Abstract
Congenital hypothyroidism with biallelic thyroglobulin (Tg protein, encoded by the TG gene) mutation is an endoplasmic reticulum (ER) storage disease. Many patients (and animal models) grow an enlarged thyroid (goiter), yet some do not. In adulthood, hypothyroid TGcog/cog mice (bearing a Tg-L2263P mutation) exhibit a large goiter, whereas adult WIC rats bearing the TGrdw/rdw mutation (Tg-G2298R) exhibit a hypoplastic thyroid. Homozygous TG mutation has been linked to thyroid cell death, and cytotoxicity of the Tg-G2298R protein was previously thought to explain the lack of goiter in WIC-TGrdw/rdw rats. However, recent studies revealed that TGcog/cog mice also exhibit widespread ER stress–mediated thyrocyte death, yet under continuous feedback stimulation, thyroid cells proliferate in excess of their demise. Here, to examine the relative proteotoxicity of the Tg-G2298R protein, we have used CRISPR–CRISPR-associated protein 9 technology to generate homozygous TGrdw/rdw knock-in mice in a strain background identical to that of TGcog/cog mice. TGrdw/rdw mice exhibit similar phenotypes of defective Tg protein folding, thyroid histological abnormalities, hypothyroidism, and growth retardation. TGrdw/rdw mice do not show evidence of greater ER stress response or stress-mediated cell death than TGcog/cog mice, and both mouse models exhibit sustained thyrocyte proliferation, with comparable goiter growth. In contrast, in WIC-TGrdw/rdw rats, as a function of aging, the thyrocyte proliferation rate declines precipitously. We conclude that the mutant Tg-G2298R protein is not intrinsically more proteotoxic than Tg-L2263P; rather, aging-dependent difference in maintenance of cell proliferation is the limiting factor, which accounts for the absence of goiter in adult WIC-TGrdw/rdw rats.
Keywords: protein misfolding, secretory pathway, cell death, ER stress, aging
Abbreviations: BiP, immunoglobulin heavy-chain binding protein; Cas9, CRISPR-associated protein 9; CHOP, CCAAT/enhancer-binding protein homologous protein; DAPI, 4′,6-diamidino-2-phenylindole; eIF2α, eukaryotic initiation factor 2α; ER, endoplasmic reticulum; PARP, poly(ADP-ribose) polymerase; PTU, propylthiouracil; Tg, thyroglobulin protein; TG, thyroglobulin gene; TSH, thyroid stimulating hormone
The vertebrate thyroid gland supplies the entire supply of thyroxine (also known as T4) to the body (1). T4 biosynthesis normally occurs upon secretion of thyroglobulin (Tg protein, encoded by the TG gene) (2) from thyroid epithelial cells into the (extracellular) thyroid follicle lumen, in which the secreted Tg protein is stored (3). The iodination of proteins contained within the follicle lumen (4) includes tyrosyl residues on secreted Tg (5), triggering a coupling reaction that promotes formation of T4 within Tg (6, 7, 8). Hundreds of different TG gene mutations altering the primary structure of the Tg protein have been linked to defective thyroid hormone biosynthesis (9). Tg is synthesized within the endoplasmic reticulum (ER); most if not all mutant Tg is thought to misfold and become entrapped within the ER (3). As Tg is the single most abundant protein within the thyrocyte translatome, the misfolding of mutant Tg induces significant ER stress (10, 11, 12, 13, 14, 15). On the one hand, primary hypothyroidism with reduced circulating thyroid hormone levels results in a compensatory upregulation of the pituitary secretion of thyroid stimulating hormone (TSH) to induce hyperplastic (i.e., proliferative) thyroid gland growth (16, 17, 18, 19, 20); on the other hand, the ER stress caused by misfolded mutant Tg has been found to be associated with thyroid cell death in both animal models and human patients (21). Indeed, we recently demonstrated that, albeit inefficient, T4 can be synthesized on mutant Tg released in the follicle lumen from dead thyrocytes, and thyroid goiter growth (proliferation in excess of cell death) helps to provide the cells that sustain this mechanism (21).
It is not guaranteed that net goiter growth will occur in all individuals with hypothyroidism from biallelic TG mutations. Whereas TGcog/cog (congenital goiter) mice (bearing Tg-L2263P) are famous for their adult goiter (22), WIC-TGrdw/rdw rats (bearing Tg-G2298R) do not develop a goiter (23); this was the first model of the disease in which thyrocyte cell death was noted (24). This absence of goiter has been attributed to increased rdw-Tg proteotoxicity (25). However, original descriptions of the thyroid phenotype of TGcog/cog mice and WIC-TGrdw/rdw rats were limited to the very different AKR/J mouse and Wistar–Imamichi rat strain backgrounds, respectively, in which species- and strain-specific genetic interactions might distinctly alter the balance of thyroid cell growth and death. JAX laboratories currently distribute the TGcog allele in the C57BL6J background, and here, we have used CRISPR/CRISPR-associated protein 9 (Cas9) technology to generate homozygous TGrdw/rdw knock-in mice in the same strain background. Our analysis indicates that the gene product of the TGrdw allele is not intrinsically more proteotoxic than that of the TGcog allele, and our data suggest an alternative explanation for the incapacity of WIC-TGrdw/rdw rats to maintain their hypothyroid goiter in adulthood.
Results
Generation of TGrdw/rdw mice
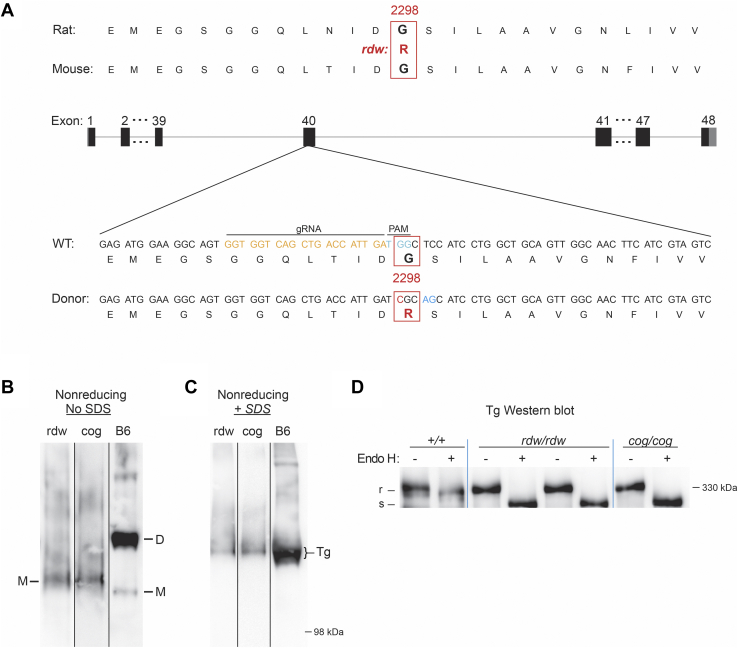
Using CRISPR/Cas9 with a suitable guide RNA, we introduced within C57BL6J mouse TG exon 40, the point mutation that encodes Tg-G2298R (Fig. 1A; in the UniProt P01266 system, this numbering would need to add 19 to account for the signal peptide), just 35 residues from the site of the cog mutation in the ChEL domain of Tg (Fig. S1). After crossing with C57BL6J, animals were bred to homozygosity so that TGrdw/rdw mice could be compared with TGcog/cog mice in the identical genetic background. Resection of the thyroid gland of adult animals was followed by tissue homogenization in buffer lacking all denaturants, followed by nondenaturing polyacrylamide gel electrophoresis (i.e., in the absence of SDS, mobility is dependent not on protein mass but rather on charge/mass ratio and hydrodynamic radius (26), and this has been used to separate unfolded and folded Tg monomers from native Tg dimers (27, 28)). After electrotransfer to nitrocellulose and immunoblotting with anti-Tg antibody, immunoreactive Tg in WT-control C57BL6J thyroids appeared primarily as two bands corresponding to folded Tg monomers (a smaller quantity) and dimers (a larger quantity, Fig. 1B marked “M” and “D”). However, mutant Tg protein from TGcog/cog and TGrdw/rdw mouse thyroids both appeared primarily as a monomer of slower electrophoretic mobility (Fig. 1B) indicating a less compact structure consistent with an unfolded state (28). When the same samples were analyzed under denaturing conditions by nonreducing SDS-PAGE—although the molecular masses of cog-Tg-L2263P, rdw-Tg-G2298R, and WT-Tg are identical—the mobility of WT-Tg still appeared slightly faster (Fig. 1C), suggesting a more tightly disulfide-linked structure (29, 30). Moreover, in WT thyroid glands, nearly all Tg proteins had migrated out of the ER so that most glycans on those Tg molecules were processed to complex sugars resistant to digestion with endoglycosidase H (Fig. 1D, marked as “R”), whereas all Tg proteins in TGcog/cog and TGrdw/rdw mouse thyroids remained fully endoglycosidase H-sensitive (Fig. 1D, “S”), indicating failure of the mutant Tg protein to arrive at the Golgi complex.
