Abstract
The bacteriocin produced by Lactococcus lactis IFPL105 is bactericidal against several Lactococcus and Lactobacillus strains. Addition of the bacteriocin to exponential-growth-phase cells resulted in all cases in bacteriolysis. The bacteriolytic response of the strains was not related to differences in sensitivity to the bacteriocin and was strongly reduced in the presence of autolysin inhibitors (Co2+ and sodium dodecyl sulfate). When L. lactis MG1363 and its derivative deficient in the production of the major autolysin AcmA (MG1363acmAΔ1) were incubated with the bacteriocin, the latter did not lyse and no intracellular proteins were released into the medium. Incubation of cell wall fragments of L. lactis MG1363, or of L. lactis MG1363acmAΔ1 to which extracellular AcmA was added, in the presence or absence of the bacteriocin had no effect on the speed of cell wall degradation. This result indicates that the bacteriocin does not degrade cell walls, nor does it directly activate the autolysin AcmA. The autolysin was also responsible for the observed lysis of L. lactis MG1363 cells during incubation with nisin or the mixture of lactococcins A, B, and M. The results presented here show that lysis of L. lactis after addition of the bacteriocins is caused by the resulting cell damage, which promotes uncontrolled degradation of the cell walls by AcmA.
Bacteriocins are antimicrobial polypeptides synthesized ribosomally by bacteria (34). Most bacteriocins from lactic acid bacteria exert their antibacterial effect by permeabilizing the target cell membrane, whereby the cells lose their viability (1, 5, 29). Apart from damaging cell membranes, some bacteriocins have also been reported to cause bacteriolysis. Bierbaum and Sahl (4) were among the first to show the involvement of autolysins in the bacteriolytic effect of a bacteriocin. Autolysins are peptidoglycan hydrolases that are capable of causing bacterial autolysis (39). The authors showed that the bacteriocins Pep5, produced by Staphylococcus epidermidis, and nisin, produced by Lactococcus lactis, activate an N-acetylmuramoyl-l-alanine amidase and an β-N-acetylglucosaminidase of S. simulans (4). Plantaricin C has been shown to be bacteriolytic for Lactobacillus fermentum LM 13554 and L. delbrueckii subsp. bulgaricus LMG 13551, while no reduction of the optical densities (ODs) of mid-exponential-phase cultures of L. sake CECT 906, L. helveticus LMG13555, or Leuconostoc mesenteroides was observed (14, 15). Microscopic analysis of L. fermentum cells treated with plantaricin C showed that changes had taken place in the cell wall. The authors suggested that cell lysis could be a secondary effect of the bacteriocin caused by a deregulation of the autolytic system of the sensitive cells resulting in destruction of the peptidoglycan layer. While no lysis of Lc. mesenteroides cells treated with plantaricin C was seen, a clear reduction of the OD was observed when these cells were incubated with pediocin AcH (3). This effect was not observed with Lactobacillus plantarum, although intracellular components were released. Transmission electron micrographic analysis of cells of both bacterial species revealed the presence of lysed ghost cells upon treatment with pediocin AcH (18). The action of nisin against Listeria monocytogenes Scott A cells resulted in the loss of cellular material following lysis, as shown by electron microscopic analysis (10). The antibacterial cyclic peptide AS-48 produced by Enterococcus faecalis S-48 also has bactericidal and bacteriolytic activity against several L. monocytogenes strains (28). These authors show that cells adapted to AS-48 have a changed fatty acid composition of their cytoplasmic membrane and a thicker cell wall and become more resistant to autolysins. For L. monocytogenes and E. faecalis growing cells, it was observed that loss of viability was much more rapid than the observed reduction of the OD. L. monocytogenes growing cells also lysed upon addition of pediocin PA-1, while the amount of bacteriocin activity added did not have a great influence on the degree of reduction of the OD (37).
A bacteriolytic effect of bacteriocins on lactococci was first reported by Kok et al. (21), who described that treatment of lactococcal cells with lactococcin A (LcnA) resulted in the release of UV-absorbing material. Using the same bacteriocin, Morgan et al. (30) obtained bacteriolysis and subsequent release of an intracellular enzyme from sensitive lactococcal cells only when LcnA acted in concert with the lactococcins B and M. Another bacteriocin which has been shown to cause lysis of sensitive L. lactis cells is the bacteriocin produced by L. lactis IFPL105 (previously identified as Lactobacillus curvatus IFPL105) (9). This secreted broad-spectrum bacteriocin has been shown to cause lysis of logarithmically growing L. lactis and Lactobacillus casei (26). The importance of the lytic effect of this bacteriocin in accelerating cheese proteolysis has been demonstrated in cheese curd slurries manufactured with sensitive strains as starter and bacteriocin-producing adjuncts (27). Increase of starter cell lysis and free amino acid concentration in Cheddar cheese have been described by Morgan et al. (31) after using as starter adjunct a lactococcal strain producing lactococcins A, B, and M.
The major autolysin activity described for lactococci and lactobacilli is that of an N-acetylmuramidase (24, 32). The gene (acmA) encoding the enzyme in L. lactis has been cloned and sequenced (6). The construction of an acmA deletion mutant by replacement recombination has allowed to demonstrate that AcmA is required for cell separation and autolysis of cells during stationary growth phase (6, 7). Several factors, such as starvation for a carbon source, reagents which cause depletion of either the electrical or pH gradients of cellular membranes or which cause disruption of these membranes, as well as proteolytic degradation, have been shown to influence the autolytic behavior of cells (8, 11, 19, 20, 24).
