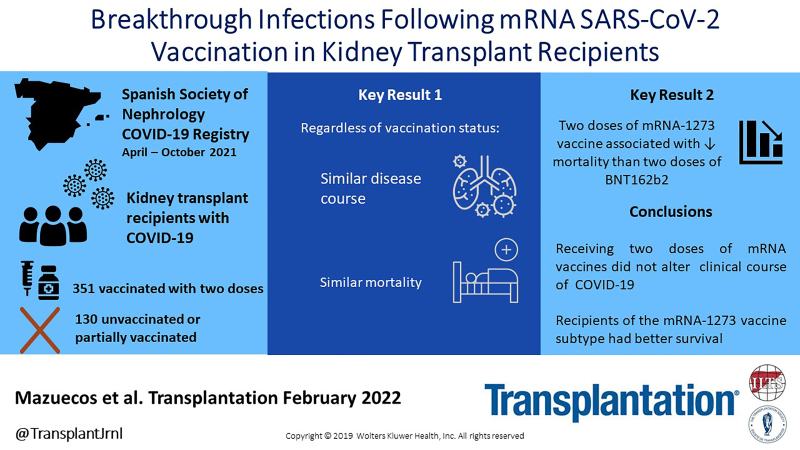
Background.
The clinical effectiveness of coronavirus disease 2019 (COVID-19) vaccination in kidney transplant (KT) recipients is lower than in the general population.
Methods.
From April to October 2021, 481 KT recipients with COVID-19, included in the Spanish Society of Nephrology COVID-19 Registry, were analyzed. Data regarding vaccination status and vaccine type were collected, and outcomes of unvaccinated or partially vaccinated patients (n = 130) were compared with fully vaccinated patients (n = 351).
Results.
Clinical picture was similar and survival analysis showed no differences between groups: 21.7% of fully vaccinated patients and 20.8% of unvaccinated or partially vaccinated died (P = 0.776). In multivariable analysis, age and pneumonia were independent risk factors for death, whereas vaccination status was not related to mortality. These results remained similar when we excluded patients with partial vaccination, as well as when we analyzed exclusively hospitalized patients. Patients vaccinated with mRNA-1273 (n = 213) showed a significantly lower mortality than those who received the BNT162b2 vaccine (n = 121) (hazard ratio: 0.52; 95% confidence interval, 0.31-0.85; P = 0.010).
Conclusions.
COVID-19 severity in KT patients has remained high and has not improved despite receiving 2 doses of the mRNA vaccine. The mRNA-1273 vaccine shows higher clinical effectiveness than BNT162b2 in KT recipients with breakthrough infections. Confirmation of these data will require further research taking into account the new variants and the administration of successive vaccine doses.
INTRODUCTION
Since vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) began, there has been a marked decrease in the rate of infections throughout the world.1 The SARS-CoV-2 mRNA vaccines, BNT162b2 (Pfizer–BioNTech) and mRNA-1273 (Moderna), have shown efficacy rates of 95% and 94.1%, respectively, in their clinical trials, defined as preventing the first occurrence of symptomatic coronavirus disease 2019 (COVID-19).2,3 Furthermore, COVID-19 mRNA vaccines have demonstrated to be highly effective against SARS-CoV-2 infection requiring hospitalization or intensive care unit (ICU) admission in the general population, and subsequently, COVID-19–related deaths have decreased to 0.004% to 0.007% in the fully vaccinated people.4-6
In Spain, vaccination was initiated on December 27, 2020, soon after the first COVID-19 vaccine (BNT162b2 mRNA) was approved, initially with the elderly and highly exposed groups.7 In March 2021, a group of high-risk patients was defined, including kidney transplant (KT) recipients, who are known to be vulnerable to COVID-19 infection and death. A widespread and rapid vaccination started in this group. Most KT recipients received their first mRNA vaccine dose in March 2021 and the second one 3 to 4 wk later.8 As a result, >75% of KT patients were fully vaccinated in May and almost 90% in July, and this percentage was exceeded in September 2021.9 In the general Spanish population, on October 14, 2021, 70 982 052 doses had been administered, which means that 87.9% of the target population had completed the 2-dose vaccination regimen.10
Likewise, the severity of the disease has been reduced in the general population. In Spain, the risk of hospitalization in the fully vaccinated population has been 9 to 16 times lower than in unvaccinated people, with a 14-fold-decreased risk of death among fully vaccinated people over 60 y of age in December 202111; however, these results have not been as favorable in KT patients. Although some initial studies published high clinical effectiveness of vaccination, others have reported the occurrence of severe COVID-19 in vaccinated KT patients with up to a 15-fold-higher risk of death.12-18 Several reasons have been proposed, especially a poor humoral response to 2-dose vaccination.19-22 Information on the clinical effectiveness of the different types of mRNA vaccines in KT patients has not been reported. Moreover, in May 2021, the B.1.617.2 (Delta) variant was introduced in Spain, becoming predominant in the last weeks of June 2021.23 This variant is characterized by some spike protein mutations that may affect immune responses, being more contagious than the previous SARS-CoV-2 variants.24 Consequently, we suffered a marked increase in the infection rate in July and August 2021, when the percentage of the Delta variant was close to 100%; however, this did not lead to a very significant increase in the number of deaths in the general population, as occurred in the epidemic waves before vaccination.25 We did not have clinical information on the effect that this epidemiological situation had on KT patients. Therefore, our objective was to analyze the characteristics and outcomes of a large series of KT with COVID-19 breakthrough infection during this period and compare them with unvaccinated patients. As a second objective, we analyzed the evolution according to the type of mRNA vaccine administered.
Our study predates the appearance of the Omicron variant in our country, which has become dominant in January 2022. This new epidemic phase is still ongoing and should be the subject of future research.
