Abstract
Decreased smell function is related to brain health, future mortality, and quality of life. Most people inflicted with the SARS-CoV-2 virus evidence some measurable smell dysfunction during its acute phase, although many are unaware of the loss. Long-term deficits occur in up to 30% of COVID-19 cases, although total anosmia is relatively rare. This review explores what is presently known about the nature and pathophysiology of olfactory dysfunction due to the SARS-CoV-2 virus, including reversible inflammation within the olfactory cleft, downregulation of olfactory receptor proteins, and long-lasting peripheral and central damage to olfactory structures. It also addresses the question as to whether long-term smell loss might increase the likelihood of future development of cognitive and neurological deficits.
Keywords: COVID-19, olfaction, anosmia, SARS-CoV-2, taste, neuropathology
Olfactory dysfunction: a marker of brain health, quality of life, future mortality, and insults from environmental microbes and xenobiotics
The smell loss associated with coronavirus disease 2019 (COVID-19) has brought to public attention the importance of olfaction in everyday life. Although largely taken for granted, this sensory system is critical for establishing the flavor of foods and beverages and for defending against such dangers as leaking natural gas, fire, spoiled food, and environmental toxins. We use smell to confirm that our clothes, homes, and offices are clean and to fully enjoy flowers, perfumes, festive occasions, personal care products, and nature (e.g., the mountains and the sea shore). It is thus not surprising that losses or distortions in the ability to smell significantly impact our safety, nutrition, and physical and psychological well-being.
Aside from aging, viruses are the primary cause of long-lasting or permanent decrements in smell function, a condition termed postviral olfactory disorder (PVOD) [1,2]. Smell deficits are associated with a number of medical conditions known to have viral underpinnings, including numerous cardiovascular, endocrine, immune, metabolic, and neurodegenerative diseases [3., 4., 5.]. Several viruses, such as hepatitis B and C, confer an elevated risk for Parkinson’s disease (PD) [5], a disorder with marked olfactory dysfunction [6]. In a case–control study using the Danish National Patient Registry data, those who had an influenza viral diagnosis were 1.73 times more likely to develop PD over a subsequent 10-year period [7]. Alzheimer’s disease (AD) is also accompanied with significant olfactory dysfunction [8]. Recently it was found that among the top ten of 5066 genes differentially expressed between 51 AD patients and 31 controls were ones associated with viral infection signaling, namely those of herpes simplex (HSV, HSV1), Epstein-Barr, and human papillomavirus [9]. Interestingly, a gene associated with olfactory transduction, adenylate cyclase 3 (ADCY3) (see Glossary), was also among this group of ten. HSV 1 is found in a high proportion of brains from elderly people and, in combination with the APOE-ε4 genotype, is a major risk for AD [10]. Remarkably, older people with smell loss have three times the likelihood of an earlier death than their peers with no smell loss, leading to the suggestion that smell function is a sign of overall brain health [11,12].
This review explores what is presently known about the nature, prevalence, and pathophysiology of olfactory dysfunction due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for COVID-19. In common with other viruses, SARS-CoV-2 subjugates the synthetic machinery of living cells to replicate its own genetic information. Similarly, its effectiveness depends upon the presence of appropriate receptors for cell attachment and entry, tropism for particular tissues or cells, efficacy of virus genome replication, and the immune status of the host. Hypotheses regarding how the virus damages olfaction-related cells and whether long-term smell loss increases the chances for later development of psychological and neurological problems are addressed.
Smell loss can be the most common symptom of COVID-19
A number of surveys find that the strongest and most consistent symptom associated with COVID-19 is decreased smell or taste function. One study evaluated 514 459 records from over 10 million respondents to three COVID-19 digital surveillance platforms employed in the United States, Israel, and Great Britain [13]. People reporting loss of smell (anosmia) or taste (ageusia) were 17 times more likely to test positive for COVID-19 than those without these symptoms. Those reporting fever were 6.5 times more likely, shortness of breath 4.7 times more likely, or cough 4.3 times more likely. However, prevalence rates of COVID-19-related smell or taste dysfunction vary considerably among surveys, ranging from 8% to 85% [14], conceivably reflecting not only response biases, but variations in such factors as age, race, gender, vaccination status, smoking behavior, genetics, time since infection onset, comorbidities, and the specific COVID-19 variant.
Early in the pandemic, much lower prevalence rates were noted in eastern Asian countries than in European countries [15], in spite of higher symptom severity in Asian countries [16]. One COVID-19 study of 624 pairs of monozygotic and 288 pairs of dizygotic twins found 19% of the individual differences in self-reported olfactory dysfunction was attributable to genetic factors, potentially reflecting differences in viral infection susceptibility and immune responsiveness [17]. Importantly, some SARS-CoV-2 variants appear to have greater effects than others. For example, a single nucleotide polymorphism from D614 to G614 in the virus’ spike protein is associated with a significantly higher prevalence of smell loss [15,18]. More recent COVID-19 variants reportedly produce relatively less smell dysfunction, although objective testing is limited and a confound with vaccination status may be present. In a study of 3431 COVID-19 cases, the relationship between self-reported smell loss and COVID-19 was lower during the period of the omicron variant peak [27 December 2021–7 February 2022; odds ratio (OR) 0.17, 95% confidence interval (CI) 0.15–0.18] than during the period of the initial/untyped SARS-CoV-2 variant (22 June–3 August 2020) [19]. This was also true, albeit not to the same degree, for the peak periods of the alpha (19 April–31 May 2021) and delta (20 September–1 November 2021) variants [respective ORs = 0.50 (95% CI, 0.45–0.55) and 0.44 (0.41–0.48; all P < 0.001].
Most surveys fail to distinguish between olfaction and taste, the latter reflecting taste bud-mediated sensations such as sweet, sour, bitter, salty, and savory/brothy (called umami). Clinically, nearly all complaints of ‘taste’ loss actually reflect olfactory disturbances [1] (see Clinician’s corner). During chewing and swallowing, volatiles from foods and beverages enter the olfactory receptor region from the oral cavity via the opening in the rear of the nasal cavity (nasopharynx), producing sensations misinterpreted as ‘taste’ (e.g., chocolate, coffee, steak sauce, mint, peach, cheese, etc.) [20]. Thus, in many cases, it is unclear whether, or to what relative degree, smell, taste, or both are compromised by infection with SARS-CoV-2. A recent meta-analysis of 241 studies concluded that taste loss is a distinct symptom of COVID-19, with a pooled prevalence rate of 39.4% [21]. However, only 2% of the studies employed validated taste tests and their findings are equivocal. Some reported no meaningful effects on average taste test scores [22., 23., 24.], whereas others reported prevalence rates of 12% [25], 18% [26], 23% [27], and 26% [28].
Clinician’s corner.
Smell dysfunction has made the public aware of the important role that olfaction plays in their everyday life (e.g., safety and determining the flavor of foods and beverages), leading them to seek help for their problem from multiple medical practitioners.
Large surveys suggest that decreased smell or taste function is the strongest and most consistent symptom associated with COVID-19, although significant geographic, ethnic, and genetic factors, including those related to the host and the virus itself, appear to be at play. The magnitude of the dysfunction, when measured quantitatively, appears to be similar to that observed in smell losses from other types of upper respiratory viruses as well as those related to Alzheimer’s disease or Parkinson’s disease.
Although the olfactory dysfunction caused by SARS-CoV-2 resolves in most people within a few weeks, long-term and likely permanent deficits appear to be present in up to 30% of those who have been infected.
COVID-19-related smell dysfunction may reflect a combination of pathophysiologic factors, including blockage of airflow to receptors due to localized inflammation and alterations in mucus within the olfactory cleft, downregulation of olfactory receptor proteins, damage to the olfactory neuroepithelium, and subtle alterations in central brain structures related to olfaction, most notably the olfactory bulb. The involvement of capillary endothelia cannot be ruled out.
With the exception of the olfactory receptor cells, SARS-CoV-2 invades all types of cells within the olfactory neuroepithelium via the angiotensin-converting enzyme II (ACE2) receptor. Entry is facilitated by cleavage of the virus’ spike protein by the cell’s transmembrane protease serine enzyme (TMPRSS2). Other receptors may also be involved, including neuropilin 1 (NRP1) and BSG, although to what degree this occurs in olfaction-related structures is not known.
It remains unknown whether SARS-CoV-2 invades the brain via the olfactory neuroepithelium.
Alt-text: Clinician’s corner
Regrettably, surveys also suffer from reliance on self-report, which is often at odds with measured test performance. A significant number of people are unaware of a taste or smell disorder prior to being objectively tested, regardless of its cause, stressing the need for empirical testing (Box 1 ) [6,8,27,29., 30., 31.]. In the case of COVID-19, a meta-analysis of 104 olfactory studies, of which 13 employed objective test measures, found those using objective measures had an estimated prevalence rate of 65.52% (95% CI, 52.26–76.74%), whereas those based solely on self-report had a prevalence rate of 38.84% (31.96–46.2%) [15]. In a pioneering study, the 40-item University of Pennsylvania Smell Identification Test (UPSIT) was administered to 60 COVID-19 patients near the end of the disease’s acute phase [41]. While nearly all (98%) exhibited some degree of measured smell dysfunction [25% anosmia (total smell loss), 33% severe microsmia (lessened smell function), 27% moderate microsmia, 13% mild microsmia], only 35% were aware of their dysfunction before testing.
