Akmeriç and Gerhardt highlight work from Coon et al., which shows that sustained laminar shear stress regulates Klf2 expression in endothelial cells via mitochondrial remodelling and mitophagy.
Abstract
Since its discovery as a mechanosensitive transcription factor in endothelial networks, Klf2’s varying expression levels under different blood flow patterns remained a mystery. In this study, Coon et al. (2022. J. Cell Biol. https://doi.org/10.1083/jcb.202109144) discover a connection between sustained laminar shear stress and mitochondrial flux that contributes to Klf2’s transcriptional dynamics.
Vascular networks continuously adapt to changing tissue requirements. Endothelial cells lining their inner surface read and integrate chemical and mechanical signals in a local manner; failure to do so drives endothelial dysfunction, causing the majority of known cardiovascular disease manifestations. A key response of endothelial cells to blood flow–mediated sustained laminar shear stress (LSS) is the induced expression of the transcription factor Krüppel-like factor 2 (Klf2), downstream elements of which activate a plethora of anti-inflammatory and anti-oxidative genetic programs, providing the cellular environment for sustained vascular homeostasis (1). Klf2 expression dramatically decreases around regions of curving or bifurcating vasculature, where the direction and speed of flow becomes disturbed, making these regions prone to inflammatory vascular diseases such as atherosclerosis (2). The underlying mechanism that mediates this change has been one of the major outstanding questions of vascular medicine over the past two decades with only a few components successfully identified.
To systematically unravel the molecular pathways affecting Klf2 expression under flow, Coon and colleagues (3) employed a high throughput CRISPR knockout (KO) approach in conjunction with in vitro perfusion endothelial cell culture and an ingenious synthetic Klf2 reporter. Expressed under the control of the Klf2 promoter, a modified GFP with a half-life similar to that of Klf2 protein allowed accurate quantification of Klf2 expression in live experiments while knocking out single genes. Using quantitative binning strategies via cell sorting, the authors generate an exhaustive list of hundreds of genes that modulate Klf2 activity. Some of the top hits include members of the MEKK2/3-MEK5-ERK5 pathway, confirming previous findings of this pathway to be a key mechanosensitive inducer of Klf2 expression (4). Intriguingly, beyond the few known genes involved in responses to LSS, the list highlights oxidative stress and mitophagy, pointing to a novel role of metabolic pathways in the regulation of Klf2 expression.
To assess potential interdependencies between the ERK5 pathway, the genes from the CRISPR screen, and Klf2 expression, the authors probe phosphorylation levels of MEKK2/3 (indicating downstream signal transduction) and Erk5 nuclear localization (indicating Erk5 activity) at high temporal resolution. They report a striking observation: while LSS triggers a sustained MEKK2/3 activation, Erk5 activity reaches a transient peak at 20 min and only becomes active in a sustained manner after a delay of 3 h. This biphasic activation profile turns out to depend on the presence of (among others) the autophagy receptor p62(Sqmt1), the mitochondrial calcium uniporter EMRE(Smdt1), and the mitophagy inducing ubiquitin kinase PINK1, suggesting that mitochondrial activity and Erk5-induced Klf2 expression are connected.
p62 is known for its involvement in autophagy, reactive oxygen species (ROS) signaling, and processing ubiquitinated proteins (5). The authors employ immunofluorescence and pulldown assays to show that p62 forms protein complexes with MEK5, NIPSNAPs, and TMEM160, the latter two of which are needed for targeting damaged mitochondria for degradation (6). With all prior findings pointing toward oxidative stress under LSS, they also determine the origin of ROS production to be mitochondrial and calcium induced under shear, using ROS sensitive protein sensors, calcium assays, and organelle-specific ROS quenchers. This mitochondrial ROS production reaches a sustained plateau around the same time the first Erk5 peak occurs at 20 min, followed by increased mitochondrial fission at 1.5 h. This elevation is then brought to lower levels than the pre-shear state through sustained mitophagy. Remarkably, this work demonstrates that LSS induces a biphasic activation response of both Erk5 and mitochondrial ROS production. The latter is driven by the mitochondrial calcium uniporter EMRE. Knocking out the ubiquitin kinase PINK1 decreased the mitophagic flux, and therefore LSS induced mitochondrial remodeling.
