Abstract
Objectives
The immune system plays a critical defence role against infections, injuries, and carcinogenic stimuli. As the macrophages of the brain resides in the innate immune system, microglia and their polarisation (M1/M2) play regulatory roles in inflammation in CNS, such as Parkinson's, Alzheimer's, dementia complex, and multiple sclerosis. Nigella sativa belongs to the Ranunculaceae family and has different anti-inflammatory and antioxidant effects. We conducted this study to evaluate the anti-inflammatory and protective properties of N. sativa oil (NSO) on the microglial cells and their polarisation (M1/M2) in the presence of LPS as a model of neuroinflammation.
Methods
The protective effects of NSO (10–40 µg/ml) were studied on the LPS-induced microglial cells, and the levels of tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, prostaglandin E2 (PGE2), and IL-10 were evaluated using both ELISA and gene expression methods. The levels of cyclooxygenase-2 (COX-2), inducible NOS (iNOS), and arginase-1 (Arg1) were also evaluated using the real-time PCR method. In addition, nitrite oxide (NO) and urea were measured using biochemical methods.
Results
NSO decreased LPS-induced toxicity at all doses (P < 0.001). NSO (10–40 μg/ml) also significantly reduced the levels of TNF-α, PGE2, IL-1β, and IL-6 in the presence of LPS (P < 0.01 to 0.001). Pretreatment with NSO attenuated the levels of iNOS but increased Arg1 (P < 0.001). The ratio of iNOS/Arg1 was also decreased in the presence of NSO (P < 0.001) than that of the LPS group (P < 0.001).
Conclusion
NSO attenuated LPS-induced inflammation and increased microglia's anti-inflammatory status. These results may prove that NSO is potentially an immunomodulator for various neurodegenerative diseases by M1 phenotype dominancy, such as Alzheimer's and Parkinson's diseases.
1. Introduction
Generally, the immune system plays a key role in defending our body against infections and injuries as well as carcinogenic stimuli. In contrast, overactivation of the immune system leads to exceeded inflammation, regarding oxidative and inflammatory mediators, and host injuries consisting of autoimmune diseases and allergic conditions as well as cancers [1–3]. Inflammatory diseases have been growing all over the world during the past two decades, which assigned a new research line to them [4]. One of the essential inflammation research targets has ideally been arranged to the immune system and the innate immune system for attributed focal diseases, such as central nervous system (CNS) inflammatory diseases, including Parkinson's and Alzheimer's diseases [4–6].
Microglia are an essential part of the brain's innate immune system and play crucial regulatory roles in CNS inflammation [7–9]. Upon stimulation with a plethora, microglial cells get activated and begin a cascade of inflammation in the CNS. These cells also contribute to the pathogenesis of some diseases, such as Parkinson's disease, Alzheimer's disease, AIDS, dementia complex, multiple sclerosis, and ischemia [10–12]. Following the microglia activation, the liberation of some inflammatory mediators, including nitric oxide (NO), inducible NO synthase (iNOS), interleukin (IL)-1β, IL-6, and tumour necrosis factor-α (TNF-α) is increased in the CNS microenvironments, in which they possess an important role in proceeding neurodegenerative diseases [9, 12, 13].
Lipopolysaccharides (LPS), by acting on toll-like receptor-4 (TLR-4), lead to the release of proinflammatory and neurotoxic agents and differentiate macrophages into inflammatory type 1 macrophages (M1) [14–19]. Given that this type of macrophages deserves an essential role against injuries and noxious stimuli, including bacterial and viral infections as well as tumour cells, overactivation of the cells causes inflammatory diseases, such as neurodegeneration, rheumatoid arthritis, multiple sclerosis, and other autoimmune diseases [5, 16, 17]. In contrast, type 2 macrophage (M2) cells, which are also known for their action as healing macrophages, produce anti-inflammatory mediators consisting of IL-10 and IL-4 as well as highly expressed arginase 1 (Arg-1) to provide urea from the catabolism of arginine [6, 12, 16, 17].
Nigella sativa belongs to the Ranunculaceae family and grows in Southwest Asia. N. sativa has been considered as an herbal medicine in Islamic culture and in Avicenna's famous book, Qanun [20]. In this regard, it has been mentioned that the seeds of N. sativa act as traditional remedies for the treatment of different neurological-based diseases, such as memory impairment, epilepsy, pain, and neurotoxicities, as well as Alzheimer's (AD) and Parkinson's (PD) diseases [20, 21]. Phytochemical analysis has reported the presence of different chemical compounds in N. sativa, such as phospholipids [22], fatty acids [23], vitamins [24], and ascorbic acid [25]. Also, other compounds have been found in N. sativa, including dithymoquinone, thymoquinone (TQ), thymol, and carvacrol, which have therapeutic effects as an analgesic [26], antioxidant [27], and anticancer as well as immune-modulatory activities [28–30]. Furthermore, recent studies have reported anti-inflammatory effects of N. sativa extract and its active compounds on different inflammation models, such as rheumatoid arthritis in rat models [31], eicosanoid generation in leukocytes [32], allergic lung inflammation in a mouse model [33], carrageenan-induced paw oedema [34], ulcerative colitis [35], mouse dendritic cells [36], allergic encephalomyelitis as an animal model for multiple human sclerosis [37], and nitric oxide production by murine macrophages [38].
LPS-induced microglia activation is an appropriate model for in vitro investigation to understand related mechanisms that play a considerable role in CNS inflammatory disease [6, 39]. Furthermore, due to the importance of microglial activation during neuroinflammatory diseases, regulating these cells can be considered a therapeutic pathway. Moreover, in the light of the knowledge that N. sativa concomitantly suggested in traditional medicines and experimental and pharmacological experiments, we conducted the current in vitro study to evaluate the direct anti-inflammatory and protective properties of N. sativa oil (NSO) on the microglial cells' polarisation (M1/M2) in the presence of LPS as a model of neuroinflammation.
