Abstract
SARS-Cov-2 infection is not limited to the respiratory tract and can involve other organs including the heart, blood vessels, kidneys, liver, gastrointestinal tract, placenta, and skin. Covid-19 patients with cardiac involvement usually have higher morbidity and mortality compared to those without cardiac involvement. The frequency and the specificity of the myocardial pathological changes in patients who die after documented infection with SARS-Cov-2 is uncertain. Macrophages can be found in the normal heart (interstitium, around the endothelial cells and in the epicardial adipose tissue), and they are considered part of the major immune cell population in the heart. In this case-control autopsy study, we compare the gross and microscopic cardiac findings, and the available clinical characteristics between a group of 10 Covid-19 decedents and a control group of 20 patients who died with non-SARS-Cov-2 severe bronchopneumonia and/or diffuse alveolar damage. The objectives of this semi-quantitative study are to study single myocyte necrosis and its relation to the strain on the heart caused by lung injury as a causative mechanism, and to study the density of myocardial and epicardial macrophages in Covid-19 hearts in comparison to the control group, and in Covid-19 hearts with single myocyte necrosis in comparison to Covid-19 hearts without single myocyte necrosis. Lymphocytic myocarditis was not identified in any of the hearts from the Covid-19 or the control group. Single myocyte necrosis is more frequent in the Covid-19 group compared to the control group, suggesting that it is unrelated to the strain on the heart caused by underlying lung injury. The density of the macrophages in the epicardium and myocardium in the hearts of the Covid-19 group is higher compared to those in the control group. The density of epicardial macrophages is higher in the Covid-19 hearts with single myocyte necrosis than in those without. These observations contribute to our increasing appreciation of the role of macrophages in the pathophysiologic response to infection by SARS-CoV-2.
Keywords: Covid-19, myocarditis, heart inflammation, macrophages, single myocyte necrosis, epicardium
1. Introduction
Infection with a new strain of coronavirus, severe acute respiratory syndrome Coronavirus 2 (SARS-Cov-2), rapidly progressed to a global public health emergency, beginning in late 2019 [1]. Respiratory tract disease is the leading cause of morbidity and mortality from infection caused by SARS-Cov-2 (Covid-19 illness) [2]; however, this disease is not limited to the respiratory tract and can have manifestations in the heart, blood vessels, gastrointestinal tract, kidneys, liver, placenta, and skin [3].
Up to 20%–30% of hospitalized patients with Covid-19 develop clinical evidence of myocardial involvement, manifested by elevated serum troponin and abnormal cardiac magnetic resonance (CMR) findings [4], [5], [6], [7], with increased morbidity and mortality compared to Covid-19 patients without myocardial involvement [7]. The true incidence of conventional viral (lymphocytic) myocarditis is difficult to discern, as endomyocardial biopsy (EMB), the diagnostic gold standard for this entity [8], is infrequently used. In the early stages of the pandemic, the diagnosis of Covid-19 related myocarditis was sometimes erroneously based on the radiological and clinical data alone [9], [10], [11].
By definition, the histological diagnosis of myocarditis is contingent upon the presence of inflammation of the heart muscle [12]. Early in the pandemic, it became clear that the clinical impression of myocarditis was not supported by the histologic finding of lymphocytic inundation of the myocardium with associated lymphocyte-mediated myocardial necrosis typical of viral myocarditis [13,16]. More recent studies on the cardiovascular complications of fatal Covid-19 illness describe subtle histologic changes in the myocardium, including non-myocarditic inflammation, single myocyte necrosis/ischemia, microvascular thrombi, pericarditis, fibrosis, edema and small vessel vasculitis [ [13], [14], [15], [16], [17], [18] ]. Single myocyte necrosis/ischemia has been described variably as: “single cell ischemia”, “myocyte damage”, “scattered individual cell myocyte necrosis”, and “focal necrosis” [13]. These non-specific findings of inflammation suggest that alternative pathways of inflammation play a role in the pathogenesis of Covid-19 related heart disease. Fox et al. [19], Basso et al. [20], and Bearse et al. [17] independently reported increased interstitial macrophage density in the myocardium of patients who died of Covid-19. The proximity of macrophages to the myocytes suggests an important role for macrophages in Covid-19 cardiac injury [21].
Fox et al. [19] studied the inflammatory cells in Covid-19 hearts and compared them to two control groups (non-Covid-19); a group of patients with acute viral myocarditis and a group of patients who died with hypertension, diabetes and chronic kidney disease. They found that typical viral myocarditis is rare in Covid-19 and that there was a subgroup of Covid-19 hearts with increased CD68 positive macrophages (when compared to both control groups). They also found that the number of T lymphocytes was significantly larger in the typical myocarditis control group in comparison to the Covid-19 and the other control group. The authors suggest that Covid-19 might be associated with a different form of myocarditis, which could be associated with increased numbers of CD68 macrophages.
Macrophages constitute the major resident immune cell population in the heart, and can be found in the interstitium as well as around endothelial cells. It has also been suggested that they play a role in cardiac conduction [21]. Furthermore, only small populations of B-cells, regulatory T-cells, innate lymphoid cells and dendritic cells reside in the heart. By flow cytometry, parabiosis studies, and lineage tracing, two types of cardiac macrophages have been identified: Chemokine Receptor-2 negative (CCR2−) and Chemokine Receptor-2 positive (CCR2+) phenotypes [22,23]. Studies have shown that CCR2+ macrophages are proinflammatory while CCR2− macrophages are involved in maintenance of hemostasis [22,23].
Angiotensin-converting enzyme 2 (ACE2), the target cellular receptor of SARS-CoV-2, is known to be present in multiple organs, including the heart [24] and adipose tissue [25,26]. In the heart, ACE2 receptors are present on cardiomyocytes, pericytes, fibroblasts, endothelial cells, and macrophages [24,27]; however, it is still not clear whether the virus directly infects any of these cells, and if so, whether that infection elicits an inflammatory response. Bearse et al. [17], using in situ hybridization, identified SARS-CoV-2 genetic material, predominantly in macrophages, but also rarely in endothelial cells and myocytes. Electron microscopy studies claim to have detected viral particles in cardiac endothelial cells [16], interstitial cells of the myocardium [28], interstitial macrophages, and in myocytes [29]. Oudit et al. [30], by means of reverse transcriptase polymerase-chain reaction (rtPCR), also claim to have detected SARS-CoV-2 genome in the heart. These results are contested, however, and have not been easily reproducible. While direct viral myocardial infection with SARS-CoV-2 is one of the suggested mechanisms of Covid-19 related cardiac injury [7,30,31], other studies suggest that epicardial adipose tissue could contribute to SARS-CoV-2 entry into the heart in the context of myocarditis and promote an augmented inflammatory response in the myocardium [25,32,33].
