Abstract
Cardiovascular defects, injuries, and degenerative diseases often require surgical intervention and the use of implantable replacement material and conduits. Traditional vascular grafts made of synthetic polymers, animal and cadaveric tissues, or autologous vasculature have been utilized for almost a century with well-characterized outcomes, leaving areas of unmet need for the patients in terms of durability and long-term patency, susceptibility to infection, immunogenicity associated with the risk of rejection, and inflammation and mechanical failure. Research to address these limitations is exploring avenues as diverse as gene therapy, cell therapy, cell reprogramming, and bioengineering of human tissue and replacement organs. Tissue-engineered vascular conduits, either with viable autologous cells or decellularized, are the forefront of technology in cardiovascular reconstruction and offer many benefits over traditional graft materials, particularly in the potential for the implanted material to be adopted and remodeled into host tissue and thus offer safer, more durable performance. This review discusses the key advances and future directions in the field of surgical vascular repair, replacement, and reconstruction, with a focus on the challenges and expected benefits of bioengineering human tissues and blood vessels.
Keywords: bioengineering, cell- and tissue-based therapy, humans, regenerative medicine, tissue engineering
Regenerative medicine and bioengineering of functional human tissues strive to fulfill the evergrowing need for organ repair, regeneration, or replacement. In the quest for performance and durability comparable to autologous tissues with no or minimal immune response, manufacturing challenges are only commensurate with the immense benefit for patients in a variety of conditions such as traumatic injuries, chronic and degenerative conditions, cancer, and autoimmune diseases. Tissue engineering, already impactful in areas such as dermatology with the manufacturing of cellular or acellular skin substitutes, has been making considerable strides in the vascular field. The development of human tissue material for vascular repair, replacement, or reconstruction aims to offer a readily available, off-the-shelf alternative to autologous conduits with characteristics similar in terms of immunogenicity and long-term functionality, while eliminating the wound care and operating time burden associated with harvesting patients’ own vessels.1,2 The number of patients requiring replacement vascular conduits is steadily growing. Cardiovascular disease is the leading cause of mortality in the United States, and in 2018 alone, 26.1 million adults had coronary heart disease, heart failure, or stroke. Often the outcome of these conditions requires surgery, and every year, ≈370 000 coronary artery bypass grafts (CABG), 156 000 heart valve operations, and 86 000 coronary endarterectomies are performed in the United States alone.3 Peripheral artery disease (PAD) and vascular injuries, including civilian and military traumatic injuries, represent another area of critical need for replacement vessels. In 2015, >200 million people globally were living with PAD3,4 with a 13% increase in prevalence in high-income countries between 2000 and 2010,3,4 while vascular injuries especially in the military setting are on the rise.5,6
Furthermore, patients on hemodialysis for end-stage renal disease (ESRD) require access to the circulatory system multiple times per week relying on either a central catheter or a superficial arteriovenous loop access created surgically. The arteriovenous conduit is most often on the upper limb, either with the patients’ vessels (arteriovenous fistula [AVF]) or by the insertion of a synthetic graft such as expanded polytetrafluoroethylene (ePTFE). The incidence of ESRD has been steadily increasing since 1980,7 with 809 000 prevalent cases in the United States as of December 2019, of which 62.5% were treated with hemodialysis.8 Although autologous AVF is the preferred option in most cases,9 as of 2019, only 15.2% of patients initiate hemodialysis with an AVF, leaving the vast majority to rely on central venous catheters or synthetic grafts, which puts them at high risk for infection and thrombosis and highlights the need for new materials.9
Vascular replacements have been used for over 60 years, initially with synthetic polymers followed by the first autologous saphenous vein (SV) graft implemented in 1967 by Favaloro et al.10 Today, vascular grafts originate from 4 broad sources: synthetic polymers, xenogeneic tissues, allogeneic or autologous tissues, and engineered tissues. While all have proven functional to a degree, each of these types of vascular replacements has vastly different outcomes in vivo, with infection, inflammation, occlusion, and degradation presenting as the most frequent failure modes.11 One of the greatest challenges to deployment of vascular grafts is their adoption by the host and ability to remodel into new tissues. Regenerative medicine focuses on the production of materials or techniques that harness the body’s own remodeling abilities, to improve these outcomes, and offer recipients the experience of fully functional organs. This review details the challenges of bioengineering human blood vessels and their expected applications, comparing their features with those of historical conduits and with the current options in development to outline potential future avenues of research.
Traditional Vascular Grafts
Traditional vascular grafts in use today include synthetic conduits, xenografts of mostly bovine or porcine origin, cadaveric allografts, and autografts obtained by harvesting a patient’s own vessels, primarily SVs or internal mammary arteries (IMAs).
Nondegradable Polymer Grafts
Synthetic nondegradable polymer vascular grafts are made of ePTFE (Gore-Tex), polyethylene terephthalate (Dacron), and polyurethane (PU); their appeal lies in their relatively inexpensive production, off-the-shelf availability, and long shelf lives.
In practice, ePTFE grafts have been classified as low porosity (<30 μm internodal distance) and high porosity (>45 μm internodal distance), with the observation that higher porosity would offer greater potential for tissue ingrowth while lower porosity means stiffer, less flexible grafts with reduced tissue ingrowth and endothelialization.12,13 Drawbacks, in small-diameter (<6 mm) settings in particular, include problematic long-term patency due to the graft’s susceptibility to thrombosis, inflammation, stenosis, and associated compliance mismatch with the host vessels.14 On par with infection, thrombosis is the other major complication of ePTFE vascular grafts, particularly at smaller diameters.12,13,15
Dacron offers another type of synthetic nondegradable polymer conduit. Textile vascular grafts made from polyethylene terephthalate fibers are either woven or knitted. While woven grafts have small pores with lower permeability and less bleeding, knitted grafts formed by looping fibers together have larger pores that promote greater tissue ingrowth and are more compliant. However, due to larger pore sizes, knitted grafts must be preclotted with a degradable coating of albumin, gelatin, or even blood to prevent plasma seepage and seroma formation after implantation, especially in high-pressure vessels like the aorta.16 Poor patency over the long-term, notably in small-diameter vessels, still limits Dacron use clinically.17 Systematic evaluation and meta-analysis of randomized controlled trials comparing Dacron and ePTFE have shown no substantial advantages of one conduit type over the other in terms of primary and secondary patency rates or infection.18
PU vascular grafts represent the other segment of synthetic grafts, constructed as copolymers of 3 different monomers: (1) hard, crystalline segments for rigidity; (2) a polymer chain extender; and (3) soft, amorphous segments for flexibility, most commonly polyester, polyether, or polycarbonate.19 Degradation of traditional PU grafts under physiological conditions is a concern, with structural failure linked to enzymatic attack, oxidative stress, and reaction to strain or stress cracking, but the main concern in clinical applications is the risk of thrombosis.19 Overall, ePTFE, Dacron, and PU grafts show little or no transmural tissue ingrowth and similar patency rates over time.11
Nondegradable synthetic grafts pose continued risk to patients in terms of patency rates, mechanical compliance, infection, and durability,15 as shown in ePTFE explant (Figure 1). Up to 90% of patients with graft thrombosis have intimal hyperplasia that led to stenosis,20 and infection is reported in up to 17% of synthetic arteriovenous grafts that are used for hemodialysis access.21 More critically, patency rates of grafts at diameters <5 mm are unacceptably low.11
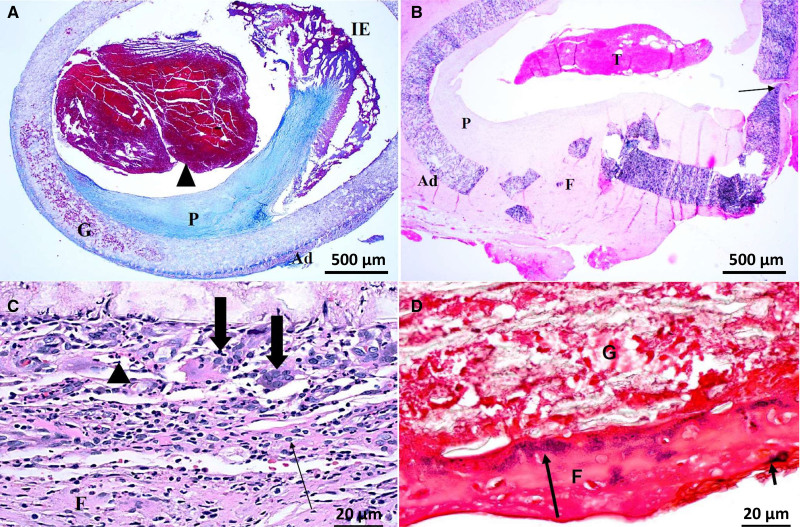
Figure 1.
Explants of expanded polytetrafluoroethylene grafts used as hemodialysis vascular access at 7 mo post-implantation. A, Thrombosis (arrowhead), inflammatory exudate (IE), adventitia (Ad), graft (G) and pannus (P) in trichrome staining. B, Fragmented wall with Ad and fibrosis in the defect (F) and thrombosis (T) in hematoxylin and eosin staining. C, Multinucleated giant cells (large arrows), macrophages (small arrow), and neutrophils (arrowhead) in hematoxylin and eosin staining. D, Gram-positive cocci (arrows) within intraluminal fibrin along luminal surface in Gram staining.
Several research strategies have been implemented with the goals of reducing infection rates, promoting endothelialization, and inhibiting inflammation. Thus far, these have failed to produce significant benefits.11 Coatings may contain heparin, create nitric oxide, utilize micro-RNAs to decrease intimal hyperplasia,22 or stimulate endothelial cell (EC) growth through factors such as VEGF-R2 (VEGF [vascular endothelial growth factor] receptor 2),13 angiogenic micro-RNAs,23 or resorbable polymers to promote endothelialization.24 These coatings have yielded unconvincing results thus far, likely because of the limited time during which these coatings are active due to passivation by contact with blood proteins, as well as host responses to the bulk foreign material of the conduit.25 Finally, seeding with ECs has been explored over the past 20 years with limited clinical success.14,19,25-28 In an effort to improve the hemocompatibility of ePTFE grafts, autologous ECs have been seeded onto the lumen.26 ECs derived from small vein biopsies were expanded and cultured on ePTFE grafts over 4 weeks, which then were implanted as femoropopliteal grafts in patients with severe PAD. In a study of >300 patients who received the endothelialized grafts, a 76% 5-year patency rate was comparable to that of native SV, making this endothelialization approach a possible avenue for exploration.26 However, the need for harvesting suitable numbers of autologous ECs from vein segments, the time and cost involved in the growth and seeding of these ECs, along with challenges with long-term EC adherence to artificial surfaces, have prevented more widespread adoption of this technology.
Autologous Blood Vessels
Of all graft options, autologous blood vessels possess physiological properties that align the most closely with that of the vasculature requiring repair (Figure 2), thus making them an appealing first option. Options frequently used in cardiovascular surgery range from small-diameter arteries (radial artery and internal thoracic artery) to greater and lesser saphenous, brachial, or other arm veins.29 In coronary artery bypass surgery, the most recent American College of Cardiology guidelines recommend the use of the autologous arteries over SVs, specifically the left internal mammary (also known as thoracic) artery followed by its right counterpart or both IMA in a procedure called bilateral IMA grafting, and the radial artery for multivessel CABG, all of which show better patency outcomes over the use of SV grafting.30 In coronary revascularization surgery, 3% to 12% of SV grafts become occluded within the first month following CABG.31,32 Long-term patency of the SVs in this setting is poor, with up to 50% of grafts failing within 10 years, mostly due to fast-progressing atherosclerosis on a foundation of neointimal hyperplasia.31,32
Figure 2.
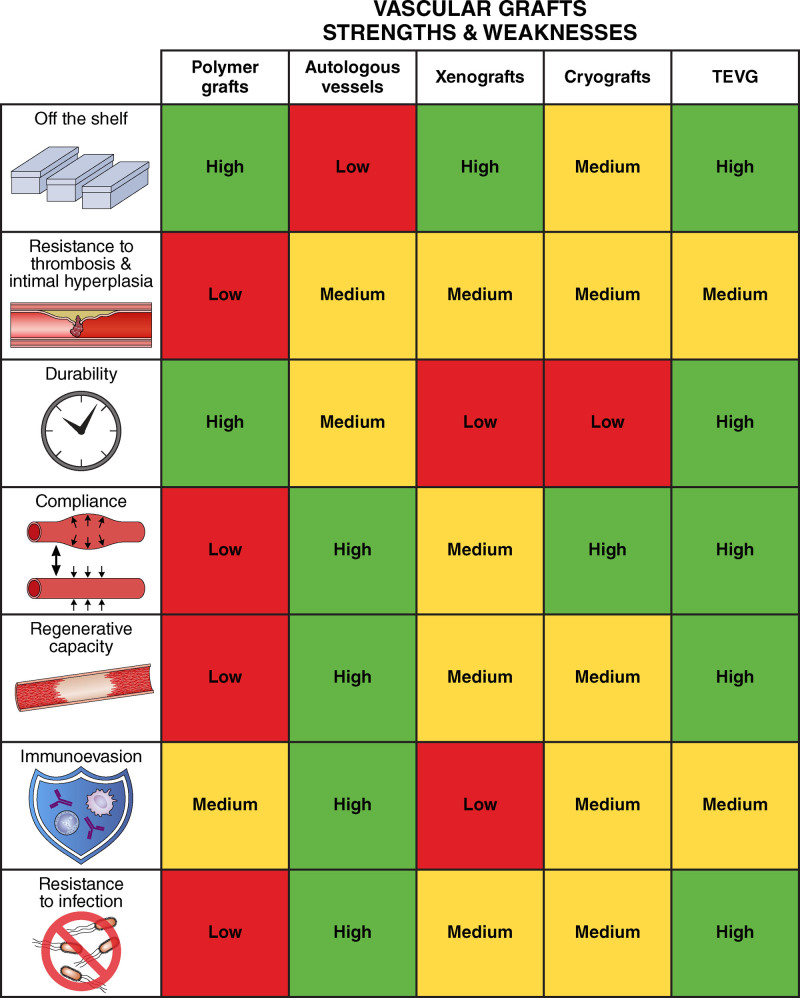
Strengths and weaknesses of different vascular graft materials. A semiquantitative heat map representing the qualities of the vascular graft materials discussed, with green indicating high, yellow indicating medium, and red indicating low relative amounts or incidence of the parameters listed on the vertical axis. TEVG indicates tissue-engineered vascular graft. Illustration credit: Patrick Lane.
