Background.
Severe acute respiratory syndrome coronavirus 2 is associated with high mortality among transplant recipients. Comparative data that define humoral responses to the Oxford-AstraZeneca (AZ) and BNT162b2 (Pfizer-BioNTech) vaccines are limited.
Methods.
We recruited 920 kidney transplant patients receiving at least 1 dose of severe acute respiratory syndrome coronavirus 2 vaccine, excluding patients with virus pre-exposure. Serological status was determined with the COVID-SeroKlir ELISA (Kantaro-EKF Diagnostics). Patients with a corrected antibody level of <0.7 AU/mL were considered seronegative.
Results.
Four hundred ninety-five AZ and 141 Pfizer patients had a sample analyzed after first dose and 593 after second dose (346 AZ versus 247 Pfizer). After first dose, 25.7% of patients seroconverted (26.6% AZ, 22.8% Pfizer). After second dose, 148 (42.8%) of AZ seroconverted compared with 130 (52.6%) of Pfizer (P = 0.02; hazard ratio, 1.48; 95% confidence interval, 1.07-2.06). When negative responders were excluded, Pfizer patients were shown to have significantly higher response than AZ patients (median 2.6 versus 1.78 AU/mL, P = 0.005).
Patients on mycophenolate had a reduced seroconversion rate (42.2% versus 61.4%; P < 0.001; hazard ratio, 2.17) and reduced antibody levels (0.47 versus 1.22 AU/mL, P = 0.001), and this effect was dose dependent (P = 0.05). Prednisolone reduced the seroconversion from 58.2% to 43.6% (P = 0.03) among Pfizer but not AZ recipients. Regression analysis showed that antibody levels were reduced by older age (P = 0.002), mycophenolate (P < 0.001), AZ vaccine (versus Pfizer, P = 0.001), and male gender (P = 0.02). Sixteen of 17 serious postvaccine infections occurred to patients who did not seroconvert.
Conclusions.
Both seroconversion and antibody levels are lower in AZ compared with Pfizer vaccinated recipients following 2 vaccine doses. Mycophenolate was associated with lower antibody responses in a dose-dependent manner. Serious postvaccine infections occurred among seronegative recipients.
INTRODUCTION
Vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become an essential part of the recovery phase of the SARS-CoV-2 pandemic. However, immunocompromised individuals, including primary and secondary immunodeficient patients, may be less likely to benefit from this intervention. This includes individuals with kidney disease and diabetes, particularly those who have or are going to receive transplants, because they receive immunosuppressive drugs to inhibit and protect from graft rejection. These drugs may variably suppress the magnitude and durability of vaccine-induced responses, rendering individuals more susceptible to SARS-CoV-2 infections.
The risk of SARS-CoV-2 infection and disease could be exacerbated by the emergence of virus variants enabling more rapid transmission and evasion of host immunity. Immunodeficient individuals may experience persistent SARS-CoV-2 with a risk of emergence of novel variants because of insufficient immunological control of virus replication.1
Altered vaccine-induced immunity has been described in transplant patients in the context of other viral pathogens and differs depending on the vaccine type. In hepatitis B for example, where antibody response is low following standard regimes, repeat boosters have been shown to lead to improved serological response.2-4 In the case of the influenza vaccine, results vary with some evidence of similar protection to the general population,5 whereas other studies suggest seroprotective response of 50% compared with healthy individuals.6 This led to the recommendations from the American Society of Transplantation for vaccination in transplant recipients.7
This variability underlines the importance of understanding vaccination responses to different vaccines in immunocompromised populations in an evolving situation with new variants that transmit more readily and may be associated with immune evasion and more severe disease. Improved understanding may inform choice of vaccine platform (adenoviral versus messenger RNA [mRNA]), timing, number of booster doses, and monitoring and defining risk groups within the patient population where alternative strategies such as monoclonal antibodies may be needed for protection.
In the context of SARS-CoV-2, there are reports8-12 that the Moderna (mRNA-1273 SARS-CoV-2) and the Pfizer Biotech (mRNA BNT162b2) mRNA vaccines provide suboptimal antibody protection after the first dose in transplant recipients with some improvement following the second dose but remaining low compared with the general population.11,13,14 However, some of these reports are limited by insufficient numbers to perform subanalysis of potential risk factors that may help explain this blunted response. Furthermore, it is unclear whether induction immunosuppression blunts serological responses even further. Finally, it is currently unknown whether different vaccine types, such as those based on mRNA (Pfizer) or adenovirus (AstraZeneca [AZ]), differentially induce anti–SARS-CoV-2 serological responses in this population.
It has been established that seroconversion protects from severe SARS-CoV-2 infection, at least in the general population.15 We also know that the level of antibody response to vaccination may correlate with the degree of protection from severe SARS-CoV-2 infection15 and therefore would be important to establish if different vaccines induce different antibody titers within the immunocompromised cohort.
PATIENTS AND METHODS
Patient Population and Samples
We recruited 920 solid organ transplant patients in South Wales, United Kingdom, receiving different, according to availability, SARS-CoV-2 vaccines and were able to stratify these by immunosuppressive drugs and induction regimens. Patients were recruited as part of an institutional Research and Development and Research Ethics Committee–approved evaluation of vaccine efficacy in transplant and waiting list patients following verbal consent and as part of the vaccine arm of the Early Novel Laboratory Insight Study (Integrated Research Application System 283297, Research Ethics Committee Bradford, Leeds, United Kingdom) following written informed consent.
