccines in clinical development, and another 195 candidate vaccines in preclinical development. Especially in protein subunit, DNA, RNA- or viral vector-based vaccines, which platforms offer the potential of rapid development during a pandemic but represent a major challenge for science and medicine [2]. As of 27 February 2022, a total of 10,585,766,316 vaccine doses have been administered [1]. Although the COVID-19 crisis is a global emergency, the benefits versus the risks of vaccination put a large emphasis on safety because vaccinating large numbers of healthy people have a significant ethical implication. The broader term ‘safety’ profile refers to all adverse events that could potentially be caused/triggered or worsened at any time after vaccination, such as anaphylactic reactions, diseases diagnosed after vaccination, and autoimmune events [3]. However, to trigger an effective adaptive immune response by vaccine antigen, a certain level of inflammation is necessary. Hence, the subset of local reactions that occur soon after vaccination (pain, redness, swelling or induration at the injection site) and systemic symptoms, such as fever, myalgia, headache, malaise, or sickness behaviors syndrome, are defined as reactogenicity [4]. These symptoms represent a physical manifestation of the inflammatory response to vaccination, typically higher after a second dose [5]. The brain processes information generated by the innate immune system through cytokine signaling [6,7]. In particular, reactogenic systemic symptoms such as hypersomnolence and reduced sleep quality could suggest destabilized arousal states. Nevertheless, the studies assessing sleep alterations after vaccination are limited [7,8].
On the other hand, reactogenicity symptoms are an outworking of the expected immune response that occurs in response to vaccination. Interestingly, an association between reactogenic fever and SARS-CoV-2 antibodies after the second dose of the BNT162b2 COVID-19 vaccine has been reported [9]. But balancing the beneficial and detrimental effects of these inflammatory events is necessary to keep reactogenicity at clinically acceptable levels [4]. Current analysis of COVID-19 homologous and heterologous vaccination schedules has shown a manageable reactogenicity profile [[10], [11], [12]].
A brief outline of sleep response in the context of COVID-19 vaccination provides some information about the frequent occurrence of sleepiness after its administration [[12], [13], [14], [15], [16]]. Then, considering that orexin (Ox) hypothalamic neurons are the key brain substrate controlling wakefulness and mediate some aspects of sickness behavior, we hypothesize its implication in reactogenic sleepiness after peripheral activation of the innate immune system by some of the COVID-19 vaccines. At the same time, this circumstance should be an alert to be considered in pharmacovigilance studies and future research to understand additional possible long-term inflammatory mechanisms to preserve confidence in these new vaccines.
2. Sleep response to acute immune activation in the context of COVID-19 vaccination
Reciprocal neuroimmune interactions, which have an impact on physiological and many pathological conditions, are recognized [[17], [18], [19]]. Particularly, sleep and immunity are bidirectionally linked, but cellular and molecular mechanisms underlying these interactions are not entirely understood [8]. Prolonged sleep deficiency can lead to chronic, systemic low-grade inflammation and is associated with various diseases that have an inflammatory component, like diabetes, atherosclerosis, cancer, and neurodegeneration [8,19]. Given that lymphocyte lymph node trafficking and adaptive immune responses shows circadian rhythmicity, post-vaccination sleep and morning timing of vaccination as possible natural immune adjuvants for COVID-19 vaccination have been proposed [20]. On the other hand, sleepiness or sleep disturbance as minor to moderate side effects after Pfizer-BioNTech COVID-19 vaccination have been reported [13,14]. Likewise, in some cases, after protein subunit candidates, a number of participants reported a desire to sleep or somnolence [12]. This fact could be a good sign that the vaccine is working because a more pronounced immune activation during an infection can induce a sleep response that in turn may support host defense and immunological memory formation [8,21]. Healthy individuals who received the hepatitis A virus or influenza A H1N1 (swine flu) virus vaccines did not appear to show any significant sleep changes in the night following the vaccination. However, sleep data was not compared to a placebo-vaccinated control group in these studies [8]. Actually, epidemiological evidence on the relationship between reactogenicity and immunogenicity of COVID-19 vaccines is still limited [9].
In response to acute immune activation, the sleep-modulating effect is triggered by pathogens' components and decomposition products, including pathogen-associated molecular patterns (PAMPs) such as endotoxins, lipids, peptides, and viral double-stranded RNA. PAMPs are recognized by their respective pattern recognition receptors (PRRs) like Toll-like receptors (TLRs) and the nucleotide-binding oligomerization domain-like receptors (Nod-like receptors, NLRs) on tissue-resident macrophages or dendritic cells. Furthermore, downstream pathways like the transcription factor NF-κB and inflammasomes, which induce cytokines and prostaglandins expression [8]. Vaccine antigens as potential pathogens are recognized, and mostly vaccination studies have examined the effects of naturally occurring or experimentally induced sleep loss on immunological responses, but studies investigating alterations of sleep itself in response to vaccination are rare [2,7]. During an infectious challenge or acute mild immune activation, decreases in rapid-eye-movement (REM) sleep and increases in non-REM (NREM) sleep amount or intensity (i.e., increased slow-wave sleep), which are related to the release of interleukin (IL) 1 (IL-1α and IL-1β) and tumor necrosis factor-α (TNF-α) have been observed [9,16]. These cytokines, in addition to adenosine (AD) and prostaglandin D2 (PGD2) are considered NREM sleep-promoting substances or somnogens [22,23]. However, several other mediators such as hormones of the hypothalamus-pituitary-adrenal (HPA) axis, classical neurotransmitters [glutamate, acetylcholine, serotonin, γ-aminobutyric acid (GABA), noradrenaline, histamine, and dopamine], neuropeptides such as orexin (Ox), and the hormone melatonin are involved in the neurobiological bases and regulation of sleep [7,8]. These molecules may interact with the immune system to promote or inhibit sleep, even more in a stressful context as the COVID-19 crisis, because an imbalance in the functional interaction between the nervous and immune systems may occur caused by destabilizing factors such as the generalized lockdown and social distancing measures [18,24].
