PURPOSE:
Smoking after a cancer diagnosis is linked to cancer-specific and all-cause mortality, among other adverse outcomes. Yet, 10%-20% of US cancer survivors are current smokers. Implementation of evidence-based tobacco treatment in cancer care facilities is widely recommended, yet rarely accomplished. This study focuses on the early outcomes of a population-based tobacco treatment program integrated within an National Cancer Institute–designated cancer center.
METHODS AND MATERIALS:
The sample consists of 26,365 patients seen at the cancer center during the first 18 months of program implementation. The study is a retrospective chart review of patients' tobacco use and, among current users, patients' treatment referral response.
RESULTS:
More than 99% of patients were screened for tobacco use. Current (past month) use was observed in 21.05% of patients; cigarettes were the most popular product. Only 17.22% of current users accepted a referral for tobacco treatment; among current users who declined, the majority were not ready to quit (65.84%) or wanted to quit on their own (27.01%). Multiple demographic variables were associated with tobacco use and treatment referral response outcomes.
CONCLUSION:
Despite cancer diagnosis presenting a teachable moment for tobacco cessation, patients with cancer may not be ready to quit or engage with treatment. Clinically proven strategies to increase motivation, prompt quit attempts, and encourage treatment use should be key components of tobacco treatment delivery to patients with cancer.
INTRODUCTION
The 2014 US Surgeon General's Report on Smoking1 articulates that cigarette smoking of patients with cancer plays a causal role in adverse outcomes. Smoking after a cancer diagnosis is causally associated with higher rates of all-cause mortality; cancer-specific mortality; and second primary plus increased risk of recurrence, poor treatment response, and severe toxicity. Those who smoke are more likely than nonsmokers to have postsurgical complications, longer hospital stays, and return to the operating room.2,3 Patients with cancer who smoke also report worse quality of life than former and never smokers.4,5 Smoking undermines health of patients with cancer.
Many US patients with cancer smoke postdiagnosis. In one large recent study (n = 33,525), 16% of patients with cancer reported smoking.6 Similarly, other population-based surveys (n = 2,060-2,527) have found that 9%-19% of patients with cancer smoke.7-9 Prevalence estimates are higher if one focuses on individuals who were smoking at cancer diagnosis.10,11 To summarize, 10%-20% of people with a history of cancer smoke, with higher rates in subgroups of the patient population.
Most health behavior guidelines for patients with cancer recommend tobacco abstinence.12-14 Guidelines also exist for hospitals and clinics to follow in their care of patients with cancer.15-17 The National Comprehensive Cancer Network Clinical Practice Guidelines for Smoking Cessation state that tobacco treatment should be standard of care, integrated throughout cancer care from workup to curative treatment to end-of-life care.18 The National Comprehensive Cancer Network recommends asking every patient with cancer at every visit about smoking status and documenting responses in the electronic medical record (EMR).18 The American Association for Cancer Research has a policy statement that includes universal assessment and documentation of tobacco use as standard of care.17 Furthermore, the American Association for Cancer Research policy statement says that cancer care providers should receive training in tobacco treatment and be incentivized for treatment referral and delivery. There are several approaches to tobacco treatment delivery, including the 5 As (ie, Ask, Advise, Assess, Assist, and Arrange), Ask Advise Refer (AAR), and Ask Advise Connect models. While the 5 As model predicates treatment delivery on patients' willingness to quit, the AAR and Ask Advise Connect models do not, although there are distinctions between how providers refer19,20 versus connect patients with treatment.21 Model differences aside, widespread delivery of tobacco treatment would promote health of patients with cancer. Unfortunately, the results of US cancer care provider surveys found that although 90% ask about tobacco use and 80% advise tobacco cessation, only 40%-45% provide treatment assistance,22,23 and a recent literature review found similar results.24 Clearly, patients with cancer do not uniformly receive the high-quality, population-based tobacco treatment recommended by the foremost cancer care organizations.
To improve cancer care facilities' provision of evidence-based tobacco treatment, the National Cancer Institute (NCI) launched the Cancer Center Cessation Initiative.25 Since 2017, 52 NCI-designated cancer centers have received funding to create or expand existing tobacco treatment programs.26 This implementation science study focuses on one of these cancer centers, one that follows the AAR model and promptly offers tobacco treatment to anyone who reports tobacco use. First, this study will describe rates of tobacco use screening plus rates of tobacco use of patients with cancer and their decision to decline versus accept a tobacco treatment referral. Second, this study aims to identify correlates of tobacco use and referral response of patients with cancer.
METHODS AND MATERIALS
Sample
The sample (N = 26,365) consisted of all outpatients age ≥ 18 years seen at Markey Cancer Center in Lexington, Kentucky, between July 1, 2018, and December 31, 2019, the first 1.5 years of program implementation. Patients are drawn from the cancer center's four outpatient clinics: breast, gynecology, hematology, and other disease site.
