Abstract
Objective
Musculoskeletal (MSK) pain is being increasingly reported by patients as one of the most common persistent symptoms in post-COVID-19 syndrome or Long COVID. However, there is a lack of understanding of its prevalence, characteristics, and underlying pathophysiological mechanisms. The objective of this review is to identify and describe the features and characteristics of MSK pain in Long COVID patients.
Methods
The narrative review involved a literature search of the following online databases: MEDLINE (OVID), EMBASE (OVID), CINAHL, PsyclNFO, and Web of Science (December 2019 to February 2022). We included observational studies that investigated the prevalence, characteristics, risk factors and mechanisms of MSK pain in Long COVID. After screening and reviewing the initial literature search results, a total of 35 studies were included in this review.
Results
The overall reported prevalence of MSK pain in Long COVID ranged widely from 0.3% to 65.2%. The pain has been reported to be localized to a particular region or generalized and widespread. No consistent pattern of progression of MSK pain symptoms over time was identified. Female gender and higher BMI could be potential risk factors for Long COVID MSK pain, but no clear association has been found with age and ethnicity. Different pathophysiological mechanisms have been hypothesized to contribute to MSK pain in Long COVID including increased production of proinflammatory cytokines, immune cell hyperactivation, direct viral entry of neurological and MSK system cells, and psychological factors.
Conclusion
MSK pain is one of the most common symptoms in Long COVID. Most of the current literature on Long COVID focuses on reporting the prevalence of persistent MSK pain. Studies describing the pain characteristics are scarce. The precise mechanism of MSK pain in Long COVID is yet to be investigated. Future research must explore the characteristics, risk factors, natural progression, and underlying mechanisms of MSK pain in Long COVID.
Keywords: post-COVID-19 syndrome, post-acute COVID-19, chronic pain
Introduction
The lasting symptoms of COVID-19, now referred to as “Long COVID”, are a public health concern. According to the National Institute for Health and Care Excellence (NICE) guidelines, “Long COVID” is defined as signs and symptoms that develop during or after an acute infection consistent with COVID-19 and persist longer than 4 weeks.1 This comprises both ongoing symptomatic COVID-19 (from 4 to 12 weeks) and post-COVID-19 syndrome (beyond 12 weeks). The guidelines emphasize that the symptoms cannot be explained by an alternative diagnosis which is in keeping with the clinical case definition proposed by the WHO that also highlight the potential fluctuations and protracted nature of post-COVID-19 symptoms.2
Long COVID presents as a clinically diverse condition, with published work reporting a plethora of symptoms, most commonly: fatigue, shortness of breath, pain, post-exertional malaise, persisting elevated temperature and cognitive dysfunction.3–6 At the beginning of December 2021, estimated 1.3 million people in UK private households had Long COVID, 64% of whom reported an adverse impact of their symptoms/diagnosis on their ability to carry out activities of daily living (ADL).3,7 Several theories have been posited to explain the etiology of Long COVID which include continued presence of the virus, re-infection with the same or possibly different strain of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), endothelial damage and hypercoagulation, dysautonomia, and dysfunctional immune response leading to chronic inflammation.8–12
It is becoming increasingly evident that musculoskeletal (MSK) pain, defined as pain arising from MSK structures, such as bones, muscles, joints, tendons and ligaments,13 is common among Long COVID patients. A previous systematic review and meta-analysis reported a total prevalence of myalgia (muscle pain) and arthralgia (joint pain) ranging from 5.65% to 18.1% and 4.6% to 12.1%, respectively, in the 4 to 12 weeks following acute COVID-19 infection onset or hospitalisation.14 The authors suggest that up to 10% of recovered COVID-19 patients will experience Long COVID pain of MSK origin.
MSK pain-related conditions are among the leading contributors to global disability, estimated to affect 1.71 billion people worldwide.15 According to the latest Versus Arthritis report, approximately a third of the UK population is living with MSK conditions, which account for 21% of years lived with disability in the UK.16 Research suggests MSK pain has higher prevalence rates in females and is more common in older people.17–20 In addition to the negative impact on an individual’s functional status and health-related quality of life,21 the substantial costs of healthcare for MSK pain and associated loss of productivity pose a huge economic burden on societies.22–24
Given the increasing number of people with Long COVID and MSK pain, there is an urgent need for a holistic understanding of MSK pain in Long COVID to inform appropriate management and mitigate the growing burden of chronic pain. This narrative review will explore the latest reported literature on MSK pain in Long COVID. The aim of this review is to describe the prevalence, risk factors and the plausible mechanisms of MSK pain in Long COVID.
Methods
Identification of relevant studies was performed through literature search of the following online databases: MEDLINE (OVID), EMBASE (OVID), CINAHL, PsyclNFO and Web of Science. The search strategy included terms related to the condition (COVID-19; SARS-CoV-2; coronavirus; 2019-nCoV; Long COVID; Post-COVID; Long haul COVID), pain symptoms (pain; musculoskeletal pain; nociceptive pain; neuropathic pain; neuralgia; radiculopathy; widespread pain; arthralgia; myalgia; acute pain; chronic pain; pain mechanisms), and study methods (observational study; cross-sectional study; cohort study; case–control study; longitudinal study). The search was restricted to studies that were published in English between December 2019 and February 2022. Reviews, case studies, expert opinions, and editorial articles were excluded. The search terms were tailored to each database, as required (Table 1). Furthermore, the bibliographies of the relevant studies were scanned for additional relevant literature. A citation manager program (Endnote) was used to import all retrieved references and remove duplicates. Duplicates not identified by the program were manually excluded. After removal of duplicates and screening of the titles and abstracts of the literature search results, a total of 112 articles underwent full-text review. Following a full-text review, 35 articles were found suitable for inclusion in this review (Figure 1).
Table 1.
Search Strategy (OVID)
| Long COVID | Pain | Observational Studies | ||
|---|---|---|---|---|
|
COVID-19* SARS-CoV-2 Coronavirus 2019-nCov “Long COVID*” Post-COVID* Post-acute “long haul*” |
AND | Pain* “Musculoskeletal pain” “Chronic pain” “acute pain” “Nociceptive pain” Neuropath* or Neuralgia Radiculopathy “widespread pain” “joint pain” or Arthralgia “muscle pain” or Myalgia Long-term Prolong* Persist* Mechanisms* Pathophysiolog* Etiolog* Aetiolog* |
AND | “observational stud*” “cross-sectional stud*” “Cohort stud*” “case-control stud*” “longitudinal stud*” |
| Combined with OR | Combined with OR | Combined with OR | ||
| Limits: Human, Adult, English language (December 2019 to February 2022) Exclude: Reviews, case studies, expert opinion, editorial articles, and pre-prints | ||||
Note: *Truncation command to search for the root of the free-text word with any alternative ending.
Figure 1.
Flow Diagram of screening and selection process.
Synthesis of Results
Prevalence of Pain
Numerous studies have reported the prevalence of MSK pain and other persistent symptoms of COVID-19. The overall reported prevalence ranged widely from 0.3% to 65.2% (Table 2). The heterogeneity of participants (in relation to the severity of acute illness) and measurements at different timepoints after the acute infection may contribute to the wide range of prevalence reported in these studies. Myalgia and arthralgia appear to be the most common MSK pain symptoms that have been reported in 18 out of 35 studies. The lowest prevalence was reported by Mohiuddin Chowdhury et al, for both mild body ache and back pain (0.3%) persisting for 4 weeks following recovery.25 The highest prevalence of 65.2% COVID-19 survivors reporting new-onset pain at 16 weeks after hospital discharge has been reported by Carvalho-Soares et al26
Table 2.
