Background.
Hepatic ischemia-reperfusion (I/R) injury is the main factor affecting the morbidity and mortality associated with perioperative complications of liver transplantation and major hepatectomy. AXL is a member of the TYRO3, AXL, MERTK family and is involved in immune and apoptosis processes in multiple organs. However, the role of AXL in hepatic I/R injury remains to be elucidated.
Methods.
Mice pretreated with rmGas6 or R428 and mice tail vein injected with adeno-associated virus knockdown suppressor of cytokine signaling protein-1 (SOCS-1) underwent liver I/R surgery to detect the function of activated AXL in vivo. Primary hepatocytes undergo hypoxic reoxygenation injury in vitro.
Results.
AXL expression was significantly upregulated, and phosphorylated-AXL was substantially downregulated in liver transplantation patients and hepatic I/R surgery mice. A mouse model of hepatic I/R injury showed that AXL activation reduced liver inflammation and liver cells apoptosis. The inhibition of AXL activation (AXL-specific inhibitor R428) aggravated hepatic I/R injury, resulted in larger areas of liver injury, aggravated inflammatory response, and increased apoptosis of liver cells. In addition, activated AXL promotes the expression level of SOCS-1 and inhibits toll-like receptor 4 and its downstream signaling pathways. Finally, SOCS-1 was knocked down with an adeno-associated virus, and activated AXL failed to protect against hepatic I/R injury.
Conclusions.
AXL activation protects the liver from I/R injury by upregulating SOCS-1 and inhibiting the toll-like receptor 4/myeloid differentiation factor-88/nuclear factor kappa-B signaling axis. Targeting AXL may be a new therapeutic option for ameliorating hepatic I/R injury.
INTRODUCTION
Hepatic ischemia-reperfusion (I/R) injury is the most common cause of complications in hepatectomy, hemorrhagic shock, and liver transplantation.1 Although the safety of liver surgery has been improved with the promotion of liver surgical techniques and surgical equipment, hepatic I/R injury could still be the major pathological factor affecting morbidity and mortality.
It is an exogenous antigen-independent and local inflammatory response in hepatic I/R injury in which damaged liver cells generate damage-associated molecular patterns (DAMPs) that subsequently trigger innate immune responses.2 Immune cells such as neutrophils, Kupffer cells, and natural killer cells exude into the intercellular space or blood circulation during liver ischemia and hypoxia, activating and releasing large amounts of cytokines such as interleukin (IL)-1β, IL-6, tumor necrosis factor-α (TNF-α), and reactive oxygen species.3 Although the blood supply to the liver is restored during the reperfusion process, various proinflammatory cytokines activate monocytes to become macrophages that are recruited to the liver, thereby further amplifying the inflammatory response and aggravating liver damage.4 On the one hand, IL-1β and TNF-α activate CD4+ T lymphocytes to produce interferon-γ and colony-stimulating factors, which further activate Kupffer cells and promote the secretion of inflammatory cytokines. On the other hand, TNF-α directly mediates hepatocyte mitochondrial toxicity, induces hepatocyte apoptosis, and activates the exogenous apoptosis pathway, aggravating the apoptosis of hepatic I/R injury.4-6 This process is similar to a positive loop: cell death triggers and aggravates inflammation, and excessive inflammation aggravates liver cell damage.7 Therefore, blocking this positive cycle and researching these key regulatory factors will help develop a new clinical treatment to alleviate hepatic I/R injury.
TAM receptor tyrosine kinases have subtypes that include AXL, TYRO3, and MERTK.8 AXL was first discovered in patients with chronic myeloid leukemia.9 The extracellular N-terminus of TAMs has 2 typical immunoglobulin-like domains and 2 type III fibronectin domains.10 The unique KW(I/L)A(I/L)ES sequence of the TAM family is located in the intracellular domain, and plays a vital role in the activity of tyrosine kinases.11 Common ligands in the TAM family include growth arrest-specific protein 6 (Gas6), galectin-3, tubby, tubby-like protein 1, and protein S, among which Gas6 has a high affinity for AXL.8,10-12 Studies have found that important downstream pathways of the Gas6/AXL axis, such as nuclear factor kappa-B (NF-κB), JAK/STAT, PI3K/AKT/mammalian target of rapamycin, RAS/RAF/MEK/ERK signaling pathways, participate in tumor cell migration, invasion, survival, tumorigenesis, antiapoptosis, drug resistance, angiogenesis, etc.12-17 Previous studies have found that activated AXL can reduce the expression of inflammatory factors such as IL-1β, IL-6, TNF-α, and cell apoptosis in myocardial, kidney, and lung I/R injury.18-21 As yet, little has been clearly studied on the function of AXL in hepaticI/R injury and its regulatory mechanism. We found that AXL expression was significantly upregulated in liver samples from liver transplantation patients in a proteomics study.22
Here, we assessed the effect of activated AXL on hepatocyte function and the innate immune response in hepatic I/R injury. Our research found that activated AXL has significant anti-inflammatory and antiapoptotic effects in hepatic I/R injury and inhibits the toll-like receptor 4 (TLR4)/myeloid differentiation factor-88 (MYD88)/NF-κB signaling pathway by inducing the expression of suppressor of cytokine signaling (SOCS)-1 rather than SOCS-3. This study provides new perspective on potential treatment strategy for hepatic I/R injury.
MATERIALS AND METHODS
Human Sample
Liver samples were collected from transplant patients and healthy donors from the First Affiliated Hospital of Anhui Medical University. Nonfatty donor organs procured according to standardized techniques were perfused and stored in the cold University of Wisconsin solution. Control liver samples were obtained from patient with hepatic hemangioma undergoing hepatectomy without hepatitis, fatty liver, and drug-induced liver injury. Liver I/R injury samples were obtained from patients who had completed liver transplantation, and liver biopsies were performed from the left lobe 3 h after portal vein reperfusion (before abdominal closure). Participants or their family members voluntarily signed an informed consent form. No samples from executed prisoners or institutionalized individuals were used in the experiment. The Review Committee of the First Affiliated Hospital of Anhui Medical University, Anhui Province, China, approved all procedures (no. 20180145) and implemented them in accordance with the principles of the Declaration of Helsinki.
Hepatic I/R Mouse Model
A stable mouse model of local warm liver ischemia was established as previously described.23 The sham-operated mice were given the same operation while their blood vessels were not clamped. The reperfusion time is different after 60 min of ischemia. After the operation, the animals were euthanized and serum and liver samples were collected immediately for further analysis.
Intraperitoneal injection of rmGas6 (R&D Systems, 8310-GS, America), PBS (vehicle), and R428 125 mg/kg (MCE, HY-15150, America) was performed 2 h before I/R injury. The specific groups were as follows: (1) sham, I/R (6 h), I/R (6 h) + vehicle, I/R (6 h) + rmGas6 (2.5 µg), I/R (6 h) + rmGas6 (5 µg), I/R (6 h) + rmGas6 (10 µg), each group (n = 4–6) and (2) I/R (6 h), I/R (6 h) + vehicle, I/R (6 h) + rmGas6 (5 µg), I/R (6 h) + R428, I/R (6 h) + rmGas6 + R428 (n = 4–6). The adeno-associated virus (AAV) was purchased from GeneChem (China) and administered to mice via tail vein injection. The specific experimental groups were as follows: I/R (6 h), I/R (6 h) + AAV-vector, I/R (6 h) + AAV-SOCS-1, I/R (6 h) + rmGas6 (5 µg), I/R (6 h) + AAV-vector, I/R (6 h) + rmGas6 (5 µg) + AAV-SOCS-1.
Hypoxia/Reoxygenation Model of Primary Hepatocytes and Cell Death Assays
Primary hepatocytes were isolated from 6- to 8-wk-old male mice using the collagenase perfusion method as previous studies described.24 rmGas6 (concentration as follows) or R428 (200 nM) was used for pretreatment 2 h before cell hypoxia and reoxygenation (H/R). The specific experimental groups were as follows: control, H/R (6 h), H/R (6 h) + vehicle, H/R (6 h) + rmGas6 (25 ng/mL), H/R (6 h) + rmGas6 (50 ng/mL), H/R (6 h) + rmGas6 (100 ng/mL), and H/R (6 h) + rmGas6 (200 ng/mL). H/R (6 h), H/R (6 h) + vehicle, H/R (6 h) + rmGas6 (200 ng/mL), H/R (6 h) + R428 (200 nM), H/R(6 h) + rmGas6 (200 ng/mL) + R428 (200 nM).
