Abstract
This work presents a case series of four children diagnosed with severe cerebrovascular disease in association with recent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, yet no patient from the group met typical diagnostic criteria for multisystem inflammatory syndrome in children. Our aim was to highlight the possible vascular involvement and coagulopathies associated with SARS-CoV-2 infection in the pediatric population. Further data are needed to better understand the pathophysiological basis of this condition in children and to ensure its optimal management.
Keywords: COVID-19, SARS-CoV-2, Children, Brain vasculopathy, Stroke
Introduction
Coronavirus disease 2019 (COVID-19) has now spread throughout the world following an outbreak in China in late 2019. Like other coronaviruses, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) primarily targets the respiratory system. In symptomatic patients, the most common symptoms are fever, fatigue, cough, and headache. Severe forms can present with pneumonia, acute respiratory distress syndrome, acute cardiac dysfunction, and multiorgan failure.1 Some studies have indicated an association between COVID-19 and cerebrovascular conditions.1, 2, 3 These case reports or case series report mainly adult patients with a severe course of the disease. Severe COVID-19 is rare in children, and the long-term consequences of the disease and its effect on the increased propensity for thrombosis and/or the development of autoimmune diseases are unclear.
The work presents a case series of children who were diagnosed with cerebrovascular disease and whose common denominator was current or previous infection with COVID-19 confirmed with antigen/polymerase chain reaction (PCR) COVID-19 test or antibody testing. All patients underwent an extensive hematologic workup including prothrombin time/international normalized ratio test, D-dimer assay, activated protein C resistance, protein C and protein S activity, antithrombin activity, specific factor activity levels, lupus anticoagulant panel, anticardiolipin antibody, anti-B2GP1, and level of folate, vitamin B12, and homocysteine. All patients underwent genetic testing for procoagulable disorders (FV Leiden G1691A-DNA and prothrombin G20210A-DNA). In some patients, cerebrospinal fluid (CSF) was examined. In addition to basic CSF biochemical and immunological examination, PCR panel of neuroviruses (Enterovirus, HSV1, HSV2, VZV, EBV, CMV, HHV6, HHV7) and anti-Borrelia antibodies including intrathecal synthesis and antibody index were examined.
Case reports are summarized in Table . All patients were treated at the University Hospital Brno, Faculty of Medicine of Masaryk University Brno, Czech Republic between 3/2020 and 12/2021. Informed consent approved by the Institutional Review Board was obtained from all the patients/parents involved in the study.
TABLE.
Patient Overview
| Patient No. | Sex | Age | Progression of COVID-19 Infection | Cerebrovascular Complications | The Time Interval of Complications After COVID-19 Infection | Antigen/PCR COVID-19 Test Result at Hospital Admission | COVID-19 Antibody Results and Time Span After Infection | Thrombotic/Hemophilic Risk Factors | Therapy | Neurological Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient I | M | 12 | Asymptomatic | Meningitis with subdural hemorrhage | Acute | Negative | Positive 2 month after infection | None | Neurosurgical, CS | Normal |
| Patient II | F | 1 | Asymptomatic | Vasculitis with brainstem involvement | 3 months | Negative | Positive 3 months after infection | Leiden heterozygous, elevated factor VIII | CS, LMWH, ASA | Mild∗ |
| Patient III | F | 11 | Repeated infection with post-COVID rhinitis, otitis media, and protanopia | Lacunar ischemic stroke | 1 month | Negative | Positive 1 month after the second infection | None | ASA | Normal |
| Patient IV | M | 4 | Asymptomatic | CVT with hemorrhage—atypical PIMS-TS | NA | Negative | NA | None | CS, IVIG, LMWH, Neurosurgical | Moderate† |
Abbreviations:
ASA = Acetylsalicylic acid
CS = Corticosteroids
CVT = Cerebral venous thrombosis
F = Female
IVIG = Intravenous immunoglobulins
LMWH = Low-molecular-weight heparin
M = Male
NA = Not applicable
PIMS-TS = Pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2
Ptosis, squint.
