Abstract
Background
Lower-sodium oxybate (LXB) is an oxybate medication with the same active moiety as sodium oxybate (SXB) and a unique composition of cations, resulting in 92% less sodium. LXB was shown to improve cataplexy and excessive daytime sleepiness in people with narcolepsy in a placebo-controlled, double-blind, randomized withdrawal study (NCT03030599). Additional analyses of data from this study were conducted to explore the effects of LXB on cataplexy, including the clinical course and feasibility of transition from other anticataplectics to LXB monotherapy.
Objective
The aim of these analyses was to evaluate cataplexy frequency during initiation/optimization of LXB and taper/discontinuation of prior antidepressant/anticataplectic medications.
Methods
Eligible participants (adults aged 18–70 years with narcolepsy with cataplexy) entered the study taking SXB only (group A), SXB + other anticataplectics (group B), or anticataplectic medication other than SXB (group C), or were cataplexy-treatment naive (group D). LXB was initiated/optimized during a 12-week, open-label, optimized treatment and titration period (OLOTTP). Other anticataplectics were tapered/discontinued during weeks 3–10 of OLOTTP. A 2-week stable-dose period (SDP; during which participants took a stable dose of open-label LXB) and 2-week double-blind randomized withdrawal period (during which participants were randomized to continue LXB treatment or switch to placebo) followed OLOTTP. Treatment-emergent adverse events (TEAEs) were recorded throughout the duration of the study.
Results
At the beginning of OLOTTP, median weekly cataplexy attacks were lower in participants taking SXB at study entry (SXB only [2.00]; SXB + other anticataplectics [0.58]) versus participants who were taking other anticataplectics (3.50) or were anticataplectic naive (5.83). Median weekly cataplexy attacks decreased during weeks 1–2 of OLOTTP in all groups. Increased cataplexy frequency was observed in participants tapering/discontinuing other anticataplectics during weeks 3–10 and was more prominent in participants taking other anticataplectics alone compared with those taking SXB plus other anticataplectics. Cataplexy frequency decreased throughout initiation/optimization in anticataplectic-naive participants. Median number of cataplexy-free days/week at the end of SDP (study week 14) was similar in all groups (6.0, 6.1, 6.0, and 6.2 in groups A, B, C, and D, respectively). During OLOTTP and SDP, TEAEs of worsening cataplexy were reported in 0%, 47.8%, 16.7%, and 2.2% of participants in groups A, B, C, and D, respectively; most TEAEs of worsening cataplexy were reported during tapering/discontinuation of other anticataplectics.
Conclusions
LXB monotherapy was effective in reducing cataplexy and increasing cataplexy-free days. These results illustrate the feasibility of switching from SXB to LXB while tapering/discontinuing other anticataplectics.
Trial Registration
A Study of the Efficacy and Safety of JZP-258 in Subjects With Narcolepsy With Cataplexy; https://clinicaltrials.gov/ct2/show/NCT03030599; clinicaltrials.gov identifier: NCT03030599.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40263-022-00926-0.
Plain Language Summary
People with narcolepsy are often sleepy during the day. They may also have sudden muscle weakness (known as cataplexy). Lower-sodium oxybate (LXB) is a narcolepsy medicine that is similar to sodium oxybate (SXB) but has 92% less sodium. A recent study found that treatment with LXB was better at reducing how often people with narcolepsy had sleepiness and cataplexy than no medicine at all (NCT03030599). This paper is about the first 12 weeks of that study, when all the people taking part in the study first tried LXB to check that they were being given the right amount. In people who only took LXB, cataplexy happened less often over time. Some people were already taking other medicines to treat their cataplexy (such as antidepressants), so they were asked to slowly stop those medicines while taking LXB. In those people, cataplexy happened more often at first as they stopped taking antidepressants and then less often later on. The increase in cataplexy when antidepressants were stopped was smaller in people who switched from SXB to LXB. This study shows that many people getting treatment for narcolepsy can switch to LXB without their cataplexy becoming worse.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40263-022-00926-0.
Key Points
| Lower-sodium oxybate (LXB) is approved in the United States for the treatment of cataplexy or excessive daytime sleepiness in patients 7 years of age and older with narcolepsy, and for idiopathic hypersomnia in adults. |
| This study enrolled participants who were taking sodium oxybate (SXB) with or without other anticataplectic agents (primarily antidepressants) and participants who were cataplexy-treatment naive; taper and withdrawal of other anticataplectics occurred during the study. |
| Participants either transitioned from SXB to LXB at a gram-to-gram dose equivalent or initiated LXB treatment. |
| LXB monotherapy reduced cataplexy frequency and increased cataplexy-free days, and the safety profile of LXB was consistent with that previously observed for SXB. |
Introduction
Narcolepsy is a rare, potentially disabling, long-term disorder of central hypersomnolence characterized by a pentad of symptoms: excessive daytime sleepiness (EDS), cataplexy, disrupted nighttime sleep, sleep-related hallucinations (hypnagogic and hypnopompic), and sleep paralysis [1, 2]. All people with narcolepsy experience EDS, the most frequent and debilitating symptom [1, 3]. The majority of patients with narcolepsy type 1 also experience cataplexy, a sudden, brief (< 2 minutes) loss of muscle tone with preserved consciousness, typically precipitated by strong emotions [1, 2, 4]. Narcolepsy with cataplexy can have a significant negative impact on quality of life, contributing to difficulties in academic and professional domains [3]. Anticipation of cataplexy can lead to avoidance of activities and can limit social interactions [1].
Antidepressants are frequently prescribed off-label for the treatment of cataplexy, although there is limited rigorous evidence regarding their efficacy and safety [2, 5–9]. However, despite lower antidepressant doses for cataplexy than are typically prescribed for the treatment of depression, tolerance to the anticataplectic effects and rebound cataplexy (an increase in the frequency and/or severity of attacks) upon abrupt discontinuation have been reported [4]. Furthermore, protracted, debilitating rebound cataplexy (status cataplecticus) has been reported even with gradual taper and discontinuation of antidepressant treatment [4, 10].
Sodium oxybate (SXB; Xyrem®) is indicated in the United States (US) for the treatment of cataplexy or EDS in patients 7 years of age and older with narcolepsy and in Europe for the treatment of narcolepsy with cataplexy in adult patients, adolescents, and children from the age of 7 years [11, 12]. Calcium, magnesium, potassium, and sodium oxybates (also referred to as lower-sodium oxybate [LXB]; Xywav®) is an oxybate medication with the same active moiety as SXB and a unique composition of cations, resulting in 92% less sodium [13, 14]. LXB was developed to provide oxybate treatment without the potential risks associated with long-term high sodium exposure, such as effects on blood pressure and increased risk of cardiovascular-related comorbidities and mortality [15]. LXB is approved in the US for the treatment of cataplexy or EDS in patients 7 years of age and older with narcolepsy, and for idiopathic hypersomnia in adults [13].
A randomized withdrawal study design was implemented to evaluate the efficacy and safety of LXB in individuals with narcolepsy with cataplexy. An important aspect of the study design was the inclusion of an initial 12-week, open-label, optimized treatment and titration period (OLOTTP), during which initiation and individualized dose titration/optimization of LXB occurred simultaneously with tapering/discontinuation of anticataplectic medications other than oxybate. This allowed enrollment of patients initially treated with antidepressant anticataplectics, which is common in current clinical practice. Participants included patients transitioning from SXB to LXB, patients tapering and discontinuing other anticataplectics, and patients who were not receiving any treatment for cataplexy. The primary outcomes of the study (ClinicalTrials.gov identifier: NCT03030599) support the efficacy and safety of LXB, and have been previously reported [16].
The analyses reported here were conducted to examine cataplexy frequency during transition to, or initiation of, LXB treatment and anticataplectic tapering/discontinuation in this study. Assessments included cataplexy frequency, as well as the number of cataplexy-free days per week during initiation and dose optimization/titration of LXB while tapering/discontinuing other anticataplectics (if present). Additional analyses examined the timing and number of LXB dose adjustments and safety of LXB during OLOTTP.