3. Place of orexin neurons in sleep-wake regulation
The inhibition-stimulation interaction between the posterior and lateral hypothalamic circuits of wakefulness and the sleep system of the anterior hypothalamus establishes the base of a reciprocal model that results in the stability of the wake or sleep states [25,26]. Wake-promoting cell types include Ox and a population of GABA-releasing neurons of the lateral hypothalamus (LH), which promote the transition to wakefulness from NREM and REM sleep [27,28]. Ox neurons, in particular, have been implicated in the facilitation and maintenance of arousal, exhibiting wake-associated discharge and quiescent during NREM and REM sleep [25]. Activation of LH GABAergic neurons in vivo rapidly drives wakefulness via direct inhibition of galaninergic neurons within the preoptic area of the hypothalamus [29]. In contrast, melanin-concentrating hormone (MCH) neurons in the perifornical-lateral hypothalamic area (PF-LHA) discharge selectively during sleep [30]. Interesting studies in Ox knockout mice indicate a critical role for Ox neurons in regulating sleep and wakefulness. These animals exhibit marked hypophagia, hypolocomotion, hypersomnolence, and an inability to adapt to restricted feeding, a phenotype resembling sickness behavior [31]. Orexin is a neuropeptide primarily synthesized and secreted by neurons of the lateral hypothalamic area (LHA) and parts of the dorsomedial nucleus of the hypothalamus (DMH), perifornical area, and posterior hypothalamus. A single orexin precursor is processed into two isoforms (orexin A [OxA] and orexin B [OxB]). Orexin neurons project widely throughout the brain onto target regions exhibits an excitatory influence on arousal-related neurons, stabilizing wakefulness. These effects are mediated by two G protein-coupled receptor subtypes located post-synaptically. Ox receptors type-1 (OX1R) acts thought to signal via Gα11 class of G-protein, leading to increases in phospholipase C and subsequent phosphatidylinositol signaling, resulting in Ca2+ influx. At the same time, Ox receptors type-2 (OX2R) is coupled to both the Gα11 excitatory and the Gi inhibitory G-protein families [32,33]. In particular, OX2R has been more involved in controlling food intake and arousal, whereas the OX1R is in reward-seeking and drug addiction [33].
Among many target areas including the serotonergic dorsal raphe (DR) nucleus, noradrenergic locus coeruleus (LC), histaminergic tuberomammillary nucleus (TMN), dopaminergic ventral tegmental area (VTA), and cholinergic basal forebrain (BF), mesopontine laterodorsal tegmental nucleus (LDT) and pedunculopontine nucleus (PPT), all of which are involved in promoting arousal [34,35]. Also, the ventral periaqueductal gray matter (vPAG) dopaminergic cells provide a powerful ascending dopaminergic waking influence [36]. On the other hand, neural inputs regulating Ox neurons arise from BF cholinergic neurons, preoptic area GABAergic neurons, serotonergic raphe neurons, lateral septum, bed nucleus of the stria terminalis, dorsomedial nucleus, periaqueductal gray, amygdala, and many hypothalamic regions, which are implicated in a dynamic reciprocal control between sleep and arousal centers [25,34]. In addition, dense projections from Ox neurons in other spinal, diencephalic and cortical regions are involved in regulating diverse behavioral and physiological functions [34]. Therefore, Ox is not only implicated in the regulation of eating, the sleep-wake cycle, and energy metabolism, but also closely associated with cardiovascular control, reproduction, stress, reward, addiction, cognition and mood, and the modulation of pain transmission [37,38].
The hypothalamic ventrolateral preoptic area (VLPO) and median preoptic nucleus (MnPO) contain neurons essential for promoting initiation and maintenance NREM sleep [39]. VLPO and MnPO sleep-active neurons are GABAergic, and VLPO neurons also produce the inhibitory neuropeptide galanin (GAL). In general, approximately 85% of these neurons contain GAL and GABA [39,40]. Both cell groups strongly innervate and inhibit arousal-promoting brain regions, including the cholinergic neurons of the BF, Ox neurons, TMN, DR, median raphe, parabrachial nucleus, and LC [25,26]. Current studies have focused on gliotransmission's impact on sleep-immune interactions [41,42]. Especially, an astroglial modulation mediated by astrocyte-derived ATP and its subsequent hydrolysis to AD on the VLPO neurons have been proposed [23,42]. It has been postulated that ATP released from the VLPO astrocytes may inhibit the VLPO interneurons via adenosine A1 receptors (A1Rs), and then disinhibit VLPO sleep-promoting neurons, possibly galaninergic, thereby increasing sleep [40,43,44]. In addition, PGD2, an extensively studied physiological sleep-promoting substance, may mediate its action by inducing leptomeningeal cells in proximity to the posterior hypothalamus to release AD. Which subsequently excite via adenosine A2 receptors (A2Rs) nearby sleep active VLPO neurons that inhibit histaminergic wake neurons in the TMN [25,45]. Direct connectivity between preoptic area GABAergic neurons and Ox neurons has been reported [28,34,46]. GABAergic neurons from sleep-promoting VLPO send inhibitory projections to Ox neurons, which express GABAA and GABAB receptors [[46], [47], [48]]. In particular, the blockade of GABAA receptors completely abolished the post-synaptic effect of GABAergic axon stimulation, suggesting that releasing other transmitters, such as galanin, is insufficient to alter Ox neuron firing [46]. Besides, Ox neurons are under inhibitory control by local GABAergic neurons [49]. Interestingly, the LHA, adjacent to well-characterized Ox and MCH neurons, comprises a large population of peptidergic neurons neurochemically defined by the presence of neurotensin (Nts). At least subpopulations of LHA Nts neurons may co-express other neuropeptides, including GAL and corticotrophin-releasing hormone, and classical neurotransmitters such as GABA and glutamate, but the precise characterization of LHA Nts neurons with different molecular signatures have yet to be fully elucidated [50,51]. Some subpopulations of these neurons co-express the long form of the leptin receptor (LepRb) and are activated by the adipose-derived hormone leptin [50]. Recent data support the role of GAL as an important mediator of leptin action to modulate nutrient reward by inhibiting orexin neurons via GAL receptor 1 (GalR1) [52]. On the other hand, Nts neurons project to dopaminergic VTA and locally to Ox neurons playing an important role in wake promotion and producing a hyperarousal state in response to acute stress [53]. However, these cells may act as a “master orchestrator” depending on the stress signals and possibly excite or inhibit orexin neurons by releasing either Nts or galanin, respectively [52,53].
