The COVID-19 pandemic has been associated with a disruption of care and worsening outcomes of patients with liver disease.1 Fortunately, receipt of 2 doses of mRNA vaccine was associated with a 78.6% reduction in COVID-19 infection in cirrhosis.2 Although mRNA vaccines have been more widely administered in the United States, viral vector vaccines that use replication-deficient viral vectors, such as adenovirus to induce transient expression of the SARS-CoV-2 spike protein, have been the mainstay of COVID-19 vaccination worldwide.3 Patients with cirrhosis have vaccine hyporesponsiveness that has been observed with hepatitis B, influenza, pneumococcal, and COVID-19 mRNA vaccines.2 Our study aimed to determine the effectiveness of the Ad.26.COV2.S vaccine in patients with cirrhosis, and to explore how this compares with mRNA vaccines administered during the same period.
This study used participants from the Veterans Outcomes and Costs Associated with Liver disease (VOCAL) cohort assembly, which has been described in prior publications.4 , 5 The date and type of COVID-19 vaccine administered from both within and outside the Veterans Affairs (VA) were identified from the VA COVID-19 shared data resource.6 , 7
We use a test-negative case-control design, where adults (age ≥18 years) with cirrhosis who had a positive SARS-CoV-2 polymerase chain reaction (PCR) test were included as cases, and those with a negative PCR, as control subjects.8 Participants who were not tested for SARS-CoV-2 infection, who had prior COVID-19 before the study period, or received a liver transplant were excluded. COVID-19 cases were classified based on individual chart review using the National Institutes of Health COVID-19 severity scale. Participants were considered fully vaccinated 14 days after completion of a single dose of the Ad.26.COV2.S vaccine or after the second dose of an mRNA vaccine at the time of the SARS CoV2 PCR testing. We used propensity score matching to match test-positive cases and test-negative control subjects 1:1 on factors associated with the outcome using the Greedy matching algorithm (Supplementary Methods).
Multinomial logistic regression models were fit for COVID-19, to estimate the odds of having symptomatic illness, moderate/severe/critical illness, severe/critical illness, among participants vaccinated with either the Ad.26.COV2.S or an mRNA vaccine, compared with unvaccinated participants.
Vaccine efficacy (VE) against COVID-19 was estimated using logistic regression, comparing the odds of COVID-19 infection of fully vaccinated with unvaccinated subjects using the equation VE = 100 × (1 – adjusted odds ratio).
Of the participants in the VOCAL cohort (n = 120,952), those who did not have a COVID-19 test in the VA during the study period (n = 114,318), liver transplant recipients (n = 191), and with prior COVID-19 infection (n = 445) were excluded. That resulted in 5998 total study subjects, 968 with a positive SARS CoV2 PCR, and 5030 with a negative PCR (Supplementary Table 1). The overall cohort was predominantly male (96.3%) and white (60.5%), with a median age of 62.6 years (interquartile range, 10.5). In the propensity score (PS) matched cohort, cases and control subjects were well matched with respect to all baseline characteristics (Supplementary Table 1).
The PS matched cohort had 1910 participants, of whom 94 (4.9%) received the Ad.26.COV2.S vaccine, 1089 (57.0%) an mRNA vaccine, and 727 (38.0%) were unvaccinated. Severe or critical COVID-19 was seen in 73 (6.7%) of participants who received the mRNA vaccine, 7 (7.4%) of those who received the viral vector vaccine, and 89 (12.2%) of those who were unvaccinated, in the PS matched cohort.
