Abstract
External stressors such as alcohol, caffeine, and vigorous exercise are known to alter cellular homeostasis, affecting the autonomic nervous system (ANS) and overall physiological function. However, little direct evidence exists quantifying the impact of these external stressors on physiological testing. We assessed the impact of the above-listed stressors on spontaneous baroreflex sensitivity (BRS), heart rate variability (HRV), heart rate asymmetry (HRA), and systolic blood pressure variability (BPV). Seventeen male university varsity American-style football athletes completed two identical assessments on separate days, once presenting with one or more stressors (recent intake of caffeine, alcohol, or exercise participation; contraindicated assessment) and another with no stressors present (repeat assessment). Both assessments were conducted within one week and at the same time of day. The testing protocol consisted of 5-min of rest followed by 5-min of a squat-stand maneuver (0.05 Hz). Continuous beat-to-beat blood pressure and electrocardiogram measurements were collected and allowed for calculations of BRS, HRV, HRA, and BPV. Significant decreases (p < 0.05) in HRV and HRA metrics (SDNN, SD2, SDNNd, SDNNa, SD2a, SD2d), HRV total power, and BRS-up sequence were found during the contraindicated assessment in comparison to the repeat assessment. When assessing those with exercise as their only stressor, high-frequency HRV and BRS-pooled were significantly decreased and increased, respectively, during the contraindicated assessment. Pre-season physiological baseline testing in sport is becoming increasingly prevalent and thus must consider external stressors to ascertain accurate and reliable data. This data confirms the need for stringent and standardized guidelines for pre-participation baseline physiological testing.
Keywords: Autonomic nervous system, Heart rate variability, Heart rate asymmetry, Baroreflex sensitivity, Blood pressure variability, External stressor
Highlights
-
•
External stressors (exercise participation, caffeine consumption, and alcohol consumption) decrease heart rate variability and the asymmetrical contribution of heart rate accelerations and decelerations (SDNN, SD2, Total Power, SDNNd, SDNNa, SD2d, and SD2a).
-
•
External stressors decrease spontaneous baroreflex sensitivity up-sequence, but no significant changes were found regarding systolic blood pressure variability.
-
•
The establishment of standardized pre-participation guidelines controlling for external stressors would increase the validity and reliability of physiological testing, improving the clinical utility of such data.
1. Introduction
Prior to a competitive athletic season, athletes may undergo physiological testing to establish a baseline level of function and identify any concerning deficits or underlying conditions. Typical components of pre-participation testing include assessing musculoskeletal function and balance, cardiovascular screening, and baseline concussion testing (Cottle et al., 2017; Dai et al., 2019; Echemendia et al., 2017; Longo et al., 2018; Neary et al., 2011), which includes the common use of the Sport Concussion Assessment Tool 5th Edition (SCAT5) (Echemendia et al., 2017). These data provide valuable information to both athletes and training staff to understand the athlete's physical status and any potential limitations in physiological function. In addition, such information allows professionals to compare data that is relative to an athlete's own unique physiology if they suffer an injury. Multiple post-injury follow-up assessments are often required for athletes who suffer musculoskeletal injuries or concussions before they are allowed to return-to-play (Neary et al., 2019; Sharma et al., 2020). These follow-up assessments include symptom reporting, physiological measurements, and neurocognitive assessments (Cottle et al., 2017; Ellis et al., 2019) to assist in the decision-making to return players to competition (McCrory et al., 2017). Therefore, to accurately assess physiological parameters, well-defined standardized conditions must be introduced to limit ambiguous results (Neary et al., 2019; Ziemssen and Siepmann, 2019). However, there is a lack of direct evidence regarding the impact of external stressors on physiological testing to inform the development of standardized pre-participation guidelines.
Some external stressors that are known to alter cellular homeostasis and metabolism include alcohol (Husain et al., 2014; Marchi et al., 2014), caffeine (Nehlig et al., 1992), and exercise (Hawley et al., 2014). These stressors can influence cardiovascular function and the autonomic nervous system (ANS) as these systems respond to internal and external stressors to maintain homeostasis (Kim et al., 2018; Ziemssen and Siepmann, 2019). As a result, these external stressors can be confounding variables, limiting the interpretability of results from physiological testing (Kim et al., 2018). By having stressor-related, ambiguous baseline data, the comparison of baseline data to any subsequent follow-up assessments loses utility. Alcohol, caffeine, and exercise increase activity in the sympathetic branch of the ANS, although through different mechanisms (Bunsawat et al., 2015; Hawley et al., 2014; Husain et al., 2014; Marchi et al., 2014; Mastorakos et al., 2005; Nehlig et al., 1992). Thus, the use of parameters such as heart rate variability (HRV), heart rate asymmetry (HRA), spontaneous baroreflex sensitivity (BRS), and systolic blood pressure variability (BPV), which assess cardiovascular-autonomic nervous system (CV-ANS) function must be measured with strict pre-participation guidelines in place to ensure true physiological baseline values are obtained during testing.