Box 1. Quantitative olfactory testing.
Quantitative olfactory tests can be divided into three general types: psychophysical, electrophysiological, and psychophysiological. Psychophysical tests directly assess the subject’s conscious perceptual function. Examples are tests of odor identification, detection, discrimination, memory, and intensity estimation [32]. Most use simple means for presenting the stimuli (e.g., ‘scratch and sniff’ odorized pads [33]; specialized hand-held wands [34]). Despite different names, nearly all such tests are strongly correlated with one another and appear to be measuring the same elements of the underling physiological substrate [35]. Identification tests usually require the subject to identify the name of a presented odorant from multiple-choice alternatives. Some are commercially available in multiple languages and can be self-administered. In addition to categorical classification of function (e.g., normal or mild, moderate, severe, or total loss), some employ sex- and age-related normative data to establish a patient’s performance as a percentile score relative to the performance of peers [33]. Threshold tests assess the lowest concentration of an odorant that can be perceived. This is commonly done using forced-choice paradigms in which the subject must identify the stronger of two randomly presented stimuli, one of which is a blank, at different concentration levels [36]. Threshold tests are popular since their metric is easy to interpret, language factors are not involved, and their auditory analog is familiar to clinicians. However, unless the perithreshold region is repeatedly sampled, reliability is low. Although threshold measures have been reported to uniquely measure receptor cell function, this is questionable since threshold values correlate with neuropsychological measures of verbal and visuospatial memory and are sensitive to lesions in higher order brain structures due to multiple sclerosis, epilepsy, AD, PD, and other diseases [37]. Odor discrimination tests typically require, on a given trial, the subject to identify the ‘odd’ odorant from a set of three odors, two of which are equivalent. Odor memory tests assess the ability to remember an odor quality over periods of time. Suprathreshold rating or magnitude estimation tests examine the build-up of perceived intensity as a function of increasing odorant concentrations. Some studies combine data from different types of tests into a global score. Although this increases test reliability, it is questionable on a number of grounds [38]. Electrophysiological tests, such as odor event-related potentials [39], and psychophysiological tests, such as autonomic system responses to odorants [40], are largely confined to laboratory settings, being less practical and more dependent upon sophisticated odorant presentation equipment.
Alt-text: Box 1
Smell loss is long-lasting and probably permanent in a number of COVID-19 cases
As occurs in other PVODs [42], some patients who have been infected with SARS-CoV-2 have long lasting, and likely permanent, deficits in their ability to smell [43]. An early study found that while 61% of 82 patients objectively retested with the UPSIT recovered by 7–8 weeks after COVID-19 onset, most of the 39% who continued to have dysfunction had only mild loss and none were anosmic [44]. In a more recent study of 268 COVID-19 patients, 21.9% reported their function had not returned to normal by 1 year after initial diagnosis [43]. A systematic review of this literature identified 44 COVID-19 studies in which full olfactory recovery was reported over time, 14 of which used quantitative test measures [45]. Of these 14, follow-up was completed by four at 1 month, seven by 2 months, and three up to 6 months. The median respective recovery rates were similar at these time points: 72.6% (range: 44.3% to 94.6%), 73.3% (0% to 79.5%), and 73.5% (58.8% to 87.3%). In aggregate, such studies suggest that approximately a quarter to a third of people inflicted with the SARS-CoV-2 virus continue to have some degree of measurable smell dysfunction for months after their infection. Based on patients who recover from olfactory dysfunction from other viruses and disorders, the amount of long-term recovery, when it occurs, will likely depend upon such factors as the subject’s age and amount of initial loss [42].
Similarities and differences in smell dysfunction between COVID-19 and other PVODs
The average magnitude of the initial smell loss induced by COVID-19, as measured by validated olfactory tests, appears to be essentially the same as that observed for other PVODs [46], as well as for such diseases as AD and PD. For example, in one study the mean UPSIT score of 100 COVID-19 patients tested during the late acute phase of the disease was 22/40 (95% CI, 21–23) [44]. This corresponds well to UPSIT scores in a study of 132 non-COVID PVODs [mean (SD) = 23/40 (9.4)] [1], a study of 81 patients with PD [mean (SD) = 22/40 (7.3)] [6], and a study of 19 patients with early-onset AD [mean (SD) = 23/40 (6.6) [47]. It is unknown whether such scores reflect common pathophysiological factors, although in some cases common substrates could be involved [48].
In general, the onset of the smell loss observed in COVID-19 is similar to that of most other PVODs, typically being noticed around the beginning of the infection. The trajectory of return of smell function appears to be similar for COVID-19 as that of most other PVOCs, in that self-report recovery from smell loss commonly occurs over a 2- to 3-week postinfection period [49]. COVID-19 tends to have a pattern of symptoms more similar to influenza than non-influenza-related PVODs like the common cold [50., 51., 52., 53.] and its viral nucleic acid shedding pattern resembles that of patients with influenza [54]. However, COVID-19 differs from influenza in having less frequent nose running, shortness of breath, and throat irritation [52].
Although widespread awareness of smell loss in COVID-19 suggests that proportionately more people with COVID-19 uniquely experience smell loss, this could be illusory, at least to some degree [55]. Thus, nearly everyone experiences reversible smell loss during or immediately after contracting a head cold [56]. This loss is rarely viewed as abnormal and is typically attributed to congestion, since the ability to smell appears to return to normal when the congestion subsides. In the case of COVID-19, noticeable congestion is the exception, rather than the rule, so in most cases the smell loss is viewed as novel and cannot be readily attributed to perceived nasal blockage. Interestingly, early in the pandemic self-reported prevalence rates of smell dysfunction appeared to be positively correlated with the amount of attention paid in the popular press to the impact of COVID-19 on the sense of smell [57].
Pathophysiological mechanisms of smell loss in COVID-19
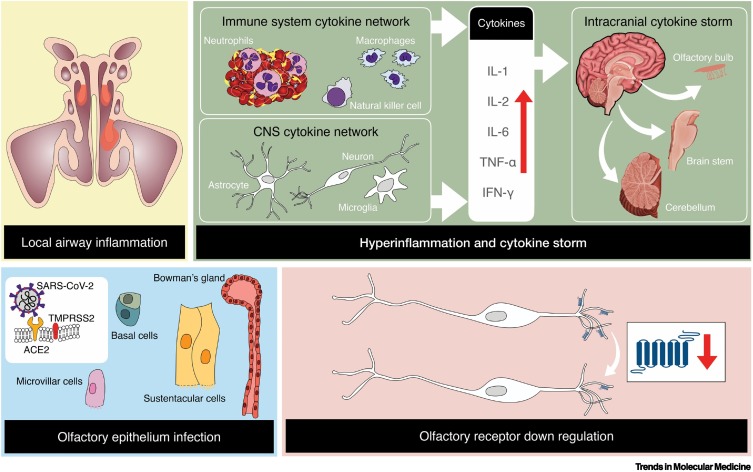
Several non-mutually exclusive causes of smell loss from the SARS-CoV-2 virus are possible (Figure 1 ), as outlined in detail in this section, including:
-
•
Blockage of transit of odorants to the olfactory receptors from local airway inflammation or changes in the volume or composition of the olfactory mucus.
-
•
Damage to the olfactory mucosa, including the ciliated olfactory receptor cells, Bowman’s glands within the lamina propria, and sustentacular cells, basal cells, and microvillar cells.
-
•
Downregulation of olfactory receptor proteins within the olfactory receptor cells.
-
•
Injury to the olfactory bulb or other central brain structures or circuits, including capillary endothelial cells, in some cases from massive activation of cytokines.
Figure 1.
Suggested mechanisms by which the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus affects the sense of smell.
These include local airway inflammation, particularly in the higher recesses of the nose, infection and damage to specific cell types within the olfactory neuroepithelium, over-reactive immune responses within the brain, and downregulation of olfactory receptor proteins located on the cilia of olfactory receptor cells. As a result of viral infection, macrophages are activated, which in turn releases a multitude of cytokines, with interleukin 6 producing the most inflammation. The listed cytokines are examples and not an inclusive list of cytokines that can influence olfactory functioning. Copyright © 2022 Shima Moein. Abbreviations: ACE2, angiotensin-converting enzyme II; TMPRSS2, transmembrane protease serine 2.
The angiotensin-converting enzyme II (ACE2) receptor is believed to be the primary entry receptor for SARS-CoV-2 [58]. This ubiquitous receptor is expressed in multiple tissues and organs in addition to the upper and lower airways, including the heart, retina, vasculature, gut, kidney, testis, and brain, although it is not as highly expressed in the brain compared with other tissues [59]. Cell entry occurs when the virus’ spike protein binds to the ACE2 receptor and becomes cleaved by the cell’s transmembrane protease serine 2 (TMPRSS2) enzyme into S1 (receptor binding) and S2 (membrane fusion) domains. However, recent evidence suggests that SARS-CoV-2 can also infect cells via neuropilin 1 (NRP1) [60] and basigin (BSG) [61] receptors and that cathepsin L ( CTSL) and Furin enzymes can also modify SARS-CoV-2 to facilitate infection [62]. Compared with ACE2 or TMPRSS2, NRP1 and BSG are more widely expressed in the human brain, including the olfactory bulb [62].
