Abstract
Covid-19 vaccination is recommended in allogeneic transplant recipients, but many questions remain regarding its efficacy. Here we studied serologic responses in 145 patients who had undergone allogeneic transplantation using in vivo T-cell depletion. Median age was 57 (range 21-79) at transplantation and 61 (range 24-80) at vaccination. Sixty-nine percent were Caucasian. One third each received transplants from HLA-identical related (MRD), adult unrelated (MUD), or haploidentical-cord blood donors. Graft-versus-host disease (GVHD) prophylaxis involved in-vivo T-cell depletion using alemtuzumab for MRD or MUD transplants and anti-thymocyte globulin for haplo-cord transplants. Patients were vaccinated between January 2021 and January 2022, an average of 31 months (range 3-111 months) after transplantation. Sixty-one percent received the BNT162b2 (bioNtech/Pfizer) vaccine, 34% received mRNA-1273 (Moderna), and 5% received JNJ-78436735 (Johnson & Johnson). After the initial vaccinations (2 doses for BNT162b2 and mRNA-1273, 1 dose for JNJ-7843673), 124 of the 145 (85%) patients had a detectable SARS-CoV-2 spike protein (S) antibody, and 21 (15%) did not respond. Ninety-nine (68%) had high-level responses (≥100 binding antibody units [BAU]/mL)m and 25 (17%) had a low-level response (<100 BAU/mL). In multivariable analysis, lymphocyte count less than 1 × 109/ mL, having chronic GVHD, and being vaccinated in the first year after transplantation emerged as independent predictors for poor response. Neither donor source nor prior exposure to rituximab was predictive of antibody response. SARS-CoV-2 vaccination induced generally high response rates in recipients of allogeneic transplants including recipients of umbilical cord blood transplants and after in-vivo T cell depletion. Responses are less robust in those vaccinated in the first year after transplantation, those with low lymphocyte counts, and those with chronic GVHD.
Key Words: Covid-19, Stem cell transplantation, Vaccination, Umbilical Cord Blood, T-cell Depletion
Graphical abstract
New York City was the epicenter of a severe outbreak of coronavirus 19 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) starting in March 2020 [1]. The initial death rates among patients with hematological malignancies were high 2, 3, 4. Although vaccination was rapidly developed, immunosuppressed patients were excluded from the initial studies and questions regarding efficacy and tolerance in these populations are only now being addressed. Responses in patients with hematological malignancies are particularly poor among patients with chronic lymphocytic leukemia and myeloma, and further weakened on exposure to daratumumab and to B cell–depleting agents [5]. Similarly, CART recipients with profound B cell suppression have poor responses to vaccination [6], as do solid organ transplant patients on lifelong immunosuppression [7]. The issue is somewhat different for stem cell transplant recipients who, after a period of profound immunosuppression, gradually regain immunocompetence.
Recent reports on COVID-19 vaccination in allogeneic transplant recipients have shown encouraging results 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30. But nearly all published data pertain to patients undergoing stem cell transplantation from adult donors. Conditioning regimens or graft versus host disease (GVHD) prophylaxis are usually not detailed. At New York Presbyterian/Weill Cornell Medical Center, we use alemtuzumab for GVHD prophylaxis in HLA-identical related and unrelated donor transplants 31, 32, 33, 34, 35, 36. Alemtuzumab is a pan-lymphocyte antibody that eliminates both T and B cells. Its use is associated with reduced acute and chronic GVHD [37,38], but an increased risk of cytomegalovirus reactivation and possibly other viral infections 39, 40, 41, 42, 43. To our knowledge, the impact of alemtuzumab on COVID-19 vaccination response has not been studied. For those lacking HLA-identical donors, we have used haplo-cord transplants in which definitive hematopoiesis is established by the cord blood graft 44, 45, 46, 47, 48, 49. Engraftment is rapid, with GVHD and relapse-free survival superior to either haplo-transplantation with post-transplantation cyclophosphamide and double umbilical cord blood transplantation 47, 48, 49, 50. Others have shown increased graft versus leukemia effects after cord blood transplantation [51,52], but concerns have been raised about delayed immune reconstitution after cord blood transplantation and over the use of anti-thymocyte globulin, which further delays T-cell reconstitution but which is required for the haplo-cord procedure 53, 54, 55. Here we report serologic responses to COVID-19 vaccination in our patient population and identify predictors of response.
METHODS
We evaluated all subjects who had undergone allogeneic transplant at New York Presbyterian Hospital/ Weill Cornell Medical College between 2012 and December 2020. A total of 804 allogeneic transplantations were performed. Among the transplantation survivors, we identified 145 patients who had received an initial COVID-19 vaccine series (2 doses of mRNA vaccine or 1 dose of J&J vaccine) between January and December 2021 and who had a serologic assessment of SARS-CoV-2 spike protein (S) antibodies after their vaccination. Patients with a clinical history of COVID-19 infection before transplantation or before vaccination were excluded.
The SARS-CoV-2 S protein antibody response to vaccination was assessed by enzyme-linked immunosorbent assay using methodology previously described (Roche Eclecsys) [56]. It is a sensitive marker of response to vaccination or infection and correlates well with virus neutralization assays [57,58]. Additionally, we determined whether any patients had serum antibodies to the SARS-CoV-2 nucleocapsid (N) protein, a marker for prior infection that is not elicited by vaccination.
In our laboratory, SARS-CoV-2 S antibody is defined as positive if > 0.8 Binding antibody units (BAU)/mL are detected, and the highest reported value is >250 BAU/mL [58,59]. Based on recently reported estimates of optimally protective levels of antibodies, we further classified those with <100 BAU/mL as “low responders” and those with ≥ 100 BAU/mL as “high responders” [60,61]. Further dilution of samples with levels >250 BAU/mL was not attempted. SARS-CoV-2 N antibody is defined as positive for >1.0 BAU/mL.
Statistics
Patients’ characteristics and clinical endpoints were summarized as median with range for a continuous variable and frequency with proportion for a categorical variable. The primary outcome was antibody response after initial vaccination, and it was categorized as a 3-level ordinal endpoint (high/low/negative). The bivariate associations with this endpoint were tested by Kruskal-Wallis rank sum test for a continuous variable and chi-squared test or Fisher's exact test for a categorical variable. To assess the association between clinical characteristics and primary outcome, an ordinal logistic regression was used.
RESULTS
Patient characteristics are summarized in Table 1 . Median age was 57 (range 21-79) at the time of transplantation and 61 (range 24-80) at the time of vaccination. Sixty-nine percent were Caucasian. One third of transplant recipients underwent transplant from HLA-identical related, adult unrelated donors or haplo-cord donors. Forty-three (29%) received conditioning with fludarabine and melphalan [62], and another 56% received fludarabine and melphalan with low dose total body irradiation (usually 400 cG) [63]. Thirteen (9%) received myeloablative regimens, and 4 received other non-myeloablative regimens. One hundred ten (76%) transplants were for patients with myeloid malignancies (acute myelogenous leukemia, myelodysplastic syndrome, and myeloproliferative disorders). There were also 14 (9%) patients with acute lymphoblastic leukemia, 17 (12%) with lymphoma, 3 (2%) with aplastic anemia, and 1 with sickle cell disease. At the time of initial vaccination, 2% had acute GVHD, and 15% had chronic GVHD, most of whom were receiving treatment. In addition, 18% of patients were receiving treatment directed at their underlying disease (e.g., low-dose decitabine, FLT3 inhibitor, IDH1, or IDH2 inhibitor) to prevent recurrence or treat minimal residual disease/early relapse.
