Abstract
Objectives
This study aims to assess the efficacy of a combination treatment of doxycycline and zinc in the primary prevention of COVID-19 infection in Tunisian health care workers compared with two control groups.
Methods
We conducted a prospective, randomized, double-blind clinical trial over 5 months to determine the efficacy of a preventive combination treatment dose of doxycycline (100 mg/day) and zinc (15 mg/day), compared with a single-dose treatment with doxycycline versus placebo. The effectiveness of preventive treatment was measured by the significant decline in the number of cases of COVID-19 infection and/or a decrease in the viral load as determined by SARS-CoV-2 cycle threshold value using reverse transcription polymerase chain reaction tests.
Results
We detected a significant decrease of SARS-CoV-2 infection in the group that received both doxycycline and zinc compared with other participants. We also demonstrated that COVID-19 infection was neither associated with diabetes (P = 0.51) nor associated with hypertension (P = 0.99), asthma (P = 0.52), and chronic obstructive pulmonary disease (P = 0.27).
Conclusion
Our findings indicated that preventive therapy reduced the risk of SARS-CoV-2. These results suggest that the combination of doxycycline and zinc has a protective effect in patients with SARS-CoV-2 infection.
Keywords: Doxycycline, Zinc, Placebo, COVID-19 infection, Prophylactic
Introduction
Prevention of COVID-19 transmission and infection includes nonpharmacologic intervention and specific protection through chemoprophylaxis or immune prophylaxis (Agrawal et al., 2020). Since the 1970s, the protective efficacy of doxycycline has been demonstrated in the prophylaxis of malaria for travelers to endemic areas (Tan et al., 2011). In Tunisia, doxycycline has been widely used in the prevention of malaria for military personnel going on missions to endemic areas (Ajili et al., 2013). It has a low cost and a favorable safety profile and has been proposed as a treatment for COVID-19 (Malek et al., 2020). Doxycycline has been used as a curative treatment for COVID-19 in Brazil and India (Butler et al., 2021), whereas in the UK, it is recommended for suspected COVID-19 pneumonia in patients who are at high risk for adverse outcomes or where bacterial infection is suspected (Butler et al., 2021).
Zinc also has antiviral effects against certain viruses (Guo et al., 2004). In fact, zinc supplementation improves the mucociliary clearance, strengthens the integrity of the epithelium, decreases viral replication, preserves antiviral immunity, attenuates the risk of hyperinflammation, supports antioxidative effects, reduces lung damage, and minimizes secondary infections (Wessels et al., 2020).
The purpose of this study was to evaluate the efficacy and safety of doxycycline with or without zinc in preventing COVID-19 infection. The study focused on Tunisian health care workers (HCWs), and the outcome measures were the decreased number of infected cases with COVID-19 and/or the decrease in the viral load, as determined by the cycle threshold (Ct) values of the reverse transcription polymerase chain reaction (RT-PCR) tests for SARS-CoV-2.
Materials and methods
Patients and methods
We conducted a prospective, randomized, double-blind clinical trial for 5 months (from November 12, 2020, through February 10, 2021). The study was conducted at different military sites of investigation (Military Hospital of Tunis, Military Hospital of Bizerte, Military Hospital of Gabes), was registered in the clinical trial database (NCT04584567 ), and approved by the local ethical committee of the General Directorate of Military Health.
The study included consenting HCWs, aged between 20 and 65 years, who did not have signs or symptoms of respiratory infection (cough, fever >38.0°C, difficulty breathing, shortness of breath, chest pains), or other symptoms associated with COVID-19, such as faintness, myalgia, headaches, and nausea or vomiting.
The study excluded HCWs with positive SARS-CoV-2 RT-PCR results or positive SARS-CoV-2 serum tests (IgM or IgG). Positive SARS-CoV-2 RT-PCR was determined by a cycle threshold (Ct) value below 33, using the Xpress SARS-CoV-2 kit (Xpert, Cepheid, Sunnyvale, CA). The GeneXpert is a system that automates and integrates sample preparation, nucleic acid extraction, amplification, and detection of the target sequences using real-time RT-PCR assays. The real-time RT-PCR reagents, including primer N2, target the virus nucleocapsid phosphoprotein (N) gene for specific detection of SARS-CoV-2 and human nucleic acid. The Cepheid Xpert Xpress SARS-CoV-2 kit detects N2 and E gene, and solely N2 indicates positive results. The presence of only E indicates presumptive positive results, whereas the presence of only A Sample Processing Control (SPC) indicates negative results. The absence of all markers indicates an invalid result (Moran et al., 2020).
