Abstract
In this study, microwave pretreatment and grinding treatment were used to enhance sulforaphane formation, then ultrasonic-assisted extraction (UAE) was applied to extract sulforaphane using simultaneous hydrolysis and extraction method. The effects of various parameters, which were ultrasonic time, ultrasonic power, solid-water ratio and solid-ethyl acetate ratio on the extraction rate of sulforaphane were investigated. The results showed that microwave pretreatment enhanced sulforaphane formation. Excessive size reduction did not increase or even reduced extraction rate of sulforaphane. Simultaneous hydrolysis and extraction significantly increased extraction rate of sulforaphane compared to hydrolysis followed by extraction. UAE accelerated mass transfer and the solubilization of the targeted compounds due to the acoustic cavitation effect, thus enhanced enzymatic hydrolysis of glucoraphanin and the extraction rate of sulforaphane. The extraction rate of sulforaphane using UAE with simultaneous hydrolysis and extraction was 4.07-fold of the conventional extraction method. UAE was an effective method to extract sulforaphane from broccoli seeds since it led to higher yield of sulforaphane in a much shorter extraction time.
Keywords: Sulforaphane, Broccoli seeds, Microwave pretreatment, Simultaneous hydrolysis and extraction, Ultrasonic-assisted extraction
1. Introduction
Sulforaphane (4-methylsulfinybutyl isothiocyanate) is known to be the most effective natural anticancer ingredient discovered so far, with broad spectrum activity against many types of cancer cells, including breast cancer, prostate cancer, colon cancer, skin cancer, gastric carcinoma, bladder cancer, chronic leukemia, etc. [1], [2], [3]. In addition, sulforaphane also exhibits prominent anti-inflammatory, anti-hypertension, cardiac protection, alleviating diabetes, fighting obesity [4], and improves schizophreni [5], autism [6], Parkinson's disease, Alzheimer's disease, etc. [7]. Importantly, recent studies have found that sulforaphane also inhibits novel SARS-CoV-2, including Delta and Omicron [8], [9]. Therefore, sulforaphane has received a great deal of attention from medical scientists, food scientists and nutritionists.
Sulforaphane, a kind of isothiocyanate, is the hydrolysis product from glucoraphanin (4-methylsulfinybutyl glucosinolate) by the action of myrosinase. The content of glucoraphanin is high in cruciferous plants and seeds, such as broccoli, kohlrabi, cauliflower, brussels sprouts and so on [10]. Especially, broccoli seeds contain the highest glucoraphanin; hence, they are a good source of preparation of sulforaphane. However, the conversion rate of glucoraphanin to sulforaphane is very low due to the presence of epithiospecifier protein (ESP) [11]. It has been reported that the heat sensitivity of ESP protein is higher than that of myrosinase. Therefore, appropriate heat treatment can reduce ESP activity and reserve myrosinase activity, so as to increase the production of sulforaphane.
Generally, the common method for preparing sulforaphane include an enzymatic conversion process, followed by an extraction process. However, suffers from the fact that it requires long time. The hydrolysis time of glucoraphanin to sulforaphane in broccoli florets, stems and leaves was 2–4 h [12]. The results of Shen et al. [13] showed that the optimum degradation time of glucoraphanin to sulforaphane was 8 h. In contrast, simultaneous hydrolysis and extraction showed a relatively greater isothiocyanates and erucin production from broccoli seeds than hydrolysis followed by extraction, and shortened extraction time [14]. On the other hand, many studies have aimed at improving the extraction efficiency of sulforaphane from different plant resources using assisted extraction methods. Tanongkankit et al. [15] suggested that microwave-assisted extraction of sulforaphane white cabbages was more effective than the conventional extraction as the former led to higher yield of sulforaphane in a much shorter extraction time. The highest extraction rate of sulforaphane from papaya seeds was obtained by high hydrostatic pressure extraction (no enzymatic conversion process), followed by UAE; but the focus was not just on sulforaphane, phenolic compounds and fatty acids were also extracted, and high hydrostatic pressure extraction required greater investment costs [16].
The UAE has been widely applied to extract bioactive compounds and shows low energy consumption. The cavitation forces resulted from ultrasound can generate localized pressure causing plant tissue rupture and enhances intracellular substances transfer into the solvent [17]. Hence, UAE can increase the yield of extracted components, decrease the extraction time. However, to the most our knowledge, little is as yet known about UAE of sulforaphane from broccoli seeds using the simultaneous hydrolysis and extraction method.