Table 1.
Patient Characteristics
| SARS-Cov-2 S antibody Response |
P Value | ||||
|---|---|---|---|---|---|
| Overall | High | Low | Negative | ||
| N | 145 | 99 | 25 | 21 | |
| Age at vaccination, median (range) | 61 (24, 80) | 59 (24, 79) | 61 (24, 75) | 66 (24, 80) | .039 |
| Gender | .29 | ||||
| Female | 69 (48%) | 51 (52%) | 11 (44%) | 7 (33%) | |
| Male | 76 (52%) | 48 (48%) | 14 (56%) | 14 (67%) | |
| Race | .052 | ||||
| Caucasian, Non-Hispanic | 100 (69%) | 62 (63%) | 21 (84%) | 17 (81%) | |
| Hispanic or non-Caucasian | 45 (31%) | 37 (37%) | 4 (16%) | 4 (19%) | |
| Underlying disease | >.99 | ||||
| ALL | 14 (9.7%) | 9 (9.1%) | 3 (12%) | 2 (9.5%) | |
| AML | 69 (48%) | 49 (49%) | 11 (44%) | 9 (43%) | |
| Lymphoma | 17 (12%) | 11 (11%) | 3 (12%) | 3 (14%) | |
| MDS and MPD | 41 (28%) | 26 (26%) | 8 (32%) | 7 (33%) | |
| Severe aplastic anemia | 3 (2.1%) | 3 (3.0%) | 0 (0%) | 0 (0%) | |
| Sickle cell disease | 1 (0.7%) | 1 (1.0%) | 0 (0%) | 0 (0%) | |
| Type of Transplant | .13 | ||||
| MRD | 47 (32%) | 35 (35%) | 8 (32%) | 4 (19%) | |
| MUD | 53 (37%) | 30 (30%) | 13 (52%) | 10 (48%) | |
| Haplo/Cord | 45 (31%) | 34 (34%) | 4 (16%) | 7 (33%) | |
| Median Days from First Vaccine Dose to initial SARS-CoV-2 S antibody, median (range) | 123 (20, 400) | 129 (20, 400) | 114 (61, 283) | 128 (65, 254) | .75 |
| Conditioning regimen | .06 | ||||
| Myeloablative | 13 (9.0%) | 9 (9.1%) | 2 (8.0%) | 2 (9.5%) | |
| FluMel | 42 (29%) | 36 (36%) | 2 (8.0%) | 4 (19%) | |
| FluMelTBI | 86 (59%) | 51 (52%) | 20 (80%) | 15 (71%) | |
| Non-myeloablative | 4 (2.8%) | 3 (3.0%) | 1 (4.0%) | 0 (0%) | |
| SARS-CoV-2 N antibody | .38 | ||||
| Negative | 84 (82%) | 53 (80%) | 16 (76%) | 15 (94%) | |
| Positive | 19 (18%) | 13 (20%) | 5 (24%) | 1 (6.2%) | |
| Missing | 42 | 33 | 4 | 5 | |
| Lymphocytes, median (range) | 1.40 (0.04, 4.96) | 1.73 (0.10, 4.96) | 0.70 (0.04, 2.13) | 0.70 (0.10, 3.20) | <.001 |
| Lymphocytes | <.001 | ||||
| < 1 × 106/mL | 47 (32%) | 17 (17%) | 16 (64%) | 14 (67%) | |
| ≥ 1 × 106/mL | 98 (68%) | 82 (83%) | 9 (36%) | 7 (33%) | |
| Months from transplant to first vaccine, median (range) | 31 (3, 111) | 38 (3, 110) | 13 (4, 71) | 11 (3, 111) | <.001 |
| Type of vaccine | .61 | ||||
| JNJ-78436735 | 8 (5.5%) | 6 (6.1%) | 1 (4.0%) | 1 (4.8%) | |
| MRNA-1273 | 48 (33%) | 36 (36%) | 8 (32%) | 4 (19%) | |
| BNT162b2 | 89 (61%) | 57 (58%) | 16 (64%) | 16 (76%) | |
| Chemotherapy | .11 | ||||
| No | 130 (90%) | 91 (92%) | 23 (92%) | 16 (76%) | |
| Yes | 15 (10%) | 8 (8.1%) | 2 (8.0%) | 5 (24%) | |
| Maintenance treatment | .066 | ||||
| No | 129 (90%) | 92 (94%) | 21 (84%) | 16 (80%) | |
| Yes | 14 (9.8%) | 6 (6.1%) | 4 (16%) | 4 (20%) | |
| Acute GVHD | .54 | ||||
| No | 143 (99%) | 98 (99%) | 24 (96%) | 21 (100%) | |
| Yes | 2 (1.4%) | 1 (1.0%) | 1 (4.0%) | 0 (0%) | |
| Chronic GVHD | <.001 | ||||
| No | 123 (85%) | 94 (95%) | 18 (72%) | 11 (52%) | |
| Yes | 22 (15%) | 5 (5.1%) | 7 (28%) | 10 (48%) | |
| GVHD Treatment | <.001 | ||||
| No | 126 (87%) | 93 (94%) | 20 (80%) | 13 (62%) | |
| Yes | 17 (12%) | 5 (5.1%) | 5 (20%) | 7 (33%) | |
| Covid after vaccination | .055 | ||||
| No | 135 (93%) | 95 (96%) | 21 (84%) | 19 (90%) | |
| Yes | 10 (6.9%) | 4 (4.0%) | 4 (16%) | 2 (9.5%) | |
| Remission | .048 | ||||
| No | 17 (12%) | 9 (9.1%) | 2 (8.3%) | 6 (29%) | |
| Yes | 127 (88%) | 90 (91%) | 22 (92%) | 15 (71%) | |
| Pretransplantation rituximab | .002 | ||||
| No | 87 (60%) | 69 (70%) | 9 (36%) | 9 (43%) | |
| Yes | 58 (40%) | 30 (30%) | 16 (64%) | 12 (57%) | |
| Time from transplantation to first vaccine | <.001 | ||||
| >12 months | 117 (81%) | 94 (95%) | 13 (52%) | 10 (48%) | |
| 6-12 months | 14 (9%) | 3 (20%) | 6 (43%) | 5 (37%) | |
| 3-6 months | 14 (9%) | 2 (14%) | 6 (43%) | 6 (43%) | |
TBI indicates total body irradiation; ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; MDS, myelodysplastic syndrome.