Cells were digested with trypsin and collected. Based on the manufacturer’s protocol, the cells were stained with PE Annexin V and 7-aminoactinomycin D for 15 min (BD Biosciences 559763). The cells were placed on ice, and Beckman Coulter CytoFlex was used for analysis.
Liver Function Analysis
Blood samples were collected from all mice and then centrifuged at 4000 rpm at 4 °C for 5 min. Serum samples were separated and stored at –80 °C. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured by colorimetry, using commercially available detection kits (Nanjing Jiancheng Institute of Biological Engineering, China).
Liver Histology and Immunohistochemistry
Liver tissue was collected for histopathological examination, fixed with 10% formalin, and sliced into 4-µm thick sections. Hematoxylin and eosin staining was used to detect the percentage of liver necrosis after hepatic I/R injury. According to the manufacturer’s instructions, LY6G (BD Biosciences, China) and AXL (Proteintech, China) were stained for immunohistochemistry (IHC) in paraffin sections.
Western Blotting
SDS-PAGE was used to separate mouse liver protein lysates (30 μg/lane) and transfer them to a polyvinylidene fluoride membrane. The blot was then incubated with the primary antibody overnight. The antibody information is shown in Table 1.
TABLE 1.
The antibody information
| Antibody name | Brand | Catalog number | Dilution |
|---|---|---|---|
| AXL | Proteintech | 13196-1-AP | 1:1000 |
| p-AXL | CST (USA) | D12B2 | 1:500 |
| Gas6 | Proteintech (China) | 13795-1-AP | 1:1000 |
| IL-1β | Proteintech (China) | 26048-1-AP | 1:1000 |
| IL-6 | Wanleibio (China) | WL02841 | 1:1000 |
| TNF-α | Wanleibio (China) | WL01581 | 1:1000 |
| BAX | Proteintech (China) | 50599-2-lg | 1:5000 |
| Bcl-2 | Wanleibio (China) | WL01556 | 1:1000 |
| c-Caspase-3 | Proteintech (China) | 19677-1-AP | 1:1000 |
| SOCS-1 | Zenbio (China) | 340946 | 1:1000 |
| SOCS-3 | Zenbio (China) | 500694 | 1:1000 |
| TLR4 | Proteintech (China) | 66350-1-lg | 1:1000 |
| TRAF6 | Proteintech (China) | 66498-1-lg | 1:1000 |
| MYD88 | Zenbio (China) | 340629 | 1:1000 |
| IκBα | Zenbio (China) | R22588 | 1:1000 |
| p-P65 | Wanleibio (China) | WL02169 | 1:1000 |
| β-Actin | Proteintech (China) | 66009-1-lg | 1:1000 |
AXL, AXL receptor tyrosine kinase; BAX, Bcl-2 associated X protein; Bcl-2, B-cell lymphoma-2; c-Caspase-3, cleaved-cysteinyl aspartatel-specific proteinase 3; Gas6, growth arrest-specific protein 6; IL, interleukin; IκBα, NF-κB inhibitor alpha; MYD88, myeloid differentiation factor-88; p-AXL, phosphorylation AXL; p-P65, phospho-NF-κB-p65; SOCS, suppressor of cytokine signaling protein; TLR4, toll-like receptor 4; TNF-α, tumor necrosis factor alpha; TRAF6, TNF receptor–associated factor 6.
Real-time Polymerase Chain Reaction
Using PerfectStart Green qPCR SuperMix (TransGen, China) and LightCycler 96 System (Roche, Switzerland), mRNA levels were measured by real-time quantitative reverse transcription polymerase chain reaction (PCR) analysis. The primers used for real-time PCR are shown in Table 2.
TABLE 2.
The primers used for real-time PCR
| Primers | |
|---|---|
| IL-1β | F: GAAATGCCACCTTTTGACAGTG |
| R: TGGATGCTCTCATCAGGACAG | |
| IL-6 | F: CTGCAAGAGACTTCCATCCAG |
| R: AGTGGTATAGACAGGTCTGTTGG | |
| TNF-α | F: CAGGCGGTGCCTATGTCTC |
| R: CGATCACCCCGAAGTTCAGTAG | |
| BAX | F: AGACAGGGGCCTTTTTGCTAC |
| R: AATTCGCCGGAGACACTCG | |
| Bcl-2 | F: GGCTGAGCACTACCTTCAGTA |
| R: TGGCGGTATCTATGGATTCCAC | |
| c-Caspase-3 | F: CTCGCTCTGGTACGGATGTG |
| R: TCCCATAAATGACCCCTTCATCA | |
| β-Actin | F: GTGACGTTGACATCCGTAAAGA |
| R: GCCGGACTCATCGTACTCC |
BAX, Bcl-2–associated X protein; Bcl-2, B-cell lymphoma-2; c-Caspase-3, cleaved-cysteinyl aspartatel-specific proteinase 3; IL, interleukin; PCR, polymerase chain reaction; TNF-α, tumor necrosis factor alpha.
Enzyme-linked Immunosorbent Assay
Blood was collected from all mice and centrifuged at 4000 rpm at 4 °C for 5 min. Enzyme- Linked Immunosorbent Assay kits purchased from Shanghai Enzyme-linked Biotechnology were used to determine the serum concentrations of inflammatory cytokines (TNF-α, IL-1β, and IL-6).
Terminal Deoxynucleotidal Transferase–mediated Biotin–deoxyuridine Triphosphate Nick-end Labeling and Immunofluorescence
As previously described,25 cell death was assessed by a terminal deoxynucleotide transferase 2'-deoxyuridine 5'-triphosphate nickel-terminal marker. As previously described,19 the expression of SOCS-1 was measured by immunofluorescence staining with an SOCS-1 antibody. The images were acquired by a fluorescence microscope (Olympus DX51, Tokyo, Japan) and CellSens standard software (version 1.6, Tokyo, Japan).
Statistical Analysis
All experiments were performed and results were analyzed blindly. SPSS version 23 software (International Business Machines Corporation, IBM, New York) was used for statistical analysis of all data. One-way analysis of variance was used for comparison between groups. Statistical significance was defined as P ≤ 0.05.
RESULTS
AXL Upregulation and Phosphorylated AXL Downregulation in Hepatic I/R Injury
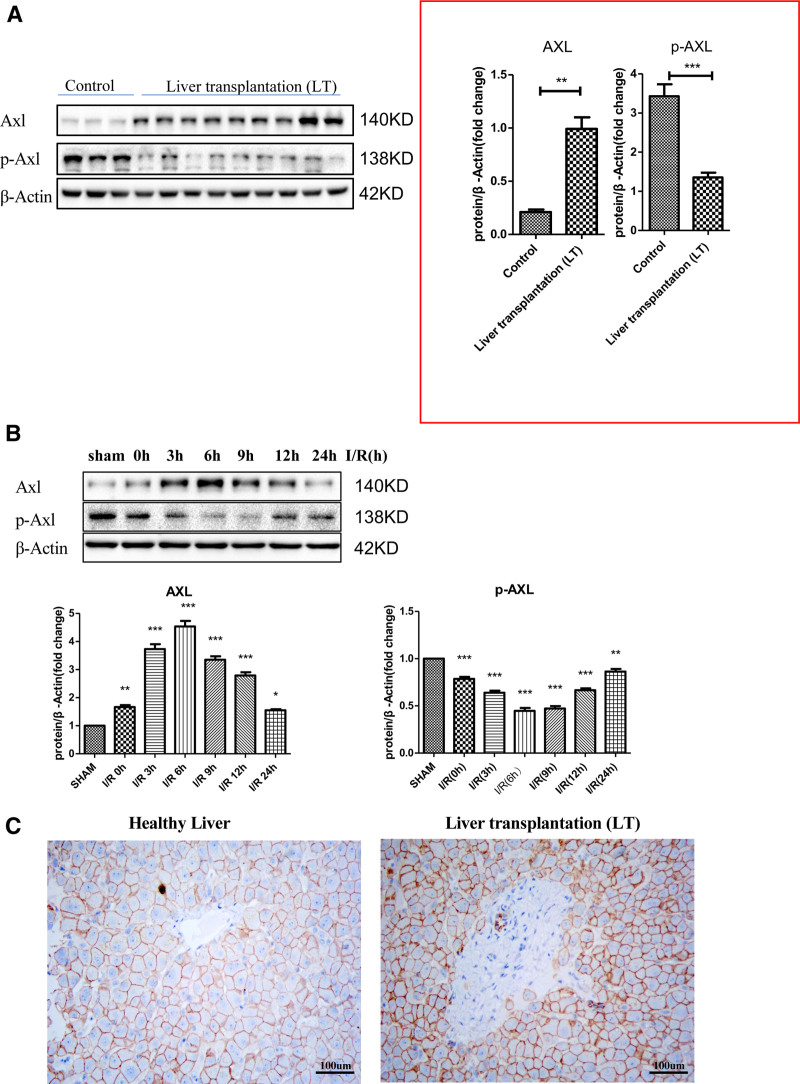
We tested the expression of the AXL in liver samples from liver transplant patients to study the potential role of AXL in hepatic I/R injury. Compared with the control group, the AXL protein level in the transplantation patient sample was significantly increased (Figure 1A). The results of IHC showed that AXL expression was upregulated in liver transplant patients compared with that in healthy livers (Figure 1C). We also measured AXL expression in the livers of wild-type mice who had been given liver I/R surgery and observed that AXL gradually increased and reached a peak at 0 to 6 h after I/R and gradually decreased 6 h later (Figure 1B). AXL is a receptor tyrosine kinase, and we tested the expression of phosphorylated AXL (p-AXL; Figure 1B). Interestingly, we found that it decreased to its lowest point within 0 to 6 h after I/R injury, and then it gradually increased within 24 h. To determine the reason for this phenomenon, we also tested the expression of endogenous Gas6 in hepatic I/R injury and found that the expression of Gas6 decreased in I/R injury (Figure S1, SDC, http://links.lww.com/TP/C421). These results demonstrated that AXL expression is relevant to hepatic I/R injury and that AXL may play an important role in this process.
