PURPOSE:
The financial toxicity of anticancer drugs is well-documented, but little is known about the costs of drugs used to manage cancer-associated symptoms.
METHODS:
We reviewed relevant guidelines and compiled drugs used to manage seven cancer-associated symptoms (anorexia and cachexia, chemotherapy-induced peripheral neuropathy, constipation, diarrhea, exocrine pancreatic insufficiency, cancer-associated fatigue, and chemotherapy-induced nausea and vomiting). Using GoodRx website, we identified the retail price (cash price at retail pharmacies) and lowest price (discounted, best-case scenario of out-of-pocket costs) for patients without insurance for each drug or formulation for a typical fill. We describe lowest prices here.
RESULTS:
For anorexia and cachexia, costs ranged from $5 US dollars (USD; generic olanzapine or mirtazapine tablets) to $1,156 USD (brand-name dronabinol solution) and varied widely by formulation of the same drug or dosage: for olanzapine 5 mg, $5 USD (generic tablet) to $239 USD (brand-name orally disintegrating tablet). For chemotherapy-induced peripheral neuropathy, costs of duloxetine varied from $12 USD (generic) to $529 USD (brand-name). For constipation, the cost of sennosides or polyethylene glycol was <$15 USD, whereas newer agents such as methylnaltrexone were expensive ($1,001 USD). For diarrhea, the cost of generic loperamide or diphenoxylate-atropine tablets was <$15 USD. For exocrine pancreatic insufficiency, only brand-name formulations were available, range of cost, $1,072 USD-$1,514 USD. For cancer-associated fatigue, the cost of generic dexamethasone or dexmethylphenidate was <$15 USD, whereas brand-name modafinil was more costly ($1,284 USD). For a 4-drug nausea and vomiting prophylaxis regimen, costs ranged from $181 USD to $1,430 USD.
CONCLUSION:
We highlight the high costs of many symptom control drugs and the wide variation in the costs of these drugs. These findings can guide patient-clinician discussions about cost-effectively managing symptoms, while promoting the use of less expensive formulations when possible.
INTRODUCTION
The high and ever rising costs of cancer drugs are well-recognized: for Medicare Part D beneficiaries, the average monthly point-of-sale price for oral anticancer drugs was nearly $14,000 US dollars (USD) in 2018, with estimated annual out-of-pocket costs for patients exceeding $10,000 USD.1 In contrast to these costs, the financial burden of symptom control drugs in oncology has received less attention.2 Traditionally, patients and clinicians often use lower-cost generics, frequently available over-the-counter, to control symptoms. Because the costs of these individual products are low relative to that of anticancer drugs, their cost-related burdens have largely escaped attention.
In recent years, newer symptom control drugs have come into more widespread use, leading to increased health care spending,3 despite narrow indications and sometimes limited benefits of these drugs over existing, predominately generic drugs.4 One example is peripheral μ-opioid receptor antagonists (PAMORAs) for opioid-induced constipation. Medicare Part D spending on PAMORAs increased from $13.6 million USD to more than $150 million USD between 2014 and 2018.3 Adding to patient-level financial toxicity, clinicians often prescribe symptom control drugs urgently in response to a symptom crisis, at a time when arranging financial support is either not considered or not feasible. For example, when a patient with cancer is actively vomiting, they need an urgent supply of antiemetics without the opportunity to consider whether a savings card, a generic formulation of the same drug, or an alternative (but reasonable) drug could provide substantial savings. Previous research suggests that even small copays can lead to prescription abandonment, with one study demonstrating that among more than 38,000 insured patients with cancer, a copay of <$10 USD was associated with a 10% rate of abandonment.5 Additionally, patients can experience significant financial burdens from being nickel-and-dimed from seemingly small but repetitive costs, such as those associated with transportation and parking.6
Perhaps more concerning, there is often limited evidence supporting the clinical benefits of many symptom control drugs, yet they are widely used. For example, data are lacking to support the routine use of gabapentinoids for chemotherapy-induced peripheral neuropathy, but clinicians often prescribe these agents.7,8 In addition to a lack of efficacy, these agents can contribute to patients' side effect profile through direct adverse events and drug-drug interactions and they may further result in financial toxicity. For most cancer-associated symptoms, several drug options often exist, with costs varying widely across treatment options. For example, the point-of-sale price for a single 8 mg dose of oral ondansetron can vary 14-fold ($6.50 USD for a generic tablet to $85 USD for a brand-name oral disintegrating tablet).9 The differences in costs across different drugs can be even more substantial.
For many patients, their prescription drug coverage is sufficiently limited that it may in effect be totally lacking, and in 2019, among adults age under 65 years, 14.7% were uninsured.10 In 2018, 45% of adults age under 65 years were inadequately insured (22.6% underinsured, 10.0% coverage gap, and 12.4% uninsured).11 Thus, we aimed to assess the average retail costs and the lowest available costs for cash-paying patients for drugs used to manage common cancer-associated symptoms. This information can help clinicians to understand the range of costs for prescribed or over-the-counter options for their patients and to inform cost-conscious prescribing when clinical benefits are similar among treatment alternatives.