4. Inflammatory regulation of the arousal. A possible implication of the orexinergic system in reactogenic sleepiness after COVID-19 vaccines
Several evidences indicate that OxA can possess immune-regulatory properties [18]. Interestingly, Ox neuron suppressions mediate inflammation-induced lethargy [54]. In addition, a localized reduction in cFos immunoreactivity of LHA after inflammatory insults has been reported, as well as that, LHA glucose-sensitive neurons reduced their electrical activity in the presence of IL-1β or TNF-α [54,55]. It has also been recognized that TNF-α acts on hypothalamic sleep regulatory circuits and the locus coeruleus to promote NREM sleep [56,57]. Several findings from animal studies established both cytokines, IL-1β and TNF-α, as substances involved in the homeostatic regulation of sleep and the sleep responses to pathologies [19,22]. IL-1β enhances the firing rate of basal forebrain and anterior hypothalamic sleep-active neurons while it inhibits wake-active neurons [22]. Blocking its biological actions reduced physiological NREM sleep amount or NREM sleep rebound after sleep deprivation. Moreover, increasing the availability of those cytokines promoted NREM sleep amount and intensity and suppressed REM sleep amount [8]. Experiments in genetically engineered mice have revealed the contribution of the IL-1 receptor type 1 (IL-1R1) and the 55 kD TNF receptor (TNF-1R) to cytokines enhanced NREMS [22,58]. Nevertheless, other cytokines, including interferon-gamma (IFN-γ), IL-2, IL-4, IL-6, IL-10, IL-13, IL-15, and IL-18, also appear to have some sleep regulatory properties but have received less attention than IL-1β and TNF-α [7,22].
On the other hand, various studies have linked vaccine-induced reactogenic side effects to producing these pro-inflammatory cytokines in humans [59]. Different inflammatory parameters associated with systemic reactogenicity following vaccination with viral antigens have been identified, such as IL-1β, IL-6, and IFN-signalling pathway [60,61]. IL-1 seems to be the predominant mediator of sickness behavior in the brain because blockade of its action by central administration of the IL-1 receptor antagonist attenuates cytokine-induced sickness behavior measured by either depression of social exploration or reduction of food intake [6]. IL-1 receptors are broadly expressed on neurons, glial cells, and endothelial cells of brain venules in many brain regions such as the hypothalamus, cortex, hippocampus, and cerebellum [58,62]. In general, vaccines produce milder and more prolonged immune responses with a favorable safety profile; but several studies have successfully used typhoid vaccine to examine sickness behavior, showing increased inflammatory cytokines, sleep disturbances, neurocognitive and psychomotor alterations, and worsened mood [63]. Also, the influenza vaccine has a more pronounced change in the affective state in patients with depression and anxiety than in mentally healthy individuals [64]. However, the underlying mechanisms of the vaccine reactogenicity remain poorly understood, an affirmation that is also valid for new COVID-19 vaccines, which are currently under research [3]. The release of pyrogenic cytokines and prostaglandins into the systemic circulation is thought to stimulate a cascade of immune and nervous system crosstalk that can lead to systemic ‘influenza-like’ symptoms, including fever after immunization [4]. Cytokines act on the brain via a relatively fast neural route represented by the primary afferent neurons that innervate the body site of the vaccine injection resulting in the production of brain pro-inflammatory cytokines by microglial cells. In addition, a slower humoral and cellular pathway that involves the production of brain pro-inflammatory cytokines in the circumventricular organs (CVOs) and choroid plexus in response to circulating PAMPs or cytokines has been recognized [62]. The vasculature in CVOs possess fenestrated capillaries with relative permeability to macromolecules, and these structures have main projections to hypothalamic nuclear groups that regulate homeostasis [17,62]. In both pathways, its actions can be mediated by prostaglandins or nitric oxide produced in endothelial cells of cerebral blood vessels and perivascular macrophages. Moreover, the entry of immune cells into the brain parenchyma via the choroid plexus and the recently discovered meningeal lymphatic drainage system in the meninges play a significant role in immune-brain networks [19,65].
Inflammatory cytokines directly modulate the activity of hypothalamic melanocortin and thermoregulatory neurons, but it has been proposed that Ox neurons are not direct targets for inflammatory signaling. Putatively a subset of perifornical LHA Nts-expressing GABAergic neurons is activated during inflammatory challenge mediating the inhibition of Ox neuron activity [54]. Like arcuate-nucleus proopiomelanocortin neurons, LHA LepRb-/GABA-/Nts-expressing neurons are considered cytokine-sensitive interneurons that play a dual role in the allostatic suppression of arousal by overnutrition and inflammation [54]. Curiously, leptin has pro-inflammatory properties and upregulates the secretion of multiple inflammatory cytokines, including TNF-α. Leptin receptors are class I cytokine receptors encoded by the db gene that vary in length due to alternative splicing, which are expressed on multiple immune cell types, making the activity of leptin influential on different immune functions [66].
Furthermore, it has been reported that IL-1β directly inhibits wake-promoting neurons, probably cholinergic, and stimulates a subset of GABAergic VLPO sleep-promoting neurons [67]. In particular, IL-1R1 has been involved in NREM sleep enhancement [22]. However, in the context of neuron-glia interactions, cytokines may also act in the anterior hypothalamus and basal forebrain by modulating the local release of AD, an essential intermediate linking inflammation and somnolence [68]. Recent studies indicate that microglia are potentially critical components of sleep regulatory mechanisms [23,42]. A possible effector of microglial influences on sleep-wake behavior may be extracellular ATP acting at the purinergic type 2 receptor P2X7 (P2X7R). Activation of glial P2X7R by ATP mediates post-translational processing of sleep regulatory pro-inflammatory cytokines [69]. Microglia induce astrocyte activation via expression of pro-inflammatory cytokines, including IL-1β, TNF-α, and IFN-γ, activated astrocytes, in turn, produce sleep regulatory substances as ATP/AD in response to immune challenge [42]. Especially, AD acting via A1R plays a role in regulating sleep by inhibiting PF-LHA neurons [70]. Ox neurons express A1R, which induce inhibitory influences, most potently via presynaptic inhibition of the glutamatergic input or excitatory post-synaptic potential [71,72]. However, A1R-mediated inhibition of other cholinergic, histaminergic and noradrenergic arousal-related neurons [25,68].
IL-1 promotes NREM sleep, in part, by inhibiting wake-promoting monoaminergic systems in dorsal raphe and locus coeruleus in a dose-dependent manner [70,84]. Hence, higher doses of IL-1, like experimental and natural inflammation, increase locus coeruleus neuronal activity [73]. Particularly, monoaminergic neurotransmitters implicated in the arousal ascending system, which are activated by Ox neurons, exert inhibitory feedback on the orexinergic system. Both serotonin (5-hydroxytryptamine, 5-HT) and noradrenaline (NA) hyperpolarize and inhibit Ox neurons by activating G-protein-regulated inwardly rectifying K+ channels. These effects are mediated by α2-adrenoreceptors and 5-HT1A receptors, each of which is expressed by Ox neurons. On the contrary, Ox neurons do not express (DA) receptors, but dopamine can inhibit Ox neurons via the α2-adrenoreceptor [74,75]. Interestingly all of these centers are stimulated by lipopolysaccharide (LPS) experimental treatment suggesting other significant inhibitory inputs on Ox neurons in a central inflammatory context [[76], [77], [78]].
