Abstract
In order to see if the biodegradative pathways for morpholine and thiomorpholine during degradation by Mycobacterium aurum MO1 could be generalized to other heterocyclic compounds, the degradation of piperidine by this strain was investigated by performing 1H-nuclear magnetic resonance directly with the incubation medium. Ionspray mass spectrometry, performed without purification of the samples, was also used to confirm the structure of some metabolites during morpholine and thiomorpholine degradation. The results obtained with these two techniques suggested a general pathway for degradation of nitrogen heterocyclic compounds by M. aurum MO1. The first step of the degradative pathway is cleavage of the C—N bond; this leads formation of an intermediary amino acid, which is followed by deamination and oxidation of this amino acid into a diacid. Except in the case of thiodiglycolate obtained from thiomorpholine degradation, the dicarboxylates are completely mineralized by the bacterial cells. A comparison with previously published data showed that this pathway could be a general pathway for degradation by other strains of members of the genus Mycobacterium.
Biodegradation of industrial organic pollutants, especially heterocyclic compounds such as morpholine, is of special environmental interest. Morpholine has great industrial importance and a wide range of applications; it is used as an anticorrosive agent in water boiling systems and as a chemical intermediate (catalyst, solvent, antioxidant, etc.) in the manufacture of rubber additives and in the textile industry. To date, only strains belonging to the genus Mycobacterium have been reported to be able to use morpholine as a sole source of carbon, nitrogen, and energy (1, 2, 4, 7, 8, 14–16, 20–22, 24). Moreover, the high water solubility of this compound and the potential for conversion to the potent mutagen and carcinogen N-nitrosomorpholine make it a xenobiotic compound of special interest from an environmental point of view (9, 11, 26).
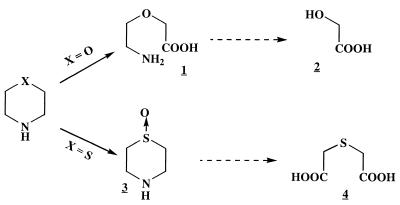
The metabolic pathway involved in biodegradation of morpholine has been very difficult to establish, because this chemical does not possess any chromophore and is highly soluble in water, which does not allow easy extraction. Consequently, no tool for direct detection of intermediates or even morpholine has been available. Only indirect strategies have been developed previously; these strategies include chemical oxygen demand, optical density, and NH3 measurements, growth on intermediates, and in vitro enzymatic assays. Recently, we developed a new approach, in which 1H-nuclear magnetic resonance (1H-NMR) spectroscopy is performed directly with culture supernatants without preliminary purification to identify some metabolic intermediates of morpholine and thiomorpholine degradation by two Mycobacterium strains, Mycobacterium aurum MO1 and Mycobacterium sp. strain RP1 (1, 7, 8, 21) (Fig. 1).
FIG. 1.
Intermediates of the morpholine and thiomorpholine biodegradation pathway in M. aurum and Mycobacterium sp. strain RP1, as shown by in situ 1H-NMR (1, 7, 8, 21). 1, 2-(2-aminoethoxy)acetic acid; 2, glycolic acid; 3, sulfoxide; 4, thiodiglycolic acid.
The result of the first step in morpholine degradation is either cleavage of the C—N bond, which leads to an amino acid, or oxidation of the sulfur atom to sulfoxide prior to the ring-opening step. By using a specific inhibitor, metyrapone, we showed previously that these reactions are initiated by a cytochrome P450 (8, 21). This finding was the first evidence of the presence of a cytochrome P450 in mycobacteria. Since then, Poupin et al. have shown that a soluble cytochrome P450 is induced during degradation of morpholine in nine bacterial strains isolated from three different environments (22). Also, cloning and characterization of the genes encoding a cytochrome P450 involved in piperidine and pyrrolidine utilization and its regulatory protein have been described recently for Mycobacterium smegmatis mc2155 (23). The complete genome sequence of Mycobacterium tuberculosis contains 20 genes that potentially code for cytochrome P450 proteins (6). All of these findings stress the key role played by cytochrome P450 in mycobacteria, especially in biodegradative processes.