METHODS
We conducted a cross-sectional, descriptive study reviewing oncology, supportive care, and gastroenterology societies' guidelines to identify patient-administered (largely oral) drugs used to manage seven common cancer-associated symptoms or conditions. Specifically, we focused on the following: (1) anorexia and cachexia, (2) cancer-associated fatigue, (3) chemotherapy-induced nausea and vomiting prophylaxis and treatment, (4) chemotherapy-induced peripheral neuropathy, (5) constipation, (6) diarrhea, and (7) exocrine pancreatic insufficiency.8,9,12-24 We selected these symptoms on the basis of their pervasiveness, impact on patients' quality of life, and the availability of several drugs to manage these symptoms.25,26 We did not include some symptoms, either because of complex management (eg, pain) or because few symptom-specific drugs are available for treatment (eg, breathlessness, mucositis, and aromatase inhibitor–associated musculoskeletal symptoms).27-32 We also included drugs commonly encountered in clinical practice, even if data to support their routine use are limited and/or guidelines do not recommend their use; we used UpToDate, Inc and authors' experience to guide the final selection of included drugs. We only included drugs focused on symptom palliation and not drugs reversing an underlying disease process (eg, we did not include corticosteroids or infliximab for managing diarrhea related to immune-medicated colitis), regardless of whether they had a US Food and Drug Administration–approved symptom control indication, as in clinical practice, clinicians often use symptom control drugs off-label. As an example, there are currently no drugs approved to treat cancer-associated anorexia and cachexia, but pharmacologic intervention is common.16,33,34 We excluded drugs not available in the United States.
We compiled all formulations of a drug (eg, tablet, capsule, and solution) commonly used in clinical practice and separately considered brand name and generic versions of each product. We excluded parenteral drugs (eg, intravenous fosnetupitant and fosaprepitant for chemotherapy-induced nausea and vomiting, subcutaneous octreotide for diarrhea, etc).13 Excluded drugs are listed in the Data Supplement (online only). Using the most commonly used dosage (strength) of that drug and the typical time course of prescription for a particular symptom using clinical guidelines and clinical judgment, we calculated a typical quantity (fill) of that drug. As examples, (1) for anorexia and cachexia, a 2-week trial of drugs is recommended16 and (2) the average requirement of pancreatic enzymes in people with exocrine pancreatic insufficiency is 250,000 USP units of lipase per day and a 2-week trial is often prescribed.12,35
We used GoodRx website,36 a nationally available drug price comparison website that provides real-time information on drug prices available to consumers at participating pharmacies in their zip code.37,38 From GoodRx website,36 we extracted the average retail price and the lowest price with coupons for each drug or formulation. The retail price (also called the cash price) is the price at a retail pharmacy for patients who either do not have prescription drug coverage or elect not to fill a prescription through insurance (most commonly because of noncoverage of the drug under the health plan or because the costs with coupons would be lower than the costs with insurance). The retail price reflects the prerebate price per fill and approximates the drug's list price. The lowest price reflects discounts offered under contracts between GoodRx36 and pharmacy benefits managers and are available to patients using pharmacy and drug-specific coupons available on the GoodRx website.36 The lowest price represents a best-case scenario of out-of-pocket costs for a patient without insurance or those electing not to use prescription drug coverage.
We collected data for a single zip code (10065, Upper East Side, Manhattan) for a single month (May 2021) to minimize geographic and temporal variation. We selected this zip code to be consistent with pricing information available in ASCO guidelines and prior methodology on cancer drug pricing.9,13,16,39
Institutional review board approval was not required because the study did not involve human subjects research. We used Microsoft Excel v16.0 (Redmond, WA) and GraphPad Prism v7.0 (San Diego, CA) for analyses.
RESULTS
We present the results for each symptom individually in graphical form (Figs 1-3 and Appendix Figs A1-A5, online only). For each symptom, we present details about the drugs (formulations, dosages, use patterns, and typical fill or quantity) in the Data Supplement. We highlight key results in the following text:
FIG 1.
Range of costs of drugs used to manage anorexia and cachexia for a typical fill (2 weeks). USD, US dollars.
FIG 3.