Characteristics of Relevant Studies
| Author, Year Country | Sample Size (Male/Female) | Hospitalization | Type of Study | Time Point(s) | MSK Pain Type and Prevalence at Follow-Up(s) | Pain Characterizations and Location | Risk Factors | Hypothesized Mechanisms |
|---|---|---|---|---|---|---|---|---|
|
Adnan et al (2021)27 Pakistan |
55 (35–20) | No | Cross-sectional study | Not clear | Myalgia and Arthralgia (63.63%) in male (36.36%) in female |
Back ache 45.45% Knee joint 18.18% Mixed 18.18% Hip joint 9.09% Ankle joint 5.45% |
The prevalence of Myalgia and Arthralgia increase with aging and was higher in the lower socio-economic status. | Not available |
|
Bai et al (2021)28 Italy |
377 (240–137) | Yes | Prospective cohort study | Median of 6 weeks from symptom onset | Musculoskeletal pain 21.2% | Joint pain or myalgia No, ever (57%) Ongoing (21.2%) Resolved (19.6%) Unknown (2.1%) |
Female gender, older age and active smoking were associated with long COVID syndrome, but not severity of the acute disease. | Not available |
|
Bellan et al (2021)89 Italy |
200 (122/78) | Yes | Prospective longitudinal study | Baseline, 17 weeks and 52 weeks. | Arthralgia/myalgia Baseline (19.3%) At week 17 (6.5%) At week 52 (21.9%) |
Not available | Not available | Not available |
|
Bileviciute-ljungar et al (2022)31 Sweden |
100 (18/82) | No (90%) Yes (10%) |
Cross-sectional study | A mean of 47 weeks since the start of the infection. | Head/face 27% Throat/neck 5% Shoulder/arms 5% Chest 16% Legs 12% Pain sites varied 15% |
The mean value of pain intensity during the last week was 4.4/10 75% reported generalized pain. |
Comorbidities Wealthy middle-aged women |
Not available |
|
Carfì et al (2020)90 Italy |
143 (90/53) | Yes | Case series | Mean of 7 weeks after onset of the first COVID-19 symptoms | Arthralgia 27.3% | Not available | Not available | Not available |
|
Carvalho-Soares et al (2021)26 Brazil |
46 (21/25) | Yes | Controlled cross-sectional study | Mean 16 week after hospital discharge | De novo pain 65.2% De novo chronic pain 19.6% Location of de novo pain: Head and neck 66.7% Upper limbs 16.7% Thorax and/or abdomen 16.7% Dorsal and/or low back 46.7% Lower limbs 36.7% Widespread pain 23.3% |
COVID-19 pain was more frequently located in the head/neck and lower limbs Frequency of de novo pain: <15 days/month 13.3% ≥ 15 days/month 50% Trend of de novo pain after discharge: Improved 17.2% Unchanged 44.8% Worsened 0% Not informed 37.9% |
Not available | Not available |
|
Carvalho-Schneider et al (2021)36 France |
150 (66/84) | Yes | Prospective cohort study | 1 week, 4 weeks and 8.5 Weeks | Chest pain Onset (14%) Day 30 (18%) Day 60 (13.1%) Arthralgia Onset (-) Day 30 (9.8%) Day 60 (16.3%) |
Not available | Not available | Not available |
|
Ferna ´ndez-de-las-Pen ~as et al (2021)38 Spain |
738 Patients reporting myalgia at admission (n=369) (176/193) Control: Patients without myalgia at admission (n=369) (176/193) |
Yes | Case-control study | A mean of 31 weeks after hospital discharge | Overall prevalence of MSK post-covid pain of 38%, 31 weeks post-discharge -With myalgia group (42.5%) -without myalgia group (34.5%) |
Musculoskeletal pain Locations (Cervical spine, Thorax-chest, Lumbar spine, Widespread pain, Upper extremity, Shoulder area, Wrist-elbow, Lower extremity, Hip region, Knee) |
Presence of myalgia at the onset of SARS-cov-2 and hospital admission | Prolonged inflammatory response associated with Covid-19 (cytokine mediated), viral neurotropic properties, Lead to hyperexcitability of peripheral and central nervous systems (nociplastic pain), Emotional and social factors (psychosocial mechanisms) |
|
Galal et al (2021)91 Egypt |
430 (156/274) | Yes | Cross-sectional study | Follow-up mean 5 weeks | Myalgia 60% Arthralgia 57.2% |
Not available | Not available | Not available |
|
Goërtz et al (2020)32 Netherlands |
2113 (310/1803) | 112 hospitalized 2001 non-hospitalized |
Cross-sectional study | Mean 11 weeks | Myalgia (36%) Pain between shoulder blades (33%) Arthralgia (22%) |
Not available | Not available | Not available |
|
Graham et al (2021)44 USA |
100 (30/70) (n=50 lab. +ve PCR Control n=50 -ve PCR) |
No | Prospective study | Baseline and an average of 20 weeks after symptom onset | Myalgia Overall 55% +ve 60% -ve 50% Pain other than chest Overall 43% +ve 40% -ve 46% |
Not available | Not available | Not available |
|
Havervall et al (2021)92 Sweden |
323 (55/268) | No | Cross-sectional study | At the 34-week follow-up Participants reported symptoms ≥8, ≥17 and ≥34 weeks |
Myalgia/Arthralgia ≥8 weeks (2.2%) ≥17 weeks (1.9%) ≥34 weeks (0.6%) |
Not available | Not available | Not available |
|
Horwitz et al (2021)93 USA |
126 (75/51) | Yes | Prospective observational study | 4 weeks and 26 weeks post-hospital discharge | Muscle/body ache (38%) Arthralgia (33%) Chest pain (21%) |
Not available | Not available | Not available |
|
Huang et al (2021)61 China |
1276 (681/595) | Yes | Ambi-directional cohort study | 26 weeks and 52 weeks after symptoms onset. | Myalgia 26 weeks (3%) 52 weeks (4%) Arthralgia 26 weeks (11%) 52 weeks (12%) Chest pain 26 weeks (5%) 52 weeks (7%) |
Not available | Not available | Not available |
|
Iqbal et al (2021)41 Pakistan |
158 (71/87) | No | Cross-sectional study | Mean of 5 weeks since recovery (from acute phase) | Arthralgia (47.5%) Chest pain (35.4%) |
Not available | Not available | Not available |
|
Jacobs et al (2020)94 Italy |
183 (112/71) | Yes | Prospective cohort study | Baseline and 5 weeks post-hospitalization | Myalgia 51% Arthralgia 54.7% |
Not available | Not available | Not available |
|
Jacobson et al (2021)33 USA |
118 (63/55) | Hospitalized n=22 Non-hospitalized n=96 |
Cross-sectional study | Median of 17 weeks | Myalgia Total 17.9% Hospitalized 22.7% Non-hospitalized 16.8% Chest pain Total 13.7% Hospitalized 9.1% Non-hospitalized 14.7% |
Not available | Not available | Not available |
|
Kamal et al (2020)95 Egypt |
287 (103/184) | Yes | Cross-sectional study | Median of 8.5 weeks | Arthralgia (31.4%) Chest pain (28.9%) |
Not available | Not available | Not available |
|
Karaarslan et al (2022)30 Turkey |
285 (172/173) | Yes | Prospective cohort study | 13 weeks and 26 weeks following the hospitalization. | Myalgia 13 weeks (40.55%) 26 weeks (15.09%) Arthralgia 13 weeks (39.18%) 26 weeks (18.59%) Low back pain 13 weeks (24.74%) 26 weeks (11.23%) Back pain 13 weeks (31.62%) 26 weeks (14.39%) Neck pain 13 weeks (20.62%) 26 weeks (9.47%) |
Severity, type, and locations of rheumatic and musculoskeletal symptoms Arthralgia and myalgia were mostly widespread (64.2% and 69.8%, respectively); if regional, arthralgia was mostly in the knee, foot-ankle, and shoulder, and myalgia was mostly in the lower leg, arm, and shoulder girdle. |
Female patients were more likely to have myalgia and joint pain at 26 weeks. | Immune response and pro-inflammatory cytokines generated after infection/direct invasion/injury of musculoskeletal cells by SARS-cov-2 through the angiotensin-converting enzyme 2 (ACE2) receptor Cellular invasion by SARS-COV-2, inflammatory and the immune response, and sequelae of post-critical illness, transforming growth factor beta (TGF-β) overexpression causing a prolonged state of immunosuppression and fibrosis |