In order to see if the biodegradation pathways for morpholine and thiomorpholine observed with M. aurum MO1 could be generalized to other saturated nitrogen heterocyclic compounds, we performed new experiments in which 1H-NMR was used. A new technique, direct ionspray mass spectrometry of the incubation supernatants, was also used to confirm the structures of some metabolites during morpholine and thiomorpholine degradation. The results obtained with these two techniques suggested a general pathway for biodegradation of nitrogen heterocyclic compounds by M. aurum MO1.
MATERIALS AND METHODS
Chemicals.
Morpholine, thiomorpholine, piperidine, dioxane, 2,6-dimethylmorpholine, diglycolic acid, glycolic acid, and glutaric acid were purchased from Aldrich, and tetradeuterated sodium trimethylsilylpropionate (TSPd4) was purchased from EurisoTop (Saint Aubin, France).
Growth conditions.
M. aurum MO1 was isolated by Cech et al. (4) and was grown in 100-ml portions of Trypticase soy broth (bioMérieux, Marcy l'Etoile, France) in 500-ml Erlenmeyer flasks incubated at 30°C with agitation at 200 rpm. The cells were harvested after 48 h of incubation.
Incubation with xenobiotic compounds.
Cells were harvested by centrifugation at 9,000 × g for 15 min at 5°C, and the pellet was washed twice with Knapp buffer (containing [per liter of distilled water] 1 g of KH2PO4, 1 g of K2HPO4, 4 mg of FeCl3, and 40 mg of MgSO4 · 7H2O; pH 6.6) and then resuspended in this buffer (5 g of wet cells in 50 ml of buffer). The cells were incubated with a xenobiotic compound at a concentration of 10 mM as the only source of energy in a 500-ml Erlenmeyer flask at 30°C with agitation (200 rpm). The negative controls consisted of preparations incubated under the same conditions without a substrate or cells. Samples (1 ml) were taken regularly and centrifuged at 12,000 × g for 5 min. The supernatants were isolated and immediately frozen until an NMR analysis was performed.
For ionspray mass spectrometry experiments, Knapp buffer was replaced by distilled water. In general, the use of a nonvolatile buffer (Knapp buffer) is strictly prohibited in routine applications.
1H-NMR spectroscopy.
The methods used to prepare NMR samples and the methods used to obtain a spectrum with a Bruker model Avance 300 DSX spectrometer (Larmor frequency, 300.13 MHz) at 21°C with 5-mm diameter tubes have been described previously (7), as has the method used for quantification of the metabolites.
Ionspray mass spectrometry. (i) Preparation of samples.
Supernatant (500 μl) was filtered (Analypore; pore size, 0.22 μm; Fischer Scientific), and the pH was measured.
(ii) Ionspray spectra.
The ionspray mass spectrometry analysis was performed with a Perkin-Elmer model Sciex API 165 mass spectrometer. The instrument was operated with pressure ionization by utilizing a PE Sciex Turboionspray interface. An Apple Macintosh System 8.1 computer with the Mass Chrom v1.0 application was used for data acquisition and processing.
The data were acquired in full-scan mode at a range of 20 to 250 amu by using a step of 0.2 amu and a dwell time of 15 ms/scan. The ionspray voltage was adjusted to 5,500 V in the positive mode and to −4,500 V in the negative mode. The heater gas flow rate was 7 liters/min. The nebulizer gas was at position 10, and the curtain gas was at position 11. The voltages on the curtain plate orifice (COR) were in the positive mode 20V, 60 V, and the rings (RNG) were at 220 and 230 V.
The samples were introduced with an infusion pump (Harvard Apparatus Canada, St. Laurent, Quebec, Canada) at a flow rate of 0.6 ml/h.
RESULTS AND DISCUSSION
Degradation of piperidine by M. aurum MO1 as determined by 1H-NMR.
The spectra for the supernatants collected after 2 and 6 h of incubation of M. aurum MO1 with 10 mM piperidine are shown in Fig. 2A and B, respectively. The spectra which we obtained were compared with the spectra for reaction mixtures without cells or substrates.
FIG. 2.