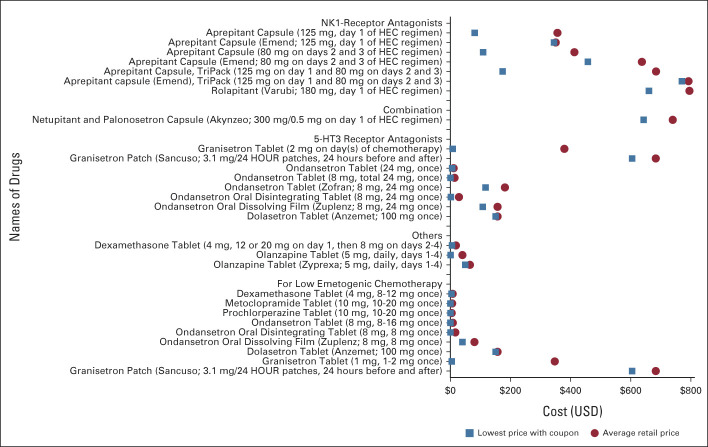
Range of costs of drugs used to prevent chemotherapy-induced nausea and vomiting for a typical fill (1 dose/cycle of chemotherapy). 5-HT3, serotonin; HEC, highly emetogenic chemotherapy; NK1, Neurokinin-1; USD, US dollars.
Anorexia and Cachexia
We included seven drugs available in 20 formulations prescribed to treat cancer-associated anorexia and cachexia (Fig 1). For a 2-week fill, average retail prices were lowest for generic metoclopramide and mirtazapine tablets ($16 USD and $20 USD, respectively) and highest for brand-name solutions of megestrol acetate (Megace ES) and dronabinol (Syndros; $729 USD and $1,440 USD, respectively). For cash-paying patients with coupons, the lowest price for these drugs was $5 USD/fill for generic olanzapine and mirtazapine tablets and $606 USD and $1,156 USD for brand-name megestrol acetate and dronabinol solutions, respectively. Costs also varied widely by formulations of the same drug or dosage: for 15 units of olanzapine 5 mg, from $5 USD (generic olanzapine tablet) to $239 USD (brand-name orally disintegrating tablet).
Cancer-Associated Fatigue
We included four drugs available in eight formulations for managing cancer-associated fatigue (Fig 2). For a 2-week supply, the average retail price was lowest for generic dexamethasone tablets ($26 USD) and highest for brand-name modafinil tablets ($1,492 USD). Mirroring the retail prices, for cash-paying patients with coupons, the lowest prices for these drugs were $12 USD for dexamethasone tablets and $1,284 USD for brand-name modafinil tablets. For generic modafinil tablets, the average retail price was $565 USD and the lowest price was $17 USD.
FIG 2.
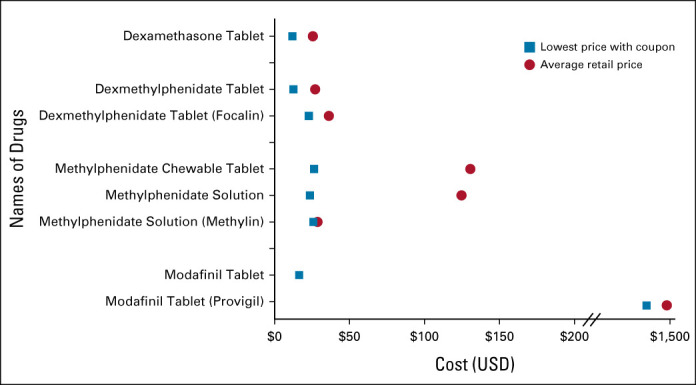
Range of costs of drugs used to manage cancer-associated fatigue for a typical fill (2 weeks). USD, US dollars.
Chemotherapy-Induced Nausea and Vomiting Prophylaxis and Treatment
For chemotherapy-induced nausea and vomiting prophylaxis, we included 10 drugs in 21 formulations (Fig 3). For Neurokinin-1-(NK1)-receptor antagonists, administered with highly emetogenic chemotherapy, options included generic aprepitant, brand-name aprepitant, brand-name rolapitant, and brand-name combined NK1-receptor and serotonin (5HT3)-receptor antagonists (netupitant and palonosetron capsule). For a single chemotherapy cycle, the average retail price of these products ranged from $684 USD to $795 USD. The lowest cost of generic aprepitant was $174 USD, whereas the lowest price of brand-name products was $643 USD-$770 USD.
Among 5HT3-receptor antagonists, for a single chemotherapy cycle, the average retail price ranged from $11 USD (generic ondansetron tablet) to $683 USD (brand-name granisetron transdermal patch). Mirroring this, the cash price with coupons was lowest for generic ondansetron tablet ($1 USD) and highest for brand-name granisetron transdermal patch ($605 USD).
Different combinations of these drugs are used for prophylaxis depending on the emetogenic potential of the chemotherapy agent(s). The Data Supplement demonstrates that for a 4-drug antiemetic regimen for a highly emetogenic chemotherapy (eg, cisplatin), consisting of an NK1-receptor antagonist (days 1-3 if aprepitant used), a 5HT3-receptor antagonist (day 1), dexamethasone (days 1-4), and olanzapine (days 1-4), the cash price with coupons can vary from $181 USD to $1,430 USD.