|
Leite et al (2021)34 Brazil |
1696 (745/951) | Yes | Cross-sectional study | 4 weeks after hospital discharge | Pain Total (28.5) 26.4%-30.8% ICU (33.9) 29.0%-39.1% Ward (27.1) 24.7%-29.6% |
Not available | Not available | Not available |
|
Leth et al (2021)37 Denmark |
49 (21/28) | Yes | Prospective longitudinal study | Baseline, 6 weeks, and 12 weeks | Myalgia Baseline (47%) 6 weeks (16%) 12 weeks (35%) Chest pain Baseline (17%) 6 weeks (10%) 12 weeks (20%) |
Not available | Not available | Not available |
|
Lu et al (2020)35 China |
60 (34/26) | Yes | Prospective study | Baseline and 13 weeks after hospital discharge | Myalgia At acute stage (15%) At follow-up (25%) |
Not available | Not available | Not available |
|
Magdy et al (2021)39 Egypt |
90 45 patients with post-COVID pain (15/30) 45 recovered from COVID-19 without pain (16/29) |
Yes | Case-control study | Recovery duration 8.5 weeks | VAS [median] 8 (6–9) Frequency (days per week) 7 (3–7) Site of pain: Hands and feet (20%) Arms and legs (66.7%) Radicular (13.3%) |
Pain character (n=45) Burning (33.3%) Painful cold (13.3%) Electric shock (37.8%) Burning and electric shock (11.1%) Burning and painful cold (4.4%) Associated symptoms: Tingling (15.6%) Numbness (42.2%) Itching (8.9%) Pins and needles (13.3%) Numbness and itching (4.4%) Numbness and pins and needles (8.9%) Pins, needles and tingling (2.2%) Pins, needles, numbness and tingling (4.4%) Hypothesia to touch (68.9%) Hypothesia to prick (31.1%) Allodynia: Yes (37.8%) No (62.2%) |
Depression, Azithromycin use, moderate and severe COVID-19 are independent predictors of persistent post-COVID-19 pain. Serum NFL may serve as a potential biomarker for persistent neuropathic pain after COVID-19. | Direct neuro- invasive potential of SARS-cov-2. And massive release of pro- inflammatory mediators (cytokine storm) The higher the chance of exposure to the injurious effect of the virus, either through a longer duration of the COVID-19 infection or the severity of the infection, the greater the likelihood of pain. Both depression and neuropathic pain may arise from a common underlying inflammatory process induced by the cytokine storm. |
|
Mahmud et al (2021)96 Bangladesh |
355 (207/148) N=162 with post-covid syndrome (symptomatic) N=193 no post-covid symptoms |
Yes | Prospective cohort study | 4 weeks after clinical improvement | Myalgia Total (0.6%) Symptomatic (1.2%) Arthralgia Total (1.4%) Symptomatic (4.8%) Chest pain Total (0.8%) Symptomatic (1.8%) |
Not available | Not available | Not available |
|
Mohiuddin Chowdhury et al (2021)25 Bangladesh |
313 (251/62) | Yes (n=62) No (n=251) |
Prospective multicenter cross-sectional study | 4 weeks following recovery. | Arthralgia (0.6%) Mild body ache (0.3%) Back pain (0.3%) |
Not available | Not available | Not available |
|
Moradian et al (2020)97 Iran |
200 (160/40) | Yes | Cross-sectional study | 6 weeks after discharge | Myalgia 8% | Not available | Not available | Not available |
|
Moreno-Perez et al (2021)98 Spain |
277 (146/131) | Severe (hospitalized) Mild (hospital follow-up) |
Prospective covid study | 8–12 weeks | Myalgias-arthralgias 19.6% | Not available | Not available | Not available |
|
Ong et al (2021)66 Singapore |
288 (243/45) | Yes | Prospective longitudinal multicenter cohort study | 4 weeks, 12 weeks, and 25 weeks post-symptom onset | Myalgia at 12 weeks or 25 weeks (22.7%) | Not available | Not available | Not available |
|
Stavem et al (2021)99 Norway |
451 (198/253) | No | Cross-sectional study | 6–26 weeks after symptom onset | Arthralgia (9%) Myalgia (8.5%) |
Not available | Not available | Not available |
|
Sykes et al (2021)42 UK |
134 (88/46) Ward-based n=107 ICU n=27 |
Yes | Cross-sectional study | Median of 16 weeks | Myalgia Total 51.5% Ward-based 49.5% ICU 59.3% |
Not available | Not available | Not available |
|
Taquet et al (2021)40 USA |
273,618 (121,461/152,157) | No | Retrospective cohort study | From 1 day to 26 weeks post-diagnosis From 13 weeks to 26 weeks post-diagnosis |
Myalgia From 1 day to 26 weeks (3.24%) From 13 weeks to 26 weeks (1.54%) Pain From 1 day to 26 weeks (11.60%) From 13 weeks to 26 weeks (7.19%) |
Not available | Females were significantly more likely to have myalgia **p < 0.01/Patients with more severe illnesses (as proxied by hospitalization, ITU admission, or leukocytosis) were less likely to have myalgia/myalgia was more common in women and in younger patients, and notably so in those who had been less acutely ill | Not available |
|
Venturelli et al (2021)100 Italy |
767 (515/252) | Yes | Cross-sectional study | Median of 11.5 weeks after discharge | Myalgia Female (3.6%) Male (3.9%) Chest pain Female (3.6%) Male (2.9%) |
Not available | Not available | Not available |
|
Wahlgren et al (2022)29 Sweden |
158 (97/61) | Yes | Descriptive ambidirectional cohort study | 21 weeks | Pain (34.8%) Neuropathic type pain (5.1%) Nociceptive type pain (31.0%) Headache (17.7%) Pain in extremities (10.1%) Generalized pain (3.8%) Trunk (2.5%) |
34.8% reported new or aggravated pain. Nociceptive-type pain was considerably more common than neuropathic-type pain For nociceptive pain, headache was the most common pain localization. |
Not available | Not available |
|
Xiong et al (2021)101 China |
538 (245/293) | Yes | Retrospective cohort study | 12 weeks | Arthralgia 7.6% Myalgia 4.5% Chest pain 12.3% |
Not available | Not available | Not available |
|
Zhou et al (2021)102 China |
89 (46/43) | Yes | Longitudinal study | 3 weeks after discharge | Myalgia and arthralgia (2.2%) | Not available | Not available | Not available |
Characteristics of Pain
Only 8 studies described the characteristics of pain, 4 of which found pain localized to a particular region to be most common,26–29 while others reported a higher prevalence of widespread and generalized pain.30,31 Of the studies that reported localized pain symptoms, cervical spine and lower extremities were the most affected regions followed by lumbar spine and upper extremities.26 One study showed that nociceptive-type pain was considerably more common than neuropathic-type pain.29 Another study reported that the most common regional areas for arthralgia are knee joint, ankle joint and shoulder joint, while the most common areas for myalgia are lower leg, arm, and shoulder girdle.30
Most studies so far have focused on patients who were severely ill requiring hospitalization (24 studies). Only 6 studies evaluated patients recovering at home and did not require hospitalization. The remaining 5 studies included both hospitalized and non-hospitalized patients with only 2 comparing the difference in MSK pain prevalence rates between hospitalized and non-hospitalized patients. Goërtz et al compared hospitalized patients with three subgroups of non-hospitalized patients (confirmed, suspected and symptom-based COVID-19) and reported a higher prevalence of MSK pain in non-hospitalized patients than hospitalized patients (Table 2).32 Similarly, Jacobson et al noted chest pain in 14.7% of non-hospitalized patients in comparison to only 9.1% in hospitalized patients.33 In contrast, there was a higher prevalence of myalgia in hospitalized patients (22.7%) compared to non-hospitalized patients (16.8%). Another important factor to consider is the difference in MSK complications between severely ill hospitalized patients who require admission to the intensive care unit (ICU) and those who were admitted to the ward due to COVID-19 infection. This has been investigated by Leite et al, where patients admitted to the ICU reported a higher prevalence of persistent pain (33.9%) than those in the ward (27.1%).34