MATERIALS AND METHODS
A national registry to collect information regarding dialysis and KT patients with COVID-19 in Spain started on March 18, 2020, promoted by the Spanish Society of Nephrology (www.senefro.org). Its characteristics have been previously described.26
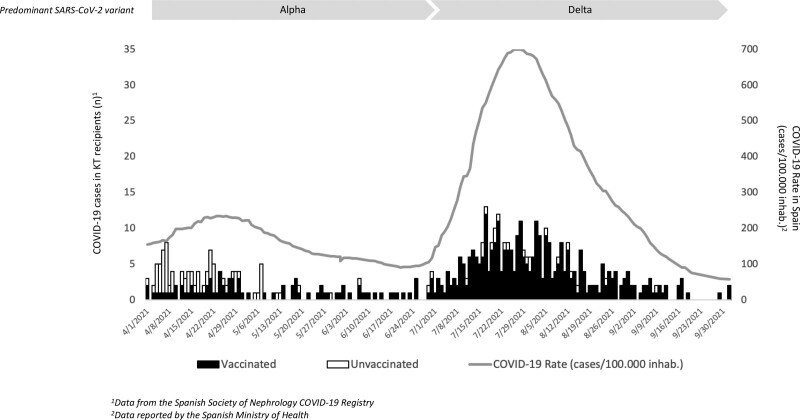
The analyzed period included from April 01, 2021, when the vaccination of transplant patients started on a massive scale, until October 02, 2021. During this period, a new epidemic wave occurred in Spain (infection rate: April 01, 2021, 154 cases/100 000 inhabitants; peak, July 27, 2021, 702 cases/100 000 inhabitants; October, 02, 2021, 58 cases/100 000 inhabitants), and the Delta variant was introduced in our country, becoming in a few weeks the predominant variant (Figure 1).23 All the centers that had reported KT cases in the registry during the study period were contacted and invited to participate, requesting additional information: to review the data communicated, to report all the COVID-19 cases that occurred among their KT patients during those months, and to complete additional clinical and vaccination data against SARS-CoV-2.
FIGURE 1.
Distribution of COVID-19 cases in KT recipients and COVID-19 rate in Spain during the study period. COVID-19, coronavirus disease 2019; KT, kidney transplant; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Variables Collected and Definitions
The variables included in the study are provided in Table 1. Outcomes were assessed as COVID-19–related mortality or recovery until the end of the study. Time to events (death or recovery) was defined as days from COVID-19 diagnosis and recovery as clinical improvement with negative reverse-transcriptase–polymerase-chain-reaction and/or SARS-CoV-2 positive IgG serology. Breakthrough infections were considered in fully vaccinated patients: those who had received either 2 doses of an mRNA vaccine (mRNA-1273 Moderna or BNT162b2 Pfizer-BioNTech), 2 doses of the Oxford–AstraZeneca ChAdOx1 vaccine, or a single dose of the Ad26.COV2.S Janssen vaccine. Patients with partial vaccination were those who only received a single dose of mRNA or ChAdOx1 vaccines.
TABLE 1.
Characteristics of all kidney transplant patients included in the study: Comparison between those fully vaccinated and those partially vaccinated or unvaccinated
| Variables | All (N = 481) | Fully vaccinated (N = 351) | Partially vaccinated or unvaccinated (N = 130) | P |
|---|---|---|---|---|
| Males, n (%) | 298 (62) | 220 (66.7) | 78 (60) | 0.59 |
| Recipient age (y), median [IQR]a | 58 [48, 68] | 58 [48, 68] | 58 [47, 67] | 0.20 |
| DM as cause of kidney disease, n (%) | 49 (10.2) | 37 (10.5) | 12 (9.2) | 0.47 |
| ACEIs treatment, n (%) | 83 (17.2) | 61 (17.3) | 22 (16.9) | 0.59 |
| ARBs treatment, n (%) | 116 (24.1) | 84 (23.9) | 32 (24.6) | 0.71 |
| Time post-KT to COVID-19 (mo), median [IQR]a | 65 [25, 133] | 61 [25, 130] | 77 [25, 139] | 0.72 |
| Time from vaccination to COVID-19 (d), median [IQR] | NA | 81 [57, 105] | NA | NA |
| Type of vaccine | ||||
| mRNA-1273 Moderna, n (%) | NA | 213 (60.7) | NA | NA |
| BNT162b2 Pfizer-BioNTech, n (%) | NA | 121 (34.5) | NA | NA |
| Oxford–AstraZeneca, n (%) | NA | 14 (4) | NA | NA |
| Ad26.COV2.S Janssen, n (%) | NA | 3 (0.8) | NA | NA |
| Immunosuppressive therapy at COVID-19 diagnosis | ||||
| Prednisone, n (%) | 419 (87.1) | 310 (83.3) | 109 (83.8) | 0.19 |
| Tacrolimus, n (%) | 439 (91.3) | 320 (91.2) | 119 (91.5) | 0.89 |
| Mycophenolate, n (%) | 380 (79) | 281 (80.1) | 99 (76.2) | 0.35 |
| mTOR inhibitors, n (%) | 71 (14.8) | 51 (14.5) | 20 (15.4) | 0.81 |
| Cyclosporine, n (%) | 22 (4.6) | 17 (4.8) | 5 (3.8) | 0.64 |
| Belatacept, n (%) | 4 (0.8) | 1 (0.3) | 3 (2.3) | 0.03 |
| Combined immunosuppressive regimen | 0.49 | |||
| Tacrolimus only, n (%) | 32 (6.7) | 25 (7.1) | 7 (5.4) | |
| Tacrolimus + mycophenolate, n (%) | 354 (73.6) | 259 (73.8) | 95 (73.1) | |
| Tacrolimus + mTOR inhibitors, n (%) | 54 (11.2) | 37 (10.5) | 17 (13.1) | |
| Cyclosporine + mycophenolate, n (%) | 11 (2.3) | 10 (2.8) | 1 (0.8) | |
| Other, n (%) | 30 (6.2) | 20 (5.7) | 10 (7.7) | |
| Immunosuppressive therapy within 2 y pre–COVID-19 diagnosisb | ||||
| Thymoglobulin, n (%) | 32 (6.9) | 28 (8.1) | 4 (3.4) | 0.08 |
| Basiliximab, n (%) | 45 (9.7) | 34 (9.8) | 11 (9.2) | 0.85 |
| Rituximab, n (%) | 10 (2.2) | 8 (2.3) | 2 (1.7) | 0.68 |
| Clinical features | ||||
| Asymptomatic, n (%) | 55 (11.4) | 35 (10) | 20 (15.4) | 0.10 |
| Fever, n (%) | 332 (69) | 283 (69.2) | 49 (68.5) | 0.87 |
| Cough, expectoration and/or rhinorrhea, n (%) | 335 (69.6) | 243 (69.2) | 92 (70.8) | 0.74 |
| Gastrointestinal symptoms, n (%) | 141 (29.3) | 108 (30.8) | 33 (25.4) | 0.25 |
| Pneumonia, n (%) | 275 (57.2) | 199 (56.7) | 76 (58.5) | 0.72 |
| COVID-19 management | ||||
| Hospitalized, n (%) | 315 (65.5) | 232 (66.1) | 83 (63.8) | 0.64 |
| Ventilator support, n (%) | 101 (26.9) | 71 (26.6) | 30 (29.7) | 0.85 |
| ICU admission, n (%) | 103 (21.4) | 79 (22.5) | 24 (18.5) | 0.33 |
| Glucocorticoids, n (%) | 291 (60.4) | 213 (60.6) | 78 (60) | 0.79 |
| Tocilizumab, n (%) | 72 (15) | 52 (14.8) | 20 (15.4) | 0.96 |
| Non anti-COVID-19 therapy, n (%) | 109 (22.6) | 78 (22.2) | 31 (23.8) | 0.89 |
| Length of COVID-19 episode (d), median [IQR]a | 16 [11, 24] | 16 [11, 23] | 16 [10, 24] | 0.82 |
| COVID-19 outcomes | ||||
| Dead, n (%) | 103 (21.4) | 76 (21.7) | 27 (20.8) | 0.83 |
aMood’s median test according to order of appearance in the table: P = 0.96, P = 0.42, and P = 0.65, respectively.