Although such receptor entry may be the genesis of, or contribute to, the pathophysiology of cells specifically involved in olfactory system function, olfactory system pathology may also occur from SARS-CoV-2 entry into cells not directly involved in such function. For example, the so-called cytokine storm induced by systemic infection or infection of nonolfactory cells could result in inflammatory damage to cells critical for olfactory function [63]. Moreover, non-cell-autonomous genetic influences on receptor protein regulation may also occur within olfactory receptor cells [64]. Wide-spread disease of fine blood vessels (microangiopathy) induced by SARS-CoV-2 may also impact blood flow to neurons involved in olfactory perception. The brain microvascular endothelial cells, which are a major component of the blood–brain barrier, express ACE2 [65] and NRP1 receptors [66].
Blockage of transit of odorants to the olfactory receptors
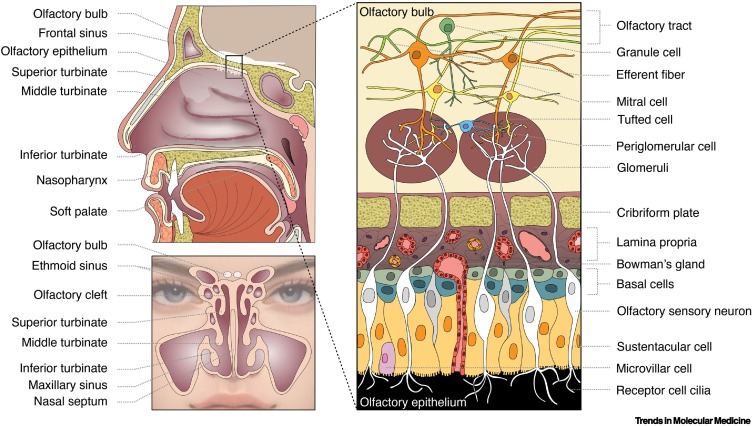
To reach the olfactory receptors, odorant-laden air must first traverse the upper airway, penetrate the narrow olfactory cleft, and absorb into a specialized mucus that covers the neuroepithelium in which the ciliated olfactory receptors are embedded (Figure 2 ). The olfactory cleft is the opening located above the middle and superior turbinates, the uppermost set of vascularized nasal structures critical for warming, humidifying, and cleansing the incoming airstream. When inflamed or diseased, the turbinates can block or decrease airflow to the receptors. The olfactory mucus, when desiccated or altered in composition, can also attenuate the movement of odorants to the olfactory receptor cells (Box 2 ). Nasal mucociliary clearance, phagocytic activity, and immune responses can be impeded by numerous factors, including cold temperatures [79,80]. Such impediment can increase the duration or presence of the virus within the nose and olfactory receptor region.
Figure 2.
Basic anatomy of the nose, olfactory epithelium, and olfactory bulb.
Left: Air, including aerosols and odorants, enters into the nasal cavity and a portion reaches the olfactory neuroepithelium located within the olfactory clefts on sectors of the nasal septum and superior and middle turbinates. The highly vascular turbinates warm, cleanse, and filter the air. Right: Olfactory receptor proteins are located on the olfactory cilia. The olfactory neuroepithelium is comprised of multiple cell types, which germinate from basal cells. The axons of the receptor cells extend through the bony cribriform plate to synapse with other cell types in globe-like structures within the olfactory bulb, the first relay station of the olfactory pathway. Copyright © 2022 Shima Moein.
Box 2. Constituents of olfactory mucus.
Among the constituents of the olfactory mucus are odorant binding proteins that shepherd hydrophobic molecules through the aqueous environment to the receptors [67] and numerous antioxidants, growth factors, immune factors, peptides, and antimicrobial and regulatory proteins, a number of which rapidly metabolize odorants and xenobiotics at a rate that can impact smell function [68]. Over 2500 proteins have been identified in the olfactory mucus, some of which are altered by aging and disorders associated with smell loss (e.g., chronic rhinosinusitis) [69,70]. For example, odorants with functional groups such as aldehydes and esters can be rapidly converted to the corresponding acids and alcohols [71]. Altered levels of some olfactory mucus cytokines are correlated with olfactory test scores and appear to impact the function of olfactory receptor cells and possibly their ability to regenerate [72]. This mucus affords a degree of protection of the neuroepithelium from viruses and other xenobiotics, which, in some cases, can invade the brain via receptor cells, perineural spaces, and lymphatics [73,74]. Beta-adrenergic, cholinergic, and peptidergic mechanisms control the secretory activity of the mucus [75., 76., 77., 78.].
Alt-text: Box 2
Although the nasal epithelium exhibits a higher SARS-CoV-2 viral load than does the lower respiratory tract [81], the majority of COVID-19 patients report having no blocked nose, nasal congestion, or nasal discharge [82]. Nevertheless, human imaging studies have found localized inflammation within the olfactory cleft early in the SARS-CoV-2 infection process, inflammation which subsides in most cases in a matter of weeks. A case–control magnetic resonance imaging (MRI) study of 20 COVID-19 patients with smell loss and 20 age-matched healthy controls found 19 of the patients (95%) displayed complete obstruction of the olfactory cleft when assessed a mean of 5.3 days (range 1–13 days) after the onset of smell loss [83]. None of the controls exhibited such obstruction. One month later, only seven of the 20 patients (35%) continued to have obstruction, a percentage similar to that reported for the prevalence of post-COVID-19 cases of olfactory dysfunction [45]. In accord with the time-related resolution of the inflammation, another study [84] noted, in 12 patients evaluated 2 weeks after anosmia onset, bilateral olfactory cleft blockage in six (50%) and partial blockage of one cleft in two (17%). No blockage was apparent in the other patients. Those with bilateral blockage exhibited lower olfactory test scores (44% correct) than those with only one-sided blockage (52% correct). A more recent study reported that total olfactory cleft opacification occurred in only one of 23 COVID-19 patients tested 1–4 months after anosmia onset, although partial opacification occurred in over two-thirds (69.6%) [85]. In accord with the lack of noticeable nasal blockage is a case study of a patient with smell loss who had no apparent nasal congestion but, upon MRI evaluation, exhibited bilateral obstructive inflammation of the olfactory clefts [86].
Expression of ACE2 receptors is not as strong in the respiratory epithelium as in the olfactory epithelium, possibly explaining the lack of significant nasal blockage. For example, less than half (nine of 19) of the biopsies of respiratory epithelium collected in one study yielded ACE2-positive epithelial cells, in contrast to all of those collected within the olfactory neuroepithelium (13/13) [87]. A possible contributing mechanism for the apparent selective inflammation of the olfactory cleft region is the differential dispersion of aerosols during inhalation. SARS-CoV-2 is largely carried in the air by aerosols. A study using inhaled fluorescein-labeled 0.5-5 μM droplets found deposition to occur primarily within the olfactory cleft [88]. Minimal or no deposition occurred in the nasal respiratory epithelium. Interestingly, a study of 24 anosmic and 26 normosmic COVID-19 patients tested 1–34 months after infection onset found the olfactory clefts to be wider and of greater volume in the anosmics [89]. The authors suggested that people with more patent olfactory clefts may be more likely to contract long-lasting and presumably neurologically based smell dysfunction from COVID-19.
Damage to the olfactory neuroepithelium
The olfactory neuroepithelium, which extends over the cribriform plate and anteriorly over segments of the superior and middle turbinates (Figure 2), has become the focus for studies seeking to understand the biological basis of the smell loss associated with COVID-19. This avascular pseudostratified columnar structure contains the 6–10 million ciliated bipolar receptor cells that harbor the olfactory receptor proteins to which odorants bind, along with sustentacular cells, microvillar cells, basal stem cells, and duct cells from Bowman’s glands [90]. The receptor cell axons coalesce into hundreds of ensheathed bundles or fila, collectively termed cranial nerve I (CN I), that project through the basal lamina and fine passages (fenestra) of the cribriform plate into the olfactory bulbs, the first relay stations of the olfactory system. Microglia and macrophages located within these bundles phagocytize bacteria and other foreign agents [91,92] and provide one barrier to the migration of viruses and other foreign agents from the nasal cavity into the brain through these extracellular spaces.
The location of the neuroepithelium at the interface of the environment places its cells at risk from damage from viruses [74], bacteria [93], nanoparticles [94], and other airborne xenobiotics [95]. Such damage is well established [96], with some viruses being capable of penetrating the brain from this region [74] (Box 3 ). Even though damaged receptor cells and other elements of the olfactory neuroepithelium can be reconstituted from basal cells if the basal cell layer is not severely damaged, full recovery of the epithelial sheet rarely occurs, leading to cumulative and pot-marked damage of the epithelium. This is one basis for age-related decreases in smell function [78].
Box 3. Viruses and the olfactory system.
