Graphical abstract
Keywords: Parent spectrum extraction, Absorbance extraction, Peak-amplitude extraction, Ratio extraction, Spectralprint recognition, Eco-friendly
Abstract
Recent studies have reported that using certain antihypertensive therapies such as angiotensin II receptor blockers (ARBs) and calcium channel blocker (CCBs) is associated with reduction of fatal outcomes and improving clinical characteristics of patients suffering from hypertension during coronavirus pandemic. Thus, in the current work an effective, innovative and eco-friendly spectrophotometric manner namely, parent spectrum extraction (PSE)was established for evaluation of recommended triple antihypertensive combination therapies incorporate valsartan (VAL) as ARBs, amlodipine besylate as CCBs (AML) and hydrochlorothiazide (HCT)as diuretic into single-pill in challengeable ratio. PSE manner composed of two complementary steps, auxiliary resolution coupled with data analysis resolution(DAR)and it is characterized by resolving the spectral bands of the drugs and extraction of their discrete parent spectra (D0); accordingly, enabling determination of each analyte at its λmax. Auxiliary resolution of AML in triple mixture was applied to decrease complexity of overlapped spectra via constant multiplication (CM) followed by spectrum subtraction (SS) to obtain resolved mixture of VAL and HCT while data analysis resolution (DAR) of this binary mixture was applied via one of three novel methods namely, absorbance extraction (AE), peak-amplitude extraction (PE) and ratio extraction (RE) along with SS method. The proposed methods had analyzed VAL, AML and HCT in the range of 4.0–44.0 µg/mL, 4.0–40.0 µg/mL and 2.0–24.0 µg/mL, respectively with an excellent correlation coefficient (r ≥ 0.9999). Further, the proposed methods in PSR manner were validated as stated by ICH guidelines and it was found that accuracy and precision results are within the acceptable limit. The suggested procedures were effectively utilized for the concurrent quantification of VAL, AML and HCT in synthetic mixtures and tablets. The greenness of the proposed spectrophotometric methods was evaluated by National Environmental Methods Index (NEMI), the Analytical Eco-Scale, the Green Analytical Procedure Index (GAPI) and Analytical greenness metric (AGREE) where the four tools affirmed the eco-friendly nature of the proposed methods. A comparison between the outcomes of the studied methods with the official and reported ones was performed and no statistical difference was arisen between the methods regarding to accuracy and precision. The achieved results along with the simplicity, affordability and low-cost of the proposed methods recommended their appropriateness for the regular quality control examination and analysis of pure materials and pharmaceutical formulations as well as their applicability for the spectralprint recognition of the studied drugs.
1. Introduction
Cardiovascular disease is considered all over the world, as one of the most prevalent reasons of morbidity and mortality. Management and control of cardiovascular disease is performed by utilizing a combination of drugs, whereas each drug functions with a different and unique mechanism of action to succeed and accomplish the therapy’s goal. Hypertension is a major worldwide problem that affects about 30.0% of the world's population. Hypertension is the cause of more than 12.8% of total deaths annually. The incidence of hypertension is growing globally as a result of ageing of the population and it increases in exposure to risk factors of lifestyle containing unhealthy diets (i.e. highly consumption of sodium and lower potassium diet) and also lacking of physical activity. Nevertheless, hypertension treatment was improved and enhanced significantly over the last two decades because of multiple combination therapies [1], [2].
Patients with high blood pressure possess a two-fold greater risk and probability of death from the coronavirus COVID-19 in comparison to other patients lacking the condition. It further detected that hypertensive patients who were not on medicines to manage their condition had even increased probability and risk of mortality [3].
Valsartan (VAL) (Fig. 1a), is chemically designated as (2S)-3-methyl-2-[pentanoyl-[[4-[2-(2Htetrazol-5-yl) phenyl] phenyl] methyl] amino] butanoic acid [4]. Valsartan (VAL) is angiotensin II receptor blockers (ARBs) [5].
Fig. 1.
The chemical structures of the proposed drugs: (a) Valsartan, (b) Amlodipine besylate and (C) Hydrochlorothiazide.
Various recent studies have reported that the use of available angiotensin receptor 1 (AT1R) blockers, such as VAL, can reduce the aggression and mortality associated with SARS-CoV-2 virus infection. This idea is relied on the observation that ACE2 is one of the enzymes that involved in renin-angiotensin system (RAS) cascade. Moreover, the virus spike protein binds and attaches to ACE2 to make a complex which is appropriate for cellular internalization. The ACE2 down regulation produces excessive angiotensin II accumulation, and it has been observed that the stimulation of the angiotensin II type 1a receptor (AT1R) leading to increase permeability of the pulmonary vascular, clarifying the increased lung pathology whenACE2 activity is decreased. At present, available AT1R blockers (ARBs) like valsartan, possess the potential to hinder and block this pathological process caused by angiotensin II. Currently, there are two complementary proposed mechanisms: 1) ARBs can block the excessive amount of angiotensin mediated AT1R activation, while 2) they upregulate ACE2, which lead to production of angiotensin II concentrations and increasing the formation of angiotensin 1-7 protective vasodilator. In a nut-shell, ARBs may prevent the development of acute respiratory distress syndrome (ARDS) and eschew morbidity (admittance to intensive care unit (ICU) and mechanical ventilation) and mortality [6], [7], [8], [9], as shown in Diagram 1 .
Diagram 1.
Representing the effect of antihypertensive medicines on clinical characteristics and fatal findings in COVID-19 patients.
Amlodipine besylate (AML) (Fig. 1b), is one of calcium channel blocker CCBs, chemically designated as; benzene sulfonic acid;3-O-ethyl 5-O-methyl 2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate [4]. It is utilized for hypertension and angina pectoris’ management; it exerts its effect via blocking the influx transmembrane of calcium ions into cardiac and vascular smooth muscles [5]. Recent research focus on the vasodilation effects of CCBs in the pulmonary and systemic vasculature which could palliate the effects of hyper coagulation, edema, local vasoconstriction, and inflammation produced as a response to the infection with SARS-CoV-2, thus enabling oxygen delivery and existence of host cells [10], [11], [12].
Hydrochlorothiazide (HCT) (Fig. 1c) is diuretic, chemically designated as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine7-sulfonamide1,1-dioxide [4]. It is a thiazide diuretic utilized to control and manage hypertension, and it is also effective for the treatment of edema which coupled with mild heart failure and with hepatic and renal disorders [5].
Single-pill combinations, which incorporate two antihypertensive drugs or more into one pill is recommended to achieve blood pressure goals, including significant reductions in cardiovascular morbidity and mortality without increasing the risk of adverse events. For the effective pharmacological action of the proposed drugs, they are co-formulated in the dosage form (Exforge HCT® tablet) with two formulas with different ratios, the first one is 32.0:1.0:2.5 and for the second one is 16.0:1.0:2.5, of VAL, AML and HCT, respectively. Nowadays, these triple antihypertensive medicines attract the attention of researchers due to its dual pharmacological effects including the drug's capability to reduce levels of the blood pressure (BP), which is the main goal factor to evade cardiovascular complications in these patients as well as lowering risk of coronavirus hospitalization and reduce the fatal outcomes of patients suffering from hypertension.
Literatures review for these triple antihypertensive formulations including spectrophotometry [13], [14], [15], [16], [17], [18], [19], [20], chromatography [16], [17], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33] and electrochemical methods [34], [35].
Mathematical spectrophotometric methods using built in spectrophotometer’s software are now being utilized more frequently for evaluation of drugs in pharmaceutical formulations [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49] owing to their inherent benefits such as simplicity, accuracy, reduction of expensive reagents and eschewing of time consuming or special software. The objective of the current study was to develop a simple, precise, eco-friendly and economical parent spectrum resolution (PSR) manner for concurrent evaluation of VAL,AML and HCT in their triple antihypertensive medicines with trade name Exforge HCT® tablets with critical ratios so, sample enrichment technique [47], [50] should be applied to increase the concentration of the minor component. The innovative PSR manner including novel methods namely, absorbance extraction (AE), peak-amplitude extraction (PE) and ratio extraction (RE) coupled with well-established constant multiplication (CM) and spectrum subtraction methods (SS) allowing the extraction of the parent absorption spectra (D0) of the studied drugs, separately. In addition, a comparable study has been conducted between the results attained by the proposed methods versus those attained by official and reported methods to ascertain the efficiency. Purity evaluation via spectralprint recognition index is a potential interpretation of the absorbance data obtained by methods in PSR manner using extracted parent spectrum of each drug in the combination which added superior advantages regarding human safety. Introducing of the developed methods’ greenness evaluation was performed in this study to avoid any hazardous and risky exposure to the environment or analyst while using and applying the proposed methods.
2. Theoretical background
2.1. Concept of parent spectrum extraction (PSE) manner
Parent spectrum extraction (PSE) manner focusing on the use of the recorded data (constant value) or mathematically analyzed data (analyzed absorbance, analyzed peak-amplitude and amplitude difference) of the studied mixture to extract the parent spectrum [zero order (D0)] of each of co-formulated drug in the mixture. The proposed methods based on this concept using constant value [constant multiplication method (CM)] as well as absorbance [absorbance extraction method (AE)], peak -amplitude [peak-amplitude extraction method (PE)] and amplitude difference [ratio extraction method (RE)] are applied to evaluate each drug in the mixture via their maxima with superlative accuracy and precision. In addition, the extracted parent spectrum (D0) acts as the spectralprint of each component of interest which affirms its purity. Upon analysis of ternary mixtures, two complementary resolution steps namely, auxiliary resolution (using constant value) followed by data analysis resolution (DAR) through utilizing (analyzed absorbance, analyzed peak-amplitude, and amplitude difference) are applied for as follows:
2.1.1. Auxiliary resolution of ternary mixture
This auxiliary resolution is a powerful tool utilized in spectral analysis for resolving ternary mixture, where one of its components showing extension, to eliminate the extended one and get the resolved binary mixture in zero order or ratio spectra which can be analyzed by less complicated spectrophotometric methods with minimum requirement and limitation.