The object of this work was to investigate whether bacteriolysis by the bacteriocin produced by L. lactis IFPL105 on different strains of lactococci and lactobacilli was a direct or indirect effect of the bacteriocin. The results show that bacteriolysis is observed only when active autolysins are present in the sensitive cells. The bacteriocin does not activate the autolysin AcmA of L. lactis. Rather, depletion of cellular energy causes an imbalance in the control of the action of the autolysin, resulting in cell wall degradation and, thus, lysis of cells.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacteriocin producer L. lactis IFPL105 and its mutant L. lactis IFPL1053 (Bac− Imm−) (9) are from the Culture Collection of the Instituto del Frío, Madrid, Spain. The bacteriocin-sensitive microorganisms used in this study are listed in Table 1. L. casei JCL1227 and L. rhamnosus JCL1211 were kindly provided by Juan Jimeno (FAM Sektion Biochemie, Liebefeld CH-3003, Bern, Switzerland). Other lactococcal strains used were L. lactis subsp. cremoris MG1363 (12), its derivative MG1363acmAΔ1 (6), and L. lactis subsp. cremoris 9B4 (41), which produces lactococcins A, B, and M. Culture media were M-17 broth (Adsa-Micro, Pharmafaster SA, Barcelona, Spain) containing lactose or glucose (5 g/liter) for lactococci and MRS broth (Adsa-Micro) for lactobacilli. The incubation temperature for all strains was 30°C. The microorganisms were stored at −80°C in reconstituted skim milk (100 g/liter).
TABLE 1.
Cell viability and lysis after 3 h of incubation at 37°C in 20 mM sodium phosphate buffer (pH 6.8) of suspensions of log-phase cells of Lactococcus and Lactobacillus strains with or without bacteriocin
| Strain | Initial countsa (CFU/ml) | Incubation in buffer
|
Incubation with bacteriocin (150 AU/ml)
|
||
|---|---|---|---|---|---|
| Counts (CFU/ml) | Lysisb (%; mean ± SE) | Counts (CFU/ml) | Lysis (%; mean ± SE) | ||
| Lactococcus lactis IFPL359 | 3.3 × 109 | 1.0 × 108 | 32.5 ± 2.3 | 4.8 × 103 | 51.3 ± 1.2 |
| L. lactis T1 | 1.5 × 109 | 5.0 × 107 | 22.4 ± 2.8 | 3.8 × 103 | 56.4 ± 3.5 |
| L. lactis IFPL22 | 2.0 × 109 | 5.0 × 107 | 15.6 ± 1.3 | 3.6 × 103 | 28.4 ± 0.9 |
| L. lactis IFPL1053 | NDc | ND | 4.3 ± 1.1 | ND | 29.8 ± 1.3 |
| Lactobacillus casei IFPL731 | 1.0 × 109 | 1.0 × 108 | 14.9 ± 1.5 | 8.7 × 103 | 25.7 ± 0.8 |
| L. casei JCL1227 | 5.9 × 108 | 1.0 × 108 | 16.3 ± 1.6 | 1.1 × 103 | 30.2 ± 0.8 |
| L. plantarum IFPL935 | 9.9 × 108 | 1.0 × 108 | 15.2 ± 4.8 | 2.3 × 105 | 74.5 ± 4.4 |
| L. rhamnosus JCL1211 | 6.8 × 108 | 1.0 × 108 | 15.6 ± 1.3 | 3.0 × 105 | 74.5 ± 3.7 |
Cell viability expressed as the number of CFU after plating two appropriate dilutions of the cell suspensions in M-17 agar (lactococci) and MRS-agar (lactobacilli).
Percent lysis = 100 − (A1/A2 × 100), where A1 is the lowest and A2 is the highest value of the OD600 measured during incubation of the cell suspensions.
ND, not determined.
Isolation of bacteriocins from the culture supernatant.
The bacteriocin was isolated from a 1-liter culture of L. lactis IFPL105 grown to the late exponential-early stationary phase. Cells were removed by centrifugation at 6,000 × g for 15 min at 4°C, and 400 g of ammonium sulfate was added to the culture supernatant. The protein precipitate was pelleted by centrifugation at 8,000 × g for 20 min at 4°C, solubilized in 15 ml of 20 mM phosphate buffer (pH 6.8), and loaded onto C18 Sep-Pak cartridges (Millipore Co., Bedford, Mass.). Cartridges were washed with 25% 2-propanol in 0.1% trifluoroacetic acid (TFA), and bacteriocin activity was eluted with 40% 2-propanol in 0.1% TFA. Lactococcins A, B, and M were concentrated by ammonium sulfate precipitation from a 1-liter culture supernatant of L. lactis 9B4 as described above. The solubilized precipitate in phosphate buffer of the lactococcins A, B, and M and the bacteriocin from L. lactis IFPL105 (crude bacteriocin) was autoclaved (121°C, 10 min) to avoid residual autolysin AcmA activity. Nisin was purchased from Sigma Chemical Co., St. Louis, Mo.).
The titer of bacteriocin activity (arbitrary units [AU]) was assayed by a serial twofold dilution test as described previously (9), using L. lactis IFPL359 or MG1363 as the indicator strain.
Analysis of the bacteriocin effect by plate counting and by measuring OD600 reduction and the release of peptidase activity.
Exponentially growing cultures (OD at 600 nm [OD600] of 0.7) in M-17 or MRS broth were harvested by centrifugation at 10,000 × g for 10 min at 4°C. Pellets were washed and suspended in 20 mM sodium phosphate buffer (pH 6.8) containing 150 AU of bacteriocin per ml. Lysis was monitored during 3 h of incubation at 37°C by recording the decrease in OD600 using a Spectronic 20 D (Milton Roy Co., Rochester, N.Y.). Percentage of lysis was determined as 100 − (A1/A2 × 100), where A1 is the lowest and A2 is the highest value of the OD600 measured during incubation (23). Cell lysis was also analyzed after direct addition of the bacteriocin (500 AU/ml) to logarithmically growing cultures (OD600 of 0.7) at 30°C in M-17 or MRS broth, by monitoring the decrease in OD600 during further growth. Sensitivity of the strains to the bacteriocin was examined at regular intervals by plate counting on M-17 or MRS agar plates.