Methods
Study Design and Participants
Study design and results of primary and key secondary endpoints have been reported in detail previously [16] and are summarized here. Eligible participants were adults 18–70 years of age with narcolepsy with cataplexy and a history of at least 14 cataplexy attacks in a typical 2-week period prior to receiving narcolepsy treatment. Participants currently taking medication(s) for narcolepsy were eligible if the medication regimen was stable for at least 2 months prior to study entry. Participants taking SXB at study entry were required to have documented prior improvement in cataplexy and EDS with SXB treatment. Participants taking wake-promoting agents or stimulants at study entry were to remain on the same dose and regimen throughout the duration of the study. Participants were taking either SXB but not other anticataplectics (SXB only; group A); SXB and other anticataplectics (SXB + other anticataplectics; group B); anticataplectics other than SXB (other anticataplectics; group C); or were cataplexy-treatment naive (anticataplectic naive; group D). Anticataplectics other than SXB included antidepressants and pitolisant.
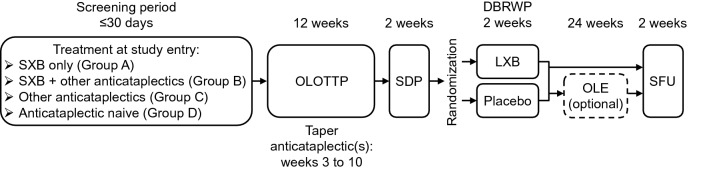
The main study consisted of a screening period; a 12-week OLOTTP; a 2-week stable-dose period (SDP); a 2-week double-blind randomized withdrawal period (DBRWP); and a 2-week safety follow-up (for those who did not proceed to an optional open-label extension study; Fig. 1). Participants initiated LXB treatment during OLOTTP (week 1), with individualized dose titration as needed to optimize efficacy and tolerability. Participants taking SXB at study entry (groups A and B) were initiated on LXB at a gram-to-gram dose equivalent to their nightly dose of SXB and remained on that dose of LXB for the first 2 weeks of OLOTTP. If needed, dose optimization occurred during the following 8 weeks at a rate of up to 1.5 g/night per week (maximum dose: 9 g/night, split across two doses; Table 1) [13]. Participants naive to SXB (groups C and D) initiated LXB at a dose of 4.5 g/night (split across two doses), after which titration to an optimal dose occurred over a minimum of 2 weeks. Dose adjustments proceeded at the same rate as groups A and B: up to 1.5 g/night (total, split across two doses) at weekly intervals (maximum dose: 9 g/night, split across two doses). Participants taking other anticataplectics at study entry, with or without SXB (groups B and C), continued taking their other anticataplectics at a stable dose for the first 2 weeks of OLOTTP. Tapering and discontinuation of prior antidepressant/anticataplectic medications began at week 3, with discontinuation by week 10 or earlier; the rate of tapering within that period was at investigator discretion. Additional time for tapering and withdrawal of prior antidepressant/anticataplectic medications was permitted, if needed, through week 12. Targeting antidepressant/anticataplectic discontinuation by the end of week 10 allowed for LXB monotherapy at the individually optimized dose during the final 2 weeks of OLOTTP, prior to completion of formal efficacy assessments during SDP. During the SDP that followed, participants took their individually optimized dose of LXB, established during OLOTTP, for 2 weeks. At the end of SDP, participants were randomized 1:1 to continue LXB treatment or receive placebo during the 2-week DBRWP.
Fig. 1.
Study design. DBRWP double-blind randomized withdrawal period, LXB lower-sodium oxybate, OLE open-label extension, OLOTTP open-label optimized treatment and titration period, SDP stable-dose period, SFU safety follow-up, SXB sodium oxybate
Table 1.
Dosing of LXB and other anticataplectics during OLOTTP
| Group | Treatment at study entry | Treatment during OLOTTP | OLOTTP | ||
|---|---|---|---|---|---|
| Weeks 1–2 | Weeks 3–10 | Weeks 11–12 | |||
| A | SXB only | LXB | Same dose as for prior SXB | Titrate as needed | Steady optimal dose; adjust dose only if necessary |
| B | SXB + other anticataplectics | LXB | Same dose as for prior SXB | Titrate as needed | Steady optimal dose; adjust dose only if necessary |
| Other anticataplectic | Same dose as before | Taper and withdraw | N/Aa | ||
| C | Other anticataplectics | LXB | 4.5 g/night (split across 2 doses), titrated as needed | Titrate as needed | Steady optimal dose; adjust dose only if necessary |
| Other anticataplectic | Same dose as before | Taper and withdraw | N/Aa | ||
| D | Anticataplectic naive | LXB | 4.5 g/night (split across 2 doses), titrated as needed | Titrate as needed | Steady optimal dose; adjust dose only if necessary |
LXB lower-sodium oxybate, N/A not applicable, OLOTTP open-label optimized treatment and titration period, SXB sodium oxybate
aAdditional time for tapering and withdrawal of prior anticataplectics was permitted if needed
The study was approved by institutional review boards or ethics committees at all sites and was performed in accordance with the International Conference on Harmonisation Guideline for Good Clinical Practice and the Declaration of Helsinki. All participants provided written informed consent.
Assessments of Cataplexy Frequency and Safety
Participants completed daily cataplexy frequency diaries to record the number of cataplexy attacks they had each day, from the beginning of OLOTTP (week 1, after initiation of LXB) through to the end of DBRWP. Participants were informed in the cataplexy diary that cataplexy attacks “1) can cause you to actually fall down or you would have fallen if you did not support yourself, or 2) make part of your body feel weak.” Participants who did not complete the cataplexy diary on at least 10 out of the 14 days in SDP were discontinued from the study.
Treatment-emergent adverse events (TEAEs), defined as any adverse events that started or worsened in severity on or after the first dose of open-label study drug in OLOTTP up to the last dose of study drug during any study period (main study or open-label extension) + 30 days, were recorded throughout the duration of the study.
Analyses
The safety population included all participants who took at least one dose of study medication. The efficacy population consisted of all randomized participants who took at least one dose of double-blind study medication and completed at least one set of post-randomization efficacy assessments.
Unless otherwise noted, cataplexy analyses reported in this publication are based on the efficacy population. Cataplexy analyses included median weekly cataplexy frequency during OLOTTP and SDP, based on treatment at study entry (pre-planned analysis), and cataplexy-free days per week during OLOTTP, SDP, and DBRWP, based on treatment at study entry (post-hoc analysis). Weekly cataplexy attacks were calculated/imputed as (total number of cataplexy attacks within a week)/(number of days with non-missing data within that week) × 7 days. Cataplexy-free days per week were calculated as (number of days with 0 cataplexy attacks)/(number of days with diary data) × 7 days.
Analyses of optimization of LXB dosing and tapering/discontinuation of other anticataplectics were descriptive. Summaries of dose adjustments of other anticataplectics and final LXB dose compared with SXB dose at study entry were based on data from participants in the safety population who entered the study taking other anticataplectics or SXB, respectively. Analyses of LXB dose optimization (total nightly dose, time to reach a stable total nightly dose, and number of dose adjustments) were based on the efficacy population.
Cataplexy data were not normally distributed; therefore, median weekly cataplexy frequency was reported, rather than mean, and linear regression fitting of median data was used to estimate trends in cataplexy frequency changes. A non-parametric method, the Mann-Whitney test, was implemented to compare the differences among groups. Location shift, an estimate of the median difference between LXB and placebo, together with the 95% confidence interval were calculated with the use of the Hodges-Lehmann approach. Likewise, analysis of cataplexy-free days was performed with the use of a Mann-Whitney test, and location shift and the 95% confidence interval were calculated with the use of the Hodges-Lehmann approach. No adjustments for multiple comparisons were made; as such, nominal p values are reported.
TEAEs occurring during OLOTTP and SDP were summarized by treatment at study entry (post-hoc analysis; safety population).