Piperidine degradation by M. aurum MO1. Resting cells (5 g [wet weight] of cells in 50 ml of Knapp buffer [1 g of KH2PO4 per liter, 1 g of K2HPO4 per liter, 4 mg of FeCl3 per liter, 40 mg of MgSO4 · 7H2O per liter; pH 6.6]) were incubated with 10 mM piperidine at 30°C with agitation (200 rpm). Samples (1 ml) were collected every hour for 12 h and from time to time until 30 h; after centrifugation, the supernatants of these samples were analyzed by 1H-NMR spectroscopy at 300.13 MHz. TSPd4 was used as a reference compound for chemical shifts and quantification. (A) 1H-NMR spectrum of a sample collected at 2 h (B) 1H-NMR spectrum of a sample collected at 6 h. (C) Time courses for the concentrations of piperidine (□) and glutarate (×). Compounds were quantified by integrating the signals in 1H-NMR spectra relative to the area for the reference compound TSPd4.
In Fig. 2A (time, 2 h), the three signals belonging to piperidine are visible. Also, a singlet that belongs to the methyl groups of TSPd4 was detected at 0 ppm (data not shown); this signal was used as an internal reference for calibration of chemical shifts and integrals.
In Fig. 2B (time, 6 h), the signals for piperidine are completely absent, but two new signals are present. These two signals were assigned to the protons of C-2 and C-3 of glutarate. This assignment was confirmed by adding authentic glutarate to the sample.
Quantitative analysis of the kinetics of degradation of piperidine (Fig. 2C) was performed by integrating the signals of the different metabolites in 1H-NMR spectra; the measured areas were compared to the integral of the TSPd4 signal in order to calculate the different concentrations of metabolites and the parent molecule.
Piperidine was exhausted after 3 h of incubation. The glutarate concentration increased with time until 5 h and then decreased. No glutaric acid was detected after 20 h, and no other metabolite appeared. Consequently, glutaric acid was degraded in the cells; presumably, it entered the pathway for amino acid metabolism, especially lysine metabolism. Large and Robertson described the route of l-lysine breakdown by Candida tropicalis via 5-aminovalerate and glutarate as metabolic intermediates (19).
The metabolic pathway described above is consistent with the pathway observed previously for metabolism of morpholine, thiomorpholine (7), and pyrrolidine (24) in M. aurum MO1 and Mycobacterium sp. strain RP1 and/or MORG; opening of the piperidine ring (C—N bond cleavage) should lead to production of 5-aminovaleric acid, which is then quickly deaminated and oxidized, yielding glutaric acid (Fig. 3). The C—N bond cleavage is likely to be initiated by a cytochrome P450 activity. We showed previously that this protein was induced by this substrate (8) and also that it was involved in this cleavage reaction. Biodegradation of morpholine was inhibited by adding metyrapone, a specific inhibitor, to the culture medium. The total mineralization of piperidine by M. aurum MO1 is also consistent with the ability of this strain to grow on piperidine as a sole source of carbon and nitrogen (20).
FIG. 3.
General metabolic pathway for degradation of nitrogen heterocyclic compounds by M. aurum MO1.
The following two other heterocyclic compounds were also tested: dioxane (a heterocyclic compound without a nitrogen atom) and 2,6-dimethylmorpholine (which has a substituent in both positions adjacent to the nitrogen). Even after 31 h of incubation with each of these substrates under the conditions described previously for piperidine, degradation by M. aurum MO1 was not observed (data not shown). The same results were obtained with Mycobacterium sp. strain RP1 (unpublished results).
It has been reported previously (20, 21) that two Mycobacterium strains were not able to grow in the presence of tetrahydropyrane or tetrahydrofurane, which suggested that these compounds were not degraded. In addition, these substrates did not induce the activity of a cytochrome P450.
These results suggest that the absence of a C—N bond or substitution at the α position by a methyl group prevents opening of the ring.
Identification of morpholine and thiomorpholine metabolites by ionspray spectrometry.