Then, considering the role of inflammatory regulation of hypothalamic neurons in sickness behavior, and because the most often investigated cytokines in the context of sleep research are the acute phase cytokines TNF-α and IL-1, we speculate that following vaccination with COVID-19 vaccines, these pro-inflammatory cytokines may propagate an inflammatory response to the hypothalamus promoting sleepiness through these pathways. Although the sleep-promoting effect of central nervous system (CNS) cytokines results from altered neuronal activity throughout several nuclei comprising the ascending arousal system, this hypothesis is focused on hypothalamic Ox neurons because its crucial role is to reinforce arousal systems [34,46]. Given that this subset of inflammation-sensitive, arousal-associated Ox neurons in the perifornical area is involved in anorectic and lethargic responses to sickness [54], points to these cells as possible targets during inflammatory challenge after vaccination (Fig. 1 A–B). However, further investigations are necessary to corroborate our hypothesis in the complex context of the reciprocal regulation of sleep and innate immunity. In addition, because specific sleep parameters, particularly slow-wave sleep intensity, are considered predictive of the magnitude of an antigen-specific immune response [21], the impact of reactogenic sleep on immunogenicity of COVID-19 vaccines should be studied. Nevertheless, considering the multifunctionality of Ox neurons in the modulation of vital physiological processes, this assumption could also be valid to explain other transient reactogenic symptoms such as general weakness, loss of appetite, headache, myalgia and joint pain after COVID vaccination [15,37,38].
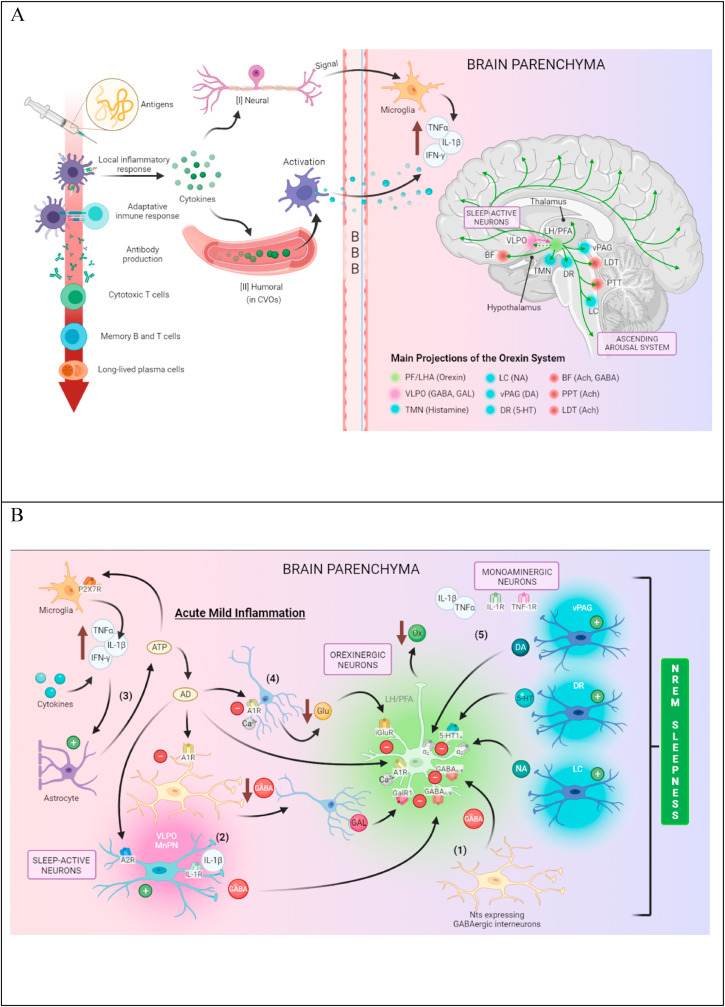
Fig. 1.
Schematic diagram illustrating the hypothetical implication of the orexinergic system in reactogenic sleepiness after COVID-19 vaccines. (A) After vaccination, inflammation is triggered by antigen and potential adjuvant present in the vaccine formulation. Then pro-inflammatory cytokines are released by activated resident innate immune cells at the periphery, which recruit immune cells, mainly hematogenous neutrophils, releasing directly acting hyperalgesic mediators on nociceptors. This fast neural route via the spinal nociceptive pathway transmits the signal to the brain neurons, producing pro-inflammatory cytokines by microglial cells. In addition, pathogen-associated molecular patterns (PAMPs) and circulating cytokines (slower humoral pathway) activate phagocytic and endothelial cells in the circumventricular organs (CVOs) and choroid plexus, respectively, leading to the production of brain cytokines that diffuse through the deficient blood-brain barrier (BBB) into the brain parenchyma. In both cases, the action of brain pro-inflammatory cytokines can be mediated by prostaglandins or nitric oxide. Brain parenchyma inflammatory milieu (increased of pro-inflammatory cytokines as somnogenic TNF-α and IL-1β) indirectly inhibits the activity of orexin (Ox) neurons during periods of wakefulness and arousal, leading to sleepiness. (B) Several central pathways could subserve its effects on wake-promoting neurons. (1) Lateral hypothalamic area (LHA) neurotensin (Nts)-expressing GABAergic neurons could mediate the suppression of Ox neuron activity, leading to hypersomnolence, resembling hypothalamic inflammation that induces sickness-associated lethargy. (2) Somnogenic IL-1β could directly activate a subset of GABAergic ventrolateral preoptic area (VLPO) sleep-promoting neurons inhibiting Ox neurons. (3) Pro-inflammatory cytokines stimulate astrocyte activation, and astrocyte-derived ATP may be hydrolyzed into adenosine (AD) by ATP-degrading enzymes in VLPO, perifornical (PF)-LHA, and other cholinergic, histaminergic and noradrenergic arousal-related neurons, promoting sleep. Adenosine via adenosine A2 receptors (A2R) directly activates GABAergic VLPO neurons as well as via adenosine A1 receptors (A1R) inhibit GABAergic interneurons, which disinhibit VLPO sleep-promoting neurons, possibly galaninergic. ATP via P2X7 receptors induces IL-1β, INF-γ, and TNF-α release from microglia increasing brain pro-inflammatory cytokines. (4) AD also induces inhibitory influence via A1R on Ox neurons at presynaptic terminals reducing calcium-dependent glutamate release and at the soma of Ox neurons, inhibiting voltage-dependent calcium channels. (5) Inhibitory inputs (inhibitory feedback) on Ox neurons under a central inflammatory context could also be provided from the serotonergic dorsal raphe (DR) nucleus, the noradrenergic locus coeruleus (LC), the dopaminergic ventral periaqueductal gray (vPAG), which are activated by lipopolysaccharide (LPS) experimental treatment.