A number of viruses, as well as ionized metals and other xenobiotics, can penetrate the brain via the olfactory neuroepithelium, largely bypassing the blood–brain barrier [74]. Movement of viruses from the nose to the brain can occur via olfactory receptor cell incorporation, lymphatic channels, or fluid-filled perineural channels created by ensheathing cells of the olfactory fila through which regenerating receptor cell axons are guided into the brain [74,97,98]. Numerous lymphatic ducts surround the olfactory cell nerve roots, facilitating a direct connection between the cerebral spinal fluid and lymph in the olfactory submucosa [99]. Viruses associated with the 1916–1930 epidemic known as von Economo’s encephalitis in which parkinsonism was common, include western equinine, coxsackie, and Japanese B viruses. Among viruses capable of entering the brain in animal models via the olfactory nerves or perineural spaces are the Japanese encephalitis virus, influenza A virus, herpesviruses, poliovirus, paramyxoviruses, vesicular stomatitis virus, rabies virus, parainfluenza virus, adenoviruses, West Nile virus, chikungunya virus, La Crosse virus, mouse hepatitis virus, and bunyaviruses [74,98]. In case of the arthropod-borne St. Louis encephalitis virus, entry into olfactory receptor cells occurs even when the virus is instilled intravenously, subdurally, or interperitoneally [100]. Once incorporated into a cell, virus-induced cellular apoptosis occurs in some cases before the replication cycle can be completed [101]. A number of viruses can suppress neuronal apoptosis by antiapoptotic genes, facilitating their replication and brain invasion [102]. Although many viruses are eliminated by neuroprotective immune responses within the olfactory bulb [103], others are not. Some viruses, once in the olfactory bulb, can target specific brain neurotransmitter systems. For example, HSV-1 inoculated into the rat olfactory bulb ultimately infects cholinergic neurons in the horizontal limb of the diagonal band, serotonergic neurons in the dorsal and medial raphe nuclei, and noradrenergic neurons in the locus coeruleus [104].
Alt-text: Box 3
There is general consensus that ACE2 receptors are expressed in all types of cells within the olfactory neuroepithelium, with the exception of receptor cells [105]. Sustentacular cells appear to be the most infected [106], although not all are positive for ACE2. The positive cells are often isolated or present in small clusters intermingled with negative sustentacular cells and, in rare instances, some respiratory epithelial cells [107]. Since the basal cells from which the olfactory receptors arise also appear to express ACE2 receptors, it has been suggested that the differentiated receptor cells can therefore be carrying the SARS-CoV-2 virus without expressing ACE2 receptors themselves [62]. If this is true, it is surprising that the SARS-CoV-2 virus is not found in large numbers of olfactory receptor cells [106]. Conceivably the infected cells may have undergone virus-induced cellular apoptosis before being able to differentiate into olfactory receptor cells [101].
It has recently been shown that SARS-CoV-2 markedly downregulates olfactory receptor proteins and associated signaling components in the olfactory receptor cells of both hamsters and humans via a non-cell-autonomous process [64]. A day after intranasal SARS-CoV-2 inoculation, viral RNA was found in about 5% of the cells of hamster olfactory epithelia; 40% of these cells were sustentacular cells and 6% olfactory receptor cells. Two days after inoculation, genes related to olfactory function, such as ADCY3, were significantly downregulated, whereas the opposite was true for genes associated with antiviral responses. The downregulation peaked at 4 days and continued through 10 days when other olfactory sensory neuron markers had recovered. SARS-CoV-2 transcripts were also found in microglia and other immune cells by three postinoculation days. Autopsied human olfactory epithelia mirrored the marked decreases of olfactory receptors and the olfactory receptor signaling gene transcription, as well as the reduction of interchromosomal olfactory receptor contacts.
Damage to the olfactory bulb and other olfaction-related central nervous system structures
The olfactory receptor cell axons synapse with the major output neurons of the olfactory bulb within globe-like structures termed glomeruli (Figure 2). These output neurons, the mitral and tufted cells, send axonal projections to other brain structures such as the piriform cortex and entorhinal cortex. The receptor cell activity is modified, in part, by local neurons termed periglomerular cells. Mitral and tufted cell activity is also modulated by other cells, including by small inhibitory granule cells located in the core of the bulb. The microglia within the bulb are uniquely pre-set to a primed state in which cytokine production is mediated by the expression of Toll-like receptor 2 (TLR2), potentially serving as sensors or modulators of brain inflammation in general [108]. For example, in mice, ischemic brain injury far from the bulb activates olfactory bulb microglia hours before microglia are activated near the injury, activation that remains for months after the injury [108]. The intranasal introduction of a small single dose of a bacterial endotoxin was found to produce a widespread wave of TLR2 activation from the bulb to higher brain regions.
As measured by MRI, the average size of the olfactory bulbs and tracts are smaller in COVID-19 cases than in controls. For example, one study [109] compared the olfactory bulb volumes, olfactory tract lengths, and olfactory sulcus depths of 36 COVID-19 patients with smell loss [mean (SD) age = 37.33 (7.38)] to those of 80 healthy controls [mean (SD) age = 35.74 (8.38)]. The mean (SD) volumes of the olfactory bulbs (left and right combined) were 82.34 (31.29) mm3 in the patients and 131.50 (32.27) mm3 in the controls (P < 0.001), with total atrophy being noted in four cases. Significant reductions were also present in the olfactory tract lengths and sulcus depths. Nevertheless, one study found the decrement in olfactory bulb size of 31 COVID-19 patients evaluated over 1 month since illness onset was less pronounced than that seen in 97 non-COVID-19 PVOD cases [110].
The basis for the decrease in olfactory bulb size is not clear. However, such a decrease need not be due to direct viral invasion of the bulb. Thus, disruption of the receptor cells of the olfactory epithelium can result in decreased olfactory bulb volume, possibly due to loss of trophic factors from the decreased numbers of incoming receptor cell axons. Decrements in olfactory bulb sizes have been reported in elderly people [78], cigarette smokers [111], and in patients with AD [112], PD [113], head trauma [114], multiple sclerosis [115], schizophrenia [116], and chronic rhinosinusitis [117].
That being said, postmortem studies using tests to localize the distribution of SARS-CoV-2 RNA in specific brain regions have found such RNA in the olfactory bulbs, with one study reporting a 53% bulbar positivity rate (eight of 15) [118] and another a 40% positivity rate (eight of 20) [119]. The RNA was absent from the neural and glial bulbar compartments, as well as from the olfactory tubercles and lateral olfactory tract. This absence, as well as the viral presence in the endothelia of such structures, suggested that hematogenous viral spread may have occurred via the common arterial supply of the bulb [118]. If systemic hematogenous involvement was present, wider viral spread into other brain regions would have been expected. Nevertheless, the analysis they performed of homogenized autopsy specimens is unable to specifically identify whether the RNA comes from the sampled tissue, per se, or from embedded fragments of endothelial cells or more distally infected tissue [120].
The neuropathological features of 43 COVID-19 brains were evaluated in one postmortem study [121]. Immunological staining was made for activated astrocytes and microglia, as well as cytotoxic T lymphocytes in the olfactory bulb, basal ganglia, brainstem, and cerebellum. The neuropathological changes were generally mild and no association between the severity of the neuropathological findings and the presence of SARS-CoV-2 in the central nervous system (CNS) was present. Most pathology was found in the brainstem and cerebellum. All olfactory bulbs had some degree of astrogliosis (five severe, 25 moderate, 13 slight) and all but one had microgliosis (five severe, 27 moderate, ten slight). Cytotoxic T lymphocytes were evident in the bulbs of all but three of the 42 cases for which data were available (zero severe, 35 slight, four moderate). It is noteworthy that the subjects were at an age when some degree of smell loss is common [median (interquartile range) = 76 (70–86) years].
In general, significant COVID-19-related pathology in CNS structures, including olfaction-related structures, appears to be relatively rare. For example, in one study of 20 COVID-19 brains, clear evidence of classic neuropathology of viral CNS infections was absent in 18 (i.e., viral inclusions, focal demyelination, lymphocytic leptomeningitis or encephalitis, microglial nodules, or pronounced or frequent perivascular lymphocytic cuffing) [119]. Only one exhibited notable pathologic features and only four exhibited either acute microscopic or macroscopic hemorrhages.
Neurological and psychiatric sequelae in COVID-19 cases evidencing persistent smell loss
A broad spectrum of acute and chronic neurological problems has been associated with COVID-19. These include confusion, dizziness, fatigue, headache, ischemic strokes, syncope, seizures, insomnia, neuropathic pain, myalgia, and Guillain-Barre syndrome [122]. Although there are a few sporadic reports based on small samples that several movement disorders may be associated with SARS-CoV-2 infection, including PD, action tremor, poor muscle control, involuntary muscle twitching, and some oculomotor disorders, these disorders are generally lacking in large multicenter studies [123]. Nonetheless, it is noteworthy that plasma levels of inflammatory mediators associated with a number of such symptoms, such as interleukin-6, are elevated during the acute phase of COVID-19 and are correlated with the degree of self-reported smell loss [124]. However, not all studies have observed such associations [125].