The data from 7 longitudinal studies following patients at multiple time points showed no consistent pattern of progression of MSK pain symptoms over time. Karaarslan et al followed-up patients at 3 months and 6 months post-discharge and reported a decline in the prevalence of myalgia, arthralgia, low back pain and neck pain (Table 2).30 In contrast, Lu et al observed an increase in myalgia symptoms at 13 weeks post-discharge (25%) comparing to baseline (15%).35 Similarly, Carvalho-Schneider et al’s study revealed an increase from 9.8% to 16.3% in the prevalence of arthralgia from day 30 to day 60 post-onset.36 Nevertheless, the same study observed a fluctuating pattern for chest pain with prevalence values of 14%, 18% and 13.1% at onset of COVID-19 infection, day 30 and day 60 after symptom onset, respectively.36 A fluctuating pattern has also been reported by Leth et al, for myalgia and chest pain symptoms, with a decrease in the prevalence from the baseline to 6 weeks after onset, followed by an increase at 12 weeks (Table 2).37
Risk Factors
Only 6 of the 35 studies reported risk factors associated with the prevalence of MSK symptoms in Long COVID patients.27,30,31,38–40 Sex differences were found in 3 studies, with females being significantly more likely to report myalgia (p = 0.022 in Iqbal et al);41 p < 0.01 in Taquet et al,40 and arthralgia (p < 0.05 in Sykes et al)42 than males. Two studies reported opposite trends for the association between age and prevalence of MSK pain of different origin, with increasing age associated with increased prevalence of arthralgia (p < 0.001 in Iqbal et al),41 and decreased prevalence of myalgia (p < 0.05 in Taquet et al).40
Karaarslan et al found no associations with age, sex or length of hospital stay after controlling for other variables.30 Nevertheless, the study found a statistically significant association between higher body mass index (BMI) and increased risk of presenting persistent arthralgia (p = 0.012) and myalgia (p = 0.015),30 consistent with the findings by Sykes et al (p = 0.012 for myalgia).42
The 2 studies investigating the severity of acute COVID-19 illness (as indicated by admission to ICU) showed contradicting findings: Leite et al found that admission to ICU related to higher prevalence of pain at 1 month after hospitalization,34 whereas Taquet et al found a lower prevalence of myalgia at 6 months.40 The presence of myalgia at hospital admission or acute COVID-19 infection onset was identified as a risk factor for MSK pain in Long COVID patients by Fernández-de-las-Peñas et al, with 42.5% of those with myalgia during acute phase reporting Long COVID MSK pain compared to 34.5% of those without myalgia (OR 1.41 95% CI 1.04–1.904, p = 0.02).38 No studies investigated the impact of ethnicity and/or pre-existing conditions on the development of MSK pain after acute COVID-19 infection.
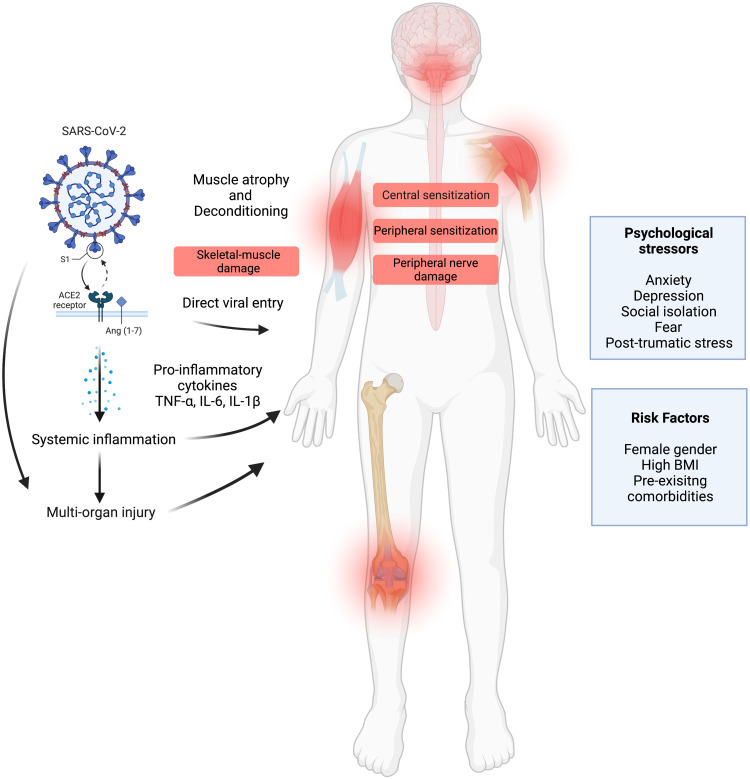
Proposed Pathophysiological Mechanisms
No dedicated mechanistic studies on MSK pain were found in relation to Long COVID. Nevertheless, 2 of the 35 studies proposed underlying mechanisms in reference to research on previous coronavirus outbreaks or inferred knowledge from the basic molecular and cellular mechanisms of SARS-CoV-2 infection.30,43 Both studies suggested the involvement of virus-induced prolonged inflammatory response, associated pro-inflammatory cytokines and immune cell hyperactivation. Another hypothetical mechanism proposed by the authors was the direct viral entry of cells of the MSK and nervous systems mediated by angiotensin-converting enzyme 2 (ACE2) receptor.30,43 Fernández-de-las-Peñas et al also proposed the potential role of inflammation- and neurotropic virus-induced neuronal hyperexcitability of peripheral and central nervous systems in promoting MSK nociplastic pain.38 The authors also mentioned the potential contribution of psychosocial factors.38 A few studies noted similarities between Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), including common symptoms of fatigue and myalgia, which may be suggestive of common pathological mechanisms.42,44
Discussion
This review summarizes current research on the prevalence and characteristics of MSK pain in Long COVID patients, potential risk factors and mechanisms underlying the development of persistent post-COVID MSK symptoms. Evidence supports that MSK pain is common among Long COVID patients, with studies reporting an overall prevalence of from 0.3% to 65.2%. Only a few studies described the characteristic features of MSK pain in Long COVID. To our knowledge, there has been no study so far that has performed a true longitudinal follow-up to understand the progression of MSK pain symptoms. In addition, as far as we are aware, no study is yet to investigate the underlying pathophysiological mechanisms other than proposing plausible hypotheses of direct viral entry, systemic inflammation, central and peripheral sensitization, and psychosocial factors. Female sex, increasing age, higher BMI and severity of acute COVID-19 illness were identified as potential risk factors in some studies. The current literature mostly used a cross-sectional approach to investigate the sequelae of COVID-19. Future studies need to focus on longitudinal assessment of MSK pain in Long COVID patients at multiple time points after the acute phase of infection. The repeated-measures design of longitudinal studies will facilitate monitoring and evaluation of the natural progression of MSK pain in Long COVID highlighting whether pain symptoms improve, worsen, or remain the same over time. It is important to also capture the fluctuations in the symptoms as Long COVID is recognized as a relapsing and remitting condition with a protracted course.12
It will be useful to determine whether Long COVID MSK pain symptoms evolve into a chronic pain syndrome, a condition characterized by sustained or recurring pain persisting beyond 3 months as defined by the World Health Organization (2018).45 An increasing number of studies have reported similarities between the clinical presentation of Long COVID and that of known chronic pain syndromes.42,44 For instance, a significant proportion of Long COVID patients have been found to meet the diagnostic criteria for fibromyalgia.46 Although fibromyalgia remains a poorly understood condition that lacks effective treatment, these similarities may point to common pathophysiological mechanisms and inform potential therapeutic strategies for MSK pain in Long COVID. Commonly recommended strategies for fibromyalgia combine pharmacological, physical and psychological therapies in a multidisciplinary approach. Advances in the scientific understanding of Long COVID MSK pain may have applicability to those suffering from fibromyalgia and other syndromes that share similarities with Long COVID, such as ME/CFS.