In vivo examination of the mouse aorta mirrors in vitro observations, with areas such as the inner curvature of the aortic arch (IC) that inherently has low/disturbed shear and low Klf2 expression also having low Erk5 activation. In PINK KO mice, Erk5/Klf2 activity is selectively reduced in aortic regions experiencing LSS. Whereas previous studies identified that the autophagy machinery is important for endothelial function under shear stress (7), Coon and colleagues now show that mitochondrial remodelling, and specifically mitophagy, is essential for high Klf2 expression, protecting endothelial function.
To understand the detailed molecular connection between mitophagy and increased Erk5 activity, the authors turn their attention to p62, specifically its PB1 domain with acidic and basic patches (8). In p62 KO lines with reduced Klf2 expression, they show that while WT p62 expression can rescue Klf2 levels through MEK5 binding, expression of a p62 mutant with a basic patch neutralized PB1 domain could not. They further characterize the expression and cellular profile of p62 and observe its increased expression following LSS, as well as increased accumulation of p62 around mitochondria. This is shown to be induced by redox-sensitive NRF2, which is also upregulated by LSS. However, KO of NRF2 only partially reduces p62 accumulation around mitochondria, suggesting the involvement of a post-transcriptional mechanism. p62 is known to be an inactive dimer until it binds to its ubiquinated cargo (9). Using an antibody that is specific for this cargo interaction, the authors observe increased signal levels under LSS. These experiments collectively demonstrate the molecular mechanism through which active p62, bound to damaged mitochondria, upregulates Erk5 following the onset of mitophagy (schematized in Fig. 1).
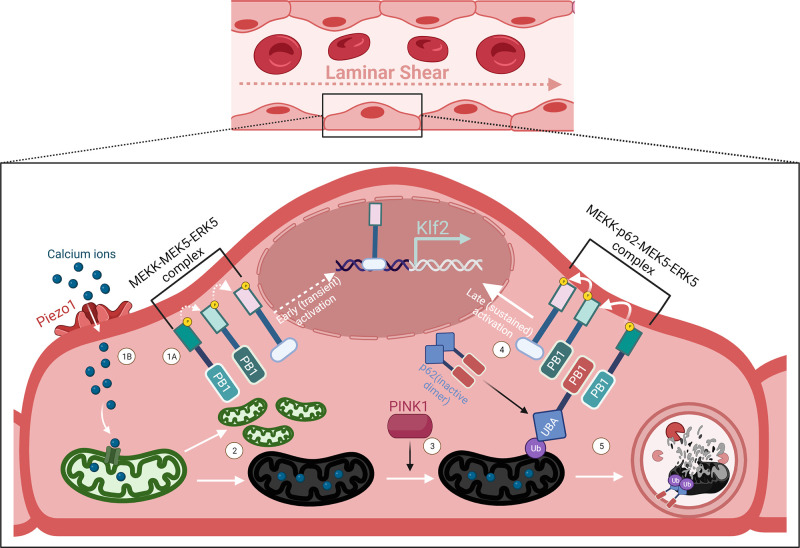
Figure 1.
