Abstract
Several cases of cancer patients with 18-fluorodeoxyglucose (18FDG) Positron Emission Tomography/Computed Tomography (PET/CT) evidence of metabolically active axillary lymph nodes after COVID-19 vaccination have been described, creating a diagnostic dilemma and sometimes leading to further unnecessary examinations. A 62-year-old male, diagnosed with prostate cancer, treated with hormone-therapy and radiotherapy of the prostate 2 years before, underwent fluorine-18 choline (F-FCH) PET/CT for restaging purpose, less than 3 weeks after he had received the second dose of the Pfizer BioNTech-BNT162b2 mRNA COVID-19 vaccine. This exam showed an increased F-FCH uptake and an enlargement of the left axillary, paratracheal, para-aortic, subcarinal, and hilar bilateral lymph nodes. Fourteen weeks later, the patient underwent a new F-FCH PET-CT scan, displaying an almost complete regularization of the FCH uptake in all the previously involved regions. The patient was not treated after the first PET-CT scan, thus, the aforementioned PET/CT findings represented inflammatory vaccine-related lymph nodes. This case highlights the significance of knowing vaccination history to correctly interpret imaging findings and to avoid false-positive reports.
Keywords: Choline, COVID-19, PET/CT, Vaccination, Prostate Cancer
Introduction
World-wide healthcare systems have been profoundly affected by the COVID-19 virus pandemic, and in Italy a national mass vaccination using the Pfizer BNT162b2 mRNA vaccine initiated in December 2020, with early attempt to prioritize cancer patients. As soon as mass COVID-19 vaccination started worldwide, Vaccine-Associated Hypermetabolic Lymphadenopathy (VAHL) began to be noticed as a possible side effect [1].
Several authors reported cancer patients with 18-fluorodeoxyglucose (18FDG) positron emission tomography/computed tomography (PET/CT) evidence of metabolically active axillary lymphnodes after COVID-19 vaccination. In all cases reported, in the clinical context of cancer history lymph node enlargement and metabolic uptake generate a diagnostic dilemma and might lead to further unnecessary examinations [2], [3], [4], [5].
Benign lymphadenopathy in the vaccine injection site nearest regions is considered a very common consequence, not only after COVID vaccination; it is also reported as an effect of H1N1 and human papillomaviruses vaccines [6,7]. Hypermetabolic lymphadenopathy is usually identified as an increased lymph nodes uptake on PET-CT occurring not only in cancer-related situations but also during an occurring inflammatory process [8].
Case report
A 62-year-old male, diagnosed with prostate cancer (Gleason 9) in 2019, with lymph nodes and bone metastasis, was initially treated with hormone-therapy and radiotherapy of the prostate (80 Gy) and pelvic lymph nodes (45 Gy), and thereafter started a regular follow-up, without evidence of recurrence for 2 years. Subsequently, the patient underwent a new follow-up F-FCH PET-CT, less than 3 weeks after he had received the second dose of the Pfizer BioNTech-BNT162b2 mRNA COVID-19 vaccine with an intramuscular left deltoid injection.
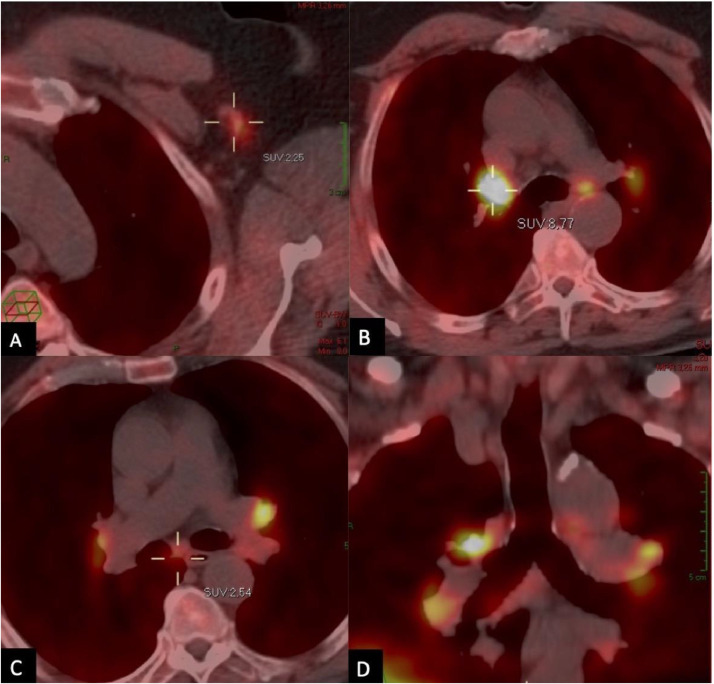
The PET-CT showed a slight F-FCH uptake and enlargement of the left axillary lymph nodes (Fig. 1), findings already reported by other authors following COVID-19 vaccine [16].