2. Materials and Methods
2.1. Chemicals, Reagents, and Kits
DMEM/F12 media culture, penicillin plus streptomycin (pen/strep), amphotericin B, FBS, DMSO, Ficoll, DNAse I, Dispase II, LPS, and other cell culture materials were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). RBC lysis buffer (10x, Cat No: 420301) was purchased from Biolegend company (San Diego, CA, USA). Proliferation assay kit (MTT) and ELISA kits (PGE2, IL-6, IL-1β, IL-10, and TNF-α) were purchased from Roche Diagnostics (Mannheim, Germany) and eBioscience (San Diego, CA, USA), respectively. All the other materials were of analytical and cell culture grade and were prepared from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
2.2. Preparation of N. sativa Seed Oil Extract (NSO)
N. sativa seeds were purchased from the local market in Mashhad, Iran. 10 g of the dried and powdered seeds were weighed, and its oil was prepared using a cold press with no solvent or heat exposure. The pressing process was carried out at a temperature lower than 35°C in chrome-nickel cold press oil squeezing machines (Household Oil Press, Oily® YD-ZY-03A, Germany). The oil was filtered through 100% cotton filters with a thickness of 2 mm, which was free from dense particles and kept at −20°C until use. The efficiency was 29.5% w/w, which is due to the weight of the dried leaves. 50 mg of extract was dissolved in 1 ml of complete DMEM/F12 (10% FBS + 1% Pen/Strep) plus 1% DMSO to prepare stock at 50 mg/ml concentration. Experimental concentrations were prepared from this stock with DMEM/F12 enriched and 10% FBS and 1% Pen/Strep. The final level of DMSO was lower than 0.1% v/v for tested concentrations.
2.3. Mice Microglia Isolation and Cell Culture
Primary microglial cells were prepared in accordance with the previously described Lee and Tansey method [12, 14, 17, 18]. Microglia cells were cultured in DMEM/F12 plus 1% v/v of Pen/Strep (100×) and 10% v/v of heat-inactivated FBS supplemented with 0.5 µg/mL amphotericin B and 2 mL glutamine. Cells were maintained in a humidified incubator at 37°C and 5% v/v CO2.
2.4. Protocol of the Experimental Procedure
Inflammatory condition on microglia was induced by the addition of LPS (1 µg/ml). The following groups indicate study groups:
Group one: microglia + vehicle (control group)
Group two: microglia + LPS (1 µg/ml)
Group three: microglia + highest concentration of NSO (40 µg/ml)
Groups four to seven: microglia + NSO (10, 20, and 40 µg/ml) + after two hours LPS (1 µg/ml)
The final concentration of DMSO was lower than 0.1% v/v for all experiments.
2.5. Cell Proliferation Assay
The effects of various concentrations of NSO were examined in the presence or absence of LPS on microglia. In brief, about 5000 cells were seeded in a 96-well plate and treated with NSO (0–160 µg/ml) and NSO (0–160 µg/ml) + LPS (1 µg/ml) and incubated for 48 hours at 37°C in a 5% v/v CO2 incubator. After 48 hours, 10 μl of MTT reagent (5 mg/ml) was added to each well, which was incubated for the next three hours. Formazan crystals were dissolved in 100 μL DMSO, and the absorbance was read using a StatFAX 2100 ELISA plate reader (Awareness Inc, USA) at 570 nm in reference with 620 nm.
2.6. Cytokine Assays
Indexing of the inflammatory state induced by LPS and inflammatory cytokines including TNF-α, IL-1β, and IL-6, as well as PGE2, were examined using the sandwich ELISA method based on the manufacturer's instructions. The microglial cells were cultured at a density of 106 cells per 6-well plate and incubated with different concentrations of NSO, based on the experimental design section. The supernatant was collected after 48 hours of incubation, and various cytokines were measured.
2.7. Quantitative Real-Time PCR (qRT-PCR)
To evaluate the effect of different concentrations of NSO on gene expression, the levels of related mRNA of TNF-α, IL-1β, IL-6, COX-2, iNOS, and Arg1 were also examined. GAPDH was considered the housekeeping gene. The primer sequences are mentioned in Table 1. The relative quantity of each mRNA was normalised to the relative quantity of GAPDH mRNA. The PCR conditions were as follows: 95°C for three minutes, followed by 40 cycles of 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds, respectively [14, 40]. The values for gene expression levels were examined using the ΔCt method, and fold-change values were reported as 2−(ΔΔCt).
Table 1.
The primers for real-time PCR.
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) | Ref. |
|---|---|---|---|
| Arg1 | CATGGGCAACCTGTGTCCTT | TCCTGGTACATCTGGGAACTTTC | [19] |
| iNOS | GACGAGACGGATAGGCAGAG | GTGGGGTTGTTGCTGAACTT | [19] |
| TNF-α | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC | [64] |
| IL-1β | GGAGAACCAAGCAACGACAAAATA | TGGGGAACTCTGCAGACTCAAAC | [40] |
| IL-6 | GTTTTCTGCAAGTGCATCATCG | GGTTTCTGCAAGTGCATCATCG | [40] |
| COX-2 | GCTGCCCGACACCTTCAACATT | CACATTTCTTCCCCCAGCAACC | [40] |
| GAPDH | GGAGGAACCTGCCAAGTATG | TGGGAGTTGCTGTCCTGGGCTGCACT | [40] |
2.8. Nitric Oxide and Urea Assay
The amount of nitrite was measured as an indicator of the concentration of the produced nitric oxide by using the method of Griess as described previously [41]. The supernatants, which were collected for cytokines assay, were used to examine the concentration of nitrite produced by the microglia cells at 540 nm using Griess reagent (G4410 SIGMA) in a spectrophotometer. The nitrite concentration was determined using the sodium nitrite standard curve [1]. Based on the manufacturer's instructions, urea was also detected using a colorimetric assay kit from Abcam (catalogue no. ab83362).