For analysis of X, Y and Z ternary mixture, upon dividing the mixture with a concentration of pure Z as a divisor so, component Z showing a constant all over the curve and parallel to the wavelength axis and its value in the mixture could be determined in the extended region. The constant value of each mixture is subtracted from its corresponding gross ratio spectrum of ternary mixture to get the ratio spectrum of the resolved binary mixture [X + Y] /Z and saved in the computer. The constant value of Z is multiplied byD0spectrum of Z divisor using constant multiplication method (CM) to restore D0 of Z followed by subtraction from its corresponding D0 gross spectrum of ternary mixture to get the D0 spectrum of the resolved binary mixture [X + Y] and saved in the computer.
2.1.2. Data analysis resolution (DAR) scheme of the resolved binary mixture
This resolution scheme based on using the data of the resolved binary mixture to extract thier parent spectra.Thus, the analyst can use resolution tools as numerical factor or mathematic difference to eliminate the spectral influence of disturbing co-formulated component and calculate response value representing the target component. This extracted response is multiplied by factorized spectrum of drug of interest to get its corresponding parent spectrum either directly or via further manipulation. For extracting co-formulated component’s parent spectrum; spectrum subtraction of the extracted parent spectrum from the equivalent mixture is performed. Finally, the concentrations of the components in the binary mixture [X + Y] can be calculated, separately via utilizing their regression equations at their maxima.
The data analysis resolution (DAR) for the resolved binary mixture can be performed via different methods based on the extent of overlapping as follows:
2.1.2.1. Absorbance extraction method (AE)
Principle:
This novel method can be utilized for the analysis of a saved resolved mixture of D0 of the two analytes (X and Y) which possessing partially overlapped spectra where D0 of Y is extended over that of X. By selection discrete pair of wavelengths (λ1, λ2) where X and Y having absorbance at (λ1) while X doesn’t display any contribution at (λ2).
Resolution tools:
-
(1)
Factorized D0spectrum at (λ1) (FSAλ1): The scanned D0 of pure analyte X is divided by the value of its recorded absorbance at (λ1) using spectrophotometer's software.
| (1) |
-
(2)
Numerical absorbance factor: This factor representing the average of the calculated ratio between the absorbance values of different concentrations of pure component Y at λ1(A1) to those at λ2(A2).
Manipulation Steps of mixture:
For analysis of resolved D0 spectrum of binary mixture via data analysis scheme, the absorbance of the mixture at λ2 is recorded, then the actual absorbance of Y at λ1canbecalculated via the numerical absorbance factor equation as follows:
| (2) |
where is the absorbance factor of different concentrations of pure Y at λ1andλ2and A2(X + Y) is the mixture’s absorbance at λ2.
Multiplication of the obtained actual absorbance value Aλ1of Y in each mixture by the factorized spectrum of Y(Y/Aλ1) was performed to restore its parent spectrum (D0). For extracting the parent spectrum of X; spectrum subtraction of the extracted parent spectrum Y and equivalent mixture (X + Y) is carried out.
2.1.2.2. Peak-amplitude extraction method (PE)
Principle:
This novel method used for the saved resolved ratio spectra of mixture composed of the two analytes {(X + Y) / Z'} which possessing partially overlapping spectra where ratio spectrum of Y/Z' is extended over that of X/Z'. By selection discrete pair of wavelengths (λ1, λ2) where X and Y having amplitudes at (λ1) while X doesn’t display any contribution at (λ2).
Resolution tools:
-
(1)
Factorized ratio spectrum of pure Y using Pλ1 (FSPλ1): The scanned D0 of pure component Y is divided by proper divisor of Z to attain ratio spectra Y/Z'. Afterwards, division of the attained ratio spectrum by the recorded amplitude at λ1was carried out using spectrophotometer's software.
| (3) |
-
(2)
Numerical amplitude factor: This factor representing the average of the calculated ratio between the amplitudes values of different concentrations of pure component Y at λ1(P1) to those at λ2(P2).
Manipulation steps of mixture:
For analysis of resolved ratio spectrum of binary mixture {(X + Y)/ Z} after auxiliary resolution of Z in the ratio spectrum, the amplitude of the mixture at λ2 is recorded, then the actual amplitude value of Y at λ1can be calculated via the numerical amplitude factor equation as follows:
| (4) |
where is the amplitude factor of different concentrations of pure Y at λ1 and λ2 and P2(X + Y) is the mixture’s amplitude at λ2.
Multiplication of the obtained actual amplitude value Pλ1 of Y in each mixture by the factorized spectrum of Y was performed to restore its ratio spectrum Y/Z' then multiplied this ratio spectrum by the divisor Z 'to restore parent D0 of Y. For extracting the parent spectrum of X; spectrum subtraction of the extracted parent spectrum Y and equivalent mixture (X + Y) is carried out.
2.1.2.3. Ratio extraction method (RE)
Principle:
This novel method for analyzing resolved D0 of mixture of the two analytes (X, Y) which possessing completely overlapped spectra then, the ratio spectrum is originated using Y' as divisor. By selection discrete pair of wavelengths (λ1,λ2) where X show equal amplitude values at these wavelengths while component of Y has amplitude value so cancellation of the contribution of X component will be achieved with nil difference.
Resolution tools.
-
–
Factorized ratio spectrum of X using ΔP (FSΔP): is prepared via the ratio spectrum of pure component of X using D0 of Y as a divisor. Afterwards, the obtained ratio spectrum is divided by the calculated amplitude difference between the two selected wavelengths (λ1 and λ2).
| Factorized ratio spectrum of X = (X/Y')/ΔP | (5) |
Manipulation steps of mixture:
For analysis of mixture of X and Y, the ratio spectra of each mixture are obtained using Y as a divisor which is previously used in the preparation of (FSΔP). The amplitude difference at the two selected wavelengths (λ1 and λ2) is calculated then multiplication of this difference by the previously prepared factorized ratio spectrum of X representing ΔP. was performed to get the ratio spectrum of X/Y' originally then multiplied this ratio spectrum by the divisor Y 'to restore parent spectrum of X. For extracting the parent spectrum of Y; spectrum subtraction of the extracted parent spectrum X and equivalent mixture (X + Y) is carried out.
3. Experimental
3.1. Apparatus and operational system:
Spectrophotometric measurements were conducted via using double beam spectrophotometer; Shimadzu (UV-1800, Japan) using quartz cells; 1.00 cm. Scans were accomplished from 200 to 400 nm at 0.1 nm intervals. By using Shimadzu UV-Probe 2.43 system software, spectra were automatically obtained.
3.2. Materials
3.2.1. Authentic materials
Pure VAL, AML (as amlodipine besylate) and HCT samples were supplied kindly by Egyptian International Pharmaceutical Industries (EIPICO), Tenth of Ramadan City, Sharqia Governorate, Egypt. They are provided with purities of 99.79 ± 0.65%, 99.74 ± 0.52% and 99.51 ± 0.55%, respectively, for VAL, AML and HCT by applying their official USP methods [51].
3.2.2. Pharmaceutical formulations
Exforge HCT® tablets, manufactured by Novartis, EL Amiria, Cairo. Two formulas with different concentrations are available, the first formula comprising 160.0 mg VAL, 5.0 mg AML free-base and 12.5 mg HCT per tablet (B.N.BKN07), while the second one comprising 160.0 mg VAL, 10.0 mg AML free-base and 25.0 mg HCT, per tablet (B.N.BKM12). Purchasing of the mentioned pharmaceutical formulations was performed from local market.
3.2.3. Chemicals and solvents
Ethanol of spectroscopic analytical grade was obtained from Sigma-Aldrich, Darmstadt-Germany.
3.3. Standard solutions
3.3.1. Standard stock solutions
Stock solutions (1000.0 µg/mL) of all proposed drugs were prepared and made by dissolving 100.0 mg of each of the proposed drugs, separately in ethanol in 100-mL volumetric flask, afterwards adjusting the volume to the mark was conducted through utilizing the same solvent and it was remained at the refrigerator at 4 °C.
3.3.2. Working solutions
Preparation of freshly prepared working solutions was carried out through dilution from the stock solutions to attain a concentration 100.0 μg/mL for all the proposed drugs.
3.3.3. Synthetic mixtures
Different mixtures were prepared via transferring accurate portions with different ratios of the studied drugs from their standard solutions and transferring them to a series of 10-mL volumetric flasks using ethanol as a solvent. Scanning of previously prepared mixtures’ spectra were performed at wavelength region (200.0–400.0 nm) and being saved at the computer.
4. Procedure
Into three individual sets of volumetric flasks (10-mL), preparation of various standard solutions in ethanol was conducted over a concentration range of 4.0–44.0 µg/mL for VAL, 4.0–40.0 µg/mL for AML and 20.0–240.0 µg/mL for HCT. Spectrophotometrically scanning of the solutions in the range of 200.0 nm − 400.0 nm was performed using ethanol as blank and saved at the computer.
4.1. Spectral characteristic
The scanned standard solutions of VAL (16.0 µg/mL), AML (6.0 µg/mL) and HCT (2.5 µg/mL) were overlaid spectrophotometrically and being saved at the computer.
4.2. Calibration graphs’ construction
Utilizing the average of three experiments, calibration graphs were performed. Construction of calibration graphs of (VAL), (AML) and (HCT) was conducted through plotting their absorbance at their maxima 250.0 nm, 237.5 nm and 270.5 nm, respectively against their correlative concentrations, afterwards accurate computation of regression equations was performed.
4.3. Resolving tools
4.3.1. Factorized spectra (FS)
-
–
Factorized D0spectrum of HCT for (AE) method:
Zero order absorption spectra (D0) of concentration of pure HCT within its linearity range was divided by the recorded value of the absorbance at 270.5 nm using arithmetic function in spectrophotometer's software and saved on the computer.
-
–
Factorized ratio spectrum of HCT using AML as a divisor for (PE) method:
The obtained ratio spectrum of concentration of pure HCT within its linearity range utilizing pure AML (28.0 µg/mL) as a divisor is divided by the value of the recorded amplitude at 274.5 nm using arithmetic function in spectrophotometer's software and saved on the computer.
-
–
Factorized ratio spectrum of HCT using VAL as a divisor for (RE) method:
The obtained ratio spectrum of concentration of pure HCT within its linearity range utilizing pure VAL (28.0 μg/ml) as a divisor is divided by the aforementioned recorded amplitude difference (ΔP 275.0–250.0 nm) using arithmetic function in spectrophotometer's software and saved on the computer.
4.3.2. Mathematical numerical factors
-
–
Absorbance factor for (AE) method: it was calculated using the average of the calculated numerical values of recorded absorbance of D0 of various concentrations of pure HCT within its linearity range at 270.5 nm and 330.0 nm (A270.5 nm/A330.0 nm).