HRV refers to the variations in the time interval between successive R-waves of QRS complexes in the cardiac cycle (Piskorski and Guzik, 2011). HRV is used to assess the ANS, providing information on the relative contributions of the sympathetic and parasympathetic branches (Piskorski and Guzik, 2011; Rajendra Acharya et al., 2006). HRV can be analyzed using several techniques, including time-domain, frequency-domain, and non-linear methods (Shaffer et al., 2014; Tarvainen et al., 2014). HRA is a physiological parameter that assesses the contribution of accelerations (shortening) and decelerations (lengthening) to the variability in R-R interval duration, commonly represented by a Poincaré plot (Piskorski and Guzik, 2011). Since HRA is a component of HRV and has been demonstrated to correlate with ANS activity, it is thought that HRA may be a valuable parameter for analyzing CV-ANS function (Karmakar et al., 2012; Piskorski and Guzik, 2011; Porta et al., 2008).
The arterial baroreflex is a key mechanism partially responsible for regulating CV-ANS function (Chapleau et al., 2001). The baroreflex regulates blood pressure through a negative feedback mechanism initiated by alterations in blood pressure sensed by the baroreceptors in the carotid sinus and aortic arch (Chapleau et al., 2001). BRS is a surrogate measure of this process (Chapleau et al., 2001; La Fountaine et al., 2019). BPV is another physiological metric that provides valuable information concerning cardiovascular regulation and the relative contributions of the parasympathetic and sympathetic branches of the ANS (Laitinen et al., 1999; Pagani et al., 1997; Singh et al., 2022). BPV represents the asymmetry due to short-term fluctuations in blood pressure (Guzik et al., 2010).
This study aimed to examine the impact of external stressors on BRS, HRV, HRA and BPV. This study was conducted using a retrospective observational approach with data obtained from a subset of athletes who presented to complete their pre-season physiological baseline testing with the presence of one or more external stressors (contraindicated) and then completed a subsequent assessment one week later without any external stressors (repeat). Alcohol, caffeine, and exercise were the external stressors examined in this analysis. We hypothesized that the presence of external stressors would depress these CV-ANS parameters, limiting the utility of these parameters as baseline physiological values for comparison to subsequent follow-up assessments.
2. Methods
2.1. Participants
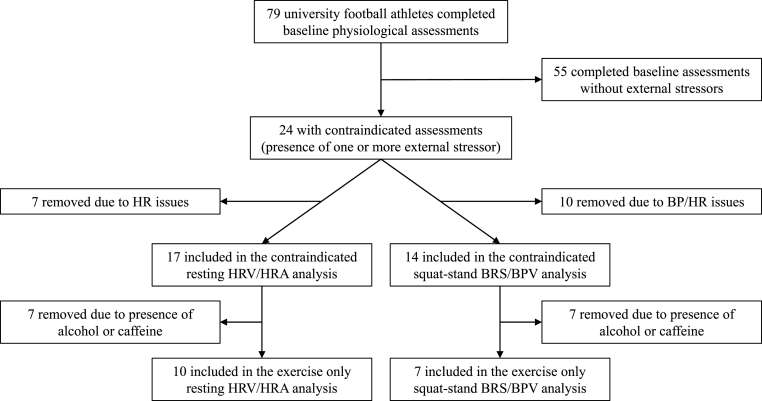
All varsity athletes at the University of Regina undergo pre-season baseline physiological assessments. Prior to testing, all athletes are instructed to refrain from alcohol, caffeine, and exercise. Seventy-nine university varsity male, American style football athletes completed baseline physiological assessments. Out of this group, a subset of 17 athletes completed their initial assessment with the presence of one or more external stressors (the contraindicated assessment; alcohol consumption within 24 h, caffeine consumption within 9 h, and exercise within 6 h). After completing their contraindicated assessment, with one or more external stressors present, these athletes were then asked to return within 1 week at the same time of day to repeat the assessment without any external stressors present (the repeat assessment). Further information regarding how the data was obtained is available in Fig. 1.
Fig. 1.
Flowchart depicting how the data was collected and stratified for the different analyses.
Contraindicated = the presence of one or more external stressor (caffeine, alcohol, exercise); HR = heart rate; BP = blood pressure; HRV = heart rate variability; HRA = heart rate asymmetry; BRS = spontaneous baroreflex sensitivity; BPV = systolic blood pressure variability.
2.2. Data Collection
Ethics approval was provided by the University of Regina Research Ethics Board (REB#55R-1213). All participants provided their written informed consent after reviewing information about the research. Using finger photoplethysmography, continuous beat-to-beat blood pressure data was collected with a Finometer Pro (Finapres Medical Systems BV, Enschede, Netherlands). The finger cuff was affixed to the left middle finger, with the height correction unit attached to the participant's shirt at heart level. HRV was assessed using a 3-lead electrocardiogram (ECG) (Finapres Medical Systems, the Netherlands). Raw data signals were simultaneously collected and displayed using PowerLab and LabChart software (AD Instruments, Colorado, USA). Participants completed a 5-min seated rest to establish baseline physiology followed by 5-min of a paced squat-stand maneuver consisting of repeated 10-s squat holds followed by 10-s of standing rest (0.05 Hz) (Neary et al., 2019; Smirl et al., 2015).