Hemiparesis, facial nerve paresis, vocal cord paresis.
Patient Description 1
A 12-year-old boy was admitted to the hospital for a sudden development of paresthesia of the right upper extremity and dysarthria. An acute head computed tomography (CT) scan revealed acute nontraumatic subdural hematoma on the left side. The patient underwent urgent surgical hematoma evacuation on the day of admission. He had a negative COVID-19 antigen and PCR test when he was admitted to the hospital (Fig 1 ).
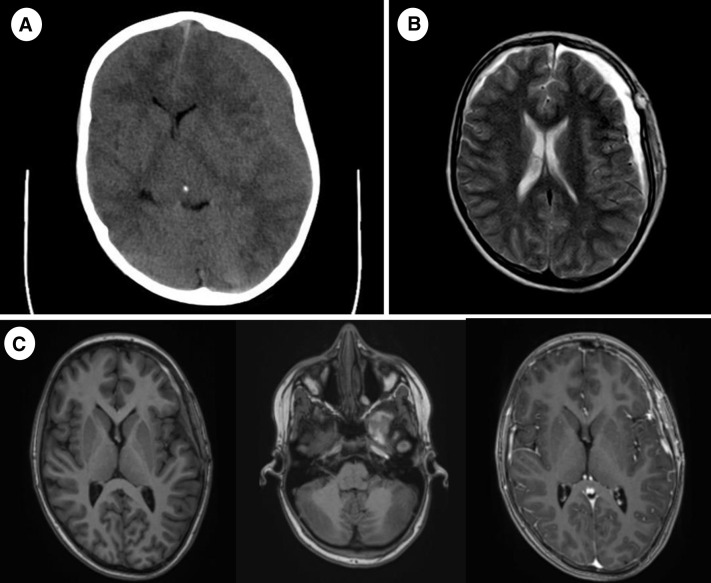
FIGURE 1.
(A) Noncontrast brain CT, at onset (axial plane): Left-sided subdural hematoma. (B) Brain MRI, day 6 after surgery, T2 tse (axial plane): Bilateral subdural hematoma. (C) Brain MRI, 1-month follow-up, T1 mprage precontrast and postcontrast (axial plane): Left temporal arachnoideal cyst with older hemorrhagic component, bilateral residual subdural hematoma and pachymeningeal contrast enhancement more pronounced on the left side. CT, computed tomography; MRI, magnetic resonance imaging.
On a head CT scan 2 days after the surgery, no further bleeding was observed. Six days after the intervention, a brain magnetic resonance imaging (MRI) scan showed a new subdural hematoma in the right frontal region and an arachnoid cyst in the left temporal lobe with signs of fresh hemorrhage. Surgical treatment was not indicated, and the patient was discharged home in good clinical condition.
However, on the second day after release, the patient was readmitted for paresthesia and weakness of the left upper extremity, left facial droop, and dysarthria. An acute CT scan identified fresh subdural bleeding in the left temporal region along with the aforementioned older subdural hematomas and arachnoid cyst. His hematologic workup showed no bleeding disorder. The patient received treatment with etamsylate and was discharged from the hospital.
One week after the second rehospitalization, the patient was yet again admitted for the development of paresthesia and weakness of the right upper arm, right facial droop, and dysarthria. An acute brain MRI showed a new intracerebral hematoma in the left temporal lobe and, importantly, meningeal contrast enhancement on the left side.
CSF manifested clear signs of inflammation (monocytes 22/1 μl). After a thorough hematological workup, no bleeding disorder was identified. The patient's family history of COVID-19 was approximately one 2 months before the first clinical symptoms associated with bleeding appeared; he was not tested at that time. During the third hospitalization, the patient showed high titers of antibodies against SARS-CoV-2 (1288 U/ml). Due to these findings, the patient was diagnosed with SARS-CoV-2–associated meningoencephalitis with subdural and intracerebral hemorrhage.