Based on data collected in our previous and present studies, a general pathway for degradation of nitrogen heterocyclic compounds by M. aurum MO1 can be suggested (Fig. 3). Cleavage of the C—N bond leads to formation of an amino acid, which undergoes deamination and oxidation. In the case of thiomorpholine and piperidine, a dicarboxylic acid was produced under these conditions (thiodiglycolic acid and glutaric acid, respectively). In the case of morpholine, a singlet resonating at 3.95 ppm was assigned to glycolate (by coincidence after the commercial compound was added to the sample). We also showed that this intermediate was integrated in central metabolism (7). However this raises the following question: Is diglycolate an intermediate of degradation, as observed for the other heterocyclic compounds? Unfortunately, the 1H chemical shift of diglycolate is the same (difference, less that 5 × 10−3 ppm) as that of glycolate whatever the pH (so 1H-NMR is not suitable for detecting this metabolite). In contrast, the two compounds have different 13C chemical shifts (64.19 and 72.37 ppm for the methylene groups of glycolate and diglycolate, respectively), but the concentration of metabolites was too low to detect 13C resonance either directly (one-dimensional spectra) or indirectly (two-dimensional HMQC experiments). Consequently, we used a new approach to detect the presence of diglycolic acid. Again, this technique had to be performed directly with the culture medium as no purification of the products was possible; it also had to be rather noninvasive (so the metabolites were not destroyed). Ionspray mass spectrometry was an ideal tool, except that the presence of nonvolatile ions, particularly phosphate ions (concentration in Knapp buffer, 15 mM), had to be avoided.
Incubation of M. aurum MO1 in the absence of inorganic phosphate.
Our first attempts to eliminate phosphates, in which we used barium hydroxide precipitation followed by Dowex exchange, were unsuccessful. We decided to incubate Mycobacterium cells directly in distilled water. Under these new conditions, degradation of morpholine (10 mM) by M. aurum MO1 was monitored as previously described (7). Figure 4 shows a comparison of two spectra collected after 12 h of incubation in Knapp buffer (pH 7.31) (Fig. 4A) and after 13 h of incubation in distilled water (pH 7.35) (Fig. 4B). The kinetics of degradation and the observed metabolites were the same as those obtained previously with Knapp buffer. Similar results were obtained for degradation of thiomorpholine (data not shown). Mycobacterium cells are able to grow without inorganic phosphate. This is probably due to the presence of inorganic phosphate polymers present in various bacteria, particularly in members of the genus Mycobacterium (17, 18, 25). These polymers can be used by the cells under Pi-limited conditions.
FIG. 4.
Morpholine degradation by M. aurum MO1 cells. 1H-NMR spectra of samples were obtained after 12 h of incubation in Knapp buffer (spectrum A) and after 13 h of incubation in distilled water (spectrum B).
Degradation of morpholine.
Mycobacterium cells (5 g [wet weight] of cells in 50 ml of distilled water) were incubated as previously described in the presence of 10 mM morpholine. Each sample was analyzed in parallel by 1H-NMR and ionspray mass spectrometry. For the latter technique, after centrifugation of the samples, the supernatants were filtered through a 0.22-μm-pore-size membrane to eliminate the cell debris before injection and analysis with the mass spectrometer. Ionspray mass spectra were recorded under a positive mode and under a negative mode.
As an example, Fig. 5 shows the ionspray mass spectra of a sample obtained after 13 h of incubation of M. aurum MO1 cells with morpholine. Under a positive mode, signals at m/z 120, m/z 142, and m/z 158, corresponding to the [M+H]+, [M+Na]+ and [M+K]+ adducts of 2-(2-aminoethoxy)acetate, respectively, were clearly detected. These signals were not present at time zero, while a signal at m/z 88 corresponding to the [M+H]+ adduct of morpholine was the sole signal (data not shown). Formation of this intermediary amino acid, which resulted from cleavage of the C—N bond of morpholine, was in complete agreement with the results obtained previously by 1H-NMR. Also, Swain et al. suggested that 4-aminobutyrate was produced from degradation of pyrrolidine by Mycobacterium sp. strain MORG (24); this suggestion was based on the scheme for Pseudomonas fluorescens described previously by Jacoby and Fredericks (12).
FIG. 5.
Ionspray mass spectra recorded under negative (A) and positive (B) ionization with an infusion pump for a sample collected after 13 h of incubation of M. aurum MO1 cells (100 g liter−1) in distilled water supplemented with 10 mM morpholine.
