Abstract
BACKGROUND
Effective strategies are needed to facilitate the prompt diagnosis and treatment of tuberculosis in countries with a high burden of the disease.
METHODS
We conducted a cluster-randomized trial in which Ugandan community health centers were assigned to a multicomponent diagnostic strategy (on-site molecular testing for tuberculosis, guided restructuring of clinic workflows, and monthly feedback of quality metrics) or routine care (on-site sputum-smear microscopy and referral-based molecular testing). The primary outcome was the number of adults treated for confirmed tuberculosis within 14 days after presenting to the health center for evaluation during the 16-month intervention period. Secondary outcomes included completion of tuberculosis testing, same-day diagnosis, and same-day treatment. Outcomes were also assessed on the basis of proportions.
RESULTS
A total of 20 health centers underwent randomization, with 10 assigned to each group. Of 10,644 eligible adults (median age, 40 years) whose data were evaluated, 60.1% were women and 43.8% had human immunodeficiency virus infection. The intervention strategy led to a greater number of patients being treated for confirmed tuberculosis within 14 days after presentation (342 patients across 10 intervention health centers vs. 220 across 10 control health centers; adjusted rate ratio, 1.56; 95% confidence interval [CI], 1.21 to 2.01). More patients at intervention centers than at control centers completed tuberculosis testing (adjusted rate ratio, 1.85; 95% CI, 1.21 to 2.82), received a same-day diagnosis (adjusted rate ratio, 1.89; 95% CI, 1.39 to 2.56), and received same-day treatment for confirmed tuberculosis (adjusted rate ratio, 2.38; 95% CI, 1.57 to 3.61). Among 706 patients with confirmed tuberculosis, a higher proportion in the intervention group than in the control group were treated on the same day (adjusted rate ratio, 2.29; 95% CI, 1.23 to 4.25) or within 14 days after presentation (adjusted rate ratio, 1.22; 95% CI, 1.06 to 1.40).
CONCLUSIONS
A multicomponent diagnostic strategy that included on-site molecular testing plus implementation supports to address barriers to delivery of high-quality tuberculosis evaluation services led to greater numbers of patients being tested, receiving a diagnosis, and being treated for confirmed tuberculosis. (Funded by the National Heart, Lung, and Blood Institute; XPEL-TB ClinicalTrials.gov number, NCT03044158.)
Prompt diagnosis and treatment of tuberculosis are essential to achieving the elimination of this disease.1 However, loss of patients to follow-up during the diagnostic process represents a clear health-system failure that is pervasive in countries with a high burden of tuberculosis. A systematic review of published studies showed that 13 to 18% of patients with positive results on sputum-smear microscopy are lost to follow-up before treatment initiation.2 Patients with smear-negative tuberculosis are even less likely to complete the diagnostic cascade of care and be linked to treatment.
A principal reason is the lack of sensitive and rapid testing for tuberculosis at community health centers. Sputum-smear microscopy, the most commonly available test, is dependent on the skill of the technician and has suboptimal sensitivity, identifying only approximately 50% of patients with tuberculosis.3 Furthermore, multiple visits are often required for testing to be completed,4,5 and many patients do not return because of high direct and indirect costs.6
To address these limitations, there has been substantial donor investment in the Xpert MTB/RIF assay (Xpert), a semiautomated molecular assay that is conducted on the GeneXpert platform (Cepheid).7 The Xpert assay identifies 85% of cases of pulmonary tuberculosis in adults, has a 2-hour turnaround time, and can be conducted with minimal training.8 However, because of high device costs and infrastructure requirements,7,9 most countries have adopted a hub-and-spoke implementation model, in which several community health centers (spokes) are linked to a central Xpert testing site (hub). Three previous randomized trials evaluating such centralized Xpert testing models showed no improvement in the speed or overall initiation of treatment among patients with confirmed tuberculosis.10–12
A new generation of molecular diagnostics is emerging that has strong potential to be deployed at community health centers. For example, GeneXpert Edge is a compact version of the GeneXpert platform (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). It has lower power requirements that enable full operation for a period of 8 hours on a rechargeable battery. Portable molecular testing platforms, such as Truelab (Molbio Diagnostics), offer further potential to decentralize molecular testing.13,14
Although newer molecular diagnostic platforms help to address certain barriers to diagnosis (e.g., accuracy and speed of testing), some new technologies often do not produce the intended results because of contextual factors in the broader health system that influence their implementation.15 Such factors are of particular concern where health systems are weak, as is often the case in countries with a high prevalence of tuberculosis. Thus, identification and testing of multicomponent interventions that target multiple barriers to the diagnosis of tuberculosis and linkage to treatment are essential if patient outcomes are to be improved in real-world settings.16 Here, we present the results of the XPEL-TB trial, which evaluated whether decentralized (i.e., on-site) molecular testing, coupled with guided restructuring of clinic work-flows and monthly performance feedback to address health center–level barriers to providing high-quality tuberculosis evaluation services,17 could lead to a greater number of patients receiving a diagnosis of and being treated for tuberculosis.