Treatment with dexamethasone for antiedematous indication at a dose of 0.2 mg/kg/day for 7 days was immediately initiated. A repeat brain MRI after 7 weeks showed a complete regression of the meningeal enhancement and did not reveal any new bleeding.
Patient Description 2
A one-year-old girl with a negative medical history was admitted with acute incomplete external ophthalmoplegia with progression of eyelid ptosis over the next few hours. She was tested negative for COVID-19 at hospital admission (PCR test) (Fig 2 ).
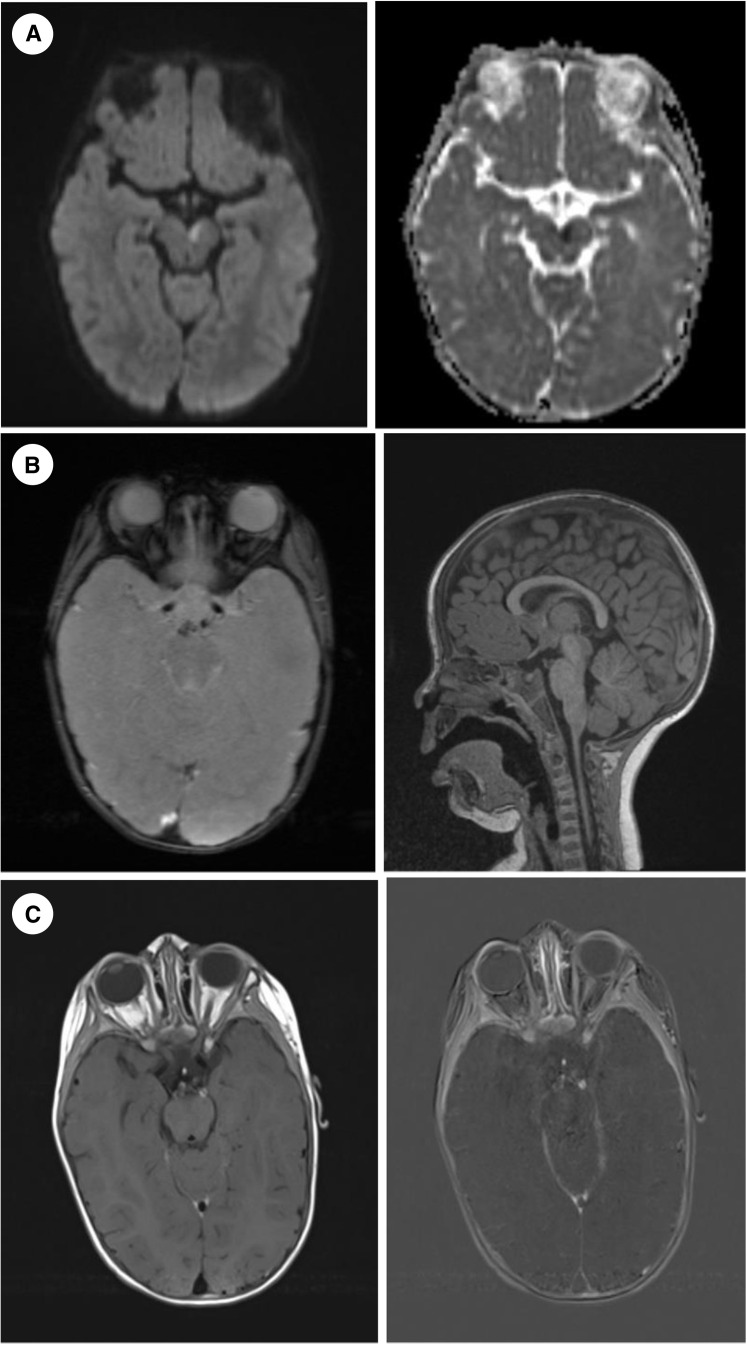
FIGURE 2.