Patients in this cohort were vaccinated between January 2021 and January 2022. Sixty-one percent received the BNT162b2 (bioNtech/Pfizer, New York, NY) vaccine, 34% received mRNA-1273 (Moderna, Cambridge, MA), and 5% received JNJ-78436735 (Johnson & Johnson, New Brunswick, NJ). Initial measurement of serologic response was done after the second dose of vaccine. Eighty-six patients (59%) also had received a third dose either as a late boost or as part of the initial vaccination set. Three patients had received a fourth dose.
The median time elapsed from transplant was 31 months (range 3-111) at the time of initial vaccination and the median lymphocyte count was 0.80 × 106/mL (range 0.04 -4.96). One hundred four patients had SARS-Cov-2 N antibody tests before vaccination, of whom 20 (19%) had detectable antibody, suggesting that they may have had previous asymptomatic infection, although passive acquisition through plasma or immunoglobulin infusion cannot be excluded in some.
Response and Predictors of Response after initial vaccination
After the initial vaccination series (2 doses for BNT162b2 and mRNA-1273, 1 dose for JNJ-78436735) 124 of the 145 (85%) patients had a detectable SARS-Cov-2 S antibody. Ninety-nine (68%) had high-level responses, and 25 (17%) had low-level responses. Twenty-one (15%) did not respond.
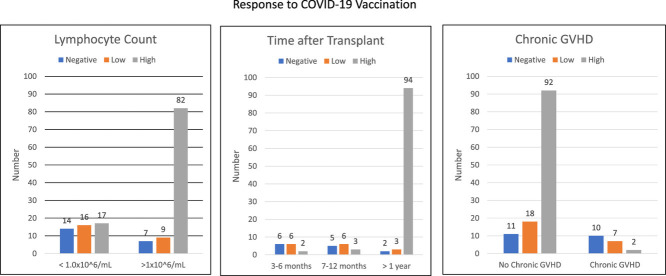
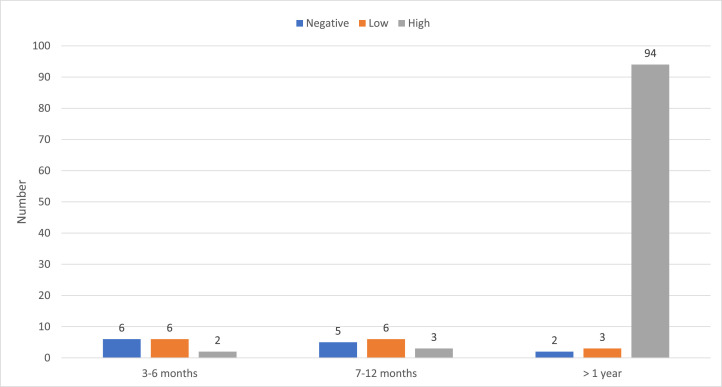
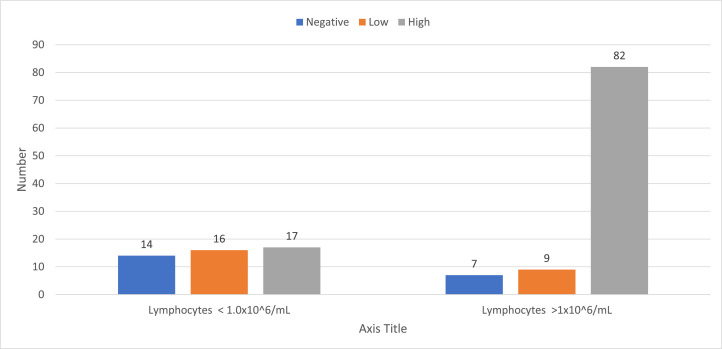
In univariable analysis (Table 1) increased time elapsed since transplantation and a higher absolute lymphocyte count at time of vaccination were associated with serologic response. Among 14 patients vaccinated in the first 3 to 6 months after transplantation, 2 (14%) had a high-level response, 6 (43%) had a low-level response, and 6 (43%) did not respond. Among 14 patients vaccinated between 6 months and 1 year after transplantation, 3 (20%) had a high- level response, 6 (43%) had a low-level response, and 5 (37%) did not respond. Among 117 patients vaccinated more than 1 year after transplantation, 90 (81%) had a high-level response, 13 (11%) had a low-level response, and 10 (8%) did not respond (Figure 1 ). Similarly, response was much lower among patients with lower lymphocyte counts. Among 56 patients with a lymphocyte count less than 1.0 × 109/mL, 25 (45%) had a high-level response, 17 (30%) had a low-level response, and 14 (25%) did not respond. Among 89 patients with a lymphocyte count greater than 1.0 × 109/mL, 73 (82%) had a high-level response, 9 (10%) had a low-level response, and 7 (8%) did not respond. Other predictors were having GHVD, being treated for GVHD, or receiving any post-transplantation disease-directed treatment. Having received pre-transplantation rituximab also was associated with decreased response in univariate analysis (P= .002), as was older age at the time of vaccination (P= .039). Donor type had no effect on response to vaccination.
Figure 2.
Time after transplantation and initial vaccine response.
Figure 1.
Lymphocyte count and initial vaccine response.
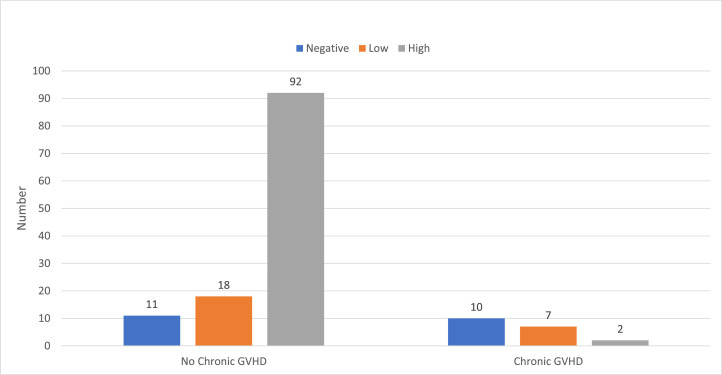
In multivariate analysis lymphocyte count equal to or greater than 1 × 106/mL (odds ratio 4.01 [1.63-9.92], P= .0024), not having chronic GVHD (odds ratio 5.6 [2.13-14.2], P= .0005) and being vaccinated more than 1 year after transplantation (odds ratio 3.85 [1.43-10.0], P=.0077) emerged as independent predictors for response to vaccination (Table 2 and Figures 1 to 3 ). Age or pretransplantation exposure to rituximab were no longer significant.
Table 2.