METHODS
TRIAL DESIGN AND OVERSIGHT
We conducted this highly pragmatic, cluster-randomized, parallel-group trial at 20 community health centers in Uganda. The trial included a 12-month prerandomization period and a 16-month intervention period. After the prerandomization period, health centers were randomly assigned to either the intervention group or the control (routine care) group at a public randomization ceremony.18 The protocol, which is available at NEJM.org and has been published previously,19 was approved by institutional review boards at the University of California, San Francisco, and Makerere University. An independent trial steering committee periodically reviewed the conduct of the trial and approved all changes to the protocol. A waiver of informed consent was obtained to extract patients’ data from health center data sources. The data were analyzed by the trial statistician (penultimate author) and the data manager (second author). All the authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. Additional details regarding the trial design and analysis are provided in the Supplementary Methods section in the Supplementary Appendix.
SELECTION OF HEALTH CENTERS AND PARTICIPANTS
To be eligible, health centers were required to perform on-site sputum-smear microscopy as the primary method of tuberculosis diagnosis at the time of trial initiation and to be linked to a central health facility that performed Xpert testing. We included data on all adults (≥18 years of age) who underwent evaluation for tuberculosis, which was defined as having been entered into the National Tuberculosis and Leprosy Program (NTLP) presumptive tuberculosis, tuberculosis laboratory, or tuberculosis treatment registers. These three NTLP registers include data on patients who screen positive for tuberculosis symptoms (presumptive), are tested for tuberculosis (laboratory), and are treated for tuberculosis (treatment).
RANDOMIZATION AND INTERVENTIONS
Cluster randomization was used to minimize the risk of between-group contamination. To help achieve balance across the trial groups, we grouped health centers into two strata on the basis of the median proportion of patients who were treated for confirmed tuberculosis within 14 days after presentation to the health center for evaluation during the prerandomization period.19 Restriction was done to further ensure balance of important characteristics, including health center region, location (urban or rural), number of patients evaluated for tuberculosis, distance to Xpert testing hub, and prevalence of human immunodeficiency virus (HIV) infection among patients with tuberculosis.19
The multicomponent intervention strategy was designed in collaboration with local stakeholders to address key barriers to tuberculosis diagnosis and treatment that were identified during formative work.17 The theoretical underpinnings of the intervention have been described previously,19 and details of each intervention component are provided in the Supplementary Methods section. In brief, health centers that were randomly assigned to the intervention group were each provided with one GeneXpert Edge device (Fig. S1) to enable on-site molecular testing with Xpert as the first-line test for tuberculosis. To facilitate same-day molecular testing and treatment initiation, a structured process was used to guide the intervention health centers to redesign and streamline their clinic, laboratory, and pharmacy workflows. In addition, to encourage continuous quality improvement, a monthly report card with performance indicators related to tuberculosis diagnostic evaluation was provided. Health centers that were randomly assigned to the control group continued to follow national guidelines for tuberculosis diagnostic evaluation (on-site sputum-smear microscopy, plus referral of sputum samples obtained from high-risk patients to Xpert testing hubs).
DATA COLLECTION AND MANAGEMENT
Photographs of the tuberculosis registers (Figs. S3, S4, and S5) were taken every 2 weeks by health center staff and were uploaded to a secure server. Research staff abstracted demographic, clinical, and outcome data from the photographs and entered each patient’s data into a unique record in the trial database.20
OUTCOMES
The primary outcome was the number of patients who were treated for confirmed tuberculosis (defined as a positive result on sputum-smear microscopy or molecular testing) within 14 days after presentation to the health center for tuberculosis evaluation (i.e., within 14 days after the earliest date recorded in any of the NTLP tuberculosis registers for each patient) during the 16-month intervention period. This outcome reflected a protocol change that was made 6 months after the trial began. The originally defined primary outcome was the proportion of patients who were treated for confirmed tuberculosis within 14 days after presentation among those who had undergone evaluation for tuberculosis. The independent trial steering committee approved the change because the number of patients treated for confirmed tuberculosis, rather than the proportion of patients treated for confirmed tuberculosis among those evaluated, was thought to better reflect the intended effect of the multicomponent intervention strategy, which had been designed to improve diagnosis and treatment of tuberculosis by closing gaps across the entire tuberculosis diagnostic evaluation cascade of care (see the Outcomes section in the Supplementary Appendix). Secondary outcomes were related to key steps along the tuberculosis diagnostic evaluation cascade of care and included the numbers of patients who were tested for tuberculosis according to national guidelines, who received a diagnosis of confirmed tuberculosis on the same day or within 14 days after presentation, who were treated for confirmed tuberculosis on the same day, and who were treated for tuberculosis (confirmed or clinical) on the same day or within 14 days; and the proportions of patients who were tested for tuberculosis according to national guidelines, who received a diagnosis of confirmed tuberculosis on the same day or within 14 days after presentation, who were treated for confirmed tuberculosis on the same day or within 14 days, and who were treated for tuberculosis (confirmed or clinical) on the same day or within 14 days. In addition, we assessed the time to diagnosis of tuberculosis and the time to treatment for tuberculosis (see the Supplementary Methods section and the statistical analysis plan of the protocol).