-
–
Amplitude factor for (PE) method: it was calculated using the average of the calculated numerical amplitude values of different ratio spectra of various concentrations of pure HCT within its linearity range using pure AML (28.0 µg/mL) as a divisor at 274.5 nm and 330.0 nm (P274.5 nm/P330.0 nm).
4.4. Analysis of synthetic mixtures of ternary mixture (VAL, AML and HCT):
Division of D0 of each synthetic mixture of the ternary mixture (VAL, AML and HCT) by D0 of standard solution of AML (28.0 µg/mL) was performed. Afterwards, the constant of each mixture was accurately measured in the plateau region from (380.0 nm − 400.0 nm).
Determination and elimination of AML: The measured constant’s value was multiplied by the divisor’s spectrum (AML 28.0 µg/mL) to get parent spectrum of AML (constant multiplication method).
Auxiliary resolution of ternary mixture (VAL, AML and HCT) to get D0 resolved binary mixture (VAL-HCT): Subtraction the extracted spectrum D0 of AML from its corresponding ternary mixture was conducted to get resolved D0 of binary mixture (VAL + HCT), then saved in the computer then data analysis resolution (DAR) was applied on resolved D0 of VAL-HCT mixture as follows:
Absorbance extraction (AE) method: The absorbance at 270.5 nm and 330.0 nm was recorded for each mixture. The absorbance of HCT at 270.5 nm was calculated using the absorbance factor of pure HCT (A270.5nm/A330.0nm). The parent spectrum of HCT in the mixture could be extracted by multiplication of the calculated A(270.5 nm) by the previously prepared factorized D0 spectrum of HCT. The parent spectrum of VAL could be extracted after spectrum subtraction of obtained HCT from the obtained resolved binary mixture.
Auxiliary resolution of ternary mixture (VAL, AML and HCT) to get resolved ratio binary mixture (VAL-HCT): Subtraction of the previously measured constant’s value from its corresponding ratio spectrum of the ternary mixture (VAL, AML and HCT) using AML (28.0 µg/mL) as a divisor was conducted to get the resolved ratio spectrum of binary mixture of VAL and HCT/ AML, then saved in the computer then data analysis resolution (DAR) was applied on resolved ratio of VAL-HCT mixture as follows:
-
(I)
Peak-amplitude extraction (PE) method: The amplitudes at 274.5 nm and 330.0 nm were recorded for each mixture. The amplitude of HCT at 274.5 nm was calculated using the amplitude factor of pure HCT (P274.5 nm/P330.0 nm). The parent spectrum of HCT in the mixture could be extracted by multiplication of the calculated P(274.5nm) by the previously prepared factorized ratio of (HCT/AML) then finally, multiplied by the divisor’s D0spectrum AML (28.0 µg/mL). Then parent spectrum of VAL could be extracted by spectrum subtraction of the attained parent spectrum of HCT from the resolved D0 of binary mixture (VAL and HCT).
-
(II)
Ratio extraction (RE) method: The resolved ratio spectrum of binary mixture of (VAL + HCT)/ AML was multiplied by D0 of AML (28.0 µg/mL) to get D0 of binary mixture of VAL and HCT followed by division by D0 VAL (28.0 µg/mL) to get ratio spectrum of (VAL + HCT)/ VAL, then saved in the computer then data analysis resolution (DAR) was applied on resolved ratio. The amplitudes at 275.0 nm and 250.0 nm were recorded for each mixture followed by calculation the difference between them. The parent spectrum of HCT could be extracted by multiplication of the calculated amplitude difference (ΔP275.0-250.0nm) by the previously prepared factorized ratio HCT to get (HCT/VAL) in the mixture then finally, multiplied by the divisor’s spectrum (VAL (28.0 µg/mL). The parent spectrum of VAL could be extracted after spectrum subtraction of the attained D0 of HCT from the resolved D0 of binary mixture (VAL and HCT).
For all of the proposed methods, the concentrations of VAL, AML and HCT in each mixture were calculated through substituting in the correlating regression equations at their maxima 250.0 nm, 237.5 nm and 270.5 nm, respectively.
4.5. Pharmaceutical formulations’ assessment:
4.5.1. Exforge HCT® tablets (Two formulas)
Accurately weighing of ten tablets followed by grinding was performed. An accurate weight of the mixed sample equivalent to one tablet from each of the two formulas, (formula I comprising 160.0 mg VAL, 5.0 mg AML free base and 12.5 mg HCT per tablet while formula II comprising, 160.0 mg VAL, 10.0 mg AML free base and 25.0 mg HCT per tablet) was separately transferred into two beakers, 50-mLethanol were added with continuing magnetic stirring for about 10 min. Each solution was, separately, filtered through a 0.45 µm membrane filter paper into two 100-mL volumetric flasks using ethanol. Accurately transferring of 2.0 mL of formula I solution and 1.0 mL of formula II into two separate 100-mL volumetric flasks was performed then AML was enriched for each formula with 500 µg standard of AML from its standard solution (100 µg/mL), then complete to the mark with ethanol to get solution with final concentration claimed to be 32.0 μg/mL, 6.0 μg/mL and 2.5 μg/mL of VAL, AML free base and HCT regarding formula I and 16.0 μg/mL, 6.0 μg/mL and 2.5 μg/mL of VAL, AML free base and HCT regarding formula II, respectively. Analysis and determination of the proposed drugs in their pharmaceutical formulations were achieved by using the presented methods under analysis of synthetic mixtures. The concentrations of VAL, AML and HCT in enriched sample with AML added concentration were calculated through substituting in the correlating regression equations at their maxima 250.0 nm, 237.5 nm and 270.5 nm, respectively. Calculation of AML claimed concentration in each mixture was carried out after subtraction of AML added concentration which prepared and analyzed separately using the same experimental procedures.
4.5.2. Spectralprint recognition index
The above stated and described procedures were performed for each entire tablet (10 tablets were utilized) to extract the parent spectra of VAL, AML and HCT. Recording the absorbance value at wavelengths pairs 250.0 nm and 280.0 nm for VAL, 234.5 nm and 360.0 nm for AML and 270.5 nm and 257.6 nm for HCT for extracted spectrum of pharmaceutical formulations and authentic spectrum of the raw drugs was performed using the proposed methods.
5. Results and discussion
Investigations of pharmaceuticals analysis field and their quality control depend upon analytical manner /methods which have excellent implementation characteristics, where an analyst can choose the most suitable one for analysis. The major objective is to develop validated innovative resolution manner namely, parent spectrum resolution (PSR) including two complementary steps, auxiliary resolution (AR)via CM coupled with SS to decrease complexity of the ternary mixture and data analysis resolution(DAR) via novel spectrophotometric methods using factorized spectrum of cited drugs as a resolving spectrum. This PSR manner is able to obtain the parent spectra of each drug which considered as their spectralprint and the concentration of each component is obtained via constructed regression equation at their λmax. with maximum accuracy and precision. Validation of the developed spectrophotometric methods as stated by ICH guidelines [52], was the main purpose of the present work. The linearity ranges according to beer's law of the studied drugs were applied at their maxima and found to be 4.0–44.0 µg/mL, 4.0–40.0 µg/mL and 2.0–24.0 µg/mL for VAL, AML and HCT, respectively.
5.1. Experimental optimization
To illustrate the power of this innovative PSR manner, the triple antihypertensive medicines consisting of VAL, AML and HCT in their challenge ratios, 32.0:1.0:2.5 and 16.0:1.0:2.5 µg/mL for VAL, AML and HCT, respectively were taken as models. By scanning standard solutions of the proposed drugs in these studied ratios within 200–400 nm, it was found that AML is out of its linearity range (4.0–40.0 µg/mL) and it show extension at wavelength region (351.0–400.0 nm) without any contribution of VAL and HCT while HCT is partially overlapped with VAL and show extension over VAL at wavelength region (330.0–350.0 nm).Thus, pre-analyzed sample should be enriched via spiking using 5.0 µg/mL of pure AML before applying proposed procedures, Fig. 2 . Upon analysis of synthetic mixtures containing higher concentration of VAL > 40 µg/mL, its spectrum is completely interfering with HCT and AML spectra in wavelength region up to 379 nm thus wavelength region (380.0–400.0 nm) is the suitable region for analysis AML alone without any contribution of VAL and HCT.
Fig. 2.
Parent zero order absorption spectra of VAL (----) 16.0 µg/mL, AML (.....) 6.0 µg/mL and HCT (—) 2.5 µg/mL.
This overlapping prevented the direct resolution of the studied triple drugs so advanced mathematical manner is used composed of two complementary resolution steps namely, auxiliary resolution(AR) and data analysis resolution (DAR). The auxiliary resolution applied on ternary mixture to eliminate extended drug via constant multiplication coupled with spectrum subtraction (CM-SS) method to decrease complexity of the mixture while data analysis resolution performed for the resolved binary mixture including three novel methods namely, absorbance extraction (AE), peak-amplitude extraction (PE) and ratio extraction (RE).
During PE and RE methods development, it was found that, divisor concentration was one of the major parameters which can impact the shape of the ratio spectra of the proposed drugs. The chosen divisor should achieve compromising between maximum sensitivity and minimal noise. The divisor concentrations were tested within linearity range of AML in case of (CM-SS) method and VAL in case of RE method. As the concentration of the divisor increased or decreased, there was a correlative decrease or increase in the produced ratio values, respectively. Nevertheless, the positions of the peaks and troughs remain not affected. Using a divisor concentration of 28.0 µg/mL for both AML and VAL gave the best results concerning accuracy, reproducibility and recovery in synthetic mixtures and various pharmaceutical formulations.
5.2. Analysis of synthetic mixtures of ternary mixture (VAL, AML and HCT):
Division of D0 of each synthetic mixture of triple drugs by D0 of standard solution of AML (28.0 µg/mL) was performed. Afterwards, the constant of each mixture was accurately measured in the plateau region from (380.0–400.0 nm).
5.2.1. Determination of AML via CM method:
This method has the capability to restore the D0of AML and analysed via its maxima.The measured constant’s value was multiplied by the divisor’s spectrum (AML 28.0 µg/mL) to get parent spectrum of AML. The concentration of AML in each mixture is calculated by utilizing its correlating regression equation which is representing linear relationship amongst the absorbance values of AML at 237.5 nm against their correlating concentrations in the range (4.0–40.0 mg/mL).