In a thorough review of microscopic alterations in the hearts of 40 patients who died of Covid-19, [18] Vander Heide and Fox propose that SARS-CoV-2 infection may cause small elevations in troponin through individual myocyte necrosis. They suggest three possible pathophysiologic pathways to explain single myocyte necrosis: 1-Increased strain on the heart due to severe injury to the lungs; 2-Viral infection of the endothelium/pericyte which could cause endothelial dysfunction or damage resulting in local platelet/fibrin clotting and subsequent microinfarcts; and 3- Monocytes and/or monocyte-derived macrophages carry the virus to the heart, where the virus subsequently causes localized endothelial damage which in turn leads to microinfarcts of myocardial tissue.
In this paper, we explore three hypotheses suggested by the literature on epicardial and myocardial involvement as sequelae of SARS-CoV-2 infection: a) Single myocyte necrosis in the myocardium as described by Vander Heide and Fox [18] is caused by increased strain on the heart due to severe injury to the lungs, irrespective of etiology; b) Epicardial macrophage density is elevated to the same degree in diffuse alveolar damage and bronchopneumonia irrespective of etiology; and c) Epicardial and myocardial macrophage densities are elevated to the same degree in Covid-19 decedents whose hearts show single myocyte necrosis compared to those without single myocyte necrosis.
2. Materials and methods
2.1. Subjects
From 2016 to 2021, the pathology department conducted 453 adult and pediatric autopsies. For this study, we retrospectively reviewed the autopsies of adult decedents who died of pulmonary complications during this time period. The study population was divided into “cases” comprised of autopsies performed on 10 Covid-19 patients during 2020 and 2021, and a control group which included 20 non-consecutive autopsies performed between 2016 and 2020, in which the decedents had non-Covid-19 severe bronchopneumonia and/or diffuse alveolar damage at the time of death. The inclusion criteria for Covid-19 cases were: SARS-CoV-2 diagnosis confirmed by an antemortem positive polymerase chain reaction (PCR) test, and cause of death related to severe pulmonary manifestations of Covid-19. The PCR test was performed on a nasopharyngeal swab which detects the spike and nucleocapsid proteins. There was no further testing to identify the specific SARS-CoV-2 variant [34,35]. Moreover, these cases were collected prior to the advent of the Omicron variant of SARS-CoV-2. All autopsies were performed at MetroHealth Medical Center (Cleveland, OH) following institutional protocols. The study is exempt from institutional review board approval.
2.2. Chart review
A detailed chart review was performed on the cases and controls to evaluate demographic characteristics including age and sex, duration of hospitalization and symptoms, clinical characteristics including body mass index (BMI), underlying medical conditions, use of pressors, use of renal replacement therapy (dialysis), last SpO2 (oxygen saturation) measured before death, mechanical ventilation, cardiac arrest or ischemic encephalopathy during the last hospitalization, intubation and duration of intubation, imaging data, and laboratory data including Troponin I, D-dimer, and baseline creatinine. For Troponin I and D-dimer, the highest documented level during the last hospitalization was utilized. The baseline creatinine is the first creatinine measurement for the patient in the last admission. Only limited imaging and laboratory data were available in both groups.
2.3. Postmortem examination
The autopsy reports of all cases in the study and control groups were reviewed for the presence of pleural effusion or pleuritis at the time of the autopsy. Hearts were dissected and sampled per routine autopsy protocol at our institution. This includes recording heart weight, thickness of the left and right ventricular walls measured approximately 2 cm distal to the atrioventricular valves, and percentage of coronary artery occlusion by atherosclerosis. Three sections are routinely taken from the myocardium (right ventricle, left ventricle, and septum). For the purpose of this study, each section from each heart was scored independently for myocarditis, single myocyte necrosis, edema, and myocytolysis. The findings for each heart were analyzed as a group and recorded as present or absent, regardless of the provenance of the section. Single myocyte necrosis was a rare and subtle finding and even one focus found was scored as present. All the slides of the Covid 19 and the control hearts were systematically examined on high power (40X objective) by at least two of the investigators, independently of one another. Discrepancies in interpretation were discussed together at a multiheaded microscope. Equivocal cases were counted as “absent.” The diagnosis of myocarditis was reserved for the presence of any inflammatory cells in the myocardium that are known to cause myocarditis – neutrophils, eosinophils, giant cells, or lymphocytes [12]. Myocardial edema was defined as accumulation of frothy fluid or fibromyxoid change between myocytes (Fig. 1 ) [[36], [37]]. Clear spaces between myocytes were attributed to postmortem or processing artefact, and not counted as edema. Myocytolysis was defined as the presence of myocardial vacuolization (Fig. 2 ). Since assessing the grade of edema and myocytolysis is subjective, we categorized these histologic changes in a binary fashion as transmural or non-transmural.
Fig. 1.
Interstitial edema of myocardium is characterized by frothy fluid or myoxid changes between myocytes. (400X; Hematoxylin and Eosin stain)
Fig. 2.
Myocytolysis: vacuolization or cytoplasmic edema of myocytes (400X; Hematoxylin and Eosin stain)
All the heart sections except those from two controls (which were inaccessible) were subsequently stained for CD68 as a macrophage marker. The density of macrophages was quantified in the myocardium and epicardium on the heart tissue blocks of the Covid-19 and control groups on the basis of this staining. The cell density for CD68 positive macrophages in the myocardium was determined by counting the number of cells in 10 high power fields (HPFs)/400× (each 0.95 mm2) in areas with a maximum number of positively-stained cells. Quantification in the myocardium was done manually, and the findings presented as cells/mm2. The cell density for CD68 positive macrophages in the epicardium was determined by counting the number of cells in five regions (each 3 mm2) at 10X power field in areas with a maximum number of cells using imageJ analysis software 1.53c (developed by the National Institutes of Health; ImageJ (nih.gov)). CD68-positive cell density was enumerated using three built-in functions in imageJ: First, background subtraction was done using rolling ball method (radius of 12.0 pixels was used). Then, the image was processed to increase the brightness of the positively staining cells using the color threshold tool. After that, the “Analyze the Particles” tool was used to count the positively staining cells. The counts were expressed as cells/mm2. Enumeration (manual and digital) was performed by one investigator who was blinded to whether the section was from a case or control heart during the analysis. Since the areas in which the cells were counted were selected on a subjective assessment of “maximum number of positively-stained cells” instead of counting all the macrophages present on the slide, we consider our study to be semi-quantitative.
We grouped our findings for the macrophage density in the epicardium instead of reporting them based on the specific anatomic location because of the variability of the amount of epicardium present in each section and because the epicardial fat was not represented in some heart sections. Similarly, the macrophage counts in the myocardium were grouped for each heart instead of being reported based on the specific anatomic location because previous studies reported no differences in the inflammatory density between the left and right ventricles [20], and because there are no anatomical barriers between the left ventricular, right ventricular or septal myocardium. The macrophages in the myocardium were counted manually, unlike those in the epicardium where imageJ was used, in order to subtract intravascular macrophages, and to avoid counting myocytes with prominent nuclei as CD68 positive macrophages by imageJ.