2.9. Statistical Analysis
Data were prepared as means ± SEM and analysed by GraphPad Prism® 6 software (GraphPad Software, San Diego, CA). Comparisons between groups were performed by using a one-way analysis of variance (ANOVA) with the Tukey–Kramer post hoc test. The significance was approached at P < 0.05, <0.01, and 0.01, respectively. Raw data were also reported in Tables 2 and 3.
Table 2.
The raw data (mean ± SEM) for MTT assays in the presence and absence of LPS stimulation.
| Control | LPS | NSO (μg/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 5 | 10 | 20 | 40 | 80 | 160 | |||
| MTT | |||||||||
| Mean | 103.2 | — | 100.7 | 100.2 | 101.3 | 101.7 | 100.8 | 100.8 | 101.2 |
| SEM | 4.102 | — | 1.256 | 1.376 | 1.202 | 1.282 | 1.327 | 2.330 | 2.386 |
|
| |||||||||
| MTT + LPS | |||||||||
| Mean | 101.2 | 67.47 | 82.82 | 85.56 | 101.2 | 100.0 | 100.8 | 101.2 | 102.5 |
| SEM | 1.138 | 2.415 | 2.631 | 2.816 | 1.138 | 1.342 | 2.330 | 2.386 | 2.513 |
Table 3.
The raw data (mean ± SEM) for ELISA and gene expression and biochemical assays in the presence of LPS stimulation.
| Control | LPS | LPS + NSO (μg/ml) | NSO (μg/ml) | |||
|---|---|---|---|---|---|---|
| 10 | 20 | 40 | 40 | |||
| PGE2 | ||||||
| Mean | 43.50 | 413.3 | 329.3 | 276.2 | 205.8 | 42.17 |
| SEM | 5.536 | 20.67 | 15.26 | 17.09 | 21.70 | 5.952 |
|
| ||||||
| TNF-α | ||||||
| Mean | 154.2 | 1113 | 847.5 | 621.2 | 353.2 | 145.0 |
| SEM | 11.73 | 64.11 | 33.85 | 30.51 | 32.57 | 13.14 |
|
| ||||||
| IL-1β | ||||||
| Mean | 117.2 | 885.0 | 658.2 | 395.0 | 219.0 | 118.2 |
| SEM | 5.759 | 29.90 | 19.17 | 11.50 | 14.67 | 8.332 |
|
| ||||||
| IL-6 | ||||||
| Mean | 171.0 | 1050 | 773.8 | 588.2 | 413.3 | 174.8 |
| SEM | 6.826 | 34.13 | 13.88 | 20.77 | 6.427 | 7.414 |
|
| ||||||
| TNF-α gene | ||||||
| Mean | 1.000 | 7.420 | 5.650 | 4.141 | 2.354 | 0.9667 |
| SEM | 0.07821 | 0.4274 | 0.2257 | 0.2034 | 0.2171 | 0.08760 |
|
| ||||||
| COX-2 gene | ||||||
| Mean | 1.000 | 8.267 | 6.587 | 5.523 | 4.117 | 0.8433 |
| SEM | 0.1107 | 0.4133 | 0.3053 | 0.3419 | 0.4341 | 0.1190 |
|
| ||||||
| IL-1β gene | ||||||
| Mean | 1.000 | 8.850 | 6.580 | 3.948 | 2.190 | 1.182 |
| SEM | 0.05759 | 0.2990 | 0.1920 | 0.1152 | 0.1467 | 0.08332 |
|
| ||||||
| IL-6 gene | ||||||
| Mean | 1.000 | 6.560 | 4.837 | 3.675 | 2.583 | 1.093 |
| SEM | 0.04272 | 0.2135 | 0.08645 | 0.1298 | 0.04017 | 0.04633 |
|
| ||||||
| iNOS gene | ||||||
| Mean | 1.000 | 6.220 | 4.586 | 3.485 | 2.449 | 1.036 |
| SEM | 0.04050 | 0.2024 | 0.08196 | 0.1231 | 0.03809 | 0.04393 |
|
| ||||||
| Arg-1 gene | ||||||
| Mean | 1.006 | 0.6854 | 1.614 | 1.986 | 2.958 | 0.9970 |
| SEM | 0.04350 | 0.03525 | 0.06462 | 0.07948 | 0.1184 | 0.04415 |
|
| ||||||
| iNOS/Arg-1 | ||||||
| Mean | 1.000 | 8.200 | 2.850 | 1.750 | 0.8333 | 1.050 |
| SEM | 0.05164 | 0.5727 | 0.1648 | 0.09574 | 0.04216 | 0.04282 |
|
| ||||||
| NO | ||||||
| Mean | 4.500 | 16.75 | 12.17 | 9.625 | 6.333 | 4.792 |
| SEM | 0.2661 | 0.8490 | 0.4014 | 0.2940 | 0.2108 | 0.6338 |
|
| ||||||
| Urea | ||||||
| Mean | 6.877 | 1.336 | 1.660 | 2.788 | 5.319 | 7.154 |
| SEM | 0.3452 | 0.2399 | 0.05658 | 0.08570 | 0.1708 | 0.5853 |
|
| ||||||
| NO/urea | ||||||
| Mean | 0.6648 | 14.83 | 7.382 | 3.465 | 1.185 | 0.6824 |
| SEM | 0.05456 | 2.726 | 0.3716 | 0.1325 | 0.06169 | 0.08993 |
3. Results
3.1. Effects of NSO on Cell Viability with or without LPS
The cells were incubated with different concentrations (2–160 µg/ml) of NSO for 48 hours, and cell viability was evaluated. The MTT assay showed NSO alone did not decrease cell viability (Figure 1(a)). As shown in Figure 1(b), cell viability decreased in the presence of LPS (P < 0.0001). NSO decreased LPS-induced toxicity in all doses (P < 0.001).