5.2.2. PSR - manner
5.2.2.1. PSR – Manner via D0 spectrum
Step I: Auxiliary resolution (AR)
Auxiliary resolution was applied to resolve this complicated mixture to get resolved D0 spectrum of binary mixture of (VAL + HCT) with partially overlapped spectra via subtraction of extracted D0 of AML from its corresponding D0 of ternary mixture using spectrum subtraction method, Fig. 3 .
Fig. 3.
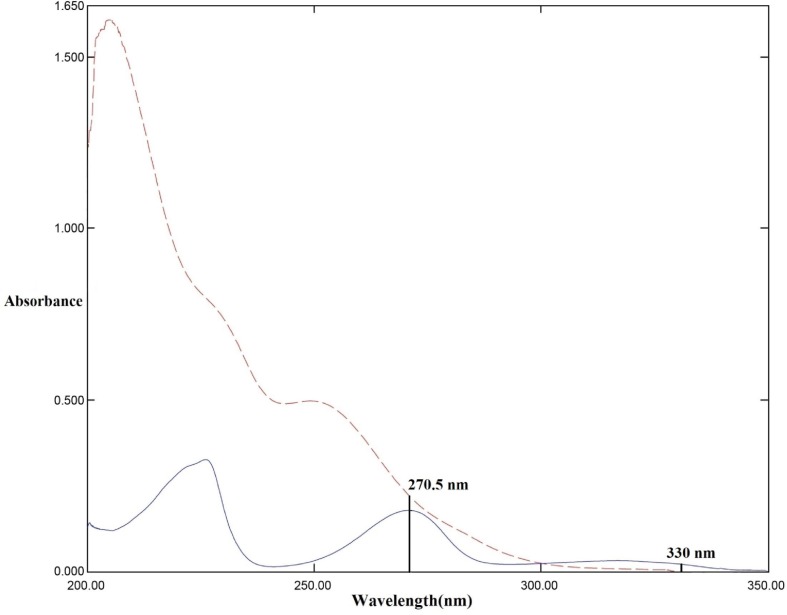
Resolved zero order absorption spectra of the binary mixture of VAL (----) 16.0 µg/mL and HCT (—) 2.5 µg/mL showing absorbance factor using λ1 (270.5 nm) and λ2 (330 nm).
Step II: Data Analysis Resolution via absorbance extraction method (DAR-AE)
This novel resolution method based on coupling numerical absorbance factor with the factorized D0spectrum of one of the proposed drugs. AE method starts with a simple mathematical manipulation to calculate numerical factor for HCT. The two selected wavelengths for numerical factor calculation were optimized and they found to be 270.5 nm and 330.0 nm without any contribution from VAL up to 40 µg/mL.
Absorbance factor was calculated representing the average of absorbance value of various concentrations of pure HCT at 270.5 nm and 330.0 nm [abs(270.5nm) / abs330.0nm], Fig. 3 and it found to be equal 9.5.
The actual absorbance of HCT in the mixture at 270.5 nm was attained as illustrated by the following equation:
where A(270.5 nm) is the actual absorbance of HCT in the mixture at λ(270.5 nm), is the absorbance factor of various concentrations of pure HCT at 270.5 nm to 330.0 nm and is the absorbance of the mixture at λ(330.0 nm).
Thus, parent D0 of HCT could be obtained by multiplication of the obtained A (270.5 nm) value by the previously prepared factorized D0 spectrum of pure HCT which prepared by using the same experimental specification. Consequently, D0 of VAL could be successfully resolved by utilizing the method of spectrum subtraction, whereas the extracted D0 of HCT in each mixture was subtracted from the corresponding resolved binarymixture’sD0 absorption spectrum.
VAL and HCT concentrations were calculated by utilizing their correlating regression equations representing linear relationship amongst their absorbance values at 250.0 nm for VAL and 270.5 nm for HCT against their correlating concentrations in the range (4.0–44.0 µg/mL) and (2.0–24.0 µg/mL) for VAL and HCT respectively.
5.2.2.2. PSR - manner via ratio spectrum of ternary mixture using AML as a divisor
Step I: Auxiliary resolution (AR)
The ratio spectra of VAL + HCT/AML of each synthetic mixture’s ratio spectrum could be attained by subtraction of the previously recorded constant value of (AML/AML') for each mixturefrom its corresponding ratio spectra of gross spectra of the ternary mixture to get resolved ratio spectrum of (VAL + HCT/ AML), Fig. 4 .
Fig. 4.
Resolved ratio spectrum of the binary mixture of VAL (----) 16.0 µg/mL and HCT (—) 2.5 µg/mL using AML 28.0 µg/mL as a divisor showing amplitude factor using λ1(274.5 nm) and λ2 (330 nm).
Step II: Data analysis resolution via peak-amplitude extraction method (DAR-PE)
This novel resolution method based on coupling numerical amplitude factor with the factorized ratio spectrum of one of the proposed drugs. PE method starts with a simple mathematical manipulation to calculate numerical factor for HCT. The two selected wavelengths for numerical factor calculation were optimized and they found to be 274.5 nm and 330.0 nm for PE where 330.0 nm showed no contribution from VAL up to 40 µg/mL, Fig. 4.
Amplitude factor is calculated representing the average of amplitude value of various concentrations of pure HCT at 274.5 nm and 330.0 nm [P(274.5nm)/P330.0nm]. Calculation of the amplitude factor was performed, and it found to be equal 16.
The actual amplitude of HCT in the mixture at 274.5 nm was attained as illustrated by the following equation:
where is the amplitude factor of various concentrations of pure HCT/AML at 274.5 nm to 330.0 nm and is the amplitude of the mixture at .
Ratio spectrum of HCT/AML could be obtained by multiplying the obtained P by the previously prepared factorized HCT prepared by using the same experimental specification. Then, by multiplying the ratio spectrum of HCT/AML by the AML divisor, parent D0 of HCT was obtained. Consequently, D0 of VAL could be successfully resolved by utilizing the method of spectrum subtraction, whereas the extracted D0 of HCT in each mixture was subtracted from the corresponding mixture’s D0 absorption spectrum of VAL + HCT.
VAL and HCT concentrations were calculated by utilizing their correlating regression equations which are representing and offering a linear relationship amongst their absorbance values at 250.0 nm for VAL and 270.5 nm for HCT against their correlating concentrations in the range (4.0–44.0 mg/mL) and (2.0–24.0 mg/mL) for VAL and HCT respectively.
5.2.2.3. PSR-manner via ratio spectrum of ternary mixture using AML and VAL as divisors
Step I: Auxiliary resolution (AR)
Auxiliary resolution was applied to get resolved D0 spectrum of binary mixture of (VAL + HCT) with partially overlapped spectrum via subtraction of extracted D0 of AML from its corresponding D0 of ternary mixture using spectrum subtraction method, Fig. 3.
Step II: Data analysis resolution via ratio extraction method (DAR-RE)
This novel resolution method based on coupling amplitude difference with the factorized ratio spectrum of target component. This amplitude difference is applied at two selected wavelengths λ1&λ2 where the ratio spectrum of the co-formulated component displays the same amplitudes (constant) and the component of interest displays significant amplitude difference. This method is valid for determination of all mixtures containing VAL up to 44.0 µg/mL.
The resolved D0 of the resolved binary mixture of (VAL + HCT) is divided by D0 VAL (28.0 µg/mL) to get ratio spectrum of (VAL + HCT) / VAL represents + constant . By selection two wavelengths for HCT ratio spectra and applying subtraction of these two amplitudes ()1 - ()2, cancellation of the constant will be achieved, and the divisor analyte’s interference will be omitted while the other analyte’s interference will be directly related to the calculated difference, The optimum wavelength pair was studied and found to be Maximum ΔP values were using peak maxima at 275.0 nm and peak trough at 250.0 nm, Fig. 5 .
Fig. 5.
Resolved ratio spectrum of the binary mixture of VAL (----) 16.0 µg/mL and HCT (—) 2.5 µg/mL using VAL 28.0 µg/mL as a divisor showing the selected λ1 (275 nm) and λ2 (250 nm).
The ratio spectrum of HCT using VAL as a divisor in each mixture was obtained by multiplication of the value of the amplitude difference (ΔP275.0-250.0nm) by the aforementioned prepared factorized HCT prepared by using the same experimental specification, Fig. 5.
The parent D0of HCT in each mixture was obtained by multiplication of the ratio spectrum of by VAL divisor. Consequently, D0 of VAL could be successfully resolved by utilizing the method of spectrum subtraction, whereas the attained D0 of HCT in each mixture was subtracted from the corresponding resolved mixture’s D0 spectrum of (VAL + HCT).
VAL and HCT concentrations were calculated by utilizing their correlating regression equations which are representing and offering a linear relationship amongst their absorbance values at 250.0 nm for VAL and 270.5 nm for HCT against their correlating concentrations in the range (4.0–44.0 mg/mL) and (2.0–24.0 mg/mL) for VAL and HCT respectively.
5.3. Method validation
Validation was conducted in accordance with the guidelines of ICH [52] as revealed in Table 1 .
Table 1.
Regression parameters and validation sheet for determination of pure VAL, AML and HCT by the proposed methods.
| Drug | VAL | AML | HCT |
|---|---|---|---|
| Method |
D0 (λmax250.0 nm) |
D0 (λmax237.5 nm) |
D0 (λmax270.5 nm) |
|
Linearity range (µg/mL) |
4.0–44.0 | 4.0–40.0 | 2.0–24.0 |
| Regression equation Parameters | |||
| Slope | 0.0318 | 0.0366 | 0.068 |
| Intercept | 0.0112 | 0.002 | 0.0062 |
| Correlation Coefficient (r) | 1.0000 | 1.0000 | 0.9999 |
|
Accuracya (Mean ± SD) |
100.19 ± 0.59 | 99.67 ± 0.59 | 100.03 ± 0.89 |
|
Precision (±%RSD) | |||
| Repeatabilityb | 0.482 | 0.585 | 0.660 |
| Intermediate precision c | 0.654 | 0.850 | 0.896 |
Accuracy was checked using concentrations (6.0 µg/mL, 24.0 µg/mL and 40.0 µg/mL), (6.0 µg/mL, 25.0 µg/mL and 38.0 µg/mL) and (4.0 µg/mL, 16.0 µg/mL and 23.0 µg/mL) for, VAL, AML and HCT respectively.
and c are repeatability and intermediate precision respectively (n = 9) relative standard deviation of three different concentrations in triplicate (8.0 µg/mL, 28.0 µg/mL and 36.0 µg/mL) for both VAL and AML, while (6.0 µg/mL, 14.0 µg/mL and 22.0 µg/mL) for HCT.