Procedures
The tobacco treatment program was prospectively standardized, and this study is a retrospective review of patients' deidentified EMR. In the six months before program implementation, tobacco use was documented 64.0% of the time. Implemented as standard of care for outpatient visits, new intake procedures required clinical service technicians to ask all adults about their tobacco use and document all responses in the EMR. Questions allowed patients to be classified as never, former, or current (past month) tobacco users. Information about tobacco product was obtained if applicable. Patients identified as current tobacco users received an offer of assistance with tobacco cessation. Patients who accepted the offer were automatically e-referred to the Psych-Oncology Service where tobacco treatment specialists (TTSs) were charged with arranging treatment and follow-up (eg, providing counseling and making pharmacotherapy recommendations). If a TTS was available that day, they would meet the patient in clinic and make a treatment plan. If not, the TTS would make at least two attempts to contact the patient via phone to discuss their treatment options. Patients who declined the referral while in clinic were asked for a rationale and advised to consider tobacco treatment in the future. Procedures for this retrospective chart review, which include waivers of Health Insurance Portability and Accountability Act authorization and informed consent documentation, were approved by the University of Kentucky Institutional Review Board (Protocol 52059).
Measures
Data extracted from patients' EMR included the following: (1) demographic characteristics (age, sex, race, ethnicity, relationship status, and insurance), (2) clinical parameters (clinic and distress rating [0 = no distress to 10 = extreme distress27]), and (3) tobacco use outcomes.28 Tobacco use outcomes included rates of (1) lifetime, past, and current tobacco use; (2) tobacco use by product type; (3) tobacco treatment referral response among current tobacco users; and (4) reasons for decline (reportedly already in treatment, wants to quit on their own, or not ready to quit), among the relevant subsample of current tobacco users. Implementation outcomes are current tobacco use, referral response, and reason for decline.
Data Analysis
Descriptive statistics describe the sample and implementation outcomes. Binomial (current tobacco use and referral response) and ordinal (reason for decline) logistic regression models were fit to examine the relationship between the implementation outcomes and covariates (demographic and clinical characteristics).29,30 Covariates were entered simultaneously into regression equations to assess their independent association with each implementation outcome; see the Data Supplement (online only) for more information. Model-adjusted odds ratios (ORs) and 95% CIs are reported. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Sample Characteristics
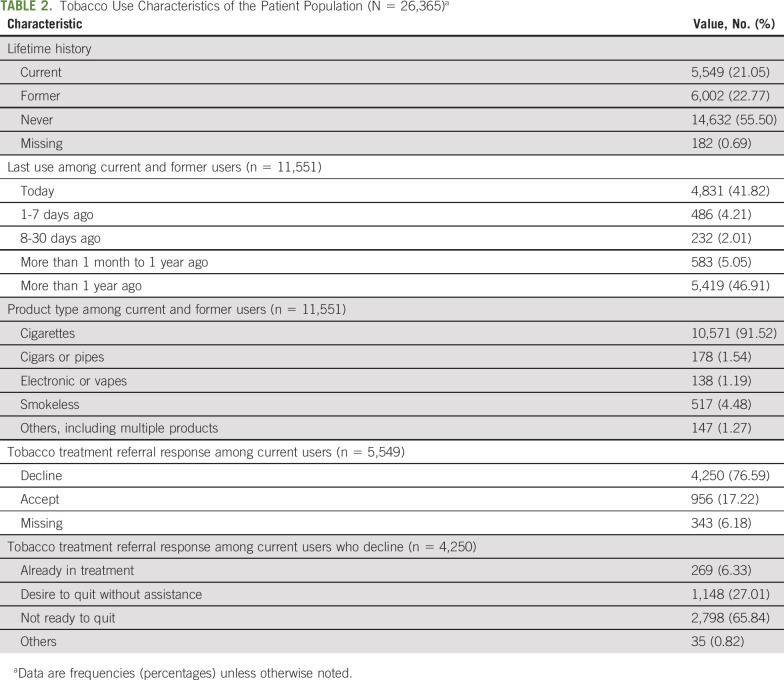
Table 1 details the sample's (N = 26,365) demographic and clinical characteristics. Patients represent an array of disease sites. About one third were male (36.43%, n = 9,604). Most patients were White non-Hispanic (93.11%, n = 24,150). Just more than half of patients were married or partnered (57.95%, n = 9,664). Medicare was the primary type of insurance (44.24%, n = 7,631). The mean age was 59.32 ± 14.34 years. The average distress level was 3.28 ± 3.12, with 24.7% (n = 6,504) reporting clinically significant distress.27
TABLE 1.