Patient samples were obtained, when possible, before the first vaccine dose, then at various time points between the 2 vaccination doses, and at multiple points after second dose, including monthly samples thereafter. Serum samples collected from January 21, 2021, to June 7, 2021, were analyzed using enzyme-linked immunosorbent assay (ELISA) as described below.
Patient demographics, transplant details, induction, and maintenance immunosuppression were obtained through a prospective clinical database (Vital Data). Vaccination status (type of vaccine, date of first and second doses) was obtained through the Welsh Clinical Information Service. Previous SARS-CoV-2 clinical infection information was gained through a central, prospective database for all infected transplanted patients in South Wales. The recent immunosuppression regimes of the Cardiff Transplant Unit involve T cell–depleting induction with either alemtuzumab or thymoglobulin and maintenance with tacrolimus and mycophenolate with or without steroids. We decided, during this period, to defer vaccination in patients transplanted before being vaccinated for 2 mo (following discussion with Infectious Disease expert colleagues from the American Society of Transplantation to allow for some recovery from the acute immunosuppression).
Antibody Testing for SARS-CoV-2
Serological status was determined using samples analyzed with the COVID-SeroKlir 2-step ELISA (Kantaro Biosciences, New York, NY; supplied by EKF Diagnostics, United Kingdom). The assay has 97.8% sensitivity and 99.6% specificity for detecting SARS-CoV-2–specific immunoglobulin G antibodies against 2 virus antigens, the full-length spike protein and its receptor-binding domain.16,17 The 96-well plate ELISA was performed on an automated platform (Dynex Technologies) according to the manufacturer’s instructions using the anti–receptor-binding domain assay. The assay relies on an assay-specific calibrator to report the ratio of the specimen absorbance to the calibrator absorbance to calculate a cutoff index value. Additionally, we used the term “seroconversion” if the assay value was above the arbitrary cutoff of 0.7 AU/mL, and for the purpose of this article, we defined nonresponders as patients whose antibody levels remained <0.7 AU/mL. Values >10 AU/mL were recorded as 10 AU/mL. The cutoff value of 0.7 AU/mL comes from studies of infected patients.16,17
Statistical Analysis
The vaccine response positive or negative was correlated with the type of vaccine, the time interval posttransplantation (<6 mo compared with >6 mo), the demographics of the cohort population, induction (thymoglobulin versus alemtuzumab), and maintenance immunosuppression. We used nonparametric tests (eg, Mann-Whitney U test) for nonnormally distributed data. False discovery rate corrections were undertaken using the Benjamini-Hochberg method. The Fisher exact test was used to compare categorical variables.
Binary regression analysis was used to identify factors independently associated with seroconversion, univariate analysis to identify factors contributing to the antibody level, and linear regression analysis to identify the relative impact of factors on the antibody levels as measured by the assay. This was performed after both the first and second doses. Results were further analyzed in patients who had a sample taken at least 14 or 21 d following the first and the second doses to account for antibody generation. The study was powered at a level of 80% to detect a 25% difference on vaccine-induced antibody titers between vaccines (with at least 212 patients in each vaccine group).
RESULTS
Patient Characteristics and Vaccination Regimes
By the end of June 2021, out of the transplant follow-up population (n = 1093), 722 (66%) had received at least the first dose of the AstraZeneca (AZ) vaccine, whereas 326 (29.8%) received Pfizer vaccination. Forty-five (4.1%) patients did not receive a vaccination or this was not recorded. Six hundred eighty patients had received both doses of the AZ vaccine compared with 321 who received 2 Pfizer vaccinations. There have been 2211 samples collected to date, out of which 1128 samples were after the second dose in 894 patients. Out of those, we have tested 1179 samples thus far. Two hundred ten patients had a sample taken before any vaccination, 636 patients had a sample taken after first vaccination, and 593 had at least 1 sample taken after the second vaccination. The distribution of patients who received at least 1 dose with samples analyzed according to timing and type of vaccine is presented in Table 1. The median interval between the 2 doses in this cohort was 77 (range, 15–132) d for the AZ and 47 (range, 28–97) for the Pfizer vaccine (P < 0.0001). The demographics of both vaccine groups are presented in Table 2.
TABLE 1.
Number of patients according to the type of vaccine received and the timing of analyzed samples in relation to vaccination
| Oxford-AstraZeneca, n (%) | Pfizer-BioNTech (mRNA BNT162b2), n (%) | |
|---|---|---|
| Before first dose | 147 (70) | 63 (30) |
| After first dose | 495 (77.8) | 141 (22.2) |
| After second dose | 346 (58.3) | 247 (41.7) |
TABLE 2.
