Abstract
Cardiovascular magnetic resonance has been utilized in the management and care of pediatric patients for nearly 40 years. It has evolved to become an invaluable tool in the assessment of the littlest of hearts for diagnosis, pre-interventional management and follow-up care. Although mentioned in a number of consensus and guidelines documents, an up-to-date, large, stand-alone guidance work for the use of cardiovascular magnetic resonance in pediatric congenital 36 and acquired 35 heart disease endorsed by numerous Societies involved in the care of these children is lacking. This guidelines document outlines the use of cardiovascular magnetic resonance in this patient population for a significant number of heart lesions in this age group and although admittedly, is not an exhaustive treatment, it does deal with an expansive list of many common clinical issues encountered in daily practice.
Keywords: cardiac magnetic resonance, congenital heart disease, pediatrics, children, acquired pediatric heart disease
Introduction
Background
The role of imaging and the modalities utilized in pediatric and congenital heart disease (CHD) is continually evolving. Cardiovascular magnetic resonance (CMR) is now a standard modality in imaging CHD and is considered a “one-stop-shop” with the capability of visualizing anatomy and assessing ventricular function, blood flow and tissue characterization. It is utilized in conjunction with other imaging modalities in almost all instances including echocardiography, invasive angiography, cardiac computed tomography (CT) and nuclear medicine. The spectacular improvements in diagnosis, treatment and follow-up in this patient population is in part due to the use of this multimodality imaging approach.
There is significant literature supporting the use of CMR in pediatric CHD and acquired heart disease, however, there is wide practice variation among centers for which patients undergo CMR. Availability, diagnostic accuracy, economics and patient burden all play a role in which imaging modality is utilized for various diagnostic categories in the different centers. Echocardiography has been and remains the front line imaging modality for most CHD patients, however, the objectives and frequency of use of echocardiography have changed with the increased utilization, established and evolving capabilities of CMR and cardiac CT.
Although there are current guidelines in adult CHD which involve CMR,1 pediatric CHD and acquired pediatric heart disease are unique and distinct entities which has different requirements and needs such as smaller structures, higher heart rates, complex unknown anatomy and the more pressing concern of avoiding ionizing radiation. Currently, there is only a consensus document that is available on CMR which is dedicated to pediatric CHD and pediatric acquired heart disease,2 an old CMR consensus documents with only small sections on pediatrics and CHD3,4 and an old appropriate use criteria (AUC) document with again, only small sections on pediatrics and CHD.5 A document describing technical protocols has been published but does not set forth guidelines or indications.6 Finally, CMR is included in the most recent AUC for multimodality imaging in the follow-up care of patients with CHD with given scenarios which is different than guidelines document for the use of CMR at all stages of care.7
Purpose of this Guidelines Manuscript
The primary objective of this document is to present guidelines based on the existing literature supporting CMR for commonly encountered pediatric CHD and acquired pediatric heart disease. It is beyond the scope of this paper to delineate CMR physics, technical details and protocols focused on imaging children, as there are excellent guidelines for this published elsewhere.6,8 Where literature is sparse or non-existent, consensus opinion of the writing group is presented. The document includes both disease specific (e.g., single ventricle) and technique specific (e.g, ventricular function) sections focused on pediatric CHD and acquired heart disease. Each section includes a brief introduction followed by a review of the literature supporting use of CMR with formal recommendations for indications at the end of the section.
These guidelines are intended to assist providers in the decision to utilize CMR. They represent an extensive review of the available current scientific evidence. Many clinical scenarios are complex and some may not be covered exactly by the document; final judgement as to whether CMR is appropriate for a particular patient requires individualized decision making. In those situations, clinical decision making should consider the quality and availability of data in the area where care is provided. When these CMR guidelines are used as a basis for either regulatory or payer decisions, the goal should be improvement in quality of care. The writing group acknowledges that there may be some institutions that do not have access to or have expertise in CMR performance and as such, other imaging modalities such as cardiac CT may be considered.
This document is not a multimodality or cross-sectional imaging guideline for all the diseases mentioned or an in depth analysis of a comparison between imaging modalities. It is primarily a work on CMR indications. Where appropriate, a few comments are made regarding other imaging modalities. As a general rule, in emergency situations (e.g., pulmonary thromboembolism, shunt occlusion, and other unstable conditions) and in relative or absolute contraindications of CMR (e.g., presence of a pacemaker, a defibrillator, metals causing severe artifact or in claustrophobic patients who do not wish to be sedated) cardiac CT or cardiac catheterization may be considered.
Selection of Writing Committee Members
A panel of acknowledged CMR experts was selected and rigorously reviewed by the Society for Cardiovascular Magnetic Resonance (SCMR) to develop these guidelines, to grade the level of clinical evidence and to write recommendations based on current knowledge of CMR and other imaging modalities. The writing group was composed of pediatric cardiologists and radiologists from both North America and Europe, representing different geographical regions, gender, ethnicities, races, perspectives and scopes of clinical practice. Representatives from the American Heart Association (AHA), the American Academy of Pediatrics and the Society for Pediatric Radiology were included in the writing group. Representation by an outside organization does not necessarily imply endorsement.
Document Development Process
Relationships with Industry
Each member of the writing committee reported all relationships with industry and other entities relevant to pediatric CMR. Every effort was made by members to avoid actual, potential or perceived conflicts of interest.
Committee Meetings, Evidence and Literature Review
After numerous planning meetings, various sections of this document were written and developed by multiple committee members. Each section was distributed to the entire committee, reviewed, extensively discussed and edited at monthly meetings. All committee members had the opportunity to question and respond which allowed for rigorous debate. Final guideline recommendations were made by consensus agreement of the writing committee; the vast majority of recommendations were unanimous. When all sections were drafted, they were merged and sent out for review to the committee for final approval. Following peer review, the writing committee chair engaged authors to address reviewer comments and finalize the document for approval by participating organizations.
The recommendations listed in this document are evidence-based whenever possible. An extensive evidence review was conducted through March 2021. The literature searches were limited to studies conducted in human subjects and published in English. The references selected for this document are representative and not all-inclusive.
Document Approval
The final version of the document was submitted to the SCMR publications committee and the SCMR Board of Trustees for review and approval. After their comments were incorporated and the document approved, the document was circulated to those organizations who contributed representatives to the writing committee (AHA, the American Academy of Pediatrics and the Society for Pediatric Radiology) along with the North American Society for Cardiac Imaging, the European Association of Cardiovascular Imaging and the American Society of Echocardiography to review this document and to give their approval.
Class of Recommendation and Level of Evidence
These guidelines are classified using a standard evidence-based methodology developed by the AHA/ American College of Cardiology (ACC) Task Force.9 The class of recommendation (COR) is indicative of the strength of the recommendation which takes into account the estimated magnitude and certainty of benefit, in this case, medically relevant diagnostic information relative to the risk of CMR. The level of evidence (LOE) rates the quality of scientific evidence that supports the COR based on type, quantity and consistency of data from imaging studies. COR and LOE are determined independently. See Table 1.
Table 1.
Class of Recommendation and Level of Evidence
The driving force for the development of these guidelines is based on an appreciation of the increasing use of CMR and a realization that the indications for pediatric CMR lack global consensus. Given the historical predominance of catheter based angiograms which chronologically was followed by echocardiography and the emergence of cardiac CT and CMR, it is clear there is a need for guidelines to optimize use of CMR. Although the guideline committee was aware of the lack of high levels of evidence with supporting randomized trials regarding pediatric CMR for many indications which is common for imaging modalities, a guideline document based on expert consensus with supporting literature nonetheless was deemed to be clinically useful.
Diseases
Single Ventricle
Background
The patient born with single ventricle (SV), where only one pumping chamber effectively exists, is one of the most complex of all CHD. Nearly all patients require reconstructive surgery or heart transplantation. During reconstructive surgery, which ultimately leads to the Fontan procedure,10 varying loads and physiology are imposed on the ventricle. To further complicate matters, SVs are not one lesion but rather a collection of many different types which fall under the same diagnostic category.
As an umbrella category for a vast array of lesions and with different terminology, it is difficult to state the exact incidence precisely. In one of the most comprehensive collections of studies on incidence, per million live births, a mean of 266 for hypoplastic left heart complexes, 222 for hypoplastic right heart complexes, 132 for pulmonary atresia, 79 for tricuspid atresia and 120 for “single ventricle” whose details were not delineated in studies was found.11 Hypoplastic left heart syndrome (HLHS) has been noted to occur in 0.016–0.36% of all live births and in pathologic series, represents 1.4–3.8% of CHD12–14 Tricuspid atresia prevalence ranges from 0.3 to 0.7% of all patients with CHD and occurs in ~ 1 in 15,000 live births.15
One of the major problems with a unified imaging strategy of SVs as a group is the variable anatomy; for example: (A) D-loop vs L-loop, (B) right (RV) vs left ventricle (LV), or (C) anatomic true SV versus a “functional” SV. As can clearly be seen, there can be a seemingly hopeless number of complex combinations, however, the underlying theme is that only one usable ventricle is present or both ventricles are connected in such a way that separating them into 2 pumping chambers is impossible.
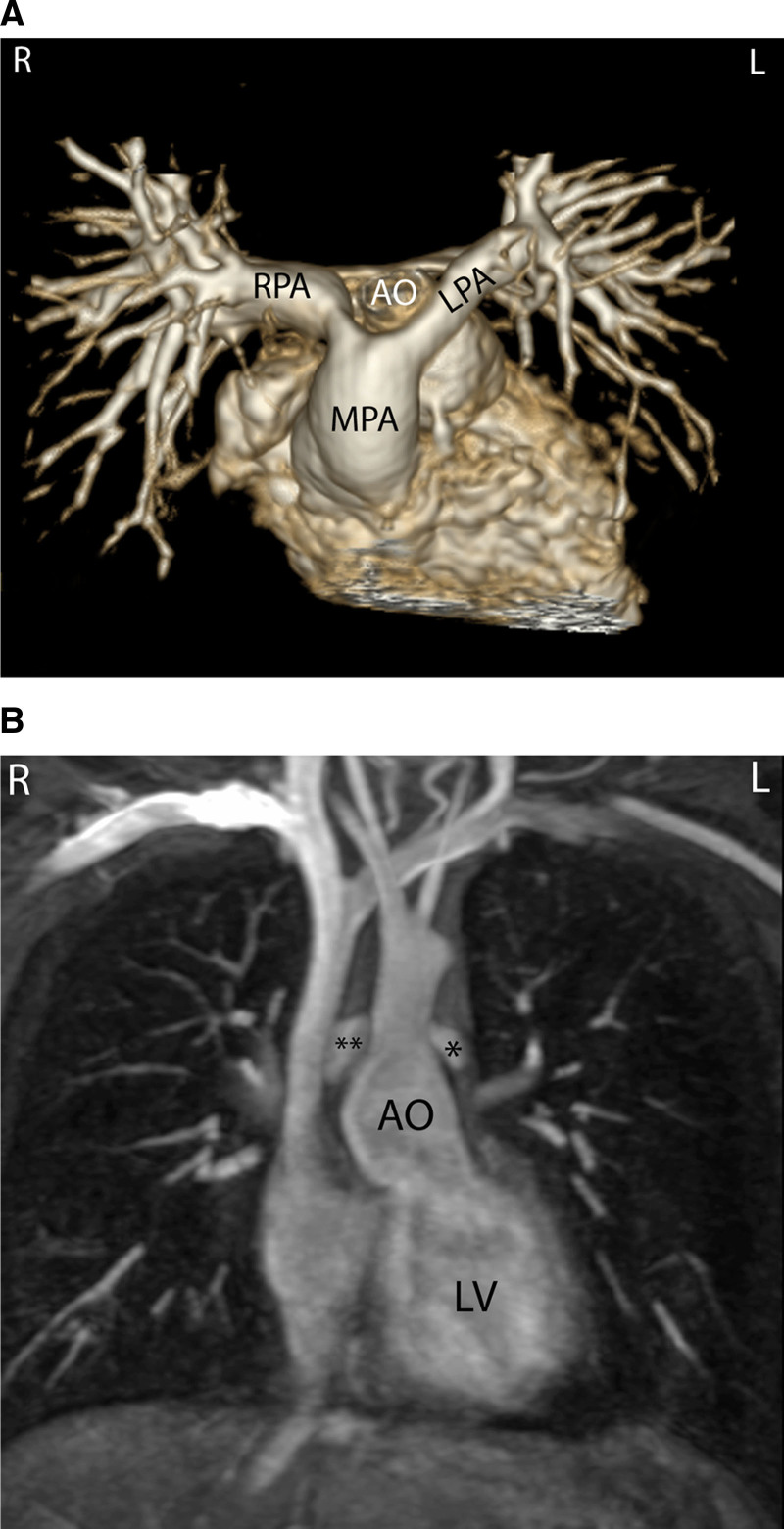
Another issue with a unified imaging strategy of SVs as a group is that during the various stages of surgical reconstruction, as noted above, the physiology of the cardiovascular system changes dramatically. The ultimate goal of surgery is to completely separate the systemic and pulmonary circulations and place them in a “series circuit.” In the native state, some patients, such as those with HLHS will always require surgical intervention—the Norwood Stage I procedure,16 which includes a systemic to pulmonary artery or ventricular to pulmonary artery (Sano)17–21 shunt (Figure 1), an atrial septectomy, and an aortic to pulmonary anastomosis. The SV pumps to both the systemic and pulmonary circulation in parallel, imposing a volume overload. Once pulmonary vascular resistance has dropped adequately (~ 3–6 months of age), a bidirectional superior cavopulmonary connection is performed. Since blood needs to go to the head/arms first before entering the pulmonary circulation, the ventricle does not pump directly to the pulmonary circulation and is therefore not technically volume loaded; it has been demonstrated, however, that systemic to pulmonary collaterals are present22 which can be quantified by CMR and puts a volume load on the ventricle.23 At approximately 2–5 years of age, directing inferior vena cava (IVC) blood into the lungs is performed to complete the Fontan operation.
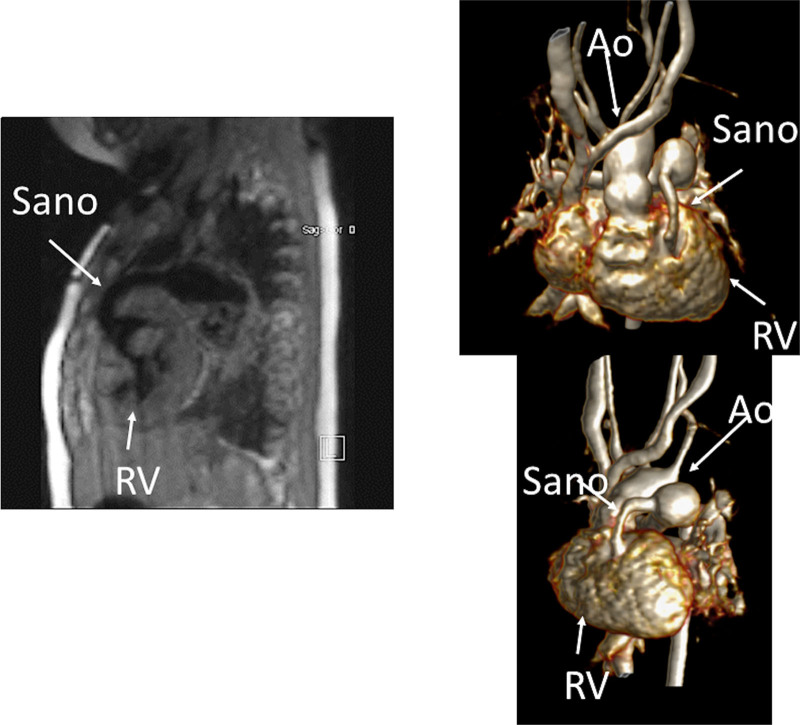
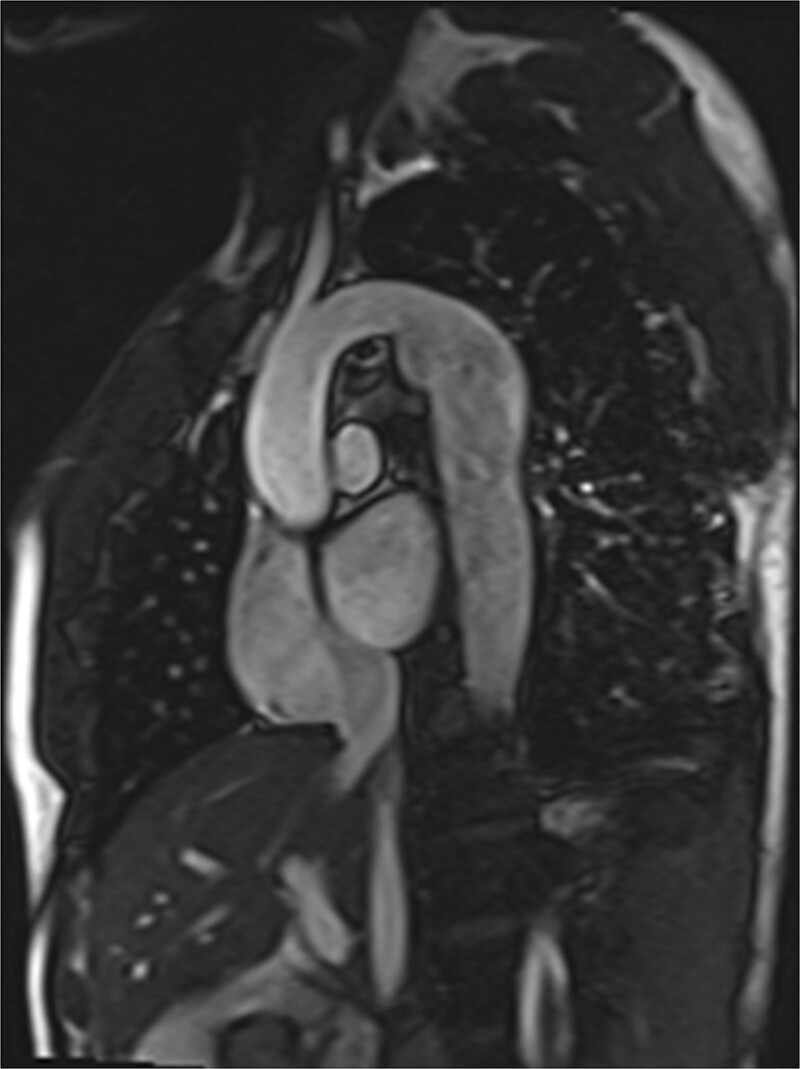
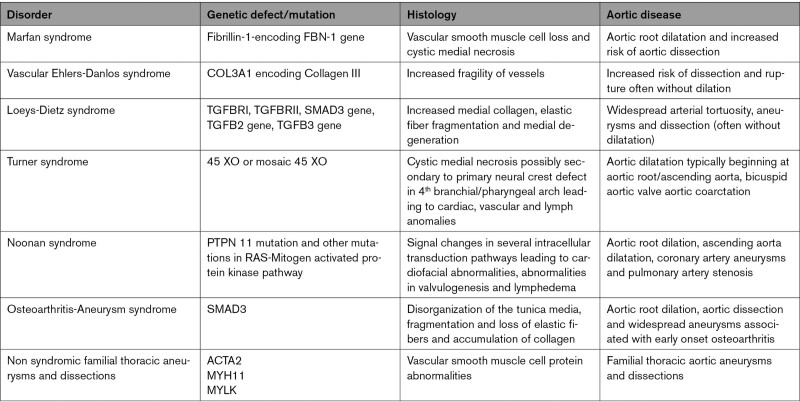
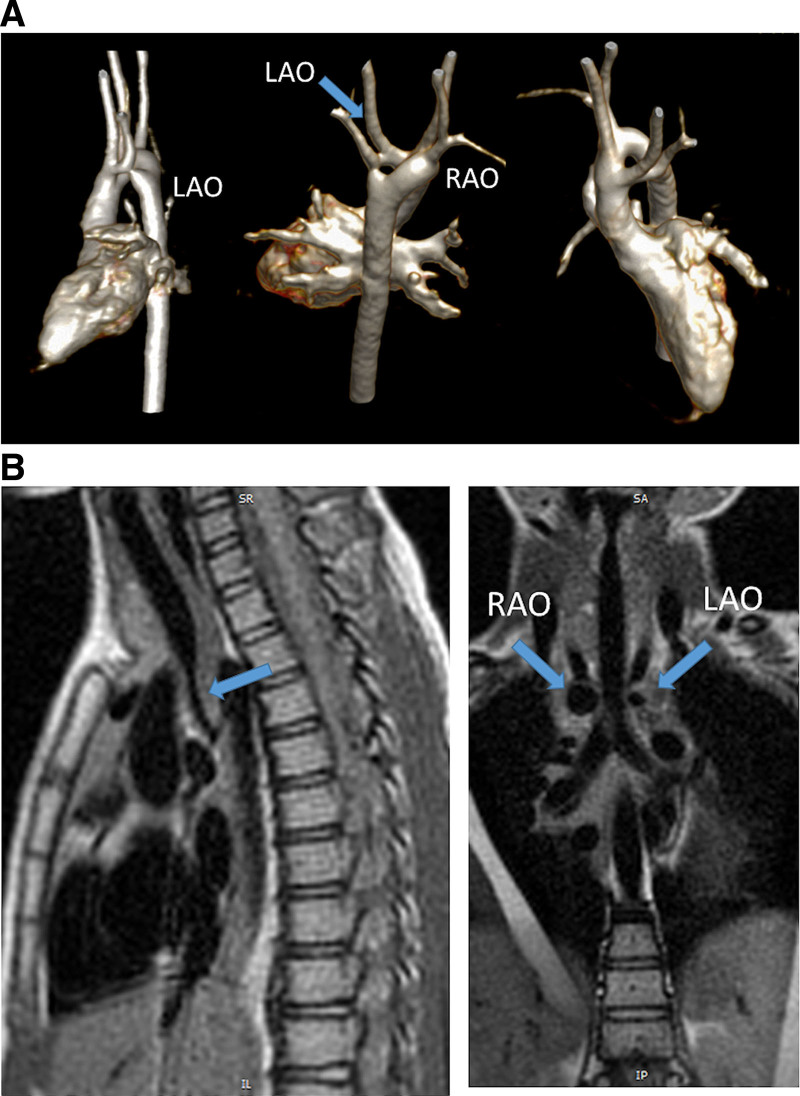
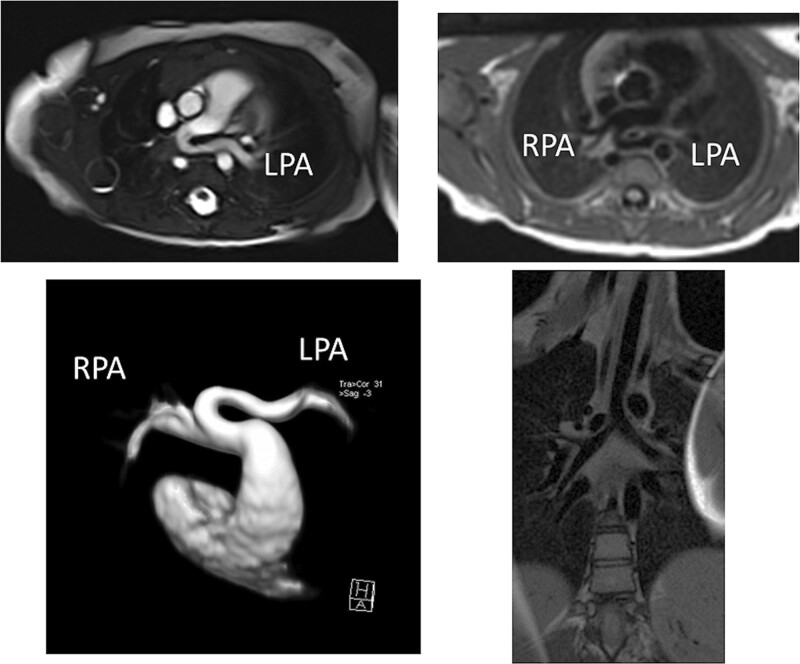
Figure 1.
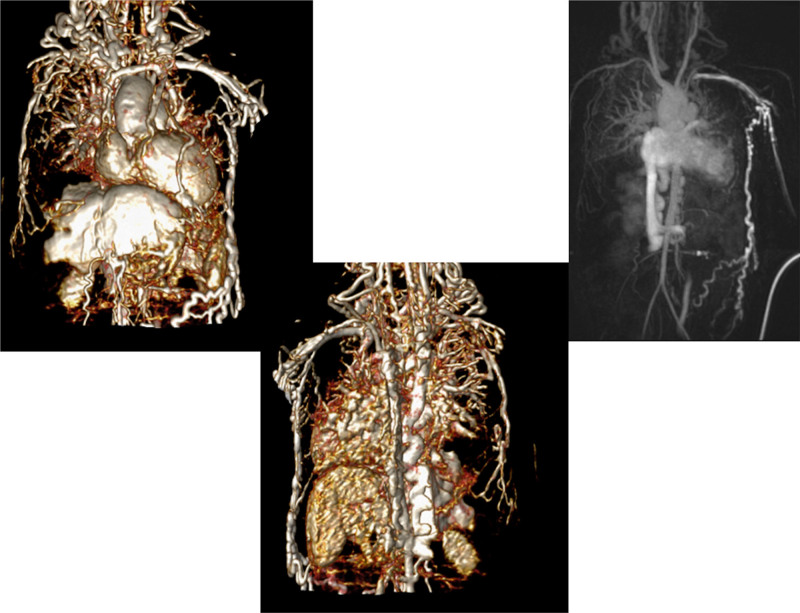
Hypoplastic left heart syndrome after Sano (right ventricle [RV] to pulmonary artery conduit). Left panel is a dark blood sagittal view and the right panels are 3D reconstructions demonstrating the entire length of the Sano shunt. Ao indicates aorta.
Finally, a third issue with a unified imaging strategy is that surgical reconstruction can vary greatly. To perform an aortic to pulmonary anastomosis, a Norwood or Damus-Kaye-Stansel procedure can be used. For a bidirectional superior cavopulmonary connection, a hemiFontan or bidirectional Glenn (BDG) can be performed. For a Fontan, a myriad of ways have been employed as modifications such as a lateral wall tunnel, an extracardiac conduit, or an atrio-pulmonary connection (not performed anymore), all with or without a fenestration.
Indication of CMR in SV
Prior to any surgery, CMR is not used frequently in the native state; generally, echocardiography is sufficient to allow for anatomic and hemodynamic characterization. Occasionally, if certain aspects of the anatomy are not delineated by echocardiography, such as pulmonary artery (PA) or pulmonary venous anatomy, CMR will be employed at this juncture (cardiac CT may be considered as an alternative if ventricle function, flow or tissue characterization information is not needed [ie anatomy alone], keeping in mind the radiation risk). In addition, if a “borderline” ventricle is present, CMR may be used to aid in the decision of a 1- versus 2-ventricle repair (Figure 2).
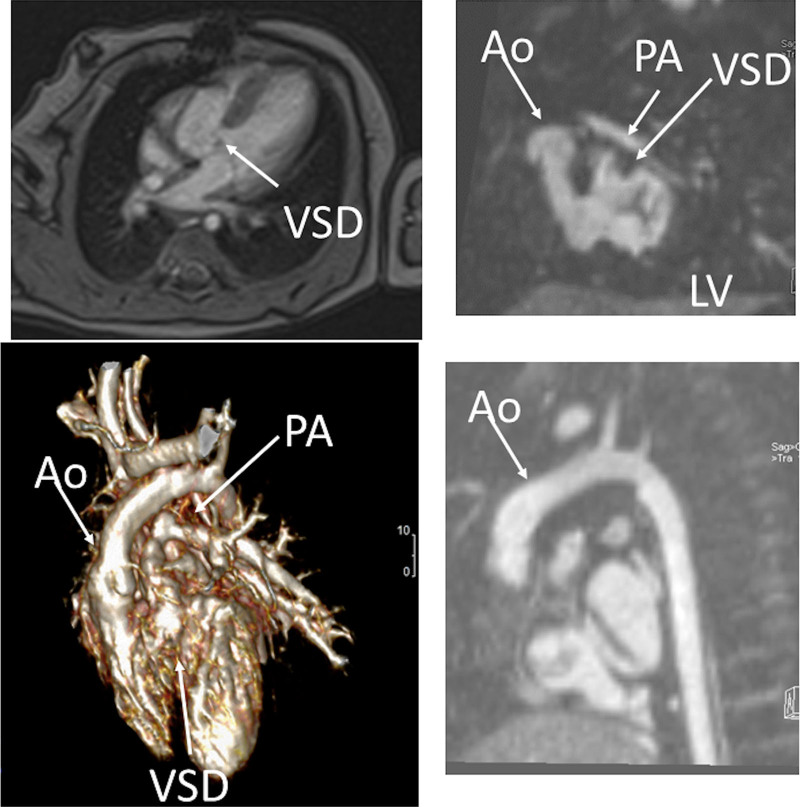
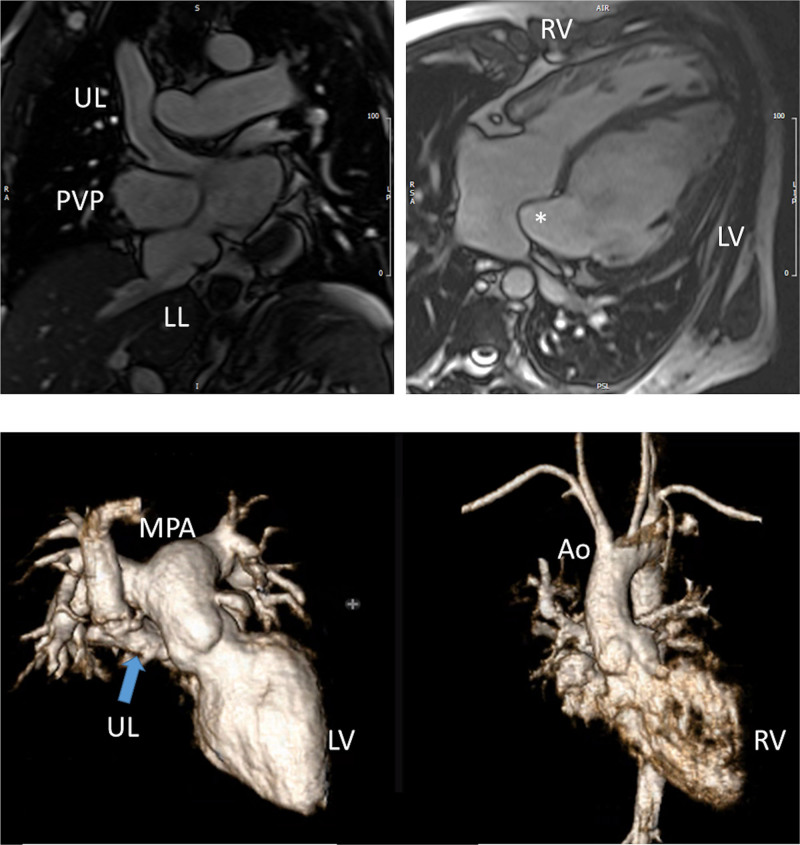
Figure 2.
Three month old with double outlet right ventricle (DORV) being considered for a 1 versus 2 ventricular repair.Upper panels are 2 orthogonal views of the left ventricle (LV) to aortic (Ao) pathway through the ventricular septal defect (VSD). Lower left panel is a 3D model demonstrating a “4-chamber” view and DORV while lower right panel shows the anterior Ao. PA indicates pulmonary artery.
At all surgical stages, echocardiography is universally employed and at younger ages, this may be adequate. However, in older individuals, echocardiography many not be sufficient because of poor acoustic windows. In addition, cardiac catheterization may be used at all stages for diagnosis, however, it is invasive, incurs radiation and is not feasible to be utilized for routine follow-up.
Anatomy
CMR has been used for many years to evaluate the anatomy of the SV patient and has been validated against catheterization and surgical observation.24–28 This is performed in both 2D, 3D and now 4D formats with or without contrast media (Figure 1). For all stages of surgical reconstruction, CMR should be utilized to assess patients whose echocardiogram has not definitively demonstrated the anatomy listed in Table 2 for surgical planning. CMR should be utilized in place of invasive angiography for this anatomy unless an intervention is planned. CMR has been utilized for many years, dating back to the late 1980’s and early 1990’s, to delineate native viscero-atrial situs, intracardiac anatomy29 and ventriculoarterial connections and is now considered standard of care. Generally, when performing each stage of surgery, however, echocardiography for anatomy is almost always supplemented by another imaging modality such as CMR27 or in some institutions, catheterization or cardiac CT (Figures 1 and 2).
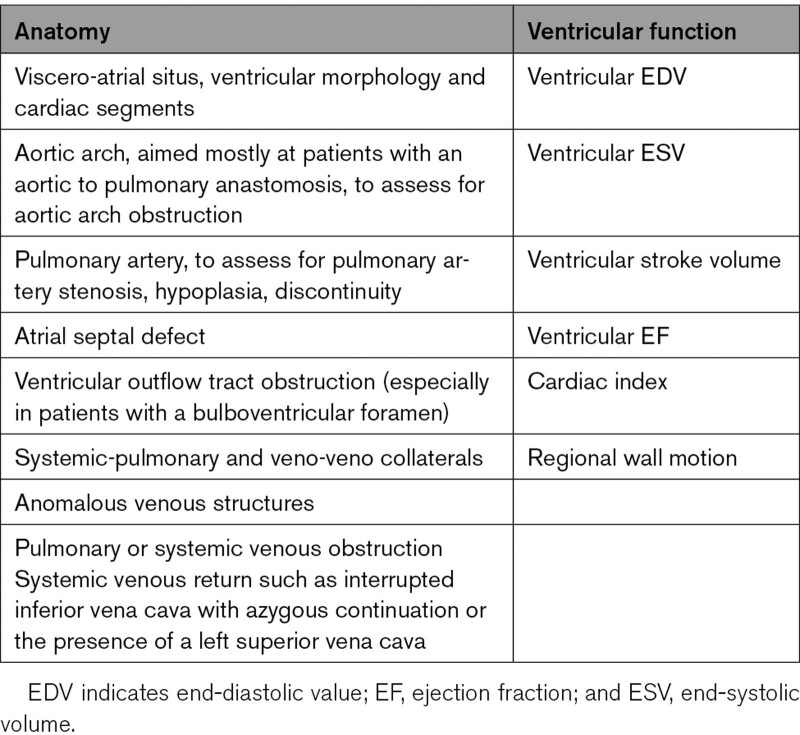
Table 2.
Anatomy and Ventricular Function Assessment in Single Ventricles
At each stage of surgical reconstruction, in addition, certain aspects are focused on. Prior to the BDG/hemiFontan stage, CMR is directed towards evaluation of the aortic arch to assess for coarctation and the aortic to pulmonary anastomosis (if present). Further, pulmonary blood flow is delineated by visualization of the systemic to pulmonary or Sano shunt (if present), pulmonary stenosis, the pulmonary arteries and aortic to pulmonary collaterals. At the BDG/hemiFontan stage, besides reassessment of the aortic arch, the superior cava connections (e.g., right or left superior venae cavae (SVC) or Kawashima connections to the PAs) are visualized along with the pulmonary arteries, aortic to pulmonary and veno-venous collaterals (Figure 3). Finally, after the Fontan, the entire systemic venous pathway, especially the IVC to PA connection is focused on, including the branch pulmonary arteries.
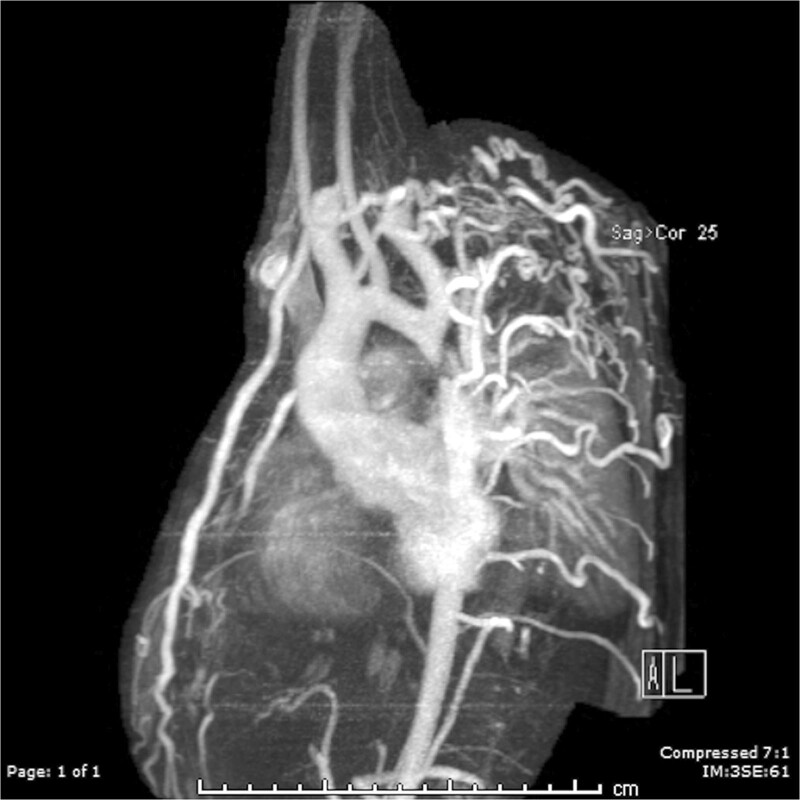
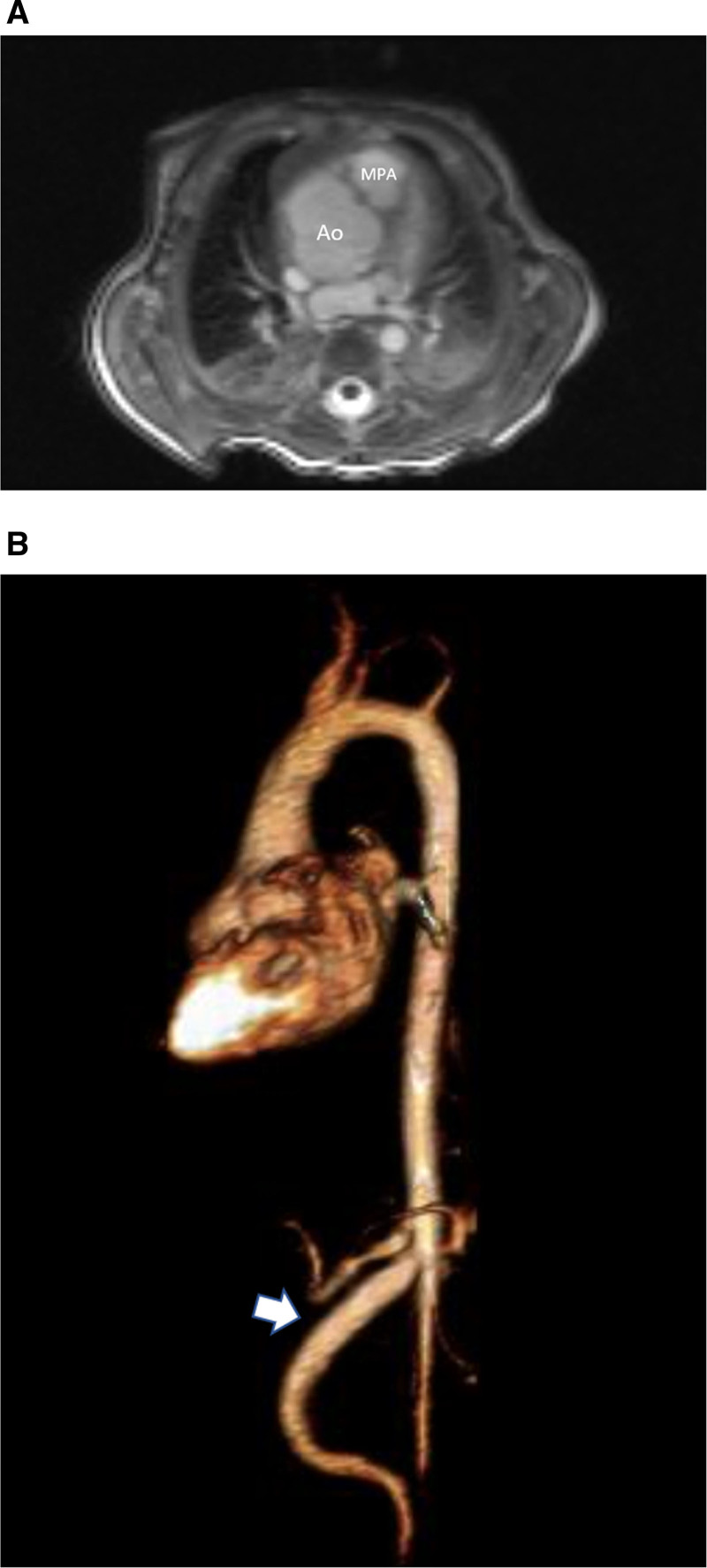
Figure 3.
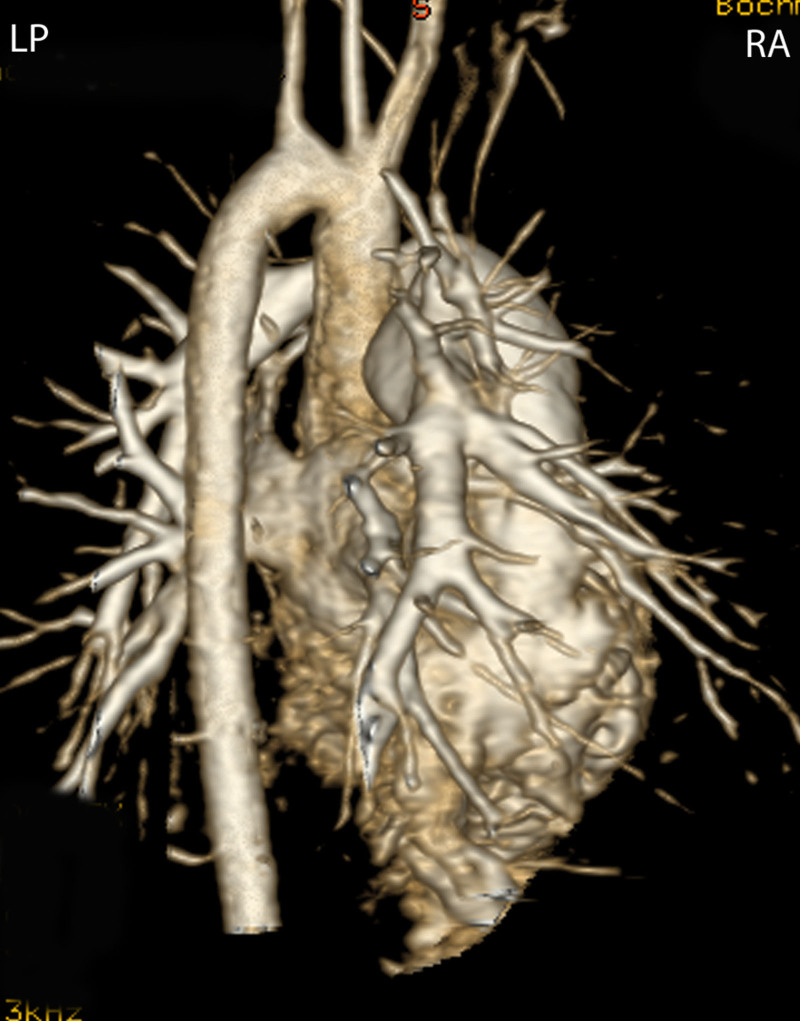
Massive systemic-to-pulmonary and venovenous collaterals in a 4 year old with pulmonary atresia and intact ventricular septum. Upper panels are maximum projection (right) and 3D reconstruction (left) of these collaterals viewed anteriorly while the lower panel is a 3D reconstruction of the collaterals as viewed from posterior.
Ventricular and Valve Function
CMR should be utilized to quantify 3D function which can be followed on a routine basis throughout all stages of surgical reconstruction and beyond. This includes regional wall motion abnormalities, ventricular volumes and mass, ejection fraction and cardiac index as delineated in Table 2. CMR has been the gold standard for biventricular volumes and function for many years and has been applied many times to the SV patient throughout staged surgical reconstruction (Figure 4).30–35 Ventricular performance parameters have been demonstrated to correlate with exercise performance34 and has been shown to correlate with transplant free survival after Fontan.36
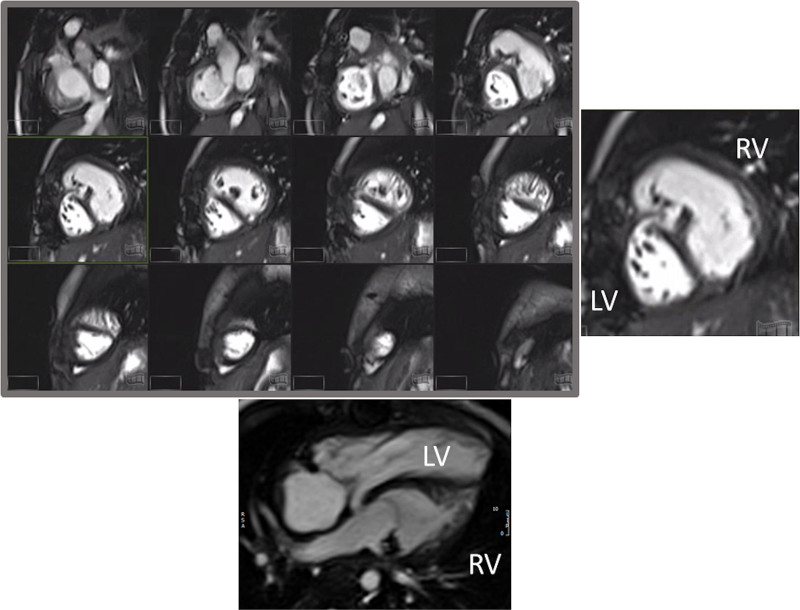
Figure 4.
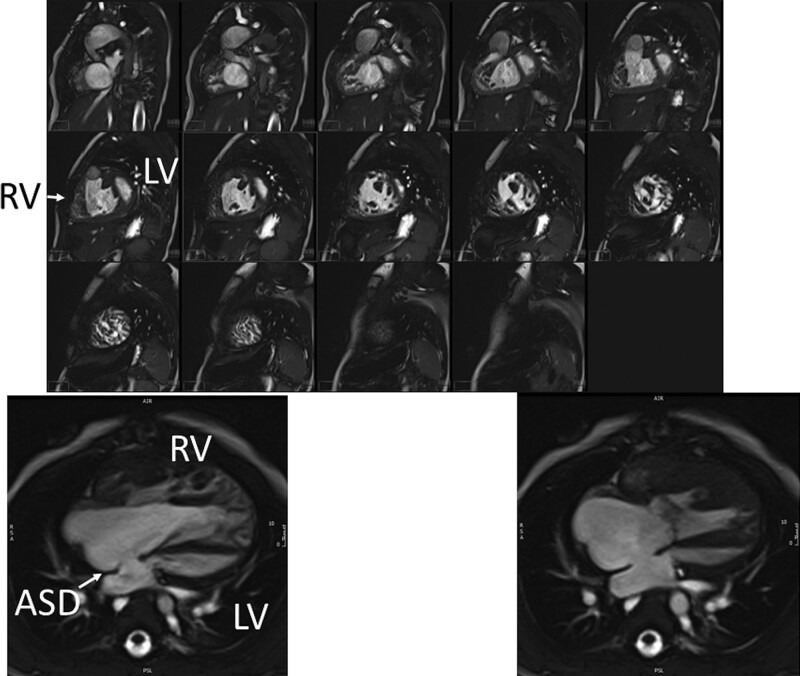
Ventricular function of an 18 month old with hypoplastic left heart syndrome. Upper panel is a short axis stack in diastole. Lower panels is a “3-chamber” view at end-diastole (left) and end-systole (right). ASD indicates atrial septal defect; LV, left ventricle; and RV, right ventricle.
Valve function, including atrioventricular and semilunar valve regurgitant volume and fractions, using phase contrast CMR (PC-CMR) or a combination of PC-CMR with ventricular volumes, should be assessed. PC-CMR has been used in the past to quantify valve function in CHD.37–39 Valve function is a significant issue in SV patients. For example, Mahle et al. has demonstrated that 6% of patients have moderate to severe atrioventricular valve regurgitation.40 Cohen et al. has shown that neoaortic regurgitation was present in 61% of patients up to 21 years of followup with progression in 49%.41
Physiology and Hemodynamics
PC-CMR has been used extensively in SV patients42,43 to assess physiology and hemodynamics. Important indices in the care of the SV patient are cardiac index as this is generally decreased, pulmonic flow (Qp)/systemic flow (Qs) which generally is close to one, flows to both lungs and systemic to pulmonary collateral flow23,44–48 which has been linked to short term outcomes such as hospital stay and presence of pleural effusions (see Qp/Qs and collateral flow section).49 In the BDG stage, cardiac catheterization cannot assess Qp because of systemic to pulmonary collaterals.43 Flows to both lungs are important parameters to determining the need for branch PA dilation, especially in SV patients where a patulous aortic reconstruction can compress the central PA. As mentioned in the forgoing paragraphs, PC-CMR is also used in the measurement of valve function.
Tissue Characterization For Myocardial Scarring
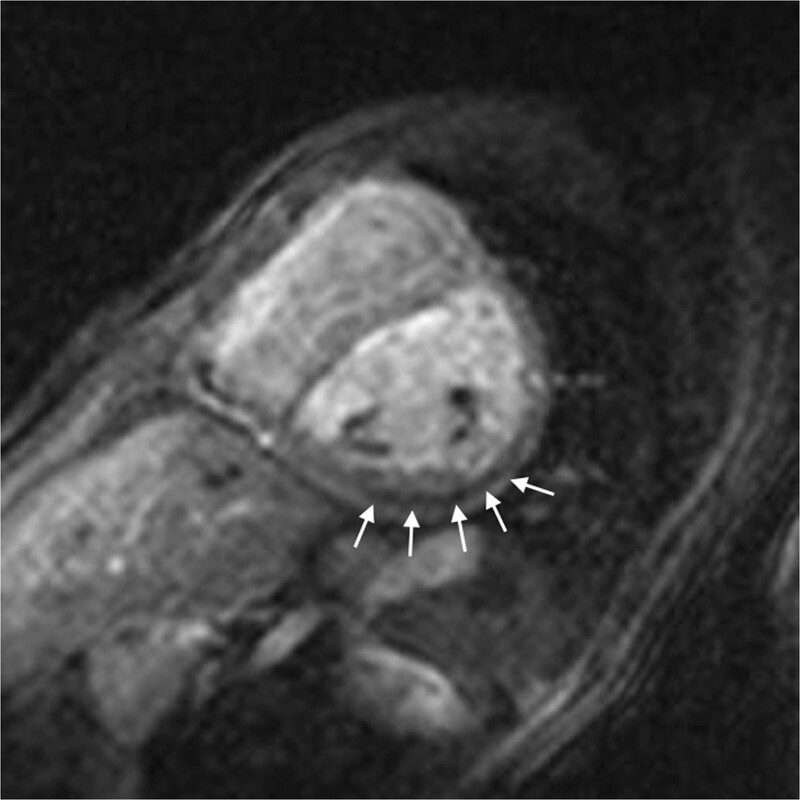
CMR has been utilized to evaluate both discrete myocardial scarring50 as well as diffuse fibrosis.51 Myocardial scarring may be an etiology for regional wall dysfunction as well as a nidus for arrhythmia. For example, diffuse fibrosis has negatively correlated with strain51 while discrete fibrosis has been linked to adverse ventricular mechanics and ventricular tachycardia.50 Myocardial scarring is commonly found around the os of the Sano shunt with accompanying regional wall motion abnormalities (Figure 5).
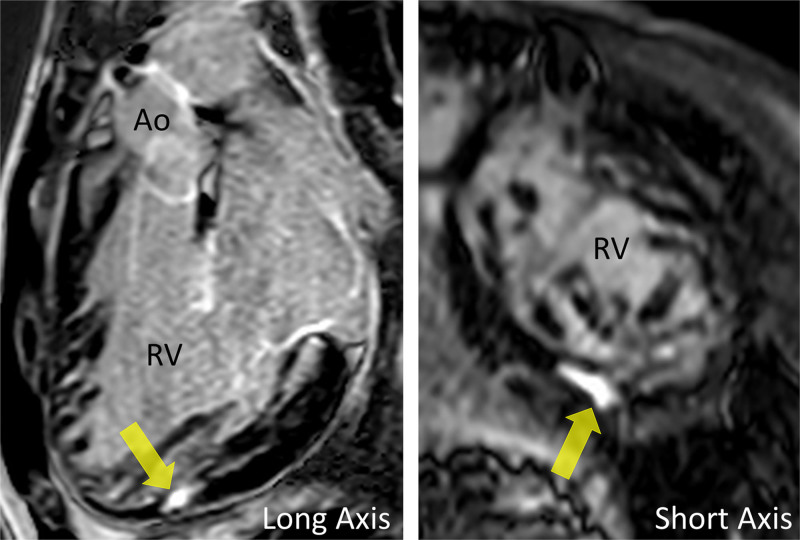
Figure 5.
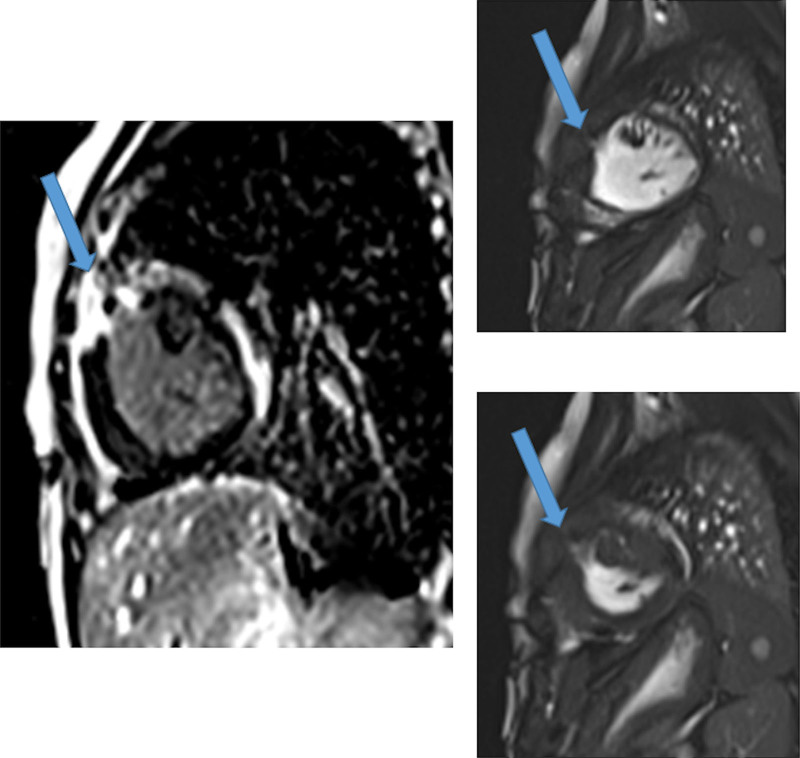
Myocardial scarring and regional wall motion abnormality in a 5 month old after a Sano shunt. Left panel is a phase sensitive viability image demonstrating the scar which is signal intense in the myocardium which should be signal poor (blue arrows). Right upper (diastole) and lower panels (systole) is the corresponding short axis view demonstrating the regional wall akinesia in the region of scar.
In part, because of the comprehensive assessment of anatomy, ventricular function, hemodynamics and tissue characterization that can be performed by CMR, a recent scientific statement from the AHA has recommended CMR be performed every 2–3 years after reaching the Fontan stage for evaluation.52
CMR Prior to BDG and Fontan Reconstructions
In the past, a pre-operative echocardiography and cardiac catheterization prior to BDG and Fontan was the standard of care. In the past 15 years, however, it has been demonstrated that a select groups of patients can undergo CMR and echocardiography alone to safely undergo surgery.
In a retrospective study prior to BDG,53 Brown et al. studied the utility of cardiac catheterization in 114 SV patients, 51 of which were without suspected issues requiring catheterization after non-invasive imaging but nevertheless underwent the procedure. Only two had unsuspected findings, both of which were branch PA stenosis that could’ve been diagnosed by CMR. Twenty-five percent had complications from catheterizations, most of which were transient with 24% requiring transfusions and 14% needing an intensive care unit stay.
In a follow-up prospective trial, Brown et al.54 randomized 81 routine SV patients prior to BDG to CMR or cardiac catheterization and assessed the outcome after surgery. The cardiac catheterization group had more minor adverse events (75% vs 5%, P < 0.001), higher cost ($34,447 vs $14,921) and longer preoperative stay (2 vs 1 day) relative to the CMR group. There was one major adverse event in the CMR group in a patient with a Blalock-Taussig shunt who developed shunt thrombosis and required cardiopulmonary resuscitation and extracorpeal membrane oxygenation (ECMO); 4 days later the patient underwent routine BDG and was in good clinical status at 3-month follow-up. The operative course, the number of successful BDG and the frequency of postoperative complications were similar. At 3-month follow-up, there was no differences in clinical status, oxygen saturation or frequency of reintervention.
Prior to Fontan, Ro et al.55 studied 99 SV patients retrospectively and listed a set of criteria to determine who might benefit from cardiac catheterization and who may be able to safely proceed to surgery without it. These criteria were clinical as well as echocardiographic based and 46 fell into the category of those who could forgo catheterization. The criteria identified all patients who died or did not proceed to Fontan as well as 9 of 11 who required intervention; it had a negative predictive value of 93% (those who can forgo catheterization) with a sensitivity of 81%. However, the positive predictive value was only 25% and the specificity only 52% and the authors thought that this may be partly due to the inability of echocardiography to adequately assess the branch PAs. They suggested the addition of CMR would substantially increase pre-operative predictive values.