Therefore, in this study, we firstly investigated the effect of microwave pretreatment, grinding degree and hydrolysis time on extraction rate of sulforaphane from broccoli seeds. Subsequently, ultrasonic time, ultrasonic power, solid-water ratio, solid-ethyl acetate on the extraction rate of sulforaphane were investigated using the simultaneous hydrolysis and extraction method.
2. Materials and methods
2.1. Material and chemicals
The broccoli seeds (Brassica oleracea var.italica) were purchased from Weifang Shouhe Seed Co. (Shandong, China). Sulforaphane standard was purchased from Hebei Bailingwei Hyperfine Material Co. Chromatographic pure acetonitrile was purchased from Tianjin Komio Chemical Reagent Co. Both ethyl acetate and petroleum ether were produced by Tianjin Fuyu Fine Chemical Co.
2.2. Sulforaphane analysis
Sulforaphane was analyzed with a Thermo Ultimate 3000 HPLC (Thermo Fly, USA), equipped with a ZORBAX Eclipse XDB-C18 column (Agilent Technology, USA; 150 mm × 4.6 mm, 3 μm). The solvent system consisted of 10% acetonitrile in ultrapure water, and then changed linearly over 10 min to 60% acetonitrile from 0 to 20 min, and maintained 100% acetonitrile for 2 min to purge the column. The temperature of the column box was set at 30 °C. The flow rate was 0.6 mL/min, and 20 μL portions were injected into the column. Sulforaphane was detected by UV 254 nm. A series of standard sample solutions of sulforaphane concentration were prepared for HPLC analysis.
2.3. Effect of microwave pretreatment on sulforaphane formation in broccoli seeds
Broccoli seeds were treated in a microwave oven (P70D20N1P-G5, Guangdong Galanz Microwave Household Appliances Manufacturing Co., LTD, China) for 1, 2, 3 and 4 min at low power, respectively. After grinding and degreasing, 4 mL water was added to enzymatic hydrolyze for 3 h, then added 4 mL ethyl acetate to extract sulforaphane for 40 min. After centrifugation at 10,000 g for 10 min, the ethyl acetate ethane fraction was dried and dissolved with 10% acetonitrile, then analyzed by HPLC. The result was expressed as mg/g broccoli seeds.
2.4. Effect of pre-hydrolysis time on the extraction rate of sulforaphane
The microwave-treated broccoli seeds were ground and degreased, then hydrolyzed with 4 mL water for 0, 1, 2 and 3 h, respectively. Hydrolysis for 0 h was expressed as simultaneous hydrolysis and extraction. After that, ethyl acetate was added to extract sulforaphane and then analyzed.
2.5. Seed grinding treatment
2.5.1. Effects of seed grinding degree on the extraction rate of sulforaphane from broccoli seeds
The microwave-treated samples were placed in the automatic grinder (JXFSTPRP-CL, Shanghai Jingxin Industrial Development Co., LTD, China) at 30, 40, 50, 60 and 70 Hz for grinding 5, 10, 20, 30 and 40 s respectively, then were degreased. Afterwards, 4 mL distilled water and 4 mL ethyl acetate were added to extract sulforaphane, then analyzed by HPLC.
2.5.2. Particle size measurement
The particle size of broccoli seed meals was measured according to the reported of Xing et al. [18] using the laser diffraction particle size analyzer (Microtrac S3500, Microtrac Inc, USA).
2.5.3. Scanning electron microscopy (SEM)
The morphology of the ground broccoli seed meals was observed by scanning electron microscopy (JSM-7500F, JEOL, Japan). In brief, samples were mounted with double-sided carbon adhesive tabs on aluminum stubs, and sputtered with a thin coating of gold. The acceleration voltage was 2 kV, and the magnification was 500 x.
2.6. UAE experiment
Microwave-treated broccoli seeds were grinded and degreased, followed by simultaneous hydrolytic and extraction for UAE. The ultrasonic probe device is shown in Fig. 1. The ultrasonic probe was inserted into the liquid level 2 cm, and the interval of ultrasonic running and stopping was set to 5 s. The temperature range of the mixture was maintained at 25–35 ℃. Simultaneous hydrolysis and extraction for 40 min by at 25 ℃ was used as control. The UAE was carried out at the set power of 300 W, and the processing time was 5, 10, 20, 30 and 40 s, respectively.