For treating breakthrough chemotherapy-induced nausea and vomiting, we included 12 drugs in 23 formulations (Appendix Fig A5). For a 1-week fill, the average retail price was lowest for generic metoclopramide ($8 USD) and promethazine ($11 USD) tablets and high for brand-name dronabinol solution ($1,440 USD), dolasetron tablets ($1,060 USD), and brand-name ondansetron oral dissolving film ($662 USD). The cash price with coupons was lowest for generic tablets of olanzapine ($2 USD), metoclopramide, ondansetron, promethazine, and lorazepam ($3 USD each) and generic ondansetron oral disintegrating tablets ($5 USD).
The cash price with coupons also varied widely by formulations of the same drug or dosage: for 15 units of ondansetron 4 mg, it varied from $3 USD (generic tablet), $5 USD (generic, oral disintegrating tablets), $349 USD (brand-name tablet), to $516 USD (brand-name oral dissolving film).
Chemotherapy-Induced Peripheral Neuropathy
We included six drugs available in 13 formulations used to treat chemotherapy-induced peripheral neuropathy (Appendix Fig A1). For a 30-day fill, the average retail price of duloxetine formulations was $241 USD (generic capsule) and $637 USD (brand-name capsule, Cymbalta). For cash-paying patients with coupons, the lowest price for duloxetine formulations was $12 USD (generic capsule) and $529 USD (brand-name capsule, Cymbalta). Generic formulations of all drugs were less expensive (lowest price, < $15 USD), whereas brand-name formulations were very costly (eg, for brand-name nortriptyline capsules, average retail price $1,426 USD and lowest price $1,168 USD).
Constipation
We included 11 drugs available in 19 formulations used to treat constipation (Appendix Fig A2). For a 2-week fill, average retail prices were lowest for over-the-counter, traditional laxatives such as bisacodyl ($4 USD), docusate ($4 USD), sennosides ($6 USD), and magnesium-containing solutions ($6 USD). These drugs also had low lowest prices ($1 USD-$3 USD). Novel drugs used to treat laxative-refractory opioid-induced constipation (such as PAMORAs and lubiprostone) were more costly: the average retail price and lowest price for methylnaltrexone, a PAMORA, were $1,170 USD and $1,001 USD, respectively.
Diarrhea
We included three drugs available in six formulations used to treat diarrhea (Appendix Fig A3). For a 1-week fill, average retail prices were lowest for generic loperamide capsule ($26 USD) and generic diphenoxylate and atropine tablets ($28 USD) and highest for diphenoxylate and atropine formulations (brand-name tablets, $122 USD, and solution, $114 USD). For cash-paying patients with coupons, the lowest price for these drugs was $6 USD for generic cholestyramine packets and $95 USD for brand-name diphenoxylate and atropine tablets.
Exocrine Pancreatic Insufficiency
We included five formulations of PERT for managing exocrine pancreatic insufficiency (Appendix Fig A4). All formulations were brand-name products, and there were no generic formulations. For a 15-day supply, average retail prices ranged from $1,288 USD to $1,860 USD. For cash-paying patients with coupons, the lowest price for these drugs ranged from $1,072 USD-$1,514 USD.
DISCUSSION
In this cross-sectional, descriptive study, we noted a wide range in costs of drugs used to manage common symptoms experienced by patients with cancer, which could add to the financial burden of patients. As expected, the use of brand-name preparations and more complex formulations (solutions or oral disintegrating tablets) was very costly for patients. Although many patients have insurance that helps to lower the out-of-pocket costs, when both generic and brand alternatives are available, the brand-name alternatives may not be covered by a patient's insurance, requiring patients to pay full retail prices for these products if the brand name is prescribed.40 Given this, clinicians should consider the potential for high costs to patients and prescribe a lower-cost drug when clinically appropriate. Financial toxicity in cancer care has received widespread attention over the past decade,2,41 and our current work underscores that symptom control drugs can contribute to economic hardship for patients with cancer.