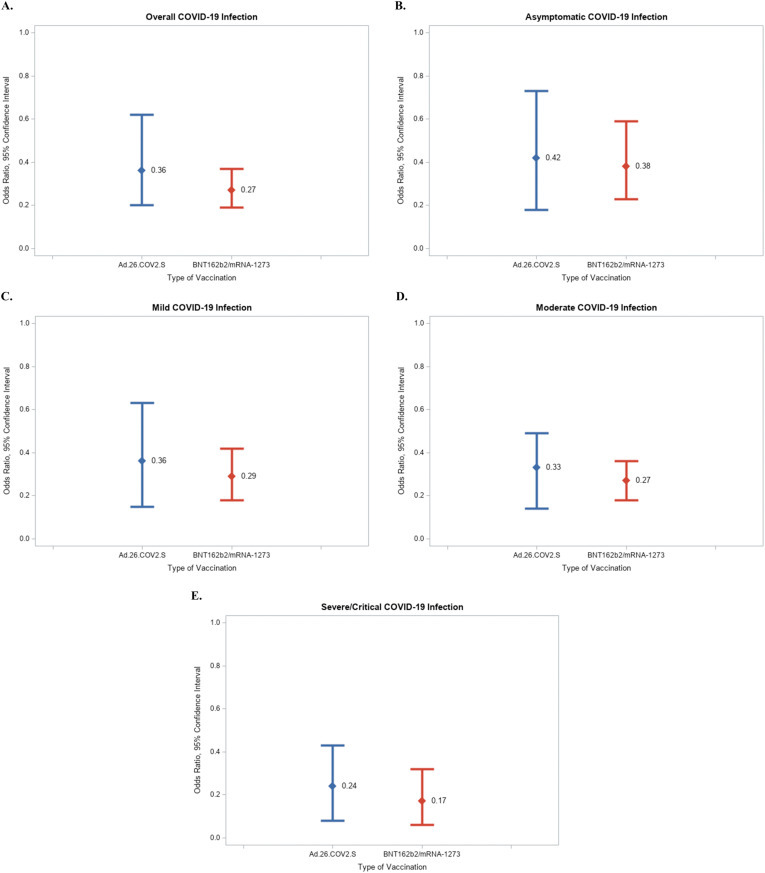
VE of the Ad.26.COV2.S vaccine against COVID-19 was 64% (odds ratio [OR], 0.36; 95% confidence interval [CI], 0.20–0.62; P = .005) (Supplementary Table 2 and Figure 1 ). Effectiveness was 68% against symptomatic illness (OR, 0.32; 95% CI, 0.21–0.41; P = .03), 70% against moderate/severe/critical COVID-19 (OR, 0.30; 95% CI, 0.26–0.37; P = .046), and 72% against severe/critical COVID-19 (OR, 0.28; 95% CI, 0.16–0.42; P = .006).
Figure 1.
Odds ratio (95% confidence interval) of (A) COVID-19, (B) symptomatic COVID-19, (C) moderate/severe/critical COVID-19, and (D). severe/critical COVID-19 among participants with cirrhosis who received the Janssen Ad.26.COV2.S vaccine, or the Moderna 1273-mRNA/Pfizer BNT162b2 mRNA vaccines.
VE of the mRNA vaccines against COVID-19 was 73% (OR, 0.27; 95% CI, 0.19–0.37; P < .0001). Effectiveness was 78% against symptomatic illness (OR, 0.22; 95% CI, 0.16–0.38; P = .03), 80% against moderate/severe/critical COVID-19 (OR, 0.20; 95% CI, 0.08–0.32; P = .003), and 82% against severe/critical COVID-19 (OR, 0.18; 95% CI, 0.07–0.31; P = .002) (Supplementary Table 2 and Figure 1).
We also performed a noninferiority analysis for each of the outcomes, to compare the 2 vaccines directly. We identified the noninferiority margin as 90% of the lower limit of the 95% CI of the BNT162b2/mRNA-1273 VE. Using the 90% noninferiority margin, we conclude that Ad.26.COV2.S is noninferior to the mRNA vaccines for outcomes of symptomatic and moderate/severe/critical COVID-19. However, if we use a more liberal noninferiority margin (75%), we conclude noninferiority against outcomes of symptomatic, moderate/severe/critical, and severe/critical COVID-19 (Supplementary Table 2).
In this study of participants with cirrhosis, we observe that vaccination with a single dose Ad.26.COV2.S vaccine is modestly effective (64%) against overall COVID-19 and performs better against severe/critical COVID-19 (72%). These numbers are comparable with the effectiveness of mRNA vaccines against both COVID-19 (73%) and severe/critical COVID-19 (82%). We also observe no statistically significant differences between the viral vector and mRNA vaccines. The findings are consistent with data reporting high immunogenicity of the COVID-19 vaccines in patients with cirrhosis.9 , 10
Strengths of this study include its test-negative case-control study design, which has been used to estimate vaccine effectiveness against medically attended, laboratory-confirmed SARS-CoV-2 infection among patients who receive testing for SARS-CoV-2 infection.