Another study assessed 3 groups prior to Fontan27 (119 patients in total); all patients underwent echocardiography, however, 41 patients underwent CMR only, 41 patients underwent catheterization only and 37 patients underwent both catheterization and CMR. No clinically significant differences were noted in patient characteristics, hemodynamics or clinical status prior to or after surgery between the CMR only and the catheterization only groups with CMR adding information in 82% of patients. Parameters such as cardiopulmonary bypass time, circulatory arrest time, days in the intensive care unit, other surgical procedures, surgical complications, interventions after Fontan, the incidence of pleural effusions, length of stay in the hospital and oxygen saturation at discharge were similar in all 3 groups. Diagnostic success at surgery relative to all imaging modalities was ≥ 95%. In the group that had both CMR and catheterization, measurements of blood vessels were similar and there were no discrepant findings. Echocardiography could not delineate completely the pulmonary arterial anatomy in 46–53% of patients.
Summary of Recommendations
Preoperatively or prior to commitment to either a univentricular or biventricular circulation, CMR is reasonable to determine anatomy, physiology and ventricular function not elucidated by echocardiography or to aid in determining one vs. two ventricle repair (Class IIa, Level of evidence B).
Prior to BDG, if there is no primary indication for an intervention or there is no indication of increased pressures or pulmonary vascular resistance by echocardiography, CMR is indicated to determine anatomy, physiology, hemodynamics and ventricular function for use in surgical planning in routine cases (Class I, Level of evidence B). See Table 2
Prior to Fontan, if there is no primary indication for an intervention or there is no indication of increased pressures (e.g., end-diastolic or Fontan pressures) or pulmonary vascular resistance by echocardiography, CMR is indicated for use in surgical planning in routine cases (Class I, Level of evidence B) (See Table 2).
After Fontan, CMR is beneficial to follow asymptomatic patients routinely (Class I, Level of evidence B) every 2–3 years, especially when they reach the teenage years and is indicated in the symptomatic patient if there is no primary indication for an intervention or there is no indication of increased pressures (eg end-diastolic or Fontan pressures) or pulmonary vascular resistance by echocardiography
Prior to surgery or at any stage of surgical reconstruction, CMR can be useful to evaluate anatomy and ventricular function including volumes and mass and valve function (Class I, Level of evidence B). Tissue characterization such as late gadolinium enhancement (LGE) may be useful in prognostication (Class I, Level of Evidence B)
Prior to surgery or at any stage of surgical reconstruction, CMR can be useful to evaluate hemodynamics such as flows, cardiac index, Qp/Qs, flows to both lungs, fenestration flow (if Fontan) and systemic to pulmonary collateral flow (Class I, Level of evidence B).
Tetralogy of Fallot
Background
Tetralogy of Fallot (TOF)56 is the most common cyanotic CHD and has a prevalence of ~ 6% of all CHDs57 and an average incidence of 32.6 per 100,000 live births11 (~ 1660 babies born each year with TOF in the United States58). The main pathologic basis is antero-cephalad deviation of the developing conal septum which causes a malalignement type ventricular septal defect (VSD), resulting in an “overriding aorta’’ and right ventricular (RV) outflow tract (RVOT) obstruction, ultimately leading to RV hypertrophy. Repair typically consists of VSD closure and relief of RVOT obstruction, typically by placement of a transannular patch, which in most instances results in severe pulmonary regurgitation (PR) from disruption of pulmonary valve integrity; RV volume overload typically ensues.59 Another commonly used approach is placement of an RV to pulmonary artery conduit instead of a transannular patch which may also result in PR and RV volume overload. Definitive repair is generally performed in infancy with survival rates of > 98% in multiple series.60–65 Because of the high success rate in childhood, the number of repaired TOF patients has been increasing over the years with adult survivors of TOF repair now outnumbering children in a number of regions.66 The 30 year survival rate is > 90%67,68
Despite these successes, complications related to residual anatomic and hemodynamic abnormalities are nearly universal. In the vast majority of patients, as mentioned, relief of the RVOT obstruction leads to PR and RV volume overload with resultant reduced RV and LV performance and are at risk for poor clinical outcomes. Multiple studies that have investigated resting RV and LV function after TOF repair69–74 consistently found diminished RV and LV performance with decreased RV ejection fraction (RVEF) and LV ejection fraction (LVEF), mostly in patients with PR. Patients with RV volume overload are at risk for sudden death, ventricular arrhythmias, increased New York Heart Association (NYHA) class and decreased exercise performance.
Exercise capacity is significantly decreased in TOF survivors and deserves special attention.75–77 This exercise incompetence may result from either primary LV dysfunction or by “ventricular-ventricular” interaction, where the dilated RV impinges on LV geometry causing poor performance.78–88 When TOF patients were studied at rest and during exercise testing, the incremental exercise response of LVEF in TOF patients was depressed relative to controls and LVEF during exercise correlated with both RV end diastolic volume index (RVEDVI) and the severity of PR.77 When comparing exercise performance in TOF patients and controls, significant differences exist in peak workload, maximal heart rate and systolic blood pressure.76 A review of 22 exercise studies89 found that 14 showed a significant relationship between PR with abnormal RV function and decreased exercise capacity. Further implicating RV volume overload are studies that demonstrate once the RV volume overload is abolished by pulmonary valve replacement (PVR), exercise tolerance improved.87,88
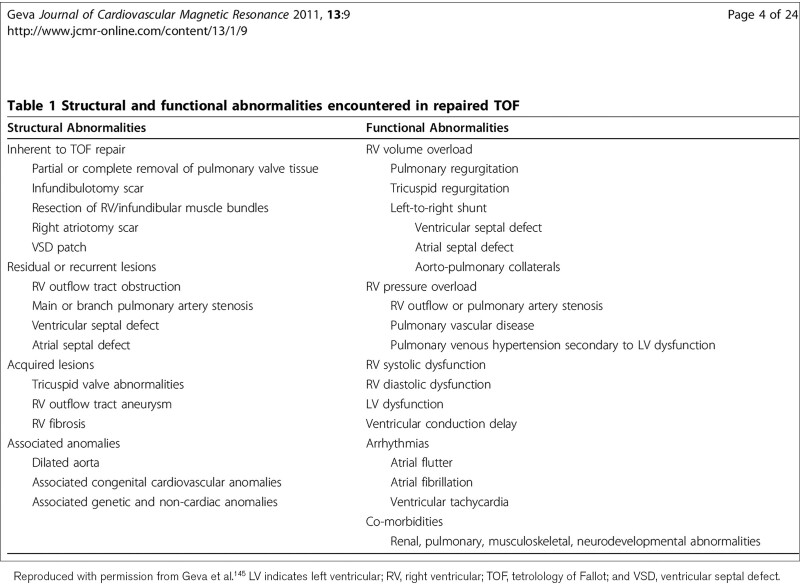
Numerous other residua can be present. Residual or recurrent RVOT obstruction or pulmonary stenosis may be present at any age and commonly occur in the first several years after the initial repair; RV to PA conduits commonly need to be upsized as the patient grows and later on may become calcified and stenotic. Scar tissue from surgical relief of the infundibulotomy as well as the use of a patch to enlarge the RVOT results in non-contractile myocardium which may progress to aneurysm formation. Residual atrial septal defects (ASD) or VSD, branch PA stenosis, tricuspid regurgitation as well as aortic dilation and aortic valve regurgitation may all occur. Arrhythmia and conduction disturbances are commonly encountered.90 A recent study suggests that TOF survivors have a higher degree of RV and LV diffuse fibrosis compared to normal, raising the possibility of an etiology for conduction disturbances or decreased exercise performance91,92; the degree and time course of this fibrosis has yet to be defined. Table 3 lists complications commonly seen in TOF.
Table 3.
Complications of Repaired Tetralogy of Fallot
Indication and the Role of CMR in TOF
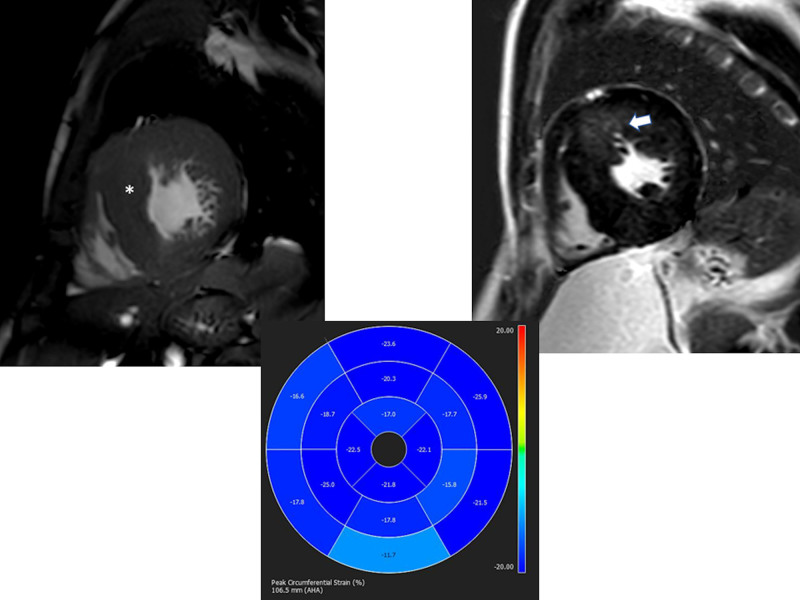
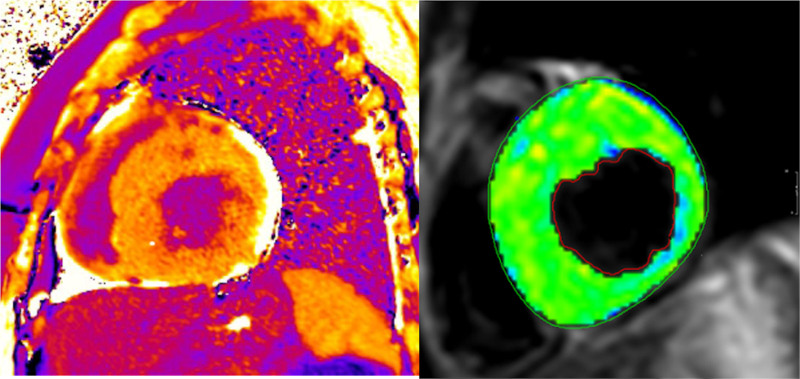
CMR has been utilized for years to assess anatomy (Figure 6), ventricular function including ventricular volumes (Figure 7), blood flow (Figure 8) and myocardial tissue characterization (Figure 9) in TOF survivors91,92,95,96,98 Multiple CMR techniques have been utilized for anatomical assessment of the RVOT, branch pulmonary arteries (PAs) (Figure 6) and aorta including electrocardiographically (ECG) gated balanced steady state free precession (bSSFP), unbalanced gradient echo imaging, dark blood imaging (which is much less susceptible to metal artifact) and contrast enhanced imaging to create 3D image sets. CMR is the gold standard for reliably and accurately measuring 3D ventricular volumes and performance generally utilizing bSSFP cine imaging and is the imaging modality of choice (Figure 7). PC-CMR93 is employed to measure flow and velocity, focused on PR (Figure 8), flow to both lungs, cardiac index, Qp/Qs, tricuspid regurgitation (alone or in combination with cine imaging) and aortic to pulmonary collateral flow. Parametric native T1 mapping94 can determine diffuse fibrosis and recent studies in children with repaired TOF have demonstrated extracellular volume (ECV) expansion91,92; in adult, TOF survivors showed a higher rate of adverse clinical events in TOF patients with ECV ≥ 30% than those with < 30% (Figure 9).95 Finally, myocardial strain by CMR using feature and tissue tracking allows for strain measurements with standard cine96 and has recently demonstrated to be prognostic in adult TOF survivors.96 Normal values for pediatric strain has recently been published.97
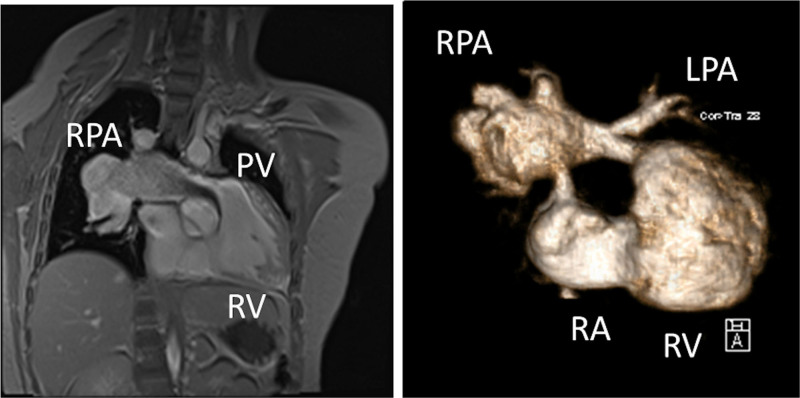
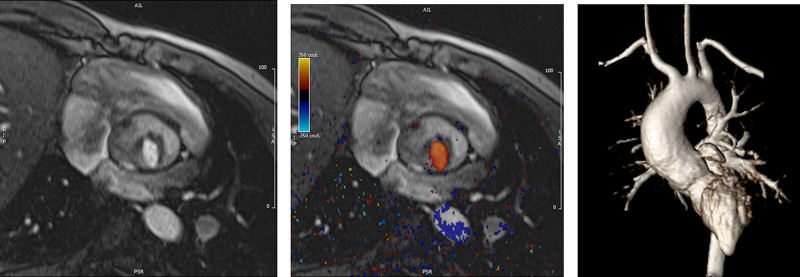
Figure 6.
Severe right pulmonary artery (RPA) aneurysm in a 14 year old patient with tetralogy of Fallot and pulmonic stenosis. Left panel is an unbalanced gradient echo cine image and the right panel is a 3D reconstruction; note the turbulence in the main pulmonary artery from the stenotic pulmonary valve (PV). LPA indicates left pulmonary artery; RA, right atrium; and RV, right ventricle.
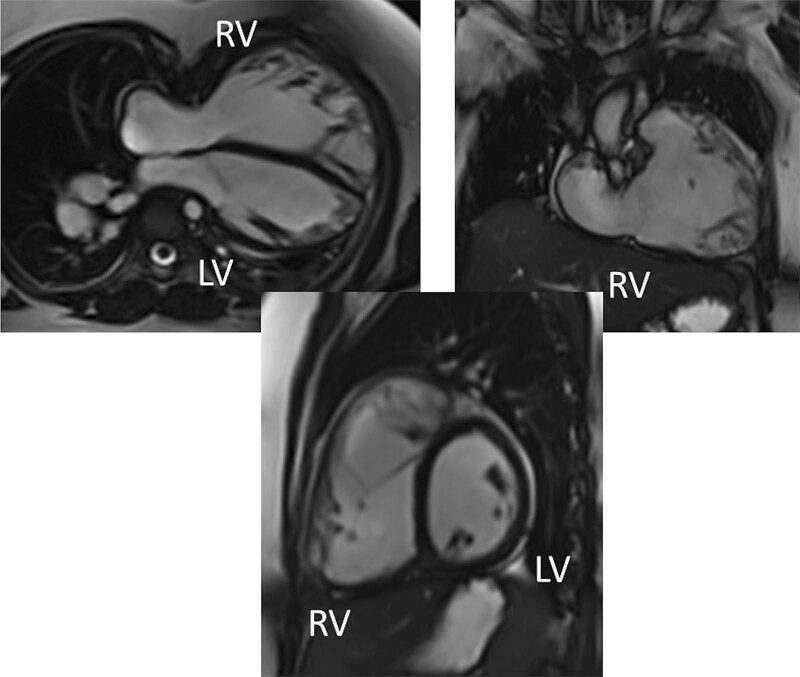
Figure 7.
Ventricular function and volumes in tetralogy of Fallot. The 4-chamber (upper left), right ventricle (RV) two chamber (upper right) and short axis (lower panel) views of the patient in Figure 6 with volume overload of the RV. LV indicates left ventricle.
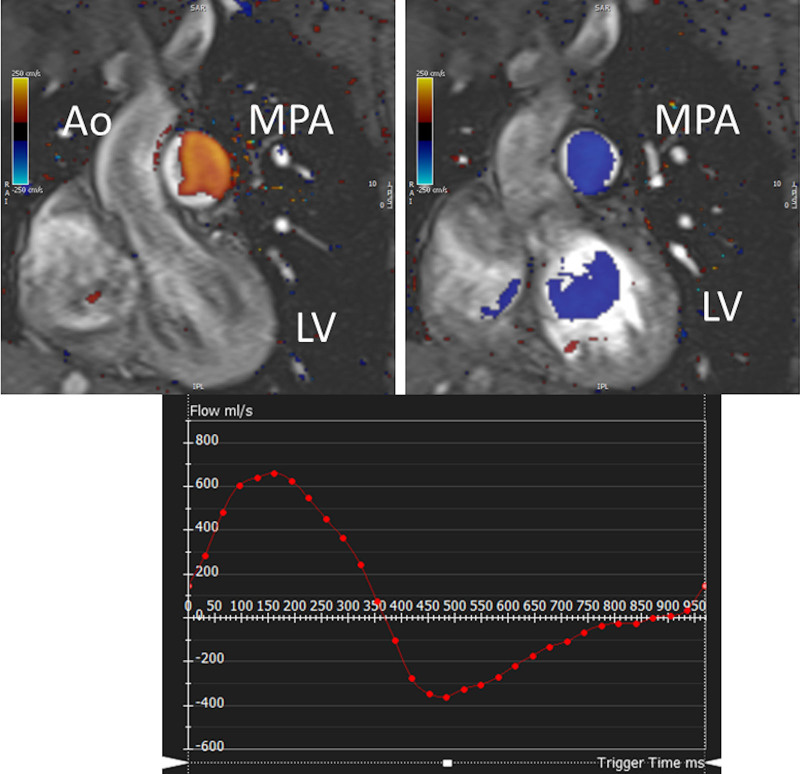
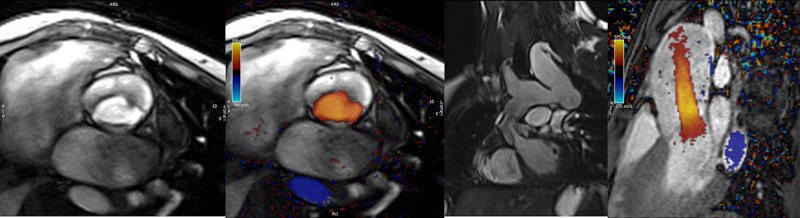
Figure 8.
Color coded through plane PC-CMR of the main pulmonary artery (MPA) in systole (upper left, orange) and diastole (upper right, blue) demonstrating antegrade (orange) and retrograde flow (blue) signifying severe pulmonary regurgitation (PR).Lower panel is a flow (Y-axis) time (X-axis) curve demonstrating PR and antegrade end diastolic flow (after 900 mseconds). Ao indicates aorta; LV, left ventricle; ml/s, milliliters/second; and ms, milliseconds.
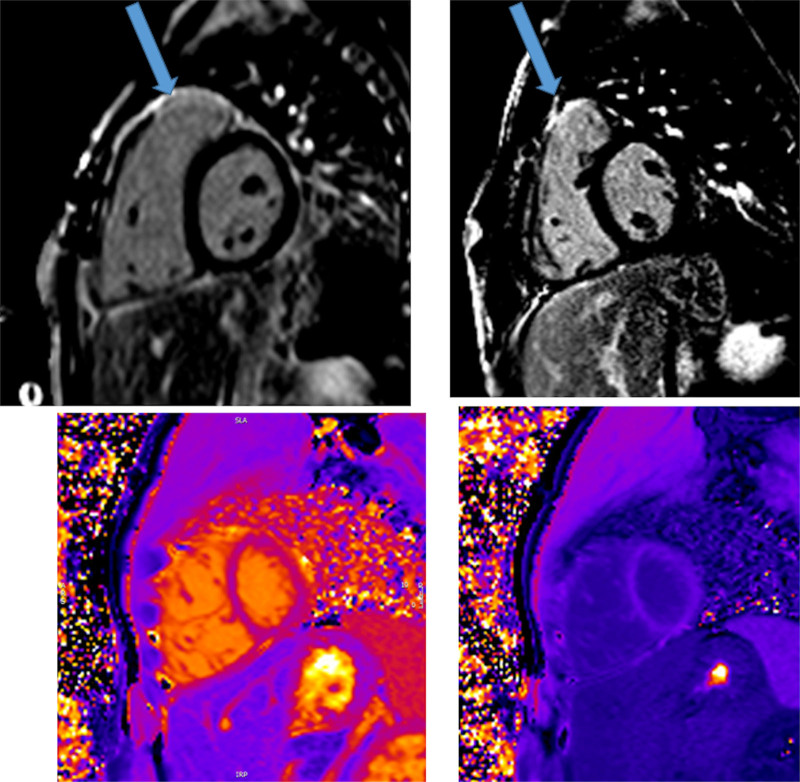
Figure 9.
Discrete (upper panels, arrows) and diffuse fibrosis (lower panels) in a patient with tetralogy of Fallot. Two separate patients are demonstrated in the upper panels showing the areas of the transannular patch. Utilizing T1 mapping before (lower left) and after (lower right) gadolinium administration, extracellular volume can be quantified.
Prior to surgery in young children, echocardiography is primarily utilized for the management and care of the patient with TOF and CMR is generally not routinely indicated. There are a few exceptions such as:
Lack of visualization of various structures such as the branch PAs by echocardiography
aortic arch anomalies
discontinuous branch PAs
aorto-pulmonary collaterals (Figure 3)
complex TOF or situs anomalies
inconsistent clinical data that may indicate the need for an intervention other than routine repair.
After surgical repair, numerous sequelae can be present and CMR is indicated to assess nearly all of them:
PR (Figure 8) PR is a major issue and CMR is the only technique that allows for accurate quantification of not only of regurgitant volumes but regurgitant fraction as well (using PC-CMR)98,99 with echocardiography only having a modest correlation with CMR.37 It has been utilized since the early to mid 90 s for this evaluation98,100 and has been demonstrated to positively correlate with RV end-diastolic volume (RVEDV).101,102 In the absence of residual intracardiac shunts and other valve insufficiency, the difference in ventricular stroke volumes would equal the PR volume by PC-CMR.
RV (Figure 7) Cine CMR is the gold standard in determining quantitative biventricular size and mass and has been so for many decades.32,103–106 PR results in RV dilation with decreased function, risking morbidity and mortality,107 and the effects of RV dilation on LV function108 are important to follow by CMR. In a large cohort of patients spanning the gamut of ages, RV hypertrophy relative to RV volume was predictive of death and ventricular tachycardia.109 RVEF has been associated with impaired exercise performance.110 Typical values for RV dilation and hypertrophy in TOF have been published by many groups.102,111–113
It has been known for a number of years that intrinsic regional RV wall function is decreased in TOF survivors using CMR.114 Relatively recently, both RV and LV strain from routine cine CMR has been performed using either CMR feature tracking or tissue tracking of the myocardium. Both RV global longitudinal strain (GLS) and LV global circumferential strain (GCS) by CMR have emerged as predictors of poor outcome across a wide gamut of age ranges including pediatric and adolescents and may be useful in prognostication.96
Fibrosis has been noted by CMR in TOF survivors and has clinical implications. LGE or discrete fibrosis, has been utilized to assess viability of the myocardium for many years115 and in the TOF population, has been found to be present in both the RV and the LV. This increased signal intensity also occurs at the site of patch material such as the VSD and the transannular patch (Figure 9).116 Patients with poor ventricular performance, exercise intolerance and arrhythmias have demonstrated increased amounts of LGE throughout all age ranges117,118 and LGE in children positively correlates with increasing RVEDV and PR.119 RV diffuse fibrosis using T1 mapping has also been shown to be increased in TOF survivors in children,91 however, the significance is unknown at this time.
A published recommendation from the American Society of Echocardiography, developed in collaboration with SCMR and the Society for Pediatric Radiology recommends yearly CMRs based on RV performance parameters (eg RVEDVI ≥ 150 cc/m2, RVEF ≤ 48%) and every 3 years if the RV does not fall into these ranges for anyone 10 years of age or older; for those younger, it is ordered to address specific questions not addressed by echocardiography.120
Left Ventricle As mentioned above, numerous studies have documented LV dysfunction in repaired TOF patients for a few reasons and therefore, CMR evaluation of the LV takes on a key position in evaluation. CMR has demonstrated that this dysfunction is directly related to adverse outcomes such as ventricular tachycardia and death across all age ranges.121 LVEF has been associated with impaired exercise performance110 and as mentioned above, LV GCS has correlated with poor outcome.96 In addition, a small study has shown that LV diffuse fibrosis in children is associated not only with biventricular enlargement but is also associated with poor exercise performance122 and impaired LV mechanics123; long term clinical outcomes have yet to be elucidated.
Anatomy Important elements to image by CMR are residual lesions of the RVOT (e.g., RVOT aneurysm, the presence of an RV muscle bundle and RVOT and annular obstruction (Figure 7)), the branch PAs and surgical reconstructions such as RV to PA conduits.124 CMR in many instances is able to visualize these structures with higher fidelity than echocardiography, especially in the older child and adolescent. Although echocardiography is generally utilized to estimate the RV systolic pressure by measuring the peak tricuspid regurgitation (TR) velocity and the pressure drop across the RVOT and annulus by assessing the peak velocity by Doppler, in-plane PC-CMR may be utilized for this, although uncommon.
Since the mid to late 90’s CMR has been known to be a highly sensitive technique to assess the branch PAs in TOF.125 It has been validated against X-ray angiography126 and is superior to echocardiography.127 Branch PA stenosis or dilation (such as in TOF with absent pulmonary valve leaflets) should be noted. Physiologically, using PC-CMR, differential PA blood flow is obtained by CMR and has shown to be accurate128–130 even in the presence of stents,131 and may be used as a component in the decision making process to determine the need for intervention on the branch PAs.
Left Sided Structures Aortic root and ascending aortic dilation are known phenomenon seen in TOF and not only can significantly dilate in a high proportion of patients in the late teens and adulthood132 but also may cause considerable pathology.133 In addition, right aortic arches occur in ~25% of TOF along with branching abnormalities and the occasional vascular ring. These structures are routinely and easily imaged by CMR with and without contrast. Aortic regurgitation (AR), associated with aortic root and ascending aortic dilation, occurs in TOF134 and should be quantified by CMR38 using PC-CMR.
Residual Shunting Residual ASD and VSD flow can be present after surgical repair and can be diagnosed by echocardiography. CMR has utility not only visualizing these structures when inadequate echocardiography windows are present, but the strength of the modality is to quantify net shunting via PC-CMR with internal checks (see Qp/Qs section). In addition, in TOF patients with pulmonary atresia and multiple aortic to pulmonary collaterals, CMR again can visualize and quantify the shunt which has been performed since the 1990s.135,136
Other Considerations TR occurs not uncommonly in TOF and is also generally seen by echocardiography. CMR can quantify atrioventricular valve regurgitation in 2 separate ways for internal consistency and accuracy. Spatial relationships of the cardiovascular system and the airways can be important such as in TOF with absent pulmonary valve leaflets along with the relationship of the sternum in case of reoperation and CMR is useful in defining this anatomy. Coronary artery anatomy, for years a staple of CMR, can be defined as well in case of stenting the RVOT and main PA (see Coronary Artery section).
It should be noted that in certain circumstances, where the necessary airway or coronary anatomy cannot be obtained by CMR, or if visualization within a stent is needed for delineation of size, cardiac CT may be considered as an alternative.
Pulmonary Valve Replacement PVR deserves special attention in that it eliminates PR, decreases RV volume overload and improves symptoms including TR and exercise intolerance87,88,137,138 but the threshold ventricular volumes above which a PVR should be performed is unknown.139–144 Indexed end-diastolic volumes have ranged in various studies from 140 to 180 cc/m2. Other parameters to consider for PVR include large RVOT aneurysms, RVOT obstruction, sustained tachyarrhythmias related to RV volume overload, left to right shunt with a Qp/Qs > 1.5, severe AR or dilation.145 CMR has played a major role in attempting to determine the optimal timing of PVR and is indicated for baseline and follow-up evaluation of the TOF patient for PVR.
Summary of Recommendations
Prior to definitive TOF surgery, CMR can be useful to delineate various anatomic structures when there is a lack of visualization by echocardiography. In addition, it can be beneficial to delineate, aortic arch anomalies, discontinuous branch PAs, aorto-pulmonary collaterals and complex TOF anatomy or situs anomalies as an adjunct to echocardiography (Class IIA, level of evidence C).
After definitive TOF repair, CMR is reasonable to delineate anatomy, physiology, blood flow, ventricular function and tissue characterization. In specific, assessing biventricular performance (ventricular volumes, ejection fraction, cardiac index), valve function (PR, TR, AR) and flows to both lungs are crucial to quantify (Class I, level of evidence B). RVOT, branch PA and aortic root/aortic anatomy are important to evaluate and measure (Class I, level of evidence B). Discrete myocardial scarring is important to identify (Class I, level of evidence B).
CMR is indicated to evaluate RV volumes as a baseline, every 2–3 years if not dilated and ≥ 10 years of age or yearly if dilated and in the range to be considered for PVR (Class I, level of evidence B).
Annual CMR is useful when surgery is being considered to evaluate RVOT aneurysms or obstruction, sustained tachyarrhythmias related to RV volume overload, left to right shunt with a Qp/Qs > 1.5, severe AR or dilation if being considered for PVR (Class IIA, level of evidence B).
If the child requires sedation or anesthesia for CMR, this modality is reasonable to delineate anatomy, physiology, blood flow, ventricular function and tissue characterization when echocardiography suggests pathology or cannot visualize structures (Class IIA, level of evidence B). This can be performed as a baseline in childhood and prior to reaching the teenage years (Class IIB, level of evidence C).
Myocardial strain (Class IIA, level of evidence B) and diffuse fibrosis (Class IIB, level of evidence C) by CMR might be considered for prognostication.
Transposition of the Great Arteries
Background
Transposition of the great arteries (TGA) is anatomically defined as a ventriculo-arterial discordance and is the second most frequent cyanotic CHD with a prevalence of 0.2–0.3 / 1000 livebirths with a male predominance of 1.5–3:1,13 accounting for 5–7% of all CHD.146 This section will focus on TGA with D-looped ventricles with repair using the arterial switch operation (ASO);L-looped TGA and repair with an atrial inversion operation is in the section on systemic RVs. The ASO is nowadays the surgical technique of choice for repair of TGA147 consisting of (1) transecting the aorta and the main PA at the level of the sinotubular junction, (2) removing the coronary ostia from the original aortic root and transferring them with a piece of surrounding tissue (button) to the neo aortic (pulmonary) root, (3) relocating the PA anteriorly and connecting it to the previous aortic root, and (4) relocating the aorta posteriorly and anastomosing it to the neoaortic root (native pulmonary root). With this technique, the branch PAs most commonly straddle the ascending aorta (LeCompte maneuver). Any additional intracardiac communication is closed during the surgery. This procedure allows both anatomical and functional repair restoring ventriculo-arterial concordance.
ASO can be performed successfully with low mortality rate.148 Nevertheless, potential postoperative complications include supravalvar and branch PA stenosis, coronary ostial occlusion/narrowing with subsequent myocardial ischemia and LV dysfunction, AR and neo-aortic root dilatation.149 Coronary artery complications after ASO have been reported in up to 10% of cases.150,151 Early detection of coronary artery lesions is essential for preventing ischemia and potentially life-threatening events. Notably, hearts after the ASO operation are denervated, and chest pain is not a reliable symptom of ischemia in these patients.152
Advanced imaging in patients with TGA after ASO is targeted to detect all potential residual findings requiring medical or surgical treatment. These include ventricular dysfunction, supravalvar pulmonary or aortic stenosis, branch PA stenosis, coronary artery stenosis/occlusion, neo-aortic or pulmonary valve regurgitation and, neoaortic root dilation.153 Even though there is one meta-analysis that concludes that coronary surveillance is not needed,154 multiple studies have concluded otherwise.150–152,155
Indications for CMR
Prior to ASO
Echocardiography is the first line imaging modality prior to ASO and in most cases, CMR is not indicated. Occasionally there may be anatomic or physiologic abnormalities not delineated by echocardiography (e.g., branch PAs) and for those few cases, CMR is useful to delineate this missing information prior to surgery. When it is necessary to delineate the coronary anatomy or if echocardiography fails to do so, CMR has become more utilized; however, at the current time, it is not widespread and standard of care remains cardiac catheterization with cardiac CT as a backup.
After ASO
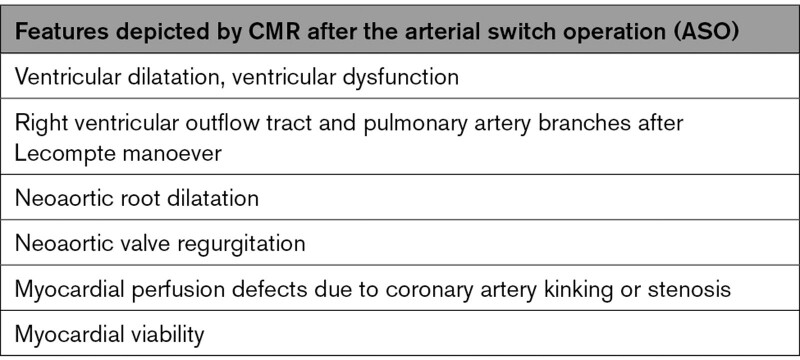
CMR is indicated and can depict almost all common potential residual findings after ASO (Table 4).
Table 4.
Features Depicted by CMR After the Arterial Switch Operation
Ventricular Function
CMR is considered the modality of choice for quantification of biventricular volumes and function, especially the RV (Table 5).6,8,156,157 CMR has a high accuracy and reproducibility and is therefore the ideal modality for repeated measurements during follow up.158,159 In ASO patients, CMR can recognize diminished ventricular function in ASO patients at times when echocardiography fails to do so (Table 5).160 Moreover, advanced imaging with CMR can provide potential causes of ventricular dysfunction in the same examination (eg myocardial scarring, myocardial perfusion imaging if an adenosine stress CMR is being performed).
Table 5.
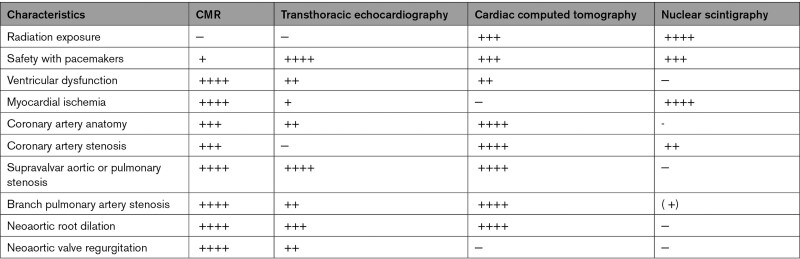
Comparison of CMR with Other Imaging Modalities as it Relates to Transposition of the Great Arteries
Mild biventricular dilation is not uncommon after the ASO operation. LV dysfunction has been observed in up to 20% of the cases and is correlated to clinical symptoms.161 In presence of ventricular dysfunction, concomitant evaluation of myocardial perfusion and scar imaging are essential for assessing coronary artery obstruction. RV dysfunction is rarer but can occur in combination with RVOT obstruction or stenosis of the branch PAs. Therefore, in presence of RV dysfunction, imaging the RVOT and the PAs is mandatory. Due to the position of the RVOT located immediately posterior to the sternum and of the branch PAs straddling the ascending aorta, visualization by transthoracic echocardiography (TTE) is rarely sufficient as patients grow and postoperative scar tissue often limit clear visualization of these structures.
Coronary Arteries, Myocardial Perfusion and Viability
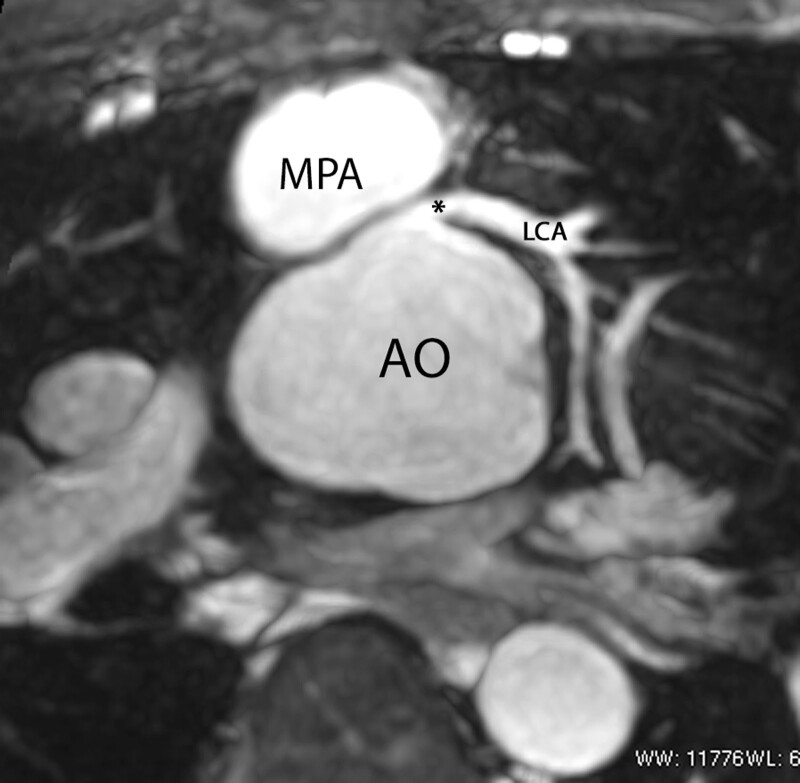
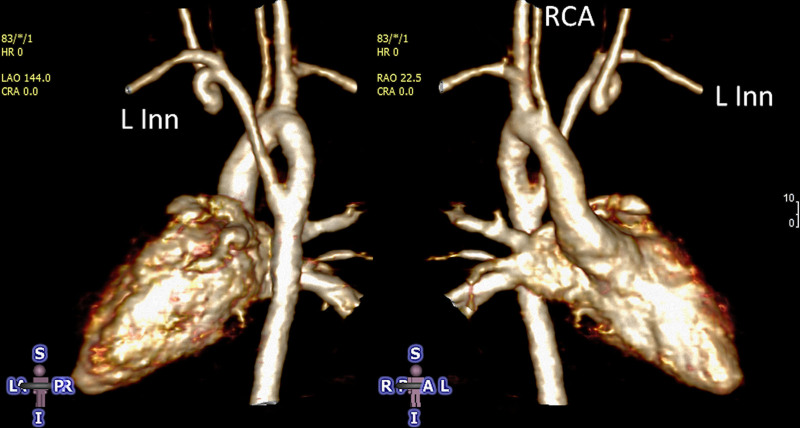
After coronary artery transfer by the ASO, the origin of both coronary arteries is usually in a different position than normal, facing the anteriorly positioned neopulmonary artery (Figure 10). Depending on its individual position, the proximal left coronary artery may show a tangential course which must be distinguished from true coronary artery obstruction. Moreover, it is still unclear whether this steep angle of origin may promote stenosis long term.162,163 Whole-heart CMR (3D balanced bSSFP, contrast enhanced inversion recovery gradient echo imaging using gadolinium or ferumoxytol) enables accurate detection of the abnormal origin and course of the coronary arteries even in very young patients with CHD164,165 and patients with TGA after ASO are no exception.166 Thus, evaluation of the coronary origins and courses routinely added to the CMR protocol167 (see section on CMR for coronary arteries).
Figure 10.
The coronary arteries after the arterial switch operation. 3D balanced steady state free precession (bSSFP) reconstructed image of the origin of the left coronary artery (LCA). The origin of the LCA (*) is occasionally wedged between the main pulmonary artery (MPA) and the aortic root (AO).
In the cases with symptoms, LV dysfunction or coronary narrowing, evaluation of first-pass perfusion and viability can be performed by CMR.168 Myocardial perfusion, typically with the vasodilator adenosine,169,170 can be safely and accurately performed in children.171–177 In 56 myocardial first-pass perfusion scans performed in children, a sensitivity of 87% and a specificity of 95% have been described when compared with coronary angiography171 (Figure 11). Another group reported on 64 first-pass perfusion exams in 48 children and found a positive predictive value of 80% and a negative predictive value of 88% for detecting coronary lesions.173 There are some studies of TGA after ASO which did not find any scar or perfusion defects,178 however, there are others, using regadenoson as a vasodilator stress agent, which detected myocardial perfusion defects in up to 30% with very good agreement with coronary angiography.179
Figure 11.
First-pass perfusion imaging. First-pass perfusion image showing a decrease intake of contrast-medium in the perfusion segments of the circumflex coronary artery in a 9-year-old boy after the arterial switch operation. The finding were confirmed at invasive coronary angiography.
LGE can be found in up to 20% of the patients with TGA after ASO, some of which occur in a non-coronary pattern with small focal enhancement in the septal-free wall junction (possibly residuals from thromboembolic events during Rashkind maneuver and/or cardiopulmonary by-pass). Elevated diffuse myocardial fibrosis has been observed in a cohort of pediatric ASO patients178,180; the prognostic significance of this finding remains unclear.
Pulmonary Arteries
CMR is effective and superior to echocardiography for detecting complications of the PAs after the Lecompte maneuver.181–183 As the cross-section of the PAs is ellipsoid, the antero-posterior dimension is usually smaller than the supero-inferior one184 (Figure 12). PC-CMR measurements provide accurate quantitative differential lung perfusion and add crucial hemodynamic information to the anatomical images.185–187 An unbalanced lung perfusion > 70:30 is usually taken as cut off for the need of an intervention in the PA branches.188 By combining CMR anatomic findings with flow measurement, CMR has demonstrated that orientation of the neo-pulmonary root and diameter of the neo-aortic root are major determinants of the degree of branch PA stenosis.189 With 4D flow, the hemodynamics in the main PA and in branch PAs can be even better understood.190,191
Figure 12.
Geometry of the pulmonary bifurcation after Lecompte maneuver. A, Volume rendered 3D reconstruction showing that the pulmonary side branches runs around the aortic root. An external impression on the proximal course of the pulmonary arteries can occur. B, Coronal view of the geometric relation between ascending aorta and pulmonary arteries (RPA **, LPA *). Note the oval shape of the pulmonary arteries, with the broader diameter in the cranio-caudal direction and slimmer diameter in the lateral direction. Ao indicates ascending aorta; LPA, left pulmonary artery; and RPA, right pulmonary artery.
Neoaortic Root Dilation
Neoaortic root dilatation is a common finding during long term follow up and has been described by CMR in up to 76% of the patients.161 Neoaortic root dilatation progresses over time and is strongly associated with significant semilunar valve regurgitation. CMR is superior to other modalities for quantification of neoaortic valve regurgitation.192 Older age at time of ASO, presence of VSD, and previous PA banding are described risk factors for neoaortic valve regurgitation.193–195 CMR data has also demonstrated that aortic arch geometry (Figure 13) has a significant influence on the severity of neoaortic root dilation, with more acute aortic angles associated with larger neoaortic root and higher incidence of regurgitation.196
Figure 13.
Geometry of the aortic arch after the arterial switch operation. Right-posterior view of 3D volume rendered angiography images showing the typical form of the aortic arch, consisting of a higher convexity, after the arterial switch operation.
Even though reoperation on the neoaortic valve in the currently studied adult patients was rarely necessary, the potential progression of both neoaortic root dilatation and valve regurgitation should have accurate imaging follow up.197 CMR is the ideal modality as it provides both diameters of the neoaortic root measured in different planes and reproducible quantification of the neoaortic valve regurgitation (Figure 14).
Figure 14.
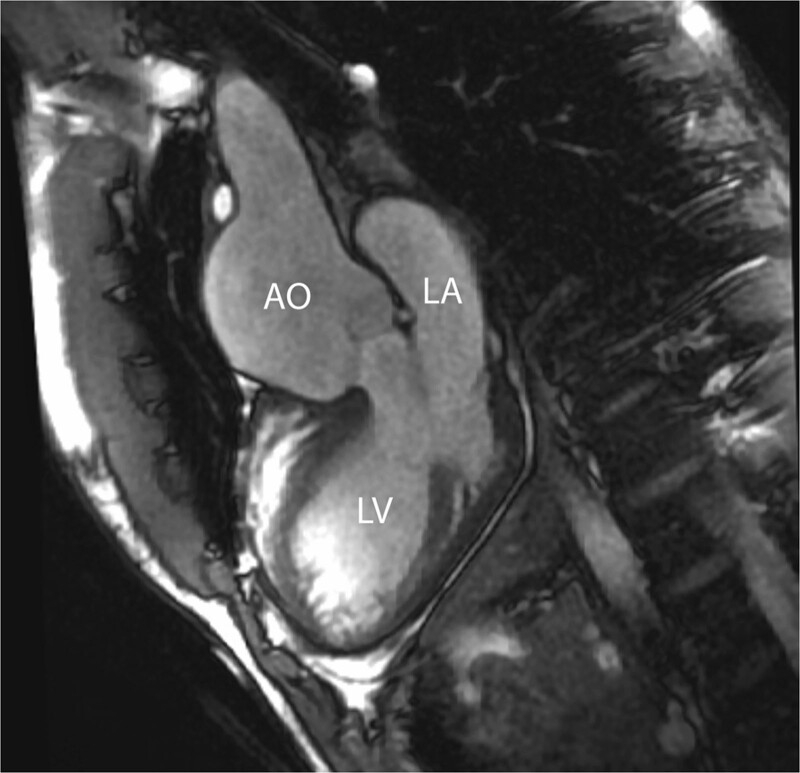
Aortic root dilation. bSSFP cine image in a vertical long-axis view through the inlet and outlet of the LV demonstrates a significant dilation of the aortic root. Ao indicates aorta; LA, left atrium; and LV, left ventricle.
Summary of Recommendations
Prior to surgery, CMR is useful in evaluating anatomy and physiology required for medical or surgical management in patients with TGA which is not delineated by echocardiography (Class I, Level of evidence C).
A comprehensive CMR examination should be performed during routine follow-up of patients who received an ASO and is complimentary to echocardiography (Class I, Level of evidence B)
CMR is beneficial for quantification of biventricular volumes and function in TGA after the ASO (Class I, Level of evidence B).
CMR is beneficial for visualization of the coronary arteries in TGA after the ASO (Class I, Level of evidence B).
CMR is recommended for evaluation of the main PA and branch PA stenosis with assessment of differential pulmonary flow (Class I, Level of evidence B)
CMR is recommended for measure of neoaortic root enlargement and quantification of neoaortic valve regurgitation (Class I, Level of evidence B)
Vasodilator stress perfusion CMR imaging is useful in symptomatic patients to test for ischemia (Class I, Level of Evidence B) and may be considered as an initial, non-invasive screening test for myocardial perfusion defects and therefore detection of potential coronary artery obstruction. (Class IIB, Level of evidence B)
In the case of suspected myocardial perfusion defects, CMR may be considered for visualization of coronary ostial stenosis (Class IIB, Level of evidence B)
CMR is useful in screening for myocardial scarring with LGE (viability imaging) or in confirming the diagnosis in cases of symptomatic individuals, given manipulation of the coronary arteries in this lesion (Class I, level of evidence C).
Pulmonary Venous Anomalies
Background
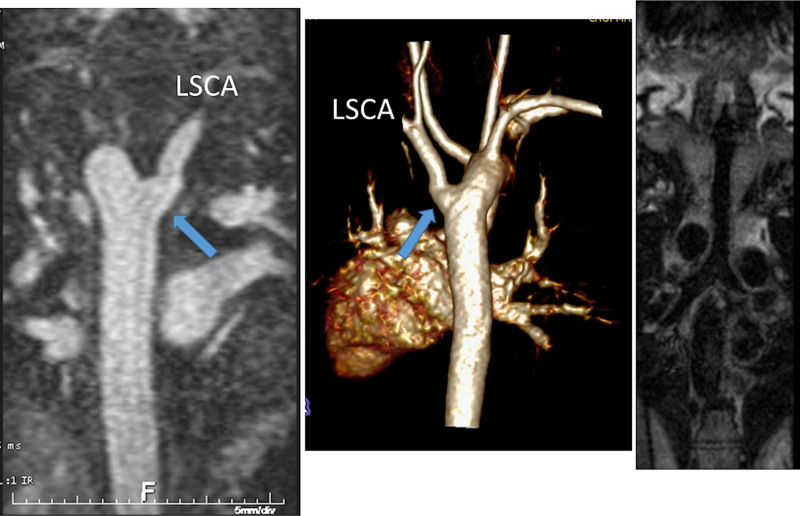
Anomalies of the pulmonary veins (PVs) can be congenital or acquired after an intervention or during the progression of a disease. Congenital PV lesions are rare and occur with a prevalence of 0.6–1.2 / 10 000 livebirths.11,198 Partial anomalous PV connection (PAPVC) is the most frequent observed lesion and can occur in isolation but more frequently in association with an ASD (specifically a sinus venosus ASD) (Figure 15). In presence of a sinus venous ASD of the SVC type, a right upper PV connecting to the SVC is common whereas in a sinus venosus ASD of the IVC type, the right lower PV will connect to the inferior margin of the right atrium. Another condition associated with PAPVC is Turner Syndrome in which typically the left upper PV connects to the innominate vein.199 In Scimitar syndrome, usually all right PVs connect anomalously to the IVC200,201 which may occur below the diaphragm (Figure 16).
Figure 15.
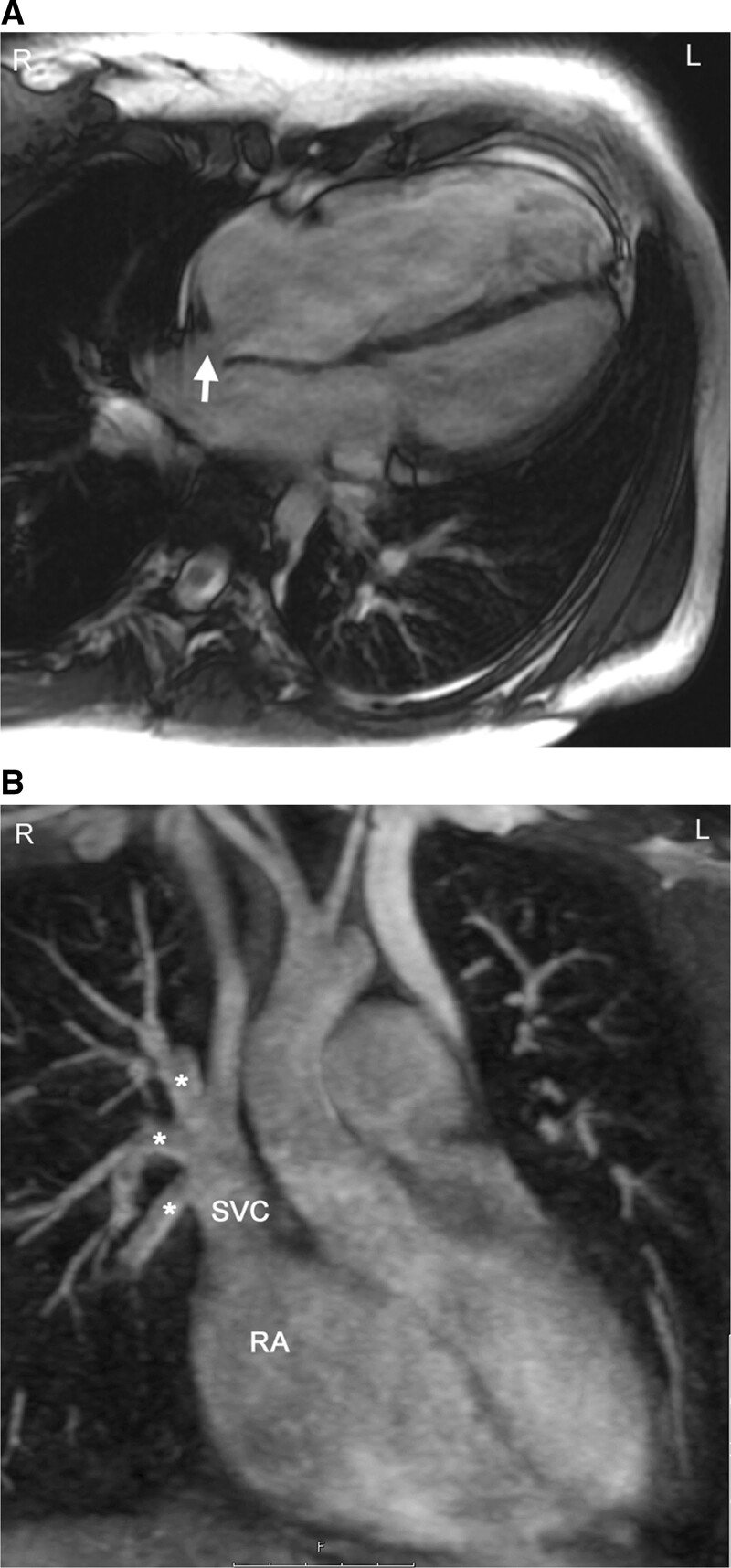
Sinus venosus atrial septal defect with partial anomalous venous connection. A, bSSFP cine image in a horizontal long axis view showing a dilation of the right atrium and right ventricle. The arrow indicates the septal defect of sinus venosus type. B, The right upper and at least one branch of the right middle pulmonary vein (stars) are connected to the superior vena cava (SVC). This is a reconstructed maximum intensity projection image from contrast-enhanced cardiac magnetic resonance angiography. RA indicates right atrium.
Figure 16.
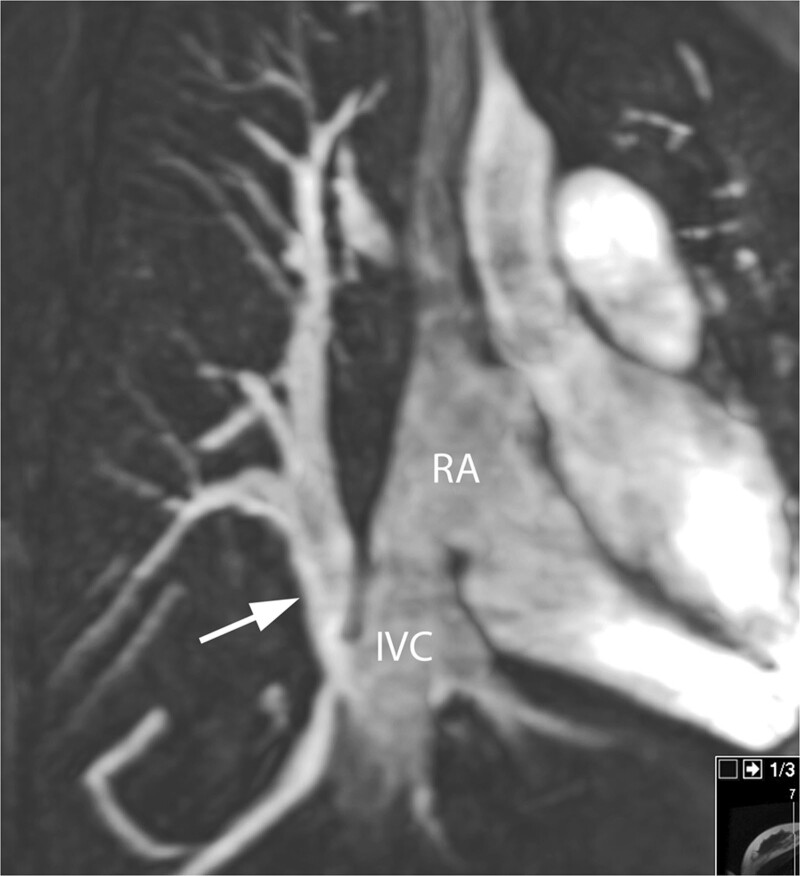
Scimitar syndrome. All venous drainage from the right lung is connected (arrow) to the inferior vena cava (IVC) at the entrance in the right atrium (RA). Reconstructed maximum intensity projection image from contrast-enhanced CMR angiography.