Our findings of the remarkably high costs of certain symptom control drugs should be interpreted in the context of widespread overuse of several of these drugs, especially when less expensive alternatives may be available.42 Of the 10 ASCO Choosing Wisely recommendations published in 2012 and 2013, two focused on appropriate use of supportive care drugs (antiemetics and growth factors).43,44 Notably, the evidence to support the use of certain symptom control drugs is limited, even for some widely used drugs, leading to a disconnect between true clinical benefit, putative clinical benefit, and out-of-pocket costs. As an example, megestrol acetate, which is commonly prescribed off-label for anorexia and cachexia,45 lacks data supporting a meaningful improvement in quality of life and is associated with physical toxicity (eg, edema, thromboembolism, and adrenal insufficiency).33 In the current work, we also now demonstrate the financial toxicity of megestrol acetate, as a prescription for brand-name megestrol acetate (Megace ES) can cost patients approximately $600 USD out of pocket for a 2-week fill. Costs to the health care system are also significant; Medicare part D spent close to $30 USD million on megestrol acetate in 2018.33 In the case of multiple potential treatment options of similar or equal efficacy, out-of-pocket costs affect decision making.46 Our findings highlight that when drug costs are high and expected clinical benefit is absent or low (eg, most drugs for anorexia and cachexia), the most appropriate action may be to not prescribe any drug. This do-nothing action can prevent financial toxicity, especially in patients with advanced cancer who may have limited time.46
For several symptoms, nonpharmacologic interventions may be more effective than some of the drugs commonly used and should preferentially be used first. For example, with anorexia and cachexia, appropriate counseling and addressing patient and caregiver concerns about eating and weight loss are more likely to improve distress than drugs.34 Additionally, for fatigue, addressing comorbid conditions and optimizing sleep and exercise are likely to be more effective than costly modafinil.47 Payer noncoverage and high out-of-pocket costs of nonpharmacologic interventions (such as acupuncture or exercise regimens) for symptom control are partly responsible for their underutilization.48 Our data demonstrate that even simple, seemingly innocuous drug prescriptions can cause significant economic consequences to patients. Patients are often given a handful of prescriptions; apart from cost considerations, reflexive prescription of symptom control drugs can contribute to polypharmacy, medication burden and nonadherence, and drug-drug interactions.
Our findings have several salient takeaways for clinicians to consider. First, we recognize that not all drugs for managing a particular symptom are bioequivalent. Costlier agents may very well be more appropriate, may improve patient quality of life, and prevent downstream health care use. Just like overuse, underuse of costly but appropriate antiemetics can also occur.49,50 Thus, we call for appropriate use of symptom control drugs and not abandoning the use of costly agents altogether. Second, appropriate symptom control (often preventive) can help limit downstream spending on more costly drugs. As an example, most patients receiving opioids do not receive an appropriate prophylactic bowel regimen to prevent opioid-induced constipation; these patients can then develop refractory constipation, requiring the use of costly PAMORAs.51-53 Third, payers commonly design prescription drug plans to cover generics over brand-name formulations.40 Prescribing a generic over a brand-name drug can greatly influence costs. As an example, the lowest price of a 30-day prescription of generic versus brand-name duloxetine for chemotherapy-induced peripheral neuropathy would cost $12 USD versus $529 USD. Clinician-pharmacist partnerships and, in particular, leveraging the electronic medical record and the computerized provider order entry provide ideal opportunities to test and implement such interventions. Previous work has demonstrated that redesigning the electronic medical record default options can significantly increase generic prescriptions.54 In addition to electronic health record–embedded Real-Time Benefit Tools available to clinicians,55 pharmacist-led medication management strategies such as therapeutic (and generic) substitutions can reduce costs. When only brand-name drugs are available, as in the case of pancreatic enzymes, costs can be significant (>$1,000 USD for just a 2-week supply). Clinicians and patients attempt to counter these high costs by rationing drugs, using nonregulated ineffective preparations, and using drug coupons.35,56 Fourth, clinicians should be aware that costs of the same drug can vary substantially between different formulations: as an example, the lowest price for 15 units of 4 mg of generic metoclopramide is $3 USD for tablet and $60 USD for oral disintegrating tablet.
This study has several limitations. First, we describe costs for patients without (or those electing not to use) prescription drug coverage and we did not collect or analyze payer or coverage details for these drugs. Actual out-of-pocket costs for patients who have and choose to use prescription drug coverage will vary by the plan, product coverage, and benefit phase. However, since 45% of US adults age < 65 years are inadequately covered, we believe that these data are widely applicable11 Second, costs are dynamic and we provide only cross-sectional data from May 2021. Third, we present data for a single zip code (New York City) to be consistent with pricing information in ASCO guidelines. Drug costs are higher in large coastal cities, and although cost of living accounts for some of the geographical price variation, it is also related to the prevalence of the types of pharmacies in a region. In general, prices at big box and large chain pharmacies (such as Costco, Walgreens) are significantly lower than those at small or independent pharmacies.38 The lowest cost for a patient in an area is likely to be at large pharmacy. The variation in lowest costs across zip codes is significantly less than the variation in the average of the average retail price (cash price) across a zip code, which is more affected by markups and the proportion of the types of pharmacies.38
In conclusion, our findings highlight the high costs of many symptom control drugs and there is wide variation in the costs of drugs available to treat a particular cancer symptom. This work suggests that spending on symptom control can contribute substantially to patients' financial toxicity given the number of these products used and frequency of use among patients with cancer. These data may be helpful for patients and clinicians to re-evaluate the risk-benefit ratio of a prescription, especially in the context of the limited data to support the use of some of these symptom control drugs.
APPENDIX
FIG A1.