STATISTICAL ANALYSIS
The trial was designed to have 80 to 90% power to detect an absolute difference of at least 6 percentage points in the percentage of patients who were treated for confirmed tuberculosis within 14 days after presentation, assuming 10 health centers per group, a harmonic mean number of patients per health center of 268 (determined on the basis of the number of patients expected to undergo evaluation for tuberculosis over a period of 18 months), and a coefficient of variation of 0.36. Revised calculations after the change in the primary outcome estimated that the trial would have 80 to 90% power to detect a rate ratio of 1.30 or higher for the relative difference between the intervention group and the control group in the total numbers of patients who were treated for confirmed tuberculosis within 14 days after presentation to the health center for evaluation during the 16-month intervention period (see the Supplementary Methods section).
We performed cluster-level analyses, taking into account the stratified randomization, to assess the effect of the intervention strategy on outcomes.21 We analyzed count-based outcomes, including the primary outcome, using a negative binomial regression model, with the natural logarithm of months enrolled as the offset. We adjusted for cluster-level covariates, including the stratification variable used for randomization and the number of patients who had been treated for confirmed tuberculosis during the 12-month prerandomization period. Further details, including information about the analysis of secondary proportion-based and time-to-event outcomes, are provided in the Supplementary Methods section and the statistical analysis plan.
Patients who had missing data on age were excluded from the trial population, which included only patients 18 years of age or older. Patients with unknown HIV infection status were excluded from the adjusted analyses of secondary outcomes. Data that were analyzed for the primary outcome reflect the tuberculosis test result and treatment information as recorded in the NTLP registers.
RESULTS
TRIAL POPULATION