Ct is a value that can broadly classify the concentration of viral genetic material as low, medium, or high in a patient sample, using RT-PCR test. It tells us approximately how much viral genetic material is in the sample. A low Ct indicates a high concentration of viral genetic material, which is associated with high risk of infection. A high Ct indicates a low concentration of viral genetic material, which is associated with a lower risk of infection.
For the qualitative detection of antibodies against SARS-CoV-2, we used the Biosynex COVID-19 Ag+ BSS test (Illkirch-Graffenstaden, France).
Additionally, we excluded HCWs who had a known hypersensitivity to doxycycline or zinc. HCWs with comorbidities (gastric bypass, epilepsy, cardiovascular disease, renal failure) or being treated with vitamin A during the study were excluded. Women who were pregnant or nursing were also excluded.
Eligible participants were randomized in three parallel groups as follows:
Group A: doxycycline (100 mg/ day) and zinc (15 mg/ day) combined orally for 6 weeks.
Group B: doxycycline (100 mg /day) orally for 6 weeks.
Group C: placebo orally for 6 weeks.
Allocated study groups were randomly assigned by an interactive web response system (Dacima), using simple 1:1 nonstratified sequence and a block size of six. Allocation sequences are set automatically by the interactive web response systems software.
Doxycycline was manufactured by Philadelphia Pharmaceutical (batch number 12927222). Placebo was also produced by the same manufacturer (Philadelphia Pharmaceutical). Placebo contains the same excipients and no active substances. Zinc (zinc bisglycinate) was manufactured by Albion human nutrition (batch number F001).
The participants were screened 72 hours before enrollment. They were examined physically, tested for SARS-CoV-2 RT-PCR and serum test (IgM/IgG). All personal data were anonymized, and signed written informed consent forms were obtained before the beginning of the study. The participants were monitored at days 21, 42, and 49 after enrollment. At each follow-up visit, participants were asked about their medical compliance and drug safety, underwent a clinical examination, and had an RT-PCR with calculated Ct values. After screening for patient's eligibility, a balanced randomization was done on the same day by the investigator. Allocated study drug was provided to the patient by the Military Hospital Pharmacist, according to the allocation details provided by the interactive web response systems. The patient received the drug on the same day of the randomization. Study blindness was maintained by a blind allocation, and drugs bottles were labeled anonymously by the pharmacist. Statistical analysis of the database was performed with blinded groups. Unblinding was performed at the final statistical report review. The Dacima Clinical Suite software (Montréal, Québec, Canada) was used to collect the data and to randomize participants on the basis of the Food and Drug Administration 21 Code of US Federal Regulations part 11, Health Insurance Portability and Accountability Act, and International Conference on Harmonization requirements.
Study end points
The first end point of this study was to assess how doxycycline with low dose can be used as a preventive treatment to decrease the number of cases of COVID-19 in the active arms compared with the placebo. Participants for each randomized treatment arm (doxycycline-only or combined with zinc) were compared with placebo. The second end point was intended to quantify by RT-PCR the number of infected cases with COVID-19 and the viral ribonucleic acid load after each treatment.
Sample size
Sample size was estimated by the Cochran-Armitage test for linear trend in proportions. Considering “a sample power of 80%”, an expected proportion of the primary end point of 8% in group A, 10% in group B, and 14% in group C, with a confidence level of 95%. The expected sample size was estimated up to 1100 overall eligible subjects. However, due to the pandemic situation and the rush for nationwide vaccination against the SARS-CoV-2, the study was ended after including 172 randomized patients.
Evaluation
During the follow-up period (6 weeks), we monitored the SARS-CoV-2 infection by RT-PCR tests at days 21, 42, and 49. The last evaluation was done by day 49 to determine if a potential infection occurred after the treatment stopped at day 42.
Statistical analysis
For statistical analysis, qualitative data were described by frequency and percentage of valid values. Continuous variables were used to describe mean and standard deviation (SD). Chi-square, Mann-Whitney U test, and Kruskal-Wallis tests (one-way analysis of variance) were used to make inferential comparisons, with a 5% significance level as a threshold. Differences were considered significant if P ≤ 0.05. No further statistical analysis was performed due to the study's premature interruption.
