Abstract
Objectives
This study aims to better characterize potential responders of Y-90-radioembolization at baseline through analysis of clinical variables and contrast enhanced (CE) MRI tumor volumetry in order to adjust therapeutic regimens early on and to improve treatment outcomes.
Methods
Fifty-eight HCC patients who underwent Y-90-radioembolization at our center between 10/2008 and 02/2017 were retrospectively included. Pre- and post-treatment target lesion volumes were measured as total tumor volume (TTV) and enhancing tumor volume (ETV). Survival analysis was performed with Cox regression models to evaluate 65% ETV reduction as surrogate endpoint for treatment efficacy. Univariable and multivariable logistic regression analyses were used to evaluate the combination of baseline clinical variables and tumor volumetry as predictors of ≥ 65% ETV reduction.
Results
Mean patients’ age was 66 (SD 8.7) years, and 12 were female (21%). Sixty-seven percent of patients suffered from liver cirrhosis. Median survival was 11 months. A threshold of ≥ 65% in ETV reduction allowed for a significant (p = 0.04) separation of the survival curves with a median survival of 11 months in non-responders and 17 months in responders. Administered activity per tumor volume did predict neither survival nor ETV reduction. A baseline ETV/TTV ratio greater than 50% was the most important predictor of arterial devascularization (odds ratio 6.3) in a statistically significant (p = 0.001) multivariable logistic regression model. The effect size was strong with a Cohen’s f of 0.89.
Conclusion
We present a novel approach to identify promising candidates for Y-90 radioembolization at pre-treatment baseline MRI using tumor volumetry and clinical baseline variables.
Key Points
• A decrease of 65% enhancing tumor volume (ETV) on follow-up imaging 2–3 months after Y-90 radioembolization of HCC enables the early prediction of significantly improved median overall survival (11 months vs. 17 months, p = 0.04).
• Said decrease in vascularization is predictable at baseline: an ETV greater than 50% is the most important variable in a multivariable logistic regression model that predicts responders at a high level of significance (p = 0.001) with an area under the curve of 87%.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00330-022-08585-x.
Keywords: Carcinoma, hepatocellular; Treatment outcome; Survival analysis; Tumor burden; Yttrium radioisotopes
Introduction
Yttrium-90 (Y-90) radioembolization is a minimally invasive intra-arterial therapy for unresectable liver tumors such as advanced hepatocellular carcinoma (HCC) or liver metastases [1]. Selected patients with intermediate to advanced stage, unresectable HCC benefit substantially more from Y-90 radioembolization than others which demands further investigation to improve patient selection [2].
HCC is the third most common cause of tumor-related death worldwide [3]. Despite the widespread use of screening programs, 60–70% of HCCs are detected at intermediate and advanced stages when curative treatment approaches such as surgical therapy or ablation are precluded [4, 5]. Y-90 radioembolization is performed interchangeably with TACE for intermediate stage HCC at some centers or as a second-line therapy following TACE failure for intermediate and advanced stage HCC [1, 6, 7]. During radioembolization, radionuclides such as Yttrium-90 or Holmium-166 embedded in microspheres are applied via branches of the hepatic artery. Given the predominantly arterial blood supply of HCC nodules, the microspheres accumulate in the tumor micro-vasculature and emit high-energy, low-penetration beta radiation to the tumor [8]. The technique proved non-inferior to TACE in a series of phase 2 trials [6, 9–12]. Both the SIRveNIB and the SARAH trials, the first randomized controlled phase 3 trials confirming the safety and efficacy of Y-90 radioembolization in patients with locally advanced HCC, showed Y-90 radioembolization to be better tolerated than chemotherapy with sorafenib but failed to prove a superiority of Y-90 radioembolization in terms of overall and progression-free survival [13, 14]. Recent evidence underlines that treatment response is strongly dependent on the actual, deployed radiation dose within the tumor which, in turn, is highly correlated with the arterial tumor vascularization before treatment [15–18].
This study aims to better characterize potential responders of Y-90 radioembolization through analysis of contrast enhanced (CE) MRI tumor volumetry. Regression analysis is used to control for confounding clinical variables and for building an exemplary multivariable prediction model of MR morphologic treatment response at baseline.
Materials and methods
Study cohort
This retrospective single-institution study was approved by the institutional ethics committee. Written informed consent was waived due to retrospective character of the study. All therapies were endorsed by an interdisciplinary tumor board in accordance with current recommendations [19, 20]. All patients who underwent their first Y-90 radioembolization session between 10/2008 and 02/2017 were evaluated for inclusion. Exclusion criteria were missing baseline MRI in a 60-day timeframe before radioembolization or missing follow-up MRI between day 60 and 90 afterwards, disseminated disease that impeded segmentation, and poor imaging quality. The patient selection process is presented as a flowchart in Fig. 1.
Fig. 1.
Flowchart of the patient selection process
Y-90 radioembolization protocol
Board-certified radiologists (B.G., G.W., F.S., D.S., all with > 10 years of experience, and D.G., with 1 year of experience after certification) performed the interventions according to a standard protocol (Supplement 1). At our institution, Y-90 radioembolization is performed with Yttrium 90 (Y-90) resin microspheres (SIR-Spheres®, Sirtex Medical Pty. Ltd.). The prescribed activity of Y-90 resin microsphere was determined according to body surface area (BSA) method. There was no dose reduction performed for patients with signs of liver cirrhosis.