The trial was conducted from October 2018 through February 2020. Of 1514 microscopy centers that were affiliated with the Uganda NTLP at the time of trial design, 1430 were excluded (Fig. 1). Among 84 eligible health centers, 20 were selected for inclusion in the trial (Tables 1 and S7). No health center was lost to follow-up. The trial period was shortened by 52 days (from 18 months to approximately 16 months) owing to anticipated effects of restrictions related to coronavirus disease 2019 on the care and treatment of patients with tuberculosis.
Figure 1. Randomization and Trial Eligibility.
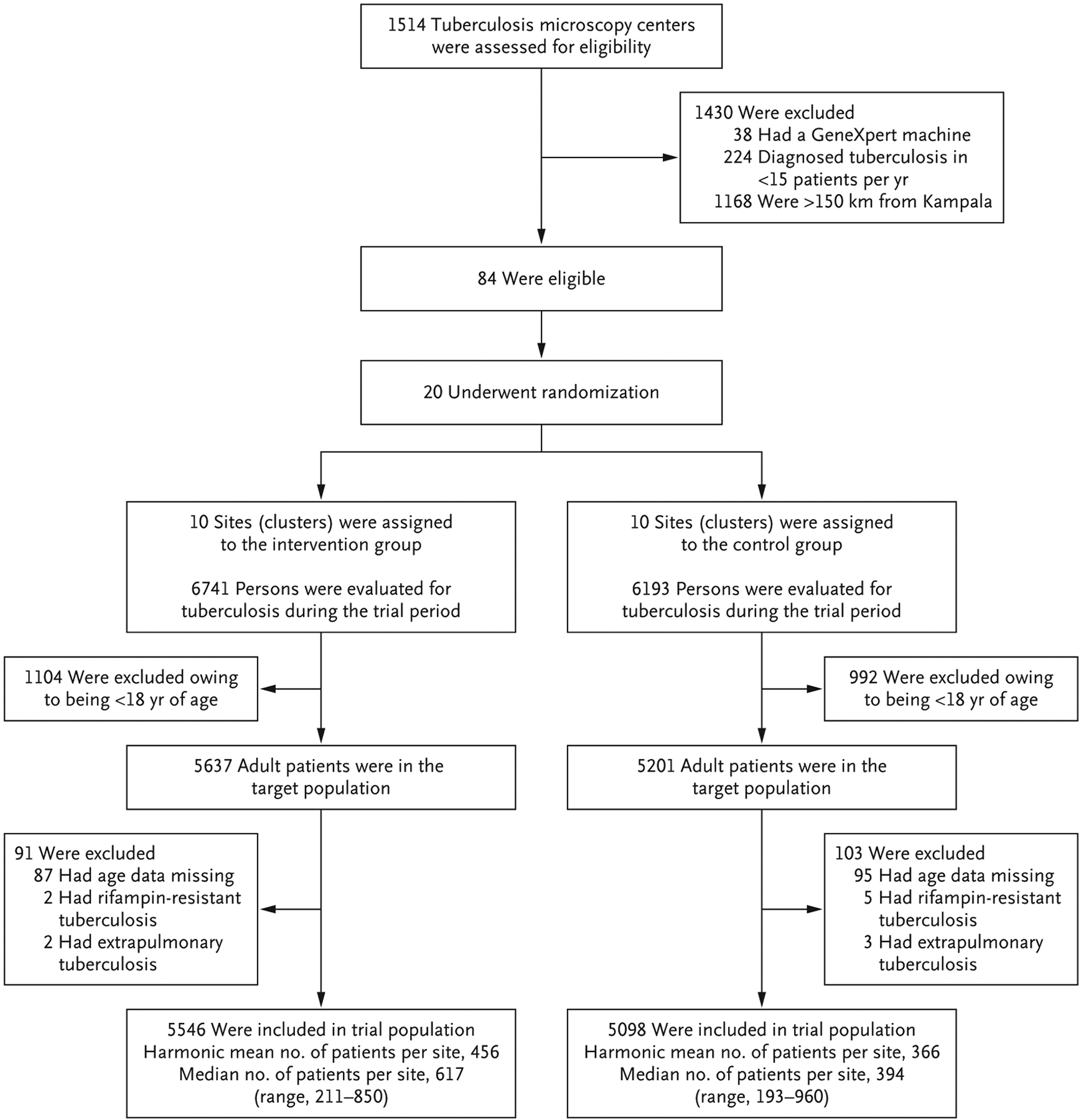
In this cluster-randomized trial, 20 community health centers in Uganda were assigned to implement a multicomponent diagnostic strategy (on-site molecular testing for tuberculosis, guided restructuring of clinic workflows, and monthly feedback of quality metrics) or to continue routine care (on-site sputum-smear microscopy and referral-based molecular testing). The trial included data on all adults (≥18 years of age) who were evaluated for tuberculosis, which was defined as having been entered into national registers regarding presumptive tuberculosis, laboratory testing for tuberculosis, or tuberculosis treatment. The trial intervention period was from October 2018 through February 2020.
Table 1.
Patient-Level and Cluster-Level Characteristics.*
| Patient-level characteristics | ||
| Female sex — no. (%) | 3289 (59.3) | 3112 (61.0) |
| Age at evaluation | ||
| Median age (IQR) — yr | 40 (30–52) | 38 (27–50) |
| Distribution — no. (%) | ||
| 18–29 yr | 1309 (23.6) | 1539 (30.2) |
| 30–39 yr | 1267 (22.8) | 1149 (22.5) |
| 40–49 yr | 1163 (21.0) | 1060 (20.8) |
| ≥50 yr | 1807 (32.6) | 1350 (26.5) |
| HIV infection status — no./total no. (%)† | ||
| Positive | 2285/5273 (43.3) | 1905/4290 (44.4) |
| Negative | 2988/5273 (56.7) | 2385/4290 (55.6) |
| Cluster-level characteristics | ||
| No. of health centers | 10 | 10 |
| Level of health center — no. (%)‡ | ||
| III | 6 (60) | 8 (80) |
| IV | 4 (40) | 2 (20) |
| Location — no. (%) | ||
| Rural | 7 (70) | 8 (80) |
| Urban | 3 (30) | 2 (20) |
| Median distance to molecular testing hub (IQR) — km | 20.5 (6–32) | 15 (13–23) |
| Median prevalence of HIV infection in prerandomization period (IQR) — % of patients | 34.3 (26.0–43.2) | 35.0 (29.9–47.7) |
| Health center region — no. (%) | ||
| Central Uganda | 7 (70) | 6 (60) |
| Eastern Uganda | 3 (30) | 4 (40) |
IQR denotes interquartile range, and HIV human immunodeficiency virus.