Fig. 1.
June 2021. PET-CT axial images showing a slight F-FCH uptake and an enlargement of the left axillary (A), hilar bilateral (B) and subcarinal (C) lymph nodes. (D) Coronal PET-CT reconstruction better displaying the findings.
At the same time, the scan also revealed an increased F-FCH uptake in paratracheal, para-aortic, subcarinal, and hilar bilateral lymph nodes. This outcome was unexpected, considering the clinical history of the patient, without evidence of pulmonary disease.
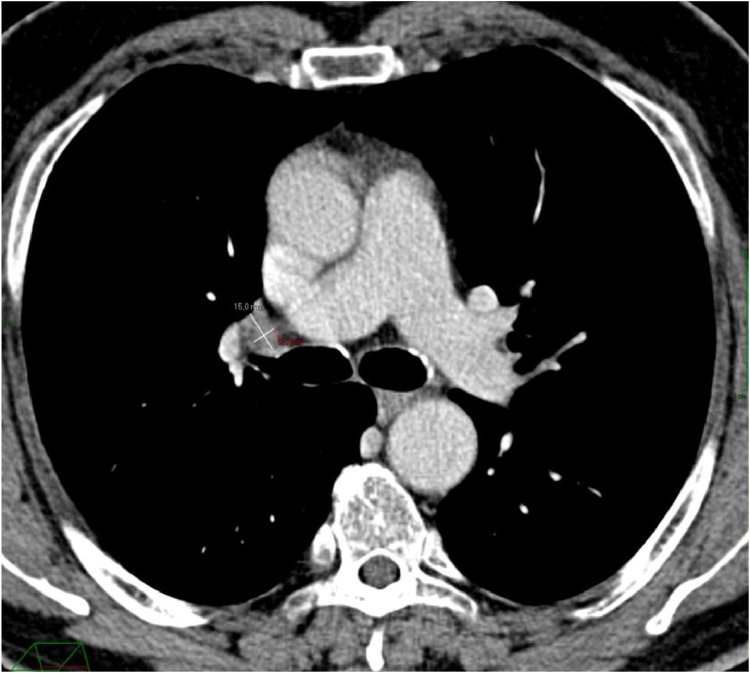
There was no evidence of increase in the PSA levels (1.17 ng/mL) and the comparison of the imaging findings with the staging CT scan performed 2 years earlier (Fig. 2), revealed the morphological stability of lymph nodes.
Fig. 2.
September 2019. Axial portal-phase CT scan, revealing the dimensional stability of the right hilar lymph node.