Demographic and Clinical Characteristics of the Patient Population (N = 26,365)a
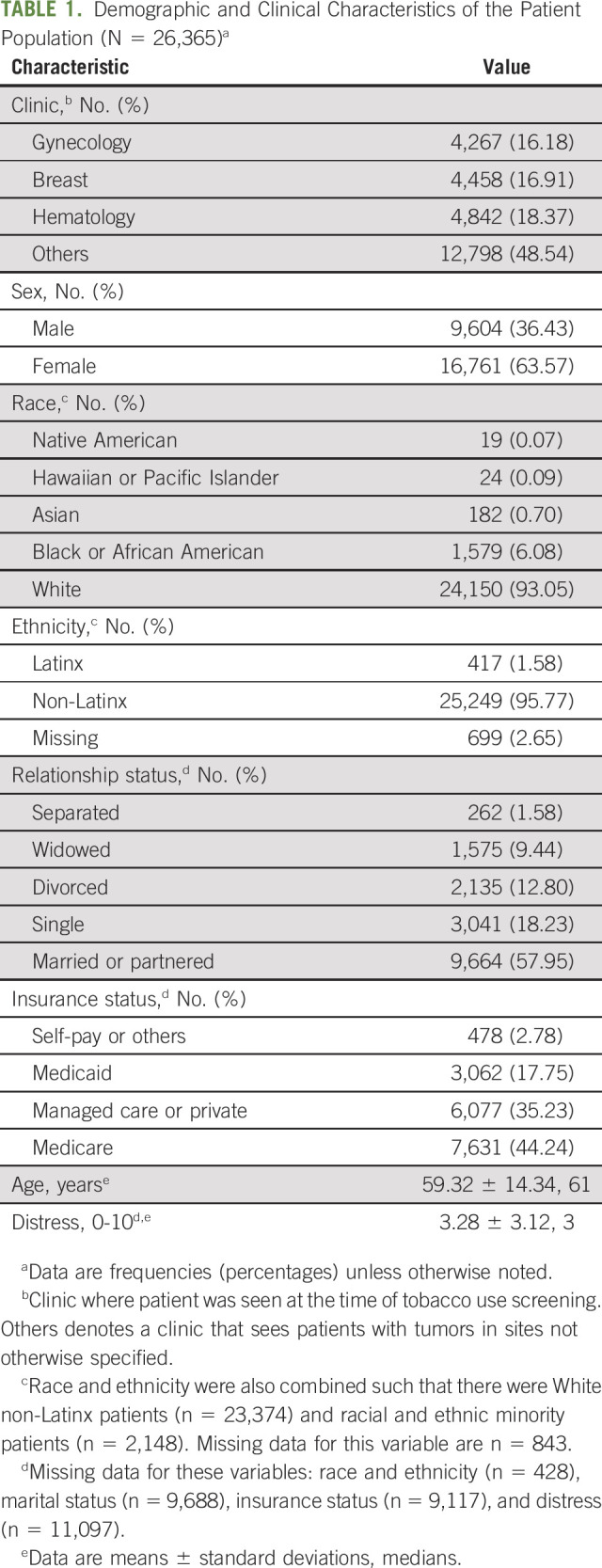
Table 2 presents the sample's tobacco use characteristics. Nearly all (99.3%, n = 26,183) patients' tobacco use status was documented in the EMR. Lifetime tobacco use was reported by 43.82% (n = 11,551) of patients, and cigarettes were most popular (91.52%, n = 10,571). Current tobacco use was observed in 48.04% (n = 5,549) of lifetime users or 21.05% (n = 5,549) of the full sample. Seventy-six percent (n = 4,250) of current users actively declined the offer of tobacco treatment, and another 6.18% (n = 343) of current users were nonresponders or passive refusers. Of those who declined, most (65.84%, n = 2,798) were not ready to quit.
TABLE 2.
Tobacco Use Characteristics of the Patient Population (N = 26,365)a
Associations With Implementation Outcomes
Current tobacco use.
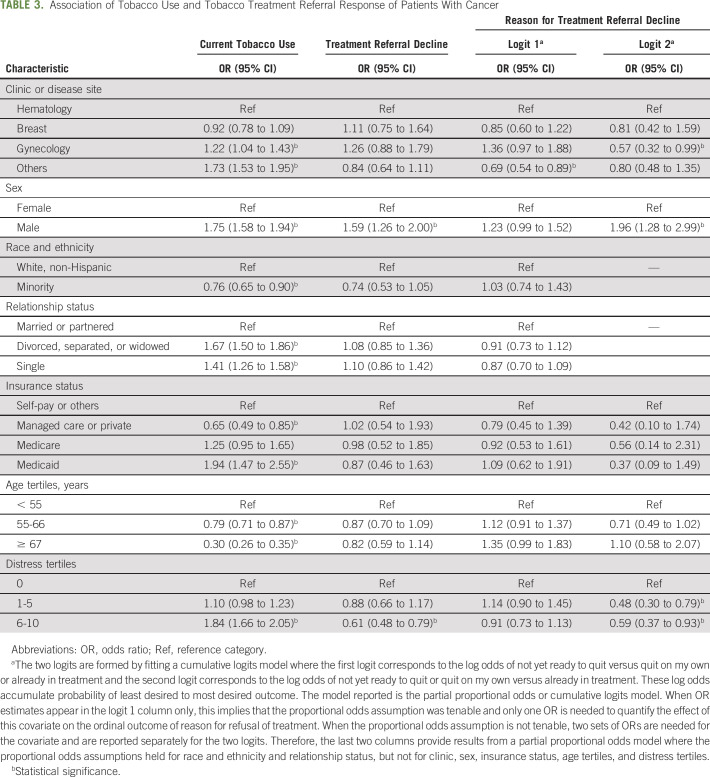
Patients from the gynecology clinic (OR = 1.22; 95% CI, 1.04 to 1.43) and other clinic (OR = 1.73; 95% CI, 1.53 to 1.95) were more likely to use tobacco than patients from the hematology clinic (Table 3). Males were almost twice as likely than females to use tobacco (OR = 1.75; 95% CI, 1.58 to 1.94). Racial and ethnic minorities were less likely than Whites to use tobacco (OR = 0.76; 95% CI, 0.65 to 0.90). Compared with patients in a relationship, those who were single (OR = 1.41; 95% CI, 1.26 to 1.58) and those who were divorced, separated, or widowed (OR = 1.67; 95% CI, 1.50 to 1.86) were about one-and-a-half times more likely to use tobacco. Regarding insurance, compared with self-pay patients, those with Medicaid were nearly twice as likely to use tobacco (OR = 1.94; 95% CI, 1.47 to 2.55), whereas those with managed care or private insurance were much less likely (OR = 0.65; 95% CI, 0.49 to 0.85). As age increased, patients were less likely to use tobacco (OR = 0.79; 95% CI, 0.71 to 0.87; OR = 0.30; CI, 0.26 to 0.35). Finally, those with distress scores ≥ 6 were nearly twice as likely to use tobacco than those with no distress (OR = 1.84; 95% CI, 1.66 to 2.05).