Imaging technique
Every patient received cross-sectional imaging before and after Y-90 radioembolization. According to the standard protocols at our institution, CE MRI was performed with a 1.5-T (Siemens Avanto or Aera) imaging unit. A phased-array torso coil and 0.1 mL per kilogram body weight of intravenous dinatrium gadoxetat (Eovist/Primovist, Bayer Healthcare) were used. The MRI protocol included breath-hold unenhanced and CE T1-weighted 3D fat-suppressed spoiled gradient-echo imaging (section thickness, 2.5 mm; receiver bandwidth, 64 kHz; flip angle, 10°) in the arterial phase (delay of 15 s after bolus tracking), the portal venous phase (delay of 70 s), the delayed phase (delay 3 min), and the hepatobiliary excretion phase (delay of 20 min after administration). Arterial phase was used for volumetric evaluation.
Quantitative measurement of arterial tumor vascularization on CE MRI
For all measurements, a semi-automated quantification software (Philips IntelliSpace Portal) was used (Fig. 2). Accuracy and reproducibility were previously demonstrated [7, 8, 13, 17–19]. Two readers with 6 and 7 years of experience supervised the measurements. A signal intensity greater two standard deviations than the average signal intensity measured within a region of interest (ROI) of 10 × 10 × 10 mm in the musculus erector spinae was defined an arterial vascularization. As most patients had multifocal disease with more than 3 lesions in 97% of the cases, a maximum of three dominant lesions were analyzed and combined as surrogate total tumor volume (TTV) and enhancing tumor volume (ETV). In each case, the same lesions were measured before and after treatment. The measurements are given in milliliters. Relative vascularization was calculated as the ratio of ETV and TTV and expressed as percentage.
Fig. 2.
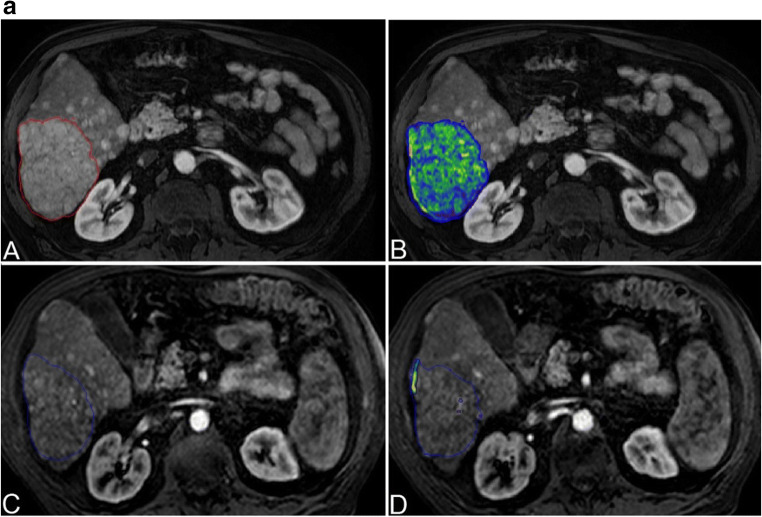
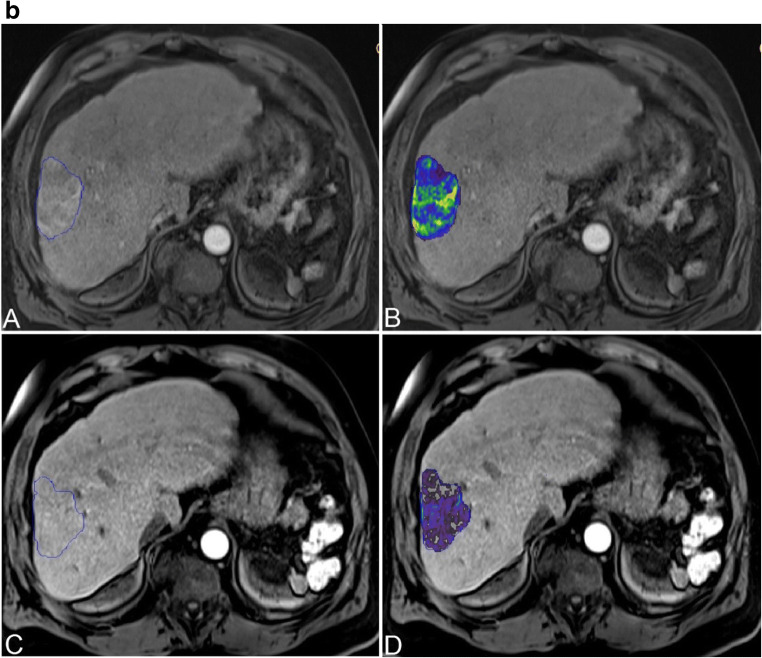
Quantification of total tumor volume and enhancing tumor volume on baseline and follow-up imaging. a Patient with highly vascularized HCC. Images only show representative tumor measurements in one layer. Target lesions were volumetrically assessed. A Preinterventional MRI: tumor tissue appears highly vascularized compared to surrounding liver parenchyma. B Colored enhancing tumor volume/total tumor volume (ETV/TTV) overlay. C Postinterventional MRI: the tumor appears to be de-vascularized after treatment. D ETV/TTV overlay. b Patient with intermediately vascularized HCC. A Preinterventional MRI: Intermediate vascularization of tumor; relative arterial vascularization is color-coded in (B). C Postinterventional MRI: a tumor with initially intermediate vascularization loses hyper-vascularization compared to surrounding liver tissue. D ETV/TTV overlay
Variables for the prediction of survival and MR morphologic response
To assess MR morphologic treatment response, we employed the quantitative European Association for the Study of the Liver (qEASL) criteria of ≥ 65% decrease in enhancing tumor volumes on follow-up MRI [21]. Alongside qEASL response, we evaluated measurements and derived indices of TTV and ETV at baseline as well as routinely available clinical variables as potential predictors of patient survival. Clinical variables were demographic data of gender and age, underlying liver disease, previous systemic or local treatments, and the radioembolization approach with respect to whole liver vs. sequential therapy or dose reduction. Baseline imaging and clinical variables were also assessed as predictors of qEASL response.