Under a negative mode, a strong signal was present at m/z 133, and this signal was assigned to the [M−H]− anion of diglycolic acid. This assignment was confirmed by the presence of the [M−2H+Na]− and [M−2H+K]− adducts at m/z 155 and m/z 171, respectively. A signal corresponding to the [M−H]− adduct of glycolic acid was also detected at m/z 75. This signal could have come either from the presence of this compound as a metabolite in the biodegradative process or from breakdown of diglycolic acid in the mass spectrometer. None of these signals were detected in spectra at time zero, indicating that they corresponded to metabolites of morpholine. The metabolites observed by ionspray mass spectrometry are in complete agreement with those observed by 1H-NMR. This was true for the other samples all along the kinetics (data not shown). These experiments clearly showed that diglycolic acid is an intermediate in morpholine metabolism; this diacid is further cleaved to form glycolic acid and is completely mineralized by the cells. To confirm this last finding, M. aurum cells were incubated with diglycolic acid (5 mM), and the kinetics of this degradation was monitored by 1H-NMR. This compound was completely degraded in 20 h (data not shown).
We showed previously that glycolate was degraded by M. aurum MO1 and Mycobacterium sp. strain RP1 (7, 21). Swain et al. found evidence of enzymes of the glycolate branch in Mycobacterium sp. strain MORG. These results clearly show that the ethanolamine branch proposed previously (20, 24) as a downstream pathway for morpholine degradation is not present. Indeed, cleavage of 2-(2-aminoethoxy)acetate in ethanolamine would be accompanied by production of oxalate without diglycolate. Actually, although the enzymes of this pathway branch are naturally present in mycobacteria (24), no intermediate of ethanolamine degradation was found when the cells were incubated with morpholine (7, 21). Also, Swain et al. (24) showed that only the enzymes of the glycolate branch were knocked out in Mycobacterium sp. strain MORG mutants that were not able to degrade morpholine (Mor−), while the enzymes of the ethanolamine branch were not modified. These results are consistent with a unique route via glycolate.
Degradation of thiomorpholine.
The ionspray mass spectrometry method used to study morpholine degradation was applied to thiomorpholine degradation. Our aims were (i) to validate this new approach as a general technique, (ii) to confirm the structure of the metabolites deduced by 1H-NMR analyses, and (iii) to eventually identify new metabolites.
An 1H-NMR spectrum recorded after 14 h of incubation of M. aurum MO1 with 10 mM thiomorpholine in distilled water is shown in Fig. 6A). The signals corresponding to the sulfoxide of thiomorpholine, whose synthesis and NMR shifts have been described previously (8), and to thiodiglycolate are indicated on the spectrum; thiomorpholine was completely metabolized at the time examined. Note that these metabolites are the same metabolites observed when the cells were incubated in Knapp buffer (8).
FIG. 6.
Results of an analysis performed after 14 h of incubation of M. aurum MO1 cells (100 g liter−1) in distilled water in the presence of thiomorpholine (10 mM). (A) 1H-NMR spectrum. (B) Mass spectrometry spectra obtained under positive (upper spectrum) and negative (lower spectrum) ionization with an infusion pump. In the positive mode, the OR voltage applied was 20 V and the RNG voltage was 220 V. In the negative mode, the OR voltage was −60 V and the RNG voltage was −330 V.
Figure 6B shows the corresponding ionspray mass spectra. Under a positive mode, an intense signal was detected at m/z 120, corresponding to the [M+H]+ adduct of the sulfoxide of thiomorpholine. The [M+K]+ adduct was also detected at m/z 158. These signals were not present at time zero, while a signal at m/z 104 corresponding to the [M+H]+ adduct of thiomorpholine was present. Under a negative mode, a signal at m/z 149 was assigned to the [M−H]− anion of thiodiglycolic acid; this signal was not present at time zero.
In conclusion, we were able to use ionspray mass spectrometry in the case of thiomorpholine. We clearly identified the sulfoxide of thiomorpholine and thiodiglycolate, which confirmed unambiguously the assignments of 1H-NMR resonances.
Conclusions.