Predictors of SARS-Cov-2 S Antibody Response—Univariable and Multivariable Ordinal Logistic Regression Analysis
| Predictors | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Lymphocytes | <.0001 | .0024 | ||
| <1 | — | — | ||
| ≥1 | 8.08 (3.82-17.7) | 4.01 (1.63-9.92) | ||
| CGVHD | <.0001 | .0005 | ||
| Yes | — | — | ||
| No | 10 (4-25) | 5.6 (2.13-14.2) | ||
| Time from transplantation to vaccination | <.0001 | .0077 | ||
| 0-12 months | — | — | ||
| >12 months | 12.5 (5.3-25.0) | 3.85 (1.43-10.0) | ||
OR indicates odds ratio; CI, confidence interval.
Figure 3.
Chronic GVHD and initial vaccine response.
Response to third vaccination dose
Among 25 patients with low SARS-Cov-2 S antibody titers after initial vaccination, 19 received a third vaccine. SARS-Cov-2 S antibody titers were retested in 13 such patients and were high in 10 and remained low in 3. Among 21 nonresponders to initial vaccination, 18 received a third vaccine dose. Twelve developed positive response to the third vaccine (6 high-level and 6 low-level).
Only 6 patients (4%) remain without serologic response after completing both initial- and third-dose vaccines (Table 3 ). All had low lymphocyte counts, 5 were vaccinated in the first half year after transplantation, and one at 8 months. Six had chronic GVHD and were receiving treatment. Only 1 patient, a 79-year-old, almost 10 years after transplantation had no obvious reason other than age and low lymphocyte count for her failure to respond.
Table 3.
Nonresponders After Initial Vaccine and Third Vaccine Dose
| Gender + F:H: | Age at vaccination | General disease | Type of Donor | Conditioning regimen (READ ONLY) | Lymphocytes | Month Tx Vaccine | Type | CGVHD | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Female | 78 | AML | URD | Flu/Mel | 0.70 | 111 | BNT162b2 | No | In remission, no GVHD |
| Male | 51 | AML | URD | FluMelTBI | 0.20 | 5 | BNT162b2 | Yes | Chronic GVHD on Jakafi Tacro Belumosudil |
| Female | 67 | AML | HaploCord | FluMelTBI | 0.11 | 5 | BNT162b2 | Yes | Mild GVHD budesonide/beclo methasone – on maintenance decitabine |
| Female | 63 | MDS | MRD | FluMelTBI | 0.10 | 8 | BNT162b2 | Yes | Chronic GVHD ruxolitinib and low-dose steroids |
| Male | 77 | MF | URD | FluMelTBI | 0.12 | 5 | mRNA-1273 | Yes | Chronic GVHD on Jakafi Tacro Belumosudil |
| Female | 67 | MF | URD | FluMelTBI | 0.14 | 4 | mRNA-1273 | Yes | Second transplant -on eltrombopag for poor engraftment |
Subsequent Risk for infection (during delta and omicron)
We had the opportunity to follow the patients through the delta and omicron waves in New York City. One patient contracted the delta variant within a few days after the third dose of vaccine. Symptoms were mild and resolved without treatment in a few days. Since mid-December 2021, during the highly transmissible omicron wave, 8 patients were diagnosed with COVID-19 after vaccination. Seven were prior responders to vaccines (3 low responders, and 4 high responders). One was a nonresponder. None of the Covid-19 cases were severe—only upper respiratory tract infections were documented. Three patients did not receive any treatment, three patients were treated with Sotrovimab, and 2 were treated with 3 days of Remdesivir. Only 1 patient required hospitalization for hypoxia.
DISCUSSION
COVID-19 has a high rate of hospitalization and death in unvaccinated patients with hematological malignancies. Vaccination is recommended by all major organizations including ASH/ASTCT and the European Group for Blood and Marrow Transplantation, but data on their efficacy is only recently emerging [64,65]. Here we report outcomes of COVID-19 vaccination among adult patients receiving in vivo T-cell–depleted transplant and/or umbilical cord blood grafts—such transplants have low rates of GVHD but delayed immune reconstitution. Response rates to vaccination were encouraging, with 85% of patients having a serologic response after initial vaccination series and 68% having a high-level response. Responses were not dependent on graft source—CBU recipients had similar responses to adult donor transplant recipients. In univariable analysis, poor or absent response was associated with (1) time after transplantation, (2) low lymphocyte count, (3) having GVHD, (4) receiving treatment for GHVD, (5) receiving disease-directed treatment (e.g., low-dose decitabine, FLT3 inhibitors), (6) older age at time of vaccination, and (7) prior exposure to rituximab. In multivariable analysis, lymphocyte count less than 1 × 106/mL, chronic GVHD, and vaccination in the first year after transplantation were independent predictors of poor response.
These predictors are similar to those reported by others. Responses after allogeneic transplantation have previously been shown to be less robust compared to healthy volunteers [6]. As summarized in Table 4 , responses are consistently worse in those vaccinated within the first 6 months to 1 year after transplantation [8,9,14,16,18,22, 23, 24, 25,30,66]. Low lymphocyte counts [8,23,66], low B cell [22,24,25,28] or natural killer cell counts, low CD4 counts [28], low CD/CD8 ratio [24], or low immunoglobulin levels [12,28,29] are all associated with poor response. Worse responses are found in those with GVHD [9,10,23,30], those receiving immunosuppressants (particularly ibrutinib and ruxolitinib) [8,11,12,17,21,22,26,30,66], and those under active treatment for their underlying disease [8,66]. Other less frequently mentioned or examined predictors include recent exposure to rituximab [10,23], reduced-intensity conditioning [26], receiving anti-thymocyte globulin in conditioning [16,18], older recipient age [10,16,18,22], and male gender [15,24]. Having had a prior episode of COVID-19 may be a predictor of better response [14]. Many previous reports have limitations related to their mostly retrospective nature and often limited patient numbers. Nearly all reports to date are from adult donor grafts with mostly HLA-identical donors. But the overall picture that emerges is that vaccine responsiveness depends on a functioning immune system and absence of GVHD or its treatment.
Table 4.