After comparing the macrophage densities in the myocardium and epicardium between the case and control groups, the Covid-19 group was further stratified into two subgroups: hearts with single myocyte necrosis in at least one section, and those with no single myocyte necrosis. The gross and microscopic findings, including macrophage density in the myocardium and epicardium, were compared between these two subgroups.
2.4. Statistical analysis
Statistical analysis was performed using two-tailed T-test (Microsoft Excel) for the numerical data. Two-tailed Fisher exact test (two-tailed) (Prism 6; GraphPad software, Inc., La Jolla, CA) was used to analyze the categorical data. Correlation analysis was performed using Spearman rho test. Significance was set at a P value of <0.05.
3. Results
3.1. Demographics, clinical characteristics, imaging, and laboratory data
There were no significant differences in age or sex between the Covid-19 and control groups; in the Covid-19 group, there were 3 females and 7 males with a mean age of 63.8 years (range, 43–82 years) and in the control group, there were 8 females and 12 males with a mean age of 58.6 years (range, 34–83 years). There were more patients who experienced cardiac arrest during the last hospitalization in Covid-19 cases without single cell necrosis compared to Covid-19 cases with single cell necrosis (P = 0.047). The duration of intubation was longer in Covid-19 patients with single cell necrosis compared to those without (P = 0.049; Table 2). There were no significant differences between the study groups regarding all other available clinical characteristics or laboratory data (Tables 1 and 2 ). Bronchopneumonia and/or diffuse alveolar damage were either the immediate cause of death or directly related to the immediate cause of death in both groups.
Table 2.
Baseline patient characteristics of Covid-19 patients with single myocyte necrosis compared to Covid-19 patients without single myocyte necrosis
| Characteristic | Covid-19 decedents with myocyte necrosis (N = 5) | Covid-19 decedents without myocyte necrosis (N = 5) | P value |
|---|---|---|---|
| Age | 60 (53–70) | 68 (43–82) | 0.33 |
| Gender MaleFemale | 2 (40) 3 (60) | 5 (100)0 (0) | 0.16 |
| Duration of symptoms | 26.4 (13–40) | 18 (5–28) | 0.22 |
| Duration of hospitalization | 22 (8–35) | 10.8 (5–23) | 0.08 |
| Obesity (BMI >30 kg/m^2) | 4 (80) | 3 (60) | 1.00 |
| Hypertension | 4 (80) | 3 (60) | 1.00 |
| Diabetes mellitus | 3 (60) | 3 (60) | 1.00 |
| Hyperlipidemia | 2 (40) | 3 (60) | 1.00 |
| Underlying CHF | 2 (40) | 2 (40) | 1.00 |
| Cardiac arrest (during the last admission) | 0 (0) | 4 (80) | 0.047 |
| Ischemic encephalopathy | 0 (0) | 0 (0) | 1 |
| peripheral circulatory support (ECMO) | 0 (0) | 0 (0) | 1 |
| Intubation | 5 (100) | 4 (80) | 1 |
| Mean duration of intubation | 15.6 (6-26), n=5 | 5.5 (1-9), n=4 | 0.049 |
| Mechanical ventilation | 5 (100) | 4 (80) | 1 |
| SpO2 (last measured) | 85.6 (59–100), n = 5 | 75.2 (55–100), n = 5 | 0.39 |
| Renal replacement therapy (dialysis) | 4 (80) | 1 (20) | 0.2 |
| Use of pressors | 5 (100) | 2 (40) | 0.16 |
| Cases with elevated troponin | 2 (40) | 1 (20), n = 4 | 1.00 |
| Peak troponin (ng/L)a | 3 (0.02–15.2) | 0.077 (0.03–0.18), n = 4 | 0.37 |
| Cases with decreased ejection fraction | 1 (20), n = 4 | 1 (20), n = 3 | 1.00 |
| Cases with elevated D-dimer | 5 (100) | 4 (100), n = 4 | 1.00 |
| Baseline creatinine (mg/dL) | 2.3 (0.9–4.99) | 1.9 (0.7–3.4) | 0.63 |
| Immediate cause of death due to bronchopneumonia/ diffuse alveolar damage | 3 (60) | 4 (800) | 1.00 |
| Other immediate causes of death | Sepsis: 2 (40) | PE: 1 (20) |
Data refer to number of patients (percentage) or mean (range) unless specified.
CHF, congestive heart failure. CAD, coronary artery disease. SpO2, oxygen saturation.
For each characteristic, when information regarding the characteristic was not available for all of the patients in the group, the number of patients is noted as n = x.
Highest troponin value available in the last admission.
Table 1.
Baseline patient characteristics in Covid-19 patients compared to controls
| Characteristic | Covid-19 decedent (N = 10) | Non Covid-19 decedent (N = 20) | P value |
|---|---|---|---|
| Age | 63.8 (43-82) | 58.6 (34-83) | 0.28 |
| GenderMaleFemale | 7 (70) 3 (30) | 12 (60)8 (40) | 0.7 |
| Duration of symptoms (days) | 22.2 (5–40) | 16.9 (7–58), n = 12 | 0.34 |
| Duration of hospitalization (days) | 16.4 (5–35) | 9.1(1–28), n = 18 | 0.06 |
| Obesity (BMI >30 kg/m^2) | 7 (70) | 9 (45) | 0.26 |
| Hypertension | 7 (70) | 12 (60) | 0.44 |
| Diabetes mellitus | 6 (60) | 4 (20) | 0.11 |
| Hyperlipidemia | 5 (50) | 9 (45) | 1.00 |
| Underlying CHF | 4 (40) | 3 (15) | 0.18 |
| Cardiac arrest (during the last admission) | 4 (40) | 5 (25) | 0.43 |
| Ischemic encephalopathy | 0 (0) | 1 (5) | 1 |
| peripheral circulatory support (ECMO) | 0 (0) | 0 (0) | 1 |
| Intubation | 9 (90) | 13 (65) | 0.2 |
| Mean duration of intubation | 11.1 (1–26), n = 9 | 6.3 (1–26), n = 13 | 0.051 |
| Mechanical ventilation | 9 (90) | 13 (65) | 0.2 |
| SpO2 (last measured) | 80.4 (55–100), n = 10 | 73.2 (40–100) n = 18 | 0.37 |
| Renal replacement therapy (dialysis) | 5 (50) | 6 (30) | 0.42 |
| Use of pressors | 7 (70) | 13 (65) | 1 |
| Cases with elevated troponin | 3 (30), n = 9 | 6 (30), n = 15 | 0.66 |
| Peak troponin (ng/L)a | 1.7 (0.02–15.2), n = 9 | 0.13 (0–0.4), n = 15 | 0.46 |
| Cases with decreased ejection fraction | 2 (20), n = 6 | 1 (5), n = 16 | 0.16 |
| Cases with elevated D-dimer | 9 (90), n = 9 | 6 (30), n = 8 | 0.2 |
| Baseline creatinine (mg/dL) | 2.1 (0.78–4.99) | 2.4 (0.3–8.2), n = 18 | 0.72 |
| Immediate cause of death due to bronchopneumonia/ diffuse alveolar damage | 7 (70) | 14 (70) | 1.0 |
| Other immediate causes of death | Sepsis: 2 (20) | Sepsis: 6 (30) | |
| PE: 1 (10) |
Data refer to number (percentage) of patients or mean (range).
