Abstract
Podoplanin is a small cell-surface mucin-like glycoprotein that participates in multiple physiological and pathological processes. Podoplanin exerts an important function in the immune response and is upregulated in fibroblasts, macrophages, T helper cells, and epithelial cells during inflammation. Herein, we summarize the latest knowledge on the functional expression of podoplanin in the immune system and review the contribution of podoplanin to several inflammatory diseases. Furthermore, we discuss podoplanin as a novel therapeutic target for various inflammatory diseases.
Keywords: podoplanin, immune system, inflammation
Introduction
The immune system and inflammation are essential for clearing pathogens and damaged cells, as well as tissue repair. Acute inflammation is a protective reaction that is beneficial to the organism. However, consistent and uncontrolled immune response and inflammation underly various degenerative diseases.1 Clinical treatments of these diseases lack biological agents to modify specific inflammatory and effector pathways, which is a promising therapeutic strategy. Podoplanin (PDPN) is a small cell-surface mucin-like glycoprotein, which is widely expressed in different tissues and cell types, such as glomerular podocytes (hence its name), type I alveolar cells, mesothelial cells, and different types of fibroblasts.2 Podoplanin is involved in multiple physiological and pathological processes, such as embryonic development, immune response, and cancer. Recent evidence indicates that podoplanin expression in immune cells participates in regulation of inflammation during different inflammation-related diseases.2 Interaction between podoplanin and its protein partners is the mechanism of regulating immune response and mediating inflammatory diseases, which is critical for the clinical treatment of inflammatory diseases.
Podoplanin
Podoplanin is a type I transmembrane sialomucin-like glycoprotein, with a heavily O-glycosylated extracellular domain and a cytoplasmic tail consisting of 9 amino acids.2,3 The structure of podoplanin lacks obvious enzymatic motifs and hence, it most likely exerts its cellular functions through protein-protein interactions.2 Among the many protein ligands of podoplanin, C-type lectin-like receptor 2 (CLEC-2), ezrin, and moesin are studied comprehensively.
CLEC-2 belongs to the family of the non-classical C-type lectins, expressed on dendritic cells, neutrophils, and platelets and to date, podoplanin is the only known endogenous ligand for CLEC-2.2,4–8 The interaction between platelet CLEC-2 and podoplanin is the key for platelet activation and its multiple roles besides hemostasis.5 The combination of CLEC-2 with the platelet aggregation-stimulating domains in the extracellular domain of podoplanin induces platelet activation through the Src, Syk and SLP-76 kinase pathway.9–11 Furthermore, the combination with CLEC-2 is also one of the important ways for podoplanin to play an inflammatory regulatory role, which we elaborate in detail in the following sections.
Ezrin and moesin, which belong to the ERM (ezrin, radixin, moesin) protein family, can bind to the cytoplasmic domain of podoplanin, involved in cell polarity, adhesion, and migration.12 The interaction with ERM proteins is crucial for the modulation of small Rho GTPases and podoplanin-mediated rearrangement of the actin cytoskeleton, participating in many physiologic and pathologic processes, such as embryonic development, immune response, and epithelial-mesenchymal transition (EMT).13–15
The main protein partners of podoplanin are presented in Table 1.
Table 1.
The Main Protein Partners of Podoplanin
| Ligands | Binding sites with Podoplanin | Functions | References |
|---|---|---|---|
| CLEC-2 | Extracellular Domain | Platelet activation Thrombosis Blood vascular integrity Lymphangiogenesis Immune response Cancer invasion and metastasis |
[2,4,16–18] |
| CCL21 | Extracellular Domain | Immune response Development of nature Treg cells Tumor immune escape |
[2,19,20] |
| Galectin-8 | Extracellular Domain | Lymphangiogenesis | [21] |
| CD9 | Transmembrane Domain | Inhibition of podoplanin-CELC-2 interaction | [22] |
| CD44 | Transmembrane Domain | Modulation of cell migration | [23] |
| ERM | Cytoplasmic Tail | Embryonic development Immune response Cancer invasion and metastasis Epithelial-mesenchymal transition |
[13–15] |
Podoplanin Expression in the Innate and Adaptive Immune Systems
Inflammation underlies many chronic and degenerative disease, triggered by noxious stimuli, such as infection and tissue injury.1,24 Generally, a controlled inflammatory response is beneficial, but it can become detrimental if dysregulated. The acute inflammatory response induced by infection or tissue damage is triggered by the innate immune system, in which tissue-resident macrophages and mast cells mediate the initial recognition of stimuli and, subsequently, lead to the production of inflammatory mediators that elicit the exudate of plasma protein and leukocytes (mainly neutrophils).1 When acute inflammation eliminates harmful stimuli, it enters a resolution and repair phase mediated by tissue-resident and recruited macrophages, which is the main source of growth factors and cytokines that modulate tissue repair.25 Moreover, the adaptive immune system also plays a critical role in resisting pathogen invasion or insult. The adaptive immune response originates in secondary lymphoid organs (lymph nodes and spleen), generally under innate immune system signals, which are provided either by circulating pathogens directly or indirectly by antigen-presenting cells (APCs) migrating to the secondary lymphoid organs.26 Then lymphocytes including T and B lymphocytes emigrating from the spleen and lymph nodes travel to many sites in the body to exert effector functions.26 However, the persistence of pathogens or failure to resolve inflammation may lead to chronic inflammation. Inappropriate adaptive immune response can lead to development of autoimmune diseases, such as rheumatoid arthritis, psoriasis, and multiple sclerosis.27 Furthermore, lymphangiogenesis is also associated with inflammation. The lymphatic system plays a crucial role in immune defense against infection by transporting immune cells from peripheral tissues to lymph nodes, especially in the rapid activation of adaptive immunity during inflammatory response.28
It has been well documented that podoplanin expression exists in the innate and adaptive immune systems during inflammation. Podoplanin expression has been reported in CD4+ effector T cells29,30 and macrophages.31 In addition, podoplanin on lymphatic endothelial cells (LECs) and fibroblast-like reticular cells (FRCs) has attracted equal attention.
Macrophages
Macrophages are the most plastic cells of the hematopoietic system, found in all tissues and showing good functional diversity.32 Podoplanin expression exists in particular subsets of macrophages, and this glycoprotein was identified as a marker of a subset of highly phagocytic F4/80+ macrophages in the spleen and peritoneal cavity.33 Podoplanin is unable to be detected on bone marrow-derived macrophages (BMDMs) and tissue-resident macrophages.31 Rather, podoplanin expression was upregulated in BMDMs following inflammatory stimulation, such as lipopolysaccharide (LPS), Toll-like receptors agonists and tumor necrosis factor (TNF), which suggests that podoplanin is expressed on inflammatory but not tissue-resident macrophages and its expression is upregulated in response to Toll-like receptor (TLR) stimulation and some inflammatory cytokines.31
Studies show that podoplanin expression functionally affects macrophages, including polarization and mobility. Depending on the microenvironment, macrophages can acquire distinct functional phenotypes during inflammation. Two well-established polarization phenotypes are usually called classical activated macrophages (M1 macrophages) and alternatively activated macrophages (M2 macrophages).34 Fei et al reported podoplanin expression was corelated with mice microglia (macrophage in the brain and spinal cord) activation and phenotypes during neuroinflammation after traumatic brain injury.35 With hemoglobin treatment as inflammatory stimuli in vitro, knockdown of podoplanin could decrease the proportion of M1-like microglia and increase the proportion of M2-like microglia with reduced secretion of IL-1β and TNF-α and increased secretion of IL-10 and TGF-β.35 This finding demonstrated that podoplanin could influence the inflammatory phenotype of macrophages.