(A) Brain MRI, at onset, DWI and ADC maps (axial plane): Area of cytotoxic edema of left mesencephalon. (B) Brain MRI, at onset, GRE (axial plane) and T1 mprage (sagittal plane): Discrete subarachnoideal hemorrhage in the prepontine cistern. (C) Brain MRI, at onset, T1 tse blood suppression postcontrast and subtracted (axial plane): Left P1 of ACP wall enhancement. ACP, arteria cerebri posterior; ADC, apparent diffusion coefficient; DWI, diffusion weighted imaging; GRE, multiecho gradient recalled echo; MRI, magnetic resonance imaging.
On an acute brain MRI, cytotoxic edema of the left mesencephalon with minor hemorrhage in the prepontine cistern was reported. Sequences for vasculitis (precontrast and postcontrast T1 spin echo blood suppression) showed arterial wall enhancement of the left P1 posterior cerebral artery basin. An examination of the CSF did not show any sign of inflammation.
The patient was treated with 5 pulses of corticoids at a dose of 30 mg/kg/day. Subsequently, prednisone was administered orally at 2 mg/kg/day for three months. Low-molecular-weight heparin was introduced into prophylaxis with the continuation of aminosalicylic acid treatment at 2 mg/kg/day in accord with latest American College of Clinical Pharmacy consensus for the treatment of central nervous system (CNS) ischemia.4
The follow-up brain MRI performed 6 weeks after the clinical onset was negative. The patient showed thrombophilic risks (FV Leiden heterozygous and increased factor VIII), and high titers of antibodies against SARS-CoV-2 virus (934 U/ml) were detected 3 months after the occurrence of COVID-19 in a close family. The patient herself had no symptoms of COVID-19 disease and was not tested at that time. The condition was concluded as post-COVID-CNS vasculitis with primary brainstem involvement in a patient with a thrombophilic predisposition.
Patient Description 3
An 11-year-old girl with a history of repeated infection of COVID-19, post-COVID rhinitis, otitis media, and protanopia was admitted for an episode of right upper extremity paresthesia, right facial drooping, and dysarthria; the episode lasted for 5 minutes in total and terminated spontaneously. Her first COVID-19 infection occurred in December 2020, and the second one in September 2021, one month before this episode. The patient had a negative COVID-19 antigen test when admitted to the hospital (Fig 3 ).
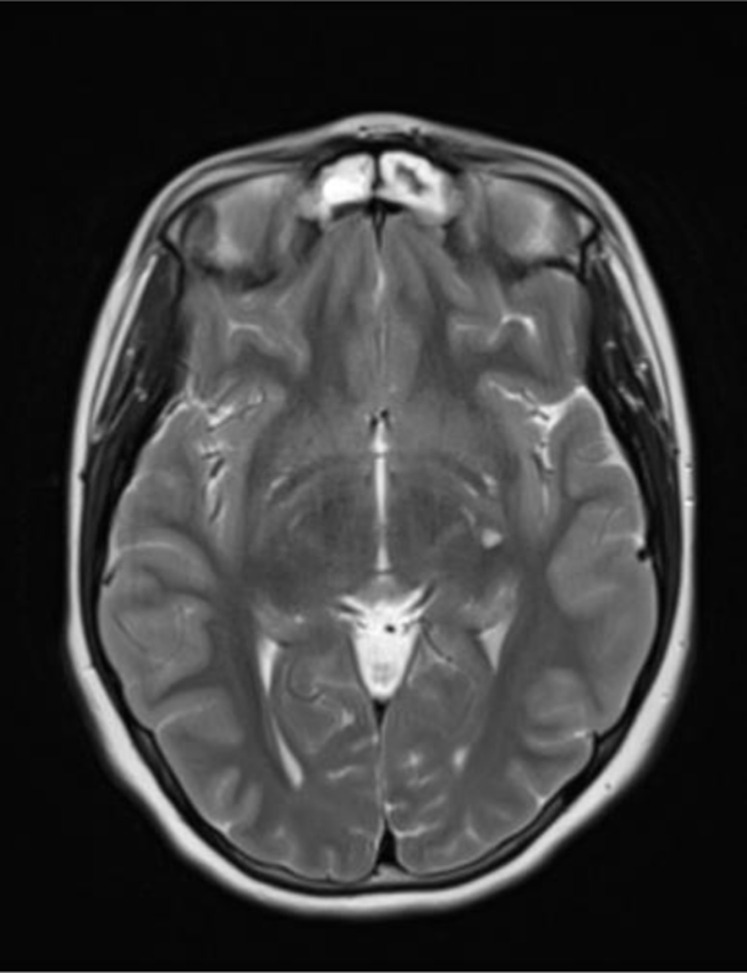
FIGURE 3.