CHF, congestive heart failure; CAD, coronary artery disease; SpO2, oxygen saturation.
For each characteristic, when information regarding the characteristic was not available for all of the patients in the group, the number of patients is noted as n = x.
Highest troponin value available in the last admission.
3.2. Findings on postmortem examination
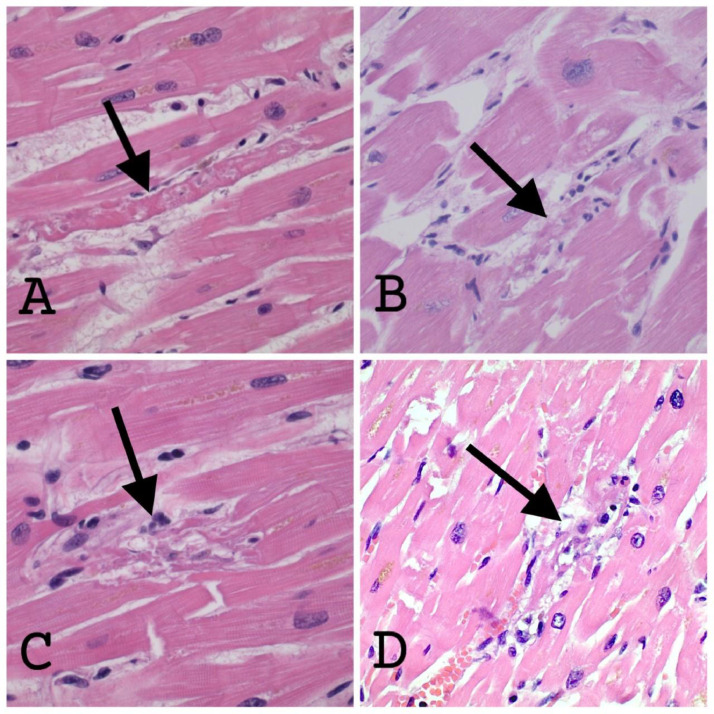
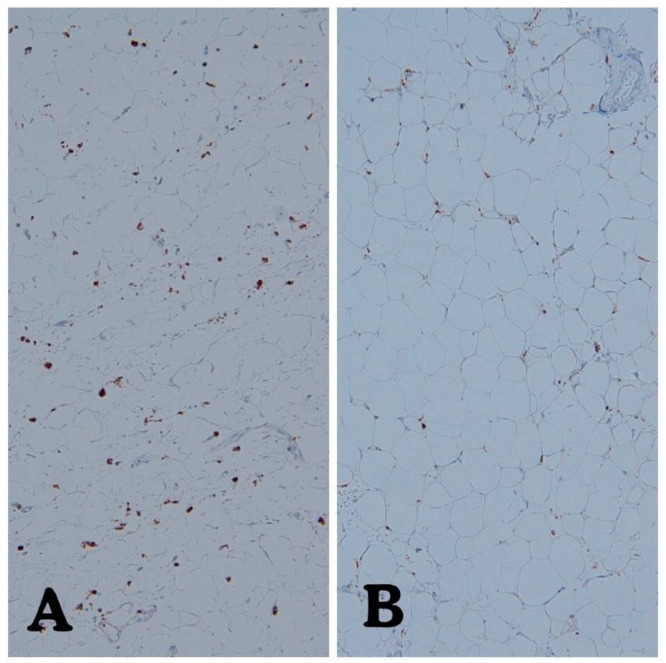
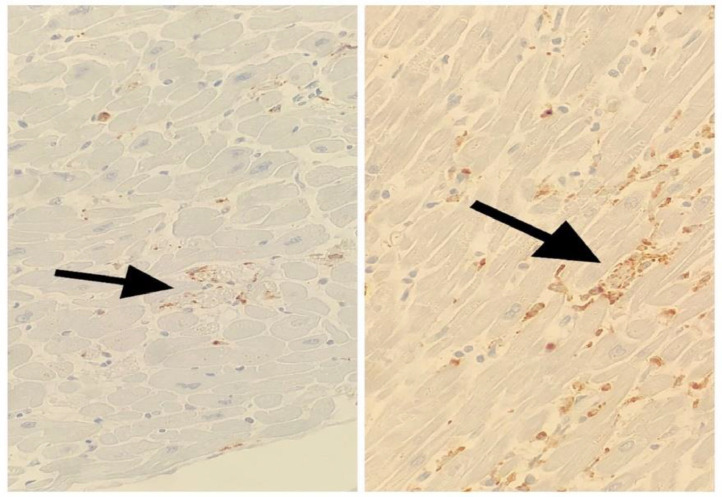
There were no significant differences between the Covid-19 and control groups in the following pathological (gross and microscopic) characteristics: pleural effusion, pleuritis, heart weight, degree of coronary atherosclerosis, thickness of left ventricle, thickness of right ventricle, or the presence of thrombosis (pulmonary, brachiocephalic vein or portal vein; Table 3 ). Transmural edema was identified in 9/10 (90%) Covid-19 patients compared to 10/20 (50%) in the control group (P = 0.04). Transmural myocytolysis was identified in 9/10 (90%) Covid-19 patients compared to 10/20 (50%) in the control group (P = 0.04). Single myocyte necrosis was identified in 5/10 Covid-19 hearts compared to 1/20 control hearts (Fig. 3 , Table 3; P = 0.008). The heart sections did not show zones of myocyte necrosis, nor did they show coagulative necrosis or contraction bands. Single myocyte necrosis was a subtle and rare finding in all but one of the Covid 19 cases in which it was observed; in this one case, single myocyte necrosis was present in multiple foci. Single myocyte necrosis was identified in a single focus in one of three sections of one of the control hearts. The appearance of single myocyte necrosis was similar in the Covid and control groups. Microvascular thrombosis was not observed in any of the heart sections. No cases of lymphocytic, eosinophilic, neutrophilic, or giant cell myocarditis were identified in the Covid-19 or control group. The density of macrophages was higher in the epicardium of the Covid-19 hearts compared to the control hearts, P = 0.008 (Figs. 4 and 5 ). The density of macrophages was also higher in the myocardium of Covid-19 hearts compared to the myocardium of control hearts, P = 0.008 (Figs. 6 and 7 ). There were two Covid-19 cases in which there was single myocyte necrosis associated with an adjacent CD68 positive macrophage infiltrate (Figure 8 ).