Fig. 1.
Schematic diagram of ultrasonic device.
After that, under the optimal time, ultrasonic power was carried out at 200 W, 300 W, 400 W and 500 W, respectively. Next, at optimum ultrasonic time and power, the solid-water ratio (g:mL) was set as 1:10, 1:20, 1:30, 1:40 and 1:50. And the solid-ethyl acetate ratio (g:mL) was set as 1:10, 1:20, 1:30, 1:40 and 1:50.
2.7. Statistical analysis
All experiments were carried out in triplicate and the results were expressed as mean ± standard deviation. SPSS 18.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis of the results and significant differences were analyzed using Duncan's multiple range tests. Differences at p < 0.05 were considered as statistically significant.
3. Results and discussions
3.1. HPLC identification
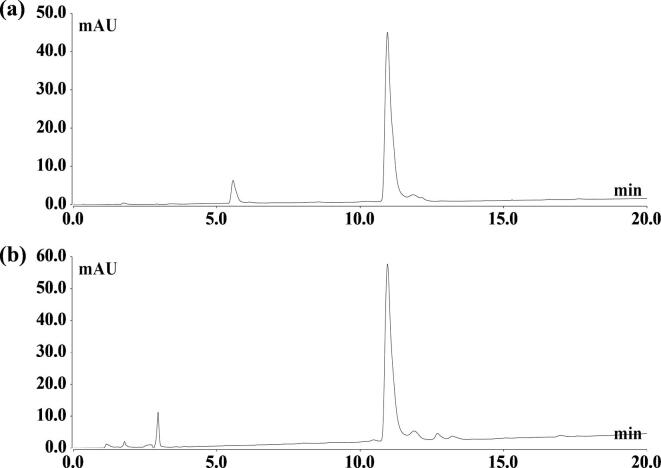
HPLC chromatograms of sulforaphane standard and sulforaphane extracted from seeds by ultrasound are shown in Fig. 2, At 11.25 min, single peaks appeared in both the standard and sample with no interference peaks on both sides of the target peak. As can be seen from Fig. 2b, there were fewer impurity compounds in broccoli seeds, the peak shape was the same as that of the standard, and there were no other peaks with similar shapes before and after time. Therefore, it could be used for quantitative analysis of sulforaphane in broccoli seeds.
Fig. 2.
The HPLC Chromatograms of the sulforaphane standard (a) and sulforaphane extracted from broccoli seeds (b).
3.2. Effect of microwave pretreatment on sulforaphane formation
Proper heating treatment can reduce ESP activity and the production of sulforaphane nitrile as well as allow for more production of sulforaphane [19]. It has been reported that ESP was inactivated after heating for 5–10 min at 60–70 ℃, while myrosinase was inactivated after heating for 5–15 min at 100℃ [20]. Steaming for 1–3 min resulted in more sulforaphane from broccoli than microwave and boiling [21]. Lu et al. [19] reported that microwave heating increased the yield of sulforaphane by about 80% compared to traditional heating at 60 ℃. In addition, microwave as a mild heating treatment might induce more cell lysis, resulting in the diffusion of greater amounts of glucosinolates and myrosinase, thus promoted the formation of sulforaphane [22]. In the present study, microwave pretreatment significantly improved sulforaphane yield in broccoli seeds compared with the control (Fig. 3). Sulforaphane yield reached the highest when microwave pretreatment for 3 min, which was 1.5 times that of control. However, sulforaphane production decreased after microwave treatment for 4 min. These results indicated that microwave treatment for 3 min could effectively inactivate ESP and retain myrosinase in broccoli seeds; but treatment for 4 min might lead to partial myrosinase inactivation, thus terminated the enzymatic process of glucoraphanin to sulforaphane. Similar to our study, Tabart et al. [23] also reported that sulforaphane formation in broccoli gradually decreased after microwave heating for 3 min.
Fig. 3.
Effect of microwave pretreatment time on sulforaphane formation in broccoli seeds. Values not sharing the same letter are significantly different at p < 0.05.