In this paper we describe a new method in which we performed ionspray mass spectrometry directly with incubation medium without purification. Until now, only liquid chromatography or gas chromatography coupled with mass spectrometry has been used to analyze biological fluids containing natural metabolites or xenobiotic compounds (3, 5, 10, 13). In our study, heterocyclic compounds could not be purified by chromatography, so we had to develop a direct approach. Using this technique, we found a new metabolite, diglycolate, which is not detectable by 1H-NMR. Identification of this dicarboxylate was essential for the proposal for a general pathway for heterocyclic compound degradation by Mycobacterium strains. Also, we could confirm unambiguously the identities of other morpholine and thiomorpholine metabolites.
This method described here is limited by the presence of large amounts of nonvolatile ions, such as phosphate ions. We overcame this problem by incubating M. aurum MO1 cells in pure water; we showed that the metabolites obtained under these conditions were the same as the metabolites obtained in the presence of buffer. We believe that our approach can be applied in many situations as many microorganisms contain large amounts of polyphophates (17, 18).
This work provides new information, but it also confirms the findings obtained by 1H-NMR concerning the metabolic pathways involved in biodegradation of morpholine and its analogues by M. aurum MO1. A general pathway can be proposed for degradation of nitrogen heterocyclic compounds; the metabolic routes are present in various Mycobacterium strains (MO1, RP1, and MORG) and might be general routes for members of this genus.
The tandem 1H-NMR–ionspray mass spectrometry method performed directly with the incubation medium is a powerful tool for studying cell metabolism. 1H-NMR allows workers to monitor the biodegradation process like a camera, while ionspray mass spectrometry allows workers to focus on a frame of film (zoom) in order to perform a more precise study.
ACKNOWLEDGMENTS
We thank J. S. Cech for the gift of M. aurum MO1. We also acknowledge the Service de Chimie Analytique of RL-CERM for providing help.
REFERENCES
- 1.Besse P, Combourieu B, Poupin P, Sancelme M, Truffaut N, Veschambre H, Delort A M. Degradation of morpholine and thiomorpholine by an environmental Mycobacterium involves a cytochrome P450. Direct evidence of the intermediates by in situ1H-NMR. J Mol Biocatal B Enz. 1998;5:403–409. [Google Scholar]
- 2.Brown V R, Knapp J S, Heritage J. Instability of the morpholine degradative phenotype in mycobacteria isolated from activated sludge. J Appl Bacteriol. 1990;69:54–62. [Google Scholar]
- 3.Cassada D A, Monson S J, Snow D D, Spalding R F. Sensitive determination of RDX, nitroso-RDX metabolites, and other munitions in ground water by solid-phase extraction and isotope dilution liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. J Chromatogr A. 1999;844:87–95. doi: 10.1016/s0021-9673(99)00310-6. [DOI] [PubMed] [Google Scholar]
- 4.Cech J S, Hartman P, Slosarek M, Chudoba J. Isolation and identification of a morpholine-degrading bacterium. Appl Environ Microbiol. 1988;54:619–621. doi: 10.1128/aem.54.2.619-621.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole R B. Electrospray ionization mass spectrometry. New York, N.Y: John Wiley & Sons; 1997. [Google Scholar]
- 6.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver S, Osborne J, Quail M A, Rajandream M A, Rogers J, Rutter S, Seeger K, Skelton S, Squares R, Squares S, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 7.Combourieu B, Besse P, Sancelme M, Veschambre H, Delort A M, Poupin P, Truffaut N. Morpholine degradation pathway of Mycobacterium aurum MO1: direct evidence of intermediates by in situ 1H nuclear magnetic resonance. Appl Environ Microbiol. 1998;64:153–158. doi: 10.1128/aem.64.1.153-158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Combourieu B, Poupin P, Besse P, Sancelme M, Veschambre H, Truffaut N, Delort A M. Thiomorpholine and morpholine oxidation by a cytochrome P450 in Mycobacterium aurum MO1. Evidence of the intermediates by in situ 1H NMR. Biodegradation. 1998;9:433–442. doi: 10.1023/a:1008321610465. [DOI] [PubMed] [Google Scholar]