Table 3.
Microscopic and gross pathological findings in hearts from Covid-19 decedents compared to hearts from control group
| Characteristic | Covid-19 decedents (N = 10) | Non Covid-19 decedents (N = 20) | P value |
|---|---|---|---|
| Heart weight (grams) | 463 (150–650) | 460 (320–890) | 0.96 |
| Thickness of left ventricular wall (cm) | 1.56 (1.3–1.9) | 1.74 (1–2.6) | 0.11 |
| Thickness of right ventricular wall (cm) | 0.55 (0.4–0.7) | 0.57 (0.3–1.7) | 0.78 |
| Mean percentage of coronary artery stenosis | 43.5 (10–80) | 40.1 (0–90) | 0.87 |
| Pleural effusion | 7 (70) | 14 (70) | 1 |
| Pleuritis | 0 (0) | 2 (10) | 1 |
| Thrombosis | 4 (40); 2 PE and 2 brachiocephalic vein thrombosis | 3 (15); 2 PE and 1 portal vein thrombosis | 0.18 |
| Epicardial macrophages per mm2 | 80.4 (18–112) | 47.8 (14-115), n = 18 | 0.008 |
| Myocardial macrophages per mm2 | 62.4 (10.2–130.6) | 26.8 (6.8-60.1), n = 18 | 0.008 |
| Cases with single myocyte necrosis | 5 (50) | 1 (5) | 0.008 |
| Cases with transmural myocytolysis | 9 (90) | 10 (50) | 0.04 |
| Cases with transmural edema | 9 (90) | 10 (50) | 0.04 |
| Cases with microvascular thrombosis | 0 (0) | 0 (0) | 1 |
Data refer to number (percentage) or mean (range) unless specified.
PE, pulmonary embolism.
For each characteristic, when information regarding the characteristic was not available for all of the patients in the group, the number of patients is noted as n = x.
Fig. 3.
(400X; Hematoxylin and Eosin stain): (A & B) The arrows point to single myocytes with degenerating cytoplasm in the background of hypertrophic but otherwise viable cells. There are no inflammatory cells associated with the degenerating cells. Edema fluid is present in the interstitium. (C) The arrow is pointing to haphazardly arranged, dark pink fragments of myocyte cytoplasm. Adjacent myocytes are hypertrophic but appear viable. Inflammatory cells may be present, although it would be difficult to call this a “myocarditic” focus on the basis of H&E stain alone. (D) Focus of single myocyte necrosis in a heart from the “control” group. The arrow is pointing to a degenerating myocyte, in which the nucleus can still be identified but the cytoplasm is disrupted. Surrounding myocytes are hypertrophic and show paranuclear lipofuscin granules, but there is no evidence of contraction bands or coagulative necrosis. A few inflammatory cells are present in the necrotic focus, although it is difficult to tell which are “innocent bystanders” or in transit through adjacent capillaries in this H&E photograph.
Fig. 4.
The epicardium of a representative case (A) and control (B) heart, stained with CD68, photographed at 100×. Even by subjective comparison, there are more CD68 positive cells in the adipose tissue of the epicardium in the Covid-19 decedent.
Fig. 5.
Macrophage density in epicardium of Covid-19 hearts versus control hearts (P = 0.008).
Fig. 6.
A: CD68 stain in a section of myocardium from a Covid-19 decedent (A) in comparison to a section of myocardium from a control heart (both at 400×).
Fig. 7.
Macrophage density in myocardium of Covid-19 hearts versus control hearts (P = 0.008).
Fig. 8.
In these two images, arrows point to a focal condensation of CD68 positive staining in areas of individual myocyte necrosis (400X; CD68 stain).
The number of myocardial CD68 positive macrophages showed a positive correlation with the epicardial CD68 positive macrophages across Covid-19 and control hearts, when grouped together (rs = 0.59214, P = 0.0009). The number of myocardial CD68 positive macrophages showed a positive correlation with the epicardial CD68 positive macrophages in the Covid-19 hearts alone (rs = 0.74164, P = 0.014). There was no correlation between the number of myocardial CD68 positive macrophages and epicardial CD68 positive macrophages in the control hearts alone (rs = 0.2, P = 0.43184).
There is a correlation between the epicardial macrophages and the BMI in control hearts (rs = 0.4, P = 0.05) but no correlation was noted in the Covid-19 hearts or both Covid-19 and control hearts combined (rs = 0.2, P = 0.6; rs = 0.5, P = 0.09 respectively). The number of myocardial macrophages in the 18 hearts with transmural edema were not significantly higher than in the 9 hearts with non-transmural edema (44 vs. 32, P = 0.26).
Among the 5 Covid-19 hearts with single myocyte necrosis, the density of macrophages in the epicardium was higher compared to the 5 hearts without single myocyte necrosis (Table 4 , Fig. 9 ). There was no significant difference in the density of myocardial macrophages between the two groups (Table 4, Fig. 10 ). There were no differences between the groups in the rest of the pathological (gross and microscopic) characteristics (Table 4).
Table 4.
Microscopic and gross pathological findings in Covid-19 patients with single myocyte necrosis compared to Covid-19 patients without single myocyte necrosis.
| Characteristic | Covid-19 decedent with myocyte necrosis (N = 5) | Covid-19 decedent without myocyte necrosis (N = 5) | P value |
|---|---|---|---|
| Heart weight (grams) | 436 (300–640) | 490 (150–650) | 0.63 |
| Thickness of left ventricular wall (cm) | 1.62 (1.3–1.9) | 1.5 (1.3–1.7) | 0.34 |
| Thickness of right ventricular wall (cm) | 0.52 (0.4–0.6) | 0.58 (0.4–0.7) | 0.36 |
| Mean percentage of coronary artery stenosis | 50 (10–80) | 37 (15–50) | 0.47 |
| Pleural effusion | 5 (100) | 2 (40) | 0.16 |
| Thrombosis | 2 (40) | 2 (40) | 1 |
| Epicardial macrophages per mm2 | 99.2 (80–112) | 61.6 (18–81) | 0.02 |
| Myocardial macrophages per mm2 | 75.8 (41–130.6) | 49 (10.2–77.7) | 0.22 |
| Cases with transmural myocytolysis | 4 (80) | 5(100) | 1 |
| Cases with transmural edema | 5 (100) | 4 (80) | 1 |
| Cases with microvascular thrombosis | 0 (0) | 0 (0) | 1 |
Data refer to number of patients (percentage) or mean (range) unless specified.
