Abstract
Acute cardiac manifestions of COVID-19 have been well described, while chronic cardiac sequelae remain less clear. Various studies have shown conflicting data on the prevalence of new or worsening cardiovascular disease, myocarditis or cardiac dysrhythmias among patients recovered from COVID-19. Data are emerging that show that patients recovering from COVID-19 have an increased incidence of myocarditis and arrhythmias after recovery from COVID-19 compared with the control groups without COVID-19. The incidence of myocarditis after COVID-19 infection is low but is still significantly greater than the incidence of myocarditis from a COVID-19 vaccine. There have been several studies of athletes who underwent a variety of screening protocols prior to being cleared to return to exercise and competition. The data show possible, probable or definite myocarditis or cardiac injury among 0.4–3.0% of the athletes studied. Recent consensus statements suggest that athletes with full recovery and absence of cardiopulmonary symptoms may return to exercise and competition without cardiovascular testing. In conclusion, patients with COVID-19 may be expected to have an increased risk of cardiovascular disease, myocarditis or arrhythmias during the convalescent phase. Fortunately, the majority of patients, including athletes may return to their normal activity after recovery from COVID 19, in the absence of persisting cardiovascular symptoms.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has had a major impact on the practice of cardiovascular medicine. The origin of the outbreak has been traced back to December 2019, but it was not until March 2020 that the World Health Organization declared COVID-19 a pandemic [1]. The infection can be asymptomatic or manifest with a wide spectrum of symptoms, ranging from mild respiratory discomfort to life threatening sepsis [2]. Acute cardiac involvement has been well documented, but its long-term significance is still evolving. One report of concern identified myocardial inflammation in 60% of infected patients during convalescence from COVID-19 [3]. The extent to which COVID-19 causes cardiac abnormalities during the convalescent or chronic phase of recovery remains undetermined.
Many patients infected with SARS-CoV-2 remain symptomatic with a variety of sequelae following the infection and have been described in the lay press as ‘long haulers.’ Chronic respiratory involvement and/or pertubations in autonomic neural function appear more prevalent among the ‘long haulers.’ In this manuscript, the rationale for assessment of acute or chronic cardiac involvement is analyzed, along with the potential long-term sequelae that justify an approach for long term cardiac surveillance and tracking of outcomes. Patients with new or worsening cardiopulmonary symptoms during convalescent phase of COVID19 should have assessment for cardiac injury and ascertainment of cardiac arrhythmias as clinically indicated.
Acute cardiac manifestations of COVID-19
Myocardial involvement, as reflected by elevated troponin levels, has been identified in 20–35% of patients hospitalized with SARS-CoV-2 infection [4,5]. Multiple pathophysiologic mechanisms may be involved in acute cardiac involvement, including myocarditis, secondary effects from cytokine storm, ischemia due to demand-supply mismatch, and thrombosis, among others [6]. The long-term consequences of acute myocardial involvement are unknown [6], as is the incidence of myocardial involvement in patients not hospitalized, presumably with asymptomatic or less severe acute illness.
COVID-19 and acute myocarditis
Multiple viral agents may cause acute myocarditis, including cardiotropic viruses such as adenovirus, enterovirus and others [7,8]. Some viruses trigger myocarditis by immune-mediated mechanisms, including HIV, hepatitis C, and influenza A and B. Acute myocarditis appears to be an infrequently diagnosed complication during the acute phase of COVID-19 [9]. Hospital administrative data have shown that there is a 16-fold greater risk of myocarditis among patients with COVID-19 compared to the COVID-19 negative population [10]. Before SARS CoV2 was identified, myocarditis-associated cardiac injury was rarely associated with coronavirus infections. However, myocardial injury had been described with the SARS CoV virus in 2003 [11], and Middle East Respiratory Syndrome [12] in 2012.
The mechanisms of cardiac injury related to SARS CoV-2 infection are multifactorial, including direct viral myocyte infiltration, induction of thrombosis/inflammation, hyperinflammatory state associated with catecholamine surge, immune activation and cytokine release syndrome [13]. In addition to having potential effects on myocardial function, these abnormalities may also produce the electrophysiologic milieu for acute arrhythmias, including atrial and ventricular arrhythmias, as have been noted during acute SARS-CoV-2 infection.
Vaccine associated myocarditis
There have been several reports demonstrating an association between vaccines for COVID-19 and myocarditis or cardiac arrhythmias, particularly among young males. Data from the British Health Service showed excess risk of myocarditis within 28 days after vaccination with two doses of the mRNA vaccines of 10/million, which compares with an excess risk of 40/million for those with a positive SARS CoV2 test [14]. The excess risk was particularly notable in males under the age of 40 years. In a study from Israel, there was a relative risk of myocarditis of 3.24 (1.55-12.44) with the mRNA vaccine (BNT162b2). However, this is significantly less than the relative risk of 18.28 (3.95-25.12) for those infected with SARS CoV-2. There was an excess of 2.7 cases of myocarditis/100,000 after the vaccine compared with an excess of 11 cases of myocarditis/100,000 patients infected with SARS CoV-2 [15].
In one study of adolescents and young adults, the presentation and clinical course of myocarditis associated with vaccine included presentation with chest pain and elevated troponin levels shortly after vaccination (median of 2 days) [16]. Most patients had an abnormal ECG, including dysrhythmias. No patient died and all patients with follow-up had normalized cardiac function [16]. Vaccine associated myopericarditis has been reported after other vaccines with an incidence of 0.1% of the reports received by the Vaccine Adverse Event Reporting System between 1990 and 2018 [17]. Therefore, although there is an association between COVID-19 vaccines and myocarditis, the available data demonstrate that the incidence of myocarditis is significantly greater when associated with an actual COVID-19 infection rather than the vaccine.
COVID-19 and acute myocardial infarction (AMI)
There have been several reports of AMI-associated with COVID-19. Cytokine storm activates the coagulation cascade, which promotes platelet activation by IL-6 and TNF, and consequent formation of microthrombi [13]. An autopsy study of the hearts of 40 patients with COVID-19 [18] showed that 35% had evidence of cardiac injury, identified by the presence of myocyte necrosis, with 64.3% caused by microthrombi. In addition, COVID-19 may unmask pre-existing CAD among some patients. AMI occurring during the convalescent phase of COVID-19 has been described in case reports [18], [19], [20]. This highlights the importance of adept surveillance to prevent further cardiac complications from the infection.
Cardiac manifestations of COVID-19 during the early convalescent phase
The identification of cardiac injury or myocarditis in asymptomatic or minimally symptomatic patients during the early phase following COVID-19 infection can be challenging. CMR criteria were developed for acute evaluation of symptomatic patients [21]. Moreover, CMR findings consistent with athlete's heart may be a confounding factor in young athletic individuals in whom it is important to properly identify myocarditis. Among middle aged or older adults, co-morbidities such as hypertension, diabetes, or coronary artery disease can affect CMR findings. Lingering cardiac injury or myocarditis in the convalescent phase could have long-term implications, depending on the patterns of healing.
The extent and type of surveillance testing influences the diagnostic yield of cardiac injury or myocarditis in the early convalescent phase. Even timing of the evaluation may be important. In a group of 26 athletes evaluated with cardiac magnetic resonance (CMR) [22], 4 were found to have evidence of myocarditis. Of 3 athletes evaluated <15 days from initial COVID-19 diagnosis, 2 (67%) were diagnosed with myocarditis. Of 23 athletes evaluated ≥15 days following initial diagnosis, 2 (9%) were diagnosed with myocarditis (p < 0.05). This supports the concept that CMR findings may be more likely to show inflammation during the acute phase compared with the convalescent phase.