2.3. Data analysis
After inspection for any artifacts, all data files containing the blood pressure waveform were imported into the Ensemble-R software (Elucimed Ltd., Wellington, NZ) to assess BRS and BPV. BRS was evaluated by partitioning the BRS-up and BRS-down sequences and quantifying the pooled BRS. BPV was assessed using spectral analysis and categorized into high (HF-BPV) and low (LF-BPV) frequency power components along with total power (TP-BPV). The BRS and BPV analyses were conducted during the repeated squat-stand maneuver. The ECG signal was inspected for ectopic beats. The R-R intervals were analyzed, and power spectral density was estimated using the Lomb method (Laguna et al., 1998). HRV was then assessed through spectral analysis (LF, HF, LF/HF ratio, and total power), time-domain methods (SDNN), and statistics derived from Poincaré plots (SD1 and SD2). For both HRV and BPV, LF power was measured at 0.04–0.15 Hz, while HF power was measured at 0.15–0.40 Hz. Finally, HRA was analyzed by separating information on a Poincaré plot into the accelerations or decelerations of HR, allowing for the calculation of geometric HRV parameters, including SD1a, SD1d, SD2a, SD2d, SDNNa, and SDNNd (Piskorski and Guzik, 2011). The R-R intervals were analyzed using an independent, in-house software to separate the decelerating and accelerating characteristics (Piskorski and Guzik, 2011). HRV and HRA were assessed during the 5-min of seated rest.
2.4. Statistical analysis
A paired sample t-test (p < 0.05) was used to compare BRS, BPV, HRA and HRV in the group with one or more external stressors (contraindicated) to the same individuals one week later without the presence of any external stressors (repeat) (Table 2, Table 3). Furthermore, prior exercise was the most prevalent external stressor in this data set (70.6%; n = 12). As such, to understand the influence of exercise alone on BRS, BPV, HRA and HRV, a paired sample t-test (p < 0.05) was applied to those with prior exercise as their only external stressor (exercise only) compared to the same participants one-week later without any external stressors (repeat) (Table 4, Table 5). In addition, for all analyses, absolute effect sizes are reported using Cohen's D (Cohen, 1988).
Table 2.
Poincaré plot and spectral analysis of R-R intervals (n = 17) during rest for the contraindicated assessment (one or more external stressors present) vs. the repeat assessment (no external stressors present).
| Parameter | Contraindicated | Repeat | t-statistic | Cohen's D | p-value |
|---|---|---|---|---|---|
| SDNN* (ms) | 53 ± 18 | 68 ± 20 | −2.50 | −0.61 | 0.02 |
| SD1 (ms) | 30 ± 18 | 36 ± 18 | −1.53 | −0.37 | 0.15 |
| SD2* (ms) | 68 ± 22 | 88 ± 25 | −2.62 | −0.64 | 0.02 |
| SDNNd* (ms) | 37 ± 12 | 47 ± 14 | −2.52 | −0.61 | 0.02 |
| SDNNa* (ms) | 38 ± 14 | 48 ± 14 | −2.46 | −0.60 | 0.03 |
| SD1d (ms) | 22 ± 13 | 26 ± 13 | −1.39 | −0.34 | 0.20 |
| SD1a (ms) | 20 ± 12 | 25 ± 12 | −1.70 | −0.41 | 0.11 |
| SD2d* (ms) | 47 ± 13 | 61 ± 16 | −2.69 | −0.65 | 0.01 |
| SD2a* (ms) | 49 ± 18 | 63 ± 19 | −2.55 | −0.62 | 0.02 |
| LF (ms2) | 7 ± 6 | 10 ± 6 | −1.72 | −0.42 | 0.10 |
| HF (ms2) | 6 ± 8 | 8 ± 10 | −1.93 | −0.47 | 0.07 |
| LF/HF | 5 ± 5 | 3 ± 2 | 1.56 | 0.38 | 0.14 |
| Total Power* (ms2) | 18 ± 13 | 34 ± 27 | −2.62 | −0.64 | 0.02 |
* (p < 0.05).
SDNN = total variability; SD1 = short-term variability; SD2 = long-term variability; SDNNd = contribution of decelerations to total variability; SDNNa = contribution of accelerations to total variability, SD1d = contribution of decelerations to short-term variability; SD1a = contribution of accelerations to short-term variability; SD2d = contribution of decelerations to long-term variability; SD2a = contribution of accelerations to long-term variability; HF = high frequency; LF = low frequency.
Table 3.