3.3. Effect of pre-hydrolysis time on the extraction rate of sulforaphane
As shown in Fig. 4, hydrolytic process before extraction significantly reduced extraction rate of sulforaphane from broccoli seeds. Whereas, simultaneous hydrolytic and extraction significantly increased the extraction rate of sulforaphane by 42.5% compared to hydrolysis for 3 h. Erucin is a precursor isothiocyanate of sulforaphane, and they can interconvert into each other both in vitro and in vivo [24], [25], [26], [27]. In addition, erucin might be convert into 1-isothiocyanato-butane after formation during hydrolysis [28]. Therefore, it is speculated that the reduction of sulforaphane during hydrolysis might be due to the interconversion of sulforaphane and other isothiocyanates; and the conversion mechanism is needed to be investigated in further research.
Fig. 4.
Effect of pre-hydrolysis time on extraction rate of sulforaphane. Values not sharing the same letter are significantly different at p < 0.05.
3.4. Effect of grinding treatment on the extraction rate of sulforaphane
Grinding frequency and time significantly affected the extraction rate of sulforaphane (Fig. 5a). Except for 70 Hz, the extraction rate of sulforaphane firstly raised and then declined with the increase of grinding time at the same grinding frequency. Grinding at 40 Hz for 20 s led to the highest extraction rate of sulforaphane, which was 2.24 times of that grinding at 40 Hz for 5 s. Our results were similar to the findings of Xing et al. [18], who found that after high-pressure homogenization, larger particles were destroyed, reduced the broccoli seed meals particle size, greatly increased the seed tissue specific surface area, then accelerated the contact of glucoraphanin and myrosinase, thus produced more sulforaphane. This observation was also confirmed by size distribution curve (Fig. 5b) in our study. A bimodal size distribution curve from 1 μm to 1000 μm was observed in broccoli seeds meals, the first peak of the sample size distribution should represent the seed core (approximately 20 μm) and the second peak referred the seed shells (approximately 500 μm) [18]. During the grinding process, the particle size distribution peak gradually moved to the left, indicating that the average particle size gradually decreased, and the seed meals with uniform particle size distribution and small particle size was obtained (Fig. 5b). Meanwhile, the images of samples with different grinding degrees (Supplementary Fig. S1) showed that with the increase of grinding pressure, the particle size of samples was observed to be more uniform and the seed meals gradually changed from bicolor to monochrome.
Fig. 5.
Effect of grinding frequency and time on extraction rate of sulforaphane (a) and particle size distribution of broccoli seeds ground for 20 s at different frequencies (b). Values not sharing the same letter are significantly different at p < 0.05.
However, higher grinding frequency and time did not increase even decreased the extraction rate of sulforaphane (Fig. 5a), which might be due to the irreversible destruction and aggregation of the polymer, thus reduced the extraction rate sulforaphane [18], [29]. Another possible reason might be that partial myrosinase of broccoli seed meals inactivated under higher frequency and longer time grinding. The relevant mechanism needs to be further studied.
In order to understand the relationship between grinding degree and seed cells, broccoli seeds after grinding for 20 s under different frequencies were analyzed by SEM (Fig. 6). It was observed that when the grinding frequency was low, broccoli seeds would be broken into irregular particles. The fragments were large, evenly distributed, with a thin lamellar structure, rough surface, no adhesion between particles, clear spacing (Fig. 6a). With the increase of grinding frequency, broccoli seeds were broken down into smaller spherical fragments and randomly recombined to form new aggregates. Furthermore, the cells were observed to be highly twisted and densely packed (Fig. 6d and e). The results of Hua et al. [30] showed that tomato residue fibers could be broken into micro-fragments by pressure and then aggregated into large aggregates. This was consistent with our observation that high pressure treatment led to polymer aggregation, resulting in the lower extraction rate of sulforaphane.
Fig. 6.
Scanning electron microscope image of broccoli seed meals after grinding for 20 s at 30 Hz (a), 40 Hz (b), 50 Hz (c), 60 Hz (d) and 70 Hz (e).
3.5. Effect of UAE on the extraction rate of sulforaphane
With the increase of ultrasonic time, the extraction rate of sulforaphane from broccoli seeds firstly increased and then tended to remain constant (Fig. 7a). This phenomenon could be explained by the fact that all plant cells would gradually burst due to the acoustic cavitation effect generated by ultrasound in the early stage of extraction [31]; meanwhile, the number of cavitation microbubbles produced by ultrasound increased with the extension of extraction time. The bursting of bubbles facilitated the penetration of water into seed cells, resulting in a larger contact area between water and seed cells, and more sulforaphane was produced by hydrolysis. At the same time, ethyl acetate rapidly extracted the formed sulforaphane from the water phase, thus improving the extraction rate. The extraction rate reached the maximum value of 16.95 mg/g at 25 min, which was 1.37 times of the control (extraction for 40 min without ultrasound treatment), indicating that sulforaphane had been fully extracted at 25 min. Hence, with the extension of extraction time, the extraction rate of sulforaphane did not increase and tended to be constant. The optimal extraction time was 25 min.