Early detection of myocardial injury in COVID-19 patients
Early detection of cardiac involvement in post-acute COVID-19 patients may identify patients at risk for cardiomyopathies or arrhythmias. CMR has been proposed as a screening tool for diagnosing myocarditis or subtle myocardial changes [23]. The Lake Louise Criteria (LLC) were developed for diagnosis of acute myocarditis, specifically assessing abnormalities in T1, T2, and late gadolinium enhancement (LGE), with diagnostic accuracy up to 90% [21]. Lake Louise Criteria have moderate positive predictive value with poor diagnostic accuracy in detecting myocarditis during the convalescent phase [24]. Nevertheless, a normal T1 value provides a very high negative predictive value. Elevated T1 relaxation time indicates diseased myocardium (with ≥5 SD higher than normal range for acute and ≥2 SD for residual changes) which helps distinguish between acute and chronic processes, along with detecting subclinically diseased myocardium [25]. In a study from Italy published in 2019, the utility of repeat CMR after acute myocarditis was assessed. The presence of persistent LGE (particularly in the mid-wall septum) at 6 months was the strongest independent predictor of long-term adverse cardiac events at follow-up [26].
Results of studies in COVID-19 among athletes
The natural history of cardiac involvement in the convalescent phase of COVID-19 in patients not requiring hospitalization has been best elucidated in athletes who have been routinely screened for COVID-19 infection, and then subjected to mandatory post-infection cardiac testing prior to being allowed to return to play. Based on multiple studies and screening protocols, data have emerged showing that SARS-CoV2 results in a low incidence of potential cardiac injury/myocarditis among college and professional athletes (Table 1 ). To the extent that there might be myocarditis or other cardiac injury, the risk of adverse cardiac events would be heightened in athletes due to training and competition [27]. Therefore, the data on athletes are relevant to the general population.
Table 1.
Current studies of SARS-CoV-2 positive athletes who underwent CMR evaluation for return-to-play.
| STUDIES | Cohort/Characteristics | Days to CMR | Abnormal CMR | ↑T1 | ↑T2 | LGE | LV Function | RV Function | Pericardial Effusion | Diagnosis of Myocarditis | Resolution of Myocarditis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brito(33) |
54 college athletes 46 (85.2%) men 37 (70%) symptomatic 48 underwent CMR |
27 |
27 (56.3%) | 9 (18.75%) | 0 | 1 (2%) | 59.91% | 53.58% | 28 (58.3%) | Not Reported | Not Studied |
| Clark(31) |
59 college athletes 37 (63%) women 46 (78%) symptomatic 59 underwent CMR |
21.5 | Not Reported | Not Reported | Not Reported | 27 (16%) | 60% | 53% | 1 (2%) | 2 (3%) | Not Studied |
| Daniels(34) |
2461 college athletes 1879 (66.9%) men Symptoms not reported 1597 underwent CMR |
22 | Not Reported | 5 (0.3%) | 31 (1.9%) | 37 (2.3%) | Not Reported | Not Reported | Not Reported | 37 (2.3%) | 100% T2 resolved 43% residual LGE 40.7% resolved LGE 27% pending imaging |
| Martinez(35) |
789 professional athletes 777 (98.5%) men 460 (58.3%) symptomatic 27 underwent CMR |
17 | Not Reported | Not Reported | Not Reported | 3 (11%) | Not Reported | Not Reported | Not Reported | 5 (0.6%) | Not Studied |
| Moulson(36) |
3018 college athletes 965 (32%) women 2022 (67%) symptomatic 317 underwent CMR |
33 | Not Reported | 7 (2%) | 7 (2%) | 12 (4%) | Not Reported | Not Reported | 13 (4%) | 15 (0.7%) | Not Studied |
| Rajpal(22) |
26 college athletes 15 (57.7%) men 12 (26.9%) symptomatic 26 underwent CMR |
11-53 | 46% | 9 (45%) | 13 (50%) | 12 (46%) | 58% | 57% | 2 (8%) | 4 (15%) | Not Studied |
| Mitrani(37) |
174 professional athletes 122 (70%) men 148 (85.1%) symptomatic 28 underwent CMR |
21 | 5 | 2 | 1 | 3 | 56.8% | 43% (3/5 reported) | 3 | (2) 1.1% | 100% |
CMR, cardiovascular magnetic resonance; LGE, late gadolinium enhancement; LV, left ventricle; RV, right ventricle; LVEDV, left ventricular end-diastolic volume
Initial recommendations to screen athletes in the post COVID-19 recovery period included obtaining 12 lead ECGs, hs-troponin levels, and transthoracic echocardiograms, prior to clearance to return-to-play [28,29]. CMR would be reserved for those with abnormal findings. Some groups have used CMR for evaluation in all athletes [22].
One confounding factor is the distinction between physiologic changes associated with athlete's hearts versus pathologic changes related to COVID-19 cardiac injury. Exercise-induced cardiac remodeling occurs as a result of the athletes’ age, gender, type of exercise (resistance versus aerobic) and intensity of training. Biventricular volumes are elevated. On CMR, delayed enhancement seen in the right ventricular insertion points is another common finding [30].
The initial small single center case series among student and professional athletes identified a wide range of abnormal CMR findings, observed among 3–46% of the athletes [22,[31], [32], [33]]. The incidence of elevated T2, a marker for myocardial inflammation and myocarditis was identified among 2–50% of athletes. Moreover, these authors identified a predilection for pericardial effusion and/or delayed enhancement. The wide range in reported patterns was likely due to differences in standards among centers for abnormal T1 and T2 mapping, scanning protocols, and timing of the CMR relative to the COVID-19 infection.
Subsequent large multicenter and single center studies and registries demonstrated a low incidence of myocarditis among athletes recovered from COVID-19 [31,33,34] (Table 1). In a report of 789 professional athletes all of whom had undergone troponin testing, ECGs and resting echocardiograms, a mean of 19 days following diagnosis, there were 30 (3.8%) athletes with abnormal findings [35]. Five (0.6%) athletes underwent CMR and were diagnosed with myocarditis or pericarditis and were restricted from play. In a report of 1,597 athletes from 13 universities in the ‘Big Ten’, who underwent CMR (864 athletes who did not undergo CMR were excluded), 37 (2.3%) were diagnosed with myocarditis, including 28 with subclinical myocarditis and 9 with symptomatic myocarditis [34]. Follow-up CMR scans revealed resolution of T2 abnormalities in 100% and resolution of delayed enhancement in 40.7%.
In a multicenter study by Moulson and colleagues, 2820 athletes who underwent testing with ECGs, troponin measurements and/or TTEs, a diagnosis of at least possible cardiac involvement was made in 0.7% [36]. Among the 198 and 119 athletes undergoing primary CMR (for routine screening) and clinically indicated CMR (based on prior abnormal results), the incidences of cardiac involvement were 3.0%, and 12.6%, respectively. These data support the notion that performing preliminary testing first does stratify risk for abnormal CMR. Performing primary CMR may identify additional athletes with potential or actual COVID-19 related cardiac injury. These data suggest that the incidence of myocarditis or cardiac injury detected during the convalescent phase of COVID-19 is in part related to the intensity of the screening protocols. Given the extremely low incidence of fatal ventricular arrhythmias reported among athletes during the convalescent phase of COVID-19, it is unclear whether a strategy of primary CMR is too sensitive and would identify and unnecessarily restrict too many athletes.
We developed the MIAMI (Multidisciplinary Inquiry of Athletes in Miami In COVID-19 recovery) [37] protocol to include biomarkers and functional testing with exercise echocardiographic stress testing performed at least 14 days post COVID-19 diagnosis. CMR was performed only for those with abnormalities on initial testing. An interdisciplinary panel reviewed each athletes’ results. All subjects in our cohort had normal troponin and resting left ventricular (LV) function during baseline testing performed a median of 18.5 days after COVID-19 diagnosis . Twenty-eight of the 174 (16.1%) athletes underwent CMR based on initial screen, and 5 of 174 (2.9%) athletes were confirmed by CMR with possible, probable or definite or myocarditis. Two (1.1%) of these 5 athletes had definite myocarditis. In the MIAMI protocol, the 5 (2.9%) of 174 patients with possible, probable or definite myocarditis had an abnormality in their EKG, echocardiogram and/or stress test which triggered the need for a CMR. Our data are in line with single center and multicenter studies and suggest a potential protocol that is sufficiently sensitive to identify potential COVID-19 associated cardiac injury without having to pursue CMR imaging in all subjects.