Limitations include possible residual confounding and the limited number of females. Despite propensity matching, the low number of participants who received the Ad.26.COV2.S vaccine is a limitation, and the viral vector vaccine group is underpowered. These findings take into account the influence of the Delta, but not the Omicron variant. There is the possibility that participants could have received the vaccine or tested positive for COVID-19 outside the VA, but this is unlikely to differ between cases and control subjects. Finally, these data were collected before the administration of booster doses of the BNT162b2 vaccine for immunocompromised patients and the more recent recommendation for boosters for all adults in the United States.
In conclusion, a single dose of the Ad.26.COV2.S vaccine has a 64% vaccine effectiveness against COVID-19, with a 72% effectiveness against severe/critical COVID-19.
Acknowledgments
The authors acknowledge data and support from the VA COVID-19 shared data resource. The authors prepared this work in their personal capacity. The opinions expressed in this article are the authors’ own and do not reflect the view of the Department of Veterans Affairs or the United States government.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Services supporting the analysis and interpretation of the data of this research project were generated by the VCU Massey Cancer Center Biostatistics Shared Resource, supported, in part, with funding from NIH-NCICancer Center Support Grant P30 CA016059. The VCU Massey Cancer Center Biostatistics Shared Resource or the NIH-NCI had no role in the design of the current study.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2022.05.038.
Supplementary Methods
Participants with a positive SARS-CoV-2 PCR test were included as cases. If a person tested positive on more than 1 occasion within 90 days, this was considered a single illness episode.
COVID-19 cases were classified based on individual chart review using the National Institutes of Health COVID-19 severity scale as asymptomatic infection, mild (signs and symptoms of COVID-19 including fever, cough, sore throat, loss of taste and smell but without dyspnea, or abnormal chest imaging), moderate (with evidence of lower respiratory illness on clinical or radiologic evaluation with oxygen saturation [Spo 2] ≥94% on room air), severe (with Spo 2 <94% on room air, respiratory rate >30 breaths/minute, or lung infiltrates >50%), and critical illness (individuals with respiratory failure, septic shock, and/or multiple organ dysfunction).
Institutional review boards at participating VA medical centers approved the study and waived the requirement for informed consent.
Participants were considered fully vaccinated 14 days after completion of a single dose of the Ad.26.COV2.S vaccine or after the second dose of an mRNA vaccine at the time of the SARS CoV2 PCR testing. The Pfizer BNT 162b2 was first available within the VA on December 18, 2020; the Moderna 1273-mRNA on December 27, 2020; and the Ad.26.COV2.S vaccine on March 1, 2021. Because the earliest date of full vaccination of a participant who received Ad.26.COV2.S vaccine was March 15, 2021, this was chosen as the start date of the study. Participants whose SARS CoV2 PCR was performed while unvaccinated or after 1 dose of a vaccine but before full vaccination were considered unvaccinated. Participants were followed up until they had a positive SARS CoV2 PCR, death, or until the end of the study period (October 3, 2021).
The effectiveness of COVID-19 vaccines was assessed with the use of a test-negative design to compare the odds of testing positive for SARS-CoV-2 among vaccinated patients with the odds among unvaccinated patients.
We used propensity score (PS) matching to match test-positive cases and test-negative control subjects on factors associated with the outcome. The propensity scores of having COVID-19 were derived from a logistic regression that adjusted for the participant’s age group; sex; race/ethnicity; location; week of COVID-19 PCR testing; body mass index; comorbidities as measured by the Cirrhosis Comorbidity Index; the presence of diabetes mellitus, hypertension, chronic kidney disease, chronic obstructive pulmonary disease; current tobacco use; alcohol as the cause of liver disease; current alcohol use (as measured by AUDIT-C Score); the severity of liver disease as measured by the electronic Child-Turcotte-Pugh score; and tests of alanine aminotransferase, platelet count, serum creatinine, total bilirubin, international normalized ratio, and MELD-Na closest to the date of PCR testing.
Descriptive statistics were compared between the case and control group for the matched samples before/after PS matching and P values were calculated using Wilcoxon tests comparing median of continuous variables, or chi-square tests for binary and categorical variables.
Supplementary Table 1.
