Abstract
Objective
The aim was to detect effects of blue light on reducing the adverse effect of heat stress in thermal manipulation (TM) of broiler embryos by subjecting embryos to heat stress during incubation development.
Methods
Eggs were assigned to four treatments in which the TM (thermal manipulation) was exposed to 40°C for 4 h daily during five successive days, if TM was operated. The treatments were (1) normal temperature with white lighting group (37°C+W), (2) normal temperature with blue lighting group (37°C+B), (3) thermal manipulation with white lighting group (40°C+W), and (4) thermal manipulation with blue lighting group (40°C+B).
Results
Blue light significantly lowered MDA and corticosterone concentrations in the embryonic liver. Additionally, the damage of embryonic liver tissue caused by heat stress could be reduced by blue light. HSPs and HSFs gene expression of chicken liver were modulated by blue light significantly, whereas the effects were different, respectively. Moreover, blue light modulated liver antioxidant enzyme activity and their gene expression in embryonic liver significantly. However, blue light did not exert significant effects on body weight, late hatch rectal temperature and tibia length of hatched chicks.
Conclusions
The results suggest that monochromatic blue light can reduce the content of MDA and corticosterone of broiler embryos in heat stress and increase the relative expression of SOD and CAT genes. Moreover, the monochromatic blue light may reduce the metabolic heat production of broilers during the embryonic stage, thus reducing the damage of broilers due to heat stress during the embryonic heat acclimation stage.
1. Introduction
For the global poultry production, climate change has gradually become a huge challenge [1]. The pecuniary losses resulting from high temperature in the poultry industry alone is up to $128 to $165 million per year. The annual pecuniary losses of the livestock of the United States is 16.9 to23.6 million dollars [2]. Finding measures to reduce the harm caused by heat stress is very important for some tropical regions of the world. Heat treatment of broiler chickens during incubation by exposing the embryo to heat stress has been shown to increase the heat resistance of broilers in the later stage [3, 4]. Broilers hatched after embryonic exposure to intermittent TM have lower calorific rates, lower weight of abdominal fat pads, larger muscle growth, and a thinner average fibre diameter under heat stress (32°C for 12 h/d) [5]. In addition, compared with the control, metabolic rate and calorific value of TM treated embryos were lower [6]. However, heat exposure reduces animal welfare and growth performance [7]. Yolk consumption and embryo growth can be lowered by embryo TM [8]. Additionally, heat stress triggers oxidative stress in the liver, increasing tissue damage [9]. Elevated blood corticosterone concentration indicates that heat exposure increases the stress response [4].
Severity of heat stress is decided by degree of destruction of the balance between cellular antioxidants and reactive oxygen species (ROS) [10–13]. Both catalase (CAT) and superoxide dismutase (SOD) belong to cellular antioxidant enzymes which have different responses with different affected tissues [10]. Additionally, heat stress will cause protein damage, which will lead to the aggregation of unfolded proteins [14, 15]. The expressions of heat shock proteins (HSPs) are increased by heat stress, which leads to protein stability and heat resistance [16]. HSP70 and HSP90 are two kinds among the HSPs. HSP70 and HSP90 work to protect affected cells, preventing the aggregation of unfolded proteins [17]. Heat shock transcription factors (HSFs) can modulate HSP expression by acting on specific DNA sequence. (heat shock element (HSE)) [18–20].
Different mitigation methods have been adopted to reduce the harm caused by heat stress, which include environmental strategies in shape of housing, ventilation, and natural or artificial shading, along with feed additives [1]. Another approach that can alleviate the heat stress deleterious effects is lighting management. Abdo et al. [9] described that susceptibility of broilers could be reduced by blue light. Reducing the stress response and improving immune response in broilers has been proved to be the effect of blue light [21]. In addition, blue light may alleviate stress response by modulating expression of interleukin-1β (IL-1β) and ratio of heterophils to lymphocytes (H/L) [22, 23]. The target of the present study was exploring function of blue light in alleviating adverse effect of thermal manipulation by exposing embryos to periodic high temperature during incubation development.
2. Materials and Methods
2.1. Incubation Management
Hatching eggs (n = 800) were selected by weight (60 ± 2 g) from Huangjiaoma broiler breeders. The breeders were from a company at Jiaxing, China. All eggs were incubated in separate environmentally controlled commercial incubators from a company at Shandong, China. The incubators were set at the incubation room, Zhejiang University, China. The relative humidity (RH) of the incubators was kept at 60 ± 1%, and temperature of the incubator was maintained at 37.8 ± 0.1°C. Eggs were turned from D1 to D18 with 2 h a day. Eggs were examined on 18th day to remove the nonviable eggs.