FIGURE 1.
AXL upregulation and p-AXL downregulation after hepatic I/R injury. A, The expression of AXL and p-AXL in liver transplant patients (n = 9) and healthy livers (n = 3). B, Western blotting analysis of the protein expression of AXL and p-AXL after mice underwent sham operation or ischemia for 1 h and then reperfusion at the specified time points (n = 6 at each time point). C, Immunohistochemistry showing the expression of AXL in liver transplant patients and healthy livers (n = 3). ***P < 0.001; **P < 0.01; *P < 0.05. AXL, AXL receptor tyrosine kinase; I/R, ischemia-reperfusion; p-AXL, phosphorylation AXL.
Activated AXL Protects the Liver From I/R Injury
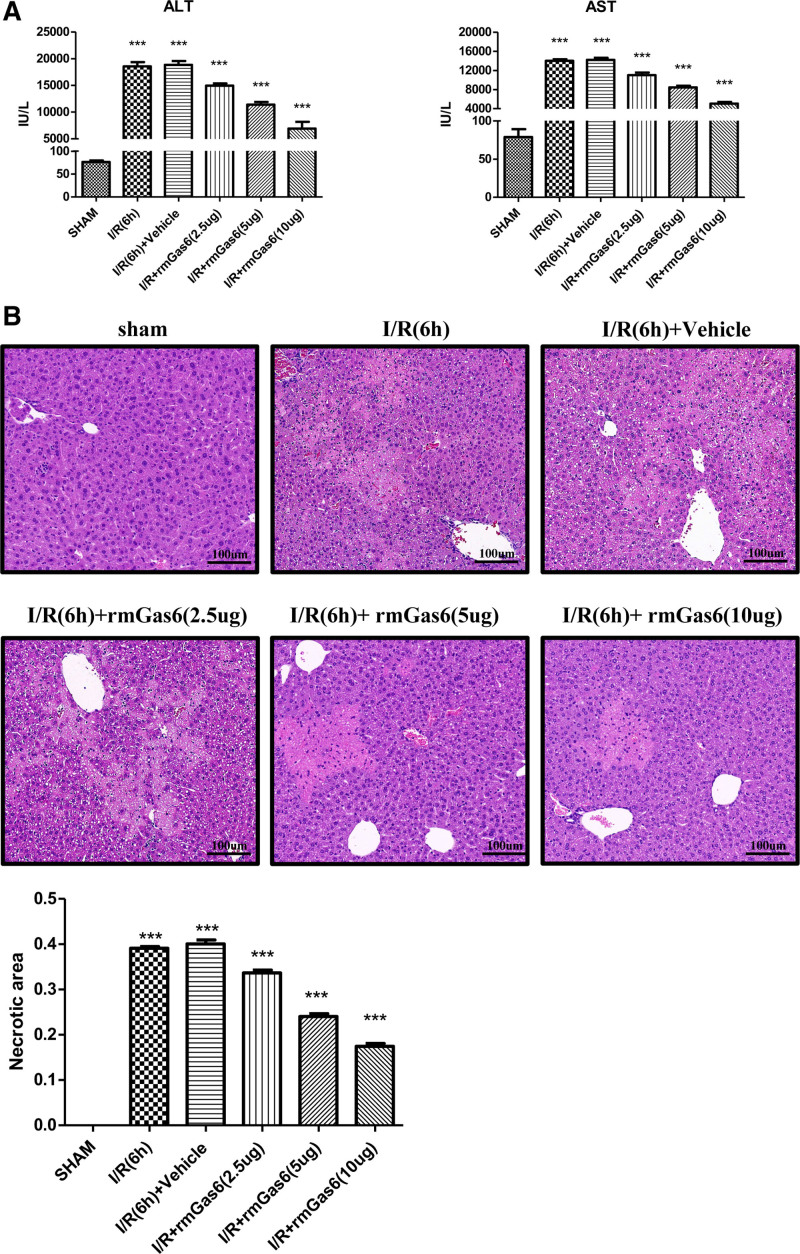
As shown in Figure 1, the expression of p-AXL was decreased in hepatic I/R injury. Therefore, we used exogenous recombinant mouse Gas6 protein (rmGas6) to activate AXL. Interestingly, after pretreatment with different doses of rmGas6, the level of transaminase (ALT/AST) in the mouse liver gradually decreased after I/R injury (Figure 2A). Hematoxylin and eosin staining showed that the area of liver necrosis in the rmGas6 pretreatment group was reduced after liver I/R injury (Figure 2B). This evidence indicated that AXL activation with rmGas6 can reduce hepatic I/R injury.
FIGURE 2.
Activated AXL protects the liver from I/R injury. A, Serum ALT and AST levels (n = 4–6) after hepatic I/R injury in the control group and the treatment group (as shown in the figure). B, Representative histological H&E-stained images showing the area of liver necrosis after hepatic I/R injury in the control group and the treatment group (n = 4–6). ***P < 0.001. ALT, alanine aminotransferase; AST, aspartate aminotransferase; AXL, AXL receptor tyrosine kinase; H&E, hematoxylin and eosin; I/R, ischemia-reperfusion; rmGas6, recombinant growth arrest-specific protein 6.
Activated AXL Ameliorates Inflammation in Hepatic I/R Injury
Inflammation is an important feature of hepatic I/R injury.3 Thus, we examined the expression of inflammatory cytokines in hepatic I/R injury. Western blotting results showed that after using different doses of rmGas6, the expression level of p-AXL in mice gradually increased during liver I/R injury. Compared with the control group, the rmGas6 pretreatment group gradually decreased the levels of inflammatory cytokines (IL-1β, IL-6, and TNF-α) in hepatic I/R injury (Figure 3A). The results showed that activated AXL reduced the expression of inflammatory cytokines in the livers of mice after I/R surgery at the mRNA and serum levels (Figure 3B and C). We used Ly6G staining IHC to verify whether rmGas6 could reduce the number of infiltrated neutrophils in liver I/R injury. The results showed that neutrophil infiltration in the rmGas6 pretreatment group was reduced compared with that in the control group (Figure 3D). These results showed that using rmGas6 to activate AXL can reduce the inflammation in hepatic I/R injury.
FIGURE 3.