Demographics of the 2 vaccine groups after second dose
| Patient characteristic | Oxford-AstraZeneca | Pfizer-BioNTech (mRNA BNT162b2) | P |
|---|---|---|---|
| Median age, y (range) | 56 (20–87) | 57 (20–87) | 0.5 |
| Latest median BMI (range) | 29.5 (18–40) | 27 (18–35) | 0.2 |
| Median transplant duration, y (range) | 7.75 (3 mo–40 y) | 7.36 (3 mo–33 y) | 0.59 |
| Male/female, n (%) | 208/138 (60.1/39.9) | 150/97 (60.4/39.6) | 0.9 |
| Ethnic group: any White/any Asian/any African background, n (%) | 266/13/3 (76.9/3.8/0.9) | 182/21/1 (73.7/8.5/0.4) | 0.13 |
| Median eGFR (range) | 48.5 (8–110) | 54 (11–120) | 0.09 |
| Diabetes as cause of renal failure, n (%) | 40 (11.5) | 21 (8.5) | 0.22 |
BMI, body mass index; eGFR, estimated glomerular filtration rate.
Prevaccine Positive Patients
Before vaccination, we identified 34 samples from 31 patients (3 duplicates) with antibody values >0.7 AU/mL. The median value of antibody measured was 3.48 AU/mL. Six subsequently received Pfizer and 25 AZ vaccines. Of them, 8 were known to have had a clinical infection before sampling.
Mycophenolate Derivatives but not Vaccine Type Influence Seroconversion Following a Single-dose SARS-CoV-2 Vaccination
We analyzed 769 samples obtained from 599 patients following the initial vaccination. Of those, 463 received AZ (77%) and 136 Pfizer-BioNTech (Pfizer) vaccines (23%). Overall, 154 patients (25.7%) seroconverted (as defined by an assay value of >0.7 AU/mL). When vaccination type was examined independently of other factors, 26.6% of AZ patients seroconverted following the first dose compared with 22.8% of Pfizer (P = 0.4). The median values of antibody responses (0.22 AU/mL) were low compared with those observed in infected patients (3.48 AU/mL), and we did not observe any difference in antibody titers between individuals receiving the different vaccines at this time. Patients receiving a transplant <6 mo before vaccination responded numerically better than patients who were vaccinated after 6 mo from transplantation (36% versus 25.4%, P = 0.3). In a binary regression model for seroconversion after 1 vaccine dose, only the presence of mycophenolate derivatives affected (negatively) the antibody response rate to the vaccine (β, 0.6; 95% confidence interval [CI], 0.41-0.94).
Pfizer Vaccination Induces Higher Seroconversion and Antibody Titers Than AZ Vaccination After 2 Doses
Five hundred ninety-three patients had at least 1 sample analyzed after second dose, 346 of them had received AZ and 247 Pfizer vaccines. Two hundred seventy-eight of 593 patients (48.5%) had seroconverted on the basis of the assay cutoff of 0.7 AU/mL at the time of their latest sample tested, representing 148 of 346 (42.8%) of AZ versus 130 of 247 (52.6%) of the Pfizer patients (P = 0.02; hazard ratio [HR], 1.48; 95% CI, 1.07-2.06).