Descriptive Statistics for Study Patients
|
Variables |
Full sample |
Matched sample |
||||
|---|---|---|---|---|---|---|
| Case (n = 968) |
Control (n = 5030) |
P value | Case (n = 955) |
Control (n = 955) |
P value | |
| Vaccination type, n (%) | ||||||
| Unvaccinated | 454 (46.9) | 1544 (30.7) | 446 (46.7) | 281 (29.4) | ||
| Pfizer BNT162b2 mRNA vaccine | 274 (28.3) | 1595 (31.7) | < .0001 | 271 (28.4) | 286 (30.0) | < .0001 |
| Moderna 1273 mRNA vaccine | 199 (20.6) | 1629 (32.4) | 198 (20.7) | 334 (35.0) | ||
| Janssen Ad.26.COV2.S vaccine | 41 (4.2) | 262 (5.2) | 40 (4.2) | 54 (5.6) | ||
| Location, n (%) | ||||||
| Northeast | 102 (10.5) | 763 (15.2) | 101 (10.6) | 107 (11.2) | ||
| Southeast | 241 (24.9) | 954 (19.0) | 237 (24.8) | 217 (22.7) | ||
| Midwest | 190 (19.6) | 1109 (22.1) | < .0001 | 188 (19.7) | 204 (21.4) | .7352 |
| South | 246 (25.4) | 1120 (22.3) | 241 (25.2) | 225 (23.6) | ||
| Northwest | 54 (5.6) | 392 (7.8) | 54 (5.7) | 57 (6.0) | ||
| Southwest | 135 (13.9) | 692 (13.8) | 134 (14.0) | 145 (15.2) | ||
| Sex, n (%) | ||||||
| Male | 925 (95.6) | 4849 (96.4) | .2048 | 923 (96.7) | 923 (96.7) | 1.0000 |
| Female | 43 (4.4) | 181 (3.6) | 32 (3.3) | 32 (3.3) | ||
| Age, y, median (IQR) | 62.1 (10.5) | 62.7 (10.5) | .0831 | 62.2 (10.6) | 62.4 (11.2) | .5306 |
| White, n (%) | 598 (61.8) | 3030 (60.2) | .8353 | 595 (62.3) | 595 (62.3) | 1.0000 |
| BMI, median (IQR) | 30.0 (8.1) | 29.0 (7.8) | < .0001 | 30.0 (8.0) | 30.0 (8.5) | .9635 |
| Diabetes, n (%) | 579 (59.8) | 2481 (49.3) | < .0001 | 574 (60.1) | 550 (57.6) | .2645 |
| Current smoker, n (%) | 586 (60.5) | 3296 (65.5) | .0029 | 577 (60.4) | 563 (59.0) | .5137 |
| Alcohol, n (%) | 254 (26.2) | 1331 (26.5) | .8862 | 250 (26.2) | 239 (25.0) | .5641 |
| AUDIT-C score, n (%) | ||||||
| Low | 768 (79.3) | 3809 (75.7) | .0155 | 759 (79.5) | 754 (79.0) | .7780 |
| High | 200 (20.7) | 1221 (24.3) | 196 (20.5) | 201 (21.0) | ||
| Cirrhosis comorbidity, n (%) | ||||||
| 0 | 97 (10.0) | 483 (9.6) | .0568 | 97 (10.2) | 107 (11.2) | .8551 |
| 1 + 0 | 214 (22.1) | 1346 (26.8) | 205 (21.5) | 200 (20.9) | ||
| 1 + 1 | 259 (26.8) | 1311 (26.1) | 257 (26.9) | 268 (28.1) | ||
| 3 + 0 | 39 (4.0) | 208 (4.1) | 39 (4.1) | 34 (3.6) | ||
| 3 + 1 | 352 (36.4) | 1660 (33.0) | 350 (36.7) | 342 (35.8) | ||
| 5 + 0 | 1 (0.1) | 1 (0.02) | 1 (0.1) | 0 (0.0) | ||
| 5 + 1 | 6 (0.6) | 21 (0.4) | 6 (0.6) | 4 (0.4) | ||
| eCTP class, n (%) | ||||||
| A | 763 (78.8) | 4058 (80.7) | .3952 | 752 (78.7) | 769 (80.5) | 1.0000 |
| B | 182 (18.8) | 856 (17.0) | 180 (18.9) | 167 (17.5) | ||
| C | 23 (2.4) | 116 (2.3) | 23 (2.4) | 19 (2.0) | ||
| Baseline laboratory results, median (IQR) | ||||||
| Alanine aminotransferase, IU/mL | 40.0 (45.2) | 42.0 (49.0) | .1304 | 40.0 (45.5) | 43.0 (47.0) | .0905 |
| Platelet count, × 10E9/L | 147.0 (91.0) | 151.0 (90.0) | .1471 | 147.0 (91.2) | 151.0 (88.8) | .3632 |