Brain MRI, 1 week after onset, T2 tse (axial plane): Lacunar lesion in the left basal nuclei. MRI, magnetic resonance imaging.
CT and CT angiography brain scans were performed immediately with negative results. Within a few days, brain MRI showed lacunar infarction in the left basal nuclei. Magnetic resonance angiography and diffusion-weighted MRI sequences were negative.
No thrombotic risk factors (inherited as well as acquired) were identified, despite a family history of her father dying of an ischemic stroke at the age of 30 years. The patient had high anti-SARS-CoV-2 antibody titers (9807 U/ml) one month after the second COVID-19 infection.
Due to the temporal association with COVID-19 reinfection and absence of other thrombotic risk factors, a diagnosis of post-COVID lacunar ischemic stroke was established. The patient was started on acetylsalicylic acid at a dose of 100 mg per day as recommended by latest American College of Clinical Pharmacy consensus for ischemic CNS lesions.4
Patient Description 4
A 4-year-old boy with a history of headache, fever, and subsequent vomiting was admitted due to somnolence and positive upper meningeal irritation signs. On fundoscopic examination, incipient papilledema was found. The antigen and PCR COVID-19 tests were negative at hospital admission. CSF examination confirmed a high concentration of red blood cells (7000/1 μl) and mild pleocytosis (15 monocytes/1 μl and 125 polymorphonuclear cells/1 μl). Blood tests showed severe thrombocytopenia (52 × 109/l), anemia (69 g/l), and elevated D-dimers (11 mg/l). The patient developed generalized convulsions that were terminated with 200 mg of phenobarbital. An acute brain CT showed extensive central venous thrombosis of the left internal jugular vein and left cerebral sinuses, including the typical radiology dense vein sign. This was accompanied by secondary intracranial hemorrhage in the left temporal, occipital, and parietal lobe, with a large hypodense area and mild midline shift. Due to intracranial hypertension, the patient underwent an emergency decompressive craniectomy. A brain MRI confirmed an extensive lesion of hemorrhagic venous infarction due to the left-sided cerebral venous sinus thrombosis, passing into the left internal jugular vein (Fig 4 ).