When antibody titers were quantified, antibody levels increased from a median of 0.22 AU/mL after first dose to 0.62 AU/mL after the second dose, with patients receiving Pfizer vaccination (0.79 AU/mL) having a higher response than patients receiving AZ vaccination (0.52 AU/mL; Mann-Whitney P = 0.006).
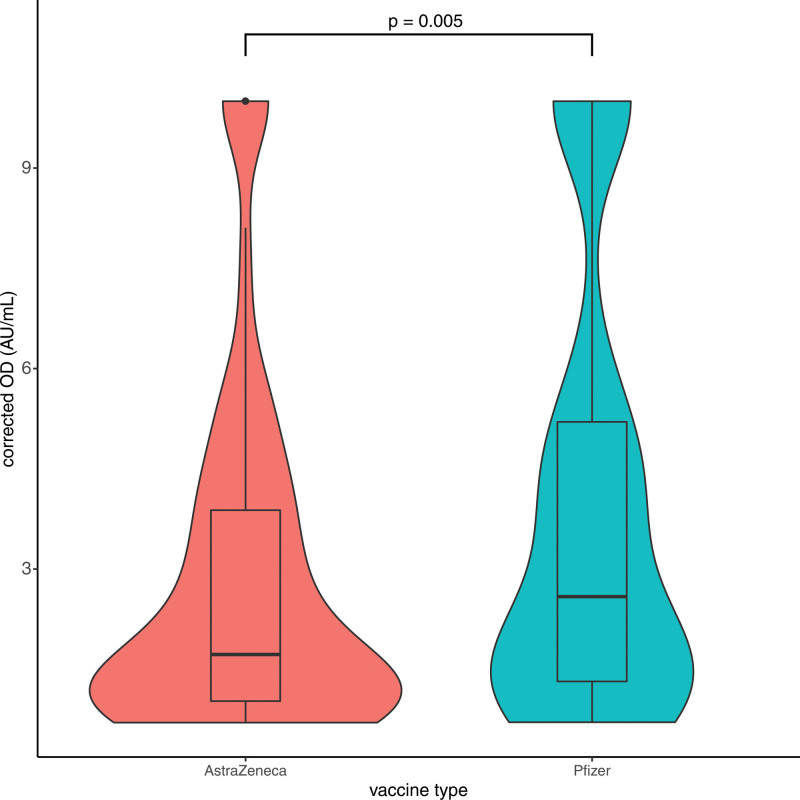
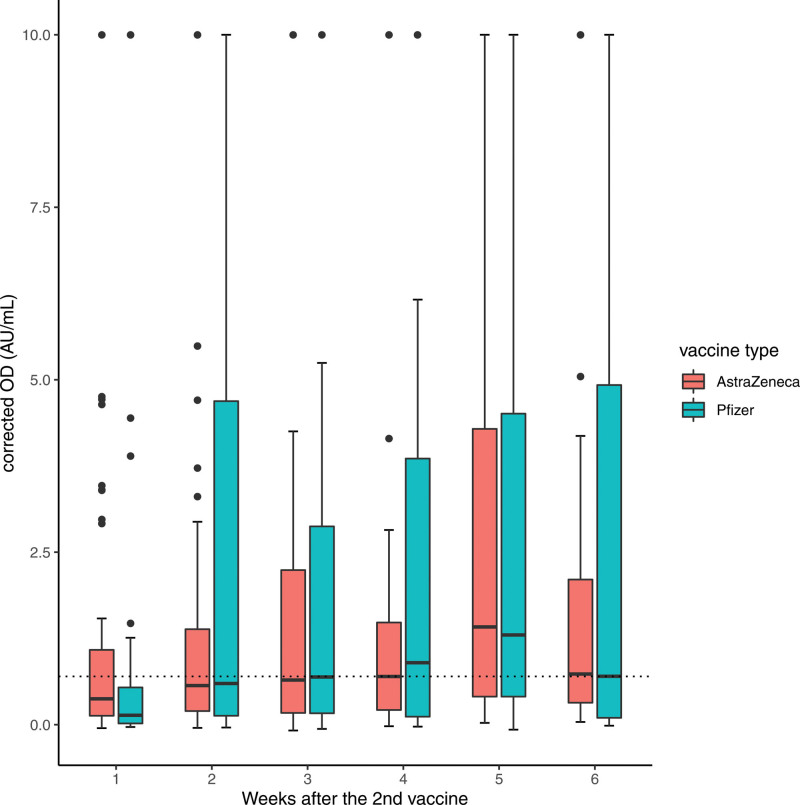
When negative responders were excluded, Pfizer patients were shown to have a significantly higher response than AZ patients (median 2.6 versus 1.78 AU/mL, P = 0.005; Figure 1). Moreover, the majority of these positive responders of AZ were concentrated within the lower quartile of antibody levels (0.7–1.1 AU/mL). Looking at the data longitudinally following the second dose, the antibody levels increase in both vaccines to week 5 after dose (Figure 2). The increase is sharper for the Pfizer vaccine, but the timing between the 2 vaccines might have affected that. Thus, our data suggest that Pfizer vaccination induces increased seroconversion and antibody titers following a 2-dose vaccination protocol.
FIGURE 1.
Antibody levels following the second dose according to the vaccine type. Patients receiving Pfizer vaccination (n = 247) had a higher antibody response than patients receiving AstraZeneca vaccination (n = 346) following the second dose, when nonresponders were excluded (median 2.6 vs 1.78 AU/mL, Mann-Whitney P = 0.005). Moreover, the majority of the antibody values of the AstraZeneca group were concentrated in the lower quartile (0.7–1.1). Box plot with dots representing outliers. Corrected OD indicates adjusted values of antibody as measured by the assay. OD, optical density.
FIGURE 2.
Antibody levels according to the week of testing after the second vaccine dose and the vaccine used. Longitudinal data following the second dose of the vaccine show that the antibody levels increase in both vaccines to week 5 postdose. It should be noted not all patients are represented in all weeks. Box plot with dots representing outliers. Values >10 were amalgamated to 10 AU/mL. Corrected OD indicates adjusted values of antibody as measured by the assay. AZ, AstraZeneca vaccine; OD, optical density; Pfizer, Pfizer-BioNTech vaccine.
Maintenance Immunosuppression Impacts Vaccine Immunogenicity After the Second Dose
We assessed whether immunosuppression impacted on immunogenicity of SARS-CoV-2 vaccination. Patients in <6 mo posttransplantation (n = 35) demonstrated comparable seroconversion rate (60%) compared with patients after 6 mo (n = 407, 47.4%, P = 0.15) and no difference in antibody titer (1.06 versus 0.62 AU/mL, P = 0.6). There was no difference in the response between patients receiving thymoglobulin compared with alemtuzumab within this group. Thus, although numbers are small, our data indicate no impact of induction immunosuppression on antibody seroconversion.