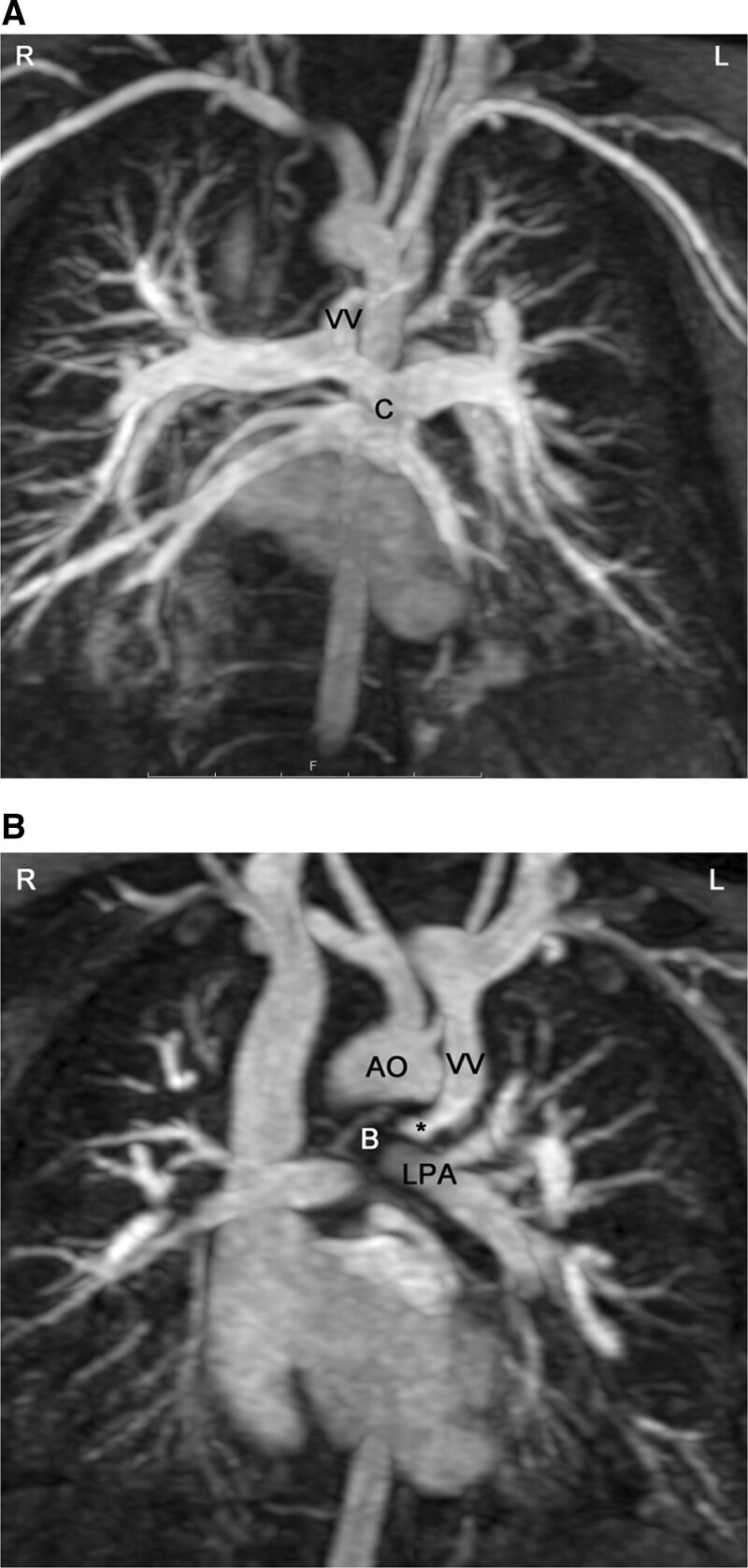
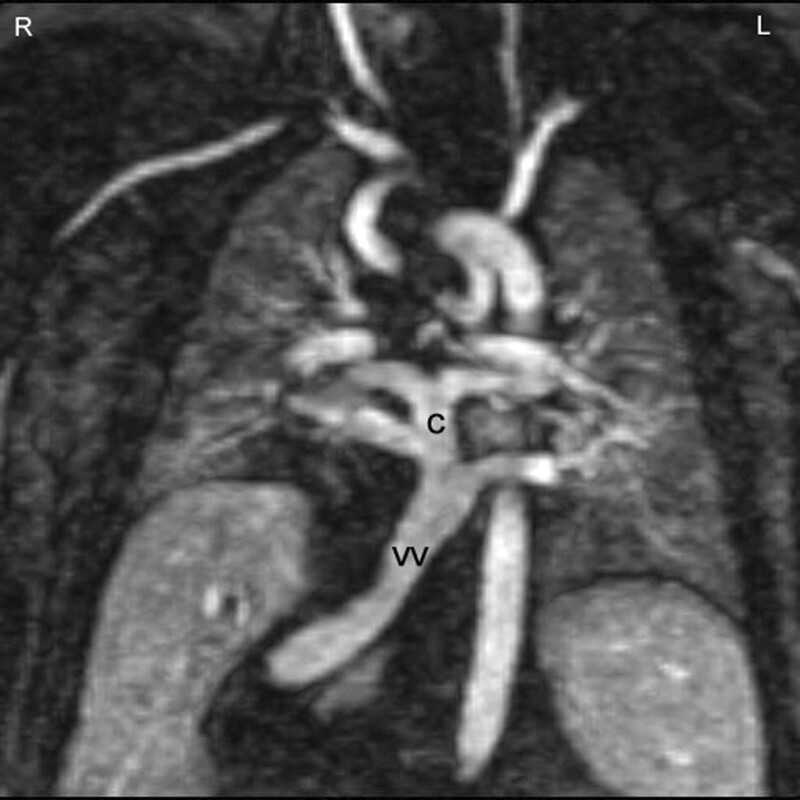
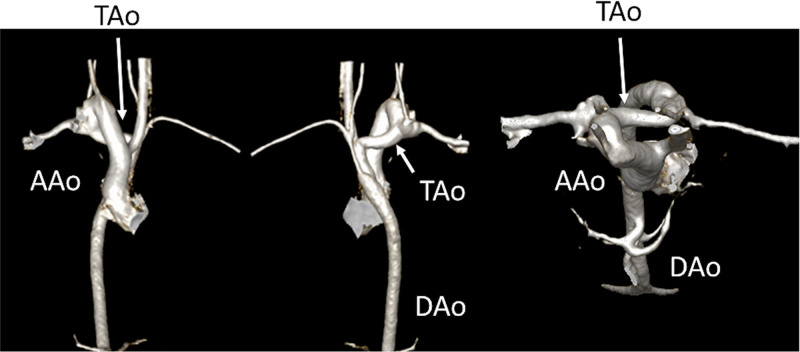
Total anomalous pulmonary venous connection (TAPVC) occurs in four different formations defined by the location of the connection of the PVs to the right-sided circulation. In order of prevalence, the sites of connection are: supracardiac (Figure 17), infradiaphragmatic (Figure 18), cardiac, and mixed type. TAPVC can occur in isolation or in association with complex CHD such as heterotaxy syndrome.202–204 As variations of the course of the PVs are not infrequent, accurate imaging of each PV is mandatory prior to surgery. Moreover, obstruction in the PV pathway is not rare and can be caused by (a) stenosis of each PV, (b) stenosis at the site of connection, (c) extrinsic compression of the connecting channel, (d) compression of the vertical vein in between the left bronchus and the left PA (supracardiac type) (Figure 17), (e) in the infradiaphragmatic type, compression in the small esophageal hiatus, in the ductus venosus or in the capillary system of the liver (Figure 18) or other solid parenchymal organ.205 TAPVC with obstruction of the PV drainage causes severe symptoms of pulmonary congestion in the first days of life and requires immediate surgical or catheter based relief. Unobstructed TAPVC usually leads to significant left to right shunt and heart failure in the first weeks of life.
Figure 17.
Total anomalous pulmonary venous connection of supracardiac type. A, All 4 pulmonary veins are connected to a collector (c), which is draining to a vertical vein (vv). B, Along its course to the innominate vein, the vertical vein (vv) passes between the left main bronchus (B) and the left pulmonary artery (LPA). This represents an anatomic vice (*) which may cause obstruction of flow. Ao indicates aorta.
Figure 18.
Total anomalous pulmonary venous connection (TAPVC) of infracardiac type. In infracardiac type the collector (c) is draining to a vertical vein connected to the liver portal system. This contrast-enhanced CMR angiography was acquired in a patient with heterotaxy syndrome, typically associated with TAPVC.
Finally, congenital stenosis of one or more PVs can occur, a progressive disease which leads to a dismal outcome (Figure 19).206 This can occur with or without associated CHD, and unilateral PV stenosis can lead to flow asymmetry to the lungs (i.e., decreased flow to the lung associated with the PV stenosis). It is not uncommon to have recurrent and/or progressive PV obstruction or even death after surgical repair.207
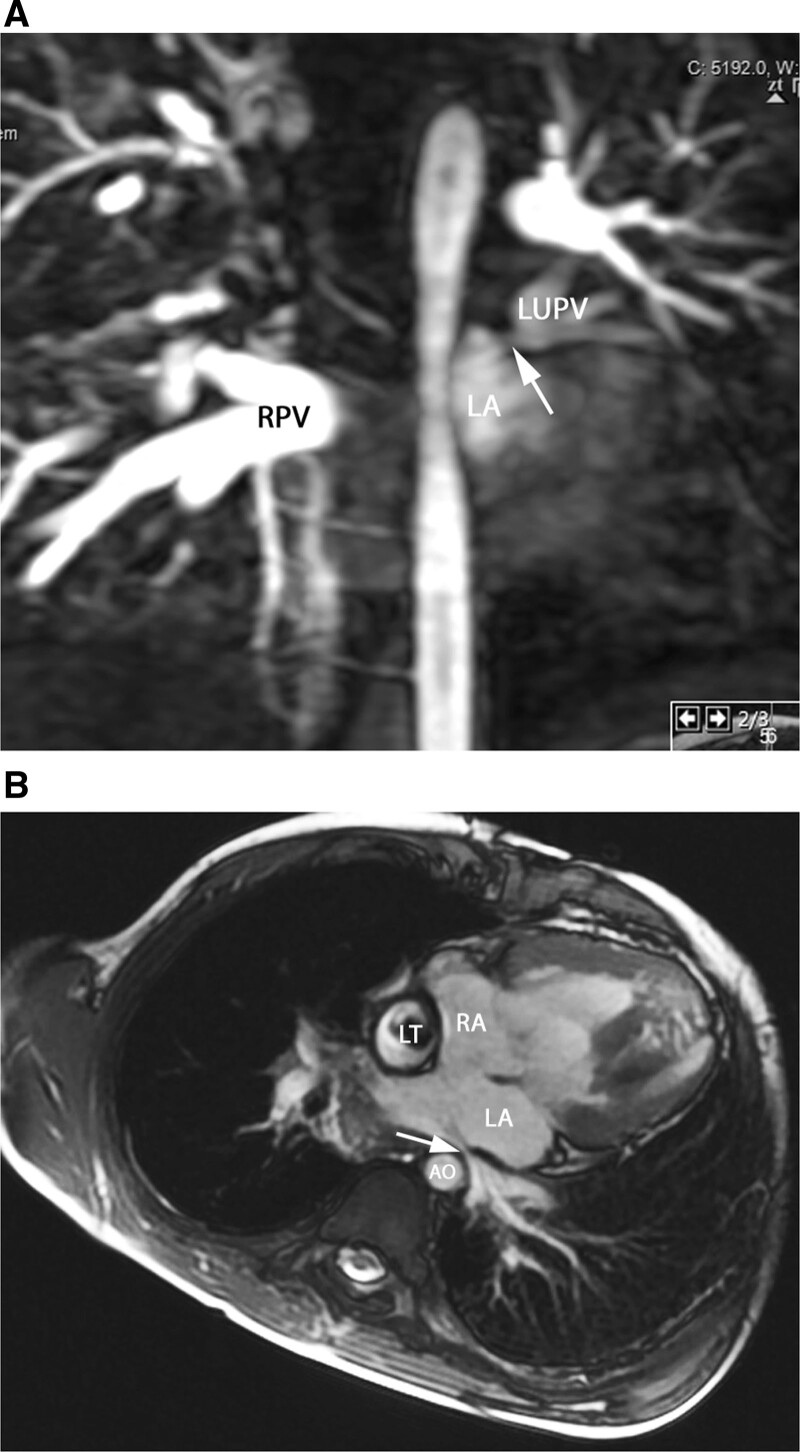
Figure 19.
Pulmonary vein stenosis. A, Contrast-enhanced CMR angiography depict an intrinsic stenosis (arrow) of the left upper pulmonary vein (LUPV). Note the difference of contrast between the left and the right pulmonary veins (RPV), as well as the large diameter of the right pulmonary veins, probably caused by flow redistribution between the two lungs. B, Pulmonary vein (PV) stenosis in the Fontan patient is demonstrated in this bSSFP cine image in an axial view; external compression of the left lower pulmonary vein visualized which is impinged (arrow) between the left atrium and the descending aorta (AO) and spine. LA indicates left atrium; LT, lateral wall tunnel; and RA, right atrium.
CMR Indication in PV Anomalies
CMR is nearly always performed after an echocardiography for all PV anomalies (Figures 15, 16, 17, 18, 19). In presence of an ASD, particularly of the sinus venosus type (Figure 15), CMR is indicated for ruling out a PAPVC whenever TTE is not conclusive.208,209 Greater than 4 PVs may be present and therefore, echocardiography delineation of only 4 PVs without searching for others in lesions associated with PAPVC is not sufficient and CMR is mandatory. In patients with limited acoustic windows, CMR is indicated to delineate other PVs even if 4 are visualized on echocardiography. Besides providing clear information on the exact location of PV connection and drainage required for proper planning of all surgical corrections such as the Warden operation,210,211 CMR is useful to assess size and function of the dilated right heart, assessing the contribution of the anomalous vein(s) to the left to right shunt and to measure Qp/Qs (Figure 15). In case of isolated PAPVC, quantification of Qp/Qs and right heart dilation is a major determinant of indication for surgical repair.
CMR is the modality of choice for evaluation of Scimitar syndrome,212,213 demonstrating not only the anomalous drainage of all right PVs into the IVC (Figure 16) with the typical shape of an Arabic sword (scimitar) but also the associated anomalies including dextrocardia, right lung hypoplasia, horseshoe lung, aberrant systemic arterial blood supply to the right lower lung. In addition, in TAPVC, CMR is an important tool for visualization of each single pulmonary vein, their exact site of insertion (especially in mixed TAPVC), drainage, and if present, site of obstruction. (Figure 17). Moreover, shunt fraction and blood flow distribution to each lung can be quantified.
In congenital stenosis of one or more PVs, CMR angiography provides exact depiction of the location of stenosis, number of veins affected and can be repeated during follow up (Figure 19). PV stenosis is also a possible complication after surgical TAPVC repair214,215 and if all PV are not affected, pulmonary flow redistributes among the different lung segments. Recognition of the severity of obstruction can be difficult by echocardiography as severely diminished flow may not induce turbulence seen by color flow Doppler at the site of stenosis; on the other hand, turbulence may be visualized at a PV vein which is normal size but has increased flow across it. For congenital PV stenosis and after surgical repair for TAPVC, CMR can clearly depict PV stenosis216,217 and delineating not only peak velocities in them (with PC-CMR) but also flows in each PV and PA.
Flows in the PVs can be assessed quantitatively218 and qualitatively.219 The normal PV flow curve consists of 2 forward waves during systole and early diastole as well as a short wave of reverse flow during late diastole at atrial contraction. This normal flow profile can be altered in several conditions or if atrial compliance is disturbed. In presence of unilateral PV stenosis, a redistribution of flow occurs within the lung and can be assessed by measuring the flow in the pulmonary arteries by CMR; this is correlated with particular changes in flow profile.220,221 Flow profiles are also affected directly within the PVs; proximal to a focal stenosis, flow loses its triphasic profile similarly as observed by using Doppler echocardiography.219 On the other hand, peak PV flow velocities > 100 cm/s indicate significant obstruction.217
In complex CHD, particularly heterotaxy syndrome, PV anomalies are frequent and occur in a wide anatomic variety.204 Due to anatomic complexity, echocardiography is often insufficient to describe all diagnostic features required for surgical and medical management. In these lesions, CMR has an important role for surgical planning or staged palliation222,223 especially in SV patients. The PV may become impinged between the dilated heart and the spine or the descending aorta (Figure 19). PV occlusion may increase the overall resistance to pulmonary flow which has a negative impact on the Fontan circulation and ultimately clinical outcome.
In general, in comparison to other modalities, cross sectional imaging is superior to echocardiography or conventional angiography due to 3D data acquisition which enables a targeted multiplane reformatting and therefore, visualization of each single PV without superimposition of other vascular structures.224,225 CT has the same ability to delineate anatomy. CMR has been validated against lung perfusion scintigraphy for measurement of differential lung perfusion and has been shown to be a similarly accurate and robust modality.187,226 In patients with CHD, CMR flow has been shown to be even more accurate (especially in the presence of systemic to pulmonary collaterals) and to overcome some pitfalls associated with scintigraphy.188
Summary of Recommendations
In patients with PV stenosis or suspected anomalous PV connection, whether PAPVC or TAPVC, CMR should be performed for anatomic evaluation whenever echocardiography is insufficient (Class I, Level of evidence B).
CMR is useful to understand the hemodynamics of PV anomalies such as calculating any shunt (Qp/Qs) caused by anomalous PV connection and associated intracardiac lesions as an indication for surgical repair (Class I, Level of evidence B) as well as quantifying differential lung perfusion with flow redistribution (Class I, Level of evidence B).
CMR examination should be performed for assessing PV anatomy in cases with complex CHD when there is a clinical or imaging suspicion of anomalies of PV connection or drainage, particularly heterotaxy syndrome (Class I, Level of evidence B)
CMR angiography should be performed for surgical planning of repair of PV anomalies (Class I, Level of evidence B)
It is reasonable to perform at least one CMR examination during follow up after surgical repair for PV anomalies (Class IIA, Level of evidence B).
Coronary Artery Disease
Background
Categorically, pediatric coronary artery pathologies can be either congenital or acquired. Acquired lesions can also be sub-categorized as either “disease” based or “surgically” based. Congenital lesions would include those related to anomalous aortic origin of a coronary artery (AAOCA) from an inappropriate sinus (e.g., anomalous origin of the left coronary from the right sinus of Valsalva), anomalous origin from a different vessel such as anomalous origin of the left coronary from the PA, and/or anomalous course of a coronary artery (eg intraseptal) or exit (eg coronary cameral fistulae as seen in pulmonary atresia with intact ventricular septum). “Acquired disease” based lesions would include Kawasaki disease whereas “surgically” based lesions would include alterations of the locations of the coronary ostia or their proximal courses related to corrective surgeries such as the arterial switch operation (Jatene procedure)227 or Ross procedure.228 Clearly, many of these diseases lend themselves to potentially decrease myocardial perfusion, possibly resulting in ischemia and infarction.
Echocardiography, CT, and CMR are the most commonly used non-invasive imaging modalities for the evaluation of pediatric patients with coronary artery pathologies. Echocardiography is the most easily available with its inherent mobility and high temporal resolution and remains the front-line imaging modality. In many instances, echocardiography is utilized as a screening tool for progression of disease (e.g., Kawasaki’s disease) or may suggest a pathology as an incidental finding (e.g., an echocardiography for evaluation of physical examination findings of a murmur that suggests AAOCA).
Cardiac CT, with state-of-the-art dual-source or volume CT scanners, compared to CMR performed on 1.5 T or 3 T CMR scanners, has slightly higher isotropic spatial resolution (0.5–0.6 mm), faster total examination and scanning time and can, at times, accommodate high heart rates (> 120 bpm) despite a modest temporal resolution for nearly all scanners of 75 ms, although the fastest scanner can obtain a resolution of 66 ms. The drawbacks are that CT requires ionizing radiation and rapid bolus intravenous injection of iodinated contrast agent along with possibly administering medication to slow the heart rate (e.g. propranolol). Cardiac CT typically is limited to morphologic imaging due to radiation exposure concern.
CMR is generally utilized to confirm the diagnosis by echocardiography as well as to allow for visualization of longer segments with whole-heart coverage229 in addition to assessing myocardial function (both regional and global), perfusion177 and infarction (ie viability imaging).50,172 Further, CMR adds anatomic information as well in the same patient such as those with TGA after ASO.172 CMR can obtain in-plane resolution of 0.5–0.6 mm230 at 3 T in children and can usually obtain 1–1.2 mm isotropic resolution at 1.5 T. CMR can also obtain coronary images without the need for contrast in the pediatric age range165,231,232 in multiple different formats (eg bright blood or dark blood)233 although contrast can enhance the imaging.234 Newer techniques,235,236 currently utilized in several pediatric centers, take advantage of the significant increase signal afforded by the iron-particle blood pool contrast agent, ferumoxytol, and have shown very promising results with sub-mm isotropic whole-heart coverage even in infants with high heart rates (Figure 20) (0.6-0.8 mm in-plane resolution at 1.5 T with slightly lower resolution in the z axis).
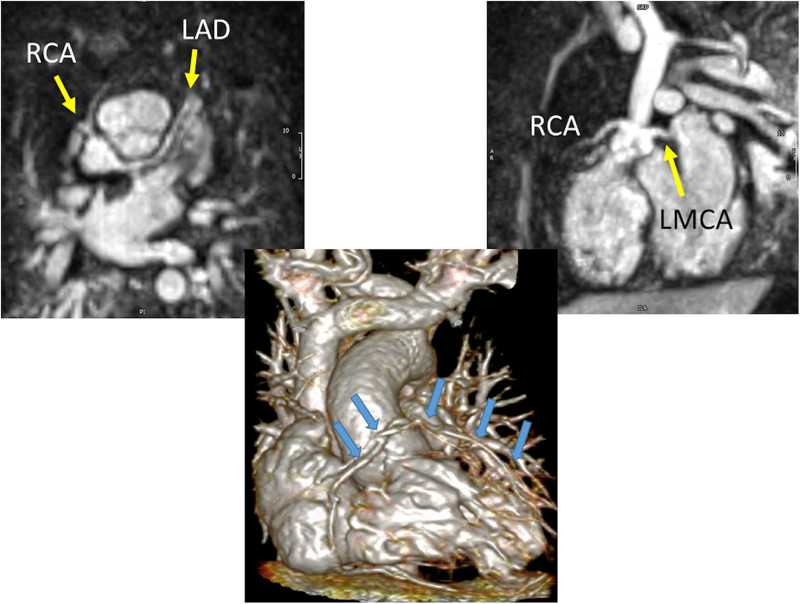
Figure 20.
Coronary artery imaging in infants utilizing ferumoxytol. The top two panels demonstrated normal origins and courses from a 2 day old with Taussig Bing anomaly and hypoplastic aortic root and ascending aorta in the off-axis axial (left) and sagittal views (right). The lower image is a 3D reconstruction from a 5 day old with tetralogy of Fallot and pulmonary atresia demonstrating a single right coronary artery (RCA); arrows outline the RCA and left main (LMCA) and anterior descending coronary arteries (LAD).
Indications for CMR to Assess Coronary Arteries
Congenital
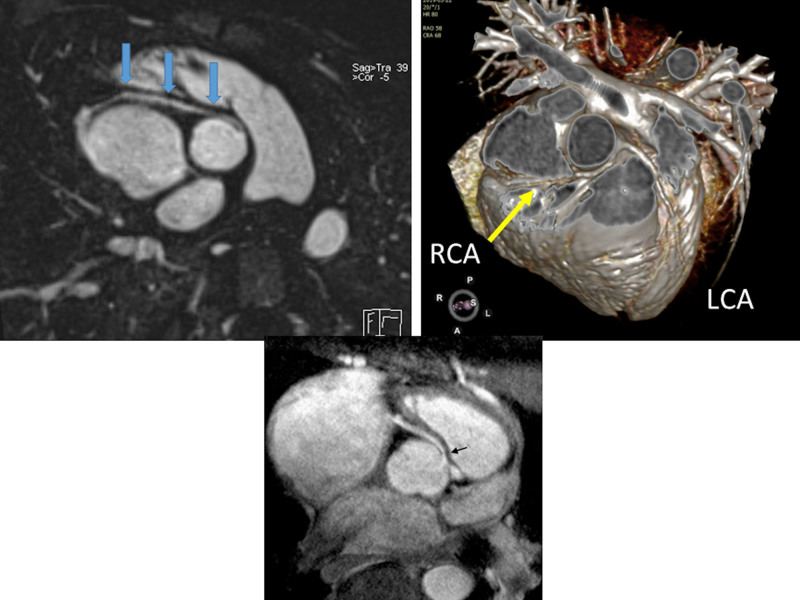
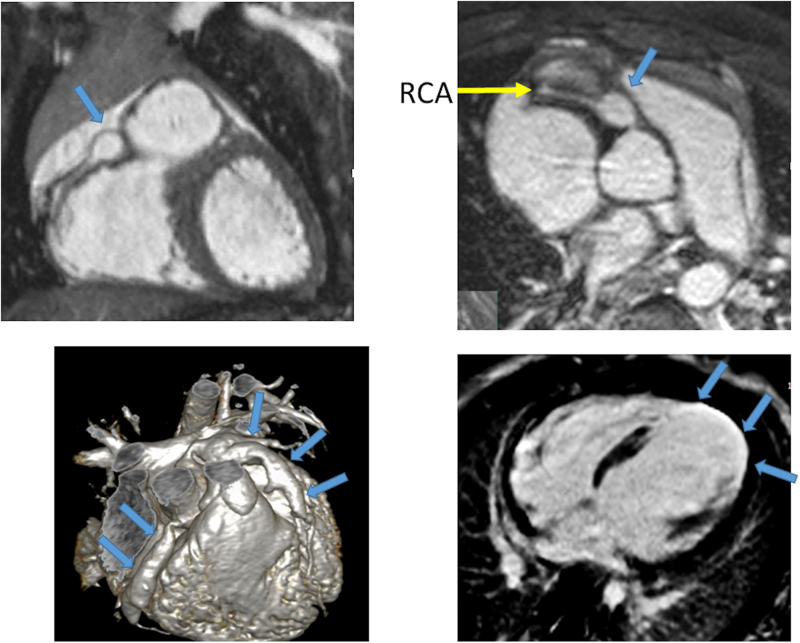
Following screening echocardiography for suspicion or diagnosis of AAOCA, CMR should be used to confirm the presence of AAOCA and further characterize the location and shape of the ostium and proximal and mid-segment course of the anomalous coronary artery (Figures 21, 22). It should also be used to assess biventricular function both globally as well as regionally to assess regional wall motion abnormalities which may be due to AAOCA. Finally, viability can be performed on a routine basis to determine if there is any discrete LGE due to myocardial infarction. Vasodilator stress perfusion CMR should be reserved for special cases.
Figure 21.
Anomalous origin of the right coronary artery (RCA) from the opposite sinus in an off axis axial view (top left with arrows demonstrating the RCA course) and with a 3D reconstruction (top right). Lower panel is high resolution from a 3 T CMR scanner demonstrating the same with arrow showing origin of an intramural course.
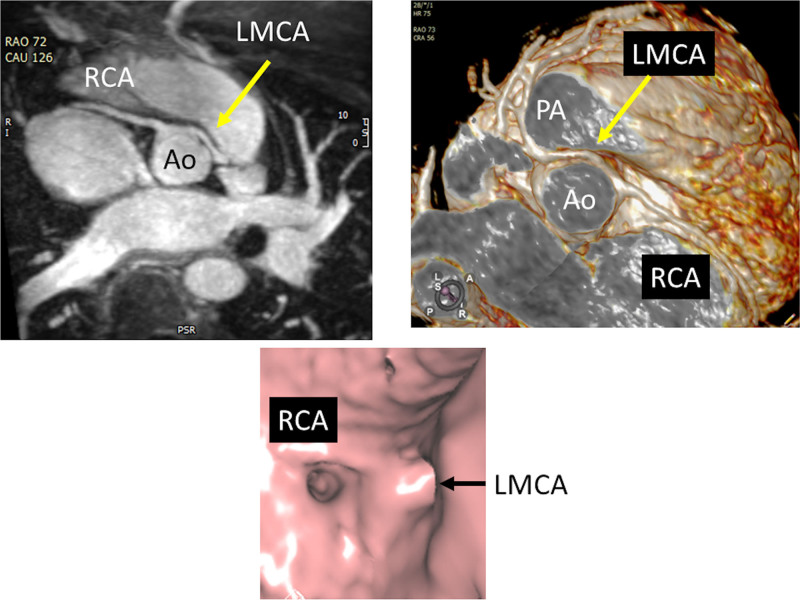
Figure 22.
Anomalous origin of the left main coronary artery (LMCA) from the right sinus in an off axis axial view (top left) and with a 3D reconstruction (top right). Lower panel is an endoscopic view of the same patient demonstrating the orifice origins and shapes; note the round right coronary artery (RCA) os and the oval LMCA os.
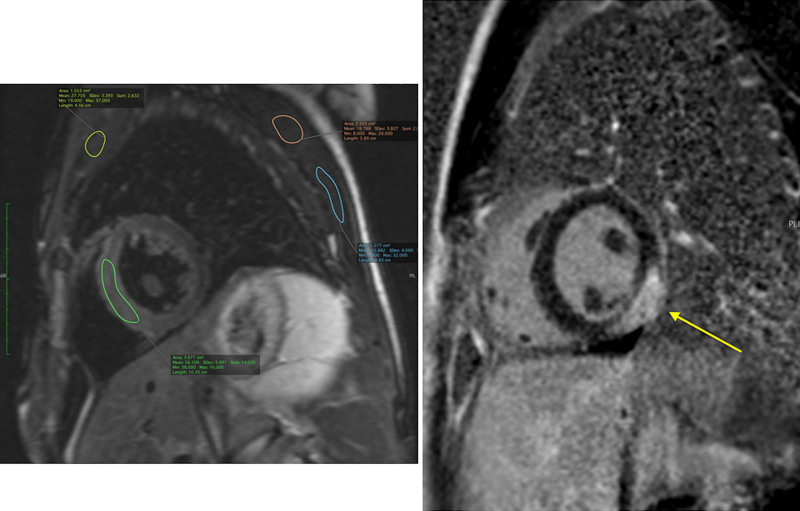
In a prospective study of 50 patients (age range, 18 days to 18 years), Hussain et al.229showed that whole-heart coronary artery CMR has a success rate of 94% for the detection of coronary origins. Brothers et al. demonstrated prospectively in a small group of patients the multifaceted utility of CMR by characterizing stenosis, perfusion and fibrosis both prior to and after surgery in children with AAOCA.167 The latest techniques using ferumoxytol overcomes previous spatial limitations (Figure 20) even in small infants. Detailed depiction of the anomalous coronary artery and its proximal course can also be achieved with high in-plane sub-mm spatial resolution coronary CMR imaging with a targeted approach and 3D endovascular view of the morphology of the ostium can be performed (Figure 22).237 Most recently, in one institution, a large cohort of 5,169 asymptomatic volunteers (11—18 years of age) were screened with CMR for cardiomyopathy and anomalous coronary artery origin from the opposite sinus with an intramural segment (ACAOS-IM). There were 23 such cases (6 left-ACAOS-IM and 17 right-ACAOS-IM)238,239 establishing a prevalence of 0.4%.
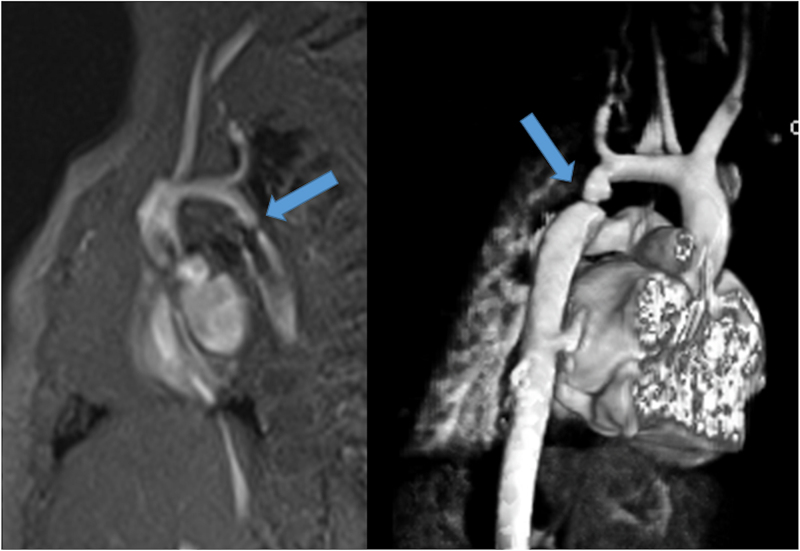
Other important anatomic findings of the coronary arteries can be detected by CMR such as a conal branch coursing anteriorly across the RVOT and/or position of left main coronary and proximal left anterior descending (LAD) artery with respect to RVOT, significant in patients with TOF.240 In a prospective study of whole-heart coronary CMR in 100 patients (age 2 months–11 years; median 3 years), of the 58 patients who underwent surgery, all CMR coronary artery findings were confirmed including 4 cases of coronary anomalies.165 In addition, coronary anomalies of “course” such as an intraseptal or retroaortic course, are important to delineate and have been demonstrated by CMR.237 Finally, anomalies of exit such as those with pulmonary atresia with intact ventricular septum with coronary cameral fistulae or a RV dependent coronary circulation can be delineated by CMR, especially important because of the sequelae of myocardial infarction (Figure 23).
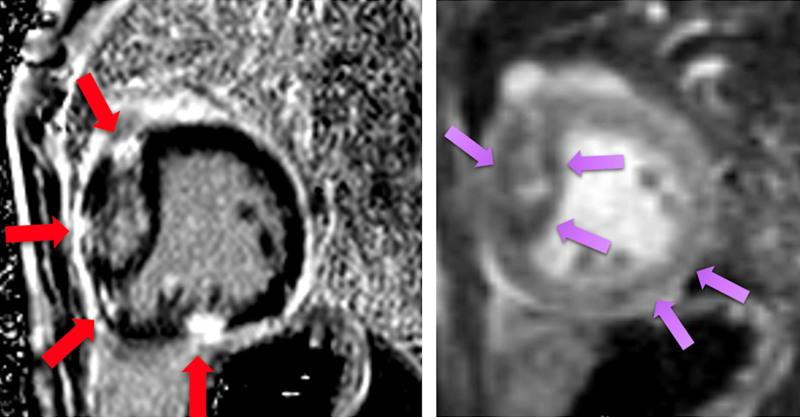
Figure 23.
A 3 month old with pulmonary atresia with intact ventricular septum with coronary cameral fistulae and a RV dependent coronary circulation. The right panel is an adenosine stress perfusion CMR demonstrating perfusion defects (arrows) while the left panel demonstrates myocardial scarring in that same patient (arrows).
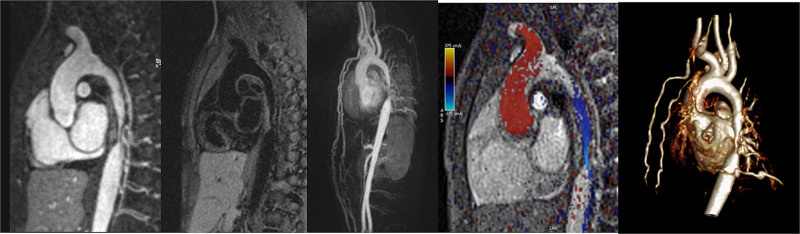
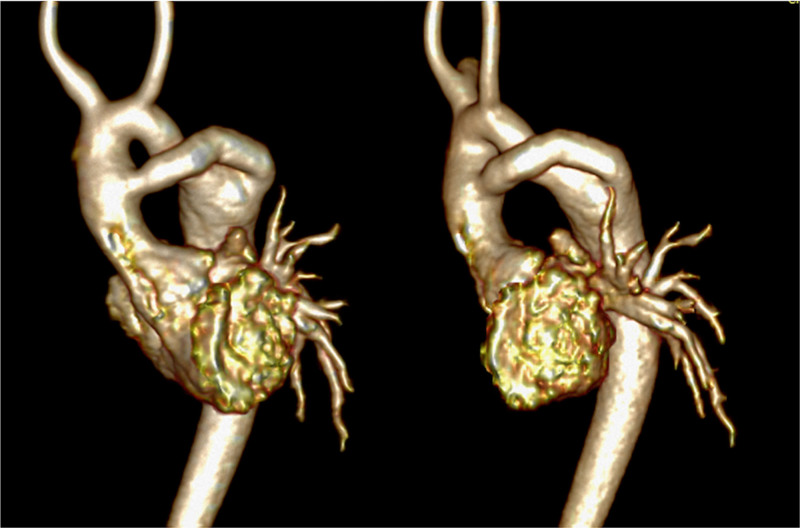
Acquired
CMR can be used to assess for the morphology including size, shape, and location of coronary aneurysms in diseases such as Kawasaki’s disease (Figure 24). Similar to other CHD, it should also be used to assess global and regional ventricular performance, viability and perfusion (perfusion in select cases).
Figure 24.
Kawasaki’s disease. Top panels demonstrate a discrete right coronary artery (RCA) aneurysm (arrow) in a 3 year old. The bottom left panel is from a 2 year with diffuse aneurysms of both the right and left coronary systems (arrows); clots were found in the left coronary systems the resultant infarct and rounding of the left ventricular apex (arrows) is seen from the 4-chamber viability imaging (right).
Mavrogeni et al.241 has shown in a prospective study of 16 patients (age range 3–8 years) that coronary CMR correlated completely with invasive cath for size and location of aneurysms. There were no cases of stenoses for comparison. Suzuki et al.242 and in related work by Takemura et al.242 showed in a retrospective study with a larger cohort of 106 Kawasaki disease patients (median age 13 years, range: 4 months–33 years) and a smaller cohort of 35 consecutive pediatric KD patients (under 6 years of age) that imaging of coronary arteries in pediatric patients with Kawasaki disease can routinely be performed with a 96% success rate. High sub-millimeter spatial resolution imaging that is used for AAOCA has been successful in young patients with Kawasaki disease.243,244 In addition, CMR should also assess for global and regional ventricular dysfunction, coronary perfusion, and myocardial scarring/LGE during the convalescence and follow up of the Kawasaki disease patients.245,246 Advanced imaging of characterization of the coronary vessel wall has also been shown to be possible.246
CMR can be used to characterize the anatomy of the coronary arteries following surgery in which the position and/or origin of the native coronary arteries have been altered such as after the Ross procedure or TGA after ASO (Figure 10). Taylor et al. reported in a prospective study of 50 asymptomatic pediatric TGA patients after ASO (age range 6–16 years) a comprehensive CMR examination including coronary artery CMR, cine imaging for ventricular function, and myocardial characterization for scarring.172 In 100 patients after ASO, Rodriguez et al. studied 100 whole heart 3D CMRs and found coronary artery stenosis in nearly 11%.166
Imaging coronary artery walls for vasculopathy in transplant patients247 including those in the pediatric age range248 and in other diseases such as Takayasu’s arteritis249 is an emerging application of CMR coronary imaging but should be considered experimental at this time.
Summary of Recommendations
For patients with suspected AAOCA or other congenital anomalies of origin, course or exit, CMR is recommended to depict the origin and detailed anatomy of the vessels for both diagnosis and pre-operative planning (Class I, Level of evidence B).
For patients with Kawasaki disease or other acquired “diseased” based coronary pathology, CMR is recommended to accurately depict the size, shape, and location of coronary aneurysms (Class I, Level of evidence B).
For patients with acquired “surgically” based coronary pathology such as TGA after ASO or Ross procedure, CMR is recommended to evaluate the post-operative coronary anatomy as part of a clinically indicated comprehensive CMR examination. (Class I, Level of evidence B)
CMR should be utilized to assess the secondary effects of congenital coronary anomalies or acquired pathology such as effects on myocardial function (e.g., regional wall motion abnormalities, end-diastolic volume, ejection fraction), perfusion and infarction (Class I, Level of evidence B) both prior to and after repair (if surgery) or in followup.
Coarctation of the Aorta and Bicuspid Aortic Valve
Background
Coarctation of the aorta is the most common left sided obstructive heart lesion with a mean incidence of 409 per million live births11 and a prevalence of ~ 7% of patients with CHD.198 Coarctation is a discrete or relatively discrete narrowing of the proximal descending thoracic aorta, most commonly located in the juxtaductal region, immediately distal to the left subclavian artery (Figure 25) although it may occur anywhere in the thoracic (Figure 26) and abdominal aorta. It can be simple or complex with arch hypoplasia and tortuosity and associated with other significant intracardiac CHD such as HLHS, TGA or truncus arteriosus. Bicuspid aortic valve (BAV) is frequently identified in patients with coarctation with studies reporting an incidence of > 50% and up to 85% (Figure 27).250 Coarctation is part of a spectrum of aortopathies; genetic aortopathies are addressed elsewhere in this manuscript.
Figure 25.
Coarctation of the aorta in the juxtaductal region in a 4 month old utilizing unbalanced gradient echo cine imaging (left) and 3D reconstruction (right). Note the turbulent jet on cine. Arrows point to the coarctation.
Figure 26.
Coarctation of the aorta in the mid-thorax. This figure demonstrates the multiple ways a coarctation can be imaged by CMR. From left to right, there is 2D bright blood gadolinium enhanced imaging, a multiplanar reformat of a 3D dark blood sequence, a maximum intensity projection 3D image, inplane velocity mapping with color coding of the flow through the coarctation and a 3D volume rendered display. Note the collaterals on the maximum intensity projection and 3D volume rendered display. Color coding of the inplane velocity map is red cephalad and blue caudad.
Figure 27.
Volume rendered image of an ascending to descending aortic conduit from anterior (left) and lateral (right) views to repair a coarctation utilizing gadolinium enhanced 3D imaging by CMR.
If not detected prenatally or with pulse oximetry screening, neonates with coarctation of the aorta may present in clinical distress with significant metabolic acidosis and respiratory failure at the time of ductal closure.251 The lesion may be ductal dependent, requiring prostaglandin infusion and other supportive measures for resuscitation and survival of the patient until more definitive treatment can be undertaken. Accurate detailed diagnosis is essential prior to any intervention. Echocardiography is the first line imaging modality and may be all that is needed in infants with simple coarctation or associated intracardiac defects. If portions of the aorta proximal or distal to the coarctation or branching vessels are not well visualized, CMR can provide full anatomical details and may be superior to echocardiography.252
Coarctation may also present later in childhood or early adulthood, usually in the setting of referral for hypertension or heart murmur and diminished lower extremity pulses. Depending on the age of the patient, chest X-ray may demonstrate rib notching such as when significant large collateral vessels may be present. When a native coarctation of the aorta is first diagnosed in an older child or adult by echocardiography, CMR is then used to define the anatomical and hemodynamic severity in preparation for treatment by surgery or in the cardiac cath lab. Echocardiography may demonstrate the unusual arch anatomy and abnormal Doppler flow patterns through the obstruction, but due to limited acoustic windows, may not allow for full visualization of the coarctation or its proximal and distal segments, aortic arch branching abnormalities, or the presence and magnitude of associated collateral vessels which are all important pieces of clinical information to aid in determining the appropriate method of intervention.
In infancy and early childhood, coarctation of the aorta is most commonly repaired surgically with methods including subclavian flap repair, patch aortoplasty and simple or extended end-to-end anastomosis. In some situations, coarctation may be initially treated with balloon angioplasty with or without placement of a stent. In more unusual complex anatomical situations, bypassing the severe arch obstruction with a LV apical or ascending to descending aorta conduit may be rarely undertaken (Figure 27). Regardless of method of initial repair, long-term complications that require monitoring include residual coarctation, recoarctation, associated arch hypoplasia or tortuosity, aneurysm formation near the site of repair (Figure 28), dissection, hypertension and endocarditis. Abnormalities of the aorta will often impact LV function. Long-standing hypertension leads to LV hypertrophy and ventricular dysfunction, as well as increased incidence of coronary heart disease and multiple other sequelae eventually leading to premature morbidity and mortality.253,254
Figure 28.
Candy cane view of a 12 year old after subclavian flap angioplasty repair of coarctation of the aorta with a moderate sized aneurysm formation.
Indication for CMR
Important anatomic and hemodynamic data is obtained with CMR having been utilized for many years in the assessment of coarctation of the aorta. Multiple techniques, including non-contrast and contrast CMR sequences are used to provide a complete anatomical 3D analysis (Figure 26). Collateral vessels can be visualized providing flow to the descending aorta distal to the obstruction and CMR can be utilized to quantify this flow both prior to and after intervention (See Colateral flow section of this document) (Figure 29).255–260 Anatomic measurements throughout the aorta can be applied and compared to published normative data.261 Cine bSSFP provides visualization of the flow disturbance in the narrowed and dilated regions of the aorta and PC-CMR is used to assess flow, estimate gradients (Figure 26)262 and quantify collateral flow255,256,258–260 (see Qp/Qs and collateral flow section).
Figure 29.
Maximum intensity projection of a patient with coarctation and multiple aortic collaterals. Flow in these collaterals can be quantified (see text).
CMR is used as preplanning for intervention, as well as continued surveillance of the entire aorta and function of the systemic ventricle.263 After surgical coarctation repair, CMR is the preferred imaging modality.252,264,265 Monitoring for restenosis is important and visualization of collaterals and measurement of flow volume increase from the coarctation repair site to the diaphragm aorta level is more reliable for assessment of recoarctation than arm-leg blood pressure drop.259 Aneurysms may occur at the repair site (Figure 28) and patients with poorly controlled hypertension may be at risk for dissection. The most cost-effective approach to care of patients with coarctation after balloon angioplasty or surgical repair is clinical assessment and CMR in every patient.266 In adults, guidelines recommend CMR imaging of coarctation repair for routine follow up at intervals as much as every 1–3 years depending on the patient’s physiologic state1 and similar reasoning may be thought of in older children and young adults.
After stent placement, CMR is safe and may be chosen to evaluate areas around the stent and other vascular or intracardiac concerns, including BAV, as well as the effects of residual abnormalities such as LV hypertrophy, cardiac index and collateral flow but is not absolutely dependable for visualizing the interior of the stent although dark blood imaging can visualize patency (but not exact dimensions). With the advent of ferumoxytol, in stent visualization has improved for bright blood imaging but exact dimensions remains still elusive. If visualization of the interior of the stent is needed, CT and invasive angiography may be performed.
Besides collateral flow measurements and cardiac index, 4-dimensional (4D) flow CMR imaging has been utilized to determine flow and visualize 4D flow patterns in coarctation of the aorta both prior to and after repair.267–269 There has also been recent work using 4D flow CMR to measure pressure drop in the aorta of patients with coarctation.270,271 Although promising, 4D flow is not yet routinely performed in clinical practice across all centers.
Physiologic assessment of the effects of coarctation on the LV can be assessed by CMR as it is the gold standard for this evaluation.272 LV mass, ventricular volumes, global and regional ventricular dysfunction should be routinely assessed. LV myocardial strain can be assessed using tissue or feature tracking and can be abnormal with LV hypertrophy and systemic hypertension.273 Diffuse fibrosis and increased ECV may be increased in hypertensive heart disease,94,274 although not routinely performed clinically. All of these methods for determining LV status will provide important data for making clinical decisions.
Summary of Recommendations
At the initial diagnosis of coarctation by echocardiography, CMR is recommended to provide conclusive anatomical and functional details needed prior to treatment or to decide if treatment is needed including anatomy of the coarctation, LV hypertrophy, cardiac index and the presence of a BAV etc. if these are not fully delineated by echocardiography (Class I, level of evidence B).
CMR is reasonable to assess collateral flow in patients with coarctation of the aorta, especially if it is unclear from other criteria that treatment is needed (Class IIA, level of evidence B).
After surgical repair of coarctation, CMR is indicated to monitor the status of the aorta and to visualize restenosis or aneurysm formation (Class I, level of evidence B).
After stent placement, CMR can be useful to provide anatomic and hemodynamic assessment of the aorta surrounding the stent, but not to accurately visualize in-stent stenosis (Class IIA, level of evidence B).
After coarctation repair, CMR is recommended to assess the aorta and LV function every 1–3 years in children and adolescents, similar to adults, if echocardiography is insufficient or pathology is suspected (Class I, level of evidence B).
CMR holds the potential to assess LV myocardial strain and diffuse fibrosis/increased ECV in patients with coarctation to assess effects on the LV and may be considered for this purpose (Class IIB, level of evidence B).
Bicuspid Aortic Valve
Background
BAV is the most common congenital cardiac abnormality with a prevalence estimated from 0.5 to 2% in the general population and has a 2:1 male to female ratio.11,275,276 Some families demonstrate an autosomal dominant inheritance pattern.277 Unless associated with significant stenosis or regurgitation or other LV outflow tract lesions, young individuals are often asymptomatic (Figure 30). Critical and moderate to severe aortic stenosis associated with abnormal valve morphology often requires intervention during infancy or early childhood. Later in life, patients may be at risk for associated complications such as ascending aortic dilation with aneurysms (Figure 31) and dissection, aortic stenosis, AR, and endocarditis, all of which could eventually require aortic valve replacement and other aortic surgery. Many cases of BAV diagnosed after childhood may not need intervention until after the fourth decade and beyond.
Figure 30.
Bicuspid aortic valve with fusion of the right and non-coronary cusps using through plane phase contrast-cardiovascular magnetic resonance (PC-CMR) (left without color and 2nd from left with color). Second from the right panel is an bSSFP image in the LV outflow tract demonstrating the limited excursion of the valve leaflets while the right panel is an inplane phase contrast (PC-CMR demonstrating a peak velocity of 3.5 m/s.
Figure 31.
Unicuspid aortic valve with only the left coronary-non coronary commissure open without (left) and with color (middle). Ascending aortic dilation is seen on the right panel
There are multiple types of valve morphology under the rubric of BAV in children and adolescents.278 The most common type is fusion of the right and left coronary commissures (70%) followed by fusion of the right and non-coronary commissures (28%); fusion of the left and non-coronary commissures is the least common. The vast majority of patients with coarctation have fusion of the right and left coronary commissures while fusion of the right and non-coronary commissures is most commonly associated with aortic regurgitation.278 Other types of morphology include partial or complete fusion of one or more of the commissures of the coronary cusps with associated cusp asymmetry and complete or incomplete raphe, as well as “true” symmetric BAV cusps without raphe. Some severely malformed valves are labeled “unicommissural” which may or may not be classified under this label (Figure 31).279,280
Indication for CMR in BAV
Historically, TTE, when acoustic windows are adequate, has been reported as highly sensitive and specific for diagnosing BAV.281 If not well visualized by echocardiography, CMR can determine aortic valve anatomy (Figures 30, 31). In a more recent analysis comparing pathology specimens with TTE and CMR images, the ability to assess valve morphology was higher with CMR when compared to TTE (96% vs 73%).282 Valve morphology has not been definitely associated with severity of disease other than a small subgroup of patients with preferential dilation of the sinuses of Valsalva who have right-left coronary cusp fusion. Flow abnormalities associated with the different types of aortic valve have been characterized, but clinical implications are still being identified.279
For aortic valve morphology, CMR planimetry for aortic valve area has been demonstrated to have the best sensitivity and specificity of all non-invasive methods when compared to invasive catheterization and has high reliability and reproducibility.283–285 Valve area by CMR can be obtained using velocity time integrals and compares favorably to echocardiography.286 Other ways to measure aortic valve area by CMR include Hakki’s formula287 and the continuity equation.
CMR can assess hemodynamic effects of associated stenosis and AR from aortic valve disease. This includes LV hypertrophy and dilation as well as functional volumetric analysis, changes in strain or fibrosis and ECV expansion. In those with significant aortic valve dysfunction, CMR is advantageous for serial evaluation of LV volumes and mass. The standard for determining ventricular sizes and volumes is CMR.272 Though quantitative evaluation of AR volume can be calculated by the difference between RV and LV volumes (assuming no intracardiac shunting, atrioventricular valve insufficiency or PR), PC-CMR sequences allow for direct measurement of forward and reverse volume used to calculate a regurgitant fraction, as well as peak velocity of the jet in the prescribed plane286; checks on the data include subtracting the flow of caval return, main PA flow or the sum of branch PA flowfrom aortic forward flow making CMR more accurate. CMR also allows for assessment of myocardial blood flow with first-pass perfusion at rest and stress, as well as tissue characterization with myocardial LGE for fibrosis and viability. All this information by CMR provides assistance in determining timing for aortic valve intervention or replacement.3
CMR allows for evaluation of the ascending aorta (Figure 31) as it relates to BAV disease and should be utilized when the morphology of the aortic root or ascending aorta cannot be viewed well or measured accurately by echocardiography. Once the aorta is dilated, serial yearly evaluation should occur.288 Because of its strong association with coarctation of the aorta, patients diagnosed with a BAV should also undergo imaging for coarctation of the aorta.250 CMR has also been used to evaluate shear wall stress of the aorta in patients with BAV.289 See Genetic Aortopathy section and the 4D flow imaging subsection.
Summary of Recommendations
If echocardiography is unable to visualize morphology of a BAV or visualize the aortic root or ascending aorta adequately, CMR is indicated to provide the needed information. (Class I, level of evidence B)
CMR should be used for serial monitoring in the setting of BAV valve with associated aortic stenosis or AR and to quantify these hemodynamics (Class I, level of evidence B).
CMR is useful for serial monitoring of the size of the aortic root and ascending aorta in the setting of BAV. (Class I, Level of Evidence B)
CMR is recommended for serial monitoring of the effects of aortic stenosis and AR on the LV (e.g. LV volumes, EF and mass) (Class I, Level of Evidence B) and to obtain direct measurement of AR volume, peak flow velocity and aortic valve area when determining need for aortic valve/aortic surgery (Class IIA, Level of Evidence B).
CMR is beneficial to determine lesions associated with BAV such as coarctation of the aorta, interrupted aortic arch other left sided obstructed lesions (Class I, Level of Evidence B).
Genetic Aortopathy
Background
Genetic disorders associated with aortopathy include Marfan syndrome (MFS), autosomal dominant Vascular Ehlers-Danlos (EDS), Loeys-Dietz (LDS), aneurysms-osteoarthritis syndromes, Turner syndrome, Noonan Syndrome and non-syndromic familial thoracic aortic aneurysms and dissections (FTAAD). These group of disorders are associated with altered connective tissue composition leading to a combination of cardiovascular, skeletal and often ocular manifestations.
The cardiovascular features include valvular and vascular disease, in particular aortic dilatation with increased risk of dissection, aneurysm, rupture and death. The aortic dilation most often affects the aortic root and ascending aorta but can also affect the more distal aorta including the transverse arch and descending aorta. These disorders have variable effect on other cardiac structures. For example, mitral valve prolapse (MVP) is a feature of MFS and LDS, but not EDS, which in turn has a higher risk of coronary artery dissections than the other aortopathies. Turner syndrome is due to the partial absence of an X chromosome, and phenotypically has short stature and ovarian insufficiency, in addition to aortic dilatation, BAV, PAPVR and aortic coarctation. Noonan Syndrome is considered a renin-angiotensin system (RAS)opathy, a group of disorders which have mutations in the signaling proteins for RAS/mitogen activated protein kinase (MAPK) pathway and results in cardiovascular, facial and lymphatic anomalies. Inheritance and penetrance of all these conditions is variable, with many patients representing new sporadic mutations. The diagnosis of these conditions is most often in infancy, due to the combination of musculoskeletal, facial and cardiovascular anomalies. However, the progressive nature of the associated vasculopathy means that lifelong cardiovascular imaging surveillance is required. Table 6 contains a summary of the genetic aortopathies.
Table 6.
Summary of the Genetic Aortopathies
Indications for CMR
Aortic Size Measurements
A small study comparing reproducibility of aortic measurements at multiple levels in both pediatric BAV and genetic aortopathy patients showed that echocardiography derived measurements were systematically smaller by 5–7% and were less reproducible than CMR measurements, particularly in dilated aortic roots and in the descending aorta.290 Most of the genetic aortopathy patients have the potential for complex abnormalities that may affect the entire aorta necessitating comprehensive evaluations. Furthermore, the presence of concomitant skeletal abnormalities renders echocardiography less reliable due to poor acoustic windows. The need for accurate and reproducible aortic size measurements is of utmost importance as aortic size is considered a surrogate for aortic dissection, surveillance recommendations and surgical interventions in genetic aortopathies.291 The 2010 published guidelines for diagnosis and management of thoracic aortic diseases including genetic aortopathies with surgical repair recommendations for genetic aortopathies are purely based upon aortic size.291
The aortopathy in Turner syndrome is complicated by the presence of short stature necessitating a Turner specific z-score (TSZ) developed for 2D echocardiography.292 In patients > 15 years of age, a unique index, the aortic size index (ASI), was proposed (ASI = maximum aortic diameter/bodysurface area) and has been shown to be a better predictor of vascular complications than traditional Turner z score analysis.293 The AHA recently published a scientific statement on the management of Turner syndrome and recommends CMR at diagnosis and CMR surveillance due to the high incidence of undiagnosed vascular anomalies such as coarctation, PAPVC, systemic venous anomalies and coronary anomalies. In low risk Turner syndrome patients, imaging surveillance can be performed every 5–10 years, but with increased frequency of surveillance in patients with high ASIs (> 2.3 cm/m2).294
The natural history of most genetic aortopathies is one of a thinned aortic wall which progressively dilates and loses distensibility thereby heightening the risks of aneurysm formation and dissection throughout its length, but particularly at the root.295 Cohort studies have shown that nearly 60% of MFS patients will have aortic root dilatation by age 35 years and that the aorta increases further with age.296–298
Valvular Disease
As the root dilates, aortic leaflets fail to fully coapt and AR increases. Echocardiography is the mainstay of AR surveillance but a systematic review of 11 articles comparing quantification of AR by CMR to semiquantitative evaluation with echocardiography confirmed that direct aortic measurements using PC-CMR at the sinotubular junction/aortic valve are highly reproducible and accurate and predicts those who progress to surgery with high overall sensitivity and specificity.299 Similarly, the 2014 AHA/ACC guidelines recommend CMR for quantification of AR in patients with moderate or severe AR with suboptimal echocardiography images and evaluation of LV systolic function and volumes.300 Atrioventricular valve disease is more common in MFS compared to other genetic aortopathies with some studies demonstrating > 65% prevalence of mitral valve disorders. Infants and children with MFS present early with tricuspid and mitral valve fibroxanthomatous thickening and prolapse.301,302 CMR has emerged as an important noninvasive modality to diagnose and characterize MVP and help quantify mitral regurgitation.303–306
Left Ventricular Function
Dilated cardiomyopathy is an under-recognized clinical feature of aortopathy syndromes. Myocardial dysfunction is often considered to be the consequence of valvular insufficiency and ventricular volume overload,307 however, in a subgroup of aortopathy patients without significant valvular dysfunction or aortic dilatation, subclinical cardiac dysfunction has been described,308 attributed to a combination of increased aortic wall stiffness and extracellular matrix abnormalities. CMR is the reference standard for evaluating biventricular function as mentioned previously3,309 and can be used to evaluate ventricular performance.