Activated AXL ameliorates inflammation in hepatic I/R injury. A, Western blotting used to test the expression of the inflammatory factors IL-1β, IL-6, and TNF-α after hepatic I/R injury in the control group and the treatment group. B, ELISA to test the levels of the serum IL-1β, IL-6, and TNF-α after hepatic I/R injury in the control group and the treatment group. C, RT-qPCR used to detect the mRNA levels of IL-1β, IL-6, and TNF-α after hepatic I/R injury in the control group and the treatment group. D, A representative control group and treatment group with LY6G immunohistochemical staining of liver lobes after hepatic I/R injury. For (A–D), each group n = 4–6. ***P < 0.001; **P < 0.01. AXL, AXL receptor tyrosine kinase; ELISA, enzyme-linked immunosorbent assay; IL, interleukin; I/R, ischemia-reperfusion; p-AXL, phosphorylation AXL; rmGas6, recombinant growth arrest-specific protein 6; RT-qPCR, quantitative reverse transcription polymerase chain reaction; TNF-α, tumor necrosis factor-alpha.
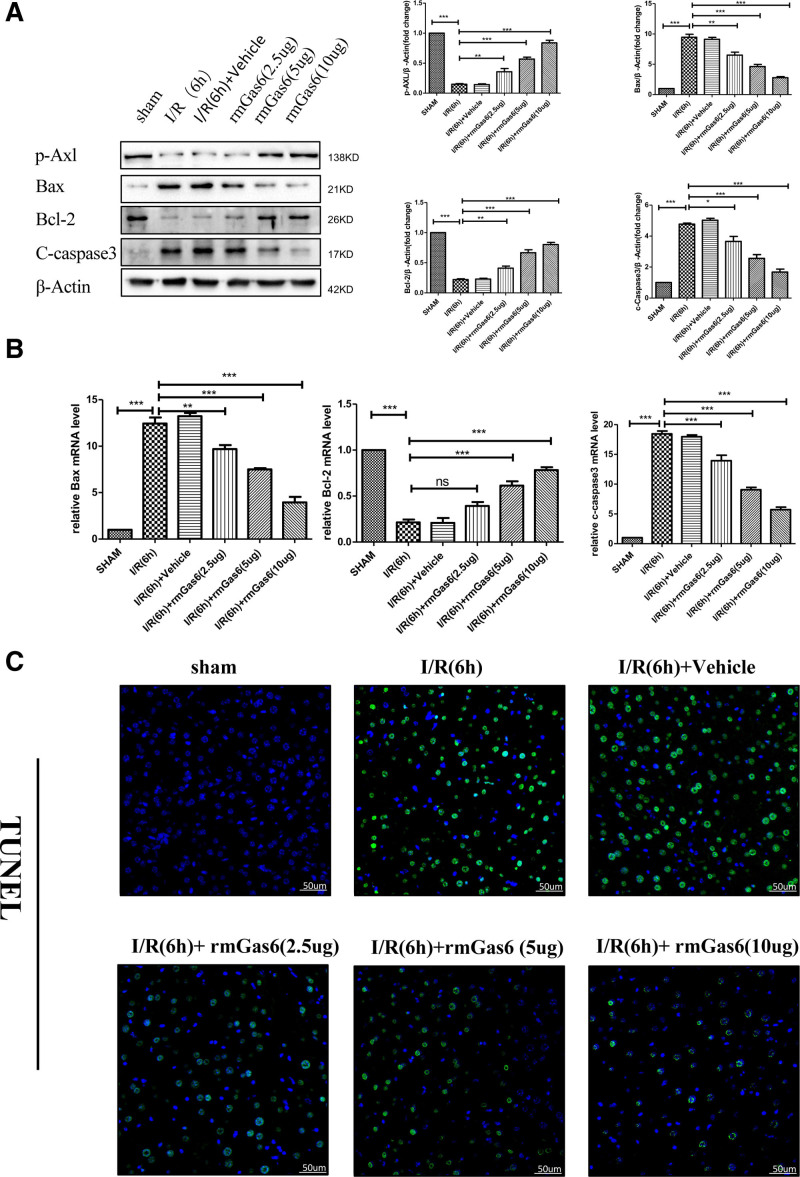
Activated AXL Ameliorates Hepatocyte Apoptosis in Hepatic I/R Injury
Apoptosis is another significant feature of hepatic I/R injury.1 We found that rmGas6 pretreatment increased the expression of p-AXL after hepatic I/R injury while reducing the proapoptotic proteins BAX and cleaved caspase-3 expression and increasing the expression of the antiapoptotic protein Bcl-2 (Figure 4A). RT-qPCR was used to detect Bcl-2, BAX, and cleaved caspase-3 expression at the mRNA level. This result was consistent with the Western blotting results (Figure 4B). We also used terminal deoxynucleotidal transferase–mediated biotin–deoxyuridine triphosphate nick-end labeling to verify the apoptosis level of hepatocytes in hepatic I/R injury, and the results showed that AXL activation reduced the apoptosis level of hepatocytes after hepatic I/R injury (Figure 4C). Primary hepatocytes were used for in vitro H/R experiments (Figure S2, SDC, http://links.lww.com/TP/C421). Our results showed that pretreatment with rmGas6 to activate AXL can reduce hepatocyte apoptosis in hepatic I/R injury.
FIGURE 4.
Activated AXL ameliorates hepatocyte apoptosis in hepatic I/R injury. A, Western blotting used to detect the expression of BAX, c-caspase-3, and Bcl-2 after hepatic I/R injury in the control group and the treatment group. B, RT-qPCR used to detect the mRNA levels of BAX, Bcl-2, and c-caspase-3 after hepatic I/R injury in the control group and the treatment group. C, Representative TUNEL staining showing the level of liver cell apoptosis after hepatic I/R in the control group and the treatment group. For (A–C), each group n = 3–6. ***P < 0.001; **P < 0.01; *P < 0.05. AXL, AXL receptor tyrosine kinase; BAX, Bcl-2 associated X protein; Bcl-2, B-cell lymphoma-2; c-caspase-3, cleaved-cysteinyl aspartatel-specific proteinase 3; I/R, ischemia-reperfusion; ns, not significant; p-AXL, phosphorylation AXL; rmGas6, recombinant growth arrest-specific protein 6; RT-qPCR, quantitative reverse transcription polymerase chain reaction; TUNEL, terminal deoxynucleotidal transferase–mediated biotin–deoxyuridine triphosphate nick-end labeling.