Baroreflex sensitivity and blood pressure variability (n = 14) during the repeated squat-stand maneuver for the contraindicated assessment (one or more external stressors present) vs. the repeat assessment (no external stressors present).
| Parameter | Contraindicated | Repeat | t-statistic | Cohen's D | p-value |
|---|---|---|---|---|---|
| BRS-down (msec/mmHg) | 5 ± 2 | 6 ± 3 | 0.60 | 0.16 | 0.60 |
| BRS-up* (msec/mmHg) | 8 ± 4 | 11 ± 5 | 2.97 | 0.79 | 0.01 |
| BRS-pooled (msec/mmHg) | 6 ± 3 | 8 ± 3 | 1.67 | 0.45 | 0.12 |
| HF-BPV (mmHg2) | 44 ± 18 | 40 ± 15 | −0.72 | −0.19 | 0.49 |
| LF-BPV (mmHg2) | 1259 ± 485 | 1361 ± 624 | 0.59 | 0.16 | 0.56 |
| TP-BPV (mmHg2) | 1329 ± 496 | 1428 ± 643 | 0.55 | 0.15 | 0.59 |
* (p < 0.05).
HF = high frequency; LF = low frequency; TP = total power; BRS = spontaneous baroreflex sensitivity, BPV = systolic blood pressure variability.
Table 4.
Poincaré plot and spectral analysis of R-R intervals (n = 10) during rest for prior exercise only vs. the repeat assessment (no external stressors present).
| Parameter | Exercise Only | Repeat | t-statistic | Cohen's D | p-value |
|---|---|---|---|---|---|
| SDNN* (ms) | 50 ± 20 | 72 ± 20 | −2.7 | −0.85 | 0.02 |
| SD1 (ms) | 26 ± 15 | 36 ± 15 | −1.9 | −0.60 | 0.09 |
| SD2* (ms) | 65 ± 26 | 94 ± 26 | −2.7 | −0.85 | 0.02 |
| SDNNd* (ms) | 35 ± 13 | 50 ± 14 | −2.7 | −0.85 | 0.01 |
| SDNNa* (ms) | 36 ± 16 | 51 ± 15 | −2.7 | −0.85 | 0.02 |
| SD1d (ms) | 19 ± 12 | 26 ± 11 | −1.6 | −0.51 | 0.14 |
| SD1a (ms) | 17 ± 10 | 25 ± 10 | −2.2 | −0.70 | 0.05 |
| SD2d* (ms) | 45 ± 15 | 65 ± 18 | −2.8 | −0.89 | 0.02 |
| SD2a* (ms) | 47 ± 22 | 68 ± 20 | −2.6 | −0.82 | 0.03 |
| LF (ms2) | 7 ± 8 | 11 ± 7 | −1.47 | −0.46 | 0.18 |
| HF* (ms2) | 4 ± 4 | 8 ± 8 | −2.43 | −0.77 | 0.04 |
| LF/HF | 5 ± 4 | 2 ± 2 | 1.52 | 0.48 | 0.16 |
| Total Power* (ms2) | 16 ± 11 | 39 ± 30 | −2.51 | −0.79 | 0.03 |
* (p < 0.05).
SDNN = total variability; SD1 = short-term variability; SD2 = long-term variability; SDNNd = contribution of decelerations to total variability; SDNNa = contribution of accelerations to total variability, SD1d = contribution of decelerations to short-term variability; SD1a = contribution of accelerations to short-term variability; SD2d = contribution of decelerations to long-term variability; SD2a = contribution of accelerations to long-term variability; HF = high frequency; LF = low frequency.
Table 5.
Baroreflex sensitivity and blood pressure variability (n = 7) during the repeated squat-stand maneuver for prior exercise only vs. the repeat assessment (no external stressors present).
| Parameter | Exercise Only | Repeat | t-statistic | Cohen's D | p-value |
|---|---|---|---|---|---|
| BRS-down (msec/mmHg) | 7 ± 4 | 5 ± 2 | 1.8 | 0.68 | 0.12 |
| BRS-up* (msec/mmHg) | 14 ± 4 | 9 ± 4 | 5 | 1.89 | <0.01 |
| BRS-pooled* (msec/mmHg) | 9 ± 4 | 6 ± 3 | 2.5 | 0.94 | 0.04 |
| HF-BPV (mmHg2) | 36 ± 18 | 39 ± 15 | −0.4 | −0.15 | 0.69 |
| LF-BPV (mmHg2) | 1154 ± 572 | 1260 ± 569 | −0.4 | −0.15 | 0.69 |
| TP-BPV (mmHg2) | 1214 ± 594 | 1319 ± 580 | −0.4 | −0.15 | 0.71 |
* (p < 0.05).
HF = high frequency; LF = low frequency; TP = total power; BRS = spontaneous baroreflex sensitivity, BPV = systolic blood pressure variability.