Furthermore, podoplanin expression also influences the mobility of macrophages. Using a mice sepsis model of cecal ligation and puncture (CLP), Rayes et al demonstrated that podoplanin expression on hematopoietic cells played a role in regulating macrophage recruitment to the infected peritoneum, modulating bacterial load in the peritoneal cavity and limiting subsequent organ damage.36 Moreover, Bourne et al reported that crosslinking podoplanin using recombinant CLEC-2-Fc limited the inflammatory environment by reducing inflammatory macrophage accumulation in the inflamed tissue during murine peritonitis.37 Platelet CLEC-2 induces a loss of phosphorylation of the serine residues in the intracellular podoplanin tail, which is associated with a rapid translocation of podoplanin from intracellular stores to the surface of inflammatory macrophages.38 Additionally, the interaction between podoplanin and CLEC-2 induced the reorganisation of the actin cytoskeleton and increased podoplanin interaction with ERM proteins and CD44, promoting macrophage migration.37 Cheok et al also proposed that during Helicobacter pylori infection, podoplanin drives motility of active macrophages via regulating Filamin C, a member of actin-crosslinking protein family that stabilizes and links actin web to the cell membrane.39,40 They demonstrated that podoplanin may promote Filamin C transactivation through interacting with the ERM complex and activating Rho GTPase signaling pathway, but further investigation is required to confirm this notion.39
CD4+ Effector T Cells
Podoplanin is preferentially expressed on differentiated Th17 cells, rather than on other effector T cell subsets.41 Interleukin 17-producing helper T (Th17) cells play pathogenic roles in chronic inflammatory and autoimmune diseases, characterized by the production of IL-17A and IL-17F.42 Podoplanin expression has been confirmed on Th17 cells in the inflamed tissues of mice models of spontaneous SKG arthritis, autoimmune encephalomyelitis, and inflammatory bowel disease.30 And in vitro, a subset of naive CD4+ T cells expressed significant levels of podoplanin under Th17-polarizing conditions.30,43 By immunohistochemical analysis of inflamed synovium in the mice SKG arthritic model, notable numbers of podoplanin-expressing Th17 cells were found to infiltrate these tissues and these podoplanin-positive cells produce IL-17A with destruction of cartilage and bone.30 However, Nylander et al found that unlike in mice, human podoplanin+ T cells induced under classic Th17-polarizing conditions express transcription factors associated with Th17 cells but do not reflect IL-17 secretion characteristics.43 In contrast, by qPCR quantitative analysis, the mRNA expression profile of podoplanin+ Th17 cells is consistent with that of nonpathogenic Th17 cells and may be more regulatory.43 They express higher levels of nonpathogenic Th17 cells genes, including IL10, IL9, Ikzf3, Ahr, and IL1rn,43 and several of these genes are involved in the regulation of IL-10.44 Additionally, the differentiation of Th17 cells is promoted toward a pathogenic phenotype under a pro-inflammatory condition, such as high salt concentration, with the reduced podoplanin expression and increased IL-17 production.43 These results were contrary to those reported in mice, suggesting that human podoplanin-positive Th17 cells had a protective effect on inflammation, rather than promoting it.
In order to maintain peripheral and tissue tolerance, self-reactive T effector cells that escape negative selection in the thymus are actively controlled in the periphery by expression of inhibitory cell surface receptors, such as cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1).45 Podoplanin is a novel co-inhibitory receptor on effector T cells.46,47 In contrast to PD-1 and CTLA-4, podoplanin does not have an immunoreceptor tyrosine-based inhibitory motif (ITAM) in its short cytoplasmic tail, which is required for the former to modulate TCR signaling.45 The TCR signaling is essential for T-cell activation and therefore, podoplanin may not directly interfere with this process. On the other hand, cytokine IL-7 permits long-term survival of memory-precursor CD4+T cells after acute activation signals fade.48 Peters et al used global and T-cell-specific Pdpn-deficient mice to demonstrate that podoplanin inhibits T cell responses by reducing responsiveness to IL-7 survival signal.46 However, the role of podoplanin in Th17 cells is far from clear. Nylander et al reported that ligation of podoplanin by its ligand CLEC-2 ameliorates the Th17 inflammatory response.43 As podoplanin does not possess an ITAM domain on its cytoplasmic tail to mediate intracellular signaling, it is unknown if there are other intracellular pathways that play a role in intracellular signaling in CD4+ T cells in response to ligation of podoplanin by CLEC-2, and it is possible that podoplanin may be involved in multiple parallel downstream mechanisms resulting in Th17 regulation.
Lymphatic Endothelial Cells (LECs) and Fibroblast-Like Reticular Cells (FRCs)
LECs and FRCs are essential for the migration of antigen-presenting dendritic cells (DCs) from periphery to regional lymph nodes, which initiates adaptive immunity and tolerance. In this process, tissue-resident DCs enter the blind-ended afferent lymphatic vessels, moving along the lymphatic endothelium to reach lymph nodes. Subsequently, DCs crawl along the collagen-based reticular network formed by FRCs to present antigen to effector T cells.49,50 Podoplanin is expressed on both LECs and FRCs and participates in the intravasation of DCs into afferent lymphatic vessels and DC migration to and within lymph nodes by several mechanisms. First, podoplanin on LECs immobilizes the chemokine CCL21 in the lymphatic endothelium and upregulates the expression of the chemokine receptor CCR7 on DCs, which can bind CCL21 to promote DC adhesion to the endothelium and transmigration into the lumen of the vessel.19 Second, podoplanin on LECs and FRCs, which link frameworks that support migratory DCs from tissues to lymph nodes, can interact with CLEC-2 on DCs, coordinately stimulating Rac1 and reducing RhoA GTPase activity via a signaling pathway downstream of CLEC-2 similar to that of platelet activation. This process leads to changes in the dynamics of the actin cytoskeleton, resulting in DC spreading along stromal cell scaffolds and migration.17
Furthermore, podoplanin appears to functionally affect LECs and FRCs. Previous research revealed that platelets inhibit LEC proliferation and migration through the CLEC-2-podoplanin interaction, in which BMP-9, a member of the bone morphogenetic protein (BMP) subfamily of TGF-β growth factors, plays a crucial role.51 Knockdown of podoplanin in human LEC inhibits polarization and migration mediated by upregulation of Cdc42 and downregulation of RhoA GTPase activity.52,53 Additionally, Kasinath et al studied the role of podoplanin-positive FRCs in nephrotoxic serum nephritis (NTN), a classic experimental anti–glomerular basement membrane antibody-mediated model of crescentic glomerulonephritis.54 Neutralization of podoplanin by an anti-podoplanin antibody inhibited the constitutive extracellular matrix fiber-secreting properties of FRCs, resulting in a disorganized fiber network and reduced expansion of lymphatic vasculature, which prevents the activation of CD4+ T cells.54 Astarita et al demonstrated that podoplanin appears to affect physical elasticity of lymph nodes by regulating FRCs function in the reticular network.55 Under resting conditions, when FRCs are unlikely to encounter mature DCs expressing CLEC-2, podoplanin induces actomyosin contractility in FRCs via activation of RhoA/C and downstream Rho-associated protein kinase (ROCK). During inflammation, CLEC-2 on mature DCs rapidly uncouples podoplanin from RhoA/C activation, relaxing the actomyosin cytoskeleton and permitting FRC stretching. Podoplanin-CLEC-2 interaction allows FRC network stretching and lymph node expansion, which is a critical hallmark in adaptive immunity.55,56
Podoplanin in Tissue and Organ Injury