Fig. 7.
Effects of ultrasonic time (a), ultrasonic power (b), solid-water ratio (c) and solid-ethyl acetate ratio (d) on the extraction rate of sulforaphane. Values not sharing the same letter are significantly different at p < 0.05.
With the increase of ultrasonic power, the extraction rate of sulforaphane increased slightly, and no significant difference was observed among the ultrasonic power at 300, 400 and 500 W (Fig. 7b). Strong cavitation effect and mechanical effect produced by ultrasonic waves can destroy the plant cells and make the solvent penetrate into the plant cells. When the power increased, the amplitude of ultrasonic extraction medium was large, resulting in more bubble rupture [32], which would destroy the seed cell wall [33]. At the same time, more violent shock waves and high-speed jets were generated, which accelerated mass transfer, enhanced the permeability of solvent to tissues, and improved the extraction rate of sulforaphane. The results of Dabbour et al. [34] showed that ultrasonic-assisted enzymolysis greatly improved the enzymolysis efficiency of sunflower meal protein. Therefore, in the present study, UAE method also might accelerate the process of glucoraphanin hydrolysis by myrosinase via enhancing the contact of myrosinase and the substrate glucoraphanin. UAE technology can quickly extract compounds into the solvent in a shorter time than traditional methods, thus shorten extraction time, increase extraction rate [35], [36], [37].
3.6. Effect of solid-water ratio and solid-ethyl acetate ratio on the extraction rate of sulforaphane
As shown in Fig. 7c, with the increase of water content, the extraction rate of sulforaphane firstly increased and then decreased. The solid-water ratio of 1:10 (g/mL) led to the highest extraction rate of sulforaphane, which was 1.47 times of the solid-water ratio of 1:40. On one hand, it might be that more water decreased the concentration of sulforaphane in the hydrolysate, so that ethyl acetate was not completely extract sulforaphane from all the hydrolysate, reducing the extraction rate of sulforaphane. On the other hand, as the concentration of sulforaphanin and myrosinase decreased gradually, the reaction rate decreased, and the rate of sulforaphanin decreased.
Solid-liquid ratio is a routine parameter that significantly affects the extraction efficiency. In the present study, with the increase of ethyl acetate, the extraction rate of sulforaphane increased gradually and then stable (Fig. 7d). When the solid-ethyl acetate ratio was 1:50, the extraction rate reached the highest, which was enhanced by 29.2% compared to the ratio of 1:30. No significant difference was found among the solid-ethyl acetate ratio of 1:50, 1:60 and 1:70. It has been reported that less solvent resulted in higher viscosity of the extraction, thus reduced the intensity of cavitation, since the formation of cavitation required the negative pressure in the rarefaction region of wave function overcome the natural cohesive forces [38], [39]. Therefore, cavitation is more difficult to produce in viscous liquids, where the cohesive forces are stronger [39], [40]. In the present study, with the increase of ethyl acetate content, the viscosity and concentration of the extraction system decreased, and the cavitation effect of ultrasonic on the seed meals also increased, which led to the increase of extraction rate. On the other hand, with the amount of ethyl acetate increase, the concentration of sulforaphane in solvent was low, which led to a big difference between active constituent concentration in material and solvent boundary layer and high active diffusion force [41], thus increased the extraction efficiency of sulforaphane.
4. Conclusions
This study employed ultrasonic technique to assist in extraction of sulforaphane from broccoli seeds after microwave pretreatment. Microwave pretreatment before extraction significantly increased sulforaphane formation in broccoli seeds. Interestingly, the analysis of the relationship between particle size and sulforaphane extraction yield indicated that the extraction rate of sulforaphane did not increase continuously with increasing grinding frequency and time. Simultaneous hydrolysis and extraction saved time and enhanced extraction rate. Under simultaneous hydrolysis and extraction, UAE further shortened extraction time and improved extraction rate of sulforaphane from broccoli seeds, which was attributed to the high efficiency of enzymolysis process and solubilization of the targeted compounds induced by strong cavitation effect and mechanical effect. Therefore, ultrasonic-assisted simultaneous hydrolytic extraction has wonderfully potential application in extraction of sulforaphane from broccoli seeds.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 31801457) and Postgraduate Innovation Program of Qingdao Agricultural University (No. QNYCX21080).