Determining the mechanisms involved in the etiology of MSK pain in Long COVID is a key step in identifying potential biomarkers for targeted therapeutic interventions. There is evidence to support the hypotheses raised by the authors of some studies included in this review. Cells of the MSK and nervous systems, such as skeletal muscle cells and sensory neurons, are known to express ACE2,47 thus suggesting they are prone to direct SARS-CoV-2-induced damage. Muus et al showed that the expression of mRNA ACE2 in skeletal muscle tissue is higher in females than in males due to differences in fat percentage, which may contribute to the sex differences observed in MSK pain prevalence.48 Nevertheless, evidence of direct skeletal muscle viral entry is rare and still controversial with few postmortem studies reporting low levels49,50 or absence51,52 of viral SARS-CoV-2 RNA in skeletal muscle autopsy tissue. Hooper et al suggested that muscle injury in COVID-19 is more likely to be a secondary outcome of damage to skeletal muscle microvasculature rather than direct invasion into myocytes.53
Serum levels of creatinine kinase, a marker of muscle damage, are increased in COVID-19, particularly in patients that present with myalgia symptoms suggestive of elements of muscle injury contributing to muscle pain.54–56 Indirect laboratory findings of muscle damage have been confirmed by histological and histochemical examination of skeletal muscle tissue obtained from autopsy of COVID-19-infected patients. The studies detected pathological features consistent with myopathies, as well as indications of peripheral neuropathic changes in some cases.51,52,57 These findings were further corroborated by electromyography and nerve conduction studies.58,59 For instance, a recent study evaluating 17 patients with Long COVID for peripheral neuropathy confirmed small-fiber neuropathy diagnosis in most of the patients in this group.60 Myopathy and peripheral neuropathy could also contribute to myalgia and neuropathic pain in Long COVID.
SARS-CoV-2 is known to elicit an extensive inflammatory response marked by a so-called cytokine storm.61–65 Ong et al reported that the abnormally elevated levels of proinflammatory cytokines (including interleukin-1β) persisted at 6 months after symptom onset, even in patients that remained asymptomatic during the acute phase of the disease.66 Prolonged exposure to proinflammatory mediators, most notably tumor necrosis factor-α, interleukin-6, interleukin-1β and chemokines, is known to promote “peripheral sensitization” and hyperexcitability of nociceptors via interactions with membrane receptors.67–72 It may also contribute to heightened responsiveness in the spinal cord and higher brain centers involved in pain processing, also referred to as “central sensitisation”.73–75 A self-report study by Goudman et al identified the presence of symptoms indicative of central sensitization in over 70% of recovered COVID-19 patients using the Central Sensitization Inventory (CSI) questionnaire.76
Another possible explanation for the development of MSK manifestations of Long COVID not mentioned in the included studies is a process of muscular deconditioning that occurs following long periods of immobility such as bedrest, hospitalization and prolonged periods of inactivity,7,77–79 although this theory has been challenged as it does not explain long-term MSK symptoms in milder cases of COVID-19 infection.80
The presence of emotional stressors in the context of COVID-19 may lead to worsening of pre-existing MSK pain conditions or put individuals at increased risk of developing new-onset MSK pain after acute infection. For example, high levels of anxiety, depression, social isolation, fear and post-traumatic stress symptoms are well documented in pain literature to exacerbate the experience of pain and predispose individuals to pain chronification.81–83 The contribution of risk factors, such as age, sex, ethnicity, mental health status, comorbidities and others, must be further evaluated in such a way that they may be predictive of the development of MSK pain or predispose to exacerbation of pre-existing pain following COVID-19 infection. Figure 2 summarizes the hypothesized mechanisms and risk factors of MSK pain in Long COVID.
Figure 2.
Hypothesized mechanisms and risk factors of MSK pain in Long COVID (Created with BioRender.com).
Although there is evidence to suggest the multifactorial nature of MSK pain in Long COVID, mechanistic studies are needed to confirm the causal relationship between central, peripheral, and psychological factors and MSK pain outcomes in Long COVID. Quantitative sensory testing (QST) protocols can be used to detect sensory alterations that reflect both peripheral and central sensitizations in Long COVID patients.84 Structural and functional neuroimaging studies (for instance, magnetic resonance imaging, electroencephalography and transcranial magnetic stimulation) can help understand central mechanisms involved in the etiology of Long COVID MSK pain. Mechanistic studies will not only provide novel insights into the pathological processes underlying the development of MSK pain but also allow the identification of targetable biological markers of MSK pain in Long COVID. Early detection of biomarkers may help identify “at risk” patients who are likely to develop MSK pain following COVID-19 infection. This may help inform potential therapeutic interventions to relieve painful symptoms and prevent the development of chronic MSK pain in Long COVID.
Assessing the clinical significance of persistent MSK pain symptoms in Long COVID is crucial to estimate the burden on individuals and evaluate their rehabilitation needs. Studies should explore the severity of MSK symptoms and their impact on quality of life, functional status, and psychological wellbeing. Clinical and research studies must use condition-specific outcome measures that capture all aspects of this novel condition using the WHO International Classification of Functioning, Disability and Health (ICF) framework as a guide for the selection of measures.85–88 This will inform clinical decision-making on allocation of resources to provide appropriate care and estimate the cost-effectiveness of the care.
Several limitations to this narrative review need to be acknowledged. The heterogeneity in the methodology of the studies reporting the prevalence of MSK pain in Long COVID made assimilation of findings from the studies challenging. Studies utilized different study designs; some used a prospective cohort method to follow-up patients over time, some used a cross-sectional method, and others used a retrospective design. Moreover, the studies used inconsistent definitions for Long COVID and reported the prevalence of symptoms at different time periods. In addition, most studies required evidence of a positive antigen or polymerase chain reaction (PCR) test resulting in their inclusion criteria. Therefore, the experience of patients who were not tested in the early stages of the pandemic due to the unavailability of the testing programs were not captured in these studies. However, this issue has been overcome in some recent studies that included cases of self-reported Long COVID without confirmatory evidence of COVID-19 infection.