The secondary inflammation after tissue injury is correlated with the expression of podoplanin. Kolar et al found that podoplanin was upregulated in reactive astrocytes in tumor and non-tumor brain injury models.57 Moreover, Song et al found upregulation of podoplanin in neurons rather than astrocytes involved in neuronal apoptosis in a LPS-induced neuronflammatory model.58 These differences could be explained by the different models used in the two studies but still fail to further reveal the role of podoplanin in neuroinflammation. Using a mouse traumatic brain injury model, Fei et al confirmed the upregulated podoplanin on microglia rather than neurons or activated astrocytes in vivo, and in vitro, podoplanin in primary microglia was substantially elevated by inflammatory stimuli, such as ATP, LPS, H2O2, and hemoglobin (Hb).35 Interestingly, as we referred before, knockdown of podoplanin in primary microglia prevented the induction of the M1 phenotype and elevated the proportion of M2 phenotype cells after stimuli of Hb, with the reduced secretion of IL-1β and TNF-α and increased secretion of IL-10 and TGF-β, which suggests podoplanin might act as an inflammation sensor in microglia.35
Growing evidence shows that LPS could induce upregulation of podoplanin on inflammatory macrophages and podoplanin-positive inflammatory macrophages play a key role in regulating inflammation via directly contacting platelets. Lax et al reported the podoplanin-CLEC-2 interaction protects against lung injury in a mouse model of LPS-induced acute respiratory distress syndrome. They reported a decline in arterial oxygen saturation and an augmentation of proinflammatory cytokines in bronchoalveolar lavage (BAL) in response to IT-LPS in hematopoietic-specific podoplanin-deficient mice, which indicates that podoplanin expression on inflammatory alveolar macrophages (iAM) prevents exaggerated lung inflammation.59 Further, Lax et al speculated that in alveolar space, CLEC-2 expressed on platelets interacts with podoplanin on inflammatory alveolar macrophages (iAM) to regulate matrix metalloproteinase (MMP)-12 release, which can cleave and inactivate CXC chemokines, resulting in “off” signals during acute lung inflammation and limiting lung injury.59,60 Similarly, using two mouse models of sepsis–intraperitoneal lipopolysaccharide and cecal ligation and puncture, Rayes et al demonstrated a protective role for podoplanin-CLEC-2 interaction in regulating the inflammatory reaction and preserving organ function in sepsis.36 In vitro, expression of podoplanin on inflammatory macrophages regulates the expression of TNF-α, with disruption of the podoplanin-CLEC-2 interaction by anti-podoplanin antibody in vivo altering cytokine and chemokine expression by inflammatory macrophages, including CXCL-1, CCL-2, and IL-636. These studies suggest that podoplanin on inflammatory macrophages appears to exert a protective or regulatory role in inflammation against pathogen insult. The upregulation of podoplanin on macrophages is common in several models of LPS-induced tissue injury. However, the intracellular pathway of podoplanin upregulation remains unclear and further studies are necessary to reveal the downstream mechanism of podoplanin affecting macrophage function.
Ischemia-reperfusion injury (IRI) contributes to a wide range of pathologies, such as myocardial infarction, ischemic stroke, and acute kidney injury, and it is also a major challenge during organ transplantation and cardiothoracic, vascular, and general surgery.61 Studies have shown the upregulation of podoplanin expression after ischemia in various tissues. Meng et al reported the role of the podoplanin‑CLEC‑2 axis in promoting the inflammatory response after ischemic stroke in mice via a mouse middle cerebral artery occlusion (MCAO) model.62 Podoplanin on both neurons and microglia was notably upregulated after ischemia-reperfusion and anti-podoplanin antibody pretreatment notably attenuated neurological deficits and decreased infarct volume, significantly decreasing the expression of IL-18 and IL-1β.62 Besides, Meng et al speculated that the production of IL-18 and IL-1β is attributed to the activation of NLRP3 inflammasome via the podoplanin-CELC-2 axis.62 In kidney, Kasinath et al recently found that the immunofluorescent signal of podoplanin fades in the glomerulus and intensifies in the tubulointerstitial compartment of the kidney shortly after ischemia-reperfusion injury, which is mediated by the podoplanin-positive podocytes and spindle-shaped cells.63 The podocytes may encapsulate podoplanin in extracellular vesicles and expel it into the urine immediately after IRI, while podoplanin-positive spindle-shaped cells, which morphologically resemble fibroblasts, may support the growing vasculature in the kidney during the repair phase after IRI.63 More importantly, podoplanin can be detected in the urine immediately after IRI and increases with the severity of ischemia, which suggests that the measurement of urine podoplanin represents a promising possible solution to the current important unmet clinical need for the expedient identification of renal IRI.
In addition, during tissue injury, monocytes-macrophages are able to functionally affect platelets through the podoplanin-CLEC-2 axis. In a mouse model of systemic Salmonella typhimurium infection, Hitchcock et al demonstrated that infection-induced IFN-γ release increases podoplanin expression on monocytes and Kupffer cells in the hepatic parenchyma and perivascular sites via TLR4 pathway, which activates platelets and results in inflammatory thrombosis.64 Moreover, Xie et al revealed another aspect of the podoplanin-CLEC-2 axis: increased podoplanin on monocytes binds to platelets CLEC-2, inducing the activation of platelets and complement inhibitor release to protect liver function during early sepsis.65 Notably, podoplanin binds CLEC-2 rather than GPVI to activate platelets, which may represent a possible therapeutic strategy to regulate inflammation without bleeding risk.
Podoplanin in Chronic Inflammatory Autoimmune Diseases
Autoimmune diseases, such as multiple sclerosis (MS), rheumatoid arthritis (RA), and colitis, are characterized by the dysregulation of the immune system which finally results in the break of tolerance to self-antigen. Immunosuppressive agents tried in autoimmune disease patients nonspecifically suppress the immune response, but often cause various side effects. Utility of biological agents to modify specific inflammatory and effector pathways is a promising therapeutic strategy in treating autoimmune diseases. The agents of blocking TNF-α were approved firstly for therapeutic drugs. However serious side effects result in unsatisfactory long-term prognoses and confined availability. Therefore, it is urgent to explore new therapeutic targets and strategies for chronic inflammatory autoimmune diseases.
CD4+ T helper cells play pivotal roles in the tissue destruction of many inflammatory autoimmune diseases, such as MS, RA, systemic sclerosis, and inflammatory bowel diseases.42 Autoimmune diseases are driven by self-reactive T helper Th1 and Th17 cells, and the latter can be heterogeneous and differentiate into two functional subtypes depending on the environmental stimuli. During inflammatory autoimmune diseases, Th17 cells can be highly proinflammatory, which induces irreversible tissue damage. Commonly, an association is made between Th17 cells and the secretion of IL-17, which is a pro-inflammatory cytokine involved in several chronic inflammatory diseases.66,67 A recent study indicates that interaction among activated lymphocytes and RA synoviocytes is critical for a high IL-17 secretion depending on the co-culture experiments with phytohemagglutinin-activated peripheral blood mononuclear cells (PBMCs) and synoviocytes isolated from patients with RA.68 However, both Th17 cells and synoviocytes express podoplanin with the respective involvement in IL-17 secretion. As we mentioned before, podoplanin can negatively regulate CD4+ effector T cell functions through podoplanin-CLEC-2 interaction and a high podoplanin expression was found on nonpathogenic Th17 lymphocytes.43,46 Hence, podoplanin displays two divergent functions which may depend on different ligands. Ligands such as CLEC-2 may mediate Th17 cell inhibition while another ligand, perhaps unknown, could promote inflammation by stimulating pro-inflammatory cytokine production.