Results
Participant Disposition, Demographics, and Baseline Characteristics
A total of 201 participants were enrolled and treated (safety population; 52, 23, 36, and 90 participants in groups A, B, C, and D, respectively); among them, 45 (86.5%), 14 (60.9%), 26 (72.2%), and 70 (77.8%) completed OLOTTP. A total of 134 participants took one or more doses of double-blind study drug during DBRWP and had one or more post-randomization efficacy assessments (efficacy population; 41 [78.8%], 14 [60.9%], 21 [58.3%], and 58 [64.4%] in groups A, B, C, and D, respectively). Participant disposition and discontinuations during OLOTTP and prior to taking randomized drug in DBRWP are presented in Online Resource 1 (see electronic supplementary material [ESM]). In the efficacy population, 69 participants were randomized to continue taking LXB in DBRWP and 65 were randomized to placebo.
Demographic and baseline characteristics of the safety population were described previously [16]. Demographic and baseline characteristics by treatment at study entry are presented in Table 2.
Table 2.
Baseline characteristics, by treatment at study entry (safety population)
| Characteristics | Group A | Group B | Group C | Group D |
|---|---|---|---|---|
| SXB only (n = 52) | SXB + other anticataplectics (n = 23) | Other anticataplectics (n = 36) | Anticataplectic naive (n = 90) | |
| Age (years) | ||||
| Mean (SD) | 39.4 (13.0) | 39.0 (12.5) | 35.7 (12.2) | 36.0 (11.6) |
| Median (minimum, maximum) | 41.0 (18, 68) | 38.0 (19, 65) | 34.0 (18, 62) | 34.5 (18, 70) |
| Sex, n (%) | ||||
| Female | 27 (51.9) | 11 (47.8) | 23 (63.9) | 61 (67.8) |
| Male | 25 (48.1) | 12 (52.2) | 13 (36.1) | 29 (32.2) |
| Body mass index (kg/m2) | ||||
| Mean (SD) | 28.5 (6.5)a | 29.8 (5.3) | 29.0 (5.4) | 28.5 (6.3) |
| Median (minimum, maximum) | 26.8 (20.0, 42.8)a | 28.8 (20.7, 47.1) | 28.0 (20.5, 43.6) | 26.8 (18.7, 47.9) |
| Race, n (%) | ||||
| White | 48 (92.3) | 18 (78.3) | 35 (97.2) | 76 (84.4) |
| Black or African American | 1 (1.9) | 1 (4.3) | 0 | 9 (10.0) |
| Asian | 2 (3.8) | 0 | 0 | 1 (1.1) |
| American Indian or Alaska Native | 0 | 0 | 0 | 0 |
| Native Hawaiian or other Pacific Islander | 0 | 0 | 0 | 0 |
| Multiple | 0 | 1 (4.3) | 0 | 1 (1.1) |
| Missing | 1 (1.9) | 3 (13.0) | 1 (2.8) | 3 (3.3) |
| Region, n (%) | ||||
| Europe | 27 (51.9) | 18 (78.3) | 28 (77.8) | 49 (54.4) |
| North America | 25 (48.1) | 5 (21.7) | 8 (22.2) | 41 (45.6) |
| Past narcolepsy symptoms prior to any narcolepsy treatment, n (%)b | ||||
| Disrupted nighttime sleep | 30 (57.7) | 18 (78.3) | 23 (63.9) | 56 (62.2) |
| Hypnagogic and/or hypnopompic hallucinations | 29 (55.8) | 14 (60.9) | 22 (61.1) | 55 (61.1) |
| Sleep paralysis | 28 (53.8) | 15 (65.2) | 19 (52.8) | 58 (64.4) |
| Cardiovascular/cardiometabolic comorbidities: system organ class, n (%) | ||||
| Cardiac disorders | 1 (1.9) | 0 | 1 (2.8) | 3 (3.3) |
| Atrial fibrillation | 1 (1.9) | 0 | 0 | 0 |
| Bundle branch block right | 0 | 0 | 0 | 1 (1.1) |
| Mitral valve incompetence | 0 | 0 | 1 (2.8) | 0 |
| Palpitations | 0 | 0 | 0 | 1 (1.1) |
| Sinus tachycardia | 0 | 0 | 0 | 1 (1.1) |
| Supraventricular extrasystoles | 0 | 0 | 1 (2.8) | 0 |
| Tricuspid valve incompetence | 0 | 0 | 1 (2.8) | 0 |
| Ventricular extrasystoles | 0 | 0 | 1 (2.8) | 0 |
| Metabolism and nutrition disorders | ||||
| Obesity | 2 (3.8) | 1 (4.3) | 5 (13.9) | 6 (6.7) |
| Hypercholesterolemia | 3 (5.8) | 2 (8.7) | 1 (2.8) | 3 (3.3) |
| Diabetes mellitus | 4 (7.7) | 1 (4.3) | 1 (2.8) | 0 |
| Hyperlipidemia | 4 (7.7) | 0 | 1 (2.8) | 1 (1.1) |
| Type 2 diabetes mellitus | 0 | 2 (8.7) | 1 (2.8) | 3 (3.3) |
| Vitamin D deficiency | 2 (3.8) | 1 (4.3) | 1 (2.8) | 0 |
| Dyslipidemia | 0 | 2 (8.7) | 0 | 0 |
| Glucose tolerance impaired | 2 (3.8) | 0 | 0 | 0 |
| Hypertriglyceridemia | 0 | 1 (4.3) | 0 | 1 (1.1) |
| Gluten sensitivity | 0 | 0 | 0 | 1 (1.1) |
| Gout | 1 (1.9) | 0 | 0 | 0 |
| Hyperglycemia | 0 | 0 | 0 | 1 (1.1) |
| Insulin resistance syndrome | 1 (1.9) | 0 | 0 | 0 |
| Vascular disorders | ||||
| Hypertension | 11 (21.2) | 8 (34.8) | 5 (13.9) | 11 (12.2) |
| Deep vein thrombosis | 0 | 0 | 0 | 1 (1.1) |
| Raynaud’s phenomenon | 1 (1.9) | 0 | 0 | 0 |
| Anticataplectic/antidepressant treatment other than SXB, n (%)c | ||||
| SSRI | ||||
| Fluoxetine | 0 | 1 (4.3) | 6 (16.7) | 0 |
| Escitalopram | 0 | 2 (8.7) | 3 (8.3) | 0 |
| Paroxetine | 0 | 0 | 1 (2.8) | 0 |
| Sertraline | 0 | 0 | 1 (2.8) | 0 |
| SNRI | ||||
| Venlafaxine | 0 | 8 (34.8) | 12 (33.3) | 0 |
| Reboxetine | 0 | 0 | 1 (2.8) | 0 |
| SSNRI | ||||
| Duloxetine | 0 | 3 (13.0) | 0 | 0 |
| Tricyclic/tetracyclic | ||||
| Clomipramine | 0 | 5 (21.7) | 7 (19.4) | 0 |
| Protriptyline | 0 | 1 (4.3) | 0 | 0 |
| Mirtazapine | 0 | 0 | 1 (2.8) | 0 |
| Atypical | ||||
| Agomelatine | 0 | 1 (4.3) | 0 | 0 |
| Other | ||||
| Baclofen | 0 | 0 | 0 | 0 |
| Pitolisant | 0 | 4 (17.4) | 2 (5.6) | 0 |
SD standard deviation, SNRI selective norepinephrine reuptake inhibitor, SSNRI selective serotonin and norepinephrine reuptake inhibitor, SSRI selective serotonin reuptake inhibitor, SXB sodium oxybate
an = 50
bParticipants could have been included in more than one category
cParticipants could have taken more than one anticataplectic/antidepressant treatment
Cataplexy Frequency
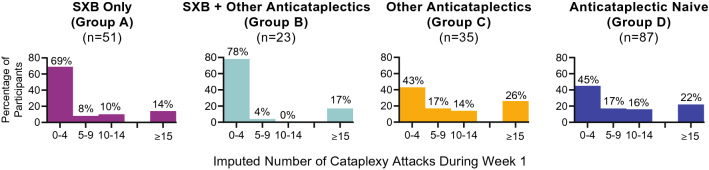
The percentage of participants experiencing low levels of cataplexy (0–4 attacks) at baseline (i.e., during week 1 of OLOTTP) was greater in those taking SXB at study entry (groups A and B; 69% and 78%, respectively) compared with groups C and D (43% and 45%, respectively; Fig. 2 [safety population]). Some participants had higher frequencies of cataplexy attacks (in groups A, B, C, and D, respectively: 8%, 4%, 17%, and 17% who experienced 5–9 attacks; 10%, 0%, 14%, and 16% who experienced 10–14 attacks; and 14%, 17%, 26%, and 22% who experienced ≥ 15 attacks).