Controls for components other than the bacteriocin included culture supernatant from L. lactis IFPL1053 (Bac−) precipitated with 40% ammonium sulfate, loaded on C18 cartridges, and eluted with 40% 2-propanol in 0.1% TFA as described for the parental strain.
Incubations were performed in triplicate, and results were statistically compared by using one-way analysis of variance to determine significant differences (P < 0.05) in percentage of lysis among incubation conditions and strains.
The lytic effect of the bacteriocin produced by L. lactis IFPL105 was also tested by the addition of the autoclaved crude bacteriocin (300 AU/ml) to exponential-phase cultures (OD600 of 0.7) of L. lactis MG1363 or MG1363acmAΔ1. Lysis was followed during incubation at 30°C by monitoring the OD600 decrease and the release of intracellular X-prolyl dipeptidyl aminopeptidase (PepX) activity as described before (8). PepX activity was measured in culture supernatants (100 μl) filtered through a 0.22-μm-pore-size filter (Millipore Co.), using as substrate 100 μl of 1 mM Gly-Pro-p-nitroanilide (Sigma) solution in 50 mM phosphate buffer (pH 7.0). The total volume of the reaction mixture was brought to 1 ml with phosphate buffer, and incubation was at 37°C using a Peltier CPS-240A temperature controller in a model UV-1601 spectrophotometer (Shimadzu Inc., Columbia, Md.). Release of p-nitroaniline was measured as the increase in absorbance at 410 nm (E410 = 8,800), and PepX activity was expressed as units of supernatant per milliliter.
The effects of the bacteriocin produced by L. lactis IFPL105, the mixture of lactococcins A, B, and M, and nisin were compared after addition of each of the bacteriocin samples (300 AU/ml) to cell suspensions of L. lactis MG1363 or MG1363acmAΔ1 in the supernatant fraction of an overnight culture of L. lactis MG1363acmAΔ1 (OD600 of 2) filtered through a 0.22-μm-pore-size filter. Cell suspensions were obtained by centrifugation (10,000 × g for 10 min) of exponential-phase cultures (OD600 of 0.7) of the two strains. Lysis was examined after 3 h of incubation at 37°C by monitoring the OD600 decrease and the release of PepX activity.
Effect of metal ions and chemical reagents on bacteriolysis.
The effect of Co2+ on the action of the bacteriocin of L. lactis IFPL105 was measured by adding the chloride salt (1 mM, final concentration) to L. lactis IFPL359 and L. rhamnosus JCL1211 cell suspensions in 20 mM phosphate buffer (pH 6.8), obtained as described above. Bacteriocin (500 AU/ml) was added to the cells, and the lysis was monitored during incubation at 37°C by recording the decrease in OD600. The effects of sodium dodecyl sulfate (SDS; 0.40 mg/ml) and cardiolipin (0.04 mg/ml) were analyzed by their addition to exponentially growing cultures (OD600 of 0.6 to 0.7) of the two strains incubated for 30 min with 500 AU of bacteriocin per ml. Results were expressed as percentage of decrease in cell density measured at 600 nm during the following incubation at 30°C.
Detection and determination of cell wall hydrolytic activity.
Autolysin activity of Lactobacillus and Lactococcus strains was assayed after addition of bacteriocin (500 AU/ml) to cell suspensions in buffer or to growing cultures. Samples of 5 ml were obtained at different intervals and centrifuged at 10,000 × g for 5 min at 4°C. Portions (0.1 ml) of the supernatants were tested for cell wall hydrolytic activity, using as substrate 0.9 ml of 0.2% (wt/vol) autoclaved Micrococcus lysodeikticus cells (Sigma) for L. lactis and autoclaved cells of the tested strain for Lactobacillus, in 20 mM phosphate buffer, pH 6.8 or 7.5. The reaction mixture was incubated at 30 or 37°C (depending on the strain) for 3 h. Activity was determined by the decrease in the OD600 of the cell suspension per minute.
Lytic activities of the strains were also tested by renaturing SDS-polyacrylamide gel electrophoresis (PAGE) (zymograms) as described by Potvin et al. (36), using SDS–12.5% polyacrylamide gels containing 0.2% (wt/vol) autoclaved cells. M. lysodeikticus cells were used as substrate for L. lactis samples, while samples of the Lactobacillus strains were assayed on autoclaved cells of the tested strain. Samples (5 ml) were obtained at different intervals during incubation of cell suspensions in buffer or broth cultures, with or without bacteriocin, and centrifuged (10,000 × g, 5 min, 4°C). Before loading, the samples (cell pellets and lyophilized supernatants) were treated with Laemmli buffer (22) as described by Valence and Lortal (40). Electrophoresis was done in a Mini-Protean II cell unit (Bio-Rad Laboratories, Hercules, Calif.) at 180 V for 1 h. Gels were washed with distilled water, and proteins were renatured in 25 mM Tris-HCl (pH 7.0, 7.5, or 8.0, depending on the strain tested) containing 1% Triton X-100. The renatured cell wall hydrolytic activities appeared as clear bands on the opaque background. The contrast was enhanced by staining the gels with 1% methylene blue in 0.01% KOH and destaining in distilled water.
Effect of the bacteriocin produced by L. lactis IFPL105 on autolysin activity was also tested by mixing 300 AU of bacteriocin per ml with a lactococcal cell wall fraction derived from L. lactis MG1363 or MG1363acmAΔ1 and suspended (to give a final OD600 of 0.7) in the supernatant fraction of overnight cultures of the two strains (6). Native cell walls were obtained at 4°C from exponentially growing cells, harvested by centrifugation (8,000 × g, 15 min), suspended in 50 mM potassium phosphate buffer (pH 7.0), mixed (1:1, vol/wt) with glass beads (150 to 212 μm in diameter; Sigma), and disrupted for 16 min (four intervals of 4 min each) in a Mini Blend (Sunbeam-Oster Co. Inc., Miami, Fla.). Whole cells were removed by centrifugation at 1,000 × g for 15 min, and the cell walls were recovered from the supernatant by centrifugation at 14,000 × g for 15 min at 4°C. The cell wall fragments were suspended in 20 mM sodium phosphate buffer (pH 6.8) containing 0.02% sodium azide. Reduction of the OD600 of the cell wall suspensions during incubation at 37°C was followed over time using a Shimadzu UV-1601 spectrophotometer.