The ectopic lymphoid follicle-like structure, characterized by the compartmentation of T and B cells and the presence of lymphatic vessels and high endothelial venules (HEVs), is a key factor in the pathogenesis of autoimmune diseases.69 The presence of ectopic lymphoid follicle-like structures is associated with the expression of podoplanin. In experimental autoimmune encephalomyelitis (EAE), the animal model of MS, development of ectopic lymphoid follicles was partly dependent on IL-17 and podoplanin, which was expressed on Th17 cells. Anti-podoplanin polyclonal antibody treatment reduced the number of ectopic lymphoid follicles.41 However, whether the blocking of podoplanin on Th17 cells or on other cell types including macrophages, stromal, and parenchymal cells leads to the reduction in ectopic lymphoid follicles remains to be determined. Moreover, in both clinical and experimental RA, podoplanin-expressing Th17 cells are associated with the formation of synovial ectopic lymphoid follicles and IL-27 inhibited the differentiation of podoplanin-expressing Th17 cells, negatively regulating ectopic lymphoid follicles development.70 Besides Th17 cells, expression of podoplanin in synoviocytes is also associated with ectopic lymphoid follicles and inflammation.71 Activated fibroblast-like synoviocytes (FLSs) in RA are key mediators of synovial tissue transformation and joint destruction.72,73 These aggressive cells share many characteristics with tumor cells, such as upregulated expression of proto-oncogenes and promigratory adhesion molecules, increased production of proinflammatory cytokines and matrix-degrading enzymes,74 as well as increased resistance to apoptosis.75,76 This is reminiscent of podoplanin upregulated in the invasive front of several human cancers, which is associated with epithelial-mesenchymal transition, cell migration and increased tissue invasion.77–79 In fact, podoplanin is highly expressed in FLSs of the invading synovial tissue in RA and pro-inflammatory mediators increased the podoplanin expression in cultured RA-FLS.80 Podoplanin-positive human synovial fibroblasts also exhibit characteristics of highly invasive myofibroblasts.81,82 In addition, in a severe combined immunodeficiency mouse model of RA, podoplanin-expressing synovial fibroblasts are associated with early fibroblast migration and cartilage erosion.83 These results together indicate that podoplanin-expressing synoviocytes play a critical role in joint destruction.
Podoplanin in Inflammatory Lymphangiogenesis
Lymphatic vessels play a critical role in fluid balance, nutrient absorption, and immune surveillance. Lymphangiogenesis is actively involved in a number of pathological processes including tissue inflammation and tumor dissemination.84 The key regulatory signaling axis that induces lymphangiogenesis is vascular endothelial growth factor receptor-3 (VEGFR-3) and its ligand, VEGF-C.85 Improved knowledge of the molecular mechanisms controlling lymphangiogenesis is attributed to progress in the identification of regulatory molecules and markers specific to the lymphatic endothelium. Physiologically, podoplanin expressed by LECs promotes blood-lymph separation and mice lacking podoplanin have leaky lymphatic vessels and congenital lymphedema.86,87 It has been reported that podoplanin is expressed in two lymphatic endothelial sublineages in human skin, which were defined by their cell surface densities of podoplanin and were designated LEC podo-low and LEC podo-high. LEC podo-low exists in precollector lymphatic vessels that secrete CCL27 and attract inflammatory CCR10-positive T lymphocytes, whereas LEC podo-high is restricted to initial lymphatic vessels, which produce CCL21 and attract CCR7-positive dendritic cells and regulatory T cells (Tregs).88 The differential podoplanin expression in both lymphatic endothelial sublineages appears to be negatively regulated by Notch signaling.89
Galectin-8 is a tandem-repeat type member of the galectin family and in vitro studies have shown that galectin-8 binds to podoplanin and that the lectin promotes adhesion and haptotaxis of LECs.90 A recent work showed that podoplanin is a key player in VEGF-C-induced lymphangiogenesis. Chen et al found the upregulated galectin-8 expression in the inflamed human and mice corneas working as a key medium for VEGF-C signal transduction and an effective lymphangiogenesis factor in vivo through a mechanism involving the interaction with podoplanin and integrin.21 They demonstrated that galectin-8 has a unique dual-faceted mechanism of action to promote lymphangiogenesis, where galectin-8-mediated interactions between lymphangiogenic integrins (α1ß1/α5ß1) and podoplanin are sufficient to activate the integrins and trigger the process of lymphangiogenesis without the involvement of VEGFR-3, but in the presence of VEGF-C/VEGFR-3, podoplanin-galectin-8-integrin interactions substantially increase the magnitude of the lymphangiogenic pathway by potentiating VEGF-C/VEGFR-3 signaling.21
Additionally, a previous study found that human CD34+ cells derived from fetal liver expressed podoplanin and VEGFR-3, which constitute a phenotypically and functionally distinct population of endothelial stem and precursor cells that may play a role in postnatal lymphangiogenesis.91 Lee et al also reported a novel type of bone-marrow-derived podoplanin-positive cells, which function as lymphatic endothelial progenitor cells and participate in postnatal lymphatic neovascularization through both vasculogenesis and lymphangiogenesis.92 Subsequent research found that induced by inflammatory stimuli including fibronectin, VEGF-C, TNF-α, LPS, or IL-3 in vitro, monocytes can transdifferentiate into lymphatic endothelial cells, expressing podoplanin and other lymphatic endothelial markers.93 Moreover, Maruyama et al reported that the CD11b+ and F4/80+ macrophages of mice, which express podoplanin, LYVE-1 and VEGFR-3 lymphatic markers, incorporate into lymphatic vessels in the inflamed cornea, playing a critical role in inflammatory lymphangiogenesis.94,95 Similar to corneal inflammation, the same researchers also found that lymphatic vessels that form during wound repair in diabetic mice are comprised largely of F4/80+ macrophages cells, which express LYVE-1 and podoplanin.96 These studies together indicate that podoplanin-positive cells from circulation and bone marrow function as progenitors of LECs, which are essential for lymphatic neovascularization under pathological conditions. On the other hand, Cimini et al firstly described a heterogeneous podoplanin-expressing cardiac interstitial cells population associated with inflammatory responses, fibrogenesis, and lymphangiogenesis after myocardial infarction.97 In fact, these podoplanin-expressing cells also contain a population with progenitor capabilities and distinctly display epitopes of fibrogenic and endothelial commitment, suggesting an alternative ability to lymphangiogenesis and pro-fibrosis.97,98
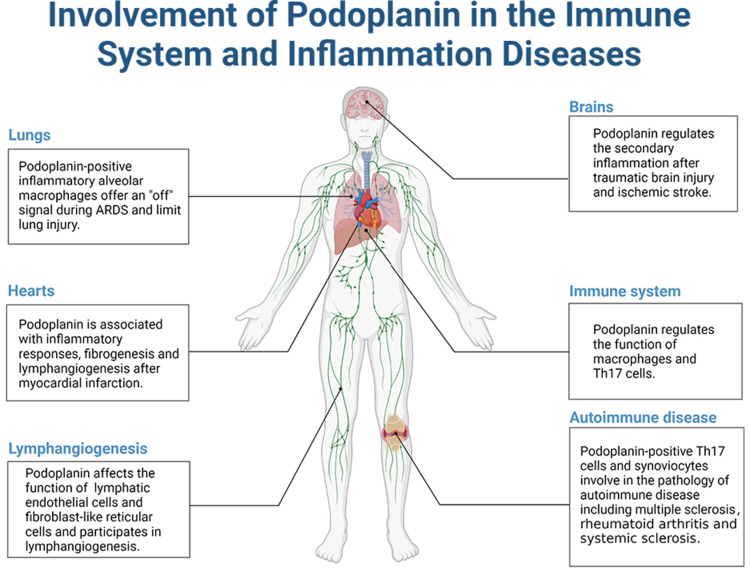
The main inflammatory diseases podoplanin is involved in are summarized in Figure 1.