Fig. 2.
Distribution of cataplexy frequency during week 1, by treatment at study entry (safety population). n numbers of participants with available data, SXB sodium oxybate
A few participants experienced very high weekly frequencies (≥ 55) of cataplexy attacks during week 1; among them, one was in group B with 85 attacks, one was in group C with 159.6 attacks (imputed based on the patient having recorded 50, 30, 18, 12, and 4 cataplexy attacks on 5 days of the 7-day period), and one was in group D with 239 attacks. The participant with 85 cataplexy attacks recorded during week 1 was a 39-year-old male who was taking SXB and another anticataplectic at study entry (clomipramine 75 mg QD, which was discontinued by study day 66). The participant with 159.6 cataplexy attacks recorded during week 1 was an 18-year-old female who was taking a non-SXB anticataplectic (venlafaxine 75 mg QD) at study entry, which was discontinued by study day 40. The participant with 239 cataplexy attacks recorded during week 1 was a 51-year-old male who was treatment naive at study entry.
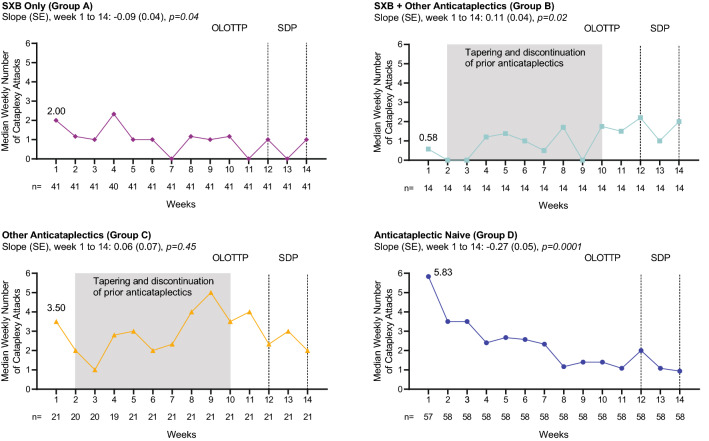
Median weekly cataplexy attack frequency during all weeks of OLOTTP and SDP, by treatment at study entry (efficacy population), is presented in Fig. 3. Median weekly cataplexy attack frequency at the start of OLOTTP was lower in participants taking SXB at study entry (groups A and B combined; median [minimum, maximum]: 1.4 [0, 85]) compared with participants not taking SXB at study entry (groups C and D combined; median [minimum, maximum]: 5.6 [0, 239]), with a location shift of −2.0 (p < 0.01). In group A, median weekly cataplexy attacks decreased over the OLOTTP and SDP (slope [standard error, SE]: −0.09 [0.04]; p = 0.04). In group B, median weekly cataplexy attacks increased from the start of tapering and discontinuation of other anticataplectics in OLOTTP and stabilized by the end of SDP (slope [SE]: 0.11 [0.04]; p = 0.02). In group C (other anticataplectics), median weekly cataplexy attacks decreased as LXB was titrated, then increased during taper and discontinuation of prior treatments and stabilized during SDP (slope [SE]: 0.06 [0.07]; p = 0.45), with no overall change in weekly cataplexy attacks observed. In the anticataplectic-naive group (group D), median weekly cataplexy attacks decreased from the first week of titration and throughout the course of OLOTTP and SDP (slope [SE]: − 0.27 [0.05]; p = 0.0001). Most participants tapering/discontinuing other antidepressant/anticataplectic medications (groups B and C) demonstrated worsening of cataplexy associated with taper and discontinuation. Variability in weekly cataplexy attacks during OLOTTP was greatest during cross-titration in participants who had been taking anticataplectics other than SXB at study entry.
Fig. 3.
Median weekly cataplexy attack frequency during OLOTTP and SDP, by treatment at study entry (efficacy population). Linear regression fitting of median data was used to estimate trends in cataplexy frequency changes; p-values associated with slopes are based on t tests. p-values are nominal. OLOTTP open-label optimized treatment and titration period, SDP stable-dose period, SE standard error, SXB sodium oxybate
Over the duration of OLOTTP and SDP (weeks 1–14), notably high cataplexy frequency (≥ 100 weekly cataplexy attacks) was observed in four participants. Weekly attack frequencies were examined in these individuals to assess the evolution of high cataplexy rates over time. Two of these participants were taking SXB plus other anticataplectics at entry (group B); one was taking other anticataplectics at entry (group C); and one was treatment naive (group D). One of the participants in group B experienced increased cataplexy frequency during weeks 5–11, with a peak of 189 cataplexy attacks during week 7. The other participant in group B withdrew from the study during week 8, when 165.2 cataplexy attacks (imputed) were recorded. The participant in group C was the same participant mentioned earlier, with 159.6 cataplexy attacks (imputed) during week 1; for this participant, weekly cataplexy attacks decreased to 16.3 during week 3, then increased to 210 during week 5 and 186.7 during week 6 before declining to 0 during week 12. The participant in group D was the same participant mentioned earlier with 239 cataplexy attacks during week 1. This participant experienced a rapid decline in the number of weekly cataplexy attacks following initiation of LXB treatment (4.5 g/night) at study entry, to 106 during week 2 and 81 during week 3. The dosage of LXB for this participant was adjusted to 5.5 g/night on day 8 (week 2), 6.5 g/night on day 22 (week 4), and 7.5 g/night on day 43 (week 7); a dose increase beyond 7.5 g/night was not attempted during the study period.
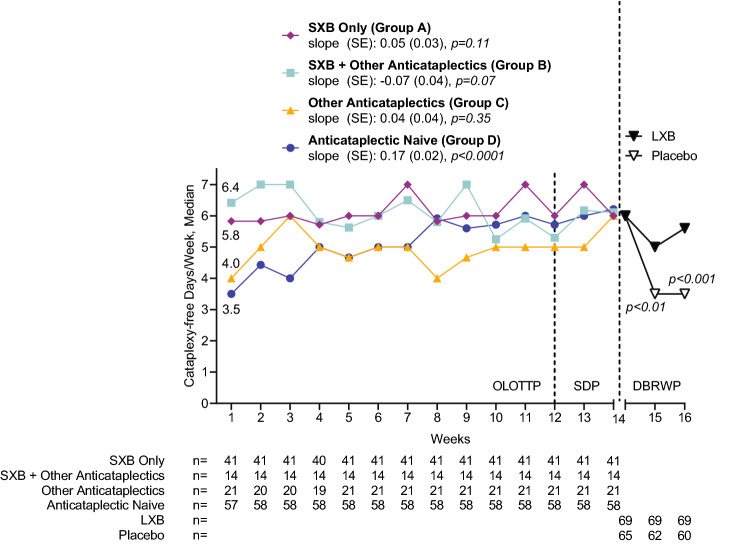
Cataplexy-Free Days Per Week
Results of cataplexy-free days per week analysis during OLOTTP, SDP, and DBRWP (efficacy population) are presented in Fig. 4. During OLOTTP week 1, the median number of cataplexy-free days per week was higher in participants who were taking SXB at study entry (groups A [5.8] and B [6.4]) compared with participants who were not taking SXB at study entry (groups C [4.0] and D [3.5]). During weeks 3–12 of OLOTTP, cataplexy-free days per week varied among the groups but stabilized in all groups toward the end of OLOTTP, then remained so during SDP. Linear regression showed that cataplexy-free days per week in previously anticataplectic-naive participants increased over the course of week 1 to week 14 (slope [SE]: 0.17 [0.02]; p < 0.0001), but an increase was not observed in the other groups (p > 0.05). At the end of SDP, cataplexy-free days were high in all groups (median: 6.0, 6.1, 6.0, and 6.2 in groups A, B, C, and D, respectively).
Fig. 4.