Figure 1.
Generation of TGrdw/rdwmice.A, schematic illustration of CRISPR/Cas9-mediated gene editing to knock-in rdw (Tg-G2298R) mutation within the exon 40 of the mouse TG gene. The target Tg protein sequences and the target site of gRNA are indicated. B and C, polyacrylamide gel electrophoresis of thyroid homogenates from B6, TGrdw/rdw, and TGcog/cog mice in the absence (B) or the presence (C) of SDS, followed by immunoblotting with an mAb anti-Tg (three repeats). D, endoglycosidase H digest and Tg Western blotting of thyroid from B6, TGrdw/rdw, and TGcog/cog mice (n = 3 animals per group). Cas9, CRISPR-associated protein 9; gRNA, guide RNA; mAb, monoclonal antibody; TG, thyroglobulin gene; Tg, thyroglobulin protein.
Thyroid histology of TGrdw/rdw mice
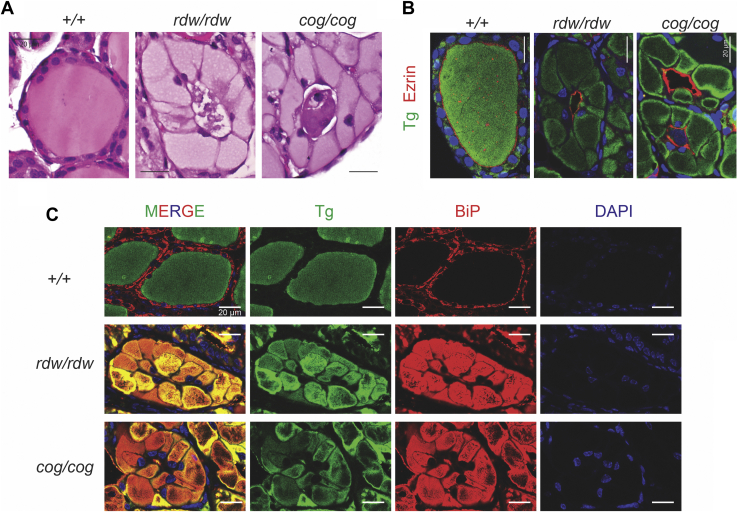
Careful examination of the histology of TGrdw/rdw and TGcog/cog mouse thyroid glands revealed important differences with that of the WT thyroid. While all three tissues exhibited prominent eosinophilic staining consistent with accumulation of Tg protein, in WT (TG+/+) thyroid, the Tg protein was primarily localized extracellularly in the follicle lumen, whereas in TGrdw/rdw and TGcog/cog mouse thyroid glands, most Tg proteins were found in an expanded and a distorted cytoplasm within the thyrocytes (Fig. 2A), although there was some aberrant material in the follicle lumen (that we have recently established is comprised of the detritus of dead cells (21)). From multiple images of thyroid sections derived from 3-month-old animals of each genotype, the cross-sectional area of individual thyrocytes was abnormally expanded approximately eightfold in TGrdw/rdw and TGcog/cog mouse thyroid glands (Fig. S2A) with a fraction of total cellular area occupied by cytoplasm increased to >90% (Fig. S2B) plus an increase of cross-sectional nuclear area (Fig. S2C). With the huge expansion of cytoplasm, the number of thyrocytes actually accommodated within each follicle profile shrank nearly in half (Fig. S2D).
Figure 2.
Thyroid histology of TGrdw/rdwmice.A, representative H&E of thyroid glands from B6, TGrdw/rdw, and TGcog/cog mice (n = 5–8 animals per group), showing thyrocyte distention in TGrdw/rdw and TGcog/cog mice. The scale bars represent 20 μm. B, immunofluorescence of anti-Tg (green) and antiezrin (red) of thyroid glands from B6, TGrdw/rdw, and TGcog/cog mice (n = 5 animals per group), with DAPI counterstaining (blue). The scale bars represent 20 μm. C, immunofluorescence of Tg (green) and anti-BiP (red) of thyroid glands from B6, TGrdw/rdw, and TGcog/cog mice (n = 5 animals per group), with DAPI counterstaining (blue). The scale bars represent 20 μm. BiP, immunoglobulin heavy-chain binding protein; DAPI, 4′,6-diamidino-2-phenylindole; TG, thyroglobulin gene; Tg, thyroglobulin protein.
To independently confirm whether Tg protein in mouse thyroid glands was primarily extracellular or intracellular, we coimmunostained these tissues for Tg and ezrin, which delimits the follicle lumen at the apical plasma membrane of thyrocytes. Whereas Tg in WT glands was contained within the ezrin ring (i.e., extracellular, in the follicle lumen), the mutant Tg protein in TGrdw/rdw and TGcog/cog mice was largely excluded from within the ezrin ring, indicating that it had not been transported from thyrocytes to the follicle lumen (Figs. 2B and S3). Indeed, in WT (TG+/+) thyroid, the Tg protein distribution was largely nonoverlapping with the ER molecular chaperone, BiP (immunoglobulin heavy-chain binding protein); whereas in TGrdw/rdw and TGcog/cog mouse thyroid glands, the Tg protein was essentially exclusively contained within the swollen ER, colocalized with BiP (Fig. 2C).
Hypothyroidism of TGrdw/rdw mice
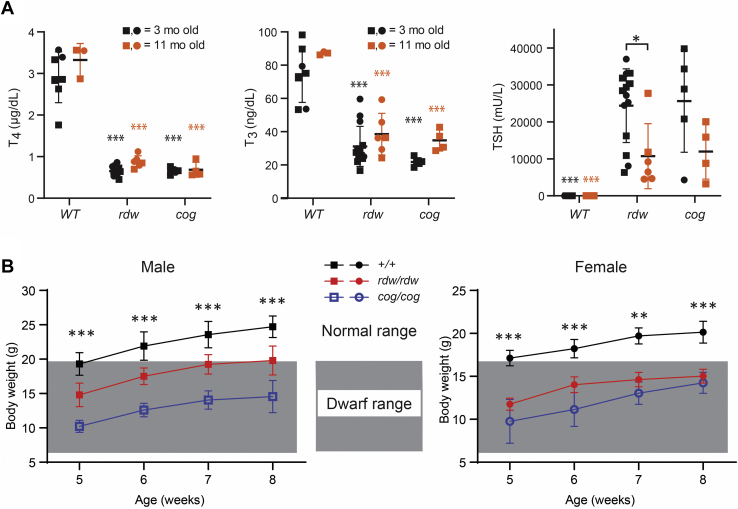
As TGcog/cog mice have goiter from hypothyroidism (31), we wished to determine if TGrdw/rdw mice were even more hypothyroid by comparing them to TGcog/cog mice at 3 months of age and again at 11 months. At 3 months, both sets of mutant animals had >75% inhibition of circulating total T4 and >65% inhibition of circulating total triiodothyronine (T3), accompanied by dramatic elevation of murine TSH that averaged ∼25,000 mU/l (Fig. 3A). Accompanying the thyroid hormone defect was body growth retardation for both sets of mutant animals (Fig. 3B), which has been reported in other models of congenital hypothyroidism (32).
Figure 3.