Results
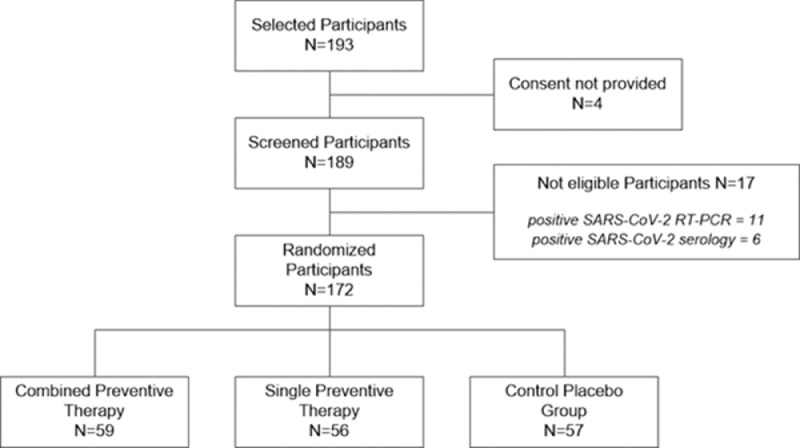
From the 193 selected HCWs, 189 were screened. Four subjects declined to participate in the study. The screening phase excluded 17 subjects who were infected with COVID-19: positive SARS-CoV-2 RT-PCR (n = 11) and positive SARS-CoV-2 serology (n = 6). Participants were not symptomatic at the time of RT-PCR/serology testing at inclusion (Figure 1 ).
Figure 1.
Design and analysis of the study. A total of 172 eligible participants were randomized into the three study arms: 59 in the combined doxycycline and zinc arm, 56 in the doxycycline-only arm, and 57 in the placebo arm. RT-PCR, reverse transcription polymerase chain reaction.
The remaining 172 eligible participants were randomized to one of the three study arms: 59 in the combined doxycycline and zinc arm (group A), 56 in the doxycycline-only arm (group B), and 57 in the placebo arm (group C).
The mean age was 38.4 ± 10.7 years (20 to 60 years). In groups A, B, and C, the average age was 38.0 ± 10.6, 38.5 ± 10.0, and 38.7 ± 11.4 years, respectively (P = 0.934) (Table 1 ).
Table 1.
Demographic characteristics of the investigated groups.
| All participants N = 172 | Group A N = 59 | Group B N = 56 | Group C N = 57 | P-value | |
|---|---|---|---|---|---|
| Age | 38.4 ± 10.7 | 38.0 ± 10.6 | 38.5 ± 10.0 | 38.7 ± 11.4 | 0.936 |
| Gender ratio | 1.6 (105 M/67 F) | 2.5 (42 M/17 F) | 1.2 (31 M/25 F) | 1.3 (32 M/25 F) | 0.143 |
| Comorbidities, n (%) | 14 | 17 | 19 | ||
| Hypertension | 7 (4.1%) | 0 (0%) | 2 (3.6%) | 5 (8.8%) | 0.056 |
| Diabetes | 4 (2.3%) | 0 (0%) | 1 (1.8%) | 3 (5.3%) | 0.161 |
| Asthma | 2 (1.2%) | 0 (0%) | 1 (1.8%) | 1 (1.8%) | 0.589 |
| COPD | 1 (0.6%) | 0 (0%) | 0 (0%) | 1 (1.8%) | 0.363 |
| History of COVID-19 infection | 0 | 0 | 0 | 0 | _ |
| Vaccination | Vaccine not yet available |
COPD, chronic obstructive pulmonary disease; F, female; M, male; NS, not significant.
The sex ratio was 1.6 (males = 5, females = 67), with no significant differences between the three study groups (P = 0.143). The study arms did not differ significantly for history of influenza (n = 43; 25.0%; P = 0.927), hypertension (n = 7; 4.1%; P = 0.056), diabetes (n = 4; 2.3%; P = 0.162), asthma (n = 2; 1.2%; P = 0.590), and chronic obstructive pulmonary disease (COPD) (n = 1; 0.6%; P = 0.363) (Table 1).