Statistical analysis
All statistical analyses were conducted with IBM SPSS STATISTICS, version 25 (IBM Corporation). Testing for normality was performed with the Shapiro–Wilk test, and skewed data were transformed with the natural logarithm for further analysis. Normally distributed, continuous data are presented with mean and standard deviation and compared with the two-sample t test. Non-normally distributed data are expressed as median and interquartile range (IQR) and compared with the Mann–Whitney U test or the Wilcoxon signed-rank test. Agreement of two was measured with the Pearson correlation coefficient (PCC) statistic. Receiver operating characteristic (ROC) curves and Youden’s J statistic were used to evaluate thresholds for binary classifiers. Survival is reported as median with a 95% confidence interval and was analyzed with the Cox proportional hazard model, Kaplan-Meier curves, and the log-rank test. Median overall survival (mOS) was defined from the date of the first Y-90 radioembolization session until death. Patients who were either lost to follow-up or alive at the end-of-observation date were censored. To identify predictors for MRI morphologic treatment response at baseline, the effects of the abovementioned variables were investigated with logistic regression analysis. Model building for logistic regression analysis was informed by the resulting impact on Nagelkerke’s pseudo-R2. Multivariable Cox regression models were compared with the log-likelihood ratio statistic. Regression coefficients, hazard ratios (HR), and odds ratios (OR) as well as p values are presented. As this is an explorative study, no adjustment for multiple testing was applied. Thus, p values < 0.05 are reported as significant, but are only given as an orientation and to be interpreted cautiously and not as confirmatory.
Results
Patient characteristics
Fifty-eight patients were included for tumor volumetry and further analysis in predictive modeling. Patient inclusion process is shown in Fig. 1. Patient characteristics are summarized in Table 1 and contrasted with the 100 excluded patients in Supplement 2. About 65% of the patients had Barcelona clinic liver cancer (BCLC) stage B; liver cirrhosis was present in 67% and portal vein thrombosis in 26% of the included patients. All patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1.
Table 1.
Patient characteristics at baseline
| Patient characteristics | ||
|---|---|---|
| Demographics | ||
| Number of patients | 58 | |
| Age (years), mean (SD) | 66 (8.7) | |
| Sex: male/female (% male) | 46/12 (79%) | |
| Median survival in months (95% CI)* | 11 [8–14] | |
| Liver disease | ||
| BCLC stage | B | 38 (66%) |
| C | 20 (34%) | |
| Child Pugh Score | A | 56 (97%) |
| B | 2 (3%) | |
| C | 0 | |
| Cirrhosis | 39/58 (67%) | |
| Hepatitis (B, C) | 21/58 [6, 15] (36%) | |
| Portal vein thrombosis | Proximal occlusion | 7 (12%) |
| Proximal partial occlusion | 1 (2%) | |
| Left | 3 (5%) | |
| Right | 4 (7%) | |
| Previous treatments (possible multiple therapies) | ||
| Resection | 10 (17%) | |
| TACE | 16 (28%) | |
| Brachytherapy | 15 (26%) | |
| RFA | 4 (7%) | |
| PEI | 1 (2%) | |
| Sorafenib | 16 (28%) | |
| Other systemic therapies | 2 (3%) | |
| Specifics of Y-90 radioembolization | ||
| Sequential/whole liver therapy | 35/23 | |
| Hepatopulmonary shunt %, mean (SD) | 8.6% (4.7%) | |
| Median tumor volumes in mL (IQR) | 209 (326) | |
| Median activity in mBq (IQR) | 1.7 (0.53) | |
*Kaplan–Meier estimator
BCLC, Barcelona Clinic liver cancer stage (BCLC); CI, confidence interval; ECOG, Eastern Cooperative Oncology Group performance (ECOG); IQR, interquartile range; PEI, percutaneous ethanol injection; RFA, radiofrequency ablation; SD, standard deviation; TACE, transarterial chemoembolization
MRI tumor segmentation at baseline and 3 months after treatment
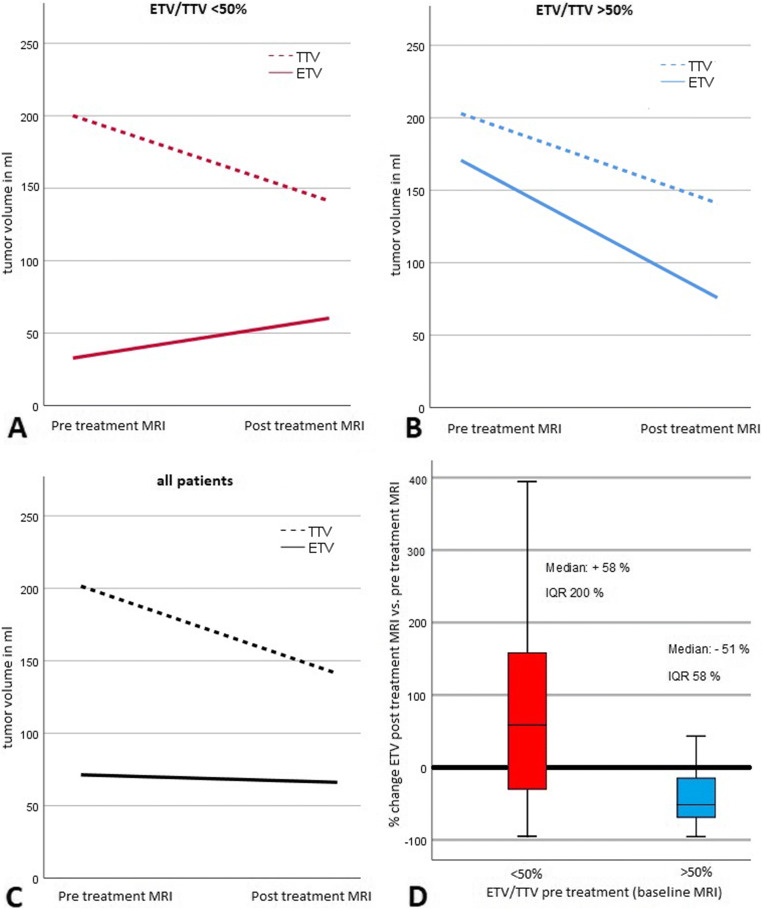