Hypothyroidism of TGrdw/rdwmice.A, serum total T4, T3, and TSH levels of B6, TGrdw/rdw, and TGcog/cog mice at 3 months (black) and 11 months (red) of age (males shown as squares and females as circles). Data are mean ± SD; ∗p < 0.05, #p < 0.001 compared with B6 mice of 3 months old, &p < 0.001 compared with B6 mice of 11 months old. B, body weight of B6 (black), TGrdw/rdw, (red) and TGcog/cog mice (blue). The shaded area indicates the body weights of dwarf mice. Data are mean ± SD; ∗∗p < 0.01, ∗∗∗p < 0.001 compared with B6 mice. T3, triiodothyronine; T4, thyroxine; TG, thyroglobulin gene; TSH, thyroid stimulating hormone.
In both TGrdw/rdw and TGcog/cog mice, the highly elevated TSH levels tended to decline by more than 50% by 11 months (Fig. 3), either as an aging-related phenomenon or possibly consistent with a small improvement in thyroid hormone output by the mutant thyroid glands over time (which, in the case of TGcog/cog mice, is known to be accompanied by goiter growth (22)). Notably, when compared with TGcog/cog mice, TGrdw/rdw mice never had more elevated TSH (or diminished T4 or T3) levels or more severe growth retardation, indicating that TGrdw/rdw mice did not have more severe hypothyroidism than TGcog/cog mice at any age examined (Fig. 3).
Chronic ER stress in the thyroid glands of TGrdw/rdw mice
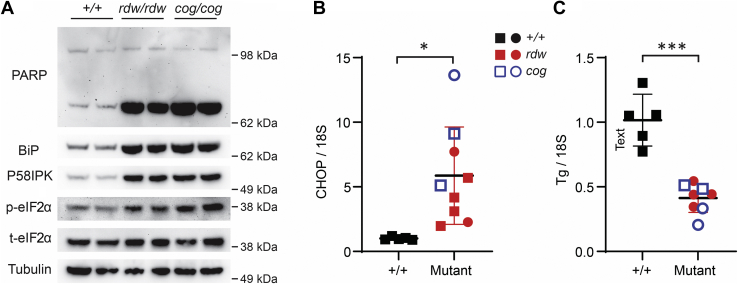
In the thyroid gland at 3 months of age, TGrdw/rdw mice showed elevated levels of BiP (normalized to tubulin as a loading control), as was also the case in TGcog/cog mice (Fig. 4A)—indicative of ongoing ER stress. In addition, the BiP cochaperone p58ipk was also clearly elevated in the mutant thyroid glands compared with WT (Fig. 4A). The ratio of phosphorylated eukaryotic initiation factor 2α (phospho-eIF2α) to total eIF2α, suggestive of activation of the ER stress sensor, PERK—was elevated in the thyroid glands of both mouse mutants. Importantly, none of these ER stress markers were more elevated in the thyroid glands of TGrdw/rdw mice than in TGcog/cog mice (Fig. 4A). Compared with WT mice, elevation of CCAAT/enhancer-binding protein homologous protein (CHOP) mRNA level in the thyroid gland was increased in both TGrdw/rdw mice and TGcog/cog mice (Fig. 4B) but again was not more elevated in TGrdw/rdw mice (4.1 ± 2.2 fold) than in TGcog/cog mice (13.2 ± 8.7 fold). Moreover, because chronic continuous ER stress has been associated with subtle signs of dedifferentiation in thyrocytes (11, 33) as well as Ire1-mediated mRNA decay (in various cell types (34, 35, 36)), we examined thyroidal TG mRNA levels and observed an average decrease of 56 ± 8% of WT levels in TGrdw/rdw mice and 62 ± 14% in TGcog/cog mice—both significantly different from WT but not different from each other (Fig. 4C). Finally, in conjunction with the chronically increased ER stress, cell death signaling as measured by poly(ADP-ribose) polymerase (PARP) cleavage was apparent in the thyroid glands of both mutant animals—but with no evidence of greater PARP cleavage in TGrdw/rdw mice than in TGcog/cog mice (Fig. 4A).
Figure 4.
Chronic ER stress in the thyroid glands of TGrdw/rdwmice.A, BiP, p58ipk, phospho-eIF2α, and PARP (by Western blotting) in the thyroid glands of B6, TGrdw/rdw, and TGcog/cog mice (n = 3 animals per group, two of which are shown in the panel). B, CHOP mRNA levels (normalized to 18S) in the thyroid glands of B6, TGrdw/rdw (purple), and TGcog/cog (orange) mice (n = 3–6 animals per group; each point represents a single animal; square = male, circle = female). Data are shown as mean ± SD; ∗p < 0.05. C, Tg mRNA levels (normalized to 18S) in the thyroid glands of B6 (black symbols), TGrdw/rdw (red symbols), and TGcog/cog (blue symbols) mice (n = 4–5 animals per group; each point represents a single animal; square = male, circle = female). Data are shown as mean ± SD; ∗∗∗p < 0.001. BiP, immunoglobulin heavy-chain binding protein; CHOP, CCAAT/enhancer-binding protein homologous protein; eIF2α, eukaryotic initiation factor 2α; ER, endoplasmic reticulum; PARP, poly(ADP-ribose) polymerase; TG, thyroglobulin gene; Tg, thyroglobulin protein.
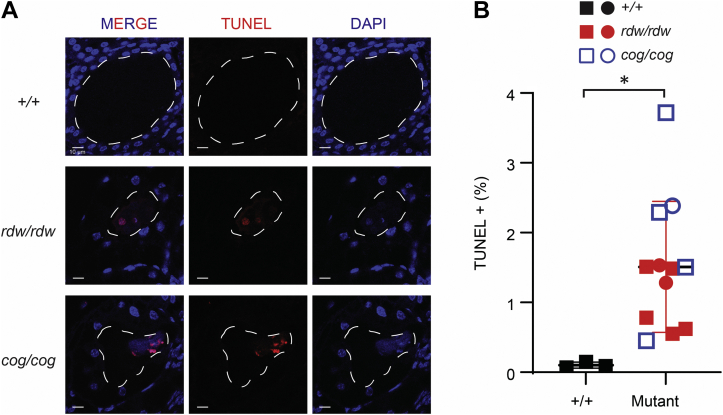
Because both TGcog/cog mice and WIC-TGrdw/rdw rats are known to exhibit intrathyroidal cell death (21), we performed TUNEL staining on the newly engineered TGrdw/rdw mice. As expected, the thyroid glands of TGrdw/rdw mice revealed 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen)–positive nuclear material within the lumen of thyroid follicles, associated with positive TUNEL staining (Fig. 5A). While thyrocyte cell death in the mutant mice was significantly greater than background TUNEL staining seen in WT mouse thyroid glands, the TGrdw/rdw mice did not exhibit greater TUNEL staining than that found in TGcog/cog thyroid (Fig. 5B). Altogether from the results of Figs. 4 and 5, although homozygous expression of Tg-G2298R does indeed produce hypothyroidism, we found no evidence to suggest that this mutant Tg yields more ER stress or more proteotoxicity than the Tg-L2263P expressed in TGcog/cog mice.
Figure 5.
Thyroid cell death in TGrdw/rdwmice.A, representative TUNEL labeling (red) with DAPI counterstain (blue) in the thyroid sections of B6, TGrdw/rdw, and TGcog/cog mice (n = 3–7 animals per group). A dashed white line delimits the thyroid follicle lumen. The scale bars represent 10 μm. B, quantification of A presented as TUNEL-positive nuclei in the proportion of total nuclei in the thyroids of B6 (black symbols), TGrdw/rdw (red symbols), and TGcog/cog (blue symbols) mice (n = 3–7 animals per group; each point represents a single animal; square = male, circle = female). Data are mean ± SD; ∗p < 0.05. DAPI, 4′,6-diamidino-2-phenylindole; TG, thyroglobulin gene.