3. Results
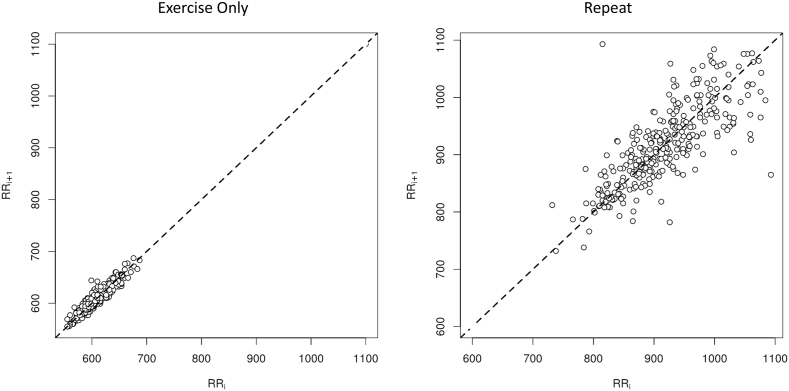
In the resting R-R interval analyses, 17 participants were included (BMI = 29.9 ± 5.6; age = 20.2 ± 1.7). Significant decreases (p < 0.05) were found from the R-R interval data for SDNN, SD2, SDNNd, SDNNa, SD2d, and SD2a for the Poincaré plot parameters in the contraindicated group. There was a decrease (p < 0.05) in total power in the contraindicated group, as observed in the spectral data. All Poincaré plot and spectral results during rest for the contraindicated analysis are presented in Table 2. Of the 17 participants, 3 were not included in the contraindicated squat-stand BRS and BPV analysis due to poor blood pressure signal quality. BRS-up sequence was decreased (p < 0.05) in the contraindicated group. No other significant differences were found in the contraindicated BRS and BPV analysis. All BRS and BPV results for the contraindicated analysis are presented in Table 3. When separating the data by exercise as the only external stressor, the results were similar to the contraindicated analyses, along with a significant decrease in HF HRV (Table 4) and an increase in BRS-pooled (Table 5) during the contraindicated assessment. Fig. 2 is a representative Poincaré plot of a participant who exercised prior to the assessment, thus depicting the changes in HRA and HRV as a result of exercise.
Fig. 2.
Poincaré plots for exercise only and a repeat assessment for a representative participant.
Note: This figure illustrates the significant impact of exercise only on heart rate variability (HRV) and heart rate asymmetry (HRA) during rest, compared to a repeat assessment.
4. Discussion
This retrospective, observational study was conducted to assess the impact of external stressors on physiological function during baseline testing. Although some previous research has demonstrated that alcohol, caffeine, and exercise independently alter metabolic function (Hawley et al., 2014; Husain et al., 2014; Marchi et al., 2014; Nehlig et al., 1992), limited data is available on how these stressors can alter ANS function as applied to physiological baseline athletic testing and return-to-play protocols. Our results indicated that the external stressors examined in this study significantly impact BRS, HRV, and HRA during rest and when undergoing a repeated squat-stand maneuver. The magnitudes of these changes are considered a medium effect, with the absolute effect size at significant intervals being at least d = 0.60 (Cohen, 1988). These results speak to the importance of developing strict pre-participation guidelines to control for external stressors before conducting baseline testing of athletes prior to their competitive sport season.
External stressors generally affect the ANS by increasing sympathetic activity, albeit through slightly different mechanisms (Kim et al., 2018). In general, the limbic system becomes activated, stimulating the hypothalamic-pituitary-adrenal axis, which increases activity in the sympathetic branch of the ANS (Chand et al., 2020). Specifically relating to the physiological effect of the three stressors discussed, alcohol consumption increases activity in the sympathetic branch of the ANS, as demonstrated through increased blood pressure and decreased baroreflex activity following alcohol consumption (Husain et al., 2014; Marchi et al., 2014). Caffeine is a potent stimulator of the sympathetic branch of the ANS, as evidenced by increased release of plasma catecholamines, elevated BP, decreased HRV, and decreased BRS following consumption (Bunsawat et al., 2015). Finally, exercise is well known to increase sympathetic activity due to its ability to trigger the release of catecholamines (Mastorakos et al., 2005) and can significantly affect cardiovascular indices (Neary and Wenger, 1985).
We add further evidence here that physiological alterations can occur as a result of alcohol intake, caffeine intake, or exercise participation. Specifically, we showed that the BRS-up sequence and Poincaré plot accelerations and decelerations were altered due to these external stressors. The Poincaré plot visually depicts R-R intervals plotted on a cartesian plane distributed around a central line of identity (Karmakar et al., 2012; Piskorski and Guzik, 2011). The distribution of the R-R intervals around the line of identity allows for depictions of heart rate accelerations and decelerations, represented by points below and above the line of identity, respectively (Piskorski and Guzik, 2011). This analysis clearly shows the decreased HRV seen with one or more external stressors present and the marked effects on the acceleration and deceleration properties of the R-R intervals on the Poincaré plots, representative of HRA (Fig. 2) (Chand et al., 2020; Piskorski and Guzik, 2011).
Although measures of ANS function, such as HRV, exhibit a degree of day-to-day variation, this variability is not statistically significant if multiple recordings are collected under similar conditions (Burma et al., 2021). For example, ANS activity fluctuates throughout the day as rhythmic circadian changes modulate endogenous physiological mechanisms (Smolensky et al., 2017). Importantly, we controlled for the potential confounding impact of circadian changes by ensuring both assessments were completed at the same time of day for each participant. As demonstrated in Table 1, there were no differences in the SCAT5 symptom totals between the contraindicated and repeat assessments, demonstrating that the participants did not subjectively feel different. Furthermore, external stressors, as previously discussed, can influence ANS activity. However, during the repeat assessment, there was strict adherence to pre-participation guidance to avoid alcohol, caffeine, and exercise before the assessment.