bData of 465 patients: 346 fully vaccinated and 119 partially vaccinated/unvaccinated.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; COVID-19, coronavirus disease 2019; DM, diabetes mellitus; IQR, interquartile range; ICU, intensive care unit; KT, kidney transplantation; NA, not applicable.
The study was conducted according to the guidelines dictated by the Declaration of Helsinki. All data were recorded anonymously. Approval was granted by the Scientific Committee of the Spanish Society of Nephrology COVID-19 Registry and the Regional Ethical Committee of Asturias.
Statistical Analyses
Categorical variables were summarized as counts and percentages and continuous variables as median with interquartile range. Unvaccinated or partially vaccinated patients were compared with fully vaccinated patients. In addition, patients vaccinated with mRNA vaccines were compared according to the type of vaccine (mRNA-1273 or BNT162b2). Categorical variables were compared using the Fisher exact test or chi-square test and continuous variables using the t test or the Mann-Whitney U test, as appropriate. Mood’s median test was performed to compare median scores. Survival curves were plotted using the Kaplan-Meier method and compared between patients according to vaccination status and type of mRNA vaccine by log-rank test.
Univariable and multivariable Cox proportional hazard regression analyses were assessed for independent risk factors of COVID-19–related death. In the multivariable analysis, demographic variables, those covariates with a P < 0.10, and vaccination status or type of mRNA vaccine (according to the comparison analyzed) were included. Proportionality assumption in the model was assessed by visual inspection of the log-log survival plots. Covariates included in the model did not violate the proportionality assumption. Differences in anti-COVID-19 therapy were not considered for multivariable analysis, as they were consequences and not factors causing the severity of the infection. Survival time was considered until death, 90 d after a COVID-19 episode, or the end of the study period for both the Cox and the Kaplan-Meier analyses. This 90-d period was chosen because those cases with a new positive reverse-transcriptase–polymerasechain-reaction that had a confirmed SARS-CoV-2 infection >90 d before are considered reinfected.27 Results are expressed as hazard ratios with their 95% confidence intervals. Sensitivity analyses were done: (1) excluding patients with partial vaccination, (2) defining vaccine breakthrough infections according to Centers for Disease Control and Prevention criteria (≥14 d after completing all recommended vaccine doses), and (3) considering only hospitalized patients. We used SPSS version 25.0 (SPSS Inc) for statistical analysis. A P < 0.05 was considered statistically significant.
RESULTS
Characteristics of KT Patients With COVID-19 and Comparison According to Vaccination Status
From April 01, 2021, to October 02, 2021, 500 KT recipients with COVID-19 were included in the registry. Recipients with incomplete data about the type of vaccine or the number of doses (n = 8) and those who had not reported an outcome for the episode (n = 11) were excluded. Finally, 481 adequately documented patients were included: 351 patients had been fully vaccinated and 130 partially vaccinated (n = 40) or unvaccinated (n = 90). Comparison between these 2 groups is shown in Table 1.
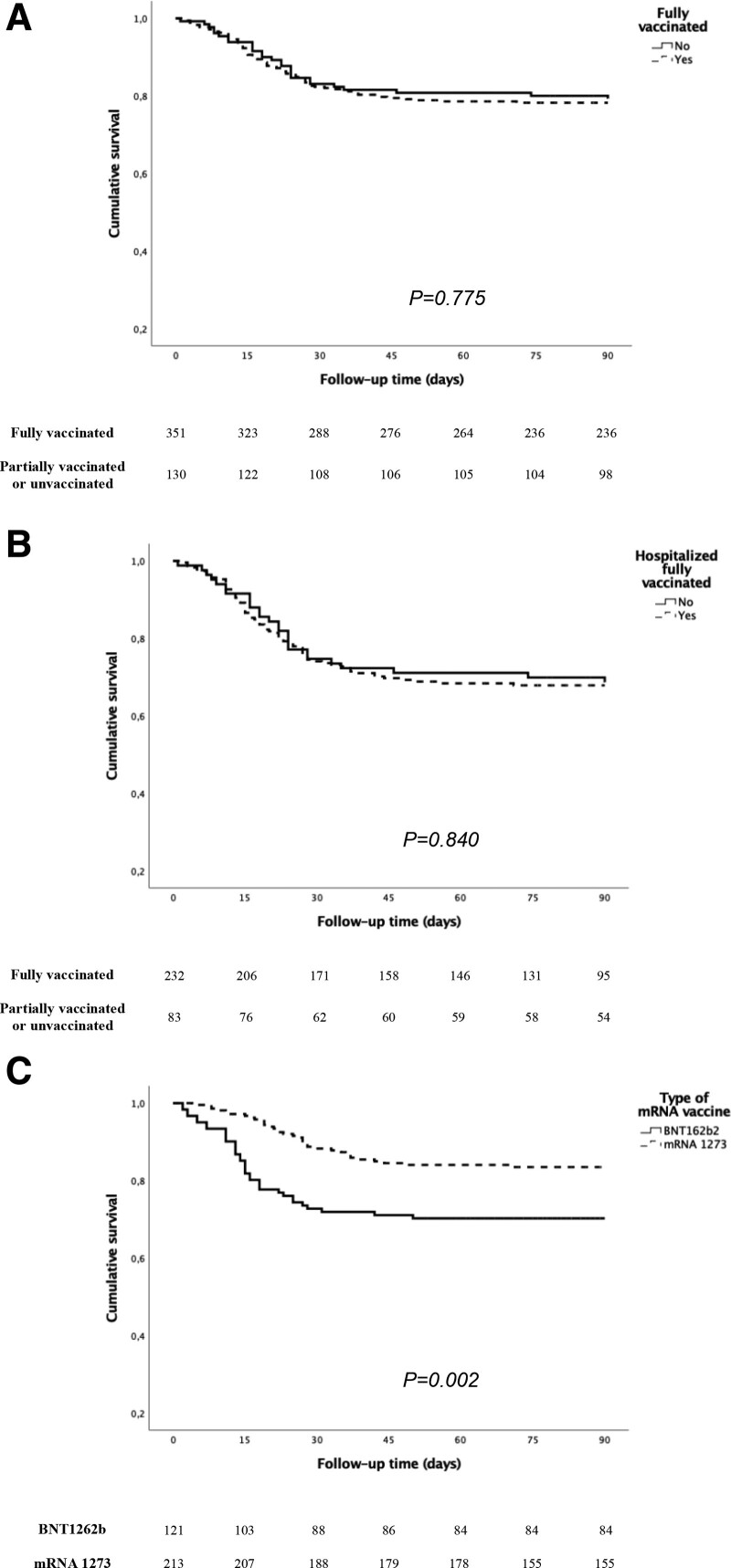