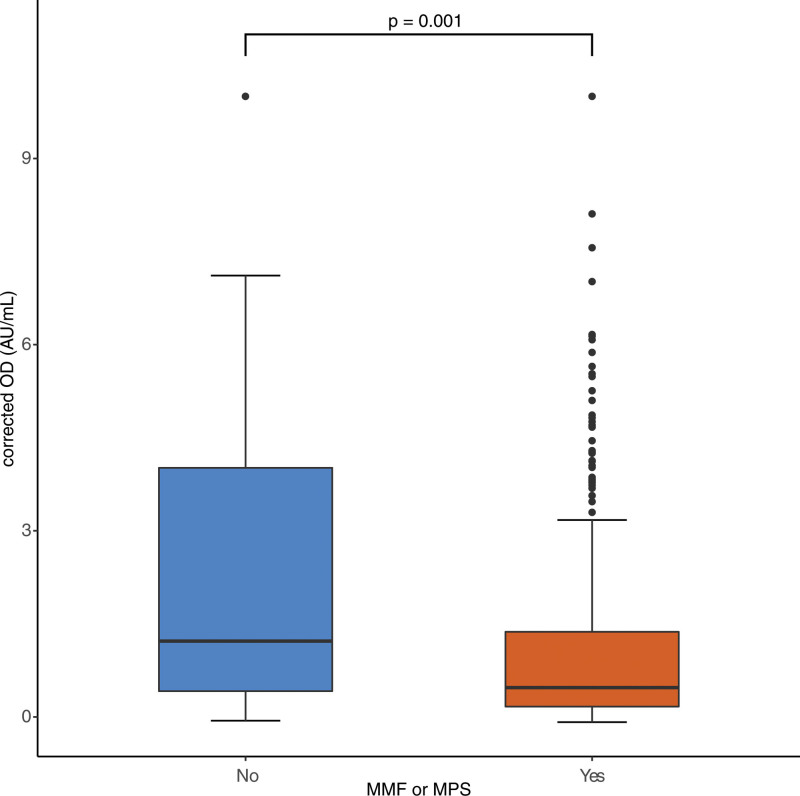
Next, we investigated whether maintenance immunosuppression impacted vaccine immunogenicity after the second dose. We observed that the 335 patients receiving mycophenolate derivatives (mycophenolate mofetil or mycophenolate sodium) who were tested exhibited a lower seroconversion rate as compared with the 108 patients tested not receiving mycophenolate (42.5 versus 61.3%, Fisher exact P < 0.001). The demographics of the groups receiving mycophenolate derivatives or not are included in Table 3. The level of antibody response was also reduced by mycophenolate derivatives (Figures 3; 0.45 versus 1.25 AU/mL, P < 0.001). Furthermore, higher maintenance doses of mycophenolate mofetil (and equivalent of mycophenolate sodium) from 250 to 750 mg were associated with lower antibody responses (0.61–0.18 AU/mL, P = 0.06, test for linearity P = 0.07).
TABLE 3.
Demographics of patients according to mycophenolate derivatives use
| Patient characteristic | No mycophenolate | Mycophenolate | P |
|---|---|---|---|
| Median age, y (range) | 60 (20–87) | 55.5 (20–87) | 0.001 |
| Latest median BMI | 28.5 | 30 | 0.9 |
| Median transplant duration, y (range) | 16.2 (3 mo–40 y) | 6.08 (2 mo–29 y) | 0.001 |
| Male/female, n (%) | 73/72 (50.3/49.7) | 285/163 (63.6/36.4) | 0.005 |
| Median eGFR (range) | 43 (11–110) | 54 (8–120) | 0.001 |
| Diabetes as cause of renal failure, n (%) | 10 (6.9) | 51 (11.3) | <0.0001 |
BMI, body mass index; eGFR, estimated glomerular filtration rate.
FIGURE 3.
Antibody levels following the second dose of the vaccine according to mycophenolate. The level of antibody response was reduced in patients who were on mycophenolate derivatives (0.47 vs 1.22 AU/mL, Mann-Whitney P = 0.001). Patients on mycophenolate also had a lower seroconversion rate as compared with those not receiving mycophenolate (42.2% vs 61.4%, P < 0.001). Box plot with dots representing outliers. Values >10 were amalgamated to 10 AU/mL. Number of patients not receiving mycophenolate: 145 (24.5%); number of patients receiving mycophenolate: 448 (75.5%). Corrected OD indicates adjusted values of antibody as measured by the assay. MMF, mycophenolate mofetil; MPS, mycophenolate sodium; OD, optical density.
Next, we assessed the impact of prednisolone on seroconversion. We found that 44.2% of the 190 patients receiving prednisolone seroconverted compared with 51.4% of the 253 who were not receiving prednisolone (P = 0.14). Further analysis revealed that the presence of prednisolone had no effect on the seroconversion rate among AZ-vaccinated patients (45.5% versus 48.3%), whereas it suppressed seroconversion rate among patients who received Pfizer (from 54% to 42.9%, P = 0.1; Table 4). Furthermore, patients receiving both mycophenolate and prednisolone had the lowest seroconversion response after the second dose of 39%. In contrast, patients receiving calcineurin inhibitors only and who received Pfizer vaccination exhibit a seroconversion rate of 72%.
TABLE 4.
Seroconversion rates according to prednisolone use in the 2 vaccine groups
| Vaccine type | Antibody response | Prednisolone, n (%) | No prednisolone, n (%) |
|---|---|---|---|
| AZ | Positive (>0.7 AU/mL) | 45 (45.5) | 56 (48.3) |
| Negative (<0.7 AU/mL) | 54 (54.5) | 60 (51.7) | |
| Pfizer | Positive (>0.7 AU/mL) | 39 (42.9) | 74 (54) |
| Negative (<0.7 AU/mL) | 52 (57.1) | 63 (46) |
The negative effect of prednisolone on seroconversion rate seems to be limited to the Pfizer group.