Figure 1.
The involvement of podoplanin in the immune system and inflammatory diseases.
Podoplanin as a Potential Therapeutic Target in Inflammatory Disease
Previous studies showed that CAR-T cells, antibodies and lectins that target podoplanin can inhibit the growth and progression of several cancers in animal models, such as mesothelioma, glioma, and squamous cell carcinoma.99–102 In view of the critical role of podoplanin in the immune system and inflammatory disease, targeted podoplanin to regulate inflammation also represents a novel therapeutic strategy. The majority of podoplanin structure consists of a highly glycosylated extracellular domain that impacts podoplanin signaling and can be targeted by antibodies and lectins. Firstly, Galectins can bind podoplanin and the interaction between podoplanin and Galectin-8 is involved in pathological lymphangiogenesis.21,90 Hence, using plant lectins to interfere with the interaction between galectins and podoplanin may impede activation of downstream signaling pathways.103,104 The extracellular domain of podoplanin contains oligosaccharides, which are modified by the addition of sialic acid.105 The lectin MASL present in the seeds of Maackia amurensis binds to sialylated glycoproteins,106,107 such as podoplanin, by recognition of terminal α-2,3-sialylated oligosaccharides.108 Notably, MASL can preserve the structure and function of cartilage under osteoarthritis insults by interfering with the function of α-2,3-sialylated transmembrane receptors podoplanin.109 On the other hand, antibodies can be utilized to disrupt podoplanin-CLEC-2 interaction. Treatment with podoplanin-neutralizing antibody inhibits the interaction between podoplanin and CLEC-2 expressing immune cells in the heart, which can enhance the cardiac performance, regeneration, and angiogenesis after MI.97 Moreover, pharmacologic inhibition of the podoplanin-CLEC-2 axis by the anti-podoplanin antibody mAb 8.1.1 regulates the inflammatory reaction and immune cell infiltration during sepsis without inducing bleeding in the peritoneal cavity.36 These observations together suggest podoplanin as a novel anti-inflammatory target regulating immune cell recruitment and activation.
Conclusion and Outlook
Researchers have studied podoplanin for over 30 years. In this review, we elaborate the podoplanin expression in immune system and the regulatory role of podoplanin in inflammation. However, the pathological role of the glycoprotein in inflammation is far from elucidated. It remains to be established which is the additional ligand of podoplanin and the identification of novel ligands is beneficial to better explain the mechanism of podoplanin in inflammation immune cell recruitment, polarization and activation. In addition, the upregulation of podoplanin in inflamed tissue is common in several models of inflammatory tissue injury, but the intracellular pathway of podoplanin upregulation remains unclear. The function of podoplanin in immune response remains controversial and further studies should put more effort in revealing the function of podoplanin in macrophages polarization, regulation of effector T cells and the interaction between lymphocytes and mesenchymal cells. Also, the involvement of podoplanin in lymphangiogenesis deserves more in-depth research. These will notably improve our knowledge on the function of podoplanin in immunity and inflammation and lay the foundation for developing new therapeutic strategies.
Funding Statement
This work was supported by the National Natural Science Foundation of China [grant numbers: 81871601 and 82170107] and the Foundation of the Science and Technology Commission of Shanghai Municipality (19ZR1443000).
Abbreviations
PDPN, podoplanin; CLEC-2, C-type lectin-like receptor 2; EMT, epithelial-mesenchymal transition; APC, antigen-presenting cell; LEC, lymphatic endothelial cell; FRC, fibroblast-like reticular cell; BMDM, bone marrow-derived macrophage; LPS, lipopolysaccharide; TNF, tumor necrosis factor; TLR, Toll-like receptor; CLP, cecal ligation and puncture; Th17 cell, interleukin 17-producing helper T cell; ITAM, immunoreceptor tyrosine-based inhibitory motif; DC, dendritic cell; BMP, bone morphogenetic protein; NTN, nephrotoxic serum nephritis; Hb, hemoglobin; BAL, bronchoalveolar lavage; iAM, inflammatory alveolar macrophage; MMP, matrix metalloproteinase; IRI, ischemia-reperfusion injury; MCAO, middle cerebral artery occlusion; MS, multiple sclerosis; RA, rheumatoid arthritis; PBMC, peripheral blood mononuclear cell; HEV, high endothelial venules; EAE, experimental autoimmune encephalomyelitis; FLS, fibroblast-like synoviocyte; VEGFR, vascular endothelial growth factor receptor; Treg, regulatory T cell.
Disclosure
The authors have declared no conflicts of interest in relation to this work.
References
- 1.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi: 10.1091/mbc.E10-06-0489 [DOI] [PubMed] [Google Scholar]
- 2.Quintanilla M, Montero-Montero L, Renart J, Martin-Villar E. Podoplanin in inflammation and cancer. Int J Mol Sci. 2019;20(3):707. doi: 10.3390/ijms20030707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ester M-V, Scholl FG, Carlos G, et al. Characterization of human PA2.26 antigen (T1alpha-2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas. Int J Cancer. 2005;113(6). doi: 10.1002/ijc.20656 [DOI] [PubMed] [Google Scholar]
- 4.Ozaki Y, Tamura S, Suzuki-Inoue K. New horizon in platelet function: with special reference to a recently-found molecule, CLEC-2. Thromb J. 2016;14(Suppl 1):27. doi: 10.1186/s12959-016-0099-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki-Inoue K, Tsukiji N, Shirai T, Osada M, Inoue O, Ozaki Y. Platelet CLEC-2: roles beyond hemostasis. Semin Thromb Hemost. 2018;44(2):126–134. doi: 10.1055/s-0037-1604090 [DOI] [PubMed] [Google Scholar]
- 6.Colonna M, Samaridis J, Angman L. Molecular characterization of two novel C-type lectin-like receptors, one of which is selectively expressed in human dendritic cells. Eur J Immunol. 2000;30(2):697–704. doi: [DOI] [PubMed] [Google Scholar]
- 7.Kerrigan AM, Dennehy KM, Mourão-Sá D, et al. CLEC-2 is a phagocytic activation receptor expressed on murine peripheral blood neutrophils. J Immunol. 2009;182(7):4150–4157. doi: 10.4049/jimmunol.0802808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acton SE, Astarita JL, Malhotra D, et al. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity. 2012;37(2):276–289. doi: 10.1016/j.immuni.2012.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki-Inoue K, Fuller G, García A, et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107(2):542–549. doi: 10.1182/blood-2005-05-1994 [DOI] [PubMed] [Google Scholar]
- 10.Fuller G, Williams J, Tomlinson M, et al. The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade. J Biol Chem. 2007;282(17):12397–12409. doi: 10.1074/jbc.M609558200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki-Inoue K, Inoue O, Ozaki YJ. Novel platelet activation receptor CLEC-2: from discovery to prospects. J Thromb Haemost. 2011;9:44–55. doi: 10.1111/j.1538-7836.2011.04335.x [DOI] [PubMed] [Google Scholar]
- 12.Martín-Villar E, Megías D, Castel S, Yurrita M, Vilaró S, Quintanilla MJ. Podoplanin binds ERM proteins to activate RhoA and promote epithelial-mesenchymal transition. J Cell Sci. 2006;119(21):4541–4553. doi: 10.1242/jcs.03218 [DOI] [PubMed] [Google Scholar]