Reports of neuropsychiatric symptoms are common in COVID-19 patients during and after their discharge from hospital. One large cohort study of largely males employed mental health data from the US Department of Veterans Affairs national healthcare databases [126]. Data from 153 848 COVID-19 ‘long-haul’ survivors was compared with that from 5 637 840 never infected contemporaneous controls and 5 859 251 historical controls who predated the COVID-19 pandemic. Relative to the controls, the long haulers exhibited increased neurocognitive decline [hazard ratio (95% CI) = 1.80 (1.72–1.89)], incident sleep disorders [1.41 (1.38–1.45)], depressive disorders [1.39 (1.34–1.43)], and stress and adjustment disorders [1.38 (1.34–1.43)]. In a systematic review of 66 studies of long haulers, screened from a total of 1725 studies, 61% reported the presence of anxiety and/or depression, 48% fatigue, 41% cognitive deficits, 35% sleep disturbances, and 30% symptoms of post-traumatic stress disorder [127]. Examples of the cognitive deficits are problems with attention, concentration, short-term and general memory, language, verbal encoding, and verbal fluency. To what degree such symptoms reflect pre-existing conditions, CNS-related viral infection, systemic reactions to the virus (e.g., coagulopathy, sepsis, autoimmune responses), or other factors, including smell or taste dysfunction, remains unclear.
A recent MRI study of 401 patients before and after contracting COVID-19, along with similarly longitudinally tested 384 non-COVID-19 controls, was designed to minimize the effects of pre-existing conditions on the brain measures [128]. This study found modest (from 0.2% to ~2%) COVID-19-related decrements in global brain size and grey matter thickness in the orbitofrontal cortex and parahippocampal gyrus, regions involved in olfactory processing. Increases in relative changes in markers of tissue damage in these and other brain regions functionally connected to piriform cortex and related areas were also noted, although no olfactory testing was performed.
The question arises as to whether COVID-19 patients with long-lasting smell loss are more likely than those who regain smell function to experience later psychological disabilities. Few studies have addressed this issue. In an online survey of 322 COVID-19 positive subjects who had experienced a loss of smell or taste, 43% reported depression and 37% loss of weight. Notably, 87% reported reduced enjoyment of food, 56% decreased enjoyment of life in general, and 55% loss of appetite [129]. One study identified subjects from user-generated posts from a Reddit subforum dedicated to people with COVID-19 [130]. Those with a history of anosmia/ageusia-related posts and who self-identified as COVID-19 positive had a 30% higher instantaneous risk of suicidal ideation or depression on posts made in suicide- or depression-related forums. Another study compared scores on an anxiety and depression scale of 84 COVID-19 patients reporting olfactory dysfunction to those of 19 COVID-19 patients who reported no olfactory dysfunction [131]. Similar comparisons were made for 25 patients reporting no taste dysfunction and 78 reporting taste dysfunction. In both the taste and smell groups, those reporting dysfunction had higher anxiety scores than those not reporting such dysfunction (P = 0.018 and 0.002, respectively). However, depression scores were only significant for those reporting taste dysfunction, a phenomenon also seen for a measure of distress. It is unknown to what extent the reported taste dysfunction reflected deficits in flavor sensations due to decreased retronasal olfactory function [20].
Concluding remarks
As the pandemic spread of COVID-19 developed, smell loss became the most salient initial symptom of COVID-19. Over the past 2 years, much has been learned about such loss. First, in most cases it is not total when measured by objective quantitative tests. Second, the majority of those who are inflicted can expect to regain normal function within 4 to 6 weeks after being infected by the SARS-CoV-2 virus. Third, while the magnitude of the measured smell loss seems to be similar to that observed for other types of PVODs, the sheer number of infected people is demonstrably greater given the high prevalence of COVID-19 cases. Fourth, while the neurotropism of this virus does not appear to be as great as other coronaviruses, its influences on the brain remain enigmatic. Fifth, short-term smell loss associated with COVID-19 may reflect inflammatory processes, whereas long-term loss may reflect alterations in neurological structures, including the downregulation of olfactory receptors. Sixth, it remains to be determined whether having had COVID-19 predisposes persons to neurological disorders, including those catalyzed by subsequent exposures to solvents, pesticides, or other toxic agents. Finally, much more research is needed to fully understand how SARS-CoV-2 influences the olfactory sensory system (see Outstanding questions).
Outstanding questions.
To what degree are viral entry proteins other than ACE2 involved in the production of COVID-19-related smell dysfunction? What differences exist between variants of COVID-19 in terms of their impact on the sense of smell not confounded by vaccination status?
Are some cases of COVID-19 smell loss, particularly those which reverse themselves over the course of a few weeks, simply due to epithelial inflammatory processes that resolve or do they reflect other factors?
Do cases in which smell or taste dysfunction linger for long periods of time reflect significant neural damage to elements of the olfactory system, notably cells within the olfactory neuroepithelium? Is the degree of resolution in such cases related to the extent of damage to the stem cell layer of the olfactory epithelium? Is SARS-CoV-2 neurotropic?
Given that up to 30% of COVID-19 cases appear to continue to have some smell dysfunction long after the initial infection, how many, if any, of these cases will ultimately regain normal function?
Are COVID-19 patients more susceptible to later neurological disorders, such as AD or PD? If so, is such susceptibility associated with the degree of smell loss, as well as genetic predispositions or environmental exposures to pesticides or other toxic agents?
Do other types of viral upper respiratory infections that impact olfactory function, such as the common cold or influenza, utilize the same viral entry receptor proteins as SARS-CoV-2 to infect olfactory eloquent cells?
Alt-text: Outstanding questions
Acknowledgments
Acknowledgments
The author wishes to thank Dr Shima Moein for providing the artwork for this review and for critically reading an earlier version of the manuscript. The preparation of this work was not funded by any outside agency.
Declaration of interests
The author is a consultant to Eisai Co, Merck Pharmaceuticals, the Michael J. Fox Foundation for Parkinson's Research, and Johnson & Johnson. He receives royalties from Cambridge University Press, Johns Hopkins University Press, and John Wiley & Sons, and is president of, and a major shareholder in, Sensonics International, a manufacturer and distributor of smell and taste tests.
Glossary
- Adenylate cyclase 3 (ADCY3)
an encoding gene that influences olfaction by modulating intracellular cAMP concentrations.
- APOE-ε4
a genetic variant of the apolipoprotein E gene that is major risk factor for Alzheimer’s disease.
- Apoptosis
self-destruction of a cell by an appropriate trigger.
- Astrocyte
a star-shaped glial cell common in the CNS.
- Astrogliosis
an abnormal increase in astrocytes.
- Basal cell
the primary stem cell within the olfactory neuroepithelium.
- Basigin (BSG)
a widely expressed receptor with multiple functions, including regulating responsiveness to lymphocytes and serving as receptor for SARS-CoV-2.
- Bowman’s gland
specialized gland that secretes into the olfactory mucus.
- Cathepsin L (CTSL)
an enzyme with multiple functions, including facilitation of SARS-CoV-2 spike protein cleavage and virus entry.
- Coagulopathy
a condition that impairs the ability of blood to clot.
- Cytokine
a protein involved in immune system activity.
- Cytokine storm
inflammatory syndrome involving markedly elevated levels of circulating cytokines.
- Endothelial cell
main cell type lining the heart and blood and lymph vessels.
- Entorhinal cortex
a sector of the medial temporal lobe that receives input from the olfactory bulb and the piriform cortex; plays an important role in odor memory.
- Furin
an enzyme that catalyzes the proteolytic maturation of prohormones and proproteins.
- Granule cells
most common inhibitory neuron in the olfactory bulb.
- Hazard ratio
a measure of how often an event happens in one group compared to how often it occurs in another group.
- Lymphocyte
an immune cell made in bone marrow and found in blood and lymph tissue.
- Macrophage
a white blood cell that kills microorganisms, removes dead cells, and activates other immune system cells.
- Microglia
a type of cell that serves as an immune system scavenger in the CNS.
- Microgliosis
increase in number of microglia.
- Microvillar cell
a small cell within the olfactory neuroepithelium of unknown function that sends microvillae into the mucus.
- Neuropilin 1 (NRP1)
a receptor involved in vascular processes; facilitates entry of SARS-CoV-2 into cells.
- Odds ratio (OR)
the odds that an outcome will occur given a particular exposure (e.g., COVID-19), compared to that of the outcome occurring in the absence of that exposure.
- Olfactory sulcus
a groove underneath the frontal lobe that harbors the olfactory bulb.
- Piriform cortex
largest olfactory processing and information coding brain region.
- Polymorphism
a genetic variation upon which natural selection can operate.
- Sustentacular cell
a major cell type in the olfactory neuroepithelium that provides structural and metabolic support to the receptor cells.
- Toll-like receptor 2 (TLR2)
a receptor that recognizes components of gram-positive bacteria.
- Transmembrane protease serine 2 (TMPRSS2)
an endothelial cell surface enzyme that facilitates viral fusing to the ACE2 receptor.
- University of Pennsylvania Smell Identification Test (UPSIT)
a 40-item self-administered microencapsulated odorant smell test.