Recent Reports on COVID Vaccination in Allo-Transplant
| Author | No. | Age | Donor Type | Conditioning |
% CGVHD | Time from Transplantation to Vaccination (mo) | Vaccine Type | RESP | Predictors of Poor Vaccine Response | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRD | URD | Haplo | UCB | MAC | RIC | BNT 162b2 | mRNA 1273 | Other | |||||||
| Beerlage [8] | 182 | 56 (21-80) | 36 | 56 | 9 | 0 | 51 | 39 (3-410) | 48 | 52 | 92% | Less than 1 year after transplantation, immunosuppressive therapy, lymphopenia, ongoing antitumor therapy | |||
| Bergman [9] | 87 | 74%<65 | 52 | 84% | Less than 1 year after transplantation, chronic GVHD | ||||||||||
| Canti [10] | 40 | 60 | 8 | 27 | 5 | 0 | 8 | 32 | 22% | 31 (5-51) | 40 | 86% | Rituxan, GVHD, older age | ||
| Chevalier [66] | 112 | 57 (20-75) | 26 | 51 | 35 | 0 | 83 | 26 | 51% | 20 (3-206) | 112 | 55% | Less than 2 years after transplantation, lymphopenia, immunosuppressive therapy, or chemotherapy | ||
| Chiarucci [11] | 12 | 50% | Cyclosporine | ||||||||||||
| Dhakal [12] | 71 | 64 (25-70) | 68% | Hypogammaglobulinemia, prednisone | |||||||||||
| Einarsdottir Blood Adv [13] | 50 | 54 (29-78) | 15 | 34 | 1 | 0 | 25 | 25 | 92 (7-340) | 76% | No predictors identified | ||||
| Huang [14] | 110 | 57 | 32 | 57 | 21 | 0 | 36 | 74 | 26% | 20 (3-420) | 94 | 16 | Less than 1 year after transplantation, pre-vaccination COVID, NOT chronic GVHD | ||
| Lindemann [15] | 117 | 59 (21-77) | NS | 68% | 30 (5-391_ | 111 | Male gender | ||||||||
| Maillard [16] | 687 | 59 (IQR 46-66) | 30 | 51 | 20 | 0 | 213 | 474 | 38% | 27 (IQR 14-56) | 660 | 78% | Less than 1 year after transplantation, immunosuppressive treatment, B-CD19 count <100/mm3, lymphocyte count <1000/mm3 | ||
| Majcherek [17] | 64 | 52 (20-68) | 7 | 52 | 5 | 0 | 21% | 23 (3-1112) | 63 | 87% | Treatment with calcineurin inhibitors |
||||
| Mamez [18] | 63 | 54 (18-78) | 13 | 28 | 22 | 1 | 14(3-150) | 17 | 63 | Age, time since transplantation, and ATG | |||||
| Maneikis [19] | 122 | 48 | 76 | 122 | Less than 6 months after transplantation, receiving ATG, age over 60 | ||||||||||
| Matkowska-Kojan [20] | 65 | 21 (18-31) | 126(36-324) | 65 | 96% | None | |||||||||
| Morsink [21] | 70 | 60 (24-76) | 10 | 51 | 9 | 0 | 49 | 21 | 28 (1-50) | 8 | 54 | 90% | Ruxolitinib, ibrutinib for GVHD | ||
| Pabst [22] | 167 | 60 | 40 (3-303) | 133 | 7 | 81% | Age, number of immunosuppressants (≥2), B cell counts, type of vaccine (mRNA better), and interval from vaccination | ||||||||
| Pinara [23] | 311 | 57 (18-80) | 127 | 102 | 76 | 6 | 133 | 178 | 26% | 98 (4-646) | 47 | 261 | 3 | 79% | Less than 1 year after transplantation, lymphocytes less than<1.0 × 106/mL, active GVHD vaccine, B-cell NHL |
| Ram [24] | 66 | 65 | 17 | 46 | 3 | 40 | 26 | 62% | 32 (3-263) | 65 | 75% | Less time after transplantation, lower CD19 counts, male gender; NOT: immune suppression or GVHD | |||
| Redjoul [25] | 88 | 60 (26-77) | 26 | 46 | 16 | 23 (3-213) | 88 | 78% | Lymphocytes <1.0 × 106/mL, Immunosuppressive therapy | ||||||
| Sherman [27] | 20 | 66 | 14 | 6 | 82% | ||||||||||
| Shem-tov [26] | 152 | 58 (22-82) | 62 | 84 | 6 | 21 | 131 | 44% | 38 (IQR24-75) | 152 | 78% | Immunosuppressive therapy, reduced-intensity conditioning | |||
| Tamari [28] | 149 | 66 | 37 (2-172) | 87% | Less time after transplantation, Low CD4 counts, Low CD19 counts, Low IgG level | ||||||||||
| Watanabe [29] | 25 | 55 (23-71) | 9 | 16 | 53 (5-137) | 25 | 76% | Low lymphocytes, steroids, Low IgG | |||||||
| Yeshurun [30] | 106 | 65 (23-80) | 39 | 58 | 3 | 69 | 37 | 75% | 41 (4-439) | 106 | 85% | Time BMT to vaccine <4.5 years, AGVHD CGVHD, immunosuppression | |||
Rituximab has been shown to have an adverse effect on vaccine responsiveness in lymphoma and chronic lymphocytic leukemia patients [5], and recent exposure to rituximab has also been found in some studies to be detrimental in the transplantation setting [10,23]. We and others have shown that pretransplantation treatment with rituximab is protective of Epstein-Barr virus (EBV)–post-transplantation lymphoproliferative disorder, and since early 2018 we have given 1 dose of rituximab before transplantation to prevent EBV reactivation in our haplo-cord recipients [67,68]. In late 2019, several months before the COVID pandemic affected New York, we extended the prophylactic administration of rituximab to related and unrelated donor transplant recipients. As such, all the most recent transplants and recipients of vaccination in the first year after transplantation were also recipients of pretransplantation rituximab, and we could not attribute any independent effect of pretransplantation rituximab on the efficacy of vaccination.
The general effects of a third vaccination dose in stem cell transplant recipients have been studied by only a few groups. Maillard et al. [16] found that 41% of those without a previous response mounted a detectable response after boost and response improved in 85% of those with a low response [69]. Redjoul et al. [69] offered a third vaccine within 4 weeks after the second dose to patients who had not sufficiently responded. Among 42 participants, the third dose increased the levels of SARS-CoV-2 antibodies. But only 20 (48%) reached the protective threshold. Le Bourgeois et al. [62] found that high level of antibodies were achieved in 81% of recipients of a third dose compared with 50% in recipients of 2 doses. Still, about 11% remained negative after 3 doses. Our data are similar with about two thirds of failures to initial vaccination responding to a third dose.
Our patients have been followed throughout the recent delta and omicron waves and several have contracted Covid during that time period. None have had more than a mild infection. Numbers are small, and additional mild cases might have been missed, but it is unlikely that we would have remained unaware of life-threatening cases. This suggests that the vaccine was generally effective.
In allogenic transplant patients, mRNA vaccinations have been usually well tolerated, but skin reactions, exacerbations of GVHD [24], cytopenias [24], and even graft rejection [24] have been occasionally reported. Other risks include myocarditis (particularly in young patients) and vaccine-induced thrombosis (mostly with J&J and Astra Zeneca Vaccines) [9,24,65]. Our retrospective analysis did not focus on side effects. The baseline incidence and severity of chronic GVHD is low in our patients, and we are aware of only one case of worsening GVHD after vaccination—in a patient who received donor lymphocyte infusion.
Our study has many limitations including its retrospective nature, the unplanned testing schedule with variable intervals between vaccination and testing, the absence of exact titer determination at levels above 250, the absence of lymphocyte subset analysis, and the lack of T-cell assays of response. Many of these limitations are inherent to clinical research during the pandemic and some relate to questions of assessment of vaccine response. Serologic responses are not the only measure of vaccine response and T-cell responses measured by Elispot can show discordant results [15,24,70]. Einarsdottir et al. [13] found deficient T-cell responses in patients with low-level serologic response. But Clémenceau et al. [71] regularly found T-cell responsiveness in the absence of serologic response. Others have shown that vaccine-induced SARS-Cov-2 S antibody levels in transplant patients and other patients with hematological malignancies are lower than those of healthy volunteers [5,6,23]. Vice-versa, emerging data suggest that thresholds levels of S-antibodies correlate well with protection [60,72]. The excellent clinical outcome of our patients, especially their rapid recovery after omicron or delta support the clinical relevance of S-antibody levels.