All patients included were older than 18 y, and median age was 58 y. Most fully vaccinated patients received the mRNA-1273 vaccine (n = 213, 60.7%), followed by the BNT162b2 vaccine (n = 121, 34.5%). Tacrolimus-based immunosuppression was the most common drug combination (91.5%) above all tacrolimus with mycophenolate (73.6%). The median time from the second dose to breakthrough infection was 81 d. Age, gender, diabetes as primary kidney disease, baseline immunosuppressive treatment, previous use of thymoglobulin or antibody immunosuppressive therapy, and KT vintage were similar between fully vaccinated patients and the comparison group. We did not observe significant differences in clinical features, the COVID-19 management, and the outcomes: 21.7% of fully vaccinated patients and 20.8% of unvaccinated or partially vaccinated patients died (P = 0.775) (Figure 2A). Table 2 summarizes the univariable and multivariable Cox regression analysis for COVID-19–related death in the whole cohort. Age and pneumonia were independent risk factors, whereas vaccination status was not related to mortality.
FIGURE 2.
Survival function for COVID-19–related death according to vaccination status (A); only in hospitalized patients (B); and patients who received mRNA-1273 or BNT1622b vaccines (C). COVID-19, coronavirus disease 2019.
TABLE 2.
Univariable and multivariable Cox regression analyses for death after COVID-19 in all kidney transplant recipients included in the study
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (y) | 1.06 (1.04-1.08) | 0.000 | 1.05 (1.03-1.08) | 0.000 |
| Sex (male) | 1.16 (0.77-1.74) | 0.463 | 1.00 (0.65-1.54) | 0.974 |
| DMa | 1.91 (1.13-3.23) | 0.015 | 1.36 (0.80-2.31) | 0.246 |
| Fever | 2.65 (1.55-4.51) | 0.000 | 1.58 (0.90-2.80) | 0.110 |
| Respiratory symptomsb | 1.78 (1.10-2.87) | 0.018 | 0.76 (0.44-1.30) | 0.328 |
| Pneumonia | 22.59 (8.31-61.40) | 0.000 | 14.16 (5.02-39.88) | 0.000 |
| Vaccination statusc | 1.06 (0.68-1.65) | 0.953 | 0.95 (0.60-1.50) | 0.836 |
Demographic variables, vaccination status, and all covariates with a P <0.10 in univariable analysis were included. Similar results were observed when the model was adjusted for time from KT.
aDM and kidney disease.
bCough, expectoration, and/or rhinorrhea.
cReference: fully vaccinated.
CI, confidence intervals; COVID-19, coronavirus disease 2019; DM, diabetes mellitus; HR, hazard ratio; KT, kidney transplantation.
Due to the differences in the definition of breakthrough infections, to avoid bias as much as possible, we performed a first sensitivity analysis excluding patients with partial vaccination (fully vaccinated, n = 351, versus unvaccinated patients, n = 90). A second comparison considered only patients infected ≥14 d after completing all recommended vaccine doses as fully vaccinated (fully vaccinated, n = 331, versus unvaccinated or partially vaccinated patients, n = 130). Similar results were obtained in both analyses to those observed in the analysis of the entire population (Supplemental Tables S1–S4, SDC, http://links.lww.com/TP/C390).
In addition, a third comparison was done exclusively in hospitalized patients (n = 315; 232 vaccinated versus 83 partially vaccinated or unvaccinated recipients). This approach matches the disease severity of both vaccinated and unvaccinated groups because the criteria for hospital admission have remained unchanged throughout the study period in our country.28 A higher proportion of women were hospitalized in the unvaccinated/partially vaccinated group, whereas the rest of the variables and outcomes were similar (Supplemental Table S5, SDC, http://links.lww.com/TP/C390). The death rates were 31.3% and 31.9% in hospitalized fully vaccinated patients and those unvaccinated or partially vaccinated, respectively (P = 0.840) (Figure 2B). The same factors associated with COVID-19–related death were identified, and a trend toward a greater risk was also observed in patients treated with mycophenolate at COVID-19 diagnosis (Table 3).
TABLE 3.