PE, pulmonary embolism.
For each characteristic, when information regarding the characteristic was not available for all of the patients in the group, the number of patients is noted as n = x.
Fig. 9.
Macrophage density in epicardium of Covid-19 hearts with single myocyte necrosis versus Covid-19 hearts without single myocyte necrosis (P = 0.02).
Fig. 10.
Macrophage density in myocardium of Covid-19 hearts with single myocyte necrosis versus Covid-19 hearts wthout single myocyte necrosis (P = 0.22).
4. Discussion
This study demonstrates that single myocyte necrosis, transmural edema, and transmural myocytolysis are significantly more common in the hearts of Covid-19 decedents compared to those from a control group of patients who died with severe bronchopneumonia or diffuse alveolar damage unrelated to Covid-19. Second, the density of macrophages in the epicardium and myocardium is significantly higher in the hearts of Covid-19 decedents compared to non-Covid-19 control patients. Finally, we demonstrate a positive correlation between the density of epicardial and myocardial macrophages in the hearts of Covid-19 decedents and the presence of single myocyte necrosis.
Vander Heide and Fox [18] suggest that single myocyte necrosis in the myocardium of patients who die with Covid-19 may be due to increased strain on the heart in the setting of severe pulmonary pathology. We designed the first experiment to test this hypothesis by comparing the occurrence of single myocyte necrosis in Covid-19 decedents to its occurrence in a control group of patients who died with severe bronchopneumonia and/or diffuse alveolar damage unrelated to Covid-19 illness. There were significantly more patients with single myocyte necrosis in the Covid-19 group compared to the control group. This suggests that single myocyte necrosis is a direct manifestation of Covid-19 illness rather than a secondary effect of severe pulmonary impairment due to underlying bronchopneumonia or diffuse alveolar damage. However, single myocyte necrosis does not occur in all patients with Covid-19 infection. In the literature, the occurrence of single myocyte necrosis in patients who died of Covid-19 varies from 14%–100% [[13], [14], [15], [16],18]. Also, single myocyte necrosis was present in one (5%) of our non-Covid-19 decedents, and, since our attention was called to this finding, we subsequently found it in two additional non-Covid-19 decedents who died as a result of pulmonary embolization (not included in this study). Expanding the control group to include patients who died of pulmonary emboli unrelated to Covid-19 illness may yield further insights into clinicopathologic characteristics that correlate with the development of single myocyte necrosis. In the present study, only one of the Covid-19 patients died of pulmonary emboli, and hence the potential role of thromboemboli as a risk factor for myocyte necrosis was not assessed. Based on our observations, single myocyte necrosis should not be considered a specific hallmark of SARS-CoV-2 associated cardiac injury.
Interstitial edema and myocytolysis are commonly found at autopsy in agonal hearts, however, we observed that the edema and myocytolysis associated with Covid-19 infection tend to be transmural, as opposed to only subendocardial or limited in extent. The observation of global edema in the heart correlates with published premortem imaging studies utilizing advanced imaging modalities that show edematous hearts [37] in Covid-19 patients. Although transmural edema and myocytolysis are more commonly seen in the hearts of Covid-19 decedents than in the control group, 50% of patients in the control group also demonstrated global cardiac edema.
Some investigators have implicated myocardial macrophages in the pathophysiology of Covid-19 related cardiac injury [17,19,20]. Concordant with these prior studies, we also found the density of macrophages to be significantly (P = 0.008) higher in the myocardium of Covid-19 decedents (average of 62.4 cells per mm2) compared to the myocardium in the control group (average of 26.8 cells per mm2). Azzawi et al. [38] report a median number of up to 5 macrophages per HPF in “normal” hearts (from patients who died of sudden cardiac death). The macrophage density in our control group would be roughly equivalent to that reported by Azzawi et al. after approximately converting their HPF to mm2.
Macrophages are cells of the mononuclear phagocyte system that play integral roles in different phases of inflammatory responses, and whose activity is tightly regulated by complex cytokine signaling pathways [39]. The heart normally contains both yolk sac-derived and monocyte-derived macrophages. The monocyte-derived macrophages are further subdivided into fetal monocyte-derived macrophages (liver origin) and adult monocyte-derived macrophages (bone marrow origin). The major population of macrophages resident in the heart is from yolk sac and fetal monocyte (liver) origin. These are known to have low surface expression of chemokine receptor 2 (CCR2−), and to be involved in tissue repair, angiogenesis and phagocytosis [22,23]. In the setting of infection, the resident macrophages of the myocardium secrete chemokines that attract bone-marrow derived monocytes, which differentiate into CCR2+ macrophages with pro-inflammatory function [22,23]. The increased numbers of myocardial macrophages observed in this and other studies in Covid-19 patients could be due to increased systemic levels of proinflammatory cytokines. Systemic cytokine elevations may also be responsible for the elevated numbers of macrophages we document in some control hearts, as several of these patients died with overwhelming pulmonary infection and/or sepsis.
Other investigators have suggested that epicardial adipose tissue could be implicated in the pathophysiology of cardiac involvement in Covid-19 illness [32,33]. Iacobellis et al. [40] compared the epicardial attenuation (EAT) from CT scans of patients who were admitted for Covid-19 illness. The degree of EAT attenuation is presumed to reflect inflammatory changes. The authors divided the study population into four groups based on the severity of Covid-19 illness, and found that patients with severe and critical Covid-19 illness had significantly greater EAT attenuation than those with mild and moderate infection. Similarly, in our study, the density of macrophages in the epicardium was significantly higher in the hearts of Covid-19 decedents compared to the hearts in the control group, and in the hearts of Covid-19 decedents with single myocyte necrosis compared to the hearts of Covid-19 decedents without single myocyte necrosis. These findings support the impression that infiltration of the epicardium by CD68-positive macrophages plays a role in the pathophysiology of cardiac decompensation in the setting of SARS-CoV-2 infection.
In terms of clinical parameters that may have contributed to our observations, we showed that the number of cases experiencing cardiac arrest during their last hospitalization was higher in Covid-19 cases without single cell necrosis compared to Covid-19 cases with single cell necrosis. On the other hand, the duration of intubation was longer in Covid-19 patients with single cell necrosis compared to those without. Larger studies are required to show whether these correlations may indeed have a basis in pathophysiologic processes.