The analysis excluded 1081 patients with unknown status regarding HIV infection.
Level III and IV health centers are at the lowest levels of the health system where tuberculosis diagnostic and treatment services are provided. Level III health centers offer outpatient medical services only and are managed by a senior clinical officer with a diploma in clinical medicine. Level IV health centers offer those services in addition to emergency surgical services and are managed by a medical officer with a bachelor’s degree in medicine.
Of the 12,934 patients who underwent evaluation for tuberculosis during the intervention period, 2096 (16.2%) were younger than 18 years of age. Of the remaining 10,838 patients, 182 (1.7%) were excluded because data on age were missing and 12 (0.1%) because they either had rifampin resistance that was identified on molecular testing (7 patients) or were classified as having extrapulmonary tuberculosis (5 patients). Of the remaining 10,644 patients who were included in the trial, 5546 were evaluated for tuberculosis at health centers in the intervention group and 5098 at health centers in the control group. The median age of the patients was 40 years, 60.1% were women, and 43.8% had HIV infection. The harmonic mean number of patients per health center was 456 in the intervention group and 366 in the control group, and the median number of patients per health center was 617 and 394, respectively (Fig. 1). This finding suggests that the intervention increased the number of patients who underwent evaluation for tuberculosis. Patients in the two trial groups were similar with respect to sex, age, and HIV infection status (Table 1).
PRIMARY OUTCOME
During the 16-month intervention period, more patients were treated for confirmed tuberculosis within 14 days after presenting for evaluation at health centers in the intervention group than at health centers in the control group. A total of 342 patients were treated for confirmed tuberculosis across the 10 intervention health centers, as compared with 220 patients across the 10 control health centers (adjusted rate ratio, 1.56; 95% confidence interval [CI], 1.21 to 2.01) (Figs. 2 and S2 and Tables S1 and S8).
Figure 2. Forest Plot of Ratio Effect Measures for Tuberculosis Diagnosis and Treatment in Count-based and Proportion-based Outcomes.

Panel A shows a forest plot of the relative differences, with 95% confidence intervals, in count-based tuberculosis diagnosis and treatment outcomes. Analysis of count-based outcomes was done at the cluster (health center) level with adjustment for randomization strata (fixed effect, two levels) and the number of patients treated for confirmed tuberculosis within 14 days after presentation during the 12-month prerandomization period (linear term). The primary outcome was treatment for confirmed tuberculosis within 14 days after presentation. The rate ratio is the number of persons with the outcome per observation day in the intervention group as compared with the control group. Panel B shows a forest plot of relative differences in proportion-based outcomes. Analysis of proportion-based outcomes was done at the cluster level with adjustment for cluster-level covariates (randomization strata [fixed effect, two levels] and the proportion of patients who were treated for confirmed tuberculosis within 14 days after presentation during the 12-month prerandomization period [linear term]) and patient-level covariates (age, sex, and human immunodeficiency virus [HIV] infection status [yes or no; persons with unknown status were excluded]). Adjustment for patient-level covariates was conducted with the use of a two-stage approach.21 The denominator for all the proportion-based outcomes was the number of patients evaluated for tuberculosis. The numerator for “Tested in accordance with national guidelines” was the number of patients who completed recommended testing (one valid Xpert Ultra result or, for patients without known HIV infection, one positive or two negative results on sputum-smear microscopy). The numerator for “Received diagnosis of confirmed tuberculosis” was the number of patients who received a diagnosis of confirmed tuberculosis within 1 day (same day) or 14 days after presentation. The numerator for “Treated for confirmed tuberculosis” was the number of patients treated for confirmed tuberculosis within 1 day (same day) or 14 days after presentation. The numerator for “Treated for tuberculosis” was the number of patients who were treated for tuberculosis within 1 day (same day) or 14 days after presentation.
SECONDARY OUTCOMES
The trial intervention improved yield and timeliness at each step of the tuberculosis diagnostic evaluation cascade of care. The number of patients who were tested for tuberculosis in accordance with national guidelines was higher at health centers in the intervention group than at health centers in the control group (adjusted rate ratio, 1.85; 95% CI, 1.21 to 2.82), as were the numbers of patients who received a diagnosis of confirmed tuberculosis on the same day (adjusted rate ratio, 1.89; 95% CI, 1.39 to 2.56) or within 14 days after presentation (adjusted rate ratio, 1.28; 95% CI, 0.99 to 1.66), who were treated for confirmed tuberculosis on the same day (adjusted rate ratio, 2.38; 95% CI, 1.57 to 3.61), and who were treated for tuberculosis (confirmed or clinical) on the same day (adjusted rate ratio, 1.90; 95% CI, 1.21 to 2.98) or within 14 days (adjusted rate ratio, 1.48; 95% CI, 1.04 to 2.12) (Fig. 2 and Table S1).