Conclusion
The literature supports that MSK pain is common among Long COVID patients. The currently available literature suggests that MSK pain symptoms in Long COVID may present as localized pain in a particular region or generalized and widespread. Female gender and higher BMI could be potential risk factors for Long COVID MSK pain, with no clear association between age and ethnicity. The literature proposes different plausible pathophysiological mechanisms that contribute to the development of MSK pain in Long COVID including direct viral entry, systemic inflammation, central and peripheral sensitization, and psychosocial factors, although mechanistic studies are still lacking. Much of the current literature on Long COVID pays particular attention to reporting the prevalence alone of persistent MSK pain symptoms. Studies describing the pain features and characteristics are scarce. The longitudinal progression and understanding of the precise mechanisms of MSK pain in Long COVID remains to be properly researched. Further studies are required to fully characterize MSK pain in Long COVID, identify risk factors, study the natural evolution, and investigate the underlying mechanisms to identify targetable biomarkers of MSK pain in Long COVID. Researchers and clinicians should aim to mitigate the long-term burden of chronic MSK pain that seems likely to emerge from Long COVID.
Acknowledgments
The authors would like to acknowledge the support of the Leeds NIHR Biomedical Research Centre and Prof Paul Emery from the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.National Institute for Health and Care Excellence, Practitioners RC of G, Scotland HI. COVID-19 rapid guideline: managing the long-term effects of COVID-19. NICE guidel; 2020:1–35. Available from: https://www.nice.org.uk/guidance/ng188/resources/covid19-rapid-guideline-managing-the-longterm-effects-of-covid19-pdf-51035515742. Accessed June 9, 2022. [PubMed]
- 2.WHO Headquarters (HQ). A clinical case definition of post COVID-19 condition by a Delphi consensus; 2021. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1. Accessed June 9, 2022. [DOI] [PMC free article] [PubMed]
- 3.Ayoubkhani D, Pawelek P. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK; 2022. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/alldatarelatingtoprevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk. Accessed June 9, 2022.
- 4.Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. SSRN Electron J. 2021. doi: 10.2139/ssrn.3820561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370. doi: 10.1136/bmj.m3026 [DOI] [PubMed] [Google Scholar]
- 6.Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2020;93:1013–1022. doi: 10.1002/jmv.26368 [DOI] [PubMed] [Google Scholar]
- 7.Wright J, Astill SL, Sivan M. The relationship between physical activity and long COVID: a Cross-Sectional Study. Int J Environ Res Public Heal. 2022;19(9). doi: 10.3390/ijerph19095093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker-Davies RM, O’Sullivan O, Senaratne KPP, et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br J Sports Med. 2020;54(16):949–959. doi: 10.1136/bjsports-2020-102596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doykov I, Hällqvist J, Gilmour KC, Grandjean L, Mills K, Heywood WE. The long tail of Covid-19” - The detection of a prolonged inflammatory response after a SARS-CoV-2 infection in asymptomatic and mildly affected patients. F1000Research. 2020;9:1349. doi: 10.12688/f1000research.27287.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salmon-Ceron D, Slama D, De Broucker T, et al. Clinical, virological and imaging profile in patients with prolonged forms of COVID-19: a cross-sectional study. J Infect. 2021;82(2):E1–E4. doi: 10.1016/j.jinf.2020.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sivan M, Parkin A, Makower S, Greenwood DC. Post-COVID syndrome symptoms, functional disability, and clinical severity phenotypes in hospitalized and nonhospitalized individuals: a cross-sectional evaluation from a community COVID rehabilitation service. J Med Virol. 2021. doi: 10.1002/jmv.27456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perrot S, Cohen M, Barke A, Korwisi B, Rief W, Treede R-D. The IASP classification of chronic pain for ICD-11: chronic secondary musculoskeletal pain. Pain. 2019;160(1):77–82. doi: 10.1097/j.pain.0000000000001389 [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-de-las-Penas C, Gómez-Mayordomo V, Cuadrado ML, et al. The presence of headache at onset in SARS-CoV-2 infection is associated with long-term post-COVID headache and fatigue: a case-control study. Cephalalgia. 2021;41(13):1332–1341. doi: 10.1177/03331024211020404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cieza A, Causey K, Kamenov K, Hanson SW, Chatterji S, Vos T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2021;396(10267):2006–2017. doi: 10.1016/S0140-6736(20)32340-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Versus Arthritis. The state of musculoskeletal health 2021; 2021. Available from: https://www.versusarthritis.org/policy/resources-for-policy-makers/musculoskeletal-calculator/. Accessed June 9, 2022.
- 17.Bergman S, Herrström P, Högström K, Petersson IF, Svensson B, Jacobsson LT. Chronic musculoskeletal pain, prevalence rates, and sociodemographic associations in a Swedish population study. J Rheumatol. 2001;28(6):1369–1377. [PubMed] [Google Scholar]
- 18.Picavet HSJ, Schouten JSAG. Musculoskeletal pain in the Netherlands: prevalences, consequences and risk groups, the DMC(3)-study. Pain. 2003;102(1–2):167–178. doi: 10.1016/s0304-3959(02)00372-x [DOI] [PubMed] [Google Scholar]
- 19.Cimas M, Ayala A, Sanz B, Agulló-Tomás MS, Escobar A, Forjaz MJ. Chronic musculoskeletal pain in European older adults: cross-national and gender differences. Eur J Pain. 2018;22(2):333–345. doi: 10.1002/ejp.1123 [DOI] [PubMed] [Google Scholar]
- 20.Sivan M, Kinnear L, Tan A, McGonagle D. Risk factors and predictors of long covid or post-COVID-19 syndrome. J R Coll Physicians. 2022;45:e34. [Google Scholar]
- 21.Tüzün EH. Quality of life in chronic musculoskeletal pain. Best Pract Res Clin Rheumatol. 2007;21(3):567–579. doi: 10.1016/j.berh.2007.03.001 [DOI] [PubMed] [Google Scholar]
- 22.Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain. 2000;84(1):95–103. doi: 10.1016/S0304-3959(99)00187-6 [DOI] [PubMed] [Google Scholar]
- 23.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715–724. doi: 10.1016/j.jpain.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 24.Sivan M, Rayner C, Delaney B. Fresh evidence of the scale and scope of long covid. BMJ. 2021;373:n853. doi: 10.1136/bmj.n853 [DOI] [PubMed] [Google Scholar]
- 25.Mohiuddin Chowdhury ATM, Karim MR, Ali MA, Islam J, Li Y, He S. Clinical characteristics and the long-term post-recovery manifestations of the COVID-19 patients—A prospective multicenter cross-sectional study. Front Med. 2021;8:1–10. doi: 10.3389/fmed.2021.663670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soares FHC, Kubota GT, Fernandes AM, et al. Prevalence and characteristics of new-onset pain in COVID-19 survivors, a controlled study. Eur J Pain. 2021;25:1342–1354. doi: 10.1002/ejp.1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adnan JW, Saleemi H, Shafqat A, Arif M, Hamid K. Tendency of post COVID muscle and joint pains. Med Forum Mon. 2021;32(4):161–163. [Google Scholar]