Convalescent phase cardiac manifestations
Symptoms related to COVID-19 may extend into the convalescent and chronic post-COVID-19 periods. Post-Acute COVID syndrome, generally includes symptoms such as fatigue and shortness of breath beyond 3-4 weeks after acute infection [38]. The majority of patients do not have clinically detectable cardiac involvement. Some of the preliminary studies assessing persistence of cardiopulmonary symptoms are described in Table 2 [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49]. These studies had limited followup, ranging from a few weeks to several months. Moreover, there was a preponderance of hospitalized patients in these cohorts. It should be noted that cardiac testing was not routinely performed in many studies.
Table 2.
Current studies describing symptom and disease manifestations in the post-COVID-19 period.
| STUDIES | F/U | F/U Time | n | CP* | Palpitations* | SOB* | Inc HR* | Arrhythmia* | Syncope* | HTN* | CM* | Pericarditis* | Myopericarditis* | Myocarditis* | DD* | PulmHTN* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carfi (42) | F2F | 60d | 143 | 21.7 | - | 43.4 | - | - | - | - | - | - | - | - | - | - |
| Carvahlo (43) | Tele | 30d; 60d |
150 | 18.0 13.1 | 6.5; 10.9 |
10.7; 7.7 | - | - | - | - | - | - | - | - | - | - |
| Garrigues (44) | Tele | 3-4m | 120 | 10.8 | - | 41.7 | - | - | - | - | - | - | - | - | - | - |
| Halpin (45) | Tele | 4-8w | 68h 32I |
- | - | 42.6 65.6 |
- | - | - | - | - | - | - | - | - | - |
| Chopra(46) | Tele | 60d | 488 | - | - | 16.5 | - | - | - | - | - | - | - | - | - | - |
| Fayol(41) | F2F | 6m | 48 | 6 | - | 56 | - | - | - | - | - | - | - | - | 8 | 6.8 |
| Davis (38) | Survey | 1-6m | 3762 | 54.7 | 68.8 | 77.4 | - | 62.9 | 12.9 | - | - | - | - | - | - | - |
| Huang (40) | F2F | 6m | 1733 | 5 | 9 | 23 | - | - | - | - | - | - | - | - | - | - |
| Dennis(47) | Survey | 4w | 201 | 76 | - | 88 | - | - | - | - | 9 | - | - | 19.4** | - | - |
| Xiong(48) | Tele | 3m | 538 | 12.3 | 4.8 | 26.1 | 11.2 | - | - | - | - | - | - | - | - | - |
| Eiros(39) | F2F | 4.4-10.4w | 139 | 19 | 14 | 26 | - | 26 | - | - | 5 | 3 | 11 | 26 | - | - |
| Finn(49) | F2F | 1-11m | 13 | - | - | - | - | - | - | - | 38.4 | - | - | - | - | - |
F2F- face to face; h-hospitalized but not in ICU; I-ICU admission; Tele- telephone survey; w- weeks; d- days; m-months; *All findings are reported as percentages; ** This may be falsely elevated as the study used abnormal T1 on CMR as the sole criteria to diagnose myocarditis.
Among the studies that examined cardiac manifestations during the convalescent phase of COVID-19, some suggested a high incidence of myocarditis or pericarditis during the convalescent phase of COVID-19 [39,50]. Eiros reported an incidence of myocarditis or pericarditis of up to 30.9% in healthcare workers (mean age 52 years) assessed by CMR 10 weeks post index infection [39]. The patients in this cohort were not hospitalized. The CMR criteria for myocarditis or pericarditis included T1 and/or T2 measurements. Another study by Puntmann et al. [50] also identified worrisome findings in a cohort of adult patients recovered from COVID-19. CMR was performed a mean of 71 days following COVID-19 diagnosis in 100 patients, 67% of whom were not hospitalized. CMR abnormalities were identified in 78% of patients. Abnormal T1 and T2 were identified in 60% of patients. A minority of patients had pre-existing cardiovascular disease and/or cardiac risk factors. Finally, a small study from China examined 34 patients with CMR, 6 months after the patients were hospitalized due to COVID-19 [51]. Among patients with cardiac abnormalities during the acute phase, the 6 month follow-up CMR showed 70% of patients had persistent myocardial edema and 100% had CMR evidence for fibrosis.
However, other studies have shown much lower incidence of myocarditis and its sequelae. In a case-control study, 74 healthcare workers seropositive for SARS CoV2 with mild or asymptomatic COVID-19 infection underwent CMR 6 months post infection. The control group included 75 age-, sex-, and ethnicity-matched seronegative healthcare workers. The prevalence of abnormalties on CMR or biomarkers was low and not significantly different between groups [52]. The investigators assessed left ventricular ejection fraction, global longitudinal shortening, and CMR-measurements of T1 and T2. Interestingly, there were findings of delayed gadolinium enhancement in 9% of the entire cohort, with no difference between controls and those who had COVID-19. The authors concluded that in patients with asymptomatic or mild infection with COVID-19, there was no measurable impact on left ventricular function, structure or fibrosis.
Analysis of the English National Immunization database was done in order to determine excess frequency of vaccine-associated myocarditis or pericarditis [14] They also studied the frequency of excess myocarditis or pericarditis following infection with COVID-19. In this analysis, the authors identified only an extra 40 cases of myocarditis per 1 million patients in the 28 days following index infection [14].
In a prospective, multicenter observational study from Austria, 145 patients, mean age 57 years had structured follow-up visits at 60 and 100 days post-COVID-19 infection. The visits included history and physical exam, lung function testing, echocardiography and thoracic low-dose CT scan. In this cohort, 75% of patients had been hospitalized and 40% of patients had pre-existing cardiovascular disease. During follow-up, 36% of patients still complained of persistent dyspnea, including 4% with severe dyspnea [53]. The main findings of this study were the demonstration of persistent CT scan abnormalities, as well as impaired lung function testing, in up to 42% of patients. Echocardiographic data at 60 and 100 days showed a high prevalence of diastolic dysfunction (60% and 55%, respectively). There was systolic dysfunction in only 3% at 60 and 100 days, and pericardial effusion in 6% at 60 days which decreased to 1% at 100 days. These findings suggest that the post COVID-19 pulmonary function test abnormalities likely accounted for persisting symptoms of dyspnea in a cohort of patients that had been hospitalized. The extent to which the patients’ abnormal cardiac findings were new or exacerbated by COVID-19, and to what extent they caused symptoms, is unclear.