2.2. Experimental Design
Before incubation, the hatching eggs were disinfected and equally divided into four groups (n = 200), with four replicates in each group (n = 50). Two groups (W+37.6°C and B+37.6°C) were incubated at normal temperature under white light or blue light. The other two groups (W+40°C and B+40°C) received specific TM. On this point, TM was carried out from 14th day to 18th day in which eggs were exposed to 40 ± 1°C for 4 h a day. After TM, the temperature was lowered to 37.6°C till hatch. The intensity of blue light was 27 lux and the wavelength was 450 nm.
2.3. Sample Collection
Twenty-four eggs were randomly taken from each group for specimen gathered after TM (after day 18 heat acclimatization). Cervical dislocation was used to kill the embryos before liver specimens of each bird were collected. Formalin (10%) was used to preserve one-third of liver specimens for histopathological examination; for RNA extraction, one-third of liver specimens were frozen in liquid nitrogen; for antioxidant enzyme activity analysis, one-third of liver specimens were preserved at −20°C.
2.4. Histopathological Examination
The tissues were treated with paraffin. Each specimen was sectioned and mounted on a slide. The tissues were stained with hematoxylin and eosin (H&E), and then, the liver sections were examined with a light microscope (200x) [24].
2.5. Corticosterone and Malondialdehyde (MDA) Content Determination
Liver samples were placed in phosphate buffer (pH 7.2-7.4). The supernatant was obtained by centrifuging liver homogenate (5000 rpm at 4°C for 15 min). The concentration of malondialdehyde was measured using a biological diagnostic kit (Biodiagnostic, A003-1, China). The content of malondialdehyde was determined by ultraviolet visible spectrophotometer at 532 nm. Liver corticosterone content was assayed using corticosterone ELISA kit (Biodiagnostic, MB-5253A, China), measuring absorbance (450 nm) by spectrophotometer (Ahmed et al., 2020).
2.6. Antioxidant Enzyme Activity Determination
SOD activity was measured using diagnostic kit (Biodiagnostic, MB-9428A, China). CAT activity was determined by spectrophotometry based on the spectrophotometric using diagnostic kits (MB-9429A, China), measuring absorbance (450 nm).
2.7. RNA Extraction and cDNA Synthesis
RNA was extracted from the liver tissue (30 mg) (n = 8 from each treatment) by RNA purification kit (Norgen Biotek Corporation, Canada). RNA integrity was verified by gel electrophoresis of rRNA strips. Additionally, UV-Vis spectrophotometer (Nanodrop ND1000) was used to determine the concentration of RNA. RNA sample was reverse transcribed finally.
2.8. Real-Time PCR
Real-time PCR (qPCR) was performed using the QuantiFast SYBR® Green Supermix (Qiagen, Germany). The reaction mixture included 10 μl of 2×SYBR Green, 2 μl of cDNA, and 0.5 μM of each prime. All the genes were denatured initially at 95°C for 15 min followed by 40 cycles at 95°C for 15 s and annealed at 60°C for 1 min. Dissociation curve analysis started at 65°C and ended at 95°C to verify the specificity of PCR products (with an increase of 0.5°C every 5 s). There was only one peak in the dissociation curve analysis of all tested genes at a specific melting temperature, indicating the specificality of the PCR product amplification. Genes of eight birds were tested repeatedly. The “fold change” calculation included CT values of each sample [25].
2.9. Statistical Analysis
The data were analyzed by GraphPad Prism 7 software. Statistically significant differences of temperature and light treatment effects on MDA, corticosterone, antioxidant enzymes and gene expression of HSPs and HSFs, and embryonic body temperature were detected by two-way ANOVA.
3. Results
3.1. Blue Light Significantly Lowers MDA and Corticosterone Concentrations in Embryonic Liver
The effects of light and temperature on MDA concentration were statistically significant (P < 0.0001 for light and P < 0.05 for temperature). Because MDA is an index of lipid peroxidation, MDA level (Figure 1(a)) increased significantly in W+40°C and B+40°C compared to W+37.6°C in chick embryos (for W+40°C P < 0.001 and for B+40°C P < 0.05). Significantly, compared to W+40°C, the concentration of MDA was lower in B+40°C (P < 0.01).
Figure 1.