TABLE 3.
Association of Tobacco Use and Tobacco Treatment Referral Response of Patients With Cancer
Referral decline.
Neither clinic, race and ethnicity, relationship status, insurance type, age, nor distress were associated with patients' decision to decline or accept tobacco treatment (Table 3). Males were more likely to decline than females (OR = 1.59; 95% CI, 1.26 to 2.00), and patients with distress scores ≥ 6 were less likely to decline treatment (OR = 0.61; 95% CI, 0.48 to 2.05).
Reason for referral decline.
Neither race and ethnicity, relationship status, insurance type, nor age were associated with reason for decline (Table 3). Relative to hematology clinic patients, those from the other clinic were less likely to report not being ready to quit (OR = 0.69; 95% CI, 0.54 to 0.89) and patients from the gynecology clinic were less likely to report not being ready to quit or preferring to quit without assistance (OR = 0.57; 95% CI, 0.32 to 0.99). Males were more likely to report not being ready to quit or preferring to quit without assistance than females (OR = 1.96; 95% CI, 1.28 to 2.99). Finally, relative to patients with less distress, patients with more distress were less likely to report not being ready to quit or preferring to quit without assistance (distress scores 1-5: OR = 0.48; 95% CI, 0.30 to 0.79; distress scores 6-10: OR = 0.59; 95% CI, 0.37 to 0.93).
DISCUSSION
Previous studies suggest recommendations by the foremost cancer care organizations to conduct population-based tobacco use assessment and provide evidence-based tobacco treatment, which are inadequately met by some cancer care facilities, and smoking rates among some patients with cancer remain high.17,31 This study aimed to determine rates and correlates of tobacco use, tobacco treatment referral decline, and reasons for decline at an NCI-designated cancer center, with the goal of better understanding how to optimize the reach of tobacco treatment in cancer settings. This cancer center's population-based approach increased the percentage of patients with cancer screened for tobacco use from 64% to 99%. This 50% increase reflects nearly universal documentation of tobacco use status in the EMR, a necessary first step to high-quality tobacco treatment delivery.
Three major study findings emerge. First, 20% of adult patients with cancer reported tobacco use. This converges with the upper limits of US population-based survey data6-9 and data from 13 NCI-designated cancer centers, where current smoking rates ranged from 4% to 22%.32-36 In addition to reinforcing concerns about cigarette smoking of patients with cancer, this study highlights the occurrence of noncigarette tobacco use. Although only 1% of patients with cancer in this study engaged in this behavior, other studies have found that 3%-25% of patients with cancer are current users of electronic cigarettes,37,38 a number that may increase with time,39 in part because smokers believe that these products aid smoking cessation.40 Tobacco use is a deeply entrenched behavior that cancer care providers cannot ignore because of fears of upsetting patients or perceptions of inadequate training.22,41 The aforementioned tobacco use rates underscore the need to ask every patient at every visit about tobacco use and advise tobacco users to quit consistent with quality health care delivery42 and best practices for cancer care.17,31 There might even be sufficient reason to extend core items of the Cancer Patient Tobacco Use Questionnaire to include questions about noncigarette tobacco use13,17 and to extend eligibility for clinical trials and treatment programs to all patients with cancer who use tobacco, not just those who smoke cigarettes.43-45 In trying to reach the target audience for tobacco treatment, study results point toward a focus on patients with cancer who are male, are not in a relationship, have Medicaid insurance, and report high distress, consistent with previous studies on correlates of smoking in patients with cancer7,46,47; the results for disease site or clinic were mixed, so firm conclusions are untenable.