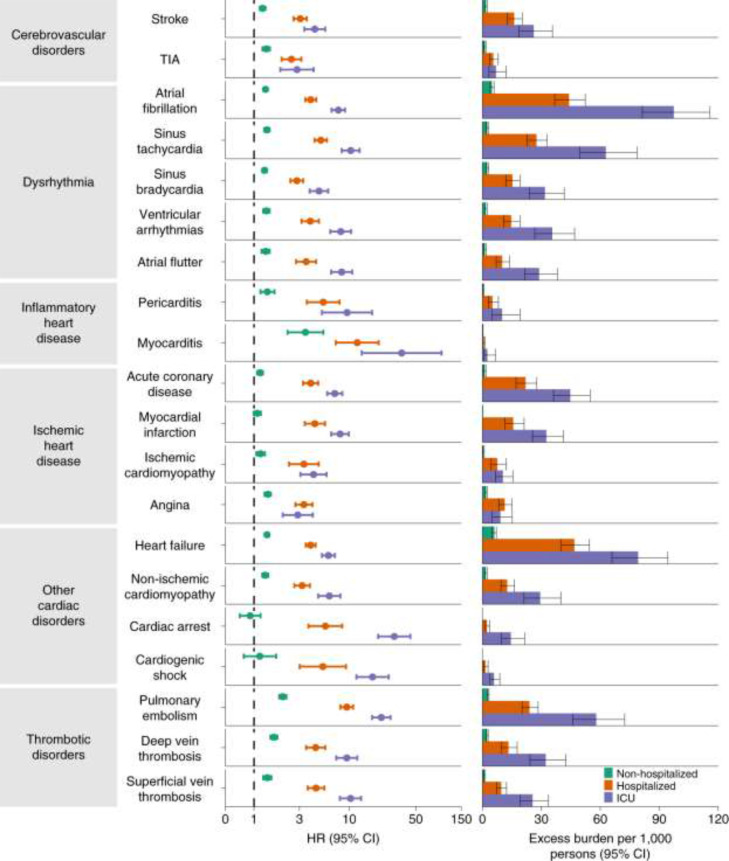
The clinical relevance of the diagnostic studies showing evidence of cardiac injury or myocarditis in the convalescent phase of COVID-19 in some patients is demonstrated by the prognostic impact in a recent study from the Veterans Affairs database which showed an increased incidence of cardiovascular disease among veterans with COVID-19 [54] (Fig. 1 ). This large data set compared 153,760 veterans with COVID-19 to 2 sets of controls (contemporary and historical), each consisting of over 5 million veterans. Outcomes were assessed after 30 days post-infection to one year follow-up. The groups were matched. The outcomes showed increases in several cardiovascular endpoints, including stroke (HR 1.5), heart failure (HR1.7), cardiomyopathy (HR 1.6), myocarditis (HR 5.38) and pericarditis (HR 1.85). An analysis of major adverse cardiovascular events (myocardial infaction, stroke all-cause mortality) identified an excess burden of 23.5 /1000 patients with COVID-19 infection. The 12 month burden of cardiovascular disease, inflammatory heart disease, and ischemic heart disease showed that the hazard ratio and excess disease burden were related to the acuity of the initial infection (Fig. 1). Whereas patients with COVID-19 who were not hospitalized had excess cardiovascular disease burden, those who were hospitalized had substantially more disease burden and those who required intensive care subsequently had the highest hazard ratio and excess disease burden.
Fig. 1.
Observational data from VA database showed increase in cardiovascular, and dysrhythmic disorders in veterans with COVID-19 compared with matched Veterans without COVID-19. Risks and excess burdens were assessed at 12 months stratified by acuity of initial COVID-19 infection: non-hospitalized (green), hospitalized but not in intensive care for COVID-19 (orange) and admitted to intensive care (blue). Within the COVID-19 cohort, non-hospitalized (n = 131,612), hospitalized (n = 16,760), admitted to intensive care (n = 5388) were compared with contemporary control cohort (n = 5,637,647). Adjusted HRs and 95% CIs are presented. The length of the bar represents the excess burden per 1000 persons at 12 months.
In summary, data are now emerging on new or worsening cardiovascular abnormalities following COVID-19. Among patients with mild courses of COVID-19 infection, there appears to be a low incidence of new significant cardiac disease, while patients who had acute cardiac manifestations may have detectable cardiac abnormalites during long-term followup. In addition, there is potential for COVID-19 to accelerate or aggravate pre-existing cardiac disease. Given the enormous population burden of COVID-19 infection, even a low incidence of cardiac issues may represent a large number of patients. The presence of pre-existing disease (hypertension, subclinical CAD), the co-existance of persistent pulmonary disease related to COVID-19 and possibly the persistence of an enhanced inflammatory state needs to considered when following and managing these patients.
COVID-19 and arrhythmias
Cardiac arrhythmias are commonly present with COVID-19 [55] often manifested clinically by palpitations. Numerous studies have reported atrial arrhythmias, including atrial fibrillation (AF)/flutter and supraventricular tachycardias (SVT), bradyarrhythmias, including sinus bradycardia and atrioventricular blocks (AVB), ventricular arrhythmias (VA), and sudden cardiac death (SCD) associated with COVID-19 infection [55], [56], [57], [58], [59], [60]. In one study of 270 patients with COVID-19 and arrhythmia, AF or flutter was noted in 166 patients, including 101 patients with newly diagnosed atrial arrhythmia [56]. This same study reported that patients with arrhythmias had significantly more in-hospital complications, and that any arrhythmia was independently associated with 30-day mortality.
Over the course of the pandemic, atrial arrhythmias have been the most prevalent arrhythmia noted in patients with COVID-19. In an analysis of 4526 patients hospitalized with COVID-19 early in the pandemic in 2020, 827 (18.3%) patients developed arrhythmias, predominantly atrial arrhythmias [55]. This is consistent with findings from an earlier Heart Rhythm Society survey sent to electrophysiologists worldwide, reporting atrial fibrillation as the most commonly seen tachyarrhythmia [57].
The incidence of bradyarrhythmias varies across studies, but in one study of hospitalized patients early in the pandemic, bradyarrhythmias were noted in 22.6% of COVID-19 patients [55]. Published case reports describe both transient and persistent sinus node dysfunction, as well as AV block. Five (71%) of 7 patients presenting with bradyarrhythmias during COVID-19 infection died during or within 3 months of admission [58]. While bradyarrhythmias may be transient in some, other patients have required implantation of a cardiac implantable electrical device (CIED). Among patients hospitalized with COVID-19, one study, utilizing surveys, identified a crude rate of CIED implant of 1.16 per 100,000 hospitalized patients [61]. The predominant indications for CIED were high degree AV block (65%), sick sinus syndrome (21%), device generator change (4.2%), and secondary prevention for sudden death (4.8%). The 30 day complication rate was substantial at 13.9% and with a mortality rate of 9.6% among the 166 CIED procedures in this report. Patients with new CIED during their acute illness need to be followed to determine whether they develop new cardiomyopathies related to the COVID-19, or right ventricular pacing, and also to determine whether they improve such that they no longer have the indication that promped the CIED during their hospitalization.
There has been a low incidence of life-threatening arrhythmias associated with acute COVID-19. However, among those patients hospitalized with COVID-19 with concomitant diagnosis of VT, the mortality rate has been substantially higher [59]. In a study by Russo et al. [59], among 414 patients hospitalized with COVID-19, ventricular tachycardia (VT) occurred in 3.4%, and of the patients who did not survive, the incidence of VT was 8.4%.
During the pandemic, sudden cardiac death (SCD) and, specifically, out-of-hospital cardiac arrest has significantly increased [60]. One study demonstrated a threefold increase in cardiac arrest in New York City [60]. The SARS CoV-2 virus can directly affect the inflammatory and coagulation pathways eliciting endothelial damage, plaque destabilization, and ultimately SCD and out-of-hospital cardiac arrest (OHCA) [62]. These factors along with the societal impact of the pandemic, including, but not limited to, stay-at-home orders, overburdening of the EMS system and increased emergency response time, decrease in bystander CPR, time attempted to achieve ROSC and an overwhelming fear of seeking medical care, have all contributed to the increases seen in OHCA [63].
Potential arrhythmic sequelae during the convalescent phase
The long-term cardiac arrhythmic sequelae of COVID-19 are currently unknown, but may be anticipated to depend on the extent of acute cardiac injury and the host response/recovery to the acute infection. Patients who have clinically manifest cardiac involvement can be evaluated and treated as any patient with structural heart disease. The challenging scenario is the patient with subclinical disease. Myocarditis may have a heterogeneous clinical presentation with a heterogeneous pattern of recovery (Fig. 2 ). Moreover, the direct and indirect effects of COVID-19 on progression of pre-existing cardiovascular disease needs to be considered.