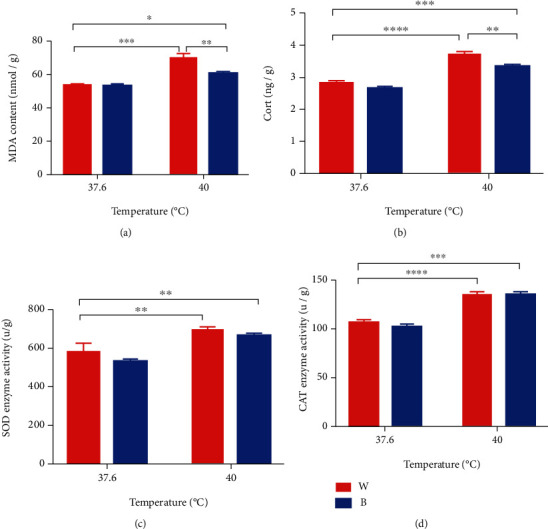
Concentration of MDA and corticosterone as well as activities of antioxidant enzymes in the liver of chick embryo under different conditions: (a) MDA content, (b) corticosterone concentration, (c) activity of SOD, and (d) activity of CAT. Mean ± SEM is shown. ∗, ∗∗, ∗∗∗, and ∗∗∗∗ denote statistical significance (two-way ANOVA) with P < 0.05, P < 0.01, P < 0.001, and P < 0.0001, respectively.
The effects of light on corticosterone contents were significant (P < 0.0001 for light and P = 0.001 for temperature). The content of corticosterone (Figure 1(b)) increased significantly in W+40°C and B+40°C compared to W+37.6°C (for W+40°C P < 0.0001 and for B+40°C P = 0.0001). Significantly, compared with W+40°C, B+40°C induced a lower concentration of corticosterone (P < 0.01).
3.2. Blue Light Can Alleviate the Increase of Liver Interstitial Cracks Caused by Heat Stress
Figures 2(a) and 2(c) show that the cells of embryonic liver were closely arranged and structurally intact in the case of W+37.6°C and B+37.6°C. Figure 2(b) shows that there were more cracks and loose arrangement in embryonic liver tissue in the case of W+40°C; moreover, severe mononuclear cell infiltration and focal necrosis were found, which was consistent with the discovery of Abdo et al. [9] that heat stress under high temperature can lead to some degree of fat changes, perivascular monocyte infiltration, and focal necrosis. Figure 2(d) shows that there were fewer cracks and the liver tissue structure was more compact in the case of B+40°C compared to W+40°C.
Figure 2.
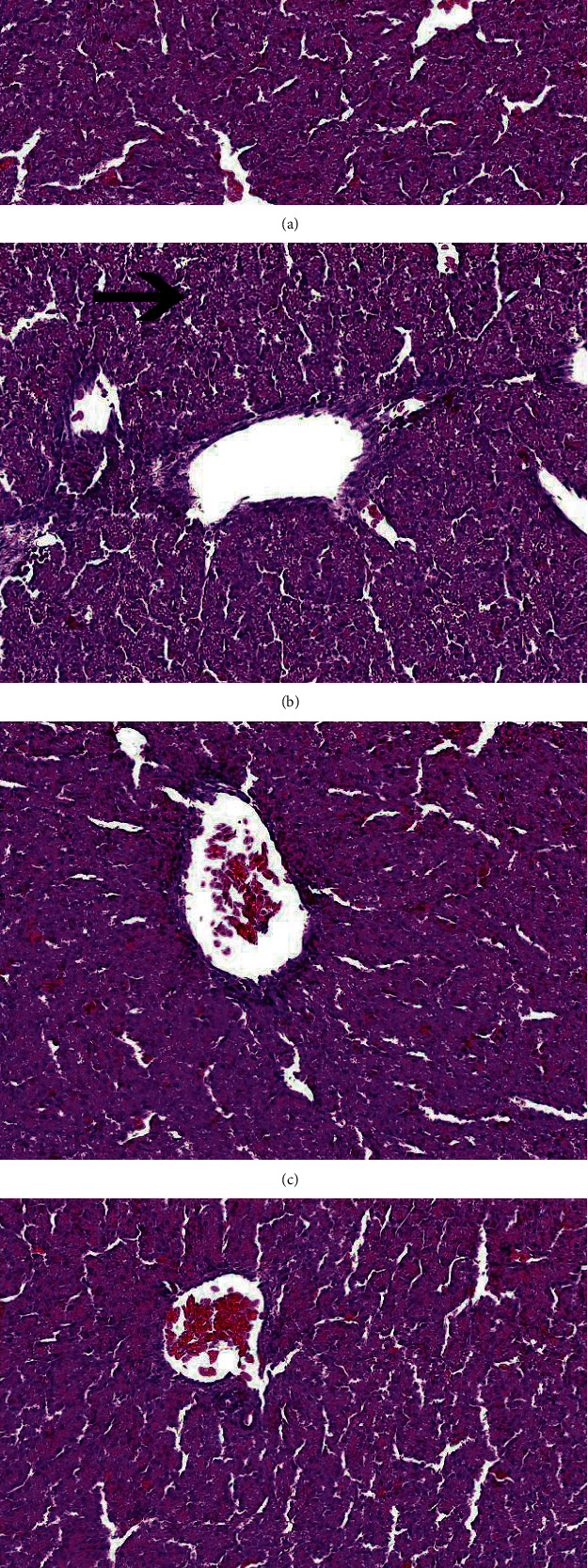
Liver histomorphology of Huangjiaoma chicken: (a) white light (W+37.6°C), (b) white light+H (W+40°C), (c) blue light (B+37.6°C), and (d) blue light+H (B+40°C). Arrows point the interstitial cracks.