Range of costs of drugs used to manage chemotherapy-induced peripheral neuropathy for a typical fill (1 month). USD, US dollars.
FIG A2.
Range of costs of drugs used to manage constipation for a typical fill (2 weeks). USD, US dollars.
FIG A3.

Range of costs of drugs used to manage diarrhea for a typical fill (7 days). USD, US dollars.
FIG A4.

Range of costs of drugs used to manage exocrine pancreatic insufficiency for a typical fill (2 weeks). USD, US dollars.
FIG A5.
Range of costs of drugs used to treat chemotherapy-induced nausea and vomiting for a typical fill (7 days). 5-HT3, serotonin; USD, US dollars.
PRIOR PRESENTATION
Presented as abstract form at the 2021 ASCO Quality Care Symposium, September 24-25, 2021, held in Boston, MA.
SUPPORT
A.G. and R.S. were supported by individual Conquer Cancer/the ASCO Foundation Young Investigator Awards. H.M.P. reports support from NIH P30 CA77598 Masonic Cancer Center and the Leukemia and Lymphoma society for unrelated work.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at DOI https://doi.org/10.1200/OP.21.00466.
AUTHOR CONTRIBUTIONS
Conception and design: Arjun Gupta, Ramy Sedhom, Helen M. Parsons, Anne H. Blaes, Ishwaria M. Subbiah, Ryan D. Nipp, Stacie B. Dusetzina
Financial support: Arjun Gupta
Administrative support: Arjun Gupta
Provision of study materials or patients: Arjun Gupta
Collection and assembly of data: Arjun Gupta, Udhayvir S. Grewal, Stacie B. Dusetzina
Data analysis and interpretation: Arjun Gupta, Leonce Nshuti, Ramy Sedhom, Devon K. Check, Helen M. Parsons, Anne H. Blaes, Beth A. Virnig, Maryam B. Lustberg, Ishwaria M. Subbiah, Ryan D. Nipp, Sydney M. Dy, Stacie B. Dusetzina
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Financial Burden of Drugs Prescribed for Cancer-Associated Symptoms
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Devon K. Check
Research Funding: AstraZeneca
Maryam B. Lustberg
Honoraria: Novartis, bioTheranostics
Consulting or Advisory Role: PledPharma, Disarm Therapeutics, Pfizer
Other Relationship: Cynosure/Hologic
Ishwaria M. Subbiah
Consulting or Advisory Role: MedImmune (I)
Research Funding: Bayer (I), Novartis (I), GlaxoSmithKline (I), NanoCarrier (I), Celgene (I), Northwest Biotherapeutics (I), Incyte (I), Fujifilm (I), Pfizer (I), Amgen (I), AbbVie (I), Multivir (I), Exelixis (I), Loxo (I), Blueprint Medicines (I), Takeda (I)
Travel, Accommodations, Expenses: Bayer (I), PharmaMar (I), Novartis (I), MedImmune (I)
Stacie B. Dusetzina
Other Relationship: Institute for Clinical and Economic Review, Arnold Ventures, Leukemia and Lymphoma Society, The Commonwealth Fund, West Health, National Academy of State Health Policy, Robert Wood Johnson Foundation, Medicare Payment Advisory Commission
No other potential conflicts of interest were reported.
REFERENCES
- 1.Dusetzina SB, Huskamp HA, Keating NL: Specialty drug pricing and out-of-pocket spending on orally administered anticancer drugs in Medicare Part D, 2010 to 2019. JAMA 321:2025-2027, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sedhom R, Chino F, Gupta A: Financial toxicity and cancer care #409. J Palliat Med 24:453-454, 2021 [DOI] [PubMed] [Google Scholar]
- 3.Premnath N, Sumarsono A, Sedhom R, et al. : Use of peripheral mu-opioid receptor antagonists for treating opioid-induced constipation among US Medicare beneficiaries from 2014 to 2018. J Palliat Med 24:1236-1239, 2021 [DOI] [PubMed] [Google Scholar]
- 4.Gupta A, Wang P, Ali SA, et al. : Use of bone-modifying agents among Medicare beneficiaries with multiple myeloma. JAMA Oncol 6:296-298, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doshi JA, Li P, Huo H, et al. : Association of patient out-of-pocket costs with prescription abandonment and delay in fills of novel oral anticancer agents. J Clin Oncol 36:476-482, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Premnath N, Grewal US, Gupta A: Park the parking. JCO Oncol Pract 16:215-217, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gewandter JS, Kleckner AS, Marshall JH, et al. : Chemotherapy-induced peripheral neuropathy (CIPN) and its treatment: An NIH collaboratory study of claims data. Support Care Cancer 28:2553-2562, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hershman DL, Lacchetti C, Dworkin RH, et al. : Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 32:1941-1967, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Hesketh PJ, Kris MG, Basch E, et al. : Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 35:3240-3261, 2017 [DOI] [PubMed] [Google Scholar]