All participants worked at the Military Hospital of Tunis, which has been active since the beginning of the COVID-19 pandemic. Among the participants, there were different levels of exposure to COVID-19 infection in the workplace, including invasive bedside procedures with patients with COVID-19 (n = 40; 23.3%), intraoperative procedure (n = 19; 11.0%), and routine care (n = 113; 65.7%), with no significant differences between the studied arms (P = 0.2).
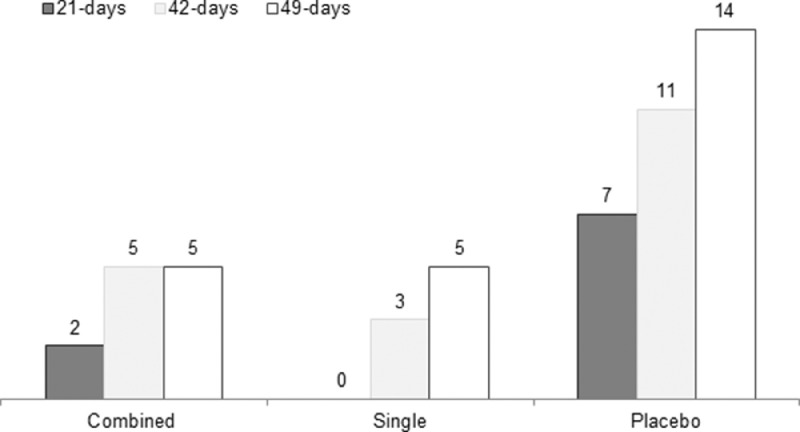
At the end of the study, a total of 24 subjects tested positive for SARS-CoV-2 (14.0%): 5 (8.5%) in group A, 5 (8.9%) in group B, and 14 (24.6%) in group C, with a significant difference between the three arms (P = 0.018) (Figure 2 ).
Figure 2.
Cumulative positive reverse transcription polymerase chain reaction results for SARS-CoV-2 between the three groups, during follow-up (P = 0.018). Each group's histogram represents the number of days during follow-up; 24 days are represented by black, 42 days by gray, and 49 days by white.
In the first group (group A), most participants (4 of 5) were asymptomatic, 1 participant developed symptoms with a persistent fever for 3 days. In the second (doxycycline-only) group (group B), 2 participants were asymptomatic and 3 developed moderate symptoms with fever, headache, and mild nonpersistent cough. In the third (placebo) group (group C), 6 of 14 participants developed symptoms such as fever, cough, and headache. For 2 of them, the cough was persistent, and corticosteroid treatment was prescribed. None of the participants in the three groups required oxygen (Table 2 ).
Table 2.
Clinical symptoms of COVID-19–infected participant.
| Symptoms | Combined treatments (Group A) | Single treatment (Group B) | Placebo (Group C) | Total |
|---|---|---|---|---|
| Asymptomatic | 04 | 02 | 08 | 14 |
| Mild to Moderate | 01 | 03 | 06 | 10 |
| Total | 5 | 5 | 14 | 24 |
This difference was also found from day 21 of the follow-up, when 9 subjects tested positive for SARS-CoV-2 RT-PCR (5.2%), as opposed to 2, 0, and 7 in the A, B, and C groups, respectively (P = 0.01). Figure 2 shows the cumulative positive RT-PCR frequency trend among the three groups during the follow-up visits.
The average Ct for participants with a positive SARS-CoV-2 RT-PCR was 22.6 ± 4.7. Comparison between the study arms revealed a significant difference in Ct levels (P < 0.001), with the highest mean level of Ct in group A (29.0 ± 1.3), then group B (22.8 ± 4.0), then group C (19.4 ± 2.5). Table 3 shows Ct profiles of the three groups during the study.
Table 3.
Total cycle threshold profile of the three groups during the study.
| Study arm | Mean ± SD | 95% CI | P-value |
|---|---|---|---|
| Combined Therapy | 29.0 ± 1.3 | 27.7-30.3 | <0.001 |
| Single Therapy | 22.8 ± 4.0 | 18.6-26.9 | |
| Control Group | 19.4 ± 2.5 | 17.8-21.0 | |
| Overall | 22.6 ± 4.7 | 20.6-24.7 |
CI, confidence interval; SD, standard deviation.