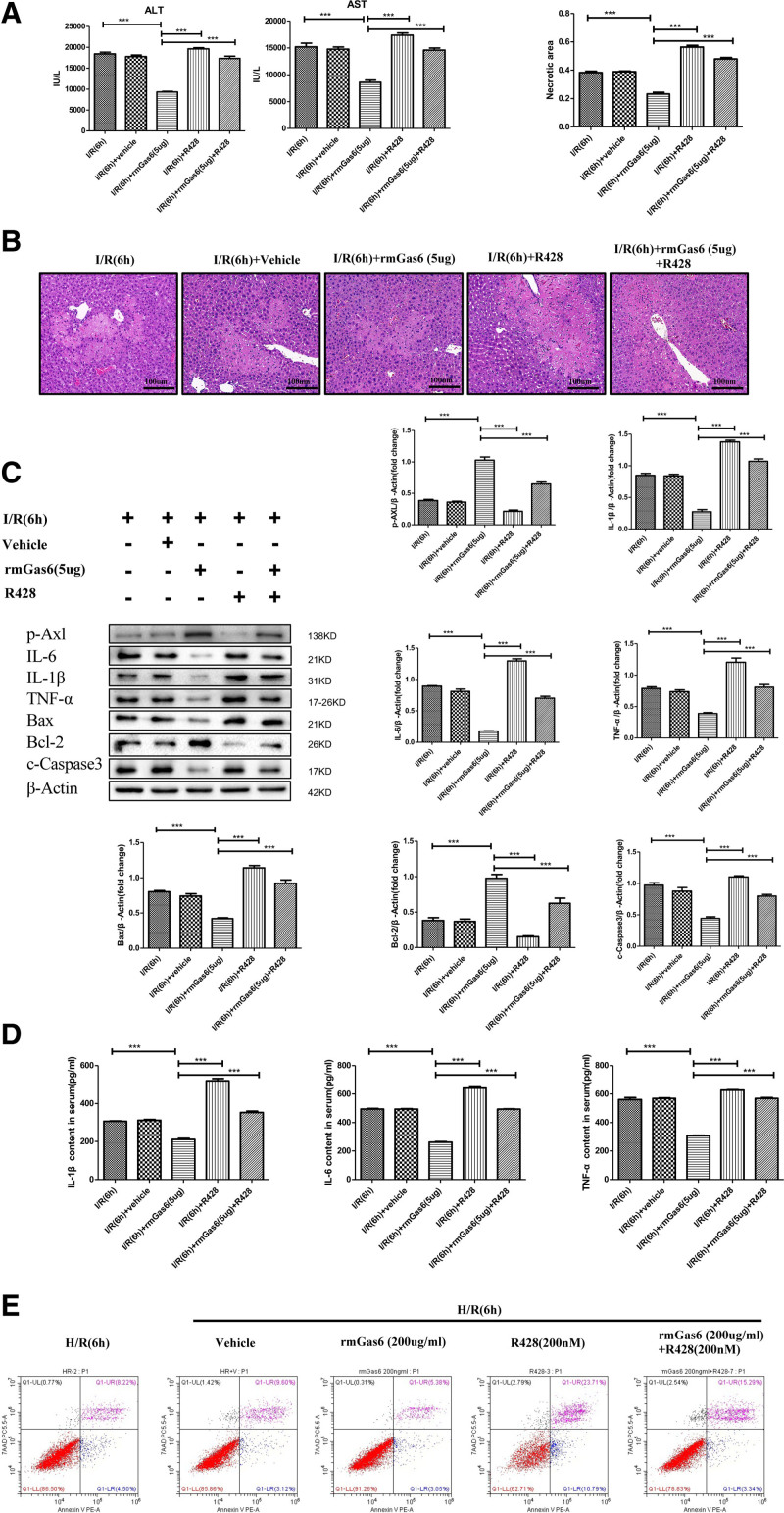
Inhibition of AXL Activation Aggravates Hepatic I/R Injury
We used the AXL-specific inhibitor R428 to inhibit the activation of AXL to further verify the function of activated AXL in hepatic I/R injury. The results showed that the R428 pretreatment group had higher ALT and AST levels and a larger areas of liver damage in hepatic I/R injury (Figure 5A and B). After pretreatment with R428, the expression of the inflammatory cytokines (IL-1β, IL-6, and TNF-α) increased, the expression of the BAX and c-caspase-3 increased, and the expression of the Bcl-2 decreased in hepatic I/R injury. At the same time, R428 + rmGas6, the results showed that R428 reversed the effects of rmGas6 in reducing the inflammatory response and liver cell apoptosis in hepatic I/R injury (Figure 5C and D). In vitro, we used cell apoptosis flow cytometry to verify the apoptosis of hepatocytes, and the results showed that hepatocyte apoptosis increased after R428 pretreatment (Figure 5E). Our results further illustrate that AXL activation protects the liver from I/R injury.
FIGURE 5.
Inhibition of AXL activation aggravates hepatic I/R injury. A, Serum ALT and AST activities of the treatment group (as shown in the figure) after hepatic I/R injury. B, Representative histological H&E-stained images showing the area of liver necrosis after hepatic I/R injury in the control group and the treatment group. C, Western blotting used to detect the expression of inflammatory factors and apoptosis-related proteins after hepatic I/R injury in the control group and the treatment group. D, ELISA used to detect the levels of the serum IL-1β, IL-6, and TNF-α after hepatic I/R injury in the control group and the treatment group. E, Flow cytometry experiment showing the apoptosis level of primary liver cells in hypoxia-reoxygenation experiment. For (A–E) each group n = 4–6. ***P < 0.001. ALT, alanine aminotransferase; AST, aspartate aminotransferase; AXL, AXL receptor tyrosine kinase; BAX, Bcl-2 associated X protein; Bcl-2, B-cell lymphoma-2; c-caspase-3, cleaved-cysteinyl aspartatel-specific proteinase 3; ELISA, enzyme-linked immunosorbent assay; H&E, hematoxylin and eosin; H/R, hypoxia and reoxygenation; IL, interleukin; I/R, ischemia-reperfusion; p-AXL, phosphorylation AXL; rmGas6, recombinant growth arrest-specific protein 6; TNF-α, tumor necrosis factor-alpha.
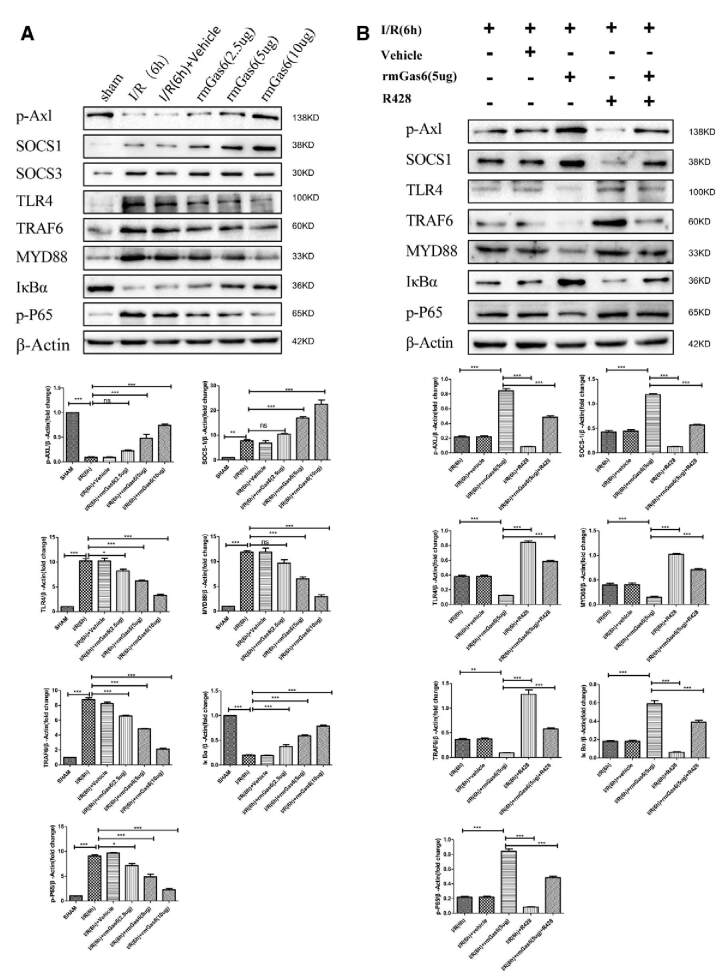
Activated AXL Inhibits the TLR4/MYD88/NF-κB Pathway by Upregulating SOCS-1
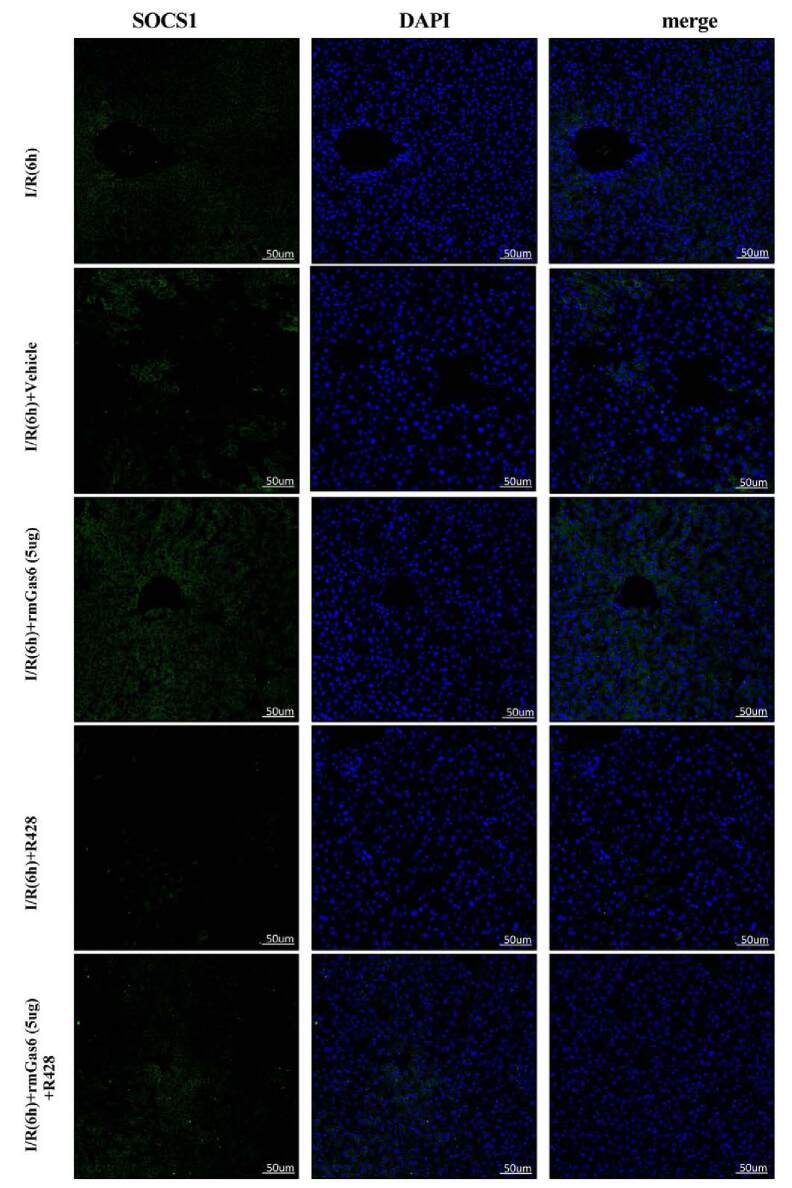
In liver I/R injury, necrotic liver cells release endogenous DAMPs, which activate the TLR4 signaling pathway and produce an inflammatory cascade.3 As shown in Figure 6A, rmGas6 upregulated the expression of SOCS-1 and reduced the expression of TLR4, MYD88, TRAF6, and phospho-NF-κB-p65. Interestingly, we also detected the expression of SOCS-3 that did not change (Figure 6A). R428 pretreatment inhibited the expression of SOCS-1 and activated the TLR4/MYD88/NF-κB signaling axis. The results of the rmGas6 + R428 group showed that R428 reversed the inhibitory effect of activated AXL on the TLR4/MYD88/NF-κB signaling axis (Figure 6B). Immunofluorescence showed that the expression of SOCS-1 was upregulated after rmGas6 pretreatment. R428 pretreatment inhibited the expression of SOCS-1 in hepatic I/R injury and reversed the upregulation of SOCS-1 by AXL (Figure 6C). This evidence indicates that AXL activation can inhibit the TLR4/MYD88/NF-κB signaling pathway by upregulating SOCS-1.