Median cataplexy-free days per week during OLOTTP and SDP, by treatment at study entry, and during DBRWP, by randomized treatment (efficacy population). Slope is from week 1 to week 14. Linear regression fitting of median data was used to estimate trends in cataplexy frequency changes; p-values associated with slopes are based on t tests. p-values are nominal. DBRWP double-blind randomized withdrawal period, LXB lower-sodium oxybate, OLOTTP open-label optimized treatment and titration period, SDP stable-dose period, SE standard error, SXB sodium oxybate
During DBRWP, in participants randomized to placebo, median cataplexy-free days per week declined (3.5 and 3.5 in DBRWP weeks 1 and 2, respectively, vs 6.0 in SDP week 2). In participants randomized to continue LXB treatment, median cataplexy-free days per week were stable (5.0 and 5.6 in DBRWP weeks 1 and 2, respectively, vs 6.0 in SDP week 2). The number of cataplexy-free days per week was greater in participants randomized to continue LXB treatment compared with participants randomized to placebo during both DBRWP week 1 (Mann-Whitney U: − 3.03; p < 0.01) and DBRWP week 2 (Mann-Whitney U: − 4.05; p < 0.001; Fig. 4).
Tapering/Discontinuation of Other Anticataplectics and Optimization of LXB Dosing
A total of 59 participants (29.4%; 23 in group B and 36 in group C) entered the study taking other anticataplectics (primarily antidepressants), namely venlafaxine (34.8% and 33.3% in groups B and C, respectively), clomipramine (21.7% and 19.4% in groups B and C, respectively), fluoxetine (4.3% and 16.7% in groups B and C, respectively), or other antidepressants (30.3% and 19.5% in groups B and C, respectively); in addition, 17.4% in group B and 5.6% in group C were taking pitolisant. As reported previously [16], of the 59 participants in groups B and C, all but three remaining in the study at week 10 of OLOTTP completed tapering/discontinuation of antidepressant/anticataplectic medications; of these three participants, two discontinued the study because of this protocol deviation and one continued tapering during weeks 11–12. In groups B and C, the median (minimum, maximum) time to taper/discontinue antidepressant/anticataplectic medications was 42.5 (22, 64) and 23.0 (4, 74) days, respectively.
In the efficacy population, 55 participants entered the study taking SXB, either alone (group A, n = 41) or in combination with another anticataplectic (group B, n = 14). Among them, the median (minimum, maximum) dose was 8.0 (4.5, 9.0) g/night. Most (50/55; 90.9%) reached a stable total nightly dose within one titration step (i.e., ± 1.5 g/night) of their dose at study entry. Of the 55 participants, 2 (3.6%) decreased dose by ≤ 1.5 g/night, 37 (67.3%) had no change, 11 (20.0%) increased dose by ≤ 1.5 g/night, 2 (3.6%) increased by 2 g/night, 2 (3.6%) increased by 3 g/night, and 1 (1.8%) increased by 4.25 g/night. Eighteen of the 55 were at the maximum recommended total nightly dose of 9 g/night at study entry. Eight participants (6 in group A and 2 in group B) were taking the minimum recommended effective dose of SXB (6 g/night) at study entry, and five of those eight titrated to a higher dose of LXB.
Total nightly dose of LXB during SDP, time to reach a stable total nightly dose of LXB, and number of dose adjustments to reach a stable total nightly dose are summarized in Table 3 (efficacy population). Overall, the median (minimum, maximum) total nightly stable dose during SDP was 7.5 (3.0, 9.0) g/night, the median (minimum, maximum) time to reach a stable dose of LXB was 29 (1, 84) days, and the median (minimum, maximum) number of dose adjustments required to reach a stable total nightly dose was 2 (0, 8). In participants who entered the study taking SXB as well as other anticataplectics (group B), the average total nightly dose of LXB during SDP was higher compared with groups A, C, and D (median dose [g/night]: 9.0 vs 7.5, 7.5, and 7.0, respectively). Both time to reach a stable dose and number of dose adjustments were lower in participants taking SXB at study entry (groups A and B) compared with those who were not (groups C and D; Table 3). However, there was considerable variability in time to reach a stable total nightly dose.
Table 3.
Average total nightly dose of LXB during SDP, time to reach stable total nightly dose, and number of LXB dose adjustments in participants who achieved a stable dose of LXB, by treatment at study entry (efficacy population)
| Group A | Group B | Group C | Group D | Total (N = 134) | |
|---|---|---|---|---|---|
| SXB only (n = 41) | SXB + other anticataplectics (n = 14) | Other anticataplectics (n = 21) | Anticataplectic naive (n = 58) | ||
| Average total nightly dose of LXB (g/night) | |||||
| Mean | 7.6 | 8.3 | 7.4 | 6.9 | 7.3 |
| SD | 1.4 | 1.1 | 1.3 | 1.5 | 1.4 |
| Median | 7.5 | 9.0 | 7.5 | 7.0 | 7.5 |
| Minimum, maximum | 4.5, 9.0 | 6.0, 9.0 | 4.5, 9.0 | 3.0, 9.0 | 3.0, 9.0 |
| Time to reach stable total nightly dose (days) | |||||
| Mean | 14.5 | 15.7 | 45.5 | 39.0 | 30.1 |
| SD | 21.37 | 24.59 | 18.57 | 20.86 | 24.56 |
| Median | 1.0 | 1.0 | 50.0 | 36.5 | 29.0 |
| Minimum, maximum | 1, 84 | 1, 64 | 5, 73 | 1, 81 | 1, 84 |
| Number of dose adjustments to reach stable total nightly dose | |||||
| Mean | 1.0 | 0.8 | 3.5 | 2.6 | 2.1 |
| SD | 1.99 | 1.48 | 1.47 | 1.35 | 1.87 |
| Median | 0.0 | 0.0 | 3.0 | 3.0 | 2.0 |
| Minimum, maximum | 0, 8 | 0, 5 | 1, 7 | 0, 6 | 0, 8 |
LXB lower-sodium oxybate, SD standard deviation, SDP stable-dose period, SXB sodium oxybate
Safety
TEAEs during OLOTTP and SDP in the overall safety population and by treatment at study entry are summarized in Table 4. Overall safety has been reported in detail elsewhere [16] and is summarized in this publication. TEAEs relevant to cataplexy are the focus of these safety results.
Table 4.