The superior performance of the IMA may be explained by its anatomic structure. It is an elastic artery with elastin unevenly distributed in its structure: while the origin, proximal, and middle parts of the IMA contain 5 to 10 elastin laminae, the distal segment has a predominately muscular tunica media containing a limited number of elastic lamellae33; when used as a vascular graft, the presence of distensible elastin in the media correlates with considerably less intimal thickening as compared with other muscular arteries.33 IMA grafts placed in the aorta-coronary circulation are inherently accustomed to the high-pressure arterial flow, and the size similarity between the grafts and the coronary arteries may play a role in protecting the IMA from development of atherosclerosis and may contribute to long-term patency.34,35
The ECs of the IMA are densely stacked and firmly anchored to the subendothelium and encounter minimal disturbance during IMA harvesting. The IMA endothelium displays fewer fenestrations and lower intercellular junctional permeability as compared with the endothelium of SV, which might prevent lipoproteins from entering the subendothelial space and instigating inflammation and intimal hyperplasia. The ECs of the IMA are also rich in heparin sulfate and eNOS (endothelial NO synthase), which may help with maintaining patency after CABG.35
Early IMA graft failure is most commonly attributed to technical errors with harvesting and graft anastomosis. When thickening of the intima happens, it consists primarily of smooth muscle cells (SMCs), proteoglycan, collagen, and elastin fibers with luminal ECs and is generally observed at the anastomotic suture site between 1 week to 2 months.35 Studies suggest that intimal thickening does not increase with time.35
In patients experiencing PAD and requiring surgical revascularization, the use of autologous SV is recommended over synthetic materials (ePTFE; Dacron) due to better patency rates and reduced risk of reintervention.36 Multiple studies and meta-analyses have demonstrated superiority of SV grafts in femoropopliteal bypass, particularly at longer points of follow-up to synthetic and xenogeneic alternatives.37,38
The SV structure is that of a typical vein, with intimal, medial, and adventitial layers. The intima is lined by an endothelium that lies on a fenestrated basement membrane. This endothelium may be more susceptible than that of the IMA to injury during vessel harvesting, storage, and implantation because it is less firmly anchored to the subendothelium.39 The thin media is composed of SMCs arranged in ≥2 circular layers and interlaced between bundles of collagen and elastic fibers and separated from the intima by a small internal elastic membrane. A relatively thick adventitia shows a loose network of longitudinally oriented collagen bundles and embedded vasa vasorum.40
A vein inserted into the arterial circulation develops intimal hyperplasia.41 Increased wall tension becomes the significant driver of development of intimal thickening after implantation into the arterial environment, and deformation of SMCs by pulsatile stretch stimulates their proliferation while deformation of the vessel wall in a circumferential direction is associated with medial thickening.42-44 Dysfunctional endothelial regulation exacerbates the development of intimal hyperplasia, while EC loss induces platelet adhesion and accumulation of inflammatory cells in the denuded areas, which in turn stimulates the release of cytokines including PDGF-BB (platelet-derived growth factor BB), bFGF (basic FGF [fibroblast growth factor]), and IGF-1 (insulin-like growth factor 1). These signals stimulate SMC proliferation in the intima.31 Attenuation of EC production of antimitotic agents including prostacyclin, NO, and adenosine further contributes to SMC migration and replication.31 All these elements combine to allow activated SMCs to synthesize and release collagen and other ECM (extracellular matrix) proteins in the intima, leading to intimal hyperplasia.31,45
After implantation into arterial environments, SV grafts rapidly remodel with a change in lumen size and wall thickness,31,44 mostly within the first 30 days but then continuing thereafter, to gradually stabilize in response to hemodynamic stress.46-48 In addition to intimal hyperplasia, atherosclerotic disease progresses rapidly in SV grafts explaining the disappointing long-term patency of SV grafts in CABG and in peripheral vascular surgery.45,49
With the goal of limiting the damage inflicted to the endothelium of harvested veins, research efforts have focused on the protection of vessels harvested for use in revascularization surgery. DuraGraft is an example of a flushing and storage solution that is approved in Europe and was developed to protect the structural and functional integrity of the vascular endothelium. It is an ionically and pH-balanced physiological salt solution containing L-glutathione, L-ascorbic acid, L-arginine, and other additives, designed to minimize the risk of neointimal hyperplasia stemming from injury to the vein’s endothelium during harvesting and storage.50 Haime et al compared the experimental solution to heparinized saline in patients undergoing CABG with SV (1036 versus 1400 patients, respectively). Long-term follow-up showed a potential benefit over heparinized saline with a 45% lower occurrence of nonfatal myocardial infarction after 1000 days and a significantly lower risk for revascularization and MACE but failed to show differences in the rate of events on the short term; mortality was comparable between groups.50
Allogeneic and Xenogeneic Materials
Cryopreserved Grafts
Cryopreserved cadaveric allogeneic veins (CVGs) have been used for >2 decades as an alternative when synthetic grafts are undesirable, such as in the case of an infected field and when autologous veins are unavailable. The need for cryopreservation and specialized storage and the related planning, thawing, and preparation time, all represent practical limitations to the use of such conduits. In addition, cryopreserved veins have accumulated insults inflicted during harvesting and storage that decrease the functional performance of the endothelium. Most importantly, the presence of viable allogeneic cells elicits an immune response from the recipient that not only jeopardizes patency but also impacts the future chance of successful organ transplant in the case of end-stage kidney disease.51 Overall, patency and performance reported in the literature are mixed, across indications and use, due to a combination of these factors.51-55 Multiple studies have investigated the use of CVGs as an alternative in surgical procedures including infrainguinal bypass, CABG, hemodialysis access, and portal vein reconstruction when autologous SV is unavailable.51-55 Primary patency rates are poor in the majority of studies, with thrombotic occlusion as the primary cause of graft failure.52,53,55 In a series of CVGs used for CABG, only 1 of 17 remained patent after 12 months.55 In peripheral revascularization procedures, highly variable primary patency rates (17%–53%) at 1 year were reported in patients with infrainguinal bypass using CVG.52,53 Long-term patency rates were 19% to 22% and 3% at 2 years and 4 years, respectively.53 Again, occlusion resulting from thrombosis was the primary cause of CVG failure.52,53 Carpenter et al56 examined 19 explanted samples from a prospective study of 40 patients undergoing below-knee lower extremity revascularization. Of the explanted CVGs, 47% had moderate or severe intimal thickening and with a trend of intimal thickening increasing with time after implantation. A cellular infiltrate, which was evenly distributed throughout the intima, media, and adventitia, was detected in all explanted grafts with moderate or severe cellular infiltration in one-third of grafts. Leukocytes, T cells (CD3 [cluster of differentiation 3] and CD8), and HLA II-DR positive markers were identified that suggested that an adaptive immune response might be driving allograft failure.56 However, low-dose immunosuppression therapy did not improve allograft patency significantly, and evidence for involvement of immunologic rejection in cryopreserved vein failure is variable and inconsistent in other studies.57
Xenogeneic Cardiovascular Grafts
ECM grafts of xenogeneic origin combine the benefits of abundant supply with a biological tissue that is strong, pliable, and durable (Figure 2). Cardiovascular xenografts are derived from varying mammalian tissue sources, often bovine or porcine. All vascular xenografts currently utilized are decellularized, with the intention of lowering the immunogenic potential of the graft. Xenograft efficacy and durability are heavily influenced by their specific application. Whether utilizing CorMatrix porcine small intestinal submucosa,58,59 bovine carotid artery (Artegraft),60-64 or mesenteric vein (ProCol)65,66 for arteriovenous access for hemodialysis, some studies have shown adequate performance compared with autologous or synthetic alternatives. The overall lower pressures applied to arteriovenous grafts, as compared with arterial grafts, may underlie some of the performance benefits observed with xenografts in dialysis access.
Beyond hemodialysis, xenografts have also been utilized for vascular repair with satisfactory patency and long-term performance,67,68 even when compared with autologous grafts.69,70 Not all vascular applications of xenograft ECM have shown desirable efficacy, however. While some successes have been documented,71,72 implementation of xenografts to repair cardiac defects and damage have produced higher rates of reintervention and failure in both neonatal/pediatric populations,73–75 as well as adults.76,77 Utilization of xenografts to repair and reconstruct arteries has produced notable mechanical failures of the materials.78–82 These adverse events suggest that not all cardiac and vascular sites, especially those that experience mechanical stress or high pressure, are equally suited for xenograft-based repairs. Serum samples collected from patients who received a decellularized allograft showed a slight increase in IgG levels in the first 4 months after implantation. But IgG responses to Matrix P valves were 6× higher than for allografts, indicating a sustained immune response against the xenograft and providing a potential basis for xenograft ECM degradation and graft failure.82
A major appeal of utilizing biological materials for cardiovascular repair is the putative ability of the material to remodel and to be adopted as a functional tissue in the host. Although some studies have shown evidence of remodeling over time,83-85 the responses observed to vascular xenografts may not accurately recapitulate the typical range of response to these ECM materials, as almost all instances of histological examination occur after the failure of an implanted graft. To obtain a better perspective of xenograft ECM remodeling over time, an explanted postmortem series of 10 CorMatrix xenografts from neonatal and pediatric patching of the great vessels or coronary arteries was reported.86 In these samples, an intima formed around the ECM xenograft and progressively thickened over time (1 week to 26 months). The new tissue was lined by an endothelium within 1 week of implantation, and CD31+ capillaries formed on the luminal side of the patch. During the time course, the ECM xenografts degraded and remained acellular, suggesting that such materials were not remodeled per se but instead function as a scaffold for new tissue growth surrounding the original xenograft implant. In this way, xenogeneic tissues may mimic the remodeling of synthetic materials to a degree, with limited host cell infiltration into the implant, and formation of extensive host tissue surrounding the graft.86
While some histological data demonstrate partial recellularization of xenografts, many clinical reports of explanted specimens provide evidence of inflammation, calcification, and neointima formation without cellularization of the implanted ECM. Failed CorMatrix grafts used as valve replacements in the aorta and pulmonary artery showed a foreign body giant cell response, no cell infiltration, and gradual degradation of the ECM.71,72 An inflammatory response comprised of eosinophils, macrophages, and giant cell reactions has been repeatedly demonstrated in explants of CorMatrix and CardioCel.76,80,87 In limited observation of a single patient with 2 CorMatrix patches, 1 patch generated a marked inflammatory response while the other did not, indicating that such responses could be tissue specific or related to the surgical procedure.86 In many but not all cases, explanted xenografts showing inflammation also show fibrosis and calcification, processes that are undesirable for development of true functional neotissues.80,87,88 In a study where 15% (9/60) CardioCel heart valve grafts from both adults and children failed, all explanted grafts showed matrix degeneration and a lack of recellularization.89
In summary, xenografts for cardiovascular repair and reconstruction offer potential benefits and have been successfully deployed in some surgical applications. However, graft failures due to loss of graft integrity, inflammation, and fibrosis have repeatedly been observed, often in environments experiencing mechanical stress or high pressures. And despite evidence of recellularization in preclinical models, most observed xenograft explants from patients do not show recellularization of the graft itself and instead demonstrate repopulation of cells around the periphery of the graft. When such perigraft tissue growth is outpaced by degradation of the xenogeneic ECM, these grafts can fail.
Current and Future Research Avenues
Autologous grafts still remain the preferred option for vessel replacement or repair, eliminating the risk of rejection presented by xenografts and allogeneic vein grafts, offering a resistance to infection better than that of synthetic materials and displaying mechanical properties most closely aligned with the conduit to be replaced. However, they face limitations with regard to availability, risk of thrombosis due to damaged endothelium, intimal hyperplasia, and accelerated atherosclerosis.29 New research avenues focus on the generation of innovative materials that would decrease the risk of infection, thrombosis, and rejection and either undergo remodeling by the host or induce regeneration and repair of the host own tissues (Figure 3).
Figure 3.