References
- 1.Elkashty O.A., Tran S.D. Sulforaphane as a promising natural molecule for cancer prevention and treatment. Cree. Med. Sci. 2021;41(2):250–269. doi: 10.1007/s11596-021-2341-2. [DOI] [PubMed] [Google Scholar]
- 2.Cheng A.-C., Shen C.-J., Hung C.-M., Hsu Y.-C. Sulforaphane decrease of SERTAD1 expression triggers G1/S arrest in breast cancer cells. J. Med. Food. 2019;22(5):444–450. doi: 10.1089/jmf.2018.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S., Yang Y., Sargsyan D., Wu R., Yin R., Kuo H.-C., Yang I., Wang L., Cheng D., Ramirez C.N., Hudlikar R., Lu Y., Kong A.-N. Epigenome, transcriptome, and protection by sulforaphane at different stages of UVB-Induced skin carcinogenesis. Cancer Prev. Res. 2020;13(6):551–562. doi: 10.1158/1940-6207.CAPR-19-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel B., Mann G.E., Chapple S.J. Concerted redox modulation by sulforaphane alleviates diabetes and cardiometabolic syndrome. Free Radical Bio. Med. 2018;122:150–160. doi: 10.1016/j.freeradbiomed.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Akihiro S., Nobuhisa K., Tsuyoshi S., Yasunori O., Tasuku H., Tadashi H., Taisuke Y., Masaomi I., Kenji H. An open study of sulforaphane-rich broccoli sprout extract in patients with schizophrenia. Clin. Psychopharm. Neu. 2015;13:62–67. doi: 10.9758/cpn.2015.13.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nadeem A., Ahmad S.F., Al-Harbi N.O., Attia S.M., Bakheet S.A., Ibrahim K.E., Alqahtani F., Alqinyah M. Nrf2 activator, sulforaphane ameliorates autism-like symptoms through suppression of Th17 related signaling and rectification of oxidant-antioxidant imbalance in periphery and brain of BTBR T+tf/J mice. Behav. Brain Res. 2019;364:213–224. doi: 10.1016/j.bbr.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 7.Schepici G., Bramanti P., Mazzon E. Efficacy of sulforaphane in neurodegenerative diseases. Int. J. Mol. Sci. 2020;21:8637. doi: 10.3390/ijms21228637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bousquet J., Le Moing V., Blain H., Czarlewski W., Zuberbier T., de la Torre R., Pizarro Lozano N., Reynes J., Bedbrook A., Cristol J.P., Cruz A.A., Fiocchi A., Haahtela T., Iaccarino G., Klimek L., Kuna P., Melen E., Mullol J., Samolinski B., Valiulis A., Anto J.M. Efficacy of broccoli and glucoraphanin in COVID-19: From hypothesis to proof-of-concept with three experimental clinical cases, World Allergy Organ. J. 2021;14 doi: 10.1016/j.waojou.2020.100498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ordonez A.A., Bullen C.K., Villabona-Rueda A.F., Thompson E.A., Turner M.L., Merino V.F., Yan Y., Kim J., Davis S.L., Komm O., Powell J.D., D'Alessio F.R., Yolken R.H., Jain S.K., Jones-Brando L. Sulforaphane exhibits antiviral activity against pandemic SARS-CoV-2 and seasonal HCoV-OC43 coronaviruses in vitro and in mice. Commun Biol. 2022;5:242. doi: 10.1038/s42003-022-03189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West L.G., Meyer K.A., Balch B.A., Rossi F.J., Schultz M.R., Haas G.W. Glucoraphanin and 4-hydroxyglucobrassicin contents in seeds of 59 cultivars of broccoli, raab, kohlrabi, radish, cauliflower, brussels sprouts, kale, and cabbage. J. Agr. Food Chem. 2004;52(4):916–926. doi: 10.1021/jf0307189. [DOI] [PubMed] [Google Scholar]
- 11.Matusheski N.V., Juvik J.A., Jeffery E.H. Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli. Phytochemistry. 2004;65(9):1273–1281. doi: 10.1016/j.phytochem.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Ares A.M., Bernal J., Martín M.T., Bernal J.L., Nozal M.J. Optimized formation, extraction, and determination of sulforaphane in broccoli by liquid chromatography with diode array detection. Food Anal. Method. 2014;7(3):730–740. [Google Scholar]