3.3. Gene Expression of HSPs and HSFs Can Be Modulated by Blue Light Significantly in Chicken Liver
The damage of heat stress to liver could be reduced by blue light by reducing the concentration of MDA and corticosterone in chicken embryo (Figures 1(a) and 1(b), resp.). Figure 3 presents the relative gene expression (compared to W+37.6°C) profiles of HSPs and HSFs from 8 birds for each treatment. The expression levels of HSP70 and HSP90 were regulated by blue light in chick embryos without temperature differences (P < 0.0001).
Figure 3.
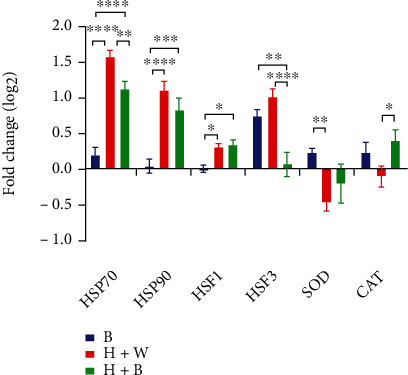
Expression of HSPs, HSFs, and antioxidant enzyme genes. The expression levels were presented as log2 fold change and shown in the figure as mean ± SEM. ∗, ∗∗, ∗∗∗, and ∗∗∗∗ denote statistical significance (two-way ANOVA) with P < 0.05, P < 0.01, P < 0.001, and P < 0.0001, respectively.
In the liver, heat stress significantly increased HSP70 gene expression in W+40°C and B+40°C, whereas blue light at normal temperature only slightly increased its expression (for W+40°C P < 0.0001 and P < 0.0001 for B+40°C). Interestingly, in B+40°C, compared with W+40°C, the expression of HSP70 gene was significantly downregulated by using blue light (P = 0.0094).
The expression pattern of HSP90 (Figure 3) is similar to that of HSP70. There was no temperature difference, only the difference caused by light treatment, and there was no interaction between light and temperature (P > 0.05 for interaction and P < 0.0001 for light treatment). B+ 37°C upregulated gene expression level of HSP90 insignificantly. In addition, compared with normal condition, W+40°C and B+40°C stimulated more HSP90 gene expression (P < 0.0001 and P = 0.0003, resp.). However, in comparison with W+40°C, the expression of HSP90 gene in B+40°C did not decrease significantly (P > 0.05).
For HSF1 gene, difference due to temperature+light interaction was not significant (P > 0.05). W+40°C upregulated the expression of HSF1 significantly, but the change was not significantly different with that induced by B+40°C (P = 0.7444). Furthermore, B+37.6°C nonsignificantly upregulated the expression levels of HSF1 (P = 0.9534).
For HSF3 gene, interaction of two conditions had a significant effect (P < 0.0001). W+40°C increased expression of HSF3 significantly, whereas a slight increase appeared in B+40°C. Nevertheless, in B+37.6°C, it induced a significant increase (P = 0.0007).
To sum up, the expression of HSPs and HSFs can be regulated by blue light under heat stress. Changes that occurred on HSP70 and HSP90 were similar. However, changes of gene expression of HSF1 and HSF3 have different patterns.
3.4. Blue Light Modulates Activities and Gene Expression of Antioxidant Enzymes Significantly in Embryonic Liver
Figures 1(c) and 1(d), respectively, show the effects of different temperature and light conditions on antioxidant enzyme activity. Compared with W+37.6°C, the activity of SOD increased significantly in W+40°C and B+40°C (for W+40°C P < 0.01 and for B+40°C P < 0.01). However, in B+37.6°C, it caused a slight downregulation (P = 0.1168). In addition, CAT enzyme also had similar response after heat stress. CAT activity increased significantly in W+40°C and B+40°C compared to W+37.6°C (for W+40°C P = 0.0007 and for B+40°C P = 0.0004).