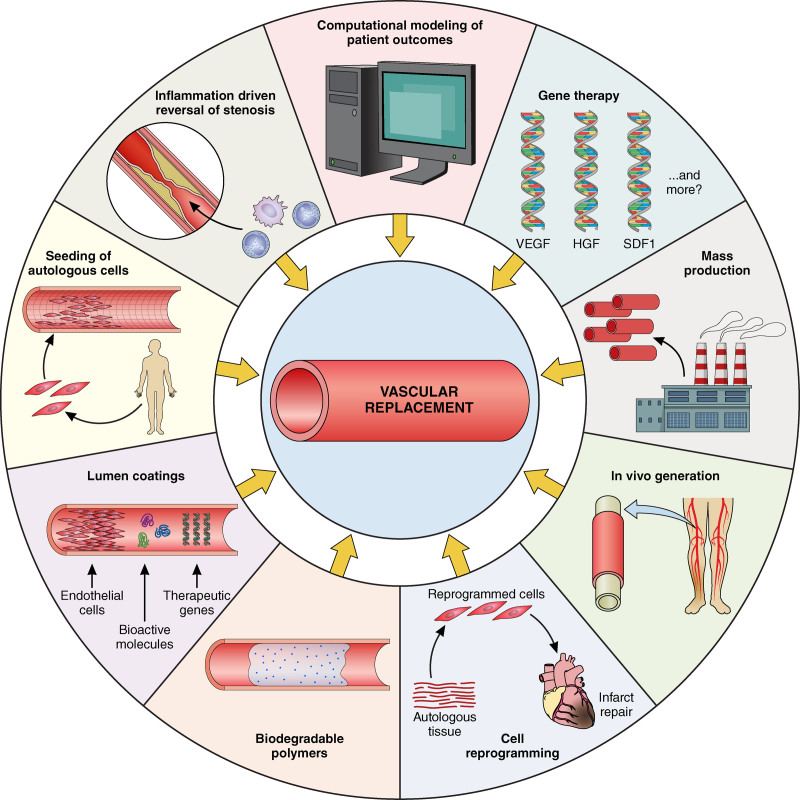
The future of tissue-engineered vascular grafts. Multiple avenues of research continue to advance the field of engineering of vascular grafts. Some of the approaches undertaken and discussed in this article are depicted herein. Illustration credit: Patrick Lane.
Gene Therapy
Gene therapy is being explored in a variety of diseases, from cancer to genetic, infectious, and degenerative diseases. Therapeutic genes are brought to the cells by vectors, including nonviral (plasmids, bacterial) and viral vectors. Alternatively, patients’ own cells can be harvested, genetically modified, and returned to the patients. More recently, CRISPR (clustered regularly interspaced short palindromic repeats) and other technologies and editing human genes directly to repair mutations that cause diseases represent a potential evolution of the technology.90
In PAD, a Cochrane systematic review published in 2018 looked at 17 randomized and quasi-randomized studies that evaluated gene therapy versus no gene therapy (excluding cell-based therapies) in people with PAD, providing data for a total of 1988 participants.91 Growth factor-encoding gene therapy was the most frequently studied with 6 studies using VEGF-encoding genes, 4 using HGF (hepatocyte growth factor)-encoding genes, and 3 using FGF-encoded genes. In addition, HIF-1L (hypoxia-inducible factor 1-alpha) gene therapy was evaluated in 2 studies and developmental endothelial locus-1 gene therapy and SDF-1 (stromal cell-derived factor-1) gene therapy in one study each (Figure 3). None of these gene therapy studies in PAD showed clear differences in the key outcomes of amputation-free survival, major amputation, and all-cause mortality between the two groups (gene therapy versus no gene therapy). Studies did not allow conclusions to be reached on impact of gene therapy on wound healing, quality of life, ankle-brachial index, symptom scores, or claudication distance.91
In the cardiovascular area specifically, several products designed to trigger neoangiogenesis and address critical limb ischemia, intermittent claudication, or ischemic heart disease have been studied in clinical trials. In a 2013 review of gene therapy for the revascularization of the ischemic myocardium, Kaminsky et al92 point out that a number of angiogenic mediators such as VEGF, FGF, HGF, and others encoded by DNA plasmids or adenovirus vectors have been extensively studied with disappointing results. Aside from angiogenic outcomes, other goals of gene therapy can include reduction of fibrosis, induction of replication of endogenous cardiomyocytes, or expansion of existing cardiac stem cells, though these outcomes have not yet been significantly demonstrated. Some of the issues faced by angiogenesis-based therapeutic approaches and gene therapy products lay in the identification of the most effective route of delivery, dosing, and frequency of treatment that are needed to achieve results. In addition, the field needs progress in decreasing immunogenicity of delivering vectors, identifying nonintegrative vectors for enhanced patient safety, minimally invasive delivery techniques for genetic delivery payloads, as well as gaining a better understanding of off-target effects of gene products or gene integration sites, are needed.
Despite many years of exploration and relative lack of clinical benefits, there is a resurgence of interest in newer gene therapy approaches, led by cell reprogramming, though these efforts remain largely nascent and preclinical.93
Cellular Reprogramming
Postmyocardial infarction, for example, the most promising option seems to be the cellular reprogramming approach that aims at regenerating cardiomyocytes directly from endogenous scar fibroblasts, bypassing the typical cardiomyocyte progenitor state. In animal models, this approach to cardiac regeneration by injection of fibroblast reprogramming factors (Gata4, Mef2c, and Tbx5 [GMT])94,95 shows 10% to 25% improvement from baseline in postinfarct ejection fraction and reduction in fibrosis by almost 50%.96-100 Cardiac gene therapy that aims to reconstitute myocardium, as opposed to simply increasing angiogenesis, may help resolve some of the obstacles that myocardial cell therapy faces, including poor electromechanical integration and low implanted cell survival.101
Biodegradable Vascular Grafts
The concept of biodegradable vascular grafts was proposed as early as 1919.102 Biodegradable vascular grafts comprise a synthetic 3-dimensional framework that aims to elicit cell adhesion, infiltration, and proliferation, thus allowing cells to produce their own ECM and finally transform into native tissue without residual polymers.103 Multiple biodegradable polymers for vascular grafts have been investigated, including polyglycolic acid (PGA), polylactic acid, poly(ε-caprolactone), polydioxanone, poly(glycerol-sebacate), poly-4-hydroxybutyrate, and their copolymers and polymer combinations.104 This field has struggled to achieve the appropriate balance between premature and late polymer scaffold degradation. Finding this balance is a critical consideration for the design of biodegradable scaffolds and remains a challenge for clinical application, since premature degradation leads to early loss of structural integrity and increased risks of rupture, while late degradation causes foreign body response and prevents adaptive neotissue formation. Many researchers have utilized biodegradable polymers as a scaffold for generating a vascular graft in vitro, in particular, in pediatric cardiac surgery indications to potentially repair congenital defects.105-108
Xeltis AG developed a highly porous polymer-based degradable scaffold composed of chain-extending poly-caprolactone with 2-ureido-4[1H]-pyrimidinone (a bioabsorbable supramolecular polyester),109 designed to be immediately functional while offering physiochemical characteristics thought to enable cell infiltration and subsequent endogenous tissue formation. Following encouraging functional results in animal models,110 the graft was implanted in a feasibility trial in one center in Moscow in 5 pediatric patients (aged 4–12 years) as an extracardiac connection between the inferior vena cava (IVC) and the pulmonary artery. All patients were doing well at 24 months of follow-up, with no graft-related serious adverse events, and transthoracic echocardiography demonstrated adequate function of the conduit in all patients while magnetic resonance imaging showed anatomic and functional stability of the restorative grafts.111 Early outcomes of a preliminary global, multicenter clinical study in 18 patients, using the material as an implantable replacement cardiac valve showed early failure of the implanted valves. The early mechanical valve failures led to structural modifications of the implant and highlighted the need for further evaluation of the material.112
Incremental advances have somewhat improved the performance of nonbiodegradable synthetic grafts made of ePTFE, Dacron, or PU, which compared with degradable grafts are relatively inexpensive to produce and have long shelf lives but carry a significant risk of failure due to thrombosis, stenosis, and infection. The biodegradable polymer grafts have been developed with the goal of transforming in situ from a polymeric tube to a living, vessel-like structure, producing a broadly available therapy for patients without autologous alternatives. The immune response to synthetic scaffolds is poorly understood and requires further investigation (Figure 3).113
Finding the proper pore size affects the remodeling outcomes. Pore size may be more critical for materials that degrade slowly or have poor cell adhesion. In general, smaller pores in the size range of 25 to 40 μm tend to promote macrophage infiltration better than larger pores.114 Many synthetic grafts are electrospun—a process that typically yields small pores.
From the perspective of mechanical properties, both currently available nondegradable and biodegradable synthetic grafts are stiffer than native arteries. The mechanical discordance at the anastomoses induces disruptions of transmural stress and shear stress at the intima level, negatively impacting both ECs and SMCs, resulting in turbulent flow and anastomotic or neointimal hyperplasia, ultimately leading to thrombosis. Although an attractive concept, potential pitfalls of surgically implanting synthetic biodegradable scaffolds include degradation times that are not aligned with the rate of remodeling in the host, leading to risk of aneurysms, early structural and mechanical failure, as well as the risk of chronic inflammatory responses and fibrosis with stenosis and thrombosis associated risks.
To try and resolve some of these issues, many researchers have turned to biodegradable polymers as scaffolds for generating a vascular graft in vitro, and these approaches are discussed in depth in the next section.115-117
Tissue-Engineered Vascular Grafts
Development of the next generation of bioengineered vascular grafts should answer in practice the needs of both patients and the health care professionals and hospital setting by eliminating altogether the growth time, wound care, or operating time burden of harvesting autologous cells, veins, or arteries. Simple standard storage on-site, for example, is critical for emergent revascularization procedures and eliminates any preprocedure delay due to shipping, thawing rinsing, or coating. The mechanical and structural properties of the replacement vessels must allow for surgical handling and tolerate the arterial system high-pressure environment with low permeability and offer functional performance on par or superior to synthetic grafts and autologous vasculature in particular, with regard to risk of infection, thrombosis, or mechanical failure. To provide a meaningful alternative to autologous veins or arteries, the bioengineered replacement vessels must show minimal immunogenic potential to avoid triggering rejection of the implant and preserve patients’ future opportunities of organ transplant, therefore, eliminating the use of xenogeneic or allogeneic cellular material.1
The first engineering approach to develop a biological vascular graft was the formation of patient-specific tissue tubes by Charles Sparks in the 1970s. These autologous tubes were formed by the foreign body response to a silicone mandril—called the Sparks mandril—that was implanted subcutaneously into the patient’s leg. The fibrous tissue surrounding the mandril, created by a foreign body response, could be later harvested as an autologous tubular graft. However, this approach was abandoned due to low patency rates and aneurysmal remodeling in preclinical and clinical trials.118 The first tissue-engineered vascular graft (TEVG) produced in vitro with vascular cells was reported in a seminal study by Weinberg and Bell119 in 1986. This structure was made of a collagen gel tube supported by a Dacron sleeve as a protective jacket. Although this first TEVG resembled a biological artery with its SMC-populated wall, EC lining in the lumen, and fibroblast-populated adventitia layer, its mechanical properties without the Dacron support did not allow for in vivo trials. Since these pioneering studies, vascular tissue engineering has improved remarkably by utilizing multidisciplinary approaches.
The first TEVG transplantation in a human was performed by Shin’oka et al120 in 1999. The patient was a 4-year-old girl who had undergone pulmonary artery angioplasty and the Fontan procedure due to a single right ventricle and pulmonary atresia at the age of 3 years. Complete blockage of the pulmonary artery was detected 7 months after the Fontan procedure. Shin’oka et al reconstructed the blocked pulmonary artery with a tubular scaffold made of a poly(L-lactide-co-e-caprolactone) sponge reinforced with PGA fiber seeded with the patient’s own cells (Figure 4A). Approximately 3 months before the surgery, a 2-cm segment of peripheral vein of the patient was harvested, and the cells from the vessel wall were expanded in vitro. Ten days before transplantation, the tubular polymeric structure (2 cm length, 1 cm diameter, 1 mm wall thickness) was seeded with the autologous expanded cells to form the TEVG. This method of seeding the scaffolds was improved for subsequent patients, by using either autologous bone marrow cells or mononuclear bone marrow cells to seed the graft on the day of implantation.121
Figure 4.