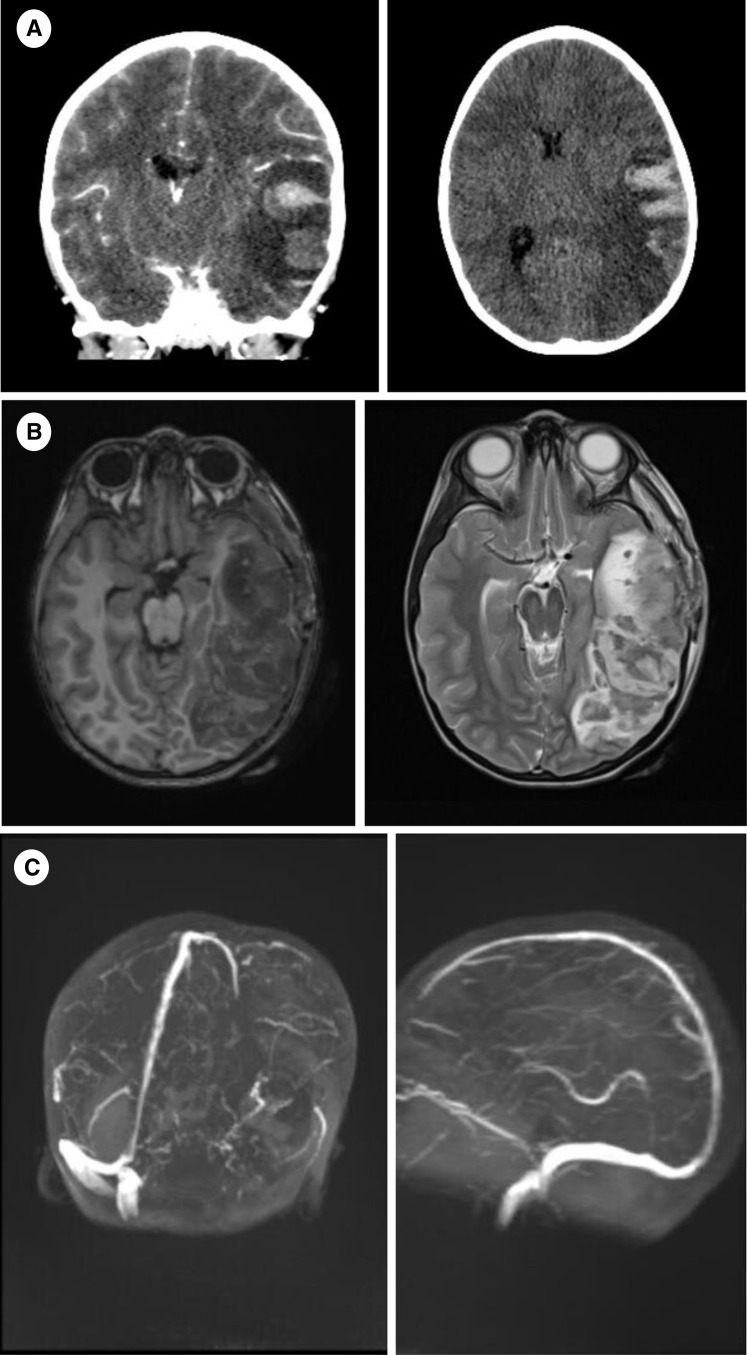
FIGURE 4.
(A) Brain CT, at onset (coronary and axial plane): Large hypodense ischemic area of left temporal, occipital and parietal lobe with profound hemorrhagic component and moderate midline shift. (B) Brain MRI, day 1 follow-up, T1 tse and T2 tse (axial plane): Extensive hemorrhagic venous infarction of left temporal, occipital and parietal lobe. (C) Brain MRI, day 1 follow-up, TOF 3D venography (3D rec): Left-sided cerebral venous sinus thrombosis propagating into the left internal jugular vein. CT, computed tomography; MRI, magnetic resonance imaging; TOF, time-of-flight.
The patient was started on slowly escalating anticoagulation therapy with low-molecular-weight heparin in coordination with a hematologist and a neurosurgeon to treat the thrombosis without progression of the bleeding complication. High titers of antibodies against SARS-CoV-2 S (680 U/ml) were detected 3 weeks after the onset of first symptoms. Other broad examinations did not reveal any other causative agent. The possibility of atypical pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 was considered. Treatment using high doses of corticosteroids (30 mg/kg given for two days followed by a maintenance dose of 2 mg/kg) and immunoglobulins (1 g/kg for two days) was initiated as per local pediatric inflammatory multisystem syndrome protocol, followed by a gradual normalization of thrombocytopenia.
After awakening from the medically induced coma, the clinical presentation dominated with severe right-sided hemiparesis, including facial nerve palsy, anisocoria, speech impairment, and dysphagia. Two months later, the condition was complicated by vocal cord paralysis with the need for tracheostomy.