5.3.1. Linearity and range
Estimation of the linearity of the studied methods was conducted via analyzing various concentrations of VAL, AML and HCT, ranging from 4.0–44.0 µg/ml, 4.0–40.0 µg/mL and 2.0–24.0 µg/mL, respectively. Repetition of every concentration was carried out three times. The analysis was applied in accordance with aforementioned experimental circumstances. Demonstration of linear equations were performed, Table 1.
Calibration’s range was carried out through utilizing the practical range in accordance with Beer’s law and the concentration of the proposed drugs which existing in their combination as dosage form to provide linear, accurate, and precise results, Table 1 .
5.3.2. Accuracy
Accuracy was studied and investigated through implementing the studied methods for analysis and determination of various models of the cited drugs. Attaining the proposed drugs’ concentrations were conducted utilizing the relative regression equations, Table 1.
5.3.3. Precision
5.3.3.1. Repeatability and Intermediate precision
To check the precision (Repeatability and Intermediate precision), three concentrations (8.0 µg/mL, 28.0 µg/mL and 36.0 µg/mL) for both VAL and AML while (6.0 µg/mL, 14.0 µg/mL and 22.0 µg/mL) for HCT were separately examined and investigated three times intra-daily and inter-daily on three various days utilizing the studied methods. Computation of the correlated standard deviations which is associated to each concentration was performed, Table 1.
5.3.4. Specificity
Specificity was ascertained and checked through using various synthetic mixtures’ analysis comprising of studied drugs with different ratio within the range of linearity. Good results were revealed in Table 2 .
Table 2.
Determination of the studied drugs in the synthetic mixtures by the proposed spectrophotometric methods.
| Ternary Mixture | |||||||
|---|---|---|---|---|---|---|---|
| Recovery%*± SD | |||||||
| VAL:AML: HCT | VAL(D0) |
AML(D0) | HCT(D0) |
||||
| Ratio (µg/mL) | SS of D0 HCT via AE | SS of D0 HCT via PE |
SS of D0 HCT via RE |
CM | AE | PE | RE |
| (32.0:6.0:2.5) ** | 98.86 | 100.23 | 99.80 | 100.24 | 100.51 | 100.36 | 99.26 |
| (16.0:6.0:2.5) ** | 100.36 | 99.45 | 99.28 | 99.38 | 99.46 | 99.52 | 99.84 |
| (10.0:10.0:10.0) | 99.57 | 100.68 | 100.25 | 100.35 | 100.82 | 99.87 | 100.41 |
| (42.0:7.0:14.0) | — | — | 100.44 | 99.44 | — | — | 100.25 |
| (20.0:10.0:20.0) | 100.44 | 100.55 | 98.56 | 99.58 | 100.23 | 100.24 | 99.56 |
| Mean ± SD | 99.81 ± 0.74 | 100.23 ± 0.55 | 99.67 ± 0.76 | 99.80 ± 0.46 | 100.26 ± 0.58 | 100.00 ± 0.38 | 99.86 ± 0.48 |
* Average of 3 replicates.
** Ratio as dosage forms enriched with 5.0 µg/mL AML.
5.4. Application of the developed methods for assaying Exforge HCT® tablets
Determination of the proposed drugs’ concentrations in Exforge HCT® tablets was carried out utilizing the proposed spectrophotometric methods and all proposed methods showed acceptable percentage recoveries. As a consequence, this permit their utilizing for proposed drugs’ regular analysis and determination in their combined formulation, Table 3 . The accuracy of pharmaceutical formulations was certified by making comparison between the obtained results from studied methods and those of the reported method [16], Table 4 .
Table 3.
Quantitative estimation of valsartan, amlodipine and hydrochlorothiazide in the two formulas of Exforge HCT® Tablets.
| Pharmaceutical dosage form | VAL (D0) |
AML (D0) |
HCT (D0) |
||||
|---|---|---|---|---|---|---|---|
| SS of D0 HCT via AE |
SS of D0HCT via PE |
SS of D0HCT via RE |
CM | AE | PE | RE | |
| Pharmaceutical dosage forma (Exforge HCT®) Formula I (B.N.BKM12) (32.0 VAL: 1.0 AML: 2.5 HCT)* (Found% ± SD) |
100.16 ± 0.57 | 100.66 ± 0.45 | 99.89 ± 0.53 | 99.10 ± 0.64 | 99.20 ± 0.54 | 99.58 ± 0.70 |
99.80 ± 0.42 |
| Pharmaceutical dosage forma (Exforge HCT®) Formula II (B.N.BKN07) (16.0 VAL: 1.0 AML: 2.5 HCT)*(Found% ± SD) |
100.41 ± 0.50 | 100.33 ± 0.37 | 100.08 ± 0.64 | 100.12 ± 0.67 | 100.80 ± 0.55 | 100.93 ± 0.67 | 100.82 ± 0.52 |
Average of six experiments.
Enriched with 5.0 µg/mL of AML before being analyzed by the proposed methods.
Table 4.
Statistical comparison of the results obtained by the proposed spectrophotometric methods and those obtained by the reported one for the determination of the cited drugs in the two studied pharmaceutical formulations.
| Parameter | Exforge HCT® Formula I (VAL: AML: HCT) (32.0: 1.0:2.5) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VAL (D0) |
AML(D0) |
HCT(D0) |
||||||||
| Reported method [16]a | SS of D0 HCT via AE |
SS of D0 HCT via PE |
SS of D0 HCT via RE |
Reported method [16]a | CM | Reported method [16]a | AE | PE | RE | |
| Mean | 100.50 | 100.16 | 100.66 | 99.89 | 98.66 | 99.10 | 99.19 | 99.20 | 99.58 | 99.80 |
| S.D | 0.60 | 0.57 | 0.45 | 0.53 | 0.48 | 0.64 | 0.56 | 0.54 | 0.70 | 0.42 |
| n | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Variance | 0.3600 | 0.3249 | 0.2025 | 0.2809 | 0.2304 | 0.4096 | 0.3136 | 0.2916 | 0.4900 | 0.1764 |
| Student's t-test b (2.228) | – | 1.006 | 0.522 | 1.866 | – | 1.347 | – | 0.031 | 1.066 | 2.134 |
| F test b (5.05) |
– | 1.11 | 1.78 | 1.28 | – | 1.78 | – | 1.08 | 1.56 | 1.78 |
| Exforge HCT® Formula II (VAL: AML:HCT) (16.0:1.0:2.5) | ||||||||||
| VAL | AML | HCT | ||||||||
| Parameters | Reported method [16]a | SS of D0 HCT via AE |
SS of D0 HCT via PE | SS of D0 HCT via RE | Reported method [16]a | CM | Reported method [16]a | AE | PE | RE |
| Mean | 100.71 | 100.41 | 100.33 | 100.08 | 100.05 | 100.12 | 101.40 | 100.80 | 100.93 | 100.82 |
| SD | 0.43 | 0.50 | 0.37 | 0.64 | 0.68 | 0.67 | 0.81 | 0.55 | 0.67 | 0.52 |
| n | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Variance | 0.1849 | 0.2500 | 0.1369 | 0.4096 | 0.4624 | 0.4489 | 0.6561 | 0.3025 | 0.4489 | 0.2704 |
| Student's t-test b (2.228) | – | 1.114 | 1.641 | 2.002 | – | 0.180 | – | 1.501 | 1.095 | 1.476 |
| F test b (5.05) |
– | 1.72 | 1.35 | 2.22 | – | 0.97 | – | 2.17 | 1.46 | 2.43 |
Reported UHPLC method using C18 column and mobile phase consisting of acetonitrile-methanol-phosphate buffer, 25:50:25, by volume, pH 2.8.
The figures in parenthesis are the corresponding theoretical values at P = 0.05.
5.5. Statistical analysis
Statistical comparison based on student t-test and f-test was performed between the results obtained by developed methods and those obtained after applying the official methods [51] and reported method [16]. It was found that at p-value 0.05, computed t and F values were less than their relative theoretical ones revealing insignificant difference between the developed methods and official and reported ones with respect to accuracy and precision as shown in Table 4 and Table 5 . To compare The capability of the developed methods for assaying each component in the ternary mixture of (VAL,AML and HCT),statistical analysis was implemented between the results of developed methods and those of official methods [51] using one way ANOVA test. The results attained from ANOVA analysis’s revealing insignificant difference between the developed methods for authentic samples and the official methods as shown in Table 6 .
Table 5.
Statistical analysis for the results obtained by the proposed methods and the official HPLC methods for analysis of the cited drugs in their pure forms.
| Drug | VAL |
AML |
HCT |
|||
|---|---|---|---|---|---|---|
| parameters | D0 at λmax (250.0 nm) |
Official method [51]a | D0 at λmax (237.5 nm) |
Official method [51]a | D0 at λmax (270.5 nm) |
Official method [51]a |
| Mean | 100.08 | 99.51 | 100.21 | 99.74 | 99.95 | 99.79 |
| SD | 0.62 | 0.55 | 0.39 | 0.52 | 0.68 | 0.65 |
| n | 6 | 6 | 6 | 6 | 7 | 6 |
| Variance | 0.3844 | 0.3025 | 0.1521 | 0.2704 | 0.8424 | 0.4225 |
| Student's t-test (tab.) b | 2.228 | – | 2.228 | — | 2.201 | – |
| Student's t-test (cal.) | 1.685 | – | 1.772 | — | 0.433 | – |
| F (tab.) b | 5.05 | – | 5.05 | — | 4.95 | – |
| F (cal.) | 1.27 | – | 1.78 | — | 1.99 | – |
Official USP methods for determination of VAL, AML and HCT (RP-HPLC).
The figures in parenthesis are the corresponding theoretical values at P = 0.05.
Table 6.