- 13.Astarita J, Acton S, Turley SJ. Podoplanin: emerging functions in development, the immune system, and cancer. Front Immunol. 2012;3:283. doi: 10.3389/fimmu.2012.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaime R, Patricia C-R, Beatriz F-M, et al. New insights into the role of podoplanin in epithelial-mesenchymal transition. Int Rev Cell Mol Biol. 2015;317. doi: 10.1016/bs.ircmb.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 15.Harini K, Julie R, Tomoyuki M, et al. Podoplanin: an emerging cancer biomarker and therapeutic target. Cancer Sci. 2018;109(5). doi: 10.1111/cas.13580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herzog B, Fu J, Wilson S, et al. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature. 2013;502(7469):105–109. doi: 10.1038/nature12501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acton S, Astarita J, Malhotra D, et al. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity. 2012;37(2):276–289. doi: 10.1016/j.immuni.2012.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hur J, Jang JH, Oh I-Y, et al. Human podoplanin-positive monocytes and platelets enhance lymphangiogenesis through the activation of the podoplanin/CLEC-2 axis. Mol Ther. 2014;22(8):1518–1529. doi: 10.1038/mt.2014.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tal O, Lim H, Gurevich I, et al. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J Exp Med. 2011;208(10):2141–2153. doi: 10.1084/jem.20102392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuertbauer E, Zaujec J, Uhrin P, et al. Thymic medullar conduits-associated podoplanin promotes natural regulatory T cells. Immunol Lett. 2013;154(1–2):31–41. doi: 10.1016/j.imlet.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 21.Chen WS, Cao Z, Sugaya S, et al. Pathological lymphangiogenesis is modulated by galectin-8-dependent crosstalk between podoplanin and integrin-associated VEGFR-3. Nat Commun. 2016;7(1):11302. doi: 10.1038/ncomms11302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Youya N, Shigeo S, Mikihiko N, et al. Tetraspanin family member CD9 inhibits Aggrus/podoplanin-induced platelet aggregation and suppresses pulmonary metastasis. Blood. 2008;112(5). doi: 10.1182/blood-2007-11-124693 [DOI] [PubMed] [Google Scholar]
- 23.Ester M-V, Beatriz F-M, Maddy P, et al. Podoplanin associates with CD44 to promote directional cell migration. Mol Biol Cell. 2010;21:24. doi: 10.1091/mbc.E10-06-0489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016;529(7586):307–315. doi: 10.1038/nature17039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–1197. doi: 10.1038/ni1276 [DOI] [PubMed] [Google Scholar]
- 26.Bonilla FA, Oettgen HC. Adaptive immunity. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S33–S40. doi: 10.1016/j.jaci.2009.09.017 [DOI] [PubMed] [Google Scholar]
- 27.Gray KJ, Gibbs JE. Adaptive immunity, chronic inflammation and the clock. Semin Immunopathol. 2022;44(2):209–224. doi: 10.1007/s00281-022-00919-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438(7070):946–953. doi: 10.1038/nature04480 [DOI] [PubMed] [Google Scholar]
- 29.Peters A, Pitcher L, Sullivan J, et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity. 2011;35(6):986–996. doi: 10.1016/j.immuni.2011.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyamoto Y, Uga H, Tanaka S, et al. Podoplanin is an inflammatory protein upregulated in Th17 cells in SKG arthritic joints. Mol Immunol. 2013;54(2):199–207. doi: 10.1016/j.molimm.2012.11.013 [DOI] [PubMed] [Google Scholar]
- 31.Kerrigan AM, Navarro‐nuñez L, Pyz E. Podoplanin-expressing inflammatory macrophages activate murine platelets via CLEC-2. J Thromb Haemost. 2012;10(3):484–486. doi: 10.1111/j.1538-7836.2011.04614.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wynn T, Chawla A, Pollard JJN. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. doi: 10.1038/nature12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou T, Bystrom J, Sherlock J, et al. A distinct subset of podoplanin (gp38) expressing F4/80+ macrophages mediate phagocytosis and are induced following zymosan peritonitis. FEBS Lett. 2010;584(18):3955–3961. doi: 10.1016/j.febslet.2010.07.053 [DOI] [PubMed] [Google Scholar]
- 34.Lawrence T, Natoli GJ. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11(11):750–761. doi: 10.1038/nri3088 [DOI] [PubMed] [Google Scholar]
- 35.Fei M, Wang H, Zhou M, Deng C, Zhang L, Han Y. Podoplanin influences the inflammatory phenotypes and mobility of microglia in traumatic brain injury. Biochem Biophys Res Commun. 2020;523(2):361–367. doi: 10.1016/j.bbrc.2019.12.003 [DOI] [PubMed] [Google Scholar]
- 36.Rayes J, Lax S, Wichaiyo S, et al. The podoplanin-CLEC-2 axis inhibits inflammation in sepsis. Nat Commun. 2017;8(1):2239. doi: 10.1038/s41467-017-02402-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bourne J, Beristain-Covarrubias N, Zuidscherwoude M, et al. CLEC-2 prevents accumulation and retention of inflammatory macrophages during murine peritonitis. Front Immunol. 2021;12:693974. doi: 10.3389/fimmu.2021.693974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishnan H, Ochoa-Alvarez JA, Shen Y, et al. Serines in the intracellular tail of Podoplanin (PDPN) regulate cell motility. J Biol Chem. 2013;288(17). doi: 10.1074/jbc.C112.446823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheok Y, Tan G, Fernandez K, et al. Podoplanin drives motility of active macrophage via regulating Filamin C during Helicobacter pylori infection. Front Immunol. 2021;12:702156. doi: 10.3389/fimmu.2021.702156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stossel T, Condeelis J, Cooley L, et al. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol. 2001;2(2):138–145. doi: 10.1038/35052082 [DOI] [PubMed] [Google Scholar]
- 41.Peters A, Pitcher LA, Sullivan JM, et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity. 2011;35(6):986–996. doi: 10.1016/j.immuni.2011.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yasuda K, Takeuchi Y, Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin Immunopathol. 2019;41(3):283–297. doi: 10.1007/s00281-019-00733-8 [DOI] [PubMed] [Google Scholar]