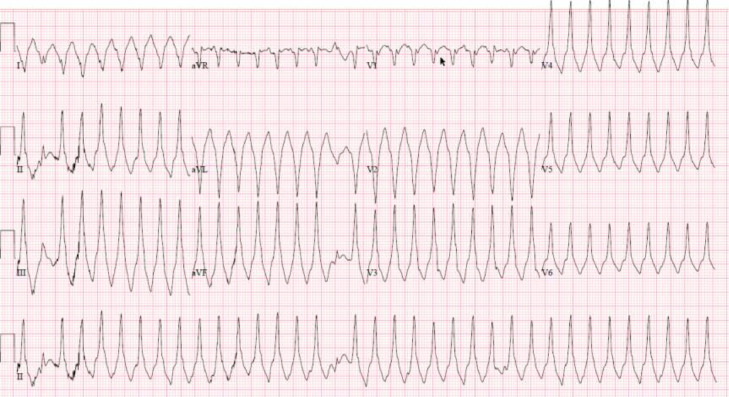
Fig 2.
This ECG is from a 35 year old woman who had a mild course of COVID 19 six weeks prior to presentation with syncope and incessant ventricular tachycardia. Subsequent CMR evaluation showed elevation elevation in T1 and T2 with delayed enhancement in a nonischemic pattern consistent with myocarditis. The patient was treated with steroids, beta blockers and amiodarone. CMR-cardiac MRI
There are limited studies on arrhythmias during the convalescent phase of COVID-19. One review from the VA health system showed a higher arrhythmia burden among veterans who had COVID-19 (RR 1.7) [64]. In another study from the VA database [54], there was a significant increase in dysrhythmias and cardiac arrest between 30 days and 12 months following infection (Fig. 1) [54]. The risk was proportional to severity of illness stratified by non-hospitalized, hospitalized and ICU status (Fig. 1) [54]. Cardiac arrest was not increased for patients who were not hospitalized, but there was an increased hazard ratio and excess burden among those hospitalized, particularly those patients requiring critical care. Atrial fibrillation, atrial flutter and undefined ventricular arrhythmias were increased among all patients post COVID -19, with excess burden proportional to the severity of the disease.
Studies in patients with myocarditis not related to COVID-19 may provide clues as to potential sequelae from SARS-CoV-2 infection. Multiple series of patients with myocarditis show diminished survival and sudden cardiac death [65,66]. A case report describes a patient recovered from myocarditis who had a monitored ventricular fibrillation event two months later when left ventricular function was normal [67]. Peretto et al. [68] recently reported on ventricular arrhythmias in 185 patients with active (n = 123) versus previous (n = 62) myocarditis and ventricular arrhythmia; mean ejection fraction was 47–50%. From a different perspective, autopsy series [68], [69], [70] of patients with sudden cardiac death have found myocarditis as a potential explanation in a significant number of cases, even in the setting of a grossly normal appearing heart. The finding of myocarditis as a significant cause of sudden death in the young [70] is notable and relevant to survivors of COVID-19.
One study from the Veterans Administration database compared cardiovascular diagnoses among veterans hospitalized with COVID-19 to those hospitalized with seasonal influenza after six months (Table 3 ). Interestingly, there was a higher incidence of myocarditis among patients hospitalized with seasonal influenza compared with patients hospitalized with COVID-19. However, there was a trend for more dysrhythmias and there was a higher incidence of cardiac arrest at 6 months among patients hospitalized with COVID-19 compared with seasonal influenza [54].
Table 3.
Comparison of incident rate at 6 months in patients hospitalized with COVID-19 compared with patients hospitalized with seasonal influenza.
| Diagnosis | P value | Hazard ratio (95% Confidence interval) | Incident rate per 1000 at 6-months in hospitalized COVID-19 (95% confidence interval) | Incident rate per 1000 at 6-months in hospitalized seasonal influenza (95% confidence interval) |
|---|---|---|---|---|
| Myocarditis and cardiomyopathy | 0.002 | 0.75 (0.63, 0.90) | 23.61 (19.77, 28.19) | 31.30 (26.23, 37.34) |
| Pericarditis/pericardial disease | 0.20 | 1.27 (0.88, 1.85) | 6.69 (4.62, 9.70) | 5.27 (3.63, 7.64) |
| Conduction disease | 0.84 | 0.98 (0.82, 1.17) | 27.15 (22.76, 32.37) | 27.66 (23.19, 32.98) |
| Cardiac dysrhythmias | 0.089 | 1.10 (0.99, 1.23) | 85.92 (77.31, 95.44) | 78.40 (70.51, 87.13) |
| Cardiac Arrest | 0.020 | 1.58 (1.07, 2.32) | 6.38 (4.35, 9.36) | 4.05 (2.76, 5.94) |
| Heart failure | 0.20 | 0.93 (0.82, 1.04) | 69.85 (62.39, 78.17) | 75.24 (67.23, 84.17) |
Adapted from Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med (2022). https://doi.org/10.1038/s41591-022-01689-3. Permission granted by Creative Commons License: http://creativecommons.org/licenses/by/4.0/.
While myocarditis is typically considered for its manifestations on the ventricle, the atrium is also involved [71]. A Taiwanese study 72] found an adjusted odds ratio of 1.182 (p = 0.03) for developing atrial fibrillation in individuals who had had influenza versus those who did not. This is consistent with the finding of increased atrial fibrillation among veterans with COVID-19 between 30 days and 1 year post infection [54] (Fig. 1).
These findings support the notion that patients who have “recovered” from myocarditis may still have sequelae that predispose to developing atrial fibrillation or lethal ventricular arrhythmias in follow-up. While the excess incidence is likely very low, the high population burden of COVID-19 will translate to a substantial excess prevalence.
Treatment and follow-up of COVID-19 related myocarditis
Treatment of COVID-19 related myocarditis is based on limited clinical trials performed since the beginning of the pandemic [73]. Patients with non-viral myocarditis may benefit from immunosuppressive therapy [74]. On the other hand, viral myocarditis treatment depends on virus isolation at endomyocardial biopsy as some viruses have specific anti-viral therapies [8,75]. Nonsteroidal anti-inflammatory drugs may cause renal impairment, sodium retention, and further aggravate acute ventricular dysfunction in those with myocarditis and therefore are not generally recommended [76]. In fulminant myocarditis, stabilizing the patient with inotropes and/or vasopressors may be necessary at the beginning of treatment [77]. For COVID-19 myocarditis, expert opinion recommends high dose steroids and intravenous immunoglobulins (IVIG) based on the idea that in late stages of COVID-19 there is a diffuse non-specific immune activation [4,73]. Different anti-viral agents were evaluated for hospitalized patients with COVID-19 such as remdesivir, hydroxychloroquine, lopinavir/ritonavir, and interferon beta-1α. These drugs had little effect on decreasing mortality and duration of hospital stay. The efficacy of anti-viral agents for COVID-19 myocarditis is still lacking [73], and emerging potent antiviral drugs (e.g. molnupiravir and paxlovid) have not yet been specifically tested for the indication of acute COVID-19 myocarditis.
Treatment of convalescent heart failure remains directed by the current guidelines for heart failure patients. Low dose beta blockers should be considered among all patients recovering from myocarditis [78]. Avoiding QT prolonging agents is recommended [79].