Univariable and multivariable Cox regression analyses for death after COVID-19 in the subgroup of hospitalized kidney transplant recipients
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (y) | 1.05 (1.03-1.07) | 0.000 | 1.05 (1.03-1.07) | 0.000 |
| Sex (male) | 1.12 (0.74-1.69) | 0.565 | 1.01 (0.67-1.53) | 0.948 |
| Gastrointestinal symptoms | 0.68 (0.44-1.06) | 0.093 | 0.74 (0.48-1.16) | 0.196 |
| Pneumonia | 4.50 (1.65-12.2) | 0.003 | 3.45 (1.26-9.42) | 0.016 |
| Mycophenolate therapya | 1.78 (1.03-3.09) | 0.038 | 1.67 (0.96-2.90) | 0.068 |
| Vaccination statusb | 1.04 (0.67-1.63) | 0.841 | 0.88 (0.53-1.47) | 0.648 |
Demographic variables, vaccination status, and all covariates with a P <0.10 in univariable analysis were included. Similar results were observed when the model was adjusted for time from KT.
aIMMUNOSUPPRESSIVE therapy at COVID-19 diagnosis.
bReference: Fully vaccinated.
CI, confidence intervals; COVID-19, coronavirus disease 2019; HR, hazard ratio; KT, kidney transplantation.
Comparison of Fully Vaccinated KT Patients According to the Type of mRNA Vaccine
Characteristics and outcomes of KT patients fully vaccinated with mRNA-1273 and with BNT162b2 are detailed in Table 4. We did not observe differences in any of the baseline and treatment-related variables analyzed in our study. Fever was more frequent in those vaccinated with BNT162b2. The incidence of pneumonia (61.2% versus 53.5%) and the hospitalization rate (69.4% versus 62.9%) were slightly higher in those who had received the BNT162b2 vaccine, although without statistical significance. The difference in the death rate in this group compared with those vaccinated with mRNA-1273 was remarkable: 28.8% versus 16.4%, respectively (P = 0.002) (Figure 2C). Multivariable analysis confirmed a higher protective effect for COVID-19–related death of the mRNA-1273 vaccine than the BNT162b2 vaccine (hazard ratio: 0.52; 95% confidence interval, 0.31-0.85; P = 0.010) (Table 5).
TABLE 4.
Characteristics and comparison of fully vaccinated KT patients according to the type of mRNA vaccine
| Variables | mRNA-1273 Moderna (N = 213) | BNT162b2 Pfizer-BioNTech (N = 121) | P |
|---|---|---|---|
| Males, n (%) | 128 (60.1) | 81 (66.9) | 0.21 |
| Recipient age (y), median [IQR]a | 58 [49, 72] | 58.5 [47, 66] | 0.10 |
| DM as cause of kidney disease, n (%) | 18 (7.8) | 12 (9.9) | 0.38 |
| Time post-KT to COVID-19 (mo), median [IQR]a | 63 [28, 143] | 56 [23, 130] | 0.25 |
| Time from vaccination to COVID-19 (d), median [IQR]a,b | 81 [57, 102] | 82 [62, 115] | 0.11 |
| Immunosuppressive therapy at COVID-19 diagnosis | |||
| Prednisone, n (%) | 189 (88.7) | 104 (86) | 0.45 |
| Tacrolimus, n (%) | 198 (93) | 106 (87.6) | 0.10 |
| Mycophenolate, n (%) | 172 (80.8) | 94 (77.7) | 0.50 |
| mTOR inhibitors, n (%) | 28 (13.1) | 20 (16.5) | 0.39 |
| Belatacept, n (%) | 0 (0) | 1 (0.8) | 0.18 |
| Combined immunosuppressive regimen | 0.39 | ||
| Tacrolimus only, n (%) | 15 (7) | 10 (8.3) | |
| Tacrolimus + mycophenolate, n (%) | 162 (76.1) | 83 (68.6) | |
| Tacrolimus + mTOR inhibitors, n (%) | 22 (10.3) | 13 (10.7) | |
| Cyclosporine + mycophenolate, n (%) | 5 (2.3) | 4 (3.3) | |
| Other, n (%) | 9 (4.2) | 11 (9.1) | |
| Immunosuppressive therapy within 2 y pre–COVID-19 diagnosisc | |||
| Thymoglobulin, n (%) | 19 (9) | 9 (7.6) | 0.66 |
| Basiliximab, n (%) | 16 (7.6) | 11 (9.3) | 0.58 |
| Rituximab, n (%) | 4 (1.9) | 3 (2.5) | 0.69 |
| Clinical features | |||
| Asymptomatic, n (%) | 21 (9.9) | 14 (11.6) | 0.62 |
| Fever, n (%) | 139 (65.3) | 92 (76) | 0.04 |
| Cough, expectoration and/or rhinorrhea, n (%) | 142 (66.7) | 87 (71.9) | 0.32 |
| Gastrointestinal symptoms, n (%) | 68 (31.9) | 32 (26.4) | 0.29 |
| Pneumonia, n (%) | 114 (53.5) | 74 (61.2) | 0.17 |
| COVID-19 management | |||
| Hospitalized, n (%) | 134 (62.9) | 84 (69.4) | 0.23 |
| Ventilator support, n (%) | 40 (18.7) | 29 (23.9) | 0.16 |
| ICU admission, n (%) | 47 (22.1) | 29 (24) | 0.69 |
| Glucocorticoids, n (%) | 123 (57.7) | 77 (63.6) | 0.08 |
| Tocilizumab, n (%) | 30 (14) | 20 (16.5) | 0.18 |
| Non anti-COVID-19 therapy, n (%) | 48 (22.5) | 29 (23.9) | 0.87 |
| Length of COVID-19 episode (d), median [IQR]a | 16 [11,24] | 15 [10,23] | 0.11 |
| COVID-19 outcomes | |||
| Dead, n (%) | 35 (16.4) | 36 (29.8) | 0.004 |
aMood’s median test according to order of appearance in the table: P = 0.61, P = 0.45, P = 0.75, and P = 0.20, respectively.
bTime from the second dose of vaccinate to breakthrough infection.
cData of 329 patients: 211 mRNA-1273 Moderna and 118 Pfizer-BioNTech.
COVID-19, coronavirus disease 2019; DM, diabetes mellitus; IQR, interquartile range; ICU, intensive care unit; KT, kidney transplantation.
TABLE 5.