Table 1.
Participant characteristics and the proportion of the sample with each external stressor.
| Participants (n = 17) | |
|---|---|
| Age (mean ± SD) | 20.2 ± 1.7 |
| BMI (mean ± SD) | 29.9 ± 5.6 |
| Alcohol within 24 h (%) | 23.5% (n = 4) |
| Caffeine within 9 h (%) | 47.1% (n = 8) |
| Exercise within 6 h (%) | 70.6% (n = 12) |
| SCAT5 Symptom Sum (Contraindicated) (mean ± SD) | 1 ± 1 |
| SCAT5 Symptom Sum (Repeat) (mean ± SD) | 1 ± 1 |
BMI = body mass index; SCAT=Sport Concussion Assessment Tool 5th Edition.
Further analyses were completed on the group with exercise as their only stressor. Indeed, exercise was the most prevalent external stressor, present in 70.6% of the participant's initial assessments. Caffeine and alcohol were less prevalent in our sample, 47.1% and 23.5%, respectively, and rarely the only external stressor. As such, we could not assess the independent impact of caffeine and alcohol. Altogether, there were 10 participants with exercise as their only external stressor. The analysis of these 10 participants demonstrated significant HRA and HRV differences. Out of these 10 participants, 3 were excluded from the analysis of BRS and BPV due to poor BP waveforms. The BRS and BPV analysis in this sub-group showed significant differences in BRS-up and BRS-pooled.
As demonstrated by the results of these analyses, it is clear that prior to physiological testing, standardized pre-participation guidelines must be established for the utility and objectivity of the data. Only then can the medical and training staff make accurate and objective decisions for returning athletes to play. Thus, the importance of our research is for its application to follow-up testing and return-to-play protocols. For example, external stressors could impact diagnosis and treatment related to concussion. Concussion has been demonstrated to cause impairments in various measures of ANS function, such as BRS, HRV, and BPV, resembling those seen when external stressors are present (Krzyzaniak and Fatehi Hassanabad, 2021; La Fountaine et al., 2019; Singh et al., 2022). As a result, if an individual's baseline assessment were to be performed with an external stressor, any comparisons to follow-up assessments would not provide accurate and comparable data.
The findings presented in this study have important implications for establishing standardized pre-participation guidelines for baseline physiological testing and return-to-play protocols. To the author's knowledge, this is the first study of its kind to quantify the confounding impact of external stressors on BRS, HRV, HRA and BPV when compared to follow-up testing with strict adherence to pre-participation guidelines. This data provides strong evidence for creating standardized pre-participation guidelines to control for external stressors prior to physiological testing. Without such guidelines, such testing will continue to yield ambiguous results and hinder the utility of such assessments. Further research is warranted to explore the influence of these stressors on psychological and perceptual-cognitive function as well. Many return-to-play protocols also rely upon these components when assessing the ability to resume sport and activity after a concussion (Daniel et al., 1999; Randolph et al., 2005).
Limitations of this study include the small sample size, varying doses of stressors between participants, variations in when the participant introduced the stressor, and exclusively examining male participants. Furthermore, our results cannot be extrapolated to different populations given the non-randomized nature and the small sample size.
CRediT authorship contribution statement
Chase J. Ellingson: Writing – original draft, Writing – review & editing, Conceptualization, Formal analysis, Data curation. Jyotpal Singh: Writing – review & editing, Methodology, Conceptualization, Software, Formal analysis, Data curation. Cody A. Ellingson: Writing – review & editing. Ryan Dech: Conceptualization, Investigation, & Data Collection, Writing – review & editing. Jaroslaw Piskorski: Methodology, Software, Writing – review & editing. J. Patrick Neary: Writing – review & editing, Supervision, Funding acquisition, Conceptualization, Resources.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments and Funding
We are grateful to the participants that contributed their time to this study. Partial funding was provided by the University of Regina. Research funding for the Ensemble Software was provided by matching funding from a Mitacs Accelerate Grant (#IT18480) and LLA Technologies Inc.