How and when to vaccinate transplant recipients is an increasingly complex issue. Most reported experience is with the mRNA vaccines (BNT 162b2 and mRNA-1273). A consensus is emerging that these vaccines are superior, with likely some advantage for mRNA-1273 over BNT 162b2—mostly because of superior protection against the delta variant [61]. The Centers for Disease Control and Prevention recommends administration of 3 doses followed by a booster, and this is supported by the observed increase in antibody titers [64]. Because response rates and levels are lower in the early months after transplantation, the European Group for Blood and Marrow Transplantation recommends adapting the timing of vaccination to the SARS-CoV-2 infection rate in the surrounding community: “If transmission in the surrounding community is well controlled, it would be logical to wait until six months after transplantation to initiate vaccination. If the transmission rate in the surrounding community is high, vaccination could be initiated at the earliest three months after HCT” [65].
In conclusion, mRNA vaccines provide reliable protection when given more than a year after transplantation regardless of donor source or type of GVHD prophylaxis. Those in the first year after transplantation remain at risk with inferior efficacy of vaccines as do those who are immunosuppressed by GVHD, and those with low lymphocyte counts. Specifically, our patients had response rates of close to 60% in the first six months after transplant, but only 20% were high level responses. Such figures justify early vaccination during high viral prevalence times but may warrant delay in vaccination during times of low prevalence. At the current time we recommend prophylactic administration of monoclonal antibodies (currently Evusheld [tixagevimab/cilgavimab]), as well as continued vigilance and early treatment. We usually delay vaccination until at least 6 months after transplantation. This recommendation can change depending on the local prevalence of COVID and the infectivity of variants. We also follow updated recommendations from the Centers for Disease Control and Prevention, which now include 3 doses followed by a booster dose for patients with a compromised immune system. Lastly, waning of response to COVID vaccine is emerging as an increasingly important issue and could not be addressed here [73].
References
- 1.Shah MA, Emlen MF, Shore T, et al. Hematology and oncology clinical care during the coronavirus disease 2019 pandemic. CA Cancer J Clin. 2020;70:349–354. doi: 10.3322/caac.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma A, Bhatt NS, St Martin A, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;8(3):e185–e193. doi: 10.1016/S2352-3026(20)30429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shahzad M, Chaudhary SG, Zafar MU, et al. Impact of COVID-19 in hematopoietic stem cell transplant recipients: a systematic review and meta-analysis. Transplant Infect Dis. 2022;24(2):e13792. doi: 10.1111/tid.13792. [DOI] [PubMed] [Google Scholar]
- 4.Bailey AJM, Kirkham AM, Monaghan M, et al. A portrait of SARS-CoV-2 infection in patients undergoing hematopoietic cell transplantation: a systematic review of the literature. Curr Oncol. 2022;29:337–349. doi: 10.3390/curroncol29010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jurgens EM, Ketas TJ, Zhao Z, et al. Serologic response to mRNA COVID -19 vaccination in lymphoma patients. Am J Hematol. 2021;96(11):E410–E413. doi: 10.1002/ajh.26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gagelmann N, Passamonti F, Wolschke C, et al.Antibody response after vaccination against SARS-CoV-2 in adults with haematological malignancies: a systematic review and meta-analysis [e-pub ahead of print December 16, 2021]. Haematologica. doi: 10.3324/haematol.2021.280163 [DOI] [PMC free article] [PubMed]
- 7.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beerlage A, Leuzinger K, Valore L, et al. Antibody response to mRNA SARS-CoV-2 vaccination in 182 patients after allogeneic hematopoietic cell transplantation. Transpl Infect Dis. 2022;24(3):e13828. doi: 10.1111/tid.13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergman P, Blennow O, Hansson L, et al. Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. eBioMed. 2021;74 doi: 10.1016/j.ebiom.2021.103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canti L, Humblet-Baron S, Desombere I, et al. Predictors of neutralizing antibody response to BNT162b2 vaccination in allogeneic hematopoietic stem cell transplant recipients. J Hematol Oncol. 2021;14(1):174. doi: 10.1186/s13045-021-01190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiarucci M, Paolasini S, Isidori A, et al. Immunological response against SARS-COV-2 after BNT162b2 vaccine administration is impaired in allogeneic but not in autologous stem cell transplant recipients. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.737300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhakal B, Abedin S, Fenske T, et al. Response to SARS-CoV-2 vaccination in patients after hematopoietic cell transplantation and CAR T-cell therapy. Blood. 2021;138:1278–1281. doi: 10.1182/blood.2021012769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Einarsdottir S, Martner A, Waldenström J, et al. Deficiency of SARS-CoV-2 T-cell responses after vaccination in long-term allo-HSCT survivors translates into abated humoral immunity. Blood Adv. 2022;6:2723–2730. doi: 10.1182/bloodadvances.2021006937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang A, Cicin-Sain C, Pasin C, et al. Antibody response to SARS-CoV-2 vaccination in patients following allogeneic hematopoietic cell transplantation. Transplant Cell Ther. 2022;28(4):214.e1–214.e11. doi: 10.1016/j.jtct.2022.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindemann M, Klisanin V, Thümmler L, et al. Humoral and cellular vaccination responses against SARS-CoV-2 in hematopoietic stem cell transplant recipients. Vaccines. 2021;9:1075. doi: 10.3390/vaccines9101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maillard A, Redjoul R, Klemencie M, et al. Antibody response after 2 and 3 doses of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic cell transplant recipients. Blood. 2022;139:134–137. doi: 10.1182/blood.2021014232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majcherek M, Matkowska-Kocjan A, Szymczak D, et al. Two doses of BNT162b2 mRNA vaccine in patients after hematopoietic stem cell transplantation: humoral response and serological conversion predictors. Cancers. 2022;14:325. doi: 10.3390/cancers14020325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mamez A-C, Pradier A, Giannotti F, et al. Antibody responses to SARS-CoV2 vaccination in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2021;56:3094–3096. doi: 10.1038/s41409-021-01466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maneikis K, Šablauskas K, Ringelevičiūtė U, et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8(8):e583–e592. doi: 10.1016/S2352-3026(21)00169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matkowska-Kocjan A, Owoc-Lempach J, Chruszcz J, et al. The COVID-19 mRNA BNT163b2 vaccine was well tolerated and highly immunogenic in young adults in long follow-up after haematopoietic stem cell transplantation. Vaccines. 