TEAEs during OLOTTP and SDP, by treatment at study entry (safety population)
| Group A | Group B | Group C | Group D | Total (N = 201) | |
|---|---|---|---|---|---|
| SXB only (n = 52) | SXB + other anticataplectics (n = 23) | Other anticataplectics (n = 36) | Anticataplectic naive (n = 90) | ||
| Participants with ≥ 1 TEAE | 30 (57.7) | 20 (87.0) | 30 (83.3) | 72 (80.0) | 152 (75.6) |
| TEAEs leading to discontinuation | 2 (3.8) | 5 (21.7) | 6 (16.7) | 8 (8.9) | 21 (10.4) |
| Cataplexy | 0 | 4 (17.4) | 3 (8.3) | 0 | 7 (3.5) |
| Nausea | 0 | 0 | 1 (2.8) | 2 (2.2) | 3 (1.5) |
| Anxiety | 0 | 0 | 0 | 2 (2.2) | 2 (1.0) |
| Depressed mood | 0 | 0 | 1 (2.8) | 1 (1.1) | 2 (1.0) |
| Depression | 0 | 0 | 0 | 2 (2.2) | 2 (1.0) |
| Headache | 1 (1.9) | 0 | 0 | 1 (1.1) | 2 (1.0) |
| Irritability | 0 | 0 | 0 | 2 (2.2) | 2 (1.0) |
| Abnormal sleep-related event | 0 | 0 | 1 (2.8) | 0 | 1 (0.5) |
| Bile duct stone | 0 | 0 | 0 | 1 (1.1) | 1 (0.5) |
| Cognitive disorder | 1 (1.9) | 0 | 0 | 0 | 1 (0.5) |
| Decreased appetite | 0 | 0 | 0 | 1 (1.1) | 1 (0.5) |
| Fatigue | 0 | 0 | 1 (2.8) | 0 | 1 (0.5) |
| Pain in extremity | 0 | 0 | 0 | 1 (1.1) | 1 (0.5) |
| Sleep talking | 0 | 0 | 1 (2.8) | 0 | 1 (0.5) |
| Sleep-related eating disorder | 0 | 1 (4.3) | 0 | 0 | 1 (0.5) |
| Somnolence | 0 | 0 | 0 | 1 (1.1) | 1 (0.5) |
| Viral cardiomyopathy | 0 | 0 | 1 (2.8) | 0 | 1 (0.5) |
| Vomiting | 0 | 0 | 0 | 1 (1.1) | 1 (0.5) |
| TEAEs in ≥ 5% of total participants | |||||
| Headache | 8 (15.4) | 3 (13.0) | 7 (19.4) | 24 (26.7) | 42 (20.9) |
| Nausea | 2 (3.8) | 1 (4.3) | 7 (19.4) | 16 (17.8) | 26 (12.9) |
| Dizziness | 1 (1.9) | 1 (4.3) | 6 (16.7) | 13 (14.4) | 21 (10.4) |
| Cataplexya | 0 | 11 (47.8) | 6 (16.7) | 2 (2.2) | 19 (9.5) |
| Decreased appetite | 0 | 1 (4.3) | 2 (5.6) | 12 (13.3) | 15 (7.5) |
| Influenza | 5 (9.6) | 3 (13.0) | 3 (8.3) | 3 (3.3) | 14 (7.0) |
| Nasopharyngitis | 2 (3.8) | 1 (4.3) | 4 (11.1) | 6 (6.7) | 13 (6.5) |
| Diarrhea | 4 (7.7) | 0 | 0 | 7 (7.8) | 11 (5.5) |
| Vomiting | 1 (1.9) | 0 | 4 (11.1) | 5 (5.6) | 10 (5.0) |
Data presented as n (%)
aTEAEs of cataplexy were reported when cataplexy worsened compared with the study baseline visit
OLOTTP open-label optimized treatment and titration period, SDP stable-dose period, SXB sodium oxybate, TEAE treatment-emergent adverse event
During OLOTTP and SDP, no TEAEs of worsening cataplexy were reported in participants who transitioned from SXB only to LXB (group A). Worsening cataplexy was reported as a TEAE in 11 (47.8%) and 6 (16.7%) participants in groups B and C, in which tapering/discontinuing of other anticataplectics occurred, and in 2 (2.2%) participants in group D. Most TEAEs of worsening cataplexy were reported during tapering/discontinuation of other anticataplectics.
TEAEs leading to discontinuations during OLOTTP and SDP included worsening cataplexy and depressed mood or depression. Discontinuation due to worsening cataplexy occurred in seven participants (3.5%), all of whom were in groups B and C (i.e., participants who tapered and withdrew other anticataplectics). Discontinuation due to depressed mood or depression occurred in four participants (2.0%); one was in group C and three were in group D (i.e., participants not taking SXB at study entry). Discontinuation due to headache was reported by two participants (1.0%; one in group A and one in group D), and discontinuation due to irritability was reported by two participants (1.0%; both in group D). All other TEAEs leading to discontinuation occurred in a single participant each.
The proportion of participants who experienced any TEAE was lowest in the group taking SXB only at study entry (group A; 57.7%), followed by group D (80.0%), group C (83.3%), and group B (87.0%). Headache, nausea, and dizziness were more frequently observed in groups C and D compared with groups A and B.
Discussion
In the open-label optimized treatment and titration period of this placebo-controlled, double-blind, randomized withdrawal trial in adult participants with narcolepsy with cataplexy [16], LXB monotherapy reduced cataplexy frequency and increased cataplexy-free days, a measure of symptom control similar to ones used in other neurologic disease states including migraine (headache-free days) [17] and epilepsy (seizure-free days) [18]. The overall TEAE profile of LXB was consistent with that previously observed for SXB. These results demonstrate that cataplexy was reduced and/or controlled by the end of SDP in all participants, and the number of cataplexy-free days per week increased in parallel with decreases in cataplexy frequency.
Changes in cataplexy frequency observed during OLOTTP differed by cataplexy treatment at study entry. In group A (SXB only), weekly cataplexy attacks were low initially and declined slightly during titration of LXB treatment, whereas in group D (anticataplectic naive), cataplexy attacks were higher initially and decreased during titration of LXB treatment. In participants who entered the study taking other anticataplectics, tapering and discontinuation appeared to be associated with worsening cataplexy, observed as an increased number of cataplexy attacks during tapering/discontinuation; a similar phenomenon has been observed in other studies [4, 10]. This increase in weekly cataplexy attacks during tapering and discontinuation of other anticataplectics was more pronounced in participants in group C (other anticataplectics) compared with group B (SXB + other anticataplectics). In group C, weekly cataplexy attacks decreased during the latter part of OLOTTP (weeks 10–12), after taper and discontinuation of other anticataplectics was complete.
Participants entering the study taking SXB were able to optimize their treatment with LXB during OLOTTP. Titration to an efficacious and safe dose of LXB was more rapid, with fewer adjustments, in these participants compared with participants who were not taking SXB at study entry. Most (90.9%) had either no change in dose or reached a stable nightly dose within one dosing adjustment; the median number of dose adjustments was zero in both group A (SXB only) and group B (SXB + other anticataplectics). Some participants entered the study at the maximum dose of SXB (9 g/night) and could not further increase their dose, which limited the number of dose adjustments among this subset of participants. Additionally, participants taking SXB at study entry were required to have documented prior improvement in cataplexy and EDS with SXB treatment, indicating that they had already achieved an efficacious dose of SXB prior to study entry. Participants not previously taking SXB achieved a tolerable and efficacious dose of LXB after a median of three dose adjustments.
A majority of participants completed OLOTTP. The rate of study completion and incidence of TEAEs of cataplexy appeared to be related to treatment at study entry. Completion rates were lower in the groups required to taper and discontinue other prior anticataplectics compared with the groups that were not being treated with antidepressant/anticataplectic medications at study entry. A greater proportion of participants in the groups who tapered/discontinued prior anticataplectics reported TEAEs overall, as well as TEAEs of cataplexy, during OLOTTP and SDP. All seven of the participants who discontinued the study due to cataplexy were in group B or group C.
The overall safety profile of LXB during OLOTTP and SDP was consistent with that previously observed for SXB [11], and no new safety concerns were identified for LXB. As expected, a greater proportion of participants naive to SXB at entry reported TEAEs of headache, nausea, and dizziness following initiation of LXB treatment, compared with participants taking SXB at study entry who transitioned to LXB. A similar pattern of early TEAEs following initiation of SXB treatment, followed by decreased frequency of those TEAEs within several weeks, has been observed in other studies in adults with narcolepsy [19].
Antidepressants are often used off-label for the treatment of cataplexy [2, 5–9]. However, limited evidence is available from controlled clinical trials regarding the efficacy or safety of antidepressant use and tapering in people with narcolepsy, including the potential for rebound cataplexy [2, 4, 10]. Antidepressants are associated with impairment of sexual function [20], as well as other tolerability and safety concerns, including changes in blood pressure, weight gain, cardiac arrhythmia, and drug–drug interactions [21–23]; furthermore, an antidepressant discontinuation syndrome may occur when therapy is withdrawn, possibly resulting in nausea, light-headedness, chills, and aches, as well as paresthesia and insomnia [24]. Although some such events (e.g., nausea and dizziness) occurred in the present study, they occurred more commonly in groups C and D and, thus, appear to be more associated with oxybate initiation and titration.
The randomized withdrawal design employed in this study afforded several benefits. The study population included participants with narcolepsy with cataplexy treated with medication regimens reflective of current clinical practice, compared with a traditional study design in which tapering/discontinuation of prior medications occurs prior to study entry. With the inclusion of participants taking prior cataplexy treatment, the effects of initiation/optimization of LXB and simultaneous antidepressant tapering/discontinuation on cataplexy frequency could be assessed. Additionally, the randomized withdrawal period was sufficiently brief (2 weeks) to observe a statistically significant difference between treatment arms, while minimizing the duration of placebo exposure, as demonstrated in two prior oxybate studies [25, 26].
