Abstract
The coronavirus disease 2019 (COVID-19) pandemic has had a major global impact on solid organ transplantation (SOT). An estimated 16% global reduction in transplant activity occurred over the course of 2020, most markedly impacting kidney transplant and living donor programs, resulting in substantial knock-on effects for waitlisted patients. The increased severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection risk and excess deaths in transplant candidates has resulted in substantial effort to prioritize the safe restart and continuation of transplant programs over the second year of the pandemic, with transplant rates returning towards prepandemic levels. Over the past 2 y, COVID-19 mortality in SOT recipients has fallen from 20%–25% to 8%–10%, attributed to the increased and early availability of SARS-CoV-2 testing, adherence to nonpharmaceutical interventions, development of novel treatments, and vaccination. Despite these positive steps, transplant programs and SOT recipients continue to face challenges. Vaccine efficacy in SOT recipients is substantially lower than the general population and SOT recipients remain at an increased risk of adverse outcomes if they develop COVID-19. SOT recipients and transplant teams need to remain vigilant and ongoing adherence to nonpharmaceutical interventions appears essential. In this review, we summarize the global impact of COVID-19 on transplant activity, donor evaluation, and patient outcomes over the past 2 y, discuss the current strategies aimed at preventing and treating SARS-CoV-2 infection in SOT recipients, and based on lessons learnt from this pandemic, propose steps the transplant community could consider as preparation for future pandemics.
INTRODUCTION
The first reports of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), were made to the World Health Organization (WHO) on December 31, 2019. By March 11, 2020, COVID-19 had become a pandemic, and within days, cases were reported in solid organ transplant (SOT) recipients.1
Compared with COVID-19, previous pandemics have had minimal impact on global healthcare delivery systems in general and transplantation in particular.2 The 2009 H1N1 pandemic resulted in high use of intensive care beds for patients with acute lung injury, with Argentina reporting a 50% reduction in organ donors during the disease peak,3 and during the 2003 SARS-CoV-1 epidemic, an outbreak in Toronto required transplant programs to be temporarily closed.4 These limitations, however, were localized and short lived, distinguishing them from the global repercussions of COVID-19.
The impact of COVID-19 on transplantation has varied geographically and over time. The past 2 y have seen national “lockdowns” and mandated nonpharmaceutical interventions to control spread of infection, with restrictions tightening and relaxing in line with “waves” of infection and the emergence of SARS-CoV-2 variants of concern.5 Effective treatments and vaccines have provided promise, but with the pandemic ongoing 2 y later, waitlisted patients, transplant recipients, and transplant programs continue to face unique challenges.
We discuss the impact of COVID-19 on global solid organ transplantation and review the current understanding of the outcomes, treatment, and vaccination against SARS-CoV-2 in SOT recipients.
COVID-19 AND ORGAN DONATION AND TRANSPLANT ACTIVITY
Overview of Changes in Donation and Transplant Activity
At the start of the pandemic, the relative risks and benefits of transplantation in the context of COVID-19 were unknown, and early efforts were made to create risk prediction models to help determine the situations in which transplantation could continue versus being placed on hold.6 The concern of donor-derived disease transmission, adverse outcomes in immunosuppressed recipients, safety of living donors, and reduced availability of intensive care resources resulted in a widespread reduction in transplant activity, although varying approaches were taken by transplant centers within and between countries.7 A study of 22 countries comparing solid organ trasplantation (SOT) rates in 2019 and 2020 estimated a 16% global decrease in transplant activity, most notable in the first 3 mo of the pandemic.8 However, substantial differences were noted between countries, with some experiencing large reductions in transplant activity despite low COVID-19 death rates (Argentina, Japan, Chile), others demonstrating a moderate fall in transplant rates with more sizable death rates (United Kingdom, France, Germany) and some showing a smaller decline in transplant rates despite high COVID-19 deaths (United States, Italy, Belgium).
Deceased Donor Transplantation
Reductions in transplant activity have been noted at all stages of the donation process, with most reports from early in the pandemic. First, reductions in donor referrals of 12%–39% were reported in 2020.9,10 National lockdowns and travel restrictions resulted in a reduction in major trauma and road traffic accidents,11,12 and in some locations, patients were hesitant to seek medical attention for other critical conditions—perhaps relating to fear of burdening already stretched healthcare systems or of contracting SARS-CoV-2 infection themselves.13 Intensive care units were caring for a different population, evidenced by a 4.5% reduction in donors dying from trauma, 25% reduction in donors dying from road traffic accidents, and 35% increase in donors dying from substance abuse over the first wave of the pandemic.14 Restrictions on acceptable donor criteria may have further limited organ referrals,9 and the strain on intensive care clinical teams could have reduced opportunities for broaching organ donation with families.15 Furthermore, it is not uncommon for potential donors to spend an additional 36–48 h in intensive care before donation. With bed, ventilator, and staff shortages, it is possible donor evaluation could not always be accommodated.16
Consent for donation has also varied. In France and the United States, consent fell by >10%,10,14 although the United Kingdom saw a rise in consent rates in the first half of 2020.9 Extended waiting times relating to delays in donor SARS-CoV-2 testing led to withdrawal of consent from some families.15,17,18 Furthermore, many hospitals had visiting restrictions meaning family discussions were held virtually, with prepandemic studies suggesting this associates with lower consent rates.19
A 20%–25% reduction in organ recovery was reported in the first wave of the pandemic,10,14,16,20,21 although regional variation was significant with some areas experiencing reductions of 50%–80%.9,17,22 Furthermore, a US report of kidney transplantation found 21% of kidneys accepted for transplantation in 2020 were discarded, corresponding to COVID-19 surges and most frequently relating to the inability to locate an organ recipient.23 A report from New York early in the pandemic also noted issues with organ allocation, finding organs were declined because of perceived infection risk despite negative donor SARS-CoV-2 swabs,24 instead preferring local graft allocation to protect their teams and limit cold ischemic times given potential delays in organ transport because of reduced air travel.25,26
Declines in deceased donor transplant rates varied by organ, over time, and with geographical location. In Italy, a 25% reduction in transplantation was reported in the first month of the pandemic16 and reductions of 50%–90% were reported in the United States, United Kingdom, Spain, France, and the Netherlands.9,15,17,27 The greatest reduction was in kidney transplantation, with a global decrease of 19% over 2020.8 Declines were seen in all organ types; heart transplantation was least impacted with a 5% global reduction in 2020.8,10,20,21,22,28,29
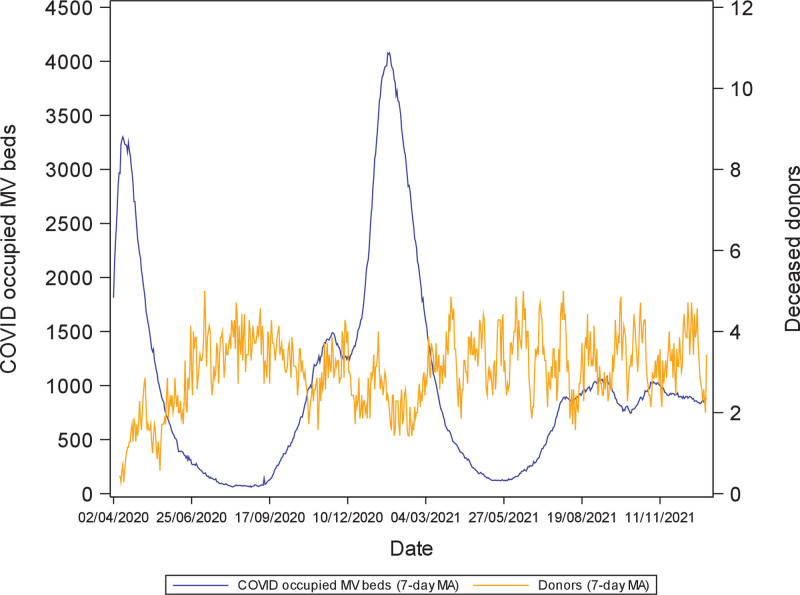
Although geographical location influenced transplant activity, this was only partly explained by local COVID-19 rates,8,21,30 with some countries experiencing greater reductions in activity despite relatively low COVID-19 incidence and others maintaining a greater ability to transplant amid high infection.17,27 Country-level variation may reflect differences in critical care bed capacity,31-33 with UK deceased donation rates following an inverse relationship to COVID-occupied mechanical ventilation beds (Figure 1) and healthcare funding and delivery structures (Table 1). Furthermore, logistical challenges disrupted transplantation even in countries less impacted by COVID-19. For example, Australia enforced strict travel restrictions and border closures. Donation and transplantation services continued but faced barriers relating to the transportation of medical teams, organs, and patients.34 Reduced commercial flights and quarantine requirements caused disruption, and at times, surgical teams were denied state entry for organ retrieval.35 Therefore, although Australia experienced a relatively low incidence of COVID-19 in 2020/2021, reductions in kidney (27%), lung (12%), and liver transplant (8%) activity still occurred.34
FIGURE 1.
Deceased donor numbers in the United Kingdom (7-d MA) by MV bed occupancy by patients with COVID-19 (7-d MA) from April 2020 to December 2021. COVID-19, coronavirus disease 2019; MA, moving average; MV, mechanical ventilation.
TABLE 1.
Critical care beds and healthcare system by country
| Country | Healthcare system | Critical care beds per 100 000 population |
|---|---|---|
| United Kingdom | Tax-based | 6.6 |
| Spain | Tax-based | 9.7 |
| France | Statutory health insurance | 11.6 |
| Italy | Tax-based | 12.5 |
| Germany | Statutory health insurance | 29.2 |
| United States | Health insurance | 34.7 |
Changes in donor type have also been noted. Some centers altered acceptance criteria of deceased donors to protect intensive care beds and maximize the use of available organs. In the United Kingdom, the maximum age for donation after brainstem death and donation after circulatory death (DCD) donors was reduced from 85 to 60 y and 80 to 50 y, respectively, during the first wave.9 This was predicted to reduce number numbers by 47%, reduce nonproceeding offers from 18% to 12%36 and increase the proportion of donation after brainstem death donors from 59% to 79%. Similar limitations to DCD age criteria were placed in Canada for liver transplant recipients,7 and reduced utilization of lower quality organs was noted in the United States.14,23 These practices result in the use of organs with a lower chance of delayed graft function, facilitating shorter hospital stays, and reduced likelihood of requiring critical care support.18 Countries with high use of DCD or extended criteria donors may therefore have been more significantly impacted. Conversely, centers that continued nonlocal organ utilization and did not limit donor criteria did not see such reductions in transplant activity, with 20% of US centers actually increasing their deceased donor transplant activity during the first wave of the pandemic and deceased donation in 2020 being 6% higher than 2019 in the United States.23,37
Living Donor Transplantation
Living donor transplantation has experienced greater reductions in activity than deceased donor transplantation, with 2020 seeing a global 40% reduction in living donor kidney and 33% reduction in living donor liver transplantation, compared with an 11% reduction in deceased donor transplantation.8
Significant reductions or complete suspension of adult living donor transplant programs occurred early in the pandemic, with the greatest reductions in areas of high COVID-19 incidence.10,15,18,22,29,38,39 Reductions related to both donor and recipient concerns and administrative factors, such as loss of access to operating theaters, the need to create safe admissions pathways with designated staff for donors, and the redeployment of transplant teams.39,40 Potentially exposing “well” individuals who do not gain physical health benefits from donation to SARS-CoV-2 infection created ethical dilemmas, particularly when the risks of infection were poorly understood. Living donor programs began to reopen after the first wave but were slow to restart even in areas where deceased donor transplantation continued.41
Recipient Selection for Transplantation During the Pandemic
Centers adopted differing approaches when selecting transplant candidates to remain active on the waiting list, based on the balance of risk of adverse COVID-19 outcomes and benefits of transplantation. Some centers restricted transplantation to their most complex patients, such as those with the most severe organ failure, limited dialysis access options, long waiting times, or high HLA sensitization.7,18,42,43 Others kept lower risk candidates active, such as those not requiring depleting induction therapy, no additional risk factors for severe COVID-19, and higher estimated posttransplant survival scores, who may be anticipated to require shorter hospital stays and be managed out with critical care.10,15,18,22,23
Waiting List Activity
Waiting list registrations decreased in the early stages of the pandemic. In the United States, registrations fell by up to 50% in the first wave,22 and in France, reductions of 27% for lung, 15% for kidney, 10% for heart, and 2% for liver transplants were seen in 2020.10 Furthermore, waitlist suspensions were up to 75% higher than prepandemic levels, with 70% relating to COVID precautions, implying that center-level risks to service delivery or individualized risk assessment decisions at a patient-level necessitated suspensions.20,28 Globally, it is estimated 48 239 waitlisted patient life-years have been lost because of the pandemic.8
Transplant Activity in 2021
With time, the impact of COVID-19 on transplant activity has lessened though not been eliminated. Transplant activity in 2021 has risen, with the most notable increases in living donor and kidney transplantation programmes,44-46 and deceased donor transplantation has generally continued during COVID-19 surges.38 Prioritized SARS-CoV-2 testing for donors and recipients,47 transporting organs instead of living donors,48 the creation of “COVID-free” hospital pathways,42 and protection of transplant teams from redeployment10 are likely to have helped. Vaccination of donors, recipients, and transplant staff may also have played a role, with perceived protection from infection potentially lowering safety concerns and encouraging the reactivation of transplant candidates on the waitlist. Furthermore, the creation of collaborative networks to facilitate transfer of transplant activity to nearby centers in the event of local outbreaks have ensured patients’ need for transplantation is prioritized.49 Ensuring availability of sufficient personal protective equipment, reducing acute bed pressures by opening off-site “field hospitals,” and recruiting additional staff are also likely to have increased centers’ resilience and ability to continue transplantation despite the challenges of COVID-19 surges.