Univariable and multivariable Cox regression analyses for death after COVID-19 in fully vaccinated KT with mRNA vaccines
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (y) | 1.07 (1.04-1.09) | 0.000 | 1.06 (1.03-1.08) | 0.000 |
| Sex (male) | 1.50 (0.90-2.50) | 0.118 | 1.12 (0.65-1.93) | 0.667 |
| Diabetes mellitus as kidney disease | 2.60 (1.44-4.70) | 0.002 | 1.92 (1.04-3.54) | 0.036 |
| Mycophenolate | 1.90 (0.94-3.83) | 0.071 | 1.66 (0.78-3.52) | 0.187 |
| Fever | 2.61 (1.37-4.97) | 0.003 | 1.35 (0.67-2.71) | 0.399 |
| Pneumonia | 15.60 (7.71-42.98) | 0.000 | 8.19 (2.90-23.15) | 0.000 |
| mRNA-1273 Modernaa | 0.48 (0.30-076) | 0.002 | 0.52 (0.31-0.85) | 0.010 |
Demographic variables, vaccination status, and all covariates with a P <0.10 in univariable analysis were included. Similar results were observed when the model was adjusted for time from KT and/or time from vaccination.
amRNA-1273 Moderna versus BNT162b2 Pfizer-BioNTech.
COVID-19, coronavirus disease 2019; CI, confidence intervals; HR, hazard ratio; KT, kidney transplantation.
DISCUSSION
Herein, we present one of the largest analyses on KT recipients with breakthrough SARS-CoV-2 infection published to date. Our national registry-based results show the great vulnerability of KT patients to COVID-19, even in those fully vaccinated. Mortality rate in this group showed no substantial differences compared with the partially vaccinated or unvaccinated patients. Besides, this is the first study to highlight that a 2-dose regimen with mRNA-1273 may offer higher protection for COVID-19–related mortality in KT recipients than the BNT162b2 vaccine during an epidemic wave with predominance of the Delta variant.
The evidence regarding the real-world clinical effectiveness of COVID-19 vaccination in KT recipients is limited so far. Some authors state that SARS-CoV-2 breakthrough infection is infrequent in KT recipients and vaccines offer almost 80% reduction in the incidence of symptomatic COVID-19. In these studies, infection rates in fully vaccinated KT recipients ranged from 0.4% to 1.5%, although data about the epidemiological situation of the countries at the time of the analyses are not provided12,15,16; however, fully vaccinated KT recipients still have a 20-fold-increased risk of breakthrough infection compared with the general population.2,3,12 In accordance with this, Quin et al have reported an 82-fold-higher risk of breakthrough infection and 405-fold-higher risk of hospitalization and death in a population of fully vaccinated solid organ transplant (SOT) patients in the United States.29 Callaghan et al also concluded that vaccination with 2 doses was not associated with a reduction in risk of testing positive for SARS-CoV-2 RNA in the United Kingdom.30
On the other hand, in terms of clinical severity, over 50% of fully vaccinated KT patients may have required hospitalization with severe SARS-CoV-2 infection in the period before the Omicron variant.14,17 In our analysis, up to 56.1% of fully vaccinated patients developed pneumonia, and 66.1% required hospitalization, confirming that severe COVID-19 was still frequent in KT recipients despite completing the vaccination regimen with 2 doses. Second, the first reports that analyzed the outcomes of COVID-19 in vaccinated SOT recipients found a fatality rate that ranged from 5.5% to 26.2% among infected patients.13,14,17,18 Some of these series included patients who only received 1 dose of the vaccine, which may explain this high mortality; however, our study shows a mortality rate of 21.7%, significantly high considering that all patients had received a complete 2-dose vaccination, only slightly lower than that observed in the Spanish KT population in other epidemic waves before vaccination.31
Two multicenter analyses from the United States and from the United Kingdom have recently published the outcomes of a cohort of SOT recipients with breakthrough SARS-CoV-2 infections, reporting COVID-19–related death rates of 9.1% and 9.8%.29,30 These differences observed with respect to our study may be explained by several reasons. First, it is known that there is a reduction in the mRNA vaccine efficacy in the following 4 to 6 mo after the second dose in the general population.4,32 This could have more serious consequences in KTs because the vaccine produces a lower immune response in them.19-22 Some of these reports on breakthrough infections include only the first months immediately after having completed the vaccination, with a much shorter time from vaccination to infection. Opposite, most of our cases were documented as of July 2021, with a high rate of infections, and when for most of our patients >3 mo had passed since receiving their second dose of vaccine. The waning protection of the vaccine happened around the same time the more infectious Delta variant became predominant in Spain in the middle of June 2021.24 Besides, it has been related to a more severe COVID-19 and a higher hospitalization rate in the general population.33 Finally, recipient age, one of the main risk factors for COVID-19–associated death, is not always reported in all studies. Spain has a high KT rate with a large proportion of old KT recipients.34 This combination of factors may have contributed to the lack of vaccine efficacy and the differences in the mortality rate among countries.
Given that 2 doses of SARS-CoV-2 mRNA vaccine cause an insufficient immune response rate in SOT patients, the vaccination strategy was modified in the USA and Europe in late summer 2021.19-22,35 The administration of a third and fourth dose of mRNA COVID-19 vaccine has proved to significantly increase the rate of seroconversion in KT recipients.36-39 Our results, like those reported in other countries about the real-world effectiveness of 2 vaccine doses, highlight the limited efficacy of the initial vaccination schedule and justify the need for third and subsequent doses of the vaccine in KT patients.30,40 In Spain, the Public Health Commission approved on September 16, 2021, the administration of this third dose in SOT patients, among other risk groups.35 Therefore, it is very possible that the results would have been better in vaccinated patients had they received this third dose earlier.