- 28.Bai F, Tomasoni D, Falcinella C, et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect off Publ Eur Soc Clin Microbiol Infect Dis. 2021. doi: 10.1016/j.cmi.2021.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wahlgren C, Divanoglou A, Larsson M, et al. Rehabilitation needs following COVID-19: five-month post-discharge clinical follow-up of individuals with concerning self-reported symptoms. EClinicalMedicine. 2022;43:1–14. doi: 10.1016/j.eclinm.2021.101219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karaarslan F, Güneri FD, Kardeş S. Long COVID: rheumatologic/musculoskeletal symptoms in hospitalized COVID-19 survivors at 3 and 6 months. Clin Rheumatol. 2022;41(1):289–296. doi: 10.1007/s10067-021-05942-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bileviciute-ljungar I, Norrefalk J, Borg K. Pain burden in post-COVID-19 syndrome following mild COVID-19 infection. Journal of Clinical Medicine. 2022;11(3):771. doi: 10.3390/jcm11030771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goërtz YMJ, Van Herck M, Delbressine JM, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6(4):00542–02020. doi: 10.1183/23120541.00542-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobson KB, Rao M, Bonilla H, et al. Patients with uncomplicated coronavirus disease 2019 (COVID-19) have long-term persistent symptoms and functional impairment similar to patients with severe COVID-19: a cautionary tale during a global pandemic. Clin Infect Dis. 2021;73(3):e826–e829. doi: 10.1093/cid/ciab103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leite VF, Rampim DB, Jorge VC, et al. Persistent symptoms and disability after COVID-19 hospitalization: data from a comprehensive telerehabilitation program. Arch Phys Med Rehabil. 2021;102(7):1308–1316. doi: 10.1016/j.apmr.2021.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Y, Li X, Geng D, et al. Cerebral micro-structural changes in COVID-19 patients – an MRI-based 3-month follow-up study: a brief title: cerebral changes in COVID-19. EClinicalMedicine. 2020;25(2):100484. doi: 10.1016/j.eclinm.2020.100484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carvalho-Schneider C, Laurent E, Lemaignen A, et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27(2):258–263. doi: 10.1016/j.cmi.2020.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leth S, Gunst JD, Mathiasen V, et al. Persistent symptoms in patients recovering from COVID-19 in Denmark. Open Forum Infect Dis. 2021;8(4):1–7. doi: 10.1093/ofid/ofab042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernandez-de-Las-Penas C, Rodriguez-Jimenez J, Fuensalida-Novo S, et al. Myalgia as a symptom at hospital admission by SARS-CoV-2 infection is associated to persistent musculoskeletal pain as long-term post-COVID sequelae: a case-control study. Pain. 2021. doi: 10.1097/j.pain.0000000000002306 [DOI] [PubMed] [Google Scholar]
- 39.Magdy R, Hussein M, Ragaie C, et al. Characteristics of headache attributed to COVID-19 infection and predictors of its frequency and intensity: a cross sectional study. Cephalalgia. 2020;40(13):1422–1431. doi: 10.1177/0333102420965140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18(9):1–22. doi: 10.1371/journal.pmed.1003773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iqbal A, Iqbal K, Arshad Ali S, et al. The COVID-19 sequelae: a cross-sectional evaluation of post-recovery symptoms and the need for rehabilitation of COVID-19 survivors. Cureus. 2021;2(2). doi: 10.7759/cureus.13080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sykes DL, Holdsworth L, Jawad N, Gunasekera P, Morice AH, Crooks MG. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199(2):113–119. doi: 10.1007/s00408-021-00423-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernández-de-las-Peñas C, Navarro-Santana M, Plaza-Manzano G, Palacios-Ceña D, Arendt-Nielsen L. Time course prevalence of post-COVID pain symptoms of musculoskeletal origin in patients who had survived to severe acute respiratory syndrome coronavirus 2 infection. Pain. 2021;Publish Ahead of Print. doi: 10.1097/j.pain.0000000000002496 [DOI] [PubMed] [Google Scholar]
- 44.Graham EL, Clark JR, Orban ZS, et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers.”. Ann Clin Transl Neurol. 2021;8(5):1073–1085. doi: 10.1002/acn3.51350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization. ICD-11 for mortality and morbidity statistics; 2018. Available from: https://icd.who.int/browse11/l-m/en. Accessed November 15, 2021.
- 46.Ursini F, Ciaffi J, Mancarella L, et al. Fibromyalgia: a new facet of the post-COVID-19 syndrome spectrum? Results from a web-based survey. RMD Open. 2021;7(3):e001735. doi: 10.1136/rmdopen-2021-001735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, Van, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol a J Pathol Soc Gt Britain Irel. 2004;203(2):631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muus C, Luecken MD, Eraslan G, et al. Single-cell meta-analysis of SARS-CoV-2 entry genes across tissues and demographics. Nature Medicine. 2021;27:546–559. doi: 10.1038/s41591-020-01227-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sekulic M, Harper H, Nezami BG, et al. Molecular detection of SARS-CoV-2 infection in FFPE samples and histopathologic findings in fatal SARS-CoV-2 cases. Am J Clin Pathol. 2020;154(2):190–200. doi: 10.1093/ajcp/aqaa091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong AK, Demediuk L, Tay JY, et al. COVID-19 end-of-life care: symptoms and supportive therapy use in an Australian hospital. Intern Med J. 2021;51:1420–1425. doi: 10.1111/imj.15300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aschman T, Schneider J, Greuel S, et al. Association between SARS-CoV-2 infection and immune-mediated myopathy in patients who have died. JAMA Neurol. 2021;78(8):948. doi: 10.1001/jamaneurol.2021.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suh J, Mukerji SS, Collens SI, et al. Skeletal muscle and peripheral nerve histopathology in COVID-19. Neurology. 2021;97(8):e849–e858. doi: 10.1212/WNL.0000000000012344 [DOI] [PubMed] [Google Scholar]
- 53.Hooper JE, Uner M, Priemer DS, Rosenberg A, Chen L. Muscle biopsy findings in a case of sars-cov-2-associated muscle injury. J Neuropathol Exp Neurol. 2021;80(4):377–378. doi: 10.1093/jnen/nlaa155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pitscheider L, Karolyi M, Burkert FR, et al. Muscle involvement in SARS‐CoV‐2 infection. Eur J Neurol. 2021;28(10):3411–3417. doi: 10.1111/ene.14564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Versace V, Sebastianelli L, Ferrazzoli D, et al. Myopathy in COVID-19 critically ill patients: a consequence of hyperinflammation? Front Neurol. 2021;12:66. doi: 10.3389/fneur.2021.625144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duarte‐Neto AN, Monteiro RAA, da Silva LFF, et al. Pulmonary and systemic involvement in COVID‐19 patients assessed with ultrasound‐guided minimally invasive autopsy. Histopathology. 2020;77(2):186–197. doi: 10.1111/his.14160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agergaard J, Leth S, Pedersen TH, et al. Myopathic changes in patients with long-term fatigue after COVID-19. Clin Neurophysiol. 2021;132(8):1974–1981. doi: 10.1016/j.clinph.2021.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hameed S, Khan AF, Khan S. Electrodiagnostic findings in COVID-19 patients: a single center experience. Clin Neurophysiol. 2021;132(12):3019–3024. doi: 10.1016/j.clinph.2021.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oaklander AL, Mills AJ, Kelley M, et al. Peripheral neuropathy evaluations of patients with prolonged long COVID. Neurol Neuroimmunol Neuroinflammation. 2022;9(3):e1146. doi: 10.1212/NXI.0000000000001146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398(10302):747–758. doi: 10.1016/S0140-6736(21)01755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen LW, Deng CH, Chen XH, et al. Ocular manifestations and clinical characteristics of 535 cases of COVID-19 in Wuhan, China: a cross-sectional study. Acta Ophthalmol. 2020;98(8):E951–E959. doi: 10.1111/aos.14472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han H, Ma Q, Li C, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu B, Han J, Cheng X, et al. Reduced numbers of T cells and B cells correlates with persistent SARS-CoV-2 presence in non-severe COVID-19 patients. Sci Rep. 2020;10(1):17718. doi: 10.1038/s41598-020-73955-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lucas C, Wong P, Klein J, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584(7821):463–469. doi: 10.1038/s41586-020-2588-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ong SWX, Fong S-W, Young BE, et al. Persistent symptoms and association with inflammatory cytokine signatures in recovered coronavirus disease 2019 patients. Open Forum Infectious Diseases. 2021;8(6):ofab156. doi: 10.1093/ofid/ofab156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Binshtok AM, Wang H, Zimmermann K, et al. Nociceptors are interleukin-1β sensors. J Neurosci. 2008;28(52):14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brenn D, Richter F, Schaible H. Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin‐6 in the rat: an inflammatory mechanism of joint pain. Arthritis Rheum. 2007;56(1):351–359. doi: 10.1002/art.22282 [DOI] [PubMed] [Google Scholar]