- 10.National Center for Health Statistics : Health Insurance Coverage. https://www.cdc.gov/nchs/fastats/health-insurance.htm [Google Scholar]
- 11.Collins SR, Bhupal HK, Doty MM: The Commonwealth Fund. Health Insurance Coverage Eight Years After the ACA, 2019. https://www.commonwealthfund.org/publications/issue-briefs/2019/feb/health-insurance-coverage-eight-years-after-aca [Google Scholar]
- 12.Gardner TB, Adler DG, Forsmark CE, et al. : ACG clinical guideline: Chronic pancreatitis. Am J Gastroenterol 115:322-339, 2020 [DOI] [PubMed] [Google Scholar]
- 13.Hesketh PJ, Kris MG, Basch E, et al. : Antiemetics: ASCO guideline update. J Clin Oncol 38:2782-2797, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Larkin PJ, Cherny NI, La Carpia D, et al. : Diagnosis, assessment and management of constipation in advanced cancer: ESMO clinical practice guidelines. Ann Oncol 29:iv111-iv125, 2018 [DOI] [PubMed] [Google Scholar]
- 15.McKenzie E, Zaki P, Raman S, et al. : Radiation-induced nausea and vomiting: A comparison between MASCC/ESMO, ASCO, and NCCN antiemetic guidelines. Support Care Cancer 27:783-791, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Roeland EJ, Bohlke K, Baracos VE, et al. : Management of cancer cachexia: ASCO guideline. J Clin Oncol 38:2438-2453, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Bower JE, Bak K, Berger A, et al. : Screening, assessment, and management of fatigue in adult survivors of cancer: An American Society of Clinical Oncology clinical practice guideline adaptation. J Clin Oncol 32:1840-1850, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benson AB 3rd, Ajani JA, Catalano RB, et al. : Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J Clin Oncol 22:2918-2926, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Bossi P, Antonuzzo A, Cherny NI, et al. : Diarrhoea in adult cancer patients: ESMO clinical practice guidelines. Ann Oncol 29:iv126-iv142, 2018 [DOI] [PubMed] [Google Scholar]
- 20.Crockett SD, Greer KB, Heidelbaugh JJ, et al. : American gastroenterological association Institute guideline on the medical management of opioid-induced constipation. Gastroenterology 156:218-226, 2019 [DOI] [PubMed] [Google Scholar]
- 21.Leong M, Smith TJ: Geriatrics and palliative care: All in the same family. Clin Geriatr Med 31:xi-xii, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Fabi A, Bhargava R, Fatigoni S, et al. : Cancer-related fatigue: ESMO clinical practice guidelines for diagnosis and treatment. Ann Oncol 31:713-723, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Jordan B, Margulies A, Cardoso F, et al. : Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO-EONS-EANO clinical practice guidelines for diagnosis, prevention, treatment and follow-up. Ann Oncol 31:1306-1319, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Roila F, Molassiotis A, Herrstedt J, et al. : 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 27:v119-v33, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Bubis LD, Davis L, Mahar A, et al. : Symptom burden in the first year after cancer diagnosis: An analysis of patient-reported outcomes. J Clin Oncol 36:1103-1111, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Cleeland CS: Symptom burden: Multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr:16-21, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Brown TJ, Gupta A: Management of cancer therapy-associated oral mucositis. JCO Oncol Pract 16:103-109, 2020 [DOI] [PubMed] [Google Scholar]
- 28.Gupta A, Henry NL, Loprinzi CL: Management of aromatase inhibitor-induced musculoskeletal symptoms. JCO Oncol Pract 16:733-739, 2020 [DOI] [PubMed] [Google Scholar]
- 29.Gupta A, Sedhom R, Sharma R, et al. : Nonpharmacological interventions for managing breathlessness in patients with advanced cancer: A systematic review. JAMA Oncol 7:290-298, 2021 [DOI] [PubMed] [Google Scholar]
- 30.Hui D, Bohlke K, Bao T, et al. : Management of dyspnea in advanced cancer: ASCO guideline. J Clin Oncol 39:1389-1411, 2021 [DOI] [PubMed] [Google Scholar]
- 31.Elad S, Cheng KKF, Lalla RV, et al. : MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 126:4423-4431, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feliciano JL, Waldfogel JM, Sharma R, et al. : Pharmacologic interventions for breathlessness in patients with advanced cancer: A systematic review and meta-analysis. JAMA Netw Open 4:e2037632, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown TJ, Gandhi S, Smith TJ, et al. : Lessons from spending on megestrol for cancer cachexia. Support Care Cancer 29:5553-5555, 2021 [DOI] [PubMed] [Google Scholar]