- 9.Enzmann H, Zerban H, Kopp-Schnelder A, Loser E, Bannasch P. Effects of low doses of N-nitrosomorpholine on the development of early stages of hepatocarcinogenesis. Carcinogenesis. 1995;16:1513–1518. doi: 10.1093/carcin/16.7.1513. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald R L, Rivera J D, Herold D A. Broad spectrum drug identification from urine, using liquid chromatography-tandem mass spectrometry. Clin Chem. 1999;45:1224–1234. [PubMed] [Google Scholar]
- 11.German Chemical Society. Advisory Committee on Existing Chemicals of Environmental Relevance Morpholine BUA report 56. S. Stuttgart, Germany: Hirzel; 1993. [Google Scholar]
- 12.Jacoby W B, Fredericks J. Pyrrolidine and putrescine metabolism by γ-aminobutaraldehyde dehydrogenase. J Biol Chem. 1959;234:2145–2150. [PubMed] [Google Scholar]
- 13.Kato K, Jingu S, Ogawa N, Higuchi S. Rapid characterization of urinary metabolites of pibutidine hydrochloride in humans by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 1999;13:1626–1632. doi: 10.1002/(SICI)1097-0231(19990815)13:15<1626::AID-RCM689>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 14.Knapp J S, Callely A G, Mainprize J. The microbial degradation of morpholine. J Appl Bacteriol. 1982;52:5–13. [Google Scholar]
- 15.Knapp J S, Whytell A J. The biodegradation of morpholine in river water and activated sludge. Environ Pollut. 1990;68:67–79. doi: 10.1016/0269-7491(90)90013-3. [DOI] [PubMed] [Google Scholar]
- 16.Knapp J S, Brown V R. Morpholine biodegradation. Int Biodeterior Bull. 1988;24:299–306. [Google Scholar]
- 17.Korstee G J, Appeldoorn K J, Bonting C F, van Niel E W, van Ween H W. Biology of polyphosphate-accumulating bacteria involved in enhanced biological removal. FEMS Microbiol Rev. 1994;15:137–153. doi: 10.1111/j.1574-6976.1994.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 18.Kulaev I, Vagabov V, Kulakovskaya T. New aspects of inorganic polyphosphate metabolism and function. J Biosci Bioeng. 1999;88:11–129. doi: 10.1016/s1389-1723(99)80189-3. [DOI] [PubMed] [Google Scholar]
- 19.Large P J, Robertson A. The route of lysine breakdown in Candida tropicalis. FEMS Microbiol Lett. 1991;66:209–213. doi: 10.1016/0378-1097(91)90334-7. [DOI] [PubMed] [Google Scholar]
- 20.Mazure N, Truffaut N. Degradation of morpholine by Mycobacterium aurum MO1. Can J Microbiol. 1994;40:761–765. doi: 10.1139/m94-120. [DOI] [PubMed] [Google Scholar]
- 21.Poupin P, Truffaut N, Combourieu B, Besse P, Sancelme M, Veschambre H, Delort A-M. Degradation of morpholine by an environmental strain of Mycobacterium involves a cytochrome P-450. Appl Environ Microbiol. 1998;64:159–165. doi: 10.1128/aem.64.1.159-165.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poupin P, Godon J J, Zumqtein E, Truffaut N. Degradation of morpholine, piperidine, and pyrrolidine by mycobacteria: evidences for the involvement of a cytochrome P450. Can J Microbiol. 1999;45:209–216. [PubMed] [Google Scholar]
- 23.Poupin P, Ducrocq V, Hallier-Soulier S, Truffaut N. Cloning and characterization of the genes encoding a cytochrome P450 (PipA) involved in piperidine and pyrrolidine utilization and its regulatory protein (PipR) in Mycobacterium smegmatis mc2155. J Bacteriol. 1999;181:3419–3426. doi: 10.1128/jb.181.11.3419-3426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swain A, Waterhouse K V, Venables W A, Callely A G, Lowe S E. Biochemical studies of morpholine catabolism by an environmental Mycobacterium. Appl Microbiol Biotechnol. 1991;35:110–114. [Google Scholar]
- 25.Szymona O. Studies of inorganic polyphosphate metabolism in Mycobacterium phlei. I. The incorporation of 32Pi into various phosphorous fractions of M. phlei grown on normal and modified Lowenstein media. Acta Microbiol Pol Ser A Microbiol Gen. 1974;6:231–240. [PubMed] [Google Scholar]
- 26.World Health Organization. Morpholine: health and safety guide. Geneva, Switzerland: World Health Organization; 1995. [Google Scholar]