5. Alerting elements to consider in pharmacovigilance studies in the context of neuroinflammation
On the other hand, in the context of long-term and dysregulated neuroinflammation, Ox neuropeptides exert both neuroprotective and immunomodulatory actions and becoming an emerging group of biological agents with a great potential for the treatment of immune-mediated CNS disorders such as narcolepsy, metabolic disorders, Alzheimer's disease, and multiple sclerosis [32,33]. However, sleepiness or other systemic symptoms are caused by reactive inflammation after injection of vaccines containing an adjuvant system (aluminum salts or newer adjuvants, which include virosomes, oil-in-water emulsions, as AS03 or lipid nanoparticles), should be findings of attention to researchers. Other alert elements to consider in pharmacovigilance studies must focus on possible rare events such as narcolepsy or others related to the post-vaccination inflammatory syndrome [79]. As shown previously, some of these molecules as aluminum, have a propensity to activate brain microglia and increase the production of inflammatory cytokines, thereby initiating and exacerbating inflammation and excitotoxicity in the brain [80]. Considering that glial cells are highly dynamic and responsive to the diversity of environmental stimuli, microglia, CNS resident macrophage-type immune cells, also can switch from a resting phenotype to a primed state by an initial immune stimulus that is not excessively intense but could facilitate long term neuroinflammation [81].
Although, to date, there is no evidence that COVID vaccines increase the risk to develop narcolepsy or that, in already diagnosed narcoleptic patients, its symptoms may worsen after this vaccination, the causal link between the GlaxoSmithKline's Pandemrix swine flu vaccination and the sudden significant onset of type 1 narcolepsy in some individuals has raised many questions [79]. Following investigations suggest that the formulation of H1N1 viral nucleoprotein used in Pandemrix, rather than the AS03 adjuvant, likely triggered an autoimmune assault on hypothalamic Ox-neurons in some individuals with a genetic vulnerability to developing narcolepsy [82,83]. The CoV-2 spike protein as a vaccine antigen, including in most vaccines in development against SARS-CoV-2, is structurally different from the H1N1 nucleoprotein utilized in Pandemrix. There is no rationale to support that licensed COVID vaccines should trigger an autoimmune attack on orexinergic neurons, as with Pandemrix. However, vaccine safety must be monitored after regulatory authorization, as will all medicinal products. All new vaccine has potential adverse events of special interest (AESIs) based on knowledge of previous vaccines and a vaccine's development and upon the known disease manifestations [84]. Clinical reports have confirmed that about 34% of COVID-19 patients develop neurological manifestations, which involve the peripheral nervous system and CNS [85]. Considering the routes and mechanisms of neuroinvasion of SARS-CoV-2 and the pivotal role of hypothalamic circuits in the stress, metabolic, and autonomic responses, some authors proposed that the hypothalamus is a critical player in the development of these manifestations [86]. Hypothalamic networks, also involved in regulating the sleep-wake cycle, represent a significant potential brain region that could be affected in this acute viral disease. In particular, some observations in post-acute COVID-19 syndrome patients suggest its possible contribution to neurological sequelae and residual effects of SARS-CoV-2 infection [87]. Moreover, systemic inflammatory response during COVID-19 could potentially trigger chronic autoimmune and neurodegenerative disorders like narcolepsy in susceptible individuals. Recurrence of hypersomnia after COVID-19 vaccination has been reported in a case with a history of hypersomnia secondary to infectious mononucleosis [88]. In addition to SARS-CoV-2 or other virus infections, vaccination can also cause autoimmune reactions by mechanisms such as molecular mimicry and bystander activation of T cells [89]. In this context, a dysfunction of the orexinergic system may trigger sleep disorders and different pathological conditions, including obesity, depression, cognitive disorders, and chronic pain, among others [37,38]. Subsequently, these antecedents should be an alert of special interest to be monitored in pharmacovigilance studies, like others, not a reason why some individuals choose to refuse protective vaccines.
6. Conclusion
Sleepiness side effects reported after some COVID-19 vaccines suggest a mechanistic link between reactogenic inflammatory parameters and hypothalamic circuits involved in the sleep-wake cycle. We proposed that pro-inflammatory cytokines IL-1β, INF-γ and TNF-α may propagate a peripheral inflammatory response after vaccination, which activate a subset of perifornical LHA Nts-expressing GABAergic neurons as well as inhibitory neurons from sleep-promoting areas providing an inhibitory input on wake-promoting Ox neurons. The adenosinergic modulation of sleep-wake signals in the context of neuron-glial interactions could also be implicated. However, further investigations are necessary to corroborate our hypothesis. Identifying biomarkers linked to the reactogenicity of the COVID-19 vaccines will allow further understanding of the links with its immunogenicity. At the same time, the frequent emergence of sleepiness after vaccination should be an alert to be considered in pharmacovigilance studies to preserve confidence in these new vaccines.
Contributors
Bárbara B. Garrido-Suárez: Conceptualization, Supervision, Writing - Review & Editing. Mariana Garrido-Valdes: Writing - Original Draft. Gabino Garrido: Writing - Original Draft, Review & Editing, Validation.
Declaration of competing interest
Nothing declared.
References
- 1.WHO Coronavirus (COVID-19) dashboard 2022. Available from: http://covid19.who.int Accessed 27 February, 2022.