DNA analysis and manipulation.
Genomic DNA of lactococci and lactobacilli was obtained by the method of Anderson and McKay (2). Total DNA was restricted with EcoRI (Roche, Mannheim, Germany), separated in a 0.7% agarose gel, and blotted onto positively charged nylon membranes (Roche). A 1.1-kb DNA fragment from the acmA gene of L. lactis MG1363, amplified by PCR as described by Buist et al. (6), was used as the probe. Probe labeling, hybridization, and immunological detection were performed with the DIG High prime labeling and detection kit according to the instructions of the manufacturer (Roche).
RESULTS
Cell lysis and viability of various lactococci and lactobacilli in the presence of the bacteriocin of L. lactis IFPL105.
Lysis of cells of lactococci and lactobacilli during incubation in phosphate buffer with 150 AU of a 40% 2-propanol-eluted preparation of bacteriocin produced by L. lactis IFPL105 per ml was followed by measuring the percentage of decrease in OD600 (Table 1). To ensure that the bacteriocin preparation contained no cell wall-degrading enzymes, it was subjected to SDS-PAGE in the presence of M. lysodeikticus autoclaved cells. No bands of clearing, indicative of the presence of cell wall hydrolases, were detected (data not shown).
The lytic response of the various strains to the bacteriocin was statistically different (P < 0.05). Addition of the supernatant fraction of L. lactis IFPL1053 (Bac−) had no significant effect on the reduction of cell viability and lysis compared to the incubation of cells in phosphate buffer. Autolysis of the four lactococcal strains after 3 h of incubation in phosphate buffer differed considerably, ranging from 4 to 32%. Addition of the bacteriocin and incubation over the same period resulted in 25 to 30% lysis of L. lactis IFPL22 and IFPL1053 and 50 to 55% of lysis of the L. lactis IFPL359 and T1 strains, showing that the bacteriocin-induced lysis differs among different lactococcal strains. The extents of bacteriocin-induced lysis of the two L. casei strains were found to be similar, while the highest percentage of lysis was obtained for the L. plantarum and L. rhamnosus strains.
Cell viability of the different strains 3 h after addition of the bacteriocin (150 AU/ml) to suspensions of cells taken from the logarithmic phase of growth is also shown in Table 1. Interestingly, the two Lactobacillus strains showing the highest lytic response to the bacteriocin, L. plantarum IFPL935 and L. rhamnosus JCL1211, showed the least loss of viability, whereas L. casei IFPL731 and JCL1227 exhibited a loss of viability similar to that of the Lactococcus strains.
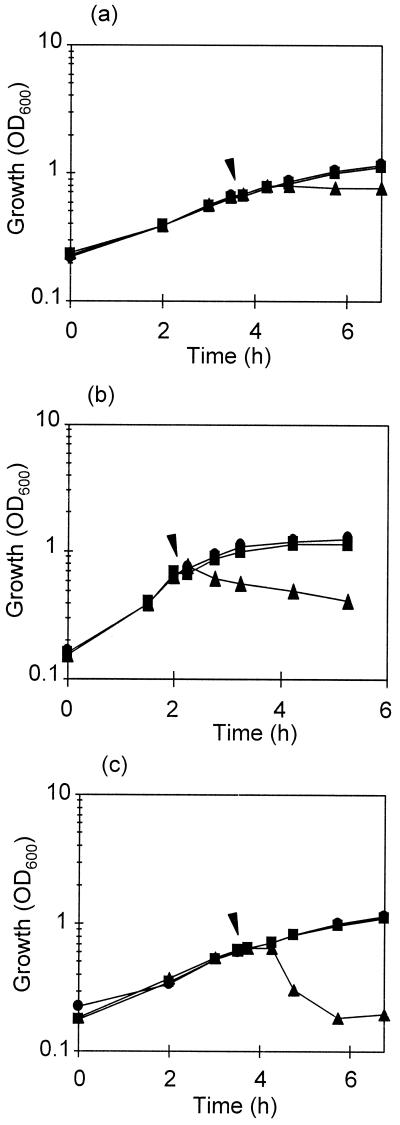
In all strains studied, reduction of cell viability and OD600 were not simultaneous. Figure 1 shows the decrease in OD600 of three representative strains after addition of the bacteriocin to exponentially growing cultures. In the case of the lactobacilli, the cell density of the cultures hardly changed within 0.5 to 1 h after addition of the bacteriocin and then decreased rapidly in L. rhamnosus JCL1211 to reach 71.3% of lysis after 2 h of further incubation.
FIG. 1.
Growth at 30°C of L. casei IFPL731 (a), L. lactis IFPL359 (b), and L. rhamnosus JCL1211 (c) in broth (●). At the time point indicated by arrowheads, bacteriocin (500 AU/ml) from L. lactis IFPL105 (▴) or the supernatant precipitate from L. lactis IFPL1053 (Bac−) culture (■) was added.
Effect of the bacteriocin of L. lactis IFPL105 on autolysin activity.