Figure 1.
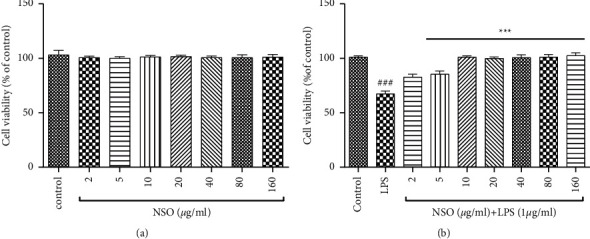
Effects of NSO on cell viability without (a) or with (b) LPS. The cell viability was evaluated in the presence of NSO alone (a), and cells were pretreated with different concentrations of NSO for 2 hours and then exposed to LPS (1 µg/mL) for 48 hours (b). The cell viability was quantitated by MTT assay. Results are mean ± SEM (n = 6).###P < 0.001 versus control; ∗∗∗P< 0.001 versus LPS.
3.2. Effects of NSO on TNF-α, PGE2, IL-1β, and IL-6 in the Presence of LPS
The cells were pretreated with NSO (10–40 µg/ml) for two hours, and then LPS was added. After 48 hours, TNF-α, PGE2, IL-1β, and IL-6 were measured by using ELISA. In comparison with control, LPS significantly increased TNF-α (P < 0.001), PGE2 (P < 0.001), IL-1β (P < 0.001), and IL-6 (P < 0.001) in the supernatant. As shown in Figures 2(a)–2(d), NSO (10–40 μg/ml) significantly reduced TNF-α (P < 0.001), PGE2 (P < 0.01 to 0.001), IL-1β (P < 0.001), and IL-6 (P < 0.001) in all of the doses in the presence of LPS.
Figure 2.

Effects of NSO on the levels of TNF-α, PGE2, IL-1β, and IL-6 in the presence of LPS; the cells were pretreated with NSO for 2 hours and then incubated with LPS for 48 hours. After 48 hours, the levels (pg/ml) of TNF-α, PGE2, IL-1β, and IL-6 were determined in the presence of LPS. ###P < 0.001 versus control; ∗∗P < 0.01 and ∗∗∗P < 0.001 versus LPS.
3.3. Effects of NSO on Gene Expression of TNF-α, COX-2, IL-1β, IL-6, iNOS, and Arg1 and Ratio of iNOS/Arg1 in the Presence of LPS
As shown in Figures 3(a)–3(d), LPS increased the gene expression of TNF-α (P < 0.001), COX-2 (P < 0.001, P < 0.01), IL-1β (P < 0.001), and IL-6 (P < 0.001). NSO (10–40 μg/ml) significantly reduced the expression of TNF-α (P < 0.001), COX-2 (P < 0.001, P < 0.01), IL-1β (P < 0.001), and IL-6 (P < 0.001) than that of the LPS group.
Figure 3.

Effects of NSO on the gene expression levels of TNF-α, COX-2, IL-1β, and IL-6 in the presence of LPS; the cells were pretreated with NSO for 2 hours and then incubated with LPS for 48 hours. After 48 hours, the expression levels of TNF-α, COX-2, IL-1 β, and IL-6 were determined in the presence of LPS. ###P < 0.001 versus control; ∗∗P < 0.01 and ∗∗∗P < 0.001 versus LPS.
As illustrated in Figures 4(a)–4(c), LPS elevated the expression of iNOS (P < 0.001), decreased Arg1 (P < 0.05), and increased the ratio of iNOS/Arg1 (P < 0.001) than that of the control group. Pretreatment with NSO (10–40 μg/ml) attenuated the levels of iNOS (P < 0.001) ,while it increased Arg1 (P < 0.001). The ratio of iNOS/Arg1 was also decreased in the presence of NSO (P < 0.001) than that of the LPS group (Figures 4(a)–4(c)).
Figure 4.

Effects of NSO on the expression of iNOS, Arg1, and the ratio of iNOS/Arg1 in the presence of LPS; the cells were pretreated with NSO for 2 hours and then were incubated with LPS for 48 hours. After 48 hours, the expression levels of iNOS, Arg1, and the ratio of iNOS/Arg1 were measured in the presence of LPS. #P < 0.05, ###P < 0.001 versus control; ∗∗∗P < 0.001 versus LPS.
3.4. Effects of NSO on NO, Urea, and Ratio of NO/Urea
As shown in Figures 5(a)–5(c), LPS increased NO (P < 0.001), decreased urea (P < 0.001), and elevated the ratio of NO/urea (P < 0.001). In contrast, NSO (10–40 μg/ml) reduced the level of NO (P < 0.001), increased urea (P < 0.05 and P < 0.001), and decreased the ratio of NO/urea (P < 0.001) than that of the LPS group.
Figure 5.

Effects of NSO on NO, urea, and ratio of NO/urea in the presence of LPS; the cells were pretreated with NSO for 2 hours and then were incubated with LPS for 48 hours. After 48 hours, the levels of NO, urea, and ratio of NO/urea were measured in the presence of LPS. ###P < 0.001 versus control; ∗P < 0.05 and ∗∗∗P < 0.001 versus LPS.