In terms of exercise restriction, it is recommended to abstain from exercise for a minimum of 3 months after a diagnosis of acute myocarditis, depending on recovery of the patient. Based upon the Eligibility and Disqualification Recommendations for Competitive Athletes with Cardiovascular Abnormalities Task Force 3, [80] before returning to sports, athletes diagnosed with myocarditis should undergo a resting echocardiogram, 24-h Holter monitoring, and an exercise 12-lead ECG within 3-6 months of diagnosis. They are eligible to return to play if the left ventricular ejection fraction and serum biomarkers have normalized, and if no clinically relevant arrhythmias are detected on Holter and ECG [29]. If a CMR was used to make the initial diagnosis of myocarditis, repeat CMR scanning may be reasonable to help guide clinical decision making regarding athletes’ return to participation [81]. Consideration should be given to managing individuals who partake in moderate to high intensity exercise, but are not competitive athletes, in the same way.
Future directions
The optimal screening protocol for patients recovered from COVID-19 is dependent on severity of the illness associated with COVID-19, pre-existing cardiovascular disease or risk factors for cardiovascular disease, and presence of new or ongoing symtpoms after recovery from the acute illness. The virulence of prevailing viral strains may also impact guidelines and recommendations.
Data from competitive professional or collegiate athletes demonstrated a very low incidence of COVID-19 related cardiac injury. This suggests that among young healthy adults with COVID-19 not requiring hospitalization, the risk for convalescent phase cardiac injury is low. Per the most recent recommendations by the ACC Sports and Exercise section on January 24, 2022, and based on recent Expert Consensus Decision Pathway [82], an individualized screening approach is recommended based on presence of persisting cardiopulmonary symptoms. Our recommendations are similar to the ACC recommendations although we include an option for exercise testing in select patients (Fig. 3 ). For most athletes, with mild or asymptomatic course of COVID-19 who recover, they may return to play without further cardiac testing after a brief period of convalescence (currently 5 days). For those with moderate or severe illness or those with persisting cardiopulmonary symptoms, a longer duration of exercise restriction is recommended and cardiac testing could be considered. For nonathletes, it would be prudent to consider pre-existing disease (hypertension, diabetes, etc), functional status, and persistence of symptoms to help guide cardiac testing. For patients with arrhythmic symptoms, cardiac monitoring can be performed. Given the overlap of autonomic disorders with cardiac symptoms, a baseline assessment of orthostatic blood pressure and heart rate may be helpful [82]. Formal head-up tilt testing can also be considered.
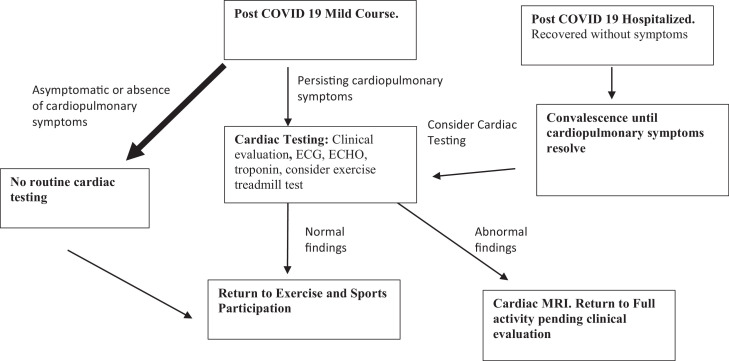
Fig. 3.
Most patients recovered from COVID 19 will be asymptomatic or will not have cardiopulmonary symptoms. For these athletes, clinical testing is not required. For athletes with persisting cardiopulmonary symptoms, we recommend clinical evaluation with triad of testing (biomarkers, ECG, and echocardiogram with consideration for exercise treadmill test). For athletes who were hospitalized with COVID-19, a period of convalescence followed by cardiac testing, depending on presence of cardiopulmonary symptoms may be required before allowing the patient to return to exercise and competition.
While cardiac MRI scans can detect subclinical myocarditis or other cardiac injury, it would be impractical to perform CMRs in a large population of COVID-19 survivors. Nevertheless, given over 84 million documented cases of COVID infections in the United States (as of May 17, 2022), there should be a low threshold to proceed with CMR when clinical data suggest cardiac abnormalities. Diagnosis of myocarditis and/or cardiac injury may guide recommendations on resumption of exercise and may also guide potential pharmacologic therapy (beta blockers and RAAS inhibitors) and need for long-term follow-up and evaluation.
Disclosures
All authors report that they have no conflicts of interest and no relationships relevant to the contents of this paper to disclose. Dr. Joshua Hare reported having a patent for cardiac cell-based therapy. He holds equity in Vestion Inc. and maintains a professional relationship with Vestion Inc. as a consultant and member of the Board of Directors and Scientific Advisory Board. JMH is the Chief Scientific Officer, a compensated consultant and advisory board member for Longeveron, and holds equity in Longeveron. JMH is also the co-inventor of intellectual property licensed to Longeveron. Longeveron LLC and Vestion Inc. did not participate in funding this work. Dr. Hare...s relationships are disclosed to the University of Miami, and a management plan is in place.
References
- 1.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 3.Puntmann V.O., Carerj M.L., Wieters I., Fahim M., Arendt C., Hoffmann J., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitrani R.D., Dabas N., Goldberger J.J. COVID-19 cardiac injury: implications for long-term surveillance and outcomes in survivors. Heart Rhythm. 2020;17:1984–1990. doi: 10.1016/j.hrthm.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tschöpe C., Ammirati E., Bozkurt B., Caforio A.L.P., Cooper L.T., Felix S.B., et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18:169–193. doi: 10.1038/s41569-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trachtenberg B.H., Hare JM. Inflammatory cardiomyopathic syndromes. Circ Res. 2017;121:803–818. doi: 10.1161/CIRCRESAHA.117.310221. [DOI] [PubMed] [Google Scholar]
- 9.Sawalha K., Abozenah M., Kadado A.J., Battisha A., Al-Akchar M., Salerno C., et al. Systematic review of COVID-19 related myocarditis: insights on management and outcome. Cardiovasc Revasc Med. 2021;23:107–113. doi: 10.1016/j.carrev.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boehmer T.K., Kompaniyets L., Lavery A.M., Hsu J., Ko J.Y., Yusuf H., et al. Association between COVID-19 and myocarditis using hospital-based administrative data - United States, March 2020-January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228–1232. doi: 10.15585/mmwr.mm7035e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oudit G.Y., Kassiri Z., Jiang C., Liu S., Poutanen J.M., Penninger, et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Investig. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alhogbani T. Acute myocarditis associated with novel middle east respiratory syndrome coronavirus. Ann Saudi Med. 2016;36:78–80. doi: 10.5144/0256-4947.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patone M., Mei X.W., Handunnetthi L., Dixon S., Zaccardi F., Shankar-Hari M., et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410–422. doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barda N., Dagan N., Ben-Shlomo Y., Kepten E., Waxman J., Ohana R., et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Truong D.T., Dionne A., Muniz J.C., McHugh K.E., Portman M.A., Lambert L.M., et al. Clinically suspected myocarditis temporally related to COVID-19 vaccination in adolescents and young adults: suspected myocarditis after COVID-19 vaccination. Circulation. 2022;145:345–356. doi: 10.1161/CIRCULATIONAHA.121.056583. [DOI] [PubMed] [Google Scholar]
- 17.Su J.R., McNeil M.M., Welsh K.J., Marquez P.L., Ng C., Yan M., et al. Myopericarditis after vaccination, vaccine adverse event reporting system (VAERS), 1990–2018. Vaccine. 2021;39:839–845. doi: 10.1016/j.vaccine.2020.12.046. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrini D., Kawakami R., Guagliumi G., Sakamoto A., Kawai K., Gianatti A., et al. Microthrombi as a major cause of cardiac injury in COVID-19: a pathologic study. Circulation. 2021;143:1031–1042. doi: 10.1161/CIRCULATIONAHA.120.051828. [DOI] [PubMed] [Google Scholar]