- 43.Nylander AN, Ponath GD, Axisa PP, et al. Podoplanin is a negative regulator of Th17 inflammation. JCI Insight. 2017;2(17). doi: 10.1172/jci.insight.92321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee Y, Awasthi A, Yosef N, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13(10):991–999. doi: 10.1038/ni.2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joller N, Peters A, Anderson A, Kuchroo VJ. Immune checkpoints in central nervous system autoimmunity. Immunol Rev. 2012;248(1):122–139. doi: 10.1111/j.1600-065X.2012.01136.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peters A, Burkett P, Sobel R, et al. Podoplanin negatively regulates CD4+ effector T cell responses. J Clin Invest. 2015;125(1):129–140. doi: 10.1172/JCI74685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chihara N, Madi A, Kondo T, et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature. 2018;558(7710):454–459. doi: 10.1038/s41586-018-0206-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198(12):1807–1815. doi: 10.1084/jem.20030725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malhotra D, Fletcher AL, Turley SJ. Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity. Immunol Rev. 2013;251(1):160–176. doi: 10.1111/imr.12023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malhotra D, Fletcher A, Astarita J, et al. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat Immunol. 2012;13(5):499–510. doi: 10.1038/ni.2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osada M, Inoue O, Ding G, et al. Platelet activation receptor CLEC-2 regulates blood/lymphatic vessel separation by inhibiting proliferation, migration, and tube formation of lymphatic endothelial cells. J Biol Chem. 2012;287(26):22241–22252. doi: 10.1074/jbc.M111.329987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Navarro A, Perez RE, Rezaiekhaligh M, Mabry SM, Ekekezie II. T1alpha/podoplanin is essential for capillary morphogenesis in lymphatic endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;295(4):L543–L551. doi: 10.1152/ajplung.90262.2008 [DOI] [PubMed] [Google Scholar]
- 53.Navarro A, Perez RE, Rezaiekhaligh MH, Mabry SM, Ekekezie II. Polarized migration of lymphatic endothelial cells is critically dependent on podoplanin regulation of Cdc42. Am J Physiol Lung Cell Mol Physiol. 2011;300(1):L32–42. doi: 10.1152/ajplung.00171.2010 [DOI] [PubMed] [Google Scholar]
- 54.Kasinath V, Yilmam O, Uehara M, et al. Activation of fibroblastic reticular cells in kidney lymph node during crescentic glomerulonephritis. Kidney Int. 2019;95(2):310–320. doi: 10.1016/j.kint.2018.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Astarita J, Cremasco V, Fu J, et al. The CLEC-2-podoplanin axis controls the contractility of fibroblastic reticular cells and lymph node microarchitecture. Nat Immunol. 2015;16(1):75–84. doi: 10.1038/ni.3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Acton S, Farrugia A, Astarita J, et al. Dendritic cells control fibroblastic reticular network tension and lymph node expansion. Nature. 2014;514(7523):498–502. doi: 10.1038/nature13814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kushal K, Moises F-A, Bechberger JF, et al. Podoplanin: a marker for reactive gliosis in gliomas and brain injury. J Neuropathol Exp Neurol. 2015;74(1). doi: 10.1097/NEN.0000000000000150 [DOI] [PubMed] [Google Scholar]
- 58.Song Y, Shen J, Lin Y, et al. Up-regulation of podoplanin involves in neuronal apoptosis in LPS-induced neuroinflammation. Cell Mol Neurobiol. 2014;34(6):839–849. doi: 10.1007/s10571-014-0060-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lax S, Rayes J, Wichaiyo S, et al. Platelet CLEC-2 protects against lung injury via effects of its ligand podoplanin on inflammatory alveolar macrophages in the mouse. Am J Physiol Lung Cell Mol Physiol. 2017;313(6):L1016–L1029. doi: 10.1152/ajplung.00023.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dean RA, Cox JH, Bellac CL, et al. Macrophage-specific metalloelastase (MMP-12) truncates and inactivates ELR+ CXC chemokines and generates CCL2, −7, −8, and −13 antagonists: potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood. 2008;112(8). doi: 10.1182/blood-2007-12-129080 [DOI] [PubMed] [Google Scholar]
- 61.Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med. 2011;17(11):1391–1401. doi: 10.1038/nm.2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meng D, Ma X, Li H, et al. A role of the podoplanin-CLEC-2 axis in promoting inflammatory response after ischemic stroke in mice. Neurotox Res. 2021;39(2):477–488. doi: 10.1007/s12640-020-00295-w [DOI] [PubMed] [Google Scholar]
- 63.Kasinath V, Yilmam O, Uehara M, et al. Urine podoplanin heralds the onset of ischemia-reperfusion injury of the kidney. Am J Physiol Renal Physiol. 2019;316(5):F957–F965. doi: 10.1152/ajprenal.00538.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hitchcock J, Cook C, Bobat S, et al. Inflammation drives thrombosis after Salmonella infection via CLEC-2 on platelets. J Clin Invest. 2015;125(12):4429–4446. doi: 10.1172/JCI79070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie Z, Shao B, Hoover C, et al. Monocyte upregulation of podoplanin during early sepsis induces complement inhibitor release to protect liver function. JCI Insight. 2020;5(13). doi: 10.1172/jci.insight.134749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361(9):888–898. doi: 10.1056/NEJMra0707449 [DOI] [PubMed] [Google Scholar]
- 67.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11(10):763–776. doi: 10.1038/nrd3794 [DOI] [PubMed] [Google Scholar]
- 68.Noack M, Ndongo-Thiam N, Miossec P. Interaction among activated lymphocytes and mesenchymal cells through podoplanin is critical for a high IL-17 secretion. Arthritis Res Ther. 2016;18(1):148. doi: 10.1186/s13075-016-1046-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weyand C, Kurtin P, Goronzy J. Ectopic lymphoid organogenesis: a fast track for autoimmunity. Am J Pathol. 2001;159(3):787–793. doi: 10.1016/S0002-9440(10)61751-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jones GW, Bombardieri M, Greenhill CJ, et al. Interleukin-27 inhibits ectopic lymphoid-like structure development in early inflammatory arthritis. J Exp Med. 2015;212(11):1793–1802. doi: 10.1084/jem.20132307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takakubo Y, Oki H, Naganuma Y, et al. Distribution of podoplanin in synovial tissues in rheumatoid arthritis patients using biologic or conventional disease-modifying anti-rheumatic drugs. Curr Rheumatol Rev. 2017;13(1):72–78. doi: 10.2174/1573397112666160331143607 [DOI] [PubMed] [Google Scholar]
- 72.Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233(1):233–255. doi: 10.1111/j.0105-2896.2009.00859.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tolboom TCA, van der Helm-Van Mil AHM, Nelissen RG, Breedveld FC, Toes REM, Huizinga TWJ. Invasiveness of fibroblast-like synoviocytes is an individual patient characteristic associated with the rate of joint destruction in patients with rheumatoid arthritis. Arthritis Rheum. 2005;52(7):1999–2002. doi: 10.1002/art.21118 [DOI] [PubMed] [Google Scholar]
- 74.Huber LC, Distler O, Tarner I, Gay RE, Gay S, Pap T. Synovial fibroblasts: key players in rheumatoid arthritis. Rheumatology. 2006;45(6):669–675. doi: 10.1093/rheumatology/kel065 [DOI] [PubMed] [Google Scholar]
- 75.Baier A, Meineckel I, Gay S, Pap T. Apoptosis in rheumatoid arthritis. Curr Opin Rheumatol. 2003;15(3):274–279. doi: 10.1097/00002281-200305000-00015 [DOI] [PubMed] [Google Scholar]
- 76.Karouzakis E, Gay RE, Gay S, Neidhart M. Epigenetic control in rheumatoid arthritis synovial fibroblasts. Nat Rev Rheumatol. 2009;5(5):266–272. doi: 10.1038/nrrheum.2009.55 [DOI] [PubMed] [Google Scholar]
- 77.Martín-Villar E, Scholl FG, Gamallo C, et al. Characterization of human PA2.26 antigen (T1alpha-2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas. Int J Cancer. 2005;113(6):899–910. doi: 10.1002/ijc.20656 [DOI] [PubMed] [Google Scholar]
- 78.Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G. Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9(4):261–272. doi: 10.1016/j.ccr.2006.03.010 [DOI] [PubMed] [Google Scholar]