Thyroid cell proliferation during chronic ER stress in the thyroid glands of animals bearing TG mutations
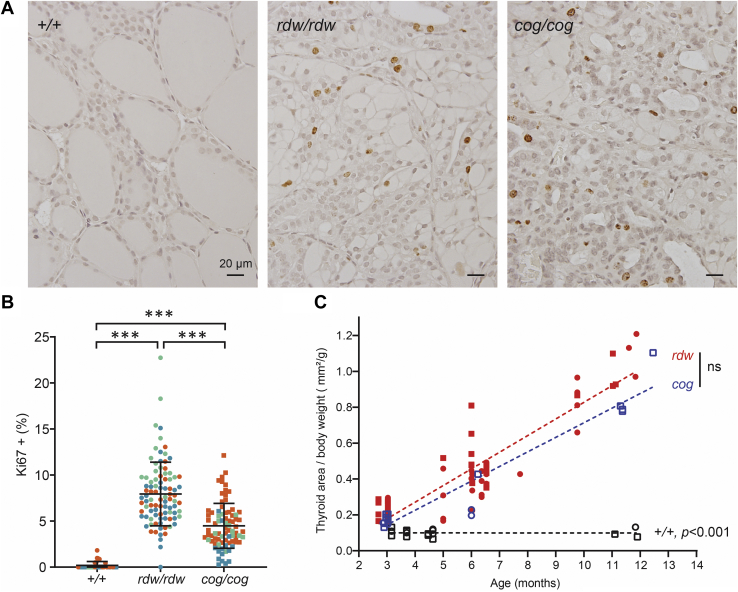
We recently reported that in WIC rats, TGrdw/rdw homozygotes do begin to develop thyroid enlargement within the first two postnatal months, but their absence of goiter in adulthood involves an inability to maintain the goiter as a function of aging (21). As TGcog/cog mice exhibit thyroid cell death (Fig. 5) but nevertheless manage to grow a large goiter in adulthood, we turned attention to thyroid cell proliferation. By Ki-67 immunostaining of mouse thyroid glands, it was apparent that both TGrdw/rdw and TGcog/cog mutants exhibited sustained enhancement of thyroid cell proliferation well into adulthood (11 months, Fig. 6A). When quantified, TGrdw/rdw mice, on average, definitely did not exhibit less thyroid cell proliferation than that observed in TGcog/cog mice—and both exhibited significantly greater proliferation than that found in the thyroids of WT animals (Fig. 6B). As ER stress–mediated cell death was not increased (Figs. 4 and 5) and thyroid cell proliferation was not diminished (Fig. 6B), there was no reason why TGrdw/rdw mice would not grow a goiter as large as that found in TGcog/cog mice. Indeed, when allowed to survive over the first postnatal year of life, multinodular goiters in TGrdw/rdw mice at least as large as those found in TGcog/cog mice appeared (Fig. S4); vastly larger than the thyroid glands seen in WT animals (Fig. 6C).
Figure 6.
Thyroid cell proliferation in TGrdw/rdwmice.A, Ki67 immunohistochemistry of the thyroid gland of WT, TGrdw/rdw, and TGcog/cog mice (11 months). There are sustained thyroid cell proliferation in both TGrdw/rdw and TGcog/cog mice. The scale bars represent 20 μm. B, quantification of images like those shown in A, presented as Ki67-positive nuclei as a proportion of total nuclei in thyroid images from 11-month-old WT, TGrdw/rdw, and TGcog/cog mice (n = 3 animals per group; each color represents a single animal; each point is an independent section; square = male, circle = female). Data are mean ± SD; ∗∗∗p < 0.001. C, thyroid gland size (normalized to body weight) as a function of age (square = male, circle = female). A dashed line shows the linear regression of the thyroid size in WT, TGrdw/rdw, and TGcog/cog mice. No significance difference was observed between thyroid glands of TGrdw/rdw and TGcog/cog mice. TG, thyroglobulin gene.
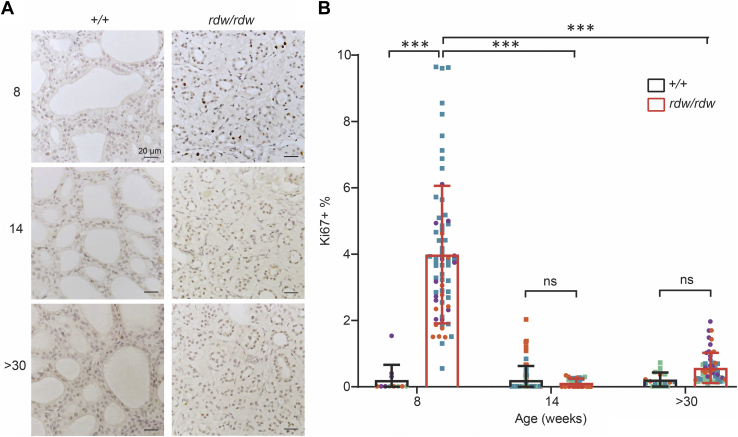
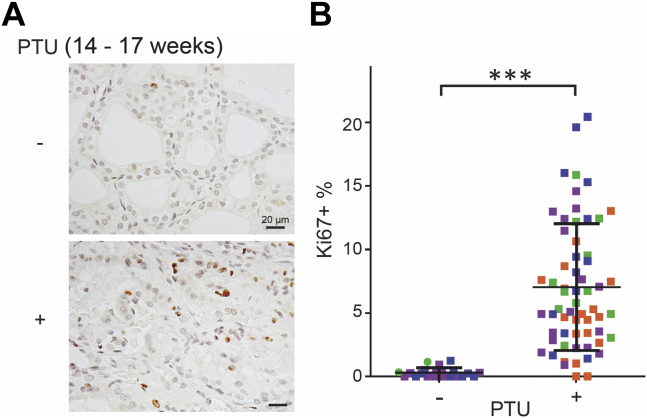
Because WIC-TGrdw/rdw rats have a dramatic loss of thyroid tissue mass during adulthood (21), the goitrogenesis in TGrdw/rdw mice seemed perplexing. We therefore returned to WIC-TGrdw/rdw rats to examine thyroid cell proliferation as a function of postnatal age. As noted previously, it is established that WIC-TGrdw/rdw rats do exhibit exuberant thyroid gland growth during the first two postnatal months of life (21); indeed, at 8 weeks of age, there was a dramatic increase of thyroidal Ki-67 immunostaining (Fig. 7, A and B). To our surprise, however, unlike in TGrdw/rdw mice (in the C57BL6J genetic background, Fig. 6, A and B), by early adulthood, the hypothyroid WIC-TGrdw/rdw rats no longer exhibited thyroid cell proliferation beyond control levels, and this feature persisted as the animals aged further (Fig. 7, A and B). It has been repeatedly reported that WIC-TGrdw/rdw rats during this period of life (18–40 weeks) do maintain markedly elevated circulating TSH levels (23, 37, 38, 39), and indeed, we found that older WIC-TGrdw/rdw rats (>30 weeks) exhibited a circulating TSH (32.7 ± 10.7 ng/ml; n = 10) that was 16.6-fold elevated over that of their WT counterparts (1.97 ± 0.11 ng/ml; n = 3). To confirm that the elevated TSH in adult WIC rats should be sufficiently bioactive to stimulate thyroid cell proliferation, we challenged 14-week-old WT WIC rats to induce hypothyroidism, by introducing propylthiouracil (PTU) continuously for 3 weeks before euthanasia and thyroid tissue analysis at 17 weeks. This treatment yielded a similar elevation of circulating TSH (35.8 ± 1.01 ng/ml; n = 3), and these older hypothyroid WT WIC rats exhibited exuberant thyroid cell proliferation well beyond control levels (Fig. 8, A and B). The data suggest that the WIC strain background is specifically deficient neither for a central nervous system response to hypothyroidism nor for the bioactivity of TSH in older animals. Thus, we conclude that the inability of WIC-TGrdw/rdw rats to expand thyroidal mass in adulthood is caused neither by a genetic deficiency of the central nervous system axis to provide adequate feedback stimulation nor solely by ER stress–mediated thyroid cell death, but rather by an inability of the adult WIC-TGrdw/rdw rat strain to maintain the TSH-driven thyroid cell proliferative response to hypothyroidism that is needed for net thyroid gland growth.
Figure 7.
Thyroid cell proliferation in WIC-TGrdw/rdwrats.A, Ki67 immunohistochemistry of the thyroid gland of WT and WIC-TGrdw/rdw rats at 8 weeks, 14 weeks, and over 30 weeks of age. The scale bars represent 20 μm. B, quantification of immunostained sections like those shown in A, presented as Ki67-positive nuclei in the proportion of total nuclei in the thyroids of WT WIC and WIC-TGrdw/rdw rats (n = 3–4 animals per group; each color represents a single animal; each point is an independent section; square = male, circle = female). Data are mean ± SD; ∗∗∗p < 0.001. TG, thyroglobulin gene.