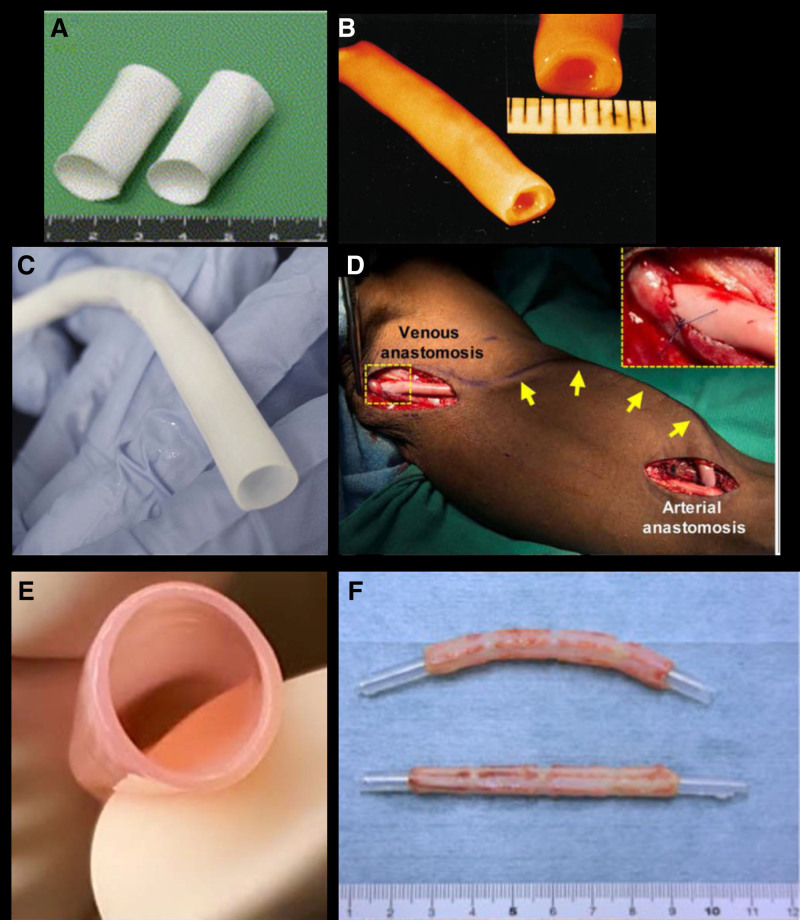
Presentation of various approaches to tissue engineered vascular grafts. A, Macroscopic image of polymeric scaffold before cell seeding. B, Gross image of biological tissue-engineered vascular graft (TEVG) produced by cell sheets rolled around a mandrel. C, A HAV ready to store/implant after decellularization.115 D, Intraoperative image of HAV implanted in the arm of a patient with end-stage renal disease as an arteriovenous conduit for hemodialysis.133 E, TEVGs produced from fibroblast-populated tubular fibrin constructs, matured in pulsatile bioreactor, and decellularized. F, Biotube made of fibrous tissue formed by foreign body reaction to the implanted mandrel. A, Reproduced from Shin’oka et al161 with permission. Copyright ©. B, Reproduced from L’Heureux et al128 with permission. Copyright ©. E, Reproduced from Syedain et al137,140 with permission. Copyright ©. F, Reproduced from Nakayama et al143 with permission. Copyright ©.
TEVGs that were produced by this method were used to divert blood flow from the IVC and the superior vena cava to the pulmonary artery in 25 pediatric patients with univentricular heart problems and resulted in remarkable clinical success.122,123 After 10 years of follow-up, some graft stenosis was observed in 30% of the patients, and in all cases, angioplasty successfully resolved the stenoses. Notably, none of the patients developed aneurysm or graft rupture.122 Animal studies showed that, 1 month after the implantation, a functional endothelium with NO production comparable to native IVC formed and the graft wall thickness increased to 2.5 mm with an inflammatory response of CD4+ cells. However, both the wall thickness and the CD4+ cell ratio gradually returned to values comparable to that of native IVC at the end of 6 months.123
Shinoka and Breuer124 used TEVGs as Fontan grafts to connect IVC to pulmonary artery in a phase I clinical trial in the United States, but advanced imaging techniques revealed a high incidence of graft stenosis within 6 months. To understand the mechanism causing the early stenosis, they built a computational model and used their 6-month imaging data to simulate vessel remodeling in an IVC interposition TEVG model. While the model successfully predicted the graft narrowing in 6 months, it surprisingly further suggested that such graft narrowing could spontaneously reverse through an inflammation-driven mechanism. They tested this simulation-generated hypothesis in an ovine IVC interposition graft model and observed the resolving stenosis in TEVGs in 1 year.125 Therefore, the inflammation-driven remodeled TEVGs may have potential for the reconstruction of congenital heart abnormalities in young patients (Figure 3).
In 1998, L’Heureux et al126 introduced the idea of creating a completely biological tissue-engineered blood vessel made of concentric layers of cell sheets that rolled to form a tubular structure. The process started with culturing confluent monolayers of fibroblasts and SMCs for 30 days in the presence of sodium ascorbate. Later, the confluent fibroblast sheets were detached from the substrate manually and rolled around an inert mandrel to form the tubular structure. After 1 week of maturation, this structure was dehydrated to form an acellular inner membrane. An SMC sheet was wrapped around the acellular inner membrane to form multiple SMC layers in the vessel wall. Silicon tubing was inserted into the lumen of the tissue-engineered blood vessel to apply periodic distension during an 8-week maturation period. Then, another fibroblast sheet was wrapped around the SMC layers to form the adventitia, and ECs were seeded to the luminal surface as the final step. The entire procedure required ≈3 months. The final structure had superior mechanical strength (burst pressure over 2500 mm Hg) compared with human SV (Figure 4B). This advanced mechanical strength was attributed to well-organized collagen and elastin bundles developed especially on the adventitia region. This technology was used as for arteriovenous shunt in 10 ESRD patients to test the effectiveness as hemodialysis access.127,128 Graft patency 3 months after the implantation was 60%, and the major failure mechanism for tissue-engineered blood vessels was dilation and subsequent thrombosis from blood infiltration between the cell sheet layers. The tissue-engineered artery at the latest stage of clinical development was first described by Niklason et al117 in 1999. A rapidly degrading PGA scaffold was wrapped around silicon tubing, and SMCs were seeded onto the PGA scaffold and were mechanically stimulated by cyclic stretch. At the end of an 8-week culture period, SMCs deposited de novo collagen matrix. The resultant tissue was decellularized to remove all immunogenic components, leaving a naturally derived ECM tube that can be stored without compromising the mechanical properties (Figure 4C).129 This method creates the potential to use allogeneic SMCs on PGA scaffolds to grow mature engineered vessels in vitro and then convert the arteries into immune-compatible, mechanically robust, off-the-shelf vascular grafts.130 Derivatives of this technology are used to produce human acellular vessels (HAVs), which are being tested as vascular grafts for hemodialysis access, PAD, and vascular reconstruction following trauma.
ESRD patients experience vascular damage due to frequent needle punctures for hemodialysis access. Currently, synthetic ePTFE grafts and AVFs are the most common vascular access options for these patients. However, high rates of infection and lack of self-healing in synthetic grafts and the excessive dilation in AVF pose major drawbacks.131 In phase 2 trials, HAVs used as hemodialysis access grafts demonstrated stable diameter and flow rate, infection resistance, functional 1-year patency of 89%, and healing capacity after needle punctures (Figure 4D).132 Some of the patients continued using the HAV for hemodialysis for >6 years. At the time points from 16 to 200 weeks, histological examination of explanted HAV samples recovered from routine surgical interventions showed that these previously acellular grafts were transformed into functional multilayered living vessels via repopulation of SMCs, ECs, and progenitor cells.133 Starting from the early time points, α-SMA+ (alpha smooth muscle actin) cells significantly infiltrated and gradually matured to form circumferentially aligned layers in the HAV wall. At the 16-week time point, these cells were supported by microvasculature formed around the neoadventitia by CD34+/CD31+ cells. Later, CD34−/CD31+ cells formed the microvascular structures in the vessel media and an endothelial lining on the lumen. While Nestin+ cells differentiated into SMCs and ECs by contributing to early vascularization and repair of HAV at earlier stages, CD90+ cell population increased in number later.133 Phase III trials of the HAV in hemodialysis are continuing. Moreover, HAV performed well as bypass conduits in PAD patients and in other clinical indications.132,134
One potentially important advantage of engineered arterial grafts is an ability to grow with a pediatric recipient. Hoerstrup et al135 was the first group to show that TEVGs grow with recipient when implanted into juvenile sheep as cardiopulmonary bypass grafts. They used PGA mesh coated with poly-4-hydroxybutyrate as a myofibroblast and EC seeding scaffold, which was implanted into animals after only 3 weeks of in vitro maturation. After 100 weeks of in vivo remodeling, the grafts grew 30% in diameter and 45% in length without any sign of dilation, thrombus, or stenosis.135,136 Syedain et al137 have developed a TEVG derived from fibroblast-populated fibrin gels cast in a tubular mold around a concentric cylindrical mandrel, which serves to form the luminal space. After 2 weeks of static culture, they remove the mandrel and apply cyclic stretch by perfusing the lumen with culture medium in bioreactors. At the end of in vitro maturation period, the TEVGs can be decellularized for long-term storage (Figure 4E). These TEVG have been implanted in 8-week-old lambs, and graft growth has been reported.138,139 When implanted as hemodialysis access grafts in a baboon model, these TEVGs showed 60% primary patency at 6 months. While the failure mechanism was either graft rupture or thrombosis, the patent grafts showed subsequent recellularization with host vascular cells without immune response or graft dilation.138,140 While bioabsorbable polymer- or natural hydrogel-based TEVGs have made immense progress, in recent years, some researchers revisited the idea of using foreign body response to create vascular grafts derived from fibrous structures formed around implanted objects, similar to the Sparks mandril.141,142 Although the Sparks mandril applications did not yield mechanically strong vascular grafts, the tissue quality has been improved by optimization of the implantation site, implant material, and length of the in vivo remodeling period.141,142 For example, Nakayama et al143 implanted the first biotubes in humans as arteriovenous shunt bypass grafts in 2 female ESRD patients. The biotubes were obtained by implanting 7-cm-long, 6-mm-wide silicone mandrels subcutaneously in the patients’ abdominal region for 2 months. After removal of the mandrels, the biotubes were implanted to bypass the stenotic venous outflow region in the patients’ arms (Figure 4F). Graft stenosis was observed in both patients 3 to 4 months after the implantations. While one of the patients needed repeated transluminal angioplasty 7 months after the implantation, angioplasty was not required over 2 years for the second patient. Hence, biotubes that are obtained utilizing the foreign body response to the implanted cylindrical objects demonstrate potential to be the next candidate for clinical trials (Figure 3).
Vessel characteristics vary by size, and the bioengineering of human conduits must adjust to these requirements: the primary role and, therefore, the anatomic and biochemical design of large vessels, typically defined by a diameter >6 mm, is in the efficient transport of blood while vessels of smaller diameter gradually switch to performing more exchange functions in lower pressure, slower flow environment.2
In the United States alone, >370 000 coronary arteries are bypassed annually,3 and such procedures require smaller caliber (<6 mm) vessels. Despite the potential high demand for small-diameter vascular grafts, current clinical research is limited to ≥6 mm TEVGs, since small-caliber grafts have heretofore failed due to acute or intermediate-term thrombosis.144,145 Importantly, however, engineered and decellularized small-caliber arteries have not yet been widely tested in vivo, and so it remains to be seen whether such conduits will function in the long term. In the absence of a functional endothelium, engineered vessel walls are exposed to the blood stream, which may trigger platelet aggregation and activation on the luminal surface and thereby initiate coagulation and thrombosis. One approach to prevent thrombosis until the de novo endothelium is restored is coating the graft lumen with ≥1 bioactive molecules. Heparin functionalization has been tested on synthetic, bioabsorbable, and decellularized native vascular grafts.146,147 Recently, heparin-like structures made of carboxymethyl chitosan and chitosan demonstrated antithrombogenic effect on bioabsorbable graft lumens.148 NO immobilization on the graft has also shown potential to prevent acute thrombosis.149 Similarly, keratin-doped poly(ε-caprolactone) vascular tubes exhibited selective function that facilitated EC repopulation while preventing SMC growth thanks to the NO production by catalyzing S-nitrosoglutathione available in the blood.150,151 In another attempt to mimic the endothelium that protects the vessel wall from the platelet attachment, a glycocalyx-like hyaluronic acid coating on the decellularized TEVG lumen reduced thrombus formation.152 Other approaches to prevent thrombosis in small-caliber grafts include in situ endothelial seeding into the graft lumen,153 coating of the lumen with agents to promote endothelialization via circulating endothelial progenitor cells or migrating ECs from the anastomosis regions,154 and coimmobilization of the ACH11 antithrombotic peptide and CAG cell-adhesive peptide to prevent platelet activation and facilitate EC adhesion at the same time (Figure 3).155 In addition to direct coating of these substances onto the graft lumen, local delivery of therapeutic genetic material also has the potential to prevent intimal hyperplasia and stenosis.156 These genes include VEGF, iNOS (inducible NO synthase), TIMP-1 (tissue inhibitor of metalloproteinase 1), and ERK2 (mitogen-activated protein kinase 1) silencing RNA. A review of these strategies in extensive detail by Gupta and Mandal157 is available. Although some of these methods demonstrated promising results in animal models, none of them have yet been confirmed by clinical studies.