References
- 1.Deems D.A., et al. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch. Otolaryngol. Head Neck Surg. 1991;117:519–528. doi: 10.1001/archotol.1991.01870170065015. [DOI] [PubMed] [Google Scholar]
- 2.Fornazieri M.A., et al. Main causes and diagnostic evaluation in patients with primary complaint of olfactory disturbances. Braz. J. Otorhinolaryngol. 2014;80:202–207. doi: 10.1016/j.bjorl.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doty R.L. Systemic diseases and disorders. Handb. Clin. Neurol. 2019;164:361–387. doi: 10.1016/B978-0-444-63855-7.00021-6. [DOI] [PubMed] [Google Scholar]
- 4.Ou Y.N., et al. Associations of infectious agents with Alzheimer's disease: a systematic review and meta-analysis. J. Alzheimers Dis. 2020;75:299–309. doi: 10.3233/JAD-191337. [DOI] [PubMed] [Google Scholar]
- 5.Smeyne R.J., et al. Infection and risk of Parkinson's disease. J. Parkinsons Dis. 2021;11:31–43. doi: 10.3233/JPD-202279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doty R.L., et al. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38:1237–1244. doi: 10.1212/wnl.38.8.1237. [DOI] [PubMed] [Google Scholar]
- 7.Cocoros N.M., et al. Long-term risk of Parkinson disease following influenza and other infections. JAMA Neurol. 2021;78:1461–1470. doi: 10.1001/jamaneurol.2021.3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doty R.L., et al. Presence of both odor identification and detection deficits in Alzheimer's disease. Brain Res. Bull. 1987;18:597–600. doi: 10.1016/0361-9230(87)90129-8. [DOI] [PubMed] [Google Scholar]
- 9.Sun X., et al. Integrated bioinformatics analysis identifies hub genes associated with viral infection and Alzheimer's disease. J. Alzheimers Dis. 2022;85:1053–1061. doi: 10.3233/JAD-215232. [DOI] [PubMed] [Google Scholar]
- 10.Lin W.R., et al. Herpesviruses in brain and Alzheimer's disease. J. Pathol. 2002;197:395–402. doi: 10.1002/path.1127. [DOI] [PubMed] [Google Scholar]
- 11.Devanand D.P., et al. Olfactory identification deficits and increased mortality in the community. Ann. Neurol. 2015;78:401–411. doi: 10.1002/ana.24447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinto J.M., et al. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS One. 2014;9 doi: 10.1371/journal.pone.0107541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sudre C.H., et al. Anosmia, ageusia, and other COVID-19-like symptoms in association with a positive SARS-CoV-2 test, across six national digital surveillance platforms: an observational study. Lancet Digit. Health. 2021;3:e577–e586. doi: 10.1016/S2589-7500(21)00115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandão Neto D., et al. Chemosensory dysfunction in COVID-19: prevalences, recovery rates, and clinical associations on a large Brazilian sample. Otolaryngol. Head Neck Surg. 2021;164:512–518. doi: 10.1177/0194599820954825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Bartheld C.S., et al. Prevalence of chemosensory dysfunction in COVID-19 patients: a systematic review and meta-analysis reveals significant ethnic differences. ACS Chem. Neurosci. 2020;11:2944–2961. doi: 10.1021/acschemneuro.0c00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian Y., et al. Distinct symptoms and underlying comorbidities with latitude and longitude in COVID-19: a systematic review and meta-analysis. Can. Respir. J. 2022;2022 doi: 10.1155/2022/6163735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams F.M.K., et al. Self-reported symptoms of COVID-19, including symptoms most predictive of SARS-CoV-2 infection, are heritable. Twin Res. Hum. Genet. 2020;23:316–321. doi: 10.1017/thg.2020.85. [DOI] [PubMed] [Google Scholar]
- 18.Butowt R., et al. Chemosensory dysfunction in COVID-19: integration of genetic and epidemiological data points to D614G spike protein variant as a contributing factor. ACS Chem. Neurosci. 2020;11:3180–3184. doi: 10.1021/acschemneuro.0c00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coelho D.H., et al. Decreasing incidence of chemosensory changes by COVID-19 variant. Otolaryngol. Head Neck Surg. 2022 doi: 10.1177/01945998221097656. Published online May 3, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burdach K.J., Doty R.L. The effects of mouth movements, swallowing, and spitting on retronasal odor perception. Physiol. Behav. 1987;41:353–356. doi: 10.1016/0031-9384(87)90400-8. [DOI] [PubMed] [Google Scholar]
- 21.Hannum M.E., et al. Taste loss as a distinct symptom of COVID-19: a systematic review and meta-analysis. Chem. Senses. 2022;47:bjac001. doi: 10.1093/chemse/bjac001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Cao A.C., et al. Objective screening for olfactory and gustatory dysfunction during the COVID-19 pandemic: a prospective study in healthcare workers using self-administered testing. World J. Otorhinolaryngol. Head Neck Surg. 2021 doi: 10.1016/j.wjorl.2021.02.001. Published online February 12, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hintschich C.A., et al. Psychophysical tests reveal impaired olfaction but preserved gustation in COVID-19 patients. Int. Forum Allergy. Rhinol. 2020;10:1105–1107. doi: 10.1002/alr.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu P.Y., Jiang R.S. Prognosis of olfactory and gustatory dysfunctions in COVID-19 patients: a case series. Clin. Case Rep. 2020;8:2744–2752. doi: 10.1002/ccr3.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Bon S.D., et al. Making scents of loss of taste in COVID-19: is self-reported loss of taste due to olfactory dysfunction? A prospective study using psychophysical testing. Int. Forum Allergy. Rhinol. 2021;11:1504–1507. doi: 10.1002/alr.22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prem B., et al. Long-lasting olfactory dysfunction in COVID-19 patients. Eur. Arch. Otorhinolaryngol. 2021;379:3485–3492. doi: 10.1007/s00405-021-07153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singer-Cornelius T., et al. Objective gustatory and olfactory dysfunction in COVID-19 patients: a prospective cross-sectional study. Eur. Arch. Otorhinolaryngol. 2021;278:3325–3332. doi: 10.1007/s00405-020-06590-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niklassen A.S., et al. COVID-19: recovery from chemosensory dysfunction. A multicentre study on smell and taste. Laryngoscope. 2021;131:1095–1100. doi: 10.1002/lary.29383. [DOI] [PubMed] [Google Scholar]
- 29.Soter A., et al. Accuracy of self-report in detecting taste dysfunction. Laryngoscope. 2008;118:611–617. doi: 10.1097/MLG.0b013e318161e53a. [DOI] [PubMed] [Google Scholar]
- 30.Wehling E., et al. Unawareness of olfactory dysfunction and its association with cognitive functioning in middle aged and old adults. Arch. Clin. Neuropsychol. 2011;26:260–269. doi: 10.1093/arclin/acr019. [DOI] [PubMed] [Google Scholar]
- 31.Nordin S., et al. Unawareness of smell loss in normal aging and Alzheimer's disease: discrepancy between self-reported and diagnosed smell sensitivity. J. Gerontol. 1995;50:187–192. doi: 10.1093/geronb/50b.4.p187. [DOI] [PubMed] [Google Scholar]
- 32.Doty R.L., Hawkes C.H. Chemosensory dysfunction in neurodegenerative diseases. Handb. Clin. Neurol. 2019;164:325–360. doi: 10.1016/B978-0-444-63855-7.00020-4. [DOI] [PubMed] [Google Scholar]
- 33.Doty R.L. 3rd edn. Sensonics; 1995. The Smell Identification TestTM Administration Manual; pp. 1–17. [Google Scholar]
- 34.Doty R.L., et al. Clinical validation of the olfactory detection threshold module of the Snap & Sniff(R) olfactory test system. Int. Forum Allergy. Rhinol. 2019;9:986–992. doi: 10.1002/alr.22377. [DOI] [PubMed] [Google Scholar]
- 35.Doty R.L., et al. Tests of human olfactory function: principal components analysis suggests that most measure a common source of variance. Percept. Psychophys. 1994;56:701–707. doi: 10.3758/bf03208363. [DOI] [PubMed] [Google Scholar]
- 36.Doty R.L. Psychophysical testing of smell and taste function. Handb. Clin. Neurol. 2019;164:229–246. doi: 10.1016/B978-0-444-63855-7.00015-0. [DOI] [PubMed] [Google Scholar]
- 37.Doty R.L. Olfactory dysfunction in Parkinson disease. Nat. Rev. Neurol. 2012;8:329–339. doi: 10.1038/nrneurol.2012.80. [DOI] [PubMed] [Google Scholar]
- 38.Doty R.L., Laing D.G. In: Handbook of Olfaction and Gustation. 3rd edn. Doty R.L., editor. Wiley-Liss; 2015. Psychophysical measurement of human olfactory function; pp. 229–261. [Google Scholar]
- 39.Osman A., Silas J. In: Handbook of Olfaction and Gustation. 3rd edn. Doty R.L., editor. Wiley-Liss; 2015. Electrophysiological measurement of olfactory function; pp. 263–279. [Google Scholar]
- 40.Tourbier I.A., Doty R.L. Sniff magnitude test: relationship to odor identification, detection, and memory tests in a clinic population. Chem. Senses. 2007;32:515–523. doi: 10.1093/chemse/bjm020. [DOI] [PubMed] [Google Scholar]
- 41.Moein S.T., et al. Smell dysfunction: a biomarker for COVID-19. Int. Forum Allergy. Rhinol. 2020;10:944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.London B., et al. Predictors of prognosis in patients with olfactory disturbance. Ann. Neurol. 2008;63:159–166. doi: 10.1002/ana.21293. [DOI] [PubMed] [Google Scholar]