Figure 8.
Thyroid cell proliferation in PTU-induced hypothyroid adult WT rats.A, Ki67 immunohistochemistry of the thyroid gland of control WT and PTU-treated WT rats at 17 weeks of age. Treated rats received PTU chow at 14 weeks of age for 21 days. The scale bars represent 20 μm. B, quantification of A presented as Ki67-positive nuclei in the proportion of total nuclei in the thyroids of control WT and PTU-treated WT rats (n = 3–4 animals per group; each color represents a single animal; each point is an independent section, males shown as squares and females as circles). Data are mean ± SD; ∗∗∗p < 0.001. PTU, propylthiouracil.
Discussion
Chronic, unremitting, and unresolved ER stress is a factor that can promote cell death (40); thus, the field of medicine is just beginning to attack ER stress–mediated cell death as a potential therapeutic approach to address various clinical disorders (41, 42). However, from studies of hypothyroid patients and animal models bearing biallelic TG mutations (10) as well as cell culture models of the disease (43), we have been mostly impressed by continued thyrocyte proliferation (11) despite the ongoing stress-mediated thyrocyte cell death (21). In such a case, the entire thyroid gland overgrows, that is, beyond normal size (22). Nevertheless, in individuals with hypothyroidism from biallelic TG mutations, net thyroid growth is not always observed. This is also the case in WIC-TGrdw/rdw rats, which initiate thyroid growth during the first one or two postnatal months (21) but in adulthood lose the ability to further expand or even maintain the goiter, leading ultimately to thyroid atrophy (23). Despite the remarkably close physical proximity of the encoded mutation from that seen in TGcog/cog mice (Fig. S1), we considered the possibility that the TGrdw-encoded Tg-G2298R protein might be intrinsically more proteotoxic than the TGcog-encoded Tg-L2263P protein. Such a hypothesis can only be tested by comparing the cell biological and physiological impact of the two mutant proteins in the identical genetic background. This was made possible using CRISPR/Cas9 technology to engineer the TGrdw allele into C57BL6J mice (Fig. 1A).
From our analysis, it was immediately apparent that TGrdw/rdw mice did not exhibit greater misfolding of the mutant Tg protein (Fig. 1, B and C) or greater failure of Tg export from the ER to that seen in TGcog/cog mice (Fig. 1D). In addition, the ER entrapment of mutant Tg led to enormous ER swelling that was comparable in the two mouse models (Fig. 2). Furthermore, TGrdw/rdw mice exhibited profound primary hypothyroidism with body growth retardation and initial elevation of circulating TSH level to ∼25,000 mU/l, which was not greater than that in TGcog/cog mice (Fig. 3). Unlike what has been reported in the original AKR/J mouse strain (22), neither TGrdw/rdw nor TGcog/cog mice in the C57BL6/J background (from JAX laboratories; fed Formulab Diet 5008 bearing 0.8–0.97 ppm iodine) could spontaneously restore circulating T4 to euthyroid levels by 11 months of age; instead showing only marginal improvement of their dramatically elevated levels of circulating TSH (Fig. 3A). Furthermore, although TGrdw/rdw mice exhibited a major increase of thyroidal ER stress markers and ER stress–mediated cell death, it was not worse than in TGcog/cog mice (Figs. 4 and 5), consistent with the notion that in both cases, endogenous thyroid hormone synthesis derived from dead thyrocytes (21) is a highly inefficient process compared with the normal hormonogenesis process occurring in WT thyroid glands. Yet despite the cell death, hypothyroid TGrdw/rdw mice exhibited exuberant thyroid cell proliferation (Fig. 6A), which was not less than that in TGcog/cog mice (Fig. 6B). Indeed, it is this feature that led ultimately to goiters in TGrdw/rdw mice that were not smaller than those observed in TGcog/cog mice (Fig. 6C). Taken together, the primary conclusion of this work is that when compared side by side in the same strain background, there is no greater thyroidal proteotoxicity caused by the mutant Tg-G2298R than by Tg-L2263P.
It is the failure to sustain thyroid cell proliferation in adult WIC-TGrdw/rdw rats (Fig. 7) that leads to the loss of thyroid tissue mass in these animals (21). What could be the cause of this failure to maintain cell proliferation in WIC-TGrdw/rdw rats? We considered the possibility that either the extent of rat TSH elevation (44)—or the bioactivity of the rat TSH protein itself (45)—might be insufficient in adult rats of the WIC strain. However, when we challenged older WIC rats with chemically induced hypothyroidism, we observed exuberant thyroid cell proliferation (Fig. 8), which seems to exclude that adults of the WIC strain background are deficient in adequate TSH production or action. Second is the possibility that under conditions of chronic continuous ER stress in adult WIC-TGrdw/rdw rats, those thyrocytes that do not die may adapt by developing a state of quiescence. Precedence for such a behavior has been noted in secretory cell tumor metastases that exist in a state of “antiproliferative dormancy” (46) with a highly activated ER stress response including markedly upregulated BiP levels (47) similar to what we have observed (this report, Fig. 4A). Indeed, dormant/latent metastatic pancreatic ductal adenocarcinoma cells exhibit a transcriptomic signature in which the single most upregulated pathway is “ER stress response” (including CHOP, linked to stress-mediated cell death; similar to what we have seen herein [Fig. 4B]), and the single most downregulated pathway is “cell division” (48). Indeed, deficiency of the MYRF transcription factor has been linked precisely to the same phenotype (49). Even in nontumorous pancreatic beta cells, sustained ER stress (from genetically encoded misfolded proinsulin—a secretory protein expressed approximately as abundantly as Tg protein is in thyrocytes) or impaired ER stress response (from defective PERK; a kinase that phosphorylates eIF2α) has been linked to impaired cell proliferation (50, 51, 52). Thus, loss of thyrocyte proliferation in adult WIC-TGrdw/rdw rats may in many ways be an expected result. What is remarkable is that neither hypothyroid TGrdw/rdw mice (this report) nor TGcog/cog mice (22)—nor many human patients with biallelic TG mutations (14, 53)—share this growth-arrest phenotype.
Why then, would the proliferative thyroid cell response to TSH be self-limited during extended concurrent ER stress in WIC-TGrdw/rdw rats? While more work is still needed to determine the difference(s) in thyroid cell proliferation in adult WIC-TGrdw/rdw rats versus TGrdw/rdw mice, we wonder about strain-specific differences in the abundance and response of stem/progenitor cells that may influence the long-term replicative/regenerative capacity of the thyroid gland in different vertebrates (54, 55, 56, 57, 58, 59) as well as the activation GLIS3 and mechanistic target of rapamycin complex 1 activity to promote the expression of various cell cycle genes in thyrocytes (60). In addition to environmental causes (such as the level of daily dietary iodide intake in patients bearing biallelic TG mutations), we speculate that differences in genetic background within the human population are likely to contribute importantly to the ultimate extent of tissue regeneration and replicative growth within tissues experiencing the chronic ER stress that underlies a variety of degenerative diseases. In the meantime, what emerges from our current studies is that in congenital hypothyroidism with biallelic TG mutation, the ultimate development and maintenance of a large goiter requires that thyroid cell proliferation must continue unabated in adulthood.
Experimental procedures
Primary antibodies
Anti-Ki67 (SP6) (catalog no.: ab16667; Abcam); monoclonal antibody anti-Tg (catalog no.: 365997, Santa Cruz; catalog no.: ab156008, Abcam); rabbit anti-Tg and rabbit anti-BiP were previously described (13, 27); rabbit anti-Ezrin (catalog no.: PA5-17518; Invitrogen); rabbit anti-p58ipk (catalog no.: 2940; Cell Signaling Technology); rabbit anti-phospho-eIF2α (Ser51) (catalog nos.: 3597 and 9721; Cell Signaling) and total eIF2α (catalog no.: 9722; Cell Signaling); mouse antitubulin (catalog no.: T5168; Sigma); and rabbit anti-PARP (catalog no.: 9542; Cell Signaling).