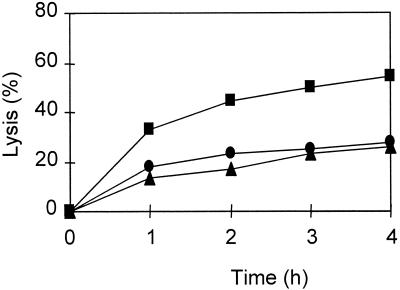
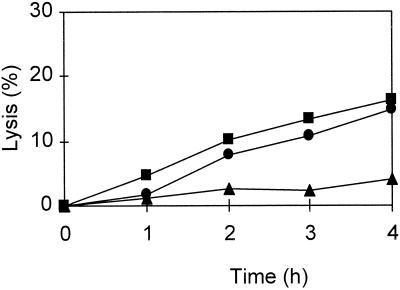
The observation that loss of viability was not concurrent with cell lysis suggested that the latter phenomenon was not directly caused by the bacteriocin but, likely, was the result of activities of other enzymes such as cell wall-degrading enzymes. The involvement of the autolytic enzymes in cell lysis was studied by adding autolysin activity inhibitors. The results showed that 1 mM Co2+ (Fig. 2) and 0.40 mg of SDS per ml (Fig. 3) strongly reduced the lytic response of L. lactis IFPL359 to the bacteriocin. Results of previous experiments showed that autolysis of lactococci is severely reduced upon the addition of Co2+ (35). No reduction of lysis was observed when these components were added to a mixture of L. rhamnosus JCL1211 cells and the bacteriocin. In this case, a 50% reduction of lysis was observed during the incubation with bacteriocin (500 AU/ml) in the presence of 0.04 mg of cardiolipin per ml.
FIG. 2.
Lysis during incubation of logarithmic-phase cells of L. lactis IFPL359 suspended in 20 mM sodium phosphate buffer (pH 6.8) (●), with 500 AU of bacteriocin from L. lactis IFPL105 per ml (■), and with 1 mM Co2+ plus bacteriocin (500 AU/ml) (▴).
FIG. 3.
Lysis during incubation of logarithmic-phase cells of L. lactis IFPL359 in M-17 broth with 500 AU of bacteriocin from L. lactis IFPL105 per ml (●) and after addition of cardiolipin (0.04 mg/ml) (■) or SDS (0.40 mg/ml) (▴).
Detection of the cell wall hydrolytic activities.
Results of the analysis by renaturing SDS-PAGE of the cell wall hydrolytic activities present in the cell and supernatant fractions of all lactococcal strains showed a banding pattern similar to that of AcmA (6). No other activities could be detected (results not shown). Hybridization experiments using a PCR probe directed against acmA showed that the gene was present in all lactococcal strains. The cell wall hydrolytic activity patterns obtained for the three Lactobacillus strains were all different. A clearing band of 110 kDa obtained for the L. plantarum strain used was of the same size as that obtained for several strains of this species (25). The bands present in the samples of L. rhamnosus and L. casei were 35 and 70 kDa, respectively (data not shown).
Cell wall hydrolytic activity of the strains in the presence of the bacteriocin from L. lactis IFPL105 was analyzed spectrophotometrically and by renaturing SDS-PAGE using autoclaved M. lysodeikticus cells or autoclaved cells of the tested strain as a substrate for L. lactis and Lactobacillus strains, respectively. Total autolysin activity did not increase when the bacteriocin was present in the assays (results not shown).
AcmA is responsible for bacteriolysis of lactococci.
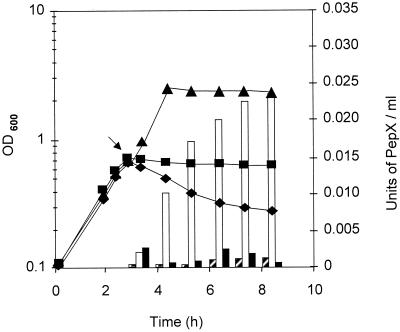
The involvement of the autolysin AcmA in cell lysis after loss of viability was investigated by comparing the effects of the bacteriocin on lysis of L. lactis MG1363 and its mutant L. lactis MG1363acmAΔ1, which cannot produce autolysins. The addition of a crude extract of autoclaved bacteriocin of L. lactis IFPL105 (300 UA/ml) to logarithmic-phase cells of L. lactis MG1363 growing in broth resulted in a sharp decrease in OD (56% of lysis after 5 h of incubation with the bacteriocin [Fig. 4]). This lysis was concomitant with the release of intracellular material (0.022 U of PepX activity per ml). No lysis or release of PepX was observed after addition of a crude extract from the supernatant of L. lactis IFPL1053 (Bac−) or after addition of the autoclaved bacteriocin preparation to logarithmic-phase cells of L. lactis MG1363acmAΔ1 (Fig. 4). However, cell counts carried out over the same period resulted in a reduction of cell viability to 103 CFU/ml for both Lactococcus strains (MG1363 and MG1363acmAΔ1) 3 h after addition of the bacteriocin.
FIG. 4.
Evolution of OD600 (lines) and release of PepX (bars) during growth at 30°C of L. lactis MG1363 in M-17 broth (▴; striped bars) and during incubation with 300 AU of the bacteriocin of L. lactis IFPL105 per ml of logarithmic-phase cells of L. lactis MG1363 (⧫; open bars) and L. lactis MG1363acmAΔ1 (■; solid bars). The arrow indicates point of bacteriocin addition.
The bacteriocin of L. lactis IFPL105 does not activate AcmA activity.
To study whether the effect of the bacteriocin on cell lysis was caused by direct activation of the autolysin AcmA, autoclaved bacteriocin (300 AU/ml) was added to native cell walls of L. lactis MG1363 suspended in a supernatant of an overnight culture of L. lactis MG1363acmAΔ1. The results of cell wall degradation, as determined by decrease in OD600 of the mixture after 4 h of incubation at 37°C, are shown in Table 2. Hydrolysis of L. lactis MG1363 cell walls was not influenced by the presence of the bacteriocin, nor was activation of AcmA found when native cell walls of L. lactis MG1363acmAΔ1, suspended in L. lactis MG1363 supernatants containing extracellular AcmA, were incubated with the bacteriocin (Table 2). No significant OD600 reduction was observed after incubation of native cell walls of L. lactis MG1363acmAΔ1 in a supernatant without AcmA activity.