- 2.WHO . 2022. Draft landscape of COVID-19 candidate vaccines. 25 February.https://who.int/publications/m/item/draftlandscape-of-covid-19-candidate-vaccines [Google Scholar]
- 3.Di Pasquale A., Bonanni P., Garçon N., et al. Vaccine safety evaluation: practical aspects in assessing benefits and risks. Vaccine. 2016;34:6672–6680. doi: 10.1016/j.vaccine.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Hervé C., Laupèze B., Del Giudice G., et al. The how's and what's of vaccine reactogenicity. NPJ Vaccines. 2019;4:39. doi: 10.1038/s41541-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canedo-Marroquín G., Saavedra F., Andrade C.A., et al. SARS-CoV-2: immune response elicited by infection and development of vaccines and treatments. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.569760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelley K.W., Kent S. The legacy of sickness behaviors. Front Psychiatr. 2020;11 doi: 10.3389/fpsyt.2020.607269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irwin M.R., Opp M.R. Sleep health: reciprocal regulation of sleep and innate immunity. Neuropsychopharmacol Rev. 2017;42:129–155. doi: 10.1038/npp.2016.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Besedovsky L., Lange T., Haack M. The sleep-immune cross-talk in health and disease. Physiol Rev. 2019;99:1325–1380. doi: 10.1152/physrev.00010.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto S., Fukunaga A., Tanaka A., et al. Association between reactogenicity and SARS-CoV-2 antibodies after the second dose of the BNT162b2 COVID-19 vaccine. medRxiv. 2021 doi: 10.1101/2021.07.19.21260744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borobia A.M., Carcas A.J., Pérez-Olmeda M., et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398:121–130. doi: 10.1016/s0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toledo-Romani M.E., Verdecia-Sánchez L., Rodriguez-González M., et al. Safety and immunogenicity of anti-SARS CoV-2 vaccine SOBERANA 02 in homologous or heterologous scheme. medRxiv. 2021 doi: 10.1101/2021.11.14.21266309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernández-Bernal F., Ricardo-Cobas M.C., Martín-Bauta Y., et al. Safety, tolerability, and immunogenicity of a SARS-CoV-2 recombinant spike protein vaccine: a randomised, double-blind, placebo-controlled, phase 1-2 clinical trial (ABDALA Study) medRxiv. 2021 doi: 10.1101/2021.11.30.21267047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Shitany N.A., Harakeh S., Badr-Eldin S.M., et al. Minor to moderate side effects of Pfizer-BioNTech COVID-19 vaccine among Saudi residents: a retrospective cross-sectional study. Int J Gen Med. 2021;14:1389–1401. doi: 10.2147/ijgm.s310497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulligan M.J., Lyke K.E., Kitchin N., et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 15.Kadali R.A.K., Janagama R., Peruru S., et al. Non-life-threatening adverse effects with COVID-19 mRNA-1273 vaccine: a randomized, cross-sectional study on healthcare workers with detailed self-reported symptoms. J Med Virol. 2021;93:4420–4429. doi: 10.1002/jmv.26996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakinah E.N., Nugraha M.Y., Qodar T.S., et al. COVID-19 vaccines programs: adverse events following immunization (AEFI) among medical clerkship student in Jember, Indonesia. BMC Pharmacol Toxicol. 2021;22:58–64. doi: 10.1186/s40360-021-00528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dantzer R. Neuroimmune interactions: from the brain to the immune system and viceversa. Physiol Rev. 2018;98:477–504. doi: 10.1152/physrev.00039.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korneva E.A., Shanin S.N., Novikova N.S., et al. Cell-molecular basis of neuroimmune interactions during stress. Ross Fiziol Zh Im I M Sechenova. 2017;103:217–229. [PubMed] [Google Scholar]
- 19.Garbarino S., Lanteri P., Bragazzi N.L., et al. Role of sleep deprivation in immune-related disease risk and outcomes. Commun Biol. 2021;4:1304. doi: 10.1038/s42003-021-02825-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benedict C., Cedernaes J. Could a good night's sleep improve COVID-19 vaccine efficacy? Lancet Respir Med. 2021;9:447–448. doi: 10.1016/S2213-2600(21)00126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lange T., Dimitrov S., Bollinger T., et al. Sleep after vaccination boosts immunological memory. J Immunol. 2011;187:283–290. doi: 10.4049/jimmunol.1100015. [DOI] [PubMed] [Google Scholar]
- 22.Krueger J.M., Majde J.A., Rector D.M. Cytokines in immune function and sleep regulation. Handb Clin Neurol. 2011;98:229–240. doi: 10.1016/B978-0-444-52006-7.00015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingiosi A.M., Opp M.R., Krueger J.M. Sleep and immune function: glial contributions and consequences of aging. Curr Opin Neurobiol. 2013;23:806–811. doi: 10.1016/j.conb.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salfi F., Lauriola M., D'Atri A., et al. Demographic, psychological, chronobiological, and work-related predictors of sleep disturbances during the COVID-19 lockdown in Italy. Sci Rep. 2021;11 doi: 10.1038/s41598-021-90993-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scammell T.E., Arrigoni E., Lipton J.O. Neural circuitry of wakefulness and sleep. Neuron. 2017;93:747–765. doi: 10.1016/j.neuron.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suntsova N., Guzman-Marin R., Kumar S., et al. The median preoptic nucleus reciprocally modulates activity of arousal-related and sleep-related neurons in the perifornical lateral hypothalamus. J Neurosci. 2007;27:1616–1630. doi: 10.1523/jneurosci.3498-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peyron C., Faraco J., Rogers W., et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 28.Saper C.B., Fuller P.M., Pedersen N.P., et al. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venner A., De Luca R., Sohn L.T., et al. An inhibitory lateral hypothalamic-preoptic circuit mediates rapid arousals from sleep. Curr Biol. 2019;29:4155–4168. doi: 10.1016/j.cub.2019.10.026. e5. [DOI] [PubMed] [Google Scholar]