Systemic Vascular Screen
Although aortic aneurysms generally occur at the aortic root in many patients (Figure 32), approximately 50% of patients with LDS syndrome have aneurysms remote from the aortic root, including the head and neck vessels, intracranial berry aneurysms, abdominal branch arterial aneurysms and iliac artery aneurysms. Current recommendations are that LDS patients have annual CT/CMR examination from head to pelvis. CMR, which avoids ionizing radiation and ability to combine both non-contrast and contrast enhanced angiography to achieve whole body evaluations, has distinct advantages over a full body CT with runoff, especially for repeat examinations over time. Non-vascular abnormalities including Chiari I malformation, vertebral subluxations, diaphragmatic hernias, pectus abnormalities and scoliosis are frequently unsuspected clinical findings found on whole body evaluations.310 Patients with Turner syndrome have increased incidence of PAPVC, systemic venous anomalies such as left SVC/interrupted IVC, congenital portosystemic shunts and coronary artery anomalies, many of which are not appreciated on echocardiography, yet relatively easily appreciated on CMR.
Figure 32.
A 1 day old with Loeys-Dietz syndrome. A, Axial oblique bSSFP image in aortic root plane showing massively dilated aortic root with a diameter of 2.2 cm (z score of 8.7) (B) 3D gadolinium enhanced image showing diffuse aneurysmal dilatation of the superior mesenteric artery (arrow).
Aortic Dissection
Aortic dissection is a catastrophic complication of aortic wall disease associated with high mortality and morbidity. In acute symptomatic dissection, rapid imaging diagnosis is essential. CMR can be used to diagnose dissection, distinguish aortic pseudoaneurysm from dissection and assess intimal flaps and aortic branch vessel involvement.311–313 Multiple CMR sequences, both contrast and non-contrast enhanced, all provide specific and unique information including exact positioning of the intimal flap and ability to distinguish slow flow from thrombus in the false lumen.
Vertebral Tortuosity Index (VTI)314
Increased arterial tortuosity of the head and neck vessels, particularly the vertebral arteries, has been described in LDS, MFS and Turner syndrome (Figure 33) and can be calculated easily by CMR. VTI is defined as [(actual vertebral artery length/straight vertebral artery length-1) × 100] measured from vertebral artery origin to C2 if included in the study. Higher VTIs (> 50) have been associated with major adverse clinical outcomes, including a more severely dilated aortic root, increased rate of cardiac surgery, younger age at dissection and death.314
Figure 33.
A 10 year old with Loeys-Dietz syndrome. A and B 3D gadolinium enhanced shaded surface displays showing a dilated ascending aorta, dilated bilateral carotid arteries and tortuous bilateral vertebral arteries. Vertebral tortuosity Index was 95 on right and 65 on left.
4D-Flow CMR in Aortopathies
(Figure 34) Hemodynamic quantification is a major advantage of CMR over other imaging modalities in genetic aortopathy. Multiple studies have demonstrated that BAV-related aortopathy patients, expressing abnormal wall shear stress, may develop significant alterations in elastin fiber and extracellular matrix protein compositions predisposing patients to further aortic dilatation and heightened risk for dissection.315,316 Less is known about genetic aortopathies, however, emerging 4D- flow CMR data suggests that similar findings are present in pediatric genetic aortopathy patients and become more pronounced over time.317,318
Figure 34.
A 17 year old with severe aortic dilatation. Sagittal multiplanar reformatted images from 4D-flow CMR showing severe ascending aortic dilatation extending to involve the common origin of the innominate and left common carotid arteries.
Advantages of CMR MR has been shown to be very accurate in the diagnosis of thoracic aortic disease with sensitivities and specificities that can exceed echocardiography.319–321 CMR provides a multiplanar evaluation of the thoracic aorta and its branch vessels and can conceivably cover the entire systemic arterial circulation from head to toe. CMR has the additional ability to evaluate the competence of the valves and assess LV function in addition to a detailed morphological assessment of the thoracic vessels and additional musculoskeletal abnormalities that are related to aortopathies. The modality is also very accurate in the diagnosis of aortic dissection but in cases of suspected acute dissection, CT may be preferred to speed of examination in a potentially unstable patient. CMR is also preferred for VTI calculation due to the beam hardening CT artifacts related to the intraforaminal courses of these vessels. There is a plethora of CMR literature in pediatric genetic aortopathies.290,314,317,318,322–327
Summary of Recommendations
CMR is the recommended imaging modality for aortic size measurement and surveillance in pediatric patients with genetic aortopathy, as well as assessment of valve regurgitation and LV function. (Class I, Level of evidence B).
CMR should be utilized for complete arterial screening and surveillance in pediatric patients with genetic aortopathy (Class I, Level of evidence C).
Depending upon the risk of the mutation, serial CMRs should be performed every few years (Class I, Level of evidence C) and if stenosis, dilation or aneurysm are determined to be progressing, serial CMRs should be occur more frequently (as much as every 6 months) (Class I, Level of evidence C).
CMR is indicated for assessment of aortic dissection risk: In patients with Turner syndrome with additional risk factors, including BAV, coarctation of the aorta, and/or hypertension, and in patients who attempt to become pregnant or who become pregnant, it may be reasonable to perform imaging of the heart and aorta to help determine the risk of aortic dissection. (Class I, Level of evidence C).
CMR may be beneficial for VTI measurement for risk stratification in patients with genetic aortopathy risk: Limited populations examined have suggested that VTI > 50 is associated with adverse clinical outcomes. (Class I, Level of evidence B).
Vascular Rings and Slings
Background
Vascular rings and slings are rare congenital anomalies involving the aortic arch and PAs, representing about 1–3% of CHD.328,329 The vessels encircle the trachea and esophagus and can lead to varying degrees of compression and clinical symptoms involving the respiratory and/or gastrointestinal system. Some patients are asymptomatic, diagnosed incidentally when imaging is needed for other unrelated reasons and do not require treatment.329 Visualization of the entire ring or sling can be challenging due to indirect imaging of structures with chest radiography or barium esophagram and suboptimal acoustic windows with echocardiography. Due to their significant respiratory symptoms, some patients may first undergo bronchoscopy, revealing a pulsatile stenosis and tracheomalacia, again indirect evidence of a vascular anomaly.330–334 In addition, the vascular structures completing the ring may be atretic, or unable to be opacified with any imaging modality.333,335,336
Indication for CMR in Vascular Rings and Slings
There is an extensive history of the utilization of CMR to diagnose aortic arch anomalies330,335,337–347 CMR provides many advantages for the assessment of vascular rings and slings including imaging of the airway and allowing a conclusive diagnosis to guide in therapeutic management.
Vascular Rings
The most common type of vascular ring, accounting for about 50–60% of cases, is double aortic arch, with persistence of both fourth arches. The double aortic arch may be right-dominant, left-dominant, or codominant, though right dominance occurs in the large majority (Figure 35). Portions of the double arch may also be atretic.333,334 Neonates may present with life-threatening complications of the airway.331 With significant tracheomalacia, symptoms may persist after surgical intervention, with some cases requiring further tracheal surgery.331 CMR has been demonstrated to successfully diagnose patent double arches and those with atretic portions and is superior to cardiac catheterization angiography in its ability to demonstrate associated compression of the airway.330,331,335
Figure 35.
A right dominant double aortic arch. A, Three different views from a contrast enhanced 3D volume rendering from the lateral (left), posterior angled cephalad (middle) and anterior angled caudad (right). Note the ring in the center. B, Dark blood imaging of the trachea in the sagittal (left) and coronal views (right). Note the narrowing distally on the sagittal view and how both arches can be visualized in cross-section on the coronal (arrows). LAO indicates left aortic arch; and RAO, right aortic arch.
The next most common vascular ring type involves a right aortic arch (30–35% of cases) with aberrant left subclavian artery, left ligamentum arteriosum and diverticulum of Kommerell (Figure 36). A small portion of patients with right aortic arch may have mirror image branching and a retroesophageal ductal ligament that can identified by an aortic “dimple” delineated on CMR imaging (diverticulum) in conjunction with tracheal and esophageal compression.333,348 Both double aortic arch and right aortic arches constituting vascular rings have been known to occur in conjunction with CHD such as TOF.349
Figure 36.
A right aortic arch with a diverticulum of Kommerell and an aberrant left subclavian artery (LSCA) in a 6 year old. The descending aorta is seen on the left with the diverticulum (arrow) and the LSCA originating as the last branch while the 3D shaded surface display is in the center of the image viewed posteriorly. The trachea from 3D dark blood imaging is visualized on the right.
Circumflex aortic arch is more rare type of vascular ring with contralateral locations of the transverse aortic arch over the bronchus and descending aorta where there is a “retroesophageal segment” of the aortic arch; that is to say a right aortic arch with a left descending aorta or a left aortic arch with a right descending aorta (Figure 37). Over half of patients have additional cardiac anomalies and some may also have a “cervical” aortic arch. CMR has much higher sensitivity for diagnosing the circumflex aortic arch when compared to chest radiography and echocardiography. Accurate diagnosis is essential for surgical planning.342
Figure 37.
A cervical circumflex right aortic arch as demonstrated by a contrast enhanced volume rendered display as viewed from anterior (left), posterior (middle) and from superior (right). The ascending aorta (AAo) ascends on the right and the transverse aortic arch (TAo) crosses posteriorly to the left, posterior to the trachea and esophagus and the descending aorta (DAo) descends on the left.
Other rarer vascular rings with varying location of aberrant vessels and the ligamentum arteriosum (eg right aortic arch with an aberrant left innominate vein) have all been optimally imaged with CMR (Figure 38).
Figure 38.
3D volume rending of a right aortic arch with an aberrant left innominate artery (L Inn) visualized from posterior (left) and anterior (right). RCA indicates right carotid artery.
Pulmonary Artery Slings
A PA sling is the anomalous origin of the left PA from the right PA which courses between the trachea and esophagus, creating a “sling “around the distal trachea or proximal right bronchus (Figure 39). Impingement of the airway leads to respiratory symptoms and compression of the esophagus commonly causes dysphagia and vomiting. The ability to diagnose PA sling is equivalent with CT and CMR (although to diagnose a complete tracheal ring, CT should be utilized), though CMR avoids ionizing radiation, iodinated contrast and flows to both lungs can be evaluated.331,339,340
Figure 39.
A pulmonary artery sling where the left pulmonary artery (LPA) arises from the right pulmonary artery (RPA) in a one year old. Off axis axial CMR cine (upper left), dark blood (upper right) and 3D reconstruction (lower left) demonstrate the anatomy. Lower right displays an off-axis coronal view of the trachea.
Considerations
Vascular rings and slings may present with life-threatening complications of the airway in the newborn period. Upper airway obstruction and dysphagia are common presenting symptoms during this time and later in infancy or early childhood and beyond. Prolonged or recurrent respiratory symptoms or refractory asthma may necessitate workup for vascular anomalies. While chest radiography, barium esophagography, bronchoscopy and echocardiography are suggestive of a vascular ring or sling, full details of the vascular anomaly and impacted structures (esophagus, trachea, bronchi) must be delineated further for adequate surgical management, and these can be provided by CMR. As some surgeons prefer a 3D reconstruction of the great vessels prior to operating on arch anomalies, CMR can faithfully create a 3D model using both contrast enhanced and non-contrast techniques.332 CT can create 3D reconstructions of the great vessels as well as the trachea and can be utilized for this in centers that do not have the expertise in CMR. Invasive angiography is not indicated for the sole diagnosis of vascular rings and slings and have been replaced by CMR,331,333,334 but may be utilized in the setting of additional intracardiac defects needing intervention.
Summary of Recommendations
After preliminary assessment and clinical suspicion for a vascular ring or pulmonary sling, CMR is indicated for definitive anatomic diagnosis in the management of these lesions prior to therapeutic intervention. (Class I, level of evidence B) Flows to both lungs may be assessed in pulmonary sling.
CMR is indicated for the assessment of tracheal narrowing in patients with vascular rings and slings (Class I, level of evidence B)
CMR is indicated for the assessment of associated lesions of vascular rings such as TOF (Class I, level of evidence B)
TGA With a Systemic RV (Corrected TGA or TGA After Atrial Inversion)
Background
TGA, apart from those with a SV, may occur with a systemic RV in 2 different categories:
Congenitally corrected transposition (ccTGA), also called L-looped TGA or TGA {S,L,L}, occurs when the morphologic LV is on the right side of the circulation and associated with the pulmonary valve and the morphologic RV is on the left side of the circulation and associated with the aorta.350 There is atrioventricular and ventriculoarterial discordance which results in normal hemodynamics where systemic venous returns is directed towards the lungs and pulmonary venous return is directed towards the body. These patients most commonly have no other CHD, but if CHD occurs, they most commonly have VSD, pulmonary stenosis or may have Ebstein’s anomaly of the left sided tricuspid valve.
TGA after atrial inversion, also called TGA after atrial switch, occurs when patients with D-looped TGA (i.e. {S,D,D}; the morphologic RV is on the right side of the circulation and associated with the aorta and the morphologic LV is on the left side of the circulation and associated with the pulmonary valve) undergo an atrial switch operation, either a Senning or a Mustard procedure. In D-looped TGA, there is atrioventricular concordance and ventriculoarterial discordance resulting in the systemic and pulmonary venous systems in parallel circuits; the Senning and Mustard procedures baffle venous return to the correct ventricle physiologically. Most of these operations were performed in the era prior to ASO so the vast majority of these patients are adults although in some circumstances, this is performed in the "double switch" operation for TGA {S,L,L}.
Whether the RV can tolerate a lifetime of systemic vascular resistance and pressure, whether a morphologic D-loop or L-loop, has always been debated, making study of RV performance in this patient population crucial. In a multi-institutional retrospective analysis, by 45 years of age, 67% of those with ccTGA with intracardiac lesions and 25% without intracardiac lesions had heart failure and systemic ventricular dysfunction.350 Mechanics of the systemic RV are markedly different from the single RV and from the normal systemic LV.30 Both the RVs in ccTGA and TGA after atrial inversion can be dilated, spherical and poorly functioning.351 For those with TGA after atrial inversion, after a high short term mortality rate (up to 20%), there is a longer lower mortality rate which nevertheless remains significant.352 Multiple studies have demonstrated a significant decline in RV function in adulthood, with or without symptoms.353,354 Both RV and LV in the systemic RV circulation demonstrate abnormal response to exercise.355
Indications for CMR
CMR is ideally suited for the study of the systemic RV in the setting of TGA due to its ability to overcome the issues of RV geometry and retrosternal location that challenge echocardiography. The issue of RV geometry is very germane in these diseases as the RV remodels and the anatomy changes when it becomes a systemic ventricle. In addition, the geometry of a D-looped RV (Figure 40) as in TGA after atrial inversion is very different than that of an L-looped RV as in ccTGA (Figure 41). The use of CMR for quantification of RV function and ventricular volumes in these patients have been extensively published351,355–362 including the aforementioned abnormal response to exercise. Adverse cardiac events have been associated with RV volume, RV mass index, RVEF, RV wall stress and LV volume.363–365 There is evidence to demonstrate that as they age, patients with a systemic RV have functional decline and no resting parameters correlate with exercise function.366,367 Because of the ability to quantify ventricular performance independent of geometry, CMR has been utilized in several pharmacologic trials in this patient population.368,369 Systolic strain by CMR has also been quantified using feature tracking methods370 and dyssynchrony, as measured by feature tracking, correlates well with major cardiac events in those with a systemic RV.363
Figure 40.
Transposition of the great arteries after atrial switch. A, Panel on the left is a cine of the upper limb (UL) and lower limb (LL) of the systemic venous pathway, in this case, a Mustard operation. On the right is a 4-chamber view with the asterisk denoting the distal end of the systemic venous pathway. B, 3D gadolinium volume rendering of the main pulmonary artery (MPA) arising from the left ventricle (LV) on the left and the aorta (Ao) arising from the right ventricle (RV). Note on the left that the UL of the systemic venous pathway can be seen (arrow).
Figure 41.
Corrected transposition of the great arteries (ccTGA). The upper left panel is a stack of bright blood cine images depicting the geometry of a 2 year old patient with ccTGA. The upper right panel is one slice showing the right ventricle (RV) on the left and the left ventricle (LV) on the right. The lower panel is a 4-chamber view.
In patients with TGA after atrial inversion, CMR has been utilized for several decades to assess the size, patency and leaks of intra-atrial baffles371–373 (Figure 40) as well as ventricular outflow tract obstruction.357,359,374 It is not uncommon to visualize obstruction to the superior limb of the systemic venous pathway375 or right to left shunts due to baffle leaks by cine.359 PC-CMR is utilized to determine the size of the shunt (Qp/Qs) with internal checks along with in-plane velocity mapping to visualize the leak. Ventricular outflow tract obstruction is assessed by CMR on a routine basis.376
Similar to the debate regarding whether the RV can tolerate a lifetime of systemic vascular resistance and pressure, the ability of the morphologic tricuspid valve to maintain systemic atrioventricular valve work is also questioned. TR is generally due to annular dilation and systemic RV dysfunction in ccTGA,377 however, this may also be due to Ebstein’s anomaly of the left sided tricuspid valve378; either way, it presents a volume load on the ventricle. Indeed, in the presence of significant TR and if present, deteriorating RV function, a “double switch” may be performed, combining an ASO procedure with a Senning379 and CMR is utilized not only to evaluate the intra-atrial baffle but also the coronary arteries and ventricular function. CMR, as previously noted, can measure atrioventricular valve insufficiency via 2 methods utilizing PC-CMR alone or in combination with cine CMR. There is evidence to demonstrate that the systemic RVEF cannot increase in response to increasing atrioventricular valve insufficiency.380 Ultimately, though, accurate measurements of TR by CMR are important as this has been linked to ventricular function and clinical outcomes in this patient population.381–383
Other anatomic abnormalities can be assessed by CMR including VSD repair, pulmonary stenosis and PR after repair and the size of the branch PA in patients with ccTGA (Figure 41). PC-CMR is used to determine Qp/Qs similar to baffle leaks along with quantification of flows to both lungs and PR fraction similar to TOF.
Characterization of the myocardium with CMR has demonstrated a role for the assessment of myocardial fibrosis and its link to the gradual decline of the systemic RV (Figure 42). The presence of LGE is associated with older age, RV volume and function, QRS duration, and prior arrhythmia or syncope.384 In a study of 34 patients with systemic RVs, LGE was linked not only to older age, lower RVEF and arrhythmia but also higher RV wall stress, reduced peak oxygen uptake during exercise and a worsening of clinical symptoms.365 LGE in the systemic RV is related to collagen content385 as well as ventricular dyssyncrhony.386 Diffuse fibrosis, using ECV, has also been assessed in the systemic RV in a dual chambered circulation.387,388 Importantly, the presence of myocardial fibrosis is prospectively related to a mix of adverse clinical outcomes in follow up.389,390 These types of studies have been important in understanding the role of fibrosis in the natural history of the systemic RV, and may indirectly imply a role of anti-fibrotic medical therapy.
Figure 42.
Myocardial scarring in the right ventricle (RV) in a patient with ccTGA from 2 views. Ao indicates aorta.
Summary of Recommendations
CMR is indicated for quantification of systemic RV volumes, mass, and ejection fraction (Class I, level of evidence B) and can be useful for quantification of pulmonary LV performance parameters in patients with a dual chambered circulation (Class IIA, level of evidence C)
CMR is recommended for the assessment of the systemic atrioventricular valve (left sided tricuspid valve in TGA {S,L,L} or right sided tricuspid valve in TGA {S,D,D} after atrial inversion procedure) in patients with a systemic RV (Class I, level of evidence B)
CMR is useful for detecting systemic RV myocardial fibrosis, which may have important implications for a given patient’s prognosis and therapy options (Class I, level of evidence B)
CMR is beneficial for the detection of stenosis and leaks of the interatrial baffle in those patients with systemic RVs who have undergone an atrial inversion procedure (Class I, level of evidence B) as well as for detection of the presence and severity of outflow tract obstruction (Class I, level of evidence B).
CMR is useful to assess associated lesions and the sequelae of repair such as VSD, pulmonary stenosis and PR, especially in patients with ccTGA (Class I, level of evidence B).
Hypertrophic Cardiomyopathy
Background
Hypertrophic cardiomyopathy (HCM) is a disorder of increased LV mass and may be differentiated into syndromic (with other systemic involvement) and nonsyndromic (without other systemic involvement) types. This section describes nonsyndromic disease. This type of HCM is a common genetic cardiomyopathy resulting from autosomal dominant mutations in multiple genes encoding the cardiac sarcomere; including cardiac β-myosin heavy chain, cardiac myosin binding protein C and Troponin T. There have been over 1400 mutations described.391 On histopathology, HCM is characterized by myocyte disarray, myocardial hypertrophy and fibrosis along with abnormal small intramural arterioles with thickened walls and narrowed lumen resulting in ventricular dysfunction and ventricular arrhythmias. The presentation of HCM is heterogenous with varied symptomatology including fatigue, chest pain, palpitations and even sudden cardiac death (SCD). Treatment options include medical management, surgical myomectomy or alcohol septal ablation for relief of critical LV outflow obstruction (gradient ≥ 50 mmHg), and implantable cardioverter and defibrillators (ICD) for those patients considered to be at highest risk for SCD.
Guidelines for the diagnosis and treatment of HCM have been published by both the European society of Cardiology (ESC) and the ACC/AHA.392,393 Both sets of guidelines have similar requirements regarding the diagnosis of HCM, which in adults is defined as a left ventricular wall thickness (LVWT) of > 15 mm in one or more myocardial segments, not explained by loading conditions. In children, the diagnosis requires an LVWT > 2 standard deviations from the predicted mean (z score > 2).
Risk stratification for SCD in pediatric HCM has also been extrapolated from these adult based guidelines as suggested in the current ACC/AHA guidelines.393 The ESC guidelines differ from the ACC/AHA in that the ESC uses a risk prediction model to guide use of implanted cardiodefibrillator (ICD). In addition, the ESC guidelines are only intended for adult use while the ACC/AHA guidelines are intended for both adult and pediatric use. In the ACC/AHA guidelines, focus on the presence of at least one clinical risk factor for SCD (LVWT ≥ 30 mm, syncope, nonsustained ventricular tachycardia (NSVT), family SCD history or abnormal blood pressure response to exercise) as class IIa indications for the implantation of ICD as primary SCD prevention. Recently, there have been attempts at creating risk calculators solely for pediatric use.394–396
Indications for CMR
Quantification and Distribution of LV Hypertrophy
The diagnosis of HCM is characterized by an increased diastolic LVWT in at least one segment in the presence of a nondilated LV chamber (Figure 43) which is readily identified on CMR at any age. Increased LVWT may be present at birth or may develop during childhood and adolescence despite previously normal echocardiographic evaluations, necessitating serial evaluations in patients with gene positive or suspected HCM.397 One of the critical indications for pediatric CMR in this disease is the identification of increased LVWT not visualized by echocardiography but visualized by CMR.398,399 This is not a trivial occurrence particularly when the increased LVWT is focal and limited (as is not infrequently the case in pediatrics) which is present in up to 5% of children. This leads to an HCM diagnosis that would have otherwise been missed with CMR solely responsible for diagnosis.400–402
Figure 43.
A 14 year old with hypertrophic cardiomyopathy (HCM). Upper left panel is a cine bSSFP sequence in short axis plane demonstrating diffuse LV myocardial hypertrophy affecting the basal septum the most (*) with phase sensitive inversion recovery image demonstrating corresponding late gadolinium enhancement (upper right, arrow). Myocardial Strain “bullseye” map demonstrating globally decreased LV myocardial circumferential strain (lower panel).
The most common pattern of HCM is increased LVWT in the basal anteroseptal region which is frequently associated with LVOT obstruction and mitral valve leaflet elongation. Other phenotypes include diffuse hypertrophy involving more than 50% of the myocardium, reverse septal contour, apical aneurysm403 and apical HCM which may manifest clinically with arrhythmias, diastolic dysfunction and small cavity size,404,405 all of which can be delineated comprehensively by CMR. RV hypertrophy is additionally observed in a third of adult HCM patients, most commonly at the RV septal hinge points.406 CMR derived LVWT measurements have a primary role in risk stratification with massive LV hypertrophy (LVWT ≥ 30 mm) associated with the highest risk of SCD.407,408 A meta-analysis of 25 pediatric HCM studies found a similar significant association between extreme LVWT and SCD in children and young adolescents. The hazard ratio for LVWT was 1.80 (95% CI 0.75–4.32, p = 0.19, I2 = 21%). The odds ratio estimate for extreme LV hypertrophy was 1.70 (95% CI 0.85–3.40, p = 0.13, I2 = 31%). For pediatric patients, new guidelines suggest that a maximal LVWT with a Z score ≥ 20 or > 10 in conjunction with other risk factors is reasonable as a risk factor for SCD.393
Biventricular Functional Assessment
As mentioned, CMR is the reference standard for evaluating biventricular function.3,309 bSSFP sequences performed in the standard planes provide accurate measurements of LVEF, chamber size and LV mass409 (Figure 43) which can be used serially for surveillance in suspected or confirmed HCM. Over time, a gradual transition from a hypertrophied, non-dilated LV with hyperdynamic systolic function to one of reduced systolic function can be seen in adults although this is uncommon in children. This is termed end-stage HCM and is generally regarded as having unfavorable outcomes with a mortality rate of 11% per year.406,410 In a large cohort of adult HCM patients, those with the lowest LVEF had the largest ventricular sizes and degrees of LGE by CMR, suggesting advanced remodeling in end-stage disease. Conversely, those with hyperdynamic systolic function had the lowest amount of LGE. Some pediatric HCM patients develop abnormal diastology with restriction which may manifest on CMR as left atrial dilatation, without LV dilation and is associated with poorer outcomes.411
Assessment of Dynamic Left Ventricular Outflow Tract (LVOT) Obstruction
Dynamic obstruction of the LVOT due to mitral valve systolic anterior motion (SAM) as well as due to septal hypertrophy is one of the leading causes of exercise intolerance in HCM patients and may necessitate invasive treatment measures such as myomectomy or septal ablation. LVOT gradients are conventionally measured using echocardiography, but CMR can identify the site of obstruction and assess whether there are contributing anomalies such as anomalous insertion of the anterior papillary muscle and elongated mitral valve leaflets (mitral leaflet length > 2 × the transverse dimension of the LVOT) at end systole.412 These findings are important in pre-operative planning in HCM patients who are considered surgical candidates by characterizing role of the mitral valve and sub-mitral apparatus and providing the surgeon an accurate estimate to the depth of the extended surgical resection of septal muscle necessary to achieve optimal relief of outflow obstruction.
Myocardial Fibrosis Assessment
LGE (Figure 43): The precise pathophysiologic mechanism responsible for LGE in HCM remains uncertain but is likely a combination of gadolinium deposition in areas of myocardial fibrosis and between areas of myocardial disarray. It is most prevalent in areas of hypertrophy and has been associated with increased incidence of ventricular tachyarrythmias and heart failure.413,414 LGE usually is patchy and midmyocardial in distribution. LGE has been described in 46–73% of children and adolescents with phenotypic HCM, despite preserved systolic function; it has been shown to increase annually with serial CMR surveillance, constituting on average 10.4% of LV mass.415,416 Those children with LGE were found to have greater LV mass and were at risk for adverse events including ventricular tachycardia,417 aborted SCD418 as well as having decreased ventricular strain (Figure 43).416,419–422 A 4-center study of 1,293 adult patients followed for 3.3 years showed that LGE of ≥ 15% was associated with a twofold increase in SCD event risk.423 Two subsequent meta-analyses that included that study and others confirmed that the incidence of SCD was increased in the presence of LGE, but differed in whether extent of LGE was important.424,425
Diffuse fibrosis (Figure 44): Adult patients with HCM have abnormal T1 indices concordant with diffuse myocardial disease, even in the absence of LGE.426–428 Additionally, diffuse ventricular fibrosis by T1 mapping has been shown to be a predictor of non-sustained ventricular tachycardia and aborted SCD in adult HCM patients.429 In pediatric HCM patients, studies have demonstrated increased native T1 and ECV in hypertrophied areas of myocardium compared with non-hypertrophied areas and higher in LGE positive segments.430,431 T1 mapping can also be used to distinguish HCM from other potential causes of hypertrophy including hypertensive cardiomyopathy and the athletes’ heart.432,433 Nevertheless, more studies are needed in this area.
Figure 44.
On the left is an extracellular volume map showing globally elevated elevated extracellular volume fraction (ECV) in a 14 year old patient with hypertrophic cardiomyopathy (HCM). On the right is a T2 map of left ventricular myocardium with T2 value of 69 ms ± 1.2 also in a 14 year old patient with HCM.
Exclusion of Other Diagnoses
Although HCM accounts for the majority of unexplained LV hypertrophy seen in adolescents, a number of other non-sarcomeric diseases can produce/mimic increased myocardial thickness including non-HCM causes of LVOT obstruction, hypertensive cardiomyopathy, LV noncompaction and metabolic/ infiltrative conditions including Anderson-Fabry’s disease and Friedrich’s ataxia. CMR can have a role in differentiating these conditions by identifying focal, limited hypertrophy not well visualized by echocardiography, distinguishing trabeculations from hypertrophy and identifying characteristic patterns of LGE. CMR may clarify and even alter the diagnosis in patients initially diagnosed with apical HCM who actually demonstrate LV non compaction on CMR.434
Myocardial Strain Assessment
The myocardial disarray, myocardial hypertrophy and abnormal papillary muscle orientation are all thought to contribute to abnormalities in myocardial strain by speckle tracking echocardiography in areas of hypertrophy and fibrosis. In addition, it has been associated with adverse cardiac events in pediatric and adult HCM.435,436 CMR feature tracking using standard cine bSSFP (Figure 43) can detect reduced LV myocardial mechanics (global and segmental longitudinal strain) in children and young adults with HCM and normal values in children have been published.97 This decrease in strain correlates with the degree of LVOT obstruction, LVWT and LGE420–422 as well as adverse events.421 Nevertheless, more studies are needed in this area.
Myocardial Perfusion
Adverse microvascular remodeling and coronary microvascular dysfunction (CMD) has been noted in HCM. Studies investigating myocardial perfusion in HCM patients have demonstrated abnormal perfusion metrics in both hypertrophied and non-hypertrophied segments of the LV, as well as correlation between the extent of perfusion abnormalities, LGE and degree of hypertrophy.437,438 A small pediatric study, demonstrated inducible myocardial ischemia in 7/13 pediatric HCM patients who were LGE negative suggesting that CMD may precede macroscopic fibrosis, however, it remains unknown whether CMD is an independent risk factor for SCD in HCM.439 Although promising, at present, it remains a research technique and more studies are needed.
T2 Weighted Imaging
Recent interest in T2 weighted imaging and its association with SCD has developed (Figure 44). Two studies investigating T2 signal in HCM patients are noteworthy. One demonstrated increased myocardial T2 signal intensity in patients classified as high risk for SCD and demonstrated positive correlation with the presence of > 15% LGE while the other demonstrated a positive correlation with life threatening arrhythmias.440,441 As with perfusion, although promising, at present, it remains a research technique and more studies are needed.
Coronary Artery Imaging
Myocardial bridging of the LAD is more common in HCM than in other causes of LV hypertrophy and may potentially be a mechanism for SCD, although there is no evidence to support this hypothesis. Myocardial bridging may be demonstrated by specific coronary CMR sequences (see Coronary section).
Advantages of CMR Over Other Modalities
Although TTE assessment of patients with HCM has been traditionally performed, limited acoustic windows, an inability to reliably capture focal hypertrophy and imaging plane obliquity may result in both under and overestimation of LVWT. CMR provides high contrast between bright blood and dark myocardium, excellent spatial resolution, and full biventricular coverage. In addition, short-axis images, derived perpendicular to the true LV long axis, allows LVWT measurements by CMR to be more precise and reproducible,442 enabling a diagnosis of HCM which may be missed by TTE.400–402 This holds true even in children, who have better acoustic windows than adults; a small study of pediatric HCM patients demonstrated that echocardiography derived measurements had poorer inter-observer and intra-observer reproducibility compared with CMR measurements.443 This may be due to superior visualization of the LV epicardial/endocardial borders with CMR, and visualization of all hypertrophied segments without any risk of obliquity272,400,444.
CMR is the only non-invasive modality that can assess tissue characteristics including the presence of macroscopic and global fibrosis, the former of which can be clinically utilized to risk stratify patients regarding potential ICD implantation. The emerging roles of parametric T1 mapping, myocardial perfusion imaging, and T2 imaging will likely increase our understanding of tissue characterization and may further stratify SCD risks.
Summary of Recommendations
CMR is the recommended for confirmation of HCM diagnosis, evaluation of possible apical HCM or aneurysm and surveillance of LVWT in HCM400,409,445 (Class I, Level of evidence A).This includes patients with LV hypertrophy in whom there is a suspicion of alternative diagnoses including the athlete’s heart. In children and adolescents with a diagnosis of HCM, contrast enhanced CMR surveillance should occur every 3–5 years for risk stratification, evaluation of LGE, wall thickness and ventricular performance (Class I, Level of evidence B).
CMR is the recommended to screen for HCM in patients with a family history of HCM when echocardiography is inconclusive (Class I, Level of evidence A)
CMR is beneficial to monitor ventricular function when a more accurate measure than echocardiography is needed, when there is a concern for ventricular performance by echocardiography, or in selected patients for risk stratification for ICD placement393 (Class I, Level of evidence A). CMR is also useful to monitor LVOT obstruction in pediatric HCM patients when echocardiography is inconclusive (Class I, Level of evidence B)
CMR is reasonable for the evaluation of myocardial fibrosis for risk stratification in pediatric HCM, possible ICD placement and to monitor the patient more closely than those without LGE. (Class I, Level of evidence B)
CMR can be beneficial in pediatric patients with LV hypertrophy in whom alternative diagnoses in addition to HCM are suspected. (Class I, Level of evidence B)
CMR strain measurements may be considered in pediatric HCM patients to monitor ventricular function as well as for risk stratification; patients should be monitored more closely if strain is below the lower limits of normal97 (Class IIb, Level of evidence B)
Duchenne Muscular Dystrophy
Background
Duchenne muscular dystrophy (DMD) is an X-linked genetic neuromuscular disorder resulting in dystrophin protein mutation. DMD is one of the dystrophinopathies that also include Becker muscular dystrophy (BMD), and X-linked dilated cardiomyopathy. DMD results in the more severe phenotype presenting as skeletal muscle weakness early in life and often progressing to loss of ambulation early in the second decade of life. BMD is milder with a considerably more variable phenotype. The incidence of DMD is estimated to be ~ 1 / 5000 live male births.446 The United States prevalence is estimated to be 1.4 per 10,000 males.447 DMD patients also develop respiratory insufficiency. The restrictive lung disease is a result of diaphragm and secondary respiratory muscle weakness. Historically, the most common cause of death was respiratory failure. However, improved respiratory therapies have resulted in cardiomyopathy and resultant heart failure and arrhythmias becoming the most common cause of death.448
The cardiomyopathy of DMD and BMD is characterized by progressive loss of functional myocytes leading to regional dysfunction followed by global dysfunction. Although, the cardiomyopathy has been commonly described as having a dilatated phenotype, chamber dilation occurs only in the late stages of the disease. The cardiomyopathy is variable both in age of onset and severity. Histologically, the dystrophinopathies lead to alternating areas of myocyte hypertrophy, atrophy and necrosis, and finally fibrosis with replacement of cardiomyocytes by connective tissue and fat. It is estimated that ~ 30% are symptomatic, but elucidating symptoms from patients who are non-ambulatory secondary to skeletal muscle disease is very difficult.449 Although myocardial damage is present on a cellular or histological level starting very early in life, echocardiographic abnormalities are usually delayed until the second decade of life. For DMD, risk of LV dysfunction increases significantly with age, from < 5% for boys < 10 years of age to > 75% for men > 20 years of age.450 There are patients who will have reduced ejection fraction as early as 8 years of age.451 Subtle abnormalities of deformation using strain analysis can be seen in patients as young as 5 years.452
Indications for CMR
There are several guidelines available addressing the cardiovascular care of DMD/BMD patients.453 All recommend that cardiac care begin shortly after initial diagnosis is made. Since the risk of cardiovascular involvement is low for very young children, the initial evaluation consists of clinical evaluation, baseline ECG, and TTE. Yearly TTEs are recommended until the child is old enough to undergo CMR without sedation.
TTE has limitations in neuromuscular diseases.454,455 As patients age, the image quality is degraded due to several factors including spinal and thoracic bony deformities, increased thoracic and abdominal adiposity that decreases ultrasound penetration and alteration of the inter-rib spaces decreasing the size of acoustic windows. A study evaluating TTE image quality for 31 DMD patients aged 11–34 years showed that none of the apical four-chamber image acquisitions were of diagnostic quality.456 In a study designed specifically to assess the ability of TTE to assess DMD patients,457 they found that by 13 years of age, 50% of the studies were classified as suboptimal with ≥ 30% of segments inadequately visualized, and by 15 years of age, 78% of studies were suboptimal. Consequently, in this population, TTE data may not always accurately reflect cardiac function. Some of these limitations can be overcome by administration of ultrasound contrast,458 however, contrast echocardiography has not been included in the standard clinical care guidelines and is used only in patients who have suboptimal acoustic windows and contraindications to CMR. Secondary to the known difficulty with accurate TTE assessment, the first CMR studies for DMD cardiomyopathy patients were performed to accurately measure the LVEF where echocardiographic windows were poor.
CMR is a proven modality for accurate and reproducible assessment of both RV and LV volumes and masses that is not affected by body habitus or lung artifacts,459,460 so it is not surprising that it has been utilized for accurate measures of ventricular function in children with this diagnosis454,455,461–464 and to assess the efficacy of treatment.465 Although the use of CMR is very common in this patient population, there are few direct head-to-head comparisons of CMR with echocardiography. Brunklaus et al.462 studied 35 DMD boys (12–18 years; median 15 years) who underwent both TTE and CMR, and although echocardiographic shortening fraction correlated with CMR LVEF (rs = 0.67; p < 0.001), 75% of the TTE studies had deficient ultrasound scanning windows and in 26% measurements, either significantly over- or underestimated LV systolic function compared to CMR. Buddhe et al.464 studied 35 subjects with a mean age of 13.6 years old who also had both TTE and CMR and found, similar to the previous study, weak correlation of TTE parameters with CMR EF.
CMR strain imaging has been studied in patients with DMD and has been found useful to detect occult contractile dysfunction and dyssynchrony.463,464,466–468 Hagenbuch et al. found that serial monitoring of cardiac dysfunction by serial strain measurements was able to predict progression of the disease in the absence of deterioration of LVEF.469
The first large scale clinical report of CMR in DMD with both ventricular function and myocardial characterization using LGE came from Brazil in 2007,462 although there have been a number of other studies of LGE with ventricular function in pediatrics.464,467 Silva and colleagues showed the feasibility of using CMR for routine assessment of both ambulatory and non-ambulatory DMD patients and demonstrated for the first time that DMD patients have LGE in a pattern that appears to represent the known myocardial fibrofatty replacement. They showed that even patients with normal LVEF were positive for LGE down to age 8 years and that LVEF was markedly different between the groups with and without LGE, linking LGE with decreased LV performance. They noted the LGE was predominantly seen in the inferolateral and anterolateral segments, a finding that has long been known from early pathology studies.470
The first report of CMR in a small multi-institutional retrospective study in children with DMD showed LGE was prevalent,471 finding 32% with LGE primarily involving the basal inferolateral segment of the LV in a sub-epicardial distribution. Patients with LGE were older than those without (mean age 16.4 vs 12.9 years), but most importantly, they noted a similar finding to that seen by Silva et al.461 where LGE was inversely correlated with LVEF. A larger study of 314 DMD children by Hor et al. showed the overall prevalence of LGE was ~ 36%,451 increasing from 17% of patients < 10 years to 34% of those aged 10–15 years and 59% of those > 15 years-old. Ten percent (11/113) of patients who had LGE died an average of 10.8 months after CMR. Conversely, only one patient from the LGE negative group died. Patients who died had larger LV volume and greater number of positive LGE segments compared to those who remained alive.
CMR has been associated with outcomes for the DMD/BMD population. In young adults, Florian et al. prospectively studied 88 male DMD/BMD patients472 and during a mean follow-up time of 47 ± 18 months, the primary endpoint (death or transplantation) was observed in 3% and the secondary endpoint (hospitalization and/or ventricular tachycardia) in 24%. They found that LVEF and the presence of “transmural” LGE were independent predictors for secondary endpoints. Interestingly, in the group of patients with preserved function (LVEF > 45%), patients with “transmural” LGE had a significantly lower event-free-survival compared to those without. Similarly, in children and adolescents, Menon et al. found LGE present in 78% of the boys.473 Compared with patients without LGE, those with LGE were older with lower LVEF (46 ± 12 vs 56 ± 9% respectively) and a higher incidence of ventricular tachycardia (40 vs 0%, respectively). Interestingly, during the study period, six of the subjects (19%) died. The factors associated with mortality were increased age, advanced grade of LGE, higher LV end-systolic volume, lower LVEF, and ventricular tachycardia.
There are now longitudinal studies of CMR in DMD populations that help to inform the appropriate longitudinal timing for these exams. Tandon et al. reviewed 465 serial CMR DMD studies, all of whom had ≥ 4 assessments.474 They determined that LVEF declined ~ 0.58% per year independent of LGE status. More interestingly, LVEF did not decline over time if LGE was absent but declined at a rate of ~ 2.2% per year when LGE was present. The number of LGE-positive LV segments increased with age as well.
Limitations of CMR as it Relates to DMD Patients Although CMR imaging does not suffer from the limitation of poor acoustic windows attributable to body habitus, thoracic abnormalities, or lung disease, there are factors that do limit its utility for imaging every patient with DMD. Some DMD patients cannot be comfortably positioned on the CMR table because of significant contractures, severe back pain, or immobility which is particularly true for older, non-ambulatory patients. CMR image quality is limited in patients with atrial or ventricular arrhythmias, irregular respiratory rates or motion, or inability to remain motionless in the scanner. Although some of these limitations can be overcome with sedation or anesthesia, such studies come with increased risk in this patient population secondary to compromises in respiratory or cardiac function. A thorough risk–benefit analysis is required before undertaking a sedated CMR study for a DMD patient. Finally, there is potential for artifact leading to CMR image degradation from implanted devices in DMD patients including both cardiac electrophysiologic therapeutic devices such as pacemakers and ICDs as well as skeletal devices including spinal fixation rods.
Summary of Recommendations
CMR should be used to evaluate biventricular size and systolic function of DMD and BMD patients after the age of 8 years (or when they do not need sedation) (Class I, Level of Evidence B) and may be performed every year if needed.
CMR myocardial fibrosis evaluation of DMD and BMD patients is recommended for prognostication and risk stratification (Class I, Level of Evidence B).
CMR strain analysis of DMD and BMD patients may be considered for prognostication and risk stratification (Class IIb, Level of Evidence B).
Cardiac Tumors
Background
Cardiac tumors are rare in the pediatric population, with an incidence between 0.027 and 0.3%.475,476 Management requires differentiation of specific tumor types, an objective for which imaging plays a significant role. Echocardiography remains the primary modality for initial detection and screening of cardiac tumors though has a limited ability to further characterize the mass other than its presence with the exception of echogenic and echolucent (presumably cystic) regions. In addition, echocardiography can be limited by acoustic windows and reliable differentiation of tumor from vegetation or thrombus can be difficult. Many masses are benign but still need to be distinguished from malignancies, characterized for prognosis, assessed for impact on heart/valve function and determined whether an intervention is needed. Thus, additional imaging is generally warranted.
Indications for CMR
After identification of masses by echocardiography, CMR is utilized in a number of different ways (Figure 45) and has demonstrated utility for477–482:
Figure 45.
Cardiac fibroma in an 8-year-old male characterized by cardiovascular magnetic resonance. A, Still frame of bSSFP cine shows a large mass in LV free wall. B, Phase sensitive inversion recovery (PSIR) sequence positive for late gadolinium enhancement, characteristic of a cardiac fibroma. C, Mass surgically resected, pathology confirmed fibroma.
Precise definition of anatomic location of the tumor including size, sites of myocardial attachment, and tissue layers involved.
Tissue characterization and differentiation between benign and malignant tumors as well as to differentiate the mass with thrombus.
Differentiation between tumor and thrombus483
Determining the extent of the tumor and relationship with surrounding structures to determine impact on heat and valve function and guide surgical resection (eg outflow tract obstruction).
Imaging of fetal tumors
As certain genetic syndromes are also associated with cardiac tumors, CMR is especially useful in establishing the diagnosis when cardiac masses are noted on echocardiography in that patient population.482
Given the overall low incidence of cardiac tumors and the varied types of tumors in pediatrics, relevant published CMR experience includes case reports as well as relatively small case series.484–491 Other publications492–498 have provided reviews and experiences of various centers of the utility and approach to CMR imaging for pediatric masses and how the findings may direct care.
As noted in a prior consensus statement, the utility of CMR is its ability to describe location, size, hemodynamic effects, and tissue type of cardiac masses (thrombus, benign or malignant tumor)2 upon which clinical decisions are often based. Various publications have described tissue characterization techniques for differentiating benign tumors such as rhabdomyomas499 most commonly or myxomas486,487 from others such as osteoscarcoma,490 highly vascularized tumors such as hemanginomas,488,491 or adipose tissue such as lipoma.489
In the one published multicenter study comparing pediatric CMR to histologic diagnoses in a blinded fashion to demonstrate the ability of CMR ability to accurately differentiate cardiac masses,477 97% of tumors were correctly identified compared to histologic diagnosis. Of these, a single correct diagnosis was made in 55% and a limited differential diagnosis which included the correct diagnosis was made in 42% (21% with 2 diagnoses and 23% with 3 or more diagnoses). There were only 2 cases of incorrect diagnoses, both having an atypical appearance on CMR. Generally, incorrect or incomplete diagnoses related either to atypical features of the mass, such as unusual location, or to technical imaging issues such as incomplete image acquisition or poor quality. This multicenter experience demonstrated the clinical utility of CMR in differentiating masses non-invasively, and thus its role in clinical management of affected patients (Table 7).
Table 7.
Tumor Characterization by Cardiac Magnetic Resonance
Summary of Recommendations
CMR with gadolinium based contrast is indicated for the evaluation of cardiac masses for tumor type, characterization, accurate identification of location, size or hemodynamic effects of the mass or visualization of the mass for surgical planning (Class I, Level of Evidence B)
CMR is indicated to distinguish between a benign and malignant cardiac tumor. (Class I, Level of Evidence B)
CMR is indicated to distinguish cardiac tumor from thrombus. (Class I, Level of Evidence B)
Non-contrast CMR is reasonable in patients with compromised renal function (eGFR < 30 ml/min/1.73 m2) for description of location, size, and any hemodynamic effect, but may be less useful for tissue characterization. (Class IIA, Level of Evidence C)
Myocarditis
Background
Myocarditis is a potentially life-threatening disease and a significant cause of pediatric morbidity and mortality.500,501 At the same time, it remains a difficult disease to diagnose501 with limited literature in pediatrics. Definitive diagnosis of myocarditis can be made from endomyocardial biopsy (EMB) which remains the diagnostic gold standard and can help guide therapy.501 EMB, however, is prone to low sensitivity related to sampling error,501,502 practice variation503 or adverse events due to its invasive nature.504,505 Thus, the diagnosis of myocarditis in pediatrics is often made from a combination of clinical factors including the patient’s history, symptoms, ECG changes and serologic findings.506
Non-invasive imaging plays a key role in diagnosis, almost always starting with echocardiography, with the addition CMR imaging to vastly increase the diagnostic sensitivity and specificity.506 In adults, CMR has become a well-established modality for diagnosing myocarditis507 with recommendations by the International Consensus Group on CMR (ie the “Lake Louise” criteria) published in 2009508 and updated in 2018.509 Evidence suggests CMR is indicated in the diagnosis of pediatric myocarditis; as a matter of fact, one of the first publications of CMR findings and tissue characterization in myocarditis was in children in 1991 which also compared these findings to EMB.510
CMR Indication
In the clinical setting of acute chest pain and concerning diagnostic testing (ECG, elevated cardiac enzymes or other serology, etc.), a diagnosis of myocardial inflammation can significantly affect prognosis and management.500,511 CMR is useful in the setting of suspected myocarditis for:
Confirming the presence of myocardial inflammation and edema
Differentiating ischemic (i.e. coronary) from non-ischemic causes of myocardial inflammation.
Risk stratification and prognostication
Guiding subsequent investigations
Confirming the Presence of Myocardial Inflammation
TTE is usually the 1st non-invasive imaging test in the evaluation of suspected myocarditis and allows assessment of ventricular function, size, regional wall motion abnormalities, valvular abnormalities and pericardial effusion. The common TTE findings in myocarditis are usually non-specific though TTE can help distinguish between the different myocarditis phenotypes of fulminant myocarditis (severe dysfunction), acute (non-fulminant) myocarditis512 or dilated cardiomyopathy. Beyond these findings, however, echocardiography lacks the sensitivity and specificity to further characterize myocardial inflammation and a test with higher diagnostic accuracy is required for further clinical management.
The value of CMR in myocarditis is to evaluate the tissue characteristics of myocardial inflammation; edema, hyperemia and capillary leak (using T1 and T2 weighted sequences) and necrosis or scarring of ventricular myocardium (Figure 46).500,508 Additional supportive criteria such as pericarditis, pericardia effusion or LV systolic dysfunction is also evaluated by CMR.509 The 2009 (aka “original”) Lake Louise criteria consists of:
Figure 46.
On the left is a CMR T2 weighted sequence showing > 2X enhancement of myocardium (green region of interest, ROI) compared to skeletal muscle (yellow, pink or blue ROI), meeting criteria for myocardial edema per 2009 Lake Louise criteria. On the right is a post-contrast phase sensitive inversion recovery (PSIR) sequence showing sub-epicardial late gadolinium enhancement indicating necrosis or scar (yellow arrow).
T2 weighted imaging showing the presence of myocardial edema.513,514
T1-weighted images obtained before and early after administration of gadolinium-contrast revealing early gadolinium enhancement (EGE),508 consistent with myocardial hyperemia or inflammation.
T1-weighted segmented inversion-recovery gradient-echo sequence515 showing LGE, characteristic of myocardial necrosis or scarring.
It also specified two positive findings (either T2 or T1 based) are necessary to diagnose myocarditis (Figure 46).
The Lake Louise criteria have been validated in pediatrics mainly from case reports516 or single-center series with relatively small populations.510,517–520 There has been one retrospective multicenter study that has evaluated CMR techniques in children with myocarditis which included 13 centers and 143 subjects521 and although there was variability in tissue characterization protocols among centers, overall sensitivity for CMR positive myocarditis was high (82%).
The 2009 Lake Louise criteria were updated in 2018 to include parametric mapping techniques that have significantly advanced in the diagnosis of myocardial inflammation in adult patients.507,522,523The updated recommendations included one T2 relaxation-based marker for myocardial edema (T2-mapping or T2 weighted images) and one T1 relaxation-based marker (abnormal T1, ECV or LGE) for associated myocardial injury.510 While the inclusion of multiparametric mapping is supported by a robust evidence base in adults, the mutiparametric mapping literature in pediatrics has been limited to small single-center studies.430,524–527 There is, however, emerging data about the potential added value of multiparametric mapping in the evaluation of pediatric myocarditis.528 The authors of the 2018 Lake Louise criteria currently state that “the original Lake Louise Criteria provide a good overall diagnostic performance, and thus they should remain in use in centers that have good experience with their application.” The MYKKE consortium is one effort currently underway to establish a multicenter registry with prospective data collection in pediatric myocarditis.529
Differentiating Ischemic from Non-ischemic Causes of Myocardial Inflammation
In the setting of myocarditis resembling an acute coronary syndrome with a young patient presenting acutely with chest pain, elevated cardiac enzymes and ECG abnormalities, CMR can be used to differentiate between myocarditis and myocardial infarction due to coronary artery disease:
In myocarditis, the pattern of LGE enhancement is characteristically subepicardal, may be transmural (but usually in a non-coronary distribution), and usually in a patchy distribution.530
The LGE pattern from coronary artery disease (myocardial infarction), is subendocardial or transmural and in a distribution of coronary perfusion territory.
Establishing the diagnosis of myocarditis in a patient with low risk for coronary artery disease (i.e. most pediatric patients) is helpful and avoids unnecessary cardiac catheterization for coronary angiography.
Risk Stratification and Prognostication
CMR can be useful for risk stratification with adult studies showing robust outcomes data on adverse cardiac events and arrhythmias.531–536 Similarly, CMR has been shown to be useful in evaluating outcomes in pediatrics537,538 including a large, multi-center study.539 Lastly, myocarditis has a known association with dilated cardiomyopathy (DCM), either as a precursor to DCM or as an acute illness that “unmasks” previously undetected DCM.540 Long-term studies in patients with acute myocarditis report the development of DCM in 21%, showing the utility of CMR for chronic management.541
Guiding Subsequent Investigations
Following the diagnosis of myocarditis by CMR, more detailed information may be needed from EMB to direct therapy. In this situation, there is diagnostic synergy between non-invasive CMR and invasive EMB to identify and treat particular etiologies of myocarditis. Examples may include giant cell or eosinophilic myocarditis that are typically treated with immunomodulator therapy.
Summary of Recommendations
The original Lake Louise Criteria (2009) is recommended for diagnosing myocardial inflammation for centers that have a good experience with these criteria in the pediatric age range (Class I, Level of Evidence B).
CMR with gadolinium-based contrast is indicated for evaluation of myocarditis to confirm the presence of myocardial inflammation in pediatric patients (Class I, Level of Evidence B).
CMR is beneficial in children and adolescents for differentiating ischemic from non-ischemic causes of myocardial inflammation, risk stratification/prognostication, and guiding subsequent investigations (Class I, Level of Evidence B).
CMR with multiparametric mapping can potentially add value in the evaluation of pediatric myocarditis, and more studies are needed in this population (Class IIa, Level of Evidence C).
Function and hemodynamics
Ventricular Function
Background
The end result of many congenital and pediatric heart lesions is ventricular dysfunction which may result in much of the morbidity and mortality associated with these diseases. Therefore, quantifying ventricular function is a vital aspect of continuing assessment.
At the simplest level, cardiac failure is defined as an inability to produce adequate cardiac output to supply the bodies metabolic needs. Thus, measurement of cardiac output can provide important information regarding cardiac function. The traditional reference standard method of measuring cardiac output is at cardiac catheterization using either thermodilution or the Fick method. Unfortunately, invasive assessment is associated with not insignificant risk as well as cost and is therefore not performed routinely. More importantly, most CHD patients have normal baseline cardiac output due to cardiac compensation (e.g. ventricular dilation, hypertrophy and increased filling pressures). Many symptoms are actually related to these compensatory mechanisms as well as an inability to augment cardiac output during exercise. Assessment of many of these compensatory mechanisms requires visualization of the ventricles and therefore, cardiac imaging is vital.
The most ubiquitous cardiac imaging modality used in CHD and acquired pediatric heart disease is echocardiography which has many advantages over other imaging techniques and is considered the first line method of assessing cardiac size and function. Nevertheless, echocardiography still has some significant problems such as operator dependence, poor acoustic windows and poor blood pool to myocardial contrast. In the last 20 years, as mentioned, CMR has become the reference standard method of assessing biventricular function.3,32,102–106,272,309
Indications for CMR
Ventricular Volumes and Ejection Fraction (Figures 4 and 40)
Many CHD lesions are associated with volume loading due to shunts or valvular regurgitation. This is true in not only such common shunt lesions such as ASDs, VSDs and patent ductus arteriosus but in more complex and less common lesions such as anomalous pulmonary venous connections or left SVC connected to the left atrium. Similarly, valvular regurgitation in patients with BAV, cleft mitral valve or TR associated with HLHS can cause significant volume loads. The cardiac response to volume loading is dilation which can result in increased risk of arrhythmia, increased wall stress and predisposition to dysfunction. Thus, measuring ventricular volumes is an important component of evaluating CHD.
Although less common, small ventricular volumes also need to be evaluated. Varying degrees of hypoplasia are seen in diseases such as HLHS, critical aortic stenosis, double outlet RV and malaligned atrioventricular canals to name a few. There has been a number of studies which attempt to aid in management of these patients, however, many questions remain.542–544
There are other lesions which occur in pediatric and CHD where the assessment of biventricular performance is important. Patients with anomalous coronary artery origins and courses, patients with surgically manipulated coronary arteries (see section on Coronary arteries), and patients with myocarditis (see Myocarditis section) are all examples where ventricular performance are useful.
Although ventricular volumes can be estimated by 2D echocardiography, most methods rely on significant geometric assumptions. These may hold true for the normal LV but are inadequate for the crescentic RV545 or the malformed LV in CHD. Newer 3D echocardiographic techniques do hold promise for true volumetric assessment, however, they still suffer from operator dependence and potentially inadequate acoustic windows. CMR has some distinct advantages over echocardiography. Firstly, it is not limited by body habitus and can be acquired in any patient irrespective of size. Secondly, unrestricted 2D imaging can be performed, allowing imaging of contiguous slices covering both ventricles. The result is true 3D measurement of ventricular volumes.546 Further, although there are CMR techniques that can be real-time (ie instantaneous acquisition), cine imaging for ventricular function is built over multiple heart beats, averaging the data in the image; whereas in echocardiography or catheterization, the imager is required to average the ventricular function in their minds, CMR averages it in the image itself and the quantification is a more true reflection of typical ventricular performance in the patient. Finally, modern bSSFP CMR techniques provide high blood pool myocardial contrast, aiding segmentation and further post-processing.547
An important benefit of accurate volume assessment is that it also enables precise measurement of ejection fraction which is a well-recognized measure of cardiac function and ventricular arterial coupling and an important prognostic marker in many forms of CHD and pediatric cardiovascular disease.109,548,549
Another metric that can be accurately evaluated from this sort of data is ventricular mass. Increased ventricular mass is common in patients with obstructive lesions (e.g. aortic stenosis or PA stenosis) or in for example, the RV of HLHS or TOF. As CMR allows visualization of the whole ventricular mass, especially the RV and its free wall, it enables very accurate measurement which is key to understanding the physiology and managing patients.