With rising rates of COVID-19 at the end of 2021, there have been concerns of further limitations to organ donation and transplantation. The challenges in restarting transplant programs after suspensions in 2020 means greater emphasis has been placed on maintaining activity.49 National transplantation authorities and societies have provided support and guidance to transplant centers to mitigate risk and ensure appropriate prioritization of transplantation.50-54
COVID-19 AND ORGAN DONORS
COVID-19 in Donors
SARS-CoV-2 is predominantly transmitted by airborne and droplet routes.55 However, viral RNA has been found in hepatocytes, renal tubular cells, and the myocardium of critically ill patients and on postmortem, leading to concerns that donor-derived infection could occur.56 Furthermore, the risk of contaminating operating theaters or exposing surgical teams to the virus resulted in initial hesitancy to accept SARS-CoV-2 positive donors.
Case studies have reported on the use of SARS-CoV-2 positive donors. Liver and kidney transplantation have been successfully performed.57,58 However, the risk appears greater for lung recipients, with at least 3 cases of donor-derived infection reported from donors with negative nasopharyngeal swabs but positive bronchoalveolar lavage samples at time of transplantation. One recipient died, although the nonlung recipients of organs from these donors remained well.59,60
As of January 2022, guidelines continue to recommend caution with SARS-CoV-2 positive donors and avoiding lung transplantation, balancing the risks of transmission to the recipient and transplant team against the recipient’s risk of remaining on the waitlist.60-63 However, SARS-CoV-2 RNA positivity without other signs or symptoms of COVID-19 disease is not an absolute contraindication to transplantation, and RNA levels should be reviewed in detail to determine how infectious the donor is likely to be. The number of polymerase chain reaction amplification cycles needed to detect viral genetic material (the “cycle threshold”) reflects the viral load and can provide information on the likelihood of there being transmissible live virus in the sample analyzed.64,65 There is no absolute cycle threshold that determines whether viable virus is present in the specimen, which varies depending on factors including the sample source, quality, assay used, and stage of infection. When interpreted in the correct context, however, the cycle threshold can provide useful information on the donor infectious status, with higher cycle thresholds associating with a lower likelihood of recovering viable virus. This can be seen in individuals who have recovered from SARS-CoV-2 infection, where SARS-CoV-2 RNA can be obtained for weeks or months in the absence of replicating virus in the respiratory sample. Given the complexity of these situations, input from clinical virologists or infection specialists is advisable.66
For living donors, vaccination, social distancing, and a SARS-CoV-2 RNA test shortly before donation are advised.60 If a donor contracts SARS-CoV-2 infection, recommendations are to consider avoiding surgery for 6–7 wks and ensure a negative RNA test before proceeding with donation.60,67 In India, which has a predominant living donor kidney transplant program, 31 transplants from donors who had recovered from COVID-19 were performed in 2020.40 All donors had 2 negative RNA tests and symptom resolution for 28 d before surgery. No donor complications occurred, and there was 100% patient and graft survival. Living liver donation has also been reported 4 wks after infection in asymptomatic donors, again with no complications noted.68
Donors With Vaccine-induced Thrombosis and Thrombocytopenia
In February 2021, concerns were raised over thrombotic events following SARS-CoV-2 vaccination by vaccine monitoring committees: a syndrome subsequently named vaccine-induced thrombosis and thrombocytopenia (VITT).69 Cases were of cerebral venous sinus thrombosis associated with thrombocytopenia, raised D-dimer and antibodies against platelet factor 4 (anti-PF4), often in previously healthy individuals receiving the first dose of ChAdOx1 nCoV-19 (AstraZeneca) vaccine. The risk of VITT is higher in younger adults, and some countries have since implemented age restrictions for ChAdOx1.70
The initial mortality following VITT was 25%–60%,71,72 and some affected individuals became organ donors. Studies from the United Kingdom, France, and Eurotransplant International Foundation described 19 donors proceeding to organ recovery.73-75 At least 2 livers were discarded preimplantation because of portal vein thrombosis and 1 lung required a thrombectomy, with 57 organs ultimately transplanted into 52 recipients. Follow-up times were short (1–2 mo). Four recipients experienced early graft failure requiring explants (3 livers and 1 kidney), and 1 recipient died of a presumed cardiac event. At least 3 recipients had bleeding episodes, and 6 experienced venous or arterial graft thrombosis. In the UK cohort, 3 of 13 recipients developed anti-PF4 antibodies.73 No recipients in the French cohort developed anti-PF4 antibodies.75
It is difficult to make definitive recommendations based on these small case series, but there seem to be risks of thrombosis or bleeding from donors with VITT, likely relating to preexisting graft endothelial dysfunction.76 Monitoring of recipients’ platelet count, fibrinogen, D-dimer, and anti-PF4 antibodies should be considered.77 A low threshold for biopsy of organs from VITT donors is recommended to look for microvascular thrombosis before organ acceptance.78 Given the greatest incidence in VITT is after the first vaccine dose, it is likely these cases will reduce with time.
COVID-19 IN TRANSPLANT RECIPIENTS
COVID-19 Outcomes
Outcomes in SOT Recipients
From the outset of the pandemic, there have been concerns about the risk of COVID-19 in SOT recipients, relating to their comorbid medical conditions, frequent contact with the healthcare system, and need for systemic immunosuppression. Although COVID-19 mortality has improved, relating to variations in access to testing, SARS-CoV-2 variants, effective treatments, and vaccination, SOT recipients remain at an increased risk of adverse outcomes compared with the general population and infection prevention remains key.79
Factors associated with testing positive for SARS-CoV-2 include older age, non-White ethnicity, having a kidney transplant, being transplanted within the past year, and having a deceased donor as opposed to living donor organ, whereas liver transplant recipients have reduced infection risk.80-83 Further investigation is needed to determine if the ethnic variation with risk of contracting SARS-CoV-2 is confounded by socioeconomic status.
For SOT recipients testing positive for SARS-CoV-2, numerous studies have then examined their outcomes, as summarized in previous reviews.53,84 Many studies were from the first wave and frequently were either from single centers or relied on voluntary reporting of cases when access to testing was limited, resulting in variation in hospitalization and mortality rates. In the first wave, 75%–90% of SOT recipients with COVID-19 were hospitalized,85-87 a third required intensive care or mechanical ventilation,10,85,88-91 and crude mortality rates of 20%–25% were reported.80,85,88,90,92-94
Over time, COVID-19 mortality has improved, although this is at least in part because of more widespread testing and differences in case mix reflecting greater capture of patients with less severe disease.95 When examining hospitalized SOT recipients, in whom disease severity is presumed to be similar, US studies reported reductions in 28-d mortality from 20% to 25% in March–May 2020 to 14% in the latter part of 2020, with mortality remaining lower after adjustment for case mix.89,95 Similar reductions in mortality have been seen in Spain, falling from 26% to 17% over 2020.96 Despite these improvements, the proportion of hospitalized SOT recipients requiring intensive care did not change over 2020, and in this most critically ill group mortality did not significantly improve.89,95-98 In addition to increased testing over time, the management of patients also evolved. Increased use of steroids occurred over 2020,95,96 and may also associate with improvements in outcomes.99
Reports of COVID-19 mortality from 2021 examine the vaccinated population, with crude mortality rates of around 10% in unvaccinated and 8% in vaccinated SOT recipients.100,101 Mortality varies by age, ranging from 2% to 3% in vaccinated individuals under 50 y, to 12%–17% in those >50 y depending on vaccination status and vaccine type, with a possible mortality benefit favoring ChAdOx1 over BNT162b2.100 These outcomes predate the Omicron SARS-CoV-2 variant, the impact of which is still unknown in SOT recipients.
Risk factors for mortality among SOT recipients testing positive for SARS-CoV-2 include older age, cardiovascular and respiratory comorbidities, obesity, and biochemical parameters including lymphopenia, thrombocytopenia, and raised ferritin, C-reactive protein, troponin, or D-dimer.80,85,102 Lung transplant recipients seem to be at increased risk of mortality,96,100 as are those of Black ethnicity.100 Although there is increased infection risk in patients transplanted more recently, no consistent association between transplant vintage and mortality has been observed,93,94,102,103 nor with immunosuppression regime (although immunosuppression does associate with immunological response to vaccination).
The earlier data highlight the impact of COVID-19 on SOT recipients. However, it must also be considered whether this reflects excess mortality among the SOT population, that is, whether overall mortality is greater than that from the prepandemic era. This has been illustrated by a registry analysis of the US kidney transplant population. Here, the 2020 death rate was 20% higher than in 2019, with 16% of deaths attributable to COVID-19. Recipients dying of COVID-19 were more likely to be younger, obese, of lower educational attainment and of an ethnic minority group than those dying of other causes. Furthermore, there were fewer non-COVID deaths in White recipients than previous years and almost no excess mortality in this group, contrasting with large numbers of excess deaths from COVID and non-COVID causes in Black and Hispanic recipients.104
Outcomes in Waitlisted Patients
The risk of adverse outcomes among SOT recipients with COVID-19 has helped guide decision making on whether to continue transplantation through the pandemic. However, this must be balanced against the risk to patients on the waiting list.6 The risk benefit balance varies by transplant type, availability of other treatments for organ failure, and the risks associated with these treatments. This is particularly noteworthy for patients with kidney failure in whom dialysis is an option, providing an alternative to transplantation but often at the expense of frequent healthcare contact and reduced ability to socially distance.
The risk of SARS-CoV-2 infection in waitlisted patients is 2–3 times higher than that of SOT recipients, with the highest rates in kidney, kidney pancreas, and intestinal transplant candidates.80,103 Although there may be some bias in these estimates relating to testing practice, waitlisted patients are conceivably less able to distance resulting in an increased infection risk, and SARS-CoV-2 outbreaks in dialysis units have occurred.105 COVID-19 mortality is lower among waitlisted patients than SOT recipients (10% versus 25% during the first wave), but the increased incidence results in comparable overall COVID-19 mortality between groups.80,81,103
Again, the indirect effects of COVID-19 on waitlisted patients and excess mortality relating to cessation of transplant programs also needs considered. In a US study from March–May 2020, kidney transplant candidates had a 37% greater risk of all-cause mortality than prepandemic, although this finding was not replicated in other SOT candidates.106 A US kidney transplant registry study found 11% of deaths on the waiting list related to COVID-19 in 2020 and overall waitlist mortality was 26% higher than in 2019.104 These risks have tipped the balance in favor of continuing transplantation for most patients.