Contribution of Computer Modeling, Personalized Medicine, and Systems Biology Approaches
The purpose of personalized medicine and its identification of patient-specific profiles incorporating genetic and genomic data to clinical factors and environmental risks is to offer disease prevention and treatment strategies tailored to the individuals. Its application to the area of vascular grafts has explored many different diseases or conditions. Applying precision medicine and next-generation sequencing to the study of aortic aneurysms, for example, showed that despite common manifestations, genetic factors play a critical role in the risk of aortic root and ascending thoracic aortic aneurysms, as opposed to abdominal aortic aneurysms, which are primarily driven by atherosclerosis, and allowing for the identification of target pathways for potential new therapeutic approaches in these patients.158 In cardiac transplant recipients, gene expression profiling of peripheral blood cells is being evaluated to detect the risk of rejection and help in the management of immunosuppressive agents, as an alternative to repeated endomyocardial surveillance biopsies, and could possibly be applied to allogeneic and xenogeneic vascular grafts to monitor the immune response.159
Computational modeling and systems biology approaches applied to the study of vascular pathophysiology have produced several examples of multiscale models, such as for atherosclerosis, in-stent restenosis, remodeling of a vascular tissue-engineered scaffold, or venous graft remodeling. By creating these in-silico models, this approach would guide the development of new materials and devices and help avoid the limitations of current grafts. Including genomics in these complex models is anticipated to contribute to refining personalized medicine in these areas.160
Conclusions and Predicted Clinical Applications of Emerging Technologies
Vascular grafts offer immense potential to meet the ever-increasing needs of patients in nearly all fields of cardiovascular surgery, including hemodialysis access for end-stage renal disease, cardiovascular bypass, limb rescue in vascular trauma, and reconstruction and repair of congenital heart defects for pediatric patients who would benefit greatly from a graft that would grow as the patient matures. For most of these applications, autologous vascular grafts have been the standard of care for decades, but their limited supply and the side effects of excision of native arteries or veins are significant (Figure 2). Patients with end-stage renal disease on hemodialysis can benefit greatly from AVF as vascular accesses, but the time to maturation and failure of maturation in many patients result in other vascular access placements. In controlled trials, xenogeneic vascular grafts often have patency superior to synthetic conduits, but notable frequency of failure, particularly in environments experiencing mechanical stress, inflammatory responses, and inconsistent remodeling are challenges. TEVGs have the potential to meet all of the needs above, and several cellular or decellularized options are being evaluated, to produce materials that can mimic most aspects of native vasculature. Furthermore, understanding of the ability of these tissues to be remodeled by the host into a native tissue, and ability for that tissue to grow with patients who are young, will be important as the development of TEVG continues. Challenges are numerous, starting with creating the most effective process to mitigate or eliminate the immunogenic potential of these tissues, resolving the complexities of establishing bioengineering processes and platforms that follow the regulatory and quality guidelines, and achieving commercial-scale manufacturing to ultimately offer patients and surgical teams access to true replacement tissue and opening the way to multiple applications of organ replacement.
Multiple teams have made substantial preclinical and clinical progress toward these goals, but further research and development is warranted to realize the potential futures of cardiovascular tissue engineering (Figure 3). Incorporation of computational modeling and systems biology approaches help better understand the intricate details of the genesis of tissue remodeling and vascular adaptation. Ultimately, bioengineering will offer replacement human organs and tissues in response to most chronic, degenerative, and autoimmune diseases, as well as traumatic injuries.
Article Information
Acknowledgments
We acknowledge the contribution of Rob Kirkton to this article and to the responses to the Editors and Reviewers’ comments. We also acknowledge the contributions of Ryan McKee in the conceptualization of Figures 2 and 3.
Sources of Funding
None.
Disclosures
L.E. Niklason is a founder, shareholder, President, and CEO of Humacyte, Inc, and serves on Humacyte’s Board of Directors. L.E. Niklason’s spouse is a shareholder of Humacyte, Inc, and serves on the Board of Directors. Y. Li, M.H. Kural, E.A. Hugentobler, J. Wang, and K.M. Naegeli are shareholders and employees of Humacyte, Inc.
Nonstandard Abbreviations and Acronyms
- AVF
- arteriovenous fistula
- bFGF
- basic fibroblast growth factor
- CABG
- coronary artery bypass grafting
- CRISPR
- clustered regularly interspaced short palindromic repeats
- CVG
- cryopreserved vein graft
- EC
- endothelial cell
- ECM
- extracellular matrix
- eNOS
- endothelial NO synthase
- ePTFE
- expanded polytetrafluoroethylene
- ESRD
- end-stage renal disease
- FGF
- fibroblast growth factor
- HAV
- human acellular vessel
- HGF
- hepatocyte growth factor
- HIF-1L
- hypoxia-inducible factor 1-alpha
- IGF-1
- insulin-like growth factor 1
- IMA
- internal mammary artery
- iNOS
- inducible NO synthase
- IVC
- inferior vena cava
- PAD
- peripheral artery disease
- PDGF-BB
- platelet-derived growth factor BB
- PGA
- polyglycolic acid
- PU
- polyurethane
- SDF-1
- stromal cell-derived factor-1
- SMC
- smooth muscle cell
- SV
- saphenous vein
- TEVG
- tissue-engineered vascular graft
- TIMP-1
- tissue inhibitor of metalloproteinase 1
- VEGF
- vascular endothelial growth factor
- VEGF-R2
- vascular endothelial growth factor receptor 2
M.H. Kural, Y. Li, and J. Wang contributed equally.
For Sources of Funding and Disclosures, see page 122.
References
- 1.Dahl SL, Blum JL, Niklason LE. Bioengineered vascular grafts: can we make them off-the-shelf? Trends Cardiovasc Med. 2011;21:83–89. doi: 10.1016/j.tcm.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 2.Devillard CD, Marquette CA. Vascular tissue engineering: challenges and requirements for an ideal large scale blood vessel. Front Bioeng Biotechnol. 2021;9:721843. doi: 10.3389/fbioe.2021.721843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 4.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0 [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi L, Coimbra R, Goes AMO, Jr, Reva V, Santorelli J, Moore EE, Galante J, Abu-Zidan F, Peitzman AB, Ordonez C, et al. American Association for the Surgery of Trauma-World Society of Emergency Surgery guidelines on diagnosis and management of peripheral vascular injuries. J Trauma Acute Care Surg. 2020;89:1183–1196. doi: 10.1097/TA.0000000000002967 [DOI] [PubMed] [Google Scholar]
- 6.White JM, Stannard A, Burkhardt GE, Eastridge BJ, Blackbourne LH, Rasmussen TE. The epidemiology of vascular injury in the wars in Iraq and Afghanistan. Ann Surg. 2011;253:1184–1189. doi: 10.1097/SLA.0b013e31820752e3 [DOI] [PubMed] [Google Scholar]
- 7.Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen X, Gipson D, Gu H, Hirth RA, Hutton D, Jin Y, et al. US renal data system 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75:A6–A7. doi: 10.1053/j.ajkd.2019.09.003 [DOI] [PubMed] [Google Scholar]
- 8.Johansen KL, Chertow GM, Gilbertson DT, Herzog CA, Ishani A, Israni AK, Ku E, Li S, Li S, Liu J, et al. US renal data system 2021 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2022;79(4 Suppl 1):A8–A12. doi: 10.1053/j.ajkd.2022.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lok CE, Huber TS, Lee T, Shenoy S, Yevzlin AS, Abreo K, Allon M, Asif A, Astor BC, Glickman MH, et al. ; National Kidney Foundation. KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis. 2020;75:S1–S164. doi: 10.1053/j.ajkd.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 10.Favaloro RG, Effler DB, Cheanvechai C, Quint RA, Sones FM, Jr. Acute coronary insufficiency (impending myocardial infarction and myocardial infarction): surgical treatment by the saphenous vein graft technique. Am J Cardiol. 1971;28:598–607. doi: 10.1016/0002-9149(71)90104-4 [DOI] [PubMed] [Google Scholar]
- 11.Ravi S, Chaikof EL. Biomaterials for vascular tissue engineering. Regen Med. 2010;5:107–120. doi: 10.2217/rme.09.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chlupáč J, Filová E, Bačáková L. Blood vessel replacement: 50 years of development and tissue engineering paradigms in vascular surgery. Physiol Res. 2009;58(suppl 2):S119–S140. doi: 10.33549/physiolres.931918 [DOI] [PubMed] [Google Scholar]
- 13.Hytönen JP, Leppänen O, Taavitsainen J, Korpisalo P, Laidinen S, Alitalo K, Wadström J, Rissanen TT, Ylä-Herttuala S. Improved endothelialization of small-diameter ePTFE vascular grafts through growth factor therapy. Vasc Biol. 2019;1:1–9. doi: 10.1530/VB-18-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishibe T, Kondo Y, Muto A, Dardik A. Optimal prosthetic graft design for small diameter vascular grafts. Vascular. 2007;15:356–360. doi: 10.2310/6670.2007.00053 [DOI] [PubMed] [Google Scholar]
- 15.Mehta RI, Mukherjee AK, Patterson TD, Fishbein MC. Pathology of explanted polytetrafluoroethylene vascular grafts. Cardiovasc Pathol. 2011;20:213–221. doi: 10.1016/j.carpath.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 16.Cziperle DJ, Joyce KA, Tattersall CW, Henderson SC, Cabusao EB, Garfield JD, Kim DU, Duhamel RC, Greisler HP. Albumin impregnated vascular grafts: albumin resorption and tissue reactions. J Cardiovasc Surg (Torino). 1992;33:407–414. [PubMed] [Google Scholar]
- 17.Quarmby JW, Burnand KG, Lockhart SJ, Donald AE, Sommerville KM, Jamieson CW, Browse NL. Prospective randomized trial of woven versus collagen-impregnated knitted prosthetic Dacron grafts in aortoiliac surgery. Br J Surg. 1998;85:775–777. doi: 10.1046/j.1365-2168.1998.00650.x [DOI] [PubMed] [Google Scholar]
- 18.Rychlik IJ, Davey P, Murphy J, O’Donnell ME. A meta-analysis to compare Dacron versus polytetrafluroethylene grafts for above-knee femoropopliteal artery bypass. J Vasc Surg. 2014;60:506–515. doi: 10.1016/j.jvs.2014.05.049 [DOI] [PubMed] [Google Scholar]
- 19.Adipurnama I, Yang MC, Ciach T, Butruk-Raszeja B. Surface modification and endothelialization of polyurethane for vascular tissue engineering applications: a review. Biomater Sci. 2016;5:22–37. doi: 10.1039/c6bm00618c [DOI] [PubMed] [Google Scholar]
- 20.Dixon BS, Beck GJ, Vazquez MA, Greenberg A, Delmez JA, Allon M, Dember LM, Himmelfarb J, Gassman JJ, Greene T, et al. ; DAC Study Group. Effect of dipyridamole plus aspirin on hemodialysis graft patency. N Engl J Med. 2009;360:2191–2201. doi: 10.1056/NEJMoa0805840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akoh JA, Patel N. Infection of hemodialysis arteriovenous grafts. J Vasc Access. 2010;11:155–158. doi: 10.1177/112972981001100213 [DOI] [PubMed] [Google Scholar]
- 22.Wen M, Zhi D, Wang L, Cui C, Huang Z, Zhao Y, Wang K, Kong D, Yuan X. Local delivery of dual microRNAs in trilayered electrospun grafts for vascular regeneration. ACS Appl Mater Interfaces. 2020;12:6863–6875. doi: 10.1021/acsami.9b19452 [DOI] [PubMed] [Google Scholar]
- 23.Ding MH, Lozoya EG, Rico RN, Chew SA. The role of angiogenesis-inducing microRNAs in vascular tissue engineering. Tissue Eng Part A. 2020;26:1283–1302. doi: 10.1089/ten.TEA.2020.0170 [DOI] [PubMed] [Google Scholar]