Several studies have shown that CMR provides highly accurate and reproducible measurement of ventricular volumes, mass and ejection fraction data in CHD.159,550,551 For these reasons CMR is now be considered the reference standard method of measuring LV and RV volumetric data.
Several studies have demonstrated that CMR measurements of ventricular volumes, mass and ejection fraction provide important clinical information in conditions such as repaired TOF,548,549SV at different stages of the Fontan palliation (Figure 4)36 and pediatric pulmonary hypertension549 to name a few. This includes prediction of outcome (e.g. mortality or major cardiac event) and exercise tolerance, as well as response to interventions (e.g. valve replacement).552
There are some limitations in using CMR to measures ventricular volumes and ejection fraction. In some patients, particularly children, breath holding is difficult and free breathing approaches are required including signal averaged and real-time acquisitions. Real-time sequences are becoming increasingly used in pediatric imaging and with the advent of new accelerated techniques, are now reaching the quality of conventional breath hold cines.553 Studies have validated real-time CMR for measurement of ventricular volumes and they should be considered a possible alternative to conventional imaging. Real-time imaging can also be useful in patients with irregular cardiac rhythm, although no large-scale studies demonstrating utility have been performed. Motion correction techniques, now in common use, can be used to increase image quality and negate respiratory artifacts.
Ventricular Filling and Diastolic Dysfunction
Diastolic dysfunction is often overlooked as a cause of symptoms in CHD. Evaluation of diastolic dysfunction requires measurement of the dynamic aspects of ventricular filling. Thus, a limitation of conventional ventricular volume and ejection fraction assessment is that it provides little information regarding diastolic function. The traditional method of assessing diastolic function is echocardiography assessment of atrioventricular valve inflow. Specifically, the ratio of peak early to late inflow velocities (E/A ratio) is an important indicator of ventricular diastolic dysfunction. The E/A ratio can also be assess using PC-CMR and some studies have demonstrated clinical utility.554 However, this should be considered a subsidiary measurement rather than the main reason to perform CMR. A better method of assessing systolic and diastolic function may be to directly measure myocardial motion in diastole and myocardial velocities have been measured by CMR and validated against echocardiography.555
Myocardial Motion
Volumetric measurement enables evaluation of global changes to ventricular volume and function. However, assessment of volumes alone does provide the full picture of cardiac dysfunction. There is evidence that both systolic and diastolic early ventricular dysfunction is better identified by evaluating the specific aspects of local myocardial motion. Although the cardiac myocyte can only shorten, the heart has complex local motion due to the specific arrangement of myocytes. This includes longitudinal and radial contraction, twisting and wall thickening. Some or all of these metrics can be measured with echocardiography using tissue Doppler or strain imaging. However, like all echocardiographic techniques, they may suffer from inadequate windows and are always operator dependence. There are also several CMR techniques that can be used to evaluate myocardial motion such as myocardial tagging,556,557 tissue phase mapping and strain/displacement encoding. These techniques have been used to better understand pathophysiology in CHD. However, significant clinical utility has not been demonstrated and a number of sequences are still at the research stage.
More recently, strain (and strain rate) data has been derived from conventional CMR cines using feature and tissue tracking,558,559 processed using the same tools used for echocardiography strain imaging. The main benefit of this approach is that imaging acquired for conventional volumetric analysis can also provide local motion data. This has allowed retrospective analysis of historical cine data and demonstration of clinical utility. Several studies have now shown that strain metrics can independently predict outcome (e.g. death or major cardiac event) and exercise tolerance in patients with CHD.560–562 However, no large scale studies have demonstrated that CMR strain measures outperform conventional ventricular volumes or ejection fraction data. Thus, currently, strain assessment should be considered as an addition to rather than a replacement of conventional ventricular volumes.
Regional ventricular wall motion is important in pediatric heart disease patients such as AAOCA, surgically manipulated coronary arteries, myocarditis, arrhythmogenic RV cardiomyopathy and other lesions which occur in pediatric and CHD. This may be performed qualitatively on cine imaging or using quantitative measures such as myocardial strain.
Finally, tissue characterization such as LGE and diffuse fibrosis can add another dimension to the assessment of ventricular function, elucidating the etiology of regional or global myocardial dysfunction (see previous sections).
Summary of Recommendations
CMR should be performed for the evaluation of biventricular volumes, mass and ejection fraction in patients with volume or pressure overload lesions as well as with varying degrees of RV or LV hypoplasia (Class I, Level of Evidence B).
CMR is useful in assessing biventricular volumes and ejection fraction in patients with non-structural pediatric heart disease (e.g. DMD, myocarditis or pulmonary hypertension) or in those patients with congenital or surgically manipulated coronary artery lesions (Class I, Level of Evidence B).
CMR is indicated in assessing ventricular volumes and ejection fraction in patients with SV (Class I, Level of Evidence B).
CMR strain analysis may be considered in assessing ventricular function in CHD and prognostication of outcome (Class IIB, Level of Evidence B).
CMR tissue characterization is reasonable in assessing ventricular function in CHD and prognostication of outcome (Class IIA, Level of Evidence B)
Systemic to Pulmonary Blood Flow Ratio and Collateral Flow
Background
Cardiovascular shunts between the systemic and pulmonary circulations are the commonest result of CHD, accounting for approximately 50% of all cases.563 Shunts are characterized in 3 main ways: (i) location, (ii) magnitude and direction, and (iii) effect on ventricular volume and function. This section addresses evaluation of shunt magnitude and direction by calculation of the systemic and pulmonary blood flow ratio (Qp/Qs). A left-to-right shunt will result in greater blood flow to the pulmonary vasculature compared to the systemic vasculature and a Qp/Qs ratio > 1 (higher Qp/Qs indicates a larger shunt). Conversely, a right-to-left shunt will result in greater flow to the systemic vasculature and thus a Qp/Qs ratio < 1 (the closer the Qp/QS is to zero, the larger the shunt). Quantifying Qp/Qs is vital in deciding appropriate management of patients with shunt lesions and is included in most current international guidelines.564
The reference standard method of measuring Qp/Qs is invasive oximetry with measurement of oxygen saturations (or content) in the pulmonary and systemic arterial and venous systems. This method should be used if measurement of PA pressure and pulmonary vascular resistance are also required (e.g., Eisenmenger physiology). However, there are some disadvantages to catheter based evaluation of Qp/Qs. The most obvious are its invasive nature which can associated be morbidity and high cost. Furthermore, there, some technical problems with invasive oximetry that should be understood. Firstly, the requirement for measurement of oxygen saturation from multiple sampling sites during steady state can result in significant error propagation and inaccurate Qp/Qs quantification.565 Secondly, quantification of Qp/QS is only possible if sampling can be performed distal to the shunt which is not possible in some extracardiac lesions (e.g., systemic-pulmonary arterial collaterals). Finally, in some lesions such a SV after BDG, it is impossible to obtain adequate mixed systemic venous saturation as it is separated into SVC and IVC circuits.43 For all these reasons, non-invasive assessment of Qp/QS is becoming increasingly important in the assessment of shunt lesions.
The simplest non-invasive method is Doppler echocardiography with several techniques based on vessel area and blood flow velocity being described.566 The main benefit of this approach is that echocardiography is already the first line method of anatomically evaluating shunt lesions. Unfortunately, echocardiographic evaluation of shunt magnitude is prone to inaccuracy due to inadequate data acquisition and invalid assumption in the calculation.567
Collateral flow in CHD comes in 4 broad categories: (1) systemic to pulmonary (Figure 3), (2) veno-venous collaterals as found, for example, in patients with SV, (3) aortic collaterals which develop, for example, in patients with coarctation (Figure 29) and (4) coronary collaterals; these guidelines will not address coronary collaterals. Systemic-to-pulmonary collaterals have been known for many years to develop in SV patients,22 however, precise measurement of flow in these vessels remained a challenge up until the past 10 years. Their clinical impact has been debated in the past568 in part due to the challenge of quantification, although it is clear now that there is an effect on patient care and management49,569–571; it has also been demonstrated by pilot data that these collaterals can be mostly obliterated in the catheterization lab with a measured decrease in the collateral flow.44 Similarly, veno-venous collaterals are found not uncommonly in SV patients, from 20 to 33%,572,573 and interventions have some time been needed. Finally, known since the 1930s and 1940s,574 aortic collaterals develop from existing vessels such as intercostal and mammary arteries to bypass aortic obstructions such as coarctation of the aorta which clearly has clinical implications for the diagnosis and treatment of the disease.
Indications for CMR
Recently, CMR has become the non-invasive reference standard method of measuring Qp/Qs using PC-CMR which has been shown to allow highly accurate and reproducible quantification of Qp/Qs. In PC-CMR, through plane flow is directly measured without assumptions—which has been borne out by the significant number of studies that have demonstrated good agreement between PC-CMR and both direct measurement of flow in phantom studies575,576 and invasive oximetry in patient studies.577–579 A further benefit of PC-CMR over invasive oximetry is that measurement Qp and Qs is not limited to assessment of flow in the pulmonary trunk and aorta respectively. For instance, Qp and Qs can be quantified by measuring flow in the PV and vena cavae respectively. These alternative calculations are particularly useful in situations where: (i) it is not possible to measure blood flow in one of the great arteries (i.e. artifact obscuring the pulmonary trunk in a patient with prosthetic valve containing metal) and (ii) invasive oximetry fails, such as patients with systemic-to-pulmonary arterial collaterals. Finally, and maybe more importantly for the accuracy of the technique, these multiple methods of evaluating Qp and Qs can be performed in the same patient, providing key internal quality assurances.
An important additional benefit of CMR is the ability to accurately measure ventricular volumes (see Ventricular function section). It is possible to measure Qp/Qs directly using ventricular stroke volumes. However, shunt location must be taken into consideration. An ASD and VSD may both have a Qp/Qs of 2:1, but RV stroke volume will be twice the LV stroke volume in the ASD, while LV stroke volume will be twice the RV stroke volume in the VSD. Thus, without knowledge of shunt location, ventricular stroke volume should be used with caution. Furthermore, accurate assessment of Qp/Qs using ventricular stroke volume relies on competence of the atrioventricular and semilunar valves. Consequently, measurement of ventricular volumes is not the first line method of calculating Qp/QS. Nevertheless, ventricular stroke volumes do provide a way of performing internal quality assurance of the PC-CMR calculation. More importantly, evaluation of ventricular size and function allows accurate evaluation of the physiological sequelae of a shunt lesion (e.g., RV volume loading in an ASD).
Another important advantage of CMR is the ability to anatomically delineate shunts. Studies have shown that CMR can provide definitive diagnosis and evaluation, for example, of sinus venosus defects and anomalous PV connections (see PV section).580,581 This is relevant as these lesions are difficult to image with echocardiography, particularly in older patients. Several studies have also shown that CMR provides comprehensive evaluation of secundum ASDs and can determine candidacy for transcatheter or surgical closure.582–584 Patients with VSDs are generally diagnosed and managed using echocardiography, however, CMR with 3D imaging may useful for delineation of complex or multiple defects.585 Finally, CMR can be useful in delineating the anatomy of patent ductus arteriosus and aorto-pulmonary windows in older patients, where echocardiography can be insufficient.
With all the possible shunt lesions in CHD other than the ones listed above such a truncus arteriosus, double outlet RV, TOF, it is not feasible to list them all in this guidance. Suffice it to say that when clinically important shunt lesions are present, imaging with CMR is useful.
Systemic-to-pulmonary collateral vessels can be visualized by CMR most notably by administering contrast (Figure 3) and flow in these vessels was first quantified by CMR in 2009.23,45 CMR is the only methodology that can accurately quantify this flow.43 The use of CMR to quantify this flow has demonstrated that these collaterals do have a measurable effect on outcome46,49,569–571 and act to steal blood from key organs such as the brain.47 CMR has been able to quantify the short term decrease in systemic-to-pulmonary collateral flow after embolization in the catheterization lab.44
CMR has also been extensively used to quantify collateral flow around a coarctation site (Figure 29) which is calculated by measuring flow volumes in the aorta near the coarctation and at the diaphragm.255,256,258–260 An increased flow volume at the diaphragm indicates significant collateral flow into the aorta, bypassing the narrowing (see Coarctation of the aorta section).258 Venovenous collaterals are also imaged by CMR586–588 and their presence needs to be taken under consideration prior to intervention.
Summary of Recommendations
CMR should be used for the evaluation of the magnitude and direction of intracardiac shunts in children and adults such as with ASDs and VSDs as examples (Class I, Level of Evidence B).
CMR is indicated for the evaluation of the magnitude and direction of extracardiac shunts in children and adults such as with patent ductus arteriosus and systemic-to-pulmonary collaterals as in Fontan patients (Class I, Level of Evidence B).
CMR is beneficial for the anatomic and quantitative flow assessment of systemic-to-pulmonary collaterals, aortic and venovenous collaterals (Class I, Level of Evidence B).
Late Gadolinium Enhancement (LGE)
Background
CMR is an integral part of the non-invasive imaging of both acquired and CHD lesions in children for the evaluation of complex cardiac anatomy, quantification of ventricular and valvular function, and myocardial characterization. A primary mode of myocardial characterization is with post-contrast LGE imaging.115 The principle of this technique is that gadolinium-based contrast agents have increased volume of distribution in abnormal myocardium. The difference between normal and abnormal tissue distribution volume (Vd) results in a CMR signal difference. The mechanism of increased Vd is variable. In inflammatory disease such as acute myocarditis or acute ischemia, the increased volume of distribution results from myocardial cell injury or death. In chronic myocardial infarction or fibrosis in cardiomyopathies, the extracellular space is expanded.
The standard LGE imaging is performed ~10 min following intravenous administration of 0.1–0.2 mmol/kg of gadolinium-based contrast agent (GBCA). Because of increased cardiac output, LGE imaging in pediatrics can start as early as 6 min after infusion. It should be noted that due to the risk of nephrogenic systemic fibrosis, the use of GBCA should be used with caution in acute or chronic renal disease. Multiple versions of the sequence have been developed since it was first introduced.589
Indications for CMR
Masses (see Tumor section) CMR with LGE imaging is very useful in the evaluation of pediatric cardiac masses because of its tissue characterization capabilities, allowing a narrowing of differential diagnoses. Cardiac mass evaluation includes standard cine, T1-weighted, and T2-weighted, perfusion and LGE sequences. Using LGE in a comprehensive study of cardiac masses adds significantly to the differentiation of common tumor types.477 This is particularly true for fibroma that shows marked enhancement. In addition, CMR using LGE and a TI scout following gadolinium administration is highly accurate in discerning tumor versus chronic thrombus.483
Myocarditis and Other Inflammatory Diseases (See Myocarditis Section) LGE is a primary component of the CMR myocarditis evaluation..508,509 The presence of LGE in patients with suspected myocarditis has a high specificity with the signal thought to represent areas of necrosis or fibrosis. The LGE lesions are typically patchy, subepicardial, located in the basal or mid-ventricular segments, and are not in a coronary distribution. The median area under the ROC curve for LGE alone in myocarditis is 83% but that is much improved when other criteria are taken into account. It is sensitive for acute myocarditis with large severely affected areas. It is not sensitive for mild cases and does not yield information regarding acute edema or active inflammation.
CHD In a variety of both unrepaired and repaired CHD, LGE imaging has been investigated (Figures 5, 23, 42, 43) and has played a significant role.590,591 When extensive LGE is seen following surgeries for CHD, it is generally associated with adverse prognosis such as in systemic RVs (see below and ccTGA section). Scarring can be an iatrogenic complication when a coronary is injured or there is a problem with cardiopulmonary bypass or myocardial protection. The following lists some of the applications in CHD but is certainly not comprehensive.
In TOF (see TOF section), LGE is normally detected in the RVOT (and occasionally at the VSD patch), particularly following transannular patch repair, but occasionally extends beyond the site.117 A greater degree of RV LGE has been shown to be associated with arrhythmias and lower exercise capacity, all of which imply poor prognosis.118
LGE is seen in patients following both the ASO and atrial switch procedure for TGA (see TGA section).172,365 LGE can be seen because of myocardial fibrosis from either preoperative hypoxemia or from demand–supply mismatch caused by increased myocardial mass or decreased myocardial flow reserve. The extent of LGE correlates with age, RV dysfunction, QRS duration, QT dispersion and clinical events (arrhythmia and sudden cardiac death).
LGE has been seen in 28% of patients who had the Fontan operation50 and can be seen in the LV of patients with HLHS as endocardial fibroelastosis (see SV section). Presence and extent of LGE correlate with lower ejection fraction, dilated and hypertrophied systemic ventricle, regional wall motion abnormalities and NSVT; however, positive LGE is shown not to be associated with clinical endpoints (i.e. death or listing for cardiac transplant).
Congenital aortic valvular stenosis in children is caused by developmentally abnormal dysplastic aortic valve. Severe aortic stenosis can result in myocardial fibrosis manifested as LGE in a diffuse subendocardial pattern.592 Subendocardial LGE in adolescents who underwent balloon valvuloplasty in infancy corresponds to fibroelastosis on pathology and is associated with diastolic dysfunction.
Ischemic Lesions (See Coronary Section) Although rare in children and adolescents, myocardial infarctions occur as a result of cardiac surgery, thrombotic events, inflammatory heart disease (including Kawasaki disease) and hypercoagulable states.593,594 This becomes even more important with patients who have congenital coronary abnormalities (eg anomalous coronary arteries) or who have had coronary manipulation due to CHD (e.g., TGA after ASO). CMR has an important role in the evaluation of myocardial infarction and LGE is useful in establishing the diagnosis and extent of ischemic myocardial injury. LGE in a myocardial infarct is always subendocardial extending toward the epicardium and becoming transmural in severe infarctions. LGE can be used to quantify the scar either in a qualitative or quantitative manner. There is an inverse correlation between the extent of scar and recovery of contractile function.
Pericardial Disease CMR is used in the diagnosis and management of both acute and chronic pericarditis. Acute pericarditis manifests with pericardial thickening (> 4 mm) and pericardial effusion. Most standard acute pericarditis does not require CMR. LGE imaging shows focal or diffuse pericardial enhancement.517 More complicated cases of recurrent or chronic pericarditis often require advanced imaging and studies have shown the correlation between pericardial enhancement and systemic inflammation. Chronic pericarditis results in restriction from non-compliant and thickened pericardium. In patients with constrictive pericarditis who have LGE as evidence of pericardial inflammation, aggressive anti-inflammatory therapy may result in resolution without the need for surgery.
Cardiomyopathies
Hypertrophic Cardiomyopathy (See Hypertrophic Cardiomyopathy Section) LGE is seen in 40–80% of adults with HCM. Recent studies in children have shown LGE in 28–73% of patients in a similar pattern as in adults. LGE has been shown to have diagnostic importance for both diagnosis and management. It is correlated with adverse clinical outcomes including arrhythmia and SCD in both children and adults.416,417,419 There is a significant relationship between the extent of LGE and the presence of NSVT.
Dilated Cardiomyopathy CMR demonstrates linear mid-myocardial LGE in a non-vascular distribution in patients with idiopathic DCM. The presence of LGE, regardless of its extent or distribution, is associated with adverse prognosis such as SCD. In children, a study performed on a group with DCM showed the presence of LGE in only 16%.595
Neuromuscular Disease CMR is the standard for longitudinal evaluation of neuromuscular disease patients as they age and echocardiography becomes more difficult. LGE is an important part of these exams as increase in signal likely represents fibrofatty replacement of myocardium and portends onset of declining function.474
Summary of Recommendations
CMR with LGE is recommended for evaluation of cardiac tumors or masses. (Class I, Level of evidence B)
CMR with LGE is indicated for assessment of suspected myocarditis (Class I, Level of evidence B)
CMR with LGE is reasonable for assessment of post-operative CHD (Class I, Level of evidence B)
CMR with LGE is indicated for assessment of pediatric HCM, dilated cardiomyopathy and neuromuscular disease patients (Class I, Level of evidence B)
CMR with LGE should be used for myocardial viability assessment in pediatric patients with decreased ventricular function, suspicion of a CHD, acquired, or iatrogenic coronary lesions (Class I, Level of evidence B).
CMR with LGE can be beneficial for assessment of chronic pericarditis when the diagnosis or therapeutic strategy is unclear (Class IIa, Level of evidence B)
Article Information
Acknowledgements
Not applicable.
Authors’ Contributions
All authors contributed substantially to the research, writing and editing of these guidelines. All recommendations were voted on by the writing committee. All authors read and approved the final manuscript.
Sources of Funding
There was no funding for this guidelines manuscript.
Availability of Data and Materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Disclosures
The authors declare that they have no competing interests.
Footnotes
Nonstandard Abbreviations and Acronyms
- 3D
- three dimensional
- 4D
- four dimensional
- AAOCA
- anomalous aortic origin of a coronary artery
- ACAOS-IM
- anomalous coronary artery origin from the opposite sinus with an intramural segment
- ACC
- American College of Cardiology
- AHA
- American Heart Association
- AR
- aortic regurgitation
- ASD
- atrial sepal defect
- ASE
- American Society of Echocardiography
- ASI
- aortic size index
- ASO
- arterial switch operation
- AUC
- appropriate use criteria
- BAV
- bicuspid aortic valve
- BDG
- Bidirectional Glenn
- BMD
- Becker muscular dystrophy
- bSSFP
- balanced steady state free precession
- Cath
- cardiac catheterization
- ccTGA
- congenitally corrected transposition of the great arteries
- CHD
- congenital heart disease
- CMD
- coronary microvascular dysfunction
- CMR
- cardiovascular magnetic resonance
- COR
- class of recommendation
- CT
- computed tomography
- DCM
- dilated cardiomyopathy
- DF
- diffuse fibrosis
- DMD
- Duchenne’s muscular dystrophy
- EACVI
- European Association of Cardiovascular Imaging
- ECG
- Electrocardiography
- ECMO
- extracorpeal membrane oxygenation
- ECV
- extracellular volume fraction
- EDS
- Ehler’s Danlos syndrome
- EGE
- early gadolinium enhancement
- EMB
- endomyocardial biopsy
- ESC
- European Society of Cardiology
- EST
- exercise stress test
- FTAAD
- familial thoracic aortic aneurysms and dissections
- GBCA
- gadolinium based contrast agents
- GCS
- global circumferential strain
- GLS
- global longitudinal strain
- HCM
- hypertrophic cardiomyopathy
- HLHS
- hypoplastic left heart syndrome
- ICD
- implanted cardioverter defibrillator
- IVC
- inferior vena cava
- KD
- Kawasaki’s disease
- LDS
- Loey’s Deitz syndrome
- LGE
- late gadolinium enhancement
- LOE
- level of evidence
- LV
- left ventricle/left ventricular
- LVOT
- left ventricular outflow tract
- LVWT
- left ventricular wall thickness
- MAPK
- mitogen activated protein kinase
- MFS
- Marfan syndrome
- MRA
- magnetic resonance angiography
- MVP
- mitral valve prolapse
- NASCI
- North American Society of Cardiovascular Imaging
- NSVT
- non-sustained ventricular tachycardia
- NYHA
- New York Heart Association
- PA
- pulmonary artery(ies)
- PAPVC
- partial anomalous pulmonary venous connection
- PC-CMR
- phase contrast cardiovascular magnetic resonance
- PR
- pulmonary regurgitation
- PV
- pulmonary vein
- PVR
- pulmonary valve replacement
- Qp
- pulmonary blood flow
- Qs
- systemic blood flow
- RAS
- renin angiotensin system
- RV
- right ventricle/right ventricular
- RVEDV
- right ventricular end-diastolic volume
- RVEDVI
- right ventricular end-diastolic volume index
- RVEF
- right ventricular ejection fraction
- RVOT
- right ventricular outflow tract
- SAM
- systolic anterior motion
- SCD
- sudden cardiac death
- SCMR
- Society for Cardiovascular Magnetic Resonance
- SPR
- Society for Pediatric Radiology
- SV
- single ventricle
- SVC
- superior vena cava
- TAPVC
- total anomalous pulmonary venous connection
- TGA
- transposition of the great arteries
- TOF
- tetralogy of Fallot
- TR
- tricuspid regurgitation
- TTE
- transthoracic echocardiography
- TSZ
- Turner’s Z score
- VSD
- ventricular septal defect
- VTI
- vertebral tortuosity index
- Vd
- distribution volume
References
- 1.Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e698–e800. doi: 10.1161/CIR.0000000000000603 [DOI] [PubMed] [Google Scholar]
- 2.Valsangiacomo Buechel ER, Grosse-Wortmann L, Fratz S, Eichhorn J, Sarikouch S, Greil GF, Beerbaum P, Bucciarelli-Ducci C, Bonello B, Sieverding L, et al. ; EACVI. Indications for cardiovascular magnetic resonance in children with congenital and acquired heart disease: an expert consensus paper of the Imaging Working Group of the AEPC and the Cardiovascular Magnetic Resonance Section of the EACVI. Eur Heart J Cardiovasc Imaging. 2015;16:281–297. doi: 10.1093/ehjci/jeu129 [DOI] [PubMed] [Google Scholar]
- 3.Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG, Ho VB, Jerosch-Herold M, Kramer CM, Manning WJ, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55:2614–62. doi: 10.1016/j.jacc.2009.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pennell DJ, Sechtem UP, Higgins CB, Manning WJ, Pohost GM, Rademakers FE, van Rossum AC, Shaw LJ, Yucel EK; European Society of cardiology; Soceity for Cardiovascular Magnetic Resonance. Clinical indications for cardiovascular magnetic resonance (CMR): Consensus Panel report. J Cardiovasc Magn Reson. 2004;6:727–765. doi: 10.1081/jcmr-200038581 [DOI] [PubMed] [Google Scholar]
- 5.Hendel RC, Patel MR, Kramer CM, Poon M, Hendel RC, Carr JC, Gerstad NA, Gillam LD, Hodgson JM, Kim RJ, et al. ; American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group; American College of Radiology; Society of Cardiovascular Computed Tomography; Society for Cardiovascular Magnetic Resonance; American Society of Nuclear Cardiology; North American Society for Cardiac Imaging; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48:1475–1497. doi: 10.1016/j.jacc.2006.07.003 [DOI] [PubMed] [Google Scholar]
- 6.Fratz S, Chung T, Greil GF, Samyn MM, Taylor AM, Valsangiacomo Buechel ER, Yoo SJ, Powell AJ. Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. J Cardiovasc Magn Reson. 2013;15:51. doi: 10.1186/1532-429X-15-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sachdeva R, Valente AM, Armstrong AK, Cook SC, Han BK, Lopez L, Lui GK, Pickard SS, Powell AJ, Bhave NM, et al. ACC/AHA/ASE/HRS/ISACHD/SCAI/SCCT/SCMR/SOPE 2020 Appropriate Use Criteria for Multimodality Imaging During the Follow-Up Care of Patients With Congenital Heart Disease: A Report of the American College of Cardiology Solution Set Oversight Committee and Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Pediatric Echocardiography. J Am Coll Cardiol. 2020;75:657–703. doi: 10.1016/j.jacc.2019.10.002 [DOI] [PubMed] [Google Scholar]
- 8.Valsangiacomo Buechel ER, Grosse-Wortmann L, Fratz S, Eichhorn J, Sarikouch S, Greil GF, Beerbaum P, Bucciarelli-Ducci C, Bonello B, Sieverding L, et al. Indications for cardiovascular magnetic resonance in children with congenital and acquired heart disease: an expert consensus paper of the Imaging Working Group of the AEPC and the Cardiovascular Magnetic Resonance Section of the EACVI. Cardiol Young. 2015;25:819–838. doi: 10.1017/S1047951115000025 [DOI] [PubMed] [Google Scholar]
- 9.ACCF/AHA Task Force on Practice Guidelines. Methodology Manual and Policies from the ACCF/AHA Task Force on Practice Guidelines. http://assets.cardiosource.com/Methodology_Manual_for_ACC_AHA_Writing_Committees.pdf and http://circ.ahajournals.org/site/manual/index.xhtml. Accessed 2 Feb 2019.
- 10.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–248. doi: 10.1136/thx.26.3.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7 [DOI] [PubMed] [Google Scholar]
- 12.Stuart AG, Wren C, Sharples PM, Hunter S, Hey EN. Hypoplastic left heart syndrome: more potential transplant recipients than suitable donors. Lancet. 1991;337:957–959. doi: 10.1016/0140-6736(91)91581-e [DOI] [PubMed] [Google Scholar]
- 13.Samánek M, Slavík Z, Zborilová B, Hrobonová V, Vorísková M, Skovránek J. Prevalence, treatment, and outcome of heart disease in live-born children: a prospective analysis of 91,823 live-born children. Pediatr Cardiol. 1989;10:205–211. doi: 10.1007/BF02083294 [DOI] [PubMed] [Google Scholar]
- 14.Fyler DC. Report of the New England Regional Infant Cardiac Program. Pediatrics. 1980;65(suppl):463. [PubMed] [Google Scholar]
- 15.Rao PS. Tricuspid atresia. Mount Kisco, NY: Futura; 1982. p. 13–24. [Google Scholar]
- 16.Norwood WI, Lang P, Hansen DD. Physiologic repair of aortic atresia-hypoplastic left heart syndrome. N Engl J Med. 1983;308:23–26. doi: 10.1056/NEJM198301063080106 [DOI] [PubMed] [Google Scholar]
- 17.Sano S, Ishino K, Kawada M, Arai S, Kasahara S, Asai T, Masuda Z, Takeuchi M, Ohtsuki S. Right ventricle-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2003;126:504–9; discussion 509. doi: 10.1016/s0022-5223(02)73575-7 [DOI] [PubMed] [Google Scholar]
- 18.Tabbutt S, Dominguez TE, Ravishankar C, Marino BS, Gruber PJ, Wernovsky G, Gaynor JW, Nicolson SC, Spray TL. Outcomes after the stage I reconstruction comparing the right ventricular to pulmonary artery conduit with the modified Blalock Taussig shunt. Ann Thorac Surg. 2005;80:1582–90; discussion 1590. doi: 10.1016/j.athoracsur.2005.04.046 [DOI] [PubMed] [Google Scholar]
- 19.Cua CL, Thiagarajan RR, Gauvreau K, Lai L, Costello JM, Wessel DL, Del Nido PJ, Mayer JE, Jr, Newburger JW, Laussen PC. Early postoperative outcomes in a series of infants with hypoplastic left heart syndrome undergoing stage I palliation operation with either modified Blalock-Taussig shunt or right ventricle to pulmonary artery conduit. Pediatr Crit Care Med. 2006;7:238–244. doi: 10.1097/01.PCC.0000201003.38320.63 [DOI] [PubMed] [Google Scholar]
- 20.Mahler WT, Charade AR, Tam VKH. Early experience with a modified Norwood procedure using right ventricle to pulmonary artery conduit. Ann Thorac Surg. 2003;76:1084–9. [DOI] [PubMed] [Google Scholar]
- 21.Azakie A, Martinez D, Sapru A, Fineman J, Teitel D, Karl TR. Impact of right ventricle to pulmonary artery conduit on outcome of the modified Norwood procedure. Ann Thorac Surg. 2004;77:1727–1733. doi: 10.1016/j.athoracsur.2003.10.002 [DOI] [PubMed] [Google Scholar]
- 22.Triedman JK, Bridges ND, Mayer JE, Jr, Lock JE. Prevalence and risk factors for aortopulmonary collateral vessels after Fontan and bidirectional Glenn procedures. J Am Coll Cardiol. 1993;22:207–215. doi: 10.1016/0735-1097(93)90836-p [DOI] [PubMed] [Google Scholar]
- 23.Whitehead KK, Gillespie MJ, Harris MA, Fogel MA, Rome JJ. Noninvasive quantification of systemic-to-pulmonary collateral flow: a major source of inefficiency in patients with superior cavopulmonary connections. Circ Cardiovasc Imaging. 2009;2:405–411. doi: 10.1161/CIRCIMAGING.108.832113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fogel MA, Donofrio MT, Ramaciotti C, Hubbard AM, Weinberg PM. Magnetic resonance and echocardiographic imaging of pulmonary artery size throughout stages of Fontan reconstruction. Circulation. 1994;90:2927–2936. doi: 10.1161/01.cir.90.6.2927 [DOI] [PubMed] [Google Scholar]
- 25.Dasi LP, Krishnankuttyrema R, Kitajima HD, Pekkan K, Sundareswaran KS, Fogel M, Sharma S, Whitehead K, Kanter K, Yoganathan AP. Fontan hemodynamics: importance of pulmonary artery diameter. J Thorac Cardiovasc Surg. 2009;137:560–564. doi: 10.1016/j.jtcvs.2008.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris MA, Cosulich MT, Gillespie MJ, Whitehead KK, Liu TI, Weinberg PM, Fogel MA. Pre-Fontan cardiac magnetic resonance predicts post-Fontan length of stay and avoids ionizing radiation. J Thorac Cardiovasc Surg. 2009;138:941–947. doi: 10.1016/j.jtcvs.2008.12.051 [DOI] [PubMed] [Google Scholar]
- 27.Fogel MA, Pawlowski TW, Whitehead KK, Harris MA, Keller MS, Glatz AC, Zhu W, Shore D, Diaz LK, Rome JJ. Cardiac magnetic resonance and the need for routine cardiac catheterization in single ventricle patients prior to Fontan: a comparison of 3 groups: pre-Fontan CMR versus cath evaluation. J Am Coll Cardiol. 2012;60:1094–1102. doi: 10.1016/j.jacc.2012.06.021 [DOI] [PubMed] [Google Scholar]
- 28.Restrepo M, Tang E, Haggerty CM, Khiabani RH, Mirabella L, Bethel J, Valente AM, Whitehead KK, McElhinney DB, Fogel MA, et al. Energetic implications of vessel growth and flow changes over time in Fontan patients. Ann Thorac Surg. 2015;99:163–170. doi: 10.1016/j.athoracsur.2014.08.046 [DOI] [PubMed] [Google Scholar]
- 29.Geva T, Vick GW, 3rd, Wendt RE, Rokey R. Role of spin echo and cine magnetic resonance imaging in presurgical planning of heterotaxy syndrome. Comparison with echocardiography and catheterization. Circulation. 1994;90:348–356. doi: 10.1161/01.cir.90.1.348 [DOI] [PubMed] [Google Scholar]
- 30.Fogel MA, Weinberg PM, Fellows KE, Hoffman EA. A study in ventricular-ventricular interaction. Single right ventricles compared with systemic right ventricles in a dual-chamber circulation. Circulation. 1995;92:219–230. doi: 10.1161/01.cir.92.2.219 [DOI] [PubMed] [Google Scholar]
- 31.Fogel MA, Gupta KB, Weinberg PM, Hoffman EA. Regional wall motion and strain analysis across stages of Fontan reconstruction by magnetic resonance tagging. Am J Physiol. 1995;269(3 Pt 2):H1132–H1152. doi: 10.1152/ajpheart.1995.269.3.H1132 [DOI] [PubMed] [Google Scholar]
- 32.Fogel MA, Weinberg PM, Chin AJ, Fellows KE, Hoffman EA. Late ventricular geometry and performance changes of functional single ventricle throughout staged Fontan reconstruction assessed by magnetic resonance imaging. J Am Coll Cardiol. 1996;28:212–221. doi: 10.1016/0735-1097(96)00111-8 [DOI] [PubMed] [Google Scholar]
- 33.Margossian R, Schwartz ML, Prakash A, Wruck L, Colan SD, Atz AM, Bradley TJ, Fogel MA, Hurwitz LM, Marcus E, et al. ; Pediatric Heart Network Investigators. Comparison of echocardiographic and cardiac magnetic resonance imaging measurements of functional single ventricular volumes, mass, and ejection fraction (from the Pediatric Heart Network Fontan Cross-Sectional Study). Am J Cardiol. 2009;104:419–428. doi: 10.1016/j.amjcard.2009.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prakash A, Travison TG, Fogel MA, Hurwitz LM, Powell AJ, Printz BF, Puchalski MD, Shirali GS, Yoo SJ, Geva T; Pediatric Heart Network Investigators. Relation of size of secondary ventricles to exercise performance in children after fontan operation. Am J Cardiol. 2010;106:1652–1656. doi: 10.1016/j.amjcard.2010.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haggerty CM, Whitehead KK, Bethel J, Fogel MA, Yoganathan AP. Relationship of single ventricle filling and preload to total cavopulmonary connection hemodynamics. Ann Thorac Surg. 2015;99:911–917. doi: 10.1016/j.athoracsur.2014.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rathod RH, Prakash A, Kim YY, Germanakis IE, Powell AJ, Gauvreau K, Geva T. Cardiac magnetic resonance parameters predict transplantation-free survival in patients with fontan circulation. Circ Cardiovasc Imaging. 2014;7:502–509. doi: 10.1161/CIRCIMAGING.113.001473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mercer-Rosa L, Yang W, Kutty S, Rychik J, Fogel M, Goldmuntz E. Quantifying pulmonary regurgitation and right ventricular function in surgically repaired tetralogy of Fallot: a comparative analysis of echocardiography and magnetic resonance imaging. Circ Cardiovasc Imaging. 2012;5:637–643. doi: 10.1161/CIRCIMAGING.112.972588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kutty S, Whitehead KK, Natarajan S, Harris MA, Wernovsky G, Fogel MA. Qualitative echocardiographic assessment of aortic valve regurgitation with quantitative cardiac magnetic resonance: a comparative study. Pediatr Cardiol. 2009;30:971–977. doi: 10.1007/s00246-009-9490-6 [DOI] [PubMed] [Google Scholar]
- 39.Mercer-Rosa L, Ingall E, Zhang X, McBride M, Kawut S, Fogel M, Paridon S, Goldmuntz E. The impact of pulmonary insufficiency on the right ventricle: a comparison of isolated valvar pulmonary stenosis and tetralogy of fallot. Pediatr Cardiol. 2015;36:796–801. doi: 10.1007/s00246-014-1087-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahle WT, Cohen MS, Spray TL, Rychik J. Atrioventricular valve regurgitation in patients with single ventricle: impact of the bidirectional cavopulmonary anastomosis. Ann Thorac Surg. 2001;72:831–835. doi: 10.1016/s0003-4975(01)02893-4 [DOI] [PubMed] [Google Scholar]
- 41.Cohen MS, Marino BS, McElhinney DB, Robbers-Visser D, van der Woerd W, Gaynor JW, Spray TL, Wernovsky G. Neo-aortic root dilation and valve regurgitation up to 21 years after staged reconstruction for hypoplastic left heart syndrome. J Am Coll Cardiol. 2003;42:533–540. doi: 10.1016/s0735-1097(03)00715-0 [DOI] [PubMed] [Google Scholar]
- 42.Whitehead KK, Sundareswaran KS, Parks WJ, Harris MA, Yoganathan AP, Fogel MA. Blood flow distribution in a large series of patients having the Fontan operation: a cardiac magnetic resonance velocity mapping study. J Thorac Cardiovasc Surg. 2009;138:96–102. doi: 10.1016/j.jtcvs.2008.11.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Downing TE, Whitehead KK, Dori Y, Gillespie MJ, Harris MA, Fogel MA, Rome JJ, Glatz AC. Accuracy of conventional oximetry for flow estimation in patients with superior cavopulmonary connection: a comparison with phase-contrast cardiac MRI. Circ Cardiovasc Imaging. 2013;6:943–949. doi: 10.1161/CIRCIMAGING.113.000496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dori Y, Glatz AC, Hanna BD, Gillespie MJ, Harris MA, Keller MS, Fogel MA, Rome JJ, Whitehead KK. Acute effects of embolizing systemic-to-pulmonary arterial collaterals on blood flow in patients with superior cavopulmonary connections: a pilot study. Circ Cardiovasc Interv. 2013;6:101–106. doi: 10.1161/CIRCINTERVENTIONS.112.972265 [DOI] [PubMed] [Google Scholar]
- 45.Grosse-Wortmann L, Al-Otay A, Yoo SJ. Aortopulmonary collaterals after bidirectional cavopulmonary connection or Fontan completion: quantification with MRI. Circ Cardiovasc Imaging. 2009;2:219–225. doi: 10.1161/CIRCIMAGING.108.834192 [DOI] [PubMed] [Google Scholar]
- 46.Prakash A, Satiroglu E, Porras D, McElhinney DB, Keane JF, Lock JE, Geva T, King W, Powell AJ. Risk factors for profuse systemic-to-pulmonary artery collateral burden in hypoplastic left heart syndrome. Am J Cardiol. 2013;112:400–404. doi: 10.1016/j.amjcard.2013.03.043 [DOI] [PubMed] [Google Scholar]
- 47.Fogel MA, Li C, Wilson F, Pawlowski T, Nicolson SC, Montenegro LM, Diaz Berenstein L, Spray TL, Gaynor JW, Fuller S, Keller MS, et al. Relationship of cerebral blood flow to aortic-to-pulmonary collateral/shunt flow in single ventricles. Heart. 2015;101:1325–1331. doi: 10.1136/heartjnl-2014-307311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitehead KK, Harris MA, Glatz AC, Gillespie MJ, DiMaria MV, Harrison NE, Dori Y, Keller MS, Rome JJ, Fogel MA. Status of systemic to pulmonary arterial collateral flow after the fontan procedure. Am J Cardiol. 2015;115:1739–1745. doi: 10.1016/j.amjcard.2015.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glatz AC, Rome JJ, Small AJ, Gillespie MJ, Dori Y, Harris MA, Keller MS, Fogel MA, Whitehead KK. Systemic-to-pulmonary collateral flow, as measured by cardiac magnetic resonance imaging, is associated with acute post-Fontan clinical outcomes. Circ Cardiovasc Imaging. 2012;5:218–225. doi: 10.1161/CIRCIMAGING.111.966986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rathod RH, Prakash A, Powell AJ, Geva T. Myocardial fibrosis identified by cardiac magnetic resonance late gadolinium enhancement is associated with adverse ventricular mechanics and ventricular tachycardia late after Fontan operation. J Am Coll Cardiol. 2010;55:1721–1728. doi: 10.1016/j.jacc.2009.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kato A, Riesenkampff E, Yim D, Yoo SJ, Seed M, Grosse-Wortmann L. Pediatric Fontan patients are at risk for myocardial fibrotic remodeling and dysfunction. Int J Cardiol. 2017;240:172–177. doi: 10.1016/j.ijcard.2017.04.073 [DOI] [PubMed] [Google Scholar]
- 52.Rychik J, Atz AM, Celermajer DS, Deal BJ, Gatzoulis MA, Gewillig MH, Hsia TY, Hsu DT, Kovacs AH, McCrindle BW, et al. Evaluation and management of the child and adult with fontan circulation: a scientific statement from the American Heart Association. Circulation. 2019;140:e234–84. [DOI] [PubMed] [Google Scholar]
- 53.Brown DW, Gauvreau K, Moran AM, Jenkins KJ, Perry SB, del Nido PJ, Colan SD. Clinical outcomes and utility of cardiac catheterization prior to superior cavopulmonary anastomosis. J Thorac Cardiovasc Surg. 2003;126:272–281. doi: 10.1016/s0022-5223(03)00054-0 [DOI] [PubMed] [Google Scholar]
- 54.Brown DW, Gauvreau K, Powell AJ, Lang P, Colan SD, Del Nido PJ, Odegard KC, Geva T. Cardiac magnetic resonance versus routine cardiac catheterization before bidirectional glenn anastomosis in infants with functional single ventricle: a prospective randomized trial. Circulation. 2007;116:2718–2725. doi: 10.1161/CIRCULATIONAHA.107.723213 [DOI] [PubMed] [Google Scholar]
- 55.Ro PS, Rychik J, Cohen MS, Mahle WT, Rome JJ. Diagnostic assessment before Fontan operation in patients with bidirectional cavopulmonary anastomosis: are noninvasive methods sufficient? J Am Coll Cardiol. 2004;44:184–187. doi: 10.1016/j.jacc.2004.02.058 [DOI] [PubMed] [Google Scholar]
- 56.Fallot A. Contribution a l’anatomie pathologique de la maladie bleue (cyanose cardiaque). Marseillee Med. 1888;25:77. [Google Scholar]
- 57.Cambell M. Incidence of cardiac malformations at birth and later, and neonatal mortality. Br Heart J. 1973;35:189. doi: 10.1136/hrt.35.2.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, Anderson P, Mason CA, Collins JS, Kirby RS, et al. ; National Birth Defects Prevention Network. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004-2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–1016. doi: 10.1002/bdra.20735 [DOI] [PubMed] [Google Scholar]
- 59.Poirier RA, McGoon DC, Danielson GK, Wallace RB, Ritter DG, Moodie DS, Wiltse CG. Late results after repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 1977;73:900–908. [PubMed] [Google Scholar]
- 60.Al Habib HF, Jacobs JP, Mavroudis C, Tchervenkov CI, O’Brien SM, Mohammadi S, Jacobs ML. Contemporary patterns of management of tetralogy of Fallot: data from the Society of Thoracic Surgeons Database. Ann Thorac Surg. 2010;90:813–9; discussion 819. doi: 10.1016/j.athoracsur.2010.03.110 [DOI] [PubMed] [Google Scholar]
- 61.Sousa Uva M, Lacour-Gayet F, Komiya T, Serraf A, Bruniaux J, Touchot A, Roux D, Petit J, Planché C. Surgery for tetralogy of Fallot at less than six months of age. J Thorac Cardiovasc Surg. 1994;107:1291–1300. [PubMed] [Google Scholar]
- 62.Di Donato RM, Jonas RA, Lang P, Rome JJ, Mayer JE, Jr, Castaneda AR. Neonatal repair of tetralogy of Fallot with and without pulmonary atresia. J Thorac Cardiovasc Surg. 1991;101:126–137. [PubMed] [Google Scholar]
- 63.Kolcz J, Pizarro C. Neonatal repair of tetralogy of Fallot results in improved pulmonary artery development without increased need for reintervention. Eur J Cardiothorac Surg. 2005;28:394–399. doi: 10.1016/j.ejcts.2005.05.014 [DOI] [PubMed] [Google Scholar]
- 64.Reddy VM, Liddicoat JR, McElhinney DB, Brook MM, Stanger P, Hanley FL. Routine primary repair of tetralogy of Fallot in neonates and infants less than three months of age. Ann Thorac Surg. 1995;60(6 Suppl):S592–S596. doi: 10.1016/0003-4975(95)00732-6 [DOI] [PubMed] [Google Scholar]
- 65.Touati GD, Vouhé PR, Amodeo A, Pouard P, Mauriat P, Leca F, Neveux JY. Primary repair of tetralogy of Fallot in infancy. J Thorac Cardiovasc Surg. 1990;99:396–402; discussion 402. [PubMed] [Google Scholar]
- 66.Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–172. doi: 10.1161/CIRCULATIONAHA.106.627224 [DOI] [PubMed] [Google Scholar]
- 67.Chiu SN, Wang JK, Chen HC, Lin MT, Wu ET, Chen CA, Huang SC, Chang CI, Chen YS, Chiu IS, et al. Long-term survival and unnatural deaths of patients with repaired tetralogy of Fallot in an Asian cohort. Circ Cardiovasc Qual Outcomes. 2012;5:120–125. doi: 10.1161/CIRCOUTCOMES.111.963603 [DOI] [PubMed] [Google Scholar]
- 68.Pokorski RJ. Long-term survival after repair of tetralogy of Fallot. J Insur Med. 2000;32:89–92. [PubMed] [Google Scholar]
- 69.Bove EL, Byrum CJ, Thomas FD, Kavey RE, Sondheimer HM, Blackman MS, Parker FB, Jr. The influence of pulmonary insufficiency on ventricular function following repair of tetralogy of Fallot. Evaluation using radionuclide ventriculography. J Thorac Cardiovasc Surg. 1983;85:691–696. [PubMed] [Google Scholar]
- 70.Schamberger MS, Hurwitz RA. Course of right and left ventricular function in patients with pulmonary insufficiency after repair of Tetralogy of Fallot. Pediatr Cardiol. 2000;21:244–248. doi: 10.1007/s002460010050 [DOI] [PubMed] [Google Scholar]
- 71.Lang P, Chipman CW, Siden H, Williams RG, Norwood WI, Castaneda AR. Early assessment of hemodynamic status after repair of tetralogy of Fallot: a comparison of 24 hour (intensive care unit) and 1 year postoperative data in 98 patients. Am J Cardiol. 1982;50:795–799. doi: 10.1016/0002-9149(82)91236-x [DOI] [PubMed] [Google Scholar]
- 72.Lange PE, Onnasch DG, Bernhard A, Heintzen PH. Left and right ventricular adaptation to right ventricular overload before and after surgical repair of tetralogy of Fallot. Am J Cardiol. 1982;50:786–794. doi: 10.1016/0002-9149(82)91235-8 [DOI] [PubMed] [Google Scholar]
- 73.Cullen S, Shore D, Redington A. Characterization of right ventricular diastolic performance after complete repair of tetralogy of Fallot. Restrictive physiology predicts slow postoperative recovery. Circulation. 1995;91:1782–1789. doi: 10.1161/01.cir.91.6.1782 [DOI] [PubMed] [Google Scholar]
- 74.Gatzoulis MA, Till JA, Somerville J, Redington AN. Mechanoelectrical interaction in tetralogy of Fallot. QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation. 1995;92:231–237. doi: 10.1161/01.cir.92.2.231 [DOI] [PubMed] [Google Scholar]
- 75.Gatzoulis MA, Clark AL, Cullen S, Newman CG, Redington AN. Right ventricular diastolic function 15 to 35 years after repair of tetralogy of Fallot. Restrictive physiology predicts superior exercise performance. Circulation. 1995;91:1775–1781. doi: 10.1161/01.cir.91.6.1775 [DOI] [PubMed] [Google Scholar]
- 76.Sarubbi B, Pacileo G, Pisacane C, Ducceschi V, Iacono C, Russo MG, Iacono A, Calabrò R. Exercise capacity in young patients after total repair of Tetralogy of Fallot. Pediatr Cardiol. 2000;21:211–215. doi: 10.1007/s002460010041 [DOI] [PubMed] [Google Scholar]
- 77.Kondo C, Nakazawa M, Kusakabe K, Momma K. Left ventricular dysfunction on exercise long term after total repair of tetralogy of Fallot. Circulation. 1995;92(suppl II):II250–5. [DOI] [PubMed] [Google Scholar]
- 78.Santamore WP, Lynch PR, Heckman JL, Bove AA, Meier GD. Left ventricular effects on right ventricular developed pressure. J Appl Physiol. 1976;41:925–930. doi: 10.1152/jappl.1976.41.6.925 [DOI] [PubMed] [Google Scholar]
- 79.Feneley MP, Gavaghan TP, Baron DW, Branson JA, Roy PR, Morgan JJ. Contribution of left ventricular contraction to the generation of right ventricular systolic pressure in the human heart. Circulation. 1985;71:473–480. doi: 10.1161/01.cir.71.3.473 [DOI] [PubMed] [Google Scholar]