MANAGEMENT OF COVID-19: INFECTION PREVENTION
COVID-19 Vaccination
SARS-CoV-2 vaccines have been rapidly developed and mass vaccination programs began in December 2020. As of November 2021, 7 vaccines have WHO Emergency Use Listing, including the mRNA vaccines BNT162b2 (Pfizer/BioNTech) and mRNA-1273 (Moderna) and viral vector vaccines ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and Ad26.COV2.S (Johnson & Johnson).107 Primary vaccination courses comprise 2 doses except Ad26.COV2.S, which only requires 1 dose. In the general population, vaccine efficacy of 70%–95% is reported.108
Most countries have adopted vaccination prioritization processes, including priority for SOT recipients.109-112 Uptake has generally been good, with 93% of SOT recipients double vaccinated by September 2021 in England,100 and 80% receiving at least 1 dose by October 2021 in Italy.113 An international survey of SOT recipients in 2020 suggested 85% planned to accept vaccination,114 although uptake has varied by geographical location and ethnicity.115
Although SOT recipients are prioritized for vaccination, vaccine efficacy in this population was not tested before roll-out.116-119 SOT recipients mount lower immunological responses to vaccines than general populations,120 and breakthrough infections were reported from early 2021.121,122 Multiple studies have since examined SOT recipients’ immunological and clinical responses to vaccination.
Immunological Response to Vaccination
The threshold for protective immunity against SARS-CoV-2 is not known, with both antibody and T-cell responses being important. Anti-spike immunoglobulin G (IgG) concentration correlates with neutralizing capacity,123,124 and neutralizing capacity is predictive of immune protection.125,126 It should, however, be noted that the threshold for protection against severe infection is likely to be lower than that required to prevent infection.127
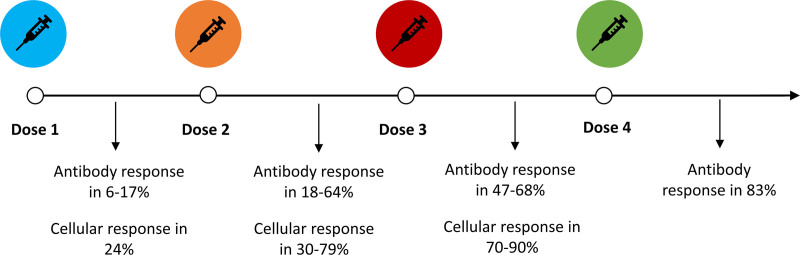
After the first and second doses of SARS-CoV-2 vaccines, immunological responses are lower in SOT recipients than the general population (Table 2; Figure 2). After 1 dose, antibody responses were detected in just 6%–17% of SOT recipients with minimal neutralizing capacity,128-134 although cellular responses were more frequently observed in around 25% of patients.133,134 After a second dose, the proportion of recipients with antibody responses rises to 18%–64%, with neutralizing capacity in two-thirds of these.131,132,135-145 Cellular responses are also higher, noted in 30%–79% of patients.133,134,140,142 Given T-cell responses can occur without detectable antibody responses, patients without antibodies could still mount a sufficient immune response to prevent severe infection. As such, the presence of antibodies should not be interpreted as indicating “immune protection” and routine antibody monitoring after vaccination is not universally recommended,146 although it may still have a role in some circumstances, by determining eligibility for clinical trials and in assisting the prioritization of patients for treatments such as monoclonal antibodies.
TABLE 2.
Studies examining immunological responses to first and second doses of a COVID-19 vaccine
| Author | Population | Vaccine | Dose | Measured response | Time postvaccine | Proportion with response | Associations with reduced response |
|---|---|---|---|---|---|---|---|
| Boyarsky et al128 | 436 SOT recipients | BNT162b2 mRNA-1273 |
First | Anti-spike IgG | 20 d | 17% | Antimetabolite Increased age BNT162b2 vaccine |
| Benotmane et al129 | 242 kidney transplant recipients | mRNA-1273 | First | Anti-spike IgG | 28 d | 10.8% | Shorter time from transplant Use of MMF Use of steroids Higher creatinine |
| Yi et al130 | 145 kidney transplant recipients | BNT162b2 MmRNA-1273 |
First | Anti-spike IgG | At second dose | 5.5% | Not examined |
| Boyarsky et al131 | 658 SOT recipients | mRNA-1273 BNT162b2 |
First Second |
Anti-spike IgG | 21–29 d | Dose 1: 15% Dose 2: 54% |
Not examined |
| Marion et al132 | 895 first dose, 367 second dose SOT recipients | BNT162b2 MmRNA-1273 |
First Second |
Anti-spike IgG | 28 d | Dose 1: 6.4% Dose 2: 33.8% |
Not examined |
| Bertrand et al133 | 45 kidney transplant recipients | BNT162b2 | First Second |
Anti-spike IgGIFN-γ producing T cells | 21–28 d | Dose 1: 2.2% humoral 24.4% cellular Dose 2: 17.8% humoral 57.8% cellular |
Humoral response: Recent transplantation Immunosuppression regime Cellular response: No significant association |
| Schmidt et al134 | 40 SOT recipients | BNT162b2M mRNA-1273ChAdOx1 |
First Second |
Anti-spike IgG Neutralizing capacity IFN-γ producing T cells |
Dose 1: 5.3% humoral 23.7% cellular 26.3% humoral or cellular Dose 2: 35.3% humoral 64.7% cellular 70.6% humoral or cellular |
Homologous vaccine regime | |
| Prendecki et al135 | 920 kidney transplant recipients | BNT162b2 ChAdOx1 |
Second | Anti-spike IgG T-cell response to spike protein |
31 d | 55% humoral 11% cellular 58% humoral or cellular |
Humoral response: ChAdOx1 vaccine Transplantation within 1 y Diabetes |
| Stumpf et al136 | 368 kidney transplant recipients | BNT162b2 mRNA-1273 |
Second | Anti-spike IgG/IgA Neutralizing capacity IFN-γ producing T cells |
28–35 d | 42% humoral response 66% with neutralizing capacit 30% cellular response |
Use of MMF Use of CNI Use of belatacept BNT162b2 vaccine Older age Shorter duration of transplant |
| Rozen-Zvi et al137 | 308 kidney transplant recipients | BNT162b2 | Second | Anti-spike IgG | 28 d | 36.4% | Higher MMF dose Higher CNI levels Lower eGFR Older age |
| Kantauskaite et al138 | 225 kidney transplant recipients | BNT162b2mRNA-1273 | Second | Anti-spike IgG Neutralizing capacity |
14 d | 24.9%68% with neutralizing capacity | Higher trough MMF concentration Lower eGFR |
| Benotmane et al139 | 205 kidney transplant recipients | mRNA-1273 | Second | Anti-spike IgG | 28 d | 47.8% | Previous kidney transplant Shorter transplant duration Lower eGFR More immunosuppression |
| Cucchiari et al140 | 148 kidney and kidney pancreas recipients | mRNA-1273 | Second | Anti-spike IgG/MIFN-γ producing T cells | 14 d | 29.9% humoral 54.7% cellular 65% humoral or cellular |
Humoral response: Immunosuppression regime Cellular response: Diabetes Lymphopenia Decreasing eGFR ATG within 1 y |
| Grupper et al141 | 136 kidney transplant recipients | BNT162b2 | Second | Anti-spike IgG | 16.5 d | 37.5% | Older age High dose steroid in past y Triple immunosuppressionUse of MMF |
| Herrera et al142 | 104 liver and heart transplant recipients | mRNA-1273 | Second | Anti-spike IgGIFN-γ producing T cells | 28 d | 64% humoral 79% cellular 90% humoral or cellular |
Humoral response: Hypogammaglobulinemia Transplant within 1 y Higher dose of MMF Cellular response: Hypogammaglobulinemia |
| Rabinowich et al143 | 80 liver transplant recipients | BNT162b2 | Second | Anti-spike IgG | 14.8 d | 47.5% | Older age High dose steroid in past y Use of MMF Triple immunosuppression Lower eGFR |
| Peled et al144 | 77 heart transplant recipients | BNT162b2 | Second | Anti-RBD IgG Neutralizing antibody |
21 d | 18% 57% with neutralizing capacity |
Use of MMF |
| Marinaki et al145 | 34 SOT recipients | BNT162b2 | Second | Anti-spike IgG | 10 d | 58.8% | Use of MMF |
This represents merely a selection of studies and is not an exhaustive list.
ATG, anti-thymocyte globulin; CNI, calcineurin inhibitor; COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate; IFN-γ, interferon gamma; Ig, immunoglobulin; MMF, mycophenolate mofetil; RBD, receptor-binding domain; SOT, solid organ transplant.
FIGURE 2.
Immunological responses to severe acute respiratory syndrome coronavirus 2 vaccination doses in solid organ transplant recipients. References for this figure are taken from studies within Tables 2 and 3.
Immunological response to vaccination varies by patient and transplant factors. The number and type of immunosuppressants appears important. Patients on mycophenolate mofetil (MMF)–containing regimes mount lower antibody responses than those not on MMF.128,129,136-138,141-145 This follows a dose dependent effect, with patients receiving under 1 g/d having a 5 times higher humoral response than patients on higher doses. Furthermore, following cessation of MMF, seroconversion has been noted in SOT recipients previously seronegative after 2 vaccine doses.147 Patients receiving belatacept are also less likely to mount immunological responses.136,148,149 Other factors associated with reduced immunological responses include increased age,129,136,137,141,143 and lower kidney function, with every 1 mL/min/1.73m2 increase in estimated glomerular filtration rate (eGFR) associating with a 3% increased likelihood of developing anti-spike IgG.128,136-138 Variation has also been observed with vaccine type. Humoral responses are more pronounced with mRNA-1273 than BNT162b2, and BNT162b2 than ChAdOx1,128,135,136 although cellular responses may be greater after viral vector vaccine priming. Heterologous second doses (ie, using a vaccine with a different mechanism of action) may also associate with greater immune responses.134
The timing of vaccination in relation to transplantation is also likely to be important. Patients with a longer duration of transplantation have better immunological responses than recipients transplanted more recently, particularly within the past year.129,133,135,136,142,143 Response to vaccination is also greater in patients on dialysis or the liver transplant waiting list than SOT recipients, with 80%–95% having antibodies after 2 doses in addition to greater T-cell responses, suggesting vaccination before transplantation is likely to be beneficial.133,136,150-152 Early reports of kidney transplant recipients who were vaccinated pretransplantation show seroconversion is sustained posttransplantation.153
Clinical Responses
Although immunological responses to standard vaccine regimes in SOT recipients may be disappointing, vaccination still improves some clinical outcomes. By April 2021, breakthrough infection had occurred in 0.83% of vaccinated SOT recipients in the United States,101,154 and by October 2021 in Scotland breakthrough infection occurred in 8% of kidney transplant recipients, with infection being more frequent in those of younger age or from areas of deprivation.155 These breakthrough rates are greater than in the general population,79,156 and a national registry study in England found that vaccination does not reduce the risk of testing positive for SARS-CoV-2 in SOT recipients. Infection was in fact more frequent in vaccinated recipients (incidence rate ratio, 1.29; 95% confidence intervals [CI], 1.03-1.61), hypothesized to relate to risk compensation behavior in vaccinated individuals.157 Of SOT recipients testing positive for SARS-CoV-2, vaccination associated with a 20% reduction in risk of death (8.2% versus 10.4%), but this was driven by a 30% mortality reduction in those receiving ChAdOx1 (hazard ratio, 0.69; 95% CI, 0.52-0.92), whereas BNT162b2 did not confer mortality benefit (hazard ratio, 0.97; 95% CI, 0.71-1.31).100 In Scotland, vaccine effectiveness of 40% at preventing infection and hospitalization has been being reported in kidney transplant recipients,155 lower than the 70%–90% vaccine effectiveness in general populations.158-160 These findings highlight the importance of additional protective measures in SOT recipients such as further vaccine doses, use of novel antivirals and monoclonal antibodies, and ongoing adherence to nonpharmaceutical interventions such as face masks and social distancing.
Repeated Doses
The reduced immunological and clinical responses to vaccination in SOT recipients have led to the investigation of third vaccine doses (Table 3; Figure 2). A randomized control trial of the mRNA-1273 vaccine found that a third dose 2 mo after the primary vaccine course resulted in a significant rise in the proportion of SOT recipients with detectable antispike IgG (55% versus 18%) and an increase in SARS-CoV-2 reactive T cells.161 Nonrandomized studies have similarly shown improvements in antibody and T-cell responses after a third dose, including improved serum neutralizing capacity and rises in antibody titers in previously seropositive patients. Between 30% and 50% of seronegative SOT recipients after 2 doses seroconverted after a third dose.162,163,164,165-168 However, by 6 mo postvaccination immunological responses can wane,169 with a 64% reduction in antibody titer and 62% reduction in T-cell activity being reported in kidney transplant recipients.170
TABLE 3.