A potential limitation of this study is that participants entering the study taking SXB were required to have shown a positive response to SXB prior to the study, as is typical for randomized withdrawal trials. Such participants would be expected to respond similarly to LXB in terms of efficacy and tolerability, whereas the response of other participants to first oxybate exposure with LXB would be less certain. It is also possible that worsening cataplexy in participants tapering other anticataplectics during OLOTTP may have continued into SDP and DBRWP (i.e., cataplexy control may not have entirely stabilized in all participants), or that some participants may have benefitted from a combination of LXB and low doses of anticataplectics to best manage cataplexy. However, reduction in cataplexy frequency with open-label LXB treatment during OLOTTP and SDP was observed in groups B and C, even in the context of anticataplectic withdrawal. As the randomized treatment groups were stratified by treatment at study entry, any effects related to prior treatment would be expected to affect the LXB and placebo groups equally. Additional limitations of this study include study discontinuations. Almost one-quarter of participants (22.9%) discontinued before completing OLOTTP, with percentages in the baseline treatment groups ranging from 13.5% (group A) to 39.1% (group B). The most frequently reported reason for discontinuation was due to TEAEs (39.1% of discontinuations), followed by protocol deviation (17.4% of discontinuations) and withdrawal by participant (13.0% of discontinuations). As limited information is available regarding the circumstances of discontinuations reported as being due to reasons other than TEAEs, it is possible that TEAEs were contributing factors. An additional limitation is that it may have been difficult for participants to report an exact number of cataplexy attacks per day in their cataplexy diaries, particularly participants with high frequencies of cataplexy attacks. Partial cataplexy may have been counted as cataplexy attacks by some participants but not all.
Lastly, rigorous evaluation of blood pressure changes was not performed in this study, which would have provided important insights into additional effects of switching from a high sodium-containing medication (SXB) to LXB, given the significant sodium reduction in the latter. Narcolepsy is associated with increased prevalence of cardiovascular and cardiometabolic comorbidities, including hypertension, hyperlipidemia, obesity, and diabetes, and increased risk of cardiovascular events [27–32]. In addition, increased risk of cardiovascular consequences following long-term treatment with high sodium-containing medications (for a variety of conditions) has been described in the literature [33, 34]. At study entry, mean systolic blood pressure values were higher, and cardiovascular/cardiometabolic comorbidities associated with narcolepsy (most notably hypertension) were reported more frequently, in participants taking SXB (groups A and B) than in participants not taking SXB (groups C and D) [16]. It is not known whether these differences were related to SXB treatment. However, the US Food and Drug Administration (FDA) recently noted that the differences in chronic sodium intake with LXB relative to SXB “at the recommended doses will be clinically meaningful in reducing cardiovascular morbidity in a substantial proportion of patients for whom the drug is indicated” for the treatment of narcolepsy [35]. Future studies of LXB involving longitudinal assessment of blood pressure under well-controlled conditions, including ambulatory blood pressure monitoring, are needed to provide more definitive data on blood pressure and cardiovascular outcomes. Likewise, the impact of lower dietary sodium intake on overall cardiovascular risk or general health was not assessed, and aspects of the study design, including duration, sample size, and lack of controlled dietary sodium intake, preclude rigorous evaluation of these issues.
Conclusions
LXB monotherapy was effective in reducing cataplexy and increasing cataplexy-free days. The results illustrate the feasibility of switching from SXB to LXB, as well as the tapering and discontinuation of antidepressants/anticataplectics, and may guide expectations regarding possible changes in cataplexy frequency with initiation of LXB treatment, switch from SXB to LXB, dose adjustments when initiating LXB treatment, and discontinuation of antidepressant/anticataplectic medications.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to thank the study team and patients for their participation in this research. Under the direction of the authors, Karyn Liu, Ph.D., and Michael J. Theisen, Ph.D., of Peloton Advantage, LLC, an OPEN Health company, provided medical writing assistance for this publication, which was funded by Jazz Pharmaceuticals.
Declarations
Funding
This study was funded by Jazz Pharmaceuticals. Jazz Pharmaceuticals was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of interest
Yves Dauvilliers is a consultant for and has participated in advisory boards for Jazz Pharmaceuticals, UCB Pharma, Flamel Technologies, Idorsia, Takeda, Theranexus, and Bioprojet. Karel Šonka has served on the speakers’ bureau for Sanofi, Angelini, and Stada and participated in advisory boards for UCB and in clinical trials for Jazz Pharmaceuticals, Flamel-Avadel, and Luitpold Pharmaceuticals. Richard K. Bogan is a shareholder of WaterMark Medical and Healthy Humming, LLC; is on the board of directors of WaterMark Medical; serves as a consultant to Jazz Pharmaceuticals, Harmony Biosciences, Avadel Pharmaceuticals, Takeda, and Oventus; conducts industry-funded research for Avadel Pharmaceuticals, Axsome Therapeutics, Bresotec Medical, Bayer, Idorsia, Suven Life Sciences, Jazz Pharmaceuticals, Balance Pharma Inc., NLS Pharmaceutics, Vanda Pharmaceuticals Inc., Merck, Eisai Co. Ltd, Philips, Fresca Medical, Takeda, LivaNova, Roche, Sanofi, Sommetrics, and Noctrix Health; and has served on the speakers’ bureau for Jazz Pharmaceuticals, Eisai Co. Ltd., and Harmony Biosciences. Markku Partinen has participated in advisory boards of AOP Orphan, Bioprojet, UCB, and Umecrine. He has participated in clinical trials for Bioprojet, Jazz Pharmaceuticals, MSD, and Umecrine. Rafael del Rio Villegas has participated in advisory boards for Bioprojet and trials for Jazz Pharmaceuticals, Bioprojet, and Takeda. Nancy Foldvary-Schaefer served as a consultant for Jazz Pharmaceuticals and receives research support from Jazz Pharmaceuticals, Suven Life Sciences, and Takeda Pharmaceuticals. Roman Skowronski is a full-time employee of Jazz Pharmaceuticals who, in the course of this employment, has received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals, plc. Abby Chen is a full-time employee of Jazz Pharmaceuticals who, in the course of this employment, has received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals, plc. Jed Black is a part-time employee of Jazz Pharmaceuticals and shareholder of Jazz Pharmaceuticals plc. Franck Skobieranda is a full-time employee of Jazz Pharmaceuticals and shareholder of Jazz Pharmaceuticals, plc. Michael J. Thorpy has received research/grant support and consultancy fees from Jazz Pharmaceuticals, Harmony Biosciences, Balance Therapeutics, Axsome Therapeutics, and Avadel Pharmaceuticals.
Ethics approval
The study was approved by institutional review boards or ethics committees at all sites and was performed in accordance with the International Conference on Harmonisation Guideline for Good Clinical Practice and the Declaration of Helsinki.
Consent to participate
All participants provided written informed consent.
Consent to publish
Not applicable.
Availability of data and material
All relevant data are provided within the manuscript and supporting files.
Code availability
Not applicable.
Author contributions
All authors had full access to all the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: RS and YD. Acquisition, analysis, or interpretation of data: YD, KS, RKB, MP, RdRV, NF-S, RS, AC, JB, FS, MJT. Drafting of the manuscript: Under the direction of the authors, Karyn Liu, PhD, and Michael J. Theisen, PhD, of Peloton Advantage, LLC, an OPEN Health company, provided medical writing assistance for this publication. Critical revision of the manuscript for important intellectual content: YD, KS, RKB, MP, RdRV, NF-S, RS, AC, JB, FS, MJT. Statistical analysis: AC. Obtaining funding: Funded by Jazz Pharmaceuticals. Administrative, technical, or material support: Peloton Advantage, LLC, an OPEN Health company. Study supervision: RKB, RS.
Footnotes
The original online version of this article was revised due to a retrospective Open access order.
Change history
6/28/2022
A Correction to this paper has been published: 10.1007/s40263-022-00933-1
References
- 1.American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien: American Academy of Sleep Medicine; 2014.