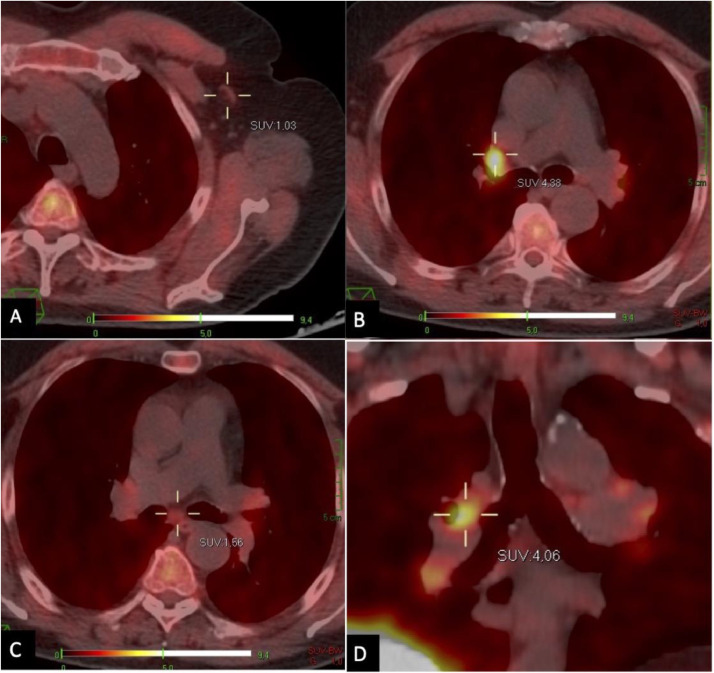
Thus, considering dimensional stability of lymph nodes and serum PSA level steadiness, no therapies were prescribed, and, 14 weeks later (Fig. 3), the patient underwent a new F-FCH PET-CT scan, displaying an important decrease of the FCH uptake in all the previously involved regions, proving that the hypermetabolic lymphadenopathy was a side effect of the COVID-19 vaccine, not associated with his oncological disease.
Fig. 3.
September 2021. PET-CT axial images revealing a significant reduction of the F-FCH uptake in left axillary (A), hilar (B) and subcarinal (C) lymph nodes. (D) Coronal PET-CT reconstruction better displaying the findings. Compare findings with figure 1.
Discussion
It is established that vaccine administration leads to a local inflammatory response in a certain number of cases, involving injection site muscles and the lymphnodes afferent to the injection site. Therefore, this is responsible of the increased 18FDG uptake of axillary lymphnodes on the PET-CT [9].
As different studies show, this condition can be challenging when occurring in oncological patients: it is hard to define whether the lymphnodes uptake is due to a cancer recurrence or not [10,11], particularly when considering fluorine-18 choline (F-FCH) PET-CT increased uptake.
More recently, the association between choline phospholipid metabolism and macrophage immune responsiveness has been recognized and it is due to the increase of the choline transporter-like protein-1 (CTL1) which allows the uptake of choline in activated macrophages that populate the pneumonia-affected lungs [12]. 18F-Fluorocholine is a synthetic analog of the natural choline, and this may help understanding the evidence of COVID-19-induced lung lesions with an amplified uptake of 18F-fluorodeoxyglucose [13,14] and 18F-fluorocholine [15,16] but there are no reports of F-FCH increased uptake after COVID-19 vaccination.
To our knowledge this is one case of vaccine-associated hypermetabolic lymphadenopathy involving lymph nodes that are located far away from the injection site, especially mediastinal and pulmonary hilar locations, which can create a diagnostic challenge in patients with hitory of prostate cancer. This case highlights the significance of knowing vaccination history to correctly interpret the findings and to avoid false-positive reports.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Patient consent
Written informed consent for publication of their case was obtained from the patients.
Footnotes
Competing Interests: All authors declare no conflict of interest.
Funding: No funding sources.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2022.05.072.