Immunological responses to third dose of a COVID-19 vaccine
| Author | Population | Vaccine | Measured response | Time postvaccine | Response rate | Associations reduced response |
|---|---|---|---|---|---|---|
| Del Bello et al162 | 396 SOT recipients | BNT162b2 | Anti-spike IgG | 28 d | 5.1% after first dose, 41.4% after second dose, 67.9% after third dose 45% seronegative patients after second dose seroconverted after third dose |
Older age Use of MMF Use of belatacept |
| Benotmane et al163 | 159 kidney transplant recipients | mRNA-1273 | Anti-spike IgG | 28 d | Only examined patients with no significant response to 2 vaccine doses 49% seroconverted after third dose |
Triple agent immune suppression |
| Kamar et al164 | 101 SOT recipients | BNT162b2 | Anti-spike IgG | 28 d | 40% after 2 doses to 68% after third dose 44% seronegative patients after second dose seroconverted after third dose |
Increased age Lower eGFR |
| Bertrand et al165 | 80 kidney transplant recipients | BNT162b2 | Anti-spike IgGIFN-γ producing spike-reactive T cells | Minimum 4 wks | Humoral response: 37.5% after second dose to 61.2% after third dose Cellular response: 51.2% after second dose to 70% after third dose |
Use of belatacept Use of MMF |
| Massa et al166 | 61 kidney transplant recipients | BNT162b2 | Anti-spike IgG Neutralizing capacity IFN-γ producing spike-reactive T cells |
28 d | 44.3% after second dose to 62.3% after third dose One-third seronegative patients after second dose seroconverted after third dose Increase in neutralizing capacity after third dose Rise in frequency of spike-reactive T cells |
Use of antiproliferative Lymphopenia |
| Werbel et al167 | 30 SOT recipients | BNT162b2 mRNA-1273 |
Anti-spike IgG | 60 d (second dose), 14 d (third dose) | 20% after second dose to 47% after third dose One-third seronegative patients after second dose seroconverted after third dose |
Not examined |
| Schrezenmeier et al168 | 25 kidney transplant recipients | BNT162b2 ChAdOx1 |
Anti-spike IgG/IgACD4 T-cell reactivity to spike peptide mix | 7–28 d | 36% seronegative patients after second dose seroconverted after third dose; 28% after homologous and 45% after heterologous vaccination Spike-specific CD4 T-cell responses in over 90% after the second and third dose |
Not examined |
COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate; IFN-γ, interferon gamma; Ig, immunoglobulin; MMF, mycophenolate mofetil; SOT, solid organ transplant.
Clinical outcomes in SOT recipients following third vaccine doses are not yet reported. In general populations, the risk of SARS-CoV-2 infection starts to rise from 90 d postvaccination,171 and third doses associate with a reduced risk of infection and mortality.172,173 In dialysis patients, 3 vaccine doses are required to protect against infection from the Omicron SARS-CoV-2 variant, although similar studies in SOT recipients are awaited.174
Based on current evidence, 3 vaccine doses are now frequently recommended as a “primary course” for SOT recipients, followed by a fourth “booster” dose.175,176 A small case series of SOT recipients receiving a fourth dose found 63% of those with negative or low-positive antibody titers after 3 doses developed high titer responses after dose 4.177
Vaccination Strategies
Based on the earlier evidence, vaccination of transplant candidates and recipients is strongly recommended in transplant guidelines, with vaccination occurring pretransplantation if possible.146,178 The optimum vaccine timing posttransplantation is unknown, although most guidelines suggest waiting for 1–3 mo to optimize vaccine responses.179-181
For transplant candidates who decline vaccination, there are ethical issues surrounding transplantation.182 The risks to the patient, their graft, transplant programs, and society need to be considered but must be balanced against autonomy and justice. Clinicians should discuss vaccination with patients, although most suggest declining vaccination not be an absolute contraindication to transplantation.183
Given the reduced responsiveness to vaccination in SOT recipients, additional doses, “ring immunization” (prioritizing household members and caregivers for vaccines), and mandatory vaccination of healthcare staff is being considered in some countries.184 Although the optimum vaccination strategy is waiting to be determined, continued adherence to nonpharmaceutical interventions is an advisable supporting strategy.
Nonvaccine Prophylaxis Against COVID-19
There is some evidence to support addition nonvaccination treatments to prevent COVID-19. The receipt of the SARS-CoV-2 monoclonal antibody casirivimab plus imdevimab after a household exposure to COVID-19 associated with a 66% relative risk reduction of developing infection and faster resolution of symptoms in clinical trials predating the emergence of the Omicron variant185 and is licensed in the United States for individuals at high risk of severe disease who are a close contact of a positive case.186 Casirivimab plus imdevimab, however, does not maintain efficacy against Omicron, and currently there are no authorized treatments with anti-Omicron activity for postexposure prophylaxis. The United States has emergency use authorization for the long-acting monoclonal antibody tixagevimab plus cilgavimab as preexposure prophylaxis in immunocompromised individuals,187 although the evidence for this also predates the Omicron variant.188 Results are awaited from further studies on novel prophylactic treatments in SOT recipients without SARS-CoV-2 exposure.187,189,190
MANAGEMENT OF COVID-19: TREATMENT OF SARS-COV-2 INFECTION
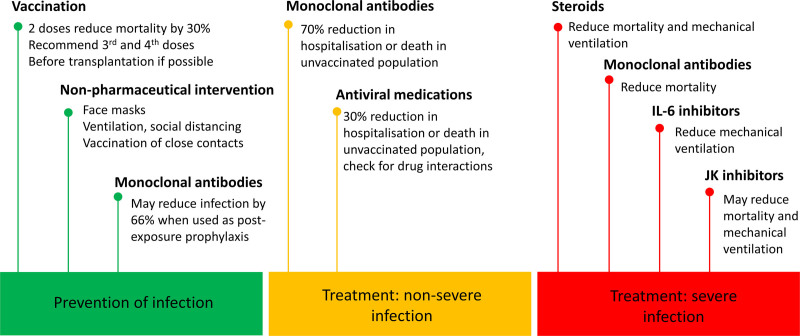
There are >5000 registered randomized control trials of treatments for COVID-19, and this field is changing rapidly. The below summarizes evidence as of December 2021. Living meta-analyses, such as those by the MAGIC Evidence Ecosystem Foundation (www.magicproject.org), provide up-to-date information. Current management of COVID-19 is illustrated in Figure 3.
FIGURE 3.
Strategies for preventing and treating severe acute respiratory syndrome coronavirus 2 infection in solid organ transplant recipients. References for this figure are quoted within the main text of the article. IL, interleukin; JK, Janus kinase.
Treatment of Patients With Nonsevere COVID-19
For patients with nonsevere COVID-19 managed in the community, studies suggest no benefit to azithromycin,191 doxycycline,192 and colchicine,193 and although inhaled corticosteroids may reduce symptom duration in older patients or those with comorbidities, their effect on hospitalization and mortality is less clear.194
In patients with risk factors for severe COVID-19, such as SOT recipients, monoclonal antibodies against the SARS-CoV-2 spike protein have shown potential in randomized controlled trials. Casirivimab plus imdevimab reduces hospitalization or death by 70% and shortens symptoms by 4 d.195 Sotrovimab administered within 5 d of symptom onset is associated with an 85% relative risk reduction of hospitalization or death at 1 mo (1% treatment group versus 7% placebo group) in a prespecified interim analysis,196 with positive outcomes predominantly driven by reductions in hospitalization.197 Similar outcomes to these large studies have been reported in small series of SOT recipients.198,199 It should be noted that these studies were performed before the emergence Omicron variant and before vaccination, although case reports of vaccinated SOT recipients infected with the Alpha, Delta, and Gamma variants suggest monoclonal antibodies could still be beneficial.200 Furthermore, casirivimab plus imdevimab is less effective against the Omicron variant, and given sotrovimab has greater proposed efficacy treatment choices may need to reflect the dominant SARS-CoV-2 variant.201 Finally, monoclonal antibodies are currently given as an intravenous or subcutaneous infusion, so pathways to facilitate their administration safely to ambulatory patients are needed. Intramuscular sotrovimab is reported to offer similar efficacy to intravenous formulations and, if confirmed, could expand delivery options and improve access to treatment.202
The oral antiviral drug molnupiravir, a competitive nucleoside analogue in RNA dependent RNA polymerase, has also shown promise in nonhospitalized patients at risk of severe COVID-19. An interim analysis of 775 patients in the MOVe-OUT study suggested a 45% reduction in hospitalization or death,203 although the full cohort analysis in 1433 patients showed a more modest relative risk reduction of 30%, with 6.8% of patients receiving molnupiravir dying or requiring hospitalization versus 9.7% in the control arm.204 Again, studies were performed before vaccination and patients with an eGFR < 30 mL/min/1.73m2 were excluded, although molnupiravir is not renally excreted and benefits in patients with renal dysfunction likely outweigh risks. Immunosuppressant drug interactions are not expected, although molnupiravir is teratogenic and contraceptive advice is required.
Other antiviral drugs also show promise. An interim analysis of the oral antiviral ritonavir-nirmatrelvir suggests an 89% reduction in hospitalization or death if taken within 3 d of symptoms in individuals at risk of severe disease and has been approved for use in the United Kingdom and United States.205 Ritonavir, however, is a CYP-450 inhibitor and interacts with calcineurin and mammalian target of rapamycin inhibitors, requiring dose reductions and close drug level monitoring.206 Careful supervision by experienced transplant professionals appears sensible, whilst studies of its safety in SOT recipients are awaited. Furthermore, results from the PINETREE study published in January 2022 show a 3-d course of intravenous remdesivir in patients at risk of severe COVID-19 reduces hospitalization or death by 87%, although again would require establishment of safe pathways to facilitate its administration to outpatients.207
Treatment of Patients With Moderate to Severe COVID-19
The WHO Guideline Development Group living systematic review and meta-analysis evaluates treatments with sufficient evidence on which to make recommendations.208 In general populations, for patients with severe disease (oxygen saturation <90%, respiratory distress, or organ support), current evidence is in favor of:
Steroids (eg, dexamethasone): these reduce death (odds ratio [OR], 0.83; 95% credible interval [CrI], 0.69-0.98, moderate certainty) and mechanical ventilation (OR, 0.76; 95% CrI, 0.59-0.99, moderate certainty).
Interleukin-6 inhibitors (eg, toculizumab, sarilumab): these reduce mechanical ventilation (OR, 0.72; 95% CrI, 0.57-0.90, moderate certainty) and length of hospital stay (−4.5 d; 95% CrI, −6.7 to −2.3) but have an uncertain effect on mortality (OR, 0.87; 95% CrI, 0.74-1.05, low certainty). Case series of tocilizumab in SOT recipients suggest it is safe to use.209,210
Monoclonal antibodies against SARS-CoV-2 (eg, casirivimab plus imdevimab, sotrovimab): these reduce mortality in patients seronegative at diagnosis.211
Janus kinase inhibitors (eg, baricitinib, ruxolitinib): these are targeted synthetic disease modifying antirheumatic drugs that interfere with cytokine signaling pathways. These may reduce mortality (OR, 0.58; 95% CrI, 0.33-1.00) and mechanical ventilation (OR, 0.57; 95% CrI, 0.33-0.95), although the certainty of benefit is low.
Recommendations are currently against the use of hydroxychloroquine, azithromycin, interferon-beta, ivermectin, lopinavir-ritonavir, and convalescent plasma (unless in a clinical trial) given a lack of clinically significant effects on outcomes.208 The antiviral remdesivir had initial positive reports, but meta-analysis shows no difference in mortality (OR, 0.90; 95% CI, 0.70-1.12) or mechanical ventilation (OR, 0.89; 95% CI, 0.76-1.03) based on low certainty evidence, resulting in a weak recommendation against its use from WHO.208 Despite this, remdesivir continues to be recommended under specific circumstances in other clinical practice guidelines,212 and more data are needed on its use in SOT recipients in whom its effect on outcomes may differ compared with the general population.