The expression levels of mRNA of antioxidant enzymes were measured to determine their regulatory patterns. For SOD (Figure 3), light treatment had significant difference, while for CAT, temperature effect had significant difference (with regard to SOD, temperature, and interaction treatment P > 0.05, light treatment P = 0.0112; with regard to CAT, temperature treatment P = 0.0214; light and interaction treatment P > 0.05). SOD gene expression was upregulated in B+37.6°C. Compared with W+40°C, the upregulation was significant, while heat stress stimulated a nonsignificant downregulation (P = 0.0543).
The reaction of CAT gene and SOD gene is different. Blue light caused a slight upregulation in B+37.6°C and significant upregulation in B+40°C (P = 0.1298 and P < 0.0239, resp.) compared to W+40°C. To sum up, blue light regulated the gene expression and the activity of antioxidant enzymes in the period of heat stress.
3.5. Blue Light Changed the Metabolic Performance and Development of Chick Embryo after Heat Stress and before Hatching
Figure 4 shows multiple metabolic parameters and developmental status of chick embryo following thermal manipulation. Figure 4(a) shows that body weight displayed a significant decrease in B+37.6°C compared to W+37.6°C (P < 0.01). Similarly, body weight displayed a significant decrease in B+40°C compared to W+40°C (P < 0.01). According to Figure 4(c), a significant increase of the ratio of yolk to body weight was noticed in B+37.6°C compared to W+37.6°C (P < 0.05). Similarly, the weight ratio (yolk to body) displayed a significant increase in B+40°C compared to W+40°C (P < 0.05). Besides, Figure 4(d) shows that heart to body weight displayed a significant increase in W+37.6°C and B+40°C compared to W+40°C (P < 0.05). Besides, no differences were observed between treatments in yolk weight and weight ratio (liver to body or digestive system to body) (P > 0.05).
Figure 4.
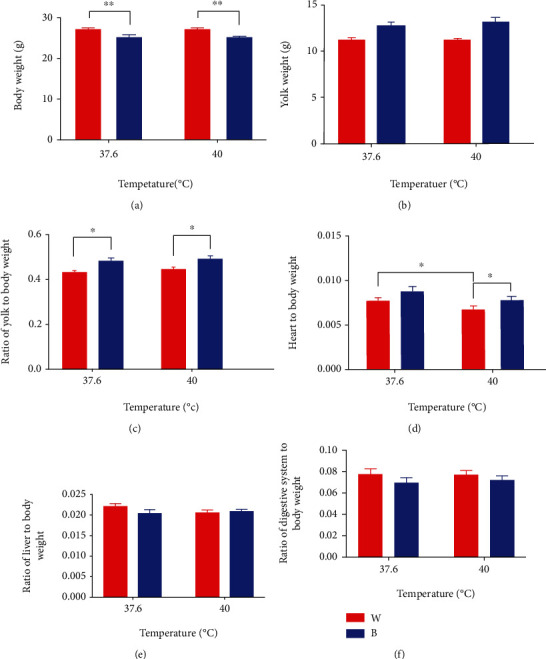
Metabolic performance and development of chick embryo following thermal manipulation (mean ± SEM): (a) body weight, (b) yolk weight, (c) weight ratio (yolk to body), (d) weight ratio (heart to body), (e) weight ratio (liver to body), and (f) weight ratio (digestive system to body). ∗ and ∗∗ denote statistical significance (two-way ANOVA) with P < 0.05 and P < 0.01, respectively.
3.6. Blue Light Did Not Affect Body Weight, Rectal Temperature, and Tibia Length of Hatched Chicks
The body weight of broilers is measured just after hatching. The body weight is presented in Figure 5(a). Differences (P > 0.05) between treatments in body weight were not observed. Figures 6 and 5(b) show the rectal temperature and tibia length of posthatch 0-day chicks. No differences (P > 0.05) in the date of late hatch rectal temperature and tibia length were observed between treatments.
Figure 5.
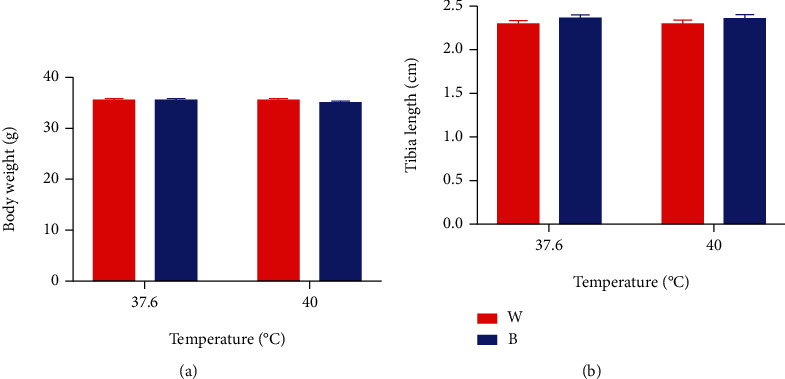
Body weight and tibia length of chicks after hatching (mean ± SEM): (a) body weight and (b) tibia length.