There are several limitations inherent in this study. We did not attempt to identify SARS-CoV-2 viral particles in the hearts of the Covid-19 patients in our “case” group, primarily because the time between diagnosis and death was prolonged and therefore the likelihood of finding the particles and subsequently establishing causality would be diminished. We did not establish cell lineage of the macrophages in the myocardium and the epicardium. This would be a logical next step to understanding the pathophysiologic role of macrophages in the course of Covid-19 related cardiac injury. Finally, the correlations that we found are not absolute; the fact that some hearts in the control group showed similar findings – of global edema and myocytolysis, single myocyte necrosis and elevated densities of macrophages in the myocardium and epicardium – suggest that the role of macrophages in the hearts of patients with severe and terminal lung injury is not entirely specific to Covid-19 illness. Nevertheless, the strength of correlations between single myocyte necrosis, global edema and myocytolysis, and densities of macrophages in the myocardium and epicardium, is striking.
We were unable to correlate our findings with clinical diagnosis of “myocarditis” based on cardiac MRI, because of the unavailability of this data. In the Covid-19 group compared to the control group, and in Covid-19 patients with myocyte necrosis compared to the Covid-19 patients without single myocyte necrosis, there was no significant difference in the level of peak troponin, D-dimer, or baseline creatinine. Previous autopsy studies of Covid-19 patients did not show significant differences in troponin levels between “myocarditis” and “non-myocarditis” cases [17,20]. The relationship of the histopathological changes in patients with Covid-19 illness and elevated clinical parameters of myocardial injury remain uncertain.
5. Conclusion
Our study adds to the evidence accumulating since the beginning of the SARS-CoV-2 pandemic in 2019 that histological evidence of myocardial inflammation in patients with severe Covid-19 illness is sparse. Classic “myocarditis” is seen in only rare cases reported in the literature, and we did not observe it any of our cases. More often, evidence of inflammation or myocardial injury is present in the form of single myocyte necrosis and transmural edema and myocytolysis. Recently, increased density of CD68-positive macrophages in the myocardium has been added to the list of “inflammatory” changes in the heart in Covid-19 decedents. We reproduce this observation and add to it by demonstrating that CD68 macrophage density is also increased in the epicardium, and that epicardial macrophages correlate both with elevated CD68-positive macrophage density in the myocardium and with the presence of single myocyte necrosis. Although we have demonstrated that these correlations are statistically significant in patients with Covid-19 illness, they are not unique to Covid-19. The role of other pathways, such as cytokine fluctuations or thrombosis, may be important factors underlying these observations.
Availability of data and material
All data is available from the corresponding author upon reasonable request.
Ethics approval
This study is exempt from IRB approval.
Footnotes
Disclosures: The authors declare no conflict of interest.
References
- 1.Li X, Wang W, Zhao X, Zai J, Zhao Q, Li Y, Chaillon A. Transmission dynamics and evolutionary history of 2019-nCoV. J Med Virol. 2020;92(5):501–511. doi: 10.1002/jmv.25701. Epub 2020 Feb 14. PMID: 32027035; PMCID: PMC7166881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babapoor-Farrokhran S, Gill D, Walker J, Rasekhi RT, Bozorgnia B, Amanullah A. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253 doi: 10.1016/j.lfs.2020.117723. Epub 2020 Apr 28. PMID: 32360126; PMCID: PMC7194533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. Epub 2020 Jul 10. PMID: 32651579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shchendrygina A, Nagel E, Puntmann VO, Valbuena-Lopez S. COVID-19 myocarditis and prospective heart failure burden. Expert Rev Cardiovasc Ther. 2021;19(1):5–14. doi: 10.1080/14779072.2021.1844005. Epub 2020 Nov 27. PMID: 33119418. [DOI] [PubMed] [Google Scholar]
- 5.Castiello T, Georgiopoulos G, Finocchiaro G, Claudia M, Gianatti A, Delialis D, et al. COVID-19 and myocarditis: a systematic review and overview of current challenges. Heart Fail Rev. 2022;27(1):251–261. doi: 10.1007/s10741-021-10087-9. Epub 2021 Mar 24. PMID: 33761041; PMCID: PMC7988375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol. 2009;53(17):1475–1487. doi: 10.1016/j.jacc.2009.02.007. PMID: 19389557; PMCID: PMC2743893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitrani RD, Dabas N, Goldberger JJ. COVID-19 cardiac injury: implications for long-term surveillance and outcomes in survivors. Heart Rhythm. 2020;17(11):1984–1990. doi: 10.1016/j.hrthm.2020.06.026. Epub 2020 Jun 26. PMID: 32599178; PMCID: PMC7319645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34(33):2636–2648. doi: 10.1093/eurheartj/eht210. 2648a-2648dEpub 2013 Jul 3. PMID: 23824828. [DOI] [PubMed] [Google Scholar]
- 9.Kim IC, Kim JY, Kim HA, Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J. 2020;41(19):1859. doi: 10.1093/eurheartj/ehaa288. PMID: 32282027; PMCID: PMC7184491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokoo P, Fonseca EKUN, Sasdelli Neto R, Ishikawa WY, Silva MMA, Yanata E, et al. COVID-19 myocarditis: a case report. Einstein. 2020;18:eRC5876. doi: 10.31744/einstein_journal/2020RC5876. Sao PauloPMID: 33111813; PMCID: PMC7575039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naneishvili T, Khalil A, O'Leary R, Prasad N. Fulminant myocarditis as an early presentation of SARS-CoV-2. BMJ Case Rep. 2020;13(9) doi: 10.1136/bcr-2020-237553. PMID: 32928810; PMCID: PMC7490957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.M.A. Seidman, B.M. McManus, Chapter 9 - Myocarditis, Editor(s): L. Maximilian Buja, Jagdish Butany, Cardiovascular Pathology (4th ed), Academic Press, 2016, Pages 341-359, ISBN 9780124202191, https://doi.org/10.1016/B978-0-12-420219-1.00009-4 (https://www.sciencedirect.com/science/article/pii/B9780124202191000094)
- 13.Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50 doi: 10.1016/j.carpath.2020.107300. Epub 2020 Oct 23. PMID: 33132119; PMCID: PMC7583586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jum'ah H, Loeffler A, Tomashefski JF. Histopathological findings in the hearts of COVID-19 autopsies: a letter to cardiovascular pathology journal editor in response to Halushka et al. 2020. Cardiovasc Pathol. 2021;52 doi: 10.1016/j.carpath.2021.107333. Epub 2021 Mar 16. PMID: 33741530; PMCID: PMC7962914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, et al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. Epub 2020 May 27. PMID: 32473124; PMCID: PMC7255143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox SE, Li G, Akmatbekov A, Harbert JL, Lameira FS, Brown JQ, et al. Unexpected features of cardiac pathology in COVID-19 infection. Circulation. 2020;142(11):1123–1125. doi: 10.1161/CIRCULATIONAHA.120.049465. Epub 2020 Jul 21. PMID: 32689809. [DOI] [PubMed] [Google Scholar]