Comparison between three groups using Kruskal-Wallis analysis. Results were considered significant if P-value <0.05
We observed that a higher risk of COVID-19 infection was not associated with diabetes (P = 0.51), hypertension (P = 0.99), asthma (P = 0.52), or COPD (P = 0.27). Table 4 shows the patient's baseline profile according to SARS-CoV-2 infection. Four of the 24 infected participants had comorbidities. In the combined treatment arm, there were no participants with comorbidities. In the single treatment arm, there was 1 patient with diabetes and 1 with hypertension. In the placebo group, there was 1 participant with asthma and 1 participant with COPD. However, in the combined treatment arm, there were no participants with comorbidities.
Table 4.
Patient baseline profile according to SARS-CoV-2 infection.
| End point | Infected patients with SARS-coV-2 | Noninfected patients | P-value |
|---|---|---|---|
| N | 24 | 148 | - |
| Diabetes | 1 (4.2%) | 3 (2.0%) | 0.518 |
| HTN | 1 (4.2%) | 6 (4.0%) | 0.999 |
| Asthma | 1 (4.2%) | 1 (0.68%) | 0.520 |
| COPD | 1 (4.2%) | 0 (0.0%) | 0.279 |
| Conveyance Own car | 8 (33.34%) | 60 (40.55%) | |
| Public transport | 16 (66.67%) | 88(75.68%) | 0.502 |
Values are presented as number (percentage).
COPD, chronic obstructive pulmonary disease; HTN, hypertension.
The safety of the doxycycline/zinc intervention was also assessed during the study follow-up. The most common adverse events reported epigastralgia (n = 14) and nausea (n = 8). In term of side effects, the difference between studied groups were significant only for nausea (P = 0.032) but not for epigastralgia (P = 0.249) (Table 5 ).
Table 5.
Safety profile of the study arms.
| Adverse event | Combined therapy | Single therapy | Control group | Overall | P-value |
|---|---|---|---|---|---|
| N | 59 | 56 | 57 | 172 | - |
| Epigastralgia | 7 (11.9%) | 5 (8.9%) | 2 (3.5%) | 14 (8.1%) | 0.249 |
| Nausea | 1 (1.7%) | 6 (10.7%) | 1 (1.8%) | 8 (4.7%) | 0.032 |
| Overall | 8 (13.6%) | 11 (19.6%) | 3 (5.3%) | 22 (12.8%) | 0.071 |
Values are presented as number (percentage).
Discussion
As of January 2022, there are 106 clinical trials underway for prophylactic alternatives to COVID-19, some of which involve antivirals, such as favipiravir or interferons (Ben-Zuk et al., 2021). To the best of our knowledge, this is the only study to evaluate the effect of doxycycline in patients infected with SARS-CoV-2.
In addition to its anti-inflammatory effects, doxycycline has also antiviral activity against several ribonucleic acid viruses in vitro (Gendrot et al., 2020). It also chelates zinc from metalloproteases (MMPs) (Sodhi and Etminan, 2020). As a result, their chelating activity may help to inhibit SARS-CoV-2 infection by limiting the virus’ ability to replicate in the host, thereby acting as a prophylactic agent (Sodhi and Etminan, 2020).
There have already been some recommendations of doxycycline for COVID-19, particularly in patients with pneumonia and those who are at high risk for complications. There is now evidence of increased use of respiratory antibiotics, including doxycycline, during the COVID-19 pandemic in both the United Kingdom and United States (de Lusignan et al., 2021).
In this randomized, double-blind, placebo-controlled study, we compared doxycycline and zinc with only doxycycline or placebo as prophylaxis for COVID-19 in Tunisian HCWs.
In this trial, however, the results are underpowered (69.2%) because we were unable to meet the scheduled sample size, which was estimated at a total of 1100 samples. Moreover, the recruitment of HCWs remains difficult because the enrollment of patients overlapped with the vaccination campaign. The study recruited only 15.6% of the estimated sample size.
The prophylactic effect observed with doxycycline may be attributed to the fact that it inhibits MMPs. In fact, it has been demonstrated that coronaviruses exploit MMPs for a range of activities (replication, cell infection, and survival). Therefore, doxycycline may have an antiviral effect on SARS-CoV-2 (Dutta and Basu, 2011).