Figure 6.
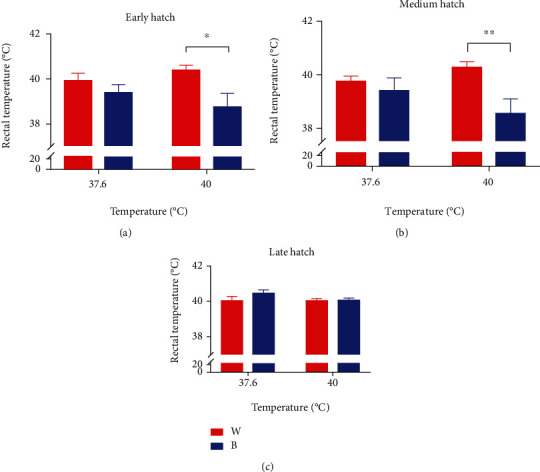
Rectal temperature of chicks after hatching (mean ± SEM): (a) early hatch, (b) medium hatch, and (c) late hatch. ∗ and ∗∗ denote statistical significance (two-way ANOVA) with P < 0.05 and P < 0.01, respectively.
4. Discussion
Meat type chickens have limited ability to face the destructive effect of heat stress. Several studies addressed heat challenge during embryogenesis enhancing adaptive capacity of broilers to cope with heat exposure. However, the heat challenge during embryonic development may trigger oxidative stress and stress response and reduce growth performance [8]. This research was aimed at exploring the effect of blue light on reducing the negative effect of TM during incubation.
With regard to MDA (Figure 1(a)), the content was significantly reduced in B+40°C compared to W+40°C. High MDA content is an indicator of lipid peroxidation, which can cause greater oxidative damage [26]. Under the condition of B+40°C, the concentration of MDA decreased, indicating that blue light may play a role in reducing negative effect caused by heat stress [27]. In addition, corticosterone (Figure 1(b) had similar response. Neuroendocrine system activities of poultry could be altered by heat stress, which contribute to activation of the hypothalamic–pituitary–adrenal axis. This disturbance causes elevation of corticosterone levels and degradation of the health status of animals [28, 29]. Elevated corticosterone levels of meat type chickens have demonstrated depressed lymphocyte number culminating in a higher H:L ratio [30]. The content of corticosterone is a sensitive indicator of stress in broilers and it is commonly used as a tool to assess the physiological stress [31]. The results of the present research indicated that exposure of eggs to heat stress significantly enhanced MDA and corticosterone content in embryonic liver, whereas supplementation of blue light in the environment was shown to restore the MDA and corticosterone level toward normal.
The effect of exposure to monochromatic blue light on liver histology was examined. Heat stress (W+40°C) caused tissue damage and increased interstitial fissures. Blue light (B+40°C) led to the loss of tissue damage compared with white light (W+40°C). Blue light can improve the resistance of broilers [23].
In the present research, when ambient temperature was normal, compared with W+37.6°C, B+37.6°C could significantly increase the expression of HSP70 and HSF3 genes, and these proteins acting as molecular chaperones to prevent protein aggregation can improve cell heat tolerance to resist high temperature, thus regulating the balance of cell survival and death. It is worth noting that compared with the condition of W+40°C, the gene expression of HSP70, Hsp90, and HSF3 in B+40°C decreased slightly, which may be due to the fact that the way of improving the heat resistance of broilers by blue light is not single, and the need for heat shock protein of embryo was reduced due to improvement of heat stress resistance under the monochromatic blue light.
Figure 1 shows that activity of SOD and CAT was higher in high temperature group (40°C) than that in normal group (37.6°C), but the activity of the two enzymes was not changed by blue light significantly, which indicated that blue light may not help to regulate the activity of antioxidant enzymes. However, according to Figure 3, monochromatic blue light could increase the relative gene expression of two antioxidant enzymes. Especially, under high temperature, the gene expressions of the two enzymes were significantly decreased, but under this condition, compared with W+40°C, B+40°C also increased the gene expression of the two enzymes. This indicated that monochromatic blue light can improve the heat tolerance of broiler embryos through modulating gene expression of antioxidant enzymes rather than their activity.
According to Figure 4, a lower body weight of embryos under blue light could be found, compared to white light with corresponding temperature. In addition, at the same ambient temperature, blue light exposure meant a higher weight percentage of egg yolk than white light exposure, which indicated that monochromatic blue light might reduce the heat production of broiler embryos by reducing the metabolic rate of broiler embryos, because egg yolk was the main energy source of hatching embryos. However, after hatching, there was no difference in body weight between blue light group and white light group, and there was no difference in tibia length. In addition, according to Figure 6, the rectal temperature of broilers in B+40°C was significantly lower than that in W+40°C at the early and middle stage after hatching, and the rectal temperature directly represented the body temperature, which may also prove that blue light can reduce the heat production of hatching embryos. This difference did not exist at the late stage after hatching.