Results of ANOVA (single factor) for comparison of the results obtained by the proposed methods and the official methods for determination of the proposed drugs in their pure powder forms.
| Source of variation | Sum of squares | DF | Mean Square | F value | P value | F crit |
|---|---|---|---|---|---|---|
| VAL | ||||||
| Between exp. | 0.9747 | 1 | 0.9747 | 2.857883 | 0.121807 | 4.964603 |
| Within exp. | 3.410566667 | 10 | 0. 341,057 | |||
| Total | 4.385266667 | 11 | ||||
| AML | ||||||
| Between exp. | 0.6627 | 1 | 0.6627 | 3.170207 | 0.10534 | 4.964603 |
| Within exp. | 2.0904 | 10 | 0.20904 | |||
| Total | 2.7531 | 11 | ||||
| HCT | ||||||
| Between exp. | 0.087958 | 1 | 0.087958 | 0.19628 | 0.666327 | 4.844336 |
| Within exp. | 4.92935 | 11 | 0.448123 | |||
| Total | 5.017308 | 12 | ||||
-At the 0.05 level.
-The population means are not significantly different.
5.6. Spectralprint recognition index (SRI)
Using advanced spectrophotometric methods based on extraction of parent spectrum of each cited drugs which act as spectralprint is advantageous due to its capability to assess and evaluate the sample purity. Assessment of purity is greatly critical respecting compounds have a biological activity as impurities’ traces with either no or great potency can probably cause undesirable action [53]. This (SRI) is a measure of the purity of the component in the pharmaceutical formulations and it is applied using discrete pairs of wavelengths all over the spectrum of parent extracted spectrum and its scanned spectrum. To assess (SRI) of the cited drugs, the ratio of the absorbance at two chosen wavelengths for the identical concentration of the extracted spectrum from the commercial formulations and the authentic spectrum of the cited drugs after applying the developed methods should be calculated (Aλ1 / Aλ2of extracted spectrum / Aλ1 / Aλ2 of authentic spectrum) and it should be around 1.000 (0.991–1.000) to ascertain the purity. The two selected wavelengths are 250.0 nm and 280.0 nm for VAL, 234.5 nm and 360.0 nm for AML and 270.5 nm and 257.6 nm for HCT. SRI was evaluated for VAL, HCT and AML in each individual Exforge HCT® tablet (utilizing 10 tablets). The purity profile was calculated via utilizing their extracted D0 spectra of VAL (32.0 µg/mL), AML free base (1.0 µg/mL) and HCT (2.5 µg/mL) from the pharmaceutical formulation (formula I) and VAL (16.0 µg/mL), AML free base (1.0 µg/mL) and HCT (2.5 µg/mL) from the pharmaceutical formulation (formula II) by the developed methods and their equivalent D0 of pure drugs of the same concentrations found in the analyzed products. The attained values of SRI from Exforge HCT® tablets were all acceptable and satisfactory verifying their purity, Table 7 .
Table 7.
Spectralprint recognition index for Valsartan (VAL), Amlodipine (AML) and Hydrochlorothiazide (HCT) in Exforge HCT® tablets.
| Tablet No. | Exforge HCT® Tablets |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Formula I |
Formula II |
|||||||||||||
| VAL |
AML |
HCT |
VAL |
AML |
HCT |
|||||||||
| Spectralprint Index |
Spectralprint Index |
|||||||||||||
| SS of D0HCT via PE |
SS of D0HCT via RE |
SS of D0HCT via AE |
CM | AE | PE | RE | SS of D0HCT via AE |
SS of D0HCT via PE |
SS of D0HCT via RE |
CM | AE | PE | RE | |
| Tablet 1 | 0.991 | 0.991 | 0.999 | 0.988 | 0.989 | 0.993 | 0.996 | 0.996 | 0.99 | 0.994 | 0.999 | 0.999 | 0.992 | 0.993 |
| Tablet 2 | 0.996 | 0.998 | 0.994 | 0.989 | 0.995 | 0.99 | 0.999 | 0.998 | 0.999 | 0.996 | 0.994 | 0.994 | 0.991 | 0.997 |
| Tablet 3 | 0.998 | 0.996 | 0.992 | 0.999 | 0.99 | 0.987 | 0.998 | 0.994 | 0.998 | 0.999 | 0.995 | 0.995 | 0.99 | 0.994 |
| Tablet 4 | 0.997 | 0.997 | 0.995 | 0.987 | 0.992 | 0.994 | 0.895 | 0.993 | 0.997 | 0.998 | 0.993 | 0.993 | 0.989 | 0.995 |
| Tablet 5 | 0.992 | 0.993 | 0.995 | 0.996 | 0.99 | 0.991 | 0.988 | 0.994 | 0.993 | 0.997 | 0.998 | 0.998 | 0.991 | 0.996 |
| Tablet 6 | 0.985 | 0.998 | 0.999 | 0.996 | 0.991 | 0.987 | 0.992 | 0.992 | 0.994 | 0.993 | 0.996 | 0.996 | 0.986 | 0.995 |
| Tablet 7 | 0.988 | 0.991 | 0.998 | 0.998 | 0.988 | 0.999 | 0.999 | 0.994 | 0.992 | 0.995 | 0.998 | 0.998 | 0.988 | 0.994 |
| Tablet 8 | 0.999 | 0.993 | 0.994 | 0.994 | 0.989 | 0.986 | 0.995 | 0.998 | 0.995 | 0.996 | 0.997 | 0.997 | 0.991 | 0.993 |
| Tablet 9 | 0.996 | 0.989 | 0.995 | 0.997 | 0.99 | 0.998 | 0.994 | 0.997 | 0.996 | 0.992 | 0.992 | 0.992 | 0.988 | 0.995 |
| Tablet 10 | 0.998 | 0.995 | 0.994 | 0.998 | 0.992 | 0.994 | 0.988 | 0.999 | 0.992 | 0.996 | 0.994 | 0.994 | 0.991 | 0.995 |
| Mean | 0.990 | 0.997 | 0.998 | 0.993 | 0.993 | 0.996 | 0.996 | 0.996 | 0.995 | 0.996 | 0.996 | 0.996 | 0.990 | 0.995 |
| SD | 0.002 | 0.002 | 0.001 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.003 | 0.002 | 0.002 | 0.002 | 0.002 | 0.001 |
5.7. Greenness evaluation of the proposed methods
The intent for replacing conventional pharmaceutical analysis techniques that rely on high volume consumption of several toxic and organic solvents, by eco-friendly green ones with comparable fulfilment characteristics, has developed considering deeply assure the compliance with the requirements of the green chemistry and the importance of environmental protection. In this study, using four various greenness evaluation techniques was performed to evaluate the proposed spectrophotometric methods’ ecofriendly nature.
5.7.1. National environmental methods Index (NEMI)
From the first used qualitative tools for prompting the greenness of an analytical methodology is NEMI approach [54]. It is applied via using a simple expression called pictogram by means of circle composing of four quadrants. Each quarter shows specific criteria, namely, the usage of PBT chemicals (persistent, bioaccumulative and toxic),consumption of hazardous chemical, corrosive criteria and the amount of waste generated [55]. Fortunately, NEMI pictograms were all green for the proposed methods and fulfilled the four quadrants’ acceptance criteria, Table 8 , since ethanol was the used solvent which is safe in terms of safety, health, and environment hazards, it has been described by the US Environmental Protection Agency as neither hazardous nor PBT [56] in addition, The pH of the study is non-corrosive (pH is not 12) and the amount of waste released is less than 50 g / sample.
Table 8.
Greenness assessment of the two proposed spectrophotometric methods according to Eco-Scale, NEMI, GAPI and AGREE tools.
| Eco-Scale assessment | NEMI tool | GAPI tool | AGREE tool | |
| Parameters | Penalty points |
 |
 |
 |
| Reagents | ||||
| Ethanol* | 4 | |||
| Instrument (spectrophotometer) | ||||
| Energy consumption | 0 | |||
| Occupational hazards | 0 | |||
| Waste | 3 | |||
| Total penalty points (PP) | 7 | |||
| Analytical EcoScale total score | 93 | |||
| Comment | Excellent green analysis | |||
*Ethanol is given a signal word ‘danger’ with two pictograms and consumed volume per sample analysis is <10 mL (sample cuvette).
5.7.2. Analytical Eco-Scale system
Another scoring tool associated with the penalty point system was presented as a semi-quantitative approach to assess the environmental impact and greenness of analytical methods [57]. The penalty points are given for each parameter of the analytical process that may affect the excellent green analysis, for instance the hazardousness and amount of the used reagents, waste generation, energy consumption, types of waste treatment and any probable occupational risks. They are subtracted from the base value of 100 of the ideal green method, above 75 for the analytical procedure is considered to be ecofriendly. The score obtained from the proposed methods was of more than 75 which affirms the excellent green performs of proposed methods, Table 8. The total of penalty points for the developed methods was 7. Accordingly, the eco-scale score for the developed spectrophotometric methods was 93 which indicate the greenness of the developed methods.
5.7.3. Green analytical procedure Index (GAPI)
(GAPI) is approach composing of hybrid of both NEMI and Eco-Scale. It has been presented to assess the whole analytical procedures’ green character [58]. This tool utilizes a certain pictogram with five pentagrams for assessing and measuring and it is color-coded extending from green through yellow to red, indicating low, medium, and high environmental impact for each level. The GAPI tool provides information regarding 15 different parameters including sample preparation and its collection, instrumentation, health and safety impact of reagents and compounds utilized, as well as waste treatment. The proposed spectrophotometric methods reveal GAPI pictograms with a greater number of shaded green and yellow sections than red shaded ones, showing the greenness of the developed methods.
5.7.4. Analytical greenness metric (AGREE)
AGREE tool [59] is a software which could be downloaded producing a twelve sections colored pictogram. It covers twelve principles and standards of green analytical chemistry. Every section is colored and shaded from deep green to deep red according to its environmental impact and the total score assessment is displayed in the middle of the circular pictogram. The score value of AGREE pictogram for the proposed methods was found to be 0.71, Table 8.
To summarize greenness evaluation of the developed methods, the success of the developed methods in decreasing the consumption of solvents and energy of instrument in addition, the ethanol utilization were obviously reflected by the high values of analytical eco-score and AGREE score with the prevalence of green color in pictograms of NEMI and GAPI. Therefore, the developed methods are considered “green methods” which can be safely applied without any vulnerable harm to either the analyst or the environment.