- 34.Kabani A, Dy SM, Gupta A: The key role of nonpharmacologic management of cachexia in persons with advanced illness: A teachable moment. JAMA Intern Med 181:978-979, 2021 [DOI] [PubMed] [Google Scholar]
- 35.Gupta A, Premnath N, Sedhom R, et al. : Projected 30-day out-of-pocket costs and total spending on pancreatic enzyme replacement therapy under Medicare Part D. Pancreatology 21:1009-1010, 2021 [DOI] [PubMed] [Google Scholar]
- 36.GoodRx, Inc : GoodRx.com
- 37.GoodRx Holdings Inc : S-1 Registration Statement. Washington, DC: US Securities and Exchange Commission, 2020 [Google Scholar]
- 38.Luo J, Kulldorff M, Sarpatwari A, et al. : Variation in prescription drug prices by retail pharmacy type: A national cross-sectional study. Ann Intern Med 171:605-611, 2019 [DOI] [PubMed] [Google Scholar]
- 39.Center for Health Policy & Outcomes. Memorial Sloan Kettering Cancer Center : Price & Value of Cancer Drug. Methods for Drug Price Calculations. https://www.mskcc.org/sites/default/files/node/25097/documents/methods-for-drug-price-calculations-081517.pdf [Google Scholar]
- 40.Dusetzina SB, Cubanski J, Nshuti L, et al. : Medicare Part D plans rarely cover brand-name drugs when generics are available. Health Aff (Millwood) 39:1326-1333, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zafar SY, Abernethy AP: Financial toxicity, Part I: A new name for a growing problem. Oncology (Williston Park) 27:80-81, 149, 2013 [PMC free article] [PubMed] [Google Scholar]
- 42.Schleicher SM, Bach PB, Matsoukas K, et al. : Medication overuse in oncology: Current trends and future implications for patients and society. Lancet Oncol 19:e200-e208, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schnipper LE, Lyman GH, Blayney DW, et al. : American Society of Clinical Oncology 2013 top five list in oncology. J Clin Oncol 31:4362-4370, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Schnipper LE, Smith TJ, Raghavan D, et al. : American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: The top five list for oncology. J Clin Oncol 30:1715-1724, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Garcia JM, Shamliyan TA: Off-label megestrol in patients with anorexia-cachexia syndrome associated with malignancy and its treatments. Am J Med 131:623-629.e1, 2018 [DOI] [PubMed] [Google Scholar]
- 46.Henrikson NB, Shankaran V: Improving price transparency in cancer care. JCO Oncol Pract 12:44-47, 2016 [DOI] [PubMed] [Google Scholar]
- 47.Popescu RA, Roila F, Arends J, et al. : Supportive care: Low cost, high value. Am Soc Clin Oncol Ed Book 41:1-11, 2021 [DOI] [PubMed] [Google Scholar]
- 48.Sedhom R, Gupta A, Wang L, et al. : Payer coverage of integrative medicine interventions for symptom control in patients with cancer. JCO Oncol Pract 17:587-590, 2021 [DOI] [PubMed] [Google Scholar]
- 49.Mahendraratnam N, Farley JF, Basch E, et al. : Characterizing and assessing antiemetic underuse in patients initiating highly emetogenic chemotherapy. Support Care Cancer 27:4525-4534, 2019 [DOI] [PubMed] [Google Scholar]
- 50.Roeland EJ, Ruddy KJ, LeBlanc TW, et al. : What the HEC? Clinician adherence to evidence-based antiemetic prophylaxis for highly emetogenic chemotherapy. J Natl Compr Canc Netw 18:676-681, 2020 [DOI] [PubMed] [Google Scholar]
- 51.Brown TJ, Keshvani N, Gupta A, et al. : Rates of appropriate laxative prophylaxis for opioid-induced constipation in veterans with lung cancer: A retrospective cohort study. Support Care Cancer 28:5315-5321, 2020 [DOI] [PubMed] [Google Scholar]
- 52.Saha S, Nathani P, Gupta A: Preventing opioid-induced constipation: A teachable moment. JAMA Intern Med 180:1371-1372, 2020 [DOI] [PubMed] [Google Scholar]
- 53.Saha S, Nathani P, Gupta A: Preventing opioid-induced constipation-reply. JAMA Intern Med 181:726-727, 2021 [DOI] [PubMed] [Google Scholar]
- 54.Patel MS, Day SC, Halpern SD, et al. : Generic medication prescription rates after health system-wide redesign of default options within the electronic health record. JAMA Intern Med 176:847-848, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Everson J, Frisse ME, Dusetzina SB: Real-time benefit Tools for drug prices. JAMA 322:2383-2384, 2019 [DOI] [PubMed] [Google Scholar]
- 56.Forsmark CE, Tang G, Xu H, et al. : The use of pancreatic enzyme replacement therapy in patients with a diagnosis of chronic pancreatitis and pancreatic cancer in the US is infrequent and inconsistent. Aliment Pharmacol Ther 51:958-967, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]