Immunosuppression Management
There is no robust evidence to guide the management of immunosuppression in the context of COVID-19, though minimization of immunosuppression is common with other viral infections.213 However, given many of the severe manifestations of COVID-19 relate to release of proinflammatory cytokines,214 the balance of immunosuppression in the setting of COVID-19 is complex.215
At present, preemptively reducing maintenance immunosuppression in SOT recipients without COVID-19 to reduce the risk of acquiring infection, progression to severe disease, or improve vaccine responses has not generally been recommended, partly because of concerns over reduced ability to follow up patients during COVID-19 surges.216 In those testing positive for SARS-CoV-2, a stepwise reduction of immunosuppression is usually performed. This typically starts with the antimetabolite, with MMF being reduced or stopped. Further reductions are suggested in patients with severe disease, although there is no comparative data to guide recommendations and decisions should be based on individualized assessment of the severity of COVID-19 and consequences of rejection.217-220
PSYCHOLOGICAL IMPACT OF THE PANDEMIC ON PATIENTS AND STAFF
The impact of the pandemic on individuals cannot be fully captured by outcomes such as mortality risk. Severe isolation from nonhousehold contacts, media reminders of their “high risk” status, and changes in access to healthcare are likely to influence patients’ wellbeing. Generally, SOT recipients have shown remarkable resilience, with many adopting positive coping strategies such as acceptance, self-distraction, and positive reframing, which they may have developed through their experience of living with organ failure.221 SOT recipients also noted that transplantation increased their attention to hygiene and infection prevention strategies.222 Despite this, half of kidney transplant recipients worried more about their health and a third wanted to postpone hospital appointments to minimize their risk of COVID-19,222,223 noting the benefits of telehealth.224
For patients on the waiting list, the suspension of transplant programs left some feeling disappointed and helpless with concerns their health could deteriorate.225 Although most patients were in favor of waiting list reactivation, this was not universal, highlighting the need for individualized discussions as the pandemic picture evolves.226
The impact of pandemic-working on the transplant multidisciplinary teams should also be noted. Burnout has been described in half of nephrology and hepatology workforces during the pandemic,227,228 and rates may be higher in intensive care environments.229 The wellbeing of staff is key to protect transplantation programs and patients, with strategies including flexible working and optimizing staffing levels being recommended.230
CONCLUSIONS
Huge advances in the management of COVID-19 have been made during the second year of the pandemic. Transplantation rates are returning to prepandemic levels, novel treatments have been identified, and vaccination of SOT recipients has been prioritized. However, further challenges are likely, emphasized by the recent rapid spread of the Omicron variant, the impact of which is unknown at the time of writing.
Important lessons have been learnt from the pandemic. Rapid research approvals, public engagement in clinical trials, streamlined registry data linkages, preprint articles and expedited publication of open-access papers have helped rapidly disseminate new knowledge. Collaborative working of transplant teams at regional, national, and international levels, and examples of strong leadership to support staff and patients have been seen. The exclusion of immunosuppressed patients from vaccine trials has, however, delayed our understanding of vaccine effectiveness in a vulnerable population in whom disease prevention is key. In the future, including such individuals in sub-studies could help inform clinical and policy decisions. Other suggested strategies to transplant teams to mitigate avoidable risks for patients in a future pandemic are shown in Table 4.
TABLE 4.
Suggested recommendations to transplant teams for research priorities for the SARS-CoV-2 pandemic, and planning and preparedness for future pandemics
| Research priorities for the SARS-CoV-2 pandemic | • Assess safety of use of SARS-CoV-2–positive donors. |
| • Determine optimal vaccination regime for SOT recipients. | |
| • Determine efficacy of novel treatments in a vaccinated population. | |
| • Assess potential demand and indications for lung transplantation for post–COVID-19 pulmonary fibrosis. | |
| • Prioritize methods to maintain wellbeing of transplant teams. | |
| Planning and preparedness for future pandemics | • Early assessment and consensus derivation on the likelihood of donor-derived infection transmission for example, based on plausibility of blood borne or respiratory transmission. |
| • Advocacy for rapid nucleic acid testing of potential organ donors and recipients. | |
| • Early identification of resources that allow safe continuation of transplant programs without overlap with resources caring for patients with active infection, for example, ring-fenced transplant unit and intensive care beds and operating theaters. | |
| • Development of risk prediction tools/calculators that utilize simulation and machine-learning approaches to assist in decision making by transplant centers. | |
| • Advocacy for SOT recipients to be included in clinical trials of novel vaccines and antiviral treatments or clinical trials exploring novel or repurposed treatments. | |
| • Establish national and international registry linkages to enable real-time assessment of infection and mortality risk in SOT recipients and waitlisted patients. | |
| • Establish infrastructure for organ donation and transplant organizations from across the world to collaborate as a consortium to rapidly derive clinical and patient facing consensus guidance. |
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOT, solid organ transplant.
The COVID-19 pandemic is likely to move to an endemic phase, with vaccination reducing COVID-19 mortality but potentially not infection rates in SOT recipients. In the event of ongoing cases, the optimal strategies for prevention and treatment of COVID-19 must be identified, and the safety of using organs from SARS-CoV-2 positive donors considered. Furthermore, it remains to be seen how transplantation will be affected as the pandemic wanes, for example whether post–COVID-19 pulmonary fibrosis will increase demand for lung transplantation.231,232 As a return to normality occurs for general populations, many SOT recipients still have concerns over their protection from SARS-CoV-2, and nonpharmaceutical precautions may still be advisable.
Footnotes
The authors declare no funding or conflicts of interest.
All authors contributed to the design, development, and writing of this article.
Supplemental Visual Abstract; http://links.lww.com/TP/C409.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Zhu L, Xu X, Ma K, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. 2020;20:1859–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huremović D. Brief history of pandemics (pandemics throughout history). Huremović D, ed. In: Psychiatry of Pandemics. Springer; 2019:7–35. [Google Scholar]
- 3.Vallejos A. The role of nephrology in the influenza A (H1N1) pandemic update Article in Spanish. Nefrologia. 2009;29:576–581. [DOI] [PubMed] [Google Scholar]
- 4.Kumar D, Tellier R, Draker R, et al. Severe Acute Respiratory Syndrome (SARS) in a liver transplant recipient and guidelines for donor SARS screening. Am J Transplant. 2003;3:977–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing 31. December 10, 2021. Available at https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1040076/Technical_Briefing_31.pdf. Accessed December 11, 2021.
- 6.Massie AB, Boyarsky BJ, Werbel WA, et al. Identifying scenarios of benefit or harm from kidney transplantation during the COVID-19 pandemic: a stochastic simulation and machine learning study. Am J Transplant. 2020;20:2997–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahn C, Amer H, Anglicheau D, et al. Global transplantation COVID report march 2020. Transplantation. 2020;104:1974–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aubert O, Yoo D, Zielinski D, et al. COVID-19 pandemic and worldwide organ transplantation: a population-based study. Lancet Public Health. 2021;6:e709–e719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manara AR, Mumford L, Callaghan CJ, et al. Donation and transplantation activity in the UK during the COVID-19 lockdown. Lancet. 2020;396:465–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Legeai C, Savoye E, Cantrelle C, et al. Impact of COVID-19 on 2020 transplant activity and waiting lists in France. J Liver Transpl. 2021;5:100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saxon L, Fazio TN, Gumm K, et al. Quality of care was not compromised during the COVID-19 pandemic at a level 1 trauma centre. ANZ J Surg. 2022;92:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hakkenbrak NAG, Loggers SAI, Lubbers E, et al. ; COVID-Trauma Collaborator Group. Trauma care during the COVID-19 pandemic in the Netherlands: a level 1 trauma multicenter cohort study. Scand J Trauma Resusc Emerg Med. 2021;29:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2871–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed O, Brockmeier D, Lee K, et al. Organ donation during the COVID-19 pandemic. Am J Transplant. 2020;20:3081–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Vries APJ, Alwayn IPJ, Hoek RAS, et al. Immediate impact of COVID-19 on transplant activity in the Netherlands. Transpl Immunol. 2020;61:101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angelico R, Trapani S, Manzia TM, et al. The COVID-19 outbreak in Italy: initial implications for organ transplantation programs. Am J Transplant. 2020;20:1780–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domínguez-Gil B, Coll E, Fernández-Ruiz M, et al. COVID-19 in Spain: transplantation in the midst of the pandemic. Am J Transplant. 2020;20:2593–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyarsky BJ, Po-Yu Chiang T, Werbel WA, et al. Early impact of COVID-19 on transplant center practices and policies in the United States. Am J Transplant. 2020;20:1809–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh JM, Ball IM, Hartwick M, et al. Factors associated with consent for organ donation: a retrospective population-based study. CMAJ. 2021;193:E1725. [Google Scholar]
- 20.DeFilippis EM, Sinnenberg L, Reza N, et al. Trends in US heart transplant waitlist activity and volume during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Cardiol. 2020;5:1048–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agopian V, Verna E, Goldberg D. Changes in liver transplant center practice in response to coronavirus disease 2019: unmasking dramatic center-level variability. Liver Transpl. 2020;26:1052–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goff RR, Wilk AR, Toll AE, et al. Navigating the COVID-19 pandemic: initial impacts and responses of the Organ Procurement and Transplantation Network in the United States. Am J Transplant. 2021;21:2100–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li MT, King KL, Husain SA, et al. Deceased donor kidneys utilization and discard rates during COVID-19 pandemic in the United States. Kidney Int Rep. 2021;6:2463–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman AL, Delli Carpini KW, Ezzell C, et al. There are no best practices in a pandemic: organ donation within the COVID-19 epicenter. Am J Transplant. 2020;20:3089–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vistoli F, Furian L, Maggiore U, et al. ; Italian National Kidney Transplantation Network; the Joint Committee of the Italian Society of Organ Transplantation and the Italian Society of Nephrology. COVID-19 and kidney transplantation: an Italian survey and consensus. J Nephrol. 2020;33:667–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strauss AT, Cartier D, Gunning BA, et al. Impact of the COVID-19 pandemic on commercial airlines in the United States and implications for the kidney transplant community. Am J Transplant. 2020;20:3123–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loupy A, Aubert O, Reese PP, et al. Organ procurement and transplantation during the COVID-19 pandemic. Lancet. 2020;395:e95–e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan EG, Chan PG, Harano T, et al. Trends in lung transplantation practices across the United States during the COVID-19 pandemic. Transplantation. 2021;105:187–192. [DOI] [PubMed] [Google Scholar]
- 29.Merola J, Schilsky ML, Mulligan DC. The impact of COVID-19 on organ donation, procurement, and liver transplantation in the United States. Hepatol Commun. 2021;5:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnol M, Smrkolj T, Avsec D, et al. An increase in kidney transplantation procedures from deceased donors during the COVID-19 epidemic in Slovenia. Transpl Int. 2020;33:1562–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhodes A, Ferdinande P, Flaatten H, et al. The variability of critical care bed numbers in Europe. Intensive Care Med. 2012;38:1647–1653. [DOI] [PubMed] [Google Scholar]
- 32.Bittner MI, Donnelly M, van Zanten AR, et al. How is intensive care reimbursed? A review of eight European countries. Ann Intensive Care. 2013;3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallace DJ, Angus DC, Seymour CW, et al. Critical care bed growth in the United States. A comparison of regional and national trends. Am J Respir Crit Care Med. 2015;191:410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chadban SJ, McDonald M, Wyburn K, et al. Significant impact of COVID-19 on organ donation and transplantation in a low-prevalence country: Australia. Kidney Int. 2020;98:1616–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.TSANZ. COVID-19—Australian transplantation and donation rapid response taskforce communique no.44. August 31, 2021. Available at https://tsanz.com.au/storage/COVID_Communiques/Website-Update---COVID-19-as-at-31-August-2021.pdf. Accessed December 14, 2021.
- 36.NHS Blood and Transplant. COVID-19 bulletin 3. March 23, 2020. Available at https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/18065/covid-19-bulletin-3-23-march-2020.pdf. Accessed December 8, 2021.
- 37.Organ Procurement and Transplantation Network. National data. Available at https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#. Accessed February 10, 2022.