- 19.Jain V., Gupta K., Bhatia K., Bansai A., Arora S., Khandelwal A.K., et al. Management of STEMI during the COVID-19 pandemic: lessons learned in 2020 to prepare for 2021. Trends Cardiovasc Med. 2021;31:135–140. doi: 10.1016/j.tcm.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranard L.S., Engel D.J., Kirtane A.J., Masoumi A. Coronary and cerebral thrombosis in a young patient after mild COVID-19 illness: a case report. Eur Heart J Case Rep. 2020;4:1–5. doi: 10.1093/ehjcr/ytaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cundari G., Galea N., De Rubeis G., et al. Use of the new lake louise criteria improves CMR detection of atypical forms of acute myocarditis. Int J Cardiovasc Imaging. 2021;37:1395–1404. doi: 10.1007/s10554-020-02097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajpal S., Tong M.S., Borchers J., Zareba K.M., Obarsi T.P., Simonetti O.P., et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6:116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vago H., Szabo L., Dohy Z., Merkely B. Cardiac magnetic resonance findings in patients recovered from COVID-19: initial experiences in elite athletes. JACC Cardiovasc Imaging. 2021;14:1279–1281. doi: 10.1016/j.jcmg.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lurz P., Eitel I., Adam J., Steiner J., Grothoff M., Desch S., et al. Diagnostic performance of CMR imaging compared with EMB in patients with suspected myocarditis. JACC Cardiovasc Imaging. 2012;5:513–524. doi: 10.1016/j.jcmg.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 25.Hinojar R., Foote L., Sangle S., Marber M., Mayr M., Carr-White G., et al. Native T1 and T2 mapping by CMR in lupus myocarditis: disease recognition and response to treatment. Int J Cardiol. 2016;222:717–726. doi: 10.1016/j.ijcard.2016.07.182. [DOI] [PubMed] [Google Scholar]
- 26.Aquaro G.D., Ghebru Habtemicael Y., Camastra G., Monti L., Dellegrottaglie S., Moro C., et al. Prognostic value of repeating cardiac magnetic resonance in patients with acute myocarditis. J Am Coll Cardiol. 2019;74:2439–2448. doi: 10.1016/j.jacc.2019.08.1061. [DOI] [PubMed] [Google Scholar]
- 27.Harmon K.G., Asif I.M., Maleszewski J.J., Owens D.S., Prutkin J.M., Salerno J.C., et al. Incidence, cause, and comparative frequency of sudden cardiac death in national collegiate athletic association athletes: a decade in review. Circulation. 2015;132:10–19. doi: 10.1161/CIRCULATIONAHA.115.015431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phelan D., Kim J.H., Elliott M.D., Wasfy M.M., Cremer P., Johri A.M., et al. Screening of potential cardiac involvement in competitive athletes recovering from COVID-19: an expert consensus statement. JACC Cardiovasc Imaging. 2020;13:2635–2652. doi: 10.1016/j.jcmg.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J.H., Levine B.D., Phelan D., Emery M.S., Martinez M.W., Chung E.H., et al. Coronavirus disease 2019 and the athletic heart: emerging perspectives on pathology, risks, and return to play. JAMA Cardiol. 2021;6:219–227. doi: 10.1001/jamacardio.2020.5890. [DOI] [PubMed] [Google Scholar]
- 30.Domenech-Ximenos B., Sanz-de la Garza M., Prat-González S., Sepúlveda-Martínez A., Crispi F., Duran-Fernandez K., et al. Prevalence and pattern of cardiovascular magnetic resonance late gadolinium enhancement in highly trained endurance athletes. J Cardiovasc Magn Reson. 2020;22:62. doi: 10.1186/s12968-020-00660-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark D.E., Parikh A., Dendy J.M., Diamond A.B., George-Durrett K., Fish F.A., et al. COVID-19 myocardial pathology evaluation in athletes with cardiac magnetic resonance (COMPETE CMR) Circulation. 2021;143:609–612. doi: 10.1161/CIRCULATIONAHA.120.052573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malek L.A., Marczak M., Milosz-Wieczorek B., Kanopka M., Braksator W., Drygas W., et al. Cardiac involvement in consecutive elite athletes recovered from COVID-19: a magnetic resonance study. J Magn Reson Imaging. 2021;53:1723–1729. doi: 10.1002/jmri.27513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brito D., Meester S., Yanamala N., Patel H.B., Balcik B.J., Casaclang-Verzosa G., et al. High prevalence of pericardial involvement in college student athletes recovering from COVID-19. JACC Cardiovasc Imaging. 2021;14:541–555. doi: 10.1016/j.jcmg.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daniels C.J., Rajpal S., Greenshields J.T., Rosenthal G.L., Chung E.H., Terrin M., et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: results from the big ten COVID-19 cardiac registry. JAMA Cardiol. 2021;6:1078–1087. doi: 10.1001/jamacardio.2021.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez M.W., Tucker A.M., Bloom O.J., Green G., DiFiori J.P., Solomon G., et al. Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening. JAMA Cardiol. 2021;6:745–752. doi: 10.1001/jamacardio.2021.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moulson N., Petek B.J., Drezner J.A., Harmon K.G., Kliethermes S.A., Patel M.R., et al. SARS-CoV-2 cardiac involvement in young competitive athletes. Circulation. 2021;144:256–266. doi: 10.1161/CIRCULATIONAHA.121.054824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitrani R.D., Alfadhli J., Lowery M.H., Best T.M., Hare J.M., Fishman J., et al. Utility of exercise testing to assess athletes for post COVID-19 myocarditis. Am Heart J Plus Cardiol Res Pract. 2022;14 doi: 10.1016/j.ahjo.2022.100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis H.E., Assaf G.S., McCorkell L., Wei H., Low R.J., Re'em Y., et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eiros R., Barreiro-Pérez M., Martín-García A., Villacorta E., Pérez-Pons A., Merchán S., et al. Pericardial and myocardial involvement after SARS-CoV-2 infection: a cross-sectional descriptive study in healthcare workers. Rev Esp Cardiol. 2021 doi: 10.1016/j.rec.2021.11.001. (Engl Ed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fayol A., Livrozet M., Boutouyrie P., Khettab H., Betton M., Tea V., et al. Cardiac performance in patients hospitalized with COVID-19: a 6 month follow-up study. ESC Heart Fail. 2021;8:2232–2239. doi: 10.1002/ehf2.13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carvalho-Schneider C., Laurent E., Lemaignen A., Beaufils E., Bourbao-Tournois C., Laribi S., et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27:258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garrigues E., Janvier P., Kherabi Y., Le Bot A., Hamon A., Gouze H., et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81:e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halpin S.J., McIvor C., Whyatt G., Adams A., Harvey O., McLean L., et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93:1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 46.Chopra V., Flanders S.A., O'Malley M., Malani A.N., Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174:576–578. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dennis A., Wamil M., Alberts J., Oben J., Cuthbertson D.J., Wooton D., et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiong Q., Xu M., Li J., Liu Y., Zhang J., Xu Y., et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27:89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finn A., Jindal A., Selvaraj V., Authelet N., Gutman N.H., Dapaah-Afriyie K. Presentations and outcomes of severe cardiac complications in COVID-19: Rhode Island experience. R I Med J. 2021;104:8–13. (2013) [PubMed] [Google Scholar]