The second key finding is that more than three quarters of tobacco users declined a referral for tobacco treatment that was integrated into the cancer care system. This study's treatment acceptance rate (17%) is much lower than that in clinical trials for smoking cessation in patients with cancer (17%-84%).43,44,48-50 This rate is also at the lower end of enrollment rates for other cancer centers' tobacco treatment programs (17%-83%).32,34,45,51-53 Even with the undesirable acceptance rate, the population-based approach resulted in the reach of thousands of patients with cancer who use tobacco. The discrepancy between this and previous studies could be due to this tobacco treatment program's proactive approach (ie, an offer of assistance to every tobacco user) compared with only offering treatment to people who ask for help or report readiness to quit, as is customary in research54 and some clinical implementation.50 By offering treatment to all comers, one would expect a high rate of decline or low rate of acceptance, as most tobacco users are interested in quitting eventually but not right now.55,56 Indeed, most patients with cancer in this study declined treatment because they were not ready to quit. Patients with cancer who use tobacco experience many barriers to quitting (eg, stress, insufficient knowledge or appreciation of smoking's impact on cancer outcomes, and regular exposure to others' tobacco use),54,57 so it may be advantageous to offer tobacco treatment alongside interventions for distress, unmet information or practical needs, and inadequate social support. Additionally, a patient-centered approach for those who want to reduce, but not stop tobacco use entirely, may be advisable and could serve as a gateway to eventual abstinence. For patients with cancer who were ready to quit, tobacco treatment referral was often declined because of the desire to quit on one's own. This result may reflect perceived practical barriers to treatment use (eg, cost and side effects)58,59 and/or a preference to rely on one's internal strength to overcome nicotine dependence,58 both issues that could undermine engagement of patients with cancer in tobacco cessation.
The final key finding concerns correlates of referral response. Patients were significantly more likely to decline tobacco treatment if they were male. This converges with previous studies where male sex predicts patients with cancer declining tobacco treatment.53,60 This study also found that patients with higher levels of distress were less likely to decline tobacco treatment, contrary to some research,60 but possibly consistent with the effective response component of the teachable moment heuristic.61,62 No other variables were significantly associated with treatment referral decline, possibly because of difficulties in predicting a high overall rate of refusal. Upon examining covariate associations with reasons for refusal, patients with cancer were more likely to report low readiness to quit or the preference to quit without assistance if they were male, and less likely to report these outcomes if they endorsed higher distress levels or their cancer site was either gynecologic or others (ie, neither hematologic nor breast). Previous studies have not found demographic or clinical variables that reliably predict readiness to quit, but tobacco use variables (eg, nicotine dependence) consistently play a role.51 To our knowledge, this study is among the first to examine distress level of patients with cancer as a correlate of tobacco treatment acceptance or utilization, with at least one study showing a positive association between negative effect and treatment use.63 By contrast, depressive symptoms and other markers of distress have demonstrated negative associations with readiness and confidence of patients with cancer to quit.48,64,65 Because many patients with cancer experience distress during the acute period of cancer diagnosis and treatment,66 integrating psychologic services into cancer care might help patients capitalize on any effect-related motivation to quit while preventing any distress levels that might impede successful engagement in tobacco treatment. As is, further elucidation of demographic and clinical variables tied to tobacco treatment outcomes is important, as it could lead to more targeted offers and tailored interventions.
Implementation outcomes must be viewed in light of the study's methodology and limitations. First, clinical service technicians were chiefly responsible for screening for tobacco use and offering tobacco treatment. On the one hand, because patients may feel pressure to accept tobacco treatment when asked by physicians or nurses,67 the referral acceptance rates observed might be especially low because of who asked the important questions. On the other hand, patients in this study might have felt more at ease and perhaps were more honest about their tobacco use and treatment readiness because of less perceived stigma or blame since the person asking about tobacco was not the person providing cancer care.68 Second, the predictive models are not comprehensive. The study relied on EMR data, so some known predictors of tobacco use and treatment acceptance (eg, nicotine dependence and risk perception) were unavailable for analysis51 and clinic was not a detailed enough variable to provide definitive answers about the role of disease site in the implementation outcomes. That said, the correlates considered herein are consistent with those in similar studies.51,55,60 Third, there was little racial and ethnic diversity in this sample, which might narrow generalizability to patients who are White, non-Hispanic. Fourth, and also related to the study design, there were sizable missing data for relationship status, insurance, and distress level. Finally, this study lacked biomedical validation of tobacco use status, so abstinence rates may be inaccurate.69
Even with its limitations, this population-based study of more than 25,000 adults provides new information about tobacco use, interest in tobacco treatment, and readiness of patients with cancer to quit. The results underscore the need for cancer care facilities to ask patients with cancer about all forms of tobacco use and among patients who report tobacco use, to stress the critical importance of tobacco cessation as an integral component of high-quality cancer treatment. The results of this study further demonstrate the feasibility of population-based implementation of tobacco use screening and proactive offers of tobacco treatment that have potential to reach wide swatches of the cancer patient population and engage people throughout the tobacco cessation process. Tobacco treatment is an integral component of cancer treatment, and this study provides one example of how the goals of the NCI Cancer Center Cessation Initiative could be met.
ACKNOWLEDGMENT
The authors would like to acknowledge the support of all cancer center administrators, providers, staff, and patients who contributed to this project.