| Creatinine, mg/dL | 0.9 (0.4) | 0.9 (0.3) | .1667 | 0.9 (0.3) | 0.9 (0.3) | .5657 |
| Total bilirubin, mg/dL | 0.9 (0.7) | 0.8 (0.7) | .3491 | 0.9 (0.7) | 0.8 (0.7) | .4925 |
| International normalized ratio | 1.1 (0.2) | 1.1 (0.2) | .1373 | 1.1 (0.2) | 1.1 (0.2) | .4592 |
| MELD-Na | 8.0 (6.0) | 8.0 (5.0) | .3463 | 8.0 (6.0) | 8.0 (6.0) | .5567 |
NOTE: Bold values indicate P value < .05.
AUDIT-C, Alcohol Use Disorders Identification Test-Concise; BMI, body mass index; eCTP, electronic Child-Pugh-Turcotte; IQR, interquartile range; MELD-Na, Model for End-Stage Liver Disease adding Serum Na Parameter.
Supplementary Table 2.
VE and Noninferiority Assessment for the Risk of Different COVID-19 Criteria in Patients Who Received Ad.26.COV2.S, BNT162b2/mRNA-1273 Vaccines Versus Unvaccinated Control Subjects
| VE = 1-OR |
Symptomatic COVID-19 | Moderate/severe/critical COVID-19 | Severe/critical COVID-19 | |
|---|---|---|---|---|
| COVID-19 | ||||
| Ad.26.COV2.S | ||||
| Upper confidence limit | 0.64 | 0.68 | 0.7 | 0.72 |
| VE | 0.8 | 0.79 | 0.74 | 0.84 |
| LCL | 0.38 | 0.59 | 0.63 | 0.58 |
| BNT162b2 / mRNA-1273 | ||||
| Upper confidence limit | 0.73 | 0.78 | 0.8 | 0.82 |
| VE | 0.81 | 0.84 | 0.92 | 0.93 |
| LCL | 0.63 | 0.62 | 0.68 | 0.69 |
| 90% NIM | 0.567 | 0.558 | 0.612 | 0.621 |
| Conclude noninferiority LCL (Ad.26.COV2.S) <90% NIM |
No | Yes | Yes | No |
| 75% NIM | 0.4725 | 0.465 | 0.51 | 0.5175 |
| Conclude noninferiority LCL (Ad.26.COV2.S) <75% NIM |
No | Yes | Yes | Yes |
LCL, lower confidence limit; NIM, noninferiority margin; VE, vaccine efficacy.
References
- 1.Shaheen A.A., et al. Clin Gastroenterol Hepatol. 2022;20:e1170–e1179. doi: 10.1016/j.cgh.2021.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.John B.V., et al. JAMA Internal Med. 2022 [Google Scholar]
- 3.WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/table Available at:
- 4.John B.V., et al. Hepatology. 2021;74:879–891. doi: 10.1002/hep.31776. [DOI] [PubMed] [Google Scholar]
- 5.John B.V., et al. Gastroenterology. 2022;162:645–647. doi: 10.1053/j.gastro.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Updates to the VA COVID-19 Shared Data Resource and its Use for Research. Available at: https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/3834-notes.pdf. Accessed January 11, 2022.
- 7.John B.V., et al. Hepatology. 2022;76:126–138. doi: 10.1002/hep.32337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean N.E., et al. N Engl J Med. 2021;385:1431–1433. doi: 10.1056/NEJMe2113151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ai J., et al. Clin Gastroenterol Hepatol. 2022;20:1516–1524. doi: 10.1016/j.cgh.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruether D.F., et al. Clin Gastroenterol Hepatol. 2022;20:162–172. doi: 10.1016/j.cgh.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]