Another factor related to poor response to vaccines in KT recipients is immunosuppressive therapy. Dialysis patients develop significantly higher anti-SARS-CoV-2 antibody levels after vaccination than KT recipients, which suggests the importance of the immunosuppression state.21,22 Specifically, belatacept and mycophenolate have been associated with unresponsiveness.40-42 In patients with severe disease, such as those who required hospitalization, we observed a trend toward a higher risk of COVID-19–related death if they were receiving mycophenolate as maintenance immunosuppression at SARS-CoV-2 infection diagnosis. Individualized strategies based on changing immunosuppression in vaccination have been proposed to improve vaccine efficacy, at least in low immunological risk patients or those at a high risk of a severe course of COVID-19.40,42,43 Our results also show differences in outcomes between the 2 types of mRNA vaccines. The probability of death was significantly lower in patients fully vaccinated with the mRNA-1273 vaccine after adjusting for other risk factors. These differences may be because a more robust and long-term immune response has been described, including against the Delta variant, with the mRNA-1273 vaccine, with a lower incidence of infections, assistance in urgent care clinics, hospitalizations, and ICU admissions.21,44,45 Several aspects have been proposed to explain these differences.39 Although both mRNA-based vaccines encode the prefusion full-length stabilized peak protein of SARS-CoV-2, they differ markedly in dosage such that each dose of the BNT162b2 vaccine releases 30 µg of mRNA, whereas each dose of mRNA-1273 contains 100 µg. Likewise, the lipid composition of nanoparticles used to carry the mRNA content is not the same, which may also contribute. The interval between shots is another variable that differs (21 versus 28 d), and some studies have shown that longer time intervals between the priming and booster doses are associated with greater efficacy. In a recent study in the general population comprising cases until August 2021, Tenforde et al have shown that the mRNA-1273 vaccine is more protective than the BNT162b2 vaccine, especially after 120 d of the 2-dose administration.46 In our experience, although no significant baseline and clinical differences were observed, the incidence of pneumonia, hospitalization, and ICU admission tended to be greater in the patients who received the BNT162b2 vaccine, which could explain the higher death rate observed in these patients. Interestingly, a lower efficacy of the BNT162b2 vaccine than the Oxford–AstraZeneca ChAdOx1-S vaccine, also preceding the appearance of the Omicron variant, has been recently reported in KT patients.30 To date, no data regarding differences in effectiveness between both mRNA vaccines in KT patients in real life had been reported. These differences should be taken with caution until larger studies and, ideally, randomized clinical trials are completed.
Our study has several limitations. We cannot ensure that all cases have been reported. Therefore, our intention has not been to establish an incidence of infections in the vaccinated and unvaccinated KT population; however, we contacted all the centers that had registered cases in the study period, and a professional from each transplant unit was responsible for reporting all the cases and the missing data. In Spain, unlike other countries, the follow-up of KT patients is centralized in the transplant centers, so it is very likely that most cases, even mild or asymptomatic, have been registered. Second, the restricted number of variables collected made the registry an easy tool to report but limited the analysis of some characteristics associated with outcomes, such as cardiovascular risk factors, obesity, or renal function. On the other hand, we do not have generalized information in all centers about the humoral or cellular response to the vaccine before breakthrough infection, which could provide very interesting information to understand the evolution of infected vaccinated patients. Another potential limitation regarding the treatment of COVID-19, is that the registry only has data on the most frequently used drugs; however, during the study period, anti-COVID therapy was standardized in all centers, according to the recommendations of the Spanish guidelines,28 so it is highly unlikely that there were differences in the management of the vaccinated and unvaccinated patients or between centers. The study period preceded the appearance of the Omicron strain, so these results cannot be inferred for infections caused by this variant in KT patients.
In conclusion, COVID-19 severity in KT patients has remained markedly high during a Delta-predominant period and has not improved despite receiving 2 doses of mRNA vaccine, highlighting the poor protection provided by this vaccination schedule to our KT patients in this epidemiological situation. Additionally, we have reported for the first time that the mRNA-1273 vaccine shows a survival advantage over BNT162b2 in KT patients with breakthrough infection. Confirmation of these data will require further research that takes into account the new variants and the administration of third and subsequent vaccine doses. Therefore, it is highly necessary to develop new immunization strategies and advance in more effective anti-COVID-19 therapies. Meanwhile, we must insist on the need for preventive measures and public health measures such as mask wearing and social distancing, especially in KT patients, during the pandemic.
ACKNOWLEDGMENTS
The authors thank members of the Scientific Committee of the Spanish Society of Nephrology COVID-19 Registry and all the physicians and nurses who took care of these patients.
Supplementary Material
Footnotes
Auxiliadora Mazuecos and Florentino Villanego share co-first authorship.
J.P. and M.C. share co-senior authorship.
The authors declare no conflicts of interest.
The Spanish COVID-19 renal registry is supported by the Spanish Society of Nephrology.
A.M., F.V., M.C., and J.P. designed the study, analyzed the data, and drafted the article. All authors revised the article, made substantial contributions, and approved the final version of the article.
Supplemental Visual Abstract; http://links.lww.com/TP/C391.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.World Health Organization. WHO Coronavirus (COVID-19) Dashborad. 2021. Available at https://covid19.who.int/. Accessed November 25, 2021.
- 2.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Ktchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Eng J Med. 2021;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas SJ, Moreira ED, Jr, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson MG, Stenehjem E, Grannis S, et al. Effectiveness of COVID-19 vaccines in ambulatory and inpatient care settings. N Engl J Med. 2021;385:1355–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grange Z, Buelo A, Sullivan C, et al. Characteristics and risk of COVID-19-related death in fully vaccinated people in Scotland. Lancet. 2021;398:1799–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.COVID-19 Vaccination Technical Working Group, of the Program Report and Registry of Vaccinations. Vaccination strategy against COVID-19 in Spain. Update 1. December 18, 2020. 2020. Available at https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/covid19/docs/COVID-19_Actualizacion1_EstrategiaVacunacion.pdf. Accessed November 16, 2021.
- 8.COVID-19 Vaccination Technical Working Group, of the Program Report and Registry of Vaccinations. Vaccination strategy against COVID-19 in Spain. Update 5. March 30, 2021. 2021. Available at https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/covid19/docs/COVID-19_Actualizacion5_EstrategiaVacunacion.pdf. Accessed November 16, 2021.
- 9.Comas J, Arcos E, Torrents A, et al. Situation of vaccination against COVID-19 in transplant patients in Catalonia. Regular updates between March and November 2021. Catalan Transplant Organization. Barcelona. Published 2021. Accessed November 17, 2021.
- 10.Ministry of Health. Government of Spain. Comprehensive management of the COVID-19 vaccination. 2021. Available at https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/vacunaCovid19.htm. Accessed November 18, 2021.
- 11.Ministry of Health. Government of Spain. Update no516. Coronavirus disease (COVID-19). December 2, 2021. 2021. Available at https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_516_COVID-19.pdf. Accessed November 17, 2021.