- 80.Santamore WP, Constantinescu M, Minczak BM, Hock CE, Papa L. Contribution of each ventricular wall to ventricular interdependence. Basic Res Cardiol. 1988;83:424–430. doi: 10.1007/BF02005828 [DOI] [PubMed] [Google Scholar]
- 81.Weber KT, Janicki JS, Shroff S, Fishman AP. Contractile mechanics and interaction of the right and left ventricles. Am J Cardiol. 1981;47:686–695. doi: 10.1016/0002-9149(81)90556-7 [DOI] [PubMed] [Google Scholar]
- 82.Louie EK, Lin SS, Reynertson SI, Brundage BH, Levitsky S, Rich S. Pressure and volume loading of the right ventricle have opposite effects on left ventricular ejection fraction. Circulation. 1995;92:819–824. doi: 10.1161/01.cir.92.4.819 [DOI] [PubMed] [Google Scholar]
- 83.Santamore WP, Constantinescu M, Vinten-Johansen J, Johnston WE, Little WC. Alterations in left ventricular compliance due to changes in right ventricular volume, pressure and compliance. Cardiovasc Res. 1988;22:768–776. doi: 10.1093/cvr/22.11.768 [DOI] [PubMed] [Google Scholar]
- 84.Brinker JA, Weiss JL, Lappé DL, Rabson JL, Summer WR, Permutt S, Weisfeldt ML. Leftward septal displacement during right ventricular loading in man. Circulation. 1980;61:626–633. doi: 10.1161/01.cir.61.3.626 [DOI] [PubMed] [Google Scholar]
- 85.Niezen RA, Helbing WA, van der Wall EE, van der Geest RJ, Rebergen SA, de Roos A. Biventricular systolic function and mass studied with MR imaging in children with pulmonary regurgitation after repair for tetralogy of Fallot. Radiology. 1996;201:135–140. doi: 10.1148/radiology.201.1.8816534 [DOI] [PubMed] [Google Scholar]
- 86.Singh GK, Greenberg SB, Yap YS, Delany DP, Keeton BR, Monro JL. Right ventricular function and exercise performance late after primary repair of tetralogy of Fallot with the transannular patch in infancy. Am J Cardiol. 1998;81:1378–1382. doi: 10.1016/s0002-9149(98)00171-4 [DOI] [PubMed] [Google Scholar]
- 87.Eyskens B, Reybrouck T, Bogaert J, Dymarkowsky S, Daenen W, Dumoulin M, Gewillig M. Homograft insertion for pulmonary regurgitation after repair of tetralogy of fallot improves cardiorespiratory exercise performance. Am J Cardiol. 2000;85:221–225. doi: 10.1016/s0002-9149(99)00640-2 [DOI] [PubMed] [Google Scholar]
- 88.Warner KG, Anderson JE, Fulton DR, Payne DD, Geggel RL, Marx GR. Restoration of the pulmonary valve reduces right ventricular volume overload after previous repair of tetralogy of Fallot. Circulation. 1993;88:189–97. [PubMed] [Google Scholar]
- 89.Wessel HU, Paul MH. Exercise studies in tetralogy of Fallot: a review. Pediatr Cardiol. 1999;20:39–47; discussion 48. doi: 10.1007/s002469900393 [DOI] [PubMed] [Google Scholar]
- 90.Le Gloan L, Guerin P, Mercier LA, Abbey S, Dore A, Marcotte F, Ibrahim R, Poirier NC, Khairy P. Clinical assessment of arrhythmias in tetralogy of Fallot. Expert Rev Cardiovasc Ther. 2010;8:189–197. doi: 10.1586/erc.09.156 [DOI] [PubMed] [Google Scholar]
- 91.Kozak MF, Redington A, Yoo SJ, Seed M, Greiser A, Grosse-Wortmann L. Diffuse myocardial fibrosis following tetralogy of Fallot repair: a T1 mapping cardiac magnetic resonance study. Pediatr Radiol. 2014;44:403–409. doi: 10.1007/s00247-013-2840-9 [DOI] [PubMed] [Google Scholar]
- 92.Yim D, Riesenkampff E, Caro-Dominguez P, Yoo SJ, Seed M, Grosse-Wortmann L. Assessment of Diffuse Ventricular Myocardial Fibrosis Using Native T1 in Children With Repaired Tetralogy of Fallot. Circ Cardiovasc Imaging. 2017;10:e005695. doi: 10.1161/CIRCIMAGING.116.005695 [DOI] [PubMed] [Google Scholar]
- 93.Henk CB, Schlechta B, Grampp S, Gomischek G, Klepetko W, Mostbeck GH. Pulmonary and aortic blood flow measurements in normal subjects and patients after single lung transplantation at 0.5 T using velocity encoded cine MRI. Chest. 1998;114:771–779. doi: 10.1378/chest.114.3.771 [DOI] [PubMed] [Google Scholar]
- 94.Liu S, Han J, Nacif MS, Jones J, Kawel N, Kellman P, Sibley CT, Bluemke DA. Diffuse myocardial fibrosis evaluation using cardiac magnetic resonance T1 mapping: sample size considerations for clinical trials. J Cardiovasc Magn Reson. 2012;14:90. doi: 10.1186/1532-429X-14-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Broberg CS, Huang J, Hogberg I, McLarry J, Woods P, Burchill LJ, Pantely GA, Sahn DJ, Jerosch-Herold M. Diffuse LV Myocardial Fibrosis and its Clinical Associations in Adults With Repaired Tetralogy of Fallot. JACC Cardiovasc Imaging. 2016;9:86–87. doi: 10.1016/j.jcmg.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 96.Orwat S, Diller GP, Kempny A, Radke R, Peters B, Kühne T, Boethig D, Gutberlet M, Dubowy KO, Beerbaum P, et al. ; German Competence Network for Congenital Heart Defects Investigators. Myocardial deformation parameters predict outcome in patients with repaired tetralogy of Fallot. Heart. 2016;102:209–215. doi: 10.1136/heartjnl-2015-308569 [DOI] [PubMed] [Google Scholar]
- 97.André F, Robbers-Visser D, Helling-Bakki A, Föll A, Voss A, Katus HA, Helbing WA, Buss SJ, Eichhorn JG. Quantification of myocardial deformation in children by cardiovascular magnetic resonance feature tracking: determination of reference values for left ventricular strain and strain rate. J Cardiovasc Magn Reson. 2016;19:8. doi: 10.1186/s12968-016-0310-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rebergen SA, Chin JG, Ottenkamp J, van der Wall EE, de Roos A. Pulmonary regurgitation in the late postoperative follow-up of tetralogy of Fallot. Volumetric quantitation by nuclear magnetic resonance velocity mapping. Circulation. 1993;88(5 Pt 1):2257–2266. doi: 10.1161/01.cir.88.5.2257 [DOI] [PubMed] [Google Scholar]
- 99.Wald RM, Redington AN, Pereira A, Provost YL, Paul NS, Oechslin EN, Silversides CK. Refining the assessment of pulmonary regurgitation in adults after tetralogy of Fallot repair: should we be measuring regurgitant fraction or regurgitant volume? Eur Heart J. 2009;30:356–361. doi: 10.1093/eurheartj/ehn595 [DOI] [PubMed] [Google Scholar]
- 100.Helbing WA, Niezen RA, Le Cessie S, van der Geest RJ, Ottenkamp J, de Roos A. Right ventricular diastolic function in children with pulmonary regurgitation after repair of tetralogy of Fallot: volumetric evaluation by magnetic resonance velocity mapping. J Am Coll Cardiol. 1996;28:1827–1835. doi: 10.1016/S0735-1097(96)00387-7 [DOI] [PubMed] [Google Scholar]
- 101.Helbing WA, de Roos A. Clinical applications of cardiac magnetic resonance imaging after repair of tetralogy of Fallot. Pediatr Cardiol. 2000;21:70–79. doi: 10.1007/s002469910009 [DOI] [PubMed] [Google Scholar]
- 102.Samyn MM, Powell AJ, Garg R, Sena L, Geva T. Range of ventricular dimensions and function by steady-state free precession cine MRI in repaired tetralogy of Fallot: right ventricular outflow tract patch vs. conduit repair. J Magn Reson Imaging. 2007;26:934–940. doi: 10.1002/jmri.21094 [DOI] [PubMed] [Google Scholar]
- 103.Didier D, Ratib O, Beghetti M, Oberhaensli I, Friedli B. Morphologic and functional evaluation of congenital heart disease by magnetic resonance imaging. J Magn Reson Imaging. 1999;10:639–655. doi: 10.1002/(sici)1522-2586(199911)10:5<639::aid-jmri7>3.0.co;2-l [DOI] [PubMed] [Google Scholar]
- 104.Mor-Avi V, Sugeng L, Weinert L, MacEneaney P, Caiani EG, Koch R, Salgo IS, Lang RM. Fast measurement of left ventricular mass with real-time three-dimensional echocardiography: comparison with magnetic resonance imaging. Circulation. 2004;110:1814–1818. doi: 10.1161/01.CIR.0000142670.65971.5F [DOI] [PubMed] [Google Scholar]
- 105.Bu L, Munns S, Zhang H, Disterhoft M, Dixon M, Stolpen A, Sonka M, Scholz TD, Mahoney LT, Ge S. Rapid full volume data acquisition by real-time 3-dimensional echocardiography for assessment of left ventricular indexes in children: a validation study compared with magnetic resonance imaging. J Am Soc Echocardiogr. 2005;18:299–305. doi: 10.1016/j.echo.2004.11.002 [DOI] [PubMed] [Google Scholar]
- 106.Kühl HP, Schreckenberg M, Rulands D, Katoh M, Schäfer W, Schummers G, Bücker A, Hanrath P, Franke A. High-resolution transthoracic real-time three-dimensional echocardiography: quantitation of cardiac volumes and function using semi-automatic border detection and comparison with cardiac magnetic resonance imaging. J Am Coll Cardiol. 2004;43:2083–2090. doi: 10.1016/j.jacc.2004.01.037 [DOI] [PubMed] [Google Scholar]
- 107.Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C, Rosenthal M, Nakazawa M, Moller JH, Gillette PC, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975–981. doi: 10.1016/S0140-6736(00)02714-8 [DOI] [PubMed] [Google Scholar]
- 108.Schwartz MC, Rome JJ, Gillespie MJ, Whitehead K, Harris MA, Fogel MA, Glatz AC. Relation of left ventricular end diastolic pressure to right ventricular end diastolic volume after operative treatment of tetralogy of fallot. Am J Cardiol. 2012;109:417–422. doi: 10.1016/j.amjcard.2011.09.028 [DOI] [PubMed] [Google Scholar]
- 109.Valente AM, Gauvreau K, Assenza GE, Babu-Narayan SV, Schreier J, Gatzoulis MA, Groenink M, Inuzuka R, Kilner PJ, Koyak Z, et al. Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired tetralogy of Fallot enrolled in the INDICATOR cohort. Heart. 2014;100:247–253. doi: 10.1136/heartjnl-2013-304958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Geva T, Sandweiss BM, Gauvreau K, Lock JE, Powell AJ. Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. J Am Coll Cardiol. 2004;43:1068–1074. doi: 10.1016/j.jacc.2003.10.045 [DOI] [PubMed] [Google Scholar]
- 111.Sarikouch S, Koerperich H, Dubowy KO, Boethig D, Boettler P, Mir TS, Peters B, Kuehne T, Beerbaum P. Impact of gender and age on cardiovascular function late after repair of tetralogy of Fallot. Percentiles based on cardiac magnetic resonance. Circ Cardiovascular Imaging. 2011;4:703–11. doi: 10.1161/CIRCIMAGING.111.963637 [DOI] [PubMed] [Google Scholar]
- 112.Bhat M, Mercer-Rosa L, Fogel MA, Harris MA, Paridon SM, McBride MG, Shults J, Zhang X, Goldmuntz E. Longitudinal changes in adolescents with TOF: implications for care. Eur Heart J Cardiovasc Imaging. 2017;18:356–363. doi: 10.1093/ehjci/jew272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mercer-Rosa L, Paridon SM, Fogel MA, Rychik J, Tanel RE, Zhao H, Zhang X, Yang W, Shults J, Goldmuntz E. 22q11.2 deletion status and disease burden in children and adolescents with tetralogy of Fallot. Circ Cardiovasc Genet. 2015;8:74–81. doi: 10.1161/CIRCGENETICS.114.000819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Menteer J, Weinberg PM, Fogel MA. Quantifying regional right ventricular function in tetralogy of Fallot. J Cardiovasc Magn Reson. 2005;7:753–761. doi: 10.1080/10976640500283439 [DOI] [PubMed] [Google Scholar]
- 115.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992 [DOI] [PubMed] [Google Scholar]
- 116.Harris MA, Johnson TR, Weinberg PM, Fogel MA. Delayed-enhancement cardiovascular magnetic resonance identifies fibrous tissue in children after surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2007;133:676–681. doi: 10.1016/j.jtcvs.2006.10.057 [DOI] [PubMed] [Google Scholar]
- 117.Wald RM, Haber I, Wald R, Valente AM, Powell AJ, Geva T. Effects of regional dysfunction and late gadolinium enhancement on global right ventricular function and exercise capacity in patients with repaired tetralogy of Fallot. Circulation. 2009;119:1370–1377. doi: 10.1161/CIRCULATIONAHA.108.816546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Babu-Narayan SV, Kilner PJ, Li W, Moon JC, Goktekin O, Davlouros PA, Khan M, Ho SY, Pennell DJ, Gatzoulis MA. Ventricular fibrosis suggested by cardiovascular magnetic resonance in adults with repaired tetralogy of fallot and its relationship to adverse markers of clinical outcome. Circulation. 2006;113:405–413. doi: 10.1161/CIRCULATIONAHA.105.548727 [DOI] [PubMed] [Google Scholar]
- 119.Ylitalo P, Pitkänen OM, Lauerma K, Holmström M, Rahkonen O, Heikinheimo M, Sairanen H, Jokinen E. Late gadolinium enhancement (LGE) progresses with right ventricle volume in children after repair of tetralogy of fallot. Int J Cardiol Heart Vessel. 2014;3:15–20. doi: 10.1016/j.ijchv.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Valente AM, Cook S, Festa P, Ko HH, Krishnamurthy R, Taylor AM, Warnes CA, Kreutzer J, Geva T. Multimodality imaging guidelines for patients with repaired tetralogy of fallot: a report from the AmericanSsociety of Echocardiography: developed in collaboration with the Society for Cardiovascular Magnetic Resonance and the Society for Pediatric Radiology. J Am Soc Echocardiogr. 2014;27:111–141. doi: 10.1016/j.echo.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 121.Knauth AL, Gauvreau K, Powell AJ, Landzberg MJ, Walsh EP, Lock JE, del Nido PJ, Geva T. Ventricular size and function assessed by cardiac MRI predict major adverse clinical outcomes late after tetralogy of Fallot repair. Heart. 2008;94:211–216. doi: 10.1136/hrt.2006.104745 [DOI] [PubMed] [Google Scholar]
- 122.Riesenkampff E, Luining W, Seed M, Chungsomprasong P, Manlhiot C, Elders B, McCrindle BW, Yoo SJ, Grosse-Wortmann L. Increased left ventricular myocardial extracellular volume is associated with longer cardiopulmonary bypass times, biventricular enlargement and reduced exercise tolerance in children after repair of Tetralogy of Fallot. J Cardiovasc Magn Reson. 2016;18:75. doi: 10.1186/s12968-016-0290-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Haggerty CM, Suever JD, Pulenthiran A, Mejia-Spiegeler A, Wehner GJ, Jing L, Charnigo RJ, Fornwalt BK, Fogel MA. Association between left ventricular mechanics and diffuse myocardial fibrosis in patients with repaired Tetralogy of Fallot: a cross-sectional study. J Cardiovasc Magn Reson. 2017;19:100. doi: 10.1186/s12968-017-0410-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schievano S, Coats L, Migliavacca F, Norman W, Frigiola A, Deanfield J, Bonhoeffer P, Taylor AM. Variations in right ventricular outflow tract morphology following repair of congenital heart disease: implications for percutaneous pulmonary valve implantation. J Cardiovasc Magn Reson. 2007;9:687–695. doi: 10.1080/10976640601187596 [DOI] [PubMed] [Google Scholar]
- 125.Bernardes RJ, Marchiori E, Bernardes PM, Monzo Gonzaga MB, Simões LC. A comparison of magnetic resonance angiography with conventional angiography in the diagnosis of tetralogy of Fallot. Cardiol Young. 2006;16:281–288. doi: 10.1017/S1047951106000370 [DOI] [PubMed] [Google Scholar]
- 126.Valverde I, Parish V, Hussain T, Rosenthal E, Beerbaum P, Krasemann T. Planning of catheter interventions for pulmonary artery stenosis: improved measurement agreement with magnetic resonance angiography using identical angulations. Catheter Cardiovasc Interv. 2011;77:400–408. doi: 10.1002/ccd.22695 [DOI] [PubMed] [Google Scholar]
- 127.Greenberg SB, Crisci KL, Koenig P, Robinson B, Anisman P, Russo P. Magnetic resonance imaging compared with echocardiography in the evaluation of pulmonary artery abnormalities in children with tetralogy of Fallot following palliative and corrective surgery. Pediatr Radiol. 1997;27:932–935. doi: 10.1007/s002470050275 [DOI] [PubMed] [Google Scholar]
- 128.Weber OM, Higgins CB. MR evaluation of cardiovascular physiology in congenital heart disease: flow and function. J Cardiovasc Magn Reson. 2006;8:607–617. doi: 10.1080/10976640600740254 [DOI] [PubMed] [Google Scholar]
- 129.Harris MA, Whitehead KK, Gillespie MJ, Liu TY, Cosulich MT, Shin DC, Goldmuntz E, Weinberg PM, Fogel MA. Differential branch pulmonary artery regurgitant fraction is a function of differential pulmonary arterial anatomy and pulmonary vascular resistance. JACC Cardiovasc Imaging. 2011;4:506–513. doi: 10.1016/j.jcmg.2011.02.011 [DOI] [PubMed] [Google Scholar]
- 130.Voser EM, Kellenberger CJ, Buechel ER. Effects of pulmonary regurgitation on distensibility and flow of the branch pulmonary arteries in tetralogy of Fallot. Pediatr Cardiol. 2013;34:1118–1124. doi: 10.1007/s00246-012-0616-x [DOI] [PubMed] [Google Scholar]
- 131.Harris MA, Avitabile CM, Fu GL, Kim DW, Kim TS, Gillespie MJ, Keller MS, Fogel MA, Whitehead KK. Accuracy and Internal Consistency of Cardiac Magnetic Resonance Imaging in Measuring Branch Pulmonary Artery Flows in Patients With Conotruncal Anomalies and Branch Pulmonary Artery Stents. Am J Cardiol. 2016;117:1160–1166. doi: 10.1016/j.amjcard.2016.01.005 [DOI] [PubMed] [Google Scholar]
- 132.Dennis M, Laarkson M, Padang R, Tanous DJ, Robinson P, Pressley L, O’Meagher S, Celermajer D, Puranik R. Long term followup of aortic root size after repair of tetralogy of Fallot. Int J Cardiol. 2014;177:136–138. doi: 10.1016/j.ijcard.2014.09.113 [DOI] [PubMed] [Google Scholar]
- 133.Schäfer M, Browne LP, Morgan GJ, Barker AJ, Fonseca B, Ivy DD, Mitchell MB. Reduced proximal aortic compliance and elevated wall shear stress after early repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2018;156:2239–49. doi: 10.1016/j.jtcvs.2018.08.081 [DOI] [PubMed] [Google Scholar]
- 134.Ordovas KG, Keedy A, Naeger DM, Kallianos K, Foster E, Liu J, Saloner D, Hope MD. Dilatation of the ascending aorta is associated with presence of aortic regurgitation in patients after repair of tetralogy of Fallot. Int J Cardiovasc Imaging. 2016;32:1265–1272. doi: 10.1007/s10554-016-0902-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gomes AS, Lois JF, Williams RG. Pulmonary arteries: MR imaging in patients with congenital obstruction of the right ventricular outflow tract. Radiology. 1990;174:51–57. doi: 10.1148/radiology.174.1.2294572 [DOI] [PubMed] [Google Scholar]
- 136.Vick GW, 3rd, Rokey R, Huhta JC, Mulvagh SL, Johnston DL. Nuclear magnetic resonance imaging of the pulmonary arteries, subpulmonary region, and aorticopulmonary shunts: a comparative study with two-dimensional echocardiography and angiography. Am Heart J. 1990;119:1103–1110. doi: 10.1016/s0002-8703(05)80241-8 [DOI] [PubMed] [Google Scholar]
- 137.Vliegen HW, van Straten A, de Roos A, Roest AA, Schoof PH, Zwinderman AH, Ottenkamp J, van der Wall EE, Hazekamp MG. Magnetic resonance imaging to assess the hemodynamic effects of pulmonary valve replacement in adults late after repair of tetralogy of fallot. Circulation. 2002;106:1703–1707. doi: 10.1161/01.cir.0000030995.59403.f8 [DOI] [PubMed] [Google Scholar]
- 138.Henkens IR, van Straten A, Schalij MJ, Hazekamp MG, de Roos A, van der Wall EE, Vliegen HW. Predicting outcome of pulmonary valve replacement in adult tetralogy of Fallot patients. Ann Thorac Surg. 2007;83:907–911. doi: 10.1016/j.athoracsur.2006.09.090 [DOI] [PubMed] [Google Scholar]
- 139.Oosterhof T, van Straten A, Vliegen HW, Meijboom FJ, van Dijk AP, Spijkerboer AM, Bouma BJ, Zwinderman AH, Hazekamp MG, de Roos A, et al. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007;116:545–551. doi: 10.1161/CIRCULATIONAHA.106.659664 [DOI] [PubMed] [Google Scholar]
- 140.Therrien J, Provost Y, Merchant N, Williams W, Colman J, Webb G. Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol. 2005;95:779–782. doi: 10.1016/j.amjcard.2004.11.037 [DOI] [PubMed] [Google Scholar]
- 141.Buechel ER, Dave HH, Kellenberger CJ, Dodge-Khatami A, Pretre R, Berger F, Bauersfeld U. Remodelling of the right ventricle after early pulmonary valve replacement in children with repaired tetralogy of Fallot: assessment by cardiovascular magnetic resonance. Eur Heart J. 2005;26:2721–2727. doi: 10.1093/eurheartj/ehi581 [DOI] [PubMed] [Google Scholar]
- 142.Frigiola A, Tsang V, Bull C, Coats L, Khambadkone S, Derrick G, Mist B, Walker F, van Doorn C, Bonhoeffer P, et al. Biventricular response after pulmonary valve replacement for right ventricular outflow tract dysfunction: is age a predictor of outcome? Circulation. 2008;118(14 Suppl):S182–S190. doi: 10.1161/CIRCULATIONAHA.107.756825 [DOI] [PubMed] [Google Scholar]
- 143.Warner KG, O'Brien PKH, Rhodes J, Kaur A, Robinson DA, Payne DD. Expanding the indications for pulmonary valve replacement after repair of tetralogy of Fallot. Ann Thorac Surg. 2003;75:1066–72. doi: 10.1016/s0003-4975(03)00748-3 [DOI] [PubMed] [Google Scholar]
- 144.Fogel MA, Sundareswaran KS, de Zelicourt D, Dasi LP, Pawlowski T, Rome J, Yoganathan AP. Power loss and right ventricular efficiency in patients after tetralogy of Fallot repair with pulmonary insufficiency: clinical implications. J Thorac Cardiovasc Surg. 2012;143:1279–1285. doi: 10.1016/j.jtcvs.2011.10.066 [DOI] [PubMed] [Google Scholar]
- 145.Geva T. Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve decision support. J Cardiovasc Mag Reson. 2011;13:9. doi: 10.1186/1532-429X-13-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Martins P, Castela E. Transposition of the great arteries. Orphanet J Rare Dis. 2008;3:27. doi: 10.1186/1750-1172-3-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sarris GE, Chairperson Greece, Balmer C, Switzerland, Bonou P, Greece, Comas JV, Spain, da Cruz E, Usa, Chiara LD, Italy, Di Donato RM, United Arab Emirates, Fragata J, Portugal, Jokinen TE, Finland, Kirvassilis G, Usa, et al. Clinical guidelines for the management of patients with transposition of the great arteries with intact ventricular septum. Eur J Cardiothorac Surg. 2017;51:e1–e32. doi: 10.1093/ejcts/ezw360 [DOI] [PubMed] [Google Scholar]
- 148.Williams W. Outcomes of 829 neonates with complete transposition of the great arteries 12–17 years after repair. Eur J Cardiothorac Surg. 2003;24:1–10. [DOI] [PubMed] [Google Scholar]
- 149.Raju V, Burkhart HM, Durham LA, 3rd, Eidem BW, Phillips SD, Li Z, Schaff HV, Dearani JA. Reoperation after arterial switch: a 27-year experience. Ann Thorac Surg. 2013;95:2105–12; discussion 2112. doi: 10.1016/j.athoracsur.2013.02.040 [DOI] [PubMed] [Google Scholar]
- 150.Legendre A, Losay J, Touchot-Koné A, Serraf A, Belli E, Piot JD, Lambert V, Capderou A, Planche C. Coronary events after arterial switch operation for transposition of the great arteries. Circulation. 2003;108 Suppl 1:II186–II190. doi: 10.1161/01.cir.0000087902.67220.2b [DOI] [PubMed] [Google Scholar]
- 151.Ou P, Mousseaux E, Azarine A, Dupont P, Agnoletti G, Vouhé P, Sidi D, Bonnet D. Detection of coronary complications after the arterial switch operation for transposition of the great arteries: first experience with multislice computed tomography in children. J Thorac Cardiovasc Surg. 2006;131:639–643. doi: 10.1016/j.jtcvs.2005.11.014 [DOI] [PubMed] [Google Scholar]
- 152.Angeli E, Formigari R, Pace Napoleone C, Oppido G, Ragni L, Picchio FM, Gargiulo G. Long-term coronary artery outcome after arterial switch operation for transposition of the great arteries. Eur J Cardiothorac Surg. 2010;38:714–720. doi: 10.1016/j.ejcts.2010.03.055 [DOI] [PubMed] [Google Scholar]
- 153.Cohen MS, Eidem BW, Cetta F, Fogel MA, Frommelt PC, Ganame J, Han BK, Kimball TR, Johnson RK, Mertens L, et al. Multimodality Imaging Guidelines of Patients with Transposition of the Great Arteries: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance and the Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr. 2016;29:571–621. doi: 10.1016/j.echo.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 154.van Wijk SWH, van der Stelt F, Ter Heide H, Schoof PH, Doevendans PAFM, Meijboom FJ, Breur JMPJ. Sudden Death Due to Coronary Artery Lesions Long-term After the Arterial Switch Operation: A Systematic Review. Can J Cardiol. 2017;33:1180–1187. doi: 10.1016/j.cjca.2017.02.017 [DOI] [PubMed] [Google Scholar]
- 155.Raisky O, Bergoend E, Agnoletti G, Ou P, Bonnet D, Sidi D, Vouhé PR. Late coronary artery lesions after neonatal arterial switch operation: results of surgical coronary revascularization. Eur J Cardiothorac Surg. 2007;31:894–898. doi: 10.1016/j.ejcts.2007.02.003 [DOI] [PubMed] [Google Scholar]
- 156.Robbers-Visser D, Boersma E, Helbing WA. Normal biventricular function, volumes, and mass in children aged 8 to 17 years. J Magn Reson Imaging. 2009;29:552–559. doi: 10.1002/jmri.21662 [DOI] [PubMed] [Google Scholar]
- 157.Sarikouch S, Peters B, Gutberlet M, Leismann B, Kelter-Kloepping A, Koerperich H, Kuehne T, Beerbaum P. Sex-specific pediatric percentiles for ventricular size and mass as reference values for cardiac MRI: assessment by steady-state free-precession and phase-contrast MRI flow. Circ Cardiovasc Imaging. 2010;3:65–76. doi: 10.1161/CIRCIMAGING.109.859074 [DOI] [PubMed] [Google Scholar]
- 158.Luijnenburg SE, Robbers-Visser D, Moelker A, Vliegen HW, Mulder BJ, Helbing WA. Intra-observer and interobserver variability of biventricular function, volumes and mass in patients with congenital heart disease measured by CMR imaging. Int J Cardiovasc Imaging. 2010;26:57–64. doi: 10.1007/s10554-009-9501-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mooij CF, de Wit CJ, Graham DA, Powell AJ, Geva T. Reproducibility of MRI measurements of right ventricular size and function in patients with normal and dilated ventricles. J Magn Reson Imaging. 2008;28:67–73. doi: 10.1002/jmri.21407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Broda CR, Shugh SB, Parikh RB, Wang Y, Schlingmann TR, Noel CV. Post-operative Assessment of the Arterial Switch Operation: A Comparison of Magnetic Resonance Imaging and Echocardiography. Pediatr Cardiol. 2018;39:1036–1041. doi: 10.1007/s00246-018-1858-z [DOI] [PubMed] [Google Scholar]
- 161.Shepard CW, Germanakis I, White MT, Powell AJ, Co-Vu J, Geva T. Cardiovascular Magnetic Resonance Findings Late After the Arterial Switch Operation. Circ Cardiovasc Imaging. 2016;9:e004618. doi: 10.1161/CIRCIMAGING.116.004618 [DOI] [PubMed] [Google Scholar]
- 162.Szymczyk K, Moll M, Sobczak-Budlewska K, Moll JA, Stefańczyk L, Grzelak P, Moll JJ, Michalak KW. Usefulness of Routine Coronary CT Angiography in Patients with Transposition of the Great Arteries After an Arterial Switch Operation. Pediatr Cardiol. 2018;39:335–346. doi: 10.1007/s00246-017-1761-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ou P, Khraiche D, Celermajer DS, Agnoletti G, Le Quan Sang KH, Thalabard JC, Quintin M, Raisky O, Vouhe P, Sidi D, et al. Mechanisms of coronary complications after the arterial switch for transposition of the great arteries. J Thorac Cardiovasc Surg. 2013;145:1263–1269. doi: 10.1016/j.jtcvs.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 164.McConnell MV, Ganz P, Selwyn AP, Li W, Edelman RR, Manning WJ. Identification of anomalous coronary arteries and their anatomic course by magnetic resonance coronary angiography. Circulation. 1995;92:3158–3162. doi: 10.1161/01.cir.92.11.3158 [DOI] [PubMed] [Google Scholar]
- 165.Tangcharoen T, Bell A, Hegde S, Hussain T, Beerbaum P, Schaeffter T, Razavi R, Botnar RM, Greil GF. Detection of coronary artery anomalies in infants and young children with congenital heart disease by using MR imaging. Radiology. 2011;259:240–247. doi: 10.1148/radiol.10100828 [DOI] [PubMed] [Google Scholar]
- 166.Marín Rodríguez C, Lancharro Zapata Á, Rodríguez Ogando A, Carrasco Muñoz S, Ruiz Martín Y, Sánchez Alegre ML, Maroto Alvaro E. Quality of 3D magnetic resonance imaging of coronary arteries in patients with D-transposition of the great arteries after the Jatene switch procedure. Radiologia. 2015;57:326–32. doi: 10.1016/j.rx.2014.05.004 [DOI] [PubMed] [Google Scholar]
- 167.Brothers JA, Kim TS, Fogel MA, Whitehead KK, Morrison TM, Paridon SM, Harris MA. Cardiac magnetic resonance imaging characterizes stenosis, perfusion, and fibrosis preoperatively and postoperatively in children with anomalous coronary arteries. J Thorac Cardiovasc Surg. 2016;152:205–210. doi: 10.1016/j.jtcvs.2015.12.057 [DOI] [PubMed] [Google Scholar]
- 168.Tobler D, Motwani M, Wald RM, Roche SL, Verocai F, Iwanochko RM, Greenwood JP, Oechslin EN, Crean AM. Evaluation of a comprehensive cardiovascular magnetic resonance protocol in young adults late after the arterial switch operation for d-transposition of the great arteries. J Cardiovasc Magn Reson. 2014;16:98. doi: 10.1186/s12968-014-0098-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nagel E, Klein C, Paetsch I, Hettwer S, Schnackenburg B, Wegscheider K, Fleck E. Magnetic resonance perfusion measurements for the noninvasive detection of coronary artery disease. Circulation. 2003;108:432–437. doi: 10.1161/01.CIR.0000080915.35024.A9 [DOI] [PubMed] [Google Scholar]
- 170.Schwitter J, Nanz D, Kneifel S, Bertschinger K, Büchi M, Knüsel PR, Marincek B, Lüscher TF, von Schulthess GK. Assessment of myocardial perfusion in coronary artery disease by magnetic resonance: a comparison with positron emission tomography and coronary angiography. Circulation. 2001;103:2230–2235. doi: 10.1161/01.cir.103.18.2230 [DOI] [PubMed] [Google Scholar]
- 171.Buechel ER, Balmer C, Bauersfeld U, Kellenberger CJ, Schwitter J. Feasibility of perfusion cardiovascular magnetic resonance in paediatric patients. J Cardiovasc Magn Reson. 2009;11:51. doi: 10.1186/1532-429X-11-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Taylor AM, Dymarkowski S, Hamaekers P, Razavi R, Gewillig M, Mertens L, Bogaert J. MR coronary angiography and late-enhancement myocardial MR in children who underwent arterial switch surgery for transposition of great arteries. Radiology. 2005;234:542–547. doi: 10.1148/radiol.2342032059 [DOI] [PubMed] [Google Scholar]
- 173.Prakash A, Powell AJ, Krishnamurthy R, Geva T. Magnetic resonance imaging evaluation of myocardial perfusion and viability in congenital and acquired pediatric heart disease. Am J Cardiol. 2004;93:657–661. doi: 10.1016/j.amjcard.2003.11.045 [DOI] [PubMed] [Google Scholar]
- 174.Manso B, Castellote A, Dos L, Casaldáliga J. Myocardial perfusion magnetic resonance imaging for detecting coronary function anomalies in asymptomatic paediatric patients with a previous arterial switch operation for the transposition of great arteries. Cardiol Young. 2010;20:410–417. doi: 10.1017/S1047951109990503 [DOI] [PubMed] [Google Scholar]
- 175.Ntsinjana HN, Tann O, Hughes M, Derrick G, Secinaro A, Schievano S, Muthurangu V, Taylor AM. Utility of adenosine stress perfusion CMR to assess paediatric coronary artery disease. Eur Heart J Cardiovasc Imaging. 2017;18:898–905. doi: 10.1093/ehjci/jew151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Vijarnsorn C, Noga M, Schantz D, Pepelassis D, Tham EB. Stress perfusion magnetic resonance imaging to detect coronary artery lesions in children. Int J Cardiovasc Imaging. 2017;33:699–709. doi: 10.1007/s10554-016-1041-7 [DOI] [PubMed] [Google Scholar]
- 177.Biko DM, Collins RT, 2nd, Partington SL, Harris M, Whitehead KK, Keller MS, Fogel MA. Magnetic Resonance Myocardial Perfusion Imaging: Safety and Indications in Pediatrics and Young Adults. Pediatr Cardiol. 2018;39:275–282. doi: 10.1007/s00246-017-1752-0 [DOI] [PubMed] [Google Scholar]
- 178.Grotenhuis HB, Cifra B, Mertens LL, Riessenkampff E, Manlhiot C, Seed M, Yoo SJ, Grosse-Wortmann L. Left ventricular remodelling in long-term survivors after the arterial switch operation for transposition of the great arteries. Eur Heart J Cardiovasc Imaging. 2019;20:101–107. doi: 10.1093/ehjci/jey072 [DOI] [PubMed] [Google Scholar]
- 179.Noel CV, Krishnamurthy R, Masand P, Moffett B, Schlingmann T, Cheong BY, Krishnamurthy R. Myocardial Stress Perfusion MRI: Experience in Pediatric and Young-Adult Patients Following Arterial Switch Operation Utilizing Regadenoson. Pediatr Cardiol. 2018;39:1249–1257. doi: 10.1007/s00246-018-1890-z [DOI] [PubMed] [Google Scholar]
- 180.Suther KR, Hopp E, Geier O, Brun H, Nguyen B, Tomterstad AH, Smevik B, Fiane AE, Lindberg HL, de Lange C. Diffuse myocardial fibrosis in adolescents operated with arterial switch for transposition of the great arteries - A CMR study. Int J Cardiol. 2019;276:100–106. doi: 10.1016/j.ijcard.2018.11.107 [DOI] [PubMed] [Google Scholar]
- 181.Acar P, Maunoury C, Bonnet D, Sébahoun S, Bonhoeffer P, Saliba Z, Sidi D, Kachaner J. Comparison of myocardial perfusion single-photon emission computed tomography with coronary artery angiography after arterial switch operation. Am J Cardiol. 2001;87:1425–1427. doi: 10.1016/s0002-9149(01)01571-5 [DOI] [PubMed] [Google Scholar]
- 182.Blakenberg F, Rhee J, Hardy C, Helton G, Higgins SS, Higgins CB. MRI vs echocardiography in the evaluation of the Jatene procedure. J Comput Assist Tomogr. 1994;18:749–754. doi: 10.1097/00004728-199409000-00013 [DOI] [PubMed] [Google Scholar]
- 183.Babu-Narayan S. CMR for transposition of the great arteries. In: Lombardi MPS, Petersen S, Bucciarelli-Ducci C, ValsangiacomoBuechel ER, Basso C, Ferrari V, editors. The EACVI textbook of cardiovascular magnetic resonance. Oxford, UK: Oxford University Press; 2018. p. 538–48. [Google Scholar]
- 184.Hardy CE, Helton GJ, Kondo C, Higgins SS, Young NJ, Higgins CB. Usefulness of magnetic resonance imaging for evaluating great-vessel anatomy after arterial switch operation for D-transposition of the great arteries. Am Heart J. 1994;128:326–332. doi: 10.1016/0002-8703(94)90486-3 [DOI] [PubMed] [Google Scholar]
- 185.Weiss F, Habermann CR, Lilje C, Nimz M, Rasek V, Dallmeyer J, et al. MRT der Pulmonalarterien bei Kindern nach arterieller Switch-Operation (ASO) bei Transposition der großen Gefäße (d-TGA). Fortschr Röntgenstr. 2005;177:849–55. [DOI] [PubMed] [Google Scholar]
- 186.Gutberlet M, Boeckel T, Hosten N, Vogel M, Kühne T, Oellinger H, Ehrenstein T, Venz S, Hetzer R, Bein G, et al. Arterial switch procedure for D-transposition of the great arteries: quantitative midterm evaluation of hemodynamic changes with cine MR imaging and phase-shift velocity mapping-initial experience. Radiology. 2000;214:467–475. doi: 10.1148/radiology.214.2.r00fe45467 [DOI] [PubMed] [Google Scholar]
- 187.Roman KS, Kellenberger CJ, Farooq S, MacGowan CK, Gilday DL, Yoo SJ. Comparative imaging of differential pulmonary blood flow in patients with congenital heart disease: magnetic resonance imaging versus lung perfusion scintigraphy. Pediatr Radiol. 2005;35:295–301. doi: 10.1007/s00247-004-1344-z [DOI] [PubMed] [Google Scholar]
- 188.Fratz S, Hess J, Schwaiger M, Martinoff S, Stern HC. More accurate quantification of pulmonary blood flow by magnetic resonance imaging than by lung perfusion scintigraphy in patients with fontan circulation. Circulation. 2002;106:1510–1513. doi: 10.1161/01.cir.0000029103.26029.1e [DOI] [PubMed] [Google Scholar]
- 189.Morgan CT, Mertens L, Grotenhuis H, Yoo SJ, Seed M, Grosse-Wortmann L. Understanding the mechanism for branch pulmonary artery stenosis after the arterial switch operation for transposition of the great arteries. Eur Heart J Cardiovasc Imaging. 2017;18:180–185. doi: 10.1093/ehjci/jew046 [DOI] [PubMed] [Google Scholar]
- 190.Geiger J, Hirtler D, Bürk J, Stiller B, Arnold R, Jung B, Langer M, Markl M. Postoperative pulmonary and aortic 3D haemodynamics in patients after repair of transposition of the great arteries. Eur Radiol. 2014;24:200–208. doi: 10.1007/s00330-013-2998-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rickers C, Kheradvar A, Sievers HH, Falahatpisheh A, Wegner P, Gabbert D, Jerosch-Herold M, Hart C, Voges I, Putman LM, et al. Is the Lecompte technique the last word on transposition of the great arteries repair for all patients? A magnetic resonance imaging study including a spiral technique two decades postoperatively. Interact Cardiovasc Thorac Surg. 2016;22:817–825. doi: 10.1093/icvts/ivw014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Devos DG, Kilner PJ. Calculations of cardiovascular shunts and regurgitation using magnetic resonance ventricular volume and aortic and pulmonary flow measurements. Eur Radiol. 2010;20:410–421. doi: 10.1007/s00330-009-1568-2 [DOI] [PubMed] [Google Scholar]
- 193.Co-Vu JG, Ginde S, Bartz PJ, Frommelt PC, Tweddell JS, Earing MG. Long-term outcomes of the neoaorta after arterial switch operation for transposition of the great arteries. Ann Thorac Surg. 2013;95:1654–1659. doi: 10.1016/j.athoracsur.2012.10.081 [DOI] [PubMed] [Google Scholar]
- 194.McMahon CJ, Ravekes WJ, Smith EO, Denfield SW, Pignatelli RH, Altman CA, Ayres NA. Risk factors for neo-aortic root enlargement and aortic regurgitation following arterial switch operation. Pediatr Cardiol. 2004;25:329–335. doi: 10.1007/s00246-003-0483-6 [DOI] [PubMed] [Google Scholar]
- 195.Schwartz Marcy L, Gauvreau K, del Nido P, Mayer John E, Colan SD. Long-term predictors of aortic root dilation and aortic regurgitation after arterial switch operation. Circulation. 2004;110:II-128-II–32. doi: 10.1161/01.CIR.0000138392.68841.d3 [DOI] [PubMed] [Google Scholar]
- 196.Martins D, Khraiche D, Legendre A, Boddaert N, Raisky O, Bonnet D, Raimondi F. Aortic angle is associated with neo-aortic root dilatation and regurgitation following arterial switch operation. Int J Cardiol. 2019;280:53–56. doi: 10.1016/j.ijcard.2019.01.042 [DOI] [PubMed] [Google Scholar]
- 197.Lo Rito M, Fittipaldi M, Haththotuwa R, Jones TJ, Khan N, Clift P, Brawn WJ, Barron DJ. Long-term fate of the aortic valve after an arterial switch operation. J Thorac Cardiovasc Surg. 2015;149:1089–1094. doi: 10.1016/j.jtcvs.2014.11.075 [DOI] [PubMed] [Google Scholar]
- 198.Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J Peds. 2008;153:807–13. doi: 10.1016/j.jpeds.2008.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ho Vincent B, Bakalov Vladimir K, Cooley M, Van Phillip L, Hood Maureen N, Burklow Thomas R, Bondy CA. Major vascular anomalies in Turner syndrome. Circulation. 2004;110:1694–700. doi: 10.1161/01.CIR.0000142290.35842.B0 [DOI] [PubMed] [Google Scholar]
- 200.Canter CE, Martin TC, Spray TL, Weldon CS, Strauss AW. Scimitar syndrome in childhood. Am J Cardiol. 1986;58:652–654. doi: 10.1016/0002-9149(86)90296-1 [DOI] [PubMed] [Google Scholar]
- 201.HALASZ NA, HALLORAN KH, LIEBOW AA. Bronchial and arterial anomalies with drainage of the right lung into the inferior vena cava. Circulation. 1956;14:826–846. doi: 10.1161/01.cir.14.5.826 [DOI] [PubMed] [Google Scholar]
- 202.Fredoom RM, Mawson J, Yoo SJ, Benson LN. Abnormalities of pulmonary venous connections including subdivided left atrium. Congenital heart disease: textbook of angiocardiography. Armonk, NY: Futura; 1997. p. 665–705. [Google Scholar]
- 203.Seale AN, Uemura H, Webber SA, Partridge J, Roughton M, Ho SY, McCarthy KP, Jones S, Shaughnessy L, Sunnegardh J, et al. ; British Congenital Cardiac Association. Total anomalous pulmonary venous connection: morphology and outcome from an international population-based study. Circulation. 2010;122:2718–2726. doi: 10.1161/CIRCULATIONAHA.110.940825 [DOI] [PubMed] [Google Scholar]
- 204.Gilljam T, McCrindle BW, Smallhorn JF, Williams WG, Freedom RM. Outcomes of left atrial isomerism over a 28-year period at a single institution. J Am Coll Cardiol. 2000;36:908–916. doi: 10.1016/s0735-1097(00)00812-3 [DOI] [PubMed] [Google Scholar]
- 205.Freedom RM, Yoo SJ, Coles JG, Konstantinov I. Total anomalous pulmonary venous connection. In: Freedom RM, Yoo SJ, Mikailian H, Williams WG, editors. The natural and modified history of congenital heart disease. New York: Futura; 2004. p. 282–9. [Google Scholar]
- 206.Breinholt JP, Hawkins JA, Minich LA, Tani LY, Orsmond GS, Ritter S, Shaddy RE. Pulmonary vein stenosis with normal connection: associated cardiac abnormalities and variable outcome. Ann Thorac Surg. 1999;68:164–168. doi: 10.1016/s0003-4975(99)00311-2 [DOI] [PubMed] [Google Scholar]
- 207.Song MK, Bae EJ, Jeong SI, Kang IS, Kim NK, Choi JY, Kim SJ, Kim YH, Kim WH. Clinical characteristics and prognostic factors of primary pulmonary vein stenosis or atresia in children. Ann Thorac Surg. 2013;95:229–234. doi: 10.1016/j.athoracsur.2012.08.104 [DOI] [PubMed] [Google Scholar]
- 208.Riesenkampff EM, Schmitt B, Schnackenburg B, Huebler M, Alexi-Meskishvili V, Hetzer R, Berger F, Kuehne T. Partial anomalous pulmonary venous drainage in young pediatric patients: the role of magnetic resonance imaging. Pediatr Cardiol. 2009;30:458–464. doi: 10.1007/s00246-008-9367-0 [DOI] [PubMed] [Google Scholar]
- 209.Festa P, Ait-Ali L, Cerillo AG, De Marchi D, Murzi B. Magnetic resonance imaging is the diagnostic tool of choice in the preoperative evaluation of patients with partial anomalous pulmonary venous return. Int J Cardiovasc Imaging. 2006;22:685–693. doi: 10.1007/s10554-005-9070-7 [DOI] [PubMed] [Google Scholar]
- 210.Gustafson RA, Warden HE, Murray GF. Partial anomalous pulmonary venous connection to the superior vena cava. Ann Thorac Surg. 1995;60(6 Suppl):S614–S617. doi: 10.1016/0003-4975(95)00854-3 [DOI] [PubMed] [Google Scholar]
- 211.Said SM, Burkhart HM, Dearani JA, Eidem B, Stensrud P, Phillips SD, Schaff HV. Outcome of caval division techniques for partial anomalous pulmonary venous connections to the superior vena cava. Ann Thorac Surg. 2011;92:980–4; discussion 985. doi: 10.1016/j.athoracsur.2011.04.110 [DOI] [PubMed] [Google Scholar]
- 212.Khan MA, Torres AJ, Printz BF, Prakash A. Usefulness of magnetic resonance angiography for diagnosis of scimitar syndrome in early infancy. Am J Cardiol. 2005;96:1313–1316. doi: 10.1016/j.amjcard.2005.06.079 [DOI] [PubMed] [Google Scholar]
- 213.Grosse-Wortmann L, Al-Otay A, Goo HW, Macgowan CK, Coles JG, Benson LN, Redington AN, Yoo SJ. Anatomical and functional evaluation of pulmonary veins in children by magnetic resonance imaging. J Am Coll Cardiol. 2007;49:993–1002. doi: 10.1016/j.jacc.2006.09.052 [DOI] [PubMed] [Google Scholar]
- 214.Karamlou T, Gurofsky R, Al Sukhni E, Coles JG, Williams WG, Caldarone CA, Van Arsdell GS, McCrindle BW. Factors associated with mortality and reoperation in 377 children with total anomalous pulmonary venous connection. Circulation. 2007;115:1591–1598. doi: 10.1161/CIRCULATIONAHA.106.635441 [DOI] [PubMed] [Google Scholar]
- 215.Caldarone CA, Najm HK, Kadletz M, Smallhorn JF, Freedom RM, Williams WG, Coles JG. Relentless pulmonary vein stenosis after repair of total anomalous pulmonary venous drainage. Ann Thorac Surg. 1998;66:1514–1520. doi: 10.1016/s0003-4975(98)00952-7 [DOI] [PubMed] [Google Scholar]
- 216.Greenway SC, Yoo SJ, Baliulis G, Caldarone C, Coles J, Grosse-Wortmann L. Assessment of pulmonary veins after atrio-pericardial anastomosis by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2011;13:72. doi: 10.1186/1532-429X-13-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Videlefsky N, Parks WJ, Oshinski J, Hopkins KL, Sullivan KM, Pettigrew RI, Fyfe D. Magnetic resonance phase-shift velocity mapping in pediatric patients with pulmonary venous obstruction. J Am Coll Cardiol. 2001;38:262–267. doi: 10.1016/s0735-1097(01)01338-9 [DOI] [PubMed] [Google Scholar]
- 218.Goo HW, Al-Otay A, Grosse-Wortmann L, Wu S, Macgowan CK, Yoo SJ. Phase-contrast magnetic resonance quantification of normal pulmonary venous return. J Magn Reson Imaging. 2009;29:588–594. doi: 10.1002/jmri.21691 [DOI] [PubMed] [Google Scholar]
- 219.Valsangiacomo ER, Barrea C, Macgowan CK, Smallhorn JF, Coles JG, Yoo SJ. Phase-contrast MR assessment of pulmonary venous blood flow in children with surgically repaired pulmonary veins. Pediatr Radiol. 2003;33:607–613. doi: 10.1007/s00247-003-0983-9 [DOI] [PubMed] [Google Scholar]
- 220.Roman KS, Kellenberger CJ, Macgowan CK, Coles J, Redington AN, Benson LN, Yoo SJ. How is pulmonary arterial blood flow affected by pulmonary venous obstruction in children? A phase-contrast magnetic resonance study. Pediatr Radiol. 2005;35:580–586. doi: 10.1007/s00247-004-1399-x [DOI] [PubMed] [Google Scholar]
- 221.Kellenberger CJ, Macgowan CK, Roman KS, Al-Habshan F, Benson LN, Redington AN, Yoo SJ. Hemodynamic evaluation of the peripheral pulmonary circulation by cine phase-contrast magnetic resonance imaging. J Magn Reson Imaging. 2005;22:780–787. doi: 10.1002/jmri.20447 [DOI] [PubMed] [Google Scholar]
- 222.Foerster SR, Gauvreau K, McElhinney DB, Geva T. Importance of totally anomalous pulmonary venous connection and postoperative pulmonary vein stenosis in outcomes of heterotaxy syndrome. Pediatr Cardiol. 2008;29:536–544. doi: 10.1007/s00246-007-9128-5 [DOI] [PubMed] [Google Scholar]
- 223.Hong YK, Park YW, Ryu SJ, Won JW, Choi JY, Sul JH, Lee SK, Cho BK, Choe KO. Efficacy of MRI in complicated congenital heart disease with visceral heterotaxy syndrome. J Comput Assist Tomogr. 2000;24:671–682. doi: 10.1097/00004728-200009000-00002 [DOI] [PubMed] [Google Scholar]
- 224.Valsangiacomo ER, Levasseur S, McCrindle BW, MacDonald C, Smallhorn JF, Yoo SJ. Contrast-enhanced MR angiography of pulmonary venous abnormalities in children. Pediatr Radiol. 2003;33:92–98. doi: 10.1007/s00247-002-0789-1 [DOI] [PubMed] [Google Scholar]
- 225.Geva T, Greil GF, Marshall AC, Landzberg M, Powell AJ. Gadolinium-enhanced 3-dimensional magnetic resonance angiography of pulmonary blood supply in patients with complex pulmonary stenosis or atresia: comparison with x-ray angiography. Circulation. 2002;106:473–478. doi: 10.1161/01.cir.0000023624.33478.18 [DOI] [PubMed] [Google Scholar]
- 226.Powell AJ, Maier SE, Chung T, Geva T. Phase-velocity cine magnetic resonance imaging measurement of pulsatile blood flow in children and young adults: in vitro and in vivo validation. Pediatr Cardiol. 2000;21:104–110. doi: 10.1007/s002469910014 [DOI] [PubMed] [Google Scholar]
- 227.Jatene AD, Fontes VF, Paulista PP, Souza LC, Neger F, Galantier M, Sousa JE. Anatomic correction of transposition of the great vessels. J Thorac Cardiovasc Surg. 1976;72:364–370. [PubMed] [Google Scholar]
- 228.ROSS DN. Homograft replacement of the aortic valve. Lancet. 1962;2:487. doi: 10.1016/s0140-6736(62)90345-8 [DOI] [PubMed] [Google Scholar]
- 229.Hussain T, Mathur S, Peel SA, Valverde I, Bilska K, Henningsson M, Botnar RM, Simpson J, Greil GF. Coronary artery size and origin imaging in children: a comparative study of MRI and trans-thoracic echocardiography. BMC Med Imaging. 2015;15:48. doi: 10.1186/s12880-015-0095-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Biko DM, Chung C, Hitt DM, Kurio G, Reinhartz O, Chung T. High-resolution coronary MR angiography for evaluation of patients with anomalous coronary arteries: visualization of the intramural segment. Pediatr Radiol. 2015;45:1146–1152. doi: 10.1007/s00247-015-3302-3 [DOI] [PubMed] [Google Scholar]
- 231.Uribe S, Hussain T, Valverde I, Tejos C, Irarrazaval P, Fava M, Beerbaum P, Botnar RM, Razavi R, Schaeffter T, et al. Congenital heart disease in children: coronary MR angiography during systole and diastole with dual cardiac phase whole-heart imaging. Radiology. 2011;260:232–240. doi: 10.1148/radiol.11101659 [DOI] [PubMed] [Google Scholar]
- 232.Hussain T, Lossnitzer D, Bellsham-Revell H, Valverde I, Beerbaum P, Razavi R, Bell AJ, Schaeffter T, Botnar RM, Uribe SA, et al. Three-dimensional dual-phase whole-heart MR imaging: clinical implications for congenital heart disease. Radiology. 2012;263:547–554. doi: 10.1148/radiol.12111700 [DOI] [PubMed] [Google Scholar]
- 233.Greil G, Tandon AA, Silva Vieira M, Hussain T. 3D Whole Heart Imaging for Congenital Heart Disease. Front Pediatr. 2017;5:36. doi: 10.3389/fped.2017.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tandon A, Hashemi S, Parks WJ, Kelleman MS, Sallee D, Slesnick TC. Improved high-resolution pediatric vascular cardiovascular magnetic resonance with gadofosveset-enhanced 3D respiratory navigated, inversion recovery prepared gradient echo readout imaging compared to 3D balanced steady-state free precession readout imaging. J Cardiovasc Magn Reson. 2016;18:74. doi: 10.1186/s12968-016-0296-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhou Z, Han F, Rapacchi S, Nguyen KL, Brunengraber DZ, Kim GHJ, Paul Finn J, Hu P. Accelerated ferumoxytol-enhanced 4D multiphase, steady-state imaging with contrast enhancement (MUSIC) cardiovascular MRI: validation in pediatric congenital heart disease. NMR Biomed. 2019;30: e3663. doi: 10.1002/nbm.3663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Han F, Zhou Z, Han E, Gao Y, Nguyen KL, Finn PJ, et al. Self-gated 4D multiples, steady-state imaging with contrast enhancement (MUSIC) using rotating cartesian k-space (ROCK): validation in children with congenital heart disease. MRM. 2017;78:472–83. doi: 10.1002/mrm.26376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Brothers JA, Whitehead KK, Keller MS, Fogel MA, Pardon SM, Weinberg PM, Harris MA. Cardiac MRI and CT: differentiation of normal ostium and intraseptal course from slit-like ostium and inter arterial course in anomalous left coronary artery in children. AJR. 2015;204:W104–9. doi: 10.2214/AJR.14.12953 [DOI] [PubMed] [Google Scholar]
- 238.Angelini P, Cheong BY, Lenge De Rosen VV, Lopez A, Uribe C, Masso AH, Ali SW, Davis BR, Muthupillai R, Willerson JT. High-Risk Cardiovascular Conditions in Sports-Related Sudden Death: Prevalence in 5,169 Schoolchildren Screened via Cardiac Magnetic Resonance. Tex Heart Inst J. 2018;45:205–213. doi: 10.14503/THIJ-18-6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Angelini P, Cheong BY, Lenge De Rosen VV, Lopez JA, Uribe C, Masso AH, Ali SW, Davis BR, Muthupillai R, Willerson JT. Magnetic Resonance Imaging-Based Screening Study in a General Population of Adolescents. J Am Coll Cardiol. 2018;71:579–580. doi: 10.1016/j.jacc.2017.11.051 [DOI] [PubMed] [Google Scholar]
- 240.Beerbaum P, Sarikouch S, Laser KT, Greil G, Burchert W, Körperich H. Coronary anomalies assessed by whole-heart isotropic 3D magnetic resonance imaging for cardiac morphology in congenital heart disease. J Magn Reson Imaging. 2009;29:320–327. doi: 10.1002/jmri.21655 [DOI] [PubMed] [Google Scholar]
- 241.Mavrogeni S, Papadopoulos G, Douskou M, Kaklis S, Seimenis I, Baras P, Nikolaidou P, Bakoula C, Karanasios E, Manginas A, et al. Magnetic resonance angiography is equivalent to X-ray coronary angiography for the evaluation of coronary arteries in Kawasaki disease. J Am Coll Cardiol. 2004;43:649–652. doi: 10.1016/j.jacc.2003.08.052 [DOI] [PubMed] [Google Scholar]
- 242.Takemura A, Suzuki A, Inaba R, Sonobe T, Tsuchiya K, Omuro M, Korenaga T. Utility of coronary MR angiography in children with Kawasaki disease. AJR Am J Roentgenol. 2007;188:W534–W539. doi: 10.2214/AJR.05.1414 [DOI] [PubMed] [Google Scholar]
- 243.Kim JW, Goo HW. Coronary artery abnormalities in Kawasaki disease: comparison between CT and MR coronary angiography. Acta Radiol. 2013;54:156–163. doi: 10.1258/ar.2012.120484 [DOI] [PubMed] [Google Scholar]
- 244.Mavrogeni S, Papadopoulos G, Douskou M, Kaklis S, Seimenis I, Varlamis G, Karanasios E, Krikos X, Giannoulia A, Cokkinos DV. Magnetic resonance angiography, function and viability evaluation in patients with Kawasaki disease. J Cardiovasc Magn Reson. 2006;8:493–498. doi: 10.1080/10976640600604773 [DOI] [PubMed] [Google Scholar]
- 245.Tacke CE, Romeih S, Kuipers IM, Spijkerboer AM, Groenink M, Kuijpers TW. Evaluation of cardiac function by magnetic resonance imaging during the follow-up of patients with Kawasaki disease. Circ Cardiovasc Imaging. 2013;6:67–73. doi: 10.1161/CIRCIMAGING.112.976969 [DOI] [PubMed] [Google Scholar]
- 246.Greil GF, Seeger A, Miller S, Claussen CD, Hofbeck M, Botnar RM, Sieverding L. Coronary magnetic resonance angiography and vessel wall imaging in children with Kawasaki disease. Pediatr Radiol. 2007;37:666–673. doi: 10.1007/s00247-007-0498-x [DOI] [PubMed] [Google Scholar]