Discussion
Few publications have addressed cerebrovascular involvement or complications of COVID-19 disease in children. These are mostly case reports or case series; larger cohorts have been aimed at adult patients.1, 2, 3, 4, 5, 6, 7, 8 One possible complication of COVID-19 in children is pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2,8 , 9 which has relatively accurate diagnostic criteria with multisystem involvement.9, 10, 11, 12 No patient from our group met this typical diagnostic criteria. We focused on patients with cerebrovascular disease whose clinical manifestations shared a common denominator of COVID-19 infection. Evidence of SARS-CoV-2 infection was defined as “confirmed COVID-19” if PCR respiratory samples (nasopharyngeal swab) or if serology was positive for anti-SARS-CoV-2 IgM or IgG. We are aware that this may have been a mere coincidence of two diseases, but no other etiological cause was found; due to the pathophysiological findings on vascular involvement and coagulopathies associated with COVID-19,1 this cause is very likely. We are aware of the limitations of the study. The incidence of COVID-19 in children in our region (South Moravian Region of the Czech Republic; 1.2 million inhabitants) is 34388/100000. At this incidence, there is a risk of false positive results in terms of correlation of clinical symptoms with laboratory evidence of SARS-CoV-2 infection.
SARS-CoV-2 infects the host through its CoV spike glycoprotein, which binds to the angiotensin-converting enzyme 2 (ACE2) receptor, which is expressed in the lungs, heart, and kidneys and in endothelial cells. Endothelial cell involvement with COVID-19 has recently been demonstrated across vascular beds.1 In general, pathophysiological processes after COVID-19 infection can be divided into two groups: (1) vascular affliction and microvascular dysfunction and (2) coagulation abnormalities.2 , 8 Experimental evidence and clinical data suggest that the pathogenesis of neurovascular disease due to SARS-CoV-2 rests on a multifactorial process that involves primary direct infection with subsequent inflammatory responses causing vasculitis, intravascular coagulation, and thromboembolic events, resulting in secondary ischemic and hemorrhagic strokes.2 Systemic viremia and subsequent endothelial dysfunction may make the bridging veins of the subdural space more vulnerable to bleeding following a minor trauma even to the point of sneezing, coughing, or a Valsalva manoeuver.13 Prolonged hypoxia of endothelial cells and proinflammatory state due to cytokine storm are the other possible causes that can result in endothelial damage and hemorrhage.14 The hypercoagulable state of COVID-19 may be associated with overwhelming endothelial cell activation, which may disrupt coagulation and fibrinolytic systems.15 , 16
Patient 1 developed a neurological impairment as part of an acute SARS-CoV-2 infection, although we only detected IgG and IgM antibodies against this infection. We diagnosed meningoencephalitis with an inflammatory finding in the cerebrospinal fluid and leptomeningeal enhancement on brain MRI. The complication was the development of subdural hematomas and intracerebral hemorrhage, which required neurosurgical intervention. A similar case was reported in an adult patient.13 Patient 2 had brainstem involvement with evidence of vasculitis on special MRI sequences, with a history of an asymptomatic course of COVID-19 infection. The patient had inherited thrombophilic risks. Similarly, in Patient 3, the COVID-19 infection was very inconspicuous, but a small transient ischemic attack occurred several weeks later. No thrombophilic risks were found, though. Patient 4, with a fulminant course and severe neurological impairment, was admitted 1 month after a respiratory infection, when antigen and PCR tests on admission were negative. Only high levels of antibodies, thrombocytopenia, and elevated D-dimers were detected. Brain CT showed extensive venous infarction, but no thrombophilic risks were confirmed. Clinically, it is the most reminiscent of thrombosis syndrome with thrombocytopenia, which is described after vaccination with vector vaccines, but our patient was not vaccinated.
Conclusion
It is generally believed that COVID-19 infection in children usually has an asymptomatic benign course. However, in connection with the COVID-19 pandemic, we are more likely to encounter diseases that have rarely been seen before that share a common denominator of past COVID-19 infections. These cases are intended to show that the course of the disease may not always be benign and a longer period will be needed to assess the consequences of the infection. Further studies will certainly be needed to address the pathophysiological basis of this infection in an effort to ensure its optimal management.