- 38.Strauss AT, Boyarsky BJ, Garonzik-Wang JM, et al. Liver transplantation in the United States during the COVID-19 pandemic: National and center-level responses. Am J Transplant. 2021;21:1838–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lentine KL, Vest LS, Schnitzler MA, et al. Survey of US living kidney donation and transplantation practices in the COVID-19 era. Kidney Int Rep. 2020;5:1894–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kute VB, Godara S, Guleria S, et al. Is it safe to be transplanted from living donors who recovered from COVID-19? Experience of 31 kidney transplants in a multicenter cohort study from India. Transplantation. 2021;105:842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.NHS Blood and Transplant. Kidney advisory group living donor kidney transplantation: recommendations to restart/ expand programmes. July 13, 2020. Available at https://www.odt.nhs.uk/covid-19-advice-for-clinicians/re-opening-of-transplant-programmes/#. Accessed December 21, 2021.
- 42.Rodrigo E, Miñambres E, Gutiérrez-Baños JL, et al. COVID-19-related collapse of transplantation systems: a heterogeneous recovery? Am J Transplant. 2020;20:3265–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gori A, Dondossola D, Antonelli B, et al. Coronavirus disease 2019 and transplantation: a view from the inside. Am J Transplant. 2020;20:1939–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.NHS Blood and Transplant Organ Donation. Statistics about organ donation. Available at https://www.organdonation.nhs.uk/helping-you-to-decide/about-organ-donation/statistics-about-organ-donation/. Accessed December 15, 2021.
- 45.Organ Procurement and Transplantation Network. Data reports. Available at https://optn.transplant.hrsa.gov/data/view-data-reports/. Accessed December 15, 2021.
- 46.Deutsche Stiftung Organtransplantation. Statistiken zur Organspende. Available at https://www.dso.de/organspende/statistiken-berichte/organspende. Accessed December 22, 2021.
- 47.Lieberman JA, Mays JA, Wells C, et al. Expedited SARS-CoV-2 screening of donors and recipients supports continued solid organ transplantation. Am J Transplant. 2020;20:3106–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGregor TB, Sener A, Yetzer K, et al. The impact of COVID-19 on the Canadian Kidney Paired Donation program: an opportunity for universal implementation of kidney shipping. Can J Surg. 2020;63:E451–E453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.NHS Blood and Transplant. INF1596/1 – winter pressures in the NHS in 2021 and its potential effect on transplantation—a discussion document. October 29, 2021. Available at https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/25221/inf1596.pdf. Accessed January 23, 2022.
- 50.NHS Blood and Transplant. Organ donation and transplantation. Available at https://www.odt.nhs.uk/. Accessed January 18, 2022.
- 51.Australian Organ and Tissue Authority. Available at https://www.donatelife.gov.au. Accessed January 18, 2022.
- 52.Organización Nacional de Trasplantes. Available at http://www.ont.es/Paginas/Home.aspx. Accessed January 18, 2022.
- 53.Danziger-Isakov L, Blumberg EA, Manuel O, et al. Impact of COVID-19 in solid organ transplant recipients. Am J Transplant. 2021;21:925–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar D, Manuel O, Natori Y, et al. COVID-19: a global transplant perspective on successfully navigating a pandemic. Am J Transplant. 2020;20:1773–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang R, Li Y, Zhang AL, et al. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A. 2020;117:14857–14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shah MB, Lynch RJ, El-Haddad H, et al. Utilization of deceased donors during a pandemic: argument against using SARS-CoV-2-positive donors. Am J Transplant. 2020;20:1795–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koval CE, Poggio ED, Lin YC, et al. Early success transplanting kidneys from donors with new SARS-CoV-2 RNA positivity: a report of 10 cases. Am J Transplant. 2021;21:3743–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romagnoli R, Gruttadauria S, Tisone G, et al. Liver transplantation from active COVID-19 donors: a lifesaving opportunity worth grasping? Am J Transplant. 2021;21:3919–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaul DR, Valesano AL, Petrie JG, et al. Donor to recipient transmission of SARS-CoV-2 by lung transplantation despite negative donor upper respiratory tract testing. Am J Transplant. 2021;21:2885–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.American Society of Transplantation. SARS-CoV-2 (coronavirus, 2019-nCoV): recommendations and guidance for organ donor testing. July 7, 2021. Available at https://www.myast.org/sites/default/files/Donor%20Testing%20Document_07.07.21.pdf. Accessed December 8, 2021.
- 61.NHS Blood and Transplant. POL304/2—SARS-CoV-2 assessment and screening in organ donors and recipients. November 6, 2020. Available at https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/20342/pol304.pdf. Accessed January 12, 2022.
- 62.American Society of Transplant Surgeons. Prior COVID-19 and organ donation. February 23, 2021. Available at https://asts.org/advocacy/covid-19-resources/asts-covid-19-strike-force/prior-covid-19-and-organ-donation#.Yd6yl2jP02w. Accessed January 12, 2022.
- 63.International Society of Heart and Lung Transplantation. Deceased donor and recipient selection for cardiothoracic transplantation during the COVID-19 pandemic. April 12, 2021. Available at https://ishlt.org/ishlt/media/documents/COVID-19_GuidanceDocument_Deceased-donor-and-recipient-selection-for-cardiothoracic-transplantation.pdf. Accessed January 12, 2022.
- 64.Jefferson T, Spencer EA, Brassey J, et al. Viral cultures for coronavirus disease 2019 infectivity assessment: a systematic review. Clin Infect Dis. 2021;73:e3884–e3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim MC, Cui C, Shin KR, et al. Duration of culturable SARS-CoV-2 in hospitalized patients with covid-19. N Engl J Med. 2021;384:671–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Organ Procurement Transplantation Network. Summary of current evidence and information– donor SARS-CoV-2 testing & organ recovery from donors with a history of COVID-19. Available at https://optn.transplant.hrsa.gov/media/kkhnlwah/sars-cov-2-summary-of-evidence.pdf. Accessed January 12, 2022.
- 67.El-Boghdadly K, Cook TM, Goodacre T, et al. SARS-CoV-2 infection, COVID-19 and timing of elective surgery. Anaesthesia. 2021;76:940–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kulkarni AV, Parthasarathy K, Kumar P, et al. Early liver transplantation after COVID-19 infection: the first report. Am J Transplant. 2021;21:2279–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greinacher A, Selleng K, Palankar R, et al. Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. Blood. 2021;138:2256–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klok FA, Pai M, Huisman MV, et al. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2022;9:e73–e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pavord S, Scully M, Hunt BJ, et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385:1680–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Greenhall GHB, Ushiro-Lumb I, Pavord S, et al. ; UK Donor VITT Transplant Study Group. Organ transplantation from deceased donors with vaccine-induced thrombosis and thrombocytopenia. Am J Transplant. 2021;21:4095–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Bruchem M, van Rosmalen M, Warmerdam A, et al. Outcome after organ transplantation from brain-dead donors after a cerebral insult following SARS-CoV-2 vaccination within the Eurotransplant Region. Transplantation. 2022;106:e100–e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Loupy A, Goutaudier V, Jacquelinet C, et al. Solid organ procurement and transplantation from deceased donors with vaccine-induced thrombosis and thrombocytopenia. Am J Transplant. 2021;21:4098–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hann A, Hartog H, Nutu A, et al. Liver graft outcomes from donors with vaccine induced thrombosis and thrombocytopenia (VITT): United Kingdom multicenter experience. Am J Transplant. 2022;22:996–998. [DOI] [PubMed] [Google Scholar]
- 77.NHS Blood and Transplant. INF1569/3.1—organ donation and transplantation from patients with vaccine induced thrombosis and thrombocytopenia (VITT). June 16, 2021. Available at https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/23766/inf1569.pdf. Accessed December 8, 2021.
- 78.Wolfe C, Humar A. Buyer beware: The risks of donor-derived vaccine-induced thrombosis and thrombocytopenia. Am J Transplant. 2021;21:3829–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hippisley-Cox J, Coupland CA, Mehta N, et al. Risk prediction of covid-19 related death and hospital admission in adults after covid-19 vaccination: national prospective cohort study. BMJ. 2021;374:n2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ravanan R, Callaghan CJ, Mumford L, et al. SARS-CoV-2 infection and early mortality of waitlisted and solid organ transplant recipients in England: a national cohort study. Am J Transplant. 2020;20:3008–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clarke C, Lucisano G, Prendecki M, et al. ; ICHNT Renal COVID Group. Informing the risk of kidney transplantation versus remaining on the waitlist in the coronavirus disease 2019 era. Kidney Int Rep. 2021;6:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cravedi P, Mothi SS, Azzi Y, et al. COVID-19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20:3140–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sharma P, Chen V, Fung CM, et al. COVID-19 outcomes among solid organ transplant recipients: a case-control study. Transplantation. 2021;105:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Azzi Y, Bartash R, Scalea J, et al. COVID-19 and solid organ transplantation: a review article. Transplantation. 2021;105:37–55. [DOI] [PubMed] [Google Scholar]
- 85.Kates OS, Haydel BM, Florman SS, et al. ; UW COVID-19 SOT Study Team. Coronavirus disease 2019 in solid organ transplant: a multicenter cohort study. Clin Infect Dis. 2021;73:e4090–e4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coll E, Fernández-Ruiz M, Sánchez-Álvarez JE, et al. ; Spanish Group for the Study of COVID-19 in Transplant Recipients. COVID-19 in transplant recipients: the Spanish experience. Am J Transplant. 2021;21:1825–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heldman MR, Kates OS. COVID-19 in solid organ transplant recipients: a review of the current literature. Curr Treat Options Infect Dis. 2021;13:67–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raja MA, Mendoza MA, Villavicencio A, et al. COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis of current literature. Transplant Rev (Orlando). 2021;35:100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jering KS, McGrath MM, Mc Causland FR, et al. Excess mortality in solid organ transplant recipients hospitalized with COVID-19: a large-scale comparison of SOT recipients hospitalized with or without COVID-19. Clin Transplant. 2022;36:e14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pereira MR, Arcasoy S, Farr MA, et al. Outcomes of COVID-19 in solid organ transplant recipients: a matched cohort study. Transpl Infect Dis. 2021;23:e13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Craig-Schapiro R, Salinas T, Lubetzky M, et al. COVID-19 outcomes in patients waitlisted for kidney transplantation and kidney transplant recipients. Am J Transplant. 2021;21:1576–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hilbrands LB, Duivenvoorden R, Vart P, et al. ; ERACODA Collaborators. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35:1973–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kremer D, Pieters TT, Verhaar MC, et al. A systematic review and meta-analysis of COVID-19 in kidney transplant recipients: Lessons to be learned. Am J Transplant. 2021;21:3936–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kulkarni AV, Tevethia HV, Premkumar M, et al. Impact of COVID-19 on liver transplant recipients-a systematic review and meta-analysis. Eclinicalmedicine. 2021;38:101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heldman MR, Kates OS, Safa K, et al. ; UW COVID-19 SOT Study Team. Changing trends in mortality among solid organ transplant recipients hospitalized for COVID-19 during the course of the pandemic. Am J Transplant. 2022;22:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coll E, Fernández-Ruiz M, Padilla M, et al. ; Spanish Group for the Study of COVID-19 in Transplant Recipients. COVID-19 in solid organ transplant recipients in Spain throughout 2020: catching the wave? Transplantation. 2021;105:2146–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Intensive Care National Audit and Research Centre. COVID-19 report. Available at https://www.icnarc.org/our-audit/audits/cmp/reports. Accessed December 10, 2021.