FIGURE 6.
Activated AXL inhibits the TLR/MYD88/NF-κB pathway by upregulating SOCS-1. A, Western blotting analysis of the expression of SOCS-1, SOCS-3, TLR4, MYD88, TRAF6, IκBα, and p-P65 in the rmGas6 treatment group and the control group. B, Western blotting used to detect the expression of SOCS-1, TLR4, MYD88, TRAF6, IκBα, and p-P65 in the R428 treatment group. C, Representative immunofluorescence pictures showing the expression of SOCS-1. For (A–C) each group n = 3–6. ***P < 0.001; **P < 0.01; *P < 0.05. AXL, AXL receptor tyrosine kinase; DAPI, 4′,6-diamidino-2-phenylindole; IκBα, NF-κB inhibitor alpha; I/R, ischemia-reperfusion; MYD88, myeloid differentiation factor-88; NF-κB, nuclear factor kappa-B; ns, not significant; p-AXL, phosphorylation AXL; p-P65, phospho-NF-κB-p65; rmGas6, recombinant growth arrest-specific protein 6; SOCS, suppressor of cytokine signaling protein; TLR4, toll-like receptor 4; TRAF6, tumor necrosis factor receptor–associated factor 6.
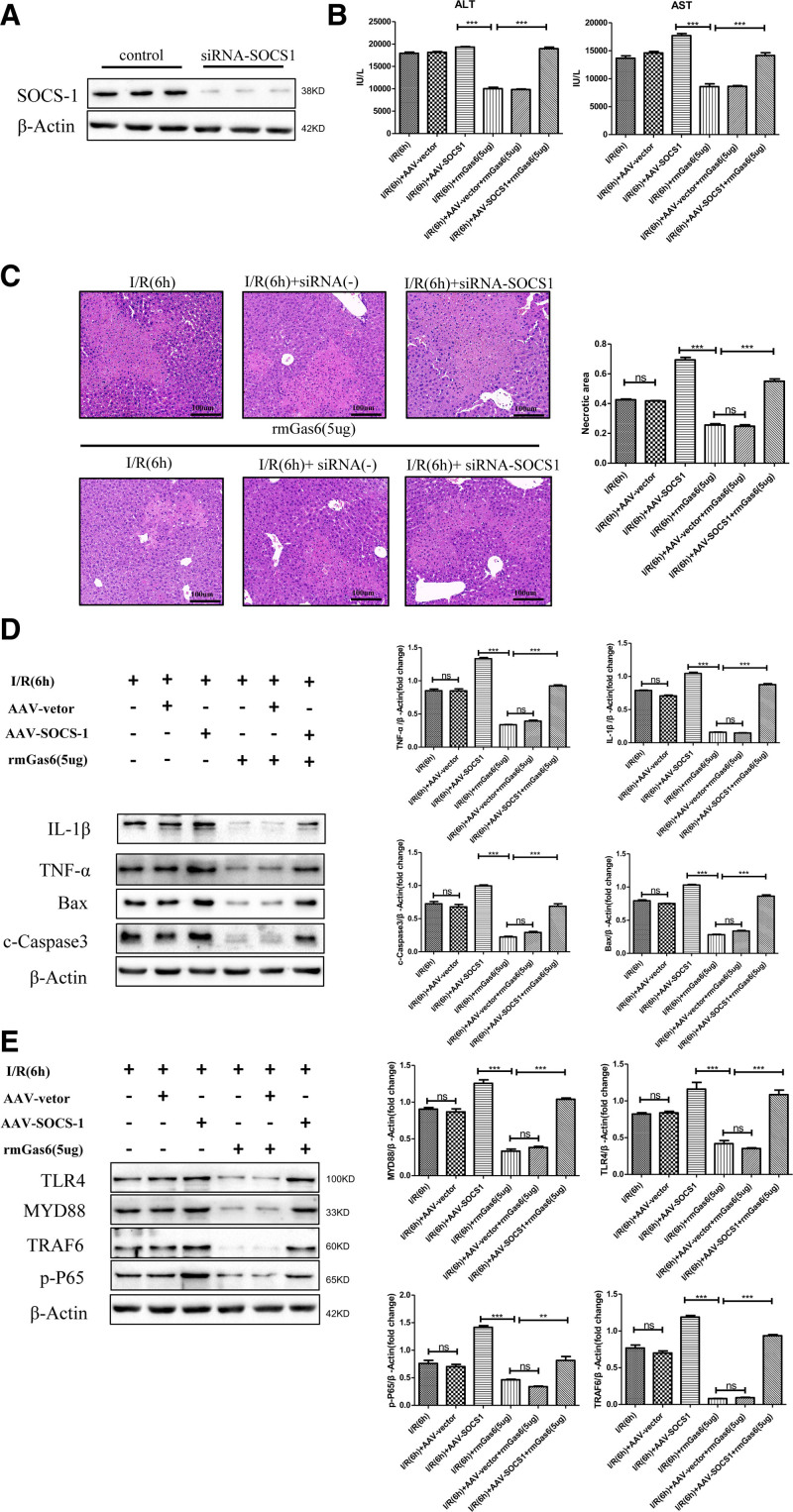
SOCS-1 Mediates the Protective Effect of Activated AXL Against Hepatic I/R Injury
To better explain how SOCS-1 mediates the protective effect of activated AXL on hepatic I/R injury, we used an AAV to specifically knock down SOCS-1 in mouse liver. As shown in the figure, compared with the I/R group, the SOCS-1 knockdown group had higher serum transaminase levels and a larger liver damage area. Despite the use of rmGas6, the SOCS-1 knockdown group did not show reduced liver damage (Figure 7B and C). At the same time, we tested the changes in inflammation and apoptosis of hepatic I/R injury after SOCS-1 was knocked down. Compared with the I/R group, the SOCS-1 knockdown group showed aggravated inflammation and increased cell apoptosis. However, activation of AXL after SOCS-1 knockdown did not improve inflammation or apoptosis in hepatic I/R injury and failed to inhibit the TLR4/MYD88/NF-κB signaling pathway. Compared with the I/R + rmGas6 group, the I/R + rmGas6 + AAV-SOCS-1 group showed increased expression of BAX and c-caspase-3 and levels of inflammatory factors, whereas the levels of TLR4, MYD88, TRAF6, and phospho-NF-κB-p65 were increased (Figure 7D and E). These results indicate that SOCS-1 mediates AXL activation and plays a protective role in hepatic I/R injury.