- 12.Aslam S, Adler E, Mekeel K, et al. Clinical effectiveness of COVID-19 vaccination in solid organ transplant recipients. Transpl Infect Dis. 2021;23:e13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caillard S, Chavarot N, Bertrand D, et al. ; French Society of Transplantation. Occurrence of severe COVID-19 in vaccinated transplant patients. Kidney Int. 2021;100:477–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tau N, Yahav D, Schneider S, et al. Severe consequences of COVID-19 infection among vaccinated kidney transplant recipients. Am J Transplant. 2021;21:2910–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadei HM, Gonwa TA, Leoni JC, et al. COVID-19 infection in solid organ transplant recipients after SARS-CoV-2 vaccination. Am J Transplant. 2021;21:3496–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malinis M, Cohen E, Azar MM. Effectiveness of SARS-CoV-2 vaccination in fully-vaccinated solid organ transplant recipients. Am J Transplant. 2021;21:2916–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali NM, Alnazari N, Mehta SA, et al. Development of COVID-19 infection in transplant recipients after SARS-CoV-2 vaccination. Transplantation. 2021;105:e104–e106. [DOI] [PubMed] [Google Scholar]
- 18.Tsapepas D, Paget K, Mohan S, et al. Clinically significant COVID-19 following SARS-CoV-2 vaccination in kidney transplant recipients. Am J Kidney Dis. 2021;78:314–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villanego F, Cazorla JM, Vigara LA, et al. Protecting kidney transplant recipients against SARS-CoV-2 infection: a third dose of vaccine is necessary now. Am J Transplant. 2022;22:1275–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21:2727–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quiroga B, Soler MJ, Ortiz A, et al. Safety and immediate humoral response of COVID-19 vaccines in chronic kidney disease patients: the SENCOVAC study. Nephrol Dial Transplant. [Epub ahead of print. November 12, 2021]. doi:10.1093/ndt/gfab313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crespo M, Barrilado-Jackson A, Padilla E, et al. Negative immune responses to two-dose mRNA COVID-19 vaccines in renal allograft recipients assessed with simple antibody and interferon gamma release assay cellular monitoring. Am J Transplant. 2022;22:786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ministry of Health. Government of Spain. Quick Risk Assesment. Circulation of major impact and interest for public health SARS-CoV-2 variants in Spain. Update June 7, 2021. 2021. Available at https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/20210608-EER.pdf. Accessed November 18, 2021.
- 24.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385:585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ministry of Health. Government of Spain. Update nº435. Coronavirus disease (COVID-19). August 8, 2021. 2021. Available at https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_435_COVID-19.pdf. Accessed November 25, 2021.
- 26.Crespo M, Mazuecos A, Rodrigo E, et al. ; Spanish Society of Nephrology COVID-19 Group. Respiratory and gastrointestinal COVID-19 phenotypes in kidney transplant recipients. Transplantation. 2020;104:2225–2233. [DOI] [PubMed] [Google Scholar]
- 27.Ministry of Health. Government of Spain. Strategy for early detection, surveillance and COVID-19 control. Update December 18, 2020. 2020. Available at https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/COVID19_Estrategia_vigilancia_y_control_e_indicadores.pdf. Accessed November 25, 2021.
- 28.The Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC). Recommendations of the SEIMC for the clinical management of patients with COVID-19. 2021. Available at https://covid19.seimc.org. Accessed November 25, 2021.
- 29.Quin CX, Morre LW, Anjan S, et al. Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients. Transplantation. 2021;105:e-265–e266. doi:10.1097/TP.0000000000003907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Callaghan CJ, Mumford L, Curtis RMK, et al. ; NHSBT Organ and Tissue Donation and Transplantation Clinical Team. Real-world effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S vaccines against SARS-CoV-2 in solid organ and islet transplant recipients. Transplantation. 2022;106:436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villanego F, Mazuecos A, Pérez-Flores IM, et al. Predictors of severe COVID-19 in kidney transplant recipients in the different epidemic waves: analysis of the Spanish Registry. Am J Transplant. 2021; 21:2573–2582. doi:10.1111/ajt.16579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheik A, McMenamim J, Taylor B, et al. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2462. doi:10.1016/S0140-6736(21)01358-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ministry of Health. Government of Spain. Activity of donation and transplantation in Spain, 2020. 2021. Available at http://www.ont.es/infesp/Memorias/ACTIVIDAD%20DE%20DONACI%C3%93N%20Y%20TRASPLANTE%20ESPA%C3%91A%202020_26042021.pdf. Accessed January 22, 2022.
- 35.COVID-19 Vaccination Technical Working Group, of the Program Report and Registry of Vaccinations. Vaccination strategy against COVID-19 in Spain. Update 9 modified. November 2, 2021. Available at https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/covid19/docs/COVID-19_Actualizacion9_Modificada_EstrategiaVacunacion.pdf.
- 36.Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abedon AT, Alejo JL, Kim JD, et al. 6-mo antibody kinetics and durability after 3 doses of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Transplantation. 2022;106:e281–e283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alejo JL, Mitchell J, Chiang TP, et al. Antibody response to a fourth dose of a SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Transplantation. 2021;105:e280–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chapman JR, Wigmore SJ. Simple vaccination is not enough for the transplant recipient. Transplantation. 2022;106:447–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ou MT, Boyarsky BJ, Chiang TPY, et al. Immunogenicity and reactogenicity after SARS-CoV-2 mRNA vaccination in kidney transplant recipients taking belatacept. Transplantation. 2021;105:2119–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Magicova M, Zahradka I, Fialova M, et al. Determinants of immune response to anti–SARS-CoV-2 mRNA vaccines in kidney transplant recipients: a prospective cohort study. Transplantation. 2022;106:842–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morishita T, Sakai A, Matsunami H. Seroconversions after withdrawal from mycophenolate mofetil in solid organ transplant recipients without a third dose of BNT162b mRNA coronavirus disease 2019 vaccine: a case series. Transplantation. 2022;106:e238–e239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grannis SJ, Rowley EA, Ong TC, et al. ; VISION Network. Interim estimates of COVID-19 vaccine effectiveness against COVID-19-associated emergency department or urgent care clinic encounters and hospitalizations among adults during SARS-CoV-2 B.1.617.2 (Delta) variant predominance - nine states, June-August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1291–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dickerman BA, Gerlovin H, Madenci AL, et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. veterans. N Engl J Med. 2022;386:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tenforde MW, Self WH, Adams K, et al. ; Influenza and Other Viruses in the Acutely Ill (IVY) Network. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA. 2021;326:2043–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]