- 13.Shen L., Su G., Wang X., Du Q., Wang K. Endogenous and exogenous enzymolysis of vegetable-sourced glucosinolates and influencing factors. Food Chem. 2010;119(3):987–994. [Google Scholar]
- 14.Lv C., Zhang Y., Zou L., Sun J., Song X., Mao J., Wu Y. Simultaneous hydrolysis and extraction increased erucin yield from broccoli seeds. ACS Omega. 2021;6(9):6385–6392. doi: 10.1021/acsomega.0c06319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanongkankit Y., Sablani S.S., Chiewchan N., Devahastin S. Microwave-assisted extraction of sulforaphane from white cabbages: Effects of extraction condition, solvent and sample pretreatment. J. Food Eng. 2013;117:151–157. [Google Scholar]
- 16.Briones-Labarca V., Plaza-Morales M., Giovagnoli-Vicuña C., Jamett F. High hydrostatic pressure and ultrasound extractions of antioxidant compounds, sulforaphane and fatty acids from Chilean papaya (Vasconcellea pubescens) seeds: Effects of extraction conditions and methods, LWT-Food. Sci. Technol. 2015;60(1):525–534. [Google Scholar]
- 17.Tian Y., Xu Z., Zheng B., Martin Lo Y. Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) seed oil. Ultrason. Sonochem. 2013;20(1):202–208. doi: 10.1016/j.ultsonch.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Xing J.-J., Cheng Y.-L., Chen P., Shan L., Ruan R., Li D., Wang L.-J. Effect of high-pressure homogenization on the extraction of sulforaphane from broccoli (Brassica oleracea) seeds. Powder Technol. 2019;358:103–109. [Google Scholar]
- 19.Lu Y., Pang X., Yang T. Microwave cooking increases sulforaphane level in broccoli. Food Sci. Nutr. 2020;8(4):2052–2058. doi: 10.1002/fsn3.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones R.B., Faragher J.D., Winkler S. A review of the influence of postharvest treatments on quality and glucosinolate content in broccoli (Brassica oleracea var. italica) heads. Postharvest Biol. Tec. 2006;41(1):1–8. [Google Scholar]
- 21.Wang G.C., Farnham M., Jeffery E.H. Impact of thermal processing on sulforaphane yield from broccoli (Brassica oleracea L. ssp. italica) J. Agr. Food Chem. 2012;60(27):6743–6748. doi: 10.1021/jf2050284. [DOI] [PubMed] [Google Scholar]
- 22.Nugrahedi P.Y., Verkerk R., Widianarko B., Dekker M. A mechanistic perspective on process-induced changes in glucosinolate content in brassica vegetables: A review. Crit. Rev. Food Sci. 2015;55(6):823–838. doi: 10.1080/10408398.2012.688076. [DOI] [PubMed] [Google Scholar]
- 23.Tabart J., Pincemail J., Kevers C., Defraigne J.-O., Dommes J. Processing effects on antioxidant, glucosinolate, and sulforaphane contents in broccoli and red cabbage. Eur. Food Res. Technol. 2018;244(12):2085–2094. [Google Scholar]
- 24.Bricker G.V., Riedl K.M., Ralston R.A., Tober K.L., Oberyszyn T.M., Schwartz S.J. Isothiocyanate metabolism, distribution, and interconversion in mice following consumption of thermally processed broccoli sprouts or purified sulforaphane. Mol. Nutr. Food Res. 2014;58(10):1991–2000. doi: 10.1002/mnfr.201400104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bheemreddy R.M., Jeffery E.H. The metabolic fate of purified glucoraphanin in F344 rats. J. Agric. Food Chem. 2007;55(8):2861–2866. doi: 10.1021/jf0633544. [DOI] [PubMed] [Google Scholar]