Appendix. Supplementary materials
References
- 1.Cohen D., Krauthammer S.H., Wolf I., Even-Sapir E. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA COVID-19 vaccine: incidence assessed by [18F]FDG PET-CT and relevance to study interpretation. Eur J Nucl Med Mol Imaging. 2021;48(6):1854–1863. doi: 10.1007/s00259-021-05314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nawwar A.A., Searle J., Hopkins R., Lyburn I.D. False-positive axillary lymph nodes on FDG PET/CT resulting from COVID-19 immunization. Clin Nucl Med. 2021;46(12):1004–1005. doi: 10.1097/RLU.0000000000003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Özütemiz C., Krystosek L.A., Church A.L., Chauhan A., Ellermann J.M., Domingo-Musibay E., et al. Lymphadenopathy in COVID-19 vaccine recipients: diagnostic dilemma in oncologic patients. Radiology. 2021;300(1) doi: 10.1148/radiol.2021210275. E296-E300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mortazavi S. Coronavirus Disease (COVID-19) Vaccination associated axillary adenopathy: imaging findings and follow-up recommendations in 23 women. AJR Am J Roentgenol. 2021;217(4):857–858. doi: 10.2214/AJR.21.25651. [DOI] [PubMed] [Google Scholar]
- 5.Seely J.M. Correction to: the Canadian Society of Breast Imaging/Canadian Association of Radiologists' Recommendations for the management of axillary adenopathy in patients with recent COVID-19 vaccination. Can Assoc Radiol J. 2021;73(2):432. doi: 10.1177/08465371211029086. [DOI] [PubMed] [Google Scholar]
- 6.Burger I.A., Husmann L., Hany T.F., Schmid D.T., Schaefer N.G. Incidence and intensity of F-18 FDG uptake after vaccination with H1N1 vaccine. Clin Nucl Med. 2011;36(10):848–853. doi: 10.1097/RLU.0b013e3182177322. [DOI] [PubMed] [Google Scholar]
- 7.Coates E.E., Costner P.J., Nason M.C., Herrin D.M., Conant S., Herscovitch P., et al. Lymph node activation by PET/CT following vaccination with licensed vaccines for human papillomaviruses. Clin Nucl Med. 2017;42(5):329–334. doi: 10.1097/RLU.0000000000001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez Portilla A., Onaindia E., Larrañaga M., López de Heredia E., Echenagusía V. Periprosthetic seroma with false-positive FDG PET-CT reactive nodes mistaken for metastases in a patient previously treated of metastasic melanoma. Potential source of diagnostic errors. Int J Surg Case Rep. 2017;38:66–68. doi: 10.1016/j.ijscr.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenberg M.T., Tingey C., Fulton O., Owen J., Snyder T. Quadrilateral space region inflammation and other incidental findings on shoulder MRI following recent COVID-19 vaccination: three case reports. Radiol Case Rep. 2021;16(10):3024–3028. doi: 10.1016/j.radcr.2021.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landete E., Gómez-Fernández I., González-Gascón-Y-Marín I., Durán-Barquero C., Churruca J., Infante M.S., et al. Hypermetabolic abdominal and cervical lymph nodes mimicking Hodgkin lymphoma relapse on FDG PET/CT after adenovirus-vectored COVID-19 vaccine. Hum Vaccin Immunother. 2021;17(12):5129–5132. doi: 10.1080/21645515.2021.2008215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gullotti D.M., Lipson E.J., Fishman E.K., Rowe S.P. Acute axillary lymphadenopathy detected shortly after COVID-19 vaccination found to be due to newly diagnosed metastatic melanoma. Radiol Case Rep. 2022;17(3):878–880. doi: 10.1016/j.radcr.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savelli G., Bonacina M., Rizzo A., Zaniboni A. Activated macrophages are the main inflammatory cell in COVID-19 interstitial pneumonia infiltrates. Is it possible to show their metabolic activity and thus the grade of inflammatory burden with 18F-Fluorocholine PET/CT? Med Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.109885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin C., Liu F., Yen T.C., Lan X. 18F-FDG PET/CT findings of COVID-19: a series of four highly suspected cases. Eur J Nucl Med Mol Imaging. 2020;47(5):1281–1286. doi: 10.1007/s00259-020-04734-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albano D., Bertagna F., Bertolia M., Bosio G., Lucchini S., Motta F., et al. Incidental findings suggestive of COVID-19 in asymptomatic patients undergoing nuclear medicine procedures in a high prevalence region. J Nucl Med. 2020;61(5):632–636. doi: 10.2967/jnumed.120.246256. [DOI] [PubMed] [Google Scholar]
- 15.García Vicente A.M., Soriano Castrejón Á. Incidental COVID-19 pneumonia on 18F-Fluorocholine PET/CT. Clin Nucl Med. 2020;45(8):e376–e377. doi: 10.1097/RLU.0000000000003189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albano D., Volpi G., Dondi F., Giubbini R., Bertagna F. COVID-19 vaccination manifesting as unilateral lymphadenopathies detected by 18F-Choline PET/CT. Clin Nucl Med. 2022;47(2):e187–e189. doi: 10.1097/RLU.0000000000003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.