Among all the patients who were evaluated for tuberculosis, the trial intervention led to greater proportions of patients than the control strategy with regard to the completion of testing in accordance with national guidelines (adjusted rate ratio, 1.57; 95% CI, 1.39 to 1.78), diagnosis of confirmed tuberculosis on the same day (adjusted rate ratio, 1.42; 95% CI, 0.99 to 2.02), and treatment for confirmed tuberculosis on the same day (adjusted rate ratio, 2.18; 95% CI, 1.17 to 4.05) (Fig. 2). Although the differences in the proportions of patients who received a diagnosis and were treated were not significant at 14 days (Fig. 2), the adjusted geometric mean number of days to the diagnosis of tuberculosis and to treatment for tuberculosis were 51% (95% CI, 38 to 61) lower and 65% (95% CI, 44 to 79) lower, respectively, in the intervention group than in the control group. In addition, among the 706 patients with confirmed tuberculosis, a higher proportion at health centers in the intervention group than at health centers in the control group received a diagnosis on the same day (adjusted rate ratio, 1.50; 95% CI, 1.25 to 1.80), were treated on the same day (adjusted rate ratio, 2.29; 95% CI, 1.23 to 4.25), and were treated within 14 days after presentation (adjusted rate ratio, 1.22; 95% CI, 1.06 to 1.40). Details are provided in Tables S2, S3, and S4.
SUBGROUP ANALYSES
Overall, there was no evidence that trial outcomes differed according to sex or HIV infection status. The numbers of patients who were treated for confirmed tuberculosis within 14 days after presentation were similarly greater in the intervention group than in the control group among both men (adjusted rate ratio, 1.59; 95% CI, 1.21 to 2.09) and women (adjusted rate ratio, 1.46; 95% CI, 1.03 to 2.07) and among both persons with HIV infection (adjusted rate ratio, 1.78; 95% CI, 1.15 to 2.77) and those without HIV infection (adjusted rate ratio, 1.46; 95% CI, 0.98 to 2.18) (Table 2). With regard to secondary outcomes at each step of the tuberculosis diagnostic evaluation cascade of care, the proportions were similarly greater in the intervention group than in the control group among both men and women and among both persons with HIV infection and those without HIV infection (Tables S5 and S6).
Table 2.
Subgroup Analysis of Treatment for Confirmed Tuberculosis within 14 Days after Presentation (Primary Outcome).*
| Subgroup | Intervention | Control | Unadjusted Rate Ratio (95% CI)† | Adjusted Rate Ratio (95% CI)‡ |
|---|---|---|---|---|
| All patients | 342 | 220 | 1.55 (1.16–2.08) | 1.56 (1.21–2.01) |
| Sex | ||||
| Male | 234 | 147 | 1.59 (1.17–2.17) | 1.59 (1.21–2.09) |
| Female | 108 | 73 | 1.48 (1.02–2.15) | 1.46 (1.03–2.07) |
| HIV infection status§ | ||||
| Positive | 134 | 75 | 1.79 (1.13–2.83) | 1.78 (1.15–2.77) |
| Negative | 206 | 144 | 1.43 (0.94–2.18) | 1.46 (0.98–2.18) |
Shown are the counts of patients who received treatment for confirmed tuberculosis within 14 days after presentation in the 16-month intervention period (497 days). The rate ratio is the number of patients with the outcome per observation day in the intervention group as compared with the control group.
The unadjusted analysis was at the cluster level with adjustment for randomization strata (fixed effect, two levels).
The adjusted analysis was at the cluster level with adjustment for cluster-level covariates (randomization strata [fixed effect, two levels] and the number of patients treated for confirmed tuberculosis within 14 days after presentation during the 12-month prerandomization period [linear term]).
The analysis excluded 1081 patients with unknown status regarding HIV infection.
DISCUSSION
In this cluster-randomized trial conducted at 20 community health centers in Uganda, we found that the multicomponent XPEL-TB strategy led to a 56% higher rate of treatment for confirmed tuberculosis within 14 days after presentation than the control strategy. In addition, the trial intervention improved the completion and timeliness of earlier steps along the cascade of care, which led to more patients being tested for tuberculosis in accordance with national guidelines and receiving a diagnosis of confirmed tuberculosis.