Erk5-induced Klf2 expression follows a biphasic expression profile under flow. At the onset of laminar blood flow, Erk5-induced Klf2 expression occurs in two distinct pathways. The first occurs through MEKK2/3-MEK5-ERK5 complex’s phosphorylation cascade and Erk5 nuclear shuttling (1A), upstream of which is hypothesized to be mechanosensitive. This transient signal reaches an activity maximum following 20 min of LSS and then subsides. Around the same time, LSS induces opening of mechanosensitive calcium channels such as Piezo1 (1B). Mitochondrial calcium uniporters open in this calcium rich environment, causing the organelle to rapidly divide at the expense of increased mitochondrial ROS, resulting in some mitochondria to lose function (2). Through PINK1 signaling, these damaged mitochondria become ubiquitinated, enabling the autophagosome receptor p62 to switch to its active monomer form by binding to its ubiquitinated cargo (3). In this form, p62 enhances Erk5 signaling by binding to both MEKK2/3 and MEK5 through its PB1 domain, providing sustained Erk5-induced Klf2 expression (4). p62 subsequently shuttles its bound mitochondria to autophagosome, producing mitophagic flux under LSS (5).
Putting their LSS induced mitophagy model to test in different flow conditions, Coon and colleagues observe that while MEKK2/3 is equally activated in LSS and oscillatory shear stress (OSS) conditions, Erk5 activity is highly impaired in OSS conditions. Strikingly, they observe that Erk5 activity, relative p62 accumulation at mitochondria, and MEKK2-MEK5 binding are all highly disrupted in OSS conditions. These findings identify that oxidized mitochondria, which are not cleared in endothelial cells exposed to OSS, disrupt Klf2 expression levels.
This paper serves as a comprehensive exposé on how metabolic systems not only crosstalk with blood flow sensitive signaling in vessels, but also serve to amplify their signals. Mechanotransduction is a process involving major deformations, cytoskeletal rearrangements, and active migration, requiring a high amount of energy that produces oxidation (10). In the case of laminar flow induced atheroprotection, the downstream signals of Klf2 and Nrf2 serve to reduce inherently oxidative effects of this process, corroborating previous findings that LSS increases expression of mitochondrial antioxidant enzymes, while establishing the mechanism through which this occurs (11). As the authors also point out, this transient increase in cellular oxidation could be a general adaptive stressor required for downstream homeostasis, explaining why high ROS-producing conditions such as hyperglycemia are often major risk factors for atherosclerosis.
Overall, the proposed mechanistic model for Klf2 activation and function by Coon and colleagues establishes an intriguing connection between metabolic and biomechanical effectors of shear stress and will undoubtedly stimulate further characterization of the functional importance of cellular recycling processes such as mitophagy in regulating and maintaining endothelial homeostasis.
Acknowledgments
The authors declare no competing financial interests.
The schematic figure has been created with BioRender.com.
References
- 1.SenBanerjee, S., et al. 2004. J. Exp. Med. 10.1084/jem.20031132 [DOI] [Google Scholar]
- 2.Davies, P.F., et al. 2013. Cardiovasc. Res. 10.1093/cvr/cvt101 [DOI] [Google Scholar]
- 3.Coon, B.G., et al. 2022. J. Cell. Biol. 10.1083/jcb.202109144 [DOI] [Google Scholar]
- 4.Parmar, K.M., et al. 2006. J. Clin. Invest. 10.1172/jci24787 [DOI] [Google Scholar]
- 5.Sánchez-Martín, P., and Komatsu, M.. 2018. J. Cell Sci. 10.1242/jcs.222836 [DOI] [PubMed] [Google Scholar]
- 6.Abudu Y.K., et al. 2019. Autophagy. 10.1080/15548627.2019.1637642 [DOI] [Google Scholar]
- 7.Vion, A.C., et al. 2017. Proc. Natl. Acad. Sci. USA. 10.1073/pnas.1702223114 [DOI] [Google Scholar]
- 8.Nakamura, K., et al. 2010. J. Biol. Chem. 10.1074/jbc.m109.065102 [DOI] [Google Scholar]
- 9.Long, J., et al. 2010. J. Mol. Biol. 10.1016/j.jmb.2009.11.032 [DOI] [Google Scholar]
- 10.Chatterjee, S. 2018. Front Physiol. 10.3389/fphys.2018.00524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu, L. 2017. J. Cell Physiol. 10.1002/jcp.26375 [DOI] [Google Scholar]