- 30.Hassani O.K., Lee M.G., Jones B.E. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci U S A. 2009;106:2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chemelli R.M., Willie J.T., Sinton C.M., et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 32.Couvineau A., Voisin T., Nicole P., et al. Orexins as novel therapeutic targets in inflammatory and neurodegenerative diseases. Front Endocrinol. 2019;10:709. doi: 10.3389/fendo.2019.00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang C., Wang Q., Ji B., et al. The orexin/receptor system: molecular mechanism and therapeutic potential for neurological diseases. Front Mol Neurosci. 2018;11:220. doi: 10.3389/fnmol.2018.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inutsuka A., Yamanaka A. The physiological role of orexin/hypocretin neurons in the regulation of sleep/wakefulness and neuroendocrine functions. Front Endocrinol. 2013;4:18. doi: 10.3389/fendo.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eban-Rothschild A., Rothschild G., Giardino W.J., et al. VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat Neurosci. 2016;19:1356–1366. doi: 10.1038/nn.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu J., Jhou T.C., Saper C.B. Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J Neurosci. 2006;26:193–202. doi: 10.1523/jneurosci.2244-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chieffi S., Carotenuto M., Monda V., et al. Orexin system: the key for a healthy life. Front Physiol. 2017;8:357. doi: 10.3389/fphys.2017.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang X., Tang H., Liu Y., et al. Research progress on the mechanism of orexin in pain regulation in different brain regions. Open Life Sci. 2021;16:46–52. doi: 10.1515/biol-2021-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherin J.E., Shiromani P.J., McCarley R.W., et al. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 40.Kroeger D., Absi G., Gagliardi C., et al. Galanin neurons in the ventrolateral preoptic area promote sleep and heat loss in mice. Nat Commun. 2018;9:4129. doi: 10.1038/s41467-018-06590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haydon P.G. Astrocytes and the modulation of sleep. Curr Opin Neurobiol. 2017;44:28–33. doi: 10.1016/j.conb.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deurveilher S., Golovin T., Hall S., et al. Microglia dynamics in sleep/wake states and in response to sleep loss. Neurochem Int. 2021;143 doi: 10.1016/j.neuint.2020.104944. [DOI] [PubMed] [Google Scholar]
- 43.Kim J.H., Choi I.S., Jeong J.Y., et al. Astrocytes in the ventrolateral preoptic area promote sleep. J Neurosci. 2020;40:8994–9011. doi: 10.1523/jneurosci.1486-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morairty S., Rainnie D., Mc Carley R., et al. Disinhibition of ventrolateral preoptic area sleep-active neurons by adenosine: a new mechanism for sleep promotion. Neuroscience. 2004;123:451–457. doi: 10.1016/j.neuroscience.2003.08.066. [DOI] [PubMed] [Google Scholar]
- 45.Kumar S., Rai S., Hsieh K.C., et al. Adenosine A2A receptors regulate the activity of sleep regulatory GABAergic neurons in the preoptic hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2013;305:R31–R41. doi: 10.1152/ajpregu.00402.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saito Y.C., Tsujino N., Hasegawa E., et al. GABAergic neurons in the preoptic area send direct inhibitory projections to orexin neurons. Front Neural Circ. 2013;7:192. doi: 10.3389/fncir.2013.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sergeeva O.A., Andreeva N., Garret M., et al. Pharmacological properties of GABAA receptors in rat hypothalamic neurons expressing the epsilon-subunit. J Neurosci. 2005;25:88–95. doi: 10.1523/jneurosci.3209-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie X., Crowder T.L., Yamanaka A., et al. GABAB receptor-mediated modulation of hypocretin/orexin neurones in mouse hypothalamus. J Physiol. 2006;574(2):399–414. doi: 10.1113/jphysiol.2006.108266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrari L.L., Park D., Zhu L., et al. Regulation of lateral hypothalamic orexin activity by local GABAergic neurons. J Neurosci. 2018;38:1588–1599. doi: 10.1523/jneurosci.1925-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leinninger G.M., Opland D.M., Jo Y.-H., et al. Leptin action via neurotensin neurons controls orexin neurons, the mesolimbic dopamine system and energy balance. Cell Metabol. 2011;14:313–323. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown J.A., Woodworth H.L., Leinninger G.M. To ingest or rest? Specialized roles of lateral hypothalamic area neurons in coordinating energy balance. Front Syst Neurosci. 2015;9:9. doi: 10.3389/fnsys.2015.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laque A., Yu S., Qualls-Creekmore E., et al. Leptin modulates nutrient reward via inhibitory galanin action on orexin neurons. Mol Metabol. 2015;4:706–717. doi: 10.1016/j.molmet.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naganuma F., Kroeger D., Bandaru S.S., et al. Lateral hypothalamic neurotensin neurons promote arousal and hyperthermia. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grossberg A.J., Zhu X.X., Leinninger G.M., et al. Inflammation-induced lethargy is mediated by suppression of orexin neuron activity. J Neurosci. 2011;31:11376–11386. doi: 10.1523/JNEUROSCI.2311-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plata-Salamán C.R., Oomura Y., Kai Y. Tumour necrosis factor and interleukin-1 beta: suppression of food intake by direct action in the central nervous system. Brain Res. 1988;448:106–114. doi: 10.1016/0006-8993(88)91106-7. [DOI] [PubMed] [Google Scholar]
- 56.Kubota T., Li N., Guan Z., et al. Intrapreoptic microinjection of TNF-alpha enhances non-REMS in rats. Brain Res. 2002;932:37–44. doi: 10.1016/s0006-8993(02)02262-x. [DOI] [PubMed] [Google Scholar]
- 57.De Sarro G., Gareri P., Sinopoli V.A., et al. Comparative, behavioural and electrocortical effects of tumor necrosis factor-alpha and interleukin-1 microinjected into the locus coeruleus of rat. Life Sci. 1997;60:555–564. doi: 10.1016/S0024-3205(96)00692-3. [DOI] [PubMed] [Google Scholar]
- 58.Parnet P., Kelley K.W., Bluthe R.-M., et al. Expression and regulation of interleukin-1 receptors in the brain. Role in cytokines-induced sickness behavior. J Neuroimmunol. 2002;125:5–14. doi: 10.1016/s0165-5728(02)00022-x. [DOI] [PubMed] [Google Scholar]
- 59.Talaat K.R., Halsey N.A., Cox A.B., et al. Rapid changes in serum cytokines and chemokines in response to inactivated influenza vaccination. Influenza Other Respi Viruses. 2018;12:202–210. doi: 10.1111/irv.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Athearn K., Sample C.J., Barefoot B.E., et al. Acute reactogenicity after intramuscular immunization with recombinant vesicular stomatitis virus is linked to production of IL-1β. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burny W., Marchant A., Hervé C., et al. Inflammatory parameters associated with systemic reactogenicity following vaccination with adjuvanted hepatitis B vaccines in humans. Vaccine. 2019;37:2004. doi: 10.1016/j.vaccine.2019.02.015. 15. [DOI] [PubMed] [Google Scholar]