5. Conclusions
The results suggest that monochromatic blue light can reduce the content of MDA and corticosterone in broiler embryos after TM and increase the relative expression of SOD and CAT genes. Moreover, the monochromatic blue light may reduce the metabolic heat production of broilers during the embryonic stage, thus reducing the damage of broilers due to heat stress during the embryonic heat acclimation stage.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (Grant No. 31972609) and China Agriculture Research System (CARS-40).
Contributor Information
Jian Li, Email: lijiannp@zju.edu.cn.
Jinming Pan, Email: panhouse@zju.edu.cn.
Data Availability
The data that support the findings of this study are openly available in Figshare repository (10.6084/m9.figshare.17000221.v1).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Li Zeng and Qiong Liu contributed equally to this work and are co-first authors.
References
- 1.Nawab A., Ibtisham F., Li G. H., et al. Heat stress in poultry production: mitigation strategies to overcome the future challenges facing the global poultry industry. Journal of Thermal Biology . 2018;78:131–139. doi: 10.1016/j.jtherbio.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Lara L., Rostagno M. Impact of heat stress on poultry production. Animals . 2013;3(2):356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yahav S., Collin A., Shinder D., Picard M. Thermal manipulations during broiler chick embryogenesis: effects of timing and temperature. Poultry Science . 2004;83(12):1959–1963. doi: 10.1093/ps/83.12.1959. [DOI] [PubMed] [Google Scholar]
- 4.Yahav S., Rath R. S., Shinder D. The effect of thermal manipulations during embryogenesis of broiler chicks (Gallus domesticus) on hatchability, body weight and thermoregulation after hatch. Journal of Thermal Biology . 2004;29(4-5):245–250. doi: 10.1016/j.jtherbio.2004.03.002. [DOI] [Google Scholar]
- 5.Piestun Y., Halevy O., Shinder D., Ruzal M., Druyan S., Yahav S. Thermal manipulations during broiler embryogenesis improves post-hatch performance under hot conditions. Journal of Thermal Biology . 2011;36(7):469–474. doi: 10.1016/j.jtherbio.2011.08.003. [DOI] [Google Scholar]
- 6.Piestun Y., Zimmerman I., Yahav S. Thermal manipulations of turkey embryos: the effect on thermoregulation and development during embryogenesis. Poultry Science . 2015;94(2):273–280. doi: 10.3382/ps/peu047. [DOI] [PubMed] [Google Scholar]
- 7.Loyau T., Bedrani L., Berri C., et al. Cyclic variations in incubation conditions induce adaptive responses to later heat exposure in chickens: a review. Animal . 2015;9(1):76–85. doi: 10.1017/S1751731114001931. [DOI] [PubMed] [Google Scholar]
- 8.Willemsen H., Kamers B., Dahlke F., et al. High- and low-temperature manipulation during late incubation: effects on embryonic development, the hatching process, and metabolism in broilers. Poultry Science . 2010;89(12):2678–2690. doi: 10.3382/ps.2010-00853. [DOI] [PubMed] [Google Scholar]
- 9.Abdo S. E., El-Kassas S., El-Nahas A. F., Mahmoud S. Modulatory effect of monochromatic blue light on heat stress response in commercial broilers. Oxidative medicine and cellular longevity . 2017;2017:13. doi: 10.1155/2017/1351945.1351945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akbarian A., Michiels J., Degroote J., Majdeddin M., Golian A., De Smet S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. Journal of animal science and biotechnology . 2016;7(1) doi: 10.1186/s40104-016-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng J., Zhang M., Zheng S., Xie P., Ma A. Effects of high temperature on multiple parameters of broilers in vitro and in vivo. Poultry Science . 2008;87(10):2133–2139. doi: 10.3382/ps.2007-00358. [DOI] [PubMed] [Google Scholar]
- 12.Halliwell B., Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? British journal of pharmacology . 2004;142(2):231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahmoud K. Z., Edens F. W. Influence of selenium sources on age-related and mild heat stress-related changes of blood and liver glutathione redox cycle in broiler chickens (Gallus domesticus) Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology . 2003;136(4):921–934. doi: 10.1016/S1096-4959(03)00288-4. [DOI] [PubMed] [Google Scholar]