- 247.Yeon SB, Sabir A, Clouse M, Martinezclark PO, Peters DC, Hauser TH, Gibson CM, Nezafat R, Maintz D, Manning WJ, et al. Delayed-enhancement cardiovascular magnetic resonance coronary artery wall imaging: comparison with multislice computed tomography and quantitative coronary angiography. J Am Coll Cardiol. 2007;50:441–447. doi: 10.1016/j.jacc.2007.03.052 [DOI] [PubMed] [Google Scholar]
- 248.Hussain T, Fenton M, Peel SA, Wiethoff AJ, Taylor A, Muthurangu V, Razavi R, Botnar RM, Burch M, Greil GF. Detection and grading of coronary allograft vasculopathy in children with contrast-enhanced magnetic resonance imaging of the coronary vessel wall. Circ Cardiovasc Imaging. 2013;6:91–98. doi: 10.1161/CIRCIMAGING.112.975797 [DOI] [PubMed] [Google Scholar]
- 249.Schneeweis C, Schnackenburg B, Stuber M, Berger A, Schneider U, Yu J, Gebker R, Weiss RG, Fleck E, Kelle S. Delayed contrast-enhanced MRI of the coronary artery wall in takayasu arteritis. PLoS One. 2012;7:e50655. doi: 10.1371/journal.pone.0050655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Roos-Hesselink JW, Schölzel BE, Heijdra RJ, Spitaels SE, Meijboom FJ, Boersma E, Bogers AJ, Simoons ML. Aortic valve and aortic arch pathology after coarctation repair. Heart. 2003;89:1074–1077. doi: 10.1136/heart.89.9.1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Engel MS, Kochilas LK. Pulse oximetry screening: a review of diagnosing critical congenital heart disease in newborns. Med Devices (Auckl). 2016;9:199–203. doi: 10.2147/MDER.S102146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Didier D, Saint-Martin C, Lapierre C, Trindade PT, Lahlaidi N, Vallee JP, Kalangos A, Friedli B, Beghetti M. Coarctation of the aorta: pre and postoperative evaluation with MRI and MR angiography; correlation with echocardiography and surgery. Int J Cardiovasc Imaging. 2006;22:457–475. doi: 10.1007/s10554-005-9037-8 [DOI] [PubMed] [Google Scholar]
- 253.Vigneswaran TV, Sinha MD, Valverde I, Simpson JM, Charakida M. Hypertension in Coarctation of the Aorta: Challenges in Diagnosis in Children. Pediatr Cardiol. 2018;39:1–10. doi: 10.1007/s00246-017-1739-x [DOI] [PubMed] [Google Scholar]
- 254.Rajbanshi BG, Joshi D, Pradhan S, Gautam NC, Timala R, Shakya U, Sharma A, Biswakarma G, Sharma J. Primary surgical repair of coarctation of the aorta in adolescents and adults: intermediate results and consequences of hypertension. Eur J Cardiothorac Surg. 2019;55:323–330. doi: 10.1093/ejcts/ezy228 [DOI] [PubMed] [Google Scholar]
- 255.Gutberlet M, Hosten N, Vogel M, Abdul-Khaliq H, Ehrenstein T, Amthauer H, Hoffmann T, Teichgräber U, Berger F, Lange P, et al. Quantification of morphologic and hemodynamic severity of coarctation of the aorta by magnetic resonance imaging. Cardiol Young. 2001;11:512–520. doi: 10.1017/s1047951101000749 [DOI] [PubMed] [Google Scholar]
- 256.Ajlan AM. Previously undetected severe aortic coarctation in an adult: value of MRI. Ann Saudi Med. 2016;36:153–154. doi: 10.5144/0256-4947.2016.28.3.1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Konen E, Merchant N, Provost Y, McLaughlin PR, Crossin J, Paul NS. Coarctation of the aorta before and after correction: the role of cardiovascular MRI. AJR Am J Roentgenol. 2004;182:1333–1339. doi: 10.2214/ajr.182.5.1821333 [DOI] [PubMed] [Google Scholar]
- 258.Steffens JC, Bourne MW, Sakuma H, O’Sullivan M, Higgins CB. Quantification of collateral blood flow in coarctation of the aorta by velocity encoded cine magnetic resonance imaging. Circulation. 1994;90:937–943. doi: 10.1161/01.cir.90.2.937 [DOI] [PubMed] [Google Scholar]
- 259.Araoz PA, Reddy GP, Tarnoff H, Roge CL, Higgins CB. MR findings of collateral circulation are more accurate measures of hemodynamic significance than arm-leg blood pressure gradient after repair of coarctation of the aorta. J Magn Reson Imaging. 2003;17:177–183. doi: 10.1002/jmri.10238 [DOI] [PubMed] [Google Scholar]
- 260.Pujadas S, Reddy GP, Weber O, Tan C, Moore P, Higgins CB. Phase contrast MR imaging to measure changes in collateral blood flow after stenting of recurrent aortic coarctation: initial experience. J Magn Reson Imaging. 2006;24:72–76. doi: 10.1002/jmri.20613 [DOI] [PubMed] [Google Scholar]
- 261.Kaiser T, Kellenberger CJ, Albisetti M, Bergsträsser E, Valsangiacomo Buechel ER. Normal values for aortic diameters in children and adolescents–assessment in vivo by contrast-enhanced CMR-angiography. J Cardiovasc Magn Reson. 2008;10:56. doi: 10.1186/1532-429X-10-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mohiaddin RH, Kilner PJ, Rees S, Longmore DB. Magnetic resonance volume flow and jet velocity mapping in aortic coarctation. J Am Coll Cardiol. 1993;22:1515–1521. doi: 10.1016/0735-1097(93)90565-i [DOI] [PubMed] [Google Scholar]
- 263.Muzzarelli S, Meadows AK, Ordovas KG, Higgins CB, Meadows JJ. Usefulness of cardiovascular magnetic resonance imaging to predict the need for intervention in patients with coarctation of the aorta. Am J Cardiol. 2012;109:861–865. doi: 10.1016/j.amjcard.2011.10.048 [DOI] [PubMed] [Google Scholar]
- 264.Mühler EG, Neuerburg JM, Rüben A, Grabitz RG, Günther RW, Messmer BJ, von Bernuth G. Evaluation of aortic coarctation after surgical repair: role of magnetic resonance imaging and Doppler ultrasound. Br Heart J. 1993;70:285–290. doi: 10.1136/hrt.70.3.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Tsai SF, Trivedi M, Boettner B, Daniels CJ. Usefulness of screening cardiovascular magnetic resonance imaging to detect aortic abnormalities after repair of coarctation of the aorta. Am J Cardiol. 2011;107:297–301. doi: 10.1016/j.amjcard.2010.09.016 [DOI] [PubMed] [Google Scholar]
- 266.Therrien J, Thorne SA, Wright A, Kilner PJ, Somerville J. Repaired coarctation: a “cost-effective” approach to identify complications in adults. J Am Coll Cardiol. 2000;35:997–1002. doi: 10.1016/s0735-1097(99)00653-1 [DOI] [PubMed] [Google Scholar]
- 267.Frydrychowicz A, Markl M, Hirtler D, Harloff A, Schlensak C, Geiger J, Stiller B, Arnold R. Aortic hemodynamics in patients with and without repair of aortic coarctation: in vivo analysis by 4D flow-sensitive magnetic resonance imaging. Invest Radiol. 2011;46:317–325. doi: 10.1097/RLI.0b013e3182034fc2 [DOI] [PubMed] [Google Scholar]
- 268.Hope MD, Meadows AK, Hope TA, Ordovas KG, Reddy GP, Alley MT, Higgins CB. Images in cardiovascular medicine. Evaluation of bicuspid aortic valve and aortic coarctation with 4D flow magnetic resonance imaging. Circulation. 2008;117:2818–2819. doi: 10.1161/CIRCULATIONAHA.107.760124 [DOI] [PubMed] [Google Scholar]
- 269.Hope MD, Meadows AK, Hope TA, Ordovas KG, Saloner D, Reddy GP, Alley MT, Higgins CB. Clinical evaluation of aortic coarctation with 4D flow MR imaging. J Magn Reson Imaging. 2010;31:711–718. doi: 10.1002/jmri.22083 [DOI] [PubMed] [Google Scholar]
- 270.Mirzaee H, Henn T, Krause MJ, Goubergrits L, Schumann C, Neugebauer M, Kuehne T, Preusser T, Hennemuth A. MRI-based computational hemodynamics in patients with aortic coarctation using the lattice Boltzmann methods: Clinical validation study. J Magn Reson Imaging. 2017;45:139–146. doi: 10.1002/jmri.25366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Riesenkampff E, Fernandes JF, Meier S, Goubergrits L, Kropf S, Schubert S, Berger F, Hennemuth A, Henneumuth A, Kuehne T. Pressure fields by flow-sensitive, 4D, velocity-encoded CMR in patients with aortic coarctation. JACC Cardiovasc Imaging. 2014;7:920–926. doi: 10.1016/j.jcmg.2014.03.017 [DOI] [PubMed] [Google Scholar]
- 272.Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, Pennell DJ. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0 [DOI] [PubMed] [Google Scholar]
- 273.Kuruvilla S, Janardhanan R, Antkowiak P, Keeley EC, Adenaw N, Brooks J, Epstein FH, Kramer CM, Salerno M. Increased extracellular volume and altered mechanics are associated with LVH in hypertensive heart disease, not hypertension alone. JACC Cardiovasc Imaging. 2015;8:172–180. doi: 10.1016/j.jcmg.2014.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Taylor AJ, Salerno M, Dharmakumar R, Jerosch-Herold M. T1 Mapping: Basic Techniques and Clinical Applications. JACC Cardiovasc Imaging. 2016;9:67–81. doi: 10.1016/j.jcmg.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 275.Larson EW, Edwards WD. Risk factors for aortic dissection: a necropsy study of 161 cases. Am J Cardiol. 1984;53:849–855. doi: 10.1016/0002-9149(84)90418-1 [DOI] [PubMed] [Google Scholar]
- 276.Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol. 2010;55:2789–2800. doi: 10.1016/j.jacc.2009.12.068 [DOI] [PubMed] [Google Scholar]
- 277.Huntington K, Hunter AG, Chan KL. A prospective study to assess the frequency of familial clustering of congenital bicuspid aortic valve. J Am Coll Cardiol. 1997;30:1809–1812. doi: 10.1016/s0735-1097(97)00372-0 [DOI] [PubMed] [Google Scholar]
- 278.Fernandes SM, Sanders SP, Khairy P, Jenkins KJ, Gauvreau K, Lang P, Simonds H, Colan SD. Morphology of bicuspid aortic valve in children and adolescents. J Am Coll Cardiol. 2004;44:1648–1651. doi: 10.1016/j.jacc.2004.05.063 [DOI] [PubMed] [Google Scholar]
- 279.Michelena HI, Prakash SK, Della Corte A, Bissell MM, Anavekar N, Mathieu P, Bossé Y, Limongelli G, Bossone E, Benson DW, et al. ; BAVCon Investigators. Bicuspid aortic valve: identifying knowledge gaps and rising to the challenge from the International Bicuspid Aortic Valve Consortium (BAVCon). Circulation. 2014;129:2691–2704. doi: 10.1161/CIRCULATIONAHA.113.007851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005;111:920–925. doi: 10.1161/01.CIR.0000155623.48408.C5 [DOI] [PubMed] [Google Scholar]
- 281.Chan KL, Stinson WA, Veinot JP. Reliability of transthoracic echocardiography in the assessment of aortic valve morphology: pathological correlation in 178 patients. Can J Cardiol. 1999;15:48–52. [PubMed] [Google Scholar]
- 282.Joziasse IC, Vink A, Cramer MJ, van Oosterhout MF, van Herwerden LA, Heijmen R, Sieswerda GT, Mulder BJ, Doevendans PA. Bicuspid stenotic aortic valves: clinical characteristics and morphological assessment using MRI and echocardiography. Neth Heart J. 2011;19:119–125. doi: 10.1007/s12471-010-0060-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kupfahl C, Honold M, Meinhardt G, Vogelsberg H, Wagner A, Mahrholdt H, Sechtem U. Evaluation of aortic stenosis by cardiovascular magnetic resonance imaging: comparison with established routine clinical techniques. Heart. 2004;90:893–901. doi: 10.1136/hrt.2003.022376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Friedrich MG, Schulz-Menger J, Poetsch T, Pilz B, Uhlich F, Dietz R. Quantification of valvular aortic stenosis by magnetic resonance imaging. Am Heart J. 2002;144:329–334. doi: 10.1067/mhj.2002.124057 [DOI] [PubMed] [Google Scholar]
- 285.John AS, Dill T, Brandt RR, Rau M, Ricken W, Bachmann G, Hamm CW. Magnetic resonance to assess the aortic valve area in aortic stenosis: how does it compare to current diagnostic standards? J Am Coll Cardiol. 2003;42:519–526. doi: 10.1016/s0735-1097(03)00707-1 [DOI] [PubMed] [Google Scholar]
- 286.Caruthers SD, Lin SJ, Brown P, Watkins MP, Williams TA, Lehr KA, Wickline SA. Practical value of cardiac magnetic resonance imaging for clinical quantification of aortic valve stenosis: comparison with echocardiography. Circulation. 2003;108:2236–2243. doi: 10.1161/01.CIR.0000095268.47282.A1 [DOI] [PubMed] [Google Scholar]
- 287.Puymirat E, Chassaing S, Trinquart L, Barbey C, Chaudeurge A, Bar O, Blanchard D. Hakki’s formula for measurement of aortic valve area by magnetic resonance imaging. Am J Cardiol. 2010;106:249–254. doi: 10.1016/j.amjcard.2010.03.019 [DOI] [PubMed] [Google Scholar]
- 288.Bonow RO, Carabello BA, Kanu C, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84–231. doi: 10.1161/CIRCULATIONAHA.106.176857 [DOI] [PubMed] [Google Scholar]
- 289.Rose MJ, Rigsby CK, Berhane H, Bollache E, Jarvis K, Barker AJ, Schnell S, Allen BD, Robinson JD, Markl M. 4-D flow MRI aortic 3-D hemodynamics and wall shear stress remain stable over short-term follow-up in pediatric and young adult patients with bicuspid aortic valve. Pediatr Radiol. 2019;49:57–67. doi: 10.1007/s00247-018-4257-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Nejatian A, Yu J, Geva T, White MT, Prakash A. Aortic Measurements in Patients with Aortopathy are Larger and More Reproducible by Cardiac Magnetic Resonance Compared with Echocardiography. Pediatr Cardiol. 2015;36:1761–1773. doi: 10.1007/s00246-015-1231-4 [DOI] [PubMed] [Google Scholar]
- 291.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Jr, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American College of Radiology; American Stroke Association; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society of Thoracic Surgeons; Society for Vascular Medicine. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369. doi: 10.1161/CIR.0b013e3181d4739e [DOI] [PubMed] [Google Scholar]
- 292.Quezada E, Lapidus J, Shaughnessy R, Chen Z, Silberbach M. Aortic dimensions in Turner syndrome. Am J Med Genet A. 2015;167A:2527–2532. doi: 10.1002/ajmg.a.37208 [DOI] [PubMed] [Google Scholar]
- 293.Matura LA, Ho VB, Rosing DR, Bondy CA. Aortic dilatation and dissection in Turner syndrome. Circulation. 2007;116:1663–1670. doi: 10.1161/CIRCULATIONAHA.106.685487 [DOI] [PubMed] [Google Scholar]
- 294.Silberbach M, Roos-Hesselink JW, Andersen NH, Braverman AC, Brown N, Collins RT, De Backer J, Eagle KA, Hiratzka LF, Johnson WH, Jr, et al. ; American Heart Association Council on Cardiovascular Disease in the Young; Council on Genomic and Precision Medicine; and Council on Peripheral Vascular Disease. Cardiovascular Health in Turner Syndrome: A Scientific Statement From the American Heart Association. Circ Genom Precis Med. 2018;11:e000048. doi: 10.1161/HCG.0000000000000048 [DOI] [PubMed] [Google Scholar]
- 295.Keane MG, Pyeritz RE. Medical management of Marfan syndrome. Circulation. 2008;117:2802–2813. doi: 10.1161/CIRCULATIONAHA.107.693523 [DOI] [PubMed] [Google Scholar]
- 296.Saleh RS, Finn JP, Fenchel M, Moghadam AN, Krishnam M, Abrazado M, Ton A, Habibi R, Fonkalsrud EW, Cooper CB. Cardiovascular magnetic resonance in patients with pectus excavatum compared with normal controls. J Cardiovasc Magn Reson. 2010;12:73. doi: 10.1186/1532-429X-12-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Alpendurada F, Mohiaddin R. Prevalence of cardiovascular manifestations in patients with Marfan syndrome: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2008;10:A164. [Google Scholar]
- 298.van Karnebeek CD, Naeff MS, Mulder BJ, Hennekam RC, Offringa M. Natural history of cardiovascular manifestations in Marfan syndrome. Arch Dis Child. 2001;84:129–137. doi: 10.1136/adc.84.2.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Lee JC, Branch KR, Hamilton-Craig C, Krieger EV. Evaluation of aortic regurgitation with cardiac magnetic resonance imaging: a systematic review. Heart. 2018;104:103–110. doi: 10.1136/heartjnl-2016-310819 [DOI] [PubMed] [Google Scholar]
- 300.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252–289. doi: 10.1016/j.jacc.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 301.Pyeritz RE, Wappel MA. Mitral valve dysfunction in the Marfan syndrome. Clinical and echocardiographic study of prevalence and natural history. Am J Med. 1983;74:797–807. doi: 10.1016/0002-9343(83)91070-7 [DOI] [PubMed] [Google Scholar]
- 302.Sisk HE, Zahka KG, Pyeritz RE. The Marfan syndrome in early childhood: analysis of 15 patients diagnosed at less than 4 years of age. Am J Cardiol. 1983;52:353–358. doi: 10.1016/0002-9149(83)90138-8 [DOI] [PubMed] [Google Scholar]
- 303.Gelfand EV, Hughes S, Hauser TH, Yeon SB, Goepfert L, Kissinger KV, Rofsky NM, Manning WJ. Severity of mitral and aortic regurgitation as assessed by cardiovascular magnetic resonance: optimizing correlation with Doppler echocardiography. J Cardiovasc Magn Reson. 2006;8:503–507. doi: 10.1080/10976640600604856 [DOI] [PubMed] [Google Scholar]
- 304.Chuang ML, Hibberd MG, Salton CJ, Beaudin RA, Riley MF, Parker RA, Douglas PS, Manning WJ. Importance of imaging method over imaging modality in noninvasive determination of left ventricular volumes and ejection fraction: assessment by two- and three-dimensional echocardiography and magnetic resonance imaging. J Am Coll Cardiol. 2000;35:477–484. doi: 10.1016/s0735-1097(99)00551-3 [DOI] [PubMed] [Google Scholar]
- 305.Han Y, Peters DC, Salton CJ, Bzymek D, Nezafat R, Goddu B, Kissinger KV, Zimetbaum PJ, Manning WJ, Yeon SB. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc Imaging. 2008;1:294–303. doi: 10.1016/j.jcmg.2008.01.013 [DOI] [PubMed] [Google Scholar]
- 306.Delling FN, Kang LL, Yeon SB, Kissinger KV, Goddu B, Manning WJ, Han Y. CMR predictors of mitral regurgitation in mitral valve prolapse. JACC Cardiovasc Imaging. 2010;3:1037–1045. doi: 10.1016/j.jcmg.2010.06.016 [DOI] [PubMed] [Google Scholar]
- 307.Bradley TJ, Bowdin SC, Morel CF, Pyeritz RE. The Expanding Clinical Spectrum of Extracardiovascular and Cardiovascular Manifestations of Heritable Thoracic Aortic Aneurysm and Dissection. Can J Cardiol. 2016;32:86–99. doi: 10.1016/j.cjca.2015.11.007 [DOI] [PubMed] [Google Scholar]
- 308.Takeda N, Yagi H, Hara H, Fujiwara T, Fujita D, Nawata K, Inuzuka R, Taniguchi Y, Harada M, Toko H, et al. Pathophysiology and Management of Cardiovascular Manifestations in Marfan and Loeys-Dietz Syndromes. Int Heart J. 2016;57:271–277. doi: 10.1536/ihj.16-094 [DOI] [PubMed] [Google Scholar]
- 309.Klem I, Shah DJ, White RD, Pennell DJ, van Rossum AC, Regenfus M, Sechtem U, Schvartzman PR, Hunold P, Croisille P, et al. Prognostic value of routine cardiac magnetic resonance assessment of left ventricular ejection fraction and myocardial damage: an international, multicenter study. Circ Cardiovasc Imaging. 2011;4:610–619. doi: 10.1161/CIRCIMAGING.111.964965 [DOI] [PubMed] [Google Scholar]
- 310.Kalra VB, Gilbert JW, Malhotra A. Loeys-Dietz syndrome: cardiovascular, neuroradiological and musculoskeletal imaging findings. Pediatr Radiol. 2011;41:1495–504; quiz 1616. doi: 10.1007/s00247-011-2195-z [DOI] [PubMed] [Google Scholar]
- 311.García A, Ferreirós J, Santamaría M, Bustos A, Abades JL, Santamaría N. MR angiographic evaluation of complications in surgically treated type A aortic dissection. Radiographics. 2006;26:981–992. doi: 10.1148/rg.264055082 [DOI] [PubMed] [Google Scholar]
- 312.Kunz RP, Oberholzer K, Kuroczynski W, Horstick G, Krummenauer F, Thelen M, Kreitner KF. Assessment of chronic aortic dissection: contribution of different ECG-gated breath-hold MRI techniques. AJR Am J Roentgenol. 2004;182:1319–1326. doi: 10.2214/ajr.182.5.1821319 [DOI] [PubMed] [Google Scholar]
- 313.Dake MD, Thompson M, van Sambeek M, Vermassen F, Morales JP; DEFINE Investigators. DISSECT: a new mnemonic-based approach to the categorization of aortic dissection. Eur J Vasc Endovasc Surg. 2013;46:175–190. doi: 10.1016/j.ejvs.2013.04.029 [DOI] [PubMed] [Google Scholar]
- 314.Morris SA, Orbach DB, Geva T, Singh MN, Gauvreau K, Lacro RV. Increased vertebral artery tortuosity index is associated with adverse outcomes in children and young adults with connective tissue disorders. Circulation. 2011;124:388–396. doi: 10.1161/CIRCULATIONAHA.110.990549 [DOI] [PubMed] [Google Scholar]
- 315.Bollache E, Guzzardi DG, Sattari S, Olsen KE, Di Martino ES, Malaisrie SC, van Ooij P, Collins J, Carr J, McCarthy PM, et al. Aortic valve-mediated wall shear stress is heterogeneous and predicts regional aortic elastic fiber thinning in bicuspid aortic valve-associated aortopathy. J Thorac Cardiovasc Surg. 2018;156:2112–2120.e2. doi: 10.1016/j.jtcvs.2018.05.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Guzzardi DG, Barker AJ, van Ooij P, Malaisrie SC, Puthumana JJ, Belke DD, Mewhort HE, Svystonyuk DA, Kang S, Verma S, et al. Valve-Related Hemodynamics Mediate Human Bicuspid Aortopathy: Insights From Wall Shear Stress Mapping. J Am Coll Cardiol. 2015;66:892–900. doi: 10.1016/j.jacc.2015.06.1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Geiger J, Hirtler D, Gottfried K, Rahman O, Bollache E, Barker AJ, Markl M, Stiller B. Longitudinal Evaluation of Aortic Hemodynamics in Marfan Syndrome: New Insights from a 4D Flow Cardiovascular Magnetic Resonance Multi-Year Follow-Up Study. J Cardiovasc Magn Reson. 2017;19:33. doi: 10.1186/s12968-017-0347-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.van der Palen RL, Barker AJ, Bollache E, Garcia J, Rose MJ, van Ooij P, Young LT, Roest AA, Markl M, Robinson JD, et al. Altered aortic 3D hemodynamics and geometry in pediatric Marfan syndrome patients. J Cardiovasc Magn Reson. 2017;19:30. doi: 10.1186/s12968-017-0345-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.François CJ, Carr JC. MRI of the thoracic aorta. Cardiol Clin. 2007;25:171–84, vii. doi: 10.1016/j.ccl.2007.02.005 [DOI] [PubMed] [Google Scholar]
- 320.Moore AG, Eagle KA, Bruckman D, Moon BS, Malouf JF, Fattori R, Evangelista A, Isselbacher EM, Suzuki T, Nienaber CA, et al. Choice of computed tomography, transesophageal echocardiography, magnetic resonance imaging, and aortography in acute aortic dissection: International Registry of Acute Aortic Dissection (IRAD). Am J Cardiol. 2002;89:1235–1238. doi: 10.1016/s0002-9149(02)02316-0 [DOI] [PubMed] [Google Scholar]
- 321.Bansal RC, Chandrasekaran K, Ayala K, Smith DC. Frequency and explanation of false negative diagnosis of aortic dissection by aortography and transesophageal echocardiography. J Am Coll Cardiol. 1995;25:1393–1401. doi: 10.1016/0735-1097(94)00569-C [DOI] [PubMed] [Google Scholar]
- 322.Lanzarini L, Larizza D, Prete G, Calcaterra V, Meloni G, Sammarchi L, Klersy C. Aortic dimensions in Turner’s syndrome: two-dimensional echocardiography versus magnetic resonance imaging. J Cardiovasc Med. 2007;8:428–37. doi: 10.2459/01.JCM.0000269716.33435.d3 [DOI] [PubMed] [Google Scholar]
- 323.Johnson RK, Premraj S, Patel SS, Wahle A, Stolpen A, Sonka M, Scholz TD. Quantitative assessment of the entire thoracic aorta from magnetic resonance images. Cardiol Young. 2011;21:170–177. doi: 10.1017/S1047951110001678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Schäfer M, Browne LP, Truong U, Jaggers JJ, Mitchell MB, Malone L, Morgan G, Chatfield K, McLennan D, Turbendian H, et al. Aortic stiffness in adolescent Turner and Marfan syndrome patients. Eur J Cardiothorac Surg. 2018;54:926–32. doi: 10.1093/ejcts/ezy168 [DOI] [PubMed] [Google Scholar]
- 325.Prakash A, Adlakha H, Rabideau N, Hass CJ, Morris SA, Geva T, Gauvreau K, Singh MN, Lacro RV. Segmental Aortic Stiffness in Children and Young Adults With Connective Tissue Disorders: Relationships With Age, Aortic Size, Rate of Dilation, and Surgical Root Replacement. Circulation. 2015;132:595–602. doi: 10.1161/CIRCULATIONAHA.114.014934 [DOI] [PubMed] [Google Scholar]
- 326.Merlocco A, Lacro RV, Gauvreau K, Rabideau N, Singh MN, Prakash A. Longitudinal Changes in Segmental Aortic Stiffness Determined by Cardiac Magnetic Resonance in Children and Young Adults With Connective Tissue Disorders (the Marfan, Loeys-Dietz, and Ehlers-Danlos Syndromes, and Nonspecific Connective Tissue Disorders). Am J Cardiol. 2017;120:1214–1219. doi: 10.1016/j.amjcard.2017.06.064 [DOI] [PubMed] [Google Scholar]
- 327.van der Linde D, van de Laar IM, Bertoli-Avella AM, Oldenburg RA, Bekkers JA, Mattace-Raso FU, van den Meiracker AH, Moelker A, van Kooten F, Frohn-Mulder IM, et al. Aggressive cardiovascular phenotype of aneurysms-osteoarthritis syndrome caused by pathogenic SMAD3 variants. J Am Coll Cardiol. 2012;60:397–403. doi: 10.1016/j.jacc.2011.12.052 [DOI] [PubMed] [Google Scholar]
- 328.Marmon LM, Bye MR, Haas JM, Balsara RK, Dunn JM. Vascular rings and slings: long-term follow-up of pulmonary function. J Pediatr Surg. 1984;19:683–692. doi: 10.1016/s0022-3468(84)80353-x [DOI] [PubMed] [Google Scholar]
- 329.Loomba RS. Natural History of Asymptomatic and Unrepaired Vascular Rings: Is Watchful Waiting a Viable Option? A New Case and Review of Previously Reported Cases. Children (Basel). 2016;3:E44. doi: 10.3390/children3040044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Beekman RP, Beek FJ, Hazekamp MG, Meijboom EJ. The value of MRI in diagnosing vascular abnormalities causing stridor. Eur J Pediatr. 1997;156:516–520. doi: 10.1007/s004310050651 [DOI] [PubMed] [Google Scholar]
- 331.Eichhorn J, Fink C, Delorme S, Ulmer H. Rings, slings and other vascular abnormalities. Ultrafast computed tomography and magnetic resonance angiography in pediatric cardiology. Z Kardiol. 2004;93:201–208. doi: 10.1007/s00392-004-0026-z [DOI] [PubMed] [Google Scholar]
- 332.Kellenberger CJ. Aortic arch malformations. Pediatr Radiol. 2010;40:876–884. doi: 10.1007/s00247-010-1607-9 [DOI] [PubMed] [Google Scholar]
- 333.Gould SW, Rigsby CK, Donnelly LF, McCulloch M, Pizarro C, Epelman M. Useful signs for the assessment of vascular rings on cross-sectional imaging. Pediatr Radiol. 2015;45:2004–16; quiz 2002. doi: 10.1007/s00247-015-3424-7 [DOI] [PubMed] [Google Scholar]
- 334.Smith BM, Lu JC, Dorfman AL, Mahani MG, Agarwal PP. Rings and slings revisited. Magn Reson Imaging Clin N Am. 2015;23:127–135. doi: 10.1016/j.mric.2014.09.011 [DOI] [PubMed] [Google Scholar]
- 335.Schlesinger AE, Krishnamurthy R, Sena LM, Guillerman RP, Chung T, DiBardino DJ, Fraser CD, Jr. Incomplete double aortic arch with atresia of the distal left arch: distinctive imaging appearance. AJR Am J Roentgenol. 2005;184:1634–1639. doi: 10.2214/ajr.184.5.01841634 [DOI] [PubMed] [Google Scholar]
- 336.Kaldararova M, Simkova I, Varga I, Tittel P, Kardos M, Ondriska M, Vrsanska V, Masura J. Double aortic arch anomalies in Children: A Systematic 20-Year Single Center Study. Clin Anat. 2017;30:929–939. doi: 10.1002/ca.22955 [DOI] [PubMed] [Google Scholar]
- 337.Weinberg PM. Aortic arch anomalies. J Cardiovasc Magn Reson. 2006;8:633–643. doi: 10.1080/10976640600713756 [DOI] [PubMed] [Google Scholar]
- 338.Julsrud PR, Ehman RL. Magnetic resonance imaging of vascular rings. Mayo Clin Proc. 1986;61:181–185. doi: 10.1016/s0025-6196(12)61846-1 [DOI] [PubMed] [Google Scholar]
- 339.Pawade A, de Leval MR, Elliott MJ, Stark J. Pulmonary artery sling. Ann Thorac Surg. 1992;54:967–970. doi: 10.1016/0003-4975(92)90661-m [DOI] [PubMed] [Google Scholar]
- 340.van Son JA, Julsrud PR, Hagler DJ, Sim EK, Puga FJ, Schaff HV, Danielson GK. Imaging strategies for vascular rings. Ann Thorac Surg. 1994;57:604–610. doi: 10.1016/0003-4975(94)90552-5 [DOI] [PubMed] [Google Scholar]
- 341.Midiri M, Finazzo M, Pilato M, Lagalla R, De Maria M. Right aortic arch with aberrant left innominate artery: MR imaging findings. Eur Radiol. 1999;9:311–315. doi: 10.1007/s003300050672 [DOI] [PubMed] [Google Scholar]
- 342.Philip S, Chen SY, Wu MH, Wang JK, Lue HC. Retroesophageal aortic arch: diagnostic and therapeutic implications of a rare vascular ring. Int J Cardiol. 2001;79:133–141. doi: 10.1016/s0167-5273(01)00402-8 [DOI] [PubMed] [Google Scholar]
- 343.Hernandez RJ. Magnetic resonance imaging of mediastinal vessels. Magn Reson Imaging Clin N Am. 2002;10:237–251. doi: 10.1016/s1064-9689(01)00011-3 [DOI] [PubMed] [Google Scholar]
- 344.Greil GF, Kramer U, Dammann F, Schick F, Miller S, Claussen CD, Sieverding L. Diagnosis of vascular rings and slings using an interleaved 3D double-slab FISP MR angiography technique. Pediatr Radiol. 2005;35:396–401. doi: 10.1007/s00247-004-1376-4 [DOI] [PubMed] [Google Scholar]
- 345.Johnson TR, Goldmuntz E, McDonald-McGinn DM, Zackai EH, Fogel MA. Cardiac magnetic resonance imaging for accurate diagnosis of aortic arch anomalies in patients with 22q11.2 deletion. Am J Cardiol. 2005;96:1726–1730. doi: 10.1016/j.amjcard.2005.07.084 [DOI] [PubMed] [Google Scholar]
- 346.Fleenor JT, Weinberg PM, Kramer SS, Fogel M. Vascular rings and their effect on tracheal geometry. Pediatr Cardiol. 2003;24:430–435. doi: 10.1007/s00246-002-0385-z [DOI] [PubMed] [Google Scholar]
- 347.Ismat FA, Weinberg PM, Rychik J, Karl TR, Fogel MA. Right aortic arch and coarctation: a rare association. Congenit Heart Dis. 2006;1:217–223. doi: 10.1111/j.1747-0803.2006.00038.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Hanneman K, Newman B, Chan F. Congenital Variants and Anomalies of the Aortic Arch. Radiographics. 2017;37:32–51. doi: 10.1148/rg.2017160033 [DOI] [PubMed] [Google Scholar]
- 349.Virdi IS, Keeton BR, Shore DF, Monro JL. Surgical management in tetralogy of Fallot and vascular ring. Pediatr Cardiol. 1987;8:131–134. doi: 10.1007/BF02079470 [DOI] [PubMed] [Google Scholar]
- 350.Graham TP, Jr, Bernard YD, Mellen BG, Celermajer D, Baumgartner H, Cetta F, Connolly HM, Davidson WR, Dellborg M, Foster E, et al. Long-term outcome in congenitally corrected transposition of the great arteries: a multi-institutional study. J Am Coll Cardiol. 2000;36:255–261. doi: 10.1016/s0735-1097(00)00682-3 [DOI] [PubMed] [Google Scholar]
- 351.Morcos M, Kilner PJ, Sahn DJ, Litt HI, Valsangiacomo-Buechel ER, Sheehan FH. Comparison of systemic right ventricular function in transposition of the great arteries after atrial switch and congenitally corrected transposition of the great arteries. Int J Cardiovasc Imaging. 2017;33:1993–2001. doi: 10.1007/s10554-017-1201-4 [DOI] [PubMed] [Google Scholar]
- 352.Vejlstrup N, Sørensen K, Mattsson E, Thilén U, Kvidal P, Johansson B, Iversen K, Søndergaard L, Dellborg M, Eriksson P. Long-Term Outcome of Mustard/Senning Correction for Transposition of the Great Arteries in Sweden and Denmark. Circulation. 2015;132:633–638. doi: 10.1161/CIRCULATIONAHA.114.010770 [DOI] [PubMed] [Google Scholar]
- 353.Roos-Hesselink JW, Meijboom FJ, Spitaels SE, van Domburg R, van Rijen EH, Utens EM, McGhie J, Bos E, Bogers AJ, Simoons ML. Decline in ventricular function and clinical condition after Mustard repair for transposition of the great arteries (a prospective study of 22-29 years). Eur Heart J. 2004;25:1264–1270. doi: 10.1016/j.ehj.2004.03.009 [DOI] [PubMed] [Google Scholar]
- 354.Szymański P, Klisiewicz A, Lubiszewska B, Lipczyńska M, Michałek P, Janas J, Hoffman P. Application of classic heart failure definitions of asymptomatic and symptomatic ventricular dysfunction and heart failure symptoms with preserved ejection fraction to patients with systemic right ventricles. Am J Cardiol. 2009;104:414–418. doi: 10.1016/j.amjcard.2009.03.057 [DOI] [PubMed] [Google Scholar]
- 355.Roest AA, Lamb HJ, van der Wall EE, Vliegen HW, van den Aardweg JG, Kunz P, de Roos A, Helbing WA. Cardiovascular response to physical exercise in adult patients after atrial correction for transposition of the great arteries assessed with magnetic resonance imaging. Heart. 2004;90:678–684. doi: 10.1136/hrt.2003.023499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Lorenz CH, Walker ES, Graham TP, Jr, Powers TA. Right ventricular performance and mass by use of cine MRI late after atrial repair of transposition of the great arteries. Circulation. 1995;92(9 Suppl):II233–II239. doi: 10.1161/01.cir.92.9.233 [DOI] [PubMed] [Google Scholar]
- 357.Chung KJ, Simpson IA, Glass RF, Sahn DJ, Hesselink JR. Cine magnetic resonance imaging after surgical repair in patients with transposition of the great arteries. Circulation. 1988;77:104–109. doi: 10.1161/01.cir.77.1.104 [DOI] [PubMed] [Google Scholar]
- 358.Groenink M, Mulder BJ, van der Wall EE. Value of magnetic resonance imaging in functional assessment of baffle obstruction after the Mustard procedure. J Cardiovasc Magn Reson. 1999;1:49–51. doi: 10.3109/10976649909080833 [DOI] [PubMed] [Google Scholar]
- 359.Theissen P, Kaemmerer H, Sechtem U, Luhmer I, Smolarz K, Kallfelz HC, Schicha H. Magnetic resonance imaging of cardiac function and morphology in patients with transposition of the great arteries following Mustard procedure. Thorac Cardiovasc Surg. 2008;39(Suppl 3):221–4. doi: 10.1055/s-2007-1020023 [DOI] [PubMed] [Google Scholar]
- 360.Driessen MM, Baggen VJ, Freling HG, Pieper PG, van Dijk AP, Doevendans PA, Snijder RJ, Post MC, Meijboom FJ, Sieswerda GT, et al. Pressure overloaded right ventricles: a multicenter study on the importance of trabeculae in RV function measured by CMR. Int J Cardiovasc Imaging. 2014;30:599–608. doi: 10.1007/s10554-014-0367-2 [DOI] [PubMed] [Google Scholar]
- 361.Winter MM, Bernink FJ, Groenink M, Bouma BJ, van Dijk AP, Helbing WA, Tijssen JG, Mulder BJ. Evaluating the systemic right ventricle by CMR: the importance of consistent and reproducible delineation of the cavity. J Cardiovasc Magn Reson. 2008;10:40. doi: 10.1186/1532-429X-10-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Jimenez-Juan L, Joshi SB, Wintersperger BJ, Yan AT, Ley S, Crean AM, Nguyen ET, Deva DP, Paul NS, Wald RM. Assessment of right ventricular volumes and function using cardiovascular magnetic resonance cine imaging after atrial redirection surgery for complete transposition of the great arteries. Int J Cardiovasc Imaging. 2013;29:335–342. doi: 10.1007/s10554-012-0083-8 [DOI] [PubMed] [Google Scholar]
- 363.Shiina Y, Inai K, Takahashi T, Taniguchi K, Watanabe E, Fukushima K, Niwa K, Nagao M. Inter- and intra-ventricular dyssynchrony in the systemic right ventricle is a surrogate marker of major cardiac events in mildly symptomatic patients. Heart Vessels. 2018;33:1086–1093. doi: 10.1007/s00380-018-1144-2 [DOI] [PubMed] [Google Scholar]
- 364.van der Bom T, Winter MM, Groenink M, Vliegen HW, Pieper PG, van Dijk AP, Sieswerda GT, Roos-Hesselink JW, Zwinderman AH, Mulder BJ, et al. Right ventricular end-diastolic volume combined with peak systolic blood pressure during exercise identifies patients at risk for complications in adults with a systemic right ventricle. J Am Coll Cardiol. 2013;62:926–936. doi: 10.1016/j.jacc.2013.06.026 [DOI] [PubMed] [Google Scholar]
- 365.Giardini A, Lovato L, Donti A, Formigari R, Oppido G, Gargiulo G, Picchio FM, Fattori R. Relation between right ventricular structural alterations and markers of adverse clinical outcome in adults with systemic right ventricle and either congenital complete (after Senning operation) or congenitally corrected transposition of the great arteries. Am J Cardiol. 2006;98:1277–1282. doi: 10.1016/j.amjcard.2006.05.062 [DOI] [PubMed] [Google Scholar]
- 366.Helsen F, De Meester P, Van De Bruaene A, Gabriels C, Santens B, Claeys M, Claessen G, Goetschalckx K, Buys R, Gewillig M, et al. Right ventricular systolic dysfunction at rest is not related to decreased exercise capacity in patients with a systemic right ventricle. Int J Cardiol. 2018;260:66–71. doi: 10.1016/j.ijcard.2018.03.029 [DOI] [PubMed] [Google Scholar]
- 367.Schaefer A, Tallone EM, Westhoff-Bleck M, Klein G, Drexler H, Röntgen P. Relation of diastolic and systolic function, exercise capacity and brain natriuretic peptide in adults after Mustard procedure for transposition of the great arteries. Cardiology. 2010;117:112–117. doi: 10.1159/000320097 [DOI] [PubMed] [Google Scholar]
- 368.van der Bom T, Winter MM, Bouma BJ, Groenink M, Vliegen HW, Pieper PG, van Dijk AP, Sieswerda GT, Roos-Hesselink JW, Zwinderman AH, et al. Effect of valsartan on systemic right ventricular function: a double-blind, randomized, placebo-controlled pilot trial. Circulation. 2013;127:322–330. doi: 10.1161/CIRCULATIONAHA.112.135392 [DOI] [PubMed] [Google Scholar]
- 369.van Dissel AC, Winter MM, van der Bom T, Vliegen HW, van Dijk APJ, Pieper PG, Sieswerda GT, Roos-Hesselink JW, Zwinderman AH, Mulder BJM, et al. Long-term clinical outcomes of valsartan in patients with a systemic right ventricle: Follow-up of a multicenter randomized controlled trial. Int J Cardiol. 2019;278:84–87. doi: 10.1016/j.ijcard.2018.11.027 [DOI] [PubMed] [Google Scholar]
- 370.Tutarel O, Orwat S, Radke RM, Westhoff-Bleck M, Vossler C, Schülke C, Baumgartner H, Bauersachs J, Röntgen P, Diller GP. Assessment of myocardial function using MRI-based feature tracking in adults after atrial repair of transposition of the great arteries: Reference values and clinical utility. Int J Cardiol. 2016;220:246–250. doi: 10.1016/j.ijcard.2016.06.108 [DOI] [PubMed] [Google Scholar]
- 371.Muzzarelli S, Ordovas KG, Higgins CB, Meadows AK. Collateral flow measurement by phase-contrast magnetic resonance imaging for the assessment of systemic venous baffle patency after atrial switch repair for transposition of the great arteries. J Thorac Imaging. 2012;27:175–178. doi: 10.1097/RTI.0b013e31823fb9a0 [DOI] [PubMed] [Google Scholar]
- 372.Fogel MA, Hubbard A, Weinberg PM. A simplified approach for assessment of intracardiac baffles and extracardiac conduits in congenital heart surgery with two- and three-dimensional magnetic resonance imaging. Am Heart J. 2001;142:1028–1036. doi: 10.1067/mhj.2001.118469 [DOI] [PubMed] [Google Scholar]
- 373.Fogel MA, Hubbard A, Weinberg PM. Mid-term follow-up of patients with transposition of the great arteries after atrial inversion operation using two- and three-dimensional magnetic resonance imaging. Pediatr Radiol. 2002;32:440–446. doi: 10.1007/s00247-001-0637-8 [DOI] [PubMed] [Google Scholar]
- 374.Sampson C, Kilner PJ, Hirsch R, Rees RS, Somerville J, Underwood SR. Venoatrial pathways after the Mustard operation for transposition of the great arteries: anatomic and functional MR imaging. Radiology. 1994;193:211–217. doi: 10.1148/radiology.193.1.8090893 [DOI] [PubMed] [Google Scholar]
- 375.Campbell RM, Moreau GA, Johns JA, Burger JD, Mazer M, Graham TP, Jr, Kulkarni MV. Detection of caval obstruction by magnetic resonance imaging after intraatrial repair of transposition of the great arteries. Am J Cardiol. 1987;60:688–691. doi: 10.1016/0002-9149(87)90383-3 [DOI] [PubMed] [Google Scholar]
- 376.Stauber A, Wey C, Greutmann M, Tobler D, Wustmann K, Wahl A, Valsangiacomo Buechel ER, Wilhelm M, Schwerzmann M; SACHER Investigators. Left ventricular outflow tract obstruction and its impact on systolic ventricular function and exercise capacity in adults with a subaortic right ventricle. Int J Cardiol. 2017;244:139–142. doi: 10.1016/j.ijcard.2017.06.050 [DOI] [PubMed] [Google Scholar]
- 377.Said SM, Burkhart HM, Schaff HV, Dearani JA. Congenitally Corrected Transposition of Great Arteries: Surgical Options for the Failing Right Ventricle and/or Severe Tricuspid Regurgitation. World J Pediatr Congenit Heart Surg. 2011;2:64–79. doi: 10.1177/2150135110386977 [DOI] [PubMed] [Google Scholar]
- 378.Wallis GA, Debich-Spicer D, Anderson RH. Congenitally corrected transposition. Orphanet J Rare Dis. 2011;14:6–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Devaney EJ, Charpie JR, Ohye RG, Bove EL. Combined arterial switch and Senning operation for congenitally corrected transposition of the great arteries: patient selection and intermediate results. J Thorac Cardiovasc Surg. 2003;125:500–507. doi: 10.1067/mtc.2003.158 [DOI] [PubMed] [Google Scholar]
- 380.Lewis M, Ginns J, Rosenbaum M. Is systemic right ventricular function by cardiac MRI related to the degree of tricuspid regurgitation in congenitally corrected transposition of the great arteries? Int J Cardiol. 2014;174:586–589. doi: 10.1016/j.ijcard.2014.04.129 [DOI] [PubMed] [Google Scholar]
- 381.Kral Kollars CA, Gelehrter S, Bove EL, Ensing G. Effects of morphologic left ventricular pressure on right ventricular geometry and tricuspid valve regurgitation in patients with congenitally corrected transposition of the great arteries. Am J Cardiol. 2010;105:735–739. doi: 10.1016/j.amjcard.2009.10.066 [DOI] [PubMed] [Google Scholar]
- 382.Scherptong RW, Vliegen HW, Winter MM, Holman ER, Mulder BJ, van der Wall EE, Hazekamp MG. Tricuspid valve surgery in adults with a dysfunctional systemic right ventricle: repair or replace? Circulation. 2009;119:1467–1472. doi: 10.1161/CIRCULATIONAHA.108.805135 [DOI] [PubMed] [Google Scholar]
- 383.Dobson R, Danton M, Nicola W, Hamish W. The natural and unnatural history of the systemic right ventricle in adult survivors. J Thorac Cardiovasc Surg. 2013;145:1493–501; discussion 1501. doi: 10.1016/j.jtcvs.2013.02.030 [DOI] [PubMed] [Google Scholar]
- 384.Babu-Narayan SV, Goktekin O, Moon JC, Broberg CS, Pantely GA, Pennell DJ, Gatzoulis MA, Kilner PJ. Late gadolinium enhancement cardiovascular magnetic resonance of the systemic right ventricle in adults with previous atrial redirection surgery for transposition of the great arteries. Circulation. 2005;111:2091–2098. doi: 10.1161/01.CIR.0000162463.61626.3B [DOI] [PubMed] [Google Scholar]
- 385.Ladouceur M, Baron S, Nivet-Antoine V, Maruani G, Soulat G, Pereira H, Blanchard A, Boutouyrie P, Paul JL, Mousseaux E. Role of myocardial collagen degradation and fibrosis in right ventricle dysfunction in transposition of the great arteries after atrial switch. Int J Cardiol. 2018;258:76–82. doi: 10.1016/j.ijcard.2018.01.100 [DOI] [PubMed] [Google Scholar]
- 386.Babu-Narayan SV, Prati D, Rydman R, Dimopoulos K, Diller GP, Uebing A, Henein MY, Kilner PJ, Gatzoulis MA, Li W. Dyssynchrony and electromechanical delay are associated with focal fibrosis in the systemic right ventricle - Insights from echocardiography. Int J Cardiol. 2016;220:382–388. doi: 10.1016/j.ijcard.2016.06.090 [DOI] [PubMed] [Google Scholar]
- 387.Plymen CM, Sado DM, Taylor AM, Bolger AP, Lambiase PD, Hughes M, Moon JC. Diffuse myocardial fibrosis in the systemic right ventricle of patients late after Mustard or Senning surgery: an equilibrium contrast cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging. 2013;14:963–968. doi: 10.1093/ehjci/jet014 [DOI] [PubMed] [Google Scholar]
- 388.Shehu N, Meierhofer C, Messroghli D, Mkrtchyan N, Martinoff S, Ewert P, Stern H. Diffuse fibrosis is common in the left, but not in the right ventricle in patients with transposition of the great arteries late after atrial switch operation. Int J Cardiovasc Imaging. 2018;34:1241–1248. doi: 10.1007/s10554-018-1338-9 [DOI] [PubMed] [Google Scholar]
- 389.Rydman R, Gatzoulis MA, Ho SY, Ernst S, Swan L, Li W, Wong T, Sheppard M, McCarthy KP, Roughton M, et al. Systemic right ventricular fibrosis detected by cardiovascular magnetic resonance is associated with clinical outcome, mainly new-onset atrial arrhythmia, in patients after atrial redirection surgery for transposition of the great arteries. Circ Cardiovasc Imaging. 2015;8:e002628. doi: 10.1161/CIRCIMAGING.114.002628 [DOI] [PubMed] [Google Scholar]
- 390.Broberg CS, Valente AM, Huang J, Burchill LJ, Holt J, Van Woerkom R, Powell AJ, Pantely GA, Jerosch-Herold M. Myocardial fibrosis and its relation to adverse outcome in transposition of the great arteries with a systemic right ventricle. Int J Cardiol. 2018;271:60–65. doi: 10.1016/j.ijcard.2018.04.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Roma-Rodrigues C, Fernandes AR. Genetics of hypertrophic cardiomyopathy: advances and pitfalls in molecular diagnosis and therapy. Appl Clin Genet. 2014;7:195–208. doi: 10.2147/TACG.S49126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–79. doi: 10.1093/eurheartj/ehu284 [DOI] [PubMed] [Google Scholar]
- 393.Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2020;76:3022–3055. doi: 10.1016/j.jacc.2020.08.044 [DOI] [PubMed] [Google Scholar]
- 394.Miron A, Lafreniere-Roula M, Steve Fan CP, Armstrong KR, Dragulescu A, Papaz T, Manlhiot C, Kaufman B, Butts RJ, Gardin L, et al. A Validated Model for Sudden Cardiac Death Risk Prediction in Pediatric Hypertrophic Cardiomyopathy. Circulation. 2020;142:217–229. doi: 10.1161/CIRCULATIONAHA.120.047235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Norrish G, Ding T, Field E, Ziólkowska L, Olivotto I, Limongelli G, Anastasakis A, Weintraub R, Biagini E, Ragni L, et al. Development of a Novel Risk Prediction Model for Sudden Cardiac Death in Childhood Hypertrophic Cardiomyopathy (HCM Risk-Kids). JAMA Cardiol. 2019;4:918–927. doi: 10.1001/jamacardio.2019.2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Östman-Smith I, Sjöberg G, Rydberg A, Larsson P, Fernlund E. Predictors of risk for sudden death in childhood hypertrophic cardiomyopathy: the importance of the ECG risk score. Open Heart. 2017;4: e000658. doi: 10.1136/openhrt-2017-000658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Maron BJ, Spirito P, Wesley Y, Arce J. Development and progression of left ventricular hypertrophy in children with hypertrophic cardiomyopathy. N Engl J Med. 1986;315:610–614. doi: 10.1056/NEJM198609043151003 [DOI] [PubMed] [Google Scholar]
- 398.Maron MS, Maron BJ, Harrigan C, Buros J, Gibson CM, Olivotto I, Biller L, Lesser JR, Udelson JE, Manning WJ, et al. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J Am Coll Cardiol. 2009;54:220–228. doi: 10.1016/j.jacc.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 399.Moon JC, Fisher NG, McKenna WJ, Pennell DJ. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart. 2004;90:645–649. doi: 10.1136/hrt.2003.014969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Rickers C, Wilke NM, Jerosch-Herold M, Casey SA, Panse P, Panse N, Weil J, Zenovich AG, Maron BJ. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation. 2005;112:855–861. doi: 10.1161/CIRCULATIONAHA.104.507723 [DOI] [PubMed] [Google Scholar]
- 401.Bois JP, Geske JB, Foley TA, Ommen SR, Pellikka PA. Comparison of Maximal Wall Thickness in Hypertrophic Cardiomyopathy Differs Between Magnetic Resonance Imaging and Transthoracic Echocardiography. Am J Cardiol. 2017;119:643–650. doi: 10.1016/j.amjcard.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 402.Corona-Villalobos CP, Sorensen LL, Pozios I, Chu L, Eng J, Abraham MR, Abraham TP, Kamel IR, Zimmerman SL. Left ventricular wall thickness in patients with hypertrophic cardiomyopathy: a comparison between cardiac magnetic resonance imaging and echocardiography. Int J Cardiovasc Imaging. 2016;32:945–954. doi: 10.1007/s10554-016-0858-4 [DOI] [PubMed] [Google Scholar]
- 403.Rowin EJ, Maron BJ, Haas TS, Garberich RF, Wang W, Link MS, Maron MS. Hypertrophic Cardiomyopathy With Left Ventricular Apical Aneurysm: Implications for Risk Stratification and Management. J Am Coll Cardiol. 2017;69:761–773. doi: 10.1016/j.jacc.2016.11.063 [DOI] [PubMed] [Google Scholar]
- 404.Maron MS. Clinical utility of cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. 2012;14:13. doi: 10.1186/1532-429X-14-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Nguyen A, Schaff HV, Nishimura RA, Geske JB, Dearani JA, King KS, Ommen SR. Apical myectomy for patients with hypertrophic cardiomyopathy and advanced heart failure. J Thorac Cardiovasc Surg. 2019;152:145–52. doi: 10.1016/j.jtcvs.2019.03.088 [DOI] [PubMed] [Google Scholar]
- 406.Keeling AN, Carr JC, Choudhury L. Right ventricular hypertrophy and scarring in mutation positive hypertrophic cardiomyopathy. Eur Heart J. 2010;31:381. doi: 10.1093/eurheartj/ehp528 [DOI] [PubMed] [Google Scholar]
- 407.Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342:1778–1785. doi: 10.1056/NEJM200006153422403 [DOI] [PubMed] [Google Scholar]
- 408.Nagueh SF, Bierig SM, Budoff MJ, Desai M, Dilsizian V, Eidem B, Goldstein SA, Hung J, Maron MS, Ommen SR, et al. ; American Society of Echocardiography; American Society of Nuclear Cardiology; Society for Cardiovascular Magnetic Resonance; Society of Cardiovascular Computed Tomography. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: Endorsed by the American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr. 2011;24:473–498. doi: 10.1016/j.echo.2011.03.006 [DOI] [PubMed] [Google Scholar]
- 409.Olivotto I, Maron MS, Autore C, Lesser JR, Rega L, Casolo G, De Santis M, Quarta G, Nistri S, Cecchi F, et al. Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008;52:559–566. doi: 10.1016/j.jacc.2008.04.047 [DOI] [PubMed] [Google Scholar]
- 410.Harris KM, Spirito P, Maron MS, Zenovich AG, Formisano F, Lesser JR, Mackey-Bojack S, Manning WJ, Udelson JE, Maron BJ. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation. 2006;114:216–225. doi: 10.1161/CIRCULATIONAHA.105.583500 [DOI] [PubMed] [Google Scholar]
- 411.Maskatia SA, Decker JA, Spinner JA, Kim JJ, Price JF, Jefferies JL, Dreyer WJ, Smith EO, Rossano JW, Denfield SW. Restrictive physiology is associated with poor outcomes in children with hypertrophic cardiomyopathy. Pediatr Cardiol. 2012;33:141–149. doi: 10.1007/s00246-011-0106-6 [DOI] [PubMed] [Google Scholar]
- 412.Kwon DH, Setser RM, Thamilarasan M, Popovic ZV, Smedira NG, Schoenhagen P, Garcia MJ, Lever HM, Desai MY. Abnormal papillary muscle morphology is independently associated with increased left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Heart. 2008;94:1295–1301. doi: 10.1136/hrt.2007.118018 [DOI] [PubMed] [Google Scholar]
- 413.Adabag AS, Maron BJ, Appelbaum E, Harrigan CJ, Buros JL, Gibson CM, Lesser JR, Hanna CA, Udelson JE, Manning WJ, et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1369–1374. doi: 10.1016/j.jacc.2007.11.071 [DOI] [PubMed] [Google Scholar]
- 414.Cheng S, Fang M, Cui C, Chen X, Yin G, Prasad SK, Dong D, Tian J, Zhao S. LGE-CMR-derived texture features reflect poor prognosis in hypertrophic cardiomyopathy patients with systolic dysfunction: preliminary results. Eur Radiol. 2018;28:4615–4624. doi: 10.1007/s00330-018-5391-5 [DOI] [PubMed] [Google Scholar]
- 415.Axelsson Raja A, Farhad H, Valente AM, Couce JP, Jefferies JL, Bundgaard H, Zahka K, Lever H, Murphy AM, Ashley E, et al. Prevalence and Progression of Late Gadolinium Enhancement in Children and Adolescents With Hypertrophic Cardiomyopathy. Circulation. 2018;138:782–792. doi: 10.1161/CIRCULATIONAHA.117.032966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Chaowu Y, Shihua Z, Jian L, Li L, Wei F. Cardiovascular magnetic resonance characteristics in children with hypertrophic cardiomyopathy. Circ Heart Fail. 2013;6:1013–1020. doi: 10.1161/CIRCHEARTFAILURE.113.000414 [DOI] [PubMed] [Google Scholar]
- 417.Spinner JA, Noel CV, Denfield SW, Krishnamurthy R, Jeewa A, Dreyer WJ, Maskatia SA. Association of Late Gadolinium Enhancement and Degree of Left Ventricular Hypertrophy Assessed on Cardiac Magnetic Resonance Imaging With Ventricular Tachycardia in Children With Hypertrophic Cardiomyopathy. Am J Cardiol. 2016;117:1342–1348. doi: 10.1016/j.amjcard.2016.01.032 [DOI] [PubMed] [Google Scholar]
- 418.Windram JD, Benson LN, Dragelescu A, Yoo SJ, Mertens L, Wong D, Grosse-Wortmann L. Distribution of Hypertrophy and Late Gadolinium Enhancement in Children and Adolescents with Hypertrophic Cardiomyopathy. Congenit Heart Dis. 2015;10:E258–E267. doi: 10.1111/chd.12286 [DOI] [PubMed] [Google Scholar]
- 419.Smith BM, Dorfman AL, Yu S, Russell MW, Agarwal PP, Mahani MG, Lu JC. Clinical significance of late gadolinium enhancement in patients<20 years of age with hypertrophic cardiomyopathy. Am J Cardiol. 2014;113:1234–1239. doi: 10.1016/j.amjcard.2013.12.034 [DOI] [PubMed] [Google Scholar]
- 420.Mazurkiewicz Ł, Ziółkowska L, Petryka J, Śpiewak M, Małek Ł, Kubik A, Marczak M, Misko J, Brzezińska-Rajszys G. Left-ventricular mechanics in children with hypertrophic cardiomyopathy A CMR study. Magn Reson Imaging. 2017;43:56–65. doi: 10.1016/j.mri.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 421.Smith BM, Dorfman AL, Yu S, Russell MW, Agarwal PP, Ghadimi Mahani M, Lu JC. Relation of strain by feature tracking and clinical outcome in children, adolescents, and young adults with hypertrophic cardiomyopathy. Am J Cardiol. 2014;114:1275–1280. doi: 10.1016/j.amjcard.2014.07.051 [DOI] [PubMed] [Google Scholar]