TTV and ETV on CE MRI both at baseline and 3 months after treatment were approximated by summarizing the volumes of a maximum of three dominant lesions in 58 patients (Table 2). The inter-reader agreeability of our unidirectional and volumetric MRI measurements as measured with the Pearson correlation coefficient (PCC) statistic varied between 0.93 and 0.99 (Supplement 3) which is in line with results from previous studies [22–24]. The measurements for target lesion TTV at baseline correlated significantly with total tumor volume assessment performed routinely before radioembolization with a PCC of 0.88 (p < 0.001). After transformation with the natural logarithm, baseline TTV and ETV followed a log-normal distribution. The non-logarithmic ETV/TTV ratio showed a pronounced bimodal distribution with frequency peaks between 10 and 30% and above 80% relative arterial tumor vascularization (Fig. 3). ROC analysis and Youden’s J statistic revealed an ETV/TTV threshold of 50% to be most suitable to stratify patients in two significantly different (p < 0.001) groups in terms of ETV change as assessed with the Mann–Whitney U test. Patients with a baseline ETV/TTV ratio greater than 50% (25/58 patients) showed a median reduction in ETV by 51% and less variability with an IQR of 58%. Patients with a ratio less than 50% (33/58 patients) had a median increase in ETV of 58% with an IQR of 200% (Fig. 4). Apart from a higher proportion of BCLC C patients in the group with a baseline ETV/TTV ratio < 50% (45% of the patients vs. 20% of the patients in the ETV/TTV ≥ 50% group), the two groups did not show significant differences with respect to baseline clinical variables (Supplement 4).
Table 2.
Quantitative target lesion analysis on contrast-enhanced MRI before and after Y-90 radioembolization
| Quantitative target lesion analysis (median, IQR) | |||
|---|---|---|---|
| Baseline | Follow-up | Significance | |
| TTV in mL | 201 (370) | 141 (272) | p < 0.001 |
| ETV in mL | 71 (160) | 66 (153) | p = 0.34 |
| ETV/TTV | 46 (68) | 55 (59) | p = 0.34 |
| ETV reduction in % | −15 (129) | ||
ETV, enhancing tumor volume; IQR, interquartile range; ml, milliliter; TTV, total tumor volume
Fig. 3.
Frequency distribution of relative vascularization at baseline. The enhancing tumor volume/total tumor volume (ETV/TTV) ratio showed a pronounced bimodal distribution with frequency peaks between 10 and 30% and above 80% relative arterial tumor vascularization
Fig. 4.
Impact of relative vascularization at baseline on later decrease in enhancing tumor volumes. Line charts in A to C depict the development of enhancing tumor volume (ETV) and total tumor volume (TTV) between baseline and follow-up MRI for patients with low tumor vascularization (ETV/TTV < 50% in baseline MRI), high tumor vascularization (ETV/TTV > 50% in baseline MRI), and the whole cohort. The change of median ETV over time in both subgroups is compared with boxplots in D
Prediction of survival based on qEASL response, baseline imaging, and clinical variables
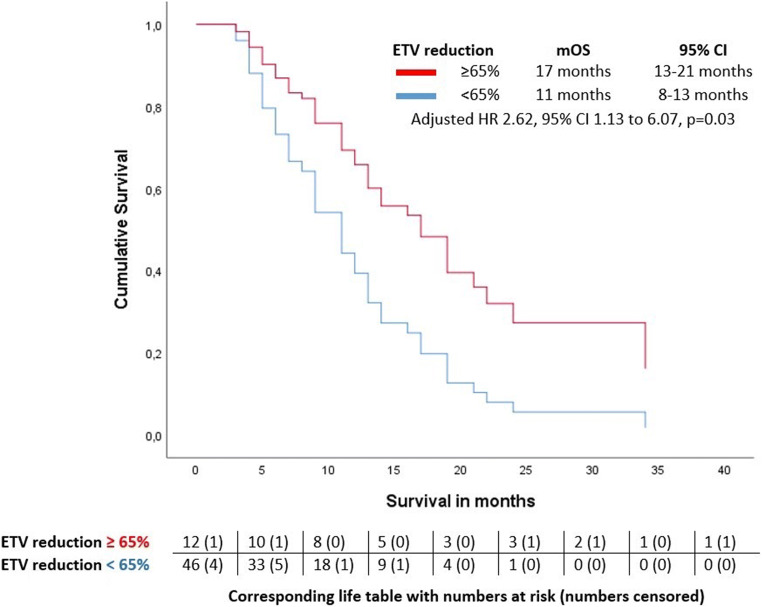
The population included for tumor volumetry had a mOS of 11 months (95% CI 8–14 months). In univariable Cox regression, age above 70 years and the presence of liver cirrhosis significantly decreased patient survival; prior ablation, cTACE, or systemic therapy were no significant predictors, nor was response according to qEASL criteria on follow-up MRI with a p value of 0.06 (Table 3). In multivariable Cox regression, survival was best explained by a model that included qEASL response, the status on cirrhosis, and patient age above or below 70 years as predictors (chi-squared test, p = 0.001), whereas qEASL response was the single strongest predictor with a HR of 2.6 (p = 0.03) (Table 3). Survival curves for the stratification into MRI morphologic responders and non-responders are shown in Fig. 5. Median survival in non-responders was 11 months (95% CI 8–14 months) and 17 months in responders (95% CI 13–21 months).