- 62.Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin. 2009;29(2):247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharpley A.L., Cooper C.M., Williams C., et al. Effects of typhoid vaccine on inflammation and sleep in healthy participants: a double-blind, placebo-controlled, crossover study. Psychopharmacology. 2016;233(18):3429–3435. doi: 10.1007/s00213-016-4381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harper J.A., South C., Trivedi M.H., et al. Pilot investigation into the sickness response to influenza vaccination in adults: effect of depression and anxiety. Gen Hosp Psychiatr. 2017;48:56–61. doi: 10.1016/j.genhosppsych.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Da Mesquita S., Fu Z., Kipnis J. The meningeal lymphatic system: a new player in neurophysiology. Neuron. 2018;100:375–388. doi: 10.1016/j.neuron.2018.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.La Cava A. Leptin in inflammation and autoimmunity. Cytokine. 2017;98:51–58. doi: 10.1016/j.cyto.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alam M.N., McGinty D., Bashir T., et al. Interleukin-1beta modulates state-dependent discharge activity of preoptic area and basal forebrain neurons: role in sleep regulation. Eur J Neurosci. 2004;20:207–216. doi: 10.1111/j.1460-9568.2004.03469.x. [DOI] [PubMed] [Google Scholar]
- 68.Hong Z.-Y., Huang Z.-L., Qu W.-M., et al. An adenosine A receptor agonist induces sleep by increasing GABA release in the tuberomammillary nucleus to inhibit histaminergic systems in rats. J Neurochem. 2005;92:1542–1549. doi: 10.1111/j.1471-4159.2004.02991.x. [DOI] [PubMed] [Google Scholar]
- 69.Choi H.B., Ryu J.K., Kim S.U., et al. Modulation of the purinergic P2X7 receptor attenuates lipopolysaccharide-mediated microglial activation and neuronal damage in inflamed brain. J Neurosci. 2007;27:4957–4968. doi: 10.1523/jneurosci.5417-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alam M.N., Kumar S., Rai S., et al. Role of adenosine A1 receptor in the perifornical-lateral hypothalamic area in sleep-wake regulation in rats. Brain Res. 2009;1304:96–104. doi: 10.1016/j.brainres.2009.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Z.W., Gao X.B. Adenosine inhibits activity of hypocretin/orexin neurons by the A1 receptor in the lateral hypothalamus: a possible sleep-promoting effect. J Neurophysiol. 2007;97:837–848. doi: 10.1152/jn.00873.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thakkar M.M., Winston S., McCarley R.W. Orexin neurons of the hypothalamus express adenosine A1 receptors. Brain Res. 2002;944:190–194. doi: 10.1016/s0006-8993(02)02873-1. [DOI] [PubMed] [Google Scholar]
- 73.Borsody M.K., Weiss J.M. Alteration of locus coeruleus neuronal activity by interleukin-1 and the involvement of endogenous corticotropin releasing hormone. Neuroimmunomodulation. 2002;10:101–121. doi: 10.1159/000065186. [DOI] [PubMed] [Google Scholar]
- 74.Yamanaka A., Muraki Y., Tsujino N., et al. Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochem Biophys Res Commun. 2003;303:120–129. doi: 10.1016/S0006-291X(03)00299-7. [DOI] [PubMed] [Google Scholar]
- 75.Yamanaka A., Muraki Y., Ichiki K., et al. Orexin neurons are directly and indirectly regulated by catecholamines in a complex manner. J Neurophysiol. 2006;96:284–298. doi: 10.1152/jn.01361.2005. [DOI] [PubMed] [Google Scholar]
- 76.Borsody M.K., Weiss J.M. The effects of endogenous interleukin-1 bioactivity on locus coeruleus neurons in response to bacterial and viral substances. Brain Res. 2004;1007:39–56. doi: 10.1016/j.brainres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 77.Hollis J.H., Lightman S.L., Lowry C.A. Lipopolysaccharide has selective actions on sub-populations of catecholaminergic neurons involved in activation of the hypothalamic-pituitary-adrenal axis and inhibition of prolactin secretion. J Endocrinol. 2005;184:393–406. doi: 10.1677/joe.1.05839. [DOI] [PubMed] [Google Scholar]
- 78.Hollis J.H., Evans A.K., Bruce K.P.E., et al. Lipopolysaccharide has indomethacin-sensitive actions on Fos expression in topographically organized subpopulations of serotonergic neurons. Brain Behav Immun. 2006;20:569–577. doi: 10.1016/j.bbi.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 79.Mignot E., Black S. Narcolepsy risk and COVID-19. J Clin Sleep Med. 2020;16(10):1831–1833. doi: 10.5664/jcsm.8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tomljenovic L., Shaw C.A. Aluminum vaccine adjuvants: are they safe? Curr Med Chem. 2011;18:2630–2637. doi: 10.2174/092986711795933740. [DOI] [PubMed] [Google Scholar]
- 81.Giannotta G., Giannotta N. Post-vaccination inflammatory syndrome: a new syndrome. Clin Case Rep Rev. 2019;5:1–12. doi: 10.15761/CCRR.1000454. [DOI] [Google Scholar]
- 82.Kornum B.R., Faraco J., Mignot E. Narcolepsy with hypocretin/orexin deficiency, infections and autoimmunity of the brain. Curr Opin Neurobiol. 2011;21:897–903. doi: 10.1016/j.conb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 83.Jiang W., Birtley J.R., Hung S.C., et al. In vivo clonal expansion and phenotypes of hypocretinspecific CD4(+) T cells in narcolepsy patients and controls. Nat Commun. 2019;10(1):5247. doi: 10.1038/s41467-019-13234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li X., Ostropolets A., Makadia R., et al. Characterising the background incidence rates of adverse events of special interest for covid-19 vaccines in eight countries: multinational network cohort study. BMJ. 2021;373:n1435. doi: 10.1136/bmj.n1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schirinzi T., Landi D., Liguori C. COVID-19: dealing with a potential risk factor for chronic neurological disorders. J Neurol. 2021;268:1171–1178. doi: 10.1007/s00415-020-10131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mussa B.M., Srivastava A., Verberne A.J.M. COVID-19 and neurological impairment: hypothalamic circuits and beyond. Viruses. 2021;13:498. doi: 10.3390/v13030498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu M., Li S.X., Xue P., et al. COVID-19 Vaccine could trigger the relapse of secondary hypersomnia. Nat Sci Sleep. 2021;13:2267–2271. doi: 10.2147/NSS.S345801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mobasheri L., Nasirpour M.H., Masoumi E., et al. SARS-CoV-2 triggering autoimmune diseases. Cytokine. 2022;154 doi: 10.1016/j.cyto.2022.155873. [DOI] [PMC free article] [PubMed] [Google Scholar]