- 98.Carbonell R, Urgelés S, Rodríguez A, et al. ; COVID-19 SEMICYUC Working Group. Mortality comparison between the first and second/third waves among 3,795 critical COVID-19 patients with pneumonia admitted to the ICU: a multicentre retrospective cohort study. Lancet Reg Health Eur. 2021;11:100243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Docherty AB, Mulholland RH, Lone NI, et al. ; ISARIC4C Investigators. Changes in in-hospital mortality in the first wave of COVID-19: a multicentre prospective observational cohort study using the WHO Clinical Characterisation Protocol UK. Lancet Respir Med. 2021;9:773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Callaghan CJ, Mumford L, Curtis RMK, et al. ; NHSBT Organ and Tissue Donation and Transplantation Clinical Team. Real-world effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S vaccines against SARS-CoV-2 in solid organ and Islet transplant recipients. Transplantation. 2022;106:436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Qin CX, Moore LW, Anjan S, et al. Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients. Transplantation. 2021;105:e265–e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Udomkarnjananun S, Kerr SJ, Townamchai N, et al. Mortality risk factors of COVID-19 infection in kidney transplantation recipients: a systematic review and meta-analysis of cohorts and clinical registries. Sci Rep. 2021;11:20073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thaunat O, Legeai C, Anglicheau D, et al. ; French nationwide Registry of Solid Organ Transplant Recipients with COVID-19. IMPact of the COVID-19 epidemic on the moRTAlity of kidney transplant recipients and candidates in a French Nationwide registry sTudy (IMPORTANT). Kidney Int. 2020;98:1568–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mohan S, King KL, Husain SA, et al. COVID-19-associated mortality among kidney transplant recipients and candidates in the United States. Clin J Am Soc Nephrol. 2021;16:1695–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Corbett RW, Blakey S, Nitsch D, et al. ; West London Renal and Transplant Centre. Epidemiology of COVID-19 in an Urban dialysis center. J Am Soc Nephrol. 2020;31:1815–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miller J, Wey A, Musgrove D, et al. Mortality among solid organ waitlist candidates during COVID-19 in the United States. Am J Transplant. 2021;21:2262–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.World Health Organization. Coronavirus disease (COVID-19): vaccines. Available at https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-(covid-19)-vaccines?adgroupsurvey={adgroupsurvey}&gclid=CjwKCAiAzrWOBhBjEiwAq85QZz4IiGG7MqLFqA1l17qymHH_0TzmoipDgZ-u5r6U1AQcNFKQHwLH7hoCBEkQAvD_BwE. Accessed December 31, 2021.
- 108.Kim JH, Marks F, Clemens JD. Looking beyond COVID-19 vaccine phase 3 trials. Nat Med. 2021;27:205–211. [DOI] [PubMed] [Google Scholar]
- 109.Stephenson J. National Academies report advises on allocation priorities for a COVID-19 vaccine. JAMA Health Forum. 2020;1:e201288. [DOI] [PubMed] [Google Scholar]
- 110.Department of Health and Social Care. Joint Committee on Vaccination and immunization: advice on priority groups for COVID-19 vaccination. Available at https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-30-december-2020/joint-committee-on-vaccination-and-immunisation-advice-on-priority-groups-for-covid-19-vaccination-30-december-2020. Accessed December 31, 2021.
- 111.International Long-term Care Policy Network. A brief overview of the current German COVID-19 vaccination strategy. December 18, 2020. Available at https://ltccovid.org/2020/12/18/a-brief-overview-of-the-current-german-covid-19-vaccination-strategy/. Accessed December 31, 2021.
- 112.European Cente for Disease Prevention and Control. COVID-19 vaccination and prioritisation strategies in the EU/EEA. December 22, 2020. Available at https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-vaccination-and-prioritisation-strategies.pdf. Accessed January 4, 2022.
- 113.Italian National Transplant Centre. COVID-19 vaccine for transplanted patients. Available at https://www.trapianti.salute.gov.it/trapianti/archivioDatiCnt.jsp. Accessed December 29, 2021.
- 114.Ou MT, Boyarsky BJ, Zeiser LB, et al. Kidney transplant recipient attitudes toward a SARS-CoV-2 vaccine. Transplant Direct. 2021;7:e713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.NHS Blood and Transplant/British Transplantation Society. NHS Blood and Transplant/British Transplantation Society latest advice on COVID-19 vaccination in transplant recipients and patients waiting for a transplant. August 19, 2021. Available at https://bts.org.uk/wp-content/uploads/2021/08/NHSBT-BTS-Joint-Statement-on-COVID-19-Vaccine-Efficacy-19th-Aug-2021.pdf. Accessed December 14, 2021.
- 116.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Voysey M, Clemens SAC, Madhi SA, et al. ; Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Glenn DA, Hegde A, Kotzen E, et al. Systematic review of safety and efficacy of COVID-19 vaccines in patients with kidney disease. Kidney Int Rep. 2021;6:1407–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Eckerle I, Rosenberger KD, Zwahlen M, et al. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS One. 2013;8:e56974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wadei HM, Gonwa TA, Leoni JC, et al. COVID-19 infection in solid organ transplant recipients after SARS-CoV-2 vaccination. Am J Transplant. 2021;21:3496–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Caillard S, Chavarot N, Bertrand D, et al. ; French Society of Transplantation. Occurrence of severe COVID-19 in vaccinated transplant patients. Kidney Int. 2021;100:477–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jahrsdörfer B, Kroschel J, Ludwig C, et al. Independent side-by-side validation and comparison of 4 serological platforms for SARS-CoV-2 antibody testing. J Infect Dis. 2021;223:796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tan CW, Chia WN, Qin X, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38:1073–1078. [DOI] [PubMed] [Google Scholar]
- 125.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. [DOI] [PubMed] [Google Scholar]
- 126.Legros V, Denolly S, Vogrig M, et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol. 2021;18:318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Feng S, Phillips DJ, White T, et al. ; Oxford COVID Vaccine Trial Group. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325:1784–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Benotmane I, Gautier-Vargas G, Cognard N, et al. Weak anti-SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int. 2021;99:1487–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yi SG, Knight RJ, Graviss EA, et al. Kidney transplant recipients rarely show an early antibody response following the first COVID-19 vaccine administration. Transplantation. 2021;105:e72–e73. [DOI] [PubMed] [Google Scholar]
- 131.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Marion O, Del Bello A, Abravanel F, et al. Safety and immunogenicity of anti-SARS-CoV-2 messenger RNA vaccines in recipients of solid organ transplants. Ann Intern Med. 2021;174:1336–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bertrand D, Hamzaoui M, Lemée V, et al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32:2147–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schmidt T, Klemis V, Schub D, et al. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am J Transplant. 2021;21:3990–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Prendecki M, Thomson T, Clarke CL, et al. ; Imperial Renal COVID-19 vaccine study group in collaboration with the OCTAVE Study Consortium. Immunological responses to SARS-CoV-2 vaccines in kidney transplant recipients. Lancet. 2021;398:1482–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stumpf J, Siepmann T, Lindner T, et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021;9:100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rozen-Zvi B, Yahav D, Agur T, et al. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clin Microbiol Infect. 2021;27:1173.e1–1173.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kantauskaite M, Müller L, Kolb T, et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS-CoV-2 vaccination in kidney transplant recipients. Am J Transplant. 2022;22:634–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Benotmane I, Gautier-Vargas G, Cognard N, et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99:1498–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21:2727–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21:2719–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Herrera S, Colmenero J, Pascal M, et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am J Transplant. 2021;21:3971–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75:435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Peled Y, Ram E, Lavee J, et al. BNT162b2 vaccination in heart transplant recipients: Clinical experience and antibody response. J Heart Lung Transplant. 2021;40:759–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Marinaki S, Adamopoulos S, Degiannis D, et al. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am J Transplant. 2021;21:2913–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.American Society of Transplantation. AST statement about vaccine efficacy in organ transplant recipients. August 13, 2021. Available at https://www.myast.org/sites/default/files/ast%20ishlt%20guidance%20vaccine%2008132021FINAL%20DRAFT2.pdf. Accessed December 15, 2021.
- 147.Morishita T, Sakai A, Matsunami H. Seroconversions after withdrawal from mycophenolate mofetil in solid organ transplant recipients without a third dose of BNT162b2 mRNA coronavirus disease 2019 vaccine: a case series. Transplantation. 2022;106:e238–e239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chavarot N, Morel A, Leruez-Ville M, et al. Weak antibody response to three doses of mRNA vaccine in kidney transplant recipients treated with belatacept. Am J Transplant. 2021;21:4043–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chavarot N, Ouedrani A, Marion O, et al. Poor Anti-SARS-CoV-2 humoral and T-cell responses after 2 injections of mRNA vaccine in kidney transplant recipients treated with belatacept. Transplantation. 2021;105:e94–e95. [DOI] [PubMed] [Google Scholar]
- 150.Sattler A, Schrezenmeier E, Weber UA, et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest. 2021;131:150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hasmann S, Paal M, Füeßl L, et al. Humoral immunity to SARS-CoV-2 vaccination in haemodialysis patients: (Response to: Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine.). Lancet Reg Health Eur. 2021;10:100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Calleri A, Saracco M, Pittaluga F, et al. Seroconversion after coronavirus disease 2019 vaccination in patients awaiting liver transplantation: fact or fancy? Liver Transpl. 2022;28:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Magicova M, Zahradka I, Fialova M, et al. Determinants of immune response to anti–SARS-CoV-2 mRNA vaccines in kidney transplant recipients: a prospective cohort study. Transplantation. 2022;106:842–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Malinis M, Cohen E, Azar MM. Effectiveness of SARS-CoV-2 vaccination in fully vaccinated solid organ transplant recipients. Am J Transplant. 2021;21:2916–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bell S, Campbell J, Lambourg E, et al. The impact of vaccination on incidence and outcomes of SARS-CoV-2 infection in patients with kidney failure in Scotland. J Am Soc Nephrol. 2022;33:677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Green AC, Curtis HJ, Hulme WJ, et al. The OpenSAFELY Collaborative. Describing the population experiencing COVID-19 vaccine breakthrough following second vaccination in England: a cohort study from OpenSAFELY. medRxiv. [Epub ahead of print. November 8, 2021]. doi:10.1101/2021.11.08.21265380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Iyengar KP, Ish P, Botchu R, et al. Influence of the Peltzman effect on the recurrent COVID-19 waves in Europe. Postgrad Med J. 2022;98:e110–e111. [DOI] [PubMed] [Google Scholar]
- 158.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Office of National Statistics. Coronavirus (COVID-19) infection survey technical article: impact of vaccination on testing positive in the UK, October 2021. October 18, 2021. Available at https://www.ons.gov.uk/releases/coronaviruscovid19infectionsurveytechnicalarticleimpactofvaccinationontestingpositiveintheukoctober2021. Accessed January 12, 2022.
- 160.UK Health Security Agency. COVID-19 vaccine surveillance report week 47. Available at https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1036047/Vaccine_surveillance_report_-_week_47.pdf. Accessed January 12, 2022.
- 161.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Del Bello A, Abravanel F, Marion O, et al. Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients. Am J Transplant. 2022;22:322–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021;326:1063–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bertrand D, Hamzaoui M, Lemée V, et al. Antibody and T-cell response to a third dose of SARS-CoV-2 mRNA BNT162b2 vaccine in kidney transplant recipients. Kidney Int. 2021;100:1337–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Massa F, Cremoni M, Gérard A, et al. Safety and cross-variant immunogenicity of a three-dose COVID-19 mRNA vaccine regimen in kidney transplant recipients. Ebiomedicine. 2021;73:103679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174:1330–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schrezenmeier E, Rincon-Arevalo H, Stefanski AL, et al. B and T cell responses after a third dose of SARS-CoV-2 vaccine in kidney transplant recipients. J Am Soc Nephrol. 2021;32:3027–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Abedon AT, Alejo JL, Kim JD, et al. 6-mo antibody kinetics and durability after 3 doses of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Transplantation. 2022;106:e281–e283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bertrand D, Lemée V, Laurent C, et al. Waning antibody response and cellular immunity 6 months after third dose SARS-Cov-2 mRNA BNT162b2 vaccine in kidney transplant recipients. Am J Transplant. 2022;22:1498–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Israel A, Merzon E, Schäffer AA, et al. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection: test negative design study. BMJ. 2021;375:e067873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to covid-19. N Engl J Med. 2021;385:2413–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bar-On YM, Goldberg Y, Mandel M, et al. Protection against covid-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385:2421–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Spensley K, Gleeson S, Martin P, et al. Comparison of vaccine effectiveness against the Omicron (B.1.1.529) variant in patients receiving haemodialysis. Kidney Int Rep. [Epub ahead of print. April 13, 2022]. doi:10.1016/j.ekir.2022.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Centres for Disease Prevention and Control. COVID-19 vaccines for moderately or severely immunocompromised people. Available at https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html. Accessed January 4, 2022.
- 176.UK Department of Health and Social Care. Joint Committee on Vaccination and Immunisation (JCVI) advice on third primary dose vaccination. September 1, 2021. Available at https://www.gov.uk/government/publications/third-primary-covid-19-vaccine-dose-for-people-who-are-immunosuppressed-jcvi-advice/joint-committee-on-vaccination-and-immunisation-jcvi-advice-on-third-primary-dose-vaccination. Accessed January 4, 2022.