FIGURE 7.
SOCS-1 mediates the protective effect of activated AXL in hepatic I/R injury. A, Western blotting analysis of the knockdown efficiency of SOCS-1 by adeno-associated virus (n = 3). B, Serum ALT and AST levels of the SOCS-1 knockdown treatment group (as shown in the figure) after hepatic I/R injury. C, Representative histological H&E-stained images showing the area of liver necrosis after hepatic I/R injury in the SOCS-1 knockdown treatment group. D, Western blotting detection of IL-1β, TNF-α, BAX, and c-caspase-3 expression in the SOCS-1 knockdown treatment group (as shown in the figure). E, Western blotting detection of TLR4, MYD88, TRAF6, and p-P65 in the SOCS-1 knockdown treatment group. For (B–E) each group n = 3–6. ***P < 0.001; **P < 0.01. AAV, adeno-associated virus; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AXL, AXL receptor tyrosine kinase; BAX, Bcl-2 associated X protein; c-caspase-3, cleaved-cysteinyl aspartatel-specific proteinase 3; H&E, hematoxylin and eosin; IL, interleukin; I/R, ischemia-reperfusion; MYD88, myeloid differentiation factor-88; ns, not significant; p-P65, phospho-NF-κB-p65; rmGas6, recombinant growth arrest-specific protein 6; SOCS, suppressor of cytokine signaling protein; TLR4, toll-like receptor 4; TNF-α, tumor necrosis factor alpha; TRAF6, receptor–associated factor 6.
DISCUSSION
The inflammation and apoptosis play important roles in the pathogenesis of liver I/R injury. In hepatic I/R injury, damaged liver cells produce DAMPs that activate TLR4 signaling, trigger inflammation, and aggravate hepatocyte apoptosis. Identifying the key regulatory factors of these signaling pathways may be a promising strategy for preventing or intervening in hepatic I/R injury. This study suggested that AXL plays a critical role in hepatic I/R injury.
In our group’s previous studies, proteomic analysis through quantitative high-throughput mass spectrometry has shown that AXL expression is upregulated in hepatecomy with I/R injury.22 This result was confirmed in this study by observing donor liver specimens and a mouse model of hepatic I/R injury (Figure 1). Rankin et al26 found that hypoxia inducible factor-1 and hypoxia inducible factor-2 directly facilitate AXL expression by binding to hypoxia response elements of the AXL proximal promoter, which may account for AXL expression in I/R injury. AXL is a receptor tyrosine kinase, and Gas6 is the high-affinity ligand of AXL. Interestingly, we observed that p-AXL expression was inhibited in a mouse model of I/R injury despite AXL upregulation. To explore the reasons, we detected the expression of endogenous Gas6 in a mouse liver I/R injury model, and the results showed that the expression of Gas6 was inhibited in liver I/R injury (Figure S1, SDC, http://links.lww.com/TP/C421). Therefore, the reason for this interesting phenomenon may be that the decreased expression of endogenous Gas6 in the AXL high-affinity ligand led to decreased activation of AXL in hepatic I/R injury, which also provides evidence for the use of mouse recombinant Gas6 protein (rmGas6) to activate AXL in our subsequent experiments.
We observed that, compared with the control group, AXL activated by exogenous rmGas6 reduced the liver injury area and transaminase level of mice (Figure 2), as well as the inflammatory response and hepatocyte apoptosis (Figures 3 and 4). Furthermore, inhibition of AXL activation with the AXL-specific inhibitor, R428, reversed protective effects of AXL in hepatic I/R injury (Figure 5). Consistently, previous studies have also observed that AXL activation can reduce the expression of inflammatory factors and inhibit apoptosis in lung and kidney I/R injury.18-20 The inhibition of AXL in chronic lymphocytic leukemia promotes apoptosis and reduces the level of antiapoptotic protein McL-1.27 AXL activation inhibits the TLR signaling pathway by upregulating Bcl-2 and Twist, thereby limiting the expression of proinflammatory cytokines.28 These findings are consistent with our conclusion. Therefore, it is very important to further study AXL and its downstream signaling pathway regulatory mechanisms.
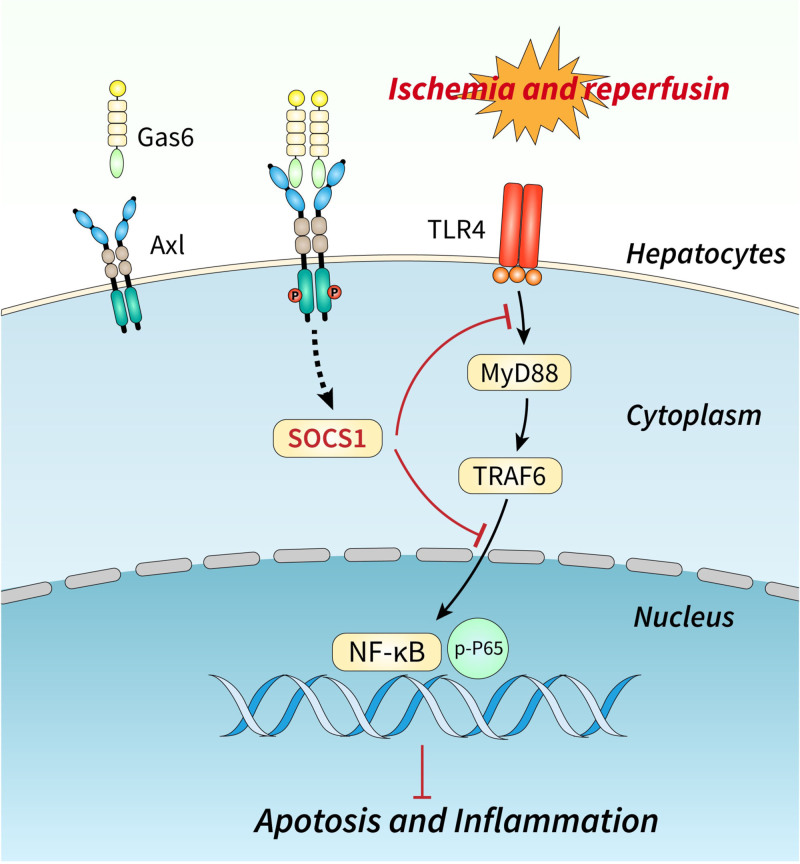
SOCS proteins inhibit cytokine transduction during immune and inflammatory responses.29 We found that AXL activation inhibited TLR4 and its downstream NF-κB signaling pathway by promoting SOCS-1 expression (Figure 6). Previous results in TLR4 knockout mice found that TLR4-deficient mice showed a more severe inflammatory response and increased cell death in hepatic I/R injury,30-32 which is consistent with our findings. Nevertheless, we observed that the expression of SOCS-3 did not change after AXL activation (Figure 6). In some studies on the inflammatory response, SOCS-3 is considered to be a key factor affecting the intensity of cytokine signaling, whereas SOCS-1 mainly regulates the duration of cytokine signal transduction.33,34 In mild liver I/R injury (I/R 20 min), SOCS-3 acts as an initial regulatory mechanism involved in the acute regulation of liver I/R injury and may be sufficient to control cytokine expression during mild injury.35 As the degree of hepatic I/R injury exacerbates, SOCS-1 induction provides an important means of injury control when SOCS-3–mediated cytokine inhibition is insufficient to control I/R injury.35 To better investigate how SOCS-1 mediates the protective effect of activated AXL in hepatic I/R injury, we used AAV-specific knockdown of SOCS-1 in the mouse liver. The results showed that after SOCS-1 knockdown, hepatic I/R injury did not improve, even when AXL was activated. This evidence suggested that activated AXL plays a protective role against hepatic I/R injury by promoting SOCS-1 expression (Figure 8).
FIGURE 8.
Activated AXL protects against hepatic ischemia-reperfusion injury by upregulating SOCS-1 expression. Activated AXL exerts a protective effect against hepatic I/R injury through SOCS-1-TLR4/MYD88/NF-κB signaling pathway. AXL, AXL receptor tyrosine kinase; Gas6, growth arrest-specific protein 6; I/R, ischemia/reperfusion; MYD88, myeloid differentiation factor-88; NF-κB, nuclear factor kappa-B; SOCS, suppressor of cytokine signaling protein; TLR4, toll-like receptor 4; TRAF6, tumor necrosis factor receptor–associated factor 6.