References
- Bunsawat K., White D.W., Kappus R.M., Baynard T. Caffeine delays autonomic recovery following acute exercise. Eur. J. Prev. Cardiol. 2015;22(11):1473–1479. doi: 10.1177/2047487314554867. [DOI] [PubMed] [Google Scholar]
- Burma J.S., Lapointe A.P., Soroush A., Oni I.K., Smirl J.D., Dunn J.F. The validity and reliability of an open source biosensing board to quantify heart rate variability. Heliyon. 2021;7(6) doi: 10.1016/j.heliyon.2021.e07148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand T., Li M., Jamalabadi H., Wagner G., Lord A., Alizadeh S., Danyeli L.V., Herrmann L., Walter M., Sen Z.D. Heart rate variability as an index of differential brain dynamics at rest and after acute stress induction. Front. Neurosci. 2020;14:645. doi: 10.3389/fnins.2020.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapleau M.W., Li Z., Meyrelles S.S., Ma X., Abboud F.M. Mechanisms determining sensitivity of baroreceptor afferents in health and disease. Ann. N. Y. Acad. Sci. 2001;940:1–19. doi: 10.1111/j.1749-6632.2001.tb03662.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. second ed. Routledge; 1988. Statistical Power Analysis for the Behavioral Sciences. [DOI] [Google Scholar]
- Cottle J.E., Hall E.E., Patel K., Barnes K.P., Ketcham C.J. Concussion baseline testing: preexisting factors, symptoms, and neurocognitive performance. J. Athl. Train. 2017;52(2):77–81. doi: 10.4085/1062-6050-51.12.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai B., Layer J., Vertz C., Hinshaw T., Cook R., Li Y., Sha Z. Baseline assessments of strength and balance performance and bilateral asymmetries in collegiate athletes. J. Strength Condit Res. 2019;33(11):3015–3029. doi: 10.1519/jsc.0000000000002687. [DOI] [PubMed] [Google Scholar]
- Daniel J.C., Olesniewicz M.H., Reeves D.L., Tam D., Bleiberg J., Thatcher R., Salazar A. Repeated measures of cognitive processing efficiency in adolescent athletes: implications for monitoring recovery from concussion. Neuropsychiatry Neuropsychol. Behav. Neurol. 1999;12(3):167–169. [PubMed] [Google Scholar]
- Echemendia R.J., Meeuwisse W., McCrory P., Davis G.A., Putukian M., Leddy J., Makdissi M., Sullivan S.J., Broglio S.P., Raftery M., Schneider K., Kissick J., McCrea M., Dvorak J., Sills A.K., Aubry M., Engebretsen L., Loosemore M., Fuller G., et al. The sport concussion assessment Tool 5th edition (SCAT5): background and rationale. Br. J. Sports Med. 2017;51(11):848–850. doi: 10.1136/bjsports-2017-097506. [DOI] [PubMed] [Google Scholar]
- Ellis M.J., Bauman S., Cowle S., Fuselli P., Tator C.H. Primary care management of concussion in Canada. Paediatr. Child Health. 2019;24(3):137–142. doi: 10.1093/pch/pxy171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik P., Piskorski J., Krauze T., Narkiewicz K., Wykretowicz A., Wysocki H. Asymmetric features of short-term blood pressure variability. Hypertens. Res. 2010;33(11):1199–1205. doi: 10.1038/hr.2010.138. [DOI] [PubMed] [Google Scholar]
- Hawley J.A., Hargreaves M., Joyner M.J., Zierath J.R. Integrative biology of exercise. Cell. 2014;159(4):738–749. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- Husain K., Ansari R.A., Ferder L. Alcohol-induced hypertension: mechanism and prevention. World J. Cardiol. 2014;6(5):245–252. doi: 10.4330/wjc.v6.i5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar C., Khandoker A., Palaniswami M. Investigating the changes in heart rate asymmetry (HRA) with perturbation of parasympathetic nervous system. Australas. Phys. Eng. Sci. Med. 2012;35(4):465–474. doi: 10.1007/s13246-012-0173-x. [DOI] [PubMed] [Google Scholar]
- Kim H.G., Cheon E.J., Bai D.S., Lee Y.H., Koo B.H. Stress and heart rate variability: a meta-analysis and review of the literature. Psychiatr. Invest. 2018;15(3):235–245. doi: 10.30773/pi.2017.08.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzaniak H., Fatehi Hassanabad A. 2021. Cardiovascular Sequelae of Sports-Related Concussions. Pm r. [DOI] [PubMed] [Google Scholar]
- La Fountaine M.F., Hohn A.N., Testa A.J., Weir J.P. Attenuation of spontaneous baroreceptor sensitivity after concussion. Med. Sci. Sports Exerc. 2019;51(4):792–797. doi: 10.1249/mss.0000000000001833. [DOI] [PubMed] [Google Scholar]
- Laguna P., Moody G.B., Mark R.G. Power spectral density of unevenly sampled data by least-square analysis: performance and application to heart rate signals. IEEE Trans. Biomed. Eng. 1998;45(6):698–715. doi: 10.1109/10.678605. [DOI] [PubMed] [Google Scholar]
- Laitinen T., Hartikainen J., Niskanen L., Geelen G., Länsimies E. Sympathovagal balance is major determinant of short-term blood pressure variability in healthy subjects. Am. J. Physiol. 1999;276(4):H1245–H1252. doi: 10.1152/ajpheart.1999.276.4.H1245. [DOI] [PubMed] [Google Scholar]
- Longo U.G., Risi Ambrogioni L., Ciuffreda M., Maffulli N., Denaro V. Sudden cardiac death in young athletes with long QT syndrome: the role of genetic testing and cardiovascular screening. Br. Med. Bull. 2018;127(1):43–53. doi: 10.1093/bmb/ldy017. [DOI] [PubMed] [Google Scholar]