- 43.Boscolo-Rizzo P., et al. Self-reported smell and taste recovery in coronavirus disease 2019 patients: a one-year prospective study. Eur. Arch. Otorhinolaryngol. 2021;279:515–520. doi: 10.1007/s00405-021-06839-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moein S.T., et al. Prevalence and reversibility of smell dysfunction measured psychophysically in a cohort of COVID-19 patients. Int. Forum Allergy. Rhinol. 2020;10:1127–1135. doi: 10.1002/alr.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jafar A., et al. Olfactory recovery following infection with COVID-19: a systematic review. PLoS One. 2021;16 doi: 10.1371/journal.pone.0259321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chapurin N., et al. Population differences between COVID-19 and other postviral olfactory dysfunction: results from a large case-control study. Int. Forum Allergy. Rhinol. 2022 doi: 10.1002/alr.22955. Published online January 8, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Velayudhan L., et al. Smell identification function in early-onset Alzheimer's disease and mild cognitive impairment. Int. Psychogeriatr. 2019;31:1065–1070. doi: 10.1017/S1041610218001503. [DOI] [PubMed] [Google Scholar]
- 48.Doty R.L. Olfactory dysfunction in neurodegenerative diseases: is there a common pathological substrate? Lancet Neurol. 2017;16:478–488. doi: 10.1016/S1474-4422(17)30123-0. [DOI] [PubMed] [Google Scholar]
- 49.Huart C., et al. Comparison of COVID-19 and common cold chemosensory dysfunction. Rhinology. 2020;58:623–625. doi: 10.4193/Rhin20.251. [DOI] [PubMed] [Google Scholar]
- 50.Khorramdelazad H., et al. Immunopathological similarities between COVID-19 and influenza: investigating the consequences of co-infection. Microb. Pathog. 2021;152 doi: 10.1016/j.micpath.2020.104554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clark N.M., Lynch J.P., 3rd Influenza: epidemiology, clinical features, therapy, and prevention. Semin. Respir. Crit. Care Med. 2011;32:373–392. doi: 10.1055/s-0031-1283278. [DOI] [PubMed] [Google Scholar]
- 52.Pormohammad A., et al. Comparison of influenza type A and B with COVID-19: a global systematic review and meta-analysis on clinical, laboratory and radiographic findings. Rev. Med. Virol. 2021;31 doi: 10.1002/rmv.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Potter M.R., et al. Olfactory dysfunction from acute upper respiratory infections: relationship to season of onset. Int. Forum Allergy. Rhinol. 2020;10:706–712. doi: 10.1002/alr.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zou L., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doty R.L. The mechanisms of smell loss after SARS-CoV-2 infection. Lancet Neurol. 2021;20:693–695. doi: 10.1016/S1474-4422(21)00202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akerlund A., et al. Olfactory threshold and nasal mucosal changes in experimentally induced common cold. Acta Otolaryngol. 1995;115:88–92. doi: 10.3109/00016489509133353. [DOI] [PubMed] [Google Scholar]
- 57.Panuganti B.A., et al. Predicting COVID-19 incidence using anosmia and other COVID-19 symptomatology: preliminary analysis using Google and Twitter. Otolaryngol. Head Neck Surg. 2020;163:491–497. doi: 10.1177/0194599820932128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffmann M., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salamanna F., et al. Body localization of ACE-2: on the trail of the keyhole of SARS-CoV-2. Front. Med. (Lausanne) 2020;7 doi: 10.3389/fmed.2020.594495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gudowska-Sawczuk M., Mroczko B. The role of neuropilin-1 (NRP-1) in SARS-CoV-2 infection: review. J. Clin. Med. 2021;10:2772. doi: 10.3390/jcm10132772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang K., et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal. Transduct. Target Ther. 2020;5:2772. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burks S.M., et al. Can SARS-CoV-2 infect the central nervous system via the olfactory bulb or the blood-brain barrier? Brain Behav. Immun. 2021;95:7–14. doi: 10.1016/j.bbi.2020.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ye Q., et al. The pathogenesis and treatment of the `cytokine storm' in COVID-19. J. Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zazhytska M., et al. Non-cell-autonomous disruption of nuclear architecture as a potential cause of COVID-19-induced anosmia. Cell. 2022;185:1052–1064. doi: 10.1016/j.cell.2022.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamming I., et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y., et al. Neuropilin-1 modulates interferon-γ-stimulated signaling in brain microvascular endothelial cells. J. Cell Sci. 2016;129:3911–3921. doi: 10.1242/jcs.190702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pevsner J., et al. Odorant-binding protein and its mRNA are localized to lateral nasal gland implying a carrier function. Proc. Natl. Acad. Sci. U. S. A. 1988;85:2383–2387. doi: 10.1073/pnas.85.7.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ijichi C., et al. Odorant metabolism of the olfactory cleft mucus in idiopathic olfactory impairment patients and healthy volunteers. Int. Forum Allergy Rhinol. 2021;12:293–301. doi: 10.1002/alr.22897. [DOI] [PubMed] [Google Scholar]
- 69.Soler Z.M., et al. Olfactory cleft mucus proteome in chronic rhinosinusitis: a case-control pilot study. Int. Forum Allergy. Rhinol. 2021;11:1162–1176. doi: 10.1002/alr.22743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshikawa K., et al. The human olfactory cleft mucus proteome and its age-related changes. Sci. Rep. 2018;8:17170. doi: 10.1038/s41598-018-35102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nagashima A., Touhara K. Enzymatic conversion of odorants in nasal mucus affects olfactory glomerular activation patterns and odor perception. J. Neurosci. 2010;30:16391–16398. doi: 10.1523/JNEUROSCI.2527-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu J., et al. Olfactory and middle meatal cytokine levels correlate with olfactory function in chronic rhinosinusitis. Laryngoscope. 2018;128:E304–E310. doi: 10.1002/lary.27112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ding X., Xie F. In: Handbook of Olfaction and Gustation. 3rd edn. Doty R.L., editor. John Wiley & Sons; 2015. Olfactory mucosa: composition, enzymatic localization, and metabolism; pp. 63–92. [Google Scholar]
- 74.Doty R.L. The olfactory vector hypothesis of neurodegenerative disease: is it viable? Ann. Neurol. 2008;63:7–15. doi: 10.1002/ana.21327. [DOI] [PubMed] [Google Scholar]
- 75.Zielinski B.S., et al. Ultrastructural localization and identification of adrenergic and cholinergic nerve terminals in the olfactory mucosa. Anat. Rec. 1989;225:232–245. doi: 10.1002/ar.1092250309. [DOI] [PubMed] [Google Scholar]
- 76.Revington M., et al. Sympathetic and parasympathetic interaction in vascular and secretory control of the nasal mucosa in anaesthetized dogs. J. Physiol. 1997;505:823–831. doi: 10.1111/j.1469-7793.1997.823ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krey L., et al. Can SARS-CoV-2 infection lead to neurodegeneration and Parkinson's disease? Brain Sci. 2021;11:1654. doi: 10.3390/brainsci11121654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Doty R.L., Kamath V. The influences of age on olfaction: a review. Front. Psychol. 2014;5:20. doi: 10.3389/fpsyg.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eccles R., Wilkinson J.E. Exposure to cold and acute upper respiratory tract infection. Rhinology. 2015;53:99–106. doi: 10.4193/Rhino14.239. [DOI] [PubMed] [Google Scholar]
- 80.Lofgren E., et al. Influenza seasonality: underlying causes and modeling theories. J. Virol. 2007;81:5429–5436. doi: 10.1128/JVI.01680-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hou Y.J., et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182:429–446. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lechien J.R., et al. Loss of smell and taste in 2013 European patients with mild to moderate COVID-19. Ann. Intern. Med. 2020;173:672–675. doi: 10.7326/M20-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eliezer M., et al. Loss of smell in patients with COVID-19: MRI data reveal a transient edema of the olfactory clefts. Neurology. 2020;95:e3145–e3152. doi: 10.1212/WNL.0000000000010806. [DOI] [PubMed] [Google Scholar]