Table 3.
Univariable and multivariable Cox regression analyses
| Variables | Univariable model | Multivariable model | ||
|---|---|---|---|---|
| Demographics | HR | Sign. | HR | Sign. |
| Sex (male) | 1.16 | 0.71 | 0.71 | |
| Age (years) % > 70 | 0.53 | 0.047 | 0.6 | 0.05 |
| Liver disease | ||||
| BCLC tumor stage (B or C) | 1.13 | 0.71 | 0.71 | |
| Hepatitis (yes/no) | 0.67 | 0.21 | 0.21 | |
| Cirrhosis (yes/no) | 0.39 | 0.01 | 0.4 | 0.01 |
| Previous treatments | ||||
| TKI (yes/no) | 1.34 | 0.40 | 0.40 | |
| Resection (yes/no) | 1.34 | 0.44 | 0.44 | |
| TACE (yes/no) | 0.64 | 0.2 | 0.20 | |
| Ablation (yes/no) | 0.09 | 0.1 | 0.09 | |
| Radioembolization approach | ||||
| Sequential lobar therapy (yes/no) | 1.63 | 0.13 | 0.13 | |
| Dose reduction (yes/no) | 1.32 | 0.46 | 0.46 | |
| Applied dose/tumor volume (mBc/mL) | 0.01 | 0.52 | 0.52 | |
| Tumor characterization | ||||
| Ln TTV (in mL) | 0.98 | 0.9 | 0.90 | |
| Ln ETV (in mL) | 0.99 | 0.94 | 0.94 | |
| ETV/TTV | 0.96 | 0.94 | 0.94 | |
| ETV/TTV > 50% | 0.93 | 0.81 | 0.81 | |
| Hepatopulmonary shunt in % | 0.97 | 0.36 | 0.36 | |
| MRI response assessment | ||||
| Reduction in ETV by 65% | 2.23 | 0.06 | 2.6 | 0.03 |
| Performance of the multivariable model | ||||
| Significance | p = 0.001 | |||
| −2 log-likelihood (range, competing models) | 249.5 (248-250) | |||
BCLC, Barcelona Clinic Liver Cancer stage; ETV, enhancing tumor volume; HR, hazard ratio; Ln, natural logarithm; ml, milliliter; Sign., significance; TACE, transarterial chemoembolization; TKI, tyrosine kinase inhibitor; TTV, total tumor volume
Fig. 5.
Comparison of survival times of MRI morphologic therapy responders and non-responders with Cox regression. Survival curves for the stratification into responders and non-responders who showed a reduction of enhancing tumor volume (ETV) greater or smaller than 65% in follow-up MRI. Median survival in non-responders was 11 months (95% confidence interval (CI) 8–14 months) and 17 months in responders (95% CI 13–21 months); the adjusted hazard ratio (HR) was 2.62. Numbers at risk and numbers censored are given at the beginning of the interval
Prediction of qEASL response at baseline
Univariable and multivariable logistic regression analyses were performed to determine the impact of the abovementioned baseline variables on the likelihood of MRI morphologic qEASL response 3 months after Y-90 radioembolization. Variables with a p value smaller than 0.25 in univariable regression were further evaluated in multivariable regression analysis which did not apply to severity of liver disease, demographic data, or previous treatments (Table 4). In multivariable logistic regression analysis, Ln TTV, hepatopulmonary shunting, and an ETV/TTV ratio greater than 50% were significant predictors of MRI morphologic treatment response with p < 0.05. Elimination of individual predictors from the multivariable model did not improve model fit with respect to the information criteria and Nagelkerke’s pseudo-R2.
Table 4.
Univariable and multivariable logistic regression analysis with respect to MRI morphologic tumor response at follow-up
| Variables | Univariable model | Multivariable model | ||
|---|---|---|---|---|
| Demographics | OR | Sign. | OR | Sign. |
| Sex (male) | 0.70 | 0.59 | ||
| Age (years) > 70 | 0.91 | 0.85 | ||
| Liver disease | ||||
| BCLC tumor stage (C vs. B) | 0.31 | 0.16 | ||
| Hepatitis (yes/no) | 1.93 | 0.37 | ||
| Cirrhosis (yes/no) | 1.03 | 0.96 | ||
| Previous treatments | ||||
| TKI (yes/no) | 0.44 | 0.23 | ||
| Resection (yes/no) | 0.56 | 0.41 | ||
| TACE (yes/ no) | 2.19 | 0.35 | ||
| Ablation (yes/no) | 4.30 | 0.18 | ||
| Radioembolization approach | ||||
| Sequential lobar therapy (yes/no) | 2.31 | 0.25 | 5.8 | 0.07 |
| Dose reduction (yes/no) | 0.73 | 0.68 | ||
| Tumor characterization | ||||
| Ln TTV (in mL) | 0.52 | 0.03 | 0.4 | 0.01 |
| Ln ETV (in mL) | 0.73 | 0.23 | ||
| ETV/TTV | 4.39 | 0.15 | ||
| ETV/TTV > 50% | 3.41 | 0.07 | 6.3 | 0.02 |
| Hepatopulmonary shunt in % | 1.08 | 0.23 | 1.3 | 0.01 |
| Performance of the multivariable model | ||||
| Significance | p = 0.001 | |||
| Nagelkerke’s pseudo-R2 (range, competing models) | 0.44 (0.30-0.44) | |||
BCLC, Barcelona Clinic Liver Cancer stage; ETV, enhancing tumor volume; HR, hazard ratio; Ln, natural logarithm; ml, milliliter; Sign., significance; TACE, transarterial chemoembolization; TKI, tyrosine kinase inhibitor; TTV, total tumor volume
The multivariable logistic regression model was statistically significant at χ2 [4] = 19.178 and p = 0.001. The model accounted for 44% (Nagelkerke’s pseudo-R2) of the variance in MRI morphologic treatment response which corresponded to a Cohen’s f of 0.89 and indicated a strong effect size (Table 4). The performance of the multivariable logistic regression model as binary classifier was verified with ROC analysis which revealed an area under the curve (AUC) of 87% (Fig. 6). An ETV/TTV ratio greater than 50% was the strongest predictor of qEASL response with an OR of 6.3.