Our study presents that preconditioning mice with rmGas6 to activate AXL can reduce the inflammatory response and apoptosis in hepatic I/R injury. Therefore, targeting AXL therapy may be a novel therapeutic strategy to prevent and treat hepatic I/R injury. Although soluble AXL is considered to be a possible serum biomarker for chronic pancreatitis, liver fibrosis, and liver cirrhosis,36,37 whether sAXL can become a novel serum biomarker for the degree of hepatic I/R injury needs to be further clarified.
In conclusion, our research found that activated AXL is a key factor in protecting hepatic I/R injury. More importantly, activated AXL may play a protective role in hepatic I/R injury by regulating SOCS-1 rather than SOCS-3. Activated AXL exerts a protective effect against hepatic I/R injury through the SOCS-1-TLR4/MYD88/NF-κB signaling pathway. Therefore, we consider that AXL may be a novel promising therapeutic target for the hepatic I/R injury.
Supplementary Material
Footnotes
Z.W., D.L., and Q.Y. have contributed equally to this work and share first authorship.
This work was supported by the National Natural Science Foundation of China (No.81970542; No.82072300, No.81871674).
The authors declare no conflicts of interest.
J.D. and L.C. designed the study. Z.W. and D.L. collated the data. Q.Y. performed data analyses. Z.W. produced the initial draft of the article. F.L., M.Z., and S.Q. contributed to drafting of the article. Q.F., L.Y., W.W., and R.Z. revised the article. All authors have read and approved the final submitted article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Zhai Y, Petrowsky H, Hong JC, et al. Ischaemia-reperfusion injury in liver transplantation—from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transplant. 2011;11:1563–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliveira THC, Marques PE, Proost P, et al. Neutrophils: a cornerstone of liver ischemia and reperfusion injury. Lab Invest. 2018;98:51–62. [DOI] [PubMed] [Google Scholar]
- 4.Ye L, He S, Mao X, et al. Effect of hepatic macrophage polarization and apoptosis on liver ischemia and reperfusion injury during liver transplantation. Front Immunol. 2020;11:1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji H, Liu Y, Zhang Y, et al. T-cell immunoglobulin and mucin domain 4 (TIM-4) signaling in innate immune-mediated liver ischemia-reperfusion injury. Hepatology. 2014;60:2052–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147:765–783.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirao H, Nakamura K, Kupiec-Weglinski JW. Liver ischaemia-reperfusion injury: a new understanding of the role of innate immunity. Nat Rev Gastroenterol Hepatol. 2022;19:239–256. [DOI] [PubMed] [Google Scholar]
- 8.Zhu C, Wei Y, Wei X. AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol Cancer. 2019;18:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu E, Hjelle B, Bishop JM. Transforming genes in chronic myelogenous leukemia. Proc Natl Acad Sci U S A. 1988;85:1952–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham DK, DeRyckere D, Davies KD, et al. The TAM family: phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat Rev Cancer. 2014;14:769–785. [DOI] [PubMed] [Google Scholar]
- 11.Linger RM, Keating AK, Earp HS, et al. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scaltriti M, Elkabets M, Baselga J. Molecular pathways: AXL, a membrane receptor mediator of resistance to therapy. Clin Cancer Res. 2016;22:1313–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grosso S, Puissant A, Dufies M, et al. Gene expression profiling of imatinib and PD166326-resistant CML cell lines identifies Fyn as a gene associated with resistance to BCR-ABL inhibitors. Mol Cancer Ther. 2009;8:1924–1933. [DOI] [PubMed] [Google Scholar]
- 14.Zhou S, Liu L, Li H, et al. Multipoint targeting of the PI3K/mTOR pathway in mesothelioma. Br J Cancer. 2014;110:2479–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umemura S, Sowa Y, Iizumi Y, et al. Synergistic effect of the inhibitors of RAF/MEK and AXL on KRAS-mutated ovarian cancer cells with high AXL expression. Cancer Sci. 2020;111:2052–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimani SG, Kumar S, Bansal N, et al. Small molecule inhibitors block Gas6-inducible TAM activation and tumorigenicity. Sci Rep. 2017;7:43908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korshunov VA. Axl-dependent signalling: a clinical update. Clin Sci (Lond). 2012;122:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu G, McBride DW, Zhang JH. Axl activation attenuates neuroinflammation by inhibiting the TLR/TRAF/NF-κB pathway after MCAO in rats. Neurobiol Dis. 2018;110:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng CK, Wu CP, Lin JY, et al. Gas6/Axl signaling attenuates alveolar inflammation in ischemia-reperfusion-induced acute lung injury by up-regulating SOCS3-mediated pathway. PLoS One. 2019;14:e0219788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen DQ, Feng YL, Chen L, et al. Poricoic acid A enhances melatonin inhibition of AKI-to-CKD transition by regulating Gas6/AxlNFκB/Nrf2 axis. Free Radic Biol Med. 2019;134:484–497. [DOI] [PubMed] [Google Scholar]
- 21.DeBerge M, Glinton K, Subramanian M, et al. Macrophage AXL receptor tyrosine kinase inflames the heart after reperfused myocardial infarction. J Clin Invest. 2021;131:139576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai H, Qi S, Yan Q, et al. Global proteome profiling of human livers upon ischemia/reperfusion treatment. Clin Proteomics. 2021;18:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J, Xie P, Dong Y, et al. Inhibition of Drp1 SUMOylation by ALR protects the liver from ischemia-reperfusion injury. Cell Death Differ. 2021;28:1174–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Lu T, Zhang C, et al. Activation of YAP attenuates hepatic damage and fibrosis in liver ischemia-reperfusion injury. J Hepatol. 2019;71:719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Mao W, Fang C, et al. Dusp14 protects against hepatic ischaemia-reperfusion injury via Tak1 suppression. J Hepatol. [Epub ahead of print. September 6, 2017]. doi:10.1016/j.jhep.2017.08.032 [DOI] [PubMed] [Google Scholar]
- 26.Rankin EB, Fuh KC, Castellini L, et al. Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proc Natl Acad Sci U S A. 2014;111:13373–13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinha S, Boysen J, Nelson M, et al. Targeted Axl inhibition primes chronic lymphocytic leukemia B cells to apoptosis and shows synergistic/additive effects in combination with BTK inhibitors. Clin Cancer Res. 2015;21:2115–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasanbasic I, Cuerquis J, Varnum B, et al. Intracellular signaling pathways involved in Gas6-Axl-mediated survival of endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H1207–H1213. [DOI] [PubMed] [Google Scholar]
- 29.Baker BJ, Akhtar LN, Benveniste EN. SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol. 2009;30:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu L, Zhou H, Ni M, et al. Innate immune regulations and liver ischemia-reperfusion injury. Transplantation. 2016;100:2601–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen XD, Ke B, Zhai Y, et al. Absence of toll-like receptor 4 (TLR4) signaling in the donor organ reduces ischemia and reperfusion injury in a murine liver transplantation model. Liver Transpl. 2007;13:1435–1443. [DOI] [PubMed] [Google Scholar]
- 32.Sosa RA, Rossetti M, Naini BV, et al. Pattern recognition receptor-reactivity screening of liver transplant patients: potential for personalized and precise organ matching to reduce risks of ischemia-reperfusion injury. Ann Surg. 2020;271:922–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brysha M, Zhang JG, Bertolino P, et al. Suppressor of cytokine signaling-1 attenuates the duration of interferon gamma signal transduction in vitro and in vivo. J Biol Chem. 2001;276:22086–22089. [DOI] [PubMed] [Google Scholar]
- 34.Croker BA, Krebs DL, Zhang JG, et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–545. [DOI] [PubMed] [Google Scholar]
- 35.Langdale LA, Hoagland V, Benz W, et al. Suppressor of cytokine signaling expression with increasing severity of murine hepatic ischemia-reperfusion injury. J Hepatol. 2008;49:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martínez-Bosch N, Cristóbal H, Iglesias M, et al. Soluble AXL is a novel blood marker for early detection of pancreatic ductal adenocarcinoma and differential diagnosis from chronic pancreatitis. Ebiomedicine. 2022;75:103797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staufer K, Dengler M, Huber H, et al. The non-invasive serum biomarker soluble Axl accurately detects advanced liver fibrosis and cirrhosis. Cell Death Dis. 2017;8:e3135. [DOI] [PMC free article] [PubMed] [Google Scholar]