- 84.Niesen M., et al. Structural and metabolic brain abnormalities in COVID-19 patients with sudden loss of smell. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:1890–1901. doi: 10.1007/s00259-020-05154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kandemirli S.G., et al. Olfactory bulb MRI and paranasal sinus CT findings in persistent COVID-19 anosmia. Acad. Radiol. 2021;28:28–35. doi: 10.1016/j.acra.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eliezer M., Hautefort C. MRI Evaluation of the olfactory clefts in patients with SARS-CoV-2 infection revealed an unexpected mechanism for olfactory function loss. Acad. Radiol. 2020;27:1191. doi: 10.1016/j.acra.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen M., et al. Elevated ACE-2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur. Respir. J. 2020;56 doi: 10.1183/13993003.01948-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Workman A.D., et al. Airborne aerosol olfactory deposition contributes to anosmia in COVID-19. PLoS One. 2021;16 doi: 10.1371/journal.pone.0244127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tekcan Sanli D.E., et al. Comparison of olfactory cleft width and volumes in patients with COVID-19 anosmia and COVID-19 cases without anosmia. ORL J. Otorhinolaryngol. Relat. Spec. 2022;84:1–9. doi: 10.1159/000518672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Menco B.P.M., Morrison E.E. In: Handbook of Olfaction and Gustation. 2nd edn. Doty R.L., editor. Marcel Dekker; 2003. Morphology of the mammalian olfactory epithelium: form, fine structure, function, and pathology; pp. 17–49. [Google Scholar]
- 91.Smithson L.J., Kawaja M.D. Microglial/macrophage cells in mammalian olfactory nerve fascicles. J. Neurosci. Res. 2010;88:858–865. doi: 10.1002/jnr.22254. [DOI] [PubMed] [Google Scholar]
- 92.Panni P., et al. Phagocytosis of bacteria by olfactory ensheathing cells and Schwann cells. Neurosci. Lett. 2013;539:65–70. doi: 10.1016/j.neulet.2013.01.052. [DOI] [PubMed] [Google Scholar]
- 93.Wang J.-J., et al. Impact of antibiotics on smell dysfunction. World J. Otorhinolaryng. Head Neck Surg. 2018;4:33–38. doi: 10.1016/j.wjorl.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Calderon-Garciduenas L., et al. Urban air pollution: influences on olfactory function and pathology in exposed children and young adults. Exp. Toxicol. Pathol. 2010;62:91–102. doi: 10.1016/j.etp.2009.02.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Genter M.B., Doty R.L. Toxic exposures and the senses of taste and smell. Handb. Clin. Neurol. 2019;164:389–408. doi: 10.1016/B978-0-444-63855-7.00022-8. [DOI] [PubMed] [Google Scholar]
- 96.Jafek B.W., et al. Postviral olfactory dysfunction. Am. J. Rhinol. 1990;4:91–100. [Google Scholar]
- 97.Forrester J.V., et al. CNS infection and immune privilege. Nat. Rev. Neurosci. 2018;19:655–671. doi: 10.1038/s41583-018-0070-8. [DOI] [PubMed] [Google Scholar]
- 98.van Riel D., et al. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J. Pathol. 2015;235:277–287. doi: 10.1002/path.4461. [DOI] [PubMed] [Google Scholar]
- 99.Johnston M., et al. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 2004;1:2. doi: 10.1186/1743-8454-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Monath T.P., et al. Mode of entry of a neurotropic virus into the central nervous system: reinvestigation of an old controversy. Lab. Investig. 1983;48:399–410. [PubMed] [Google Scholar]
- 101.Allsopp T.E., et al. Virus infection induces neuronal apoptosis: a comparison with trophic factor withdrawal. Cell Death Differ. 1998;5:50–59. doi: 10.1038/sj.cdd.4400298. [DOI] [PubMed] [Google Scholar]
- 102.Mori I., et al. Virus-induced neuronal apoptosis as pathological and protective responses of the host. Rev. Med. Virol. 2004;14:209–216. doi: 10.1002/rmv.426. [DOI] [PubMed] [Google Scholar]
- 103.Durrant D.M., et al. The olfactory bulb: an immunosensory effector organ during neurotropic viral infections. ACS Chem. Neurosci. 2016;7:464–469. doi: 10.1021/acschemneuro.6b00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McLean J.H., et al. Selective lesions of neural pathways following viral inoculation of the olfactory bulb. Exp. Neurol. 1993;122:209–222. doi: 10.1006/exnr.1993.1121. [DOI] [PubMed] [Google Scholar]
- 105.Zugaj M., et al. The effect of coronaviruses on olfaction: systematic review. Rhinology. 2021;59:226–235. doi: 10.4193/Rhin20.610. [DOI] [PubMed] [Google Scholar]
- 106.Khan M., et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell. 2021;184:5932–5949. doi: 10.1016/j.cell.2021.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fodoulian L., et al. SARS-CoV-2 receptors and entry genes are expressed in the human olfactory neuroepithelium and Brain. iScience. 2020;23 doi: 10.1016/j.isci.2020.101839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lalancette-Hebert M., et al. Live imaging of Toll-like receptor 2 response in cerebral ischaemia reveals a role of olfactory bulb microglia as modulators of inflammation. Brain. 2009;132:940–954. doi: 10.1093/brain/awn345. [DOI] [PubMed] [Google Scholar]
- 109.Altunisik E., et al. Quantitative analysis of the olfactory system in COVID-19: an MR imaging study. AJNR Am. J. Neuroradiol. 2021;42:2207–2214. doi: 10.3174/ajnr.A7278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yildirim D., et al. A comparative olfactory MRI, DTI and fMRI study of COVID-19 related anosmia and post viral olfactory dysfunction. Acad. Radiol. 2022;29:31–41. doi: 10.1016/j.acra.2021.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schriever V.A., et al. Olfactory bulb volume in smokers. Exp. Brain Res. 2013;225:153–157. doi: 10.1007/s00221-012-3356-5. [DOI] [PubMed] [Google Scholar]
- 112.Thomann P.A., et al. Reduced olfactory bulb and tract volume in early Alzheimer's disease--a MRI study. Neurobiol. Aging. 2009;30:838–841. doi: 10.1016/j.neurobiolaging.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 113.Wang J., et al. Association of olfactory bulb volume and olfactory sulcus depth with olfactory function in patients with Parkinson disease. AJNR Am. J. Neuroradiol. 2011;32:677–681. doi: 10.3174/ajnr.A2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Doty R.L., et al. Olfactory dysfunction in patients with head trauma. Arch. Neurol. 1997;54:1131–1140. doi: 10.1001/archneur.1997.00550210061014. [DOI] [PubMed] [Google Scholar]
- 115.Goektas O., et al. Olfactory bulb volume and olfactory function in patients with multiple sclerosis. Rhinology. 2011;49:221–226. doi: 10.4193/Rhino10.136. [DOI] [PubMed] [Google Scholar]
- 116.Turetsky B.I., et al. Low olfactory bulb volume in first-degree relatives of patients with schizophrenia. Am. J. Psychiat. 2003;160:703–708. doi: 10.1176/appi.ajp.160.4.703. [DOI] [PubMed] [Google Scholar]
- 117.Rombaux P., et al. Olfactory bulb volume in patients with sinonasal disease. Am. J. Rhinol. 2008;22:598–601. doi: 10.2500/ajr.2008.22.3237. [DOI] [PubMed] [Google Scholar]
- 118.Lopez G., et al. Olfactory bulb SARS-CoV-2 infection is not paralleled by the presence of virus in other central nervous system areas. Neuropathol. Appl. Neurobiol. 2022;48 doi: 10.1111/nan.12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Serrano G.E., et al. Mapping of SARS-CoV-2 brain invasion and histopathology in COVID-19 disease. medRxiv. 2021 doi: 10.1101/2021.02.15.21251511. Published online February 18, 2021. [DOI] [Google Scholar]
- 120.Xydakis M.S., et al. Post-viral effects of COVID-19 in the olfactory system and their implications. Lancet Neurol. 2021;20:753–761. doi: 10.1016/S1474-4422(21)00182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Matschke J., et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sulzer D., et al. COVID-19 and possible links with Parkinson's disease and parkinsonism: from bench to bedside. NPJ Park. Dis. 2020;6:18. doi: 10.1038/s41531-020-00123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fearon C., Fasano A. Parkinson's disease and the COVID-19 pandemic. J. Parkinsons Dis. 2021;11:431–444. doi: 10.3233/JPD-202320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cazzolla A.P., et al. Taste and smell disorders in COVID-19 patients: role of interleukin-6. ACS Chem. Neurosci. 2020;11:2774–2781. doi: 10.1021/acschemneuro.0c00447. [DOI] [PubMed] [Google Scholar]
- 125.Vaira L.A., et al. Correlations between IL-6 serum level and olfactory dysfunction severity in COVID-19 patients: a preliminary study. Eur. Arch. Otorhinolaryngol. 2021;279:811–816. doi: 10.1007/s00405-021-06868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Taquet M., et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schou T.M., et al. Psychiatric and neuropsychiatric sequelae of COVID-19 - a systematic review. Brain Behav. Immun. 2021;97:328–348. doi: 10.1016/j.bbi.2021.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Douaud G., et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604:697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Coelho D.H., et al. Quality of life and safety impact of COVID-19 associated smell and taste disturbances. Am. J. Otolaryngol. 2021;42 doi: 10.1016/j.amjoto.2021.103001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yom-Tov E., et al. Association of COVID19-induced anosmia and ageusia with depression and suicidal ideation. J. Affect. Disord. Rep. 2021;5 doi: 10.1016/j.jadr.2021.100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dudine L., et al. Investigation on the loss of taste and smell and consequent psychological effects: a cross-sectional study on healthcare workers who contracted the COVID-19 infection. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.666442. [DOI] [PMC free article] [PubMed] [Google Scholar]