- 24.Bastijanic JM, Marchant RE, Kligman F, Allemang MT, Lakin RO, Kendrick D, Kashyap VS, Kottke-Marchant K. In vivo evaluation of biomimetic fluorosurfactant polymer-coated expanded polytetrafluoroethylene vascular grafts in a porcine carotid artery bypass model. J Vasc Surg. 2016;63:1620–1630.e4. doi: 10.1016/j.jvs.2015.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mufty H, Van den Bergh M, Meuris B, Metsemakers WJ, Fourneau I. Clinical studies reporting on vascular graft coatings for the prevention of aortic graft infection: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2022;63:112–118. doi: 10.1016/j.ejvs.2021.09.020 [DOI] [PubMed] [Google Scholar]
- 26.Deutsch M, Meinhart J, Zilla P, Howanietz N, Gorlitzer M, Froeschl A, Stuempflen A, Bezuidenhout D, Grabenwoeger M. Long-term experience in autologous in vitro endothelialization of infrainguinal ePTFE grafts. J Vasc Surg. 2009;49:352–362; discussion 362. doi: 10.1016/j.jvs.2008.08.101 [DOI] [PubMed] [Google Scholar]
- 27.Taite LJ, Yang P, Jun HW, West JL. Nitric oxide-releasing polyurethane-PEG copolymer containing the YIGSR peptide promotes endothelialization with decreased platelet adhesion. J Biomed Mater Res B Appl Biomater. 2008;84:108–116. doi: 10.1002/jbm.b.30850 [DOI] [PubMed] [Google Scholar]
- 28.Antonova LV, Sevostyanova VV, Kutikhin AG, Mironov AV, Krivkina EO, Shabaev AR, Matveeva VG, Velikanova EA, Sergeeva EA, Burago AY, et al. Vascular endothelial growth factor improves physico-mechanical properties and enhances endothelialization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(ε-caprolactone) small-diameter vascular grafts in vivo. Front Pharmacol. 2016;7:230. doi: 10.3389/fphar.2016.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buxton BF, Galvin SD. The history of arterial revascularization: from Kolesov to Tector and beyond. Ann Cardiothorac Surg. 2013;2:419–426. doi: 10.3978/j.issn.2225-319X.2013.07.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW, et al. ; Writing Committee Members. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2022;79:197–215. doi: 10.1016/j.jacc.2021.09.005 [DOI] [PubMed] [Google Scholar]
- 31.Cox JL, Chiasson DA, Gotlieb AI. Stranger in a strange land: the pathogenesis of saphenous vein graft stenosis with emphasis on structural and functional differences between veins and arteries. Prog Cardiovasc Dis. 1991;34:45–68. doi: 10.1016/0033-0620(91)90019-i [DOI] [PubMed] [Google Scholar]
- 32.Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28:616–626. doi: 10.1016/0735-1097(96)00206-9 [DOI] [PubMed] [Google Scholar]
- 33.van Son JA, Smedts F, de Wilde PC, Pijls NH, Wong-Alcala L, Kubat K, Tavilla G, Lacquet LK. Histological study of the internal mammary artery with emphasis on its suitability as a coronary artery bypass graft. Ann Thorac Surg. 1993;55:106–113. doi: 10.1016/0003-4975(93)90483-x [DOI] [PubMed] [Google Scholar]
- 34.Harky A, Sankaranarayanan V, Kong QG. Internal mammary artery: the primary conduit for surgical revascularization. Coron Artery Dis. 2021;32:64–72. doi: 10.1097/MCA.0000000000000895 [DOI] [PubMed] [Google Scholar]
- 35.Otsuka F, Yahagi K, Sakakura K, Virmani R. Why is the mammary artery so special and what protects it from atherosclerosis? Ann Cardiothorac Surg. 2013;2:519–526. doi: 10.3978/j.issn.2225-319X.2013.07.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2017;135:e686–e725. doi: 10.1161/CIR.0000000000000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klinkert P, Schepers A, Burger DH, van Bockel JH, Breslau PJ. Vein versus polytetrafluoroethylene in above-knee femoropopliteal bypass grafting: five-year results of a randomized controlled trial. J Vasc Surg. 2003;37:149–155. doi: 10.1067/mva.2002.86 [DOI] [PubMed] [Google Scholar]
- 38.Pereira CE, Albers M, Romiti M, Brochado-Neto FC, Pereira CA. Meta-analysis of femoropopliteal bypass grafts for lower extremity arterial insufficiency. J Vasc Surg. 2006;44:510–517. doi: 10.1016/j.jvs.2006.04.054 [DOI] [PubMed] [Google Scholar]
- 39.Thatte HS, Khuri SF. The coronary artery bypass conduit: I. Intraoperative endothelial injury and its implication on graft patency. Ann Thorac Surg. 2001;72:S2245–S2252; discussion S2267. doi: 10.1016/s0003-4975(01)03272-6 [DOI] [PubMed] [Google Scholar]
- 40.Cheanvechai C, Effler DB, Hooper JR, Eschenbruch EM, Sheldon WC, Sones FM, Jr, Levin HS, Hawk WA. The structural study of the saphenous vein. Ann Thorac Surg. 1975;20:636–645. doi: 10.1016/s0003-4975(10)65755-4 [DOI] [PubMed] [Google Scholar]
- 41.Xenogiannis I, Zenati M, Bhatt DL, Rao SV, Rodés-Cabau J, Goldman S, Shunk KA, Mavromatis K, Banerjee S, Alaswad K, et al. Saphenous vein graft failure: from pathophysiology to prevention and treatment strategies. Circulation. 2021;144:728–745. doi: 10.1161/CIRCULATIONAHA.120.052163 [DOI] [PubMed] [Google Scholar]
- 42.Karayannacos PE, Rittgers SE, Kakos GS, Williams TE, Jr, Meckstroth CV, Vasko JS. Potential role of velocity and wall tension in vein graft failure. J Cardiovasc Surg (Torino). 1980;21:171–178. [PubMed] [Google Scholar]
- 43.Yang Z, VonSegesser L, Stulz P, Turina M, Luscher T. Pulsatile stretch and platelet-derived growth-factor (PDGF)-important mechanisms for coronary venous bypass graft disease. Circulation. 1992;86:84–84. [Google Scholar]
- 44.Dobrin PB, Littooy FN, Endean ED. Mechanical factors predisposing to intimal hyperplasia and medial thickening in autogenous vein grafts. Surgery. 1989;105:393–400. [PubMed] [Google Scholar]
- 45.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97:916–931. doi: 10.1161/01.cir.97.9.916 [DOI] [PubMed] [Google Scholar]
- 46.Kalra M, Miller VM. Early remodeling of saphenous vein grafts: proliferation, migration and apoptosis of adventitial and medial cells occur simultaneously with changes in graft diameter and blood flow. J Vasc Res. 2000;37:576–584. doi: 10.1159/000054091 [DOI] [PubMed] [Google Scholar]
- 47.Gasper WJ, Owens CD, Kim JM, Hills N, Belkin M, Creager MA, Conte MS. Thirty-day vein remodeling is predictive of midterm graft patency after lower extremity bypass. J Vasc Surg. 2013;57:9–18. doi: 10.1016/j.jvs.2012.06.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacot JG, Abdullah I, Belkin M, Gerhard-Herman M, Gaccione P, Polak JF, Donaldson MC, Whittemore AD, Conte MS. Early adaptation of human lower extremity vein grafts: wall stiffness changes accompany geometric remodeling. J Vasc Surg. 2004;39:547–555. doi: 10.1016/j.jvs.2003.09.045 [DOI] [PubMed] [Google Scholar]
- 49.Owens CD, Gasper WJ, Rahman AS, Conte MS. Vein graft failure. J Vasc Surg. 2015;61:203–216. doi: 10.1016/j.jvs.2013.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haime M, McLean RR, Kurgansky KE, Emmert MY, Kosik N, Nelson C, Gaziano MJ, Cho K, Gagnon DR. Relationship between intra-operative vein graft treatment with DuraGraft® or saline and clinical outcomes after coronary artery bypass grafting. Expert Rev Cardiovasc Ther. 2018;16:963–970. doi: 10.1080/14779072.2018.1532289 [DOI] [PubMed] [Google Scholar]
- 51.Lin PH, Brinkman WT, Terramani TT, Lumsden AB. Management of infected hemodialysis access grafts using cryopreserved human vein allografts. Am J Surg. 2002;184:31–36. doi: 10.1016/s0002-9610(02)00894-2 [DOI] [PubMed] [Google Scholar]
- 52.Harris L, O’brien-Irr M, Ricotta JJ. Long-term assessment of cryopreserved vein bypass grafting success. J Vasc Surg. 2001;33:528–532. doi: 10.1067/mva.2001.111729 [DOI] [PubMed] [Google Scholar]
- 53.Hartranft CA, Noland S, Kulwicki A, Holden CR, Hartranft T. Cryopreserved saphenous vein graft in infrainguinal bypass. J Vasc Surg. 2014;60:1291–1296. doi: 10.1016/j.jvs.2014.05.092 [DOI] [PubMed] [Google Scholar]
- 54.Randon C, Jacobs B, De Ryck F, Beele H, Vermassen F. Fifteen years of infrapopliteal arterial reconstructions with cryopreserved venous allografts for limb salvage. J Vasc Surg. 2010;51:869–877. doi: 10.1016/j.jvs.2009.11.062 [DOI] [PubMed] [Google Scholar]
- 55.Iaffaldano RA, Lewis BE, Johnson SA, Piffare R, McKiernan TL. Patency of cryopreserved saphenous vein grafts as conduits for coronary artery bypass surgery. Chest. 1995;108:725–729. doi: 10.1378/chest.108.3.725 [DOI] [PubMed] [Google Scholar]
- 56.Carpenter JP, Tomaszewski JE. Human saphenous vein allograft bypass grafts: immune response. J Vasc Surg. 1998;27:492–499. doi: 10.1016/s0741-5214(98)70323-4 [DOI] [PubMed] [Google Scholar]
- 57.Albert B, Elena H, Nicole W, Süleyman E, Ralph K, Richard K, Udo L. Neointimal hyperplasia in allogeneic and autologous venous grafts is not different in nature. Histochem Cell Biol. 2015;144:59–66. doi: 10.1007/s00418-015-1317-3 [DOI] [PubMed] [Google Scholar]
- 58.Leskovar B, Furlan T, Poznic S, Hrastelj M, Adamlje A. Using CorMatrix for partial and complete (re)construction of arteriovenous fistulas in haemodialysis patients: (re)construction of arteriovenous fistulas with CorMatrix. J Vasc Access. 2019;20:597–603. doi: 10.1177/1129729819826032 [DOI] [PubMed] [Google Scholar]
- 59.DuBose JJ, Azizzadeh A. Utilization of a tubularized cormatrix extracellular matrix for repair of an arteriovenous fistula aneurysm. Ann Vasc Surg. 2015;29:366.e1–366.e4. doi: 10.1016/j.avsg.2014.10.018 [DOI] [PubMed] [Google Scholar]
- 60.Pineda DM, Dougherty MJ, Wismer MC, Carroll C, Tyagi S, Troutman DA, Calligaro KD. Bovine carotid artery xenografts for hemodialysis access. J Vasc Surg. 2017;65:1729–1734. doi: 10.1016/j.jvs.2016.12.109 [DOI] [PubMed] [Google Scholar]
- 61.Arhuidese I, Reifsnyder T, Islam T, Karim O, Nejim B, Obeid T, Qazi U, Malas M. Bovine carotid artery biologic graft outperforms expanded polytetrafluoroethylene for hemodialysis access. J Vasc Surg. 2017;65:775–782. doi: 10.1016/j.jvs.2016.10.080 [DOI] [PubMed] [Google Scholar]
- 62.Kennealey PT, Elias N, Hertl M, Ko DS, Saidi RF, Markmann JF, Smoot EE, Schoenfeld DA, Kawai T. A prospective, randomized comparison of bovine carotid artery and expanded polytetrafluoroethylene for permanent hemodialysis vascular access. J Vasc Surg. 2011;53:1640–1648. doi: 10.1016/j.jvs.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 63.Marcus P, Echeverria A, Cheung M, Kfoury E, Shim K, Lin PH. Early cannulation of bovine carotid artery graft reduces tunneled dialysis catheter-related complications: a comparison of bovine carotid artery graft versus expanded polytetrafluoroethylene grafts in hemodialysis access. Vasc Endovascular Surg. 2019;53:104–111. doi: 10.1177/1538574418813595 [DOI] [PubMed] [Google Scholar]
- 64.Buggs J, Tanious A, Camba V, Albertson C, Rogers E, Lahiff D, Rashid T, Leone J, Pearson H, Huang J, et al. Effective arteriovenous fistula alternative for hemodialysis access. Am J Surg. 2018;216:1144–1147. doi: 10.1016/j.amjsurg.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 65.Katzman HE, Glickman MH, Schild AF, Fujitani RM, Lawson JH. Multicenter evaluation of the bovine mesenteric vein bioprostheses for hemodialysis access in patients with an earlier failed prosthetic graft. J Am Coll Surg. 2005;201:223–230. doi: 10.1016/j.jamcollsurg.2005.03.040 [DOI] [PubMed] [Google Scholar]
- 66.Senkaya I, Aytac II, Eercan AK, Aliosman A, Percin B. The graft selection for haemodialysis. Vasa. 2003;32:209–213. doi: 10.1024/0301-1526.32.4.209 [DOI] [PubMed] [Google Scholar]
- 67.Burla L, Schwegler I, Weibel P, Weber M, Zientara A, Attigah N. Intraoperatively self-made bovine pericardial graft for portomesenteric reconstruction in pancreatic surgery. Langenbecks Arch Surg. 2020;405:705–712. doi: 10.1007/s00423-020-01920-0 [DOI] [PubMed] [Google Scholar]
- 68.Papakostas JC, Avgos S, Arnaoutoglou E, Nassis C, Peroulis M, Bali C, Papadopoulos G, Matsagkas MI. Use of the vascu-guard bovine pericardium patch for arteriotomy closure in carotid endarterectomy. Early and long-term results. Ann Vasc Surg. 2014;28:1213–1218. doi: 10.1016/j.avsg.2013.10.021 [DOI] [PubMed] [Google Scholar]
- 69.Reilly B, Khan S, Dosluoglu H, Harris L, O’Brien-Irr M, Lukan J, Dryjski M, Blochle R. Comparison of autologous vein and bovine carotid artery graft as a bypass conduit in arterial trauma. Ann Vasc Surg. 2019;61:246–253. doi: 10.1016/j.avsg.2019.05.017 [DOI] [PubMed] [Google Scholar]