- 79.Wicki A, Christofori G. The potential role of podoplanin in tumour invasion. Br J Cancer. 2007;96(1):1–5. doi: 10.1038/sj.bjc.6603518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ekwall A, Eisler T, Anderberg C, et al. The tumour-associated glycoprotein podoplanin is expressed in fibroblast-like synoviocytes of the hyperplastic synovial lining layer in rheumatoid arthritis. Arthritis Res Ther. 2011;13(2):R40. doi: 10.1186/ar3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ekwall A-KH, Eisler T, Anderberg C, et al. The tumour-associated glycoprotein podoplanin is expressed in fibroblast-like synoviocytes of the hyperplastic synovial lining layer in rheumatoid arthritis. Arthritis Res Ther. 2011;13(2):R40. doi: 10.1186/ar3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mizoguchi F, Slowikowski K, Wei K, et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat Commun. 2018;9(1):789. doi: 10.1038/s41467-018-02892-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Croft A, Naylor A, Marshall J, et al. Rheumatoid synovial fibroblasts differentiate into distinct subsets in the presence of cytokines and cartilage. Arthritis Res Ther. 2016;18(1):270. doi: 10.1186/s13075-016-1156-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140(4):460–476. doi: 10.1016/j.cell.2010.01.045 [DOI] [PubMed] [Google Scholar]
- 85.D’Alessio S, Correale C, Tacconi C, et al. VEGF-C-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J Clin Invest. 2014;124(9):3863–3878. doi: 10.1172/JCI72189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schacht V, Ramirez MI, Hong Y-K, et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22(14):3546–3556. doi: 10.1093/emboj/cdg342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fu J, Gerhardt H, McDaniel JM, et al. Endothelial cell O-glycan deficiency causes blood/lymphatic misconnections and consequent fatty liver disease in mice. J Clin Invest. 2008;118(11):3725–3737. doi: 10.1172/JCI36077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wick N, Haluza D, Gurnhofer E, et al. Lymphatic precollectors contain a novel, specialized subpopulation of podoplanin low, CCL27-expressing lymphatic endothelial cells. Am J Pathol. 2008;173(4):1202–1209. doi: 10.2353/ajpath.2008.080101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kang J, Yoo J, Lee S, et al. An exquisite cross-control mechanism among endothelial cell fate regulators directs the plasticity and heterogeneity of lymphatic endothelial cells. Blood. 2010;116(1):140–150. doi: 10.1182/blood-2009-11-252270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cueni LN, Detmar M. Galectin-8 interacts with podoplanin and modulates lymphatic endothelial cell functions. Exp Cell Res. 2009;315(10):10. doi: 10.1016/j.yexcr.2009.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Salven P, Mustjoki S, Alitalo R, Alitalo K, Rafii SJB. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood. 2003;101(1):168–172. doi: 10.1182/blood-2002-03-0755 [DOI] [PubMed] [Google Scholar]
- 92.Lee J, Park C, Cho Y, et al. Podoplanin-expressing cells derived from bone marrow play a crucial role in postnatal lymphatic neovascularization. Circulation. 2010;122(14):1413–1425. doi: 10.1161/CIRCULATIONAHA.110.941468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Changming W, Xin L, Hua T, et al. Monocytes can be induced to express lymphatic phenotypes. Lymphology. 2011;44(2). [PubMed] [Google Scholar]
- 94.Maruyama K, Nakazawa T, Cursiefen C, et al. The maintenance of lymphatic vessels in the cornea is dependent on the presence of macrophages. Invest Ophthalmol Vis Sci. 2012;53(6):3145–3153. doi: 10.1167/iovs.11-8010 [DOI] [PubMed] [Google Scholar]
- 95.Kazuichi M, Masaaki I, Claus C, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115(9). doi: 10.1172/JCI23874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maruyama K, Asai J, Ii M, Thorne T, Losordo D, D’Amore P. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol. 2007;170(4):1178–1191. doi: 10.2353/ajpath.2007.060018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cimini M, Garikipati VNS, de Lucia C, et al. Podoplanin neutralization improves cardiac remodeling and function after acute myocardial infarction. JCI Insight. 2019;5. doi: 10.1172/jci.insight.126967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cimini M, Kishore R. Role of podoplanin-positive cells in cardiac fibrosis and angiogenesis after ischemia. Front Physiol. 2021;12:667278. doi: 10.3389/fphys.2021.667278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abe S, Morita Y, Kaneko MK, et al. A novel targeting therapy of malignant mesothelioma using anti-podoplanin antibody. J Immunol. 2013;190(12):6239–6249. doi: 10.4049/jimmunol.1300448 [DOI] [PubMed] [Google Scholar]
- 100.Chandramohan V, Bao X, Kato Kaneko M, et al. Recombinant anti-podoplanin (NZ-1) immunotoxin for the treatment of malignant brain tumors. Int J Cancer. 2013;132(10):2339–2348. doi: 10.1002/ijc.27919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shiina S, Ohno M, Ohka F, et al. CAR T cells targeting podoplanin reduce orthotopic glioblastomas in mouse brains. Cancer Immunol Res. 2016;4(3):259–268. doi: 10.1158/2326-6066.CIR-15-0060 [DOI] [PubMed] [Google Scholar]
- 102.Kaneko MK, Nakamura T, Kunita A, et al. ChLpMab-23: cancer-specific human-mouse chimeric anti-podoplanin antibody exhibits antitumor activity via antibody-dependent cellular cytotoxicity. Monoclon Antib Immunodiagn Immunother. 2017;36(3):104–112. doi: 10.1089/mab.2017.0014 [DOI] [PubMed] [Google Scholar]
- 103.Hasan SS, Ashraf GM, Banu N. Galectins - potential targets for cancer therapy. Cancer Lett. 2007;253(1):25–33. doi: 10.1016/j.canlet.2006.11.030 [DOI] [PubMed] [Google Scholar]
- 104.Pusztai A, Bardocz S, Ewen SWB. Uses of plant lectins in bioscience and biomedicine. Front Biosci. 2008;13(13):1130–1140. doi: 10.2741/2750 [DOI] [PubMed] [Google Scholar]
- 105.Scholl F, Gamallo C, Vilaró S, Quintanilla MJ. Identification of PA2.26 antigen as a novel cell-surface mucin-type glycoprotein that induces plasma membrane extensions and increased motility in keratinocytes. J Cell Sci. 1999;112(24):4601–4613. doi: 10.1242/jcs.112.24.4601 [DOI] [PubMed] [Google Scholar]
- 106.Geisler C, Jarvis DL. Effective glycoanalysis with Maackia amurensis lectins requires a clear understanding of their binding specificities. Glycobiology. 2011;21(8):988–993. doi: 10.1093/glycob/cwr080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang WC, Cummings RD. The immobilized leukoagglutinin from the seeds of Maackia amurensis binds with high affinity to complex-type Asn-linked oligosaccharides containing terminal sialic acid-linked alpha-2,3 to penultimate galactose residues. J Biol Chem. 1988;263(10):4576–4585. doi: 10.1016/S0021-9258(18)68821-0 [DOI] [PubMed] [Google Scholar]
- 108.Imberty A, Gautier C, Lescar J, Pérez S, Wyns L, Loris R. An unusual carbohydrate binding site revealed by the structures of two Maackia amurensis lectins complexed with sialic acid-containing oligosaccharides. J Biol Chem. 2000;275(23):17541–17548. doi: 10.1074/jbc.M000560200 [DOI] [PubMed] [Google Scholar]
- 109.Carpintero-Fernandez P, Varela-Eirin M, Lacetera A, et al. New therapeutic strategies for osteoarthritis by targeting sialic acid receptors. Biomolecules. 2020;10(4):637. doi: 10.3390/biom10040637 [DOI] [PMC free article] [PubMed] [Google Scholar]