4. Discussion
Activation of microglial cells has an essential role in the pathogenesis of some neuroinflammation diseases, such as Alzheimer's disease, Parkinson's disease, and MS [15, 16]. The induction of these cells leads to the secretion of proinflammatory cytokines, such as TNF-α, various ILs, prostaglandins, leukotrienes, and NO [42, 43]. These factors lead to neuroinflammation and cell death in neurons. Therefore, the antioxidant and anti-inflammatory agents may protect neuronal cells via inhibition of microglial activation [44, 45].
Alzheimer's disease (AD), the most incident age-related neurodegenerative disorder with cognitive impairment, is characterised by progressive brain atrophy and the presence of intracellular neurofibrillary tangles consisting of hyperphosphorylated tau protein and extracellular senile plaques composed of amyloid-beta (Aβ) peptide and neuroinflammation [46]. Moreover, Aβ plaques form in the extracellular space, thus illustrating the most obvious opportunity for direct targeting and activating by microglia [47]. In this study, we investigated the protective and anti-inflammatory effects of NSO on LPS-induced inflammatory responses in microglial cells. The studies have shown that activation of microglia leads to the production of neurotoxic factors, such as NO, which iNOS synthesises. NO at low concentrations has protective effects, but at higher levels, it is a neurotoxic agent via nitrite-free radical production [48].
Microglia might also aid in reducing Aβ, a process that has been extensively studied and reviewed [47]. Microglia (M2), by secreting proteinases, such as neprilysin, insulin-degrading enzyme, and MMP9, directly degrade and clean soluble Aβ. Generally, microglia are presumed to phagocytose and degrade Aβ. The scavenger receptor CD36 and TREM2 has been proposed as direct phagocytic receptors for Aβ or Aβ-lipoprotein complexes that direct the lysosomal degradation of Aβ [47]. Several studies indicated that N. sativa and its main bioactive constituent, thymoquinone, provide neuroprotection and attenuated disease results in AD animal models. Neuroprotective role of NSO and its fractions: hexane, ethyl acetate, and water from N. sativa were also investigated in primary cells against Aβ-induced cytotoxicity, which is a common characteristic of AD. Oil and water-fractionated, treated primary rat cerebellar granule neuron (CGN) cells treated with 3‐(4,5‐dimethylthiazol‐2‐yl)‐5‐(3‐carboxymethoxyphenyl)‐2‐(4‐sulfophenyl)‐2H‐tetrazolium, and inner salt (MTS) demonstrates higher cell viability. Hexane and ethyl acetate fractions show higher antioxidant properties against 10 µM Aβ1-40-treated primary cells [46]. Our findings showed that LPS reduced cell viability, increased TNF-α, IL-1β, IL-6, PGE2, iNOS, NO, and the ratio of iNOS/Arg1 and NO/urea. At the same time, NSO reduced all indexes in microglia cells and decreased LPS-induced inflammation. Recent studies have shown that callus (0.2 to 1.6 mg/ml) and seed (1.25 to 20 μl/ml) extracts of N. sativa reduced inflammation in rat glial cells by lowering nitric oxide [49]. Also, N. sativa and N. sativa oil decreased inflammation in rats that received LPS [50].
Parkinson's disease (PD) was firstly reported in 1817 by James Parkinson, and the second most prevalent neurodegenerative disorder with cognitive and motor deficits is characterised by a gradual loss of dopaminergic neurons in the substantia nigra pars compacta. Reactive astrocytes are present in AD and PD and around active demyelinating lesions in multiple sclerosis. Experimentally, to understand the N. sativa fatty acid function on the suborganellar level, mitochondrial membrane potential was studied in MMP+-induced apoptotic PC12 cells, which shows the protective role of fatty acids [51]. Also, N. sativa seeds prevented LPS toxicity in peritoneal macrophages by modifying NO and iNOS expressions [52]. In addition, a clinical study showed the consumption of 500 mg of N. sativa oil as a capsule by rheumatoid arthritis patients decreased MDA and NO [53]. Thymoquinone reduced Aβ-induced neurotoxicity in SK-N-SH cells via inhibition of NF-κB-p65 and reversed the expression of MAP2 [54]. Another study revealed that thymoquinone reduced LPS-toxicity in activated BV-2 microglial cells by decreasing cytokines [55]. Different mechanisms can play a role in the anti-inflammatory activity of N. sativa and thymoquinone, such as inhibition of NF-κB activation and its molecular targets [36, 56], which causes the reduction of inflammation in neurons, also attenuation of cytokines including TNF-α, IL-1β [31], nitric oxide (NO)/iNOS, IL-6, IFN-γ, prostaglandin E2 [57], TGF-β1 [58], 5-lipoxygenase activity [59], and cyclooxygenase-2 [60]. All of these studies confirmed our study and the anti-inflammatory effect of N. sativa. Therefore, this herb and its active compounds may be regarded as an anti-inflammatory drug in the future.
Although the cytokine profiles in this manuscript are roughly consistent with the phenotype shift of M1/M2, the polarisation of macrophages is generally recognised to be driven by transcription factors [61]. However, polarisation cannot be considered a fixed model, while macrophages sufficiently reserve the plasticity to integrate multiple signals, such as microbes, injured tissues, and the tissue environment [61]. In particular, two prototypic transcription factors, such as NF-κB and STAT3, are broadly involved in proinflammatory regulation [62, 63]. Therefore, as a limitation of the current study, we did not measure the transcriptional factors for confirmation of the M1/M2 polarisation. Eventually, we suggest the measurement of them in further experimental assessments.
5. Conclusion
In conclusion, the study revealed that NSO had no cytotoxicity effect on microglial cells. In addition, NSO significantly attenuated inflammatory responses of LPS and increased the anti-inflammatory status of microglia by regulating the M1/M2 ratio towards the M2 state. These results may prove that NSO is potentially an immunomodulator for various neurodegenerative diseases by M1 phenotype dominancy, such as Alzheimer's and Parkinson's diseases.