Animals
Six founder TG+/rdw mice were generated using CRISPR/Cas9: C57BL6J zygotes received injection of guide RNA (GGTGGTCAGCTGACCATTGA) and donor vector with Tg-G2298R mutation (ACCATGGAGATGGAAGGCAGTGGTGGTCAGCTGACCATTGATCGCAGCATCCTGGCTGCAGTTGGCAACTTCATCGTAGTC) targeting TG exon 40 (GemPharmatech). A 534-base PCR amplicon from genomic DNA (forward primer: 5′-GTATCAAGGCAGAGCCAGCAAGA-3’; reverse primer: 5′-CAGGACTCAAAGGAGATCCTTGG-3′) was sequenced to confirm a male F0 generation mouse that harbored the heterozygous TGrdw mutation. This male was bred with female C57BL6J mice to yield six F1 generation mice similarly confirmed to be TG+/rdw heterozygotes. These were further bred with WT C57BL6J obtained from JAX. Heterozygous crosses yielded TGrdw/rdw homozygotes. TGcog/cog mice (C57BL6J) were obtained from JAX. Mice were studied at 3 months of age unless otherwise indicated. WIC-rat TGrdw/+ heterozygotes were obtained from the National BioResource Project in Japan (rat no: 0104) and bred to homozygosity; WT animals were littermates in the same strain background. For PTU treatment, 14-week-old WT rats were fed with low-iodide chow containing 0.15% PTU (Envigo; catalog no.: TD.95125) for 21 days. All animal experiments performed were approved by the University of Michigan Institutional Animal Care and Use Committee. Both male and female animals were used (in figures, males are represented with squares and females with circles).
Western blotting
Mouse thyroid glands were mechanically homogenized either in nondenaturing lysis buffer (20 mM Tris–HCl, pH 8, 137 mM NaCl, 1% NP-40, and 2 mM EDTA) or in radioimmunoprecipitation assay buffer (25 mM Tris–HCl, pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS; Thermo Fisher Scientific). Protease and phosphatase inhibitor cocktails (Thermo Fisher Scientific) were added to cell lysis buffer. Protein concentration was measured by bicinchoninic acid yassay (Thermo Fisher Scientific). Gel sample buffer either omitted (Fig. 1B) or included SDS and boiling (Fig. 1C) and then were resolved by 3 to 8% PAGE in the absence (Fig. 1B) or the presence of SDS (Fig. 1C). For Figures 1D and 4A, lysates were boiled in SDS-gel sample buffer plus 50 mM DTT and were then resolved by SDS—straight 4.5% or gradient 4 to 12% PAGE. Proteins were electrotransferred to nitrocellulose membranes, blocked with 5% milk, immunoblotted with the indicated antibodies and appropriate horseradish peroxidase–conjugated secondary antibody, and visualized by enhanced chemiluminescence. Endoglycosidase H digestion was performed as previously described (21).
Histology and immunostaining of thyroid sections
Thyroids from mice and rats were dissected, immersion fixed with 10% formalin, paraffin embedded, sectioned, and stained with hematoxylin–eosin (Vector). For immunofluorescence, thyroid sections (6 μm) were deparaffinized in CitriSolv, followed by antigen retrieval in citrate buffer (12.3 mM, pH 6), and blocked in 1.5% normal goat serum for 30 min at room temperature before incubation with primary antibodies overnight at 4 °C. After washing, the sections were then incubated with Alexa Fluor–conjugated secondary antibodies (Thermo Fisher Scientific) for 1 h at room temperature. Sections were counterstained with Prolong-Gold and DAPI (Invitrogen) and imaged with a Nikon A1 confocal microscope. For immunohistochemistry, anti-Ki67 staining was performed as previously described (21). Images were obtained in a Leica DMI-3000B microscope (40× objective). Analysis and quantification (Ki67-positive nuclei as a proportion of total nuclei) were performed by observers blinded to the genotypes and groups.
Serum hormone measurement
Serum total T4, total T3, and TSH concentrations were measured using radioimmunoassays as previously described (61, 62).
PCR
Total RNA isolation from the mouse thyroid glands was performed using RNeasy Plus kit (Qiagen), followed by reverse transcription using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). For real-time PCR, PowerUp SYBR Green PCR Master Mix (Applied Biosystems) was used on a StepOnePlus PCR system (Thermo Fisher Scientific). CHOP and Tg mRNA levels were normalized to 18S RNA; primers are as follows: CHOP (forward: 5′-CCTGAGGAGAGAGTGTTCCAG-3’, reverse: 5′-GACACCGTCTCCAAGGTGAA-3′); Tg (forward: 5′-TGATCTGATCCACAACTACAACAG-3’, reverse: 5′-ATTCCAGTCCTGTCTCAGCC-3′); and 18S (forward: 5′-GGCGTCCCCCAACTTCTTA-3’, reverse: 5′-GGGCATCACAGACCTGTTATTC-3′).
TUNEL labeling
The In Situ Cell Death Detection Kit, Fluorescein (Roche) was used for TUNEL staining. Sections were counterstained and mounted with Prolong-Gold and DAPI. Fluorescence images were captured in a Nikon A1 confocal microscope. Quantification of TUNEL-positive nuclei in the proportion of total nuclei was performed using Imaris software (version 7.7.2; Imaris).
Thyroid gland size measurement
The areas of the thyroid glands were measured from photographic imaging at the time of dissection with an embedded millimeter rule included in each image, as previously described (21). Thyroid images for 11-month-old mice and older are shown in Fig. S4. Thyroid sizes were quantified as a fraction of body weight of each animal.
Statistics
Two-way ANOVA with Tukey's comparison sample test was used for comparison of two factors (e.g., genotype plus age) and applied for statistical analysis of serum hormones, body weight, and rat thyroid Ki67 immunostaining. Unpaired two-tailed Student’s t test was used for direct comparison of two groups (e.g., CHOP or Tg mRNA level as well as TUNEL staining). One-way ANOVA followed by Tukey's multiple comparison test was used for multigroup comparison of a single factor (e.g., effect of mouse genotype on thyroid Ki67 immunostaining). All statistical analyses were calculated with GraphPad Prism (GraphPad Software, Inc). All data were expressed as mean ± SD. Thyroid size measurements as a function of age were plotted by simple linear regression. Differences of p < 0.05 were considered significant.
Data availability
All data are contained within the article, with primary data available upon request (Dr Peter Arvan, University of Michigan, email: parvan@umich.edu).
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We acknowledge the technical support of Ms Inis Isak and the Undergraduate Research Opportunity Program.
Author contributions
P. A. conceptualization; X. Z., B. M., C. Y., and X.-H. L. methodology; X. Z., B. M., C. Y., and X.-H. L. validation; X. Z., B. M., H. Z., D. L., S. R., and P. A. formal analysis; X. Z. and P. A. writing–original draft; X. Z., B. M., C. Y., H. Z., D. L., X.-H. L., S. R., M. L., and P. A. writing–review & editing; X. Z., B. M., H. Z., D. L., S. R., and P. A. visualization; P. A., S. R., and M. L. supervision; P. A., S. R., and M. L. funding acquisition.