Acknowledgments
The authors thank Anne Johnson for grammatical assistance.
Footnotes
Conflict of interest: None of the authors has any conflict of interest to disclose. The authors confirm that they have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Hanafi R., Roger P.A., Kuchcinski G., et al. COVID-19 neurologic complications with CNS vasculitis-like pattern. AJNR Am J Neuroradiol. 2020;41:1384–1387. doi: 10.3174/ajnr.A6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirschenbaum D., Imbach L.L., Rushing E.J., et al. Intracerebral endotheliitis and microbleeds are neuropathological features of COVID-19. Neuropathol Appl Neurobiol. 2021;47:454–459. doi: 10.1111/nan.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaschetto R., Cena T., Sainaghi P.P., et al. Cerebral nervous system vasculitis in a Covid-19 patient with pneumonia. J Clin Neurosci. 2020;79:71–73. doi: 10.1016/j.jocn.2020.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens S.M., Woller S.C., Kreuziger L.B., et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545–e608. doi: 10.1016/j.chest.2021.07.055. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez Amezcua J.M., Jain R., Kleinman G., et al. COVID-19-induced neurovascular injury: a case series with emphasis on pathophysiological mechanisms. SN Compr Clin Med. 2020;2:2109–2125. doi: 10.1007/s42399-020-00598-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regev T., Antebi M., Eytan D., et al. Pediatric inflammatory multisystem syndrome with central nervous system involvement and hypocomplementemia following SARS-COV-2 infection. Pediatr Infect Dis J. 2020;39:e206–e207. doi: 10.1097/INF.0000000000002804. [DOI] [PubMed] [Google Scholar]
- 7.Varatharaj A., Thomas N., Ellul M.A., et al. CoroNerve Study Group Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beslow L.A., Linds A.B., Fox C.K., et al. Pediatric ischemic stroke: an infrequent complication of SARS-CoV-2. Ann Neurol. 2021;89:657–665. doi: 10.1002/ana.25991. [DOI] [PubMed] [Google Scholar]
- 9.Whittaker E., Bamford A., Kenny J., et al. PIMS-TS Study Group and EUCLIDS and PERFORM Consortia Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasuhara J., Watanabe K., Takagi H., Sumitomo N., Kuno T. COVID-19 and multisystem inflammatory syndrome in children: a systematic review and meta-analysis. Pediatr Pulmonol. 2021;56:837–848. doi: 10.1002/ppul.25245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaRovere K.L., Riggs B.J., Poussaint T.Y., et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78:536–547. doi: 10.1001/jamaneurol.2021.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fink E.L., Robertson C.L., Wainwright M.S., et al. Prevalence and risk factors of neurologic manifestations in hospitalized children diagnosed with acute SARS-CoV-2 or MIS-C. Pediatr Neurol. 2022;128:33–44. doi: 10.1016/j.pediatrneurol.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-olama M., Rashid A., Garozzo D. COVID-19-associated meningoencephalitis complicated with intracranial hemorrhage: a case report. Acta Neurochir (Wien) 2020;162:1495–1499. doi: 10.1007/s00701-020-04402-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parry A.H., Wani A.H., Yaseen M. Neurological dysfunction in coronavirus disease-19 (COVID-19) Acad Radiol. 2020;27:1329–1330. doi: 10.1016/j.acra.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabibkhooei A., Hatam J., Mokhtari M., Abolmaali M. COVID-19-associated spontaneous subacute subdural haematoma: report of two cases. New Microbes New Infect. 2021;40 doi: 10.1016/j.nmni.2021.100848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavalcanti D.D., Raz E., Shapiro M., et al. Cerebral venous thrombosis associated with COVID-19. AJNR Am J Neuroradiol. 2020;41:1370–1376. doi: 10.3174/ajnr.A6644. [DOI] [PMC free article] [PubMed] [Google Scholar]