Acknowledgments
This study was financially supported by a nonspecific grant from Mashhad University of Medical Sciences (Grant nos. 991660, 991593, and 990961).
Abbreviations
- AD:
Alzheimer's disease
- AGS:
Adenocarcinoma gastric cells
- Arg1:
Arginase 1
- CAT:
Catalase
- CD14:
Cluster of differentiation 14
- CNS:
Central nervous system
- COX-2:
Cyclooxygenase-2
- DSS:
Dextran sulfate sodium
- GSH:
Glutathione
- IL-1β:
Interleukin-1β
- IL-6:
Interleukin-6
- IL-10:
Interleukin-10
- IFN-γ:
Interferon-gamma
- iNOS:
Inducible nitrite oxide synthase
- LPS:
Lipopolysaccharide
- MS:
Multiple sclerosis
- MDA:
Malondialdehyde
- MTT:
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- NF-κB:
Nuclear factor κB
- NO:
Nitrite oxide
- PD:
Parkinson's disease
- PHA:
Phytohemagglutinin
- PGE2:
Prostaglandin E2
- SIF:
Stock isotonic Ficoll
- SOD:
Superoxide dismutase
- UC:
Ulcerative colitis
- TNF-α:
Tumour necrosis factor-α
- WHO:
World Health Organization.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Additional Points
Declaration of transparency and scientific rigour: this declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers, and other organisations engaged with supporting research.
Ethical Approval
Approval is not applicable as this is an in vitro study.
Consent
All authors have given consent for contribution and participation in performing the project and preparing the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
All authors equally contributed to performing the project and preparing the manuscript.
References
- 1.Askari V. R. The influence of hydro-ethanolic extract of Portulaca oleracea L. on Th1/Th2 balance in isolated human lymphocytes. Journal of Ethnopharmacology . 2016;194:1112–1121. doi: 10.1016/j.jep.2016.10.082. [DOI] [PubMed] [Google Scholar]
- 2.Rahimi V. B. Boswellia serrata has promising impact on glutamate and quinolinic acid-induced toxicity on oligodendroglia cells: in vitro study. Acta Poloniae Pharmaceutica . 2017;74(6):1803–1811. [Google Scholar]
- 3.Rahimi V. B. Comparison of honey and dextrose solution on post-operative peritoneal adhesion in rat model. Biomedicine & Pharmacotherapy . 2017;92:849–855. doi: 10.1016/j.biopha.2017.05.114. [DOI] [PubMed] [Google Scholar]
- 4.Bradford B. J. Invited review: inflammation during the transition to lactation: new adventures with an old flame. Journal of Dairy Science . 2015;98(10):6631–6650. doi: 10.3168/jds.2015-9683. [DOI] [PubMed] [Google Scholar]
- 5.Heppner F. L., Ransohoff R. M., Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nature Reviews Neuroscience . 2015;16:p. 358. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 6.Baradaran Rahimi V. The effects of hydro-ethanolic extract of Capparis spinosa (C. spinosa) on lipopolysaccharide (LPS)-induced inflammation and cognitive impairment: evidence from in vivo and in vitro studies. Journal of Ethnopharmacology . 2020;256 doi: 10.1016/j.jep.2020.112706.112706 [DOI] [PubMed] [Google Scholar]
- 7.Greter M., Merad M. Regulation of microglia development and homeostasis. Glia . 2013;61(1):121–127. doi: 10.1002/glia.22408. [DOI] [PubMed] [Google Scholar]
- 8.Harry G. J. Microglia during development and aging. Pharmacology & Therapeutics . 2013;139(3):313–326. doi: 10.1016/j.pharmthera.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shafiee-Nick R. A comprehensive review on the potential therapeutic benefits of phosphodiesterase inhibitors on cardiovascular diseases. 2017;94:541–556. doi: 10.1016/j.biopha.2017.07.084. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh A. Microglia in development and disease. Clinical and Developmental Immunology . 2013;2013 doi: 10.1155/2013/736459.736459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Scarano F., Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annual Review of Neuroscience . 1999;22(1):219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- 12.Rahimi V. B. Protective effects of hydro-ethanolic extract of Terminalia chebula on primary microglia cells and their polarisation (M1/M2 balance) Multiple Sclerosis and Related Disorders . 2018;25:5–13. doi: 10.1016/j.msard.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Lee Y.-J. Inflammation and Alzheimer’s disease. Archives of Pharmacal Research . 2010;33(10):1539–1556. doi: 10.1007/s12272-010-1006-7. [DOI] [PubMed] [Google Scholar]
- 14.Askari V. R. β-Amyrin, the cannabinoid receptors agonist, abrogates mice brain microglial cells inflammation induced by lipopolysaccharide/interferon-γ and regulates Mφ1/Mφ2 balances. Biomedicine & Pharmacotherapy . 2018;101:438–446. doi: 10.1016/j.biopha.2018.02.098. [DOI] [PubMed] [Google Scholar]
- 15.Askari V. R. Auraptene regulates Th1/Th2/TReg balances, NF-κB nuclear localisation and nitric oxide production in normal and Th2 provoked situations in human isolated lymphocytes. Phytomedicine . 2018;43:1–10. doi: 10.1016/j.phymed.2018.03.049. [DOI] [PubMed] [Google Scholar]
- 16.Askari V. R., Shafiee-Nick R. Promising neuroprotective effects of β-caryophyllene against LPS-induced oligodendrocyte toxicity: a mechanistic study. Biochemical Pharmacology . 2019;159:154–171. doi: 10.1016/j.bcp.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Askari V. R., Shafiee-Nick R. The protective effects of β-caryophyllene on LPS-induced primary microglia M1/M2 imbalance: a mechanistic evaluation. Life Sciences . 2019;219 doi: 10.1016/j.lfs.2018.12.059. [DOI] [PubMed] [Google Scholar]