- 50.Puntmann V.O., Carerj M.L., Wieters I., Fahim M., Arendt C., Hoffmann J., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fu H., Zhang N., Zheng Y., Jiang N., Xu H., Xu R., et al. Risk stratification of cardiac sequelae detected using cardiac magnetic resonance in late convalescence at the six-month follow-up of recovered COVID-19 patients. J Infect. 2021;83:125–127. doi: 10.1016/j.jinf.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joy G., Artico J., Kurdi H., Seraphim A., Lau C., Thornton G.D., et al. Prospective case-control study of cardiovascular abnormalities 6 months following mild COVID-19 in healthcare workers. JACC Cardiovasc Imaging. 2021;14:2155–2166. doi: 10.1016/j.jcmg.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sonnweber T., Sahanic S., Pizzini A., Luger A., Schwabl C., Sonnweber B., et al. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur Respir J. 2021;57 doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nature Medicine. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coromilas E.J., Kochav S., Goldenthal I., Biviano A., Garan H., Goldbarg S., et al. Worldwide survey of COVID-19-associated arrhythmias. Circ Arrhythm Electrophysiol. 2021;14 doi: 10.1161/CIRCEP.120.009458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peltzer B., Manocha K.K., Ying X., Kirzner J., Ip J.E., Thomas G., et al. Arrhythmic complications of patients hospitalized with COVID-19: incidence, risk factors, and outcomes. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.009121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gopinathannair R., Merchant F.M., Lakkireddy D.R., Etheridge S.P., Feigofsky S., Han J.K., et al. COVID-19 and cardiac arrhythmias: a global perspective on arrhythmia characteristics and management strategies. J Interv Card Electrophysiol. 2020;59:329–336. doi: 10.1007/s10840-020-00789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chinitz J.S., Goyal R., Harding M., Veseli G., Gruberg L., Jadonath R., et al. Bradyarrhythmias in patients with COVID-19: marker of poor prognosis? Pacing Clin Electrophysiol. 2020;43:1199–1204. doi: 10.1111/pace.14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russo V., Di Maio M., Mottola F.F., Pagnano G., Attena E., Verde N., et al. Clinical characteristics and prognosis of hospitalized COVID-19 patients with incident sustained tachyarrhythmias: a multicenter observational study. Eur J Clin Invest. 2020;50:e13387. doi: 10.1111/eci.13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lai P.H., Lancet E.A., Weiden M.D., Webber M.P., Zeig-Owens R., Hall C.B., et al. Characteristics associated with out-of-hospital cardiac arrests and resuscitations during the novel coronavirus disease 2019 pandemic in New York City. JAMA Cardiol. 2020;5:1154–1163. doi: 10.1001/jamacardio.2020.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tovia-Brodie O., Acha M.R., Belhassen B., Gasperetti A., Schiavone M., Forleo G.B., et al. Implantation of cardiac electronic devices in active COVID-19 patients. Results from an international survey. Heart Rhythm. 2021;19:206–216. doi: 10.1016/j.hrthm.2021.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nguyen J.L., Yang W., Ito K., Matte T.D., Shaman J., Kinney P.L. Seasonal influenza infections and cardiovascular disease mortality. JAMA Cardiol. 2016;1:274–281. doi: 10.1001/jamacardio.2016.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mitrani R.D., Goldberger J.J. Cardiac arrests during the COVID-19 pandemic: the perfect storm. JACC Clin Electrophysiol. 2021;7:12–15. doi: 10.1016/j.jacep.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 65.Anzini M., Merlo M., Sabbadini G., Barbati G., Finocchiaro G., Pinamonti B., et al. Long-term evolution and prognostic stratification of biopsy-proven active myocarditis. Circulation. 2013;128:2384–2394. doi: 10.1161/CIRCULATIONAHA.113.003092. [DOI] [PubMed] [Google Scholar]
- 66.Grun S., Schumm J., Greulich S., Wagner A., Schneider S., Bruder O., et al. Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol. 2012;59:1604–1615. doi: 10.1016/j.jacc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 67.Huang L., Zhao P., Tang D., Zhu T., Han R., Zhan C., et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13:2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Basso C., Calabrese F., Corrado D., Thiene G. Postmortem diagnosis in sudden cardiac death victims: macroscopic, microscopic and molecular findings. Cardiovasc Res. 2001;50:290–300. doi: 10.1016/S0008-6363(01)00261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tseng Z.H., Olgin J.E., Vittinghoff E., Ursell P.C., Kim A.S., Sporer K., et al. Prospective countywide surveillance and autopsy characterization of sudden cardiac death: POST SCD study. Circulation. 2018;137:2689–2700. doi: 10.1161/CIRCULATIONAHA.117.033427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maron B.J., Doerer J.J., Haas T.S., Tierney D.M., Mueller F.O. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980-2006. Circulation. 2009;119:1085–1092. doi: 10.1161/CIRCULATIONAHA.108.804617. [DOI] [PubMed] [Google Scholar]
- 71.Begieneman M.P., Emmens R.W., Rijvers L., Kubat B., Paulus W.J., Vonk A.B.A., et al. Ventricular myocarditis coincides with atrial myocarditis in patients. Cardiovasc Pathol. 2016;25:141–148. doi: 10.1016/j.carpath.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 72.Chang T.Y., Chao T.F., Liu C.J., Chen S.J., Chung F.P., Liao J.N., et al. The association between influenza infection, vaccination, and atrial fibrillation: a nationwide case-control study. Heart Rhythm. 2016;13:1189–1194. doi: 10.1016/j.hrthm.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 73.Mele D., Flamigni F., Rapezzi C., Ferrari R. Myocarditis in COVID-19 patients: current problems. Intern Emerg Med. 2021;16:1123–1129. doi: 10.1007/s11739-021-02635-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cooper L.T., Hare J.M., Tazelaar H.D., Edwards W.D., Starling R.C., Deng M.C., et al. Usefulness of immunosuppression for giant cell myocarditis. Am J Cardiol. 2008;102:1535–1539. doi: 10.1016/j.amjcard.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mason J.W., Billingham M.E., Ricci D.R. Treatment of acute inflammatory myocarditis assisted by endomyocardial biopsy. Am J Cardiol. 1980;45:1037–1044. doi: 10.1016/0002-9149(80)90174-5. [DOI] [PubMed] [Google Scholar]
- 76.Caforio A.L., Pankuweit S., Arbustini E., Basso C., Gimeno-Blanes J., Felix S.B., et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European society of cardiology working group on myocardial and pericardial diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. 48a-48d. [DOI] [PubMed] [Google Scholar]
- 77.McCarthy R.E., Boehmer J.P., Hruban R.H., Hutchins G.M., Kasper E.K., Hare J.M., et al. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med. 2000;342:690–695. doi: 10.1056/NEJM200003093421003. [DOI] [PubMed] [Google Scholar]
- 78.Rezkalla S.H., Kloner R.A. Viral myocarditis: 1917-2020: from the influenza A to the COVID-19 pandemics. Trends Cardiovasc Med. 2021;31:163–169. doi: 10.1016/j.tcm.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Manolis A.S., Manolis A.A., Manolis T.A., Apostolopoulos E.J., Papatheou D., Melita H. COVID-19 infection and cardiac arrhythmias. Trends Cardiovasc Med. 2020;30:451–460. doi: 10.1016/j.tcm.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maron B.J., Udelson J.E., Bonow R.O., Nishimura R.A., Ackerman M.J., Estes N.A., et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a scientific statement from the American heart association and American college of cardiology. Circulation. 2015;132:e273–e280. doi: 10.1161/CIR.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 81.Martinez M.W., Kim J.H., Shah A.B., Phelan D., Emery M.S., Wasfy M.M., Fernandez A.B., et al. Exercise-induced cardiovascular adaptations and approach to exercise and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78:1453–1470. doi: 10.1016/j.jacc.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 82.Gluckman T.J., Bhave N.M., Allen L.A., Chung E.H., Spatz E.S., Ammirati E., et al. 2022 ACC expert consensus decision pathway on cardiovascular sequelae of COVID-19 in adults: myocarditis and other myocardial involvement, post-acute sequelae of SARS-CoV-2 infection, and return to play: a report of the American college of cardiology solution set oversight committee. J Am Coll Cardiol. 2022;79:1717–1756. doi: 10.1016/j.jacc.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]