Researchers have suggested that doxycycline may delay COVID-19 progression through anti-inflammatory activities, including viruses that regulate the NF- κB pathway (nuclear factor kappa-light-chain-enhancer of activated B cells) and inhibit proinflammatory cytokine levels (interleukin 6 (IL-6), interleukin 1β (IL-1β), TNFα tumor necrosis factors (TNFα)) during acute respiratory distress syndrome (ARDS) in severely ill patients with COVID-19 (Anwar et al., 2020). Earlier studies demonstrated the effectiveness of chemically modified tetracyclines against SARS, preventing septic shock and ARDS development (Griffin et al., 2010). Additionally, several case reports suggest beneficial effects of doxycycline preventive treatment. In this context, a study demonstrated an improvement of clinical symptoms after treatment with standard doses of doxycycline in 4 patients with COVID-19 with a high-risk pulmonary disease (Yates et al., 2020).
Additionally, the combination of doxycycline and zinc also seems interesting because doxycycline has been shown to protect against lung infection by inhibiting MMPs, in which it is dependent on zinc (Doroszko et al., 2010). An interesting result of this clinical trial is the reduction in viral load in the combined group therapy (doxycycline and zinc) compared with other groups. This finding is supported by cumulative Ct values at day 49, which shows an inverse relationship with viral messenger ribonucleic acid (Supplementary Figure 1). These results are in agreement with other studies, which have suggested that Ct values are inversely associated with the viral load, and every threefold increase in Ct values indicates a 10-fold decrease in starting material (Tom and Mina, 2020). Low Ct values were also reported to be associated with virus growth in cell culture and infection (Platten et al., 2021). It is possible that the antiviral effect of zinc and inhibiting MMPs with doxycycline may help repair the damaged lung tissue, thus decreasing the virus’ availability in the nasal tract and enhancing recovery.
The potential effect of combined therapy may be explained by the ability of doxycycline to catalyze Zn2+ ions, which are required for the activity of MMPs, independently of its antimicrobial properties (Castro et al., 2011). Doxycycline is the most potent tetracycline derivative inhibitor of MMPs, even at low doses (25 mg) (Castro et al., 2011). It may act as an ionophore by increasing intracellular zinc concentrations, suppressing viral replication, and strengthening the immune system (Griffin et al., 2010; te Velthuis et al., 2010). In addition to doxycycline's effects, zinc also has a beneficial role in protecting against COVID-19 infection. In fact, many reports have shown that zinc can inhibit the enzymatic activity and replication of SARS-CoV-2’s ribonucleic acid polymerase and can inhibit angiotensin-converting enzyme activity (Ratia et al., 2006; Skalny et al., 2020). It was suggested that zinc can prevent fusion with the host membrane, decrease the viral polymerase function, impair protein translation and processing, block viral particle release, and destabilize the viral envelope (Wessels et al., 2020). Therefore, zinc has anti-inflammatory and antioxidative properties and underlying mechanisms, which have been the focus of numerous studies (Wessels et al., 2017).
In this study, we recruited 193 HCWs for 4 months (from November 2020 to February 2021). The percentage of HCWs who tested positive for COVID-19 was 21 (24%). A meta-analysis conducted during the first 6 months of the pandemic from January 1 to July 9, 2020 found a percentage of infected HCWs of 51.7% (Gholami et al., 2021). These results show that HCWs are at high risk for COVID-19 infection; thus, prevention strategies should be developed in this category. This will have a positive impact on patient's safety and could decrease the absenteeism level.
Our study was stopped after 4 months due to the start of the vaccination campaign, which prioritized HCWs (Kefi et al., 2021). We achieved 15.6% of the estimated sample size. Therefore, we recommend additional research on chemoprophylaxis that could enhance COVID-19 vaccine and litigation strategies to protect against COVID-19.
Although diabetes, hypertension, and asthma were considered COVID-19 risk factors (Gómez-Ochoa et al., 2021), they were not significantly associated with an increased risk of infection in the sample studied. Moreover, we did not consider the adherence of the HCWs to protective measures. The risk of COVID-19 infection directly depends on our physical preventive measures.
Despite the low number of participants in this study, the data suggest that a combined treatment of doxycycline and zinc may have a potential preventive effect. Public health suggestions can be made on the basis of this information for a wider adaptation of prophylaxis treatment in HCWs and the general population. Additional studies should be conducted considering vaccination laws.