The effects of the trial strategy on tuberculosis diagnosis and treatment outcomes were less robust at 14 days than at 1 day and were also less robust when the effects were analyzed on the basis of proportions (of persons who underwent evaluation for tuberculosis) rather than on the basis of counts. The smaller between-group differences at 14 days than at 1 day were expected, because some patients returned after the initial health center visit for additional testing or to initiate treatment. However, in the intervention group, two thirds of the patients with confirmed tuberculosis had treatment initiated at their initial health center visit, and the proportion of patients who had treatment initiated rapidly (i.e., within 14 days) was twice the proportion in the control group. The stronger effects that were seen for count-based outcomes than for proportion-based outcomes may be explained by the finding that more persons were evaluated for tuberculosis at intervention health centers than at control health centers. This hypothesis is consistent with the more uniform and larger increases from the intervention period to the prerandomization period in the numbers of patients who were evaluated for tuberculosis at the intervention health centers than at the control health centers.
The appropriate placement of molecular tests for tuberculosis is a fundamental policy question that has not yet been addressed adequately. In the context of facility-based case finding, three previous randomized trials have evaluated decentralized Xpert testing as compared with sputum-smear microscopy alone.22–24 One other trial compared decentralized with centralized Xpert testing (as was done in our trial), but that trial randomly assigned time blocks of 2 weeks at a single clinic, and there are limited data regarding the evaluation of decentralized Xpert testing along with other intervention components that might be necessary for improving patient care in the real world. Our large trial, which involved 10,644 persons, addressed the question of appropriate placement of molecular tests directly. We found that decentralized molecular testing with the use of newer-generation molecular diagnostic platforms was feasible at community health centers and, when combined with workflow redesign and performance feedback, was able to improve the quality and outcomes of tuberculosis diagnostic evaluation. The highly pragmatic trial design and implementation (involving minimal patient-eligibility criteria, a waiver of patient informed consent, no additional trial-specific tuberculosis testing, the use of only routinely collected data to assess outcomes, and a minimal presence of research staff at the health centers during the trial period) increased the likelihood that these findings would reflect what could be expected in usual care25,26 and that the trial strategy may have similar effectiveness if it is implemented in analogous settings in other countries with a high prevalence of tuberculosis.
Our trial has some limitations. First, the primary outcome was changed after the trial started from an assessment on the basis of proportions of patients to an assessment on the basis of counts of patients who were treated for confirmed tuberculosis within 14 days after presentation. The change was made with input from an external trial steering committee before any analysis of trial outcomes. Second, because of the relatively small number of health centers (clusters), it is possible that the clusters had imbalance in the underlying prevalence of tuberculosis or other factors. To minimize this possibility, we collected prerandomization data that informed stratified and restricted randomization. Finally, because we tested a multicomponent intervention, it is not possible to know whether similar results would have been observed with decentralized molecular testing alone. However, our intervention strategy reflects the situation that new tests alone are unlikely to be a silver bullet in closing gaps in the tuberculosis care cascade.15
In this highly pragmatic trial, the multicomponent XPEL-TB strategy, which included decentralized molecular testing, structured redesign of clinic-level processes to facilitate same-day testing and treatment, and monthly performance feedback, led to a greater number of patients treated for confirmed tuberculosis at community health centers in Uganda than did the control strategy. As additional platforms for decentralized molecular testing become available, this trial provides strong evidence in support of their rapid implementation at community health centers in countries with a high prevalence of tuberculosis, along with feasible strategies to promote quality improvement.
Supplementary Material
Acknowledgments
Supported by grants (R01 HL130192 and K12 HL138046) from the National Heart, Lung, and Blood Institute, National Institutes of Health.
We thank the patients and staff at the participating health centers; the staff of the Uganda National Tuberculosis and Leprosy Program and the Uganda National Tuberculosis Reference Laboratory; and the members of the trial steering committee (Drs. Gerald Friedland, Madhukar Pai, Andrew Ramsay, and Grant Theron). Access to GeneXpert Edge devices and Xpert MTB/RIF Ultra test cartridges was provided by the Foundation for Innovative New Diagnostics.