- 18.Lee J. K., Tansey M. G. Microglia isolation from adult mouse brain. Methods in Molecular Biology . 2013;1041:17–23. doi: 10.1007/978-1-62703-520-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perego C. Macrophages are essential for maintaining a M2 protective response early after ischemic brain injury. Neurobiology of Disease . 2016;96:284–293. doi: 10.1016/j.nbd.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Sharrif M. Nigella sativa traditional usages (BlackSeed) Advances in Environmental Biology . 2011;5:5–16. [Google Scholar]
- 21.Chaturvedi S. Nigella sativa and its chemical constituents: a promising approach against neurodegenerative disorders. In: Khan A., Rehman M., editors. Black Seeds (Nigella Sativa) Amsterdam, Netherlands: Elsevier; 2022. pp. 149–176. [Google Scholar]
- 22.Ramadan M. F., Morsel J.-T. Characterisation of phospholipid composition of black cumin (Nigella sativa L.) seed oil. Nahrung-Food . 2002;46(4):p. 240. doi: 10.1002/1521-3803(20020701)46:4<240::AID-FOOD240>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Nickavar B. Chemical composition of the fixed and volatile oils of Nigella sativa L. from Iran. Zeitschrift für Naturforschung C . 2003;58(9-10):629–631. doi: 10.1515/znc-2003-9-1004. [DOI] [PubMed] [Google Scholar]
- 24.Khan M. A. Chemical composition and medicinal properties of Nigella sativa Linn. Inflammopharmacology . 1999;7(1):15–35. doi: 10.1007/s10787-999-0023-y. [DOI] [PubMed] [Google Scholar]
- 25.Randhawa M. A. Black seed, Nigella sativa, deserves more attention. Journal of Ayub Medical College, Abbottabad . 2008;20(2):1–2. [PubMed] [Google Scholar]
- 26.Houghton P. J. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Medica . 1995;61(1):33–36. doi: 10.1055/s-2006-957994. [DOI] [PubMed] [Google Scholar]
- 27.Burits M., Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytotherapy Research . 2000;14(5):323–328. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 28.Kumara S. S. M., Huat B. T. K. Extraction, isolation and characterisation of antitumor principle, α-hederin, from the seeds of Nigella sativa. Planta Medica . 2001;67(1):29–32. doi: 10.1055/s-2001-10628. [DOI] [PubMed] [Google Scholar]
- 29.Niazi A., Baradaran Rahimi V., Askari N., Rahmanian-Devin P., Reza Askari V. Topical treatment for the prevention and relief of nipple fissure and pain in breastfeeding women: A systematic review. Advances in Integrative Medicine . 2021;8(4):312–321. [Google Scholar]
- 30.Niazi A., Rahimi V. B., Soheili-Far S., et al. A systematic review on prevention and treatment of nipple pain and fissure: are they curable? Journal of Pharmacopuncture . 2018;21(3):139–150. doi: 10.3831/KPI.2018.21.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tekeoglu I. Effects of thymoquinone (volatile oil of black cumin) on rheumatoid arthritis in rat models. Phytotherapy Research . 2007;21(9):895–897. doi: 10.1002/ptr.2143. [DOI] [PubMed] [Google Scholar]
- 32.Mansour M., Tornhamre S. Inhibition of 5-lipoxygenase and leukotriene C4 synthase in human blood cells by thymoquinone. Journal of Enzyme Inhibition and Medicinal Chemistry . 2004;19(5):431–436. doi: 10.1080/14756360400002072. [DOI] [PubMed] [Google Scholar]
- 33.El Gazzar M. Anti-inflammatory effect of thymoquinone in a mouse model of allergic lung inflammation. International Immunopharmacology . 2006;6(7):1135–1142. doi: 10.1016/j.intimp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Al-Ghamdi M. The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. Journal of Ethnopharmacology . 2001;76(1):45–48. doi: 10.1016/s0378-8741(01)00216-1. [DOI] [PubMed] [Google Scholar]
- 35.Mahgoub A. A. Thymoquinone protects against experimental colitis in rats. Toxicology letters . 2003;143(2):133–143. doi: 10.1016/s0378-4274(03)00173-5. [DOI] [PubMed] [Google Scholar]
- 36.Xuan N. T. Effect of thymoquinone on mouse dendritic cells. Cellular Physiology and Biochemistry . 2010;25(2-3):307–314. doi: 10.1159/000276563. [DOI] [PubMed] [Google Scholar]
- 37.Mohamed A. Improvement of experimental allergic encephalomyelitis (EAE) by thymoquinone; an oxidative stress inhibitor. Biomedical Sciences Instrumentation . 2003;39:440–445. [PubMed] [Google Scholar]
- 38.Mahmood M. S. The in vitro effect of aqueous extract of Nigella sativa seeds on nitric oxide production. Phytotherapy Research . 2003;17(8):921–924. doi: 10.1002/ptr.1251. [DOI] [PubMed] [Google Scholar]
- 39.Kim S. H., Smith C. J., Van Eldik L. J. Importance of MAPK pathways for microglial pro-inflammatory cytokine IL-1β production. Neurobiology of Aging . 2004;25(4):431–439. doi: 10.1016/S0197-4580(03)00126-X. [DOI] [PubMed] [Google Scholar]
- 40.Tu S. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilises myeloid-derived suppressor cells in mice. Cancer Cell . 2008;14(5):408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramirez F. Glucocorticoids promote a TH2 cytokine response by CD4+ T cells in vitro. The Journal of Immunology . 1996;156(7):2406–2412. [PubMed] [Google Scholar]