TABLE 2.
Degradation of L. lactis MG1363 and L. lactis MG1363acmAΔ1 cell walls suspended in an overnight culture supernatant containing or lacking AcmA activity after 4 h of incubation at 37°C with or without bacteriocin
| Cell walls from: | AcmA (supernatant) | Cell wall degradation (%; mean ± SE)
|
|
|---|---|---|---|
| Control | Bacteriocin (300 AU/ml) | ||
| L. lactis MG1363 | − | 21.9 ± 0.1 | 23.1 ± 0.9 |
| L. lactis MG1363acmAΔ1 | + | 22.8 ± 2.1 | 20.9 ± 0.5 |
| − | 3.8 ± 0.8 | 3.8 ± 1.3 | |
AcmA is also responsible for bacteriolysis observed with other lactococcal bacteriocins.
The effect of the bacteriocin of L. lactis IFPL105 on lysis of sensitive lactococcal cells was compared with that of nisin and the mixture of lactococcins A, B, and M (Table 3). Cell lysis and the subsequent release of PepX was observed with all three bacteriocin preparations on exponential-phase cells of L. lactis MG1363. Lysis was absent when the bacteriocin of L. lactis IFPL105 or nisin was added to the acmA deletion mutant, while a very small reduction of the OD600 was detected when the lactococcin mixture had been added. Also, the release of PepX from the AcmA-negative strain was much less than that obtained for the wild-type strain for all bacteriocins tested. These results indicate that the autolysin is also required for lysis of L. lactis MG1363 cells sensitive to bacteriocins other than the bacteriocin from L. lactis IFPL105.
TABLE 3.
Cell lysis and PepX activity of suspensions of exponentially growing L. lactis MG1363 or MG1363acmAΔ1 after 3 h of incubation at 30°C in the presence of bacteriocin
| Strain | Bacteriocin added (300 AU/ml) | Lysis (%)a | PepX (U/ml)b |
|---|---|---|---|
| L. lactis MG1363 | Nonec | 1 | 0.001 |
| Bacteriocin of L. lactis IFPL105 | 17.5 | 0.007 | |
| Lactococcins A, B, and M | 12 | 0.006 | |
| Nisin | 5 | 0.004 | |
| L. lactis MG1363acmAΔ1 | None | 0 | 0.001 |
| Bacteriocin of L. lactis IFPL105 | 0 | 0.001 | |
| Lactococcins A, B, and M | 2.5 | 0.002 | |
| Nisin | 0 | 0.001 |
Estimated by reduction of OD600.
1 U = 1 μmol of p-nitroaniline released/min/ml.
Cell suspensions in the supernatant fraction of an overnight culture of L. lactis MG1363acmAΔ1 (OD600 of 2) filtered through a 0.22-μm-pore-size filter, used as a control.
DISCUSSION
Addition of the bacteriocin produced by L. lactis IFPL105, nisin, or a mixture of the lactococcins A, B, and M to logarithmic-phase cultures causes effective lysis of L. lactis MG1363 cells but not in its autolysin-negative derivative L. lactis MG1363acmAΔ1 (Fig. 4 and Table 3). Apparently, the bacteriocins themselves are not capable of lysing lactococcal cells. The results presented here clearly demonstrate that cell lysis induced by addition of lactococcal bacteriocins to bacteriocin-sensitive strains is, in fact, caused by the autolytic system of these strains.
The fact that cell lysis caused by addition of the bacteriocin of L. lactis IFPL105 to Lactobacillus and Lactococcus strains was not concurrent with loss of viability (Fig. 1 and Table 1) suggests that it involves two steps. First, viability is lost due to insertion of the bacteriocin into the membrane of the sensitive cell and depletion of cellular energy (38, 41, 42). Second, a gross imbalance between cell wall buildup and degradation caused in L. lactis by AcmA leads to the observed cell lysis. Autolysis as a secondary effect of bacteriocin action has been suggested previously, but the causative agent has never been definitely pinpointed. Some delay between the decrease in cell viability and cell lysis has been observed in the mechanism of action of other bacteriocins (3, 30). Morgan et al. (30) showed that more than 99% of the cells of a lactococcal culture were killed within 10 min upon treatment with a mixture of lactococcins A, B, and M. Ten hours after addition, only 57% of the total amount of the activity of an intracellular marker was released, indicating that bacteriolysis follows loss of viability.
Similarity in the autolytic activities present in the cell and supernatant fraction of the various lactococcal strains investigated was not consistent with their lytic responses to the bacteriocin, which differed considerably. One possible explanation for this observation could be that the cell walls of the different strains have different compositions. The difference in the lytic response of the Lactobacillus strains might also be the result of the expression of different cell wall hydrolytic activities. This could also explain the different effects of the autolysin inhibitors used on L. lactis and L. rhamnosus cells.
Topological regulation of autolytic enzymes by the electrochemical potential of the cell membrane, by cell wall lipoteichoic acids, or by extracellular proteinases has been shown for several species of gram-positive bacteria (4, 8, 13, 19, 20). Incubation of the bacteriocin of L. lactis IFPL105 with native cell wall fragments of L. lactis MG1363 and its AcmA-defective mutant had no effect on lysis of these cell walls, indicating that the bacteriocin does not activate AcmA, either bound to the cell wall or in supernatants. In light of these results, direct activation of AcmA, as postulated for the autolysin N-acetylmuramoyl-l-alanine amidase of S. simulans by its replacement from the teichoic acids by cationic bacteriocins (4), does not occur.
Bacteriocins are capable of causing lesions in the cytoplasmic membrane of sensitive cells based on their small size, high hydrophobicity, and hydrophobic regions predicted to form amphipathic α helices (16, 17, 33). This originates dissipation of the proton motive force (5, 29, 42), which has a direct effect on autolysis (19, 20). This effect is shared with other substances such as holin protein of bacteriophages (11, 43). The differences in decrease in OD and in release of the intracellular PepX activity upon addition of the different bacteriocins used might be caused by the difference in effectiveness of pore formation.