- 70.Lindsey P, Echeverria A, Cheung M, Kfoury E, Bechara CF, Lin PH. Lower extremity bypass using bovine carotid artery graft (artegraft): an analysis of 124 cases with long-term results. World J Surg. 2018;42:295–301. doi: 10.1007/s00268-017-4161-x [DOI] [PubMed] [Google Scholar]
- 71.Padalino MA, Quarti A, Angeli E, Frigo AC, Vida VL, Pozzi M, Gargiulo G, Stellin G. Early and mid-term clinical experience with extracellular matrix scaffold for congenital cardiac and vascular reconstructive surgery: a multicentric Italian study. Interact Cardiovasc Thorac Surg. 2015;21:40–49; discussion 49. doi: 10.1093/icvts/ivv076 [DOI] [PubMed] [Google Scholar]
- 72.Padalino MA, Castaldi B, Fedrigo M, Gallo M, Zucchetta F, Vida VL, Milanesi O, Angelini A, Stellin G. Porcine intestinal submucosa (CorMatrix) for semilunar valve repair in children: a word of caution after midterm results. Semin Thorac Cardiovasc Surg. 2016;28:436–445. doi: 10.1053/j.semtcvs.2016.04.015 [DOI] [PubMed] [Google Scholar]
- 73.Nordmeyer S, Murin P, Schulz A, Danne F, Nordmeyer J, Kretzschmar J, Sumbadze D, Schmitt KRL, Miera O, Cho MY, et al. Results of aortic valve repair using decellularized bovine pericardium in congenital surgery. Eur J Cardiothorac Surg. 2018;54:986–992. doi: 10.1093/ejcts/ezy181 [DOI] [PubMed] [Google Scholar]
- 74.Bell D, Betts K, Justo R, Forde N, Venugopal P, Corno AF, Smith P, Caputo M, Marsico R, Karl TR, et al. Multicenter experience with 500 CardioCel implants used for the repair of congenital heart defects. Ann Thorac Surg. 2019;108:1883–1888. doi: 10.1016/j.athoracsur.2019.04.085 [DOI] [PubMed] [Google Scholar]
- 75.Pavy C, Michielon G, Robertus JL, Lacour-Gayet F, Ghez O. Initial 2-year results of CardioCel® patch implantation in children. Interact Cardiovasc Thorac Surg. 2018;26:448–453. doi: 10.1093/icvts/ivx295 [DOI] [PubMed] [Google Scholar]
- 76.Kelley TM, Jr, Kashem M, Wang H, McCarthy J, Carroll ND, Moser GW, Guy TS. Anterior leaflet augmentation with CorMatrix porcine extracellular matrix in twenty-five patients: unexpected patch failures and histologic analysis. Ann Thorac Surg. 2017;103:114–120. doi: 10.1016/j.athoracsur.2016.05.090 [DOI] [PubMed] [Google Scholar]
- 77.Christ T, Paun AC, Grubitzsch H, Holinski S, Falk V, Dushe S. Long-term results after the Ross procedure with the decellularized AutoTissue Matrix P® bioprosthesis used for pulmonary valve replacement. Eur J Cardiothorac Surg. 2019;55:885–892. doi: 10.1093/ejcts/ezy377 [DOI] [PubMed] [Google Scholar]
- 78.Dobrilovic N, Soukas P, Sadiq I, Goldstein L, Raman J. Early complications of biologic extracellular matrix patch after use for femoral artery repair. J Vasc Surg. 2017;65:705–710. doi: 10.1016/j.jvs.2016.07.131 [DOI] [PubMed] [Google Scholar]
- 79.Weber SS, Annenberg AJ, Wright CB, Braverman TS, Mesh CL. Early pseudoaneurysm degeneration in biologic extracellular matrix patch for carotid repair. J Vasc Surg. 2014;59:1116–1118. doi: 10.1016/j.jvs.2013.05.012 [DOI] [PubMed] [Google Scholar]
- 80.Erek E, Aydin S, Suzan D, Yildiz O, Demir IH, Odemis E. Early degeneration of extracellular matrix used for aortic reconstruction during the Norwood operation. Ann Thorac Surg. 2016;101:758–760. doi: 10.1016/j.athoracsur.2015.04.051 [DOI] [PubMed] [Google Scholar]
- 81.van Beynum IM, Kurul S, Krasemann T, Dalinghaus M, de Woestijne PV, Etnel JR, Bogers AJJC. Reconstruction of the aortic arch in neonates and infants: the importance of patch material. World J Pediatr Congenit Heart Surg. 2021;12:487–491. doi: 10.1177/21501351211003502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Böer U, Schridde A, Anssar M, Klingenberg M, Sarikouch S, Dellmann A, Harringer W, Haverich A, Wilhelmi M. The immune response to crosslinked tissue is reduced in decellularized xenogeneic and absent in decellularized allogeneic heart valves. Int J Artif Organs. 2015;38:199–209. doi: 10.5301/ijao.5000395 [DOI] [PubMed] [Google Scholar]
- 83.Slachman FN. Constructive remodeling of CorMatrix extracellular matrix after aortic root repair in a 90-year-old woman. Ann Thorac Surg. 2014;97:e129–e131. doi: 10.1016/j.athoracsur.2013.10.103 [DOI] [PubMed] [Google Scholar]
- 84.Stelly M, Stelly TC. Histology of CorMatrix bioscaffold 5 years after pericardial closure. Ann Thorac Surg. 2013;96:e127–e129. doi: 10.1016/j.athoracsur.2013.06.114 [DOI] [PubMed] [Google Scholar]
- 85.Bibevski S, Ruzmetov M, Ladich E, Mendoza LE, Scholl FG. Reconstruction of the neopulmonary root after coronary button harvest for arterial switch operation using 2-ply extracellular matrix (Tyke): a post-implant histology. Front Cardiovasc Med. 2020;7:562136. doi: 10.3389/fcvm.2020.562136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cox JL, Hammel JM, Radio SJ. Evaluation of cellular ingrowth within porcine extracellular matrix scaffolding in congenital heart disease surgery. Cardiovasc Pathol. 2019;39:54–60. doi: 10.1016/j.carpath.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 87.Mosala Nezhad Z, Baldin P, Poncelet A, El Khoury G. Calcific degeneration of CorMatrix 4 years after bicuspidization of unicuspid aortic valve. Ann Thorac Surg. 2017;104:e431–e433. doi: 10.1016/j.athoracsur.2017.07.040 [DOI] [PubMed] [Google Scholar]
- 88.Scholl FG, Boucek MM, Chan KC, Valdes-Cruz L, Perryman R. Preliminary experience with cardiac reconstruction using decellularized porcine extracellular matrix scaffold: human applications in congenital heart disease. World J Pediatr Congenit Heart Surg. 2010;1:132–136. doi: 10.1177/2150135110362092 [DOI] [PubMed] [Google Scholar]
- 89.Deutsch O, Bruehl F, Cleuziou J, Prinzing A, Schlitter AM, Krane M, Lange R. Histological examination of explanted tissue-engineered bovine pericardium following heart valve repair. Interact Cardiovasc Thorac Surg. 2020;30:64–73. doi: 10.1093/icvts/ivz234 [DOI] [PubMed] [Google Scholar]
- 90.U.S. Food and Drug Administration. 2022. Cellular & Gene Therapy Products [online]. Accessed May 23, 2022. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products
- 91.Forster R, Liew A, Bhattacharya V, Shaw J, Stansby G. Gene therapy for peripheral arterial disease. Cochrane Database Syst Rev. 2018;10:CD012058. doi: 10.1002/14651858.CD012058.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaminsky SM, Rosengart TK, Rosenberg J, Chiuchiolo MJ, Van de Graaf B, Sondhi D, Crystal RG. Gene therapy to stimulate angiogenesis to treat diffuse coronary artery disease. Hum Gene Ther. 2013;24:948–963. doi: 10.1089/hum.2013.2516 [DOI] [PubMed] [Google Scholar]
- 93.Rosengart TK, Fallon E, Crystal RG. Cardiac biointerventions: whatever happened to stem cell and gene therapy? Innovations (Phila). 2012;7:173–179. doi: 10.1097/IMI.0b013e318265d9f6 [DOI] [PubMed] [Google Scholar]
- 94.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- 95.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mathison M, Singh VP, Chiuchiolo MJ, Sanagasetti D, Mao Y, Patel VB, Yang J, Kaminsky SM, Crystal RG, Rosengart TK. In situ reprogramming to transdifferentiate fibroblasts into cardiomyocytes using adenoviral vectors: implications for clinical myocardial regeneration. J Thorac Cardiovasc Surg. 2017;153:329–339.e3. doi: 10.1016/j.jtcvs.2016.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tilemann L, Ishikawa K, Weber T, Hajjar RJ. Gene therapy for heart failure. Circ Res. 2012;110:777–793. doi: 10.1161/CIRCRESAHA.111.252981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, Zhang Z, Rosenberg P, Mirotsou M, Dzau VJ. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012;110:1465–1473. doi: 10.1161/CIRCRESAHA.112.269035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Inagawa K, Miyamoto K, Yamakawa H, Muraoka N, Sadahiro T, Umei T, Wada R, Katsumata Y, Kaneda R, Nakade K, et al. Induction of cardiomyocyte-like cells in infarct hearts by gene transfer of Gata4, Mef2c, and Tbx5. Circ Res. 2012;111:1147–1156. doi: 10.1161/CIRCRESAHA.112.271148 [DOI] [PubMed] [Google Scholar]
- 100.Mathison M, Singh VP, Gersch RP, Ramirez MO, Cooney A, Kaminsky SM, Chiuchiolo MJ, Nasser A, Yang J, Crystal RG, et al. “Triplet” polycistronic vectors encoding Gata4, Mef2c, and Tbx5 enhances postinfarct ventricular functional improvement compared with singlet vectors. J Thorac Cardiovasc Surg. 2014;148:1656–1664.e2. doi: 10.1016/j.jtcvs.2014.03.033 [DOI] [PubMed] [Google Scholar]
- 101.Ryan CT, Patel V, Rosengart TK. Clinical potential of angiogenic therapy and cellular reprogramming. JTCVS Open. 2021;6:108–115. doi: 10.1016/j.xjon.2020.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boretos JW, Pierce WS. Segmented polyurethane: a new elastomer for biomedical applications. Science. 1967;158:1481–1482. doi: 10.1126/science.158.3807.1481 [DOI] [PubMed] [Google Scholar]
- 103.Rosso F, Marino G, Giordano A, Barbarisi M, Parmeggiani D, Barbarisi A. Smart materials as scaffolds for tissue engineering. J Cell Physiol. 2005;203:465–470. doi: 10.1002/jcp.20270 [DOI] [PubMed] [Google Scholar]
- 104.Stowell CET, Wang Y. Quickening: translational design of resorbable synthetic vascular grafts. Biomaterials. 2018;173:71–86. doi: 10.1016/j.biomaterials.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jamshidian M, Tehrany EA, Imran M, Jacquot M, Desobry S. Poly-lactic acid: production, applications, nanocomposites, and release studies. Compr Rev Food Sci Food Saf. 2010;9:552–571. doi: 10.1111/j.1541-4337.2010.00126.x [DOI] [PubMed] [Google Scholar]
- 106.de Valence S, Tille JC, Mugnai D, Mrowczynski W, Gurny R, Möller M, Walpoth BH. Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials. 2012;33:38–47. doi: 10.1016/j.biomaterials.2011.09.024 [DOI] [PubMed] [Google Scholar]
- 107.Ulery BD, Nair LS, Laurencin CT. Biomedical applications of biodegradable polymers. J Polym Sci B Polym Phys. 2011;49:832–864. doi: 10.1002/polb.22259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sivalingam G, Vijayalakshmi S, Madras G. Enzymatic and thermal degradation of poly (ε-caprolactone), poly (d, l-lactide), and their blends. Ind Eng Chem Res. 2004;43:7702–7709. doi: 10.1021/ie049589r [Google Scholar]
- 109.Bockeria LA, Svanidze O, Kim A, Shatalov K, Makarenko V, Cox M, Carrel T. Total cavopulmonary connection with a new bioabsorbable vascular graft: first clinical experience. J Thorac Cardiovasc Surg. 2017;153:1542–1550. doi: 10.1016/j.jtcvs.2016.11.071 [DOI] [PubMed] [Google Scholar]
- 110.Kluin J, Talacua H, Smits AI, Emmert MY, Brugmans MC, Fioretta ES, Dijkman PE, Söntjens SH, Duijvelshoff R, Dekker S, et al. In situ heart valve tissue engineering using a bioresorbable elastomeric implant - from material design to 12 months follow-up in sheep. Biomaterials. 2017;125:101–117. doi: 10.1016/j.biomaterials.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 111.Bockeria L, Carrel T, Lemaire A, Makarenko V, Kim A, Shatalov K, Cox M, Svanidze O. Total cavopulmonary connection with a new restorative vascular graft: results at 2 years. J Thorac Dis. 2020;12:4168–4173. doi: 10.21037/jtd-19-739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morales DL, Herrington C, Bacha EA, Morell VO, Prodán Z, Mroczek T, Sivalingam S, Cox M, Bennink G, Asch FM. A novel restorative pulmonary valve conduit: early outcomes of two clinical trials. Front Cardiovasc Med. 2021:7:583360. doi: 10.3389/fcvm.2020.583360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fioretta ES, von Boehmer L, Generali M, Hoerstrup SP, Emmert MY. Off-the-shelf tissue-engineered vascular conduits: clinical translation. In: Walpoth BH, Bergmeister H, Bowlin GL, Kong D, Rotmans JI, Zilla P. eds. Tissue-Engineered Vascular Grafts. Springer, Cham; 2020:489–531. [Google Scholar]