- 42.Andreakos E. Distinct pathways of LPS-induced NF-κB activation and cytokine production in human myeloid and nonmyeloid cells defined by selective utilisation of MyD88 and Mal/TIRAP. Blood . 2004;103(6):2229–2237. doi: 10.1182/blood-2003-04-1356. [DOI] [PubMed] [Google Scholar]
- 43.Janeway C. A., Jr., Medzhitov R. Innate immune recognition. Annual Review of Immunology . 2002;20(1):197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 44.Agullo G. Relationship between flavonoid structure and inhibition of phosphatidylinositol 3-kinase: a comparison with tyrosine kinase and protein kinase C inhibition. Biochemical Pharmacology . 1997;53(11):1649–1657. doi: 10.1016/s0006-2952(97)82453-7. [DOI] [PubMed] [Google Scholar]
- 45.Araki E. Cyclooxygenase-2 inhibitor ns-398 protects neuronal cultures from lipopolysaccharide-induced neurotoxicity. Stroke . 2001;32(10):2370–2375. doi: 10.1161/hs1001.096057. [DOI] [PubMed] [Google Scholar]
- 46.Ismail N. Black cumin seed (Nigella sativa L.) Oil and its fractions protect against beta amyloid peptide-induced toxicity in primary cerebellar granule neurons. Journal of Food Lipids . 2008;15(4):519–533. [Google Scholar]
- 47.Perry V. H., Nicoll J. A. R., Holmes C. Microglia in neurodegenerative disease. Nature Reviews Neurology . 2010;6(4):193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 48.Brown G. C., Cooper C. E. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Letters . 1994;356(2-3):295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 49.Alemi M. Anti-inflammatory effect of seeds and callus of Nigella sativa L. extracts on mix glial cells with regard to their thymoquinone content. AAPS PharmSciTech . 2013;14(1):160–167. doi: 10.1208/s12249-012-9899-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Entok E. Anti-inflammatuar and anti-oxidative effects of Nigella sativa L.: 18FDG-PET imaging of inflammation. Molecular Biology Reports . 2014;41(5):2827–2834. doi: 10.1007/s11033-014-3137-2. [DOI] [PubMed] [Google Scholar]
- 51.Hosseinzadeh L. Bioassay-guided isolation of neuroprotective fatty acids from nigella sativa against 1-methyl-4-phenylpyridinium-induced neurotoxicity. Pharmacognosy Magazine . 2017;13(52):627–633. doi: 10.4103/pm.pm_470_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tripathi Y. B., Chaturvedi A., Pandey N. Effect of nigella sativa seeds extracts on iNOS through antioxidant potential only: crude/total extract as molecular therapy drug. Indian Journal of Experimental Biology . 2012;50(6) [PubMed] [Google Scholar]
- 53.Hadi V. Effects of Nigella sativa oil extract on inflammatory cytokine response and oxidative stress status in patients with rheumatoid arthritis: a randomised, double-blind, placebo-controlled clinical trial. Avicenna Journal of Phytomedicine . 2016;6(1):p. 34. [PMC free article] [PubMed] [Google Scholar]
- 54.Velagapudi R., Olajide O. Neuroprotective effects of thymoquinone in aβ1–42-induced toxicity in SK-N-sh neuronal cells. The FASEB Journal . 2016;30(1):p. lb509. [Google Scholar]
- 55.Taka E. Anti-inflammatory effects of thymoquinone in activated BV-2 microglial cells. Journal of Neuroimmunology . 2015;286:5–12. doi: 10.1016/j.jneuroim.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kundu J. K. Thymoquinone inhibits phorbol ester-induced activation of NF-κB and expression of COX-2, and induces expression of cytoprotective enzymes in mouse skin in vivo. Biochemical and Biophysical Research Communications . 2013;438(4):721–727. doi: 10.1016/j.bbrc.2013.07.110. [DOI] [PubMed] [Google Scholar]
- 57.Umar S. Modulation of the oxidative stress and inflammatory cytokine response by thymoquinone in the collagen induced arthritis in Wistar rats. Chemico-Biological Interactions . 2012;197(1):40–46. doi: 10.1016/j.cbi.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Ammar E.-S. M. Comparative evaluation of anti-inflammatory properties of thymoquinone and curcumin using an asthmatic murine model. International Immunopharmacology . 2011;11(12):2232–2236. doi: 10.1016/j.intimp.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 59.Landa P. Inhibition of in vitro leukotriene B4 biosynthesis in human neutrophil granulocytes and docking studies of natural quinones. Natural product communications . 2013;8(1):105–108. [PubMed] [Google Scholar]
- 60.Yang W. Effect of thymoquinone on cytosolic pH and Na+/H+ exchanger activity in mouse dendritic cells. Cellular Physiology and Biochemistry . 2012;29(1-2):21–30. doi: 10.1159/000337583. [DOI] [PubMed] [Google Scholar]
- 61.Murray P. J. Macrophage polarization. Annual Review of Physiology . 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 62.Wang L. Effects of selected resveratrol analogues on activation and polarization of lipopolysaccharide-stimulated BV-2 microglial cells. Journal of Agricultural and Food Chemistry . 2020;68(12):3750–3757. doi: 10.1021/acs.jafc.0c00498. [DOI] [PubMed] [Google Scholar]
- 63.Seung Hyeon K. Perilla extract potentiates efferocytosis by macrophages: implications for resolution of inflammation. Journal of Food Bioactives . 2020;10 [Google Scholar]
- 64.Jetten N. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis . 2014;17(1):109–118. doi: 10.1007/s10456-013-9381-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.