- 17.Bearse M, Hung YP, Krauson AJ, Bonanno L, Boyraz B, Harris CK, et al. Factors associated with myocardial SARS-CoV-2 infection, myocarditis, and cardiac inflammation in patients with COVID-19. Mod Pathol. 2021;34(7):1345–1357. doi: 10.1038/s41379-021-00790-1. Epub 2021 Mar 17. PMID: 33727695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox SE, Heide RSV. COVID-19: the heart of the matter—pathological changes and a proposed mechanism. J Cardiovasc Pharmacol Therap. 2021;26(3):217–224. doi: 10.1177/1074248421995356. [DOI] [PubMed] [Google Scholar]
- 19.Fox SE, Falgout L, Vander Heide RS. COVID-19 myocarditis: quantitative analysis of the inflammatory infiltrate and a proposed mechanism. Cardiovasc Pathol. 2021;54 doi: 10.1016/j.carpath.2021.107361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, Aubry MC, et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41(39):3827–3835. doi: 10.1093/eurheartj/ehaa664. PMID: 32968776; PMCID: PMC7543528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munshi NV. Resident macrophages: near and dear to your heart. Cell. 2017;169(3):376–377. doi: 10.1016/j.cell.2017.04.002. PMID: 28431239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavine KJ, Pinto AR, Epelman S, Kopecky BJ, Clemente-Casares X, Godwin J, et al. The macrophage in cardiac homeostasis and disease: JACC macrophage in CVD Series (Part 4) J Am Coll Cardiol. 2018;72(18):2213–2230. doi: 10.1016/j.jacc.2018.08.2149. PMID: 30360829; PMCID: PMC6209119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lafuse WP, Wozniak DJ, Rajaram MVS. Role of cardiac macrophages on cardiac inflammation, fibrosis and tissue repair. Cells. 2020;10(1):51. doi: 10.3390/cells10010051. PMID: 33396359; PMCID: PMC7824389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicin L, Abplanalp WT, Mellentin H, Kattih B, Tombor L, John D, et al. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur Heart J. 2020;41(19):1804–1806. doi: 10.1093/eurheartj/ehaa311. PMID: 32293672; PMCID: PMC7184464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao L. Obesity accompanying COVID-19: the role of epicardial fat. Obesity (Silver Spring) 2020;28(8):1367. doi: 10.1002/oby.22867. PMID: 32365275. [DOI] [PubMed] [Google Scholar]
- 26.Couselo-Seijas M, Almengló C, M Agra-Bermejo R, Luis Fernandez Á, Alvarez E, R González-Juanatey J, et al. Higher ACE2 expression levels in epicardial cells than subcutaneous stromal cells from patients with cardiovascular disease: diabetes and obesity as possible enhancer. Eur J Clin Invest. 2021;51(5):e13463. doi: 10.1111/eci.13463. Epub 2020 Dec 14. PMID: 33251580; PMCID: PMC7744875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jafarzadeh A, Chauhan P, Saha B, Jafarzadeh S, Nemati M. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: Lessons from SARS and MERS, and potential therapeutic interventions. Life Sci. 2020 Sep 15;257 doi: 10.1016/j.lfs.2020.118102. Epub 2020 Jul 18. PMID: 32687918; PMCID: PMC7367812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911–915. doi: 10.1002/ejhf.1828. Epub 2020 Apr 11. PMID: 32275347; PMCID: PMC7262276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albert CL, Carmona-Rubio AE, Weiss AJ, Procop GG, Starling RC, Rodriguez ER. The enemy within: sudden-onset reversible cardiogenic shock with biopsy-proven cardiac myocyte infection by severe acute respiratory syndrome coronavirus 2. Circulation. 2020;142(19):1865–1870. doi: 10.1161/CIRCULATIONAHA.120.050097. Epub 2020 Sep 30. PMID: 32997947. [DOI] [PubMed] [Google Scholar]
- 30.Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM, Butany J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39(7):618–625. doi: 10.1111/j.1365-2362.2009.02153.x. Epub 2009 May 6. PMID: 19453650; PMCID: PMC7163766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116(6):1097–1100. doi: 10.1093/cvr/cvaa078. Erratum in: Cardiovasc Res. 2020 Oct 1;116(12):1994. PMID: 32227090; PMCID: PMC7184507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malavazos Alexis Elias, Goldberger Jeffrey J, Iacobellis Gianluca. Does epicardial fat contribute to COVID-19 myocardial inflammation? Eur Heart J. 2020;41(24):2333. doi: 10.1093/eurheartj/ehaa471. Page. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim IC, Han S. Epicardial adipose tissue: fuel for COVID-19-induced cardiac injury? Eur Heart J. 2020;41(24):2334–2335. doi: 10.1093/eurheartj/ehaa474. PMID: 32464652; PMCID: PMC7314061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harper H, Burridge A, Winfield M, Finn A, Davidson A, Matthews D, et al. Detecting SARS-CoV-2 variants with SNP genotyping. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0243185. Published 2021 Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.John G, Sahajpal NS, Mondal AK, Ananth S, Williams C, Chaubey A, et al. Next-generation sequencing (NGS) in COVID-19: a tool for SARS-CoV-2 diagnosis, monitoring new strains and phylodynamic modeling in molecular epidemiology. Curr Issues Mol Biol. 2021;43(2):845–867. doi: 10.3390/cimb43020061. Published 2021 Jul 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dongaonkar RM, Stewart RH, Quick CM, Uray KL, Cox CS, Laine GA. Award article: Microcirculatory Society Award for Excellence in Lymphatic Research: time course of myocardial interstitial edema resolution and associated left ventricular dysfunction. Microcirculation. 2012;19(8):714–722. doi: 10.1111/j.1549-8719.2012.00204.x. PMID: 22708850; PMCID: PMC3502725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rafiee MJ, Babaki Fard F, Friedrich MG. COVID-19, myocardial edema and dexamethasone. Med Hypotheses. 2020;145 doi: 10.1016/j.mehy.2020.110307. Epub 2020 Sep 28. PMID: 33035967; PMCID: PMC7834646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azzawi M, Hasleton PS, Kan SW, Hillier VF, Quigley A, Hutchinson IV. Distribution of myocardial macrophages in the normal human heart. J Anat. 1997;191(Pt 3):417–423. doi: 10.1046/j.1469-7580.1997.19130417.x. Pt 3PMID: 9418998; PMCID: PMC1467698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4(3):281–286. doi: 10.2174/1568010054022024. PMID: 16101534. [DOI] [PubMed] [Google Scholar]
- 40.Iacobellis G, Secchi F, Capitanio G, Basilico S, Schiaffino S, Boveri S, et al. Epicardial fat inflammation in severe COVID-19. Obesity (Silver Spring) 2020;28(12):2260–2262. doi: 10.1002/oby.23019. Epub 2020 Oct 15. PMID: 32862512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is available from the corresponding author upon reasonable request.