Apart from providing fundamental insights into bacteriocin action, the result of this work is also of practical interest. Since the bacteriocin producer L. lactis IFPL105 can be used as an adjunct in cheese manufacture (27) and the bacteriocin it produces has a broad spectrum of action, this strain and bacteriocin have both technological and preservative potentials.
ACKNOWLEDGMENTS
This work was supported by research projects ALI 97-0737 (Spanish Commission for Science and Technology) and FAIR CT97-3173. The work was partially supported by contract PL98-4396 of the FAIR project of the European Community.
REFERENCES
- 1.Abee T. Pore-forming bacteriocins of gram-positive bacteria and self-protection mechanisms of producer organisms. FEMS Microbiol Lett. 1995;129:1–10. doi: 10.1016/0378-1097(95)00137-T. [DOI] [PubMed] [Google Scholar]
- 2.Anderson D G, McKay L L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983;46:549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhunia A K, Hohnson B C, Ray B, Kalchayanand N. Mode of action of pediocin AcH from Pediococcus acidilactici H on sensitive bacterial strains. J Appl Bacteriol. 1991;70:25–33. [Google Scholar]
- 4.Bierbaum G, Sahl H-G. Autolytic system of Staphylococcus simulans 22: influence of cationic peptides on activity of N-acetylmuramoyl-l-alanine amidase. J Bacteriol. 1987;169:5452–5458. doi: 10.1128/jb.169.12.5452-5458.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruno M E C, Montville T J. Common mechanistic action of bacteriocins from lactic acid bacteria. Appl Environ Microbiol. 1993;59:3003–3010. doi: 10.1128/aem.59.9.3003-3010.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buist G, Kok J, Leenhouts K J, Dabrowska M, Venema G, Haandrikman A J. Molecular cloning and nucleotide sequence of the gene encoding the major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell separation. J Bacteriol. 1995;177:1554–1563. doi: 10.1128/jb.177.6.1554-1563.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buist G, Karsens H, Nauta A, van Sinderen D, Venema G, Kok J. Autolysis of Lactococcus lactis caused by induced overproduction of its major autolysin, AcmA. Appl Environ Microbiol. 1997;63:2772–2728. doi: 10.1128/aem.63.7.2722-2728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buist G, Venema G, Kok J. Autolysis of Lactococcus lactis is influenced by proteolysis. J Bacteriol. 1998;180:5947–5953. doi: 10.1128/jb.180.22.5947-5953.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casla D, Requena T, Gómez R. Antimicrobial activity of lactic acid bacteria isolated from goat's milk and artisanal cheeses: characteristics of a bacteriocin produced by Lactobacillus curvatus IFPL105. J Appl Bacteriol. 1996;81:35–41. doi: 10.1111/j.1365-2672.1996.tb03279.x. [DOI] [PubMed] [Google Scholar]
- 10.Daeschel M A. Applications and interactions of bacteriocins from lactic acid bacteria in foods and beverages. In: Hoover D, Steenson L, editors. Bacteriocins of lactic acid bacteria. New York, N.Y: Academic Press; 1993. pp. 63–91. [Google Scholar]
- 11.Díaz E, Munthali M, Lünsdorf H, Höltje J-V, Timmis K N. The two-step lysis system of pneumococcal bacteriophage EJ-1 is functional in Gram-negative bacteria: triggering of the major pneumococcal autolysin in Escherichia coli. Mol Microbiol. 1996;19:667–681. doi: 10.1046/j.1365-2958.1996.399929.x. [DOI] [PubMed] [Google Scholar]
- 12.Gasson M J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giudicelli S, Tomasz A. Attachment of pneumococcal autolysin to wall teichoic acids, an essential step in enzymatic wall degradation. J Bacteriol. 1984;158:1188–1190. doi: 10.1128/jb.158.3.1188-1190.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González B, Arca P, Mayo B, Suarez J E. Detection, purification, and partial characterization of plantaricin C, a bacteriocin produced by a Lactobacillus plantarum strain of dairy origin. Appl Environ Microbiol. 1994;60:2158–2163. doi: 10.1128/aem.60.6.2158-2163.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.González B, Glaasker E, Kunji E R S, Driessen A J M, Suárez J E, Konings W N. Bactericidal mode of action of plantaricin C. Appl Environ Microbiol. 1996;62:2701–2709. doi: 10.1128/aem.62.8.2701-2709.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauge H H, Nissen-Meyer J, Nes I F, Eijsink V G H. Amphiphilic α-helices are important structural motifs in the α and β peptides that constitute the bacteriocin lactococcin G. Enhancement of helix formation upon α-β interaction. Eur J Biochem. 1998;251:565–572. doi: 10.1046/j.1432-1327.1998.2510565.x. [DOI] [PubMed] [Google Scholar]
- 17.Holo H, Nilssen O, Nes I F. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene. J Bacteriol. 1991;173:3879–3887. doi: 10.1128/jb.173.12.3879-3887.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jack R W, Tagg J R, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jolliffe L K, Doyle R J, Streips N. The energized membrane and cellular autolysis in Bacillus subtilis. Cell. 1981;25:753–763. doi: 10.1016/0092-8674(81)90183-5. [DOI] [PubMed] [Google Scholar]
- 20.Kemper M A, Urrutia M M, Beveridge T J, Koch A K, Doyle R J. Proton motive force may regulate cell wall-associated enzymes of Bacillus subtilis. J Bacteriol. 1993;175:5690–5696. doi: 10.1128/jb.175.17.5690-5696.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kok J, Holo H, van Belkum M J, Haandrikman A J, Nes I F. Non nisin bacteriocins in lactococci: biochemistry, genetics and mode of action. In: Hoover D, Steenson L, editors. Bacteriocins of lactic acid bacteria. New York, N.Y: Academic Press; 1993. pp. 121–150. [Google Scholar]