- 14.McCormick P. H., Chen G., Tlerney S., Kelly C. J., Bouchier-Hayes D. J. Clinically relevant thermal preconditioning attenuates ischemia-reperfusion injury. The Journal of Surgical Research . 2003;109(1):24–30. doi: 10.1016/S0022-4804(02)00035-5. [DOI] [PubMed] [Google Scholar]
- 15.Staib J. L., Quindry J. C., French J. P., Criswell D. S., Powers S. K. Increased temperature, not cardiac load, activates heat shock transcription factor 1 and heat shock protein 72 expression in the heart. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology . 2007;292(1):R432–R439. doi: 10.1152/ajpregu.00895.2005. [DOI] [PubMed] [Google Scholar]
- 16.Jaattela M. Heat shock proteins as cellular lifeguards. Annals of Medicine . 1999;31(4):261–271. doi: 10.3109/07853899908995889. [DOI] [PubMed] [Google Scholar]
- 17.Slimen I. B., Najar T., Ghram A., Abdrrabba M. Heat stress effects on livestock: molecular, cellular and metabolic aspects, a review. Journal of animal physiology and animal nutrition . 2016;100(3):401–412. doi: 10.1111/jpn.12379. [DOI] [PubMed] [Google Scholar]
- 18.Akerfelt M., Morimoto R. I., Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nature reviews Molecular cell biology . 2010;11(8):545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez-Pastor R., Burchfiel E. T., Thiele D. J. Regulation of heat shock transcription factors and their roles in physiology and disease. Nature reviews Molecular cell biology . 2018;19(1):4–19. doi: 10.1038/nrm.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morimoto R. I., Kline M. P., Bimston D. N., Cotto J. J. The heat-shock response: regulation and function of heat-shock proteins and molecular chaperones. Essays in Biochemistry . 1997;32:17–29. [PubMed] [Google Scholar]
- 21.Zhang Z. Q., Cao J., Wang Z. X., Dong Y. L., Chen Y. X. Effect of a combination of green and blue monochromatic light on broiler immune response. Journal of Photochemistry and Photobiology. B . 2014;138:118–123. doi: 10.1016/j.jphotobiol.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Mohamed R. A., Eltholth M. M., El-Saidy N. R. Rearing broiler chickens under monochromatic blue light improve performance and reduce fear and stress during pre-slaughter handling and transportation. Biotechnology in Animal Husbandry . 2014;30(3):457–471. doi: 10.2298/BAH1403457M. [DOI] [Google Scholar]
- 23.Xie D., Wang Z. X., Dong Y. L., et al. Effects of monochromatic light on immune response of broilers. Poultry Science . 2008;87(8):1535–1539. doi: 10.3382/ps.2007-00317. [DOI] [PubMed] [Google Scholar]
- 24.Bancroft J. D., Cook H. C. Manual of Histological Techniques and Their Diagnostic Application . Churchill Livingstone; 1994. [Google Scholar]
- 25.Livak K. J., Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods . 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Ib I., Al-Busadah K. A., El-Bahr S. M. Oxidative stress biomarkers and biochemical profile in broilers chicken fed zinc bacitracin and ascorbic acid under hot climate. American Journal of Biochemistry and Molecular Biology . 2013;3:202–214. [Google Scholar]
- 27.Ke Y. Y., Liu W. J., Wang Z. X., Chen Y. X. Effects of monochromatic light on quality properties and antioxidation of meat in broilers. Poultry Science . 2011;90(11):2632–2637. doi: 10.3382/ps.2011-01523. [DOI] [PubMed] [Google Scholar]
- 28.Costa-Pinto F. A., Palermo-Neto J. Neuroimmune interactions in stress. Neuroimmunomodulation . 2010;17(3):196–199. doi: 10.1159/000258722. [DOI] [PubMed] [Google Scholar]
- 29.Quinteiro W. M., Righi D. A., Palermo-Neto J. Effect of cyhalothrin on Ehrlich tumor growth and macrophage activity in mice. Brazilian Journal of Medical and Biological Research . 2009;42(10):912–917. doi: 10.1590/S0100-879X2009001000006. [DOI] [PubMed] [Google Scholar]
- 30.Siegel H. S. Stress, strains and resistance. British Poultry Science . 1995;36(1):3–22. doi: 10.1080/00071669508417748. [DOI] [PubMed] [Google Scholar]
- 31.Selvam R., Suresh S., Saravanakumar M., Chandrasekaran C. V., Prashanth D. Alleviation of heat stress by a polyherbal formulation, Phytocee (TM): impact on zootechnical parameters, cloacal temperature, and stress markers. Pharmacognosy research . 2018;10:1–8. doi: 10.4103/pr.pr_138_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in Figshare repository (10.6084/m9.figshare.17000221.v1).