Fig. 6.
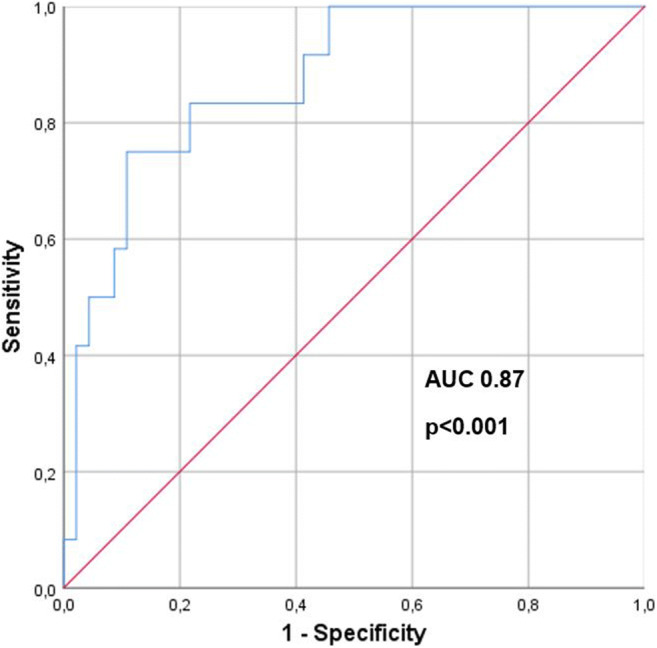
Analysis of the multivariable logistic regression model as binary classifier of MRI morphologic treatment response with ROC analysis and AUC. The performance of the multivariable logistic regression model as binary classifier was evaluated with receiver operating characteristic (ROC) analysis which revealed an area under the curve (AUC) of 87%. Significance for AUC differing from 0.50 was tested with the Mann–Whitney U test
Discussion
This retrospective, single-center study demonstrates that a decrease of 65% ETV on follow-up imaging, response according to qEASL criteria, after Y-90 radioembolization enables the early prediction of significantly and relevantly improved patient survival. An ETV/TTV ratio greater 50% before treatment is the most important variable in a multivariable logistic regression model that predicts qEASL response at baseline at a high level of significance (p = 0.001) with an AUC of 87%. Administered activity in total and per tumor volume using BSA method did neither predict patient survival nor devascularization on follow-up imaging. The baseline ETV/TTV ratio of locally advanced, unresectable HCC follows a bimodal distribution with frequency peaks of either low (10–30%) or high (> 80%) relative arterial tumor vascularization.
The median survival of our study cohort was 11 months which is in accordance with data from the protocol population of the SARAH trial (mOS of 9.9 months) [13]. As opposed to the Y-90 radioembolization protocol of the SARAH trial which stipulated sequential liver treatment, 23 out of 58 patients in our study underwent a whole liver treatment approach in one session. Most of these patients were treated before 2012 when sequential therapy was not yet universally adopted in clinical practice. In our study cohort, the MRI morphologic, volumetric partial response threshold of 65% allowed for the separation of the survival curves into one of subsequently labeled non-responders with a median survival of 11 months and one of responders with a median survival of 17 months (p = 0.04). The so-called qEASL criteria were already proven prognostically useful in HCC collectives that underwent cTACE. Data by Lewandowski et al which demonstrated partial or complete response to be the common variable in patients with an OS longer than 3 years after Y-90 radioembolization further corroborates the rationale for this threshold [21, 25, 26]. Interestingly, these results resemble the difference in mOS of 6.1 and 14.1 months that Hermann et al from the SARAH trial group demonstrated after stratification according to absorbed tumor dose lesser or greater than 100 Gy in Tc-99m-macroaggregated albumin SPECT/CT-based dosimetry [27]. The correlation of baseline arterial tumor vascularization, tumor–to–normal liver ratio on Y-90 SPECT, and tumor devascularization on follow-up imaging was demonstrated in a recent publication; an in-depth analysis of this matter was outside the scope of this study [16].
Given the significant impact of tumor devascularization on patient survival, we believed this to be a more robust surrogate measure for the efficacy and benefit of Y-90 radioembolization than mOS itself in a patient collective as complex and frail as candidates for radioembolization. Therefore, we investigated baseline imaging and clinical variables as potential predictors of devascularization. The bimodal distribution of baseline ETV/TTV ratios observed in locally advanced, unresectable HCC is probably reflective of tumor biology. The tendency to either preserve high vascularity without development of central necrosis or rapidly turn relatively hypovascular is consistent with observations made in HCC TACE collectives that were assessed with the same quantitative imaging approach [25]. Ln TTV and Ln ETV showed an inverse correlation with treatment response, probably because larger tumors have a greater tendency to turn hypoxic and develop necrosis. The much more heterogeneous effect of Y-90 radioembolization on ETV post-treatment below a threshold of 50% ETV/TTV indicates that probably a minimum relative arterial vascularization or rather tumor-to-liver ratio of dose distribution is a prerequisite for Y-90 radioembolization to consistently cause contiguous tissue necrosis. This might at least be true if the radiation dose is calculated in a traditional fashion based on BSA, liver volume, and tumor volume. A more sophisticated approach of using pre-therapeutic Tc-99m-MAA SPECT to estimate later intratumoral Y-90 distribution for individualized dose escalation showed promising preliminary results and might help to compensate for rather scarce tumor vascularity [1, 17, 18]. Hepatopulmonary shunting is known to correlate with tumor vascularization which might explain its positive correlation with treatment response [28, 29]. Nevertheless, this finding is surprising as other studies demonstrated increased hepatopulmonary shunting to be associated with poorer outcomes [30–32]. A possible explanation may be that in our collective, a positive correlation of shunting and later tumor-to-liver ratio of dose distribution outweighs the impact of dose reduction above a threshold of 10% on intratumoral dose density and subsequent devascularization on follow-up imaging. Lobar treatment also correlated positively with treatment response, probably due to a better toxicity profile [33].