- 26.Saha S., Hollands W., Teucher B., Needs P.W., Narbad A., Ortori C.A., Barrett D.A., Rossiter J.T., Mithen R.F., Kroon P.A. Isothiocyanate concentrations and interconversion of sulforaphane to erucin in human subjects after consumption of commercial frozen broccoli compared to fresh broccoli. Mol. Nutr. Food Res. 2012;56(12):1906–1916. doi: 10.1002/mnfr.201200225. [DOI] [PubMed] [Google Scholar]
- 27.Iori R., Bernardi R., Gueyrard D., Rollin P., Palmieri S. Formation of glucoraphanin by chemoselective oxidation of natural glucoerucin: A chemoenzymatic route to sulforaphane. Bioorg. Med. Chem. Lett. 1999;9(7):1047–1048. doi: 10.1016/s0960-894x(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 28.Leng C., Zhang Y., Wang M., Wang P., Gu Z., Yang R. Dynamic variation of glucosinolates and isothiocyanates in broccoli sprouts during hydrolysis. Sci. Hortic-Amsterdam. 2019;255:128–133. [Google Scholar]
- 29.Hu J.-L., Nie S.-P., Xie M.-Y. High pressure homogenization increases antioxidant capacity and short-chain fatty acid yield of polysaccharide from seeds of Plantago asiatica L. Food Chem. 2013;138(4):2338–2345. doi: 10.1016/j.foodchem.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Hua X., Xu S., Wang M., Chen Y., Yang H., Yang R. Effects of high-speed homogenization and high-pressure homogenization on structure of tomato residue fibers. Food Chem. 2017;232:443–449. doi: 10.1016/j.foodchem.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Wang X.-S., Wu Y.-F., Dai S.-L., Chen R., Shao Y. Ultrasound-assisted extraction of geniposide from Gardenia jasminoides. Ultrason. Sonochem. 2012;19(6):1155–1159. doi: 10.1016/j.ultsonch.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Quan C., Sun Y., Qu J. Ultrasonic extraction of ferulic acid from Angelica sinensis. Can. J. Chem. Eng. 2009;87(4):562–567. [Google Scholar]
- 33.Wang Y., Liu Y., Hu Y. Optimization of polysaccharides extraction from Trametes robiniophila and its antioxidant activities. Carbohyd. Polym. 2014;111:324–332. doi: 10.1016/j.carbpol.2014.03.083. [DOI] [PubMed] [Google Scholar]
- 34.Dabbour M., He R., Mintah B., Tang Y., Ma H. Ultrasound assisted enzymolysis of sunflower meal protein: Kinetics and thermodynamics modeling. J. Food Process Eng. 2018;41(7):e12865. doi: 10.1111/jfpe.2018.41.issue-710.1111/jfpe.12865. [DOI] [Google Scholar]
- 35.Esclapez M.D., García-Pérez J.V., Mulet A., Cárcel J.A. Ultrasound-assisted extraction of natural products. Food Eng. Rev. 2011;3(2):108–120. [Google Scholar]
- 36.Tiwari B.K. Ultrasound: A clean, green extraction technology, TRAC-Trend. Anal. Chem. 2015;71:100–109. [Google Scholar]
- 37.Zhang Z., Lv G., Song T., Chen J., Cai W. Recovery and analysis of phenolic extracts from Oudemansiella radicata using ultrasonic-assisted extraction. J. Food Meas. Charact. 2020;14(4):2176–2184. [Google Scholar]
- 38.Xu Y., Zhang L., Bailina Y., Ge Z., Ding T., Ye X., Liu D. Effects of ultrasound and/or heating on the extraction of pectin from grapefruit peel. J. Food Eng. 2014;126:72–81. [Google Scholar]
- 39.Majumdar S., Kumar P.S., Pandit A.B. Effect of liquid-phase properties on ultrasound intensity and cavitational activity. Ultrason. Sonochem. 1998;5(3):113–118. doi: 10.1016/s1350-4177(98)00019-4. [DOI] [PubMed] [Google Scholar]
- 40.Gogate P.R., Pandit A.B. Sonochemical reactors: scale up aspects. Ultrason. Sonochem. 2004;11(3-4):105–117. doi: 10.1016/j.ultsonch.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Stanisavljević I.T., Lazić M.L., Veljković V.B. Ultrasonic extraction of oil from tobacco (Nicotiana tabacum L.) seeds. Ultrason. Sonochem. 2007;14(5):646–652. doi: 10.1016/j.ultsonch.2006.10.003. [DOI] [PubMed] [Google Scholar]