Funding and additional information
This work was supported by the National Institutes of Health (grant no.: R01 DK132017) and from the University of Michigan Protein Folding Diseases Initiative (to P. A.) and R01 DK15070 (to S. R.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Edited by Ronald Wek
Supporting information
References
- 1.Brix K., Qatato M., Szumska J., Venugopalan V., Rehders M. In: The Thyroid and its Diseases: A Comprehensive Guide for the Clinician. Luster M., Duntas L.H., Wartofsky L., editors. Springer International Publishing; Cham: 2019. “Thyroglobulin storage, processing and degradation for thyroid hormone liberation”; pp. 25–48. [Google Scholar]
- 2.Holzer G., Morishita Y., Fini J.B., Lorin T., Gillet B., Hughes S., et al. Thyroglobulin represents a novel molecular architecture of vertebrates. J. Biol. Chem. 2016;291:16553–16566. doi: 10.1074/jbc.M116.719047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Jeso B., Arvan P. Thyroglobulin from molecular and cellular biology to clinical endocrinology. Endocr. Rev. 2016;37:2–36. doi: 10.1210/er.2015-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvalho D.P., Dupuy C. Thyroid hormone biosynthesis and release. Mol. Cell Endocrinol. 2017;458:6–15. doi: 10.1016/j.mce.2017.01.038. [DOI] [PubMed] [Google Scholar]
- 5.Dunn J.T., Dunn A.D. The importance of thyroglobulin structure for thyroid hormone biosynthesis. Biochimie. 1999;81:505–509. doi: 10.1016/s0300-9084(99)80102-3. [DOI] [PubMed] [Google Scholar]
- 6.Lamas L., Dorris M.L., Taurog A. Evidence for a catalytic role for thyroid peroxidase in the conversion of diiodotyrosine to thyroxine. Endocrinology. 1972;90:1417–1426. doi: 10.1210/endo-90-6-1417. [DOI] [PubMed] [Google Scholar]
- 7.Lamas L., Taurog A. The importance of thyroglobulin structure in thyroid peroxidase-catalyzed conversion of diiodotyrosine to thyroxine. Endocrinology. 1977;100:1129–1136. doi: 10.1210/endo-100-4-1129. [DOI] [PubMed] [Google Scholar]
- 8.Coscia F., Taler-Vercic A., Chang V.T., Sinn L., O'Reilly F.J., Izore T., et al. The structure of human thyroglobulin. Nature. 2020;578:627–630. doi: 10.1038/s41586-020-1995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pio M.G., Siffo S., Scheps K.G., Molina M.F., Adrover E., Abelleyro M.M., et al. Curating the gnomAD database: report of novel variants in the thyrogobulin gene using in silico bioinformatics algorithms. Mol. Cell Endocrinol. 2021;534:111359. doi: 10.1016/j.mce.2021.111359. [DOI] [PubMed] [Google Scholar]
- 10.Citterio C.E., Targovnik H.M., Arvan P. The role of thyroglobulin in thyroid hormonogenesis. Nat. Rev. Endocrinol. 2019;15:323–338. doi: 10.1038/s41574-019-0184-8. [DOI] [PubMed] [Google Scholar]
- 11.Morishita Y., Kabil O., Young K.Z., Kellogg A.P., Chang A., Arvan P. Thyrocyte cell survival and adaptation to chronic endoplasmic reticulum stress due to misfolded thyroglobulin. J. Biol. Chem. 2020;295:6876–6887. doi: 10.1074/jbc.RA120.012656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim P.S., Hossain S.A., Park Y.N., Lee I., Yoo S.E., Arvan P. A single amino acid change in the acetylcholinesterase-like domain of thyroglobulin causes congenital goiter with hypothyroidism in the cog/cog mouse: a model of human endoplasmic reticulum storage diseases. Proc. Natl. Acad. Sci. U. S. A. 1998;95:9909–9913. doi: 10.1073/pnas.95.17.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim P.S., Kwon O.-Y., Arvan P. An endoplasmic reticulum storage disease causing congenital goiter with hypothyroidism. J. Cell Biol. 1996;133:517–527. doi: 10.1083/jcb.133.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medeiros-Neto G., Kim P.S., Yoo S.E., Vono J., Targovnik H., Camargo R., et al. Congenital hypothyroid goiter with deficient thyroglobulin. Identification of an endoplasmic reticulum storage disease (ERSD) with induction of molecular chaperones. J. Clin. Invest. 1996;98:2838–2844. doi: 10.1172/JCI119112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baryshev M., Sargsyan E., Wallin G., Lejnieks A., Furudate S., Hishinuma A., et al. Unfolded protein response is involved in the pathology of human congenital hypothyroid goiter and rat non-goitrous congenital hypothyroidism. J. Mol. Endocrinol. 2004;32:903–920. doi: 10.1677/jme.0.0320903. [DOI] [PubMed] [Google Scholar]
- 16.Marine D. Etiology Prev. Simple Goiter Med. 1924;3:453–479. [Google Scholar]
- 17.Bychkov A. Epithelial hyperplasia is responsible for the compensatory enlargement of remaining thyroid lobe after thyroidectomy. Eur. Arch. Otorhinolaryngol. 2018;275:2417–2419. doi: 10.1007/s00405-018-4915-6. [DOI] [PubMed] [Google Scholar]
- 18.Maenhaut C., Christophe D., Vassart G., Dumont J., Roger P.P., Opitz R. In: Endotext. Feingold K.R., Anawalt B., Boyce A., Chrousos G., Dungan K., Grossman A., et al., editors. MDText.com, Inc; South Dartmouth (MA): 2000–2015. Ontogeny, anatomy, metabolism and physiology of the thyroid. [Google Scholar]
- 19.Oberlin O., Plantin-Carrenard E., Rigal O., Wilkinson C. Goitre and iodine deficiency in Afghanistan: a case-control study. Br. J. Nutr. 2006;95:196–203. doi: 10.1079/bjn20051581. [DOI] [PubMed] [Google Scholar]
- 20.Pisarev M.A., DeGroot L.J., Wilber J.F. Cyclic-AMP production of goiter. Endocrinology. 1970;87:339–342. doi: 10.1210/endo-87-2-339. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X., Kellogg A.P., Citterio C.E., Zhang H., Larkin D., Morishita Y., et al. Thyroid hormone synthesis continues despite biallelic thyroglobulin mutation with cell death. JCI insight. 2021;6 doi: 10.1172/jci.insight.148496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adkison L.R., Taylor S., Beamer W.G. Mutant gene-induced disorders of structure, function and thyroglobulin synthesis in congenital goitre (cog/cog) in mice. J. Endocrinol. 1990;126:51–58. doi: 10.1677/joe.0.1260051. [DOI] [PubMed] [Google Scholar]
- 23.Umezu M., Kagabu S., Jiang J., Sato E. Evaluation and characterization of congenital hypothyroidism in rdw dwarf rats. Lab. Anim. Sci. 1998;48:496–501. [PubMed] [Google Scholar]
- 24.Menon S., Lee J., Abplanalp W.A., Yoo S.E., Agui T., Furudate S., et al. Oxidoreductase interactions include a role for ERp72 engagement with mutant thyroglobulin from the rdw/rdw rat dwarf. J. Biol. Chem. 2007;282:6183–6191. doi: 10.1074/jbc.M608863200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vono-Toniolo J., Rivolta C.M., Targovnik H.M., Medeiros-Neto G., Kopp P. Naturally occurring mutations in the thyroglobulin gene. Thyroid. 2005;15:1021–1033. doi: 10.1089/thy.2005.15.1021. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher S.R. One-dimensional electrophoresis using nondenaturing conditions. Curr. Protoc. Protein Sci. 2018;94:e73. doi: 10.1002/cpps.73. [DOI] [PubMed] [Google Scholar]
- 27.Kim P.S., Arvan P. Folding and assembly of newly synthesized thyroglobulin occurs in a pre-Golgi compartment. J. Biol. Chem. 1991;266:12412–12418. [PubMed] [Google Scholar]