2021;9:1209. doi: 10.3390/vaccines9101209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morsink LM, Van Doesum J, Choi G, et al. Robust COVID-19 vaccination response after allogeneic stem cell transplantation using post transplantation cyclophosphamide conditioning. Blood Cancer J. 2022;12(1):6. doi: 10.1038/s41408-021-00605-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pabst C, Benning L, Liebers N, et al. Humoral responses and chronic GVHD exacerbation after COVID-19 vaccination post allogeneic stem cell transplantation. Vaccines. 2022;10:330. doi: 10.3390/vaccines10020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piñana JL, López-Corral L, Martino R, et al. SARS-CoV-2-reactive antibody detection after SARS-CoV-2 vaccination in hematopoietic stem cell transplant recipients: prospective survey from the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group. Am J Hematol. 2022;97:30–42. doi: 10.1002/ajh.26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ram R, Hagin D, Kikozashvilli N, et al. Safety and Immunogenicity of the BNT162b2 mRNA COVID-19 Vaccine in Patients after Allogeneic HCT or CD19-based CART therapy-A Single-Center Prospective Cohort Study. Transplant Cell Ther. 2021;27:788–794. doi: 10.1016/j.jtct.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Redjoul R, Le Bouter A, Beckerich F, Fourati S, Maury S. Antibody response after second BNT162b2 dose in allogeneic HSCT recipients. Lancet. 2021;398(10297):298–299. doi: 10.1016/S0140-6736(21)01594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shem-Tov N, Yerushalmi R, Danylesko I, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in haematopoietic stem cell transplantation recipients. Br J Haematol. 2022;196:884–891. doi: 10.1111/bjh.17918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherman AC, Desjardins M, Cheng C-A, et al. Severe acute respiratory syndrome coronavirus 2 messenger RNA vaccines in allogeneic hematopoietic stem cell transplant recipients: immunogenicity and reactogenicity [e-pub ahead of print November 2, 2021]. Clin Infect Dis. doi: 10.1093/cid/ciab930 [DOI] [PMC free article] [PubMed]
- 28.Tamari R, Politikos I, Knorr DA, et al. Predictors of humoral response to SARS-CoV-2 vaccination after hematopoietic cell transplantation and CAR T-cell therapy. Blood Cancer Discov. 2021;2:577–585. doi: 10.1158/2643-3230.BCD-21-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe M, Yakushijin K, Funakoshi Y, et al. The safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in Japanese patients after allogeneic stem cell transplantation. Vaccines. 2022;10:158. doi: 10.3390/vaccines10020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeshurun M, Pasvolsky O, Shargian L, et al. Humoral serological response to the BNT162b2 vaccine after allogeneic haematopoietic cell transplantation. Clin Microbiol Infect. 2022;28(2) doi: 10.1016/j.cmi.2021.10.007. 303.e301-303.e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown M, Abasov R, Salerno D, et al. Impact of alemtuzumab dosing and low-dose total body irradiation on cytomegalovirus infection in allogeneic hematopoietic stem cell transplantation. Leuk Lymphoma. 2020;61:3024–3026. doi: 10.1080/10428194.2020.1791855. [DOI] [PubMed] [Google Scholar]
- 32.van Besien K, Kunavakkam R, Rondon G, et al. Fludarabine-melphalan conditioning for AML and MDS: alemtuzumab reduces acute and chronic GVHD without affecting long-term outcomes. Biol Blood Marrow Transplant. 2009;15:610–617. doi: 10.1016/j.bbmt.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Besien K, Dew A, Lin S, et al. Patterns and kinetics of T-cell chimerism after allo transplant with alemtuzumab-based conditioning: mixed chimerism protects from GVHD, but does not portend disease recurrence. Leuk Lymphoma. 2009;50:1809–1817. doi: 10.3109/10428190903200790. [DOI] [PubMed] [Google Scholar]
- 34.Kenkre VP, Horowitz S, Smith SM, et al. Alemtuzumab-containing allogeneic hematopoietic cell transplant (HCT) for relapsed lymphomas: prognostic factors and outcome. Blood. 2011;52:214–222. [Google Scholar]
- 35.van Besien K, Artz A, Smith S, et al. Fludarabine, melphalan, and alemtuzumab conditioning in adults with standard-risk advanced acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23:5728–5738. doi: 10.1200/JCO.2005.15.602. [DOI] [PubMed] [Google Scholar]
- 36.Patel K, Parmar S, Shah S, et al. Comparison of subcutaneous versus intravenous alemtuzumab for graft-versus-host disease prophylaxis with fludarabine/melphalan-based conditioning in matched unrelated donor allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2016;22:456–461. doi: 10.1016/j.bbmt.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Besien K, de Lima M, Artz A, Oran B, Stock W, Giralt S. Alemtuzumab reduces chronic graft versus host disease (cGVHD) and treatment related mortality (TRM) after reduced intensity conditioning for AML and MDS. Blood (ASH Annual Meeting Abstracts) 2007;110(11):1076. [Google Scholar]
- 38.Chakraverty R, Orti G, Roughton M, et al. Impact of in vivo alemtuzumab dose before reduced intensity conditioning and HLA-identical sibling stem cell transplantation: pharmacokinetics, GVHD, and immune reconstitution. Blood. 2010;116:3080–3088. doi: 10.1182/blood-2010-05-286856. [DOI] [PubMed] [Google Scholar]
- 39.Kline J, Da Pollyea, Stock W, et al. Pre-transplant ganciclovir and post transplant high-dose valacyclovir reduce CMV infections after alemtuzumab-based conditioning. Bone Marrow Transplant. 2006;37:307–310. doi: 10.1038/sj.bmt.1705249. [DOI] [PubMed] [Google Scholar]
- 40.Sive JI, Thomson KJ, Morris EC, Ward KN, Peggs KS. Adenoviremia has limited clinical impact in the majority of patients following alemtuzumab-based allogeneic stem cell transplantation in adults. Clin Infect Dis. 2012;55:1362–1370. doi: 10.1093/cid/cis689. [DOI] [PubMed] [Google Scholar]
- 41.Chakrabarti S, Mackinnon S, Chopra R, et al. High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of Campath-1H in delaying immune reconstitution. Blood. 2002;99:4357–4363. doi: 10.1182/blood.v99.12.4357. [DOI] [PubMed] [Google Scholar]
- 42.Avivi I, Chakrabarti S, Milligan DW, et al. Incidence and outcome of adenovirus disease in transplant recipients after reduced-intensity conditioning with alemtuzumab. Biol Blood Marrow Transplant. 2004;10:186–194. doi: 10.1016/j.bbmt.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Chaekal O-K, Soave R, Chen Z, et al. Adenovirus viremia after in vivo T-cell depleted allo-transplant in adults: low lymphocyte counts are associated with uncontrolled viremia and fatal outcomes. Leuk Lymphoma. 2022;63:435–442. doi: 10.1080/10428194.2021.1978088. [DOI] [PubMed] [Google Scholar]