5.8. Comparative study of the developed methods along with the reported methods
The criteria for applying the proposed PSE manner including three spectrophotometric methods (AE, PE and RE) to evaluate multi component medicines, are the coupling of two complementary resolution manner namely, auxiliary and data analysis which successfully has the ability to extract the full parent spectra (D0)of the studied drugs allowing the determination of studied drugs’ concentrations at their maxima with maximum accuracy and precision using only one divisor in case of AE and PE and two divisors in case of RE with minimum manipulation steps via built-in spectrophotometric software. PE has advantage over AE since the calculated numerical factor at the two chosen wavelengths has a higher value with more accurate determination of VAL and HCT, so minimize the instrumental error. RE gave a satisfactory results for all synthetic mixtures with VAL concentration over (4.0 µg/mL- 44.0 µg/mL) while AE and PE fail to give good results for mixtures containing VAL more than 40 µg/mL, thus RE is advantageous since it has no limitation in data analysis resolution (DAR). The proposed methods offer advantages over the other reported spectrophotometric methods [13], [14], [15], [16], [17], [18], [19], [20] namely, successive ratio subtraction coupled with constant multiplication method (SS-CM) and successive derivative subtraction (SDS) which needs the availability of the two pure drugs as divisors to recover their D0 spectra (in case of CM-SS) and D1 spectra (in case SDS)as well as the extension of each spectrum over the other one. In addition, absorbance subtraction (AS), amplitude modulation (AM) and advanced amplitude centering(AAC) which based on multistep progressive resolution of iso- points responses (in case of AS and AM) and amplitude maxima(in case of AAC) to get their concentrations with multi-manipulation step using unified regression equation for determination three components. Finally, these proposed methods are applicable for the determination of spectralprint recognition index (SRI) as a measure of purity of the target components.
6. Conclusion
Findings about antihypertensive drugs and their relation with COVID-19 encouraged a statement from different medical societies counselling antihypertensive patients suffering from corona virus to continue on their current antihypertensive therapies especially those containing ARB and CCB, additionally they are considered as a good alternative for other classes of antihypertensive drugs during this pandemic. The superiority of this work is due to its ability to implement quantitative, accurate, precise, and easily used spectrophotometric methods for progressive determination and assaying of the proposed drugs in their pure powdered form, synthetic mixtures, and pharmaceutical formulations. Moreover, the proposed methods are advantageous owing to their capability of overcoming the overlapping spectra between the mentioned drugs via utilizing various manipulation steps for their measurement by restoring their parent spectra which acts as spectralprint for each studied drug indicating their purity. The best method was found to be ratio extraction (RE) due to its wide application for various mixtures with extensively overlapping spectra. The developed methods have similar separation and identification specifications to the chromatographic methods attached with diode array detector coupled with peak purity software besides they are applied with lower cost, energy, consumption of solvent and time, also it does not necessitate any complicated equipment or special computer programs. The developed methods were effectively applied to assess the analytes’ purity profiles in their tablet formulation. Additionally, the greenness profile of developed methods has verified them to be environmentally green. In the light of the above findings, the developed methods are considered to be easily applicable and appropriate for quantitative analysis and testing of the available commercial formulations in quality control laboratories.
CRediT authorship contribution statement
Sara El-Hanboushy: Conceptualization, Methodology, Software, Data curation, Software, Validation, Writing – original draft, Writing – review & editing. Hoda M. Marzouk: Visualization, Methodology, Investigation, Software, Data curation, Supervision, Writing – review & editing. Yasmin M. Fayez: Visualization, Methodology, Investigation, Software, Data curation, Supervision, Writing – review & editing. Mohamed Abdelkawy: Visualization, Methodology, Investigation, Software, Data curation, Supervision, Writing – review & editing. Hayam M. Lotfy: Conceptualization, Visualization, Methodology, Investigation, Software, Data curation, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Zhang D., Wang G., Zhang P., Fang J., Ayala C. Medical expenditures associated with hypertension in the US, 2000–2013. Am. J. Prev. Med. 2017;53(6):S164–S171. doi: 10.1016/j.amepre.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ettehad D., Emdin C.A., Kiran A., Anderson S.G., Callender T., Emberson J., Chalmers J., Rodgers A., Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. The Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 3.Gao C., Cai Y., Zhang K., Zhou L., Zhang Y., Zhang X., Li Q., Li W., Yang S., Zhao X. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur. Heart J. 2020;41:2058–2066. doi: 10.1093/eurheartj/ehaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.H.M.s.S.O. British Pharmacopoeia Commission, British Pharmacopoeia, London, United Kingdom, 2013.
- 5.S.C. Sweetman, Martindale: the complete drug reference, Pharmaceutical press London, Place, Published, 2009.
- 6.Pinto-Sietsma S.-J., Flossdorf M., Buchholz V.R., Offerhaus J., Bleijendaal H., Beudel M., Volders P.G.A., ter Bekke R.M.A., Dormans T., Zwetsloot P.-P., de Jager P., Massberg S., Rämer P., Wendtner C., Hoffmann E., Rothe K., Feihl S., Kessler T., Pinto Y.M., Schunkert H. Antihypertensive drugs in COVID-19 infection, European Heart Journal-Cardiovascular. Pharmacotherapy. 2020;6(6):415–416. doi: 10.1093/ehjcvp/pvaa058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan F., Huang F., Xu J., Yang P., Qin Y., Lv J., Zhang S., Ye L.u., Gong M., Liu Z., Wei J., Xie T., Xu K.-F., Gao G.F., Wang F.-S., Cai L., Jiang C. Antihypertensive drugs are associated with reduced fatal outcomes and improved clinical characteristics in elderly COVID-19 patients. Cell Discovery. 2020;6(1) doi: 10.1038/s41421-020-00221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang P., Zhu L., Cai J., Lei F., Qin J.-J., Xie J., Liu Y.-M., Zhao Y.-C., Huang X., Lin L. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ. Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruilope L.M., Tamargo J., Ruiz-Hurtado G. Renin–angiotensin system inhibitors in the COVID-19 pandemic: consequences of antihypertensive drugs. Eur. Heart J. 2020;41:2067–2069. doi: 10.1093/eurheartj/ehaa487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crespi B., Alcock J. Conflicts over calcium and the treatment of COVID-19, Evolution, Medicine, and Public. Health. 2021;9:149–156. doi: 10.1093/emph/eoaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alsagaff M.Y., Mulia E.P.B., Maghfirah I., Luke K., Nugraha D., Rachmi D.A., Septianda I., A'yun M.Q. Association of calcium channel blocker use with clinical outcome of COVID-19: A meta-analysis. Diabetes Metabolic Syndrome: Clin. Res. Rev. 2021;15(5) doi: 10.1016/j.dsx.2021.102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kow C.S., Ramachandram D.S., Hasan S.S. Clinical outcomes of hypertensive patients with COVID-19 receiving calcium channel blockers: a systematic review and meta-analysis. Hypertens. Res. 2022;45(2):360–363. doi: 10.1038/s41440-021-00786-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lotfy H.M., Hegazy M.A., Mowaka S., Mohamed E.H. Novel spectrophotometric methods for simultaneous determination of amlodipine, valsartan and hydrochlorothiazide in their ternary mixture. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015;140:495–508. doi: 10.1016/j.saa.2014.12.096. [DOI] [PubMed] [Google Scholar]
- 14.Saleh S.S., Lotfy H.M. Advanced Amplitude Centering as an invigorating Manipulation for Unifird Wavelength spectral resolution of ternary mixtures. Int. J. Pharm. Pharmaceut. Sci. 2017;9:43–50. [Google Scholar]
- 15.Quynh Trang N.T., Van Hop N., Giang Chau N.D., Tran T.B. Simultaneous determination of amlodipine, hydrochlorothiazide, and valsartan in pharmaceutical products by a combination of full spectrum measurement and kalman filter algorithm. Adv. Mater. Sci. Eng. 2019;2019:1–9. [Google Scholar]
- 16.Mowaka S., Hegazy M.A., Lotfy H.M., Mohamed E.H. Novel pure component contribution algorithm (PCCA) and UHPLC methods for separation and quantification of amlodipine, valsartan, and hydrochlorothiazide in ternary mixture. J. AOAC Int. 2017;100:692–699. doi: 10.5740/jaoacint.16-0195. [DOI] [PubMed] [Google Scholar]
- 17.Sharma M., Kothari C., Sherikar O., Mehta P. Concurrent estimation of amlodipine besylate, hydrochlorothiazide and valsartan by RP-HPLC, HPTLC and UV–spectrophotometry. J. Chromatogr. Sci. 2014;52(1):27–35. doi: 10.1093/chromsci/bms200. [DOI] [PubMed] [Google Scholar]
- 18.Jothieswari D., Anandakumar K., Vijay Santhi D., Vijayakumar B., Priya D., Stephen Rathinaraj B. A validated UV spectrophotometric method for the simultaneous estimation of amlodipine besylate, valsartan and hydrochlorothiazide in bulk and in combined tablet dosage form. J. Pharmaceut. Biomed. Sci. 2010;5:1–5. [Google Scholar]
- 19.Darwish H.W., Hassan S.A., Salem M.Y., El-Zeany B.A. Sequential spectrophotometric method for the simultaneous determination of amlodipine, valsartan, and hydrochlorothiazide in coformulated tablets. Int. J. Spectrosc. 2013;2013:1–8. [Google Scholar]
- 20.Darbandi A., Sohrabi M.R., Bahmaei M. Development of a chemometric-assisted spectrophotometric method for quantitative simultaneous determination of Amlodipine and Valsartan in commercial tablet. Optik. 2020;218 [Google Scholar]
- 21.Shaikh J.S.A., Raut S., Abdul A., Pathan M.A.A.K. High performance liquid chromatographic assay of amlodipine, valsartan and hydrochlorothiazide simultaneously and its application to pharmaceuticals, urine and plasma analysis. J. Chromatogr. B. 2020;1155 doi: 10.1016/j.jchromb.2020.122295. [DOI] [PubMed] [Google Scholar]
- 22.Shah J.V., Parekh J.M., Shah P.A., Shah P.V., Sanyal M., Shrivastav P.S. Application of an LC–MS/MS method for the analysis of amlodipine, valsartan and hydrochlorothiazide in polypill for a bioequivalence study. J. Pharm. Anal. 2017;7(5):309–316. doi: 10.1016/j.jpha.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaalan R.A., Belal T.S., El Yazbi F.A., Elonsy S.M. Validated stability-indicating HPLC-DAD method of analysis for the antihypertensive triple mixture of amlodipine besylate, valsartan and hydrochlorothiazide in their tablets. Arabian J. Chem. 2017;10:S1381–S1394. [Google Scholar]
- 24.Osman R., Elbashir A. Development and validation of stability indicating HPLC method for the simultaneous analysis of amlodipine, hydrochlorothiazide and valsartan in pharmaceutical formulation. J. Anal. Pharm. Res. 2017;6:00188. [Google Scholar]
- 25.USP, United States Pharmacopeia and National Formulary (USP 38-NF 33), 2015.