- 28.Kim P., Bole D., Arvan P. Transient aggregation of nascent thyroglobulin in the endoplasmic reticulum: relationship to the molecular chaperone, BiP. J. Cell Biol. 1992;118:541–549. doi: 10.1083/jcb.118.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim K., Kopylov M., Bobe D., Kelley K., Eng E.T., Arvan P., et al. The structure of natively iodinated bovine thyroglobulin. Acta Crystallogr. D Struct. Biol. 2021;77:1451–1459. doi: 10.1107/S2059798321010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J., Di Jeso B., Arvan P. Maturation of thyroglobulin protein region I. J. Biol. Chem. 2011;286:33045–33052. doi: 10.1074/jbc.M111.281337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fogelfeld L., Harel G., Beamer W.G., Schneider A.B. Low-molecular-weight iodoproteins in the congenital goiters of cog/cog mice. Thyroid. 1992;2:329–335. doi: 10.1089/thy.1992.2.329. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y., Shimizu H., Xiang Y.-Y., Sugihara J., Lu W.-Y., Liao X.-H., et al. XB130 deficiency causes congenital hypothyroidism in mice due to disorganized apical membrane structure and function of thyrocytes. Thyroid. 2021;31:1650–1661. doi: 10.1089/thy.2021.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulianich L., Mirra P., Garbi C., Cali G., Conza D., Treglia A.S., et al. The pervasive effects of ER stress on a typical endocrine cell: dedifferentiation, mesenchymal shift and antioxidant response in the thyrocyte. Front. Endocrinol. 2020;11:588685. doi: 10.3389/fendo.2020.588685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hollien J., Weissman J.S. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein responseJulie Hollien 1, Jonathan S Weissman. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 35.Lee A.H., Heidtman K., Hotamisligil G.S., Glimcher L.H. Dual and opposing roles of the unfolded protein response regulated by IRE1alpha and XBP1 in proinsulin processing and insulin secretion. Proc. Natl. Acad. Sci. U. S. A. 2011;108:8885–8890. doi: 10.1073/pnas.1105564108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coelho D.S., Domingos P.M. Physiological roles of regulated Ire1 dependent decay. Front. Genet. 2014;5:76. doi: 10.3389/fgene.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furudate S., Ono M., Shibayama K., Ohyama Y., Kuwada M., Kimura T., et al. Rescue from dwarfism by thyroid function compensation in rdw rats. Exp. Anim. 2005;54:455–460. doi: 10.1538/expanim.54.455. [DOI] [PubMed] [Google Scholar]
- 38.Sakai Y., Yamashina S., Furudate S.I. Missing secretory granules, dilated endoplasmic reticulum, and nuclear dislocation in the thyroid gland of rdw rats with hereditary dwarfism. Anat. Rec. 2000;259:60–66. doi: 10.1002/(SICI)1097-0185(20000501)259:1<60::AID-AR7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 39.Shimokawa N., Yousefi B., Morioka S., Yamaguchi S., Ohsawa A., Hayashi H., et al. Altered cerebellum development and dopamine distribution in a rat genetic model with congenital hypothyroidism. J. Neuroendocrinol. 2014;26:164–175. doi: 10.1111/jne.12135. [DOI] [PubMed] [Google Scholar]
- 40.Sano R., Reed J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Almanza A., Carlesso A., Chintha C., Creedican S., Doultsinos D., Leuzzi B., et al. Endoplasmic reticulum stress signalling - from basic mechanisms to clinical applications. FEBS J. 2019;286:241–278. doi: 10.1111/febs.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lam M., Marsters S.A., Ashkenazi A., Walter P. Misfolded proteins bind and activate death receptor 5 to trigger apoptosis during unresolved endoplasmic reticulum stress. Elife. 2020;9 doi: 10.7554/eLife.52291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morishita Y., Kellogg A.P., Larkin D., Chen W., Vadrevu S., Satin L., et al. Cell death-associated lipid droplet protein CIDE-A is a noncanonical marker of endoplasmic reticulum stress. JCI insight. 2021;6 doi: 10.1172/jci.insight.143980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li A.A., Makris S.L., Marty M.S., Strauss V., Gilbert M.E., Blacker A., et al. Practical considerations for developmental thyroid toxicity assessments: what's working, what's not, and how can we do better? Regul. Toxicol. Pharmacol. 2019;106:111–136. doi: 10.1016/j.yrtph.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 45.Menezes-Ferreira M.M., Petrick P.A., Weintraub B.D. Regulation of thyrotropin (TSH) bioactivity by TSH-releasing hormone and thyroid hormone. Endocrinology. 1986;118:2125–2130. doi: 10.1210/endo-118-5-2125. [DOI] [PubMed] [Google Scholar]
- 46.Cubillos-Ruiz J.R., Bettigole S.E., Glimcher L.H. Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell. 2017;168:692–706. doi: 10.1016/j.cell.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartkowiak K., Kwiatkowski M., Buck F., Gorges T.M., Nilse L., Assmann V., et al. Disseminated tumor cells persist in the bone marrow of breast cancer patients through sustained activation of the unfolded protein response. Cancer Res. 2015;75:5367–5377. doi: 10.1158/0008-5472.CAN-14-3728. [DOI] [PubMed] [Google Scholar]
- 48.Pommier A., Anaparthy N., Memos N., Kelley Z.L., Gouronnec A., Yan R., et al. Unresolved endoplasmic reticulum stress engenders immune-resistant, latent pancreatic cancer metastases. Science. 2018;360 doi: 10.1126/science.aao4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milan M., Balestrieri C., Alfarano G., Polletti S., Prosperini E., Nicoli P., et al. Pancreatic cancer cells require the transcription factor MYRF to maintain ER homeostasis. Dev. Cell. 2020;55:398–412.e397. doi: 10.1016/j.devcel.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 50.Riahi Y., Israeli T., Yeroslaviz R., Chimenez S., Avrahami D., Stolovich-Rain M., et al. Inhibition of mTORC1 by ER stress impairs neonatal beta-cell expansion and predisposes to diabetes in the Akita mouse. Elife. 2018;7:1–25. doi: 10.7554/eLife.38472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang W., Feng D., Li Y., Iida K., McGrath B., Cavener D.R. PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 2006;4:491–497. doi: 10.1016/j.cmet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Feng D., Wei J., Gupta S., McGrath B.C., Cavener D.R. Acute ablation of PERK results in ER dysfunctions followed by reduced insulin secretion and cell proliferation. BMC Cell Biol. 2009;10:61. doi: 10.1186/1471-2121-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siffo S., Adrover E., Citterio C.E., Miras M.B., Balbi V.A., Chiesa A., et al. Molecular analysis of thyroglobulin mutations found in patients with goiter and hypothyroidism. Mol. Cell Endocrinol. 2018;473:1–16. doi: 10.1016/j.mce.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 54.Hoshi N., Kusakabe T., Taylor B.J., Kimura S. Side population cells in the mouse thyroid exhibit stem/progenitor cell-like characteristics. Endocrinology. 2007;148:4251–4258. doi: 10.1210/en.2006-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lan L., Cui D., Nowka K., Derwahl M. Stem cells derived from goiters in adults form spheres in response to intense growth stimulation and require thyrotropin for differentiation into thyrocytes. J. Clin. Endocrinol. Metab. 2007;92:3681–3688. doi: 10.1210/jc.2007-0281. [DOI] [PubMed] [Google Scholar]
- 56.Dumont J.E., Lamy F., Roger P., Maenhaut C. Physiological and pathological regulation of thyroid cell proliferation and differentiation by thyrotropin and other factors. Physiol. Rev. 1992;72:667–697. doi: 10.1152/physrev.1992.72.3.667. [DOI] [PubMed] [Google Scholar]
- 57.Ma R., Morshed S.A., Latif R., Davies T.F. A stem cell surge during thyroid regeneration. Front. Endocrinol. (Lausanne) 2021;11:606269. doi: 10.3389/fendo.2020.606269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kimura S. Thyroid regeneration: how stem cells play a role? Front. Endocrinol. (Lausanne) 2014;5:55. doi: 10.3389/fendo.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Finkielstain G.P., Forcinito P., Lui J.C., Barnes K.M., Marino R., Makaroun S., et al. An extensive genetic program occurring during postnatal growth in multiple tissues. Endocrinology. 2009;150:1791–1800. doi: 10.1210/en.2008-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang H.S., Kumar D., Liao G., Lichti-Kaiser K., Gerrish K., Liao X.-H., et al. GLIS3 is indispensable for TSH/TSHR-dependent thyroid hormone biosynthesis and follicular cell proliferation. J. Clin. Invest. 2017;127:4326–4337. doi: 10.1172/JCI94417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dumitrescu A.M., Liao X.H., Weiss R.E., Millen K., Refetoff S. Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (mct) 8-deficient mice. Endocrinology. 2006;147:4036–4043. doi: 10.1210/en.2006-0390. [DOI] [PubMed] [Google Scholar]
- 62.Pohlenz J., Maqueem A., Cua K., Weiss R.E., Van Sande J., Refetoff S. Improved radioimmunoassay for measurement of mouse thyrotropin in serum: strain differences in thyrotropin concentration and thyrotroph sensitivity to thyroid hormone. Thyroid. 1999;9:1265–1271. doi: 10.1089/thy.1999.9.1265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the article, with primary data available upon request (Dr Peter Arvan, University of Michigan, email: parvan@umich.edu).