- 2.Kornum BR, Knudsen S, Ollila HM, Pizza F, Jennum PJ, Dauvilliers Y, et al. Narcolepsy. Nat Rev Dis Prim. 2017;9(3):16100. doi: 10.1038/nrdp.2016.100. [DOI] [PubMed] [Google Scholar]
- 3.Maski K, Steinhart E, Williams D, Scammell T, Flygare J, McCleary K, et al. Listening to the patient voice in narcolepsy: diagnostic delay, disease burden, and treatment efficacy. J Clin Sleep Med. 2017;13(3):419–425. doi: 10.5664/jcsm.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dauvilliers Y, Siegel JM, Lopez R, Torontali ZA, Peever JH. Cataplexy–clinical aspects, pathophysiology and management strategy. Nat Rev Neurol. 2014;10(7):386–395. doi: 10.1038/nrneurol.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgenthaler TI, Kapur VK, Brown T, Swick TJ, Alessi C, Aurora RN, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705–1711. doi: 10.1093/sleep/30.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swick TJ. Treatment paradigms for cataplexy in narcolepsy: past, present, and future. Nat Sci Sleep. 2015;7:159–169. doi: 10.2147/NSS.S92140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wise MS, Arand DL, Auger RR, Brooks SN, Watson NF. Treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1712–1727. doi: 10.1093/sleep/30.12.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez R, Arnulf I, Drouot X, Lecendreux M, Dauvilliers Y. French consensus. Management of patients with hypersomnia: which strategy? Rev Neurol (Paris). 2017;173(1–2):8–18. doi: 10.1016/j.neurol.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Billiard M, Bassetti C, Dauvilliers Y, Dolenc-Groselj L, Lammers GJ, Mayer G, et al. EFNS guidelines on management of narcolepsy. Eur J Neurol. 2006;13(10):1035–1048. doi: 10.1111/j.1468-1331.2006.01473.x. [DOI] [PubMed] [Google Scholar]
- 10.Ristanovic RK, Liang H, Hornfeldt CS, Lai C. Exacerbation of cataplexy following gradual withdrawal of antidepressants: manifestation of probable protracted rebound cataplexy. Sleep Med. 2009;10(4):416–421. doi: 10.1016/j.sleep.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Xyrem® (sodium oxybate) oral solution, CIII [prescribing information]. Palo Alto, CA: Jazz Pharmaceuticals, Inc.; 2020.
- 12.Xyrem [summary of product characteristics]. Brussels, Belgium: UCB Pharma; 2021.
- 13.Xywav® (calcium, magnesium, potassium, and sodium oxybates) oral solution, CIII [prescribing information]. Palo Alto, CA: Jazz Pharmaceuticals, Inc.; 2021.
- 14.Chen C, Jenkins J, Zomorodi K, Skowronski R. Pharmacokinetics, bioavailability, and bioequivalence of lower-sodium oxybate in healthy participants in 2 open-label, randomized, crossover studies. Clin Transl Sci. 2021;14(6):2278–2287. doi: 10.1111/cts.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Junnarkar G, Allphin C, Profant J, Steininger TL, Chen C, Zomorodi K, et al. Development of a lower-sodium oxybate formulation for the treatment of patients with narcolepsy and idiopathic hypersomnia. Expert Opin Drug Discov. 2022;17(2):109–119. doi: 10.1080/17460441.2022.1999226. [DOI] [PubMed] [Google Scholar]
- 16.Bogan RK, Thorpy MJ, Dauvilliers Y, Partinen M, Del Rio Villegas R, Foldvary-Schaefer N, et al. Efficacy and safety of calcium, magnesium, potassium, and sodium oxybates (lower-sodium oxybate [LXB]; JZP-258) in a placebo-controlled, double-blind, randomized withdrawal study in adults with narcolepsy with cataplexy. Sleep. 2021;44(3):zsaa206. [DOI] [PMC free article] [PubMed]
- 17.Lipton RB, Lee L, Saikali NP, Bell J, Cohen JM. Effect of headache-free days on disability, productivity, quality of life, and costs among individuals with migraine. J Manag Care Spec Pharm. 2020;26(10):1344–1352. doi: 10.18553/jmcp.2020.20103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auvin S, Williams B, McMurray R, Kumar D, Perdomo C, Malhotra M. Novel seizure outcomes in patients with Lennox-Gastaut syndrome: Post hoc analysis of seizure-free days in rufinamide Study 303. Epilepsia Open. 2019;4(2):275–280. doi: 10.1002/epi4.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Husain AM, Bujanover S, Ryan R, Scheckner B, Black J, Profant J. Incidence and duration of common, early-onset adverse events occurring during 2 randomized, placebo-controlled, phase 3 studies of sodium oxybate in participants with narcolepsy. J Clin Sleep Med. 2020;16(9):1469–1474. doi: 10.5664/jcsm.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baldwin DS, Foong T. Antidepressant drugs and sexual dysfunction. Br J Psychiatry. 2013;202:396–397. doi: 10.1192/bjp.bp.112.110650. [DOI] [PubMed] [Google Scholar]
- 21.Anafranil [package insert]. Hazelwood, MO: Mallinckrodt Inc.; 2012.
- 22.Effexor XR [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc.; 2017.
- 23.Vivactil [package insert]. Horsham, PA: Teva Pharmaceuticals USA, Inc; 2014.
- 24.American Psychiatric Association. Treating major depressive disorder: a quick reference guide. 2010 October 2010. Cited 29 October 2021. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd-guide.pdf.
- 25.Plazzi G, Strunc MJ, Parvataneni R, Wang G, Black J, Bogan R. Dosing, titration, and treatment compliance to sodium oxybate therapy in pediatric patients with narcolepsy [abstract 0765] Sleep. 2019;42(suppl 1):A307–A308. doi: 10.1093/sleep/zsz067.763. [DOI] [Google Scholar]
- 26.U.S. Xyrem Multicenter Study Group. Sodium oxybate demonstrates long-term efficacy for the treatment of cataplexy in patients with narcolepsy. Sleep Med. 2004;5(2):119–23. [DOI] [PubMed]
- 27.Cohen A, Mandrekar J, St Louis EK, Silber MH, Kotagal S. Comorbidities in a community sample of narcolepsy. Sleep Med. 2018;43:14–18. doi: 10.1016/j.sleep.2017.11.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Black J, Reaven NL, Funk SE, McGaughey K, Ohayon MM, Guilleminault C, et al. Medical comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study. Sleep Med. 2017;33:13–18. doi: 10.1016/j.sleep.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Jennum P, Ibsen R, Knudsen S, Kjellberg J. Comorbidity and mortality of narcolepsy: a controlled retro- and prospective national study. Sleep. 2013;36(6):835–840. doi: 10.5665/sleep.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohayon MM. Narcolepsy is complicated by high medical and psychiatric comorbidities: a comparison with the general population. Sleep Med. 2013;14(6):488–492. doi: 10.1016/j.sleep.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Pepin JL, Borel AL, Tamisier R, Baguet JP, Levy P, Dauvilliers Y. Hypertension and sleep: overview of a tight relationship. Sleep Med Rev. 2014;18(6):509–519. doi: 10.1016/j.smrv.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Jennum PJ, Plazzi G, Silvani A, Surkin LA, Dauvilliers Y. Cardiovascular disorders in narcolepsy: review of associations and determinants. Sleep Med Rev. 2021;58:101440. doi: 10.1016/j.smrv.2021.101440. [DOI] [PubMed] [Google Scholar]
- 33.Perrin G, Korb-Savoldelli V, Karras A, Danchin N, Durieux P, Sabatier B. Cardiovascular risk associated with high sodium-containing drugs: a systematic review. PLoS ONE. 2017;12(7):e0180634. doi: 10.1371/journal.pone.0180634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei L, Mackenzie IS, MacDonald TM, George J. Cardiovascular risk associated with sodium-containing medicines. Expert Opin Drug Saf. 2014;13(11):1515–1523. doi: 10.1517/14740338.2014.970163. [DOI] [PubMed] [Google Scholar]
- 35.Clinical superiority findings. 2021. https://www.fda.gov/industry/designating-orphan-product-drugs-and-biological-products/clinical-superiority-findings. Cited 9 July 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.