- 26.Ebeid W., Salim M., Elkady E., Elzahr A., El-Bagary R., Patonay G. Simultaneous determination of valsartan, amlodipine besylate and hydrochlorothiazide using capillary zone electrophoresis (CZE) Die Pharmazie-Int. J. Pharmaceut. Sci. 2015;70:368–373. [PubMed] [Google Scholar]
- 27.Vojta J., Jedlička A., Coufal P., Janečková L. A new, rapid, stability-indicating UPLC method for separation and determination of impurities in amlodipine besylate, valsartan and hydrochlorothiazide in their combined tablet dosage form. J. Pharm. Biomed. Anal. 2015;109:36–44. doi: 10.1016/j.jpba.2015.01.059. [DOI] [PubMed] [Google Scholar]
- 28.Lahsini R., Monser L. Development of a new UPLC method for simultaneous determination of valsartan, amlodipine besylate, and hydrochlorothiazide pharmaceutical products. Acta Chromatogr. 2015;27(3):449–460. [Google Scholar]
- 29.Osman R., Elbashir A. Development and validation of stability indicating HPLC method for the simultaneous analysis of amlodipine, hydrochlorothiazide and valsartan in pharmaceutical formulation. Pharm Methods. 2017;6:188. [Google Scholar]
- 30.Tengli A.R., Gurupadayya B.M., Soni N. Simultaneous estimation of hydrochlorothiazide, amlodipine, and losartan in tablet dosage form by RP-HPLC. Int. J. Chem. Anal. Sci. 2013;4(1):33–38. [Google Scholar]
- 31.Shaalan R.A., Belal T.S. Gradient HPLC-DAD Determination of the Antihypertensive Mixture of Amlodipine Besylate, Valsartan, and Hydrochlorothiazide in Combined Pharmaceutical Tablets. J. :Liquid Chromatogr. Related Technologies. 2012;35(2):215–230. [Google Scholar]
- 32.El-Gizawy S.M., Abdelmageed O.H., Omar M.A., Deryea S.M., Abdel-Megied A.M. Development and validation of HPLC method for simultaneous determination of amlodipine, valsartan, hydrochlorothiazide in dosage form and spiked human plasma. Am. J. Anal. Chem. 2012;03(06):422–430. [Google Scholar]
- 33.Mohammed Osman R., Elbashir A. Utilization of 1, 2-Naphthoquine-4-Sulfonate (NQS) for Development and Validation of Stability indicating HPLC method for the Simultaneous Analysis of Amlodipine, Hydrochlorothiazide and Valsartan in Pharmaceutical Formulation. Int. J. Bioanal. Methods Bioequival. Stud. 2019;5:82–92. [Google Scholar]
- 34.Farvardin N., Jahani S., Kazemipour M., Foroughi M.M. The synthesis and characterization of 3D mesoporous CeO 2 hollow spheres as a modifier for the simultaneous determination of amlodipine, hydrochlorothiazide and valsartan. Anal. Methods. 2020;12(13):1767–1778. [Google Scholar]
- 35.Mansano G.R., Pires Eisele A.P., Sartori E.R. Electrochemical evaluation of a boron-doped diamond electrode for simultaneous determination of an antihypertensive ternary mixture of amlodipine, hydrochlorothiazide and valsartan in pharmaceuticals. Anal. Methods. 2015;7(3):1053–1060. [Google Scholar]
- 36.Obaydo R.H., Al Zakri D.J., Sakur A.A., Lotfy H.M. Ultraviolet spectrophotometric methods for the determination of the minor component presented in fixed-dose pharmaceutical combinations through the last two decades (2000–2020), Future. J. Pharm. Sci. 2021;7:1–9. [Google Scholar]
- 37.Lotfy H.M., Saleh S.S. Recent development in ultraviolet spectrophotometry through the last decade (2006–2016): A review, International. J. Pharm. Pharmaceut. Sci. 2016;8:40–56. [Google Scholar]
- 38.Saleh S.S., Lotfy H.M., Tiris G., Erk N., El-Naem O.A. The Power of High Impact Amplitude Manipulation (HIAM) technique for Extracting the Basic Spectra of Two Fixed-dose Combinations (FDC)-Spectrophotometric Purity Analysis via Spectral Contrast Angle. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022;273 doi: 10.1016/j.saa.2022.121036. [DOI] [PubMed] [Google Scholar]
- 39.Rostom Y., Lotfy H.M., Öztürk M., Tiris G., Erk N., Saleh S.S. Trade-off efficacy and data processing strategy in the power of spectral resolution of co-formulated antihypertensive pharmaceuticals. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021;247 doi: 10.1016/j.saa.2020.119080. [DOI] [PubMed] [Google Scholar]
- 40.Lotfy H.M., Obaydo R.H., Sakur A.A. Evaluation of assay and in-vitro dissolution profile of certain fixed-dose combination using green analytical method Ann. Pharm. Fr. 2021;79(1):3–15. doi: 10.1016/j.pharma.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Lotfy H.M., Hagazy M.-A.-M. Comparative study of novel spectrophotometric methods manipulating ratio spectra: an application on pharmaceutical ternary mixture of omeprazole, tinidazole and clarithromycin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012;96:259–270. doi: 10.1016/j.saa.2012.04.095. [DOI] [PubMed] [Google Scholar]
- 42.Rostom Y., Wadie M., Rezk M.R., Marzouk H.M., Abdel-Moety E.M. Fingerprinting and iso-absorptive resolution techniques for the spectrally overlapping Dutasteride and Silodosin mixture: Content uniformity testing along with greenness profile assessment. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022;273:121063. doi: 10.1016/j.saa.2022.121063. [DOI] [PubMed] [Google Scholar]
- 43.Lotfy H.M., Omran Y.R. Novel absorptivity centering method utilizing normalized and factorized spectra for analysis of mixtures with overlapping spectra in different matrices using built-in spectrophotometer software. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018;200:167–178. doi: 10.1016/j.saa.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Lotfy H.M., Monir H.H., Erk N., Rostom Y. Novel feature extraction approach for achieving potential spectral resolution: Green analytical application on zofenopril calcium and hydrochlorothiazide in their spectrally overlapping binary mixture. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020;230 doi: 10.1016/j.saa.2019.117998. [DOI] [PubMed] [Google Scholar]
- 45.Lotfy H.M. Absorbance subtraction and amplitude modulation as novel spectrophotometric methods for the analysis of binary mixtures. Int. J. Pharm. Pharm. Sci. 2014;6:735–741. [Google Scholar]
- 46.Lotfy H.M., Elshahed M.S., Mohamed D. The concept of Relative Absorptivity Distribution for enhancing disbanding power of spectrophotometric technique to resolve co-formulated tablets: A tool for purity index and uniformity assessment. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020;239 doi: 10.1016/j.saa.2020.118551. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed D.A., Lotfy H.M. Evaluation of in silico and in lab sample enrichment techniques for the assessment of challengeable quaternary combination in critical ratio. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021;260 doi: 10.1016/j.saa.2021.119943. [DOI] [PubMed] [Google Scholar]
- 48.Samir A., Lotfy H.M., Salem H., Abdelkawy M. Development and validation of simultaneous spectrophotometric and TLC-spectrodensitometric methods for determination of beclomethasone dipropionate and salbutamol in combined dosage form. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014;128:127–136. doi: 10.1016/j.saa.2014.02.044. [DOI] [PubMed] [Google Scholar]
- 49.Lotfy H.M., Saleh S.S., El-Maraghy C.M. Advanced approaches for the treatment and amplification of weak spectral signals produced by critical concentrations in white multicomponent systems. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020;224 doi: 10.1016/j.saa.2019.117339. [DOI] [PubMed] [Google Scholar]
- 50.Lotfy H.M., Fayz Y.M., El-Hanboushy S., Shokry E., Abdelkawy M. Application of successive and progressive spectrophotometric resolution for the analysis of partially or completely overlapping ternary mixtures, Analytical. Chem. Lett. 2016;6(6):718–737. [Google Scholar]
- 51.USP, United States Pharmacopeia and National Formulary (USP 42-NF 37), 2019.
- 52.ICH, International Conference on Harmonization (ICH),Q2B: Validation of Analytical Procedures : Methodology, 62, US FDA Federal Register, 1997.
- 53.T. Coyle, The characterization of chemical purity. Organic compounds: Ed. by LAK Staveley, Butterworths, London, 1971, Supplement to “Pure and Applied Chemistry” Elsevier 1972.
- 54.Keith L.H., Gron L.U., Young J.L. Green analytical methodologies. Chem. Rev. 2007;107(6):2695–2708. doi: 10.1021/cr068359e. [DOI] [PubMed] [Google Scholar]
- 55.Tobiszewski M. Metrics for green analytical chemistry. Anal. Methods. 2016;8(15):2993–2999. [Google Scholar]
- 56.(Emergency Planning and Community Right-to-Know Act; Section 313 Toxic Release Inventory (TRI) Chemical List for Reporting Year 2019. (https://www.epa.gov/sites/production/files/2020-02/documents/ry_2019_tri_chemical_list_0.pdf)).
- 57.Gałuszka A., Migaszewski Z.M., Konieczka P., Namieśnik J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC, Trends Anal. Chem. 2012;37:61–72. [Google Scholar]
- 58.Płotka-Wasylka J. A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta. 2018;181:204–209. doi: 10.1016/j.talanta.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 59.Pena-Pereira F., Wojnowski W., Tobiszewski M. AGREE—Analytical GREEnness metric approach and software. Anal. Chem. 2020;92:10076–10082. doi: 10.1021/acs.analchem.0c01887. [DOI] [PMC free article] [PubMed] [Google Scholar]