- 44.Choe HK, van Besien K. Against the odds: haplo-cord grafts protect from GvHD and relapse. Bone Marrow Transplant. 2017;52:1590–1591. doi: 10.1038/bmt.2017.102. [DOI] [PubMed] [Google Scholar]
- 45.Van Besien K, Liu H, Jain N, Stock W, Artz A. Umbilical cord blood transplantation supported by third-party donor cells: rationale, results, and applications. Biol Blood Marrow Transplant. 2013;19:682–691. doi: 10.1016/j.bbmt.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jain N, Liu H, Artz AS, et al. Immune reconstitution after combined haploidentical and umbilical cord blood transplant. Leuk Lymphoma. 2013;54:1242–1249. doi: 10.3109/10428194.2012.739688. [DOI] [PubMed] [Google Scholar]
- 47.van Besien K, Hari P, Zhang MJ, et al. Reduced intensity haplo plus single cord transplant compared to double cord transplant: improved engraftment and graft-versus-host disease-free, relapse-free survival. Haematologica. 2016;101:634–643. doi: 10.3324/haematol.2015.138594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Besien K, Childs R. Haploidentical cord transplantation—The best of both worlds. Semin Hematol. 2016;53:257–266. doi: 10.1053/j.seminhematol.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Liu H, Rich ES, Godley L, et al. Reduced intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment and durable remissions in hematological malignancies. Blood. 2011;118:378–379. doi: 10.1182/blood-2011-08-372508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Besien K, Artz A, Champlin RE, et al. Haploidentical vs haplo-cord transplant in adults under 60 years receiving fludarabine and melphalan conditioning. Blood Adv. 2019;3:1858–1867. doi: 10.1182/bloodadvances.2019000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milano F, Gooley T, Wood B, et al. Cord-blood transplantation in patients with minimal residual disease. N Engl J Med. 2016;375:944–953. doi: 10.1056/NEJMoa1602074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou B, Xu M, Lu S, et al. Clinical outcomes of B cell acute lymphoblastic leukemia patients treated with haploidentical stem cells combined with umbilical cord blood transplantation. Transplant Cell Ther. 2022;28:173.e171–173.e176. doi: 10.1016/j.jtct.2021.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Politikos I, Devlin SM, Arcila ME, et al. Engraftment kinetics after transplantation of double unit cord blood grafts combined with haplo-identical CD34+ cells without antithymocyte globulin. Leukemia. 2021;35:850–862. doi: 10.1038/s41375-020-0922-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindemans CA, Te Boome LC, Admiraal R, et al. Sufficient immunosuppression with thymoglobulin is essential for a successful haplo-myeloid bridge in haploidentical-cord blood transplantation. Biol Blood Marrow Transplant. 2015;21:1839–1845. doi: 10.1016/j.bbmt.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Robin C, Bahuaud M, Redjoul R, et al. Antipneumococcal seroprotection years after vaccination in allogeneic hematopoietic cell transplant recipients. Clin Infect Dis. 2020;71(8):e301–e307. doi: 10.1093/cid/ciz1168. [DOI] [PubMed] [Google Scholar]
- 56.Ketas TJ, Chaturbhuj D, Portillo VMC, et al. Antibody responses to SARS-CoV-2 mRNA vaccines are detectable in saliva. Pathog Immun. 2021;6:116–134. doi: 10.20411/pai.v6i1.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kohmer N, Westhaus S, Ruhl C, Ciesek S, Rabenau HF. Brief clinical evaluation of six high-throughput SARS-CoV-2 IgG antibody assays. J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muench P, Jochum S, Wenderoth V, et al. Development and validation of the Elecsys Anti-SARS-CoV-2 immunoassay as a highly specific tool for determining past exposure to SARS-CoV-2. J Clin Microbiol. 2020;58:e01694. doi: 10.1128/JCM.01694-20. -01620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elecsys® Anti-SARS-CoV-2 S. In . 2022. p information on S antibody assay Roche. [Google Scholar]
- 60.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 61.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Le Bourgeois A, Coste-Burel M, Guillaume T, et al. Interest of a third dose of BNT162b2 anti-SARS-CoV-2 messenger RNA vaccine after allotransplant. Br J Haematol. 2022;196(5):e38–e40. doi: 10.1111/bjh.17911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choe HK, Gergis U, Mayer SA, et al. The addition of low-dose total body irradiation to fludarabine and melphalan conditioning in haplocord transplantation for high-risk hematological malignancies. Transplantation. 2017;101(1):e34–e38. doi: 10.1097/TP.0000000000001538. [DOI] [PubMed] [Google Scholar]
- 64.panel AAC-Ve. ASH-ASTCT: SARS-CoV-2 Vaccines in Immunocompromised Patients FAQs (V5 Posted 3/22/2022). In . 2022. p ASH/ASTCT recommendations. [Google Scholar]
- 65.EBMT. COVID-19 vaccines. Version 8, January 3, 2022. https://www.ebmt.org/sites/default/files/2022-01/COVID%20vaccines%20version%208.3%20-%202022-01-03.pdf;2022(4/2/2022): EBMT Recommendations for vaccine.
- 66.Chevallier P, Coste-Burel M, Le Bourgeois A, et al. Safety and immunogenicity of a first dose of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic stem-cells recipients. eJHaem. 2021;2:520–524. doi: 10.1002/jha2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Besien K, Bachier Rodriguez L, Satlin MJ, et al. Prophylactic rituximab prevents EBV PTLD in haplo-cord transplant recipients. Leuk Lymphoma. 2019;60:1693–1696. doi: 10.1080/10428194.2018.1543877. [DOI] [PubMed] [Google Scholar]
- 68.Burns DM, Rana S, Martin E, et al. Greatly reduced risk of EBV reactivation in rituximab-experienced recipients of alemtuzumab-conditioned allogeneic HSCT. Bone Marrow Transplant. 2016;51:825–832. doi: 10.1038/bmt.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Redjoul R, Le Bouter A, Parinet V, Fourati S, Maury S. Antibody response after third BNT162b2 dose in recipients of allogeneic HSCT. Lancet Haematol. 2021;8(10):e681–e683. doi: 10.1016/S2352-3026(21)00274-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harrington P, Doores KJ, Saha C, et al. Repeated vaccination against SARS-CoV-2 elicits robust polyfunctional T cell response in allogeneic stem cell transplantation recipients. Cancer Cell. 2021;39:1448–1449. doi: 10.1016/j.ccell.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clémenceau B, Guillaume T, Coste-Burel M, et al. SARS-CoV-2 T-cell responses in allogeneic hematopoietic stem cell recipients following two doses of BNT162b2 mRNA vaccine. Vaccines. 2022;10:448. doi: 10.3390/vaccines10030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krammer F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat Med. 2021;27:1147–1148. doi: 10.1038/s41591-021-01432-4. [DOI] [PubMed] [Google Scholar]
- 73.Leclerc M, Redjoul R, Le Bouter A, et al. Determinants of SARS-CoV-2 waning immunity in allogeneic hematopoietic stem cell transplant recipients. J Hematol Oncol. 2022;15:27. doi: 10.1186/s13045-022-01250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]