AZ, AstraZeneca; Pfizer, Pfizer-BioNTech vaccine.
Thus, overall, these data imply that maintenance immunosuppression inhibits the ability of SARS-CoV-2 vaccinations to induce antibody responses, and individuals receiving Pfizer vaccination seem to be more sensitive to the inhibitory effects of maintenance immunosuppression than AZ-vaccinated patients. This might have been affected by the overall higher response to the Pfizer vaccine.
Regression Analysis Reveals Multiple Factors That Influence SARS-CoV-2 Vaccine Immunogenicity
To control for confounders and take account of maldistribution of the multiple risk factors involved, we performed univariate and linear regression analyses. Binary regression analysis demonstrated that seroconversion was less likely in males compared with females (P = 0.025), Caucasians versus Asians (P = 0.053), older patients (aged >60 y, P = 0.048), patients on mycophenolate derivatives (P < 0.001), and AZ vaccine versus Pfizer vaccine (P = 0.028). In terms of absolute seroconversion rates, the vaccine type did not impact seroconversion. We should note, however, that we did not have sufficient patients in our study of other ethnic groups to make other valid comparisons.
In a univariate analysis performed to identify factors affecting the antibody titer, vaccine type (P = 0.001), recipient age (P = 0.006), and mycophenolate derivative use (P < 0.001) were found to be significant. We next performed linear regression analysis using as outcome of the antibody titer, as defined by the assay used. This was affected by age (negative effect, P = 0.001), mycophenolate use (negative effect, P < 0.001), the type of vaccine (Pfizer better response than AZ, P = 0.02), and gender (females better response than males, P = 0.04), whereas prednisolone use (P = 0.6), race (P = 0.14), induction immunosuppression (P = 0.37), and the time posttransplant (P = 0.17) did not affect the antibody titer (model overall is highly significant with P < 0.001; Table 5).
TABLE 5.
Linear regression model for prediction of SARS-CoV-2 antibody level response after the second vaccine dose
| β | 95% confidence interval for β | P | ||
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Recipient age, y | –0.03 (–0.17) | –0.05 | –0.01 | 0.001 |
| Transplant duration (<6 mo vs >6 mo) | –0.69 (0.07) | –1.69 | 0.3 | 0.17 |
| Mycophenolate (no vs yes) | –1.18 (–0.19) | –1.8 | –0.55 | <0.001 |
| Induction immunosuppression (thymoglobulin vs alemtuzumab) | 0.121 | –0.144 | 0.387 | 0.370 |
| Prednisolone (no vs yes) | –0.13 (–0.2) | –0.67 | 0.42 | 0.6 |
| Type of vaccine (AZ vs Pfizer) | 0.65 (0.12) | 0.10 | 1.2 | 0.02 |
| Gender group (M vs F) | 0.58 (0.10) | 0.03 | 1.14 | 0.04 |
| Ethnic group (Caucasians vs Asians) | 0.84 (0.07) | –0.28 | 1.9 | 0.14 |
AZ, AstraZeneca; F, female; M, male; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
SARS-CoV-2 Infections After Second Vaccination
As of October 1, 2021, we recorded 25 clinical infections from SARS-CoV-2 confirmed in doubly vaccinated transplant patients (before their 3d dose), as confirmed with a positive polymerase chain reaction. Of those, 16 patients had received the AZ vaccine, out of whom 14 had a negative antibody response. Of the 9 recipients of Pfizer vaccine, 6 were also negative for antibody. Six out of 14 infected AZ-vaccinated patients required admission with 1 death. Two out of the 9 infected Pfizer-vaccinated patients required admission. All admitted patients had no demonstrable antibody response before their infection.
DISCUSSION
This is the first large comparative study in renal and pancreas transplant recipients to compare patients who received either the Oxford-AZ (adenovirus-based) or the Pfizer-BioNTech (mRNA BNT162b2) vaccine. The number of samples and patients involved allowed us to make a number of valid comparisons between the 2 regimes. It is also the first study to report on the effect of AZ vaccine among immunosuppressed individuals.
The seroconversion rate following the second dose of either vaccine was low (<50%). Importantly, our data demonstrated that both the seroconversion rate and the magnitude of the antibody response in these immunosuppressed individuals were greater following the Pfizer compared with the AZ vaccine. Benotmane et al8 reported a 48% response to the Moderna (mRNA-1273 SARS-CoV-2) vaccine among 200 patients who were seronegative before vaccination. Boyarsky et al9-11 reported a seroconversion rate of 48% after the second dose in kidney transplant recipients receiving either of the 2 mRNA vaccines (mRNA-1273 SARS-CoV-2 or mRNA BNT162b2).