Conclusion
The only indication approved by the US Food and Drug Administration for the use of doxycycline in patients is as a malaria prophylaxis (<4 months). According to the portal clinicaltrials.gov, to date, there are not less than 405 clinical trials registered involving doxycycline, targeting different diseases, such as bacterial infections, cystic fibrosis, acne, vascular diseases, and HIV-AIDS. However, the prescription of long-term antibiotics must be cautious and supervised. Overuse is linked to bacterial resistance and alterations in gut microbiota, which have been related to risks of various chronic diseases. In this study, doxycycline combined or not with zinc has been used before the beginning of vaccination campaign. Nevertheless, this alternative therapy might be useful for certain HCWs and/or others who have comorbidities and are not responding to vaccines or have refused them.
Taking preventive measures decreased the risk of contamination by SARS-CoV-2. In this study, doxycycline and zinc were found to have a protective effect when taken together. Chemoprophylaxis can be used in conjunction with COVID-19 vaccination and personal protective measures, but more research is needed.
Funding
This trial was conducted with funds from the Military Hospital of Tunis.
Ethical statement
This study has been approved by the local ethics committee of the General Directorate of Military Health.
Author contributions
NS, HG, RB, MBM, MAY: protocol redaction; NS, RB, MBM, HG: conceptualization; NS, RB, MBM, HG: methodology; NS, KT, RB, HG: formal analysis; NS, KB, AD, RB, GH, RR: writing; SN, AD, BA, KB, RB, HG: review and editing; AB, RA, SH, SB, AR, NI, MG, AH, CA, AH, FG: investigation; MAY: product manager, supervision; MBM: virological testing; RR, KT: validation, resources, software, visualization, statistical analysis; MF, HG: project administration.
Conflict of interest statement
The authors have no competing interests to declare.
Acknowledgments
This project has been made possible owing to the efforts of Colonel Major Fethi Mattoussi, General Director of the Military Hospital of Tunis, and General Jalel Hmida, Director of the Care Services.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.06.016.
Appendix. Supplementary materials
References
- Agrawal S, Goel AD, Gupta N. Emerging prophylaxis strategies against COVID-19. Monaldi Arch Chest Dis. 2020;90 doi: 10.4081/monaldi.2020.1289. [DOI] [PubMed] [Google Scholar]
- Ajili F, Battikh R, Laabidi J, Abid R, Bousetta N, Jemli B, et al. Malaria in Tunisian military personnel after returning from external operation. Malar Res Treat. 2013;2013 doi: 10.1155/2013/359192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar I, El-El-dien Anwer EK, AbdAllah M. Doxycycline: a new treatment option for COVID-19. Alex J Med. 2020;56:130–131. [Google Scholar]
- Ben-Zuk N, Dechtman ID, Henn I, Weiss L, Afriat A, Krasner E, et al. Potential prophylactic treatments for COVID-19. Viruses. 2021;13:1292. doi: 10.3390/v13071292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler CC, Yu LM, Dorward J, Gbinigie O, Hayward G, Saville BR, et al. Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet Respir Med. 2021;9:1010–1020. doi: 10.1016/S2213-2600(21)00310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro MM, Kandasamy AD, Youssef N, Schulz R. Matrix metalloproteinase inhibitor properties of tetracyclines: therapeutic potential in cardiovascular diseases. Pharmacol Res. 2011;64:551–560. doi: 10.1016/j.phrs.2011.05.005. [DOI] [PubMed] [Google Scholar]
- de Lusignan S, Joy M, Sherlock J, Tripathy M, van Hecke O, Gbinigie K, et al. PRINCIPLE trial demonstrates scope for in-pandemic improvement in primary care antibiotic stewardship: a retrospective sentinel network cohort study. BJGP Open. 2021;5 doi: 10.3399/BJGPO.2021.0087. BJGPO.2021.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroszko A, Hurst TS, Polewicz D, Sawicka J, Fert-Bober J, Johnson DH, et al. Effects of MMP-9 inhibition by doxycycline on proteome of lungs in high tidal volume mechanical ventilation-induced acute lung injury. Proteome Sci. 2010;8:3. doi: 10.1186/1477-5956-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta K, Basu A. Use of minocycline in viral infections. Indian J Med Res. 2011;133:467–470. [PMC free article] [PubMed] [Google Scholar]