An exemplary multivariable logistic regression model that incorporated baseline MRI tumor segmentation data and controlled for clinical variables had a remarkably strong effect size with a Cohen f of 0.89 and predicted MRI morphologic treatment response very well. Such a prediction model is how we expect baseline imaging and clinical information to be extremely helpful in improving patient selection and treatment outcomes. Although we systematically controlled for confounding and overfitting, the model at hand cannot be used for generalization. It merely helps to illustrate the impact baseline imaging can have in a potential prediction model of response. Before we could run any model, the crucial step of cross-validation aside, one would first have to decide whether potential responders or non-responders of radioembolization were to be predicted which, not least, is an ethical matter. Second, one would have to agree on an acceptable sensitivity and specificity of threshold values for the classification as either responders or non-responders. All these steps of model specification we purposely left out because they are futile as long as a given model cannot be cross-validated. In clinical routine and with a properly cross-validated model, the values of predictor variables for an individual patient could be entered into the equation of the model. With respect to pre-defined thresholds, certain scores would inform the decision to treat patients with radioembolization, to consider them for individualized dose escalation protocols, or to evaluate them for an entirely different treatment strategy.
There are a number of considerable limitations to the generalizability of our results. First, the patient cohort was too small to perform model building and cross-validation at the same time. Although we limited the number of predictors to a maximum of four with a study population of 58 patients, we expect substantial overfitting of our model. Second, the original patient population was very inhomogeneous especially in terms of whole liver versus sequential therapy, preinterventional imaging modalities, and timing of follow-up imaging. This accounts for the relatively small proportion of patients that could be included for further evaluation. Third, there were no SPECT Y90 tumor absorbed-dose metrics performed in our cohorts which would have been very insightful to correlate with relative vascularization at baseline and could have further strengthened our hypotheses.
In summary, we present a novel approach to identify promising candidates for Y-90 radioembolization by using tumor volumetry, clinical variables, and regression modeling techniques for the prediction of tumor devascularization and associated survival benefits. This promising concept must be cross-validated in future studies in order to conceive generalizable prediction models for clinical routine. The predictive potential of tumor vascularity for later tumor-to-liver ratio of Y-90 deposition merits further investigation and should be evaluated for other potential indications of Y-90 radioembolization.
Supplementary Information
(DOCX 47 kb)
Abbreviations
- BSA
Body surface area
- CE
Contrast enhanced
- ETV
Enhancing tumor volume
- HCC
Hepatocellular carcinoma
- HR
Hazard ratio
- IQR
Interquartile range
- Ln
Natural logarithm
- mOS
Median overall survival
- OR
Odds ratio
- OS
Overall survival
- PCC
Pearson correlation coefficient
- qEASL
Quantitative European Association for the Study of the Liver (criteria)
- ROC
Receiver operating characteristic
- TACE
Transcatheter arterial chemoembolization
- TTV
Total tumor volume
- Y90
Yttrium-90(-radioembolization)
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Guarantor
The scientific guarantor of this publication is PD Dr. Gero Wieners.
Conflict of interest
Bernd Hamm declares the attached relationships of his institution (Charité) with the listed companies.
Statistics and biometry
Juliane Hardt kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was waived by the Institutional Review Board because of the retrospective character of the study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Willie Lüdemann and Johannes Kahn contributed equally and share first authorship.
Contributor Information
Willie Lüdemann, Email: Willie-magnus.luedemann@charite.de.
Johannes Kahn, Email: johannes.kahn@charite.de.