This is replicated by the 52.6% seroconversion rate among patients who received Pfizer (mRNA BNT162b2) vaccine (in patients without previous SARS-CoV-2 exposure) in our cohort. Grupper et al18,19 have shown an even lower response of 37% in kidney recipients. The rate of seroconversion among patients who received AZ vaccine in our cohort was lower than that following Pfizer at 42.8%. Of significant concern is the fact that the level of antibody response in patients who received AZ was significantly lower than in those who received Pfizer, and for both, it was significantly lower compared with the immunocompetent population.18,19 Additionally, most patients who received AZ and seroconverted had a lower positive response compared with their Pfizer counterparts. A small study of 12 patients who received a different adenovirus-based vaccine has also shown a very low response.20
The numerous outliers raise the possibility that these individuals may have been previously subclinically infected. The limited number of samples we had before the first dose show that it is fair to assume that subclinical infection was at least evenly distributed between the 2 vaccine groups. The measurement of antinucleocapsid antibody between the 2 doses will have been a more robust way to allay this concern. Because of the “real-world” nature of this study, we were not able to perform this. This represents a limitation of the study. Despite this, there is no theoretical reason that more patients would have been subclinically infected in Pfizer compared with the AZ group, given the rest of the results.
A possible concern with new transplants is that, if they receive induction with T cell–depleting agents, this might suppress their vaccine antibody response still further. It was reassuring that although numbers are still low in our cohort currently, seroconversion among early transplants who received induction T cell–depleting immunosuppression was at least equivalent to our long-term transplant patients. This result certainly means that it is the effect of chronic immunosuppression that is more important in seroconversion. It should be noted that vaccination was deferred for 2 mo posttransplantation if not vaccinated before transplantation. This pragmatic solution seems to be justified by these early results.
Additionally, at the initial phase at least, there appears not to be a difference in the response between patients who had received thymoglobulin compared with those who received alemtuzumab. Overall, these data might have implications in several other immunosuppressed groups where T-cell depletion is given, such as chronic lymphocytic leucaemia, multiple sclerosis, and others.
The impairment of antibody response because of mycophenolate derivatives, as also reported in the study of Boyarski,9-11 was significant. Because of the number of patients studied, we were able to perform an analysis of the effect of the dose that concluded that patients on higher mycophenolate doses had lower antibody responses. Furthermore, patients receiving both mycophenolate and prednisolone had the lowest seroconversion response after second dose of 39%. Our results suggest a differential effect in responses to the 2 different vaccines of patients on prednisolone with no reduction in patients who received the AZ vaccine, whereas responses to the Pfizer vaccine were impaired further. This could be a statistical anomaly, but it is worth investigating further.
It is important to note that vaccine-induced protection from SARS-CoV-2 will likely be mediated in part by T cells. T cells are implicated in control of severe COVID-19.21,22 Although transplant patients have been shown to mount robust T-cell responses following SARS-CoV-2 infection, these responses are somewhat delayed,23 suggesting immunosuppressive drug regimens may suppress SARS-CoV-2–specific T-cell immunity, including those induced by vaccination.
A very recent study that was based on linked transplant patient vaccination and mortality data from England suggested that these 2 vaccines studied did not protect patients from infection and only the Oxford AZ vaccine reduced mortality compared with unvaccinated patients in contrast with the Pfizer that did not.24 On the basis of that and our study, third and fourth vaccine doses are clearly justified and already acted upon in both England and Wales. As suggested in an editorial to this article, the failure of the mRNA vaccine to protect solid organ transplant patients from death is unexpected and further research is needed before this can be confirmed.25 This is difficult to reconcile with the results from our study and also the preliminary infection data coming from Wales included in this article. These results do suggest a significant protection from severe disease in patients with positive antibody status postvaccination.
In conclusion, a significant proportion of transplant patients does not show seroconversion following the second vaccine dose, and patients who received AZ vaccine show a significantly less antibody titer that in the general population has been associated with more severe breakthrough infections. The presence of breakthrough SARS-CoV-2 severe infections in this cohort occurring predominantly in those individuals without a demonstrable antibody response signifies that a considerable number of transplant patients remains at risk. Given our results, at least 1 extra dose, especially Pfizer, is highly recommended for the immunosuppressed population. There is evidence now that a monoclonal antibody combination (Casirivimab and Imdevimab, also known as Ronapreve in the United Kingdom and Regen-Cov in the United States) and also Sotromivab are effective in the negative antibody patients.26 Therefore, it is highly recommended that this subpopulation of immunosuppressed patients that remains at risk is identified in advance. This population also needs to remain vigilant in terms of taking safety precautions.
ACKNOWLEDGMENTS
The authors would like to acknowledge the following: Maria Coleman, Yvette Webley, and Gareth Nicol for approaching and consenting patients; Gary Hunter for providing IT support and vaccination data; EKF Diagnostics and Kantaro Biosciences for the kind donation of the ELISA kits; Kidney Wales for funding part of the study and UK Research and Innovation/National Institute for Health Research through the UK Coronavirus Immunology Consortium; and, above all, the transplant patients who participated enthusiastically in this study.
Footnotes
This study was funded by the UK Research and Innovation/National Institute for Health Research through the UK Coronavirus Immunology Consortium and by Kidney Wales. M.J.P. was supported by the Welsh Clinical Academic Training program and a Career Development Award from the Association of Clinical Pathologists, and he is a participant in the National Institutes of Health Graduate Partnership Program. I.R.H. is a Wellcome Trust Senior Research Fellow.