- Gendrot M, Andreani J, Jardot P, Hutter S, Delandre O, Boxberger M, et al. In vitro antiviral activity of doxycycline against SARS-CoV-2. Molecules. 2020;25:5064. doi: 10.3390/molecules25215064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholami M, Fawad I, Shadan S, Rowaiee R, Ghanem H, Hassan Khamis A, Ho SB. COVID-19 and healthcare workers: a systematic review and meta-analysis. Int J Infect Dis. 2021;104:335–346. doi: 10.1016/j.ijid.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Ochoa SA, Franco OH, Rojas LZ, Raguindin PF, Roa-Díaz ZM, Wyssmann BM, et al. COVID-19 in health-care workers: a living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol. 2021;190:161–175. doi: 10.1093/aje/kwaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin MO, Fricovsky E, Ceballos G, Villarreal F. Tetracyclines: a pleitropic family of compounds with promising therapeutic properties. Review of the literature. Am J Physiol Cell Physiol. 2010;299:C539–C548. doi: 10.1152/ajpcell.00047.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Carroll JW, Macdonald MR, Goff SP, Gao G. The zinc finger antiviral protein directly binds to specific viral mRNAs through the CCCH zinc finger motifs. J Virol. 2004;78:12781–12787. doi: 10.1128/JVI.78.23.12781-12787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefi HE, Kefi K, Stambouli N, Belaej R, Hmida MJ, Oumaya A. Vaccination coverage against COVID-19 in a Tunisian general hospital. Pan Afr Med J. 2021;40:101. doi: 10.11604/pamj.2021.40.101.31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek AE, Granwehr BP, Kontoyiannis DP. Doxycycline as a potential partner of COVID-19 therapies. IDCases. 2020;21:e00864. doi: 10.1016/j.idcr.2020.e00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A, Beavis KG, Matushek SM, Ciaglia C, Francois N, Tesic V, et al. Detection of SARS-CoV-2 by use of the Cepheid Xpert Xpress SARS-CoV-2 and Roche cobas SARS-CoV-2 assays. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00772-20. e00772-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platten M, Hoffmann D, Grosser R, Wisplinghoff F, Wisplinghoff H, Wiesmüller G, et al. SARS-CoV-2, CT-values, and infectivity—conclusions to be drawn from side observations. Viruses. 2021;13:1459. doi: 10.3390/v13081459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratia K, Saikatendu KS, Santarsiero BD, Barretto N, Baker SC, Stevens RC, et al. Severe acute respiratory syndrome coronavirus papain-like protease: structure of a viral deubiquitinating enzyme. Proc Natl Acad Sci U S A. 2006;103:5717–5722. doi: 10.1073/pnas.0510851103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalny AV, Rink L, Ajsuvakova OP, Aschner M, Gritsenko VA, Alekseenko SI, et al. Zinc and respiratory tract infections: perspectives for COVID19 (Review) Int J Mol Med. 2020;46:17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi M, Etminan M. Therapeutic potential for tetracyclines in the treatment of COVID-19. Pharmacotherapy. 2020;40:487–488. doi: 10.1002/phar.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Magill AJ, Parise ME, Arguin PM. Centers for Disease Control and Prevention. Doxycycline for malaria chemoprophylaxis and treatment: report from the CDC expert meeting on malaria chemoprophylaxis. Am J Trop Med Hyg. 2011;84:517–531. doi: 10.4269/ajtmh.2011.10-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velthuis AJW, van den Worm SHE, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom MR, Mina MJ. To interpret the SARS-CoV-2 test, consider the cycle threshold value. Clin Infect Dis. 2020;71:2252–2254. doi: 10.1093/cid/ciaa619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels I, Maywald M, Rink L. Zinc as a gatekeeper of immune function. Nutrients. 2017;9:E1286. doi: 10.3390/nu9121286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels I, Rolles B, Rink L. The potential impact of zinc supplementation on COVID-19 pathogenesis. Front Immunol. 2020;11:1712. doi: 10.3389/fimmu.2020.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates PA, Newman SA, Oshry LJ, Glassman RH, Leone AM, Reichel E. Doxycycline treatment of high-risk COVID-19-positive patients with comorbid pulmonary disease. Ther Adv Respir Dis. 2020;14 doi: 10.1177/1753466620951053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.