- 114.Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate JA, Cuy JL, Hauch KD, Laflamme MA, Murry CE, Ratner BD. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci USA. 2010;107:15211–15216. doi: 10.1073/pnas.1006442107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lawson JH, Glickman MH, Ilzecki M, Jakimowicz T, Jaroszynski A, Peden EK, Pilgrim AJ, Prichard HL, Guziewicz M, Przywara S, et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet. 2016;387:2026–2034. doi: 10.1016/S0140-6736(16)00557-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shoji T, Shinoka T. Tissue engineered vascular grafts for pediatric cardiac surgery. Transl Pediatr. 2018;7:188–195. doi: 10.21037/tp.2018.02.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489 [DOI] [PubMed] [Google Scholar]
- 118.Hallin RW, Sweetman WR. The Sparks’ mandril graft. A seven year follow-up of mandril grafts placed by Charles H. Sparks and his associates. Am J Surg. 1976;132:221–223. doi: 10.1016/0002-9610(76)90051-9 [DOI] [PubMed] [Google Scholar]
- 119.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816 [DOI] [PubMed] [Google Scholar]
- 120.Shin’oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med. 2001;344:532–533. doi: 10.1056/NEJM200102153440717 [DOI] [PubMed] [Google Scholar]
- 121.Matsumura G, Hibino N, Ikada Y, Kurosawa H, Shin’oka T. Successful application of tissue engineered vascular autografts: clinical experience. Biomaterials. 2003;24:2303–2308. doi: 10.1016/s0142-9612(03)00043-7 [DOI] [PubMed] [Google Scholar]
- 122.Drews JD, Miyachi H, Shinoka T. Tissue-engineered vascular grafts for congenital cardiac disease: clinical experience and current status. Trends Cardiovasc Med. 2017;27:521–531. doi: 10.1016/j.tcm.2017.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Matsumura G, Ishihara Y, Miyagawa-Tomita S, Ikada Y, Matsuda S, Kurosawa H, Shin’oka T. Evaluation of tissue-engineered vascular autografts. Tissue Eng. 2006;12:3075–3083. doi: 10.1089/ten.2006.12.3075 [DOI] [PubMed] [Google Scholar]
- 124.Shinoka T, Breuer C. Tissue-engineered blood vessels in pediatric cardiac surgery. Yale J Biol Med. 2008;81:161–166. [PMC free article] [PubMed] [Google Scholar]
- 125.Drews JD, Pepper VK, Best CA, Szafron JM, Cheatham JP, Yates AR, Hor KN, Zbinden JC, Chang YC, Mirhaidari GJM, et al. Spontaneous reversal of stenosis in tissue-engineered vascular grafts. Sci Transl Med. 2020;12:eaax6919. doi: 10.1126/scitranslmed.aax6919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.L’Heureux N, Pâquet S, Labbé R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. Faseb J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47 [DOI] [PubMed] [Google Scholar]
- 127.McAllister TN, Maruszewski M, Garrido SA, Wystrychowski W, Dusserre N, Marini A, Zagalski K, Fiorillo A, Avila H, Manglano X, et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet. 2009;373:1440–1446. doi: 10.1016/S0140-6736(09)60248-8 [DOI] [PubMed] [Google Scholar]
- 128.L’Heureux N, McAllister TN, de la Fuente LM. Tissue-engineered blood vessel for adult arterial revascularization. N Engl J Med. 2007;357:1451–1453. doi: 10.1056/NEJMc071536 [DOI] [PubMed] [Google Scholar]
- 129.Dahl SLM, Koh J, Prabhakar V, Niklason LE. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant. 2003;12:659–666. doi: 10.3727/000000003108747136 [DOI] [PubMed] [Google Scholar]
- 130.Dahl SL, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, Manson RJ, Tente WE, DiBernardo L, Hensley MT, et al. Readily available tissue-engineered vascular grafts. Sci Transl Med. 2011;3:68ra9. doi: 10.1126/scitranslmed.3001426 [DOI] [PubMed] [Google Scholar]
- 131.Pisoni RL, Zepel L, Fluck R, Lok CE, Kawanishi H, Süleymanlar G, Wasse H, Tentori F, Zee J, Li Y, et al. International differences in the location and use of arteriovenous accesses created for hemodialysis: results from the dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis. 2018;71:469–478. doi: 10.1053/j.ajkd.2017.09.012 [DOI] [PubMed] [Google Scholar]
- 132.Gutowski P, Gage SM, Guziewicz M, Ilzecki M, Kazimierczak A, Kirkton RD, Niklason LE, Pilgrim A, Prichard HL, Przywara S, et al. Arterial reconstruction with human bioengineered acellular blood vessels in patients with peripheral arterial disease. J Vasc Surg. 2020;72:1247–1258. doi: 10.1016/j.jvs.2019.11.056 [DOI] [PubMed] [Google Scholar]
- 133.Kirkton RD, Santiago-Maysonet M, Lawson JH, Tente WE, Dahl SLM, Niklason LE, Prichard HL. Bioengineered human acellular vessels recellularize and evolve into living blood vessels after human implantation. Sci Transl Med. 2019;11:eaau6934. doi: 10.1126/scitranslmed.aau6934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Morrison JJ, McMahon J, DuBose JJ, Scalea TM, Lawson JH, Rasmussen TE. Clinical implementation of the Humacyte human acellular vessel: implications for military and civilian trauma care. J Trauma Acute Care Surg. 2019;87:S44–S47. doi: 10.1097/TA.0000000000002350 [DOI] [PubMed] [Google Scholar]
- 135.Hoerstrup SP, Cummings Mrcs I, Lachat M, Schoen FJ, Jenni R, Leschka S, Neuenschwander S, Schmidt D, Mol A, Günter C, et al. Functional growth in tissue-engineered living, vascular grafts: follow-up at 100 weeks in a large animal model. Circulation. 2006;114:I159–I166. doi: 10.1161/CIRCULATIONAHA.105.001172 [DOI] [PubMed] [Google Scholar]
- 136.Cummings I, George S, Kelm J, Schmidt D, Emmert MY, Weber B, Zünd G, Hoerstrup SP. Tissue-engineered vascular graft remodeling in a growing lamb model: expression of matrix metalloproteinases. Eur J Cardiothorac Surg. 2012;41:167–172. doi: 10.1016/j.ejcts.2011.02.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Syedain ZH, Meier LA, Bjork JW, Lee A, Tranquillo RT. Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials. 2011;32:714–722. doi: 10.1016/j.biomaterials.2010.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Syedain Z, Reimer J, Lahti M, Berry J, Johnson S, Tranquillo RT. Tissue engineering of acellular vascular grafts capable of somatic growth in young lambs. Nat Commun. 2016;7:12951. doi: 10.1038/ncomms12951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Syedain ZH, Haynie B, Johnson SL, Lahti M, Berry J, Carney JP, Li J, Hill RC, Hansen KC, Thrivikraman G, et al. Pediatric tri-tube valved conduits made from fibroblast-produced extracellular matrix evaluated over 52 weeks in growing lambs. Sci Transl Med. 2021;13:eabb7225. doi: 10.1126/scitranslmed.abb7225 [DOI] [PubMed] [Google Scholar]
- 140.Syedain ZH, Graham ML, Dunn TB, O’Brien T, Johnson SL, Schumacher RJ, Tranquillo RT. A completely biological “off-the-shelf” arteriovenous graft that recellularizes in baboons. Sci Transl Med. 2017;9:eaan4209. doi: 10.1126/scitranslmed.aan4209 [DOI] [PubMed] [Google Scholar]
- 141.Geelhoed WJ, Moroni L, Rotmans JI. Utilizing the foreign body response to grow tissue engineered blood vessels in vivo. J Cardiovasc Transl Res. 2017;10:167–179. doi: 10.1007/s12265-017-9731-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Damanik FF, Rothuizen TC, van Blitterswijk C, Rotmans JI, Moroni L. Towards an in vitro model mimicking the foreign body response: tailoring the surface properties of biomaterials to modulate extracellular matrix. Sci Rep. 2014;4:6325. doi: 10.1038/srep06325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nakayama Y, Kaneko Y, Okumura N, Terazawa T. Initial 3-year results of first human use of an in-body tissue-engineered autologous “Biotube” vascular graft for hemodialysis. J Vasc Access. 2020;21:110–115. doi: 10.1177/1129729819852550 [DOI] [PubMed] [Google Scholar]
- 144.Cho SW, Lim SH, Kim IK, Hong YS, Kim SS, Yoo KJ, Park HY, Jang Y, Chang BC, Choi CY, et al. Small-diameter blood vessels engineered with bone marrow-derived cells. Ann Surg. 2005;241:506–515. doi: 10.1097/01.sla.0000154268.12239.ed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A, et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035–1040. doi: 10.1038/nm0901-1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tatterton M, Wilshaw SP, Ingham E, Homer-Vanniasinkam S. The use of antithrombotic therapies in reducing synthetic small-diameter vascular graft thrombosis. Vasc Endovascular Surg. 2012;46:212–222. doi: 10.1177/1538574411433299 [DOI] [PubMed] [Google Scholar]
- 147.Huang C, Wang S, Qiu L, Ke Q, Zhai W, Mo X. Heparin loading and pre-endothelialization in enhancing the patency rate of electrospun small-diameter vascular grafts in a canine model. ACS Appl Mater Interfaces. 2013;5:2220–2226. doi: 10.1021/am400099p [DOI] [PubMed] [Google Scholar]
- 148.Wang Y, He C, Feng Y, Yang Y, Wei Z, Zhao W. A chitosan modified asymmetric small-diameter vascular graft with anti-thrombotic and anti-bacterial functions for vascular tissue engineering. J Mater Chem B. 2019;8:568–577. doi: 10.1039/c9tb01755k [DOI] [PubMed] [Google Scholar]
- 149.Badv M, Bayat F, Weitz JI, Didar TF. Single and multi-functional coating strategies for enhancing the biocompatibility and tissue integration of blood-contacting medical implants. Biomaterials. 2020;258:120291. doi: 10.1016/j.biomaterials.2020.120291 [DOI] [PubMed] [Google Scholar]
- 150.Li P, Wang Y, Jin X, Dou J, Han X, Wan X, Yuan J, Shen J. Fabrication of PCL/keratin composite scaffolds for vascular tissue engineering with catalytic generation of nitric oxide potential. J Mater Chem B. 2020;8:6092–6099. doi: 10.1039/d0tb00857e [DOI] [PubMed] [Google Scholar]
- 151.Li P, Wang Y, Jin X, Dou J, Han X, Wan X, Yuan J, Shen J. Catalytic generation of nitric oxide from poly(ε-caprolactone)/phosphobetainized keratin mats for a vascular tissue engineering scaffold. Langmuir. 2020;36:4396–4404. doi: 10.1021/acs.langmuir.0c00579 [DOI] [PubMed] [Google Scholar]
- 152.Dimitrievska S, Wang J, Lin T, Weyers A, Bai H, Qin L, Li G, Cai C, Kypson A, Kristofik N, et al. Glycocalyx-like hydrogel coatings for small diameter vascular grafts. Adv Funct Mater. 2020;30:1908963. doi: 10.1002/adfm.201908963 [Google Scholar]
- 153.Quint C, Kondo Y, Manson RJ, Lawson JH, Dardik A, Niklason LE. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci USA. 2011;108:9214–9219. doi: 10.1073/pnas.1019506108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhao J, Feng Y. Surface engineering of cardiovascular devices for improved hemocompatibility and rapid endothelialization. Adv Healthc Mater. 2020;9:e2000920. doi: 10.1002/adhm.202000920 [DOI] [PubMed] [Google Scholar]
- 155.Zhao J, Bai L, Ren XK, Guo J, Xia S, Zhang W, Feng Y. Co-immobilization of ACH11 antithrombotic peptide and CAG cell-adhesive peptide onto vascular grafts for improved hemocompatibility and endothelialization. Acta Biomater. 2019;97:344–359. doi: 10.1016/j.actbio.2019.07.057 [DOI] [PubMed] [Google Scholar]
- 156.Cao G, Xuan X, Zhang R, Hu J, Dong H. Gene therapy for cardiovascular disease: basic research and clinical prospects. Front Cardiovasc Med. 2021;8:760140. doi: 10.3389/fcvm.2021.760140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gupta P, Mandal BB. Tissue-engineered vascular grafts: emerging trends and technologies. Adv Funct Mater. 2021;31:2100027. [Google Scholar]
- 158.Isselbacher EM, Lino Cardenas CL, Lindsay ME. Hereditary influence in thoracic aortic aneurysm and dissection. Circulation. 2016;133:2516–2528. doi: 10.1161/CIRCULATIONAHA.116.009762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kobashigawa J, Patel J, Azarbal B, Kittleson M, Chang D, Czer L, Daun T, Luu M, Trento A, Cheng R, et al. Randomized pilot trial of gene expression profiling versus heart biopsy in the first year after heart transplant: early invasive monitoring attenuation through gene expression trial. Circ Heart Fail. 2015;8:557–564. doi: 10.1161/CIRCHEARTFAILURE.114.001658 [DOI] [PubMed] [Google Scholar]
- 160.Corti A, Colombo M, Migliavacca F, Rodriguez Matas JF, Casarin S, Chiastra C. Multiscale computational modeling of vascular adaptation: a systems biology approach using agent-based models. Front Bioeng Biotechnol. 2021;9:744560. doi: 10.3389/fbioe.2021.744560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shin’oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, Sakamoto T, Nagatsu M, Kurosawa H. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330–1338. doi: 10.1016/j.jtcvs.2004.12.047 [DOI] [PubMed] [Google Scholar]