- Marchi K.C., Muniz J.J., Tirapelli C.R. Hypertension and chronic ethanol consumption: what do we know after a century of study? World J. Cardiol. 2014;6(5):283–294. doi: 10.4330/wjc.v6.i5.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastorakos G., Pavlatou M., Diamanti-Kandarakis E., Chrousos G.P. Exercise and the stress system. Hormones (Basel) 2005;4(2):73–89. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.595.856&rep=rep1&type=pdf [PubMed] [Google Scholar]
- McCrory P., Meeuwisse W., Dvorak J., Aubry M., Bailes J., Broglio S., Cantu R.C., Cassidy D., Echemendia R.J., Castellani R.J., Davis G.A., Ellenbogen R., Emery C., Engebretsen L., Feddermann-Demont N., Giza C.C., Guskiewicz K.M., Herring S., Iverson G.L., et al. Consensus statement on concussion in sport-the 5(th) international conference on concussion in sport held in Berlin, October 2016. Br. J. Sports Med. 2017;51(11):838–847. doi: 10.1136/bjsports-2017-097699. [DOI] [PubMed] [Google Scholar]
- Neary J.P., Singh J., Bishop S.A., Dech R.T., Butz M.J.A., Len T.K. An evidence-based objective study protocol for evaluating cardiovascular and cerebrovascular indices following concussion: the neary protocol. Methods Protoc. 2019;2(1) doi: 10.3390/mps2010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary J.P, MacQuarrie D.S., Jamnik V., Gledhill S., Buse E.F.G. Assessment of mechanical cardiac function in elite athletes. Open Sports Med. J. 2011;5(1):26–37. doi: 10.2174/1874387001105010026. [DOI] [Google Scholar]
- Neary J.P., Wenger H.A. The effects of prior exercise on the lactate and ventilatory thresholds. J. Sports Sci. 1985;3(3):189–195. doi: 10.1080/02640418508729751. [DOI] [PubMed] [Google Scholar]
- Nehlig A., Daval J.L., Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev. 1992;17(2):139–170. doi: 10.1016/0165-0173(92)90012-b. [DOI] [PubMed] [Google Scholar]
- Pagani M., Montano N., Porta A., Malliani A., Abboud F.M., Birkett C., Somers V.K. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation. 1997;95(6):1441–1448. doi: 10.1161/01.cir.95.6.1441. [DOI] [PubMed] [Google Scholar]
- Piskorski J., Guzik P. Asymmetric properties of long-term and total heart rate variability. Med. Biol. Eng. Comput. 2011;49(11):1289–1297. doi: 10.1007/s11517-011-0834-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta A., Casali K.R., Casali A.G., Gnecchi-Ruscone T., Tobaldini E., Montano N., Lange S., Geue D., Cysarz D., Van Leeuwen P. Temporal asymmetries of short-term heart period variability are linked to autonomic regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295(2):R550–R557. doi: 10.1152/ajpregu.00129.2008. [DOI] [PubMed] [Google Scholar]
- Rajendra Acharya U., Paul Joseph K., Kannathal N., Lim C.M., Suri J.S. Heart rate variability: a review. Med. Biol. Eng. Comput. 2006;44(12):1031–1051. doi: 10.1007/s11517-006-0119-0. [DOI] [PubMed] [Google Scholar]
- Randolph C., McCrea M., Barr W.B. Is neuropsychological testing useful in the management of sport-related concussion? J. Athl. Train. 2005;40(3):139–152. [PMC free article] [PubMed] [Google Scholar]
- Shaffer F., McCraty R., Zerr C.L. A healthy heart is not a metronome: an integrative review of the heart's anatomy and heart rate variability. Front. Psychol. 2014;5:1040. doi: 10.3389/fpsyg.2014.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Hind K., Hume P., Singh J., Neary J.P. Neurovascular coupling by functional near infra-red spectroscopy and sport-related concussion in retired rugby players: the UK rugby health project. Front. Hum. Neurosci. 2020;14:42. doi: 10.3389/fnhum.2020.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J., Bhagaloo L., Piskorski J., Neary J.P. Effects of phytocannabinoids on heart rate variability and blood pressure variability in female post-concussion syndrome patients: case series. Can. J. Physiol. Pharmacol. 2022;100(2):192–196. doi: 10.1139/cjpp-2021-0395. [DOI] [PubMed] [Google Scholar]
- Smirl J.D., Hoffman K., Tzeng Y.C., Hansen A., Ainslie P.N. Methodological comparison of active- and passive-driven oscillations in blood pressure; implications for the assessment of cerebral pressure-flow relationships. J. Appl. Physiol. 2015;119(5):487–501. doi: 10.1152/japplphysiol.00264.2015. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolensky M.H., Hermida R.C., Portaluppi F. Circadian mechanisms of 24-hour blood pressure regulation and patterning. Sleep Med. Rev. 2017;33:4–16. doi: 10.1016/j.smrv.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Tarvainen M.P., Niskanen J.P., Lipponen J.A., Ranta-Aho P.O., Karjalainen P.A. Kubios HRV--heart rate variability analysis software. Comput. Methods Progr. Biomed. 2014;113(1):210–220. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Ziemssen T., Siepmann T. The investigation of the cardiovascular and sudomotor autonomic nervous system-A review. Front. Neurol. 2019;10:53. doi: 10.3389/fneur.2019.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]