References
- 1.Mazzaferro V, Sposito C, Bhoori S, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57:1826–1837. doi: 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- 2.Teyateeti A, Mahvash A, Long JP, et al. Outcomes for Yttrium-90 transarterial radioembolization with and without sorafenib for unresectable hepatocellular carcinoma patients. J Hepatocell Carcinoma. 2020;7:117–131. doi: 10.2147/JHC.S248314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Thomas MB, Zhu AX. Hepatocellular carcinoma: the need for progress. J Clin Oncol. 2005;23:2892–2899. doi: 10.1200/JCO.2005.03.196. [DOI] [PubMed] [Google Scholar]
- 5.Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155–2166. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sangro B, Carpanese L, Cianni R, et al. European Network on Radioembolization with Yttrium-90 Resin M. Survival after Yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868–878. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 7.Salem R, Lewandowski RJ, Kulik L et al (2011) Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 140:497, e492–507 [DOI] [PMC free article] [PubMed]
- 8.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Kallini JR, Gabr A, Salem R, Lewandowski RJ. Transarterial radioembolization with Yttrium-90 for the treatment of hepatocellular carcinoma. Adv Ther. 2016;33:699–714. doi: 10.1007/s12325-016-0324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 11.Lobo L, Yakoub D, Picado O, et al. Unresectable hepatocellular carcinoma: radioembolization versus chemoembolization: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2016;39:1580–1588. doi: 10.1007/s00270-016-1426-y. [DOI] [PubMed] [Google Scholar]
- 12.Park JW, Koh YH, Kim HB, et al. Phase II study of concurrent transarterial chemoembolization and sorafenib in patients with unresectable hepatocellular carcinoma. J Hepatol. 2012;56:1336–1342. doi: 10.1016/j.jhep.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Chow PKH, Gandhi M, Tan SB, et al. Asia-Pacific hepatocellular carcinoma trials G. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol. 2018;36:1913–1921. doi: 10.1200/JCO.2017.76.0892. [DOI] [PubMed] [Google Scholar]
- 14.Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with Yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624–1636. doi: 10.1016/S1470-2045(17)30683-6. [DOI] [PubMed] [Google Scholar]
- 15.Badiyan S, Bhooshan N, Chuong MD, et al. Correlation of radiation dose and activity with clinical outcomes in metastatic colorectal cancer after selective internal radiation therapy using Yttrium-90 resin microspheres. Nucl Med Commun. 2018;39:915–920. doi: 10.1097/MNM.0000000000000887. [DOI] [PubMed] [Google Scholar]
- 16.Schobert I, Chapiro J, Nezami N, et al. Quantitative imaging biomarkers for (90)Y distribution on bremsstrahlung SPECT after resin-based radioembolization. J Nucl Med. 2019;60:1066–1072. doi: 10.2967/jnumed.118.219691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kao YH, Steinberg JD, Tay YS, et al. Post-radioembolization Yttrium-90 PET/CT - part 2: dose-response and tumor predictive dosimetry for resin microspheres. EJNMMI Res. 2013;3:57. doi: 10.1186/2191-219X-3-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garin E, Lenoir L, Edeline J, et al. Boosted selective internal radiation therapy with 90Y-loaded glass microspheres (B-SIRT) for hepatocellular carcinoma patients: a new personalized promising concept. Eur J Nucl Med Mol Imaging. 2013;40:1057–1068. doi: 10.1007/s00259-013-2395-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 21.Tacher V, Lin M, Duran R, et al. Comparison of existing response criteria in patients with hepatocellular carcinoma treated with transarterial chemoembolization using a 3D quantitative approach. Radiology. 2016;278:275–284. doi: 10.1148/radiol.2015142951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chapiro J, Duran R, Lin M, et al. Transarterial chemoembolization in soft-tissue sarcoma metastases to the liver - the use of imaging biomarkers as predictors of patient survival. Eur J Radiol. 2015;84:424–430. doi: 10.1016/j.ejrad.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chapiro J, Duran R, Lin M, et al. Early survival prediction after intra-arterial therapies: a 3D quantitative MRI assessment of tumour response after TACE or radioembolization of colorectal cancer metastases to the liver. Eur Radiol. 2015;25:1993–2003. doi: 10.1007/s00330-015-3595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahu S, Schernthaner R, Ardon R, et al. Imaging biomarkers of tumor response in neuroendocrine liver metastases treated with transarterial chemoembolization: can enhancing tumor burden of the whole liver help predict patient survival? Radiology. 2017;283:883–894. doi: 10.1148/radiol.2016160838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapiro J, Duran R, Lin M, et al. Identifying staging markers for hepatocellular carcinoma before transarterial chemoembolization: comparison of three-dimensional quantitative versus non-three-dimensional imaging markers. Radiology. 2015;275:438–447. doi: 10.1148/radiol.14141180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon AC, Gabr A, Riaz A et al (2018) Radioembolization super survivors: extended survival in non-operative hepatocellular carcinoma. Cardiovasc Intervent Radiol 41:1557–1565 [DOI] [PMC free article] [PubMed]
- 27.S.T.Group (2018) Role of 99mTc-macroaggregated albumin SPECT/CT based dosimetry in predicting survival and tumor response of patients with locally advanced and inoperable hepatocellular carcinoma (HCC) treated by selective intra-arterial radiation therapy (SIRT) with Yttrium-90 resin microspheres, a cohort from SARAH study. The International Liver Congress 2018
- 28.Powerski MJ, Erxleben C, Scheurig-Munkler C, et al. Hepatopulmonary shunting in patients with primary and secondary liver tumors scheduled for radioembolization. Eur J Radiol. 2015;84:201–207. doi: 10.1016/j.ejrad.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Das A, Riaz A, Gabr A, et al. Safety and efficacy of radioembolization with glass microspheres in hepatocellular carcinoma patients with elevated lung shunt fraction: analysis of a 103-patient cohort. Eur J Nucl Med Mol Imaging. 2020;47:807–815. doi: 10.1007/s00259-019-04517-y. [DOI] [PubMed] [Google Scholar]
- 30.Sandow T, DeVun D, Gulotta P, Bohorquez H, Kirsch D. Elevated lung shunt fraction as a prognostic indicator for disease progression and metastasis in hepatocellular carcinoma. J Vasc Interv Radiol. 2016;27:804–811. doi: 10.1016/j.jvir.2016.01.129. [DOI] [PubMed] [Google Scholar]
- 31.Deipolyi AR, Iafrate AJ, Zhu AX, Ergul EA, Ganguli S, Oklu R. High lung shunt fraction in colorectal liver tumors is associated with distant metastasis and decreased survival. J Vasc Interv Radiol. 2014;25:1604–1608. doi: 10.1016/j.jvir.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narsinh KH, Van Buskirk M, Kennedy AS, et al. Hepatopulmonary shunting: a prognostic indicator of survival in patients with metastatic colorectal adenocarcinoma treated with (90)Y radioembolization. Radiology. 2017;282:281–288. doi: 10.1148/radiol.2016152100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seidensticker R, Seidensticker M, Damm R, et al. Hepatic toxicity after radioembolization of the liver using (90)Y-microspheres: sequential lobar versus whole liver approach. Cardiovasc Intervent Radiol. 2012;35:1109–1118. doi: 10.1007/s00270-011-0295-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 47 kb)