- 177.Alejo JL, Mitchell J, Chiang TP, et al. Antibody response to a fourth dose of a SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Transplantation. 2021;105:e280–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.British Transplantation Society. British Transplantation Society latest advice on COVID-19 vaccination in transplant recipients and patients waiting for a transplant. January 4, 2022. Available at https://bts.org.uk/wp-content/uploads/2022/01/BTS-COVID-19-Vaccination-statement-4th-January-2022-FINAL.pdf. Accessed January 12, 2022.
- 179.Canadian Society of Transplantation. National Transplant Consensus guidance on COVID-19 vaccine. Available at https://profedu.blood.ca/en/organs-and-tissues/covid-19-update-organ-donation-and-transplantation-services. Accessed December 20, 2021.
- 180.American Society of Transplantation. COVID-19 vaccine FAQ sheet. Published online November 14, 2021. Available at https://www.myast.org/sites/default/files/11.14.21-VaccineFAQ-Professionals.pdf. Accessed January 26, 2022.
- 181.NHS Blood and Transplant. INF1559/7.1 – COVID-19 vaccine – Q&As for clinicians and patients. January 5, 2022. Available at https://bts.org.uk/wp-content/uploads/2022/01/INF1559-COVID-19-vaccination-QAs-for-Clinicians-and-Patients-5th-January-2022.pdf. Accessed January 26, 2022.
- 182.Kates OS, Stohs EJ, Pergam SA, et al. The limits of refusal: An ethical review of solid organ transplantation and vaccine hesitancy. Am J Transplant. 2021;21:2637–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.NHS Blood and Transplant/British Transplantation Society. DAT3911/1.1—joint OTDT & BTS guidance on SARSCoV-2 vaccination in adult solid organ and islet transplant wait-listed patients and adult living donor transplant recipients. January 22, 2021. Available at https://bts.org.uk/wp-content/uploads/2021/01/DAT3911.pdf. Accessed December 14, 2021.
- 184.Kates OS, Stock PG, Ison MG, et al. Ethical review of COVID-19 vaccination requirements for transplant center staff and patients. Am J Transplant. 2022;22:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.O’Brien MP, Forleo-Neto E, Musser BJ, et al. ; Covid-19 Phase 3 Prevention Trial Team. Subcutaneous REGEN-COV antibody combination to prevent covid-19. N Engl J Med. 2021;385:1184–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.US Food and Drug Administration. FDA authorizes REGEN-COV monoclonal antibody therapy for post-exposure prophylaxis (prevention) for COVID-19. August 10, 2021. Available at https://www.fda.gov/drugs/drug-safety-and-availability/fda-authorizes-regen-cov-monoclonal-antibody-therapy-post-exposure-prophylaxis-prevention-covid-19. Accessed January 12, 2022.
- 187.US Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes new long-acting monoclonal antibodies for pre-exposure prevention of COVID-19 in certain individuals. December 8, 2021. Available at https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-new-long-acting-monoclonal-antibodies-pre-exposure. Accessed January 5, 2022.
- 188.Levin MJ, Ustianowski A, De Wit S, et al. LB5. PROVENT: phase 3 study of efficacy and safety of AZD7442 (Tixagevimab/Cilgavimab) for pre-exposure prophylaxis of COVID-19 in adults. Open Forum Infect Dis. 2021;8(Supplement_1):S810. [Google Scholar]
- 189.ClinicalTrials.gov. PROphylaxis for paTiEnts at risk of COVID-19 infecTion -V (PROTECT-V). Available at https://clinicaltrials.gov/ct2/show/NCT04870333. Accessed January 5, 2022. [DOI] [PMC free article] [PubMed]
- 190.ClinicalTrials.gov. Phase III double-blind, placebo-controlled study of AZD7442 for pre-exposure prophylaxis of COVID-19 in adult. (PROVENT). Available at https://clinicaltrials.gov/ct2/show/NCT04625725. Accessed January 5, 2022.
- 191.PRINCIPLE Trial Collaborative Group. Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;397:1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Butler CC, Yu LM, Dorward J, et al. ; PRINCIPLE Trial Collaborative Group. Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet Respir Med. 2021;9:1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tardif JC, Bouabdallaoui N, L’Allier PL, et al. ; COLCORONA Investigators. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med. 2021;9:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mangin D, Howard M. The use of inhaled corticosteroids in early-stage COVID-19. Lancet. 2021;398:818–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Weinreich DM, Sivapalasingam S, Norton T, et al. ; Trial Investigators. REGEN-COV antibody combination and outcomes in outpatients with covid-19. N Engl J Med. 2021;385:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. ; COMET-ICE Investigators. Early treatment for covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941–1950. [DOI] [PubMed] [Google Scholar]
- 197.Siemieniuk RA, Bartoszko JJ, Díaz Martinez JP, et al. Antibody and cellular therapies for treatment of covid-19: a living systematic review and network meta-analysis. BMJ. 2021;374:n2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ahearn AJ, Thin Maw T, Mehta R, et al. A programmatic response, including bamlanivimab or casirivimab-imdevimab administration, reduces hospitalization and death in COVID-19 positive abdominal transplant recipients. Transplantation. 2022;106:e153–e157. [DOI] [PubMed] [Google Scholar]
- 199.Yetmar ZA, Beam E, O’Horo JC, et al. Monoclonal antibody therapy for COVID-19 in solid organ transplant recipients. Open Forum Infect Dis. 2021;8:ofab255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Catalano C, Servais S, Bonvoisin C, et al. Preemptive antibody therapy for vaccine breakthrough SARS-CoV-2 infection in immunocompromised patients. Transplantation. 2021;105:e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hoffmann M, Krüger N, Schulz S, et al. The Omicron variant is highly resistant against antibody-mediated neutralization – implications for control of the COVID-19 pandemic. bioRxiv. [Epub ahead of print. January 1, 2021]. doi:10.1101/2021.12.12.472286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.GlaxoSmithKline. Primary endpoint met in COMET-TAIL Phase III trial evaluating intramuscular administration of sotrovimab for early treatment of COVID-19. November 12, 2021. Available at https://www.gsk.com/en-gb/media/press-releases/primary-endpoint-met-in-comet-tail-phase-iii-trial-evaluating-intramuscular-administration-of-sotrovimab-for-early-treatment-of-covid-19/#:~:text=The%20trial’s%20primary%20endpoint%20was,days%20after%20onset%20of%20symptoms. Accessed February 15, 2022.
- 203.Mahase E. Covid-19: molnupiravir reduces risk of hospital admission or death by 50% in patients at risk, MSD reports. BMJ. 2021;375:n2422. [DOI] [PubMed] [Google Scholar]
- 204.Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. ; MOVe-OUT Study Group. Molnupiravir for oral treatment of covid-19 in nonhospitalized patients. N Engl J Med. 2022;386:509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mahase E. Covid-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375:n2713. [DOI] [PubMed] [Google Scholar]
- 206.Lange NW, Salerno DM, Jennings DL, et al. Nirmatrelvir/ritonavir use: managing clinically significant drug-drug interactions with transplant immunosuppressants. Am J Transplant. [Epub ahead of print. January 11, 2022]. doi:10.1111/ajt.16955 [DOI] [PubMed] [Google Scholar]
- 207.Gottlieb RL, Vaca CE, Paredes R, et al. ; GS-US-540-9012 (PINETREE) Investigators. Early remdesivir to prevent progression to severe covid-19 in outpatients. N Engl J Med. 2022;386:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Agarwal A, Rochwerg B, Lamontagne F, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. [DOI] [PubMed] [Google Scholar]
- 209.Trujillo H, Caravaca-Fontán F, Sevillano Á, et al. Tocilizumab use in kidney transplant patients with COVID-19. Clin Transplant. 2020;34:e14072. [DOI] [PubMed] [Google Scholar]
- 210.Pérez-Sáez MJ, Blasco M, Redondo-Pachón D, et al. ; Spanish Society of Nephrology COVID-19 Group. Use of tocilizumab in kidney transplant recipients with COVID-19. Am J Transplant. 2020;20:3182–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Horby PW, Mafham M, Peto L, et al. ; RECOVERY Collaborative Group. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv. [Epub ahead of print. June 16, 2021]. doi:10.1101/2021.06.15.21258542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.National Institutes of Health. Therapeutic management of hospitalized adults with COVID-19. Available at https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults--therapeutic-management/. Accessed February 16, 2022.
- 213.Kotton CN, Kumar D, Caliendo AM, et al. ; The Transplantation Society International CMV Consensus Group. The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2018;102:900–931. [DOI] [PubMed] [Google Scholar]
- 214.Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Abadja F, Atemkeng S, Alamartine E, et al. Impact of mycophenolic acid and tacrolimus on Th17-related immune response. Transplantation. 2011;92:396–403. [DOI] [PubMed] [Google Scholar]
- 216.Aziz F, Muth B, Parajuli S, et al. Unusually high rates of acute rejection during the COVID-19 pandemic: cause for concern? Kidney Int. 2020;98:513–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.British Transplantation Society/UK Kidney Association. Guidance on the management of transplant recipients diagnosed with or suspected of having COVID19. November 19, 2021. Available at https://bts.org.uk/wp-content/uploads/2021/11/Clinical-management-of-transplants-and-immunosuppression-19th-November-2021.pdf. Accessed December 10, 2021.
- 218.American Society of Transplantation. COVID-19 (coronavirus): FAQs for organ transplantation. August 9, 2021. Available at https://www.myast.org/sites/default/files/2021%200809%20COVID19%20FAQ.pdf. Accessed January 5, 2022.
- 219.Maggiore U, Abramowicz D, Crespo M, et al. How should I manage immunosuppression in a kidney transplant patient with COVID-19? An ERA-EDTA DESCARTES expert opinion. Nephrol Dial Transplant. 2020;35:899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Weiss MJ, Lalani J, Patriquin-Stoner C, et al. Summary of international recommendations for donation and transplantation programs during the coronavirus disease pandemic. Transplantation. 2021;105:14–17. [DOI] [PubMed] [Google Scholar]
- 221.McKay SC, Lembach H, Hann A, et al. Health-related quality of life, uncertainty and coping strategies in solid organ transplant recipients during shielding for the COVID-19 pandemic. Transpl Int. 2021;34:2122–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.De Pasquale C, Pistorio ML, Veroux P, et al. Quality of life and mental health in kidney transplant recipients during the COVID-19 pandemic. Front Psychiatry. 2021;12:645549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bennett D, De Vita E, Ventura V, et al. Impact of SARS-CoV-2 outbreak on heart and lung transplant: a patient-perspective survey. Transpl Infect Dis. 2021;23:e13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Huuskes BM, Scholes-Robertson N, Guha C, et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic. Transpl Int. 2021;34:1517–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Guha C, Tong A, Baumgart A, et al. Suspension and resumption of kidney transplant programmes during the COVID-19 pandemic: perspectives from patients, caregivers and potential living donors—a qualitative study. Transpl Int. 2020;33:1481–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Thind AK, Beckwith H, Dattani R, et al. Resuming deceased donor kidney transplantation in the COVID-19 era: what do patients want? Transplant Direct. 2021;7:e678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Selvaskandan H, Nimmo A, Savino M, et al. Burnout and long COVID among the UK nephrology workforce: results from a national survey investigating the impact of COVID-19 on working lives. Clin Kidney J. 2022;15:517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Russo MW, Kwok R, Marina S, et al. Impact of the COVID-19 pandemic on hepatology practice and provider burnout. Hepatol Commun. 2022;6:1236–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Stocchetti N, Segre G, Zanier ER, et al. Burnout in intensive care unit workers during the second wave of the COVID-19 pandemic: a single center cross-sectional Italian study. Int J Environ Res Public Health. 2021;18:6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sever MS, Ortiz A, Maggiore U, et al. Mass disasters and burnout in nephrology personnel. Clin J Am Soc Nephrol. 2021;16:829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Chen JY, Qiao K, Liu F, et al. Lung transplantation as therapeutic option in acute respiratory distress syndrome for coronavirus disease 2019-related pulmonary fibrosis. Chin Med J (Engl). 2020;133:1390–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lang C, Jaksch P, Hoda MA, et al. Lung transplantation for COVID-19-associated acute respiratory distress syndrome in a PCR-positive patient. Lancet Respir Med. 2020;8:1057–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]