Brent J. Shelton
Employment: University of KY
Research Funding: University of KY
Jamie L. Studts
Consulting or Advisory Role: J&J, AstraZeneca
Joseph Valentino
Research Funding: Rakuten Medical
No other potential conflicts of interest were reported.
DISCLAIMER
The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
PRIOR PRESENTATION
Presented in part as a poster at the 26th Annual Meeting of the Society for Research on Nicotine and Tobacco, New Orleans, LA, March 2020.
SUPPORT
Supported by the National Cancer Institute of the National Institutes of Health under Grants No. P30 CA177558 (Cancer Research Informatics Shared Resource Facility and Patient Oriented and Population Sciences Shared Resource Facility), P30 CA177558-05S5, and K07 CA181351 and the National Institute of Drug Abuse of the National Institutes of Health under Grant No. T32 DA035200.
AUTHOR CONTRIBUTIONS
Conception and design: Jessica L. Burris, Tia N. Borger, Brent J. Shelton, Audrey K. Darville, Jamie L. Studts, Joseph Valentino, Courtney Blair
Administrative support: Courtney Blair
Provision of study materials or patients: Joseph Valentino, Courtney Blair
Collection and assembly of data: Jessica L. Burris, Brent J. Shelton, Jamie L. Studts, Courtney Blair, D. Bront Davis, Joan Scales
Data analysis and interpretation: Jessica L. Burris, Tia N. Borger, Brent J. Shelton, Audrey K. Darville, Jamie L. Studts, Joseph Valentino
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cancer Patients' Tobacco Use and Tobacco Treatment Referral Response: Implementation Outcomes at a National Cancer Institute–Designated Cancer Center
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Brent J. Shelton
Employment: University of KY
Research Funding: University of KY
Jamie L. Studts
Consulting or Advisory Role: J&J, AstraZeneca
Joseph Valentino
Research Funding: Rakuten Medical
No other potential conflicts of interest were reported.
REFERENCES
- 1.US Department of Health and Human Services : The Health Consequences of Smoking- 50 Years of Progress: A Report of the Surgeon General, Executive Summary. Atlanta, GA, Centers for Disease Control and Prevention (US), 2014 [Google Scholar]
- 2.Gajdos C, Hawn MT, Campagna EJ, et al. : The adverse effects of smoking on postoperative outcomes in cancer patients: Smoking and cancer surgery outcomes. Ann Surg Oncol 5:1430-1438, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hatcher JL, Sterba KR, Tooze JA, et al. : Tobacco use and surgical outcomes in head and neck cancer patients. Head Neck 38:700-706, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aigner CJ, Cinciripini PM, Anderson KO, et al. : The association of pain with smoking and quit attempts in an electronic diary study of cancer patients trying to quit. Nicotine Tob Res 18:1449-1455, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mesquita R, Goncalves C, Hayashi D, et al. : Smoking status and its relationship with exercise capacity, physical activity in daily life and quality of life in physically independent, elderly individuals. Physiotherapy 101:55-61, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Swoboda CM, Walker DM, Huerta TR: Likelihood of smoking among cancer survivors: An updated health information national trends survey analysis. Nicotine Tob Res 21:1636-1643, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Gallaway MS, Glover-Kudon R, Momin B, et al. : Smoking cessation attitudes and practices among cancer survivors. J Cancer Surviv 13:66-74, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayer DK, Carlson J: Smoking patterns in cancer survivors. Nicotine Tob Res 13:34-40, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westmaas JL, Alcaraz KI, Berg CJ, et al. : Prevalence and correlates of smoking and cessation- related behavior among survivors of ten cancers: Findings from a nationwide survey nine years after diagnosis. Cancer Epidemiol Biomarkers Prev 23:1783-1792, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Tseng TS, Lin HY, Moody-Thomas S, et al. : Who tended to continue smoking after cancer diagnosis: The national health and nutrition examination survey 1999-2008. BMC Public Health 12:784, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burris JL, Studts JL, DeRosa AP, et al. : Systematic review of tobacco use after lung or head/neck cancer diagnosis: Results and recommendations for future research. Cancer Epidemiol Biomarkers Prev 24:1450-1461, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention : A National Plan for Cancer Survivorship: Advancing Public Health Strategies. Atlanta, GA, U.S. Department of Health and Human Services, 2004 [Google Scholar]
- 13.Doyle C, Kushi LH, Byers T, et al. : Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. CA Cancer J Clin 56:323-353, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Smith R, Andrews KS, Brooks D, et al. : Cancer screening in the United States, 2017: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 67:100-121, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Hanna N: Helping patients quit tobacco: ASCO's efforts to help oncology care specialists. J Oncol Pract 9:263-264, 2013 [DOI] [PubMed] [Google Scholar]
- 16.International Society of Nurses in Cancer Care : Position Statement Title: ISNCC Tobacco Position Statement. Vancouver, BC, International Society of Nurses in Cancer Care, 2014 [Google Scholar]