- 422.Bogarapu S, Puchalski MD, Everitt MD, Williams RV, Weng HY, Menon SC. Novel Cardiac Magnetic Resonance Feature Tracking (CMR-FT) Analysis for Detection of Myocardial Fibrosis in Pediatric Hypertrophic Cardiomyopathy. Pediatr Cardiol. 2016;37:663–673. doi: 10.1007/s00246-015-1329-8 [DOI] [PubMed] [Google Scholar]
- 423.Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, Lesser JR, Gruner C, Crean AM, Rakowski H, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130:484–495. doi: 10.1161/CIRCULATIONAHA.113.007094 [DOI] [PubMed] [Google Scholar]
- 424.Weng Z, Yao J, Chan RH, He J, Yang X, Zhou Y, He Y. Prognostic Value of LGE-CMR in HCM: A Meta-Analysis. JACC Cardiovasc Imaging. 2016;9:1392–1402. doi: 10.1016/j.jcmg.2016.02.031 [DOI] [PubMed] [Google Scholar]
- 425.Briasoulis A, Mallikethi-Reddy S, Palla M, Alesh I, Afonso L. Myocardial fibrosis on cardiac magnetic resonance and cardiac outcomes in hypertrophic cardiomyopathy: a meta-analysis. Heart. 2015;101:1406–1411. doi: 10.1136/heartjnl-2015-307682 [DOI] [PubMed] [Google Scholar]
- 426.Ho CY, Abbasi SA, Neilan TG, Shah RV, Chen Y, Heydari B, Cirino AL, Lakdawala NK, Orav EJ, González A, et al. T1 measurements identify extracellular volume expansion in hypertrophic cardiomyopathy sarcomere mutation carriers with and without left ventricular hypertrophy. Circ Cardiovasc Imaging. 2013;6:415–422. doi: 10.1161/CIRCIMAGING.112.000333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Ellims AH, Iles LM, Ling LH, Chong B, Macciocca I, Slavin GS, Hare JL, Kaye DM, Marasco SF, McLean CA, et al. A comprehensive evaluation of myocardial fibrosis in hypertrophic cardiomyopathy with cardiac magnetic resonance imaging: linking genotype with fibrotic phenotype. Eur Heart J Cardiovasc Imaging. 2014;15:1108–1116. doi: 10.1093/ehjci/jeu077 [DOI] [PubMed] [Google Scholar]
- 428.Puntmann VO, Voigt T, Chen Z, Mayr M, Karim R, Rhode K, Pastor A, Carr-White G, Razavi R, Schaeffter T, et al. Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovasc Imaging. 2013;6:475–484. doi: 10.1016/j.jcmg.2012.08.019 [DOI] [PubMed] [Google Scholar]
- 429.McLELLAN AJ, Ellims AH, Prabhu S, Voskoboinik A, Iles LM, Hare JL, Kaye DM, Macciocca I, Mariani JA, Kalman JM, et al. Diffuse Ventricular Fibrosis on Cardiac Magnetic Resonance Imaging Associates With Ventricular Tachycardia in Patients With Hypertrophic Cardiomyopathy. J Cardiovasc Electrophysiol. 2016;27:571–580. doi: 10.1111/jce.12948 [DOI] [PubMed] [Google Scholar]
- 430.Parekh K, Markl M, Deng J, de Freitas RA, Rigsby CK. T1 mapping in children and young adults with hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 2017;33:109–117. doi: 10.1007/s10554-016-0979-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Hussain T, Dragulescu A, Benson L, Yoo SJ, Meng H, Windram J, Wong D, Greiser A, Friedberg M, Mertens L, et al. Quantification and significance of diffuse myocardial fibrosis and diastolic dysfunction in childhood hypertrophic cardiomyopathy. Pediatr Cardiol. 2015;36:970–978. doi: 10.1007/s00246-015-1107-7 [DOI] [PubMed] [Google Scholar]
- 432.Hinojar R, Varma N, Child N, Goodman B, Jabbour A, Yu CY, Gebker R, Doltra A, Kelle S, Khan S, et al. T1 Mapping in Discrimination of Hypertrophic Phenotypes: Hypertensive Heart Disease and Hypertrophic Cardiomyopathy: Findings From the International T1 Multicenter Cardiovascular Magnetic Resonance Study. Circ Cardiovasc Imaging. 2015;8:e003285. doi: 10.1161/CIRCIMAGING.115.003285 [DOI] [PubMed] [Google Scholar]
- 433.Swoboda PP, McDiarmid AK, Erhayiem B, Broadbent DA, Dobson LE, Garg P, Ferguson C, Page SP, Greenwood JP, Plein S. Assessing Myocardial Extracellular Volume by T1 Mapping to Distinguish Hypertrophic Cardiomyopathy From Athlete’s Heart. J Am Coll Cardiol. 2016;67:2189–2190. doi: 10.1016/j.jacc.2016.02.054 [DOI] [PubMed] [Google Scholar]
- 434.Kelley-Hedgepeth A, Towbin JA, Maron MS. Images in cardiovascular medicine. Overlapping phenotypes: left ventricular noncompaction and hypertrophic cardiomyopathy. Circulation. 2009;119:e588–e589. doi: 10.1161/CIRCULATIONAHA.108.829564 [DOI] [PubMed] [Google Scholar]
- 435.Ganame J, Mertens L, Eidem BW, Claus P, D’hooge J, Havemann LM, McMahon CJ, Elayda MA, Vaughn WK, Towbin JA, et al. Regional myocardial deformation in children with hypertrophic cardiomyopathy: morphological and clinical correlations. Eur Heart J. 2007;28:2886–2894. doi: 10.1093/eurheartj/ehm444 [DOI] [PubMed] [Google Scholar]
- 436.Ghio S, Revera M, Mori F, Klersy C, Raisaro A, Raineri C, Serio A, Pasotti M, Visconti LO. Regional abnormalities of myocardial deformation in patients with hypertrophic cardiomyopathy: correlations with delayed enhancement in cardiac magnetic resonance. Eur J Heart Fail. 2009;11:952–957. doi: 10.1093/eurjhf/hfp122 [DOI] [PubMed] [Google Scholar]
- 437.Petersen SE, Jerosch-Herold M, Hudsmith LE, Robson MD, Francis JM, Doll HA, Selvanayagam JB, Neubauer S, Watkins H. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: new insights from multiparametric magnetic resonance imaging. Circulation. 2007;115:2418–2425. doi: 10.1161/CIRCULATIONAHA.106.657023 [DOI] [PubMed] [Google Scholar]
- 438.Yin L, Xu HY, Zheng SS, Zhu Y, Xiao JX, Zhou W, Yu SS, Gong LG. 3.0 T magnetic resonance myocardial perfusion imaging for semi-quantitative evaluation of coronary microvascular dysfunction in hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 2017;33:1949–1959. doi: 10.1007/s10554-017-1189-9 [DOI] [PubMed] [Google Scholar]
- 439.Hernandez LE. Myocardial stress perfusion magnetic resonance in children with hypertrophic cardiomyopathy. Cardiol Young. 2018;28:702–708. doi: 10.1017/S1047951118000094 [DOI] [PubMed] [Google Scholar]
- 440.Hen Y, Takara A, Iguchi N, Utanohara Y, Teraoka K, Takada K, Machida H, Takamisawa I, Takayama M, Yoshikawa T. High Signal Intensity on T2-Weighted Cardiovascular Magnetic Resonance Imaging Predicts Life-Threatening Arrhythmic Events in Hypertrophic Cardiomyopathy Patients. Circ J. 2018;82:1062–1069. doi: 10.1253/circj.CJ-17-1235 [DOI] [PubMed] [Google Scholar]
- 441.Gommans DHF, Cramer GE, Bakker J, Dieker HJ, Michels M, Fouraux MA, Marcelis CLM, Verheugt FWA, Timmermans J, Brouwer MA, et al. High T2-weighted signal intensity for risk prediction of sudden cardiac death in hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 2018;34:113–120. doi: 10.1007/s10554-017-1252-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Webb J, Villa A, Bekri I, Shome J, Teall T, Claridge S, Jackson T, Porter B, Ismail TF, Di Giovine G, et al. Usefulness of Cardiac Magnetic Resonance Imaging to Measure Left Ventricular Wall Thickness for Determining Risk Scores for Sudden Cardiac Death in Patients With Hypertrophic Cardiomyopathy. Am J Cardiol. 2017;119:1450–1455. doi: 10.1016/j.amjcard.2017.01.021 [DOI] [PubMed] [Google Scholar]
- 443.Windram JD, Dragelescu A, Benson L, Forsey J, Shariat M, Yoo SJ, Mertens L, Wong D, Grosse-Wortmann L. Myocardial Dimensions in Children With Hypertrophic Cardiomyopathy: A Comparison Between Echocardiography and Cardiac Magnetic Resonance Imaging. Can J Cardiol. 2016;32:1507–1512. doi: 10.1016/j.cjca.2016.06.014 [DOI] [PubMed] [Google Scholar]
- 444.Pons-Lladó G, Carreras F, Borrás X, Palmer J, Llauger J, Bayés de Luna A. Comparison of morphologic assessment of hypertrophic cardiomyopathy by magnetic resonance versus echocardiographic imaging. Am J Cardiol. 1997;79:1651–1656. doi: 10.1016/s0002-9149(97)00216-6 [DOI] [PubMed] [Google Scholar]
- 445.Puntmann VO, Gebker R, Duckett S, Mirelis J, Schnackenburg B, Graefe M, Razavi R, Fleck E, Nagel E. Left ventricular chamber dimensions and wall thickness by cardiovascular magnetic resonance: comparison with transthoracic echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14:240–246. doi: 10.1093/ehjci/jes145 [DOI] [PubMed] [Google Scholar]
- 446.Centers for Disease Control and Prevention. Prevalence of Duchenne/ Becker muscular dystrophy among males aged 5–24 years: four states 2007. MMWR Morb Mortal Wkly Rep. 2009;58:1119–22. [PubMed] [Google Scholar]
- 447.Sahay KM, Smith T, Conway KM, Romitti PA, Lamb MM, Andrews J, Pandya S, Oleszek J, Cunniff C, Valdez R. A Review of MD STAR net’s Research Contributions to Pediatric-Onset Dystrophinopathy in the United States; 2002-2017. J Child Neurol. 2019;34:44–53. doi: 10.1177/0883073818801704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Eagle M, Baudouin SV, Chandler C, Giddings DR, Bullock R, Bushby K. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord. 2002;12:926–929. doi: 10.1016/s0960-8966(02)00140-2 [DOI] [PubMed] [Google Scholar]
- 449.Connuck DM, Sleeper LA, Colan SD, Cox GF, Towbin JA, Lowe AM, Wilkinson JD, Orav EJ, Cuniberti L, Salbert BA, et al. ; Pediatric Cardiomyopathy Registry Study Group. Characteristics and outcomes of cardiomyopathy in children with Duchenne or Becker muscular dystrophy: a comparative study from the Pediatric Cardiomyopathy Registry. Am Heart J. 2008;155:998–1005. doi: 10.1016/j.ahj.2008.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Nigro G, Comi LI, Politano L, Bain RJ. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol. 1990;26:271–277. doi: 10.1016/0167-5273(90)90082-g [DOI] [PubMed] [Google Scholar]
- 451.Hor KN, Taylor MD, Al-Khalidi HR, Cripe LH, Raman SV, Jefferies JL, O’Donnell R, Benson DW, Mazur W. Prevalence and distribution of late gadolinium enhancement in a large population of patients with Duchenne muscular dystrophy: effect of age and left ventricular systolic function. J Cardiovasc Magn Reson. 2013;15:107. doi: 10.1186/1532-429X-15-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Ryan TD, Taylor MD, Mazur W, Cripe LH, Pratt J, King EC, Lao K, Grenier MA, Jefferies JL, Benson DW, et al. Abnormal circumferential strain is present in young Duchenne muscular dystrophy patients. Pediatr Cardiol. 2013;34:1159–1165. doi: 10.1007/s00246-012-0622-z [DOI] [PubMed] [Google Scholar]
- 453.Feingold B, Mahle WT, Auerbach S, Clemens P, Domenighetti AA, Jefferies JL, Judge DP, Lal AK, Markham LW, Parks WJ, et al. ; American Heart Association Pediatric Heart Failure Committee of the Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; Council on Cardiovascular Radiology and Intervention; Council on Functional Genomics and Translational Biology; and Stroke Council. Management of Cardiac Involvement Associated With Neuromuscular Diseases: A Scientific Statement From the American Heart Association. Circulation. 2017;136:e200–e231. doi: 10.1161/CIR.0000000000000526 [DOI] [PubMed] [Google Scholar]
- 454.Soslow JH, Xu M, Slaughter JC, Stanley M, Crum K, Markham LW, Parra DA. Evaluation of Echocardiographic Measures of Left Ventricular Function in Patients with Duchenne Muscular Dystrophy: Assessment of Reproducibility and Comparison to Cardiac Magnetic Resonance Imaging. J Am Soc Echocardiogr. 2016;29:983–991. doi: 10.1016/j.echo.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Spurney CF, McCaffrey FM, Cnaan A, Morgenroth LP, Ghelani SJ, Gordish-Dressman H, Arrieta A, Connolly AM, Lotze TE, McDonald CM, et al. Feasibility and Reproducibility of Echocardiographic Measures in Children with Muscular Dystrophies. J Am Soc Echocardiogr. 2015;28:999–1008. doi: 10.1016/j.echo.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Bahler RC, Mohyuddin T, Finkelhor RS, Jacobs IB. Contribution of Doppler tissue imaging and myocardial performance index to assessment of left ventricular function in patients with Duchenne’s muscular dystrophy. J Am Soc Echocardiogr. 2005;18:666–673. doi: 10.1016/j.echo.2004.10.003 [DOI] [PubMed] [Google Scholar]
- 457.Power A, Poonja S, Disler D, Myers K, Patton DJ, Mah JK, Fine NM, Greenway SC. Echocardiographic Image Quality Deteriorates with Age in Children and Young Adults with Duchenne Muscular Dystrophy. Front Cardiovasc Med. 2017;4:82. doi: 10.3389/fcvm.2017.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.McMahon CJ, Ayres NA, Bezold LI, Lewin MB, Alonzo M, Altman CA, Kovalchin JP, Eidem BW, Pignatelli RH. Safety and efficacy of intravenous contrast imaging in pediatric echocardiography. Pediatr Cardiol. 2005;26:413–417. doi: 10.1007/s00246-004-0795-1 [DOI] [PubMed] [Google Scholar]
- 459.Sechtem U, Pflugfelder PW, Gould RG, Cassidy MM, Higgins CB. Measurement of right and left ventricular volumes in healthy individuals with cine MR imaging. Radiology. 1987;163:697–702. doi: 10.1148/radiology.163.3.3575717 [DOI] [PubMed] [Google Scholar]
- 460.Koch JA, Poll LW, Godehardt E, Korbmacher B, Mödder U. Right and left ventricular volume measurements in an animal heart model in vitro: first experiences with cardiac MRI at 1.0 T. Eur Radiol. 2000;10:455–458. doi: 10.1007/s003300050075 [DOI] [PubMed] [Google Scholar]
- 461.Silva MC, Meira ZM, Gurgel Giannetti J, da Silva MM, Campos AF, Barbosa Mde M, Starling Filho GM, Ferreira Rde A, Zatz M, Rochitte CE. Myocardial delayed enhancement by magnetic resonance imaging in patients with muscular dystrophy. J Am Coll Cardiol. 2007;49:1874–1879. doi: 10.1016/j.jacc.2006.10.078 [DOI] [PubMed] [Google Scholar]
- 462.Brunklaus A, Parish E, Muntoni F, Scuplak S, Tucker SK, Fenton M, Hughes ML, Manzur AY. The value of cardiac MRI versus echocardiography in the pre-operative assessment of patients with Duchenne muscular dystrophy. Eur J Paediatr Neurol. 2015;19:395–401. doi: 10.1016/j.ejpn.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 463.Ashford MW, Jr, Liu W, Lin SJ, Abraszewski P, Caruthers SD, Connolly AM, Yu X, Wickline SA. Occult cardiac contractile dysfunction in dystrophin-deficient children revealed by cardiac magnetic resonance strain imaging. Circulation. 2005;112:2462–2467. doi: 10.1161/CIRCULATIONAHA.104.516716 [DOI] [PubMed] [Google Scholar]
- 464.Buddhe S, Lewin M, Olson A, Ferguson M, Soriano BD. Comparison of left ventricular function assessment between echocardiography and MRI in Duchenne muscular dystrophy. Pediatr Radiol. 2016;46:1399–1408. doi: 10.1007/s00247-016-3622-y [DOI] [PubMed] [Google Scholar]
- 465.Mavrogeni S, Giannakopoulou A, Papavasiliou A, Markousis-Mavrogenis G, Pons R, Karanasios E, Noutsias M, Kolovou G, Papadopoulos G. Cardiac profile of asymptomatic children with Becker and Duchenne muscular dystrophy under treatment with steroids and with/without perindopril. BMC Cardiovasc Disord. 2017;17:197. doi: 10.1186/s12872-017-0627-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Hor KN, Wansapura J, Markham LW, Mazur W, Cripe LH, Fleck R, Benson DW, Gottliebson WM. Circumferential strain analysis identifies strata of cardiomyopathy in Duchenne muscular dystrophy: a cardiac magnetic resonance tagging study. J Am Coll Cardiol. 2009;53:1204–1210. doi: 10.1016/j.jacc.2008.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Hor KN, Wansapura JP, Al-Khalidi HR, Gottliebson WM, Taylor MD, Czosek RJ, Nagueh SF, Akula N, Chung ES, Benson WD, et al. Presence of mechanical dyssynchrony in Duchenne muscular dystrophy. J Cardiovasc Magn Reson. 2011;13:12. doi: 10.1186/1532-429X-13-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Hor KN, Kissoon N, Mazur W, Gupta R, Ittenbach RF, Al-Khalidi HR, Cripe LH, Raman SV, Puchalski MD, Gottliebson WM, et al. Regional circumferential strain is a biomarker for disease severity in duchenne muscular dystrophy heart disease: a cross-sectional study. Pediatr Cardiol. 2015;36:111–119. doi: 10.1007/s00246-014-0972-9 [DOI] [PubMed] [Google Scholar]
- 469.Hagenbuch SC, Gottliebson WM, Wansapura J, Mazur W, Fleck R, Benson DW, Hor KN. Detection of progressive cardiac dysfunction by serial evaluation of circumferential strain in patients with Duchenne muscular dystrophy. Am J Cardiol. 2010;105:1451–1455. doi: 10.1016/j.amjcard.2009.12.070 [DOI] [PubMed] [Google Scholar]
- 470.Frankel KA, Rosser RJ. The pathology of the heart in progressive muscular dystrophy: epimyocardial fibrosis. Hum Pathol. 1976;7:375–386. doi: 10.1016/s0046-8177(76)80053-6 [DOI] [PubMed] [Google Scholar]
- 471.Puchalski MD, Williams RV, Askovich B, Sower CT, Hor KH, Su JT, Pack N, Dibella E, Gottliebson WM. Late gadolinium enhancement: precursor to cardiomyopathy in Duchenne muscular dystrophy? Int J Cardiovasc Imaging. 2009;25:57–63. doi: 10.1007/s10554-008-9352-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Florian A, Ludwig A, Engelen M, Waltenberger J, Rösch S, Sechtem U, Yilmaz A. Left ventricular systolic function and the pattern of late-gadolinium-enhancement independently and additively predict adverse cardiac events in muscular dystrophy patients. J Cardiovasc Magn Reson. 2014;16:81. doi: 10.1186/s12968-014-0081-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Menon SC, Etheridge SP, Liesemer KN, Williams RV, Bardsley T, Heywood MC, Puchalski MD. Predictive value of myocardial delayed enhancement in Duchenne muscular dystrophy. Pediatr Cardiol. 2014;35:1279–1285. doi: 10.1007/s00246-014-0929-z [DOI] [PubMed] [Google Scholar]
- 474.Tandon A, Villa CR, Hor KN, Jefferies JL, Gao Z, Towbin JA, Wong BL, Mazur W, Fleck RJ, Sticka JJ, et al. Myocardial fibrosis burden predicts left ventricular ejection fraction and is associated with age and steroid treatment duration in duchenne muscular dystrophy. J Am Heart Assoc. 2015;4:e001338. doi: 10.1161/JAHA.114.001338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Isaacs H, Jr. Fetal and neonatal cardiac tumors. Pediatr Cardiol. 2004;25:252–273. doi: 10.1007/s00246-003-0590-4 [DOI] [PubMed] [Google Scholar]
- 476.Beghetti M, Gow RM, Haney I, Mawson J, Williams WG, Freedom RM. Pediatric primary benign cardiac tumors: a 15-year review. Am Heart J. 1997;134:1107–1114. doi: 10.1016/s0002-8703(97)70032-2 [DOI] [PubMed] [Google Scholar]
- 477.Beroukhim RS, Prakash A, Buechel ER, Cava JR, Dorfman AL, Festa P, Hlavacek AM, Johnson TR, Keller MS, Krishnamurthy R, et al. Characterization of cardiac tumors in children by cardiovascular magnetic resonance imaging: a multicenter experience. J Am Coll Cardiol. 2011;58:1044–1054. doi: 10.1016/j.jacc.2011.05.027 [DOI] [PubMed] [Google Scholar]
- 478.Yinon Y, Chitayat D, Blaser S, Seed M, Amsalem H, Yoo SJ, Jaeggi ET. Fetal cardiac tumors: a single-center experience of 40 cases. Prenat Diagn. 2010;30:941–949. doi: 10.1002/pd.2590 [DOI] [PubMed] [Google Scholar]
- 479.Etesami M, Gilkeson RC, Rajiah P. Utility of late gadolinium enhancement in pediatric cardiac MRI. Pediatr Radiol. 2016;46:1096–1113. doi: 10.1007/s00247-015-3526-2 [DOI] [PubMed] [Google Scholar]
- 480.Parsai C, O’Hanlon R, Prasad SK, Mohiaddin RH. Diagnostic and prognostic value of cardiovascular magnetic resonance in non-ischaemic cardiomyopathies. J Cardiovasc Magn Reson. 2012;14:54. doi: 10.1186/1532-429X-14-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Ying L, Lin R, Gao Z, Qi J, Zhang Z, Gu W. Primary cardiac tumors in children: a center’s experience. J Cardiothoracic Surgery. 2016;11:52. doi: 10.1186/s13019-016-0448-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Lee E, Mahani MG, Lu JC, Dorfman AL, Srinivasan A, Agarwal PP. Primary cardiac tumors associated with genetic syndromes: a comprehensive review. Pediatr Radiol. 2018;48:156–164. doi: 10.1007/s00247-017-4027-2 [DOI] [PubMed] [Google Scholar]
- 483.Pazos-López P, Pozo E, Siqueira ME, García-Lunar I, Cham M, Jacobi A, Macaluso F, Fuster V, Narula J, Sanz J. Value of CMR for the differential diagnosis of cardiac masses. JACC Cardiovasc Imaging. 2014;7:896–905. doi: 10.1016/j.jcmg.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 484.Macias E, Nieman E, Yomogida K, Petrucci O, Javidan C, Baszis K, Anwar S. Rare presentation of an atrial myxoma in an adolescent patient: a case report and literature review. BMC Pediatr. 2018;18:373. doi: 10.1186/s12887-018-1313-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Chlebowski M, O’Brien J, Hertzenberg C, Wagner J. Asymptomatic Left Ventricular Myxoma in a 12-Year-Old Male. Tex Heart Inst J. 2016;43:267–269. doi: 10.14503/THIJ-15-5318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Markel ML, Waller BF, Armstrong WF. Cardiac myxoma. A review. Medicine (Baltimore). 1987;66:114–125. doi: 10.1097/00005792-198703000-00003 [DOI] [PubMed] [Google Scholar]
- 487.Grebenc ML, Rosado-de-Christenson ML, Green CE, Burke AP, Galvin JR. Cardiac myxoma: imaging features in 83 patients. Radiographics. 2002;22:673–689. doi: 10.1148/radiographics.22.3.g02ma02673 [DOI] [PubMed] [Google Scholar]
- 488.Moniotte S, Geva T, Perez-Atayde A, Fulton DR, Pigula FA, Powell AJ. Images in cardiovascular medicine. Cardiac Hemangioma Circ. 2005;112:e103–4. [DOI] [PubMed] [Google Scholar]
- 489.Friedberg MK, Chang IL, Silverman NH, Ramamoorthy C, Chan FP. Images in cardiovascular medicine. Near sudden death from cardiac lipoma in an adolescent. Circulation. 2006;113:e778–e779. doi: 10.1161/CIRCULATIONAHA.105.589630 [DOI] [PubMed] [Google Scholar]
- 490.Lurito KJ, Martin T, Cordes T. Right atrial primary cardiac osteosarcoma. Pediatr Cardiol. 2002;23:462–465. doi: 10.1007/s00246-002-0151-2 [DOI] [PubMed] [Google Scholar]
- 491.Mackie AS, Kozakewich HP, Geva T, Perez-Atayde AR, Mulliken JB. Vascular tumors of the heart in infants and children: case series and review of the literature. Pediatr Cardiol. 2005;26:344–349. doi: 10.1007/s00246-004-0717-2 [DOI] [PubMed] [Google Scholar]
- 492.Beroukhim RS, Geva T. Primary and secondary cardiac tumors. In: Da Cruz EM, Ivy D, Hraska V, Jaggers J, editors. Pediatric and congenital cardiology, cardiac surgery and intensive care. London: Springer, London; 2013. p. 2275–95. [Google Scholar]
- 493.Strecker T, Rösch J, Weyand M, Agaimy A. Primary and metastatic cardiac tumors: imaging characteristics, surgical treatment, and histopathological spectrum: a 10-year-experience at a German heart center. Cardiovasc Pathol. 2012;21:436–443. doi: 10.1016/j.carpath.2011.12.004 [DOI] [PubMed] [Google Scholar]
- 494.Paraskevaidis IA, Michalakeas CA, Papadopoulos CH, Anastasiou-Nana M. Cardiac tumors. ISRN Oncol. 2011;2011:208929. doi: 10.5402/2011/208929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Uzun O, Wilson DG, Vujanic GM, Parsons JM, De Giovanni JV. Cardiac tumours in children. Orphanet J Rare Dis. 2007;2:11. doi: 10.1186/1750-1172-2-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR. Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation. Radiographics. 2000;20:1073–103; quiz 1110. doi: 10.1148/radiographics.20.4.g00jl081073 [DOI] [PubMed] [Google Scholar]
- 497.Newman B. Thoracic neoplasms in children. Radiol Clin North Am. 2011;49:633–64, v. doi: 10.1016/j.rcl.2011.05.010 [DOI] [PubMed] [Google Scholar]
- 498.Motwani M, Kidambi A, Herzog BA, Uddin A, Greenwood JP, Plein S. MR imaging of cardiac tumors and masses: a review of methods and clinical applications. Radiology. 2013;268:26–43. doi: 10.1148/radiol.13121239 [DOI] [PubMed] [Google Scholar]
- 499.Marx GR, Moran AM. Cardiac tumors. In: Allen HD, Shaddy RE, Penny DJ, Feltes TF, Cetta F, editors. Moss and Adams heart disease in infants, adolescents, and children, including the fetus and young adult. 9th ed. Philadelphia: Wolters Kluwer; 2016. p. 1669–86. [Google Scholar]
- 500.Sagar S, Liu PP, Cooper LT, Jr. Myocarditis. Lancet. 2012;379:738–747. doi: 10.1016/S0140-6736(11)60648-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Chow LH, Radio SJ, Sears TD, McManus BM. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J Am Coll Cardiol. 1989;14:915–920. doi: 10.1016/0735-1097(89)90465-8 [DOI] [PubMed] [Google Scholar]
- 502.Hauck AJ, Kearney DL, Edwards WD. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc. 1989;64:1235–1245. doi: 10.1016/s0025-6196(12)61286-5 [DOI] [PubMed] [Google Scholar]
- 503.Yilmaz A, Kindermann I, Kindermann M, Mahfoud F, Ukena C, Athanasiadis A, Hill S, Mahrholdt H, Voehringer M, Schieber M, et al. Comparative evaluation of left and right ventricular endomyocardial biopsy: differences in complication rate and diagnostic performance. Circulation. 2010;122:900–909. doi: 10.1161/CIRCULATIONAHA.109.924167 [DOI] [PubMed] [Google Scholar]
- 504.Pophal SG, Sigfusson G, Booth KL, Bacanu SA, Webber SA, Ettedgui JA, Neches WH, Park SC. Complications of endomyocardial biopsy in children. J Am Coll Cardiol. 1999;34:2105–2110. doi: 10.1016/s0735-1097(99)00452-0 [DOI] [PubMed] [Google Scholar]
- 505.Mills KI, Vincent JA, Zuckerman WA, Hoffman TM, Canter CE, Marshall AC, Blume ED, Bergersen L, Daly KP. Is Endomyocardial Biopsy a Safe and Useful Procedure in Children with Suspected Cardiomyopathy? Pediatr Cardiol. 2016;37:1200–1210. doi: 10.1007/s00246-016-1416-5 [DOI] [PubMed] [Google Scholar]
- 506.Simpson KE, Anwar S, Canter CE. Myocarditis. In: Allen HD, Shaddy RE, Penny DJ, Feltes TF, Cetta F, editors. Moss and Adams heart disease in infants, adolescents, and children, including the fetus and young adult. 9th ed. Philadelphia: Wolters Kluwer; 2016. p. 1313–30. [Google Scholar]
- 507.Kotanidis CP, Bazmpani MA, Haidich AB, Karvounis C, Antoniades C, Karamitsos TD. Diagnostic Accuracy of Cardiovascular Magnetic Resonance in Acute Myocarditis: A Systematic Review and Meta-Analysis. JACC Cardiovasc Imaging. 2018;11:1583–1590. doi: 10.1016/j.jcmg.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 508.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, et al. ; International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072 [DOI] [PubMed] [Google Scholar]
- 510.Gagliardi MG, Bevilacqua M, Di Renzi P, Picardo S, Passariello R, Marcelletti C. Usefulness of magnetic resonance imaging for diagnosis of acute myocarditis in infants and children, and comparison with endomyocardial biopsy. Am J Cardiol. 1991;68:1089–1091. doi: 10.1016/0002-9149(91)90501-b [DOI] [PubMed] [Google Scholar]
- 511.Heymans S, Eriksson U, Lehtonen J, Cooper LT, Jr. The Quest for New Approaches in Myocarditis and Inflammatory Cardiomyopathy. J Am Coll Cardiol. 2016;68:2348–2364. doi: 10.1016/j.jacc.2016.09.937 [DOI] [PubMed] [Google Scholar]
- 512.Felker GM, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Baughman KL, Hare JM. Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol. 2000;36:227–232. doi: 10.1016/s0735-1097(00)00690-2 [DOI] [PubMed] [Google Scholar]
- 513.Boyé P, Zagrosek A, Wassmuth R, Messroghli D, Bock P, Dietz R, Friedrich MG, Schulz-Menger J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45:1815–22. doi: 10.1016/j.jacc.2004.11.069 [DOI] [PubMed] [Google Scholar]
- 514.Aletras AH, Kellman P, Derbyshire JA, Arai AE. ACUT2E TSE-SSFP: a hybrid method for T2-weighted imaging of edema in the heart. Magn Reson Med. 2008;59:229–235. doi: 10.1002/mrm.21490 [DOI] [PubMed] [Google Scholar]
- 515.Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–223. doi: 10.1148/radiology.218.1.r01ja50215 [DOI] [PubMed] [Google Scholar]
- 516.Mivelaz Y, Sekarski N, Qanadli SD, Meijboom EJ, Di Bernardo S. A noninvasive diagnostic tool to differentiate myocarditis from myocardial infarction: late gadolinium enhanced cardiac magnetic resonance. Eur J Pediatr. 2007;166:971–972. doi: 10.1007/s00431-006-0328-4 [DOI] [PubMed] [Google Scholar]
- 517.Mavrogeni S, Bratis K, Georgakopoulos D, Karanasios E, Kolovou G, Pavlides G, Papadopoulos G. Evaluation of myocarditis in a pediatric population using cardiovascular magnetic resonance and endomyocardial biopsy. Int J Cardiol. 2012;160:192–195. doi: 10.1016/j.ijcard.2011.04.019 [DOI] [PubMed] [Google Scholar]
- 518.Liu G, Yang X, Su Y, Xu J, Wen Z. Cardiovascular magnetic resonance imaging findings in children with myocarditis. Chin Med J (Engl). 2014;127:3700–3705. [PubMed] [Google Scholar]
- 519.Hales-Kharazmi A, Hirsch N, Kelleman M, Slesnick T, Deshpande SR. Utility of cardiac MRI in paediatric myocarditis. Cardiol Young. 2018;28:377–385. doi: 10.1017/S1047951117001093 [DOI] [PubMed] [Google Scholar]
- 520.Harris TH, Gossett JG. Diagnosis and Diagnostic Modalities in Pediatric Patients with Elevated Troponin. Pediatr Cardiol. 2016;37:1469–1474. doi: 10.1007/s00246-016-1459-7 [DOI] [PubMed] [Google Scholar]
- 521.Banka P, Robinson JD, Uppu SC, Harris MA, Hasbani K, Lai WW, Richmond ME, Fratz S, Jain S, Johnson TR, et al. Cardiovascular magnetic resonance techniques and findings in children with myocarditis: a multicenter retrospective study. J Cardiovasc Magn Reson. 2015;17:96. doi: 10.1186/s12968-015-0201-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Lagan J, Schmitt M, Miller CA. Clinical applications of multi-parametric CMR in myocarditis and systemic inflammatory diseases. Int J Cardiovasc Imaging. 2018;34:35–54. doi: 10.1007/s10554-017-1063-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P, Mascherbauer J, Nezafat R, Salerno M, Schelbert EB, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson. 2017;19:75. doi: 10.1186/s12968-017-0389-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Olivieri LJ, Kellman P, McCarter RJ, Cross RR, Hansen MS, Spurney CF. Native T1 values identify myocardial changes and stratify disease severity in patients with Duchenne muscular dystrophy. J Cardiovasc Magn Reson. 2016;18:72. doi: 10.1186/s12968-016-0292-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Soslow JH, Damon BM, Saville BR, Lu Z, Burnette WB, Lawson MA, Parra DA, Sawyer DB, Markham LW. Evaluation of post-contrast myocardial t1 in duchenne muscular dystrophy using cardiac magnetic resonance imaging. Pediatr Cardiol. 2015;36:49–56. doi: 10.1007/s00246-014-0963-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Soslow JH, Godown J. Non-invasive detection of myocardial fibrosis in pediatric heart transplant recipients: the role of cardiovascular magnetic resonance. Pediatr Transplant. 2017. 10.1111/petr.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Riesenkampff E, Messroghli DR, Redington AN, Grosse-Wortmann L. Myocardial T1 mapping in pediatric and congenital heart disease. Circ Cardiovasc Imaging. 2015;8:e002504. doi: 10.1161/CIRCIMAGING.114.002504 [DOI] [PubMed] [Google Scholar]
- 528.Cornicelli MD, Rigsby CK, Rychlik K, Pahl E, Robinson JD. Diagnostic performance of cardiovascular magnetic resonance native T1 and T2 mapping in pediatric patients with acute myocarditis. J Cardiovasc Magn Reson. 2019;21:40. doi: 10.1186/s12968-019-0550-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Messroghli DR, Pickardt T, Fischer M, Opgen-Rhein B, Papakostas K, Böcker D, Jakob A, Khalil M, Mueller GC, Schmidt F, et al. ; MYKKE Consortium. Toward evidence-based diagnosis of myocarditis in children and adolescents: Rationale, design, and first baseline data of MYKKE, a multicenter registry and study platform. Am Heart J. 2017;187:133–144. doi: 10.1016/j.ahj.2017.02.027 [DOI] [PubMed] [Google Scholar]
- 530.Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581–1590. doi: 10.1161/CIRCULATIONAHA.105.606509 [DOI] [PubMed] [Google Scholar]
- 531.Zagrosek A, Abdel-Aty H, Boyé P, Wassmuth R, Messroghli D, Utz W, Rudolph A, Bohl S, Dietz R, Schulz-Menger J. Cardiac magnetic resonance monitors reversible and irreversible myocardial injury in myocarditis. JACC Cardiovasc Imaging. 2009;2:131–138. doi: 10.1016/j.jcmg.2008.09.014 [DOI] [PubMed] [Google Scholar]
- 532.Grün S, Schumm J, Greulich S, Wagner A, Schneider S, Bruder O, Kispert EM, Hill S, Ong P, Klingel K, et al. Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol. 2012;59:1604–15. doi: 10.1016/j.jacc.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 533.Schumm J, Greulich S, Wagner A, Grün S, Ong P, Bentz K, Klingel K, Kandolf R, Bruder O, Schneider S, et al. Cardiovascular magnetic resonance risk stratification in patients with clinically suspected myocarditis. J Cardiovasc Magn Reson. 2014;16:14. doi: 10.1186/1532-429X-16-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Mewton N, Dernis A, Bresson D, Zouaghi O, Croisille P, Flocard E, Douek P, Bonnefoy-Cudraz E. Myocardial biomarkers and delayed enhanced cardiac magnetic resonance relationship in clinically suspected myocarditis and insight on clinical outcome. J Cardiovasc Med (Hagerstown). 2015;16:696–703. doi: 10.2459/JCM.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 535.Aquaro GD, Perfetti M, Camastra G, Monti L, Dellegrottaglie S, Moro C, Pepe A, Todiere G, Lanzillo C, Scatteia A, et al. ; Cardiac Magnetic Resonance Working Group of the Italian Society of Cardiology. Cardiac MR With Late Gadolinium Enhancement in Acute Myocarditis With Preserved Systolic Function: ITAMY Study. J Am Coll Cardiol. 2017;70:1977–1987. doi: 10.1016/j.jacc.2017.08.044 [DOI] [PubMed] [Google Scholar]
- 536.Gräni C, Eichhorn C, Bière L, Murthy VL, Agarwal V, Kaneko K, Cuddy S, Aghayev A, Steigner M, Blankstein R, et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol. 2017;70:1964–76. doi: 10.1016/j.jacc.2017.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Sachdeva S, Song X, Dham N, Heath DM, DeBiasi RL. Analysis of clinical parameters and cardiac magnetic resonance imaging as predictors of outcome in pediatric myocarditis. Am J Cardiol. 2015;115:499–504. doi: 10.1016/j.amjcard.2014.11.029 [DOI] [PubMed] [Google Scholar]
- 538.Farinha IT, Miranda JO. Myocarditis in Paediatric Patients: Unveiling the Progression to Dilated Cardiomyopathy and Heart Failure. J Cardiovasc Dev Dis. 2016;3:E31. doi: 10.3390/jcdd3040031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Ghelani SJ, Spaeder MC, Pastor W, Spurney CF, Klugman D. Demographics, trends, and outcomes in pediatric acute myocarditis in the United States, 2006 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5:622–627. doi: 10.1161/CIRCOUTCOMES.112.965749 [DOI] [PubMed] [Google Scholar]
- 540.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287 [DOI] [PubMed] [Google Scholar]
- 541.D’Ambrosio A, Patti G, Manzoli A, Sinagra G, Di Lenarda A, Silvestri F, Sciascio G. The fate of acute myocarditis between spontaneous improvement and evolution to dilated cardiomyopathy: a review. Heart. 2001;85:499–504. doi: 10.1136/heart.85.5.499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Grosse-Wortmann L, Yun TJ, Al-Radi O, Kim S, Nii M, Lee KJ, Redington A, Yoo SJ, van Arsdell G. Borderline hypoplasia of the left ventricle in neonates: insights for decision-making from functional assessment with magnetic resonance imaging. J Thorac Cardiovasc Surg. 2008;136:1429–1436. doi: 10.1016/j.jtcvs.2008.04.027 [DOI] [PubMed] [Google Scholar]
- 543.Lofland GK, McCrindle BW, Williams WG, Blackstone EH, Tchervenkov CI, Sittiwangkul R, Jonas RA. Critical aortic stenosis in the neonate: a multi-institutional study of management, outcomes, and risk factors. Congenital Heart Surgeons Society. J Thorac Cardiovasc Surg. 2001;121:10–27. doi: 10.1067/mtc.2001.111207 [DOI] [PubMed] [Google Scholar]
- 544.Colan SD, McElhinney DB, Crawford EC, Keane JF, Lock JE. Validation and re-evaluation of a discriminant model predicting anatomic suitability for biventricular repair in neonates with aortic stenosis. J Am Coll Cardiol. 2006;47:1858–1865. doi: 10.1016/j.jacc.2006.02.020 [DOI] [PubMed] [Google Scholar]
- 545.Greutmann M, Tobler D, Biaggi P, Mah ML, Crean A, Oechslin EN, Silversides CK. Echocardiography for assessment of right ventricular volumes revisited: a cardiac magnetic resonance comparison study in adults with repaired tetralogy of Fallot. J Am Soc Echocardiogr. 2010;23:905–911. doi: 10.1016/j.echo.2010.06.013 [DOI] [PubMed] [Google Scholar]
- 546.Geva T. Is MRI the preferred method for evaluating right ventricular size and function in patients with congenital heart disease?: MRI is the preferred method for evaluating right ventricular size and function in patients with congenital heart disease. Circ Cardiovasc Imaging. 2014;7:190–197. doi: 10.1161/CIRCIMAGING.113.000553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Hudsmith LE, Petersen SE, Tyler DJ, Francis JM, Cheng AS, Clarke K, Selvanayagam JB, Robson MD, Neubauer S. Determination of cardiac volumes and mass with FLASH and SSFP cine sequences at 1.5 vs. 3 Tesla: a validation study. J Magn Reson Imaging. 2006;24:312–318. doi: 10.1002/jmri.20638 [DOI] [PubMed] [Google Scholar]
- 548.Bokma JP, de Wilde KC, Vliegen HW, van Dijk AP, van Melle JP, Meijboom FJ, Zwinderman AH, Groenink M, Mulder BJM, Bouma BJ. Value of Cardiovascular Magnetic Resonance Imaging in Noninvasive Risk Stratification in Tetralogy of Fallot. JAMA Cardiol. 2017;2:678–683. doi: 10.1001/jamacardio.2016.5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Moledina S, Pandya B, Bartsota M, Mortensen KH, McMillan M, Quyam S, Taylor AM, Haworth SG, Schulze-Neick I, Muthurangu V. Prognostic significance of cardiac magnetic resonance imaging in children with pulmonary hypertension. Circ Cardiovasc Imaging. 2013;6:407–414. doi: 10.1161/CIRCIMAGING.112.000082 [DOI] [PubMed] [Google Scholar]
- 550.Beygui F, Furber A, Delépine S, Helft G, Metzger JP, Geslin P, Le Jeune JJ. Routine breath-hold gradient echo MRI-derived right ventricular mass, volumes and function: accuracy, reproducibility and coherence study. Int J Cardiovasc Imaging. 2004;20:509–516. doi: 10.1007/s10554-004-1097-7 [DOI] [PubMed] [Google Scholar]
- 551.Clarke CJ, Gurka MJ, Norton PT, Kramer CM, Hoyer AW. Assessment of the accuracy and reproducibility of RV volume measurements by CMR in congenital heart disease. JACC Cardiovasc Imaging. 2012;5:28–37. doi: 10.1016/j.jcmg.2011.05.007 [DOI] [PubMed] [Google Scholar]
- 552.Heng EL, Gatzoulis MA, Uebing A, Sethia B, Uemura H, Smith GC, Diller GP, McCarthy KP, Ho SY, Li W, et al. Immediate and Midterm Cardiac Remodeling After Surgical Pulmonary Valve Replacement in Adults With Repaired Tetralogy of Fallot: A Prospective Cardiovascular Magnetic Resonance and Clinical Study. Circulation. 2017;136:1703–1713. doi: 10.1161/CIRCULATIONAHA.117.027402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Steeden JA, Kowalik GT, Tann O, Hughes M, Mortensen KH, Muthurangu V. Real-time assessment of right and left ventricular volumes and function in children using high spatiotemporal resolution spiral bSSFP with compressed sensing. J Cardiovasc Magn Reson. 2018;20:79. doi: 10.1186/s12968-018-0500-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Paelinck BP, Lamb HJ, Bax JJ, van der Wall EE, de Roos A. MR flow mapping of dobutamine-induced changes in diastolic heart function. J Magn Reson Imaging. 2004;19:176–181. doi: 10.1002/jmri.10448 [DOI] [PubMed] [Google Scholar]
- 555.Delfino JG, Bhasin M, Cole R, Eisner RL, Merlino J, Leon AR, Oshinski JN. Comparison of myocardial velocities obtained with magnetic resonance phase velocity mapping and tissue Doppler imaging in normal subjects and patients with left ventricular dyssynchrony. J Magn Reson Imaging. 2006;24:304–311. doi: 10.1002/jmri.20641 [DOI] [PubMed] [Google Scholar]
- 556.Khalaf A, Tani D, Tadros S, Madan S. Right- and left-ventricular strain evaluation in repaired pediatric Tetralogy of Fallot patients using magnetic resonance tagging. Pediatr Cardiol. 2013;34:1206–1211. doi: 10.1007/s00246-013-0631-6 [DOI] [PubMed] [Google Scholar]
- 557.Fogel MA, Gupta KB, Weinberg PM, Hoffman EA. Regional wall motion and strain analysis across stages of Fontan reconstruction by magnetic resonance tagging. Am J Physiol. 1995;269(3 Pt 2):H1132–H1152. doi: 10.1152/ajpheart.1995.269.3.H1132 [DOI] [PubMed] [Google Scholar]
- 558.Hor KN, Gottliebson WM, Carson C, Wash E, Cnota J, Fleck R, Wansapura J, Klimeczek P, Al-Khalidi HR, Chung ES, et al. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase image analysis. J Am Coll Cardiol Imgaging. 2010;3:144–51. doi: 10.1016/j.jcmg.2009.11.006 [DOI] [PubMed] [Google Scholar]
- 559.Schuster A, Hor KN, Kowallick JT, Beerbaum P, Kutty S. Cardiovascular Magnetic Resonance Myocardial Feature Tracking: Concepts and Clinical Applications. Circ Cardiovasc Imaging. 2016;9:e004077. doi: 10.1161/CIRCIMAGING.115.004077 [DOI] [PubMed] [Google Scholar]
- 560.Menting ME, van den Bosch AE, McGhie JS, Eindhoven JA, Cuypers JA, Witsenburg M, Geleijnse ML, Helbing WA, Roos-Hesselink JW. Assessment of ventricular function in adults with repaired Tetralogy of Fallot using myocardial deformation imaging. Eur Heart J Cardiovasc Imaging. 2015;16:1347–1357. doi: 10.1093/ehjci/jev090 [DOI] [PubMed] [Google Scholar]
- 561.Kalaitzidis P, Orwat S, Kempny A, Robert R, Peters B, Sarikouch S, Beerbaum P, Baumgartner H, Diller GP; Competence Network for Congenital Heart Defects, DZHK (German Center for Cardiovascular Research). Biventricular dyssynchrony on cardiac magnetic resonance imaging and its correlation with myocardial deformation, ventricular function and objective exercise capacity in patients with repaired tetralogy of Fallot. Int J Cardiol. 2018;264:53–57. doi: 10.1016/j.ijcard.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 562.Schmidt R, Orwat S, Kempny A, Schuler P, Radke R, Kahr PC, Hellige A, Baumgartner H, Diller GP. Value of speckle-tracking echocardiography and MRI-based feature tracking analysis in adult patients after Fontan-type palliation. Congenit Heart Dis. 2014;9:397–406. doi: 10.1111/chd.12156 [DOI] [PubMed] [Google Scholar]
- 563.van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos-Hesselink JW. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241–2247. doi: 10.1016/j.jacc.2011.08.025 [DOI] [PubMed] [Google Scholar]
- 564.Kilner PJ, Geva T, Kaemmerer H, Trindade PT, Schwitter J, Webb GD. Recommendations for cardiovascular magnetic resonance in adults with congenital heart disease from the respective working groups of the European Society of Cardiology. Eur Heart J. 2010;31:794–805. doi: 10.1093/eurheartj/ehp586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Antman EM, Marsh JD, Green LH, Grossman W. Blood oxygen measurements in the assessment of intracardiac left to right shunts: a critical appraisal of methodology. Am J Cardiol. 1980;46:265–271. doi: 10.1016/0002-9149(80)90068-5 [DOI] [PubMed] [Google Scholar]
- 566.Cloez JL, Schmidt KG, Birk E, Silverman NH. Determination of pulmonary to systemic blood flow ratio in children by a simplified Doppler echocardiographic method. J Am Coll Cardiol. 1988;11:825–830. doi: 10.1016/0735-1097(88)90218-5 [DOI] [PubMed] [Google Scholar]
- 567.Dittmann H, Jacksch R, Voelker W, Karsch KR, Seipel L. Accuracy of Doppler echocardiography in quantification of left to right shunts in adult patients with atrial septal defect. J Am Coll Cardiol. 1988;11:338–342. doi: 10.1016/0735-1097(88)90099-x [DOI] [PubMed] [Google Scholar]
- 568.Bradley SM, McCall MM, Sistino JJ, Radtke WA. Aortopulmonary collateral flow in the Fontan patient: does it matter? Ann Thorac Surg. 2001;72:408–415. doi: 10.1016/s0003-4975(01)02813-2 [DOI] [PubMed] [Google Scholar]
- 569.Glatz AC, Harrison N, Small AJ, Dori Y, Gillespie MJ, Harris MA, Fogel MA, Rome JJ, Whitehead KK. Factors associated with systemic to pulmonary arterial collateral flow in single ventricle patients with superior cavopulmonary connections. Heart. 2015;101:1813–1818. doi: 10.1136/heartjnl-2015-307703 [DOI] [PubMed] [Google Scholar]
- 570.Grosse-Wortmann L, Drolet C, Dragulescu A, Kotani Y, Chaturvedi R, Lee KJ, Mertens L, Taylor K, La Rotta G, van Arsdell G, et al. Aortopulmonary collateral flow volume affects early postoperative outcome after Fontan completion: a multimodality study. J Thorac Cardiovasc Surg. 2012;144:1329–1336. doi: 10.1016/j.jtcvs.2012.03.032 [DOI] [PubMed] [Google Scholar]
- 571.Odenwald T, Quail MA, Giardini A, Khambadkone S, Hughes M, Tann O, Hsia TY, Muthurangu V, Taylor AM. Systemic to pulmonary collateral blood flow influences early outcomes following the total cavopulmonary connection. Heart. 2012;98:934–940. doi: 10.1136/heartjnl-2011-301599 [DOI] [PubMed] [Google Scholar]
- 572.Heinemann M, Breuer J, Steger V, Steil E, Sieverding L, Ziemer G. Incidence and impact of systemic venous collateral development after Glenn and Fontan procedures. Thorac Cardiovasc Surg. 2001;49:172–178. doi: 10.1055/s-2001-14339 [DOI] [PubMed] [Google Scholar]
- 573.McElhinney DB, Reddy VM, Hanley FL, Moore P. Systemic venous collateral channels causing desaturation after bidirectional cavopulmonary anastomosis: evaluation and management. J Am Coll Cardiol. 1997;30:817–824. doi: 10.1016/s0735-1097(97)00223-4 [DOI] [PubMed] [Google Scholar]
- 574.Bramwell C, Jones AM. COARCTATION OF THE AORTA: THE COLLATERAL CIRCULATION. Br Heart J. 1941;3:205–227. doi: 10.1136/hrt.3.4.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Greil G, Geva T, Maier SE, Powell AJ. Effect of acquisition parameters on the accuracy of velocity encoded cine magnetic resonance imaging blood flow measurements. J Magn Reson Imaging. 2002;15:47–54. doi: 10.1002/jmri.10029 [DOI] [PubMed] [Google Scholar]
- 576.Muthurangu V, Taylor A, Andriantsimiavona R, Hegde S, Miquel ME, Tulloh R, Baker E, Hill DL, Razavi RS. Novel method of quantifying pulmonary vascular resistance by use of simultaneous invasive pressure monitoring and phase-contrast magnetic resonance flow. Circulation. 2004;110:826–834. doi: 10.1161/01.CIR.0000138741.72946.84 [DOI] [PubMed] [Google Scholar]
- 577.Beerbaum P, Körperich H, Barth P, Esdorn H, Gieseke J, Meyer H. Noninvasive quantification of left-to-right shunt in pediatric patients: phase-contrast cine magnetic resonance imaging compared with invasive oximetry. Circulation. 2001;103:2476–2482. doi: 10.1161/01.cir.103.20.2476 [DOI] [PubMed] [Google Scholar]
- 578.Debl K, Djavidani B, Buchner S, Heinicke N, Poschenrieder F, Feuerbach S, Riegger G, Luchner A. Quantification of left-to-right shunting in adult congenital heart disease: phase-contrast cine MRI compared with invasive oximetry. Br J Radiol. 2009;82:386–391. doi: 10.1259/bjr/18500608 [DOI] [PubMed] [Google Scholar]
- 579.Hundley WG, Li HF, Lange RA, Pfeifer DP, Meshack BM, Willard JE, Landau C, Willett D, Hillis LD, Peshock RM. Assessment of left-to-right intracardiac shunting by velocity-encoded, phase-difference magnetic resonance imaging. A comparison with oximetric and indicator dilution techniques. Circulation. 1995;91:2955–2960. doi: 10.1161/01.cir.91.12.2955 [DOI] [PubMed] [Google Scholar]
- 580.Prompona M, Muehling O, Naebauer M, Schoenberg SO, Reiser M, Huber A. MRI for detection of anomalous pulmonary venous drainage in patients with sinus venosus atrial septal defects. Int J Cardiovasc Imaging. 2011;27:403–412. doi: 10.1007/s10554-010-9675-3 [DOI] [PubMed] [Google Scholar]
- 581.Valente AM, Sena L, Powell AJ, Del Nido PJ, Geva T. Cardiac magnetic resonance imaging evaluation of sinus venosus defects: comparison to surgical findings. Pediatr Cardiol. 2007;28:51–56. doi: 10.1007/s00246-006-1477-y [DOI] [PubMed] [Google Scholar]
- 582.Beerbaum P, Körperich H, Esdorn H, Blanz U, Barth P, Hartmann J, Gieseke J, Meyer H. Atrial septal defects in pediatric patients: noninvasive sizing with cardiovascular MR imaging. Radiology. 2003;228:361–369. doi: 10.1148/radiol.2282020798 [DOI] [PubMed] [Google Scholar]
- 583.Teo KS, Disney PJ, Dundon BK, Worthley MI, Brown MA, Sanders P, Worthley SG. Assessment of atrial septal defects in adults comparing cardiovascular magnetic resonance with transoesophageal echocardiography. J Cardiovasc Magn Reson. 2010;12:44. doi: 10.1186/1532-429X-12-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Thomson LE, Crowley AL, Heitner JF, Cawley PJ, Weinsaft JW, Kim HW, Parker M, Judd RM, Harrison JK, Kim RJ. Direct en face imaging of secundum atrial septal defects by velocity-encoded cardiovascular magnetic resonance in patients evaluated for possible transcatheter closure. Circ Cardiovasc Imaging. 2008;1:31–40. doi: 10.1161/CIRCIMAGING.108.769786 [DOI] [PubMed] [Google Scholar]
- 585.Bhatla P, Tretter JT, Ludomirsky A, Argilla M, Latson LA, Jr, Chakravarti S, Barker PC, Yoo SJ, McElhinney DB, Wake N, et al. Utility and Scope of Rapid Prototyping in Patients with Complex Muscular Ventricular Septal Defects or Double-Outlet Right Ventricle: Does it Alter Management Decisions? Pediatr Cardiol. 2017;38:103–114. doi: 10.1007/s00246-016-1489-1 [DOI] [PubMed] [Google Scholar]
- 586.Hauck A, Porta N, Lestrud S, Berger S. The Pulmonary Circulation in the Single Ventricle Patient. Children (Basel). 2017;4:E71. doi: 10.3390/children4080071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Mirsadraee S, Satou GM, Renella P, Hashmi A, Laks H, Finn JP. Magnetic resonance angiography in paced complex heterotaxy syndrome with Fontan conduit obstruction and venovenous collateral decompression. Congenit Heart Dis. 2013;8:E31–E35. doi: 10.1111/j.1747-0803.2011.00599.x [DOI] [PubMed] [Google Scholar]
- 588.Bellsham-Revell HR, Tibby SM, Bell AJ, Witter T, Simpson J, Beerbaum P, Anderson D, Austin CB, Greil GF, Razavi R. Serial magnetic resonance imaging in hypoplastic left heart syndrome gives valuable insight into ventricular and vascular adaptation. J Am Coll Cardiol. 2013;61:561–570. doi: 10.1016/j.jacc.2012.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Thomson LE, Kim RJ, Judd RM. Magnetic resonance imaging for the assessment of myocardial viability. J Magn Reson Imaging. 2004;19:771–788. doi: 10.1002/jmri.20075 [DOI] [PubMed] [Google Scholar]
- 590.Rathod RH, Powell AJ, Geva T. Myocardial Fibrosis in Congenital Heart Disease. Circ J. 2016;80:1300–1307. doi: 10.1253/circj.CJ-16-0353 [DOI] [PubMed] [Google Scholar]
- 591.Broberg CS, Prasad SK, Carr C, Babu-Narayan SV, Dimopoulos K, Gatzoulis MA. Myocardial fibrosis in Eisenmenger syndrome: a descriptive cohort study exploring associations of late gadolinium enhancement with clinical status and survival. J Cardiovasc Magn Reson. 2014;16:32. doi: 10.1186/1532-429X-16-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Tworetzky W, del Nido PJ, Powell AJ, Marshall AC, Lock JE, Geva T. Usefulness of magnetic resonance imaging of left ventricular endocardial fibroelastosis in infants after fetal intervention for aortic valve stenosis. Am J Cardiol. 2005;96:1568–1570. doi: 10.1016/j.amjcard.2005.07.066 [DOI] [PubMed] [Google Scholar]
- 593.Latus H, Gummel K, Rupp S, Mueller M, Jux C, Kerst G, Akintuerk H, Bauer J, Schranz D, Apitz C. Cardiovascular magnetic resonance assessment of ventricular function and myocardial scarring before and early after repair of anomalous left coronary artery from the pulmonary artery. J Cardiovasc Magn Reson. 2014;16:3. doi: 10.1186/1532-429X-16-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Schmitt B, Bauer S, Kutty S, Nordmeyer S, Nasseri B, Berger F, Alexi-Meskishvili V. Myocardial perfusion, scarring, and function in anomalous left coronary artery from the pulmonary artery syndrome: a long-term analysis using magnetic resonance imaging. Ann Thorac Surg. 2014;98:1425–1436. doi: 10.1016/j.athoracsur.2014.05.031 [DOI] [PubMed] [Google Scholar]
- 595.Latus H, Gummel K, Klingel K, Moysich A, Khalil M, Mazhari N, Bauer J, Kandolf R, Schranz D, Apitz C. Focal myocardial fibrosis assessed by late gadolinium enhancement cardiovascular magnetic resonance in children and adolescents with dilated cardiomyopathy. J Cardiovasc Magn Reson. 2015;17:34. doi: 10.1186/s12968-015-0142-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.