A.A. has received grants from Sanofi (France) for work related to thymoglobulin and from Novartis (United Kingdom) as part of various trials. He also serves as a board member of Kidney Wales. S.R.J. reports advisory board, speaker, conference, drug safety monitoring board, and project support from CSL Behring, Shire, Takeda, BioCryst Pharmaceuticals, Swedish Orphan Biovitrum, Biotest, Binding Site, LFB, Octapharma, Grifols, UCB Pharma, Sanofi, Pharming, Weatherden, and Zarodex Therapeutics Limited. S.R.J. is a member of the International Patient Organisation for Primary Immunodeficiencies Supply and Access for Everyone (SAFE) Taskforce and COVID19 Trial Group. The other authors declare no conflicts of interest.
A.A. participated in data access, responsibility, conception of the study, design, analysis, and writing of the article. L.S. has full access to all the data in the study, takes responsibility for the integrity of the data and accuracy of the data analysis, and participated in editing the article and provided database support. U.K. participated in the interface with the laboratory and editing the article. G.K. participated in design and organization of sampling and interface with the laboratory. M.J.P. participated in protocol and interface with the laboratory. C.C. participated in sample collection. K.B. and L.G. performed the serological assays. S.J.M. participated in coordination of laboratory work, refining of the assay, and editing of the article. I.R.H. participated in study design and writing and editing of the article. S.R.J. participated in conception of Early Novel Laboratory Insight Study study, design, and editing of the article. All authors approved this article for submission.
REFERENCES
- 1.Truong TT, Ryutov A, Pandey U, et al. Increased viral variants in children and young adults with impaired humoral immunity and persistent SARS-CoV-2 infection: a consecutive case series. EBioMedicine. 2021;67:103355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grob PJ, Binswanger U, Zaruba K, et al. Immunogenicity of a hepatitis B subunit vaccine in hemodialysis and in renal transplant recipients. Antiviral Res. 1983;3:43–52. [DOI] [PubMed] [Google Scholar]
- 3.Serrano B, Bayas JM, Bruni L, et al. Solid organ transplantation and response to vaccination. Vaccine. 2007;25:7331–7338. [DOI] [PubMed] [Google Scholar]
- 4.Domínguez M, Bárcena R, García M, et al. Vaccination against hepatitis B virus in cirrhotic patients on liver transplant waiting list. Liver Transpl. 2000;6:440–442. [DOI] [PubMed] [Google Scholar]
- 5.Scharpé J, Evenepoel P, Maes B, et al. Influenza vaccination is efficacious and safe in renal transplant recipients. Am J Transplant. 2008;8:332–337. [DOI] [PubMed] [Google Scholar]
- 6.Mazzone PJ, Mossad SB, Mawhorter SD, et al. The humoral immune response to influenza vaccination in lung transplant patients. Eur Respir J. 2001;18:971–976. [DOI] [PubMed] [Google Scholar]
- 7.Kumar D, Blumberg EA, Danziger-Isakov L, et al. ; AST Infectious Diseases Community of Practice. Influenza vaccination in the organ transplant recipient: review and summary recommendations. Am J Transplant. 2011;11:2020–2030. [DOI] [PubMed] [Google Scholar]
- 8.Benotmane I, Gautier-Vargas G, Cognard N, et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99:1498–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325:1784–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyarsky BJ, Ou MT, Greenberg RS, et al. Safety of the first dose of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021;105:e56–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georgery H, Devresse A, Yombi JC, et al. Very low immunization rate in kidney transplant recipients after one dose of the BNT162b2 vaccine: beware not to lower the guard! Transplantation. 2021;105:e148–e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hod T, Ben-David A, Olmer L, et al. Humoral response of renal transplant recipients to the BNT162b2 SARS-CoV-2 mRNA vaccine using both RBD IgG and neutralizing antibodies. Transplantation. 2021;105:e234–e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ou MT, Boyarsky BJ, Motter JD, et al. Safety and reactogenicity of 2 doses of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021;105:2170–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. [DOI] [PubMed] [Google Scholar]
- 16.Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26:1033–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21:2719–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grupper A, Katchman H. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus: not alarming, but should be taken gravely. Am J Transplant. 2021;21:2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyarsky BJ, Chiang TP, Ou MT, et al. Antibody response to the Janssen COVID-19 vaccine in solid organ transplant recipients. Transplantation. 2021;105:e82–e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan C, Chen L, Lu C, et al. Lung recruitability in COVID-19-associated acute respiratory distress syndrome: a single-center observational study. Am J Respir Crit Care Med. 2020;201:1294–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. ; Karolinska COVID-19 Study Group. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Favà A, Donadeu L, Sabé N, et al. SARS-CoV-2-specific serological and functional T cell immune responses during acute and early COVID-19 convalescence in solid organ transplant patients. Am J Transplant. 2021;21:2749–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callaghan CJ, Mumford L, Curtis RMK, et al. ; NHSBT Organ and Tissue Donation and Transplantation Clinical Team. Real-world effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S vaccines against SARS-CoV-2 in solid organ and islet transplant recipients. Transplantation. 2022;106:436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapman JR, Wigmore SJ. Simple vaccination is not enough for the transplant recipient. Transplantation. 2022;106:447–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.RECOVERY Collaborative Group. Casirivimab and imdevimab in patients admitted to hospital with Covid-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399:665-676. [DOI] [PMC free article] [PubMed] [Google Scholar]