- 17.Toll BA, Brandon TH, Gritz ER, et al. : Assessing tobacco use by cancer patients and facilitating cessation: An American Association for Cancer Research Policy Statement. Clin Cancer Res 19:1941-1948, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shields PG, Bierut L, Arenberg D, et al. : NCCN Clinical Practice Guidelines in Oncology: Smoking NCCN Guidelines. Version 1.2021, Cessation. 2021. [Google Scholar]
- 19.Schroeder SA: What to do with a patient who smokes. J Am Med Assoc 294:482-487, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Davidson SM, Boldt RG, Louie AV: How can we better help cancer patients quit smoking? The London Regional Cancer Program experience with smoking cessation. Curr Oncol 25:226-230, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vidrine JI, Shete S, Cao Y, et al. : Ask-advise-connect: A new approach to smoking treatment delivery in health care settings. JAMA Intern Med 173:458-464, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warren GW, Marshall JR, Cummings KM, et al. : Practice patterns and perceptions of thoracic oncology providers on tobacco use and cessation in cancer patients. J Thorac Oncol 8:543-548, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warren GW, Kasza KA, Reid ME, et al. : Smoking at diagnosis and survival in cancer patients. Int J Cancer 132:401-410, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Price SN, Studts JL, Hamann HA: Tobacco use assessment and treatment in cancer patients: A scoping review of oncology care clinician adherence to clinical practice guidelines in the U.S. Oncologist. Oncologist 24:229-238, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Cancer Institute : Cancer center cessation initiative. cancer.gov
- 26.Croyle RT, Morgan GD, Fiore MC: Addressing a core gap in cancer care—The NCI moonshot program to help oncology patients stop smoking. N Engl J Med 380:512-515, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roth AJ, Kornblith AB, Batel-copel L, et al. : Rapid screening for psychologic distress in men with a pilot study. Cancer 82:1904-1908, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Land SR, Toll BA, Moinpour CM, et al. : Research priorities, measures, and recommendations for assessment of tobacco use in clinical cancer research. Clin Cancer Res 22:1907-1913, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Box AGEP, Tidwell PW: Transformation of the independent variables. Technometrics 4:531-550, 1962 [Google Scholar]
- 30.Stokes ME, Davis CS, Koch GG: Categorical Data Analysis Using SAS (ed 3). Cary, NC, SAS Institute, 2012 [Google Scholar]
- 31.Shields PG, Herbst RS, Arenberg D, et al. : NCCN Clinical Practice Guidelines in Oncology: Smoking Cessation. 2018 [Google Scholar]
- 32.Gali K, Pike B, Kendra MS, et al. : Integration of tobacco treatment services into cancer care at Stanford. Int J Environ Res Public Health 17:2101, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramsey AT, Baker TB, Pham G, et al. : Low burden strategies are needed to reduce smoking in rural healthcare settings: A lesson from cancer clinics. Int J Environ Res Public Health 17:1728, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis JM, Thomas LC, Dirkes JEH, et al. : Strategies for referring cancer patients in a smoking cessation program. Int J Environ Res Public Health 17:6089, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.May JR, Klass E, Davis K, et al. : Leveraging patient reported outcomes measurement via the electronic health record to connect patients with cancer to smoking cessation treatment. Int J Environ Res Public Health 17:5034, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angelo HD, Rolland B, Adsit R, et al. : Tobacco treatment program implementation at NCI cancer centers: Progress of the NCI Cancer MoonshotFfunded Cancer Center Cessation Initiative. Cancer Prev Res (Phila) 12:735-740, 2019 [DOI] [PubMed] [Google Scholar]
- 37.Akinboro O, Nwabudike S, Elias R, et al. : Electronic cigarette use among survivors of smoking-related cancers in the United States. Cancer Epidemiol Biomarkers Prev 28:2087-2094, 2019 [DOI] [PubMed] [Google Scholar]
- 38.Borderud SP, Li Y, Burkhalter JE, et al. : Electronic cigarette use among patients with cancer: Characteristics of electronic cigarette users and their smoking cessation outcomes. Cancer 120:3527-3535, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Creamer MR, Wang TW, Babb S: Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Morb Mortal Wkly Rep 68:1013-1019, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.James SA, Cheney MK, Smith KM, et al. : Experiences of women with cervical dysplasia and associated diagnoses using electronic cigarettes for smoking substitution. Heal Expect 22:931-938, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nath Simmons V, Litvin EB, Jacobsen PB, et al. : Predictors of smoking relapse in patients with thoracic cancer or head and neck cancer. Cancer 119:1420-1427, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patnode CP, Henderson JT, Thompson JH, et al. : Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: A review of reviews for the U.S. Preventative Services Task Force. Ann Intern Med 163:608-621, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Dahm JL, Cook E, Baugh K, et al. : Predictors of enrollment in a smoking cessation clinical trial after eligibility screening. J Natl Med Assoc 101:450-455, 2019 [DOI] [PubMed] [Google Scholar]