Footnotes
REFERENCES
- 1.Global tuberculosis report 2019. Geneva: World Health Organization, 2019. (https://www.who.int/publications/i/item/9789241565714). [Google Scholar]
- 2.MacPherson P, Houben RM, Glynn JR, Corbett EL, Kranzer K. Pre-treatment loss to follow-up in tuberculosis patients in low- and lower-middle-income countries and high-burden countries: a systematic review and meta-analysis. Bull World Health Organ 2014; 92: 126–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cattamanchi A, Dowdy DW, Davis JL, et al. Sensitivity of direct versus concentrated sputum smear microscopy in HIV-infected patients suspected of having pulmonary tuberculosis. BMC Infect Dis 2009; 9: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chihota VN, Ginindza S, McCarthy K, Grant AD, Churchyard G, Fielding K. Missed opportunities for TB investigation in primary care clinics in South Africa: experience from the XTEND trial. PLoS One 2015; 10(9): e0138149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis J, Katamba A, Vasquez J, et al. Evaluating tuberculosis case detection via real-time monitoring of tuberculosis diagnostic services. Am J Respir Crit Care Med 2011; 184: 362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aspler A, Menzies D, Oxlade O, et al. Cost of tuberculosis diagnosis and treatment from the patient perspective in Lusaka, Zambia. Int J Tuberc Lung Dis 2008; 12: 928–35. [PubMed] [Google Scholar]
- 7.Albert H, Nathavitharana RR, Isaacs C, Pai M, Denkinger CM, Boehme CC. Development, roll-out and impact of Xpert MTB/RIF for tuberculosis: what lessons have we learnt and how can we do better? Eur Respir J 2016; 48: 516–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horne DJ, Kohli M, Zifodya JS, et al. Xpert MTB/RIF and Xpert MTB/RIF Ultra for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2019; 6(6): CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keeler E, Perkins MD, Small P, et al. Reducing the global burden of tuberculosis: the contribution of improved diagnostics. Nature 2006; 444: Suppl 1: 49–57. [DOI] [PubMed] [Google Scholar]
- 10.Churchyard GJ, Stevens WS, Mametja LD, et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Glob Health 2015; 3(8): e450–e457. [DOI] [PubMed] [Google Scholar]
- 11.Durovni B, Saraceni V, van den Hof S, et al. Impact of replacing smear microscopy with Xpert MTB/RIF for diagnosing tuberculosis in Brazil: a stepped-wedge cluster-randomized trial. PLoS Med 2014; 11(12): e1001766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mupfumi L, Makamure B, Chirehwa M, et al. Impact of Xpert MTB/RIF on anti-retroviral therapy-associated tuberculosis and mortality: a pragmatic randomized controlled trial. Open Forum Infect Dis 2014; 1(1): ofu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drain PK, Garrett NJ. The arrival of a true point-of-care molecular assay — ready for global implementation? Lancet Glob Health 2015; 3(11): e663–e664. [DOI] [PubMed] [Google Scholar]
- 14.Molecular assays intended as initial tests for the diagnosis of pulmonary and extrapulmonary TB and rifampicin resistance in adults and children: rapid communication. Geneva: World Health Organization, 2020. (https://apps.who.int/iris/handle/10665/330395). [Google Scholar]
- 15.Pai M, Schumacher SG, Abimbola S. Surrogate endpoints in global health research: still searching for killer apps and silver bullets? BMJ Glob Health 2018; 3(2): e000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochodo EA, Naidoo S, Schumacher S, et al. Improving the design of studies evaluating the impact of diagnostic tests for tuberculosis on health outcomes: a qualitative study of perspectives of diverse stakeholders. Wellcome Open Res 2019; 4: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cattamanchi A, Miller CR, Tapley A, et al. Health worker perspectives on barriers to delivery of routine tuberculosis diagnostic evaluation services in Uganda: a qualitative study to guide clinic-based interventions. BMC Health Serv Res 2015; 15: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reza TF, Nalugwa T, Nantale M, et al. Design and execution of a public randomization ceremony to enhance stakeholder engagement within a cluster randomized trial to improve tuberculosis diagnosis in Uganda. Contemp Clin Trials Commun 2021; 22: 100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reza TF, Nalugwa T, Farr K, et al. Study protocol: a cluster randomized trial to evaluate the effectiveness and implementation of onsite GeneXpert testing at community health centers in Uganda (XPEL-TB). Implement Sci 2020; 15: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) — a meta-data-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayes RJ, Moulton LH. Cluster randomised trials, 1st ed. New York: Chapman and Hall/CRC, 2009. [Google Scholar]
- 22.Cox HS, Mbhele S, Mohess N, et al. Impact of Xpert MTB/RIF for TB diagnosis in a primary care clinic with high TB and HIV prevalence in South Africa: a pragmatic randomised trial. PLoS Med 2014; 11(11): e1001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ngwira LG, Corbett EL, Khundi M, et al. Screening for tuberculosis with Xpert MTB/RIF assay versus fluorescent microscopy among adults newly diagnosed with human immunodeficiency virus in rural Malawi: a cluster randomized trial (Chepetsa). Clin Infect Dis 2019; 68: 1176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theron G, Zijenah L, Chanda D, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet 2014; 383: 424–35. [DOI] [PubMed] [Google Scholar]
- 25.Ford I, Norrie J. Pragmatic trials. N Engl J Med 2016;375: 454–63. [DOI] [PubMed] [Google Scholar]
- 26.Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015; 350: h2147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