- 69.Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992;107(3):660. doi: 10.1111/j.1476-5381.1992.tb14503.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mailhot B, Christin M, Tessandier N, et al. Neuronal interleukin-1 receptors mediate pain in chronic inflammatory diseases. J Exp Med. 2020;217(9). doi: 10.1084/jem.20191430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Silva RL, Lopes AH, Guimarães RM, Cunha TM. CXCL1/CXCR2 signaling in pathological pain: role in peripheral and central sensitization. Neurobiol Dis. 2017;105:109–116. doi: 10.1016/j.nbd.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 72.Sorkin LS, Xiao WH, Wagner R, Myers RR. Tumour necrosis factor-α induces ectopic activity in nociceptive primary afferent fibres. Neuroscience. 1997;81(1):255–262. doi: 10.1016/S0306-4522(97)00147-4 [DOI] [PubMed] [Google Scholar]
- 73.Bierle DM, Aakre CA, Grach SL, et al. Central sensitization phenotypes in post acute sequelae of SARS-CoV-2 infection (PASC): defining the post COVID syndrome. J Prim Care Community Health. 2021;12:21501327211030824. doi: 10.1177/21501327211030826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cascella M, Del Gaudio A, Vittori A, et al. COVID-pain: acute and late-onset painful clinical manifestations in COVID-19–molecular mechanisms and research perspectives. J Pain Res. 2021;14:2403. doi: 10.2147/JPR.S313978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McFarland AJ, Yousuf MS, Shiers S, Price TJ. Neurobiology of SARS-CoV-2 interactions with the peripheral nervous system: implications for COVID-19 and pain. Pain Rep. 2021;6(1):1–10. doi: 10.1097/PR9.0000000000000885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goudman L, De Smedt A, Noppen M, Moens M. Is central sensitisation the missing link of persisting symptoms after COVID-19 infection? J Clin Med. 2021;10(23):5594. doi: 10.3390/jcm10235594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cipollaro L, Giordano L, Padulo J, Oliva F, Maffulli N. Musculoskeletal symptoms in SARS-CoV-2 (COVID-19) patients. J Orthop Surg Res. 2020;15:1–7. doi: 10.1186/s13018-020-01702-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clauw DJ, Häuser W, Cohen SP, Fitzcharles M-A, Considering the potential for an increase in chronic pain after the COVID-19 pandemic. Pain. 2020;161(8):1694–1697. doi: 10.1097/j.pain.0000000000001950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kemp HI, Corner E, Colvin LA. Chronic pain after COVID-19: implications for rehabilitation. Br J Anaesth. 2020;125(4):436–440. doi: 10.1016/j.bja.2020.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vieira E, Ferreira M, Oliveira RKF. Mechanisms of exercise intolerance after COVID-19: new perspectives beyond physical deconditioning. J Bras Pneumol. 2021;47(5):19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Asmundson GJG, Katz J. Understanding the co‐occurrence of anxiety disorders and chronic pain: state‐of‐the‐art. Depress Anxiety. 2009;26(10):888–901. doi: 10.1002/da.20600 [DOI] [PubMed] [Google Scholar]
- 82.Carroll LJ, Cassidy JD, Côté P. Depression as a risk factor for onset of an episode of troublesome neck and low back pain. Pain. 2004;107(1–2):134–139. doi: 10.1016/j.pain.2003.10.009 [DOI] [PubMed] [Google Scholar]
- 83.McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106(1–2):127–133. doi: 10.1016/S0304-3959(03)00301-4 [DOI] [PubMed] [Google Scholar]
- 84.Starkweather AR, Heineman A, Storey S, et al. Methods to measure peripheral and central sensitization using quantitative sensory testing: a focus on individuals with low back pain. Appl Nurs Res. 2016;29:237–241. doi: 10.1016/j.apnr.2015.03.013 [DOI] [PubMed] [Google Scholar]
- 85.Sivan M, Halpin S, Gees J, et al. The self-report version and digital format of the COVID-19 Yorkshire rehabilitation scale (C19-YRS) for long covid or post-COVID syndrome assessment and monitoring. Adv Clin Neurosci Rehabil. 2021;20(3):8–11. doi: 10.47795/qroo4168 [DOI] [Google Scholar]
- 86.Sivan M, Wright S, Hughes S, Calvert M. Using condition specific patient reported outcome measures for long covid. BMJ. 2022;o257. doi: 10.1136/bmj.o257 [DOI] [PubMed] [Google Scholar]
- 87.Patel K, Straudi S, Sien NY, Fayed N, Melvin JL, Sivan M. Applying the who ICF framework to the outcome measures used in the evaluation of long-term clinical outcomes in coronavirus outbreaks. Int J Environ Res Public Health. 2020;17(18):1–15. doi: 10.3390/ijerph17186476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sivan M, Preston N, Parkin A, et al. The modified COVID-19 Yorkshire rehabilitation scale (C19-YRSm) patient-reported outcome measure for long covid or post-COVID syndrome. medRxiv. 2022. doi: 10.1101/2022.03.24.22272892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bellan M, Baricich A, Patrucco F, et al. Long-term sequelae are highly prevalent one year after hospitalization for severe COVID-19. Sci Rep. 2021;11(1):1–10. doi: 10.1038/s41598-021-01215-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Galal I, Hussein A, Amin MT, et al. Determinants of persistent post-COVID-19 symptoms: value of a novel COVID-19 symptom score. Egypt J Bronchol. 2021;15(1). doi: 10.1186/s43168-020-00049-4 [DOI] [Google Scholar]
- 92.Havervall S, Rosell A, Phillipson M, et al. Symptoms and functional impairment assessed 8 months after mild COVID-19 among health care workers. JAMA. 2021;325(19):2015–2016. doi: 10.1001/jama.2021.5612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Horwitz LI, Garry K, Prete AM, et al. Six-month outcomes in patients hospitalized with severe COVID-19. J Gen Intern Med. 2021;36(12):3772–3777. doi: 10.1007/s11606-021-07032-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jacobs LG, Gourna Paleoudis E, Lesky-Di Bari D, et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS One. 2020;15(12):e0243882. doi: 10.1371/journal.pone.0243882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kamal M, Abo Omirah M, Hussein A, Saeed H. Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract. 2020;75:e13746–e13746. doi: 10.1111/ijcp.13746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mahmud R, Rahman MM, Rassel MA, et al. Post-COVID-19 syndrome among symptomatic COVID-19 patients: a prospective cohort study in a tertiary care center of Bangladesh. PLoS One. 2021;16(4):e0249644. doi: 10.1371/journal.pone.0249644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moradian ST, Parandeh A, Khalili R, Karimi L. Delayed symptoms in patients recovered from COVID-19. Iran J Public Health. 2020;49(11):2120–2127. doi: 10.18502/ijph.v49i11.4729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moreno-Perez O, Merino E, Leon-Ramirez JM, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021;82(3):378–383. doi: 10.1016/j.jinf.2021.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G. Persistent symptoms 1.5–6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax. 2021;76(4):405–407. doi: 10.1136/thoraxjnl-2020-216377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Venturelli S, Benatti SV, Casati M, et al. Surviving COVID-19 in bergamo province: a post-acute outpatient re-evaluation. Epidemiol Infect. 2021;2. doi: 10.1017/S0950268821000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27(1):89–95. doi: 10.1016/j.cmi.2020.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou M, Cai J, Sun W, et al. Do post-COVID-19 symptoms exist? A longitudinal study of COVID-19 sequelae in Wenzhou, China. Ann Med Psychol. 2021;179(9):818–821. doi: 10.1016/j.amp.2021.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]