- 44.Ostroff JS, Burkhalter JE, Cinciripini PM, et al. : Randomized trial of a presurgical scheduled reduced smoking intervention for patients newly diagnosed with cancer. Health Psychol 33:737-747, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Japuntich SJ, Luberto CM, Streck JM, et al. : Integrating tobacco treatment into thoracic oncology settings: Lessons learned. J Health Psychol 21:2813-2823, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanera IM, Bolman CAW, Mesters I, et al. : Prevalence and correlates of healthy lifestyle behaviors among early cancer survivors. BMC Cancer 16:4, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Little MA, Klesges RC, Bursac Z, et al. : Correlates of smoking status in cancer survivors. J Cancer Surviv 12:828-834, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez E, Tatum KL, Weber DM, et al. : Issues related to implementing a smoking cessation clinical trial for cancer patients. Cancer Causes Control 20:97-104, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duffy SA, Scheumann AL, Fowler KE, et al. : Perceived difficulty quitting predicts enrollment in a smoking-cessation program for patients with head and neck cancer. Oncol Nurs Forum 37:349-356, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gritz ER, Carr CR, Rapkin DA, et al. : A smoking cessation intervention for head and neck cancer patients: Trial design, patient accrual, and characteristics. Cancer Epidemiol Biomarkers Prev 1:67-73, 1991 [PubMed] [Google Scholar]
- 51.Schnoll RA, Rothman RL, Newman H, et al. : Characteristics of cancer patients entering a smoking cessation program and correlates of quit motivation: Implications for the development of tobacco control programs for cancer patients. Psychooncology 358:346-358, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Amato KA, Reid ME, Ochs-balcom HM, et al. : Evaluation of a dedicated tobacco cessation support service for thoracic cancer center patients. J Public Manag Pract 24:12-19, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schnoll RA, Rothman RL, Lerman C, et al. : Comparing cancer patients who enroll in a smoking cessation program at a comprehensive cancer center with those who decline enrollment. Head Neck 26:278-286, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Westmaas JL, Berg CJ, Alcaraz KI, et al. : Health behavior theory constructs and smoking and cessation-related behavior among survivors of ten cancers nine years after diagnosis: A report from the American Cancer Society's Study of Cancer Survivors. Psychooncology 24:1286-1294, 2015 [DOI] [PubMed] [Google Scholar]
- 55.Burris JL, Wahlquist AE, Carpenter MJ: Characteristics of cigarette smokers who want to quit now versus quit later. Addict Behav 38:2257-2260, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Babb S, Malarcher A, Schauer G, et al. : Quitting smoking among adults—United States, 2000-2015. MMWR Morb Mortal Wkly Rep 65:1457-1464, 2017 [DOI] [PubMed] [Google Scholar]
- 57.Wells M, Aitchison P, Harris F, et al. : Barriers and facilitators to smoking cessation in a cancer context: A qualitative study of patient, family and professional views. BMC Cancer 17:348-362, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morphett K, Partridge B, Gartner C, et al. : Why don't smokers want help to quit? A qualitative study of smokers' attitudes towards assisted vs. unassisted quitting. Int J Environ Res Public Health 12:6591-6607, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith AL, Carter SM, Dunlop SM, et al. : The views and experiences of smokers who quit smoking unassisted: A systematic review of the qualitative evidence. PLoS One 10:e0127144, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheffer CE, Stein JS, Petrucci C, et al. : Tobacco dependence treatment in oncology: Initial patient clinical characteristics and outcomes from Roswell Park Comprehensive Cancer Center. Int J Environ Res Public Health 17:3907, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McBride CM, Ostroff JS: Teachable moments for promoting smoking cessation: The context of cancer care and survivorship. Cancer Control 10:325-333, 2003 [DOI] [PubMed] [Google Scholar]
- 62.McBride CM, Puleo E, Pollak KI, et al. : Understanding the role of cancer worry in creating a “teachable moment” for multiple risk factor reduction. Soc Sci Med 66:790-800, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee J, Cheong JW, Markham MJ, et al. : Negative affect and the utilization of tobacco treatment among adult smokers with cancer. Psychooncology 30:93-102, 2021 [DOI] [PubMed] [Google Scholar]
- 64.Duffy SA, Karvonen-Gutierrez CA, Ewing LA, et al. : Implementation of the tobacco tactics program in the department of veterans affairs. J Gen Intern Med 25:3-10, 2010. (suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schnoll RA, James C, Malstrom M, et al. : Longitudinal predictors of continued tobacco use among patients diagnosed with cancer. Soc Behav Med 25:214-221, 2003 [DOI] [PubMed] [Google Scholar]
- 66.Carlson LE, Zelinski EL, Toivonen KI, et al. : Prevalence of psychosocial distress in cancer patients across 55 North American cancer centers. J Psychosoc Oncol 37:5-21, 2019 [DOI] [PubMed] [Google Scholar]
- 67.Hoover DS, Spears CA, Vidrine DJ, et al. : Smoking cessation treatment needs of low SES cervical cancer survivors. Am J Health Behav 43:606-620, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen Johnson M, Hamann HA, Thomas AJ, et al. : Association between patient-provider communication and lung cancer stigma. Support Care Cancer 24:2093-2099, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morales NA, Romano MA, Cummings KM, et al. : Accuracy of self-reported tobacco use in newly diagnosed cancer patients. Cancer Causes Control 24:1223-1230, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]