- 22.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Langsrud T, Landaas A, Castberg H B. Autolytic properties of different strains of group N streptococci. Milchwissenschaft. 1987;42:556–560. [Google Scholar]
- 24.Lortal S, Lemée R, Valence F. Autolysis of thermophilic lactobacilli and dairy propionibacteria: a review. Lait. 1997;77:133–150. [Google Scholar]
- 25.Lortal S, Valence F, Bizet C, Maubois J-L. Electrophoretic pattern of peptidoglycan hydrolases, a new tool for bacterial species identification: application to 10 Lactobacillus species. Res Microbiol. 1997;148:461–474. doi: 10.1016/S0923-2508(97)88344-1. [DOI] [PubMed] [Google Scholar]
- 26.Martínez-Cuesta M C, Peláez C, Juárez M, Requena T. Autolysis of Lactococcus lactis ssp. lactis and Lactobacillus casei ssp. casei. Cell lysis induced by a crude bacteriocin. Int J Food Microbiol. 1997;38:125–131. doi: 10.1016/s0168-1605(97)00099-8. [DOI] [PubMed] [Google Scholar]
- 27.Martínez-Cuesta M C, Fernández de Palencia P, Requena T, Peláez C. Enhancement of proteolysis by a Lactococcus lactis bacteriocin producer in a cheese model system. J Agric Food Chem. 1998;46:3863–3867. [Google Scholar]
- 28.Mendoza F, Maqueda M, Galvez A, Martinez-Bueno M, Valdivia E. Antilisterial activity of peptide AS-48 and study of changes induced in the cell envelope properties of an AS-48-adapted strain of Listeria monocytogenes. Appl Environ Microbiol. 1999;65:618–625. doi: 10.1128/aem.65.2.618-625.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moll G, Hildeng-Hauge H, Nissen-Meyer J, Nes I F, Konings W N, Driessen A J M. Mechanistic properties of the two-component bacteriocin lactococcin G. J Bacteriol. 1998;180:96–99. doi: 10.1128/jb.180.1.96-99.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan S, Ross R P, Hill C. Bacteriolytic activity caused by the presence of a novel lactococcal plasmid encoding lactococcins A, B, and M. Appl Environ Microbiol. 1995;61:2995–3001. doi: 10.1128/aem.61.8.2995-3001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgan S, Ross R P, Hill C. Increasing starter cell lysis in Cheddar cheese using a bacteriocin-producing adjunct. J Dairy Sci. 1997;80:1–10. [Google Scholar]
- 32.Niskasaari K. Characteristics of the autolysis of variants of Lactococcus lactis subsp. cremoris. J Dairy Res. 1989;56:639–649. [Google Scholar]
- 33.Nissen-Meyer J, Holo H, Håvarstein L S, Sletten K, Nes I F. A novel lactococcal bacteriocin whose activity depends on the complementary action of two peptides. J Bacteriol. 1992;174:5686–5692. doi: 10.1128/jb.174.17.5686-5692.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nissen-Meyer J, Nes I F. Ribosomally synthesized antimicrobial peptides: their function, structure, biogenesis, and mechanism of action. Arch Microbiol. 1997;167:67–77. [PubMed] [Google Scholar]
- 35.Østlie H M, Vegarud G, Langsrud T. Autolysis of lactococci: detection of lytic enzymes by polyacrylamide gel electrophoresis and characterization in buffer systems. Appl Environ Microbiol. 1995;61:3598–3603. doi: 10.1128/aem.61.10.3598-3603.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Potvin C, Leclerc D, Tremblay G, Asselin A, Bellemare G. Cloning, sequencing and expression of a Bacillus bacteriolytic enzyme in Escherichia coli. Mol Gen Genet. 1988;214:241–248. doi: 10.1007/BF00337717. [DOI] [PubMed] [Google Scholar]
- 37.Pucci M J, Vedamuthu E R, Kunka B S, Vandenergh P A. Inhibition of Listeria monocytogenes by using bacteriocin PA-1 produced by Pediococcus acidilactici PAC 1.0. Appl Environ Microbiol. 1988;54:2349–2353. doi: 10.1128/aem.54.10.2349-2353.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruhr E, Sahl H G. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob Agents Chemother. 1985;27:841–845. doi: 10.1128/aac.27.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shockman G D, Höltje J-V. Microbial peptidoglycan (murein) hydrolases. Comprehensive biochemistry. In: Ghuysen J M, Hakenbeck R, editors. Bacterial cell wall. London, England: Elsevier; 1994. pp. 131–166. [Google Scholar]
- 40.Valence F, Lortal S. Zymogram and preliminary characterization of Lactobacillus helveticus autolysins. Appl Environ Microbiol. 1995;61:3391–3399. doi: 10.1128/aem.61.9.3391-3399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Belkum M J, Kok J, Venema G, Holo H, Nes I F, Konings W N, Abee T. The bacteriocin lactococcin A specifically increases permeability of lactococcal cytoplasmic membranes in a voltage-independent, protein-mediated manner. J Bacteriol. 1991;173:7934–7941. doi: 10.1128/jb.173.24.7934-7941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venema K, Abee T, Haandrikman A J, Leenhouts K J, Kok J, Konings W N, Venema G. Mode of action of lactococcin B, a thiol-activated bacteriocin from Lactococcus lactis. Appl Environ Microbiol. 1993;59:1041–1048. doi: 10.1128/aem.59.4.1041-1048.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walderich B, Ursinus-Wössner A, van Duin J, Höltje J-V. Induction of the autolytic system of Escherichia coli by specific insertion of bacteriophage MS2 lysis protein into the bacterial cell envelope. J Bacteriol. 1988;170:5027–5033. doi: 10.1128/jb.170.11.5027-5033.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]